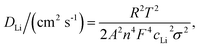Direct regeneration of cathodes from spent LIB black mass through an integrated roasting-flotation method with impurity-tailored self-decontamination
Yuchen
Li†
 ,
Zhengni
Ye†
,
Shuai
Wang
,
Hong
Zhong
,
Zhanfang
Cao
,
Zhengni
Ye†
,
Shuai
Wang
,
Hong
Zhong
,
Zhanfang
Cao
 * and
Xin
Ma
*
* and
Xin
Ma
*
Hunan Provincial Key Laboratory of Efficient and Clean Utilization of Manganese Resources, College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China. E-mail: zfcaocsu@163.com; maxin2013@csu.edu.cn
First published on 2nd December 2025
Abstract
To address the critical incompatibility between direct recovery technology and industrial battery-crushing technology, this work proposes a short-process, eco-friendly strategy for the direct regeneration of cathode materials from spent lithium-ion battery black mass (BM). In this strategy, the cathode material (designated S-NCM) with a 99.08% recovery and a 95.92% grade is first extracted greenly and nondestructively via flotation separation after roasting pretreatment. Crucially, the residual metal impurities in BM are ingeniously utilized to capture fluorinated pollutants (e.g., HF) during the roasting process, effectively eliminating hazardous gas emissions. Following targeted prelithiation and high temperature sintering, S-NCM is transformed into a regenerated cathode material (R-NCM). Electrochemical tests demonstrate that R-NCM delivers a reversible specific capacity of 139.27 mAh g−1 at 0.1C and maintains 91.85% capacity retention after 100 cycles. Techno-economic analysis confirms the strategy's significant environmental benefits and commercial viability. The total energy consumption of direct regeneration (2.88 MJ kg−1) is substantially lower than that of pyro-methods (39.12 MJ kg−1) and hydro-methods (42.97 MJ kg−1). Owing to its simplicity, low energy demand, and elimination of secondary synthesis, the regenerated cathode material can be directly used for battery remanufacturing. Consequently, the direct regeneration strategy offers 6.8- and 2.4-fold higher profitability than pyro- and hydro-methods, respectively. This study provides a sustainable solution for the large-scale direct regeneration of spent battery-derived BM.
Introduction
The power and energy storage markets are undergoing significant expansion, propelled by the ongoing pursuit of global carbon neutrality.1,2 Over the years, lithium-ion batteries have developed rapidly, with the emergence of layered nickel cobalt lithium manganate (NCM, LiNixCoyMnzO2, x + y + z = 1) ternary cathode materials becoming a prominent type of lithium-ion battery cathode material in the early market due to their distinctive advantages.3 However, lithium-ion batteries are subject to a finite lifespan.4 Current projections indicate that by the year 2030, the global market for decommissioned battery recycling will exceed 10 billion dollars; retired batteries are therefore regarded as the “second mine”.5 Moreover, China's external reliance on critical metals such as cobalt, nickel, and lithium surpasses 80%.6 The EU Battery Regulation stipulates that beginning in 2030, recycled key elements must be utilized in the fabrication of batteries with capacities exceeding 2 kWh.7 Moreover, batteries contain elevated concentrations of heavy metals and toxic components. Inadequate storage practices are likely to result in environmental contamination.8 All things considered, it is imperative to engage in the recycling of batteries. The current commercialized recovery processes are primarily pyrometallurgical or hydrometallurgical in nature,9 each boasting mature technology and high recovery efficiency, yet there are limitations, including high energy consumption and the challenges associated with wastewater treatment.10,11 With increasing upstream raw material prices12 and continuously rising performance demands for lithium-ion batteries in downstream applications,13 cost reduction and efficiency improvement have become key driving forces for the development of battery recycling and regeneration technologies.14 In this regard, there is an urgent need to develop new recycling technologies that are more cost-effective and in line with the concept of low-carbon development.At present, direct regeneration is regarded as a method with considerable potential for the non-destructive recycling of spent cathode materials.15 Chi et al.16 employed a high-temperature solid-phase method to directly regenerate NCM111, utilizing the residual lithium salt layer on the surface of the spent cathode material as a lithium source, significantly lowering the total energy consumption compared with the conventional pyro-/hydro-recycling methods. Guo et al.17 successfully obtained NCM523 in the presence of superficial degradation by direct regeneration, achieving superior greenhouse gas (GHG) reduction and profitability versus conventional pyro-/hydro-routes. However, such studies predominantly utilize artificially dismantled cathodes with negligible impurities—a paradigm misaligned with industrial realities. Manual disassembly presents inherent safety hazards and scalability constraints, while automated mechanical processes face challenges owing to diverse battery form factors.18,19 Consequently, mixed black mass (BM) from direct post-discharge crushing and screening dominates recycler feedstock, comprising interlocked anode/cathode materials, PVDF binders, residual electrolytes, conductive additives, and metal impurities (Al, Cu, etc.).20 Direct regeneration of this complex mixture could revolutionize recycling efficiency, but it remains largely unexplored due to two fundamental barriers: (1) non-destructive separation of anode and cathode components and (2) mitigation of impurity interference during regeneration. Flotation technology offers a promising route to overcoming the first challenge by exploiting the distinct surface hydrophobicity of graphite anodes and cathode materials to achieve efficient separation.
Notably, Verdugo et al.21 mixed anode and cathode materials obtained from artificial dismantling and then performed flotation experiments. The findings indicate that implementing a suitable pretreatment (e.g. roasting, pyrolysis and organic solvent method) can remarkably enhance the flotation grade of electrode materials. Although pretreatment facilitates the restoration of the hydrophilic difference between electrode materials and enhances flotation separation efficiency, it must be noted that PVDF or electrolyte undergoes decomposition during roasting or pyrolysis, thereby releasing fluorinated pollutants, such as HF.22 In recent studies, Li et al.23 and Huang et al.24 proposed that metal oxides or hydroxides can exhibit effective fluoride fixation during pyrolysis. Furthermore, the generated metal fluoride coatings have the potential to enhance the electrochemical performance of regenerated NCMs. However, due to direct crushing and screening after discharge, some metal impurities are inevitably mixed in BM.25 In summary, we propose an intriguing hypothesis. During the roasting pretreatment process, metal impurities and fluorinated pollutants in BM react with each other and are converted in situ to metal fluorides. This process may not only release agglomerated electrode materials and enhance flotation separation efficiency but also inhibit the generation of polluting gases, such as HF, by utilizing metal impurities. Concurrently, the generation of metal fluoride does not exert a detrimental influence on the subsequent direct regeneration of the cathode material.26
Hence, in this study, we especially selected the anode and cathode mixed black mass of spent NCM523 batteries, which was obtained by crushing and screening directly after discharging, as the raw material. Advanced characterization tools, such as thermogravimetric mass spectrometry (TG-MS) and thermogravimetric infrared (TG-FTIR) coupling, were used to ensure that no polluting gases, such as HF, were generated during the roasting pretreatment process. Subsequent flotation separation yielded S-NCM. Following an analysis of the degradation mechanism, the regenerated LiNi0.5Co0.2Mn0.3O2 (R-NCM) material was obtained using a high-temperature solid-state method. The material's initial discharge specific capacity was 139.27 mAh g−1 at 0.1C, after 100 cycles, with a capacity retention rate of 91.85%. These findings indicate that a simple, effective, economical, and environmentally friendly strategy for the direct recovery and regeneration of battery black mass is proposed in this study. This strategy is highly compatible with existing industrialized battery crushing technologies. It provides an environmentally sustainable solution for the direct regeneration of black mass from spent batteries, which is expected to be applied on a large scale.
Experimental section
Chemicals and materials
The anode and cathode mixed black mass of waste NCM523 batteries was provided by a battery crushing and recycling enterprise in Tianjin. The results of the X-ray diffractometry (XRD) and X-ray fluorescence detection (XRF) analyses are presented in Fig. S1 and Table S1, respectively. Lithium carbonate and N-methyl-2-pyrrolidone (NMP) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. N-Dodecane was purchased from Shanghai Macklin Biochemical Co., Ltd. 4-Methyl-2-pentanol (MIBC) was purchased from Tokyo Chemical Industry Co., Ltd. Acetone was purchased from Chengdu Kelong Chemical Co., Ltd. Polyvinylidene fluoride (PVDF) and acetylene black (Super-B) were purchased from Guangdong Canrd New Energy Technology Co., Ltd. The electrolyte (1 mol L−1 LiPF6 in DEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EC
EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC = 1
EMC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%) was purchased from DodoChem. Various appropriate concentrations of hydrochloric acid and sodium hydroxide solutions were used to adjust the pH, and distilled water was utilised throughout the experimentation process.
1 vol%) was purchased from DodoChem. Various appropriate concentrations of hydrochloric acid and sodium hydroxide solutions were used to adjust the pH, and distilled water was utilised throughout the experimentation process.
Pretreatment and flotation processes
The roasting pretreatment of BM was carried out in a muffle furnace (KSL-1100X-S). It was increased to the specified temperature at 5 °C min−1 in an air atmosphere and held for 2 h, and then, the furnace was slowly cooled. Post-roasting BM (designated as P-BM) was collected, and both pre- and post-roasting BM were passed through a 60-mesh sieve. Flotation experiments were carried out using an XFG II flotation machine with an impeller speed set at 1902 r min−1. Each flotation test was repeated thrice, and the mean value of the three flotation results was used as the final result. N-dodecane (10 mg L−1) and 4-methyl-2-pentanol (6 mg L−1) were used as the collector and frother, respectively. The flotation process is illustrated in Fig. S2. The pH was adjusted by employing 0.1 and 1 mol L−1 hydrochloric acid, and sodium hydroxide solutions that were prepared in advance to regulate the pH of the slurry to approximately 10. Subsequent to flotation separation, hydrophobic anode graphite was incorporated into the froth product, while the hydrophilic cathode material was retained in the slurry product. The products were then filtered, dried and weighed. Ultimately, the flotation recovery and grade were calculated using the formulas delineated in the SI. The collected cathode material was designated as S-NCM.Direct regeneration
S-NCM and Li2CO3 were thoroughly amalgamated in the ratio of Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M(Ni + Co + Mn) = 1.15
M(Ni + Co + Mn) = 1.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Thereafter, the mixture was transferred to a ball milling tank, and the ball milling speed was set at 400 r min−1 for 3 h. Acetone was utilized as the dispersant. Following the ball milling process, the zirconia beads were removed using tweezers, and then the ball milling tank was placed in a blast drying oven at a temperature of 60 °C (dry away acetone). The obtained well-mixed sample was manually ground, dispersed, and sieved, prior to being pressed into small discs with diameters of 18 mm using a press (PC-3). The discs were then calcined at a high temperature of 900 °C for 10 h to obtain regenerated LiNi0.5Co0.2Mn0.3O2, which was designated as RDC-NCM. After annealing at 750 °C for 1–5 h, following a cooling period, the discs were ground and dispersed, sieved, and further calcined at 900 °C for 10 h to yield regenerated LiNi0.5Co0.2Mn0.3O2, which was designated as R-NCM. All heating procedures were conducted in an air atmosphere at a heating rate of 5 °C min−1.
1. Thereafter, the mixture was transferred to a ball milling tank, and the ball milling speed was set at 400 r min−1 for 3 h. Acetone was utilized as the dispersant. Following the ball milling process, the zirconia beads were removed using tweezers, and then the ball milling tank was placed in a blast drying oven at a temperature of 60 °C (dry away acetone). The obtained well-mixed sample was manually ground, dispersed, and sieved, prior to being pressed into small discs with diameters of 18 mm using a press (PC-3). The discs were then calcined at a high temperature of 900 °C for 10 h to obtain regenerated LiNi0.5Co0.2Mn0.3O2, which was designated as RDC-NCM. After annealing at 750 °C for 1–5 h, following a cooling period, the discs were ground and dispersed, sieved, and further calcined at 900 °C for 10 h to yield regenerated LiNi0.5Co0.2Mn0.3O2, which was designated as R-NCM. All heating procedures were conducted in an air atmosphere at a heating rate of 5 °C min−1.
Material characterizations
The elemental composition and content of the cathode materials were determined using inductively coupled plasma emission spectroscopy (ICP-OES, PerkinElmer Avio500). The crystal structure of the materials was determined using an X-ray diffractometer (XRD, D8 Advance) and refined using GSAS II software. The chemical state and molecular structure of the elements were characterized using X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific/NEXSA). The micro-morphology and internal structure of the materials were examined by field emission scanning electron microscopy (FE-SEM, JEOL/JSM-7610FPlus) and transmission electron microscopy (TEM, JEOL JEM 2100 F), respectively. The contact angle test was conducted using a JC2000C contact angle analyzer. A coupled thermogravimetric mass spectrometer (TG-MS, Netzsch STA449F3/Netzsch QMS 403Q) was used to analyze the molecular weight of the gaseous substances produced during the roasting process, with a mass detection range of M/Z: 1–300 and an MS signal acquisition time of 1 s. Thermogravimetric infrared (TG-FTIR, TA TGA55/Thermo Scientific Nicolet iS50) coupling was utilized to detect the functional groups of the gaseous substances within the Fourier transform spectral range of 500–4000 cm−1 and in the temperature range of 50–600 °C.Electrochemical characterization
Conductive carbon black (Super-P) was sieved and dried in advance. Polyvinylidene fluoride (PVDF) was dissolved in N-methyl-2-pyrrolidone (NMP) to formulate a 5 wt% PVDF/NMP solution. The cathode active material and conductive carbon were thoroughly ground in an agate mortar, and the resultant mixture was transferred to a weighing bottle. The PVDF/NMP solution was then added, ensuring that the mass ratio of the cathode active material, conductive carbon (Super-P), and polyvinylidene fluoride (PVDF) was 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. An additional 550–600 µL of N-methyl-2-pyrrolidone (NMP) was added to the slurry during the process. Furthermore, magnetic stirring was conducted for 4–5 h until the slurry was well mixed and had optimal fluidity. The modulated slurry was uniformly coated onto aluminum foil with a thickness of 150 µm and dried at 60 °C in a blast drying oven and 120 °C in a vacuum drying oven. The coated sheet was then cut into circular cathode pole pieces with a diameter of 12 mm. The pieces were weighed and collected for spare use, ensuring that the mass of active material loaded on each pole piece fell within the range of 2–3 mg. The assembly of the CR2016-type coin cell was conducted in an Ar-filled glove box, with Li metal as the anode and Celgard 2400 film as the diaphragm. The electrolyte was 1 mol L−1 lithium hexafluorophosphate (LiPF6) dissolved in ethylene carbonate (EC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC) at a volume ratio of 1
1. An additional 550–600 µL of N-methyl-2-pyrrolidone (NMP) was added to the slurry during the process. Furthermore, magnetic stirring was conducted for 4–5 h until the slurry was well mixed and had optimal fluidity. The modulated slurry was uniformly coated onto aluminum foil with a thickness of 150 µm and dried at 60 °C in a blast drying oven and 120 °C in a vacuum drying oven. The coated sheet was then cut into circular cathode pole pieces with a diameter of 12 mm. The pieces were weighed and collected for spare use, ensuring that the mass of active material loaded on each pole piece fell within the range of 2–3 mg. The assembly of the CR2016-type coin cell was conducted in an Ar-filled glove box, with Li metal as the anode and Celgard 2400 film as the diaphragm. The electrolyte was 1 mol L−1 lithium hexafluorophosphate (LiPF6) dissolved in ethylene carbonate (EC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC) at a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The charge–discharge tests were performed on a LAND BT2000 battery test system at a test temperature of 28 °C with a standard current density of 160 mA g−1 and a voltage range of 2.5–4.2 V (a conservative and stable range suitable for evaluating regenerated cathode materials). Cyclic voltammetry curves (CV) and electrochemical impedance spectroscopy (EIS) were performed on a CHI760E electrochemical workstation with an AC frequency range of 10 mHz–100 kHz and a cell open-circuit potential perturbation of 5 mV at 28 °C. The voltage range and scanning rate of the CV tests were 2.5–4.2 V and 0.1 mV s−1, respectively. Z-view software was utilized to model the EIS test data. Electrochemical data were generally measured three times, and average values were taken.
1. The charge–discharge tests were performed on a LAND BT2000 battery test system at a test temperature of 28 °C with a standard current density of 160 mA g−1 and a voltage range of 2.5–4.2 V (a conservative and stable range suitable for evaluating regenerated cathode materials). Cyclic voltammetry curves (CV) and electrochemical impedance spectroscopy (EIS) were performed on a CHI760E electrochemical workstation with an AC frequency range of 10 mHz–100 kHz and a cell open-circuit potential perturbation of 5 mV at 28 °C. The voltage range and scanning rate of the CV tests were 2.5–4.2 V and 0.1 mV s−1, respectively. Z-view software was utilized to model the EIS test data. Electrochemical data were generally measured three times, and average values were taken.
Economic and environmental assessments
The energy consumption, greenhouse gas (GHG) emissions, and economic benefits of different recycling processes were evaluated and compared using the EverBatt 2023 model. Taking the example of processing 1000 tons of waste NCM523 anode- and cathode-mixed black mass annually, the modeling analysis was executed under delineated assumptions.Results and discussion
The SEM-EDS images of the raw BM show that the presence of organic impurities, such as PVDF, in the raw BM leads to significant agglomeration and coating (Fig. 1A). On the one hand, agglomeration or coating exacerbates the entrainment phenomenon in the flotation process. On the other hand, organic impurities adhere to the surface of the materials, causing a reduction in the original hydrophilicity difference between the anode and cathode materials. These are not conducive to effective flotation separation between the two. Considering that impurity metals, such as aluminum, iron and copper, are present in the raw BM (Table S1), the addition of a roasting pretreatment step prior to flotation is expected to address the above issues. Meanwhile, the polluting gases produced during the roasting process are hopefully immobilized in situ by the impurity metals or their oxides, eventually achieving a multifaceted effect. Therefore, we evaluated the extent of change and trend of the surface properties of the raw materials under three roasting temperature conditions, 525 °C, 550 °C and 600 °C, performed via SEM analysis, contact angle testing and XRD testing. During the process of contact angle testing, the powder samples were initially compressed into small discs using a press (PC-3). The surface contact angle was then measured using the hanging drop method at three distinct locations on each sample, with the average value calculated. SEM images (Fig. 1B) and contact angle test results (Fig. 1C) show that the higher the roasting temperature, the more dispersed the anode and cathode materials were. The contact angle of the 600 °C-PBM is smaller than the 525 °C-PBM and the 550 °C-PBM, which may be attributed to the oxidation loss of some hydrophobic graphites. The X-ray diffraction (XRD) patterns of pre- and post-roasting BM depicted in Fig. 1D indicate that the NaFeO2-type layered structure is preserved at 525 °C-PBM and 550 °C-PBM, and peaks related to metal fluoride are identified. However, the diffraction pattern of 600 °C-PBM exhibits three peaks at 37.6°, 43.6°, and 63.3°, which are ascribed to the (111), (200), and (220) crystal planes of NiO, respectively (PDF. 78-0643).27 The deterioration of the surface NiO-like rock salt structure hinders the direct regeneration of the cathode material in subsequent steps.28 This led to the selection of 525 °C-PBM and 550 °C-PBM for further characterization and flotation experiments. Fig. 1E shows the results of 525 °C-PBM and 550 °C-PBM utilizing 10 mg L−1 N−1-dodecane as a collector after one rougher flotation, where the lower left point is the experimental result of adding the collector alone, and the upper right point is the experimental result of adding 6 mg L−1 MIBC as a frother. The results indicate that when the roasting pretreatment temperature was set at 550 °C, the flotation recovery and grade of the cathode material that accumulated at the base of the flotation tank could attain 99.08% and 95.92%, respectively, under the combined influence of the collector and the frother. Further increasing the reagent dosage or secondary flotation only slightly increases the flotation recovery and grade of the cathode material. Thus, the cathode material obtained by flotation under these conditions, designated as S-NCM, was collected for the ensuing step of experimentation and characterization.To further investigate the presence of in situ fluoride fixation during the roasting pretreatment, TG-MS was initially employed to analyze the gas evolution of raw BM during the roasting process in an air atmosphere at temperatures ranging from 0 to 600 °C. Relevant results are presented in Fig. 2B. The TGA result indicates that the process could be divided into three stages: 0–181.13 °C, 181.13–460.63 °C and 460.63–600.00 °C. The main m/z values (mass-to-charge ratios) of ion fragments released were 12, 13, 22, 27, 29, 30, 31, 43, 44 and 45, respectively, corresponding to 12C+, 13CH+, 22CO22+, 27C2H3+, 29C2H5+, 30C2H6+, 31CH3O+, 43C3H7+/C2F+, 44CO2+/C3H8+/C2HF+ and 45C2H2F+, respectively. The absence of ion fragment peaks of 19F+ and 20HF+ throughout the roasting process suggests two possible scenarios: (1) no HF gas was produced, or (2) the HF produced was absorbed and removed by other substances present in BM. Since PVDF is difficult to oxidize or decompose below 400 °C,24 the 12C+, 27C2H3+, 29C2H5+, 30C2H6+, 31CH3O+, 43C3H7+/C2F+, 44CO2+/C3H8+/C2HF+ and 45C2H2F+ ion fragments detected below 400 °C can be attributed to the decomposition of the electrolyte, the solid electrolyte interface (SEI), Li2CO3 or a chemical reaction between PVDF and another substance, leading to the breakage of some C–C bonds or C–H bonds in the PVDF molecular chain. Furthermore, within the range of 460.63–600.00 °C, the ionic strength curves of 12C+, 44C3H8+/C2HF+ and 45C2H2F+ gradually increased. The ionic strength curves of 27C2H3+, 29C2H5+, and 30C2H6+ exhibited slight peaks. This phenomenon corresponds to the decomposition of PVDF. To further clarify the types of gases released during the roasting process, we also performed TG-FTIR characterization. As shown in Fig. 2C, the TG-FTIR spectrum displays a significant intensity change at around 2349 and 667 cm−1, which are attributed to the typical C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching vibration peak and the C–O in-plane bending vibration peak in the CO2 molecule, respectively.29 Thus, it can be determined that the gas with a molecular weight of 44 is CO2.
O asymmetric stretching vibration peak and the C–O in-plane bending vibration peak in the CO2 molecule, respectively.29 Thus, it can be determined that the gas with a molecular weight of 44 is CO2.
As the flotation procedure does not modify the elementary species and their chemical states on the material's surface, the raw BM and S-NCM were analyzed by XPS to indicate the chemical states of elemental C and F as a way to further elucidate the transformation mechanism of fluorine-containing contaminants on the material's surface before and after the roasting pretreatment. An analysis of the fitted high-resolution C 1s spectrum reveals that the –(CF![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH)–n peak in S-NCM replaces the two characteristic peaks of PVDF: –(CH2CF2)–n and –(CH2CF2)–n, and the content of the C
CH)–n peak in S-NCM replaces the two characteristic peaks of PVDF: –(CH2CF2)–n and –(CH2CF2)–n, and the content of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C peaks associated with the electrolyte decreases significantly. Furthermore, an analysis of the fitted high-resolution F 1s spectrum reveals that the characteristic peaks associated with organic fluorides, such as PVDF and electrolyte, are absent on the surface of the S-NCM, which are replaced by C–F peaks and metal-F peaks (Fig. 2D). In combination with the characteristic peaks of metal fluoride detected in the diffraction pattern of 550 °C-PBM, this suggests that the impurity metals or their oxides present in the raw BM can both undergo chemical reactions with PVDF and capture the HF gas generated by the decomposition of organic fluorinated pollutants, such as PVDF or electrolyte (the relevant calculations are provided in SI), during the roasting pretreatment process, generating metal fluoride and releasing CO2 gas.
O and C–O–C peaks associated with the electrolyte decreases significantly. Furthermore, an analysis of the fitted high-resolution F 1s spectrum reveals that the characteristic peaks associated with organic fluorides, such as PVDF and electrolyte, are absent on the surface of the S-NCM, which are replaced by C–F peaks and metal-F peaks (Fig. 2D). In combination with the characteristic peaks of metal fluoride detected in the diffraction pattern of 550 °C-PBM, this suggests that the impurity metals or their oxides present in the raw BM can both undergo chemical reactions with PVDF and capture the HF gas generated by the decomposition of organic fluorinated pollutants, such as PVDF or electrolyte (the relevant calculations are provided in SI), during the roasting pretreatment process, generating metal fluoride and releasing CO2 gas.
Following the preceding roasting pretreatment and flotation, a nearly 100% pure cathode material was successfully isolated from the complex composition of BM. We denoted this portion of the material as S-NCM, and an in-depth understanding of the specific failure mechanisms of S-NCM is important in attempting to achieve direct regeneration of it. Fig. 3B depicts a scanning electron microscope (SEM) image of the S-NCM particles. Both microcrack and secondary particle fragmentation universally occur in the particles. Then, a transmission electron microscopy (TEM) image of the subsurface area shows that the lattice stripes at the outer edges of the S-NCM are blurred and show disorder. The visible black regions are cracks and voids inside the particles. The outermost layer of the amorphous state may be related to fluoride generated during the roasting process (Fig. 3C). Fig. 3D displays the interplanar spacings of the corresponding iFFT images for regions I, II, and III in Fig. 3C. It is evident that S-NCM exhibits typical layered (003), spinel (311), and rock-salt (111) planes, with interplanar spacings of 0.474 nm, 0.231 nm and 0.245 nm, respectively.8,30,31 Remarkably, the spinel and rock-salt phases are the characteristic features of the cathode material's structure fading. Furthermore, we conducted X-ray photoelectron spectroscopy (XPS) analysis on S-NCM (Fig. 3E), and the ratio of different valence states was determined by the corresponding peak areas (Fig. 3F). It can be observed that the content of Ni3+ (54.89%) in S-NCM is higher than that of Ni2+ (45.11%) as a result of charge compensation to the deficiency of Li (≈20%).32 The O 1s fine spectrum of S-NCM can be decomposed into three peaks at 531.84, 530.13, and 529.74 eV. The binding energies located at 531.84 and 529.74 eV are attributed to Osurface and Olattice,33 respectively, while the binding energy located at 530.13 eV corresponds to the Ni–O bond,34 which further elucidates the rock salinization of the S-NCM surface. The XRD pattern indicates that S-NCM continues to possess the pure bulk phase of a NaFeO2-type layered structure. The corresponding Rietveld refinement results reveal a decrease in the a value yet an increase in the c value for S-NCM, and the Li/Ni mixing ratio exceeded 5% (Fig. 3H, Table S2). In summary, S-NCM is confirmed to be incapable of preserving the initial polycrystalline morphology and suffers from issues such as lithium loss and lattice distortion.35 The failure mechanism is illustrated in Fig. 3A.
As demonstrated by the preceding examination of the failure mechanism, it is crucial to properly replenish lithium and repair the degraded lattice structure for the successful regeneration of S-NCM. Although numerous cases of direct regeneration of spent ternary cathode materials by the molten salt method have been successfully reported, this method frequently introduces excessive molten salt, and additional washing steps are necessary to eliminate residual lithium salt on the surface after sintering.36 Unfortunately, washing not only leads to the escape of some Li+ and lattice oxygen from the material but also reduces the Ni3+ on the surface of the material to Ni2+ driven by the charge compensation effect, which makes the surface rock salinization of the material more serious.37 To alleviate the aforementioned issues, some researchers have proposed a method of tempering the material at a designated temperature after washing.38 This undoubtedly complicates the recycling process. In this study, we first adopt the routine solid-state sintering method and attempt to complete lithium replenishment and lattice restructuring through a single high-temperature reaction. The relevant steps are illustrated in Fig. 4A, and the cathode material regenerated under these circumstances is designated as RDC-NCM.
The SEM images demonstrate that the RDC-NCM is altered from the original cracked secondary particles to irregular primary particle aggregates. Moreover, the surface of the particles exhibits the presence of minute white flakes, which appear to be residual lithium salts (Fig. S4, SI). This result indicates that the original polycrystalline morphology of the cathode material is not completely restored. Although RDC-NCM displays the typical hexagonal-layered α-NaFeO2 structure with the space group of R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, the two pairs of characteristic peaks representing the crystallinity of the material, (006)/(102) and (108)/(110), do not split significantly. Moreover, the amplification of the peak area suggests that the (003) peak of the RDC-NCM shifts slightly toward lower angles compared to S-NCM (Fig. 4B). Notably, a shift of the (003) peak to a low angle often implies that the material is in a lithium-deficient state. This enhances the electrostatic repulsion between the oxygen layers, causing the c-axis to expand. This expansion negatively impacts Li+ diffusion kinetics,39 which certainly weakens the electrochemical performance of RDC-NCM (Fig. S5, SI). Consequently, it is essential to improve the regeneration strategy on this foundation, refine the morphology of S-NCM, and achieve comprehensive restoration in terms of the layered structure from the bulk phase to the surface layer at the same time.
m, the two pairs of characteristic peaks representing the crystallinity of the material, (006)/(102) and (108)/(110), do not split significantly. Moreover, the amplification of the peak area suggests that the (003) peak of the RDC-NCM shifts slightly toward lower angles compared to S-NCM (Fig. 4B). Notably, a shift of the (003) peak to a low angle often implies that the material is in a lithium-deficient state. This enhances the electrostatic repulsion between the oxygen layers, causing the c-axis to expand. This expansion negatively impacts Li+ diffusion kinetics,39 which certainly weakens the electrochemical performance of RDC-NCM (Fig. S5, SI). Consequently, it is essential to improve the regeneration strategy on this foundation, refine the morphology of S-NCM, and achieve comprehensive restoration in terms of the layered structure from the bulk phase to the surface layer at the same time.
In accordance with the above thought, we reconsidered the regeneration mechanism in the solid-state sintering process. First, Li+ is discharged from the Li2O lattice (the decomposition product of the lithium source at elevated temperatures) and migrates to the surface of the rock salt phase through solid-phase diffusion. Thereafter, it reoccupies the lithium layer site, repairs the Li+ diffusion channel, and facilitates the entry of additional Li+ into the bulk lattice, thereby replenishing the active Li lost in the cathode material. Subsequently, the sintering temperature gradually increases, providing more energy to the regeneration system, which in turn reduces grain boundary stress and promotes lattice recombination. It is hypothesized that a crystal structure with local distortion will be reordered while restoring the original polycrystalline morphology, resulting in a good regenerated cathode material. Unfortunately, RDC-NCM exhibits shown to exhibit limited lithiation, which runs counter to expectations. According to the results of the density functional theory (DFT) calculation by Huang et al.,39 it can be observed that the kinetic barriers that Li+ diffusion in rock salt structure (NiO) must overcome are considerably greater than those in the spinel structure (LiNi2O4) and layered structure (LiNiO2). This indicates that a sufficient reaction time is necessary to complete the transition from the outermost rock salt phase to the layered phase. In addition, Shi et al.40 employed DFT calculations to demonstrate that cathode materials with lithium deficiency at elevated temperatures are easily prone to the formation of oxygen vacancies, triggering the migration of cations to the lithium layer. It can therefore be inferred that during the direct heating process to 900 °C, the outermost layer rock salt phase is inadequately restored to a layered phase in total. At this time, oxygen loss occurs in the unlithiated regions under increasing temperatures, which exacerbates Li/Ni mixing. This is also precisely because the rock salt phase exhibits Li+ migration inertia, which is unfavorable for fusion and growth between grains, resulting in the failure of RDC-NCM morphology repair.
Consequently, we reset the initial sintering temperature to 750 °C (a temperature at which the surface passivation layer is well lithiated while avoiding crystal growth, Fig. S6). The experimental results (Fig. 4C) show that the degree of Li/Ni mixing in the cathode material diminishes to a minimal extent following sintering at 750 °C for 3 h. Concurrently, the pivotal indicator I(003)/I(104), employed for the assessment of cation mixing, has exhibited recovery to a comparatively maximal extent (usually 1.2 serves as its critical value).41 TEM images further verify that the cathode exhibits a consistent layered structure from the bulk phase to the surface layer (Fig. 4D). The pre-sintered sample was then calcined at a higher temperature to obtain the regenerated product (R-NCM). As shown in Fig. 4E, the R-NCM has a micron-level size, which is dense in bulk with a smooth surface. Energy dispersive spectroscopy (EDS) elemental mapping reveals a uniform distribution of Ni, Co, and Mn. More precise molar ratios of the elements were obtained using ICP-OES. The results demonstrate that the molar ratios of transition metals in R-NCM are nearly equivalent to those in S-NCM and that the Li content is effectively restored to the theoretical stoichiometric ratio (Fig. 3G and 4I). The lattice fringes of R-NCM can be observed through the enlarged region image, as depicted in Fig. 4F. The interplanar spacing is 0.474 nm, and the (003) crystal plane belonging to the layered structure is visible in the fast Fourier transform (FFT) pattern. Additionally, the Ni 2p and O 1s XPS spectra of R-NCM, as well as the ratio of different valence elements (Fig. 4G and H), show that the Ni3+ content of R-NCM decreases, and the Ni2+ content increases compared with S-NCM. Furthermore, the XPS characteristic peaks belonging to the Ni–O bond disappear, indicating that the NCM lattices have undergone complete lithium filling and structural restoration.42 It is further known that R-NCM shows a typical R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m layered structure from the XRD result. The corresponding Rietveld refinement results also confirm the successful regeneration of the cathode material. Manifestations include an increase in the lattice parameter, a decrease in the c lattice parameter, and a reduction in the Li/Ni mixing degree to 1.63% (Fig. 4J and Table S2).
m layered structure from the XRD result. The corresponding Rietveld refinement results also confirm the successful regeneration of the cathode material. Manifestations include an increase in the lattice parameter, a decrease in the c lattice parameter, and a reduction in the Li/Ni mixing degree to 1.63% (Fig. 4J and Table S2).
The electrochemical performance of S-NCM and R-NCM was evaluated by assembling a CR2016-type coin half-cell. The challenges associated with lithium loss and the degradation of the layered structure result in a higher polarization voltage and limited initial specific charge and discharge capacities of 43.4 mA g−1 and 116.4 mA g−1 at 0.1C (1C = 160 mA g−1) for S-NCM, respectively, which exhibits hardly any charge and discharge plateaus. In contrast, the initial specific charge and discharge capacities of R-NCM increase to 158.1 mA g−1 and 139.3 mA g−1, respectively, and the ICE (initial Coulomb efficiency) increases from 37.28% to 88.13% (Fig. 5A). Moreover, the differential capacity (dQ/dV) curve of R-NCM shows discernible redox peaks, which further confirms the effective restoration in the NCM crystal structure (Fig. 5B). The study of electrochemical reaction kinetics was conducted using electrochemical impedance spectroscopy (EIS). Nyquist plots reveal that EIS curves consist of the high-to-medium-frequency region semicircle and the low-frequency region straight line (Fig. 5C and D). The impedance results fitted according to the equivalent circuit diagram inserted in the figures are presented in Table 1. Rf is the solid electrolyte interface resistance, Rct is the charge transfer resistance, and Zw is the Warburg diffusion process of Li+. It can be observed that the Rct value of the S-NCM electrode is nearly 23 times that of the R-NCM electrode. The Li+ diffusion coefficient (DLi) is calculated by employing the following formula:
| Sample | 1st cycle | ||||
|---|---|---|---|---|---|
| R s/Ω | R f/Ω | R ct/Ω | Warburg factor (σ) | D Li/(cm2 s−1) | |
| S-NCM | 11.91 | 132.70 | 336.70 | 70.04 | 1.31 × 10−8 |
| R-NCM | 3.08 | — | 14.31 | 55.87 | 2.06 × 10−8 |
Ultimately, we evaluated the economic benefit and environmental impact of the direct regenerating strategy proposed in this study using the EverBatt 2023 model developed by Argonne National Laboratory, which annually treats 1000 tons of spent NCM523 anode and cathode mixed black mass as a standard. This was then compared with typical pyrometallurgical and hydrometallurgical recovery methods (Fig. 6). Specifically, the total energy consumption of direct regenerating (2.88 MJ kg−1) is notably lower than that of pyro-methods (39.12 MJ kg−1) and hydro-methods (42.97 MJ kg−1). Owing to the simple process, low energy consumption, and lack of requirement for secondary synthesis, the obtained cathode materials can be used directly. The direct regenerating strategy is 6.8- and 2.4-fold more profitable than the pyro-methods and hydro-methods, respectively. For the environmental impact, the emissions of greenhouse gases (GHGs) of the direct regenerating strategy are roughly just half of hydro-methods and one-third of pyro-methods. In this sense, our study provides a reference solution for the spent NCM battery black mass recycling industry with both benefits and a low-carbon development concept.
Conclusion
In summary, we presented a strategy to directly regenerate the cathode material from the actual crushing of battery black mass and verified its feasibility, economy, and environmental friendliness using a variety of characterization methods. This strategy allowed for employing the residual metal impurities inherent in black mass to capture fluorinated pollutants and control the transition pathway of fluorine species. Concurrently, the hydrophobicity disparity between the anode and cathode materials was restored, thereby establishing conditions conducive to subsequent efficient flotation separation. The recovery and grade of cathode material (S-NCM) in black mass could attain 99.08% and 95.92% after one rougher flotation, respectively, under the conditions of collector N-dodecane (10 mg L−1) and frother MIBC (6 mg L−1). Benefiting from the effective tailoring of the solid state sintering process, the process of prelithiation at 750 °C for 3 h and calcination at 900 °C for 10 h was available for the conversion from S-NCM to R-NCM. In particular, the microcracks, particle fragmentation, lithium loss, and lattice distortion observed in S-NCM were all repaired as expected in R-NCM. The reversible specific capacity of the half cell assembled based on R-NCM was 139.27 mAh g−1 at a 0.1C rate, and the capacity retention rate was 91.85% after 100 cycles. Even after cycling at a high rate, as the current density is restored to 0.1C, the discharge capacity could return to 137.4 mA g−1, which is 98.64% of the initial discharge capacity at 0.1C rate. Meanwhile, economic and environmental analysis results indicate that the strategy boasts significant advantages and considerable potential in terms of economic benefits, energy conservation and emission reduction. Our future studies will prioritize the enhancement of the electrochemical performance of the regenerated NCM, with a specific focus on increasing its reversible capacity and interfacial stability. In conclusion, this study provides a sustainable solution for the direct regeneration of waste battery black mass, demonstrating considerable potential for large-scale applications.Author contributions
Yuchen Li: methodology, validation, formal analysis, investigation, data curation, writing-original draft. Zhengni Ye: methodology, supervision, investigation. Shuai Wang: resources, supervision. Hong Zhong: supervision, methodology, funding acquisition. Zhanfang Cao: resources, supervision, review & editing. Xin Ma: conceptualization, methodology, writing-review & editing, resources, supervision, project administration, funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary information (SI). The data are available from the corresponding author upon reasonable request. Supplementary information: the XRD pattern and TG-DTG curves of the raw material along with its main elemental composition; the flotation flowsheet and recovery calculation details; the mass transfer behavior of metallic impurities and fluorine during calcination; initial charge–discharge curves of the RDC-NCM sample regenerated at 900 °C for 10 h; the I(003)/(104) value of the samples in a initial sintering temperature range of 300–850 °C; and the Rietveld refinement results for both S-NCM and R-NCM. See DOI: https://doi.org/10.1039/d5ta06941f.Acknowledgements
The authors are grateful for the support from the National Natural Science Foundation of China (No. 22478447), Guizhou Provincial Major Science and Technology Project (No. [2024]017), the Changsha Natural Science Foundation (No. kq2402210), Open Research Fund of State Key Laboratory of Advanced Metallurgy for Non-ferrous Metals (No. YSQH-ZD-24009), and the High Performance Computing Center of Central South University, China.Notes and references
- S. Azerblou, R. Oubah, H. Ouachtouk and E. Tace, Sustain. Energy Technol. Assessments, 2025, 75, 104245 CrossRef.
- M. E. Ölmez, I. Ari and G. Tuzkaya, Energy, 2025, 326, 136202 CrossRef.
- X. Cheng, X. Liu, L. Zhao, D. Zhang, J. Biao, Z. Chen, Y. Yuan, M. Liu, Y. B. He and F. Kang, Adv. Funct. Mater., 2023, 33, 2211171 CrossRef CAS.
- Z. Qin, T. Zhang, X. Gao, W. Luo, J. Han, B. Lu, J. Zhou and G. Chen, Adv. Mater., 2024, 36, 2307091 CrossRef CAS.
- J. Shen, M. Zhou, W. Liu, Y. Shi, W. Tang, Y. Deng, R. Liu, Y. Zuo and J. Zhang, Energy Storage Mater., 2025, 74, 103964 CrossRef.
- Y. Du, J. Li and Y. Xu, Resour. Policy, 2024, 93, 105056 CrossRef.
- Y. Wang, X. Ji, Y. Lang and Z. Zhang, Comput. Ind. Eng., 2025, 201, 110930 CrossRef.
- Z. Qin, Y. Zhang, W. Luo, T. Zhang, T. Wang, L. Ni, H. Wang, N. Zhang, X. Liu, J. Zhou and G. Chen, Angew. Chem., Int. Ed., 2023, 62, e202218672 CrossRef CAS PubMed.
- M. Rinne, H. Lappalainen and M. Lundström, Green Chem., 2025, 27, 2522–2537 RSC.
- H. Dong, H. Wang, J. Qi, J. Wang, W. Ji, J. Pan, X. Li, Y. Yin and S. Yang, ACS Sustain. Chem. Eng., 2022, 10, 11587–11596 CrossRef CAS.
- G. Jiang, L. Liu, B. Zhu, Y. Zhang, Q. Meng, Y. Zhang, P. Dong, Q. Ouyang and Z. Zhu, J. Environ. Manage., 2023, 326, 116661 CrossRef CAS.
- J. Li, H. Li, J. Jiao and Y. Xu, Environ. Impact Assess. Rev., 2025, 115, 107996 CrossRef.
- C. Liu, L. Sheng and L. Jiang, RSC Adv., 2025, 15, 7995–8018 RSC.
- M. Rezaei, A. Nekahi, A. Kumar M R, A. Nizami, X. Li, S. Deng, J. Nanda and K. Zaghib, J. Power Sources, 2025, 630, 236157 CrossRef CAS.
- Y. Liu, T. Liu, J. Zhao, Y. Shao, X. Li, Z. Li, Y. Zhang, S. Liu, Z. Lin, F. Bettels, C. Zhang, F. Ding and L. Zhang, J. Mater. Chem. A, 2025, 13, 8968–9004 RSC.
- Z. Chi, J. Li, L. Wang, T. Li, Y. Wang, Y. Zhang, S. Tao, M. Zhang, Y. Xiao and Y. Chen, Green Chem., 2021, 23, 9099–9108 RSC.
- Y. Guo, C. Guo, P. Huang, Q. Han, F. Wang, H. Zhang, H. Liu, Y. C. Cao, Y. Yao and Y. Huang, eScience, 2023, 3, 100091 CrossRef.
- M. Bruno and S. Fiore, J. Clean. Prod., 2025, 501, 145327 CrossRef CAS.
- T. Kaarlela, E. Villagrossi, A. Rastegarpanah, A. San-Miguel-Tello and T. Pitkäaho, J. Manuf. Syst., 2024, 74, 901–921 CrossRef.
- G. Zhang, T. Jiang, Y. He, H. Wang and X. Yuan, Waste Manage., 2024, 187, 244–251 CrossRef CAS.
- L. Verdugo, L. Zhang, K. Saito, W. Bruckard, J. Menacho and A. Hoadley, Sep. Purif. Technol., 2022, 301, 121885 CrossRef CAS.
- Y. Ji, C. T. Jafvert, N. N. Zyaykina and F. Zhao, J. Clean. Prod., 2022, 367, 133112 CrossRef CAS.
- P. Li, S. Luo, G. Hao, K. Sun, Q. Liu, M. Møller, D. Wang, P. K. Kristensen, L. Gurevich, L. R. Jensen, L. Wang and X. He, J. Hazard. Mater., 2025, 481, 136553 CrossRef CAS PubMed.
- H. Huang, C. Liu and Z. Sun, J. Hazard. Mater., 2023, 457, 131782 CrossRef CAS PubMed.
- Y. Wang, Y. Tu, Z. Xu, X. Zhang, Y. Chen and E. Yang, Ionics, 2022, 28, 1833–1844 CrossRef CAS.
- S. Luo, M. Gao, D. Cai, L. Zhu, C. Lai, J. Lin, Y. Peng and Z. Yuan, J. Energy Storage, 2023, 73, 108483 CrossRef.
- I. A. Poimenidis, P. A. Loukakos and M. Konsolakis, J. Phys. Chem. Solids, 2025, 196, 112365 CrossRef CAS.
- B. Wang, C. Zhu, H. Lei, H. Zhou, W. Sun, Y. Yang and P. Ge, J. Mater. Chem. A, 2025, 13, 12998–13009 RSC.
- Y. Zhang, S. Wang, X. Liu, J. Lv, X. Hao and E. Duan, Appl. Catal. B Environ. Energy, 2024, 353, 124063 CrossRef CAS.
- C. Xing, H. Da, P. Yang, J. Huang, M. Gan, J. Zhou, Y. Li, H. Zhang, B. Ge and L. Fei, ACS Nano, 2023, 17, 3194–3203 CrossRef CAS PubMed.
- M. Li, D. Shao, Z. Mao, Z. Fan, L. Xu, H. Dou, Z. Peng, B. Ding and X. Zhang, J. Mater. Chem. A, 2025, 13, 2155–2161 RSC.
- L. Chen, C. Xing, Z. Yang, S. Tao, Y. Zhang, G. Wang, P. Yang, J. Song, J. Chen and L. Fei, Adv. Funct. Mater., 2025, 35, 2411182 CrossRef CAS.
- M. Fan, X. Chang, Y. J. Guo, W. P. Chen, Y. X. Yin, X. Yang, Q. Meng, L. J. Wan and Y. G. Guo, Energy Environ. Sci., 2021, 14, 1461–1468 RSC.
- Y. Kang, C. Zhang, H. Li, Y. Li, H. Lei, R. Huang, Y. Han, W. Wei, X. Zhao and Y. Cui, ACS Catal., 2025, 15, 8768–8775 CrossRef CAS.
- W. Yin, Z. Wu, W. Tian, Y. Chen, W. Xiang, G. Feng, Y. Li, C. Wu, C. Xu, C. Bai, B. Zhong, X. Wang, J. Zhang, F. He, A. A. Alshehri and X. Guo, J. Mater. Chem. A, 2019, 7, 17867–17875 RSC.
- Z. Gao, Y. Liu, Z. M. El-Bahy, G. Chen, M. H. Helal, B. Lu, J. Han and J. Zhou, Adv. Funct. Mater., 2025, 2503674 CrossRef CAS.
- B. R. Hu, Y. Y. Yuan, Y. C. Wang and X. H. Xiong, Rare Met., 2024, 43, 87–97 CrossRef CAS.
- L. Liu, Y. Zhang, Y. Zhao, G. Jiang, R. Gong, Y. Li, Q. Meng and P. Dong, ACS Appl. Mater. Interfaces, 2022, 14, 29886–29895 CrossRef CAS.
- Q. Huang, X. Zhang, X. Lv, J. Lin, Z. Dai, E. Fan, R. Chen, F. Wu and L. Li, Adv. Mater., 2025, 37, 2419872 CrossRef CAS PubMed.
- R. Shi, J. Li, J. Zhao, H. Ji, J. Wang, W. Chen, J. Li, Y. Cao and G. Zhou, Adv. Mater., 2025, 2506423 CrossRef CAS PubMed.
- Z. Chen, J. Wang, D. Chao, T. Baikie, L. Bai, S. Chen, Y. Zhao, T. C. Sum, J. Lin and Z. Shen, Sci. Rep., 2016, 6, 25771 CrossRef CAS.
- X. Yu, S. Yu, Z. Yang, H. Gao, P. Xu, G. Cai, S. Rose, C. Brooks, P. Liu and Z. Chen, Energy Storage Mater., 2022, 51, 54–62 CrossRef.
Footnote |
| † Yuchen Li and Zhengni Ye contribute equally to the work. |
| This journal is © The Royal Society of Chemistry 2026 |