Functionalization of carbon nanotubes via Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition “click chemistry”
Sravendra
Rana
and
Jae Whan
Cho
*
Department of Textile Engineering, Konkuk University, Seoul, 143-701, South Korea. E-mail: jwcho@konkuk.ac.kr; Fax: +82 2 457 8895; Tel: +82 2 450 3513
First published on 8th October 2010
Abstract
Functionalization of carbon nanotubes is essential for achieving their mechanical, electrical, and biological functions and enhancing their dispersion in a polymer matrix. Cycloaddition reactions can play a significant role as an emerging route in this direction. This minireview focuses on covalent functionalization of carbon nanotubes using a facile approach via a Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction. Through this reaction, an enormous variety of molecules can be coupled onto carbon nanotubes in a very controlled manner, and may be utilized for many potential applications from nanoelectronics to bio-applications.
 Sravendra Rana | Sravendra Rana was born in Meerut (India) in 1982. He completed his MSc in physical chemistry (2003) from Ch. Charan Singh University, Meerut, India. In that same year, he joined National Chemical Laboratory in Pune, India, where he worked as a project fellow for an Indo-French project. He is currently a PhD student under the supervision of Professor Jae Whan Cho at Konkuk University, Seoul, Korea. His research interests include click chemistry, functionalization of carbon nanotubes, and functional polymer nanostructures and their application for shape memory materials. |
 Jae Whan Cho | Jae Whan Cho received his BSc, MSc, and PhD degrees from Seoul National University, Seoul, Korea in 1979, 1981, and 1987, respectively. He is a professor in Department of Textile Engineering at Konkuk University, Seoul, Korea. Before moving to Konkuk University in 1992, he was an assistant professor of Chonnam National University, Korea. His research focuses on shape memory polymers and nanocomposites, click chemistry and functionalization of carbon nanotubes, electroactive polymer actuators, conducting polymers and fibers, and smart nanofibers and textiles. |
Introduction
Carbon nanotubes (CNTs), the ideal building blocks in nanotechnology, have attracted intense interest from researchers owing to their unmatched electronic, chemical, and mechanical properties.1–3 In order to obtain desired properties, such as metallic or semi-conductivity, single-walled (SWNTs) or multi-walled nanotubes (MWNTs) with different diameters have enhanced their importance in a wide range of applications.4–7 Their outstanding properties, shape and small scale posses a unique combination for using small molecules as assembly molecules in the area of electronics and sensing, as well as in biodevices.8,9However, for pursuing the practical applications of CNTs, the dispersion of CNTs in different matrices continues to present challenges, largely owing to strong interaction between CNTs. A considerable portion of the recent investigations of CNTs have focused on enhancing their dispersion. To improve the dispersion of CNTs, several approaches using covalent and non-covalent functionalization methods have been developed.10–15 These different techniques render CNTs soluble not only in organic solvents,16,17 but also in aqueous media.18,19 The surface modification of CNTs through covalent functionalization is crucial for developing high-performance materials based on CNTs. Controlled CNT functionalization improves their processibility, allowing the retention of their characteristic properties, while covalent functionalization of CNTs disrupts the structural integrity of the π-system of nanotubes, affecting their electronic properties.20,21 Researchers are continuing to find simpler and more inexpensive routes for CNT functionalization in order to enhance their dispersibility.
The functionalization of CNTs via cycloaddition reactions plays an important role in this direction and covers a wide range of addition reactions such as 1,3-dipolar cycloaddtion,22 Huisgen [3 + 2] cycloaddition,23 [4 + 2] Diels–Alder reaction,24 and the [2 + 1] cycloaddition reaction,25etc. CNT functionalization using cycloaddition reactions is a controlled approach having advantages over other functionalization methods such as acid treatment, where structural integrity gets disrupted and fails to achieve a statistical distribution of functional groups onto the surface of CNTs. The present minireview focuses on CNT functionalization using the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction ‘click chemistry’ as a new approach to enhance the dispersibility of CNTs. Since its re-innovation by Sharpless et al., the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction click chemistry has been widely exploited by chemists.26
The Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction between azide and alkyne moieties is the most successful variant, forming a 1,4-substitiuted 1,2,3-triazole (Fig. 1).27 Due to its high regioselectivity and yield, easy reaction conditions, good reliability, and tolerance to a wide range of functional groups, the copper catalyzed Huisgen [3 + 2] cycloaddition reaction has emerged as a strategy for the rapid and efficient assembly of molecules in the industrial and academic realms.28–32 Some examples of CNT materials click-coupled with biomolecules, metal nanoparticles, and polymers for application in biomaterials, electronic materials, nanostructured polymers, and smart hybrid materials are presented. Regarding CNT functionalization, click chemistry may provide an ideal modular methodology by introducing a wide variety of molecules onto the CNT surface.
 | ||
| Fig. 1 Schematic representation of Cu(I)-catalyzed azide alkyne cycloaddition (from ref. 27). | ||
Functionalization of CNTs using the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction
CNT functionalization with macromolecules
Adronov et al. published the first report of CNT functionalization using the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction.23 They discussed the functionalization of SWNTs with polystyrene using a Huisgen cycloaddition reaction, as shown in Fig. 2. To achieve a high degree of functionalization, the alkyne moiety was introduced on the SWNTs’ surface using a Pschorr-type arylation.33 Polystyrene was synthesized using atom transfer radical polymerization (ATRP) and further transformed to azide-terminated polystyrene. The formation of Cu(I)-catalyzed 1,2,3-triazoles by coupling of the azide-terminated polymer and alkyne-functionalized SWNTs was found to occur in an efficient manner under a variety of favorable conditions. This reaction was extremely efficient at low reaction temperatures and with short reaction time, producing organo-soluble polymer–nanotube conjugates with a high graft density and controlled polymer molecular weight. Fig. 3 represents the transmission electron microscopy image, in which individual SWNTs were observed. The presence of such individual SWNTs signifies debundling of SWNTs as a result of polymer functionalization. Water soluble SWNTs have been realized via sulfonation of grafted polystyrene chains.34 Polystyrene-functionalized SWNTs were found to be soluble in an organic medium and completely insoluble in the aqueous layer. However, after polystyrene sulfonation, the sample was completely insoluble in the organic medium and fully soluble in the aqueous medium.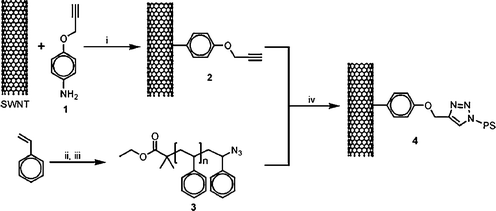 | ||
| Fig. 2 (i) Isoamyl nitrite, 60 °C; (ii) EBiB, CuBr/BPy, DMF, 110 °C; (iii) NaN3, DMF, room temperature; (iv) Cu(I), DMF (from ref. 23). | ||
Polyurethane-grafted carbon nanotubes were synthesized by coupling of alkyne moiety decorated SWNTs with the azide moiety containing polyurethane using click chemistry.35 The azide moiety containing poly(ε-caprolactone)diol was synthesized by ring-opening polymerization and further treated with 4,4′-methylenebis(phenylisocynate) to prepare the azide moiety decorated polyurethane. Due to chemical bond formation between polyurethane and SWNT at some intervals, individual SWNTs were observed. CNTs functionalization could be also controlled by changing the azide functionality in polyurethane, as the azide functionality increased as grafting percentage increased.
Carbon nanotubes functionalized with stimuli-responsive materials are expected to play a key role in environmental stimuli applications.36 Click chemistry has been applied for the preparation of nanostructured polymers composed of CNTs and stimuli-responsive materials. Li et al. prepared covalent functionalized MWNTs with thermoresponsive diblock copolymer micelles using the Huisgen cycloaddition reaction, as shown in Fig. 4.37 The alkyne-functionalized MWNTs were prepared by the reaction of isocyanate functionalized MWNTs with propargyl alcohol. The thermoresponsive diblock copolymer was composed of N,N-dimethylacrylamide (DMA) and N-isopropylacrylamide (NIPAM).38 The copolymer containing hydrophilic DMA as well as a smart NIPAM block is capable of forming micelles that respond to changes in the temperature of the aqueous solution. On the basis of NIPAM block length, the size and transition temperature can be controlled. Due to higher azide concentration on their periphery, micelles afford improved grafting efficiency and solubility of nanotubes, compared to coils in solution.
 | ||
| Fig. 4 Functionalization of MWNTs with PDMA-PNIPAM. (i) DMA, AIBN, dioxane, 60 °C; (ii) NIPAM, AIBN, dioxane, 60 °C; (iii) TDI, toluene, 80 °C; (iv) propargyl alcohol, toluene, 100 °C; (v) sodium ascorbate, copper(II) sulfate pentahydrate, H2O (from ref. 37). | ||
Many types of polymers have been grafted onto CNTs using a “grafting to” and “grafting from” approach via the Cu(I)-catalyzed Huisgen cycloaddition reaction.39 CNTs were covalently functionalized with reactive functional groups, which were used as a macroinitiator for further functionalization as well as in controlled polymerization for decorating amphiphilic polymer brushes on CNTs. Initially, the macroinitiator poly(3-azido-2-(2-bromo-2-methylpropanoyloxy)propyl methacrylate (poly BrAzPMA) was treated with alkynated CNTs using click coupling, yielding bromo as well as azido groups on the CNTs’ surface. ATRP was then applied to graft poly(n-butyl methacrylate) (poly[nBMA]) from the bromo moiety decorated CNTs. Finally, hydrophilic alkynated PEG was linked with azido group functionalized CNTs through the Huisgen cycloaddition reaction. Another route for CNT functionalization was also applied wherein the hydrophilic PEG was initially reacted with macoinitiator functionalized CNTs, followed by the grafting of polystyrene. The reaction could be easily accomplished with SWNTs and MWNTs. The most important aspect here is that both ATRP and click coupling could be achieved by a one-pot procedure, which would play a major role for functionalizing multifunctional surfaces such as polymer brushes. The same coupling reaction has been further employed to achieve the layer-by-layer functionalization of MWNTs (Fig. 5).40 Layer-by-layer is a facile approach to modify precisely the surfaces of diverse substrates. Even three-layer-functionalized CNTs can be further modified in a controlled manner, which enhances their importance as a nanoplatform for molecular design and material synthesis. The accessibility of functional groups is useful to form crosslinked polymer networks, which offer several advantages, such as high stability and good control over the quantity and thickness of the polymeric layers. Poly(2-azidoethyl methacrylate),41 was clicked with pretreated alkyne-modified MWNTs as the first polymeric layer. Poly(propargyl methacrylate) synthesized by reverse addition–fragmentation chain transfer (RAFT) polymerization containing essential alkyne side groups was subsequently coated as the second polymeric layer via click coupling. To prepare the third polymeric layer, the two layers of functionalized MWNTs were treated with poly(2-azidoethyl methacrylate). The three layers of functionalized azide moiety containing MWNTs were postmodified with alkyne moiety modified rhodamine B and alkyne terminated polystyrene via the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction.
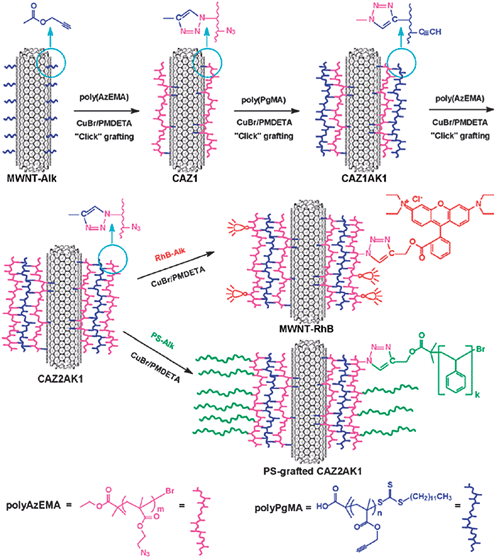 | ||
| Fig. 5 Functionalization of MWNTs by the LbL-CC approach and further modification of the functionalized MWNTs with fluorescent dye and polystyrene by click chemistry (from ref. 40). | ||
CNT functionalization with biomolecules
The click reaction also has been used for functionalization of CNTs with biological molecules to facilitate the use of nanotechnology in bio-applications, especially in drug delivery. Zheng et al. reported a β-cyclodextrin-modified SWNT nanohybrid through Huisgen cycloaddition.42 β-Cyclodextrin, an oligosaccharide, is well known to encapsulate biological molecules in its hydrophobic cavities in an aqueous solution, enhancing its utility as a drug carrier and enzyme mimic. Purified SWNTs were reacted with p-(2-propynyloxy)-benzenamine in o-dichlorobenzene (ODCB) using a diazotization-coupling procedure to produce alkyne-functionalized SWNTs and further treated with azide-functionalized cyclodextrin via Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition. The β-cyclodextrin functionalized SWNTs show good solubility in water, enhancing their biological importance for drug delivery applications. Cho et al. focused on the functionalization of SWNTs with bioactive molecules using a click chemistry approach.43 A series of well-defined chiral azides from corresponding amino acids were prepared by converting their acid part to alcohol and further converting them into azido derivatives. Azides derived from different amino acids were coupled with alkyne-functionalized SWNTs through the 1,2,3-triazole ring using the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction between alkynes and an excess of azides. As a result, a high degree of functionality on SWNTs was obtained. The applied route would be a very effective tool for functionalization of SWNTs with various biomolecules such as peptides and polysaccharides.Functionalization of CNTs with biocompatible polymers showed enormous potential in tissue engineering.44 Poly(ε-caprolactone) (PCL),45,46 a biodegradable and biocompatible polymers have been studied the most for incorporation of CNTs.47 MWNT-PCL-based composite materials may be very useful for bionanomaterials with multifunctional applications. PCL functionalized MWNTs were synthesized using click chemistry between alkyne-functionalized MWNTs and poly(α-azide-ε-caprolactone-co-ε-caprolactone) which was synthesized using ring-opening polymerization.48 Then the molecular weight of PCL was found the most crucial factor beyond CNT dispersion. Thermogravimetric analysis revealed that the weight loss increased from 27 to 52% with increasing of the molecular weight of PCL from 1300 to 3000 g mol−1, and further decreased to 24 wt%, when the molecular weight increased to 9000 g mol−1.
CNT functionalization with metal nanohybrids
Due to their synergetic properties, carbon nanotube and metal particle based hybrid nano-materials play important roles in several application areas, including electronic, optical, catalytic, and magnetic applications.49,50 Rao et al. have synthesized a novel material by functionalization of SWNTs with gold nanocrystals.51 They achieved SWNT functionalization with amidobutane containing a terminal azido group and further treated with Au nanocrystals capped with the hex-5-yne-1-thiol. This reaction yielded a SWNT–Au nanomaterial, in which the gold nanocrystals decorated the SWNTs.The properties of SWNTs depend on their metallic or semiconducting behavior. The Raman spectra have been studied for SWNTs functionalized with gold and platinum nanoparticles by microwave treatment or by click coupling.52 The G-band as well as radical breathing mode (RBM) were used to obtain the reliable proportion of the semiconducting and metallic species. For calculating the ratio of the metallic to the semiconducting species, the ratio of the areas of the 1540 (metallic, M) and 1580 cm−1 (semiconducting, S) bands were used. The M/S values showed an increase in the proportion of the metallic species by 20–100% on attachment of gold or platinum nanoparticles. The RBM band results also indicate a definitive increase in the proportion of metallic species upon interaction of SWNTs with metallic nanoparticles. The M/S ratio for SWNTs coated with nanoparticles is higher for the click reaction compared to microwave treatment. A further study was carried out by Cho et al.53 wherein they achieved functionalization of SWNTs by gold nanoparticles through the Huisgen cycloaddition reaction. Alkyne-functionalized SWNTs were prepared using the solvent-free diazotization procedure, as previously discussed.43 Gold nanoparticles containing octanethiol moieties were prepared by the reduction of tetrachloroauric acid using sodium borohydride in the presence of alkanethiol.54 The alkyl thiol-protected gold nanoparticles were further treated with azidoundecane-thiol to yield the azide-moiety containing gold nanoparticles. As a strategy for the attachment of metal nanoparticles, the 1,2,3-triazole ring was utilized as a linker between the azide-decorated nanoparticles and alkyne-functionalized SWNTs.
Gao et al. reported the preparation of nanohybrids from Fe3O4 nanoparticles and polymer-coated MWNTs (Fig. 6).55 For controlling the particle size, first they prepared mono-dispersed nanoparticles of the desired size which were then coupled with other nanomaterials. Water soluble poly(acrylic acid)-capped Fe3O4 nanoparticles (Fe3O4–COOH) were prepared according to the reported procedure.56 The carboxylic functionality containing Fe3O4 nanoparticles were coupled with 3-azidopropan-1-amine to yield azide-functionalized Fe3O4 nanoparticles (Fe3O4–N3). The MWNTs were separately treated with polymer containing an abundant level of alkyne groups (MWNTs-pAlk).39 The coated polymer layer could proliferate surface reactive groups as well as decrease the solid hindrance and collision energy when the two particles touched each other. Fe3O4–N3 were coupled with MWNTs-pAlk using Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition. The covalently coupled nanohybrids were characterized by very stable nanomaterials even under ultrasonication and were found well dispersed in water and in common organic solvents. The catalyst supported on CNT showed higher selectivity compared to a catalyst supported on commercial carbon.57 Rode et al. found that the CNT supported catalyst showed good stability behavior when recycled. Click coupling could play a major role for recycling the catalyst, where the triazole ring works as a strong linker between CNT and catalyst.
 | ||
| Fig. 6 The synthesis procedure of clicked magnetic nanohybrids: (i) (1) H2SO4–HNO3, 90–133 °C, 100 min; (2) SOCl2, 60 °C, 24 h; (3) propargyl alcohol, triethylamine, CHCl3, r.t., 24 h (from ref. 55). | ||
CNT functionalization with electron donor/acceptor molecules
Photo-induced processes between SWNTs and electron donor and electron acceptor or photoisomerizable molecules are important features for fabrication of optoelectric devices. Hybrid materials based on phthalocyanines and nanotubes are highly attractive for application in this particular research area.58,59 Campidelli et al. used the Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition reaction to synthesize phthalocyanine functionalized SWNTs.60The alkylation of SWNTs, which is an essential functionality for click coupling, was accomplished using purified SWNTs with 4-(2-trimethylsilyl)ethynylaniline in the presence of isoamyl nitrite, which were further treated with azide moiety containing zinc phthalocyanine (ZnPc) in the presence of CuSO4 and sodium ascorbate to give a nanotube–phthalocyanine assembly. Photo-induced communication between the two photoactive components (i.e. SWNT and ZnPc) was also identified. These features are helpful in incorporating the SWNT-ZnPc hybrid in a photoelectrochemical cell as a photoactive material in an ITO photoanode (Fig. 7). Porphyrin is an easily synthesizable and highly stable molecule possessing exceptional optical and electrical properties, has been largely used for preparing electron donor–acceptor hybrids via covalent and non-covalent means.61,62 Recently, the functionalization of SWNTs with zinc porphyrins (ZnP) using click chemistry has been reported.63 Purified SWNTs were treated with 4-(2- trimethylsilyl) ethynylbenzenediazonium in the presence of isoamyl nitrite to produce alkynated SWNTs. Then, the alkynated SWNTs were coupled with porphyrin derivatives in the presence of Cu(MeCN)4PF6, 2,6-lutidine, and tris- (hydroxypropyltriazolylmethyl)amine to yield SWNT-ZnP. Strong electronic coupling between the photo- and electroactive constituents led to rapid excited-state deactivation of ZnP via the charge transfer to the nanotubes. Photophysical assays by means of steady-state reveal that the selective photoexcitation of ZnP derivatives is followed by a rapid charge separation, namely, the formation of reduced SWNT and oxidized ZnP.
 | ||
| Fig. 7 Schematic representation of the photoelectrochemical cell used for the measurements (from ref. 60). | ||
Future scope
There are still opportunities and challenges to find simpler and more inexpensive route to improve the dispersion of CNTs and their applications. Selective CNT functionalization using click chemistry may satisfy such a purpose, even though it still requires more attention, as the design of azide and alkyne groups requires multi-step reactions. The presented click methodology offers a new thoroughfare, with opportunities to develop the practical applications of functionalized CNTs and enhance their significance in nanotechnology.Conclusions
The functionalization of CNTs using facile and promising Cu(I)-catalyzed Huisgen [3 + 2] cycloaddition is expected to play an important role in expanding their application from nanoelectronics to bio-nanotechnology. There are many advantages of Cu(I)-catalyzed azide-alkyne cycloaddition, such as high yield, purity, and controlled functionalization nature, as well as mild reaction conditions even at room temperature and in water medium under non-toxic conditions. Click chemistry is very useful for modifying the surface properties of CNTs through various functionalities to satisfy special needs (Table 1). For example, controlled metal nanoparticles and functionalized CNTs can represent a new class of advanced materials for catalytic applications due to specific strong metal support interactions, which can be useful to enhance catalytic activity and catalyst recovery. The methodology offers a novel and simple approach for achieving more advanced biomaterials, nanostructured polymers, and hybrids based on CNTs.| Catalyst/reagent | Nanotube type | Polymer/inorganic molecules | Ref. |
|---|---|---|---|
| CuI/1,8-diazabicyclo [5.4.0] undec-7-ene | SWNT-alkyne | Polystyrene–N3 | 23,34 |
| Polyurethane–N3 | 35 | ||
| β-cyclodextrin–N3 | 42 | ||
| MWNT-alkyne | Poly(ε-caprolactone)–N3 | 48 | |
| CuBr/N,N,N′,N′′,N′′-pentamethyldiethylenetriamine | MWNT-alkyne | Poly(ethyl methacrylate)–N3, polystyrene–alkyne, Rhodamine B–alkyne, poly(propargyl methacrylate)–alkyne | 40 |
| MWNT-alkyne, MWNT-N3 | Fe3O4–N3, Fe3O4–alkyne | 55 | |
| MWNT-alkyne, SWNT-alkyne MWNT-N3, SWNT-N3 | Poly(ethylene glycol)–alkyne, Poly(glycidyl methacrylate)–N3 | 39 | |
| CuSO4·5H2O/sodium ascorbate | MWNT-alkyne | Poly(N,N-dimethylacrylamide)–poly(N-isopropylacrylamide)–N3 | 37 |
| SWNT-alkyne | Au nanoparticles–N3 | 53 | |
| Phthalocyanine–N3 | 60 | ||
| SWNT-N3 | Au nanocrystal–alkyne | 51 | |
| Au nanoparticle–alkyne | 52 | ||
| (PPh3)3CuBr | SWNT-alkyne | Polystyrene–N3 | 23 |
| CuI/ascorbic acid/N,N′-diisopropylethylamine | SWNT-alkyne | Aminoacid–N3 | 43 |
| Cu(MeCN)4PF6/2,6-lutidine/tris-(hydroxypropyltriazolylmethyl)amine | SWNT-alkyne | Porphyrin–N3 | 63 |
Acknowledgements
This work was supported by the grant from Seoul Future Contents Convergence (SFCC) Cluster established by Seoul R&BD Program (ST090826) and the Industrial technology development program of the Ministry of Knowledge Economy (MKE) of Korea (No KEIT 10033449-2009-11).Notes and references
- P. G. Collins, A. Zettl, H. Bando, A. Thess and R. E. Smalley, Science, 1997, 278, 100 CrossRef CAS.
- S. Reich, C. Thomsen and J. Maultzsch, Carbon Nanotubes: Basic Concepts and Physical Properties, Wiley-VCH, Weinheim, 2004 Search PubMed.
- S. J. Tans, M. H. Devoret, H. J. Dai, A. Thess, R. E. Smalley, L. J. Geerligs and C. Dekker, Nature, 1997, 386, 474 CrossRef CAS.
- (a) L. Dai and A. W. H. Mau, Adv. Mater., 2001, 13, 899 CrossRef CAS; (b) H. Dai, Acc. Chem. Res., 2002, 35, 1035 CrossRef CAS.
- (a) B. S. Harrison and A. Atala, Biomaterials, 2007, 28, 344 CrossRef CAS; (b) A. Bianco, K. Kostarelos and M. Prato, Curr. Opin. Chem. Biol., 2005, 9, 674 CrossRef CAS.
- M. Yutaka, H. Masahiro, K. Shinya, K. Makoto, H. Tadashi, T. Takahiro, A. Takeshi, N. Yasuhisa, S. Tetsuo, T. Hitoshi, L. Jing and N. Shigeru, J. Mater. Chem., 2008, 18, 4189 RSC.
- J. Lee, H. Kim, S. J. Kahng, G. Kim, Y. W. Son, J. Ihm, H. Kato, Z. W. Wang, T. Okazaki, H. Shinohara and Y. Kuk, Nature, 2002, 415, 1005 CrossRef CAS.
- P. Avouris, Z. Chen and V. Perebeinos, Nat. Nanotechnol., 2007, 2, 605 CrossRef CAS.
- N. W. S. Kam and H. Dai, J. Am. Chem. Soc., 2005, 127, 6021 CrossRef CAS.
- S. Niyogi, M. A. Hamon, H. Hu, B. Zhao, P. Bhowmik, R. Sen, M. E. Itkis and R. C. Haddon, Acc. Chem. Res., 2002, 35, 1105 CrossRef CAS.
- S. Banerjee, H. T. Benny and S. S. Wong, Adv. Mater., 2005, 17, 17 CrossRef CAS.
- R. J. Chen, S. Bangsaruntip, K. A. Drouvalakis, N. W. S. Kam, M. Shim, Y. M. Li, W. Kim, P. J. Utz and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 4984 CrossRef CAS.
- S. Rana, N. Karak, J. W. Cho and Y. H. Kim, Nanotechnology, 2008, 19, 495707 CrossRef.
- J. L. Bahr and J. M. Tour, J. Mater. Chem., 2002, 12, 1952 RSC.
- S. H. Liao, C. Y. Yen, C. H. Hung, C. C. Weng, M. C. Tsai, Y. F. Lin, C. C. M. Ma, C. Pan and A. Su, J. Mater. Chem., 2008, 18, 3993 RSC.
- (a) V. Georgakilas, K. Kordatos, M. Prato, D. M. Guldi, M. Holzinger and A. Hirsch, J. Am. Chem. Soc., 2002, 124, 760 CrossRef CAS; (b) A. Hirsch, Angew. Chem., Int. Ed., 2002, 41, 1853 CrossRef CAS.
- Y. T. Liu, W. Zhao, Z. Y. Huang, Y. F. Gao, X. M. Xie, X. H. Wang and X. Y. Ye, Carbon, 2006, 44, 1613 CrossRef CAS.
- P. Wu, X. Chen, N. Hu, U. C. Tam, O. Blixt, A. Zettl and C. R. Bertozzi, Angew. Chem., Int. Ed., 2008, 47, 5022 CrossRef CAS.
- A. D. Crescenzo, D. Demurtas, A. Renzetti, G. Siani, P. D. Maria, M. Meneghetti, M. Prato and A. Fontana, Soft Matter, 2009, 5, 62 RSC.
- J. Cabana and R. Martel, J. Am. Chem. Soc., 2007, 129, 2244 CrossRef CAS.
- L. Cognet, D. A. Tsyboulski, J. D. R. Rocha, C. D. Doyle, J. M. Tour and R. B. Weisman, Science, 2007, 316, 1465 CrossRef CAS.
- V. Georgakilas, N. Tagmatarchis, D. Pantarotto, A. Bianco, J. P. Briand and M. Prato, Chem. Commun., 2002, 3050 RSC.
- H. Li, F. Cheng, A. M. Duft and A. Adronov, J. Am. Chem. Soc., 2005, 127, 14518 CrossRef CAS.
- G. Sakellariou, H. Ji, J. W. Mays, N. Hadjichristidis and D. Baskaran, Chem. Mater., 2007, 19, 6370 CrossRef CAS.
- J. Li, G. Jia, Y. Zhang and Y. Chen, Chem. Mater., 2006, 18, 3579 CrossRef CAS.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef CAS.
- J. A. Johnson, M. G. Finn, J. T. Koberstein and N. J. Turro, Macromol. Rapid Commun., 2008, 29, 1052 CrossRef CAS.
- R. F. Service, Science, 2008, 320, 868 CrossRef CAS.
- J. F. Lutz, Angew. Chem., Int. Ed., 2007, 46, 1018 CrossRef CAS.
- P. Antoni, D. Nystrom, C. J. Hawker, A. Hult and M. Malkoch, Chem. Commun., 2007, 2249 RSC.
- H. Nandivada, X. Jiang and J. Lahann, Adv. Mater., 2007, 19, 2197 CrossRef CAS.
- N. Kaval, D. Ermolatev, P. Appukkuttan, W. Dehaen, C. O. Kappe and E. V. Eycken, J. Comb. Chem., 2005, 7, 490 CrossRef CAS.
- C. A. Dyke and J. M. Tour, J. Am. Chem. Soc., 2003, 125, 1156 CrossRef CAS.
- H. Li and A. Adronov, Carbon, 2007, 45, 984 CrossRef CAS.
- S. Rana, J. W. Cho and I. Kumar, J. Nanosci. Nanotechnol., 2010, 10, 5700 CrossRef.
- C. Y. Hong and C. Y. Pan, J. Mater. Chem., 2008, 18, 1831 RSC.
- J. Liu, Z. Nie, Y. Gao, A. Adronov and H. Li, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7187 CrossRef CAS.
- A. J. Convertine, B. S. Lokitz, Y. Vasileva, L. J. Myrick, C. W. Scales, A. B. Lowe and C. L. McCormick, Macromolecules, 2006, 39, 1724 CrossRef CAS.
- Y. Zhang, H. He and C. Gao, Macromolecules, 2008, 41, 9581 CrossRef CAS.
- Y. Zhang, H. He, C. Gao and J. Wu, Langmuir, 2009, 25, 5814 CrossRef CAS.
- B. S. Sumerlin, N. V. Tsarevsky, G. Louche, R. Y. Lee and K. Matyjaszewski, Macromolecules, 2005, 38, 7540 CrossRef CAS.
- Z. Guo, L. Liang, J. J. Liang, Y. F. Ma, X. Y. Yang, D. M. Ren, Y. S. Chen and J. Y. Zheng, J. Nanopart. Res., 2008, 10, 1077 CrossRef CAS.
- I. Kumar, S. Rana, C. V. Rode and J. W. Cho, J. Nanosci. Nanotechnol., 2008, 8, 3351 CrossRef CAS.
- B. S. Harrison and A. Atala, Biomaterials, 2007, 28, 344 CrossRef CAS.
- T. Nie, Y. Zhao, Z. Xie and C. Wu, Macromolecules, 2003, 36, 8825 CrossRef CAS.
- W. J. Li, R. Tuli, C. Okafor, A. Derfoul, K. G. Danielson, D. J. Hall and R. S. Tuan, Biomaterials, 2005, 26, 599 CrossRef CAS.
- H. Zeng, C. Gao and D. Yan, Adv. Funct. Mater., 2006, 16, 812 CrossRef CAS.
- S. Rana, H. Y. Yoo, J. W. Cho, B. C. Chun and J. S. Park, J. Appl. Polym. Sci., 2010 DOI:10.1002/app.31268.
- V. Georgakilas, D. Gournis, V. Tzitzios, L. Pasquato, D. M. Guldi and M. Prato, J. Mater. Chem., 2007, 17, 2679 RSC.
- G. G. Wildgoose, C. E. Banks and R. G. Compton, Small, 2006, 2, 182 CrossRef CAS.
- R. Voggu, P. Suguna, S. Chandrasekaran and C. N. R. Rao, Chem. Phys. Lett., 2007, 443, 118 CrossRef CAS.
- R. Voggu, S. W. Pal, S. K. Pati and C. N. R. Rao, J. Phys.: Condens. Matter, 2008, 20, 215211 CrossRef.
- S. Rana, I. Kumar, H. J. Yoo and J. W. Cho, J. Nanosci. Nanotechnol., 2009, 9, 3261 CrossRef CAS.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801 RSC.
- H. He, Y. Zhang, C. Gao and J. Wu, Chem. Commun., 2009, 1655 RSC.
- J. P. Ge, Y. X. Hu, M. Biasini, C. L. Dong, J. H. Guo, W. P. Beyermann and Y. Yin, Chem.–Eur. J., 2007, 13, 7153 CrossRef CAS.
- J. M. Nadgeri, A. C. Garade, R. A. Tambe, S. P. Gokhale and C. V. Rode, Adv. Sci. Lett., 2010, 3, 313 Search PubMed.
- G. Torre, W. Blau and T. Torres, Nanotechnology, 2003, 14, 765 CrossRef CAS.
- B. Ballesteros, S. Campidelli, G. Torre, C. Ehli, D. M. Guldi, M. Prato and T. Torres, Chem. Commun., 2007, 2950 RSC.
- S. Campidelli, B. Ballesteros, A. Filoramo, D. D. Dıaz, G. Torre, T. Torres, G. M. A. Rahman, C. Ehli, D. Kiessling, F. Werner, V. Sgobba, D. M. Guldi, C. Cioffi, M. Prato and J. P. Bourgoin, J. Am. Chem. Soc., 2008, 130, 11503 CrossRef CAS.
- D. Baskaran, J. W. Mays, X. P. Zhang and M. S. Bratcher, J. Am. Chem. Soc., 2005, 127, 6916 CrossRef CAS.
- F. Cheng and A. Adronov, Chem.–Eur. J., 2006, 12, 5053 CrossRef CAS.
- T. Palacin, H. L. Khanh, B. Jousselme, P. Jegou, A. Filoramo, C. Ehli, D. M. Guldi and S. Campidelli, J. Am. Chem. Soc., 2009, 131, 15394 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |

