 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrazone fluorescent sensors for the monitoring of toxic metals involved in human health from 2014–2024
Alexander Ciupa *
*
Materials Innovation Factory, University of Liverpool, 51 Oxford Street, Liverpool L7 3NY, UK. E-mail: ciupa@liverpool.ac.uk
First published on 4th February 2025
Abstract
Hydrazone-based fluorescent sensors have been instrumental for the detection of toxic metals over the past decade due to their ease of synthesis and unique properties. This review summaries the diverse range of sensors reported for toxic metals (Al3+, Fe3+, Cu2+, Zn2+ and Hg2+) highlighting the key role this class of sensors will play in the foreseeable future.
1 Introduction
There are twenty essential metals for the maintenance of human life1 ranging from group one alkali metals (Na and K), through group two alkaline metals (Mg and Ca) to the transition metals (Cr, Mn, Fe, Co, Cu, Zn and Mo). While the regular ingestion and homeostasis of these metals is critical to life, overconsumption and dysregulation leads to disease2 confirming the Latin phase “dosis facit venenum” (the dose makes the poison). Alongside the essential metals to life, there are several heavy metals (Cd, Hg and Pb) with well-established toxicities3 therefore constant surveillance in the environment and the food supply is of paramount importance. Several analytical techniques are available to meet this challenge4 however they often require extensive time-consuming sample preparation coupled with expensive equipment to reach the required limits of detection (LoD). Fluorescence spectroscopy offers several advantages5,6 over traditional techniques including rapid analysis (seconds), low limits of detection (nanomolar range) and minimal sample volume and preparation. The development of highly selective fluorescent sensors to monitor metals within in vitro cell cultures7,8 and in vivo9 is unique to fluorescence spectroscopy. This has enabled unparalleled discovery into the role of toxic metals in human health10,11 and will likely continue for the foreseeable future. Fluorescent sensors are typically divided into two types, a “turn on” sensor12,13 in which the presence of the target analyte increases fluorescence emission at a specific wavelength (λem) or “turn off” when analyte decreases λem.14 Multiple chemical scaffolds have been utilised as fluorescent sensors15,16 with hydrazone sensors17,18 widely adopted for a myriad of different metals both in the environment and in living systems. Hydrazones can easily be synthesised by a condensation reaction between a carbonyl (typically a ketone or aldehyde) with a hydrazine derivative (Scheme 1). This well-established chemistry enables a variety of fluorophores and chelation unit (F and C in Scheme 1) combinations to achieve the desired photophysical properties. Upon binding of the target analyte (T in Scheme 1) a fluorescent response (λem) is triggered allowing detection and quantification of the analyte in question. Careful selection of the fluorophore and chelation site enables highly specific sensors functional in complex environments to be developed. The hydrazone unit is not limited to fluorescent sensors, hydrazone based molecular switches and devices,19,20 promising hydrazone drug candidates21 and its widespread use in bioconjugation22 highlight the importance of this versatile functional group.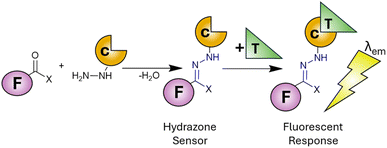 | ||
| Scheme 1 Condensation reaction between carbonyl and hydrazine to produce a hydrazone (in blue). F: fluorophore, C: chelation unit, T: target analyte and λem is fluorescence emission. | ||
This review will provide an overview of the commonly used fluorescence sensing mechanisms for hydrazone sensors while summarising hydrazone-based sensors for toxic metals over the past decade. The recent development of multi-analyte hydrazone sensors and the challenges ahead will also be discussed.
2 Sensing mechanisms
First described by de Silva in 1985,23,24 a photoelectron transfer (PET) sensor consists of three components: a fluorophore (F), a spacer (S) and an receptor (R) unit (Fig. 1).25 The sensor undergoes excitation (red arrow in Fig. 1A) resulting in transfer of an electron from the receptor to fluorophore (black arrow in Fig. 1A) in an efficient non-radiative pathway. This is photoelectron transfer (PET) from receptor to fluorophore quenching fluorescence. On binding the target analyte (T), a conformation change alters the properties between fluorophore and receptor making PET unfavourable (red cross in Fig. 1A). Return to the ground state with the emission of λem is now favourable producing a “turn on” response. A “turn off” response wherein the sensor undergoes emission of λem in the absence of analyte can also be developed (Fig. 1B). Target analyte binding improves the properties between fluorophore and receptor activating the non-radiative PET pathway, reducing λem and triggering a “turn off” response. Tsien pioneered “turn on” PET sensors for intracellular Ca2+ monitoring.26 | ||
| Fig. 1 Simplified representation of the PET mechanism23–25 for “turn on” (panel A) and “turn off” (panel B) sensors. F: fluorophore, S: spacer, R: receptor unit, T: target analyte, red arrow indicates excitation, black arrow indicates PET. | ||
An alternative to PET is internal charge transfer (ICT)27–29 involving an integrated fluorophore, acceptor and receptor site (F, A and R in Fig. 2). Note the receptor unit can be located on fluorophore, acceptor or a standalone unit. On excitation (red arrow in Fig. 2) the sensor can transfer charge (an electron) from electron-rich fluorophore to electron-deficient acceptor. Analyte binding changes the properties between donor and acceptor disrupting ICT. The release of λem is now favourable allowing return to the ground state. ICT is used for Cu2+ and Al3+ sensing.30,31
 | ||
| Fig. 2 Simplified representation of the ICT mechanism.27–29 F: fluorophore, R: receptor, A: acceptor, T: target analyte. | ||
Chelation-enhanced fluorescence (CHEF)32 is a third pathway for fluorescent sensing. On excitation (red arrow Fig. 3), a sensor (S) undergoes non-radiative decay back to the ground state, typically through vibrational rotation or solvent interactions (Fig. 3). On binding the target analyte (T) a conformation change prevents this relaxation process. The only pathway available is increased λem often of several orders of magnitude in intensity. This mechanism has been widely employed for the development of Zn2+ and Al3+ sensors. Chelation-enhanced fluorescence quenching (CHEQ) is when the sensor itself emits fluorescence. Binding of analyte disrupts this pathway producing a “turn off” response (Fig. 3). CHEQ has been developed for Hg2+ sensors.33
Aggregation-induced emission (AIE), first reported by Tang34 can be employed for fluorescence sensing. In the dilute form the sensor has a multitude of different bond rotations and vibrations (red arrows in Fig. 4) to relax to the ground state. When aggregation is triggered, for example by analyte binding, these rotations are restricted preventing non-radiative return to the ground state and triggering the radiative release of λem. AIE is often utilised in Fe3+, Cu2+, Zn2+and Hg2+ sensors.35
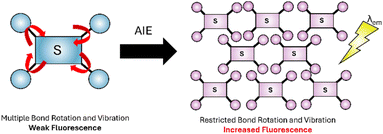 | ||
| Fig. 4 Simplified representation AIE,34 the dilute form (blue) with high degree of freedom (red arrows), the aggregated form has reduced bond rotation (purple). | ||
Förster Resonance Energy Transfer (FRET) first described by Förster36 involves two fluorophores, one acting as a donor and the other as an acceptor (D and A in Fig. 5). FRET operates over short distances, typically between 10 and 100 Å, and involves the non-radiative transfer of energy (hν) from the donor to acceptor if donor λem overlaps with acceptor λex. In a “turn on” FRET sensor, the donor and acceptor are unable to interact therefore we see only donor λem (Fig. 5A). Upon target analyte binding, a conformational change brings donor and acceptor within range to activate FRET increasing acceptor λem at the expense of donor λem. In a “turn off” sensor FRET is active until analyte binding makes FRET unfavourable, for example increasing the distance between and/or preventing the overlapping of λem and λex bands of donor and acceptor (Fig. 5B). FRET is sensitive to surroundings and has found widespread use in biomonitoring, for example proteins and peptides.37
 | ||
| Fig. 5 Simplified representation of the FRET mechanism.36 D: donor, A: acceptor, T: target analyte, hν: energy. | ||
Excited-State Intramolecular Proton Transfer (ESIPT)38 was described by Weller39 and is applicable to sensors with intramolecular hydrogen bonding, typically a OH or NH2 group. Upon excitation the hydrogen donor unit (OH in Fig. 6) becomes more acidic and the hydrogen acceptor (X in Fig. 6) more basic facilitating rapid tautomerization between enol and keto states. This process involves radiative decay back to the ground state and then regeneration of the sensor to the original form. ESIPT have been widely used for biomarker detection.40
 | ||
| Fig. 6 Simplified representation of the ESIPT mechanism.38 | ||
3 Sensors for Al3+
Aluminium is the most abundant metal in the Earth's crust and exists naturally in the trivalent form (Al3+) but does not have a physiological role in the maintenance of human health.41 Long term exposure to Al3+ has been linked to oxidative stress related disorders42 and neurodegenerative conditions such as Alzheimer's disease.43 The European Union (EU) aluminium drinking water limit is 7.4 μM44 with the World Health Organization (WHO) limit set at 33.3 μM.45 Acylhydrazone 1 (Fig. 7) demonstrated a “turn on” response at λem 456 nm with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio to Al3+ via a ESIPT and PET mechanism.46 A detection limit of 21.4 nM in 9
1 binding ratio to Al3+ via a ESIPT and PET mechanism.46 A detection limit of 21.4 nM in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O
1 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO was reported.46
DMSO was reported.46
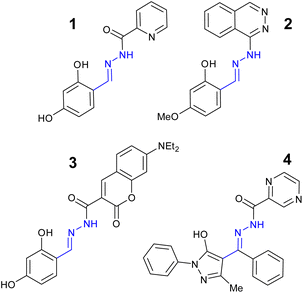 | ||
| Fig. 7 The structures of “turn on” Al3+ sensors 1–4.46–49 | ||
Quinoxaline based sensor 2 (Fig. 7) reported a similar “turn on” response for Al3+ via CHEF at λem 460 nm with detection limit of 22 nM.47 Sensor 2 displayed low toxicity to normal human hepatocytes suggesting it could be a useful sensor for the monitoring of Al3+ in biological systems such as cell culture.47 Coumarin–hydrazone 3 (Fig. 7) displayed approx. 15-fold increase in λem at 524 nm due to CHEF with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of Al3+ and a LoD of 50 nM in 3
1 binding of Al3+ and a LoD of 50 nM in 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 H2O
7 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO.48 Potential applications as logic gate were investigated.48 Pyrazine–hydrazone 4 (Fig. 7) displayed a “turn on” response at λem 500 nm with 1
DMSO.48 Potential applications as logic gate were investigated.48 Pyrazine–hydrazone 4 (Fig. 7) displayed a “turn on” response at λem 500 nm with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Al3+ complex and a LoD 0.18 μM in 2
1 Al3+ complex and a LoD 0.18 μM in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 H2O
8 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO.49 Cell culture studies confirmed 4 could detect Al3+ in vitro.49 Naphthalene–hydrazone 5 (Fig. 8) displayed “turn on” fluorescence enhancement at 435 nm due to AIE and ESIPT, a 1
DMSO.49 Cell culture studies confirmed 4 could detect Al3+ in vitro.49 Naphthalene–hydrazone 5 (Fig. 8) displayed “turn on” fluorescence enhancement at 435 nm due to AIE and ESIPT, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Al3+ ratio and LoD of 20 nM was observed.50 The ability to detect Al3+ in both river and tap water was confirmed highlighting real-world potential of simple hydrazone sensors.50 Pyrazine based 6 (Fig. 8), derived from vitamin B6, operates almost exclusively in pure water, 99
1 Al3+ ratio and LoD of 20 nM was observed.50 The ability to detect Al3+ in both river and tap water was confirmed highlighting real-world potential of simple hydrazone sensors.50 Pyrazine based 6 (Fig. 8), derived from vitamin B6, operates almost exclusively in pure water, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O
1 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO, with a “turn on” signal at 456 nM due to AIE and LoD as low as 8 nM.51 Hydroxypyrazole 7 was developed as a “turn on” sensors for Al3+ at λem 428 nm via a decrease in PET and increased CHEF effect.52 An Al3+ LoD of 5 nM in ethanol and a 1
DMSO, with a “turn on” signal at 456 nM due to AIE and LoD as low as 8 nM.51 Hydroxypyrazole 7 was developed as a “turn on” sensors for Al3+ at λem 428 nm via a decrease in PET and increased CHEF effect.52 An Al3+ LoD of 5 nM in ethanol and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio was reported.52 Naphthol derived sensor 8 is a “turn on” sensor for Al3+ due to inhibition of ESIPT and PET forming a 1
1 binding ratio was reported.52 Naphthol derived sensor 8 is a “turn on” sensor for Al3+ due to inhibition of ESIPT and PET forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with Al3+ and λem 474 nm, Al3+ LoD was 4 μM.53 In summary, multiple “turn on” sensors for Al3+ exploiting a variety of fluorescence mechanisms including PET, ESIPT, CHEF and AIE with LoD well below drinking water limits have been reported.
1 complex with Al3+ and λem 474 nm, Al3+ LoD was 4 μM.53 In summary, multiple “turn on” sensors for Al3+ exploiting a variety of fluorescence mechanisms including PET, ESIPT, CHEF and AIE with LoD well below drinking water limits have been reported.
 | ||
| Fig. 8 The structures of “turn on” Al3+ sensors 5–8.50–53 | ||
4 Sensors for Fe3+
Iron is the most abundant transition metal in the human body54 instrumental to many vital functions including the catalytic activity of enzymes,55 DNA synthesis and oxygen transport via haemoglobin.56 Two forms of iron predominant in life, ferric (Fe3+) and ferrous (Fe2+) iron and its close regulation is vital to health. Excess and unregulated iron is linked to oxidative stress through the Fenton reaction57 and medical problems including hemochromatosis and neurological diseases such as Parkinson's and Alzheimer's disease.58,59 The EU iron drinking water limit is 3.5 μM60 with the Environmental Protection Agency (EPA) in the USA limit of 5.4 μM.61 The development of probes to selectively detect and monitor iron both in the environment and in vitro is an active research area.62 Acylhydrazone–hydrazone 9 (Fig. 9) displayed a “turn off” response with approx. 11-fold reduction at λem 470 nm with Fe3+ due to inhibition of AIE.63 A LoD 1.6 μM in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 H2O
4 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) THF was calculated. Sensor 10 is a “turn off” probe for Fe3+ also due to inhibition of AIE with a LoD of 42 nM.64 Naphthol–hydrazone 11 displayed a “turn off” response due to CHEQ with 1
THF was calculated. Sensor 10 is a “turn off” probe for Fe3+ also due to inhibition of AIE with a LoD of 42 nM.64 Naphthol–hydrazone 11 displayed a “turn off” response due to CHEQ with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Fe3+ with LoD 36 nM.65 The ability to monitor and track Fe3+ in a human prostate cell lines was reported.65 Rhodamine–hydrazone 12 displayed a “turn on” response to Fe3+ at λem 579 nm with Fe3+ and LoD as low at 11 nM in a 7
1 Fe3+ with LoD 36 nM.65 The ability to monitor and track Fe3+ in a human prostate cell lines was reported.65 Rhodamine–hydrazone 12 displayed a “turn on” response to Fe3+ at λem 579 nm with Fe3+ and LoD as low at 11 nM in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 MeCN
3 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution.66
H2O solution.66
 | ||
| Fig. 9 The structures of “turn on” Fe3+ sensors 9–12.63–66 | ||
5 Sensors for Cu2+
Copper is the third most abundant transition metal in the human body67 and a key component of the immune system68 and central to the function of cytochrome C, a mitochondrial enzyme linked to cellular respiration.69 Excessive copper intakes can result in oxidative stress resulting in short term symptoms such as nausea and abdominal pain70 to long term conditions such as Parkinson's disease.71 The EU drinking water limit for copper is currently set at 31.7 μM58 and regular surveillance is essential. Anthracene derived sensor 13 (Fig. 10) displayed a strong 11-fold increase “turn on” response to Cu2+ at λem 455 nm due to ICT with LoD 0.53 nM in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO
1 DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution.72 Real-world application of the detection of Cu2+ in sewage and tap water alongside the ability to extraction Cu2+ from the environment were reported.72 Benzothiazolinone–hydrazone 14 (Fig. 10) demonstrated a “turn off” signal with 1
H2O solution.72 Real-world application of the detection of Cu2+ in sewage and tap water alongside the ability to extraction Cu2+ from the environment were reported.72 Benzothiazolinone–hydrazone 14 (Fig. 10) demonstrated a “turn off” signal with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding to Cu2+ at λem 597 nm with LoD 84.0 nM.73 Quantification of Cu2+ in a range of river and tap water environments with >98% recoveries were reported.73 Thiadiazole based hydrazone 15 (Fig. 10) demonstrated high selectivity for Cu2+ with a “turn off” response at λem 540 nm, LoD 13.6 nM in 1
1 binding to Cu2+ at λem 597 nm with LoD 84.0 nM.73 Quantification of Cu2+ in a range of river and tap water environments with >98% recoveries were reported.73 Thiadiazole based hydrazone 15 (Fig. 10) demonstrated high selectivity for Cu2+ with a “turn off” response at λem 540 nm, LoD 13.6 nM in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 H2O
3 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO.74 Coumarin–hydrazone 16 (Fig. 10) was a “turn on” sensor for Cu2+ due to inhibition of PET with a 2
DMSO.74 Coumarin–hydrazone 16 (Fig. 10) was a “turn on” sensor for Cu2+ due to inhibition of PET with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio with Cu2+ with LoD of 0.19 μM.75 Sensor 16 was shown to track and monitor Cu2+ in vitro and detect Cu2+ in real-world river samples.75
1 binding ratio with Cu2+ with LoD of 0.19 μM.75 Sensor 16 was shown to track and monitor Cu2+ in vitro and detect Cu2+ in real-world river samples.75
 | ||
| Fig. 10 The structures of “turn on” Cu2+ sensors 13–16.72–75 | ||
1,8-naphthalimide–hydrazone 17 (Fig. 11) produced a “turn on” response to Cu2+ at λem 462 nm with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Cu2+ due to LCT with a LoD of 17 nM.76 17 could detect Cu2+ in real-world samples such as beer and drinking water samples with high recoveries.76 Sensor 18 (Fig. 11) displayed a “turn off” response due to PET with a 1
1 Cu2+ due to LCT with a LoD of 17 nM.76 17 could detect Cu2+ in real-world samples such as beer and drinking water samples with high recoveries.76 Sensor 18 (Fig. 11) displayed a “turn off” response due to PET with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio Cu2+ and a LoD of 0.9 μM in 4
1 ratio Cu2+ and a LoD of 0.9 μM in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O
1 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN.77 Pyrene–hydrazone 19 (Fig. 11) displayed a strong “turn on” signal with Cu2+ at λem 466 nm with a LoD of 0.66 μM in 8
MeCN.77 Pyrene–hydrazone 19 (Fig. 11) displayed a strong “turn on” signal with Cu2+ at λem 466 nm with a LoD of 0.66 μM in 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 H2O
2 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeCN solution.78 Sensor 19 displayed low cytotoxicity to Vero cells, a kidney cell line, confirming 19 can be used to track Cu2+ in living cells.78 Julolidine–hydrazone 20 (Fig. 11) was shown to give a “turn on” signal for Cu2+ in 1
MeCN solution.78 Sensor 19 displayed low cytotoxicity to Vero cells, a kidney cell line, confirming 19 can be used to track Cu2+ in living cells.78 Julolidine–hydrazone 20 (Fig. 11) was shown to give a “turn on” signal for Cu2+ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO
1 DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution at λem 420 nm with a LoD of 0.16 μM.79 Real-world validation for the detection of Cu2+ in canal, river and rainwater samples with excellent recoveries of >98% were reported for sensor 20.79
H2O solution at λem 420 nm with a LoD of 0.16 μM.79 Real-world validation for the detection of Cu2+ in canal, river and rainwater samples with excellent recoveries of >98% were reported for sensor 20.79
 | ||
| Fig. 11 The structures of “turn on” Cu2+ sensors 17–20.76–79 | ||
6 Sensors for Zn2+
Zinc is the second most abundant transition metal in the human body and critical to enzyme maintenance,80 gene expression and neurological functions.81 Excess and unregulated zinc is associated with Parkinsons and Alzheimer's disease with the WHO recommended drinking water limit set at 46 μM.82 The Julolidine–hydrazone, 21 (Fig. 12) is a “turn on” sensor for Zn2+ at λem 610 nm attributed to CHEF on 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding Zn2+ in 6
1 binding Zn2+ in 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 H2O
4 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO.83
DMSO.83
 | ||
| Fig. 12 The structures of “turn on” Zn2+ sensors 21–24.83–86 | ||
Interestingly 21 did not response to Cu2+ despite its structural similarity to sensor 20 (Fig. 11) which was a “turn off” Cu2+ sensor. The potential of 21 as an INHIBIT logic gate and detection of Cu2+ in river and tap water real-world analysis confirmed.83 Quinoline–hydrazone 22 (Fig. 12) was a “turn on” Zn2+ sensor at λem 570 nm due to CHEF with a LoD of 0.66 μM in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 H2O
6 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH solution.84 Confocal microscopy studies confirmed 22 can monitor Zn2+ in living systems.84 Coumarin based 23 (Fig. 12) was a “turn on” sensor for Zn2+ due to AIE with LoD 3.25 μM.85 The detection and monitoring of Zn2+ in vitro in HeLa cells using confocal microscopy was confirmed for 23.85 Pyrimidine–hydrazone 24 (Fig. 12) bearing two pyridine units is a “turn on” sensor with a 2
MeOH solution.84 Confocal microscopy studies confirmed 22 can monitor Zn2+ in living systems.84 Coumarin based 23 (Fig. 12) was a “turn on” sensor for Zn2+ due to AIE with LoD 3.25 μM.85 The detection and monitoring of Zn2+ in vitro in HeLa cells using confocal microscopy was confirmed for 23.85 Pyrimidine–hydrazone 24 (Fig. 12) bearing two pyridine units is a “turn on” sensor with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Zn2+ to sensor ratio at λem 590 nm, LoD of 95.0 nM.86 This sensor also reported the ability to monitor Zn2+ in vitro in the C2C12, a mouse myoblast, cell line.86 In summary hydrazone sensors are particularly attractive as “turn on” sensors for Zn2+.The large Stokes shift is advantageous for in vitro monitoring.
1 Zn2+ to sensor ratio at λem 590 nm, LoD of 95.0 nM.86 This sensor also reported the ability to monitor Zn2+ in vitro in the C2C12, a mouse myoblast, cell line.86 In summary hydrazone sensors are particularly attractive as “turn on” sensors for Zn2+.The large Stokes shift is advantageous for in vitro monitoring.
7 Sensors for Hg2+
Mercury is a rare element in the Earth's crust, often found as the Hg2+ ion in cinnabar (mercury sulfide).87 The neurotoxicity of mercury is well known resulting in one of the strictness exposure limits of all elements88 with the WHO maximum level in drinking water set at 5 nM.44 A particular challenge with mercury sensors is selective detection of Hg2+ over other group 12 metals for example Zn2+ and Cd2+. As a result, only a few Hg2+ specific sensors have been developed.89 Fluorescein based hydrazone 25 (Fig. 13) displayed an excellent Cd2+ specific “turn on” response at λem 520 nm with no observable change with Zn2+ or Cd2+.90 Sensor 25 had a LoD of 0.23 μM in 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 H2O
2 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO solution with the ability to monitor Hg2+ in vitro.90 This sensor demonstrates it is possible to produce a useful Hg2+ sensor with real-world applications. Methoxynaphthalene–hydrazone 26 (Fig. 13) is a “turn on” Hg2+ sensor at λem 438 nm with a LoD of 6.0 μM in a 1
DMSO solution with the ability to monitor Hg2+ in vitro.90 This sensor demonstrates it is possible to produce a useful Hg2+ sensor with real-world applications. Methoxynaphthalene–hydrazone 26 (Fig. 13) is a “turn on” Hg2+ sensor at λem 438 nm with a LoD of 6.0 μM in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMSO
1 DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution.91 Interestingly neither Zn2+ or Cd2+ interfered significantly with the “turn on” response to Hg2+ which was based on hydrolysis of the hydrazone.
H2O solution.91 Interestingly neither Zn2+ or Cd2+ interfered significantly with the “turn on” response to Hg2+ which was based on hydrolysis of the hydrazone.
 | ||
| Fig. 13 The structures of “turn on” Hg2+ sensors 25–28.90–93 | ||
Isatin derived hydrazone 27 is a “turn on” sensor for Hg2+ at λem 440 in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2O
1 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol with a LoD of 3.6 μM and a 1
ethanol with a LoD of 3.6 μM and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio.92 The fluorescein–hydrazone 28 was developed as a “turn on” Hg2+ sensor with a 1
1 binding ratio.92 The fluorescein–hydrazone 28 was developed as a “turn on” Hg2+ sensor with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio and LoD as low as 137 nM in a 1
1 binding ratio and LoD as low as 137 nM in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 H2O
9 H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solution.93 The application of 28 as an INHIBIT logic gate was confirmed.
solution.93 The application of 28 as an INHIBIT logic gate was confirmed.
8 Multi-analyte sensors
One rapidly emerging area of hydrazone-based sensors is the search for a single sensor which can detect multiple analytes, a multi-analyte sensor. Benzoxazole–hydrazone sensor 29 (Fig. 14) is capable of selectively detecting and distinguishing between three different trivalent analytes: Cr3+, Al3+ and Fe3+ in aqueous environments.94 | ||
| Fig. 14 The structure of multi-analyte sensors 27–30.94–97 | ||
Sensor 29 displayed a ESIPT “turn on” response to Cr3+ at λem 563 nm, “turn on” at λem 527 nm with Al3+ and “turn off” for Fe3+ at λem 620 nm. Benzoxazole–hydrazone 29 was able to monitor Cr3+ in vitro in human mesenchymal stem cells demonstrating real-world application of this sensor.94 Pyrazine sensor 30 (Fig. 14) displayed multiple “turn on” functionality for Zn2+ at λem 545 nm, “turn on” for Al3+ at λem 525 nm and Mg2+ at λem 600 nm due to inhibition of ESIPT.93 The monitoring of Al3+ in HeLa cells was confirmed using confocal microscopy.95 Triazole–hydrazone 31 (Fig. 14) is a “turn on” sensor for Al3+, LoD 22.5 nM and Zn2+ at λem 460 due to CHEF, LoD 102.5 nM.96 Sensor 31 was confirmed to detect and monitor Zn2+ in HeLa cells via confocal microscopy.96 Rhodamine sensor 32 (Fig. 14) was developed as a dual “turn on” sensor for Al3+ at λem 588 nm and Cu2+ at λem 580 nm with LoD of 8.3 nM and 0.29 μM respectively.97
9 Conclusions
2014–2024 has been a fruitful decade for the development of hydrazone based fluorescent sensors for the detection of multiple toxic metals in human health. The hydrazone functional group enables the combination of well-established fluorophore and chelation units into a single sensor for improved properties. Typical examples include the hybridisation of established fluorescent dyes such as rhodamine and fluorescein with nitrogen-based chelators such as pyridine and pyrazine. The ease of hydrazone synthesis, typically a single step, from commercially available carbonyl and hydrazine starting materials easily facilitates this fusion approach to sensor development. Hydrazone sensors operate through a multitude of fluorescence pathways including AIE, PET, CHEF, CHEQ, ESIPT and FRET. Of the hydrazone sensors reviewed over the past decade, the vast majority are aqueous soluble ensuring these sensors find real-world applications. The detection of toxic metals in the environment, for example river and drinking water samples and in vitro monitoring of metals in cell cultures provide firm validation of the hydrazone scaffold. Current limit of detections within or below legal limits confirming they have a useful role to play in heavy metal surveillance. Recent advances in the development of multi-analyte sensors are likely to accelerate allowing researchers to detect and monitor multiple analytes concurrently. There are major challenges ahead, for example the develop of Hg2+ specific sensors with LoD below the drinking water limit of 5 nM and the detection of the group 1 and 2 biological metals in vitro. Nevertheless, hydrazone sensors will continue to be a vital addition to the sensing toolkit for the foreseeable future.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- M. A. Zoroddu, J. Aaseth, G. Crisponi, S. Medici, M. Peana and V. M. Nurchi, J. Inorg. Biochem., 2019, 195, 120 CrossRef CAS PubMed.
- K. Jomova, M. Makova, S. Y. Alomar, S. H. Alwasel, E. Nepovimova, K. Kuca, C. J. Rhodes and M. Valko, Chem. Biol. Interact., 2022, 1, 110173 CrossRef PubMed.
- X. Wu, S. J. Cobbina, G. Mao, H. Xu, Z. Zhang and L. Yang, Environ. Sci. Pollut. Res., 2016, 23, 8244 CrossRef CAS PubMed.
- L. A. Malik, A. Bashir, A. Qureashi and A. H. Pandith, Environ. Chem. Lett., 2019, 17, 1495 CrossRef CAS.
- T. Rasheed, M. Bilal, F. Nabeel, H. M. Iqbal, C. Li and Y. Zhou, Sci. Total Environ., 2018, 615, 476 CrossRef CAS PubMed.
- R. Iftikhar, I. Parveen, A. Mazhar, M. S. Iqbal, G. M. Kamal, F. Hafeez and M. Ahmadipour, J. Environ. Chem. Eng., 2023, 11, 109030 CrossRef CAS.
- T. Nagano, Proc. Jpn. Acad. Ser. B Phys. Biol. Sci., 2010, 86, 837 CrossRef CAS PubMed.
- K. Ayyavoo and P. Velusamy, New J. Chem., 2021, 45, 10997 RSC.
- J. Rao, A. Dragulescu-Andrasi and H. Yao, Curr. Opin. Biotechnol., 2007, 18, 17 CrossRef CAS PubMed.
- M. Dutta and D. Das, TrAC, Trends Anal. Chem., 2012, 32, 113 CrossRef CAS.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622 RSC.
- M. E. Jun, B. Roy and K. H. Ahn, Chem. Commun., 2011, 47, 7583 RSC.
- M. Wei, P. Yin, Y. Shen, L. Zhang, J. Deng, S. Xue, H. Li, B. Guo, Y. Zhang and S. Yao, Chem. Commun., 2013, 49, 4640 RSC.
- J. Huang, M. Liu, X. Ma, Q. Dong, B. Ye, W. Wang and W. Zeng, RSC Adv., 2014, 4, 22964 RSC.
- Selected examples (a) L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993 RSC; (b) D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105 RSC.
- X. Tian, L. C. Murfin, L. Wu, S. E. Lewis and T. D. James, Chem. Sci., 2021, 12, 3406 RSC.
- M. Jabeen and J. Turk, J. Chem. Soc. A, 2022, 9, 663 CAS.
- X. Su and I. Aprahamian, Chem. Soc. Rev., 2014, 43, 1963 RSC.
- I. Aprahamian, Chem. Commun., 2017, 53, 6674 RSC.
- L. A. Tatum, X. Su and I. Aprahamian, Acc. Chem. Res., 2014, 47, 2141 CrossRef CAS PubMed.
- S. Rollas and S. G. Kucukguzel, Molecules, 2007, 12, 1910 CrossRef CAS PubMed.
- D. K. Kölmel and E. T. Kool, Chem. Rev., 2017, 117, 10358 CrossRef PubMed.
- A. P. de Silva and R. A. D. D. Rupasinghe, J. Chem. Soc., Chem. Commun., 1985, 1669 RSC.
- Selected examples (a) B. Daly, J. Ling and A. P. de Silva, Chem. Soc. Rev., 2015, 44, 4203 RSC; (b) A. P. de Silva, T. S. Moody and G. D. Wright, Analyst, 2009, 134, 2385 RSC.
- H. Niu, J. Liu, H. M. O'Connor, T. Gunnlaugsson, T. D. James and H. Zhang, Chem. Soc. Rev., 2023, 52, 2322 RSC.
- Selected examples (a) R. Y. Tsien, Biochemistry, 1980, 19, 2396 CrossRef CAS PubMed; (b) X. Zhou, K. J. Belavek and E. W. Miller, Biochemistry, 2021, 60, 3547 CrossRef CAS PubMed.
- A. Pal, M. Karmakar, S. R. Bhatta and A. Thakur, Coord. Chem. Rev., 2021, 448, 214167 CrossRef CAS.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899 CrossRef PubMed.
- S. Sasaki, G. P. C. Drummen and G.-I. Konishi, J. Mater. Chem. C, 2016, 4, 2731 RSC.
- Z. Xu, Y. Xiao, X. Qian, J. Cui and D. Cui, Org. Lett., 2005, 7, 889 CrossRef CAS PubMed.
- S. M. Hossain, K. Singh, A. Lakma, R. N. Pradhan and A. K. Singh, Sens. Actuators, B, 2017, 239, 1109 CrossRef CAS.
- Selected examples (a) M. E. Huston, K. W. Haider and A. W. Czarnik, J. Am. Chem. Soc., 1988, 110, 4460 CrossRef CAS; (b) E. U. Akkaya, M. E. Huston and A. W. Czarnik, J. Am. Chem. Soc., 1990, 112, 3590 CrossRef CAS.
- Selected examples (a) Y. Yang, X. Gou, J. Blecha and H. Cao, Tetrahedron Lett., 2010, 51, 3422 CrossRef CAS; (b) R. Bhaskar and S. Sarveswari, ChemistrySelect, 2020, 5, 4050 CrossRef CAS.
- Selected examples (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC; (b) Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9888 CrossRef CAS PubMed.
- M. H. Chua, H. Zhou, Q. Zhu, B. Z. Tang and J. W. Xu, Mater. Chem. Front., 2021, 5, 659 RSC.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X. P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110 RSC.
- B. T. Bajar, E. S. Wang, S. Zhang, M. Z. Lin and J. Chu, Sensors, 2016, 16, 1488 CrossRef PubMed.
- Selected examples (a) A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Chem. Soc. Rev., 2018, 47, 8842 RSC; (b) V. S. Padalkar and S. Seki, Chem. Soc. Rev., 2016, 45, 169 RSC.
- D. Rehm and A. Weller, Isr. J. Chem., 1970, 8, 259 CrossRef CAS.
- H. Gu, W. Wang, W. Wu, M. Wang, Y. Liu, Y. Jiao, F. Wang, F. Wang and X. Chen, Chem. Commun., 2023, 59, 2056 RSC.
- C. Exley, Environ. Sci.: Processes Impacts, 2013, 15, 1807 RSC.
- V. Kumar and K. D. Gill, Neurotoxicology, 2014, 41, 154 CrossRef CAS PubMed.
- M. Rey and R. K. Singh, Pharmacol. Rep., 2022, 74, 439 CrossRef PubMed.
- Directive (EU) 2020/2184 Of The European Parliament And Of The Council of 16 December 2020, on the quality of water intended for human consumption, 2015, http://data.europa.eu/eli/dir/2020/2184/oj.
- Selected examples (a) W.H.O., WHO Guidelines for Drinking Water Quality, 2nd edn, 1996 Search PubMed; (b) WHO/SDE/WSH/03.04/53, Background document for development of WHO Guidelines for Drinking-water Quality, 2003, https://iris.who.int/bitstream/handle/10665/75362/WHO_SDE_WSH_03.04_53_eng.pdf.
- H. N. Peng, X. M. Peng, J. Q. Huang, A. Huang, S. J. Xu, J. J. Zhou, S. S. Huang and X. P. Cai, J. Mol. Struct., 2020, 1212, 128138 CrossRef CAS.
- Y. Y. Yang, P. Y. Ma, J. H. Xue, D. D. Yang, Y. S. Shi, X. Zhao and Q. Ma, Microchem. J., 2024, 200, 110495 CrossRef CAS.
- S. K. Ramasamy, A. Chinnathambi, S. A. Alharbi, G. Venkatesan, A. Pugazhendhi and G. Sathiyan, J. Mol. Struct., 2024, 1302, 137411 CrossRef CAS.
- F.-F. Guo, B.-B. Wang, W.-N. Wu, W.-Y. Bi, Z.-H. Xu, Y.-C. Fan, L.-Y. Bian and Y. Wang, J. Mol. Struct., 2022, 1251, 132073 CrossRef CAS.
- V. Bhardwaj, K. Bhardwaj and S. K. Sahoo, J. Fluoresc., 2023, 33, 1157 CrossRef CAS PubMed.
- M. N. Zavalishin, G. A. Gamov, G. A. Nikitin, O. A. Pimenov, V. V. Aleksandriiskii, A. K. Isagulieva and A. V. Shibaeva, J. Microchem., 2024, 197, 109791 CrossRef CAS.
- X.-Y. Cheng, R. Fang, Z.-Y. Yang, M.-F. Wang, Q.-X. Zhou, T.-R. Li and Y. Li, J. Coord. Chem., 2014, 67, 737 CrossRef CAS.
- J. C. Qin, Z. Y. Yang and P. Yang, Inorg. Chim. Acta, 2015, 432, 136 CrossRef CAS.
- Selected examples (a) A. Yiannikourides and G. O. Latunde-Dada, Medicines, 2019, 6, 85 CrossRef CAS PubMed; (b) N. Abbaspour, R. Hurrell and R. Kelishadi, Res. J. Med. Med. Sci., 2014, 19, 164 Search PubMed.
- P. C. A. Bruijnicx, G. Van Koten and R. J. M. Klein Gebbink, Chem. Soc. Rev., 2008, 37, 2716 RSC.
- A.-C. S. Vogt, T. Arsiwala, M. Mohsen, M. Vogel, V. Manolova and M. F. Bachmann, Int. J. Mol. Sci., 2021, 22, 4591 CrossRef CAS PubMed.
- C. Winterbourn, Toxicol. Lett., 1995, 82, 969 CrossRef PubMed.
- J.-L. Liu, Z.-Y. Wang and C. Guo, Front. Neurosci., 2018, 12, 411985 Search PubMed.
- K. Wojtunik-Kulesza, A. Oniszczuk and M. Waksmundzka-Hajnos, Biomed. Pharmacother., 2019, 111, 1277 CrossRef CAS PubMed.
- Directive (EU) 2020/2184 Of The European Parliament And Of The Council of 16 December 2020, on the quality of water intended for human consumption, 2015, http://data.europa.eu/eli/dir/2020/2184/oj.
- Environmental Protection Agency, Secondary drinking water regulations: Guidance for nuisance chemicals, 2013. https://www.epa.gov/sdwa/secondary-drinking-water-standards-guidance-nuisance-chemicals.
- S. K. Sahoo, D. Sharma, R. K. Bera, G. Crisponi and J. F. Callan, Chem. Soc. Rev., 2012, 41, 719 RSC.
- A. Bai, Y. Zhang, J. Tian, Y. Huang and J. Yang, Tetrahedron, 2022, 120, 132900 CrossRef CAS.
- S. Wu, J. T. Liu, S. S. Fu, J. J. Wang, P. H. Zhang, C. Y. Liu, Y. B. Wang, Q. Su, Y. Z. Sun and Q. L. Yang, New J. Chem., 2023, 47, 13152 RSC.
- B. Musikavanhu, D. Zhu, M. Tang, Z. Xue, S. Wang and L. Zhao, Spectrochim. Acta, Part A, 2023, 289, 122242 CrossRef CAS PubMed.
- V. Babagond, K. S. Katagi, M. Akki and A. Jaggal, J. Fluoresc., 2024, 9, 26 Search PubMed.
- S. K. Mustafa and M. A. Al Sharif, Am. J. Anal. Chem., 2018, 9, 15 CrossRef CAS.
- H. Tapiero, D. Townsend and K. Tew, Biomed. Pharmacother., 2003, 57, 386 CrossRef CAS PubMed.
- D. Horn and A. Barrientos, IUBMB Life, 2008, 60, 421 CrossRef CAS PubMed.
- F. Pizarro, M. Olivares, R. Uauy, P. Contreras, A. Rebelo and V. Gidi, Environ. Health Perspect., 1999, 107, 117 CrossRef CAS PubMed.
- S. Rivera-Mancia, I. Perez-Neri, C. Rios, L. Tristan-Lopez, L. Rivera-Espinosa and S. Montes, Chem.-Biol. Interact., 2010, 186, 184 CrossRef CAS PubMed.
- S. Mukherjee and S. Betal, J. Fluoresc., 2019, 29, 27 CrossRef CAS PubMed.
- Y. Jia, M. Lu, S. Cui and S. Pu, J. Photochem. Photobiol., A, 2022, 423, 113592 CrossRef CAS.
- H. Xu, A. Wang, L. Qin, M. Mo, Y. Zhou, C. Lü and L. Zou, Chem. Phys., 2022, 560, 111571 CrossRef CAS.
- L. Liu, C. Guo, Q. Zhang, P. Xu, Y. Cui, W. Zhu, M. Fang and C. Li, J. Photochem. Photobiol., A, 2022, 423, 113593 CrossRef CAS.
- S. N. K. Elmas, F. N. Arslan and D. Aydin, Analyst, 2022, 147, 2687 RSC.
- S. Mukherjee, P. Mal and H. Stoeckli-Evans, J. Lumin., 2014, 155, 185 CrossRef CAS.
- S. M. Hossain, V. P. Rakash, P. Mamidi, S. Chattopadhyay and A. K. Singh, RSC Adv., 2020, 10, 3646 RSC.
- W. Akarasareenon, S. Chanmungkalakul, L. Xiaogang and P. Rashatasakhon, J. Photochem. Photobiol., A, 2023, 427, 114422 CrossRef.
- C. T. Chasapis, C. A. Spiliopoulou, A. C. Loutsidou and M. E. Stefanidou, Arch. Toxicol., 2012, 86, 521 CrossRef CAS PubMed.
- C. J. Frederickson, BioMetals, 2001, 14, 353 CrossRef CAS PubMed.
- Zinc in Drinking-water, WHO/SDE/WSH/03.04/17, https://cdn.who.int/media/docs/defaultsource/washdocuments/wash-chemicals/zinc.pdf?sfvrsn=9529d066_4.
- V. K. Megha, P. Kaur and K. Singh, Anal. Chim. Acta, 2023, 1240, 340758 CrossRef PubMed.
- L.-L. Gao, S.-P. Li, Y. Wang, W.-N. Wu, X.-L. Zhao, H.-J. Li and Z.-H. Xu, Spectrochim. Acta, Part A, 2020, 230, 118025 CrossRef CAS PubMed.
- W.-Z. Xue, X.-F. Han, X.-L. Zhao, W.-N. Wu, Y. Wang, Z.-Q. Xu, Y.-C. Fan and Z.-H. Xu, Spectrochim. Acta, Part A, 2021, 263, 120169 CrossRef CAS PubMed.
- O. Anitha, S. Ghorai, T. Thiruppathiraja, H. Amir, A. Murugan, R. Natarajan, S. Lakshmipathi, C. Viswanathan, M. Jothi and B. Murugesapandian, Talanta, 2024, 273, 125900 CrossRef CAS PubMed.
- Y. Chen, Y. Yin, J. Shi, G. Liu, L. Hu, J. Liu, Y. Cai and G. Jiang, Crit. Rev. Environ. Sci. Technol., 2017, 47, 2415 CrossRef CAS.
- K. M. Rice, E. M. Walker, M. Wu, C. Gillette and E. R. Blough, J. Prev. Med. Public Health, 2014, 47, 74 CrossRef PubMed.
- H. Lee, H.-S. Lee, J. H. Reibenspies and R. D. Hancock, Inorg. Chem., 2012, 51, 10904 CrossRef CAS PubMed.
- M. N. Zavalishin, A. N. Kiselev, A. K. Isagulieva, A. V. Shibaeva, V. A. Kuzmin, V. N. Morozov, E. A. Zevakin, U. A. Petrova, A. A. Knyazeva, A. V. Eroshin, Y. A. Zhabanov and G. A. Gamov, Int. J. Mol. Sci., 2024, 25, 3186 CrossRef CAS PubMed.
- M. G. Choi, H. Ryu, M. S. Han and S.-K. Chang, Tetrahedron Lett., 2016, 57, 4360 CrossRef CAS.
- G. M. Ziarani, Z. Panahande, F. Mohajer, R. S. Varma, A. Badiei and S. Iravani, J. Iran. Chem. Soc., 2024, 21, 2607 CrossRef.
- S. Roy, T. Mondal, D. Dey, M. V. Mane and S. S. Panja, ChemistrySelect, 2021, 6, 10464 CrossRef CAS.
- J. F. Wang, Y. B. Li, N. G. Patel, G. Zhang, D. M. Zhou and Y. Pang, Chem. Commun., 2014, 50, 12258 RSC.
- Y. Wang, W. W. Wang, W. Z. Xue, W. N. Wu, X. L. Zhao, Z. Q. Xu, Y. C. Fan and Z. H. Xu, J. Lumin., 2019, 212, 191 CrossRef CAS.
- W. N. Wu, H. Wu, Y. Wang, X. J. Mao, B. Z. Liu, X. L. Zhao, Z. Q. Xu, Y. C. Fan and Z. H. Xu, RSC Adv., 2018, 8, 5640 RSC.
- Q. Hu, Y. Liu, Z. Li, R. Wen, Y. Gao, Y. Bei and Q. Zhu, Tetrahedron Lett., 2014, 55, 4912 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |

