Open Access Article
Open Access ArticleConfigurational control of low-symmetry heteroleptic metal–organic cages with asymmetric ligands†
Hao
Yu
,
Ziteng
Guo
,
Jie
Tang
,
Ningxu
Han
,
Junjuan
Shi
,
Meng
Li
,
Houyu
Zhang
 and
Ming
Wang
and
Ming
Wang
 *
*
State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China. E-mail: mingwang358@jlu.edu.cn
First published on 7th March 2025
Abstract
Low-symmetry metal–organic cages (MOCs) can better mimic the structure of biological enzymes compared to high-symmetry MOCs, due to their unique internal cavities that resemble the specialized and irregular active sites of enzymes. In this study, two low-symmetry heteroleptic MOCs with six Pd(II) centers, Pd6LA6LB6 and Pd6LB6LC6, were successfully constructed by combining two strategies: asymmetric ligand assembly and multi-ligand co-assembly. Crystallographic characterization and analysis revealed that Pd6LA6LB6 is a mixture of potentially 16 isomers. Introducing a methyl group at the ortho position of the coordination site of ligand LC induced steric hindrance, driving Pd6LB6LC6 to undergo a structural transformation and selectively assemble into a single dominant configuration from 13 potential isomers. This work not only demonstrates the immense potential of integrating asymmetric ligand assembly with multi-ligand co-assembly strategies but also highlights the critical role of steric effects in guiding assembly pathways and achieving precise configurational control in low-symmetry MOCs.
Introduction
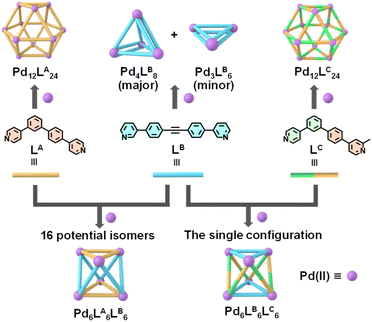
Metal–organic cages (MOCs) with discrete three-dimensional structures are formed through the self-assembly of diverse organic ligands and metal ions.1–9 MOCs have garnered significant attention from researchers, due to their highly adjustable structures and versatile functionalities, such as molecular recognition, separation, and catalysis.10–18 Consequently, they are frequently employed to emulate the functions of biological enzymes. However, most reported MOCs are constructed using symmetric ligands and metal ions, leading to highly symmetric structures. While some symmetric MOCs can exhibit high selectivity and catalytic efficiency for specific substrates due to their well-defined spatial and electronic environments,19–21 they often lack the unique and irregular internal cavities found in natural enzymes. These cavities are crucial for achieving broader substrate specificity and distinctive catalytic functions. Therefore, developing low-symmetry MOCs is essential for advancing biomimetic applications.In general, there are two approaches for constructing low-symmetry MOCs: the first approach involves reducing the symmetry of ligands, utilizing a single type of asymmetric ligands to construct low-symmetry MOCs.22–29 A representative example is Lewis's ingenious use of asymmetric ligands to assemble various low-symmetry Pd2L4 molecular cages.30,31 The second approach is employing co-assembly of two or more types of symmetric ligands to build low-symmetry MOCs.32–39 The most notable example is Clever's recent report on the co-assembly of four different ligands, resulting in the formation of a heteroleptic cage, Pd2ABCD.40 Combining these two approaches presents a valuable opportunity to develop more complex low-symmetry MOCs. However, each strategy poses specific challenges in achieving precise low-symmetry MOCs. In the case of self-assembly based on asymmetric ligands, the main challenge lies in preventing the formation of multiple isomers with similar stability. For heteroleptic systems, the difficulty is to avoid the formation of homoleptic complexes or statistical mixtures. Consequently, integrating these two approaches will inevitably complicate the assembly process. To date, as far as we know, only five reported examples have successfully integrated these approaches using different strategies. Lewis employed coordination sphere engineering,41 Preston utilized an auxiliary pairing strategy,42 Chand applied the geometric complementarity principle,43 and Bloch, Fallon,44 and Pilgrim45 used different isomeric structures with asymmetry to construct distinct dinuclear Pd(II) low-symmetry MOCs. These achievements highlight the potential of combining these methods. However, further exploration of this integration to create low-symmetry heteroleptic MOCs with higher nuclearity and achieve configurational control remains a significant challenge.
In this study, we designed and synthesized asymmetric ligands LA and LC, along with symmetric ligand LB (Fig. 1). When individually used with Pd(II) for assembly, all three ligands can form discrete structures. Co-assembly of LA, LB, and Pd(II) resulted in the formation of hexanuclear low-symmetry heteroleptic MOCs, Pd6LA6LB6. Crystallographic characterization and analysis revealed that Pd6LA6LB6 is a mixture of potentially 16 isomers. To achieve precise configurational control in the heteroleptic cage assembly, a methyl group was introduced at the ortho position of one coordination site on LC, based on the LA, to induce steric hindrance during the assembly process. This specific position was chosen to maximize control over the assembly direction and reduce the number of possible isomers. Experimental results showed that the introduction of methyl groups did not alter the overall composition of either the homoleptic or heteroleptic cages, but it positively influenced configurational control. Co-assembly of LC with LB and Pd(II) effectively controlled the assembly process, directing the formation of a single dominant configuration of the low-symmetry heteroleptic MOCs, Pd6LB6LC6, from 13 possible isomers. This study highlights the successful integration of asymmetric ligand assembly with heteroleptic co-assembly for constructing high-nuclearity, low-symmetry heteroleptic MOCs and demonstrates the effectiveness of using steric hindrance to achieve precise configurational control.
Results and discussion
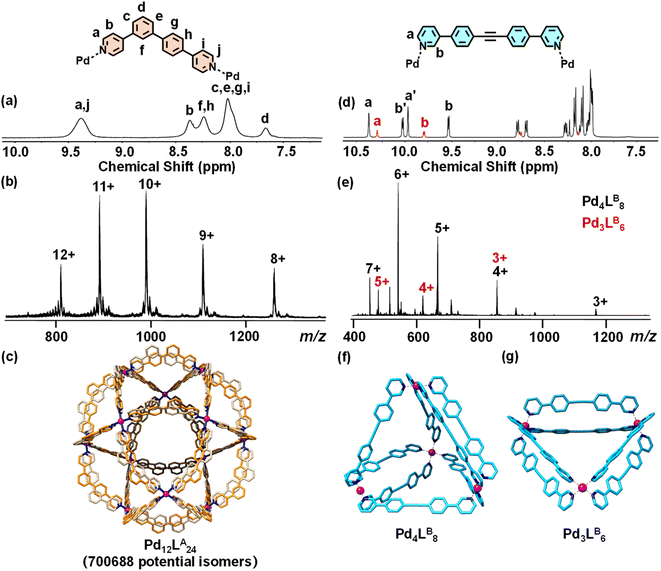
Guided by the principle of geometric complementarity, we designed LA and LB to coordinate with “naked” Pd(II) ions, enabling the formation of heteroleptic cages. To explore the role of steric effects, we further designed the other asymmetric ligand LC by introducing a methyl group at the ortho position of the nitrogen atom and studied its impact on the assembly of heteroleptic cages. The ligands LA, LB, and LC were synthesized through the efficient Suzuki and Sonogashira coupling reaction, and the detailed synthetic routes and characterizations were shown in the ESI (Schemes S1–S3 and Fig. S1–S5, S10–S14, S20–S24, and S42–S44).† We began by examining the assembly of ligands LA and LB with Pd(II) individually. To investigate this, LA was assembled with Pd(CH3CN)4(BF4)2 in DMSO-d6 at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at 70 °C overnight to form the assembly based on LA. In the 1H NMR spectrum, five broad peaks were observed, indicating the formation of a large complex with slow tumbling on the NMR timescale (Fig. 2a). Subsequently, ESI-MS was used to confirm the molecular composition of the LA assembly, revealing a series of peaks with successive charge states, corresponding to the loss of varying numbers of BF4− counterions. The observed charge fragments include m/z = 1257.76 for [Pd12LA24(BF4)16]8+, 1108.32 for [Pd12LA24(BF4)15]9+, 988.90 for [Pd12LA24(BF4)14]10+, 891.01 for [Pd12LA24(BF4)13]11+, and 809.57 for [Pd12LA24(BF4)12]12+ (Fig. 2b, S45 and S46†). By calculating the molecular ion peaks, the molecular weight of the LA assembly was determined to be 10,753.9 Da, corresponding to 24 LA, 12 Pd(II) ions, and 24 BF4−. Although these characterizations confirmed that the asymmetric ligand LA and Pd(II) could form a Pd12LA24 assembly, the exact configuration of Pd12LA24 remained unclear. To resolve this, we aim to use single-crystal characterization to determine the exact structure of the Pd12LA24 assembly. By allowing ethyl acetate vapor to diffuse into a DMF solution of (Pd12LA24)(OTf)24 over approximately two months, we obtained crystals suitable for testing diffraction experiments. The single-crystal data was collected by using synchrotron radiation (Table S1, Fig. S56 and S57†) and revealed that in Pd12LA24, each asymmetric ligand exhibits disorder and can adopt two possible orientations (shown as yellow and gray ligands in Fig. 2c). This disorder results from the absence of energetic preference for any specific isomer within the assembly, leading to multiple isomers coexisting in solution and crystallizing as a statistical mixture.46 Using Pólya's theorem, we calculated the total number of potential isomers to be 700
1 molar ratio at 70 °C overnight to form the assembly based on LA. In the 1H NMR spectrum, five broad peaks were observed, indicating the formation of a large complex with slow tumbling on the NMR timescale (Fig. 2a). Subsequently, ESI-MS was used to confirm the molecular composition of the LA assembly, revealing a series of peaks with successive charge states, corresponding to the loss of varying numbers of BF4− counterions. The observed charge fragments include m/z = 1257.76 for [Pd12LA24(BF4)16]8+, 1108.32 for [Pd12LA24(BF4)15]9+, 988.90 for [Pd12LA24(BF4)14]10+, 891.01 for [Pd12LA24(BF4)13]11+, and 809.57 for [Pd12LA24(BF4)12]12+ (Fig. 2b, S45 and S46†). By calculating the molecular ion peaks, the molecular weight of the LA assembly was determined to be 10,753.9 Da, corresponding to 24 LA, 12 Pd(II) ions, and 24 BF4−. Although these characterizations confirmed that the asymmetric ligand LA and Pd(II) could form a Pd12LA24 assembly, the exact configuration of Pd12LA24 remained unclear. To resolve this, we aim to use single-crystal characterization to determine the exact structure of the Pd12LA24 assembly. By allowing ethyl acetate vapor to diffuse into a DMF solution of (Pd12LA24)(OTf)24 over approximately two months, we obtained crystals suitable for testing diffraction experiments. The single-crystal data was collected by using synchrotron radiation (Table S1, Fig. S56 and S57†) and revealed that in Pd12LA24, each asymmetric ligand exhibits disorder and can adopt two possible orientations (shown as yellow and gray ligands in Fig. 2c). This disorder results from the absence of energetic preference for any specific isomer within the assembly, leading to multiple isomers coexisting in solution and crystallizing as a statistical mixture.46 Using Pólya's theorem, we calculated the total number of potential isomers to be 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688 (Fig. S62†), which aligns with results reported in the literature.23,47 The assembly based on LB was obtained by mixing LB and Pd(CH3CN)4(BF4)2 in DMF-d7 at a stoichiometric ratio of 2
688 (Fig. S62†), which aligns with results reported in the literature.23,47 The assembly based on LB was obtained by mixing LB and Pd(CH3CN)4(BF4)2 in DMF-d7 at a stoichiometric ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then heated at 70 °C overnight. The obtained assembly showed two sets of proton peaks (Fig. 2d) with an approximate ratio of 4
1, and then heated at 70 °C overnight. The obtained assembly showed two sets of proton peaks (Fig. 2d) with an approximate ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, indicating the presence of a dynamic equilibrium mixture of two thermodynamically similar assemblies in solution. This observation was further corroborated by DOSY measurements, which clearly resolved two distinct diffusion bands, confirming the presence of two discrete species in solution (Fig. S18†). The primary assembly (black) displayed two distinct chemical environments for its ligands, while the secondary assembly (red) showed only one. ESI-MS characterization identified that the assembly product for LB is a mixture of the octameric Pd4LB8 and the hexameric Pd3LB6, with the former being the major product (Fig. 2e). By integrating NMR, ESI-MS, and the earlier research report,48 we proposed structural models for both Pd4LB8 and Pd3LB6 (Fig. 2f and g).
1, indicating the presence of a dynamic equilibrium mixture of two thermodynamically similar assemblies in solution. This observation was further corroborated by DOSY measurements, which clearly resolved two distinct diffusion bands, confirming the presence of two discrete species in solution (Fig. S18†). The primary assembly (black) displayed two distinct chemical environments for its ligands, while the secondary assembly (red) showed only one. ESI-MS characterization identified that the assembly product for LB is a mixture of the octameric Pd4LB8 and the hexameric Pd3LB6, with the former being the major product (Fig. 2e). By integrating NMR, ESI-MS, and the earlier research report,48 we proposed structural models for both Pd4LB8 and Pd3LB6 (Fig. 2f and g).
After characterizing the assemblies of LA and LB individually, we explored their co-assembly with Pd(II). A 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of LA, LB, and Pd(CH3CN)4(BF4)2 was dissolved in DMF-d7, and the mixture was reacted at 70 °C overnight. The 1H NMR spectrum of the co-assembly exhibited distinct chemical shifts compared to the individual assemblies of LA and LB, indicating the formation of new structure (Fig. S29 and S37†). However, some weaker peaks indicating side products were also observed during the assembly process, and multiple attempts to eliminate these signals were unsuccessful. ESI-MS displayed a sequence of peaks with successive charge states, corresponding to the following m/z values: 1294.72 for [Pd6LA6LB6(BF4)8]4+, 1018.61 for [Pd6LA6LB6(BF4)7]5+, 834.36 for [Pd6LA6LB6(BF4)6]6+, 702.82 for [Pd6LA6LB6(BF4)5]7+, 604.21 for [Pd6LA6LB6(BF4)4]8+, and 527.53 for [Pd6LA6LB6(BF4)3]9+ (Fig. 3b, S52 and S53†). Calculation of the molecular ion peaks determined a molecular weight of 5521.03 Da for the co-assembly, consistent with a structure composed of six LA, six LB, six Pd(II) ions, and twelve BF4− ions. This confirms the successful construction of a mixed-ligand metal–organic cage, Pd6LA6LB6, based on asymmetric ligands. Based on our analysis and simulations, we propose that the Pd6LA6LB6 cage may exist in two different structural types, namely Pd6LA6LB6-a and Pd6LA6LB6-b (Fig. 3a). This is primarily attributed to the specific molecular angles, coordination sites, and ligand lengths of the two ligands, which promote geometric complementarity in coordination with Pd(II) ions. This complementarity directs the assembly process toward the formation of these two possible structures while preventing the formation of other isomers. Due to the disorder of the asymmetric ligand LA, both Pd6LA6LB6-a and Pd6LA6LB6-b have multiple potential isomers. Using an enumeration method, we identified 13 possible isomers for Pd6LA6LB6-a (Fig. S64†) and 16 possible isomers for Pd6LA6LB6-b (Fig. S63†). To further determine the configuration of the heteroleptic cage, we obtained the single crystal by slow diffusion of ethyl acetate into a DMF solution of Pd6LA6LB6 about three weeks (Table S2, Fig. S58 and S59†). As seen in Fig. 3c, the resulting crystal structure corresponds to the structural type Pd6LA6LB6-b. In this structure, the asymmetric ligands LA are located on opposite sides of the molecular cage, while the ligands LB are positioned in the center, connecting with each other. Additionally, each LA in Pd6LA6LB6 exhibits disorder, allowing two possible orientations, similar to the arrangement of ligands in the Pd12LA24 (shown as yellow and gray ligands in Fig. 3c). This suggests that Pd6LA6LB6 can exist in solution as a statistical mixture of up to 16 isomers, resulting in the crystallization of a variety of structures.
1 molar ratio of LA, LB, and Pd(CH3CN)4(BF4)2 was dissolved in DMF-d7, and the mixture was reacted at 70 °C overnight. The 1H NMR spectrum of the co-assembly exhibited distinct chemical shifts compared to the individual assemblies of LA and LB, indicating the formation of new structure (Fig. S29 and S37†). However, some weaker peaks indicating side products were also observed during the assembly process, and multiple attempts to eliminate these signals were unsuccessful. ESI-MS displayed a sequence of peaks with successive charge states, corresponding to the following m/z values: 1294.72 for [Pd6LA6LB6(BF4)8]4+, 1018.61 for [Pd6LA6LB6(BF4)7]5+, 834.36 for [Pd6LA6LB6(BF4)6]6+, 702.82 for [Pd6LA6LB6(BF4)5]7+, 604.21 for [Pd6LA6LB6(BF4)4]8+, and 527.53 for [Pd6LA6LB6(BF4)3]9+ (Fig. 3b, S52 and S53†). Calculation of the molecular ion peaks determined a molecular weight of 5521.03 Da for the co-assembly, consistent with a structure composed of six LA, six LB, six Pd(II) ions, and twelve BF4− ions. This confirms the successful construction of a mixed-ligand metal–organic cage, Pd6LA6LB6, based on asymmetric ligands. Based on our analysis and simulations, we propose that the Pd6LA6LB6 cage may exist in two different structural types, namely Pd6LA6LB6-a and Pd6LA6LB6-b (Fig. 3a). This is primarily attributed to the specific molecular angles, coordination sites, and ligand lengths of the two ligands, which promote geometric complementarity in coordination with Pd(II) ions. This complementarity directs the assembly process toward the formation of these two possible structures while preventing the formation of other isomers. Due to the disorder of the asymmetric ligand LA, both Pd6LA6LB6-a and Pd6LA6LB6-b have multiple potential isomers. Using an enumeration method, we identified 13 possible isomers for Pd6LA6LB6-a (Fig. S64†) and 16 possible isomers for Pd6LA6LB6-b (Fig. S63†). To further determine the configuration of the heteroleptic cage, we obtained the single crystal by slow diffusion of ethyl acetate into a DMF solution of Pd6LA6LB6 about three weeks (Table S2, Fig. S58 and S59†). As seen in Fig. 3c, the resulting crystal structure corresponds to the structural type Pd6LA6LB6-b. In this structure, the asymmetric ligands LA are located on opposite sides of the molecular cage, while the ligands LB are positioned in the center, connecting with each other. Additionally, each LA in Pd6LA6LB6 exhibits disorder, allowing two possible orientations, similar to the arrangement of ligands in the Pd12LA24 (shown as yellow and gray ligands in Fig. 3c). This suggests that Pd6LA6LB6 can exist in solution as a statistical mixture of up to 16 isomers, resulting in the crystallization of a variety of structures.
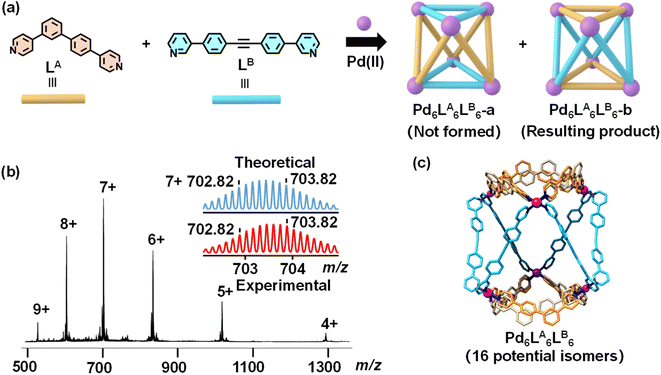 | ||
| Fig. 3 (a) Two possible structural types of Pd6LA6LB6; (b) ESI-MS spectrum of Pd6LA6LB6; (c) the crystal structure of Pd6LA6LB6 (hydrogen atoms are omitted for clarity). | ||
Employing steric group modifications on ligands to enhance spatial constraints during assembly serves as an effective strategy for controlling configuration. However, current research primarily targets simpler M2L4 molecular cages with fewer components.30,49–51 We wanted to investigate whether this strategy could be used to control the configuration of more complex heteroleptic cages. To achieve this, we utilized LC, featuring methyl group modifications on a single side of the pyridine as steric hindrance, in conjunction with LB and Pd(II) for co-assembly. The assembly method was the same as that used for Pd6LA6LB6. The 1H NMR spectrum of the co-assembly showed significant chemical shifts relative to the individual assemblies of LB and LC, indicating the formation of heteroleptic product (Fig. S33 and S38†). However, we also detected smaller proton peaks corresponding to side products. Despite multiple experimental attempts, we couldn't eliminate or reduce these signals. ESI-MS characterization confirmed the co-assembly as a heteroleptic metal–organic cage with six LB and six LC (Fig. 4b). By slowly evaporating ethyl acetate into a DMF solution of Pd6LB6LC6, the single crystals suitable for testing were successfully obtained (Table S3, Fig. S60 and S61†). The configuration of Pd6LB6LC6 may also have two structural types, namely Pd6LB6LC6-a and Pd6LB6LC6-b (Fig. 4a). Similarly, they also have 13 and 16 potential isomers, respectively. From Fig. 4c, it is evident that the observed crystal structure aligns with our prediction of the structural type Pd6LB6LC6-a, where ligand LB forms the top and bottom of the molecular cage, while the asymmetric ligand LC is located in the middle and interconnected. It is particularly noteworthy that, unlike the disordered distribution of asymmetric ligands in Pd6LA6LB6, asymmetric ligand LC in Pd6LB6LC6 exhibits a regular cis arrangement in the molecular cage due to the presence of sterically hindered methyl groups. We propose that the introduction of this steric hindrance effectively regulates intermolecular interactions and spatial organization, resulting in a more orderly assembly process and reducing isomer formation. Within each Pd(II) center of the Pd6LB6LC6-a cage, there are three unmodified pyridine groups and one methyl-modified pyridine group. This arrangement arises because the presence of two or more methyl groups on the same Pd(II) center would lead to significant steric hindrance. Consequently, the most stable configuration naturally avoids such steric clashes, and this arrangement represents the optimal binding mode achieved through coordination-driven self-assembly.
We performed Density Functional Theory (DFT) calculations on Pd6LB6LC6-a and Pd6LB6LC6-b. To align with experimental conditions, we considered solvent effects using a linearized Poisson–Boltzmann solvation model to represent the impact of DMF. The results indicated that Pd6LB6LC6-a had lower energy, with an energy difference of 7.39 kJ mol−1 compared to Pd6LB6LC6-b (Fig. 4d). This suggests that Pd6LB6LC6-a is energetically favored, which aligns well with the crystal characterization result. We hypothesize that this is due to the structure of Pd6LB6LC6-a is capable of more effectively alleviating the steric hindrance induced by the methyl group, in contrast to Pd6LB6LC6-b. By integrating NMR, ESI-MS, X-ray crystallography and DFT calculations, we observed that compared with Pd6LA6LB6, Pd6LB6LC6 not only underwent a structural transformation but also selectively assembled into a single dominant configuration from 13 possible isomers. This indicates that the strategy of introducing steric groups on asymmetric ligands has successfully achieved configurational control in low-symmetry heteroleptic MOCs. Additionally, using NMR and ESI-MS, we confirmed that the assembly product of LC and Pd(II) is Pd12LC24 (Fig. S25 and S45†). Although we didn't obtain the crystal structure to determine the precise arrangement of Pd12LC24, variable-temperature NMR and DFT calculations suggest that it might adopt a trans-Pd12LC24 configuration (Fig. S39, S65 and S66†), indicating that steric strategy might also be useful for tuning the structure of more complex large molecular cages with more components.
Conclusions
In summary, by combining asymmetric ligand assembly with heteroleptic co-assembly, we successfully synthesized six-nuclear, low-symmetry heteroleptic MOCs, Pd6LA6LB6 and Pd6LB6LC6. Crystallographic analysis revealed that Pd6LA6LB6 has 16 possible isomers. In contrast, the methyl-modified ligand LC introduced steric hindrance during assembly, leading to a structural transformation and configuration control in Pd6LB6LC6. This study highlights the potential of integrating asymmetric ligand assembly with mixed-ligand co-assembly to construct complex, high-nuclearity, low-symmetry molecular architectures. Furthermore, it underscores the crucial role of steric effects in controlling the configuration of MOCs.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
In this work, M. W. and H. Y. conceived and designed the experiments. H. Y., Z. G and J. T. completed the synthesis. H. Y. grew the crystal and collected X-ray data of the crystals. H. Z. performed the DFT calculations. H. Y. and Z. G. conducted NMR, MS characterization. M. W., H. Y., N. H, J. S. and M. L. analyzed the data and wrote the manuscript. All the authors discussed the results and commented on and proofread the final form of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the support from the National Natural Science Foundation of China (22471094 and 22271116 for M. W.), the fellowship of China Postdoctoral Science Foundation (2023M741338 for H. Y.), and Postdoctoral Fellowship Program of CPSF (GZC20230940 for H. Y.). We thank the staff at BL17B1 beamline of the National Facility for Protein Science in Shanghai (NFPS), Shanghai Advanced Research Institute, CAS, for providing technical support in X-ray diffraction data collection and analysis.References
- S. Saha, I. Regeni and G. H. Clever, Structure relationships between bis-monodentate ligands and coordination driven self-assemblies, Coord. Chem. Rev., 2018, 374, 1–14 CrossRef.
- E. G. Percástegui, T. K. Ronson and J. R. Nitschke, Design and applications of water-soluble coordination cages, Chem. Rev., 2020, 120, 13480–13544 CrossRef.
- D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Self-assembly of tetravalent Goldberg polyhedra from 144 small components, Nature, 2016, 540, 563–566 CrossRef.
- T. Wu, Z. Jiang, Q. Bai, Y. Li, S. Mao, H. Yu, L. Wojtas, Z. Tang, M. Chen, Z. Zhang, T.-Z. Xie, M. Wang, X. Li and P. Wang, Supramolecular triangular orthobicupola: Self-assembly of a giant Johnson solid J27, Chem, 2021, 7, 2429–2441 Search PubMed.
- H. Wang, L.-P. Zhou, Y. Zheng, K. Wang, B. Song, X. Yan, L. Wojtas, X.-Q. Wang, X. Jiang, M. Wang, Q.-F. Sun, B. Xu, H.-B. Yang, A. C. H. Sue, Y.-T. Chan, J. L. Sessler, Y. Jiao, P. J. Stang and X. Li, Double-layered supramolecular prisms self-assembled by geometrically non-equivalent tetratopic subunits, Angew. Chem., Int. Ed., 2021, 60, 1298–1305 CrossRef PubMed.
- S. Pullen, J. Tessarolo and G. H. Clever, Increasing structural and functional complexity in self-assembled coordination cages, Chem. Sci., 2021, 12, 7269–7293 RSC.
- J. E. M. Lewis, A. B. S. Elliott, C. J. McAdam, K. C. Gordon and J. D. Crowley, ‘Click’ to functionalise: synthesis, characterisation and enhancement of the physical properties of a series of exo- and endo-functionalised Pd2L4 nanocages, Chem. Sci., 2014, 5, 1833–1843 RSC.
- Y. Tsujimoto, T. Kojima and S. Hiraoka, Rate-determining step in the self-assembly process of supramolecular coordination capsules, Chem. Sci., 2014, 5, 4167–4172 RSC.
- Y.-S. Chen, E. Solel, Y.-F. Huang, C.-L. Wang, T.-H. Tu, E. Keinan and Y.-T. Chan, Chemical mimicry of viral capsid self-assembly via corannulene-based pentatopic tectons, Nat. Commun., 2019, 10, 3443 CrossRef.
- M. Yoshizawa, M. Tamura and M. Fujita, Diels-Alder in Aqueous Molecular Hosts: Unusual Regioselectivity and Efficient Catalysis, Science, 2006, 312, 251–254 CrossRef.
- R. Banerjee, D. Chakraborty, W.-T. Jhang, Y.-T. Chan and P. S. Mukherjee, Structural switching of a distorted trigonal metal–organic cage to a tetragonal cage and singlet oxygen mediated oxidations, Angew. Chem., Int. Ed., 2023, 62, e202305338 CrossRef PubMed.
- I. Jahović, Y. Yang, T. K. Ronson and J. R. Nitschke, Capture of singlet oxygen modulates host-guest behavior of coordination cages, Angew. Chem., Int. Ed., 2023, 62, e202309589 CrossRef PubMed.
- H. Lee, J. Tessarolo, D. Langbehn, A. Baksi, R. Herges and G. H. Clever, Light-powered dissipative assembly of diazocine coordination cages, J. Am. Chem. Soc., 2022, 144, 3099–3105 CrossRef CAS.
- L. Catti, H. Narita, Y. Tanaka, H. Sakai, T. Hasobe, N. V. Tkachenko and M. Yoshizawa, Supramolecular singlet fission of pentacene dimers within polyaromatic capsules, J. Am. Chem. Soc., 2021, 143, 9361–9367 CrossRef CAS PubMed.
- S.-J. Hu, X.-Q. Guo, L.-P. Zhou, D.-N. Yan, P.-M. Cheng, L.-X. Cai, X.-Z. Li and Q.-F. Sun, Guest-driven self-assembly and chiral induction of photofunctional lanthanide tetrahedral cages, J. Am. Chem. Soc., 2022, 144, 4244–4253 CrossRef.
- D. Chakraborty, R. Saha, J. K. Clegg and P. S. Mukherjee, Selective separation of planar and non-planar hydrocarbons using an aqueous Pd6 interlocked cage, Chem. Sci., 2022, 13, 11764–11771 RSC.
- R. Sumida, Y. Tanaka, K. Niki, Y. Sei, S. Toyota and M. Yoshizawa, Cyclic monoterpenes trapped in a polyaromatic capsule: unusual selectivity, isomerization, and volatility suppression, Chem. Sci., 2021, 12, 9946–9951 RSC.
- K. Yazaki, M. Akita, S. Prusty, D. K. Chand, T. Kikuchi, H. Sato and M. Yoshizawa, Polyaromatic molecular peanuts, Nat. Commun., 2020, 8, 15914 CrossRef PubMed.
- S. Ghosal, A. Das, D. Roy and J. Dasgupta, Tuning light-driven oxidation of styrene inside water-soluble nanocages, Nat. Commun., 2024, 15, 1810 CrossRef CAS PubMed.
- M. Yoshizawa, M. Tamura and M. Fujita, Diels-Alder in aqueous molecular hosts: unusual regioselectivity and efficient catalysis, Science, 2006, 312, 251–254 Search PubMed.
- V. A. Rinshad, M. Aggarwal, J. K. Clegg and P. S. Mukherjee, Harnessing a Pd4 water-soluble molecular capsule as a size-selective catalyst for targeted oxidation of alkyl aromatics, JACS Au, 2024, 4, 3238–3247 Search PubMed.
- D. Prajapati, J. K. Clegg and P. S. Mukherjee, Formation of a low-symmetry Pd8 molecular barrel employing a hetero donor tetradentate ligand, and its use in the binding and extraction of C70, Chem. Sci., 2024, 15, 12502–12510 RSC.
- R.-J. Li, A. Tarzia, V. Posligua, K. E. Jelfs, N. Sanchez, A. Marcus, A. Baksi, G. H. Clever, F. Fadaei-Tirani and K. Severin, Orientational self-sorting in cuboctahedral Pd cages, Chem. Sci., 2022, 13, 11912–11917 RSC.
- R.-J. Li, A. Marcus, F. Fadaei-Tirani and K. Severin, Orientational self-sorting: formation of structurally defined Pd4L8 and Pd6L12 cages from low-symmetry dipyridyl ligands, Chem. Commun., 2021, 57, 10023–10026 Search PubMed.
- D. Ogata and J. Yuasa, Dynamic open coordination cage from nonsymmetrical imidazole–pyridine ditopic ligands for turn-on/off anion binding, Angew. Chem., Int. Ed., 2019, 58, 18424–18428 Search PubMed.
- S. S. Mishra, S. V. K. Kompella, S. Krishnaswamy, S. Balasubramanian and D. K. Chand, Low-symmetry self-assembled coordination complexes with exclusive diastereoselectivity: experimental and computational studies, Inorg. Chem., 2020, 59, 12884–12894 Search PubMed.
- A. Tarzia, J. E. M. Lewis and K. E. Jelfs, High-throughput computational evaluation of low symmetry Pd2L4 cages to aid in system design, Angew. Chem., Int. Ed., 2021, 60, 20879–20887 CrossRef CAS.
- H. Yu, Z. Guo, N. Han, J. Shi, X. Jiang, Q. Bai, Z. Zhang, P. Wang and M. Wang, Construction of outward-everted metal–organic cages induced by steric hindrance groups based on dissymmetrical ligands, Cell Rep. Phys. Sci., 2023, 4, 101631 Search PubMed.
- H. Yu, J. Li, C. Shan, T. Lu, X. Jiang, J. Shi, L. Wojtas, H. Zhang and M. Wang, Conformational control of a metallo-supramolecular cage via the dissymmetrical modulation of ligands, Angew. Chem., Int. Ed., 2021, 60, 26523–26527 CrossRef CAS.
- J. E. M. Lewis, A. Tarzia, A. J. P. White and K. E. Jelfs, Conformational control of Pd2L4 assemblies with unsymmetrical ligands, Chem. Sci., 2020, 11, 677–683 RSC.
- J. E. M. Lewis, Pseudo-heterolepticity in low-symmetry metal–organic cages, Angew. Chem., Int. Ed., 2022, 61, e202212392 Search PubMed.
- W. M. Bloch, Y. Abe, J. J. Holstein, C. M. Wandtke, B. Dittrich and G. H. Clever, Geometric complementarity in assembly and guest recognition of a bent heteroleptic cis-[Pd2LA2LB2] coordination cage, J. Am. Chem. Soc., 2016, 138, 13750–13755 CrossRef CAS PubMed.
- W. M. Bloch, J. J. Holstein, W. Hiller and G. H. Clever, Morphological control of heteroleptic cis- and trans-Pd2L2L′2 cages, Angew. Chem., Int. Ed., 2017, 56, 8285–8289 CrossRef CAS.
- Y. Liu, S.-H. Liao, W.-T. Dai, Q. Bai, S. Lu, H. Wang, X. Li, Z. Zhang, P. Wang, W. Lu and Q. Zhang, Controlled construction of heteroleptic [Pd2(LA)2(LB)(LC)]4+ cages: a facile approach for site-selective endo-functionalization of supramolecular cavities, Angew. Chem., Int. Ed., 2023, 62, e202217215 CrossRef CAS PubMed.
- M. Yamashina, T. Yuki, Y. Sei, M. Akita and M. Yoshizawa, Anisotropic expansion of an M2L4 coordination capsule: host capability and frame rearrangement, Chem.–Eur. J., 2015, 21, 4200–4204 CrossRef CAS.
- X. Yan, T. R. Cook, P. Wang, F. Huang and P. J. Stang, Highly emissive platinum(II) metallacages, Nat. Chem., 2015, 7, 342–348 CrossRef CAS PubMed.
- R.-J. Li, F. Fadaei-Tirani, R. Scopelliti and K. Severin, Tuning the size and geometry of heteroleptic coordination cages by varying the ligand bent angle, Chem.–Eur. J., 2021, 27, 9439–9445 CrossRef CAS PubMed.
- S. Sudan, R.-J. Li, S. M. Jansze, A. Platzek, R. Rudolf, G. H. Clever, F. Fadaei-Tirani, R. Scopelliti and K. Severin, Identification of a heteroleptic Pd6L6L′6 coordination cage by screening of a virtual combinatorial library, J. Am. Chem. Soc., 2021, 143, 1773–1778 Search PubMed.
- T. Abe, N. Sanada, K. Takeuchi, A. Okazawa and S. Hiraoka, Assembly of six types of heteroleptic Pd2L4 cages under kinetic control, J. Am. Chem. Soc., 2023, 145, 28061–28074 Search PubMed.
- K. Wu, E. Benchimol, A. Baksi and G. H. Clever, Non-statistical assembly of multicomponent [Pd2ABCD] cages, Nat. Chem., 2024, 16, 584–591 CrossRef CAS.
- P. Molinska, A. Tarzia, L. Male, K. E. Jelfs and J. E. M. Lewis, Diastereoselective self-assembly of low-symmetry PdnL2n nanocages through coordination-sphere engineering, Angew. Chem., Int. Ed., 2023, 62, e202315451 CrossRef CAS PubMed.
- D. Preston and J. D. Evans, A lantern-shaped Pd(II) cage constructed from four different low-symmetry ligands with positional and orientational control: an ancillary pairings approach, Angew. Chem., Int. Ed., 2023, 62, e202314378 CrossRef CAS PubMed.
- M. Parbin, V. Sivalingam and D. K. Chand, Highly anisotropic Pd2L2L2 and Pd2L2L2 type cages by heteromeric completive self-sorting, Angew. Chem., Int. Ed., 2024, 63, e202410219 CrossRef CAS.
- A. P. Birvé, H. D. Patel, J. R. Price, W. M. Bloch and T. Fallon, Guest-dependent isomer convergence of a permanently fluxional coordination cage, Angew. Chem., Int. Ed., 2022, 61, e202115468 CrossRef PubMed.
- M. R. Black, S. Bhattacharyya, S. P. Argent and B. S. Pilgrim, Structural transformations of metal–organic cages through tetrazine-alkene reactivity, J. Am. Chem. Soc., 2024, 146, 28233–28241 Search PubMed.
- S. Samantray, S. Krishnaswamy and D. K. Chand, Self-assembled conjoined-cages, Nat. Commun., 2020, 11, 880 CrossRef PubMed.
- Q.-F. Sun, S. Sato and M. Fujita, An M12(L1)12(L2)12 cantellated tetrahedron: a case study on mixed-ligand self-assembly, Angew. Chem., Int. Ed., 2014, 53, 13510–13513 CrossRef.
- D. K. Chand, K. Biradha, M. Kawano, S. Sakamoto, K. Yamaguchi and M. Fujita, Dynamic self-assembly of an M3L6 molecular triangle and an M4L8 tetrahedron from naked PdII ions and bis(3-pyridyl)-substituted arenes, Chem.–Asian J., 2006, 1, 82–90 CrossRef.
- R. Zhu, W. M. Bloch, J. J. Holstein, S. Mandal, L. V. Schäfer and G. H. Clever, Donor-site-directed rational assembly of heteroleptic cis-[Pd2L2L′2] coordination cages from picolyl ligands, Chem.–Eur. J., 2018, 24, 12976–12982 CrossRef PubMed.
- R. A. S. Vasdev, D. Preston, C. A. Casey-Stevens, V. Martí-Centelles, P. J. Lusby, A. L. Garden and J. D. Crowley, Exploiting supramolecular interactions to control isomer distributions in reduced-symmetry [Pd2L4]4+ cages, Inorg. Chem., 2023, 62, 1833–1844 CrossRef PubMed.
- B. Chen, J. J. Holstein, A. Platzek, L. Schneider, K. Wu and G. H. Clever, Cooperativity of steric bulk and H-bonding in coordination sphere engineering: heteroleptic PdII cages and bowls by design, Chem. Sci., 2022, 13, 1829–1834 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and characterizations of ligands and complexes including NMR, ESI-MS, X-ray crystallographic data and DFT calculations. Moreover, it also contains the number of theoretically possible isomers of Pd12LA24, Pd6LA6LB6-a and Pd6LA6LB6-b. CCDC 2387801, 2387551 and 2387553. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc08647c |
| This journal is © The Royal Society of Chemistry 2025 |