Progress on chemical modification of cellulose in “green” solvents
Wenjiao
Ge
,
Jianbo
Shuai
,
Yuyuan
Wang
,
Yuxi
Zhou
and
Xiaohui
Wang
 *
*
State Key Laboratory of Pulp and Paper Engineering, South China University of Technology, Guangzhou 510640, China. E-mail: fewangxh@scut.edu.cn
First published on 21st December 2021
Abstract
Cellulose is an excellent candidate for the fabrication of sustainable materials owing to its good availability, renewability, biodegradability and biocompatibility. Chemical modification is an appealing way to broaden the utilization of cellulose. However, the poor solubility of cellulose in water and common organic solvents restricts chemical modification of cellulose to heterogeneous conditions or in a few limited specific systems. In recent years, advances have been made in more efficient and greener solvents for cellulose. Some of them have greatly changed the chemical modification of cellulose, and their influence on the future cellulose industry is anticipated. In this review, novel “green” solvents for cellulose dissolution, including ionic liquids (ILs), deep eutectic solvents (DESs), aqueous alkali/urea solutions and aqueous quaternary onium hydroxides (QOHs) are introduced. Recent advancements in chemical modification of cellulose in these solvents, especially those made in the past five years, are highlighted and discussed.
1. Introduction
Cellulose, as the most abundant biobased polymer, is widely distributed in diverse natural sources including plants, tunicates and microorganisms.1–3 Structurally, cellulose is a regular and linear polymer consisting of D-glucose subunits linked by β-1,4-glycosidic bonds with strong inter- and intramolecular hydrogen bonds (Scheme 1).4,5 Each cellulose chain has a reducing end behaving as an aldehyde form and a non-reducing end equipped with secondary hydroxyl groups.6 With a number of advantages like renewability, biodegradability, biocompatibility, non-toxicity, low cost, thermal and chemical stability,7,8 cellulose is considered an ideal feedstock for the production of materials, chemicals and biofuels.9 Unfortunately, owing to its extensive hydrogen bonding and high crystalline, cellulose can hardly be dissolved in common organic solvents and water. Furthermore, cellulose exhibits a melting point that is higher than its degradation temperature.10 Both characteristics seriously hamper the utility and processing of cellulose.Chemical modification is one way to improve the solubility and processability of cellulose.8 It provides a means of conferring new properties on cellulose without destroying its many intrinsic properties.11 Industrially, the synthesis of cellulose derivatives [e.g., cellulose acetate (CA), carboxymethyl cellulose (CMC), methyl cellulose (MC), nitrocellulose] still employs a heterogeneous reaction approach.12,13 The heterogeneous route involves a direct modification of cellulose under poor solvent media with insolubilization or incomplete solubilization of cellulose. The reaction process is often limited to the surface of cellulose and is hard to control, which is associated with unwanted side reactions, degradation of cellulose, a limited degree of substitution (DS) and large amounts of waste.14 On the contrary, the homogeneous approach contributes to achieving better controllable reactions with a high conversion rate.8 Cellulose molecules in homogeneous media are assumed to have full and equal accessibility of all hydroxyl groups for chemical reagents. However, efficient dissolution of cellulose is a prerequisite for homogeneous reactions. Hence, the development of cellulose solvents is an urgent need as well as a long-term goal in the cellulose research area.
In this regard, many attempts have been carried out to search for suitable solvent systems for cellulose dissolution and modification. Traditional solvents for cellulose dissolution involve several types of derivatizing solvents (e.g., CS2/NaOH) and non-derivatizing solvents [e.g., N-methylmorpholine-N-oxide (NMMO), N,N-dimethylacetamide/lithium chloride (DMAc/LiCl), tetrabutylammonium fluoride trihydrate/DMSO (TBAF/DMSO)]. However, these solvents are accompanied by significant drawbacks involving a cumbersome operation process, high energy consumption, environmental issues and inefficient recyclability.15,16 Sustainable development has promoted research into more efficient and greener substitutes for cellulose dissolution.17 In recent years, novel non-derivatizing solvent systems for cellulose dissolution have been developed, and they can be subdivided into two categories: non-aqueous solvents [i.e., ionic liquids (ILs), deep eutectic solvents (DESs)] and aqueous solvents [i.e., alkali/urea, quaternary onium hydroxides (QOHs)]. These solvents can serve as desired media for cellulose derivatization, and provide a versatile platform to fabricate functional cellulose materials. Here, the main objective of this review is to focus on recent advancements in these novel green solvents for chemical modification of cellulose. Comments on the ongoing research and perspectives on future directions of cellulose modification in green solvents will also be given.
2. A brief overview of green solvents for cellulose dissolution
Cellulose contains abundant hydroxyl groups which are easy to form extensive inter- and intramolecular hydrogen bonding. The hydrogen bonded supramolecular structure of cellulose makes it difficult to dissolve in either aqueous solutions or conventional organic solvents. To address this issue, numerous solvent systems for cellulose classified as derivatizing and non-derivatizing solvents have been developed. The dissolution process of derivatizing solvents involves the formation of derivatized cellulose intermediates and subsequent dissolution.18 A typical example of derivatizing solvents is the CS2/NaOH system which has been used on an industrial scale to produce viscose fibers. However, the viscose process is accompanied by a massive discharge of hazardous by-products (e.g., CS2, H2S, heavy metals).19 Non-derivatizing solvents, such as NMMO, are available for direct dissolution of cellulose without any derivatization. Generally, non-derivatizing solvents are more eco-friendly than derivatizing ones, because less by-products of chemical reaction are involved.20 Other solvents combining organic solvents with salts, like DMAc/LiCl, TBAF/DMSO, are well developed but have environmental concerns such as high toxicity and volatility.10Solvents are critical in the cellulose industry. As discussed above, some conventional solvents of cellulose bear several health and environmental risks. Therefore, continuing academic efforts have been made aiming to replace them with greener solvents. According to the principle of green chemistry, a green solvent should be of low waste production, less hazardous chemical syntheses, high safety, and small environmental impact. The sustainability of a solvent should also be related to recyclability, availability, stability, flammability, efficiency, and cost. Based on these considerations, we mainly introduce four types of “green” non-derivatizing solvents for cellulose in this minireview, and subdivide them into two categories, namely, non-aqueous solvents (i.e., ILs, DESs) and aqueous solvents (i.e., alkali/urea, QOHs).
2.1. Non-aqueous non-derivatizing solvents for cellulose
Recently, a combination of two single ILs is revealed to show improved dissolution capacity for cellulose. A mixture of [EMIM][Cl] and [EMIM][OAc] with 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 mol/mol was exploited to display comparable solubility (40 g per 100 g of solvent) to individual ILs such as [EMIM][Cl], [EMIM][OAc], and [BMIM][Cl].34 Moreover, research has shown that adding certain highly dipolar aprotic co-solvents (e.g., DMSO, DMF, and DMAc) into ILs can lower the viscosity of the solvent system and promote cellulose dissolution.35–41 It can be anticipated that ILs co-solvent will become an important direction in the future. Although a large number of articles and reviews have been published, most of them are devoted to the dissolution of cellulose, the attention in cellulose modification is still scarce. In addition, some critical issues associated with the industrialization of ILs in cellulose processing and modification, including the sensitivity to water, the feasibility to recycle, and the high viscosity, still need to be addressed.
70 mol/mol was exploited to display comparable solubility (40 g per 100 g of solvent) to individual ILs such as [EMIM][Cl], [EMIM][OAc], and [BMIM][Cl].34 Moreover, research has shown that adding certain highly dipolar aprotic co-solvents (e.g., DMSO, DMF, and DMAc) into ILs can lower the viscosity of the solvent system and promote cellulose dissolution.35–41 It can be anticipated that ILs co-solvent will become an important direction in the future. Although a large number of articles and reviews have been published, most of them are devoted to the dissolution of cellulose, the attention in cellulose modification is still scarce. In addition, some critical issues associated with the industrialization of ILs in cellulose processing and modification, including the sensitivity to water, the feasibility to recycle, and the high viscosity, still need to be addressed.
2.2. Aqueous non-derivatizing solvents for cellulose
2.3. Comment and comparison of the green solvents
ILs, DESs, alkali/urea, and QOHs represent the most prevalent solvents for cellulose dissolution, processing, and modification in the past decade. Compared with conventional solvents, they all present some characteristics of green chemistry, like low vapor pressure, nonflammability, and good stability. Among them, the alkali/urea systems are prominent in terms of availability, cost, and hazard potential. Before this system comes onto the market, there are many problems that need to be resolved. The relatively low solubility of high molecular weight raw materials remains a challenge. Other challenges come from the difficulty of industrialization with low temperature treatment, high energy consumption, and special instruments. From a scientific point of view, the influence of hydrogen bond network on the dissolution and reaction behavior of cellulose in alkali/urea is not clear. QOHs present a more powerful aqueous solvent of cellulose. Concerns about QOHs may come from the required high concentration, which not only leads to increased cost, but also impacts the chemical reactions in them. For example, the effect of salts is significant in many reactions. Compared with other green solvents, ILs are much more studied as reaction media for cellulose modification due to their good stability and dissolving capability. ILs are the ones most possibly being utilized in the controlled synthesis of cellulose derivatives with precisely determined structures. For future practical applications, the cost and viscosity of ILs need to be greatly reduced. DESs have shown exciting potential. The advantage of DESs majorly comes from their low cost and ease of synthesis. However, studies on the dissolution and modification of cellulose in DESs are very rare. Much more academic effort should be put into DESs in the future.3. Chemical modification of cellulose in green solvents
According to its molecular structure, cellulose comprises of repetitive anhydroglucose units (AGU) which are covalently linked through acetal functions between the OH group of C4 and C1 carbon atoms.69 Each glucose repeating unit of the cellulose chain has three hydroxyl groups, namely, a primary hydroxyl group at C6 position, and secondary hydroxyl groups at C2 and C3 positions. These hydroxyl groups along macromolecule chains are readily available for chemical modification via reacting with functional groups, thus contributing to a variety of cellulose derivatives. Furthermore, the reactivity of the three hydroxyl groups in AGU is diverse, which is affected by inherent reactive ability, reaction media, steric effects of reagents, and steric effects of the supramolecular structure of cellulose.11 To date, numerous cellulose derivatives (e.g., esters, ethers, carbamates, carbonates) have been synthesized in the aforementioned solvent systems via esterification, acylation, grafting, etherification, and carbalination.3.1. Chemical modification of cellulose in ionic liquids
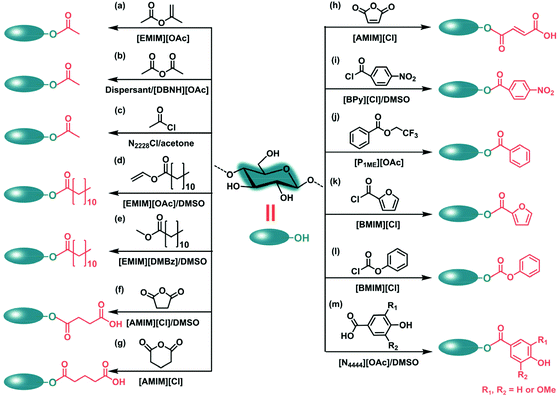
Cellulose acetate (CA), a frequently studied aliphatic cellulose ester, can be synthesized by acetylation of cellulose with reagents such as acetyl chloride (AcCl), acetic anhydride (Ac2O), isopropenyl acetate (IpeAc), vinyl acetate (VinAc) in the absence or presence of a catalyst. In this regard, the reaction can be performed in various ILs including 1-allyl-3-methylimidazolium chloride ([AMIM][Cl]),71,72 1-N-butyl-3-methylimidazolium chloride ([BMIM][Cl]),73,74 1-N-ethyl-3-methylimidazolium chloride ([EMIM][Cl]),73 1-ethyl-3-methyl-imidazolium acetate ([EMIM][OAc]),75,76 triethyloctylammonium chloride (N2228Cl),77 1,5-diazabicyclo[4.3.0]non-5-ene acetate [DBNH][OAc]78,79 and 1,8-diazabicyclo[5.4.0]undec-7-ene acetate [DBUH][OAc].80 Such solvents exhibit good cellulose solubilization behavior and some of them even can act as organocatalysts. As an example, Kakuchi et al. took advantage of the dual function of [EMIM][OAc], namely dissolving capacity and organocatalytic property, for the facile acetylation of cellulose (Scheme 2a).75 The reaction with IpeAc in the IL media can proceed smoothly and rapidly to obtain highly substituted cellulose triacetate (DS = 2.96). Kakko et al. demonstrated a superbase-derived IL (i.e., [DBNH][OAc]) for rapid and efficient acetylation of cellulose with Ac2O, IpeAc, VinAc. They carried out the reactions under mild conditions (60–70 °C, 1 h) without the assistance of catalysts.78 It was found that acetylation with IpeAc produced CA with a DS as high as 2.97, whereas the use of vinylic reagents resulted in CA products with a maximum DS of 1.58.
Besides individual IL systems, researchers also applied binary mixtures of ILs and co-solvents as acylation media. The co-solvents typically include DMSO, DMF, DMAc, acetonitrile (ACN) and acetone, which are supposed to promote the acetylation process. A gram-scale production of polysaccharide (i.e., cellulose, xylan, dextrin, pillulan) acetates was achieved in [EMIM][OAc] combined with a co-solvent of DMSO.76 The co-solvent acted as an efficient accelerator for the organocatalytic ability of the IL, is mainly due to nucleophilic assistance by IL anions of alcoholic species.81 Synthesis of CA in binary solvents of [DBNH][OAc] and dispersants (i.e., DMSO, DBN, ACN and acetone) was studied by Jogunola et al., as shown in Scheme 2b.79 In the presence of ACN, the DS of CA reached 2.68 within 0.5 h using 5 equiv./AGU at 80 °C. However, if no co-solvent was added, similar conditions only resulted in a DS of 0.9 after 3.0 h. Hanabusa et al. prepared nine protic ILs composed of amidine and acetic acid for cellulose dissolution, and validated that [DBUH][OAc] exhibited the best catalytic performance for the acetylation of cellulose.80 The addition of DMSO led to the DS of CA increasing from 2.61 to 2.87. An ammonium-based IL (N2228Cl) in combination with acetone was employed for homogeneous acetylation (Scheme 2c). The synthesized CA showed a maximum DS value of 2.79.77
By adjusting the types of reactants, cellulose esters with diverse aliphatic groups can be obtained. Among them, vinyl esters are frequently used as acylating agents for the synthesis of aliphatic cellulose esters. As an example, vinyl ester-based modification of cellulose was carried out in [EMIM][OAc] without external catalysts.82 Accordingly, aliphatic groups with chain lengths of C8 to C16 were introduced to the cellulose backbone. Vinyl laurate modified cellulose with a DS of 2.4 was obtained at 80 °C for 4 h with a molar ratio of 3 equiv./AGU. Interestingly, Milotskyi et al. adopted a rapid reactive extrusion method for cellulose acylation with vinyl laurate in [EMIM][OAc]/DMSO medium (Scheme 2d).83 The DS values of vinyl laurate synthesized at 120 °C for 10 min varied from 2.65 to 2.73. Yuan et al. performed homogeneous acylation of α-cellulose with vinyl esters in a mixture of [EMIM][OAc] and γ-valerolactone (GVL).84 After operating at 80 °C for 4 h, cellulose laurate, cellulose pivalate and cellulose chloroacetate were synthesized with DS of 3.0, 3.0 and 1.43, respectively.
Researchers still use relatively inactive but easily available alkyl esters instead of vinyl esters to produce esterified cellulose. However, reaction with alkyl esters leads to cellulose esters with lower DS values. Wang et al. designed a binary IL mixture containing a hydrophilic [BMIM][Cl] and a hydrophobic [BMIM][BF4] as a reaction medium for the acylation of cellulose.85 As reported, cellulose was acylated with methyl esters (methyl laurate, methyl palmate and methyl stearate) in the presence of a catalyst of lipase. Long-chain cellulose easters were formed and the DS values were in the range of 0.213–1.452. Acylation with methyl laurate was also attempted in another IL of 1-ethyl-3-methylimidazoium 2,4-dimethoxybenzoate ([EMIM][DMBz]). With the assistance of DMSO as a co-solvent, the reaction occurred without any external catalysts to acquire cellulose laurate with a DS of 0.52 (Scheme 2e).86
Aliphatic cellulose esters bearing a pendant carboxylic acid group could be synthesized through ring-opening esterification of cyclic anhydrides [e.g., succinic anhydride (SA), maleic anhydride (MA) and glutaric anhydride (GA)] with cellulose in ILs. In this regard, [AMIM][Cl] and [BMIM][Cl] are widely employed as the reaction media. Succinylated banana cellulose with a maximum DS of 0.37 was produced by modification with SA in AMIMCl/DMSO mixtures (Scheme 2f).87 Sun's group has made many attempts to modify cellulose with cyclic anhydrides as reagents.88–90 They proceeded with a succinylation reaction in a binary solvent of [BMIM][Cl]/DMSO using another catalyst, N-bromosuccinimide (NBS).88 Succinylated cellulose with DS values of between 0.24 and 2.31 can be attained. In the following study, three main components (cellulose, hemicellulose and lignin) were isolated from extractive-free bagasse and were homogeneously esterified with GA in [AMIM][Cl] (Scheme 2g).89 The group extended the reaction of the three main components to homogeneous maleylation (Scheme 2h).90 The maleylated cellulose showed a percentage of substitution of 13.5%.
In the case of cellulose aromatic esters, benzoyl chlorides bearing diverse p-substituted groups are widely applied for the benzoylation of cellulose. El Hamdaoui et al. described an efficient strategy with low reagent consumption and a short reaction time for the acylation of cellulose.91 Accordingly, the reaction with p-nitrobenzoyl chloride was carried out in a binary mixture of 1-butylpyridinium chloride ([BPy][Cl]) and DMSO (Scheme 2i). With processing at 80 °C for 1.30 h, cellulose p-nitrobenzoate with DS values of 0.12–1.5 was obtained. Similarly, kraft cellulose p-iodobenzoate with DS values of 0.18–2.05 was attained by the reaction with p-iodobenzoyl chloride in [BMIM][Cl].92 Homogeneous cellulose acylation with 2,2,2-trifluoroethyl benzoates proceeded in N-methyl-N-(2-methoxyethyl) pyrrolidinium acetate ([P1ME][OAc]), affording corresponding cellulose benzoates with full substitution (DS = 3) (Scheme 2j).93 In the same medium, cellulose benzoates with DS values of 2.38 and 2.67 were produced by reactions with 4-[(4-hydroxyphenyl)diazenyl]benzoate or 4-[(4-methoxyphenyl)diazenyl]benzoate. Highly selective benzoylation of cellulose at the 6-position was conducted in [AMIM][Cl].94 The DS values of cellulose 6-benzoates ranged from 1.04 to1.29. The unreacted hydroxyl groups at 2- and 3-positions of the cellulose monoesters could be further reacted with 3,5-dimethylphenyl isocyanate. Buchanan et al. prepared cellulose benzoate propionates through the phased addition of Pr2O and Bz2O to cellulose in an IL of tributylmethylammonium dimethyl phosphate (TBMADMP).95 The benzoate was selectively located at the C2 and C3 positions. Moreover, Heinze's group reported that in the presence of pyridine, fully substituted cellulose furoate and cellulose phenyl carbonate were accessible using [BMIM][Cl] as a reaction medium (Scheme 2k and l).96,97 Recently, Niu et al. selected three phenolic acids (p-hydroxybenzoic acid, vanillic acid, and syringic acid) to modify cellulose fibers. The homogeneous esterification was performed in a mixture of a recyclable IL (tetrabutylammonium acetate ([N4444][OAc]) and DMSO (Scheme 2m).98 The esterified cellulose-generated films displayed good hydrophobicity and barrier properties, although the DS of cellulose dericative was less than 0.25.
3.2. Chemical modification of cellulose in deep eutectic solvents
Moreover, DESs have also been applied as green pre-treatment routes combining with mechanical disintegration to produce derivatized nanocellulose. Anionic CNFs with diameters of 2–7 nm were produced through ring-opening esterification of succinic anhydride (SA) with hydroxyl groups of wood cellulose following mechanical treatment (Scheme 4d).127 The reaction was conducted in a non-degrading and non-dissolving urea/LiCl DES medium at 70–110 °C for 2–24 h, resulting in anionic cellulose with carboxyl content ranging from 0.34 to 0.95 mmol g−1. Another DES system consisting of imidazole and TEMACl was reported as a pre-treatment media in the anionic functionalization of groundwood pulp (GWP) with SA (Scheme 4e).128 The modified GWP treated at 70 °C for 2 h gave a carboxyl content as high as 3.34 mmol g−1, and formed highly anionic charged wood nanofibers. Such nanosized fibers could form self-standing films with good mechanical properties. More recently, Liu et al. applied a series of ChCl/carboxylic acid based DESs as both pretreatment media and reagents to produce esterified CNFs from the raw material of hardwood bleach kraft pulp.22 The presence of DES was conductive to the esterification and effective swelling of cellulose, thereby giving CNFs after mechanical treatment. The esterified CNFs showed high yields of 72%–88% and still maintained the cellulose I crystal structure.
3.3. Chemical modification of cellulose in aqueous alkali/urea solutions
Zhang and coworkers applied an aqueous 6 wt% NaOH/4 wt% urea solution as a reaction medium for the synthesis of allyl cellulose (AC) (Scheme 5a). A maximum DS of 1.65 was reached using allyl chloride as an etherification agent.140 In this case, the AC product was a good intermediate to further react with thiol compounds [n-dodecyl mercaptan (NDM), 2-aminoethanethiol hydrochloride (AET), cysteine (Cys) and monothioglycerol (MG)] through thiol–ene click reactions. Recently, an amphiphilic cellulose (MCC-C16) was designed to improve the antifouling and separation properties of poly(acrylonitrile-co-methyl acrylate) ultrafiltration membrane.141 The synthesis of MCC-C16 was achieved by the reaction of MCC with 1-bromohexadecane in the above-mentioned aqueous solution (6 wt% NaOH/4 wt% urea), as shown in Scheme 5b. An aqueous solution of 7 wt% NaOH/12 wt% urea is a favorable solvent for initiating the etherification reaction of cellulose. Cellulose dissolved in the 7 wt% NaOH/12 wt% urea aqueous solution was allowed to react with benzyl chloride under mild conditions in the absence of any catalysts (Scheme 5c).142 The DS values of benzyl cellulose ranged from 0.29 to 0.54, depending on the reaction temperature and the molar ratio of benzyl chloride to AGU. However, the high water content of the reaction medium restricts the benzylation of cellulose and makes it difficult to achieve higher DS.143
Quaternary ammonium salt-modified cellulose (QMCC) with high positive charges was prepared in an aqueous solution of 7% NaOH/12% urea.144 The QMCC-added alkaline solid polyelectrolyte membrane showed significantly improved conductivity and tensile strength, and could be applied to flexible Zn–air batteries. Using the same solvent, Qi et al. modified cellulose with allyl glycidyl ether (AGE) to obtain 3-allyloxy-2-hydroxypropylcelluloses (AHP-celluloses) with DS ranging from 0.32–0.67 (Scheme 5d).145 Furthermore, owing to the good dissolving capacity for cellulose and the inherently high content of water, aqueous alkali/urea solutions provide an appropriate aqueous environment for the preparation of cellulose-based hydrogels. These hydrogels can be fabricated by chemical cross-linking of cellulose or cellulose derivatives with the occurrence of cross-linking agents. Liu et al. synthesized cellulose hydrogels through chemical cross-linking of cellulose with 1,4-butanediol diglycidyl ether (BDE) in 6 wt% NaOH/4 wt% urea aqueous solution.146 The synthesis of cellulose ionic hydrogels (CIHs) from cellulose in NaOH/urea aqueous solution was reported by Tong et al.147 The cellulose was etherified with AGE to be endowed with double bonds and then chemically cross-linked by free radical polymerization to fabricate CIHs with high elongation and compressibility.
Benefiting from the basicity of aqueous alkali/urea systems, vinyl compounds are activated in these systems and undergo Michael addition reaction with cellulose under catalyst-free conditions. As a classical example, cyanoethylation of cellulose with acrylonitrile (AN) was accessible in 7 wt% NaOH/12 wt% urea (Scheme 5e)138 and 4.5 wt% LiOH/15 wt% urea aqueous solutions (Scheme 5f).139 In this case, the relative reactivity of hydroxyl groups was in the order of C-6 > C-2 > C-3. Cellulose polyelectrolytes bearing acylamino and carboxyl groups were homogenously synthesized in NaOH/urea aqueous solutions (Scheme 5g). The synthesis process involved two steps, Michael addition reaction with acrylamide followed by saponification of acylamino groups into carboxyl groups.148 Based on etherification of cellulose with acrylamide and benzyl chloride, an amphiphilic cellulose derivative (BCEC) containing hydrophobic benzyl and hydrophilic carboxyethyl groups was synthesized (Scheme 5h). The reaction was conducted in NaOH/urea aqueous solution without extra catalysts.149 Considering the pH responsibility and surfactant property of BCEC, microcapsules formed by cross-linking BCEC with polyurea were applied for the encapsulation and controlled release of hydrophobic methyl 1-naphthylacetate (MENA). In addition, methylenebisacrylamide (MBA) was selected as a Michael addition reagent for the cross-linking of cellulose in LiOH/urea aqueous solution. A robust hydrogel was formed and exhibited a superior water uptake capacity.150
3.4. Chemical modification of cellulose in quaternary onium hydroxides
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.153 In this case, the films were fabricated via etherification of cellulose with an expoxypropoxy-contained fluorescein molecule and subsequent cross-linking reaction. The obtained films showed excellent fluorescent property, pH sensitivity and polarized emission behavior after stretching.153
5.153 In this case, the films were fabricated via etherification of cellulose with an expoxypropoxy-contained fluorescein molecule and subsequent cross-linking reaction. The obtained films showed excellent fluorescent property, pH sensitivity and polarized emission behavior after stretching.153
4. Conclusions and prospects
Cellulose is the most abundant renewable material with fascinating properties. Homogeneous chemical modification is an efficient technique for converting cellulose into cellulose derivatives with a high degree of uniformity. However, the solubilization of cellulose is a critical step before derivatization. In this review, we have briefly overviewed four types of recent “green” solvents for cellulose dissolution, namely ILs, DESs, aqueous alkali/urea systems and aqueous QOH systems, and summarized recent examples of cellulose modification in these solvents.Although these solvents have emerged as reaction media for cellulose derivatization, they have their own advantages and disadvantages. Owing to excellent dissolution capacity and structural diversity, ILs fit well with cellulose chemistry. To date, various types of chemical modification of cellulose, such as esterification, etherification, grafting and carbalination, can be performed in ILs. ILs can efficiently dissolve both cellulose and reactants to provide a homogeneous reaction medium for cellulose functionalization. Several ILs even exhibit catalytic properties during reactions, depending on the structural characteristics of the ILs. Highly substituted cellulose derivatives are readily synthesized in ILs. Unfortunately, most ILs suffer from the drawbacks of high viscosity and high sensitivity to water. Although the incorporation of co-solvents into ILs enables an appropriate decrease in the viscosity, the water content needs to be strictly controlled during the dissolution and modification process. Besides, there are still concerns about the high cost, difficulty in preparation, potential toxicity, and non-degradability of ILs.
DESs are regarded as non- or low toxic, inexpensive and biodegradable alternatives to traditional ILs because they offer the benefits of ILs. However, examples of DESs for efficient dissolution of cellulose are limited, and the solubility of cellulose in the DESs is much lower than that in ILs. Due to the insufficient solubilization of cellulose, current cellulose derivatization using DESs as reaction media is usually performed in heterogeneous conditions.
In the case of aqueous alkali/urea systems, rapid dissolution of cellulose can be achieved, but is limited by precooling requirements and the molecular weight of cellulose. The functionalization of cellulose in alkali/urea aqueous solutions is mainly focused on etherification. Conversion of cellulose to cellulose ethers in aqueous alkali/urea is often plagued by the high water content of the solvents. Moreover, the study of cellulose modification in alkali/urea aqueous solutions is kind of limited to the group of LN Zhang. Input from a broader scientific community is needed.
Compared to aqueous alkali/urea systems, aqueous QOH solutions can rapidly dissolve cellulose at ambient temperatures. Although there are limited studies in the literature on cellulose derivatization in aqueous QOHs, these results will motivate researchers to make more attempts to modify cellulose in these systems.
In response to the 12 principles of green chemistry, important issues such as efficient preparation, toxicity assessment and recovery of cellulose solvents still need to be considered. Most of the green solvents discussed in this minireview are petroleum-based products. Taking the most widespread class of ILs as an example, the synthesis of the imidazolium cations requires a significant number of chemical reaction steps, also involving non-green reactions. Driven by the target of carbon-reduction, bio-derived solvents or solvents from renewable sources will be an important direction. Given the vast design space of ILs and DESs, many biobased molecules could be considered to make the solvents greener.
Application-oriented research is highly required in the future for the modification of cellulose in green solvents. The market is facing a significant transformation from conventional petroleum resource driven development to a more bioresource driven development. More functional cellulose chemicals and materials will be needed, which will require the cellulose modification industry to be adaptive to much richer products in the future. Future research needs to focus on the green conversion of cellulose into functional cellulose derivatives with higher efficiency, less waste/by-product and high selectivity. In addition, further research on the selection of reaction media, reactants and modification techniques is necessary.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support of the National Key Research and Development Program of China (2019YFE0114400), the Taishan Industrial Leading Talent Project (20180215) and the National Natural Science Foundation of China (22005103).References
- U. Ray, S. Zhu, Z. Pang and T. Li, Adv. Mater., 2020, 2002504, DOI:10.1002/adma.202002504.
- J. M. Rieland and B. J. Love, Resour., Conserv. Recycl., 2020, 155, 104678 CrossRef.
- F. Danafar, Cellul. Chem. Technol., 2020, 54, 327–346 CrossRef CAS.
- X. He, W. Lu, C. Sun, H. Khalesi, A. Mata, R. Andaleeb and Y. Fang, Carbohydr. Polym., 2020, 117334, DOI:10.1016/j.carbpol.2020.117334.
- M. Rincón Iglesias, E. Lizundia and S. Lanceros Méndez, Biomacromolecules, 2019, 20, 2786–2795 CrossRef PubMed.
- F. Lin, J. L. Putaux and B. Jean, Carbohydr. Polym., 2021, 257, 117618 CrossRef CAS PubMed.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- K. N. Onwukamike, S. P. Grelier, E. Grau, H. Cramail and M. A. Meier, ACS Sustainable Chem. Eng., 2018, 7, 1826–1840 CrossRef.
- O. Nechyporchuk, M. N. Belgacem and J. Bras, Ind. Crops Prod., 2016, 93, 2–25 CrossRef CAS.
- J. A. Sirviö and J. P. Heiskanen, Cellulose, 2020, 27, 1933–1950 CrossRef.
- D. Roy, M. Semsarilar, J. T. Guthrie and S. Perrier, Chem. Soc. Rev., 2009, 38, 2046–2064 RSC.
- O. A. El Seoud, H. Nawaz and E. P. Arêas, Molecules, 2013, 18, 1270–1313 CrossRef CAS PubMed.
- M. Oprea and S. I. Voicu, Carbohydr. Polym., 2020, 116683, DOI:10.1016/j.carbpol.2020.116683.
- H. Nawaz, J. Zhang, W. Tian, J. Wu and J. Zhang, Encyclopedia of Sustainability Science and Technology, 2018, pp. 1–34, DOI:10.1007/978-1-4939-2493-6_1014-1.
- L. Lucia and A. Ayoub, Chemical and Engineering Fundamentals and Industrial Applications, Springer Book, 2018, DOI:10.1007/978-3-319-56596-5.
- A. Salama and P. Hesemann, ACS Sustainable Chem. Eng., 2020, 8, 17893–17907 CrossRef CAS.
- F. Hermanutz, M. P. Vocht, N. Panzier and M. R. Buchmeiser, Macromol. Mater. Eng., 2019, 304, 1800450 CrossRef.
- T. Heinze and A. Koschella, Polímeros, 2005, 15, 84–90 CrossRef CAS.
- M. Rose and R. Palkovits, Macromol. Rapid Commun., 2011, 32, 1299–1311 CrossRef CAS PubMed.
- S. Righi, A. Morfino, P. Galletti, C. Samorì, A. Tugnoli and C. Stramigioli, Green Chem., 2011, 13, 367–375 RSC.
- S. K. Singh and A. W. Savoy, J. Mol. Liq., 2020, 297, 112038 CrossRef CAS.
- S. Liu, Q. Zhang, S. Gou, L. Zhang and Z. Wang, Carbohydr. Polym., 2021, 251, 117018 CrossRef CAS PubMed.
- S. P. G. Kelechukwu, N. Onwukamike, E. Grau, H. Cramail and M. A. R. Meier, ACS Sustainable Chem. Eng., 2019, 7, 1826–1840 CrossRef.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- T. Welton, Chem. Rev., 1999, 98(8), 2071–2083 CrossRef PubMed.
- J. Zhang, J. Wu, J. Yu, X. Zhang, J. He and J. Zhang, Mater. Chem. Front., 2017, 1, 1273–1290 RSC.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- A. Xu and F. Wang, Green Chem., 2020, 22, 7622–7664 RSC.
- M. Gericke, P. Fardim and T. Heinze, Molecules, 2012, 17, 7458–7502 CrossRef PubMed.
- M. Isik, H. Sardon and D. Mecerreyes, Int. J. Mol. Sci., 2014, 15, 11922–11940 CrossRef CAS PubMed.
- O. Kuzmina, J. Bhardwaj, S. R. Vincent, N. D. Wanasekara, L. M. Kalossaka, J. Griffith, A. Potthast, S. Rahatekar, S. J. Eichhorn and T. Welton, Green Chem., 2017, 19, 5949–5957 RSC.
- Y. Li, J. Wang, X. Liu and S. Zhang, Chem. Sci., 2018, 9, 4027–4043 RSC.
- O. Stolarska, A. Pawlowska-Zygarowicz, A. Soto, H. Rodríguez and M. Smiglak, Carbohydr. Polym., 2017, 178, 277–285 CrossRef CAS PubMed.
- A. Xu, Y. Zhang, Y. Zhao and J. Wang, Carbohydr. Polym., 2013, 92, 540–544 CrossRef CAS PubMed.
- A. Xu, L. Cao and B. Wang, Carbohydr. Polym., 2015, 125, 249–254 CrossRef CAS PubMed.
- A. Xu, X. Guo and R. Xu, Int. J. Biol. Macromol., 2015, 81, 1000–1004 CrossRef CAS PubMed.
- A. Xu, L. Chen, Y. Wang, R. Liu and W. Niu, Polymers, 2019, 11, 845 CrossRef CAS PubMed.
- Y. Tomimatsu, H. Suetsugu, Y. Yoshimura and A. Shimizu, J. Mol. Liq., 2019, 279, 120–126 CrossRef CAS.
- D. Kasprzak, E. Krystkowiak, I. Stępniak and M. Galiński, Eur. Polym. J., 2019, 113, 89–97 CrossRef CAS.
- Y. Tomimatsu, Y. Yoshimura and A. Shimizu, Aust. J. Chem., 2019, 72, 669–673 CrossRef CAS.
- F. M. Perna, P. Vitale and V. Capriati, Curr. Opin. Green Sustainable Chem., 2020, 21, 27–33 CrossRef.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- D. V. Wagle, H. Zhao and G. A. Baker, Acc. Chem. Res., 2014, 47, 2299–2308 CrossRef CAS PubMed.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich and B. W. Doherty, Chem. Rev., 2020, 121(3), 1232–1285 CrossRef PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- J. Wu, Q. Liang, X. Yu, Q. F. Lü, L. Ma, X. Qin, G. Chen and B. Li, Adv. Funct. Mater., 2021, 2011102 CrossRef CAS.
- Y. T. Tan, A. S. M. Chua and G. C. Ngoh, Bioresour. Technol., 2020, 297, 122522 CrossRef CAS PubMed.
- Y.-L. Chen, X. Zhang, T. T. You and F. Xu, Cellulose, 2019, 26, 205–213 CrossRef CAS.
- X. Tang, M. Zuo, Z. Li, H. Liu, C. Xiong, X. Zeng, Y. Sun, L. Hu, S. Liu and T. Lei, ChemSusChem, 2017, 10, 2696–2706 CrossRef CAS PubMed.
- H. Malaeke, M. R. Housaindokht, H. Monhemi and M. Izadyar, J. Mol. Liq., 2018, 263, 193–199 CrossRef CAS.
- J. Wang, Y. Wang, Z. Ma and L. Yan, Green Energy Environ., 2020, 5, 232–239 CrossRef.
- G. Sharma, K. Takahashi and K. Kuroda, Carbohydr. Polym., 2021, 118171 CrossRef CAS.
- G. Liu, W. Li, L. Chen, X. Zhang, D. Niu, Y. Chen, S. Yuan, Y. Bei and Q. Zhu, Colloids Surf., A, 2020, 594, 124663 CrossRef CAS.
- H. Qi, C. Chang and L. Zhang, Cellulose, 2008, 15, 779–787 CrossRef CAS.
- J. Cai, L. Zhang, C. Chang, G. Cheng, X. Chen and B. Chu, ChemPhysChem, 2007, 8, 1572–1579 CrossRef CAS PubMed.
- A. Lue, L. Zhang and D. Ruan, Macromol. Chem. Phys., 2007, 208, 2359–2366 CrossRef CAS.
- X. Luo and L. Zhang, Food Res. Int., 2013, 52, 387–400 CrossRef CAS.
- X. Kang, S. Kuga, L. Wang, M. Wu and Y. Huang, J. Bioresour. Bioprod., 2016, 1, 58–63 Search PubMed.
- J. Cai, Y. Liu and L. Zhang, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 3093–3101 CrossRef CAS.
- T. Ema, T. Komiyama, S. Sunami and T. Sakai, RSC Adv., 2014, 4, 2523–2525 RSC.
- J. A. Sirviö, M. Visanko and N. Hildebrandt, Eur. Polym. J., 2017, 97, 292–298 CrossRef.
- B. Swensson, A. Larsson and M. Hasani, Cellulose, 2020, 27, 101–112 CrossRef.
- B. Swensson, A. Larsson and M. Hasani, Polymers, 2020, 12, 1310 CrossRef CAS PubMed.
- E. R. D. Seiler, Y. Takeoka, M. Rikukawa and M. Yoshizawa-Fujita, RSC Adv., 2020, 10, 11475–11480 RSC.
- M. N. Nguyen, U. Kragl, D. Michalik, R. Ludwig and D. Hollmann, ChemSusChem, 2019, 12, 3458–3462 CrossRef CAS PubMed.
- C. Zhong, F. Cheng, Y. Zhu, Z. Gao, H. Jia and P. Wei, Carbohydr. Polym., 2017, 174, 400–408 CrossRef CAS PubMed.
- W. Wei, X. Wei, G. Gou, M. Jiang, X. Xu, Y. Wang, D. Hui and Z. Zhou, RSC Adv., 2015, 5, 39080–39083 RSC.
- D. W. O'Connell, C. Birkinshaw and T. F. O'Dwyer, Bioresour. Technol., 2008, 99, 6709–6724 CrossRef PubMed.
- J. Zhang, W. Chen, Y. Feng, J. Wu, J. Yu, J. He and J. Zhang, Polym. Int., 2015, 64, 963–970 CrossRef CAS.
- J. Wu, J. Zhang, H. Zhang, J. He, Q. Ren and M. Guo, Biomacromolecules, 2004, 5, 266–268 CrossRef CAS PubMed.
- H. Wang, X. Wen, X. Zhang and C. Liu, Molecules, 2017, 22, 1419 CrossRef PubMed.
- S. Barthel and T. Heinze, Green Chem., 2006, 8, 301–306 RSC.
- D. C. Ferreira, M. L. Oliveira, T. A. Bioni, H. Nawaz, A. W. King, I. Kilpeläinen, M. Hummel, H. Sixta and O. A. El Seoud, Carbohydr. Polym., 2019, 212, 206–214 CrossRef CAS PubMed.
- R. Kakuchi, M. Yamaguchi, T. Endo, Y. Shibata, K. Ninomiya, T. Ikai, K. Maeda and K. Takahashi, RSC Adv., 2015, 5, 72071–72074 RSC.
- Q. Van Nguyen, S. Nomura, R. Hoshino, K. Ninomiya, K. Takada, R. Kakuchi and K. Takahashi, Polym. J., 2017, 49, 783–787 CrossRef.
- C. Achtel and T. Heinze, Macromol. Chem. Phys., 2016, 217, 2041–2048 CrossRef CAS.
- T. Kakko, A. W. King and I. Kilpeläinen, Cellulose, 2017, 24, 5341–5354 CrossRef CAS.
- O. Jogunola, V. Eta, M. Hedenström, O. Sundman, T. Salmi and J. P. Mikkola, Carbohydr. Polym., 2016, 135, 341–348 CrossRef CAS PubMed.
- H. Hanabusa, E. I. Izgorodina, S. Suzuki, Y. Takeoka, M. Rikukawa and M. Yoshizawa-Fujita, Green Chem., 2018, 20, 1412–1422 RSC.
- R. Kakuchi, R. Ito, S. Nomura, H. Abroshan, K. Ninomiya, T. Ikai, K. Maeda, H. J. Kim and K. Takahashi, RSC Adv., 2017, 7, 9423–9430 RSC.
- L. Hinner, J. Wissner, A. Beurer, B. Nebel and B. Hauer, Green Chem., 2016, 18, 6099–6107 RSC.
- R. Milotskyi, L. Szabó, T. Fujie, K. Sakata, N. Wada and K. Takahashi, Carbohydr. Polym., 2021, 256, 117560 CrossRef CAS PubMed.
- C. Yuan, W. Shi, P. Chen, H. Chen, L. Zhang, G. Hu, L. Jin, H. Xie, Q. Zheng and S. Lu, New J. Chem., 2019, 43, 330–337 RSC.
- F. Wang, G. Zhao, X. Lang, J. Li and X. Li, J. Chem. Technol. Biotechnol., 2017, 92, 1203–1210 CrossRef CAS.
- S. B. Kusuma, D. Hirose and K. Takahashi, Chem. Lett., 2019, 48, 1122–1125 CrossRef CAS.
- W. Shang, Z. Sheng, Y. Shen, B. Ai, L. Zheng, J. Yang and Z. Xu, Carbohydr. Polym., 2016, 141, 135–142 CrossRef CAS PubMed.
- C. F. Liu, A. P. Zhang, W. Y. Li, F. X. Yue and R. C. Sun, J. Agric. Food Chem., 2009, 57, 1814–1820 CrossRef CAS PubMed.
- H. Wang, W. Chen, X. Zhang, C. Liu and R. Sun, Materials, 2017, 10, 966 CrossRef PubMed.
- H. Wang, W. Chen, X. Zhang, Y. Wei, A. Zhang, S. Liu, X. Wang and C. Liu, Polymers, 2018, 10, 433 CrossRef PubMed.
- L. El Hamdaoui, M. El Moussaouiti and S. Gmouh, Int. J. Polym. Sci., 2016, 2016, 1756971 Search PubMed.
- A. Es Said, M. El Moussaouiti and R. Bchitou, J. Polym. Res., 2019, 26, 1–9 CrossRef.
- T. Takeshita, A. Kitagawa, F. Yokosu, R. Matsumoto, T. Nokami and T. Itoh, Aust. J. Chem., 2019, 72, 61–69 CrossRef CAS.
- C. Yin, J. Zhang, L. Chang, M. Zhang, T. Yang, X. Zhang and J. Zhang, Anal. Chim. Acta, 2019, 1073, 90–98 CrossRef CAS PubMed.
- C. Buchanan, E. Guzman Morales and B. Wang, Carbohydr. Polym., 2021, 252, 117146 CrossRef CAS PubMed.
- S. Köhler and T. Heinze, Cellulose, 2007, 14, 489–495 CrossRef.
- T. Elschner, M. Kötteritzsch and T. Heinze, Macromol. Biosci., 2014, 14, 161–165 CrossRef CAS PubMed.
- X. Niu, Y. Liu, A. W. King, S. Hietala, H. Pan and O. J. Rojas, Biomacromolecules, 2019, 20, 2105–2114 CrossRef CAS PubMed.
- H. C. Arca, L. I. Mosquera-Giraldo, V. Bi, D. Xu, L. S. Taylor and K. J. Edgar, Biomacromolecules, 2018, 19, 2351–2376 CrossRef CAS PubMed.
- S. Köhler, T. Liebert, T. Heinze, A. Vollmer, P. Mischnick, E. Möllmann and W. Becker, Cellulose, 2010, 17, 437–448 CrossRef.
- Y. Lv, Y. Chen, Z. Shao, R. Zhang and L. Zhao, Carbohydr. Polym., 2015, 117, 818–824 CrossRef CAS PubMed.
- T. Kakibe, S. Nakamura, W. Mizuta and H. Kishi, Chem. Lett., 2017, 46, 737–739 CrossRef CAS.
- T. Kakibe, S. Nakamura, K. Amakuni and H. Kishi, Aust. J. Chem., 2019, 72, 101–105 CrossRef CAS.
- G. Gürdağ and S. Sarmad, in Polysaccharide based graft copolymers, Springer, 2013, pp. 15–57 Search PubMed.
- W. Ge, Y. Guo, H. Zhong, X. Wang and R. Sun, Cellulose, 2015, 22, 2365–2374 CrossRef CAS.
- C. Yan, J. Zhang, Y. Lv, J. Yu, J. Wu, J. Zhang and J. He, Biomacromolecules, 2009, 10, 2013–2018 CrossRef CAS PubMed.
- Y. Guo, X. Wang, X. Shu, Z. Shen and R.-C. Sun, J. Agric. Food Chem., 2012, 60, 3900–3908 CrossRef CAS PubMed.
- S. Liu, L. Zhang, X. Chen, T. Chu, Y. Guo and M. Niu, Eur. Polym. J., 2019, 119, 385–392 CrossRef CAS.
- Y. Guo, X. Wang, Z. Shen, X. Shu and R. Sun, Carbohydr. Polym., 2013, 92, 77–83 CrossRef CAS PubMed.
- S. Zuppolini, I. C. Maya, L. Diodato, V. Guarino, A. Borriello and L. Ambrosio, Mater. Sci. Eng., C, 2020, 108, 110385 CrossRef CAS PubMed.
- Z. Liu, M. Chen, Y. Guo, X. Wang, L. Zhang, J. Zhou, H. Li and Q. Shi, Carbohydr. Polym., 2019, 204, 214–222 CrossRef CAS PubMed.
- C. Lin, H. Zhan, M. Liu, S. Fu and L. A. Lucia, Langmuir, 2009, 25, 10116–10120 CrossRef CAS PubMed.
- L. Yang, J. Zhang, J. He, J. Zhang and Z. Gan, Chin. J. Polym. Sci., 2015, 33, 1640–1649 CrossRef CAS.
- A. Hufendiek, V. Trouillet, M. A. Meier and C. Barner-Kowollik, Biomacromolecules, 2014, 15, 2563–2572 CrossRef CAS PubMed.
- C. Tsioptsias and C. Panayiotou, Carbohydr. Polym., 2008, 74, 99–105 CrossRef CAS.
- L. Chunxiang, Z. Huaiyu, L. Minghua, F. Shiyu and Z. Jiajun, Carbohydr. Polym., 2009, 78, 432–438 CrossRef.
- X. Sui, J. Yuan, M. Zhou, J. Zhang, H. Yang, W. Yuan, Y. Wei and C. Pan, Biomacromolecules, 2008, 9, 2615–2620 CrossRef CAS PubMed.
- H. Zhong, J. Zhang, Y. Guo, L. Wang, W. Ge, M. Chen, R. Sun and X. Wang, Cellulose, 2017, 24, 889–902 CrossRef CAS.
- Y. Zhang, W. Liu, W. Huang, Y. Ding, L. Song, S. Zheng and Z. Wang, Ind. Crops Prod., 2019, 142, 111842 CrossRef CAS.
- K. Schlufter, H. P. Schmauder, S. Dorn and T. Heinze, Macromol. Rapid Commun., 2006, 27, 1670–1676 CrossRef CAS.
- R. Q. Liu, L. Y. Bai, Y. J. Zhang and Y. P. Zhang, Chem. Cent. J., 2013, 7, 1–5 CrossRef PubMed.
- C. Yin, J. Li, Q. Xu, Q. Peng, Y. Liu and X. Shen, Carbohydr. Polym., 2007, 67, 147–154 CrossRef CAS.
- Y. Zhang, H. Li, X. Li, M. E. Gibril and M. Yu, Carbohydr. Polym., 2014, 99, 126–131 CrossRef CAS PubMed.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2005, 7, 705–707 RSC.
- J. H. Park, K. W. Oh and H. M. Choi, Cellulose, 2013, 20, 2101–2114 CrossRef CAS.
- M. Lakovaara, J. A. Sirviö, M. Y. Ismail, H. Liimatainen and R. Sliz, Cellulose, 2021, 1–15 Search PubMed.
- T. Selkälä, J. A. Sirviö, G. S. Lorite and H. Liimatainen, ChemSusChem, 2016, 9, 3074–3083 CrossRef PubMed.
- J. A. Sirviö and M. Visanko, J. Mater. Chem. A, 2017, 5, 21828–21835 RSC.
- A. P. Abbott, T. J. Bell, S. Handa and B. Stoddart, Green Chem., 2006, 8, 784–786 RSC.
- S. Vuoti, K. Narasimha and K. Reinikainen, J. Water Proc. Eng., 2018, 26, 83–91 CrossRef.
- Z. Yang, T. A. Asoh and H. Uyama, Polym. Degrad. Stab., 2019, 160, 126–135 CrossRef CAS.
- J. A. Sirviö, J. Ukkola and H. Liimatainen, Cellulose, 2019, 26, 2303–2316 CrossRef.
- J. A. Sirviö and J. P. Heiskanen, ChemSusChem, 2017, 10, 455–460 CrossRef PubMed.
- P. Willberg Keyriläinen, J. Hiltunen and J. Ropponen, Cellulose, 2018, 25, 195–204 CrossRef.
- J. Zhou, L. Zhang, Q. Deng and X. Wu, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 5911–5920 CrossRef CAS.
- H. Qi, T. Liebert, F. Meister and T. Heinze, React. Funct. Polym., 2009, 69, 779–784 CrossRef CAS.
- J. Zhou, Y. Qin, S. Liu and L. Zhang, Macromol. Biosci., 2006, 6, 84–89 CrossRef CAS PubMed.
- J. Zhou, Q. Li, Y. Song, L. Zhang and X. Lin, Polym. Chem., 2010, 1, 1662 RSC.
- Q. Li, P. Wu, J. Zhou and L. Zhang, Cellulose, 2012, 19, 161–169 CrossRef CAS.
- H. Hu, J. You, W. Gan, J. Zhou and L. Zhang, Polym. Chem., 2015, 6, 3543–3548 RSC.
- N. Han, W. Zhang, W. Wang, C. Yang, L. Tan, Z. Cui, W. Li and X. Zhang, J. Membr. Sci., 2019, 591, 117276 CrossRef.
- M. F. Li, S. N. Sun, F. Xu and R. C. Sun, Eur. Polym. J., 2011, 47, 1817–1826 CrossRef CAS.
- O. Sundman, T. Gillgren and M. Broström, Cellul. Chem. Technol., 2015, 49, 745–755 CAS.
- X. You, C. Qiao, D. Peng, W. Liu, C. Li, H. Zhao, H. Qi, X. Cai, Y. Shao and X. Shi, Polymers, 2021, 13, 9 CrossRef CAS PubMed.
- H. Qi, T. Liebert and T. Heinze, Cellulose, 2012, 19, 925–932 CrossRef CAS.
- H. Liu, A. Wang, X. Xu, M. Wang, S. Shang, S. Liu and J. Song, RSC Adv., 2016, 6, 42854–42862 RSC.
- R. Tong, G. Chen, D. Pan, H. Qi, R. A. Li, J. Tian, F. Lu and M. He, Biomacromolecules, 2019, 20, 2096–2104 CrossRef CAS PubMed.
- Y. Song, J. Zhou, L. Zhang and X. Wu, Carbohydr. Polym., 2008, 73, 18–25 CrossRef CAS.
- L. Pang, Z. Gao, H. Feng, S. Wang, H. Cong, B. Yu and Y. Shen, Ind. Crops Prod., 2019, 135, 57–63 CrossRef CAS.
- J. Yang, B. Medronho, B. Lindman and M. Norgren, Polymers, 2020, 12, 373 CrossRef CAS PubMed.
- M. Abe, K. Sugimura, Y. Nishiyama and Y. Nishio, ACS Sustainable Chem. Eng., 2017, 5, 4505–4510 CrossRef CAS.
- J. A. Sirviö, M. Y. Ismail, K. Zhang, M. V. Tejesvi and A. Ämmälä, J. Mater. Chem. A, 2020, 8, 7935–7946 RSC.
- T. Shi and Y. Lu, Polymer, 2020, 189, 122167 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |