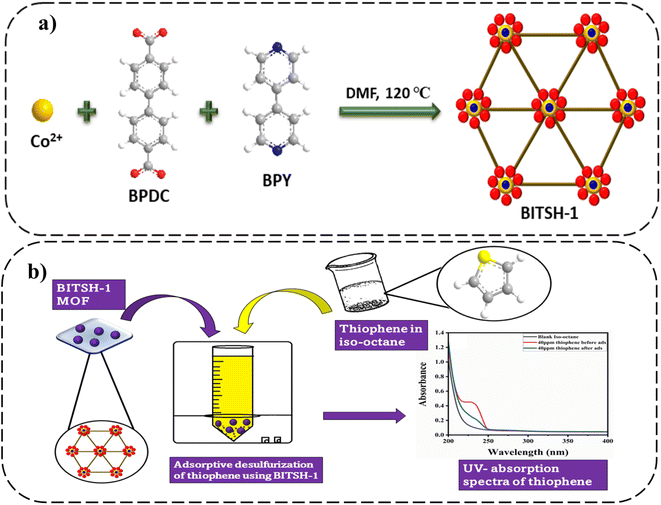
Open Access Article
Open Access ArticleCobalt-based metal–organic framework for desulfurization of thiophene as a model fuel†
M. Christina
Nilavu
,
T.
Leelasree
,
Himanshu
Aggarwal
* and
N.
Rajesh
 *
*
Department of Chemistry, Birla Institute of Technology and Science-Pilani, Hyderabad Campus, Jawahar Nagar, Hyderabad 500078, India. E-mail: nrajesh@hyderabad.bits-pilani.ac.in; himanshu.aggarwal@hyderabad.bits-pilani.ac.in
First published on 27th February 2024
Abstract
Persistent economic growth results in rising energy use, which in turn creates new environmental burdens and health risks for people due to the release of toxic gases from the combustion of fuel with sulfur-containing compounds. A cobalt-based MOF (BITSH-1) was investigated for the adsorptive desulfurization of thiophene in iso-octane as a model fuel. BITSH-1 showed a very high thiophene adsorption capacity of 95.38 mg g−1. Interestingly, BITSH-1 with a high surface area of 349.07 m2 g−1 achieves high adsorption efficiency at room temperature with substantially less time consumption without fuel oxidation or additional functionalization of the MOF, making it more feasible for an adsorptive desulfurization process. The recyclability of the MOF material showed good adsorption efficiency of thiophene for up to four cycles. The mechanistic features and possible interactions are discussed by modelling the thiophene molecule in the MOF with the help of SCXRD studies. Additionally, the mechanistic aspects of thiophene adsorption were corroborated using isotherms, kinetics, and thermodynamics, which ascertained that the reaction follows pseudo-order kinetics with spontaneity and feasibility. The overall process was found to be an exothermic reaction. Hence, the proposed adsorbent BITSH-1 could be a promising candidate for exclusive thiophenic separation from oil and could be promoted for the desulfurization of thiophenic model fuel.
1. Introduction
Fossil fuels are crucial to the advancement of research, technology, and daily lives. Nearly half of the energy from fossil fuels is provided by petroleum in the form of liquid fuel. Aromatic sulfur compounds in the fuel tend to release toxic gases, such as SO2 and SO3, on account of combustion in vehicles, impacting the ecosystem through consequences such as acid rain. SO2 emissions also induce respiratory ailments and early human mortality due to deteriorating air quality.1 Apart from pollution and health effects, high levels of sulfur content reduce the efficiency of a vehicle engine.2 According to BS(VI) emission regulations, the level of sulfur in fuel is expected to be below 10 mg L−1.1–3 The existence of sulfur in numerous forms with different properties, such as variance in size, reactivity, and polarity, makes desulfurizing fuel cumbersome. Due to their non-polar character and great stability, thiophene and its derivatives are more prevalent than aliphatic forms in petrol and are also harder to remove. Desulfurization of gasoline is an environmental challenge to be addressed on a large scale.Extractive desulfurization, bio-desulfurization, oxidative desulfurization, and adsorptive desulfurization are common approaches to desulfurization. However, these procedures have certain lacunae, such as the identification and use of organic solvents in extractive desulfurization, the instability of microorganisms at higher temperatures, and selectivity, which acts as impediments beyond the laboratory scale. High temperatures and the requirement for oxidants in the process of oxidative desulfurization also limit its usefulness.4 At the industrial level, the conventional method used is hydrodesulfurization, but requirements such as high temperature and pressure and an expensive setup restrict its application.5,6 However, adsorptive desulfurization can be advantageous over above-mentioned methods in terms of recyclability of adsorbent, high selectivity towards the targeted compound, tailorable functional properties and cost-effectiveness. It offers an alternative route for desulfurization technology considering the likelihood of physical and chemical interactions between the adsorbent and targeted adsorbate, as well as the removal of sulfur at room temperature without the consumption of additional energy.7 Proposed adsorbents for desulfurization include activated carbon, modified, functionalized and heteroatom-doped carbon, zeolites, graphitic materials, metal oxide composites and metal–organic frameworks (MOFs).8,9
Metal–organic frameworks (MOFs) are porous crystalline materials that combine organic and inorganic building blocks into a periodic networked structure. The porous nature and high specific area of MOFs make them suitable candidates for application in catalysis, sensing and separation technologies, and in gas adsorption studies.10 The pore size in MOFs could be fine-tuned by functionalization of the organic linkers towards achieving the selective adsorption of guest molecules. Additionally, MOFs possess a regular network of metal and organic moieties, which creates a cage-like structure with a sizable pore volume, increasing the likelihood of physisorption and chemisorption. Thus, separation by an adsorptive method is in vogue for MOF materials compared to other adsorbents, such as activated carbon,6,11 modified, functionalized or heteroatom-doped carbon,12 zeolites,13 graphitic materials,14 and metal oxide composites.12
MOFs have been investigated for desulfurization processes because of their advantages in terms of porosity, stability, and optimum pore sizes. However, only a few MOF structures have been explored for adsorptive desulfurization, and most of these materials display limited capacity for adsorption. Previously reported Cu-based MOFs such as MOF-199 showed a capacity of 18 mg g−1 for thiophene and 1.85 mg g−1 for sulfur from dibenzothiophene (DBT). Fe- and Cr-based MOFs were reported to have adsorption capacities of 6.50 and 32 mg g−1 for the adsorptive desulfurization of DBT. Modification of MIL-101 Cr with phosphotungstic acid (PTA) was found to give a higher adsorptive capacity of 107 mg g−1. CuCl modified MIL-101 Cr gave an adsorption capacity of 18.4 mg g−1. HKUST-1 gave an adsorption capacity of 40.6 mg g−1 for 500 μL of benzothiophene (BT) of 1500 mg L−1 concentration.13,15,16 In this study, we have explored a cobalt-based MOF (BITSH-1) for the adsorptive desulfurization of thiophene at room temperature without any modification or functionalization of the MOF. BITSH-1 was also explored for the adsorptive desulfurization of benzothiophene (BT) and dibenzothiophene (DBT) to check its selectivity with similar sulfur-based compounds present in the fuel.
2. Results and discussions
2.1. MOF synthesis and characterization
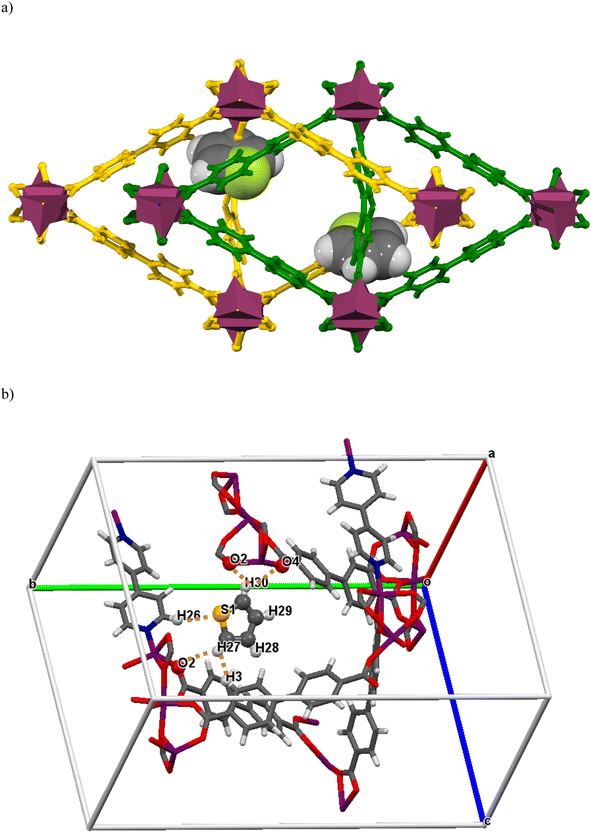
BITSH-1 was synthesized solvothermally using a mixed linker approach by reacting cobalt metal salt with biphenyl-4,4-dicarboxylic acid (BPDC) and 4,4′-bipyridine (BPY)17 (as given in Fig. 1a). BITSH-1 crystallizes in the P21/n space group with the asymmetric unit containing three cobalt (Co2+) ions, three BPDC linkers, one BPY linker and a water molecule. The as-synthesized material showed good crystallinity and bulk phase purity (Fig. S2†). TGA analyses were performed to study the thermal stability of the MOF material. The MOF material was thermally stable up to 350 °C (as shown in Fig. S4a†). N2 sorption isotherms of BITSH-1 were collected at 77 K. BITSH-1 displayed a microporous nature with a surface area of 349.07 m2 g1 and a pore diameter of 8 Å (Fig. S3b†). The high surface area along with the suitable pore size of BITSH-1 enabled us to explore this MOF for the adsorption of analytes with a desirable size up to 8 Å. Thiophene with a kinetic diameter of 4.5 Å (ref. 18) can be easily adsorbed onto BITSH-1 MOF pores. Thus, we proceeded further for the adsorptive desulfurization of thiophene. The morphology and surface nature of BITSH-1 were studied using SEM. SEM images showed distinct crystals of BITSH-1 MOF with a crystallite size of 70 to 190 μmm in a block shape. Elemental mapping and EDX confirmed the presence of Co, C, N, and O elements and the absence of S element in BITSH-1. However, sulfur was found to be present in the sample after the adsorptive desulfurization experiment (Fig. S1a–l†).2.2. Adsorptive studies of BITSH-1 for the desulfurization of thiophene
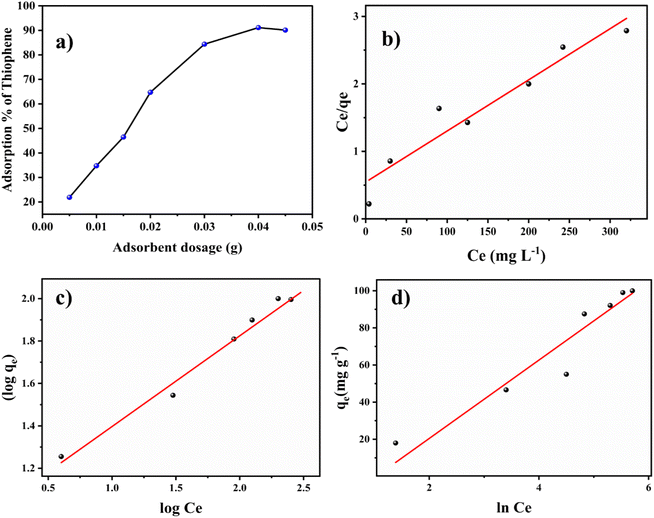
The Langmuir isotherm model gives the maximum adsorption capacity of the adsorbent from the linearly fitted values of experimental data in the linear equation,19
 | (1) |
 | (2) |
 | ||
| Fig. 4 (a) Pseudo-first-order kinetics, (b) pseudo-second-order kinetics, (c) Weber–Morris intra-particle diffusion model. (d) Effect of contact time of BITSH-1 adsorbent on adsorption efficiency. | ||
The Freundlich linear isotherm model can be given as,22
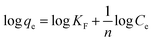 | (3) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf is the intercept value obtained by plotting log Cevs. log
Kf is the intercept value obtained by plotting log Cevs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe (Fig. 3c). Kf is the Freundlich constant, and the 1/n value (the slope) depicts the intensity of adsorption or the heterogeneity of the surface of the adsorbent since the Freundlich isotherm corresponds to multilayered adsorption on heterogenous sites. A 1/n value < 1 specifies the favourability of linear adsorption, whereas 1/n > 1 shows the unfavourability, and when 1/n = 1, it corresponds to the irreversible nature of the process. For thiophene adsorption on BITSH-1, 1/n was found to be 0.427, confirming the favourability of the adsorption. The R2 value for the Freundlich model was 0.954.
qe (Fig. 3c). Kf is the Freundlich constant, and the 1/n value (the slope) depicts the intensity of adsorption or the heterogeneity of the surface of the adsorbent since the Freundlich isotherm corresponds to multilayered adsorption on heterogenous sites. A 1/n value < 1 specifies the favourability of linear adsorption, whereas 1/n > 1 shows the unfavourability, and when 1/n = 1, it corresponds to the irreversible nature of the process. For thiophene adsorption on BITSH-1, 1/n was found to be 0.427, confirming the favourability of the adsorption. The R2 value for the Freundlich model was 0.954.
The linear Temkin isotherm equation is given as,23
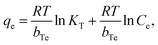 | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cevs. qe (Fig. 3d), which is related to the heat of adsorption in units of J mol−1, and T is the temperature in Kelvin. The assumption of the Temkin isotherm specifies homogeneity in the distribution of binding energy over the adsorbent BITSH-1 throughout the process provided it also specifies that the heat of adsorption tends to reduce with an increase in the occupancy of the thiophene molecules on BITSH-1 MOF throughout the process.24 The R2 value for the Temkin model was 0.881.
Cevs. qe (Fig. 3d), which is related to the heat of adsorption in units of J mol−1, and T is the temperature in Kelvin. The assumption of the Temkin isotherm specifies homogeneity in the distribution of binding energy over the adsorbent BITSH-1 throughout the process provided it also specifies that the heat of adsorption tends to reduce with an increase in the occupancy of the thiophene molecules on BITSH-1 MOF throughout the process.24 The R2 value for the Temkin model was 0.881.
The above mentioned theoretically obtained isotherm parameters from the experimental data are listed in Table S1.† Comparing the R2 values of the three isotherm models, the Langmuir isotherm model shows the highest value of 0.966, confirming that the adsorption of thiophene on BITSH-1 follows and adheres to the Langmuir isotherm model pertaining to monolayer adsorption. As the BET isotherm data shows type 1 isotherm adsorption of thiophene on BITSH-1, it clearly follows monolayer adsorption since it is a microporous material where the pore size is not much larger than the size of the adsorbate molecule (thiophene) which has a size of 4.5 Å. Therefore, these narrow pores get completely filled, corresponding to the complete formation of a molecular monolayer during the adsorption process.
The experimental data was plotted as different kinetic models according to following equations. The pseudo-first-order and pseudo-second-order equations can be linearly expressed as,25
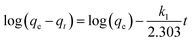 | (5) |
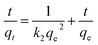 | (6) |
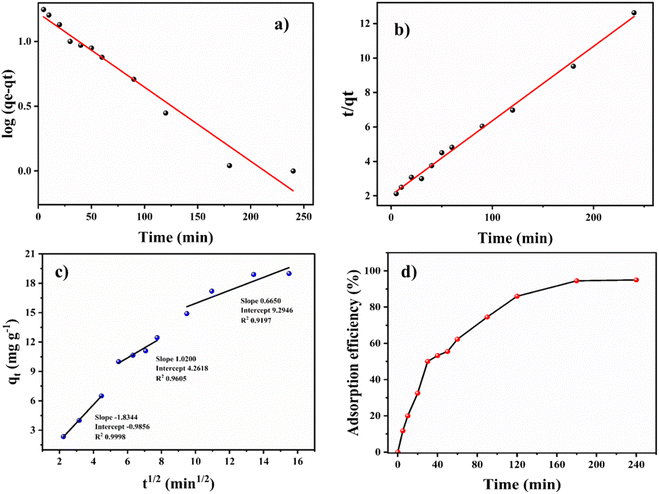
The pseudo-first-order model (Fig. 4a) was plotted between time (min) and log(qe − qt), and the pseudo-second-order model (Fig. 4b) was plotted between time (min) and t/qt. Here, qe and qt are the adsorption capacities at equilibrium and at a given time t, respectively. The R2 values for the pseudo-first-order kinetics was 0.964 and for pseudo-second-order was 0.995. The qe values obtained theoretically from the slope of the equations for first-order and second-order were 16.541 mg g−1 and 19.998 mg g−1. The experimental qe value was 20 mg g−1. Therefore, the qe value obtained from the second-order was found to be closer to the experimental qe value, ascertaining that the adsorption of thiophene follows pseudo-second-order kinetic with a higher R2 value as well. The theoretically obtained calculated data for kinetic parameters are listed in Table S2.†
An intra-particle diffusion plot for the adsorption process was drawn (given in Fig. 4c), between the square root of t and qt. The intra-particle diffusion model showed three stages of linear plot for the adsorption. The first stage of the three involves the diffusion and transfer of mass from the boundary layer to the bulk phase. This is followed by the diffusion of thiophene through the boundary layer to the surface of the BITSH-1 MOF. Then, the third step is interaction with functional groups along with the π–π interaction between thiophene and BITSH-1.26 The parameters of the Weber–Morris model were derived from the equation,
| qt = kit1/2 + C | (7) |
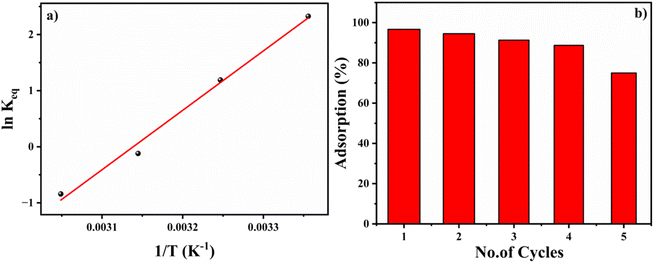
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq, where the linear form of the equation is,
Keq, where the linear form of the equation is,ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq Keq | (8) |
 | (9) |
The positive slope of the Van't Hoff plot specifies the exothermic nature of the reaction. The negative value of ΔS, −0.276 kJ mol−1 indicates a decrease in the disorderedness of the BITSH-1- thiophene interphase. The decrease in adsorption efficiency with an increase in the temperature denotes that the system is stable at standard temperature for high adsorption efficiency. The chemical potential of the thiophene is given as,
μ = μ0 + RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a | (10) |
In dilute lower concentrations of adsorbate solution at mg L−1 levels, activity (a) is directly proportional to the concentration of thiophene, which can be related to standard Gibbs's free energy and reaction Gibb's free energy as,
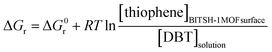 | (11) |
When ΔGr = 0 at equilibrium, this can be expressed as ΔG0r = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Keq, where
Keq, where
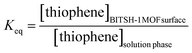 | (12) |
The change in Gibb's free energy was found to be negative (−1.423 kJ mol−1) confirming that the reaction is feasible or favourable and spontaneous with a negative change in enthalpy (−88 kJ mol−1). The adsorption of thiophene on BITSH-1 is enthalpically more favourable.
Experimental variables, such as adsorbent (MOF) dosage, time and temperature that control the adsorption process as discussed are summarized and given in Fig. 5.
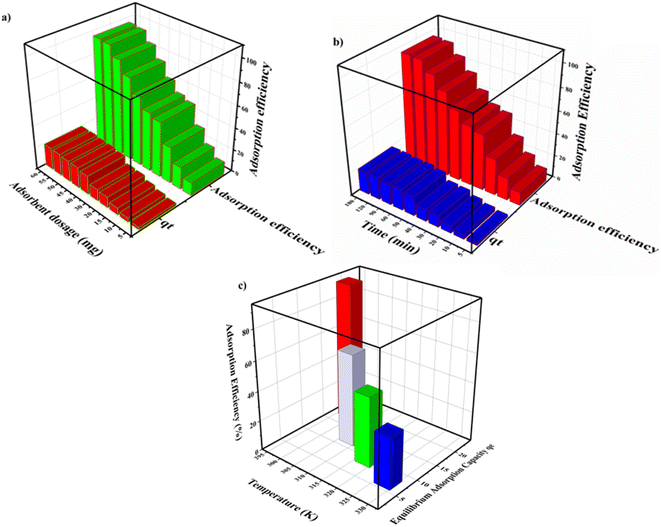 | ||
| Fig. 5 3D plots of adsorption efficiency controlled by different parameters: (a) adsorbent dosage, (b) time and (c) temperature. | ||
2.3. Comparative studies of BITSH-1 with other related materials for the desulfurization of thiophene
The proposed BITSH-1 material for thiophene adsorption was compared with previously reported MOFs and other related materials in terms of adsorption capacity, as listed in Table 1.13,23–29 It is noteworthy that BITSH-1 shows a better adsorption capacity of 95.38 mg g−1 with comparatively lower time consumption at room temperature and without any oxidation of fuel compared to various other materials. Indeed, no further functionalization of MOF is required in the present system. Hence, the proposed MOF adsorbent with reactive metal sites and an appropriate pore size with high affinity towards sulfur removal shows good potential for the desulfurization of thiophenic model fuel.| Sl. No | MOF material | Adsorbate | Condition | Adsorption capacity (qmax) mg g−1 | Reference |
|---|---|---|---|---|---|
| 1 | MOF-199 (Cu-BTC) | Thiophene | 4 h; 10 mL 0.2 g, 1000 mg L−1 | 18 (thiophene) | 16 |
| 2 | MOF-199 | DBT | 30 s, 5 mL, 0.18 g, 50 mg L−1 | 1.316 (S) | 27 |
| 3 | HKUST-1 | BT | 2 h, 20 mg 1500 mg L−1, 500 μL | 15.96 (S) | 13 |
| 4 | MIL-100 | DBT | 100 mg L−1, 50 mg, 5 mL | 6.50 | 28 |
| 5 | MIL-101 Cr, PTA-MIL 101 Cr | DBT | 500 mg L−1 S, 8 h | 32, 136.5 | 15 |
| 6 | HKUST-1 | Thiophene | 100 mg, 5 mL, 3 h, 100 mg L−1 | 27 | 29 |
| 7 | NENU-511 | BT | 5 mg, 2 mL, 1020 mg L−1 S, 12 h | 54.53 | 30 |
| 8 | Zn/Co bimetallic MOF derived porous carbon | DBT | 40 mg, 20 mL, 4 h, 500 mg L−1 | 40.6 | 31 |
| 9 | 4CuCl@MIL-101(Cr) | Thiophene | 550 mg L−1, 0.33 g | 18.4 | 32 |
| 10 | BITSH-1 | THIOPHENE | 20 mg, 3 h, 50 mg L− 1 , 10 mL | 95.38 (thiophene) | This study |
2.4. Reusability of the BITSH-1 adsorbent
An important property of any adsorbent is the ease of reusability of the adsorbent material by desorbing adsorbed molecules by different physical or chemical factors. Solvent elution methods are generally performed for the desorption of sulfur-based compounds from MOF materials.26 In the present case, thiophene was desorbed from BITSH-1 by heating the material at 100 °C (above the boiling point of thiophene, which is 84 °C) for 2 h. After heating, the structural integrity of the material was confirmed with the help of PXRD (Fig. S2†). The framework was found to be intact and suitable for carrying out the next cycle of adsorption. High adsorption efficiency was observed up to 4 cycles, which gradually decreased to 70% in the fifth cycle (shown in Fig. 6b). This could be ascribed to the active sites becoming saturated with weakening of BITSH-1 and thiophene interactions on repeated cycles.2.5. Evidence of thiophene adsorbed on BITSH-1
The adsorption process depends mostly on the surface characteristics, such as the specific surface area, pore diameter and pore volume of the MOF material. Thiophene, which has a diameter of 4.5 Å, is easily adsorbed and accumulates in the pores of the framework. The driving force for the high adsorption capacity of thiophene on the MOF can be attributed to weak interactions between the MOF and thiophene molecules, which was confirmed with FTIR, XPS, TGA, XRF and single crystal data. N2 sorption experiments were performed on BITSH-1 after the adsorptive desulfurization of thiophene. Fig. S3(c) and (d)† clearly show a decrease in the uptake of N2. This is due to the insufficient number of pores after the accumulation of the adsorbed thiophene molecules in the MOF pores. This resulted in a decrease in pore volume and specific surface area of BITSH-1 after thiophene adsorption, as given in Table 2.| Surface area (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) | Volume of N2 gas uptake (cm3 g−1) | |
|---|---|---|---|---|
| Before adsorption | 349.07 | 0.125 | 0.8 | 85.33 |
| After adsorption | 42.86 | 0.014 | 0.8 | 10.91 |
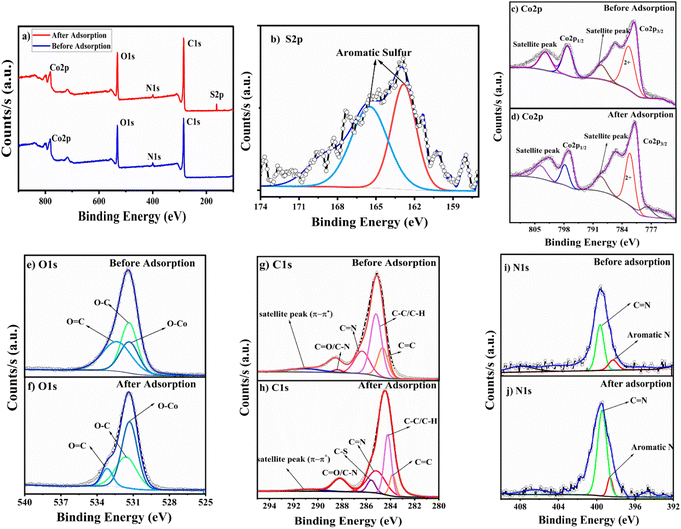
The PXRD confirmed that the crystallinity and integrity of the framework were retained even after the adsorption experiments (Fig. S2b†). The TGA graph of the thiophene-adsorbed MOF exhibits a weight loss around 83 °C attributed to the loss of thiophene molecules that have been adsorbed in the MOF pores (Fig. S4a†). This is supported by an endothermic peak at 80 °C in DTA pertaining to the release of the thiophene molecules that shows thiophene has been adsorbed by the MOF material (Fig. S4b†). XPS studies were performed to identify the elemental composition and their oxidation states. Fig. 7a shows the XPS survey spectra of BITSH-1 with characteristic binding energy peaks of Co, C, O and N elements. The XPS survey spectra of BITSH-1 after adsorption of thiophene confirms the presence of sulfur along with the other elements. Fig. 7b shows the deconvoluted spectra of sulfur S 2p with aromatic sulfur peaks at 162.89 and 165.62 eV, ascertaining the adsorption of thiophene on the MOF. The deconvoluted spectra of Co 2p in Fig. 7(c) and (d) confirm the +2-oxidation state of cobalt. The peaks at 782.63 eV and 797.29 eV in before-adsorption spectra correspond to Co 2p3/2 and Co 2p1/2.33 The shift of these peaks to 782.25 and 797.84 eV indicates the interactions of thiophene with the metal sites of the adsorbent. The deconvoluted spectra of C 1s of the adsorbent (Fig. 7g) give peaks at 284.57, 284.83, 285.84 and 288.94 eV that correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–C/C–H, C
C, C–C/C–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C
N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–N bonds. At 290.55 Ev, a satellite peak arises due to π–π* electronic transitions in the organic framework of the MOF.34 There is a noticeable increase in the intensity of this satellite peak in the spectra after adsorption, which confirms the π–π* transition due to π–π interaction between adsorbed thiophene on the MOF. The shifts in these peaks (Fig. 7h) to 284.22, 284.71, 284.21, 285.21 and 288.28 eV correspond to a decrease in binding energy due to the adsorption of thiophene. When there are weak interactions of the lone pair of electrons on the sulfur atom in the thiophene molecule with C
O/C–N bonds. At 290.55 Ev, a satellite peak arises due to π–π* electronic transitions in the organic framework of the MOF.34 There is a noticeable increase in the intensity of this satellite peak in the spectra after adsorption, which confirms the π–π* transition due to π–π interaction between adsorbed thiophene on the MOF. The shifts in these peaks (Fig. 7h) to 284.22, 284.71, 284.21, 285.21 and 288.28 eV correspond to a decrease in binding energy due to the adsorption of thiophene. When there are weak interactions of the lone pair of electrons on the sulfur atom in the thiophene molecule with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups on the framework of BITSH-1 due to the electronegative nature of sulfur, the positive charge on carbon increases, which results in the reduction in the binding energy of C
C groups on the framework of BITSH-1 due to the electronegative nature of sulfur, the positive charge on carbon increases, which results in the reduction in the binding energy of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups in the C 1s spectra of the framework.35 A new peak at 285.60 eV after adsorption in the deconvoluted spectra corresponds to the C–S bond, confirming the presence of adsorbed sulfur. The N 1s deconvoluted spectra (Fig. 7(i) and (j)) show peaks at 398.25 and 399.69 eV corresponding to aromatic or pyridinic N and C
C groups in the C 1s spectra of the framework.35 A new peak at 285.60 eV after adsorption in the deconvoluted spectra corresponds to the C–S bond, confirming the presence of adsorbed sulfur. The N 1s deconvoluted spectra (Fig. 7(i) and (j)) show peaks at 398.25 and 399.69 eV corresponding to aromatic or pyridinic N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N in the MOF.36 The O 1s deconvoluted spectra (Fig. 7(e) and (f)) show prominent peaks at 531.38, 531.41 and 532.48 eV corresponding to O–Co, O–C and O
N in the MOF.36 The O 1s deconvoluted spectra (Fig. 7(e) and (f)) show prominent peaks at 531.38, 531.41 and 532.48 eV corresponding to O–Co, O–C and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the framework.37 There was no appreciable shift in O–Co (metal–oxygen) bonds after adsorption, pertaining to the fact that the metal linking with the framework remains stronger and intact even after adsorption, keeping the framework stable. XPS studies of BITSH-1 show satellite peaks of carbon corresponding to π–π interactions with an increase in the intensity after adsorption due to π interactions between the aromatic rings of thiophene molecules and the MOF. The shift in binding energy in the Co 2p1/2 and Co 2p3/2 spectra specifies the electrostatic interaction of the Co2+ cation with the thiophene with a dipole moment of 0.55D.38
C bonds in the framework.37 There was no appreciable shift in O–Co (metal–oxygen) bonds after adsorption, pertaining to the fact that the metal linking with the framework remains stronger and intact even after adsorption, keeping the framework stable. XPS studies of BITSH-1 show satellite peaks of carbon corresponding to π–π interactions with an increase in the intensity after adsorption due to π interactions between the aromatic rings of thiophene molecules and the MOF. The shift in binding energy in the Co 2p1/2 and Co 2p3/2 spectra specifies the electrostatic interaction of the Co2+ cation with the thiophene with a dipole moment of 0.55D.38
X-ray fluorescence analysis of the as-synthesized MOF material before and after adsorption of thiophene was performed for the qualitative measurement of Co and S elements. XRF analysis of the MOF material after adsorption of thiophene gave a distinct peak at an energy of 2.4 keV, confirming the presence of sulfur in the material along with Co Kα and Co Kβ at 6.9 and 7.6 keV, respectively (Fig. 8a).
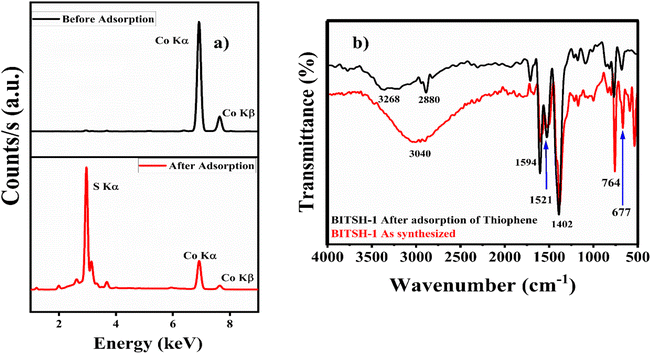 | ||
| Fig. 8 (a) XRF spectra of BITSH-1 before and after adsorption. (b) FTIR spectra of BITSH-1 before and after adsorption. | ||
The functional groups present in BITSH-1 were analysed from an FTIR study, as shown in Fig. 8b. A broad band at 3040 cm−1 corresponds to ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H and C–H stretching vibrations. There is a shift in this stretching vibration to 3268 cm−1 on adsorption of thiophene. This is due to the presence of electronegative sulfur atoms that increase the C–H stretching frequency of MOF. The vibrational bands at 1594 and 1521 cm−1 correspond to asymmetric carboxyl group stretching. Prominent bands at 764 and 677 cm−1 can be attributed the Co–O stretching frequency observed after adsorption without any further shift.39,40 A new band at 2880 cm−1 indicates the S–H bond in the thiophene moiety.41 The presence of the S–H stretching band at 2880 cm−1 in BITSH-1 after adsorption of thiophene strongly confirms the presence of adsorbed thiophene along with stretching frequency bands corresponding to C
C–H and C–H stretching vibrations. There is a shift in this stretching vibration to 3268 cm−1 on adsorption of thiophene. This is due to the presence of electronegative sulfur atoms that increase the C–H stretching frequency of MOF. The vibrational bands at 1594 and 1521 cm−1 correspond to asymmetric carboxyl group stretching. Prominent bands at 764 and 677 cm−1 can be attributed the Co–O stretching frequency observed after adsorption without any further shift.39,40 A new band at 2880 cm−1 indicates the S–H bond in the thiophene moiety.41 The presence of the S–H stretching band at 2880 cm−1 in BITSH-1 after adsorption of thiophene strongly confirms the presence of adsorbed thiophene along with stretching frequency bands corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, O–H and Co–O bands of the MOF network attributed to the interaction with thiophene. The increase in stretching frequency is due to the hydrogen-bonding interaction between the functional groups of BITSH-1 and the sulfur atom of thiophene.
O, O–H and Co–O bands of the MOF network attributed to the interaction with thiophene. The increase in stretching frequency is due to the hydrogen-bonding interaction between the functional groups of BITSH-1 and the sulfur atom of thiophene.
2.6. Single crystal XRD
Single crystal X-ray studies were performed with thiophene-exchanged crystals to obtain direct evidence of host–guest interactions at the molecular level. A few good-quality crystals were selected and immersed in a vial containing 1 mL of thiophene and 5 mL of iso-octane, for 24 h. A suitable single crystal was then chosen to collect SCXRD data, which confirmed the presence of thiophene molecules inside the MOF pores. The thiophene-loaded BITSH-1 structure (BITSH-1-T) undergoes several dramatic changes compared with the as-synthesized BITSH-1 structure.15 BITSH-1-T crystallizes in an orthorhombic crystal system with Pbcn space group (Table S4†). The asymmetric unit now contains one Co atom with full occupancy and another Co atom with 0.5 occupancy in addition to 1.5 units of BPDC and 0.5 unit of BPY linkers. One full thiophene molecule per asymmetric unit could be modelled inside the pores of the framework (Fig. 9(a) and (b), S5†). The thermal parameters of the thiophene molecule show large values owing to the movement of the molecule in channels. The thiophene molecule displays weak CH⋯HC van de Waals (vdW) interactions with the BPDC linker with a distance of 2.80 Å (Fig. 9b, Table S5†). Additionally, there are weak H-bonding interactions (2.91 Å, 2.94 Å, and 3.01 Å) present between the C–H group of the thiophene molecule and O atoms of the carboxylates of the BPDC linkers that explain the presence of thiophene closer to the cluster. Moreover, the S atom of the thiophene molecule is in proximity (3.25 Å) to the H atom of the BPY linker (Fig. 9b, Table S5†). In addition to one thiophene molecule that could be located with certainty, residual electron density was present in the channels that could not be modelled. This could be due to more thiophene molecule(s) but with a lower occupancy. The mask function of the Olex2 software40 was used to remove the residual electron density (∼30 electrons per asymmetric unit), which accounted for 0.7 thiophene molecules per asymmetric unit. Importantly, the SCXRD study confirms the presence of thiophene molecules inside the MOF channels, which explains the high selective uptake of thiophene by this MOF.2.7. Comparison of BITSH-1 adsorption for other SCCs (sulfur-containing compounds)
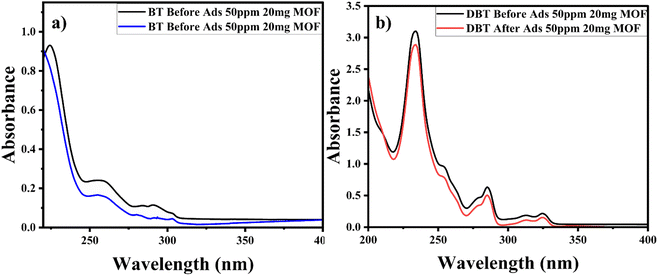
Comparative studies were carried out to check the selectivity and adsorption behaviour of BITSH-1 towards other sulfur compounds or higher thiophenic compounds, such as benzothiophene (BT) and dibenzothiophene (DBT) in the fuel. First, 50 mg L−1 of BT and DBT in a 10 mL volume iso-octane with 20 mg of adsorbent was incubated in an orbital shaker for 3 h. After adsorption, the concentration of BT and DBT in the solution was analysed through a UV absorption study, as shown Fig. 10(a) and (b). The percentage adsorption efficiency of BT on BITSH-1 was 40% and of DBT was 18%. The reduction in the adsorption efficiency can be attributed to kinetic diameters of benzothiophene (6 Å) and DBT (9 Å) with respect to the pore size of BITSH-1 (8 Å) restricting the accumulation of higher thiophene derivatives. Another reason could be the increase in electron cloud density on the S atom, which possibly restricts the interaction of the S atom with the MOF material, resulting in lower efficiency of desulfurization.423. Conclusions
The synthesized BITSH-1 MOF was successfully applied for the adsorptive desulfurization of model fuel. The framework of BITSH-1 was found to exhibit excellent stability and strong interaction for the desulfurization of thiophene. The microporous nature of the framework with a specific surface area of 349.07 m2 g−1 shows selective adsorption of thiophene with respect to other aromatic sulfur compounds. Single crystal XRD also revealed the selective uptake of thiophene. The experimental qe (19.0 mg g−1) was in good agreement with the theoretical value (19.99 mg g−1) calculated from kinetics data. The adsorption was exothermic, with negative free energy and entropy change. The maximum adsorption capacity of BITSH-1 was found to be 95.38 mg g−1, which was comparatively higher than in previous studies. This study highlights the evidence of adsorbed sulfur on the framework of BITSH-1 for up to four cycles.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge the Central Analytical Laboratory, BITS Pilani, Hyderabad Campus, India for their support in analytical characterization techniques.References
- M. A. Betiha, A. M. Rabie, H. S. Ahmed, A. A. Abdelrahman and M. F. El-Shahat, Egypt. J. Pet., 2018, 27, 715–730 CrossRef
.
- J. Wang and J. Wei, J. Mater. Chem. A, 2017, 5, 4651–4659 RSC
.
- D. Vashist, N. Kumar and M. Bindra, Arch. Curr. Res. Int., 2017, 8, 1–8 CrossRef
.
-
W. Ahmad and I. Ahmad, Mechanisms of Desulfurization by Nanomaterials, 2018 Search PubMed
.
- M. T. Timko, J. A. Wang, J. Burgess, P. Kracke, L. Gonzalez, C. Jaye and D. A. Fischer, Fuel, 2016, 163, 223–231 CrossRef CAS
.
-
M. Rezakazemi and Z. Zhang, Desulfurization materials, Comprehensive Energy Systems, 2018, vol. 2, pp. 944–979 Search PubMed
.
- G. Severa, J. Head, K. Bethune, S. Higgins and A. Fujise, J. Environ. Chem. Eng., 2018, 6, 718–727 CrossRef CAS
.
- M. Ghassa, F. Khorashe, Z. Hajjar and S. Soltanali, Energy Fuels, 2023, 37(9), 6490–6502 CrossRef CAS
.
- Q. Huo, J. Li, G. Liu, X. Qi, X. Zhang, Y. Ning, B. Zhang, Y. Fu and S. Liu, Chem. Eng. J., 2019, 362, 287–297 CrossRef CAS
.
- A. C. Elder, S. Bhattacharyya, S. Nair and T. M. Orlando, J. Phys. Chem. C, 2018, 122, 10413–10422 CrossRef CAS
.
- S. Sonal, P. Prakash, B. K. Mishra and G. C. Nayak, RSC Adv., 2020, 10, 13783–13798 RSC
.
- N. Ettekali, S. Allahyari, N. Rahemi and F. Abedini, Microporous Mesoporous Mater., 2021, 326, 111376 CrossRef CAS
.
- K. A. Cychosz, A. G. Wong-foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130(22), 6938–6939 CrossRef CAS PubMed
.
- G. Abdi, M. Ashokkumar and A. Alizadeh, Fuel, 2017, 210, 639–645 CrossRef CAS
.
- S. Jia, Y. Zhang, Y. Liu, F. Qin, H. Ren and S. Wu, J. Hazard. Mater., 2013, 262, 589–597 CrossRef CAS PubMed
.
- A. A. Alyassiry and R. T. A. Alrubaye, AIP Conf. Proc., 2020, 2213, 020090 CrossRef CAS
.
- T. Leelasree and H. Aggarwal, J. Mater. Chem. C, 2022, 10, 2121–2127 RSC
.
- C. I. Sainz-Díaz, M. Francisco-Márquez and A. Vivier-Bunge, Theor. Chem. Acc., 2009, 125, 83–95 Search PubMed
.
- M. Christina Nilavu, B. Arunraj, H. Aggarwal and N. Rajesh, Fuel, 2022, 324, 124472 CrossRef CAS
.
- B. Arunraj, V. Rajesh and N. Rajesh, J. Rare Earths, 2023, 41, 157–166 CrossRef CAS
.
- E. Ajenifuja, J. A. Ajao and E. O. B. Ajayi, Appl. Water Sci., 2017, 7, 3793–3801 CrossRef CAS
.
- M. Christina Nilavu, B. Arunraj, H. Aggarwal and N. Rajesh, Fuel, 2023, 345, 128172 CrossRef CAS
.
- B. Arunraj, S. Talasila, V. Rajesh and N. Rajesh, Sep. Sci. Technol., 2019, 54, 1620–1631 CrossRef CAS
.
- J. S. Piccin, G. L. Dotto and L. A. A. Pinto, Braz. J. Chem. Eng., 2011, 28, 295–304 CrossRef CAS
.
- J. Kodali, S. Puram, P. Srinivas, M. C. Nilavu, B. Arunraj, A. S. Krishna Kumar and R. Nagarathnam, Sep. Sci. Technol., 2023, 1–15 Search PubMed
.
- M. Daneshvar and M. R. Hosseini, Environ. Sci. Pollut. Res., 2018, 25, 28654–28666 CrossRef CAS PubMed
.
- C. Huang, R. Sun, H. Lu, Q. Yang, J. Hu, H. Wang and H. Liu, Sep. Purif. Technol., 2017, 182, 110–117 CrossRef CAS
.
- L. Wu, J. Xiao, Y. Wu, S. Xian, G. Miao, H. Wang and Z. Li, Sep. Purif. Technol., 2023, 306(Part A), 122665 Search PubMed
.
- F. Tian, Z. Fu, H. Zhang, J. Zhang, Y. Chen and C. Jia, Fuel, 2015, 158, 200–206 CrossRef CAS
.
- W. W. He, G. S. Yang, Y. J. Tang, S. L. Li, S. R. Zhang, Z. M. Su and Y. Q. Lan, Chem.–Eur. J., 2015, 21, 9784–9789 CrossRef CAS PubMed
.
- Q. Huo, J. Li, G. Liu, X. Qi, X. Zhang, Y. Ning, B. Zhang, Y. Fu and S. Liu, Chem. Eng. J., 2019, 362, 287–297 CrossRef CAS
.
- J. X. Shen, S. X. Mao, L. Wan, W. X. Wu, M. M. Jin, Y. X. Li, X. Q. Liu and L. B. Sun, Sep. Purif. Technol., 2022, 290, 120892 CrossRef CAS
.
- C. Zhang, J. Xiao, L. Qian and S. Wang, J. Mater. Chem. A, 2016, 4, 16516–16523 RSC
.
- D. N. G. Krishna and J. Philip, Appl. Surf. Sci. Adv., 2022, 12, 100332 CrossRef
.
- G. Zhou, E. Paek, G. S. Hwang and A. Manthiram, Nat. Commun., 2015, 6, 1–11 Search PubMed
.
- D. Yu, O. Suarez, A. Itzel, O. Dy, O. S. Ai, B. Av and J. Gascon, Langmuir, 2017, 33(50), 14278–14285 CrossRef PubMed
.
- G. Gao, X. Wang, Y. Ma, H. Rong, L. Lai and Q. Liu, Ionics, 2020, 26, 5189–5197 CrossRef CAS
.
- D. Peralta, G. Chaplais, A. Simon-Masseron, K. Barthelet and G. D. Pirngruber, Energy Fuels, 2012, 26, 4953–4960 CrossRef CAS
.
- T. Leelasree, S. Goel and H. Aggarwal, ACS Appl. Nano Mater., 2022, 5(11), 16753–16759 CrossRef CAS
.
- A. Mishra, P. Chaturvedi, S. Datta, S. Sinukumar, P. Joshi and A. Garg, Indian J. Med. Paediatr. Oncol., 2015, 36, 24–31 CrossRef PubMed
.
- X. Zhu, M. Su, S. Tang, L. Wang, X. Liang, F. Meng and Y. Hong, Mol. Vision, 2012, 1973–1982 CAS
.
- A. Khodadadi Dizaji, H. R. Mortaheb and B. Mokhtarani, Catal. Lett., 2019, 149, 259–271 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2289319. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3se01140b |
| This journal is © The Royal Society of Chemistry 2024 |