Photothermal chemical mechanical polishing: a synergistic mechanism for fast and atomic level surface perfection
Shichang
Wang†
a,
Chuanwang
Xing†
a,
Shenghua
Wang
a,
Chengcheng
Zhang
a,
Hailong
Feng
a,
Yuhang
Dong
a,
Yuxuan
Zhou
b,
Shuai
Yuan
ac,
Zuozuo
Wu
ac,
Zijian
Hong
 a,
Wantang
Wang
d,
Ziyi
Wang
a,
Wei
Sun
a,
Wantang
Wang
d,
Ziyi
Wang
a,
Wei
Sun
 *ace and
Deren
Yang
*ace and
Deren
Yang
 *acd
*acd
aState Key Laboratory of Silicon and Advanced Semiconductor Materials, School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: sunnyway423@zju.edu.cn; mseyang@zju.edu.cn
bInstitute of Functional Nano & Soft Materials (FUNSOM) Jiangsu Key Laboratory of Advanced Negative Carbon Technologies, Soochow University, Suzhou 215123, China
cProvincial Key Laboratory of High-Performance Silicon Material Equipment, Shangyu Institute of Semiconductor Materials, Shaoxing 312399, China
dInstitute of Advanced Semiconductors, Hangzhou Global Scientific and Technological Innovation Center, Hangzhou 311200, China
eZhejiang Provincial Key Laboratory of Optoelectronic Functional Materials and Devices, Hangzhou, 311200, China
First published on 12th December 2025
Abstract
Photo-assisted chemical mechanical polishing (PCMP) has emerged as a sustainable approach for fine polishing hard substrates. However, the light source of this approach is restricted to UV light, and the removal rate is relatively low. This study introduces a photothermal chemical mechanical polishing (PTCMP) technique adopting defect-engineered black nano-titanium dioxide (TiO2) as the photothermal catalyst. The black TiO2 demonstrates superior absorption across a broad optical range, enabling simultaneously photoinduced reactive oxygen species generation and localized photothermal heating under broadband light. This synergy in PTCMP is manifested with careful comparisons with ordinary TiO2 used in traditional PCMP under various illumination conditions. Mechanistic studies further reveal the dominant oxidative reactant is ·OH. Tunability in the polishing performance was demonstrated with varying parameters, achieving high material removal rate (MRR) and atomic-level surface roughness. This work provides an efficient method for achieving high-quality surfaces, paving the way for the sustainable processing of various substrates for advanced optics and electronics.
1. Introduction
Precision manufacturing and (opto)electronic device fabrication demand ultra-smooth surfaces to ensure optimal performance and functionality.1–5 Advanced polishing techniques are thus essential for achieving high-quality surface finishes.6–8 Conventional polishing methods, such as chemical mechanical polishing (CMP), are limited by low material removal rates and abrasive agglomeration.9,10 Recently, the photo-assisted mechanical polishing (PCMP) technique has emerged as an innovative solution to enhance efficiency and material removal rates.11–15 In this method, photocatalysts, such as TiO2 and CeO2, can absorb ultraviolet (UV) light to generate excited carriers, which subsequently produce reactive oxidative species. These mild oxidative species soften surface layers and degrade organic contaminants, facilitating material removal, ensuring environmental friendliness and high surface quality.16,17 However, traditional PCMP is constrained by reliance on UV light sources and catalysts with limited light absorption, reducing photocatalytic efficiency and material removal rates.18,19 Consequently, research into synergistic enhancements of CMP technologies is critical for advancing surface processing capabilities.Photothermal catalysis is an emerging technology that combines photochemical and thermal effects.20–25 In contrast to photocatalysis, photothermal catalysis utilizes a broad light spectrum across the UV, visible, and near-infrared wavelengths. This approach may generate abundant charge carriers to initiate redox reactions, and induce localized photothermal heating to enhance atomic and molecular motion at increased temperature and accelerate chemical reaction kinetics.26–29 Consequently, photothermal catalysis presents a highly efficient alternative to traditional photocatalysis and has obtained significant attention in environmental and energy applications, such as hazardous gas decomposition, carbon dioxide reduction, and water splitting for hydrogen production.30–36 Furthermore, the application of photothermal effects in the field of micro- and nano-fabrication has established precedents, such as the use of ultrafast laser techniques for material processing and surface control.37,38 Nevertheless, its use in polishing processes remains largely uninvestigated.
To address the limitations of conventional polishing methods, we propose a groundbreaking photothermal chemical mechanical polishing (PTCMP) technique utilizing black TiO2 synthesized via NaBH4 reduction. Black TiO2, characterized by its disordered surface layer and oxygen vacancy-rich structure, exhibits exceptional broad-spectrum light absorption and enhanced charge carrier dynamics, making it an ideal material for photothermal catalytic applications.39–43 Our work introduces the first application of defect-engineered black TiO2 in photothermal mechanical polishing, alongside a systematic investigation of its synergistic photothermal-photochemical mechanism. The light-induced temperature rise, driven by direct vibrational absorption and indirect non-radiative relaxation, dissipates excess energy as localized heat as shown in Fig. 1 for accelerating the process.44,45 The material under the broadband Xenon lamp concurrently produces abundant reactive oxygen species (ROS), which are efficiently harnessed by the substrate surface and drive the oxidative reaction.46,47 Mechanistic studies, supported by experimental and spectroscopic analyses, reveal the pivotal role of these reactive species, ranked as ·OH > ·O2− > h+ > 1O2, in facilitating efficient material removal. The PTCMP method achieves an outstanding material removal rate of 147.98 nm min−1 and a surface roughness (Sa) of 0.332 nm on silicon wafers, optimized through orthogonal experimental design. The approach was demonstrated also advantageous for other widely used substrates, e.g. SiC and quartz glass. This work establishes a highly efficient and sustainable solution for surface processing that is compatible with the existing CMP and PCMP infrastructures, with significant potential to advance precision manufacturing and semiconductor/optical technologies.
 | ||
| Fig. 1 Schematic diagram of the photothermal chemical mechanical polishing. The polished silicon wafer shows smooth surface mirroring the university emblem. | ||
2. Experimental
2.1. Materials and chemicals
All reagents were used as received without further purification. Sodium borohydride (NaBH4, AR), ethanol (C2H5OH, AR), pristine nano-titanium dioxide (P25-type TiO2, AR), aluminum oxide (Al2O3, ≥99.99%), potassium hydroxide (KOH, ≥95%), hydrogen peroxide (H2O2, AR, 30 wt% in H2O), sodium hexametaphosphate ((NaPO3)6, AR), L-histidine (≥99%), di-ammonium oxalate monohydrate ((NH4)2C2O4, GR), tert-butanol (C4H10O, ≥99.5%), and p-benzoquinone (C6H4O2, ≥99%) were procured from Aladdin Reagent (Shanghai) Co., Ltd. Silica sol (PCS-100) was sourced from Zhejiang Xinchuangna Electronic Technology Co., Ltd. Deionized water was obtained from a ultrapure water system (JISHEN). The single-crystal silicon wafers (2-inch diameter) were purchased from Regrace Technology Co., Ltd (Shenzhen). The single-crystal silicon carbide substrates (4.5 cm × 4.5 cm square) were obtained from Qianjing Semiconductor Co., Ltd (Hangzhou). The quartz glass samples (2-inch diameter) were supplied by PuriOpto Optical Materials Co., Ltd (Shanghai).2.2. Synthesis of black TiO2
Pristine TiO2 and sodium borohydride (NaBH4) were mixed in a mortar for 15 minutes at a predetermined ratio. The mixture was heated in a tubular furnace under an argon flow of 150 mL min−1 at 10 °C min−1 from 30 °C to 360 °C over 33 minutes, held for 1 hour, and cooled to room temperature. Afterwards, the sample was transferred to a centrifuge tube, dispersed in 40 mL deionized water, ultrasonicated for 10 minutes, and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 minutes. It was then washed with deionized water and absolute ethanol, and dried in a vacuum oven for 24 hours, yielding black TiO2 with photothermal catalytic properties.
000 rpm for 5 minutes. It was then washed with deionized water and absolute ethanol, and dried in a vacuum oven for 24 hours, yielding black TiO2 with photothermal catalytic properties.
2.3. Preparation of polishing slurry
A photothermal polishing slurry was prepared by combining 2.0 g sodium hexametaphosphate, 1.0 g Al2O3 particles (400 nm), and 1.0 g synthesized black TiO2 or commercial TiO2 in a beaker. To this, 700 mL deionized water, 200 mL silica sol (100 nm), and 100 mL hydrogen peroxide were added. The mixture was vigorously stirred and ultrasonicated for 15 minutes to ensure homogeneity.2.4. Polishing experiments
Silicon wafers were affixed to the polishing disk of a polishing machine (UNIPOL-1502, Shenyang Kejing Automation Equipment Co., Ltd) using wax and secured to the polishing head. A flocked non-woven polishing pad was employed. The slurry was gradually dripped onto the wafer surface for polishing. A xenon light source (PLS-SXE300E, AM 1.5G, Beijing Perfectlight Technology Co., Ltd) irradiated the photothermal polishing slurry, generating hydroxyl radicals to facilitate chemical reactions. Unless specified, the polishing experiments were conducted under the following conditions: black TiO2 concentration of 1.00 g L−1, light intensity of 220.7 mW cm−2, hydrogen peroxide concentration of 10%, silica sol concentration of 20%, polishing pressure of 300 g cm−2, rotational speed of 55 rpm, dispersant concentration of 2.00 g L−1, and a polishing duration of 30 minutes.2.5. Comparative experiments
To elucidate the photothermal coupling effect on polishing performance, seven comparative experiments were conducted with the light source varied: (I) black TiO2 polishing slurry under a xenon light source; (II) black TiO2 polishing slurry under an infrared light source; (III) black TiO2 slurry without illumination; (IV) pristine TiO2 polishing slurry under a xenon light source; (V) pristine TiO2 polishing slurry under combined black TiO2 and xenon light illumination; (VI) pristine TiO2 polishing slurry under an ultraviolet light source; (VII) black TiO2 polishing slurry under an ultraviolet light source. The xenon and ultraviolet light sources (JZ-Y02B-10040BL4A-LG-48-1, Shenzhen Jiuzhou Xinghe Technology Co., Ltd) were set to a consistent intensity of 220.7 mW cm−2, while the infrared light source (MT-810, Foshan OTL Lighting Appliance Co., Ltd) was employed to replicate the temperature achieved with black TiO2 under xenon illumination.2.6. Reactive species characterization
Aqueous solutions (0.10 g L−1) of black and pristine TiO2 were ultrasonicated for 30 minutes and illuminated for 5 minutes under an AM 1.5G xenon light source. Samples were mixed with 50 mM DMPO and analyzed in situ via electron paramagnetic resonance (EPR, Bruker A300) to detect hydroxyl radicals in black TiO2 and pristine TiO2 solutions. Superoxide radicals were tested by substituting methanol for deionized water, singlet oxygen by replacing DMPO with TEMP, and photogenerated holes by using TEMPO instead of DMPO, with all other conditions unchanged.2.7. Reactive species quenching experiments
Specific quenching experiments were performed using tert-butanol, p-benzoquinone, L-histidine, and ammonium oxalate as selective scavengers for ·OH, ·O2−, 1O2, and h+, respectively. The effect of quenching each reactive species on the polishing rate was analyzed to determine their relative contributions to polishing performance.2.8. Photoelectrical measurements of black TiO2
Working electrodes were prepared by drop-coating black and pristine TiO2 solutions of equal concentration onto ITO glass. Electrochemical measurements were performed using a workstation (CHI750E, Shanghai Chenhua Instrument Co. Ltd) with Ag/AgCl as the reference electrode, Pt as the counter electrode, and 1 M KOH as the electrolyte under AM 1.5G simulated solar illumination to obtain I–T curves and linear sweep voltammetry (LSV) profiles for black TiO2.2.9. Heat transfer stimulation
The thermal transfer properties of black TiO2 and pristine TiO2 catalysts immersed in water were simulated using COMSOL Multiphysics commercial software. A spherical model was constructed with a 15 nm diameter catalyst particle at the center, surrounded by a 500 nm diameter spherical simulation domain filled with water. The computational mesh was generated using physics-controlled settings with a normal element size in COMSOL. The simulation employed the Heat Transfer in Solids (eqn (1) and (2)) and Fluids module coupled with surface-to-surface radiation (eqn (3)–(6)). As shown in the following equations.| ρCρu·∇T + ∇·q = Q + Qted | (1) |
| q = − k∇T | (2) |
| J = εeb(T) + ρdG | (3) |
| G = Gm + Gamb + Gext | (4) |
| Gamb = Fambεambeb(Tamb) | (5) |
| eb(T) = n2σT4 | (6) |
Boundary conditions were defined as follows: a heat flux with a heat transfer coefficient of 10.00 W m−1 K−1 was applied to the outer surface of the upper hemisphere of a spherical domain (diameter 500 nm) to model heat exchange from a top-incident light source. Both the inner and outer surfaces of the spherical domain and the internal TiO2 were configured as diffuse reflective surfaces.
2.10. Characterization
Phase stability of black TiO2 across reduction temperatures was verified via X-ray diffraction (XRD, SmartLab). Absorption spectra of TiO2 powders were obtained via ultraviolet-visible spectroscopy (UV-Vis, Hitachi U-4100). High-resolution transmission electron microscopy (HRTEM, JEM-2100Plus) was used to analyze particle size and surface order changes in black TiO2 pre- and post-reduction. X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha) provided O 1s, Ti 2p, and valence band (VB) spectra to determine the diagram of density of states (DOS) for black and pristine TiO2. Low-temperature electron paramagnetic resonance (EPR) identified oxygen vacancies in black TiO2. Emission spectra of black and pristine TiO2 were compared using steady-state/transient photoluminescence spectroscopy (PL, Edinburgh FLS1000). Silicon wafer surface roughness was measured using atomic force microscopy (AFM, VEECO MultiMode) and/or white light interferometry (Zygo NewView 8200). In situ temperature variations during polishing were monitored with an infrared thermal camera (HM-TPH21PRO-3AQF, Hangzhou Hikvision Digital Technology Co., Ltd). A power meter socket (ZTY1902, CHNT Group Co., Ltd) was used to quantify the energy consumption of each comparative experiment. A light intensity meter (PL-MW2000, Beijing Perfectlight Technology Co., Ltd) was used to measure light intensity. A precision balance (PX224ZH, Changzhou OHAUS Instrument Co., Ltd) was used to measure the mass of silicon wafers before and after polishing, with the material MRR calculated using eqn (7). In this equation, Δm denotes the mass difference before and after polishing, ρ represents the material density (2.33 g cm−3 for single-crystal silicon, 3.21 g cm−3 for single-crystal silicon carbide, and 2.60 g cm−3 for quartz glass), S denotes the surface area of the substrate, and t is the polishing duration.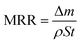 | (7) |
3. Results and discussion
3.1. Preliminary characterization and selection of black TiO2
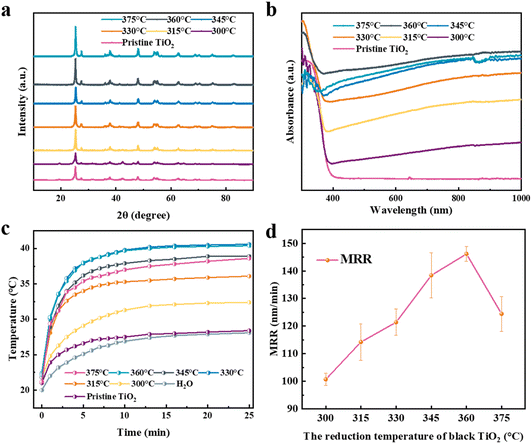
Since the synthetic approach of black TiO2 has been reported in literature, here we first focused on identifying the optimized preparation condition for accommodating our photothermal polishing scenario, based on basic material characterizations.48 Transmission electron microscopy (TEM) images (Fig. S1) of black TiO2 prepared at various reduction temperatures suggest that the nanoparticle size remains largely consistent. X-ray diffraction (XRD) patterns shown in Fig. 2a, reveal that the crystal structure of black TiO2 remains largely unchanged, displaying distinct anatase (2θ ≈ 25.3°) and rutile (2θ ≈ 27.4°) phase diffraction peaks, closely matching those of pristine TiO2. The full width at half maximum (FWHM) of diffraction peaks at 2θ ≈ 25° increases as the reduction temperature increases (Table S1). According to the Scherrer equation (eqn (8)), where β is the FWHM, crystallite size D is inversely proportional to β, suggesting a slight decrease in crystallite size. This phenomenon may arise from lattice strain induced by oxygen vacancies, disrupting long-range crystalline order. Furthermore, increasing oxygen vacancy concentrations promotes the formation of an amorphous surface layer, diminishing the effective crystallite size.46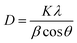 | (8) |
Ultraviolet-visible (UV-Vis) diffuse reflectance spectra of black TiO2 at various reduction temperatures, presented in Fig. 2b, demonstrate enhanced light absorption within the whole spectral region. Pristine TiO2 (P25, a mixture of anatase and rutile) exhibits minimal absorption in the visible and near-infrared regions, whereas absorption increases with reduction temperature, peaking at 360 °C for black TiO2. This enhancement marks increased oxygen vacancies or crystal defects during NaBH4 reduction.38,46 Furthermore, UV-Vis data reveal a progressive optical bandgap narrowing phenomenon with the rising reduction temperature, attributed to surface disorder and bulk defects,39,49 as detailed in Table S2 and Fig. S2.
As enhanced light absorption and reduced bandgap should induce significant photothermal effect, temperature rise was probed for black TiO2 solutions under consistent xenon light illumination (117.2 mW cm−2), as illustrated in Fig. 2c. The rise rate increases with the reduction temperature for preparation, with 360 °C corresponding to the fastest rise and the highest temperature upon stabilization, consistent with the trend in UV-Vis absorption data in Fig. 2b. The TiO2 samples reduced at these temperatures were then evaluated in a preliminary PTCMP experiment under the same conditions (described in the experimental section), with material removal rates and silicon wafer surface roughness (Saave) presented in Fig. 2d and Fig. S3, respectively. The MRR rose initially with reduction temperature but declined beyond 360 °C, again corresponding well with the light absorption trend, while the surface roughness fluctuated between 0.39 and 0.47 nm, all below the 0.5 nm threshold for atomic-level flatness. Based on the results above, black TiO2 prepared at 360 °C exhibiting the most enhanced light absorption, highest photothermal effect, and best performance for polishing, was selected for further investigations and comparisons with the pristine TiO2 used in the traditional PCMP. Hereafter, black TiO2 prepared at 360 °C will be abbreviated as black TiO2.
3.2. Photothermal chemical mechanical polishing performance
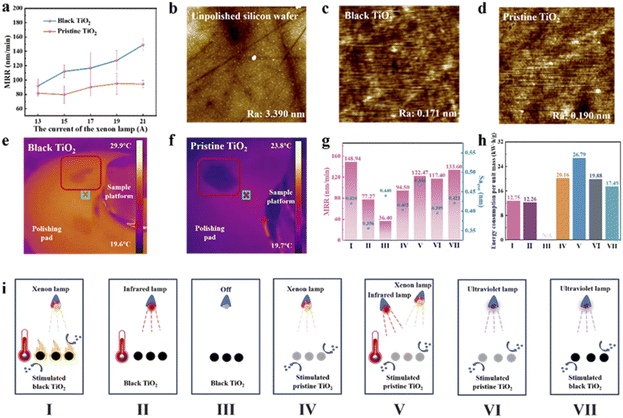
As silicon is the most widely used semiconductor material that frequently requires its surface polished, we first selected monocrystalline silicon wafers for the demonstrative polishing experiments and comparison with pristine TiO2. Black TiO2 and pristine TiO2 were incorporated in polishing slurries containing necessary ingredients commonly used for CMP (described in the experimental section). Fig. 3a reveals the material removal rates at varying light intensities using black TiO2 reduced at 360 °C and pristine TiO2 under xenon light illumination. Light intensity, adjusted via current as detailed in Table S3, correlates with increased material removal rates for both materials, driven by enhanced TiO2 photocatalytic activity. However, as the light intensity continued to increase at high levels (above ∼220 mW cm−2), the polishing rate did not exhibit a significant further improvement (Fig. S4). Black TiO2 consistently outperforms pristine TiO2 across all current levels, with the performance disparity increasing at higher intensities. Fig. 3b presents the surface morphology determined with atomic force microscopy (AFM) of an unpolished silicon wafer, while Fig. 3c and d show those of silicon wafers after 30 minutes of polishing with black TiO2 and pristine TiO2 polishing slurries, respectively. Both slurries significantly reduce surface roughness, with the black TiO2 slurry yielding lower roughness (Ra down to 0.171 nm determined with AFM) and superior polishing efficacy, consistent with its higher MRR.Since photo-induced heating is a symbolic effect for photothermal catalytic processes, we first measured real-time temperature changes during polishing using an infrared thermal camera, as shown in Fig. S5. Fig. S5a reveals that black TiO2 rapidly reached 30.5 °C, promoting localized heating that accelerates atomic and molecular motion, thereby enhancing reaction kinetics. Conversely, Fig. S5b indicates that pristine TiO2 remains around room temperature. Fig. 3e and f are infrared thermal camera snapshots that also show the higher temperature of the black TiO2 slurry throughout the polishing pad compared to pristine TiO2 slurry. As localized heating enhances photothermal catalysis by creating a temperature gradient between the catalyst and its environment, we then demonstrate the heat transfer characteristics of black TiO2 particles under xenon lamp illumination, with nanoscale simulations performed using COMSOL software. The non-adiabatic simulation domain was defined by the volume fraction of a single black TiO2 particle in the solvent, with heat transfer efficiency governed by the solvent's thermal conductivity (κ), which is considered to be 0.61 W m−1 K−1 (that of water). Surface temperature profiles for black TiO2 and pristine TiO2 were generated using boundary conditions closely matching experimental temperatures, with xenon light incident directly from the above results (Fig. S6). The simulations demonstrate a substantially greater temperature gradient for black TiO2 compared to pristine TiO2, corroborating experimental findings.
The superiority in photothermal catalysis may not solely originate from the thermal effect. To elucidate the combined advantage of photochemical contribution and photothermal effect in this system, six comparative experiments were conducted with the following conditions regarding the photocatalytic material and the light source (Fig. 3i): (I) black TiO2 polishing slurry under a xenon light source; (II) black TiO2 polishing slurry under an infrared light source; (III) black TiO2 slurry without illumination; (IV) pristine TiO2 polishing slurry under a xenon light source; (V) pristine TiO2 polishing slurry under combined xenon light and infrared light illumination; (VI) pristine TiO2 polishing slurry under an ultraviolet light source; (VII) black TiO2 polishing slurry under an ultraviolet light source. The xenon and ultraviolet light sources were set to a consistent intensity of 220.7 mW cm−2 (corresponding with a lamp current of 21A), while the infrared light source was employed to replicate the temperature achieved with black TiO2 under xenon light illumination. Comparing the results (Fig. 3g) in Experiments I and IV, a 57.6% efficiency increase for black TiO2 over pristine TiO2 under the broad-band xenon light is revealed, demonstrating the material advantage of black TiO2. Moreover, the MRR of Experiment V is lower than Experiment I, suggesting even with temperature compensation, the photochemical contribution (generation of visible-light-driven carriers and reactive oxygen species) by pristine TiO2 still underperform black TiO2. By excluding UV and visible light while compensating the temperature in Experiment II, the MRR using black TiO2 dropped by nearly 50% compared to Experiment I. This confirms the major contribution of the significant photochemical effect over 50%, considering the slurry itself can contribute to some MRR even without light (shown in Experiment III). Besides, upon changing the light source from xenon lamp in Experiment I to UV lamp (with the same total light intensity) in Experiment VII, the MRR for black TiO2 also decreased, demonstrating the advantage of the broadband light for triggering the photothermal heating to accelerate the reaction kinetics. Meanwhile, both under intense UV light only, black TiO2 (Experiment VII) also shows higher MRR than pristine TiO2 (Experiment VI). By changing the UV lamp (Experiment VI) to xenon lamp (Experiment IV) for pristine TiO2, the MRR did not increase like black TiO2, corroborating that pristine TiO2 does not possess the broadband utilization advantage. To summarize, these experiments corroborate that both photochemistry and photothermal heating have contributions to the high polishing performance with black TiO2 under broadband light source; the more significant generation of photo-generated carriers and therby reactive oxygen species are mainly responsible for the higher MRR found with black TiO2 than with pristine TiO2, while the local heating effect facilitates the kinetics. More mechanistic analysis will be discussed in the section of Chemical mechanism. A commercial colloidal SiO2 CMP slurry was also evaluated in a silicon wafer polishing test under identical conditions for comparison, yielding a material removal rates of 75.80 nm min−1. In contrast, our optimized black TiO2 slurry achieved a significantly higher MRR of 147.98 nm min−1 (Fig. S7). Meanwhile, the surface roughness (Sa) obtained using the commercial CMP slurry was 0.423 nm, whereas our PTCMP slurry also achieved a lower value of 0.332 nm, resulting in superior post-polishing surface quality. These results further substantiate the practical application potential of our PTCMP approach.
The benefit of black TiO2 for PTCMP is further manifested with respect to the lower energy consumption. A power meter socket was used to quantify the energy consumption of each comparative experiment, as shown in Fig. 3h. Among all the approaches using the different light sources and the two kinds of TiO2, Experiments I and II exhibited the lowest specific energy consumption per unit mass of material removed from silicon wafer, with an exception in Experiment III in which energy consumption by lamp is not applicable; however, Experiment II and III had significantly low material removal rate and thus too much higher time cost for practical application. Consequently, the black TiO2 polishing slurry under xenon lamp illumination achieved energy efficiency superiority with the PTCMP approach, underscoring its significant advantage over traditional PCMP.
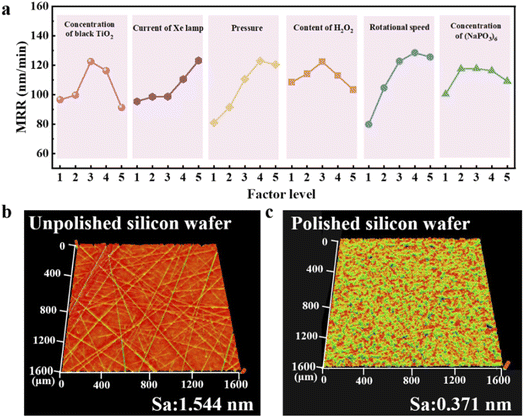
Next, we further demonstrated the tunability in MRR and roughness through adjustments to polishing slurry formula and experimental parameters. Orthogonal experiments were conducted using a six-factor, five-level design (Tables S4 and S5). Surface roughness (Sa) of 25 polished silicon wafer groups was characterized using a white light interferometer instead of AFM to evaluate the polishing quality at larger scale. Three random 100 × 100 µm regions per wafer were measured, and their average roughness values were calculated, as reported in Table S6. The white light interferometer image of the region with the lowest roughness is shown in Fig. S8. The MRR varied in a relatively wide range from 27.49 to 144.37 nm min−1, and the average surface roughness was below 0.5 nm for most conditions, in a range from 0.269 to 0.539 nm. Therefore, we focused our investigation on MRR with respect to the influence by the factors. In orthogonal experiments, for factor j at level n, Kjn denotes the sum of results, and kjn represents the mean. Kjn quantifies the effect of each level on the experimental outcomes (eqn (9))
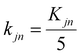 | (9) |
| Rj = kij(max) − kij(min) | (10) |
Fig. 4a illustrates the impact of each factor on the silicon wafer material removal rate. Orthogonal analysis indicates that a larger range corresponds to a greater influence on polishing performance, with factors ranked in order of significance: polishing pressure, rotational speed, black TiO2 concentration, xenon lamp current, hydrogen peroxide (H2O2) concentration and sodium hexametaphosphate ((NaPO3)6) concentration. The optimized parameters were thus determined as follows: polishing pressure of 350 g cm−2, rotational speed of 65 rpm, black TiO2 concentration of 1.00 g L−1, xenon lamp current of 21 A, H2O2 concentration of 10% and (NaPO3)6 concentration of 1.00 g L−1. Under these conditions, photothermal catalytic polishing achieved a material removal rate of 147.98 nm min−1 and an average surface roughness (Sa) of 0.332 nm. Note that Sa determined from white light interferometer for such larger area is expected to be larger than Ra determined from AFM for the small area. To reveal the high surface quality at even larger area scale, white light interferometer images of the wafer surface before and after polishing are presented in Fig. 4b and c. Within a 1600 × 1600 µm region, no scratches or obvious protrusions were observed on the smooth surface after the optimized PTCMP process, and a low Sa of 0.371 nm was obtained for this large area. Additionally, we also investigated the dependence of polishing performance on polishing time. Fig. S9a depicts variations in silicon wafer mass (Δm) and average surface roughness (Saave) over time under optimized polishing conditions. As polishing duration increases, material removal intensifies significantly during the first 30 min but slows down afterwards. Initially, surface roughness also decreases sharply due to the elimination of scratches, enhancing surface quality. However, prolonged polishing, increased roughness, likely due to abrasive particle wear or agglomeration, reducing their uniformity and cutting efficiency. To validate this hypothesis, we performed dynamic light scattering (DLS) analysis on the slurry collected from the waste liquid outlet after 10 and 60 minutes of polishing, respectively. As shown in Fig. S9b, the average particle size in the slurry increased from 102 nm to 204 nm after 60 minutes. Given that the concentration of silicon wafer abrasion debris is low and comparable across samples, we attribute the observed increase in particle size primarily to the agglomeration of the slurry particles themselves. So far, we have demonstrated tunability in the polishing performance for PTCMP via various experimental parameters in our prototypical setup, which achieved promising results and is crucial for refinement to accommodate practical processing needs. Simultaneously, we compared our polishing performance metrics with those reported in similar studies for rapid material removal (Table S8). Our approach exhibits significant advantage in achieving lower surface roughness while maintaining a competitive MRR. Even further improvement should be anticipated with more advanced polishing equipment and slurry formula.
3.3. Chemical mechanism
In the preceding sections, we demonstrated successful synthesis and selection of black TiO2 for the superior polishing performance to that of pristine TiO2, as well as the photothermal advantage. However, the origin of the enhancement and the mechanism of the chemical process remain to be clarified.The optical and chemical differences between black TiO2 and pristine TiO2 are first correlated with X-ray photoelectron spectroscopy (XPS) analysis. Deconvolution of the O 1s XPS spectra (Fig. 5a) reveals two distinct components: a peak centered at 529.68 eV, labeled OI, corresponding to the characteristic binding energy of lattice oxygen in pristine TiO2, and a broader peak centered at 530.98 eV, labeled OII, attributed to Ti–OH surface species.50,51 The Ti 2p spectra in Fig. 5b show lower binding energies for black TiO2 compared to pristine TiO2, consistent with Ti3+ formation, as Ti4+ exhibits higher binding energy due to electron loss. This increase in Ti3+ suggests elevated defect states, enhancing light absorption and photocatalytic activity.40,46 Valence band XPS (Fig. 5c) reveals a shift in the black TiO2 spectrum toward the Fermi level relative to pristine TiO2, indicating a narrowed bandgap or defect energy levels. The valence band maximum of pristine TiO2 is approximately 2.26 eV below the Fermi level, while that of black TiO2 is 2.04 eV, as determined by tangent extrapolation. Using prior UV-Vis bandgap data, the schematic diagram of density of states (DOS) for black TiO2 and pristine TiO2 is depicted in Fig. 5d. Black TiO2 exhibits shifted conduction band (−0.63 eV vs. NHE) and valence band (2.04 eV vs. NHE) positions compared to pristine TiO2 (−1.00 eV and 2.26 eV vs. NHE), suggesting a band structure optimized for photothermal synergistic catalysis.45
Fig. 5e shows that black TiO2 generates a higher photocurrent than pristine TiO2, reaching 11.31 nA cm−2 at 0.1 V compared to 6.01 nA cm−2 for pristine TiO2, a 1.88-fold increase, though peak currents decay was also observed within each illumination cycle. This enhancement likely arises from surface defects, such as oxygen vacancies and Ti3+, acting as electron capture centers to enhance photogenerated electron–hole pair separation,44 ideal for photocatalysis. Linear sweep voltammetry curves in Fig. S10 also confirm this, with black TiO2 exhibiting a significantly higher photocurrent than pristine TiO2 above 0.7 V. Photoluminescence (PL) emission spectra under 310 nm excitation (Fig. 5f) reveals lower emission intensity for black TiO2 compared to pristine TiO2, indicating superior carrier separation efficiency, corroborating earlier observations. Electron paramagnetic resonance (EPR) analysis reveals strong signals at approximately g = 2.00, confirming the presence of oxygen vacancies, as identified in prior studies49 and shown in Fig. 5g. High-resolution transmission electron microscope (HRTEM) further reveals structural differences between the black and pristine TiO2. Fig. 5h shows a black TiO2 nanoparticle, with lattice fringes analyzed in Fig. S11a revealing spacing of approximately 0.352 nm in the core regions, corresponding to the anatase (101) plane. However, at the particle edges, uneven lattice fringes indicate a highly defected surface layer. Conversely, Fig. 5i depicts pristine TiO2, displaying clear, uniformly spaced lattice fringes even at the edges, as corroborated in Fig. S11b. Again, the defects reveals benefited light absorption and photocatalytic activity.
To further investigate the origin of enhanced chemical process in the photothermal catalytic polishing mechanism, more EPR experiments were conducted to compare the reactive species concentrations in black TiO2 and pristine TiO2 polishing slurries under identical illumination conditions. Significantly higher EPR peak intensities reflecting greater reactive species concentrations of ·OH, ·O2−, and 1O2 were observed in black TiO2 than in pristine TiO2. For photogenerated hole detection, TEMPO, a persistent free radical with an inherent EPR signal, was used as the trapping agent, of which the significant diminishment of signal indicates the abundance of holes generation, unlike DMPO and TEMP. Accordingly, enhanced hole generation in black TiO2 was observed compared to pristine TiO2, consistent with the increased levels of ·OH, ·O2−, and 1O2, owing to the broadened and enhanced light absorption of black TiO2 (Fig. 6a–d).
To elucidate the contributions of reactive species in photothermal catalytic polishing, specific quenching agents were employed: tert-butanol for ·OH, p-benzoquinone for ·O2−, L-histidine for 1O2, and ammonium oxalate for h+.52–54 Results presented in Fig. 6e, show the most significant reduction in MRR when ·OH is quenched, indicating its dominant role in the process. The influence of these species on polishing performance, in descending order, is ·OH, ·O2−, h+, and 1O2. Accordingly, the main chemical mechanism is proposed.
| Si + 4·OH → SiO2 + H2O | (11) |
Hydroxyl radicals (·OH), with their potent oxidative capacity, attack silicon wafer surfaces, breaking Si–Si bonds to form a thin, porous silicon dioxide (SiO2) layer. In CMP, silica abrasives in the slurry engage this SiO2 layer through mechanical friction. The thin, loosely structured SiO2 layer is readily detached by abrasive shear forces. Furthermore, the photothermal effect of black TiO2 enhances reaction kinetics, facilitating rapid atomic-scale material removal from the wafer surface.
Since the oxidation mechanism of reactive species plays a central role in photothermal catalytic polishing, it should be extendable to other oxidizable substrates. To demonstrate this, under identical conditions, we compared the MRR of black-TiO2 and pristine TiO2 polishing slurries for silicon carbide (SiC) and quartz glass substrates, as shown in Fig. 6. For the SiC substrate (Fig. 6f), the MRR achieved with black-TiO2 slurry reached 102.56 nm h−1, significantly higher than that of pristine TiO2 slurry (71.80 nm h−1), representing a 43% improvement. Similarly, for quartz glass (Fig. 6g), the MRR of black-TiO2 slurry (231.26 nm h−1) exceeded that of pristine TiO2 slurry (160.71 nm h−1) by 44%. These results demonstrate that the black-TiO2 photothermal catalytic polishing slurry substantially enhances the MRR compared to conventional photocatalytic polishing slurries for versatile substrates.
4. Conclusion
In this study, a novel broadband-light-driven photothermal chemical mechanical polishing (PTCMP) technique was developed using defect-engineered black TiO2. Oxygen vacancies and Ti3+ species, introduced via NaBH4 reduction, enhance the optical absorption of black TiO2 across the visible to near-infrared spectrum, thereby enabling superior photochemical and photothermal performance. Unlike conventional photocatalytic polishing limited by narrow bandwidth of light and carrier recombination, or thermal methods requiring extreme conditions, PTCMP leverages localized photothermal heating and increased radical generation to achieve fast material removal and atomic-level surface roughness without thermal damage. Feasible tunability in the polishing performance was demonstrated by varying process parameters through orthogonal experiments, while radical quenching studies identified the critical role of reactive species, with their influence ranked as ·OH > ·O2− > h+ > 1O2. Based on the oxidative chemical mechanism, PTCMP demonstrates extendable advantage to the polishing of other oxidizable substrates. By integrating energy-efficient broadband light utilization with defect engineering, this approach establishes a sustainable paradigm for semiconductor manufacturing, overcoming longstanding limitations in catalytic polishing technologies.Author contributions
Shichang W., C. X. and W. S. conceived and designed the experiments. Shichang W., C. X. and Shenghua W. performed the synthesis experiments. Shichang W., C. Z., H. F. and Y. D. performed the structural characterization of catalysts. Shichang W., S. Y. and Zuozuo W. performed the polishing experiments. Shichang W., C. X. and Ziyi W. performed and analysed the EPR experiments. Y. W. and Z. H. performed the heat transfer simulation. Shichang W., W. S. wrote the paper. W. S., D. Y., Shenghua W., and S. Y. supervised the project. All authors commented on the final paper.Conflicts of interest
A Chinese patent application (application no. 202510261305.6; Inventors: W. S., S. W., C. X., Z. W., S. Y., D. Y.) related to part of this work has been authorized. The other authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ta07359f.Acknowledgements
The project was supported by the National Key R&D Program of China (2021YFF0502000), the Natural Science Foundation of Zhejiang Province (LDQ23E020002), the Inner Mongolia Science and Technology Project, and the Hohhot Science and Technology Project (2024-JBGS-G-2), the China Postdoctoral Science Foundation (2024M762820), Central Guidance Funds for Local Science and Technology Development Projects (2025ZY01012).References
- A. Matei, A. Visan and I. Negut, Micromachines, 2025, 16, 573 CrossRef PubMed.
- J. Li, H. Hu, G. Qiao, E. Qi, Y. Bai, F. Li, Z. Liu, L. Li, Z. Yang and X. Zhang, Precis. Eng., 2025, 94, 725–735 CrossRef.
- Z. Luo, Z. Zhang, F. Zhao, C. Fan, J. Feng, H. Zhou, F. Meng, X. Zhuang and J. Wang, Mater. Today Sustain., 2024, 27, 100841 Search PubMed.
- Z. Zhang, Y. Du, B. Wang, Z. Wang, R. Kang and D. Guo, Tribol. Lett., 2017, 65, 132 CrossRef.
- Y. Zhang, X. Niu, J. Zhou, J. Wang, C. Yang, Z. Hou, Y. Zhu and L. Huang, Mater. Sci. Semicond. Process., 2022, 140, 140 Search PubMed.
- W. Li, Q. Xin, B. Fan, Q. Chen and Y. Deng, Micromachines, 2024, 15, 178 CrossRef.
- J. Ji, K. Yamamura and H. Deng, Acta Phys.-Chim. Sin., 2021, 70, 068102 CrossRef.
- T. Arnold, G. Böhm, R. Fechner, J. Meister, A. Nickel, F. Frost, T. Hänsel and A. Schindler, Nucl Instrum Meth A, 2010, 616, 147–156 CrossRef CAS.
- Y. Wang, Y. Zhao, W. An, Z. Ni and J. Wang, Appl. Surf. Sci., 2010, 257, 249–253 CrossRef CAS.
- X. Zhu, J. Ding, Z. Mo, X. Jiang, J. Sun, H. Fu, Y. Gui, B. Ban, L. Wang and J. Chen, Appl. Surf. Sci., 2025, 679, 161157 CrossRef CAS.
- Z. Geng, Y. He and F. Fang, J. Manuf. Process., 2025, 134, 384–393 CrossRef.
- S. Chen and H. Lei, Friction, 2025, 13, 9440993 CrossRef CAS.
- H. Yang, Z. Jin, L. Niu, H. Wang and H. Niu, Diam. Relat. Mater., 2022, 125, 108982 CrossRef CAS.
- T. Wang, Y. Chen, A. Chen and Y. Chen, Appl. Surf. Sci., 2022, 593, 153449 CrossRef CAS.
- Z. Geng and F. Fang, J. Manuf. Process., 2025, 133, 201–210 CrossRef.
- Z. Han, B. Ran, J. Pan and R. Zhuang, Micromachines, 2025, 16, 380 CrossRef.
- J. Shao, Y. Zhao, J. Zhu, Z. Yuan, H. Du and Q. Wen, Machines, 2023, 11, 664 CrossRef.
- H. Lee, Lubricants, 2023, 11, 229 CrossRef CAS.
- J. Zhang, H. Chen, X. Duan, H. Sun and S. Wang, Mater. Today, 2023, 68, 234–253 CrossRef CAS.
- R. Ma, J. Sun, D. Li and J. Wei, Int. J. Hydrogen Energy, 2020, 55, 30288–30324 CrossRef.
- S. Luo, X. Ren, H. Lin, H. Song and J. Ye, Chem. Sci., 2021, 12, 5701–5719 RSC.
- D. Mateo, J. Cerrillo, S. Durinia and J. Gascon, Chem. Soc. Rev., 2021, 50, 2173–2210 RSC.
- J. Wang, G. Ran, J. Gao, D. Li, G. Waterhouse, R. Shi, W. Zhang, J. Tang, L. Wu, Y. Zhao and T. Zhang, Adv. Mater., 2025, 37, 2420199 CrossRef CAS.
- Y. Li, X. Bai, D. Yuan, F. Zhang, B. Li, X. San, B. Liang, S. Wang, J. Luo and G. Fu, Nat. Commun., 2022, 13, 776 CrossRef CAS PubMed.
- J. Feng, H. Huang, T. Fang, X. Wang, S. Yan, W. Luo, T. Yu, Y. Zhao, Z. Li and Z. Zou, Adv. Funct. Mater., 2019, 29, 1808389 CrossRef.
- C. Xing, H. Cai, D. Kang and W. Sun, Adv. Energy Sustainability Res., 2023, 4, 2300015 CrossRef CAS.
- C. Song, Z. Wang, Z. Yin, D. Xiao and D. Ma, Chem Catal., 2022, 2, 52–83 CAS.
- Z. Sun, X. Huang and G. Zhang, J. Clean. Prod., 2022, 381, 135156 CrossRef CAS.
- W. Li, G. Lv, M. Liu, F. Zhao, P. Shuai, Y. Feng, D. Chen and L. Liao, J. Energy Chem., 2025, 108, 332–360 CrossRef CAS.
- Y. Xiao, X. Li, T. Zheng, K. Xiao and Y. Wang, Coord. Chem. Rev., 2024, 517, 216017 CrossRef CAS.
- T. Li, Z. Zhang, D. Luo, B. Xu, R. Zhang, J. Yao, D. Li and T. Xie, Nano Res., 2024, 17, 7945–7956 CrossRef CAS.
- S. Wang, D. Zhang, W. Wang, J. Zhong, K. Feng, Z. Wu, B. Du, J. He, Z. Li, L. He, W. Sun, D. Yang and G. Ozin, Nat. Commun., 2022, 13, 5305 CrossRef CAS.
- X. Geng, Y. Xia, M. Zhu, J. Zhao, D. Yao and Y. Zeng, J. Eur. Ceram. Soc., 2025, 45, 117385 CrossRef CAS.
- C. Zhang, Z. Wu, J. Shen, L. He and W. Sun, Acta Phys.-Chim. Sin., 2024, 40, 2304004 CrossRef.
- X. Wu, S. Zhang, S. Ning, C. Yang, L. Li, L. Tang, J. Wang, R. Liu, X. Yin, Y. Zhu, S. Chen and J. Ye, Chem. Sci., 2025, 16, 4568–4594 RSC.
- D. Zhang, F. Zhao, C. Lv, M. Xu, L. Gao, S. Ning, Y. Hao, Y. Li and J. Ye, Appl. Surf. Sci., 2025, 708, 163674 CrossRef CAS.
- H. Guo, J. Xie, G. He, D. Zhu, M. Qiao, J. Yan, J. Yu, J. Li, Y. Zhao, M. Luo and H. Han, Nano Res., 2024, 17, 6212–6230 CrossRef.
- D. Zhu, P. Zuo, F. Li, H. Tian, T. Liu, L. Hu, H. Huang, J. Liu and X. Qian, Colloid Interface Sci., 2024, 59, 100770 CrossRef CAS.
- X. Chen, L. Liu and F. Huang, Chem. Soc. Rev., 2015, 44, 1861–1885 RSC.
- Q. Kang, J. Cao, Y. Zhang, L. Liu, H. Xu and J. Ye, J. Mater. Chem. A, 2013, 1, 5766–5774 RSC.
- F. Pesci, G. Wang, D. Klug, Y. Li and A. Cowan, J. Phys. Chem. C, 2013, 117, 25837–25844 CrossRef CAS PubMed.
- M. Ye, J. Jia, Z. Wu, C. Qian, R. Chen, P. O'Brien, W. Sun, Y. Dong and G. Ozin, Adv. Energy Mater., 2017, 7, 1601811 CrossRef.
- S. Fang and Y. Hu, Chem. Soc. Rev., 2022, 51, 3609–3647 RSC.
- S. Chen, Y. Xiao, Y. Wang, Z. Hu, H. Zhao and W. Xie, Nanomaterials, 2018, 16, 245 CrossRef PubMed.
- T. Lin, C. Yang, Z. Wang, H. Yin, X. Lü, F. Huang, J. Lin, X. Xie and M. Jiang, Energy Environ. Sci., 2014, 7, 967–972 RSC.
- T. Rajaraman, S. Parikh and V. Gandhi, Chem. Eng. J., 2020, 389, 123918 CrossRef CAS.
- I. Nakamura, N. Negishi, S. Kutsuna, T. Ihara, S. Sugihara and K. Takeuchi, J Mol Catal A-Chem, 2000, 161, 205–212 CrossRef CAS.
- H. Tan, Z. Zhao, M. Niu, C. Mao, D. Cao, D. Cheng, P. Feng and Z. Sun, Nanoscale, 2014, 6, 10216–10223 RSC.
- U. Diebold, Surf. Sci. Rep., 2003, 48, 53–229 CrossRef CAS.
- E. McCafferty and J. Wightman, Surf. Interface Anal., 1998, 26, 549–564 CrossRef CAS.
- X. Chen, L. Liu, P. Yu and S. Mao, Science, 2011, 331, 746–750 CrossRef CAS PubMed.
- G. Buxton, C. Greenstock, W. Helman and A. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886 CrossRef CAS.
- F. Wilkinson, W. Helman and A. Ross, J. Phys. Chem. Ref. Data, 1995, 24, 663–677 CrossRef CAS.
- P. Rao and E. Hayon, J. Phys. Chem., 1975, 79, 397–402 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |