Diazo coupling for surface attachment of small molecules to TiO2 nanoparticles†
Jennifer L.
Troiano
 ab,
Gongfang
Hu
ab,
Gongfang
Hu
 ab,
Robert H.
Crabtree
ab,
Robert H.
Crabtree
 *ab and
Gary W.
Brudvig
*ab and
Gary W.
Brudvig
 *ab
*ab
aDepartment of Chemistry, and Yale Energy Sciences Institute, Yale University, New Haven, Connecticut 06520-8107, USA. E-mail: robert.crabtree@yale.edu; gary.brudvig@yale.edu
bEnergy Sciences Institute, Yale University, West Haven, Connecticut 06516, USA
First published on 9th July 2020
Abstract
Robust surface attachment of molecular species to metal oxide semiconductors is desirable for many applications. Here, we report the interfacial diazo coupling of surface-bound amines with aromatics to bind them to the surface of TiO2 nanoparticles via a siloxane anchor and a diazo linker. The technique shows potential for the inexpensive, stable, modular and tunable attachment of molecules to metal oxide surfaces.
Attachment of molecular species to metal oxide surfaces can modify surface properties,1 introduce functionalities,2 and tune energetics.3 These interfacial assemblies offer increased control over bulk materials owing to the well-defined and tunable nature of the molecular components. Consequently, molecular surface attachment to metal oxides has found applications across many fields including nanoelectronics, optoelectronics, bioactive surfaces, semiconductor coatings, chemical or biological sensors, and solar energy conversion devices.4,5 Many such solar devices produce electricity, but the diffuseness and intermittency of solar flux means storage can be a limiting factor.6 To address this, the widely studied Dye-Sensitized Photoelectrochemical Cell (DSPEC) combines molecular catalysts with photosensitizers all bound to metal oxide semiconductors.7,8 Light-driven water-oxidation catalysis9 is carried out at the photoanode while sequential reduction events lead to the formation of a solar fuel, such as dihydrogen, at the (photo)cathode. As a result, DSPECs are an attractive approach for overcoming solar energy limitations by storing the energy in chemical bonds.
While these systems offer great promise in the field of renewable energy, stability is a key concern when integrating molecular components with heterogeneous materials.10,11 In one common method, an anchoring group covalently binds the molecular component to the oxide surface. The introduction of typical anchoring groups to the molecular species of interest is often synthetically demanding.12–14 Meyer and co-workers recently reported the functionalization of metal oxides with a silyl azide followed by azide–alkyne cycloaddition to form a surface-bound Ru(II) polypyridyl chromophore.15 Hammarström and co-workers assembled photocathodes from molecular components via amide coupling with surface-bound amines.16 Both azide–alkyne click chemistry and amide coupling avoid some of the synthetic challenges of molecular pre-installation of an anchoring group by creating a link between a surface-bound unit and the desired external molecule. With this goal in mind, we looked into diazo coupling, aiming to install a stable surface linkage for both reductive and oxidative chemistry.
In diazo coupling, a starting amine is first converted to a diazonium salt which then forms an azo link with a variety of electron-rich aromatics. This provides an inexpensive and straightforward synthetic procedure that operates under mild conditions to give an extremely strong and stable azo link of a type that often finds commercial applications.17 Additionally, it is applicable to a wide variety of substrates including benzene derivatives, anthracenes, heterocycles, and enolizable aliphatics.17 Though very common in solution chemistry, diazo coupling is rarely seen for surface attachment and, to our knowledge, never for surface attachment to a metal oxide.18 In this report, we investigate the diazo coupling method to install molecular species on a TiO2 surface via an azo linkage.
Azo linkages are common in chromophores, accounting for almost 60% of all known commercial dyes.17 Their high extinction coefficients and tuneable absorption properties suggest utility in the field of solar energy conversion devices, but this has not yet materialized. In the limited studies that exist in this area, molecular azo dyes were attached to metal oxides via pre-installed anchoring groups such as nitro, hydroxyl, pyridyl, sulfonate, and carboxylate.19–21 These groups tend to be labile on varying the pH or simply owing to weak metal oxide binding.22,23 To improve surface binding and stability, we have used the silatrane anchoring group, known to form strong, siloxane bonds with metal oxide surfaces that, for TiO2, are stable from pH 2–11.24 Charge transfer dynamics for small molecules bound to metal oxides through siloxane anchoring groups have been well studied, demonstrating their suitability for photoelectrochemical applications.25,26 The silatrane, a triethanolamine-derivatized alkoxysilane, is protected from hydrolysis and oligomerization, dramatically increasing its shelf life over its unprotected counterpart.27,28 Here, we report a novel method for the strong, stable installation of molecular species, including a chromophore, on TiO2 surfaces using a diazo coupling reaction. Complex synthetic steps are avoided by first installing an aromatic amine on the surface via a silatrane anchoring group, and next forming a stable azo dye through a diazonium–aryl coupling reaction.
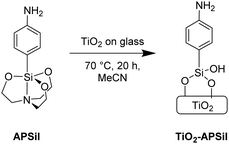
Mesoporous nanoparticulate TiO2 thin films of ∼4 μm thickness were fabricated through a doctor-blading procedure onto glass microscope slides. 4-Aminophenylsilatrane (APSil) was anchored to the TiO2 surface, providing the amino functionality needed for further reaction (Scheme 1). The reported29,30 synthesis of APSil involved refluxing commercially available p-aminophenyltrimethoxysilane in neat triethanolamine followed by a modified workup (see ESI†).
Silatranes have been shown to bind to metal oxide surfaces through loss of triethanolamine under mild heating.30 Accordingly, the TiO2 samples were soaked in 2 mM APSil in acetonitrile for 20 h at 70 °C (Scheme 1). X-ray Photoelectron Spectroscopy (XPS) confirmed APSil binding by detecting a new N 1s peak at 399.7 eV, characteristic of a C–NH2 binding mode (Fig. S1, ESI†).31 The resulting assembly, referred to as TiO2-APSil, probably involves bidentate binding of the siloxane to the TiO2 surface, as shown in Scheme 1.32
With TiO2-APSil in hand, conversion to the diazonium salt was followed by direct synthesis of an azo dye through a diazo coupling reaction (Scheme 2).33 Although diazo coupling is well established in solution, some modification was required to translate this method to interfacial chemistry. In the first step, diazotization, nitrous acid is formed from an acidic solution of sodium nitrite. Although the standard procedure calls for aqueous HCl at pH < 0,34 this is too acidic to retain silyl ether surface bonds since they are only stable in the pH range 2–11.23 To preserve TiO2-APSil surface binding while achieving a high proton concentration, the diazotization step was successfully carried out in pH 3 citric acid buffer. Citric acid is useful here because of its suitable pKa of 2.79 and high water solubility. We believe this represents the first example of implementation of these milder reaction conditions for diazo coupling. The TiO2-APSil sample was fully immersed in a citric acid buffer solution at 0 °C and NaNO2 was slowly added to form the diazonium salt.
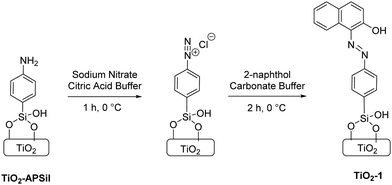 | ||
| Scheme 2 Diazo coupling reactions to form TiO2-1 including diazotization in the first step and 2-naphthol coupling in the second step. | ||
The subsequent electrophilic aromatic substitution couples the diazonium ion with a suitable nucleophilic partner. We chose 2-naphthol because it is commercially available, cheap, and forms a bright red-orange dye that facilitates quantitation of the surface-bound species.35 This step was performed in a carbonate buffer at pH 10 to maximize the rate of coupling.36 The TiO2-APSil sample was removed from the acidic solution of NaNO2 and immediately immersed in a basic solution of 2-naphthol. The characteristic color of the dye instantly appeared on the metal oxide film, but the reaction mixture was nevertheless left for two hours to promote full conversion from the diazonium ion to the surface-bound dye, denoted TiO2-1. Coupling is thought to occur at the naphthyl 1-position, as shown in Scheme 2, based on the solution formation of the analogous molecular dye, Sudan I.37 A common side reaction for diazo coupling in solution chemistry is loss of N2 leaving behind a phenyl group rather than the desired product. It is likely that this occurs to some extent in our surface bound system, but we have so far been unable to characterize this. Percent yield is also difficult to determine because surface bound APSil cannot be reliably quantified. To test if the reaction goes to completion, we subsequently repeated the diazo coupling reaction on TiO2-1. UV-vis spectroscopy showed only a 1% increase in absorbance, within experimental error, indicating there was essentially no unreacted amine left after the first coupling reaction (Fig. S2, ESI†). This suggests our reaction conditions give near-quantitative conversion of the amine to the azo compound.
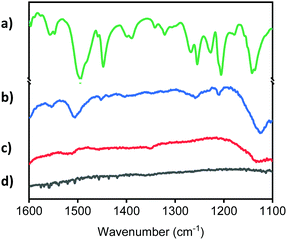
Fig. 1 compares the solid-state UV-visible absorption of the untreated TiO2 thin film (TiO2-Bare) with TiO2-APSil and TiO2-1, showing how the diazo coupling reaction of surface-bound APSil with 2-naphthol forms a new species with an absorbance in the visible region. The λmax for TiO2-1 is 480 nm, compared with 479 nm for the molecular compound 1-(phenyldiazenyl)naphthalen-2-ol.38 The molar extinction coefficient of 1-(phenyldiazenyl)naphthalen-2-ol is known to be 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1, on a par with many of the Ru(II) polypyridyl dyes that are widely used in solar energy applications.39,40 The surface loading was estimated from eqn S1 (see ESI†) to be 10.5 nmol cm−2 μm−1 using the extinction coefficient, measured absorbance and thickness, similar to reported surface coverages in dye sensitized solar cell literature.21 Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy was used as an additional method to test for the presence of the azo linkage. Spectra for TiO2-Bare, TiO2-APSil, and TiO2-1 were compared with the commercial molecular compound, Sudan I (Fig. 2). The presence of peaks characteristic to Sudan I found only on TiO2-1, including those at 1209 cm−1, 1258 cm−1, and 1504 cm−1, further confirm the formation of the surface bound azo dye. These peaks were shifted slightly, within a few wavenumbers, from those of the molecular compound, likely due to surface interactions.41 Scanning Electron Microscopy (SEM) confirmed the morphology of the TiO2 thin films was unchanged during both the sensitization process and the diazo coupling reaction (Fig. S3, ESI†).
500 M−1 cm−1, on a par with many of the Ru(II) polypyridyl dyes that are widely used in solar energy applications.39,40 The surface loading was estimated from eqn S1 (see ESI†) to be 10.5 nmol cm−2 μm−1 using the extinction coefficient, measured absorbance and thickness, similar to reported surface coverages in dye sensitized solar cell literature.21 Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR) Spectroscopy was used as an additional method to test for the presence of the azo linkage. Spectra for TiO2-Bare, TiO2-APSil, and TiO2-1 were compared with the commercial molecular compound, Sudan I (Fig. 2). The presence of peaks characteristic to Sudan I found only on TiO2-1, including those at 1209 cm−1, 1258 cm−1, and 1504 cm−1, further confirm the formation of the surface bound azo dye. These peaks were shifted slightly, within a few wavenumbers, from those of the molecular compound, likely due to surface interactions.41 Scanning Electron Microscopy (SEM) confirmed the morphology of the TiO2 thin films was unchanged during both the sensitization process and the diazo coupling reaction (Fig. S3, ESI†).
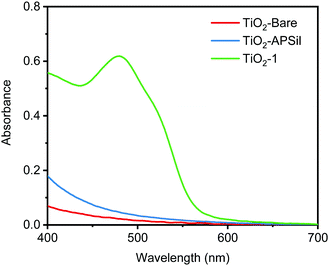 | ||
| Fig. 1 UV-vis spectra comparing the solid-state absorption spectra of TiO2-Bare, TiO2-APSil and TiO2-1. | ||
An attractive feature of this coupling method is the wide range of potentially applicable substrates, mainly activated aromatic nucleophiles containing either a hydroxyl or amino group.42 Here we aimed to demonstrate that this diazo coupling method can indeed install a variety of chemical species on surfaces. To do this, we coupled TiO2-APSil with two of the simplest examples of applicable substrates: phenol and aniline. The samples were fabricated in the same manner as TiO2-1, but 2-naphthol was replaced with either aniline or phenol to form TiO2 surface-bound 4-(phenyldiazenyl)aniline (TiO2-2, Fig. 3b) and 4-(phenyldiazenyl)phenol (TiO2-3, Fig. 3c), respectively. The coupling likely occurs at the para position, by comparison with the analogous solution reaction.43Fig. 3a shows the absorption spectra of both anchored compounds compared to that of TiO2-Bare, confirming successful coupling. 4-(Phenyldiazenyl)aniline has a maximum absorption at 375 nm causing the absorption peak for the surface-bound complex to be obscured by TiO2 absorption and scattering below 400 nm.44 Formation of the chromophore can still be evidenced by the increased absorption of TiO2-2 as compared with TiO2-Bare between 400 and 500 nm. The maximum absorption of trans-4-(phenyldiazenyl)phenol occurs at 355 nm, but any absorption at this wavelength is overwhelmed by TiO2 absorption. The cis form has a characteristic broad peak at 440 nm which can be seen in Fig. 3a. It is most plausible that both forms are present, but TiO2 absorption prevents us from confirming the trans configuration. These results serve as a proof of principle that this diazo coupling method could be applicable to a wide variety of substrates, showing potential as a widespread surface attachment technique.
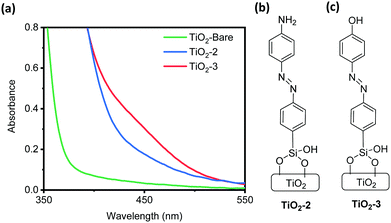 | ||
| Fig. 3 (a) UV-vis spectra comparing the solid-state absorption spectra of TiO2-Bare, TiO2-2 and TiO2-3. Structures of (b) TiO2-2 and (c) TiO2-3. | ||
In conclusion, we report a novel method for the attachment of molecular species directly on a TiO2 surface using a silatrane anchoring group that provides strong, stable, silyl ether surface bonds. The amines were derivatized through a diazo coupling reaction, forming anchored azo compounds. This method is advantageous because it avoids synthetic challenges typically associated with the stable anchorage of molecular species on metal oxide surfaces. The potential of this technique for DSPECs is established through 2-naphthol coupling that formed a strongly absorbing, noble-metal-free chromophore. An additional advantage for a photoanode is the oxidative resistance of both the anchor and the azo linkage. We can anticipate a wide substrate scope from the extensive literature examples on the synthesis of diazo dyes. We have demonstrated the generality of this method through representative reactions with two simple examples of activated aromatics, aniline and phenol. As silatranes are well known to bind strongly to indium doped tin oxide (ITO) and SnO2, it is likely that this method would be appropriate for surface attachment to these metal oxides as well. The combination of stable surface attachment, synthetic ease, modularity and tunability of the system make it an overall attractive approach for the molecular modification of metal oxide surfaces.
We thank the staff at the Yale West Campus Analytical Core and Materials Characterization Core for their help with the instrumentation. This work was supported by the U.S. Department of Energy, Chemical Sciences, Geosciences, and Biosciences Division, Office of Basic Energy Sciences, Office of Science (Grant DE-FG02-07ER15909) Additional support was provided by a generous donation from the TomKat Charitable Trust.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Gnauck, E. Jaehne, T. Blaettler, S. Tosatti, M. Textor and H.-J. P. Adler, Langmuir, 2007, 23, 377–381 CrossRef CAS PubMed.
- M. Sakeye and J.-H. Smått, Langmuir, 2012, 28, 16941–16950 CrossRef CAS PubMed.
- C. Goh, S. R. Scully and M. D. McGehee, J. Appl. Phys., 2007, 101, 114503 CrossRef.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed.
- C. Haensch, S. Hoeppener and U. S. Schubert, Chem. Soc. Rev., 2010, 39, 2323–2334 RSC.
- N. Armaroli and V. Balzani, Chem. – Eur. J., 2016, 22, 32–57 CrossRef CAS PubMed.
- J. R. Swierk and T. E. Mallouk, Chem. Soc. Rev., 2013, 42, 2357–2387 RSC.
- Z. Yu, F. Li and L. Sun, Energy Environ. Sci., 2015, 8, 760–775 RSC.
- K. J. Young, L. A. Martini, R. L. Milot, R. C. Snoeberger, V. S. Batista, C. A. Schmuttenmaer, R. H. Crabtree and G. W. Brudvig, Coord. Chem. Rev., 2012, 256, 2503–2520 CrossRef CAS PubMed.
- K. Kalyanasundaram and M. Grätzel, Coord. Chem. Rev., 1998, 177, 347–414 CrossRef CAS.
- D. L. Ashford, M. K. Gish, A. K. Vannucci, M. K. Brennaman, J. L. Templeton, J. M. Papanikolas and T. J. Meyer, Chem. Rev., 2015, 115, 13006–13049 CrossRef CAS PubMed.
- B. J. Brennan, M. J. Llansola Portolés, P. A. Liddell, T. A. Moore, A. L. Moore and D. Gust, Phys. Chem. Chem. Phys., 2013, 15, 16605–16614 RSC.
- S. K. Matta, K. Kakiage, S. Makuta, A. Veamatahau, Y. Aoyama, T. Yano, M. Hanaya and Y. Tachibana, J. Phys. Chem. C, 2014, 118, 28425–28434 CrossRef CAS.
- L. Sévery, S. Siol and S. Tilley, Inorganics, 2018, 6, 105 CrossRef.
- L. Wu, M. Eberhart, B. Shan, A. Nayak, M. K. Brennaman, A. J. M. Miller, J. Shao and T. J. Meyer, ACS Appl. Mater. Interfaces, 2019, 11, 4560–4567 CrossRef CAS PubMed.
- K. L. Materna, N. Lalaoui, J. A. Laureanti, A. P. Walsh, B. P. Rimgard, R. Lomoth, A. Thapper, S. Ott, W. J. Shaw, H. Tian and L. Hammarström, ACS Appl. Mater. Interfaces, 2020, 12, 4501–4509 CrossRef CAS PubMed.
- Y. Wu, T. Buranda, R. L. Metzenberg, L. A. Sklar and G. P. Lopez, Bioconjugate Chem., 2006, 17, 359–365 CrossRef CAS PubMed.
- S. Benkhaya, S. M’rabet and A. El Harfi, Heliyon, 2020, 6, e03271 CrossRef PubMed.
- J. Olmsted, J. Lawrence and G. G. Yee, Sol. Energy, 1983, 30, 271–274 CrossRef CAS.
- J. Ohlsson, H. Wolpher, A. Hagfeldt and H. Grennberg, J. Photochem. Photobiol., A, 2002, 148, 41–48 CrossRef CAS.
- L. Zhang, J. M. Cole and C. Dai, ACS Appl. Mater. Interfaces, 2014, 6, 7535–7546 CrossRef CAS PubMed.
- L. Zhang and J. M. Cole, ACS Appl. Mater. Interfaces, 2015, 7, 3427–3455 CrossRef CAS PubMed.
- K. L. Materna, R. H. Crabtree and G. W. Brudvig, Chem. Soc. Rev., 2017, 46, 6099–6110 RSC.
- K. L. Materna, B. Rudshteyn, B. J. Brennan, M. H. Kane, A. J. Bloomfield, D. L. Huang, D. Y. Shopov, V. S. Batista, R. H. Crabtree and G. W. Brudvig, ACS Catal., 2016, 6, 5371–5377 CrossRef CAS.
- B. Pandit, T. Luitel, D. R. Cummins, A. K. Thapa, T. Druffel, F. Zamborini and J. Liu, J. Phys. Chem. A, 2013, 117, 13513–13523 CrossRef CAS PubMed.
- J. Jiang, J. A. Spies, J. R. Swierk, A. J. Matula, K. P. Regan, N. Romano, B. J. Brennan, R. H. Crabtree, V. S. Batista, C. A. Schmuttenmaer and G. W. Brudvig, J. Phys. Chem. C, 2018, 122, 13529–13539 CrossRef CAS.
- J. N. Kinkel and K. K. Unger, J. Chromatogr. A, 1984, 316, 193–200 CrossRef CAS.
- M. Zhu, M. Z. Lerum and W. Chen, Langmuir, 2012, 28, 416–423 CrossRef CAS PubMed.
- B. J. Brennan, A. E. Keirstead, P. A. Liddell, S. A. Vail, T. A. Moore, A. L. Moore and D. Gust, Nanotechnology, 2009, 20, 505203 CrossRef PubMed.
- K. L. Materna, B. J. Brennan and G. W. Brudvig, Dalton Trans., 2015, 44, 20312–20315 RSC.
- NIST X-Ray Photoelectron Spectroscopy Database, https://srdata.nist.gov/xps/, (accessed May 2020).
- N. Iguchi, C. Cady, R. C. Snoeberger III, B. M. Hunter, E. M. Sproviero, C. A. Schmuttenmaer, R. H. Crabtree, G. W. Brudvig and V. S. Batista, Proc. SPIE, 2008, 7034, 70340C CrossRef.
- L. Abd-Alredha, R. Al-Rubaie and R. J. Mhessn, E-J. Chem., 2012, 9, 465–470 CrossRef CAS.
- J. N. Ospenson, L. G. Sillén, G. Lindstedt and P.-O. Kinell, Acta Chem. Scand., 1950, 4, 1351–1364 CrossRef.
- E. Merino, Chem. Soc. Rev., 2011, 40, 3835–3853 RSC.
- K. Hunger, P. Mischke, W. Rieper and S. Zhang, Ullmann's Encyclopedia of Industrial Chemistry, Electronic Release, Wiley-VCH, Weinheim, 2017 Search PubMed.
- D. Markovitsi, Photochem. Photobiol., 2016, 92, 45–51 CrossRef CAS PubMed.
- A. Carella, F. Borbone and R. Centore, Front. Chem., 2018, 6, 481 CrossRef CAS.
- M. Taniguchi and J. S. Lindsey, Photochem. Photobiol., 2018, 94, 290–327 CrossRef CAS PubMed.
- H. Zollinger, Color Chemistry: Syntheses, Properties and Applications of Organic Dyes and Pigments, Wiley-VCH, Weinheim, 3rd edn, 2003 Search PubMed.
- C. Bauer, P. Jacques and A. Kalt, Chem. Phys. Lett., 1999, 307, 397–406 CrossRef CAS.
- S. Chng, E. M. Parker, J.-P. Griffiths, M. G. Moloney and L. Y. L. Wu, Appl. Surf. Sci., 2017, 401, 181–189 CrossRef CAS.
- H. Dahn and H. V. Castelmur, Helv. Chim. Acta, 1953, 36, 638–645 CrossRef CAS.
- W. Liu, S. Bian, L. Li, L. Samuelson, J. Kumar and S. Tripathy, Chem. Mater., 2000, 12, 1577–1584 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Material synthesis and film preparation methods, sensitization and diazo coupling of the films, determination of surface loading, and SEM images and XPS spectra of the films. See DOI: 10.1039/d0cc03631e |
| This journal is © The Royal Society of Chemistry 2020 |