 Open Access Article
Open Access ArticleMicroneedle-integrated wearable devices for healthcare monitoring
Tianli Hu ,
Eira Beryle Ko,
Yu Song and
Chenjie Xu
,
Eira Beryle Ko,
Yu Song and
Chenjie Xu *
*
Department of Biomedical Engineering, College of Biomedicine, City University of Hong Kong, Kowloon, Hong Kong SAR, China. E-mail: chenjie.xu@cityu.edu.hk
First published on 14th January 2026
Abstract
Wearable technologies have emerged as powerful tools for non-invasive healthcare monitoring, enabling continuous detection of biomarkers to support personalized medicine and disease management. However, most biological biomarkers stay inside tissue and cannot be accessed on the skin surface through simple contact. Microneedle (MN) technology provides a solution for the wearable technologies to sample biofluid, detect biomarkers, and monitor electrophysiological signals. This article provides a comprehensive overview of the latest development in this field, highlighting MN design principles, functional integration with microfluidics, microelectronics, and artificial intelligence as well as the practical applications. We conclude by discussing the challenges in technical development, clinical validation, industrialization and regulatory compliance, as well as future prospects for MN-integrated wearable devices.
1. Introduction
Continuous healthcare monitoring facilitates personalized management of chronic conditions and improves overall wellbeing.1 Conventional practices require patients to be physically present at the clinics for sampling and diagnosis, which is inconvenient, time-consuming and offers only intermittent assessment of the patient's health status. The emergence of wearable biomedical devices has opened a new area of personalized health monitoring by accurately measuring physical states and biochemical signals without the need of presence at clinics.2,3Signals monitored by wearable devices can be classified into physical signals and chemical signals. Physical signals refer to pressures and motions, such as touch and heart pulse. They can be detected through electromechanical sensors attached on the skin.4–7 Chemical signals refer to ions/molecules that transmit information between cells (e.g. glucose, sodium ions, uric acid, antibodies or hormones).8 They exist in biofluids including blood, sweat, saliva, and tears. The sensors must access the fluid before converting them to useful signals for detection and quantification.9–13 Certain biofluids like sweat, saliva, and tears can be accessed noninvasively, but their use is limited by challenges such as low biomarker concentrations, environmental contamination, and high variability. Originating from blood plasma that diffuses through capillaries,14 skin interstitial fluid (ISF) appears as a reliable biofluid with high biomarker concentration, minimal environmental contamination, and low variability. Many studies have shown that ISF contains biomarkers that are similar in both concentration and composition to that of blood samples, making it a prime candidate for wearable biofluid sensors.15–17 The only problem is that skin ISF cannot be accessed non-invasively.18
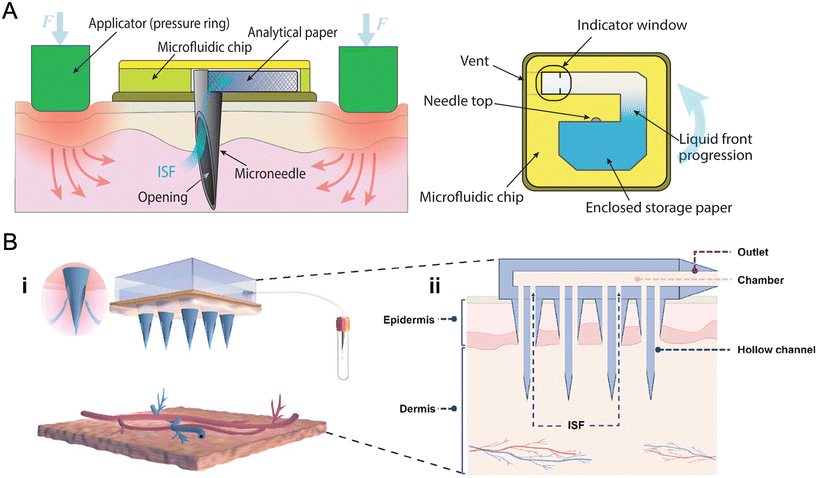
Fortunately, the development of microneedle (MN) technology provides a solution. MN devices contain one or an array of needles with lengths from 25 to 2000 μm. MNs have been categorized into solid MNs, coated MNs, hollow MNs, porous MNs and swellable MNs,19 with morphological variants such as bevelled-tip, cylindrical, pyramidal, and conical profiles20 (Fig. 1). The MN tips can pierce the stratum corneum and reach the dermis where the ISF stays,21 which permits a minimally invasive and reliable access to skin ISF. We can either extract ISF using MNs for subsequent analysis or detect the biomarkers in situ by converting them to readable signals through biosensors integrated on MN tips or the backing layer. These MN devices can be further combined with microfluidics, wearable electronics, and artificial intelligence (AI), enabling sampling, quantification, sensing, and data processing in one wearable system. Therefore, we can provide a real-time and minimally invasive approach for disease prevention, early diagnosis, and therapy optimization.
The great potentials of MN technology have driven numerous researchers and other professionals to dedicate their time and resources into MN development. Since 2012, there have been 700 articles published by searching on PubMed with the keywords “microneedle” and “monitoring”. The number of publications per year increases with an average annual growth rate of around 58% from 2021 to 2024 (Fig. 2). There are also more than 9 companies in the field of MN sensing (Table 1). In ClinicalTrials.gov, there are 9 registered clinical trials related to MN sensing and sampling technologies (Table 2).
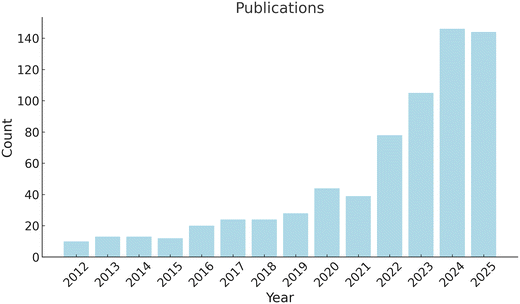 | ||
| Fig. 2 Number of publications in PubMed from 2012 to 2025 retrieved using the keywords “microneedle” and “monitoring”. | ||
| Company | Region | MN type | Technology focus | Target analytes |
|---|---|---|---|---|
| Biolinq | USA | Coated MNs | Intradermal electrochemical MN array for CGM and metabolic biomarkers | Glucose |
| Dexcom | USA | Retractable hollow MNs | CGM systems employing introducer MN and subcutaneous filament-based electrochemical sensors | Glucose |
| Abbott | USA | Retractable hollow MNs | CGM platform using introducer MN and filament-based electrochemical sensors | Glucose |
| CARI Health | USA | Not disclosed | Combining MNs for sensing with remote monitoring, potentially delivering medication on-demand | Methadone, buprenorphine and opioid |
| SIBIONICS | China | Retractable hollow MNs | CGM system using MN and filament-based electrochemical sensors | Glucose |
| PKvitality | France | Coated MNs | Smartwatch with disposable MN patch coated with glucose sensing layer | Glucose |
| WearOptimo | Australia | Solid MNs | MN electrode patch for hydration assessment | Electrolytes |
| Nutromics | Australia | Coated MNs | DNA-based electrochemical aptamer MN biosensors for multi-analyte detection | Multiple proteins, drugs, metabolites and hormones |
| Sava | UK | Coated MNs | MN biosensor platform for multi-biomarker monitoring | Glucose |
| Zimmer & Peacock | UK/EU | Solid/coated MNs | MN arrays and wearable MN sensor prototypes for translational research | Glucose, lactate, ketones, pH, etc. |
| Year | Clinical trial | Study title | Sponsor | Application(s) | Status | MN device |
|---|---|---|---|---|---|---|
| MN sensing | ||||||
| 2016 | NCT02682056 | Glucose measurement using microneedle patches (GUMP) | Emory University | ISF glucose monitoring | Completed | MN patch for ISF glucose |
| 2019 | NCT03847610 | Minimally invasive sensing of beta-lactam antibiotics | Imperial College London | Antibiotic therapeutic drug monitoring | Completed | MN biosensor (electrochemical) |
| 2019 | NCT04053140 | Closed-loop control of penicillin delivery (CLCPD) | Imperial College London | Closed-loop β-lactam monitoring and control | Recruiting | MN biosensor integrated with control algorithm |
| 2022 | NCT05546229 | Assessment of methadone and buprenorphine in interstitial fluid | CARI Health, Inc. | Opioid drug level monitoring | Recruiting | MN ISF sensing (electrochemical) |
| 2023 | NCT05998876 | Assessing dose taken in opioid use disorder with an electrochemical sensor | CARI Health, Inc. | Opioid dose adherence monitoring | Recruiting | MN electrode array |
| 2025 | NCT06977633 | Feasibility study of a hydrogel microneedle patch for continuous glucose-ketone monitoring | University of Waterloo | Metabolic monitoring (glucose, ketone) | Not yet recruiting | Hydrogel MN patch sensor |
| MN sampling | ||||||
|---|---|---|---|---|---|---|
| 2019 | NCT03795402 | Microneedle sampling in psoriasis and healthy skin | Janssen Research & Development, LLC | Biomarker/transcriptomics sampling from skin | Completed | MN patch for skin sampling |
| 2025 | NCT06934980 | Microneedle-based collection of dermal interstitial skin fluid in healthy and AD participants | Incyte Corporation | ISF sampling and biomarker collection | Recruiting | MN patch for ISF collection |
| 2025 | NCT06994988 | ARPA-H smart band-aid to measure chronic pain in women | Northwestern University | ISF sampling from the skin | Recruiting | A-band wearable MN patch |
This article overviews the latest development of MN-based wearable devices across three major application domains, including biofluid sampling, biomarker monitoring, and electrophysiological signal acquisition. For each category, we discuss the representative MN designs, integration strategies, and commercialized products, followed by a critical analysis of the key challenges and perspectives for future clinical translation. We realize there are similar articles published recently. For example, Kim et al. presented a comprehensive overview of MN-based sensing for dermal ISF analysis, encompassing skin physiology, MN design, sensing mechanisms, monitoring electronics, and practical applications.22 Li et al. also reviewed MN sensing technology for ISF analysis, covering the fundamental aspects of MNs, including materials, fabrication methods, and structural designs as well as the range of detectable biomarkers.23 Vora et al. highlighted the diverse types of MNs for biosensing, their integration with biosensors, and the clinical applications of wearable MN sensing devices aimed at advancing point-of-care testing.24 Similarly, Hu et al. introduced different classes of MN sensors based on material types, such as metals, inorganics, polymers, and hydrogels, and underscored the importance of commercialization and clinical trials in translating MN technologies into practice.25 Distinct from these articles, this review presents MN design strategies tailored to specific applications, providing a more focused perspective rather than a generalized overview of all MN sensor designs. In addition, it highlights the critical role of microfluidics in MN-based wearable devices, such as efficient fluid sample handling and transportation, emphasizing that these systems represent more than a simple combination of MNs and microelectronics. To bridge research and application, we also present commercially available products in each category, thereby providing a market-oriented view that underscores the translational potential of MN technologies.
2. MN-integrated devices for biofluid sampling
MNs can act as a sampling tool to construct a bridge between the biofluids and the reservoir, allowing biofluids like skin ISF and blood to be extracted with the assistance of negative pressure, capillary force, swelling force, or electroosmosis.2.1 Design strategies of MNs
Solid MNs, hollow MNs, porous MNs, and swellable MNs have been studied for biofluid sampling. There are three strategies to use MNs for sampling biofluids from both tissue layers (e.g. ISF and blood) and body surfaces (e.g. sweat and tears).26–29 The first is to collect biofluids through microchannels created in the skin by solid MNs. These MNs are typically fabricated from materials with high mechanical strength, such as stainless steel28,30,31 and hard resin.32 For example, Samant et al. developed a stainless-steel MN array with 5 MNs and a length of 250 μm. After creating micropores in the skin layer, a vacuum pump and gauze were employed to sample microliter volumes of ISF.29 The solid stainless-steel MNs have also been affixed with absorbent strips that utilized capillary force to collect and store ISF.28,30,31 Similarly, this methodology can be applied to extract capillary blood. Zoratto et al. developed a cost-effective MN device integrating a hidden circular array of 20–30 stainless-steel MN tips (2 mm length, 13.4° tip angle) within a silicone suction cup and a heart-shaped storage compartment containing K2-EDTA (Fig. 3A).33 The device collected roughly 195 μL of blood from piglets within 10 min by generating a negative pressure (∼−68 to −73.8 kPa) through silicone's elastic recovery post-compression. | ||
| Fig. 3 Representative examples for different types of MNs in biofluid sampling: (A) solid stainless-steel MN array creating micropores in the skin for capillary blood sampling. Reproduced from ref. 33 under the CC-BY 4.0 license. (B) (i) Osmolyte-containing swellable MN array for ISF extraction through the swelling of the hydrogel-forming polymer; (ii) comparison of extracted skin ISF between MeHA MNs and osmosis incorporated MeHA MNs at a range of time points. Reproduced with permission from ref. 36. Copyright 2020, Wiley. (C) Hollow MN array for ISF extraction via capillary force. Reproduced from ref. 46. Copyright 2025, Elsevier. (D) (i) Anisotropic porous MNs for tear sampling through the capillary force driven by the aligned channels; (ii) the volume of extracted tears by using anisotropic porous MNs and tear strips from rats with dry-eye disease. Reproduced with permission from ref. 26. Copyright 2025, Elsevier. | ||
The second strategy utilizes the swelling properties of solid MNs made from hydrogel-forming polymers such as methyl hyaluronate (MeHA),34–38 gelatin methacryloyl (GelMA),39,40 and PVA.41–43 For instance, photo-crosslinked MeHA hydrogel MNs with ∼100 MN tips successfully extracted ∼1 mg of ISF from mouse skin within 1 min.37 The incorporation of maltose as the osmolyte in MeHA MNs further improved their swelling capacity 1.5-fold and ISF extraction volume rose to ∼2 μL (mouse skin) in 1 min (Fig. 3B, i and ii).36
Finally, we can make use of the MNs' microstructure to extract biofluids. The voids and channels within the hollow or porous MN tips act as conduits, enabling the passive flow of liquid through capillary force.44 Hollow MNs are currently most popular due to the large lumen.45–47 For example, an array of 5 syringe needles (32 G, 1500 μm in length), with each needle combined with a glass capillary, achieved an extraction volume of up to 16 μL of ISF from human skin over a 1–2 h period, in contrast to 1.51 μL of ISF collected using a single needle.45 Silva et al. developed a 3D-printed device with 18 polymer hollow MNs via two-photon polymerization. Each MN is 1 mm in length with an inner diameter of 50 μm and 30 μm side openings. The 3D-printed MNs extracted approximately 1 μL of ISF through hollow channels to microfluidic chambers under 30 kPa pressure and performed consistently in more than 10 insertions (Fig. 3C).46 Porous MNs can achieve the same goal through the interconnected pores inside the needles.48–50 For instance, Takeuchi et al. fabricated a porous PDMS MN array by a combination of mold casting and salt leaching techniques, creating pores with diameters ranging from 30 to 60 μm. The MN array, consisting of 169 pyramidal tips each 1200 μm in length, achieved continuous extraction at 0.08 μL min−1 (demonstrated with PBS as an ISF substitute) when integrated with microfluidic channels.49 The aligned porous channels can improve the bioliquid extraction performance. Inspired by the wood xylem structure, we developed MNs with an anisotropic porous architecture that could be used to collect over 5 μL of tears from rat eyes within 3 min (Fig. 3D i and ii).26 Additionally, grooved MNs have been reported for ISF extraction, with the grooves on the needle surface serving as the primary liquid extraction pathway.51,52
2.2 The integration of MNs with microfluidics
The conventional role of sampling MNs is to extract biofluid such as blood, skin ISF, and tears. However, there are several limitations if MNs are used alone for biofluid extraction. Solid MNs act primarily as penetration tools and therefore typically require an additional component to collect and store the extracted fluid. Although swellable, porous, and hollow MNs can retain ISF within their tips and thereby function as self-contained sampling devices; their tip geometry inherently limits the volume of liquid that can be extracted. Consequently, the integration of a biofluid collection component is often necessary to enhance sampling efficiency and capacity, quantify the bioliquid, and ensure sufficient volume for downstream analysis.Microfluidic technology enables the manipulation of liquids at the microscale, providing precise control over fluid flow, distribution, mixing, and storage. Two main types of microfluidic platforms, paper-based and chip-based ones, have been integrated with MN devices. When paper-based microfluidics is integrated with MNs, biofluids are drawn into the paper strip by capillary forces generated from its fiber structure, thereby enhancing sampling efficiency. The absorbed liquid can be stored within predefined regions of the strip, enabling quantification of the extracted volume. Captured biomarkers can subsequently be recovered using buffer solutions for downstream laboratory analyses.53,54 For example, Ribet et al. developed a device combining a single stainless-steel hollow MN (1 mm length) with a microfluidic chip (Fig. 4A). The MN penetrated into the skin to access ISF, which was guided by microchannels to the defined piece of analytical-grade paper in the chip, enabling the collection of approximately 1.1 μL ISF in ∼5 min. The integrated paper reservoir, together with a color indicator, provided built-in volume metering, ensuring a reliable volume metering (±3.5% variability) for accurate quantification. They further analyzed analytes including caffeine, SARS-CoV-2 antibodies, and hundreds of soluble proteins by liquid chromatography-tandem mass spectrometry (LC-MS/MS) and enzyme-linked immunosorbent assay (ELISA) after recovering the analytes into buffer.54
 | ||
| Fig. 4 Integrated wearable MN devices for biofluid sampling. (A) Stainless-steel MN array with silicone suction cup for capillary blood collection. Reproduced with permission from ref. 54. Copyright 2023, Wiley. (B) (i) 3D-printed hollow MN array patch integrated with microfluids for ISF sampling driven by a connected vacuum tube (∼18 μL in 5 min); (ii) the cross-section structure of the integrated hollow MN device. Reproduced from ref. 56 under the CC-BY 4.0 license. | ||
When MNs are integrated with chip-based microfluidics, a vacuum force is typically applied to the microfluidic chip to drive biofluid to flow through the microchannels into a reservoir. This configuration enables precise quantification of the extracted fluid through well-designed microchannels and reservoirs, while the continuous pressure facilitates the sampling of larger volumes.46,55–57 For instance, Xie et al. developed a 3D-printed hollow MN array integrated with a vacuum tube, merging MN-based sampling with microfluidic chip-based transport (Fig. 4B, i and ii). This setup enhanced biofluid sampling capacity, enabling the extraction of ∼18 μL ISF from rabbit ears within 5 min.56 This integrated design of MNs and chip-based microfluidics has also been applied to sample blood in a painless manner. Blicharz et al. designed a touch-activated phlebotomy (TAP) device that combined a 30-needle stainless-steel MN array (1000 μm length, 350 μm width, 50 μm thickness) with a microfluidic system. It has a snap-dome insertion mechanism, stored vacuum, and microchannels with staggered herringbone mixers to blend blood with lithium heparin. The device collected 100 μL capillary blood painlessly in ∼3 min for diagnostics like HbA1c testing.58
2.3 Commercially available MN-based devices for blood sampling
There are several devices that have been commercialized for capillary blood sampling,59 belonging to class II medical devices under the FDA regulation. One example is the TAP from YourBio Health Inc. (formerly Seventh Sense Biosystems). Powered by HALO™ technology, the device uses a solid MN array (1 mm in length) to penetrate skin. It is significantly less invasive than traditional lancets (2 mm skin penetration depth) or venipuncture (3–10 mm skin penetration depth), avoiding nerve-rich tissue and minimizing pain. The evolution of the TAP device began with the TAP20, which is a one-step, nearly painless device for collecting 20 μL of blood for point-of-care testing released in 2013. It was followed by TAP100, the first-generation model with a 100 μL capacity, which received FDA 510(k) clearance and CE marking. To overcome limitations such as manual pipetting and manufacturing challenges, the second-generation TAP II device introduced detachable tubes for 200–300+ μL blood collection and streamlined processing. The latest model, TAP Micro Select, features modular compatibility with standard collection tubes and enables home-based capillary whole blood collection of up to 900 μL, with CE marking and FDA 510(k) clearance (Fig. 5A).Similarly, Tasso Inc. developed a capillary blood collection product, Tasso Mini (Fig. 5B). With a 1.8 mm lancet length and 1.6 mm penetration depth, it is smaller than their other product, Tasso+, which is 3.8 mm in length and 1.8 mm in depth. Its fully integrated lancet and vacuum design enables simple, nearly painless capillary blood collection. Upon activation, the device punctures the skin and gently draws blood into a connected tube. Tasso Mini is compatible with standard 13 mm × 75 mm microtainer tubes and Tasso's own dried blood spot cartridges, offering flexibility for both liquid and dried sample workflows.
Other similar products include Nanodrop and OneDraw from Drawbridge Health Inc. Nanodrop incorporates two stainless-steel needles that penetrate the skin to a depth of 1.9–2.0 mm. Its design includes a plastic housing with a hydrogel adhesive rim, a bow-shaped cavity for vacuum-assisted sampling, activation buttons for vacuum and blade deployment, a yellow lock, and auto-retracting blades after use. The OneDraw blood collection device integrates lancets and a vacuum system to draw blood onto matrix strips within a cartridge. The cartridge, which can maintain its integrity for up to 21 days at room temperature, is then placed into a transport sleeve for secure mailing to a laboratory. Compared to Nanodrop, it is a more streamlined system for sample collection, transport, and analysis.
3. MN-integrated wearable devices for biomarker monitoring
The previous section has listed the criteria and showcased a few examples using MN devices for biofluid sampling. However, to quantitatively analyze the biomarkers in the extracted biofluid, sensing components must be integrated.3.1 Design strategy for MN-based biosensors
MN-based biosensors have been designed as off-tip and on-tip biosensors. In MN devices with off-tip recognition, MNs act as the sampling component while the backing layer of the device contains the sensing element like optical or electrochemical sensors. Hollow MNs, porous MNs, and hydrogel MNs have been used for off-tip MN biosensor development. The extracted biofluid flows from the MN tip to biosensors on the backing layer. The devices with the on-tip sensors carry the recognition assay on the MN tips. Biomarkers are analyzed on the surface of coated MN tips,60 within the internal physical spaces (lumens or micropores) of hollow and porous MN tips,61–63 or within the polymer network of hydrogel MN tips.64–66 Notably, electrochemical on-tip biosensors require materials with excellent conductivity for electrical signal transmission. Conductive materials with high stability and inertness like gold and carbon materials are popular choices in the fabrication, coating, or filling of these MN-based devices.60,67,68The common sensing strategies of both off-tip or on-tip MN-based biosensors convert ISF biological signals into detectable optical or electrical signals. Optical sensing strategies include fluorescence, colorimetry, and surface-enhanced Raman spectroscopy (SERS) assays on- or off-MN tips, recognizing analytes by surface-immobilized fluorophore-conjugated recognition elements,66,69,70 target-induced chromogenic reagent color changes,51,71–73 and Raman signals from noble metal nanostructures,74,75 respectively. On the other hand, electrical biosensors rely on electrochemical signal transduction through amperometric, potentiometric, and voltammetric mechanisms.22 Amperometric MN sensors measure current from enzymatic redox reactions of ISF metabolites (e.g., glucose,76 lactate,77 ketone,78 etc.). Potentiometric MN sensors use ion-selective membranes to detect ions (e.g., Na+, K+, Ca2+) via potential differences.79,80 Voltammetric MN sensors monitor current responses to varying potentials, with analyte binding altering electron transfer kinetics of immobilized aptamers/antibodies.81–85 Notably, aptamer-functionalized MN biosensors achieve an ultra-sensitive detection limit of drugs (tobramycin: 1.41 pM; vancomycin: 0.187 nM)86,87 and metabolites (phenylalanine: fM-level; cortisol: 0.22 nM).88,89
3.2 MN wearable devices with off-tip sensors: from independent to integrated use
Off-tip sensors can function independently as wearable devices, enabling biomarker analysis in the biofluids with the biosensing component located at the backing of MNs, such as colorimetric test paper52,72,90,91 and lateral flow test strips.92–94 For example, a wax-patterned test paper was combined with swellable MeHA/HA MNs as colorimetric MN sensors. Four specific enzymes and dyes have been incorporated into the test paper to detect glucose, lactate, cholesterol, and pH in the ISF extracted by MNs, enabling visual inspection via color changes.90 Nevertheless, such detection approaches are limited to qualitative “yes or no” outputs, as the volume of the analyzed liquid cannot be quantified. Inadequate contact between the sample and the sensing interface may result in unreliable responses, and the precise concentration of target biomarkers cannot be determined.Integrating off-tip biosensors with microfluidic chips can improve biosensing performance by providing accurate fluidic control, improving analyte–sensor interactions as well as enabling reliable and quantitative assessment of biomarkers. Here, MNs function as the sampling interface, while the microfluidic chip takes roles in fluid guidance, quantification, and storage. The off-tip biosensing components are present in the chamber for converting the biomarker concentrations into readable signals.95,96 For instance, Chen et al. developed a MN wearable device combining a 6 × 6 hollow MN array with microfluidics and a colorimetric biosensing component. MNs extracted ISF through negative pressure, which was then guided by microchannels to a reaction chamber. The biosensor component used a double-antibody sandwich based on ELISA for C-peptide detection, with regeneration for 10 assays. The mobile app detected the color change and then provided the related C-peptide concentration value.95 SERS has also been used to analyze the biomarker in the collected skin ISF. Xiao et al. integrated hollow MNs (4 × 4) for skin ISF extraction with a microfluid chip for fluid transport and storage under a negative pressure from a suction cup (Fig. 6A–C). A 3D gold nanoarray SERS substrate embedded within the microfluidic chamber enabled label-free Raman detection of uric acid (Fig. 6D). When coupled with a handheld Raman spectrometer, the device achieved rapid, portable, and ultrasensitive uric acid monitoring with a detection limit of 0.51 μM.96 Electrochemical biosensors are also frequently used as the off-tip sensing component, which can be part of a circuit board (PCB) containing wireless communication components.97 For example, Abbasiasl et al. developed a wearable touch-activated device integrating polycarbonate hollow MNs, a microfluidic chip, and wireless communication elements.98 The microfluidic chip collected the ISF from MNs with the assistance of vacuum and guided the liquid to the sensing components. The wireless communication component enabled real-time data transmission to a mobile app, supporting continuous glucose and pH monitoring.98
 | ||
| Fig. 6 MN wearable devices with off-tip sensors for uric acid monitoring: (A) the design of the wearable MN sensor integrating hollow MNs, a microfluidic chip, and SERS substrate. (B) Skin ISF sampling and sensing of the wearable off-tip MN sensor. (C) The image of the integrated wearable MN device. (D) The uric acid-sensing on the SERS substrate. Reproduced with permission from ref. 96. Copyright 2023, Elsevier. | ||
3.3 MN wearable devices with on-tip sensors
On-tip biosensing works through the on-tip incorporated recognition assay which generates colorimetric, fluorescent, and SERS signals. Detection can be achieved through simple visual inspection or portable devices. For example, He et al. designed a colorimetric HA MN tattoo with segmented areas for multiplexed detection. Each area had specific reagents loaded, forming dermal tattoos upon skin insertion through tip dissolution. The design ensured >85% pattern fidelity for the simultaneous detection of pH, glucose, uric acid, and temperature through distinct color changes, readable by the naked eye or a camera.99 Researchers also developed a wearable fluorescence-based MN sensor array for continuous glucose monitoring.100 The array consisted of silk fibroin MNs that had glucose-responsive fluorescent monomers with diboronic acid (glucose-binding) and anthracene (fluorescence) moieties. Anthracene fluorescence was quenched at low glucose levels, while the binding of glucose restored the fluorescence. The fluorescence output was conveniently detected using a smartphone app, facilitating tether-free glucose monitoring with enhanced patient mobility.100On-tip biosensors can also be electrochemical and generate continuous readout of electrical signals.101,102 In these devices, the MN array serves as the working, reference, and/or counter electrodes in a two- or three-electrode system. Zhang et al. designed a wearable MN sensor device where each electrode (working, reference, and counter) was an individual MN array patch (10 × 10).103 MNs were designed in a core–shell structure with a silver paste core and an enzyme-incorporated hydrogel layer. The three-MN-patch sensor was then incorporated with PCB to allow real-time ISF glucose monitoring from a mobile phone through wireless communication.103 To minimize the size of wearable devices, fewer needles could be used for each individual electrode. For example, two 3-electrode systems (each with 6 working MN electrodes, 8 counter MN electrodes, and 1 reference MN electrode) for multiplexed sensing achieved a high degree of miniaturization and were fully integrated with a compact 5.3 cm2 PCB.104 This multiplexed sensing can be extended for sensing more analytes by using a single MN as the working electrode for single-analyte sensing. For example, Li et al. developed a self-calibrating multiplexed MN electrode array, where each electrode was a single MN (Fig. 7A). It could detect 9 analytes (glucose, cholesterol, uric acid, lactate, ROS, Na+, K+, Ca2+, pH) in ISF with self-calibration.105 If further miniaturization is required, different electrodes can be placed on one single MN tip.106 Yang et al. developed a wearable device with one MN tip containing four electrodes (two working, one reference, one counter) (Fig. 7B). This device simultaneously monitored glucose and metformin in ISF through differential pulse voltammetry (DPV) with a smartphone app for real-time analysis. This would enable personalized diabetes treatment through PK/PD evaluation.106 Finally, different electrochemical and physical sensors can be integrated into the same device. Chang et al. developed a hybrid flexible wristband with an MN sensor to analyze the ISF biomarkers, an ultrasonic transducer for blood pressure monitoring, and a biopotential electrode for ECG monitoring. The combination of these systems provided a comprehensive mapping of metabolic and cardiovascular functions simultaneously.107
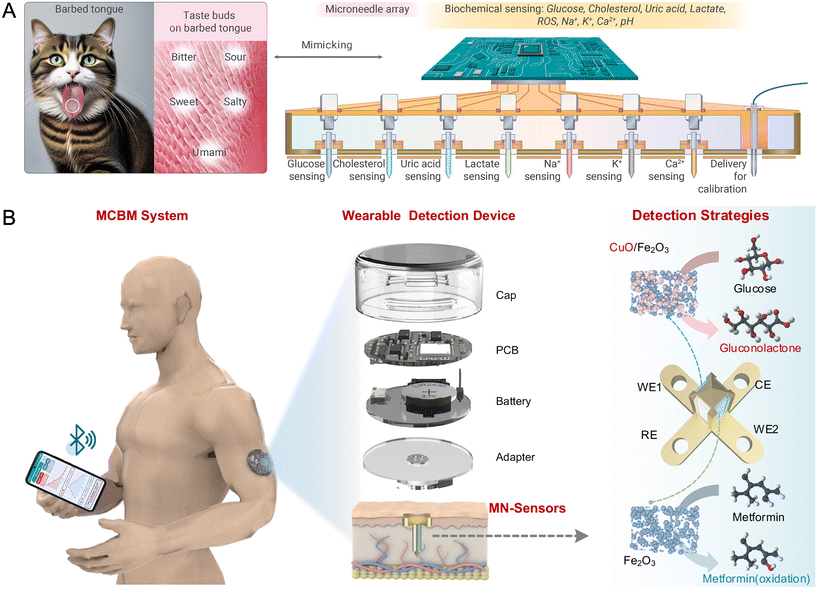 | ||
| Fig. 7 MN wearable devices with on-tip sensors: (A) self-calibrating multiplexed MN electrode array, in which each electrode is a single MN, enabling detection of nine analytes in ISF (glucose, cholesterol, uric acid, lactate, ROS, Na+, K+, Ca2+, and pH). Reproduced with permission from ref. 105. Copyright 2025, Elsevier. (B) Wearable device with one single MN containing four electrodes (two working, one reference, one counter). Reproduced from ref. 106 under the CC-BY-NC-ND license. | ||
3.4 Commercialized MN wearable devices
The most known products are devices for continuous glucose monitoring (CGM). They are class II medical devices under the FDA regulation section of integrated continuous glucose monitoring system (21 CFR 862.1355) or glucose test system (21 CFR 862.1345). CGM systems, such as the Abbott FreeStyle Libre 3 Plus and Dexcom G7, comprise two main components: a sensor module and a sensor applicator. Within the applicator, retractable hollow stainless-steel MNs are pre-loaded and serve to insert the sensor filament beneath the skin. After insertion, the needle automatically retracts, leaving the thin and flexible sensing filament in the interstitial space to access ISF. The sensing filament differs slightly between systems: the FreeStyle Libre 3 Plus employs a polymer-based filament, whereas the Dexcom G7 utilizes a platinum and tantalum core wire. In both designs, the filament tip lies in an enzyme-based electrochemical sensor, where glucose oxidase catalyzes the oxidation of glucose, generating hydrogen peroxide that is subsequently oxidized at the working electrode. The resulting current is proportional to local glucose concentration, enabling continuous, real-time glucose monitoring. Data are then transmitted via near-field communication and Bluetooth, enabling real-time display of glucose readings, trends, and customizable alerts.The Biolinq Shine Autonomous Time-in-Range Microsensor has been recently granted classification as a prescription class II medical device by the FDA under the regulation of glucose range monitoring system (21 CFR 862.1359). Unlike the Abbott FreeStyle Libre 3 Plus and Dexcom G7 using filament sensing strategy, it integrates an array of MN sensors that reside in the intradermal layer to access interstitial fluid glucose, eliminating the need for retractable subcutaneous introducer needles. Its MN sensor array works in conjunction with an accelerometer and ambient light sensor to measure and record physiological data. Different from quantitative CGMs, it features a qualitative color-changing indicator light that output glucose level trends continuously, while a paired mobile app provides more granular glucose information and correlates it with meals, rest, and activity levels. This device is designed for adults aged 22 and older who do not use insulin. It supports lifestyle and behavioral modifications for glycemic control but cannot make acute medical decisions such as insulin dosing or medication adjustments.
4. MN wearable devices for monitoring electrophysiological signals
4.1 Design of MN electrodes
As electrodes for electrophysiological signal transduction, including EEG, ECG, EMG, and ENoG, MNs rely on their conductive properties. In contrast to conventional wet electrodes and flat dry electrodes, MN-based electrophysiological monitoring electrodes increase the skin contact area, reducing signal noises and improving the quality of the acquired signals. Solid MNs and multilayer coated MNs are popular choices with innovations in improving the skin adhesion, MN conductivity, and MN patch flexibility.108 One example is a titanium-coated barbed MN array electrode using photolithography and PDMS thermal expansion. These MN electrodes could record high-quality ECG in static/dynamic states with lower impedance and higher amplitude than Ag/AgCl electrodes. The barbed structure also enhanced skin adhesion, with 3.3 times higher pull-out force than barbless ones (Fig. 8A).108 | ||
| Fig. 8 MN electrodes for electrophysiological signal monitoring. (A) Barbed MN array electrode enhancing skin adhesion for ECG monitoring. Reproduced with permission from ref. 108. Copyright 2024, Elsevier. (B) Conductive MNs made from PEDOT:PSS for EMG, ECG, EEG, and ECoG recording. Reproduced from ref. 115 under the CC-BY-NC-ND 4.0 license. (C) (i) Illustration of the Miura-ori structured MN array electrode; (ii) SEM structure of Miura-ori structured MN array; (iii) flexibility of the MN electrode. Reproduced from ref. 121 under the CC-BY 4.0 license. | ||
Metallic materials like stainless steel,109 gold, and silver110,111 are commonly used as the conductive substrate or layer of MN electrodes. Krieger et al. fabricated dry MN electrodes using medical-grade stainless steel through direct metal laser sintering. Compared to needle-free electrodes, electrode–skin contact impedance was reduced by approximately 63%.109 Gold or silver is typically deposited onto solid MNs made from material substrate like silicon,112 polyimide,100,113 epoxy,114 etc. to enable electrical signal conduction, which can enhance the mechanical strength to facilitate efficient skin penetration. Conductive polymers, such as poly(3,4-ethylenedioxythiophene) (PEDOT) and PPy113,115,116 were also used for the coating of solid MNs. For instance, Zhou et al. introduced modulus-adjustable and mechanically adaptive dry MN electrodes from PEDOT:PSS. The MN electrodes enabled high-quality EMG, ECG, EEG, and ECoG recordings, with better long-term stability than Ag/AgCl electrodes (Fig. 8B).115 MN electrodes could also be coated with conductive 2D materials, such as MXene.117,118 Chen et al. developed MXene-based MN electrodes for brain–computer interface with low impedance and high-quality EEG recording.117
The use of flexible substrate materials together with structural designs would allow MN electrodes to conform to different surface curvatures. Conductive fabrics, in particular, are commonly used as the base for MN electrodes due to their electrical conductivity. Singh et al. developed flexible conductive fabric-backed MN electrodes that acquired high-quality ECG and EMG signals without the need of wet gel or skin shaving. The conductive fabric was a nylon ripstop fabric covered by nickel, copper, silver, and a waterproof layer, providing flexibility to conform to body curves.114 Another choice is polymer, such as the hybrid of silk fibroin and polyurethane.110 The incorporation of metal serpentine interconnection configuration on polymer substrate allows the metal traces to stretch and bend without fracturing. For example, a highly flexible and stretchable MN electrode array has been developed by combining gold serpentine interconnects and elastomeric silicone substrate. This material allowed the MN array to conform to soft tissues while providing a stable interface,119 thus achieving high-quality intramuscular EMG recording and better localized sensing than planar electrodes. Moreover, the flexibility of the MN electrode substrate is adjustable through surface structure modifications, such as mesh patterns120 and Miura-ori.121 Hou et al. developed a Miura-ori-structured MN array electrode with two-directional in-plane bendability (Fig. 8C, i and ii). This unique structure not only enhanced mechanical flexibility but also mitigated metal layer peeling (Fig. 8C, iii).121
4.2 The integration of MN electrodes into wearable devices
MN electrodes can be integrated with flexible electronics into wearable devices to monitor electrophysiological signals.112,122–124 For example, Li et al. created a wearable wireless system by adding polyimide-based flexible MN array electrodes to flexible printed circuits.113 The system connected MN electrodes to front-end amplifiers, microcontrollers with Bluetooth, and power modules. Simultaneous recording of multiple physiological signals like ECG, EMG, EOG, and EEG was accomplished with low motion artifacts.113 Another example is a wireless facial biosensing system with flexible MN electrodes in a soft and wearable electronic mask (Biomask) (Fig. 9A i). This system provided facial EMG monitoring with real-time data transmission by a PCB module (Fig. 9A ii), supporting facial palsy assessment, intraoperative nerve monitoring, and long-term follow-up.122 | ||
| Fig. 9 MN wearable devices for electrophysiological signal monitoring. (A) Integration of MN electrodes with flexible electronics for facial biosensing: (i) illustration of a soft wearable electronic mask for facial EMG monitoring; (ii) design of the electrode component and PCB module. Reproduced from ref. 122 under the CC-BY 4.0 license. (B) Integration of MN electrodes as brain–computer interfaces with an EEG scalp cap: (i) Mxene MN electrode as a brain–computer interface; (ii) the wearable EEG cap. Reproduced with permission from ref. 117. Copyright 2025, American Chemical Society. (C) Integration of MN electrode devices with artificial neural networks for enhanced bioelectrical signal interpretation: (i) wearable EMG sensor for real-time monitoring of EMG signals; (ii) original and stretched wearable EMG sensor; (iii) gesture recognition by a graph neural network using transmitted data from the EMG sensor. Reproduced from ref. 126 under the CC-BY 4.0 license. | ||
The wearable devices with MN electrodes can also serve as a human–machine interface or brain–computer interface for seamless interactions between the human body and external devices.115,117,120,125 For instance, Kim et al. developed a wearable wireless system combining a stretchable MN adhesive patch with a flexible circuit for adaptability to skin deformation.125 It had a closed-loop control of exoskeleton robots, where the MN patch on lower limb muscles transmitted EMG signals wirelessly, and triggered assistance when signals exceeded the thresholds. The system reduced back muscle activation by 18.1% during lifting, showing robust performance in both pretreated and perspiring skin conditions, advancing human–machine interfaces. Additionally, these MN devices can function as a brain–computer interface115,117,120 for facilitating direct communication between the brain and computers. Detecting and interpreting neural signals through MN electrodes is promising for bridging the gap between human neural activity and digital systems. For example, a brain–computer interface framework has been integrated with MXene MN electrodes into wearable EEG cap devices (Fig. 9B, i and ii). The 1 mm2 dry MN electrodes penetrated the epidermis, delivering stable performance in vibrational and motion scenarios.117
To enhance the interpretation of bioelectrical signals, the MN wearable devices can incorporate with artificial neural networks. Lee et al. presented a stretchable array of bipolar sEMG electrodes with a self-attention-based graph neural network for gesture recognition (Fig. 9C, i).126 A sticky, hole-patterned patch provided skin-like stretchability and water vapor permeability for stable signal delivery (Fig. 9C, ii). It aimed to spatially cover skeletal muscles and acquire regional EMG activity data from 18 static and dynamic gestures, achieving ∼97% accuracy with one trial per gesture (Fig. 9C, iii). Notably, it retained ∼95% accuracy over 72 h of long-term testing and after 10 reuses, showing promise for applications in prosthetic control and smart device interaction.126 Finally, MN wearable devices can integrate with virtual reality (VR) technology. Mahmood et al. developed a wireless soft scalp electronic system with flexible EEG MN electrodes and a wireless electronic circuit as the brain–computer interface.127 A convolutional neural network was used to preprocess and classify the motor imagery brain signals delivered by the scalp device. The wearable scalp device was subsequently integrated with VR which provides visual cues in the form of animated limbs and instant feedback. This setup allows users to control a VR game using imagined movements through the brain–computer interface, while the VR component enhances task visualization and improves classification accuracy.127
5. Challenges and future perspectives for MN-based wearable devices
5.1 Technical challenges
5.2 Clinical validation
The clinical validation of MN-based measurements faces hurdles. In particular, the relationship between disease biomarkers in skin ISF and in blood has not been fully established.133 Frequent or large volumes of ISF sampling can disturb the local microenvironment and even trigger immune effects, making ISF concentrations diverge from blood levels.15 As a result, there could be diagnostic inaccuracies when detecting a target analyte with a MN sensor. Comprehensive comparative studies are therefore necessary. Long-term in vivo trials in diverse groups of people, including different ages, skin types, and health conditions, are needed to link biomarker levels in ISF with those in blood, using standard laboratory methods such as LC-MS/MS or ELISA.5.3 Industrialization and regulatory challenges
5.4 Future outlook: intelligent MN platforms
An ideal MN wearable system would continuously monitor biomarkers or electrophysiological signals and provide alerts/suggestions for the timely intervention of health conditions (Fig. 10).135 Progress toward this vision has already been demonstrated in prototype devices.136,137 For example, one recent study developed a wearable closed-loop system for diabetes in which an MN-based glucose sensor was integrated with an iontophoretic insulin delivery module.136 This device detected rising glucose levels in real time and immediately triggered a proportional insulin release, maintaining normoglycemia in diabetic rats. In principle, multi-analyte MN arrays (e.g. for lactate, electrolytes, stress hormones) could feed into a feedback controller that actuates a micro-reservoir or patch injector. This self-regulating architecture, combining sensing, electronics, and drug delivery in a single skin-conformal platform, could revolutionize chronic disease management by providing on-demand therapy without user intervention. The emergence of AI can further facilitate the realization of the ideal MN devices.135 AI and machine-learning algorithms can process the continuous streams of multi-modal data from a MN device to identify trends and forecast events.131 For instance, subtle patterns in glucose and movement data could be recognized weeks in advance of a metabolic crisis, prompting pre-emptive dietary or insulin-dosing advice. Moreover, AI-driven platforms can translate raw sensor outputs into individualized recommendations. There could be an analogous to fitness wearables that suggest hydration and activity changes, or an AI-enhanced MN patch that could advise users when to eat, take medications, or seek care based on their unique physiology. In addition, data-driven algorithms can compensate for signal artefacts and inter-subject variability. By filtering motion noise or adaptively recalibrating sensor baselines, readouts could be stabilized over time. | ||
| Fig. 10 The ideal MN wearable devices for personalized medicine. Created with https://BioRender.com. | ||
Conflicts of interest
There are no conflicts of interest to declare.Data availability
No primary research results, software, or code are included in this article, and no new data were generated or analysed in the preparation of this review.Acknowledgements
C. X. appreciates the support by the General Research Fund (CityU11100323, CityU11101324) from the Research Grants Council (RGC) of the Hong Kong Special Administrative Region China and the National Science Fund for Distinguished Young Scholars from the National Natural Science Foundation of China (T2425004).References
- Y. Wang, H. Haick, S. Guo, C. Wang, S. Lee, T. Yokota and T. Someya, Chem. Soc. Rev., 2022, 51, 3759–3793 RSC.
- S. M. A. Iqbal, I. Mahgoub, E. Du, M. A. Leavitt and W. Asghar, npj Flexible Electron., 2021, 5, 9 CrossRef.
- A. Keshet, L. Reicher, N. Bar and E. Segal, Nat. Metab., 2023, 5, 563–571 Search PubMed.
- Z. Wang, Y.-c. Lai, Y.-t. Chiang, J. M. Scheiger, S. Li, Z. Dong, Q. Cai, S. Liu, S.-h. Hsu, C.-c. Chou and P. A. Levkin, ACS Appl. Mater. Interfaces, 2022, 14, 50152–50162 CrossRef CAS PubMed.
- Z. Wang, Q. Cai, L. Lu and P. A. Levkin, Small, 2024, 20, 2305214 Search PubMed.
- T. S. Vo and K. Kim, Adv. Intell. Syst., 2024, 6, 2300730 CrossRef.
- J. Li, H. Chu, Z. Chen, C. K. Yiu, Q. a. Qu, Z. Li and X. Yu, ACS Nano, 2024, 18, 17407–17438 Search PubMed.
- K. M. Clark and T. R. Ray, ACS Sens., 2023, 8, 3606–3622 CrossRef CAS PubMed.
- F. Gao, C. Liu, L. Zhang, T. Liu, Z. Wang, Z. Song, H. Cai, Z. Fang, J. Chen, J. Wang, M. Han, J. Wang, K. Lin, R. Wang, M. Li, Q. Mei, X. Ma, S. Liang, G. Gou and N. Xue, Microsyst. Nanoeng., 2023, 9, 1 CrossRef.
- H. Li, S. Gu, Q. Zhang, E. Song, T. Kuang, F. Chen, X. Yu and L. Chang, Nanoscale, 2021, 13, 3436–3453 Search PubMed.
- M. S. Li, H. L. Wong, Y. L. Ip, Z. Peng, R. Yiu, H. Yuan, J. K. Wai Wong and Y. K. Chan, ACS Sens., 2022, 7, 1300–1314 Search PubMed.
- H.-R. Lim, S. M. Lee, S. Park, C. Choi, H. Kim, J. Kim, M. Mahmood, Y. Lee, J.-H. Kim and W.-H. Yeo, Biosens. Bioelectron., 2022, 210, 114329 Search PubMed.
- Z. Wang, Z. Lin, X. Mei, L. Cai, K.-C. Lin, J. F. Rodríguez, Z. Ye, X. S. Parraguez, E. M. Guajardo, P. C. García Luna, J. Y. J. Zhang and Y. S. Zhang, Adv. Mater., 2025, 37, 2416260 Search PubMed.
- T. Saha, S. Mukherjee, M. D. Dickey and O. D. Velev, Lab Chip, 2024, 24, 1244–1265 Search PubMed.
- Z. Wu, Z. Qiao, S. Chen, S. Fan, Y. Liu, J. Qi and C. T. Lim, Commun. Mater., 2024, 5, 33 Search PubMed.
- D. Sim, M. C. Brothers, J. M. Slocik, A. E. Islam, B. Maruyama, C. C. Grigsby, R. R. Naik and S. S. Kim, Adv. Sci., 2022, 9, 2104426 CrossRef CAS.
- M. Friedel, I. A. P. Thompson, G. Kasting, R. Polsky, D. Cunningham, H. T. Soh and J. Heikenfeld, Nat. Biomed. Eng., 2023, 7, 1541–1555 Search PubMed.
- C. Merzougui, X. Yang, D. Meng, Y. Huang and X. Zhao, Adv. Healthcare Mater., 2025, 14, 2404420 Search PubMed.
- J. Yang, H. Zhang, T. Hu, C. Xu, L. Jiang, Y. Shrike Zhang and M. Xie, Chem. Eng. J., 2021, 426, 130561 CrossRef CAS.
- M. Zheng, T. Sheng, J. Yu, Z. Gu and C. Xu, Nat. Rev. Bioeng., 2024, 2, 324–342 CrossRef CAS.
- N. Xu, W. Xu, M. Zhang, J. Yu, G. Ling and P. Zhang, Adv. Mater. Technol., 2022, 7, 2101595 CrossRef.
- G. Kim, H. Ahn, J. Chaj Ulloa and W. Gao, Med-X, 2024, 2, 15 CrossRef CAS PubMed.
- J. Li, M. Wei and B. Gao, ACS Sens., 2024, 9, 1149–1161 CrossRef CAS PubMed.
- L. K. Vora, A. H. Sabri, P. E. McKenna, A. Himawan, A. R. J. Hutton, U. Detamornrat, A. J. Paredes, E. Larrañeta and R. F. Donnelly, Nat. Rev. Bioeng., 2024, 2, 64–81 Search PubMed.
- Y. Hu, E. Chatzilakou, Z. Pan, G. Traverso and A. K. Yetisen, Adv. Sci., 2024, 11, 2306560 Search PubMed.
- T. Hu, K. S. Lui, E. B. Ko, Y. Zhao, Q. Zhang, H. Yang, M. Zheng, H. Chang, B. Guo, A. K. L. Cheung and C. Xu, Matter, 2025, 8, 102038 Search PubMed.
- X. Jiang, E. C. Wilkirson, A. O. Bailey, W. K. Russell and P. B. Lillehoj, Cell Rep. Phys. Sci., 2024, 5, 101975 Search PubMed.
- C. Kolluru, M. Williams, J. S. Yeh, R. K. Noel, J. Knaack and M. R. Prausnitz, Biomed. Microdevices, 2019, 21, 14 Search PubMed.
- P. P. Samant, M. M. Niedzwiecki, N. Raviele, V. Tran, J. Mena-Lapaix, D. I. Walker, E. I. Felner, D. P. Jones, G. W. Miller and M. R. Prausnitz, Sci. Transl. Med., 2020, 12, eaaw0285 CrossRef CAS PubMed.
- C. Kolluru, R. Gupta, Q. Jiang, M. Williams, H. Gholami Derami, S. Cao, R. K. Noel, S. Singamaneni and M. R. Prausnitz, ACS Sens., 2019, 4, 1569–1576 CrossRef CAS PubMed.
- S. Kim, M. S. Lee, H. S. Yang and J. H. Jung, Sci. Rep., 2021, 11, 14018 CrossRef CAS PubMed.
- A. H. Hung, N. U. Rajesh, A. Bermudez, S. M. Boczek, F. J. Garcia-Marqués, Y. L. Tan, J. Hwang, P. D. Sinawang, D. Ilyin, G. B. Jacobson, U. Demirci, S. P. Poplack, S. J. Pitteri and J. M. DeSimone, bioRxiv, 2025, DOI:10.1101/2025.03.13.641882.
- N. Zoratto, D. Klein-Cerrejon, D. Gao, T. Inchiparambil, D. Sachs, Z. Luo and J. C. Leroux, Adv. Sci., 2024, 11, e2308809 CrossRef.
- G. Wang, Y. Zhang, H. K. Kwong, M. Zheng, J. Wu, C. Cui, K. W. Y. Chan, C. Xu and T.-H. Chen, Adv. Sci., 2024, 11, 2306188 CrossRef CAS PubMed.
- M. Zheng, Y. Zhang, T. Hu and C. Xu, Bioeng. Transl. Med., 2023, 8, e10413 CrossRef CAS PubMed.
- M. Zheng, Z. Wang, H. Chang, L. Wang, S. W. T. Chew, D. C. S. Lio, M. Cui, L. Liu, B. C. K. Tee and C. Xu, Adv. Healthcare Mater., 2020, 9, 1901683 Search PubMed.
- H. Chang, M. Zheng, X. Yu, A. Than, R. Z. Seeni, R. Kang, J. Tian, D. P. Khanh, L. Liu, P. Chen and C. Xu, Adv. Mater., 2017, 29, 1702243 CrossRef PubMed.
- Y. Dai, J. Nolan, E. Madsen, M. Fratus, J. Lee, J. Zhang, J. Lim, S. Hong, M. A. Alam, J. C. Linnes, H. Lee and C. H. Lee, ACS Appl. Mater. Interfaces, 2023, 15, 56760–56773 Search PubMed.
- J. Zhu, X. Zhou, H.-J. Kim, M. Qu, X. Jiang, K. Lee, L. Ren, Q. Wu, C. Wang, X. Zhu, P. Tebon, S. Zhang, J. Lee, N. Ashammakhi, S. Ahadian, M. R. Dokmeci, Z. Gu, W. Sun and A. Khademhosseini, Small, 2020, 16, 1905910 Search PubMed.
- K. M. Saifullah, A. Mushtaq, P. Azarikhah, P. D. Prewett, G. J. Davies and Z. Faraji Rad, Microsyst. Nanoeng., 2025, 11, 3 CrossRef CAS PubMed.
- K. M. Saifullah, P. Azarikhah and Z. Faraji Rad, Mater. Today Chem., 2025, 45, 102661 Search PubMed.
- N. Xu, M. Zhang, W. Xu, G. Ling, J. Yu and P. Zhang, Analyst, 2022, 147, 1478–1491 RSC.
- N. G. Oh, S. Y. Hwang and Y. H. Na, ACS Omega, 2022, 7, 25179–25185 CrossRef CAS PubMed.
- C. G. Li, M. Dangol, C. Y. Lee, M. Jang and H. Jung, Lab Chip, 2015, 15, 382–390 RSC.
- P. R. Miller, R. M. Taylor, B. Q. Tran, G. Boyd, T. Glaros, V. H. Chavez, R. Krishnakumar, A. Sinha, K. Poorey, K. P. Williams, S. S. Branda, J. T. Baca and R. Polsky, Commun. Biol., 2018, 1, 173 CrossRef PubMed.
- T. Elias Abi-Ramia Silva, S. Kohler, N. Bartzsch, F. Beuschlein and A. T. Güntner, Cell Biomater., 2025, 1, 100008 CrossRef.
- Y. Li, H. Zhang, R. Yang, Y. Laffitte, U. Schmill, W. Hu, M. Kaddoura, E. J. M. Blondeel and B. Cui, Microsyst. Nanoeng., 2019, 5, 41 CrossRef PubMed.
- P. Liu, H. Du, Z. Wu, H. Wang, J. Tao, L. Zhang and J. Zhu, J. Mater. Chem. B, 2021, 9, 5476–5483 Search PubMed.
- K. Takeuchi, N. Takama, K. Sharma, O. Paul, P. Ruther, T. Suga and B. Kim, Drug Delivery Transl. Res., 2022, 12, 435–443 Search PubMed.
- G. Zhu, C. Liu, Y. Lu, X. Mo, J. Dong, K. Xu, Y. Huang, C. Chen, X. Lv, X. Yang and X. Zhao, ACS Appl. Nano Mater., 2025, 8, 7867–7875 Search PubMed.
- X.-Q. You, Q.-Y. He, T.-W. Wu, D.-Y. Huang, Z.-Z. Peng, D.-Y. Chen, Z. Chen and J. Liu, Microchem. J., 2023, 190, 108570 Search PubMed.
- F. Leng, M. Zheng and C. Xu, Exploration, 2021, 1, 20210109 Search PubMed.
- C. T. Sharkey, A. F. Aroche, I. G. Agusta, H. Nissan, T. Saha, S. Mukherjee, J. S. Twiddy, M. D. Dickey, O. Velev and M. A. Daniele, Lab Chip, 2025, 25, 4577–4587 Search PubMed.
- F. Ribet, A. Bendes, C. Fredolini, M. Dobielewski, M. Böttcher, O. Beck, J. M. Schwenk, G. Stemme and N. Roxhed, Adv. Healthcare Mater., 2023, 12, 2202564 Search PubMed.
- C. G. Li, K. Lee, C. Y. Lee, M. Dangol and H. Jung, Adv. Mater., 2012, 24, 4583–4586 Search PubMed.
- Y. Xie, J. He, W. He, T. Iftikhar, C. Zhang, L. Su and X. Zhang, Adv. Sci., 2024, 11, 2308716 Search PubMed.
- A. Trautmann, G.-L. Roth, B. Nujiqi, T. Walther and R. Hellmann, Microsyst. Nanoeng., 2019, 5, 6 Search PubMed.
- T. M. Blicharz, P. Gong, B. M. Bunner, L. L. Chu, K. M. Leonard, J. A. Wakefield, R. E. Williams, M. Dadgar, C. A. Tagliabue, R. El Khaja, S. L. Marlin, R. Haghgooie, S. P. Davis, D. E. Chickering and H. Bernstein, Nat. Biomed. Eng., 2018, 2, 151–157 CrossRef CAS PubMed.
- M. S. F. Hoffman, J. W. McKeage, J. Xu, B. P. Ruddy, P. M. F. Nielsen and A. J. Taberner, Expert Rev. Med. Devices, 2023, 20, 5–16 CrossRef CAS PubMed.
- J. Kang, K. Y. Kim, S. Kim, H. Hong, B.-S. Bae, S.-K. Kang and W. Lee, Device, 2023, 1, 100112 CrossRef.
- S. A. Ranamukhaarachchi, C. Padeste, M. Dubner, U. O. Hafeli, B. Stoeber and V. J. Cadarso, Sci. Rep., 2016, 6, 29075 CrossRef CAS PubMed.
- K. Yi, Y. Wang, K. Shi, J. Chi, J. Lyu and Y. Zhao, Biosens. Bioelectron., 2021, 190, 113404 CrossRef CAS PubMed.
- M. Dervisevic and N. H. Voelcker, ACS Mater. Lett., 2023, 5, 1851–1858 Search PubMed.
- L. He, Y. Zhou, M. Zhang, M. Chen, Y. Wu, L. Qi, L. Liu, B. Zhang, X. Yang, X. He and K. Wang, ACS Sens., 2024, 9, 6563–6571 CrossRef CAS PubMed.
- V. Behnam, A. M. McManamen, H. G. Ballard, B. Aldana, M. Tamimi, N. Milosavić, M. N. Stojanovic, M. R. Rubin and S. K. Sia, Angew. Chem., Int. Ed., 2025, 64, e202414871 CrossRef CAS PubMed.
- D. Al Sulaiman, J. Y. H. Chang, N. R. Bennett, H. Topouzi, C. A. Higgins, D. J. Irvine and S. Ladame, ACS Nano, 2019, 13, 9620–9628 CrossRef CAS PubMed.
- B. Ciui, A. Martin, R. K. Mishra, B. Brunetti, T. Nakagawa, T. J. Dawkins, M. Lyu, C. Cristea, R. Sandulescu and J. Wang, Adv. Healthcare Mater., 2018, 7, 1701264 CrossRef PubMed.
- W. Lee, S.-h. Jeong, Y.-W. Lim, H. Lee, J. Kang, H. Lee, I. Lee, H.-S. Han, S. Kobayashi, M. Tanaka and B.-S. Bae, Sci. Adv., 2021, 7, eabi6290 Search PubMed.
- X. Zhou, S. Huang, D. Zhang, W. Liu, W. Gao, Y. Xue and L. Shang, Anal. Chem., 2023, 95, 12104–12112 Search PubMed.
- Z. Wang, J. Luan, A. Seth, L. Liu, M. You, P. Gupta, P. Rathi, Y. Wang, S. Cao, Q. Jiang, X. Zhang, R. Gupta, Q. Zhou, J. J. Morrissey, E. L. Scheller, J. S. Rudra and S. Singamaneni, Nat. Biomed. Eng., 2021, 5, 64–76 CrossRef CAS PubMed.
- Z. Bao, S. Lu, D. Zhang, G. Wang, X. Cui and G. Liu, Adv. Healthcare Mater., 2024, 13, 2303511 Search PubMed.
- P. Zhang, X. Wu, H. Xue, Y. Wang, X. Luo and L. Wang, Anal. Chim. Acta, 2022, 1212, 339911 Search PubMed.
- X. Li, J. Lv, J. Zhao, G. Ling and P. Zhang, Anal. Chim. Acta, 2024, 1288, 342152 Search PubMed.
- J. Ju, C.-M. Hsieh, Y. Tian, J. Kang, R. Chia, H. Chang, Y. Bai, C. Xu, X. Wang and Q. Liu, ACS Sens., 2020, 5, 1777–1785 Search PubMed.
- R. Mei, Y. Wang, X. Zhao, S. Shi, X. Wang, N. Zhou, D. Shen, Q. Kang and L. Chen, ACS Sens., 2023, 8, 372–380 Search PubMed.
- Y. Liu, Q. Yu, X. Luo, L. Yang and Y. Cui, Microsyst. Nanoeng., 2021, 7, 75 Search PubMed.
- P. Bollella, S. Sharma, A. E. G. Cass and R. Antiochia, Biosens. Bioelectron., 2019, 123, 152–159 Search PubMed.
- C. Moonla, M. Reynoso, A. Casanova, A.-Y. Chang, O. Djassemi, A. Balaje, A. Abbas, Z. Li, K. Mahato and J. Wang, ACS Sens., 2024, 9, 1004–1013 Search PubMed.
- X. Huang, S. Zheng, B. Liang, M. He, F. Wu, J. Yang, H.-j. Chen and X. Xie, Microsyst. Nanoeng., 2023, 9, 25 CrossRef CAS PubMed.
- H. Li, G. Wu, Z. Weng, H. Sun, R. Nistala and Y. Zhang, ACS Sens., 2021, 6, 2181–2190 CrossRef CAS PubMed.
- H. Yoo, H. Jo and S. S. Oh, Mater. Adv., 2020, 1, 2663–2687 RSC.
- S. Lin, X. Cheng, J. Zhu, B. Wang, D. Jelinek, Y. Zhao, T.-Y. Wu, A. Horrillo, J. Tan, J. Yeung, W. Yan, S. Forman, H. A. Coller, C. Milla and S. Emaminejad, Sci. Adv., 2022, 8, eabq4539 CrossRef CAS PubMed.
- Y. Wu, F. Tehrani, H. Teymourian, J. Mack, A. Shaver, M. Reynoso, J. Kavner, N. Huang, A. Furmidge, A. Duvvuri, Y. Nie, L. M. Laffel, F. J. Doyle, III, M.-E. Patti, E. Dassau, J. Wang and N. Arroyo-Currás, Anal. Chem., 2022, 94, 8335–8345 CrossRef CAS PubMed.
- M. Reynoso, A.-Y. Chang, Y. Wu, R. Murray, S. Suresh, Y. Dugas, J. Wang and N. Arroyo-Currás, Biosens. Bioelectron., 2024, 244, 115802 Search PubMed.
- A. M. Downs, A. Bolotsky, B. M. Weaver, H. Bennett, N. Wolff, R. Polsky and P. R. Miller, Biosens. Bioelectron., 2023, 236, 115408 Search PubMed.
- X. Li, L. Hu, F. Xu, W. Yu, Y. Wu, J. Deng, Z. Wei, G. Shi and M. Zhang, Talanta, 2025, 295, 128312 Search PubMed.
- K. Zhao, X. Ma, M. Wang, Z. Qu, H. Chen, B. He, H. Chen and B. Zhang, Anal. Methods, 2024, 16, 5665–5675 Search PubMed.
- K. M. Cheung, K.-A. Yang, N. Nakatsuka, C. Zhao, M. Ye, M. E. Jung, H. Yang, P. S. Weiss, M. N. Stojanović and A. M. Andrews, ACS Sens., 2019, 4, 3308–3317 CrossRef CAS PubMed.
- L. Yue Jing, Y. Fan, B. Zhi Chen, D. Li, Y. Ting He, G. Liang Zhang, L. Liang, J. Du, Y. Wang and X. Dong Guo, Chem. Eng. J., 2024, 502, 157488 CrossRef.
- D. D. Zhu, L. W. Zheng, P. K. Duong, R. H. Cheah, X. Y. Liu, J. R. Wong, W. J. Wang, S. T. Tien Guan, X. T. Zheng and P. Chen, Biosens. Bioelectron., 2022, 212, 114412 CrossRef CAS PubMed.
- M. Razzaghi, J. A. Ninan, M. Azimzadeh, E. Askari, A. H. Najafabadi, A. Khademhosseini and M. Akbari, Adv. Healthcare Mater., 2024, 13, 2400881 Search PubMed.
- X. Jiang and P. B. Lillehoj, Microsyst. Nanoeng., 2020, 6, 96 Search PubMed.
- L. Bao, J. Park, B. Qin and B. Kim, Sci. Rep., 2022, 12, 10693 CrossRef CAS PubMed.
- A. Sena-Torralba, M. Parrilla, A. Hernanz-Grimalt, A. Steijlen, E. Ortiz-Zapater, C. Cabaleiro-Otero, N. López-Riquelme, S. Cerveró-Ferragut, Á. Maquieira, K. De Wael and S. Morais, Anal. Chem., 2024, 96, 20684–20692 Search PubMed.
- S. Chen, Z. Guo, B. Lu, M. Sun, S. Wang, S. Li, Y. Jiang, Q. Wei, D. Wang and X. Jiang, Sci. Adv., 2025, 11, eadw2182 Search PubMed.
- J. Xiao, S. Zhang, Q. Liu, T. Xu and X. Zhang, Sens. Actuators, B, 2024, 398, 134685 Search PubMed.
- Y. Cheng, X. Gong, J. Yang, G. Zheng, Y. Zheng, Y. Li, Y. Xu, G. Nie, X. Xie, M. Chen, C. Yi and L. Jiang, Biosens. Bioelectron., 2022, 203, 114026 Search PubMed.
- T. Abbasiasl, F. Mirlou, H. Mirzajani, M. J. Bathaei, E. Istif, N. Shomalizadeh, R. E. Cebecioglu, E. E. Ozkahraman, U. C. Yener and L. Beker, Adv. Mater., 2024, 36, e2304704 Search PubMed.
- R. He, H. Liu, T. Fang, Y. Niu, H. Zhang, F. Han, B. Gao, F. Li and F. Xu, Adv. Sci., 2021, 8, e2103030 Search PubMed.
- M. Sang, M. Cho, S. Lim, I. S. Min, Y. Han, C. Lee, J. Shin, K. Yoon, W.-H. Yeo, T. Lee, S. M. Won, Y. Jung, Y. J. Heo and K. J. Yu, Sci. Adv., 2023, 9, eadh1765 Search PubMed.
- Q. Wang, Á. Molinero-Fernandez, Q. Wei, X. Xuan, Å. Konradsson-Geuken, M. Cuartero and G. A. Crespo, ACS Sens., 2024, 9, 3115–3125 Search PubMed.
- M. Parrilla, N. Claes, C. Toyos-Rodríguez, C. E. M. K. Dricot, A. Steijlen, S. Lebeer, S. Bals and K. De Wael, Biosens. Bioelectron., 2025, 289, 117934 Search PubMed.
- Y. Zhang, G. Zhao, M. Zheng, T. Hu, C. Yang and C. Xu, Nanoscale, 2023, 15, 16493–16500 RSC.
- F. Tehrani, H. Teymourian, B. Wuerstle, J. Kavner, R. Patel, A. Furmidge, R. Aghavali, H. Hosseini-Toudeshki, C. Brown, F. Zhang, K. Mahato, Z. Li, A. Barfidokht, L. Yin, P. Warren, N. Huang, Z. Patel, P. P. Mercier and J. Wang, Nat. Biomed. Eng., 2022, 6, 1214–1224 Search PubMed.
- X. Li, S. Zheng, M. He, X. Huang, C. Yang, J. Mo, J. Yang, C. Yang, H. Chen and X. Xie, Innovation, 2025, 6, 100781 CAS.
- J. Yang, X. Gong, Y. Zheng, H. Duan, S. Chen, T. Wu, C. Yi, L. Jiang and H. Haick, Nat. Commun., 2025, 16, 6260 Search PubMed.
- A. Y. Chang, M. Lin, L. Yin, M. Reynoso, S. Ding, R. Liu, Y. Dugas, A. Casanova, G. Park, Z. Li, H. Luan, N. Askarinam, F. Zhang, S. Xu and J. Wang, Nat. Biomed. Eng., 2026, 10, 94–109 CrossRef PubMed.
- C.-W. Dong, C.-J. Lee, D.-H. Lee, S.-H. Moon and W.-T. Park, Sens. Actuators, A, 2024, 367, 115040 Search PubMed.
- K. J. Krieger, J. Liegey, E. M. Cahill, N. Bertollo, M. M. Lowery and E. D. O'Cearbhaill, Adv. Mater. Technol., 2020, 5, 2000518 CrossRef CAS.
- X. Wang, W. Qiu, C. Lu, Z. Jiang, C. Hou, Y. Li, Y. Wang, H. Du, J. Zhou and X. Y. Liu, Adv. Funct. Mater., 2024, 34, 2311535 CrossRef CAS.
- G. Stavrinidis, K. Michelakis, V. Kontomitrou, G. Giannakakis, M. Sevrisarianos, G. Sevrisarianos, N. Chaniotakis, Y. Alifragis and G. Konstantinidis, Microelectron. Eng., 2016, 159, 114–120 CrossRef CAS.
- H. Ji, M. Wang, Y. Wang, Z. Wang, Y. Ma, L. Liu, H. Zhou, Z. Xu, X. Wang, Y. Chen and X. Feng, npj Flexible Electron., 2023, 7, 46 CrossRef CAS.
- J. Li, Y. Ma, D. Huang, Z. Wang, Z. Zhang, Y. Ren, M. Hong, Y. Chen, T. Li, X. Shi, L. Cao, J. Zhang, B. Jiao, J. Liu, H. Sun and Z. Li, Nano-Micro Lett., 2022, 14, 132 CrossRef CAS PubMed.
- O. P. Singh, A. Bocchino, T. Guillerm, Y. Hu, F. Stam and C. O'Mahony, Adv. Mater. Technol., 2023, 9, 2301606 CrossRef.
- C. Zhou, G. Yao, X. Gan, K. Chai, P. Li, J. Peng, T. Pan, M. Gao, Z. Huang, B. Jiang, Z. Yan, K. Zhao, D. Yao, K. Chen and Y. Lin, npj Flexible Electron., 2025, 9, 77 CrossRef.
- A. Keirouz, Y. L. Mustafa, J. G. Turner, E. Lay, U. Jungwirth, F. Marken and H. S. Leese, Small, 2023, 19, e2206301 Search PubMed.
- Y. Chen, Z. Fan, N. Shi, B. Cheng, C. Huang, X. Liu, X. Gao and R. Liu, ACS Appl. Mater. Interfaces, 2025, 17, 33451–33464 Search PubMed.
- Y.-C. Yang, Y.-T. Lin, J. Yu, H.-T. Chang, T.-Y. Lu, T.-Y. Huang, A. Preet, Y.-J. Hsu, L. Wang and T.-E. Lin, ACS Appl. Nano Mater., 2021, 4, 7917–7924 CrossRef CAS.
- Q. Zhao, E. Gribkova, Y. Shen, J. Cui, N. Naughton, L. Liu, J. Seo, B. Tong, M. Gazzola, R. Gillette and H. Zhao, Sci. Adv., 2024, 10, eadn7202 Search PubMed.
- Z. Xiang, J. Liu and C. Lee, Microsyst. Nanoeng., 2016, 2, 16012 CrossRef CAS PubMed.
- Y. Hou, Z. Li, Z. Wang and H. Yu, Microsyst. Nanoeng., 2021, 7, 53 CrossRef CAS PubMed.
- W. Zhou, Z. Wang, Q. Xu, X. Liu, J. Li, H. Yu, H. Qiao, L. Yang, L. Chen, Y. Zhang, Z. Huang, Y. Pang, Z. Zhang, J. Zhang, X. Guan, S. Ma, Y. Ren, X. Shi, L. Yuan, D. Li, D. Huang, Z. Li and W. Jia, npj Digit. Med., 2024, 7, 13 Search PubMed.
- Z. Liu, X. Xu, S. Huang, X. Huang, Z. Liu, C. Yao, M. He, J. Chen, H. J. Chen, J. Liu and X. Xie, Microsyst. Nanoeng., 2024, 10, 72 CrossRef CAS PubMed.
- L. Xing, L. Liu, R. Jin, H. Zhang, Y. Shen, S. Zhang, Z. He, D. Li, H. Ren, Q. Huang, X. Cao, S. Zhang, S. Dong, W. Cheng and B. Zhu, ACS Appl. Mater. Interfaces, 2024, 16, 57695–57704 CrossRef CAS PubMed.
- H. Kim, J. Lee, U. Heo, D. K. Jayashankar, K.-C. Agno, Y. Kim, C. Y. Kim, Y. Oh, S.-H. Byun, B. Choi, H. Jeong, W.-H. Yeo, Z. Li, S. Park, J. Xiao, J. Kim and J.-W. Jeong, Sci. Adv., 2024, 10, eadk5260 CrossRef PubMed.
- H. Lee, S. Lee, J. Kim, H. Jung, K. J. Yoon, S. Gandla, H. Park and S. Kim, npj Flexible Electron., 2023, 7, 20 Search PubMed.
- M. Mahmood, S. Kwon, H. Kim, Y. S. Kim, P. Siriaraya, J. Choi, B. Otkhmezuri, K. Kang, K. J. Yu, Y. C. Jang, C. S. Ang and W. H. Yeo, Adv. Sci., 2021, 8, e2101129 Search PubMed.
- X. Li, S. Wan, T. S. Pronay, X. Yang, B. Gao and C. T. Lim, Nanoscale Horiz., 2025, 10, 1815–1837 Search PubMed.
- G. Kang, M. Kim, H. Yang, J. Shin, J. Sim, H. Ahn, M. Jang, Y. Kim, H. S. Min and H. Jung, Adv. Funct. Mater., 2023, 33, 2210805 Search PubMed.
- P. Rivera, F. Della Pelle, J. Stonyte, W. d. C. Martins Antunes de Melo, A. Abouhagger and R. Pauliukaite, ACS Sens., 2025, 10, 9183–9202 Search PubMed.
- K. Mahato, T. Saha, S. Ding, S. S. Sandhu, A.-Y. Chang and J. Wang, Nat. Electron., 2024, 7, 735–750 Search PubMed.
- F. Liu, A. Christou, A. S. Dahiya and R. Dahiya, Adv. Mater., 2025, 37, 2411151 CrossRef CAS PubMed.
- K. Kuruvinashetti, A. Komeili and A. Sanati Nezhad, Lab Chip, 2025, 25, 3879–3920 RSC.
- J. Lee, J. Jeong, V. P. Nguyen, S. Hong, Y. M. Paulus and C. H. Lee, NPG Asia Mater., 2025, 17, 33 CrossRef CAS PubMed.
- M. M. Paci, T. Saha, O. Djassemi, S. Wu, C. Y. X. Chua, J. Wang and A. Grattoni, Nat. Rev. Bioeng., 2025, 3, 816–834 CrossRef CAS.
- X. Li, X. Huang, J. Mo, H. Wang, Q. Huang, C. Yang, T. Zhang, H.-J. Chen, T. Hang, F. Liu, L. Jiang, Q. Wu, H. Li, N. Hu and X. Xie, Adv. Sci., 2021, 8, 2100827 CrossRef CAS PubMed.
- M. Parrilla, U. Detamornrat, J. Domínguez-Robles, S. Tunca, R. F. Donnelly and K. De Wael, ACS Sens., 2023, 8, 4161–4170 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |


