Synthesis of 2-substituted quinazolines via iridium catalysis†
Jie
Fang
a,
Jianguang
Zhou
*a and
Zhijie
Fang
b
aChemical and Analytical Development, Suzhou Novartis Pharma Technology Co. Ltd, Changshu, Jiangsu, China 215537. E-mail: jianguang.zhou@novartis.com
bSchool of Chemical Engineering, Nanjing University of Science & Technology, Nanjing, Jiangsu, China 210094
First published on 6th November 2012
Abstract
An iridium-catalyzed hydrogen transfer reaction was successfully applied in the synthesis of 2-substituted quinazolines in moderate yields starting from aldehydes or alcohols with 2-aminobenzylamines.
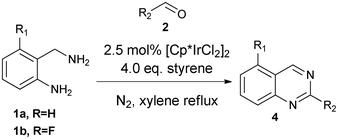
Quinazolines occur frequently in natural products and synthetic pharmaceuticals which exhibit important biological properties,1 such as antidiabetic, antibacterial, anticonvulsant and anticancer activities. For example, prazosin was an effective medicine as α-adrenergic blockers for the treatment of high blood pressure, panic disorder and anxiety,2 and lapatinib was used to treat solid tumor and breast cancer.3
Syntheses of substituted quinazolines have been widely explored,4 and many efficient methods have been developed recently. As shown in Scheme 1, one of the synthetic methods to quinazolines utilizes condensations between aldehydes 2 and 2-aminobenzylamines 1 followed by oxidation of the aminal intermediate 3. However, stoichiometric or large excess amounts of toxic oxidants were required for this oxidation; e.g., DDQ, p-chloranil,4c NaClO4k and MnO24l were used. In continuation of our work in the application of hydrogen transfer catalysis in the syntheses of quinazolinones,5 we were interested to test if a hydrogen transfer catalyst6 will catalyze the oxidation of aminal 3 to 2-substituted quinazoline 4 in one-pot as shown in Scheme 1.
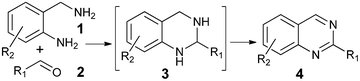 | ||
| Scheme 1 One-pot synthesis of quinazolines. | ||
Firstly, 2-aminobenzylamine 1a with benzaldehyde 2a was selected as the model substrate to test the one-pot reaction and the results are summarized in Table 1. We discovered that without a hydrogen acceptor, only 10% product 4a was formed using [Cp*IrCl2]2 (2.5 mol%) as the catalyst (Cp* = pentamethylcyclopentadienyl, entry 1). The major byproduct isolated was the N-benzylation product 57 as shown in Scheme 2.
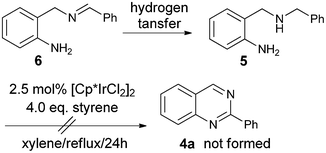 | ||
| Scheme 2 Possible pathway to 5 from hydrogenation of imine 6 and reaction of 5 under hydrogen transfer conditions. | ||
|
|
|||||
|---|---|---|---|---|---|
| Entry | Catalyst | Additive | Acceptor | Solvent | Yieldb |
| a Conditions: 1a (0.5 mmol), 2a (0.5 mmol), catalyst (2.5 mol%), styrene (4.0 eq.) in refluxing temperature of the solvent listed (1 mL) under N2, 24 h. b H-NMR yield. c Isolated yield, 12% of byproduct 5 was also isolated in entry 2. d 2.5 mol% dppf was added. | |||||
| 1 | [Cp*IrCl2]2 | No | No | xylene | 10% |
| 2 | [Cp*IrCl2]2 | No | styrene | xylene | 66%c |
| 3 | [Cp*IrCl2]2 | No | E-crotonitrile | xylene | 50%c |
| 4 | [Cp*IrCl2]2 | AcOH 0.2 eq. | styrene | xylene | 43% |
| 5 | [Cp*IrCl2]2 | KOH 0.2 eq. | styrene | xylene | 54% |
| 6 | [Cp*IrCl2]2 | t-BuONa 0.2 eq. | styrene | xylene | 60% |
| 7 | [Cp*IrCl2]2 | K2CO3 0.2 eq. | styrene | xylene | 46% |
| 8 | [Cp*IrCl2]2 | No | styrene | toluene | 35% |
| 9 | [Cp*IrCl2]2 | No | styrene | DMF | 50% |
| 10 | [Cp*IrI2]2 | No | styrene | xylene | 57% |
| 11 | RuCl2(PPh3)3 | KOH 0.2 eq. | styrene | xylene | 26% |
| 12 | [Ru(p-cymene)Cl2]2d | KOH 0.2 eq. | styrene | xylene | 52% |
This byproduct formation could have originated from hydrogen transfer8 to the imine intermediate 6. Compound 5 could not be further transformed to the product quinazoline 4a under hydrogen transfer catalysis, which accounted for the low yield of 4a in this reaction. To improve the yields of 4a, we decided to add a hydrogen acceptor to the reaction mixture. To our delight, the yields of 4a were improved to 66% with addition of styrene (entry 2) and 50% with E-crotonitrile (entry 3). Further optimizations of the reaction by using acid or base additives were also tried (entries 4 to 7), but the best yield of 60% obtained by addition of NaOtBu (entry 6) was inferior to the results of 66% without such additives in entry 2. The effects of solvents (entries 8 and 9) and catalysts (entries 10 to 12) were also examined briefly with no increase of the yield of 4a. After examining the reaction profiles, we decided to select the conditions of entry 2 (2.5 mol% [Cp*IrCl2]2 in refluxing xylene with addition of 4.0 eq. styrene) for our investigations of the substrate scope of the reaction.
Subsequently, a variety of substituted quinazolines were synthesized using our optimized conditions. As shown in Table 2, both aliphatic and aromatic aldehydes reacted with 2-aminobenzylamines to give the corresponding quinazolines 4 in moderate yields. Reactions between 1a and aromatic aldehydes with either electron-withdrawing or electron-donating groups (entries 2 to 10) showed that the yields were not affected significantly in the range of 48% to 58%. Furthermore, the reactions also performed well when 2-furyl aldehyde (55% yield, entry 11), 2-phenylacetaldehyde (49% yield, entry 12) and hexanal (57% yield, entry 13) were involed. Investigations of 2-(aminomethyl)-3-fluoroaniline 1b with several aldehydes again gave substituted quinazolines 4n to 4q in moderate yields (56% to 65%, entries 14 to 17).
|
|
|||
|---|---|---|---|
| Entry | R 1 | R 2 | Yieldb |
| a Conditions: Entries 1–13: 1a (1.0 mmol), 2 (1.0 mmol), catalyst (2.5 mol%), styrene (4.0 eq.) in refluxing xylene (2 mL) under N2, 24 h. Entries 14–17: 1b (1.0 mmol), 2 (1.0 mmol), catalyst (2.5 mol%), styrene (4.0 eq.) in refluxing xylene (2 mL) under N2, 24 h. b Isolated yield. | |||
| 1 | H | C6H5 | 4a 66% |
| 2 | H | 3–Cl–C6H4 | 4b 54% |
| 3 | H | 3–Br–C6H4 | 4c 48% |
| 4 | H | 3–NO2–C6H4 | 4d 58% |
| 5 | H | 3–Me–C6H4 | 4e 54% |
| 6 | H | 3–OMe–C6H4 | 4f 51% |
| 7 | H | 4–F–C6H4 | 4g 51% |
| 8 | H | 4–Br–C6H4 | 4h 55% |
| 9 | H | 4–NO2–C6H4 | 4i 57% |
| 10 | H | 4–Me–C6H4 | 4j 50% |
| 11 | H | Furyl | 4k 55% |
| 12 | H | Benzyl | 4l 49% |
| 13 | H | n-Pentanyl | 4m 57% |
| 14 | F | C6H5 | 4n 56% |
| 15 | F | 4–Br–C6H4 | 4o 60% |
| 16 | F | 4–Me–C6H4 | 4p 62% |
| 17 | F | n-Pentanyl | 4q 65% |
It was our next interest to test the employment of benzyl alcohol 7 instead of benzaldehyde 2a in the synthesis of quinazoline 4a. The above described conditions using benzaldehyde did not give a satisfactory yield of 4a (only 10%) when benzylalcohol 7 was used. Some optimizations (see supporting information, ESI†) identified that the addition of base additives, such as KOH (0.2 eq.) was necessary to increase the yield of 4a to 61% (Scheme 3).
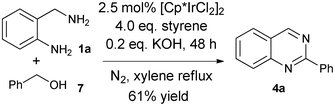 | ||
| Scheme 3 One-pot synthesis of 2-phenylquinazoline starting with benzyl alcohol. | ||
When 2-aminobenzyl alcohol 8 was used, the condensation with benzaldehydes 2a gave 2-phenyl-4H-benzo[d][1,3]oxazine 9 in 45% yield as shown in Scheme 4.9 The optimized conditions also involved the use of KOH (2 eq.) to give a better yield (see supporting information, ESI†).
![One-pot synthesis of 2-phenyl-4H-benzo[d][1,3] oxazine between 8 and 2a.](/image/article/2013/RA/c2ra22278g/c2ra22278g-s4.gif) | ||
| Scheme 4 One-pot synthesis of 2-phenyl-4H-benzo[d][1,3] oxazine between 8 and 2a. | ||
Conclusion
We have demonstrated a one-pot synthesis of 2-substituted quinazolines between 2-aminobenzylamines 1 and aldehydes 2via iridium-catalyzed hydrogen transfers using styrene as a hydrogen acceptor. The use of benzyl alcohol 7 instead of benzyaldehyde also successfully gave a quinazoline product in moderate yield. Further extension for the synthesis of 4H-3,1-benzoxazine was also demonstrated by the example using 2-aminobenzyl alcohol 8.References
- (a) J. B. Hynes and J. M. Buck, J. Med. Chem., 1975, 18, 1191 CrossRef CAS; (b) J. H. Chan, J. S. Hong, L. F. Kuyper, M. L. Jones, D. P. Baccanari, R. L. Tansik, C. M. Boytos, S. K. Rudolph and A. D. Brown, J. Heterocycl. Chem., 1997, 34, 145 CrossRef CAS; (c) J. P. Michael, Nat. Prod. Rep., 1999, 16, 697 RSC; (d) B. A. Foster, H. A. Coffrey, M. J. Morin and F. Rastinejad, Science, 1999, 286, 2507 CrossRef CAS; (e) J. P. Michael, Nat. Prod. Rep., 2002, 19, 742 RSC; (f) J. P. Michael, Nat. Prod. Rep., 2003, 20, 476 RSC; (g) L. A. Doyle and D. D. Ross, Oncogene, 2003, 22, 7340 CrossRef; (h) A. Lewerenz, S. Hentschel, Z. Vissiennon, S. Michael and K. Nieber, Drug Dev. Res., 2003, 58, 420 CrossRef CAS; (i) A. Lüth and W. Löwe, Eur. J. Med. Chem., 2008, 43, 1478 CrossRef; (j) R. Gundla, R. Kazemi, R. Sanam, R. Muttineni, J. A. R. P. Sarma, R. Dayam and N. Neamati, J. Med. Chem., 2008, 51, 3367 CrossRef CAS.
- J. F. Mendes da Silva, M. Walters, S. Al-Damluji and C. R. Ganellin, Bioorg. Med. Chem., 2008, 16, 7254 Search PubMed.
- H. A. III. Burris, Oncologist, 2004, 9, 10 Search PubMed.
- For reviews: (a) A. Witt and J. Bergman, Curr. Org. Chem., 2003, 7, 659 CAS; (b) D. J. Connolly, D. Cusack, T. P. O'Sullivan and P. J. Guiry, Tetrahedron, 2005, 61, 10153 CrossRef CAS; (c) For examples: J. J. E. Vanden, J. Godin, A. Mayence, A. Maquestiau and E. Anders, Synthesis, 1993, 867 Search PubMed; (d) T. Kitazume, F. Zulfiqar and G. Tanaka, Green Chem., 2000, 2, 133 RSC; (e) W. H. Correa, S. Papadopoulos, P. Radnidge, B. A. Roberts and J. L. Scott, Green Chem., 2002, 4, 245 RSC; (f) J. Sinkkonen, K. N. Zelenin, A. K. A. Potapov, I. V. Lagoda, V. V. Alekseyev and K. Pihlaja, Tetrahedron, 2003, 59, 1939 CrossRef CAS; (g) N. Coskun and M. Cetin, Tetrahedron Lett., 2004, 45, 8973 CrossRef CAS; (h) N. Coskun and M. Cetin, Tetrahedron, 2007, 63, 2966 Search PubMed; (i) F. Portela-Cubillo, J. S. Scott and J. C. Walton, Chem. Commun., 2008, 44, 2935 Search PubMed; (j) F. Portela-Cubillo, J. S. Scott and J. C. Walton, J. Org. Chem., 2009, 74, 4934 CrossRef CAS; (k) Y. Peng, Y. Zeng, G. Qiu, L. Cai and V. W. Pike, J. Heterocycl. Chem., 2010, 47, 1240 CrossRef CAS; (l) C. U. Maheswari, G. S. Kumar, M. Venkateshwar, R. A. Kumar, M. L. Kantam and K. R. Reddy, Adv. Synth. Catal., 2010, 352, 341 CrossRef; (m) C. Wang, S. Li, H. Liu, Y. Jiang and H. Fu, J. Org. Chem., 2010, 75, 7936 CrossRef CAS; (n) J. Zhang, D. Zhu, C. Yu, C. Wan and Z. Wang, Org. Lett., 2010, 12, 2841 CrossRef CAS; (o) B. Han, X. L. Yang, C. Wang, Y. W. Bai, T. C. Pan, X. Chen and W. Yu, J. Org. Chem., 2012, 77, 1136 CrossRef CAS.
- (a) J. Zhou and J. Fang, J. Org. Chem., 2011, 76, 7730 CrossRef CAS; (b) J. Fang and J. Zhou, Org. Biomol. Chem., 2012, 10, 2389 RSC.
- For reviews: (a) K. Fujita and R. Yamaguchi, Synlett, 2005, 4, 560; (b) T. D. Nixon, M. K. Whittlesey and J. M. J. Williams, Dalton Trans., 2009, 753 RSC; (c) M. J. Krische, Angew. Chem., Int. Ed., 2009, 48, 34 CrossRef CAS; (d) G. E. Debereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681 CrossRef CAS; (e) T. Suzuki, Chem. Rev., 2011, 111, 1825 CrossRef CAS; (f) J. Choi, A. H. R. MacArthur, M. Brookhart and A. S. Goldman, Chem. Rev., 2011, 111, 1761 CrossRef CAS.
- Compound 5 was formed in 5% under these conditions; intermediates of 3 and 6 were also detactable in LC-MS.
- For hydrogen transfer in C–N bond formations: (a) R. Yamaguchi, K. Fujita and M. W. Zhu, Heterocycles, 2010, 81, 1093 CrossRef CAS; (b) A. J. Blacker, M. M. Farah, M. I. Hall, S. P. Marsden, O. Saidi and J. M. J. Williams, Org. Lett., 2009, 11, 2039 CrossRef CAS; (c) W. X. Zhang, X. C. Dong and W. L. Zhao, Org. Lett., 2011, 13, 5386 CrossRef CAS; (d) S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem., 2011, 3, 1853 Search PubMed.
- The assay yield of intermediate 10 is 62%, the rest of compound 8 decomposed under the reaction conditions, which accounted for the overall lower yield of compound 9.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and compound characterization data. See DOI: 10.1039/c2ra22278g |
| This journal is © The Royal Society of Chemistry 2013 |