Open Access Article
Open Access ArticleDichotomous behavior of water in binary mixtures with a turning point between 40 and 60 °C†
Amiya Atahara,
Noushaba Nusrat Mafya,
Mohammad Hossain ab and
Md. Abu Bin Hasan Susan
ab and
Md. Abu Bin Hasan Susan *ac
*ac
aDepartment of Chemistry, University of Dhaka, Dhaka, 1000, Bangladesh
bDepartment of Chemistry, Bangladesh University of Engineering and Technology (BUET), Dhaka, 1000, Bangladesh
cDhaka University Nanotechnology Center (DUNC), University of Dhaka, Dhaka, 1000, Bangladesh. E-mail: susan@du.ac.bd
First published on 17th June 2025
Abstract
Water is known for its anomalous properties; however, changes in its properties between the 40–60 °C temperature range remain poorly understood, particularly in complex aqueous systems. In this study, α-cyclodextrin, urea, poly(vinyl alcohol), and an inclusion complex of β-cyclodextrin with an ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim]PF6), were found to exhibit dichotomous behavior, rather than just an anomaly or a progressive shift in water and its binary mixtures. Using temperature-dependent dynamic light scattering (DLS) analysis, physicochemical measurements, and near-infrared (NIR) spectroscopy, a sharp and additive-independent transition in hydrodynamic particle size was observed, which was centered around 50 °C. This bifurcation indicated an intrinsic reconfiguration of the hydrogen-bonding network of water. Principal component analysis (PCA) and two-dimensional correlation spectroscopy (2DCoS) of the NIR spectra revealed coordinated spectral changes across this window, confirming a discrete restructuring process rather than a gradual thermal response. Notably, features below and above ∼50 °C were spectrally and dynamically distinct, supporting a two-state-like-model of water. These results demonstrated that the 40–60 °C range marked a thermally induced, cooperative transition in the structural organization of water, which was modulated but not dominated by solute interactions. This dichotomous behavior offers critical insights into the unique responsiveness of water and highlights the importance of this temperature window in understanding solvation dynamics, reaction kinetics, and aqueous-phase behavior across scientific disciplines. Such structural duality may inspire novel thermal switches, responsive solvents, and smart hydration systems for future applications in drug delivery, nanofluidics, and green chemistry.
1. Introduction
Water is known to exhibit various anomalous behaviors across different temperature ranges, all of which lead back to its hydrogen bonding,1,2 and ultimately, the structure of water. These anomalies are primarily attributed to the dynamic hydrogen-bonding network inherent in water, which influences its structural and thermodynamic properties. Various models used to describe the water structure3–5 contradict with one another and remain incomplete.6–8 The inability to illustrate the structure of water even at ambient temperature leaves a significant gap in understanding the behavior of water at different temperatures. In recent years, anomalous characteristics have been observed in the behavior of water between 40 and 60 °C, in particular. Recent advancements in spectroscopic techniques have provided deeper insights into the structural dynamics of water. For instance, Han et al. analyzed the combined bands of bending and stretching vibrations using near-infrared (NIR) spectroscopy, revealing distinct water structures associated with varying hydrogen bond configurations.9 Similarly, Wang et al. employed temperature-dependent NIR spectroscopy combined with independent component analysis to investigate water structures in reverse micelles, highlighting the sensitivity of the hydrogen-bonded architecture of water to environmental changes.10 The concept of aquaphotomics has emerged as a potent approach to investigate the spectral patterns of water under various perturbations. Muncan et al. highlighted the potential of temperature perturbation NIR spectroscopy in monitoring water quality by showing that it could detect minute variations between native minerals in processed and aged water samples.11 Moreover, the intricate nature of the hydrogen-bonded intermolecular structure of water has been further elucidated through advanced spectroscopic methods. Recent studies employing two-dimensional infrared-Raman spectroscopy have provided a more nuanced understanding of the tetrahedral structure of water and its temperature-dependent behavior.12 Principal component analysis (PCA) and two-dimensional correlation spectroscopy (2DCoS)13 of the NIR spectra of pure water indicated a minor influence on the spectral change with respect to temperature at a turning point around 45 ± 10 °C;14,15 however, no explanation was offered. Using X-ray emission spectroscopy and X-ray Raman scattering data of water, Huang et al. suggested that the tetrahedral structure of water (low-density water) is predominant at low temperatures, and the distorted structure (high-density water) is more prominent at ambient temperature, with distortions increasing with increasing temperature.16 Water structure influences other species, such as D-(+)-glucose, with which it interacts, in addition to the characteristics of water itself.17,18Recent studies unveiled a new anomalous behavior of water. One significant observation was the existence of two ‘states’ of water19 summarized by taking into consideration the crossover temperature presented by the thermal expansion coefficient at 42 ± 5 °C, the minimum isothermal compressibility at the same temperature, and different hydration shells of alcohol in water with a crossover temperature of 60 °C.19 This structural dichotomy influences the optical and thermal behavior of nanosystems (e.g., nanoparticles and quantum dots) and may significantly affect the stability of biological macromolecules such as proteins. Another intriguing observation is the discontinuity in the dielectric constant of water near 60 °C. This anomaly is associated with a shift in the average effective dipole moment of water molecules. Below 60 °C, the dipole moment resembles that of ice, whereas above this temperature, it transitions to a value similar to that of water vapor, suggesting a fundamental change in the molecular behavior of water.20 The crossover of relative permittivity at 60 °C, thermal conductivity at 64 ± 5 °C, spin-lattice relaxation of protons at 50 ± 5 °C, and refractive index at 57 ± 5 °C are cited in recent literature studies.19,21 The work presented compelling evidence of a subtle yet significant dielectric anomaly in liquid water around 60 °C, linked to a transition in the average dipole moment from a low-temperature configuration (∼2.17 D) to a high-temperature configuration (∼1.87 D), resembling that of water vapor. This structural transformation in water influences the optical extinction characteristics of metallic nanoparticles dispersed in it. Notably, it causes a shift in the longitudinal surface plasmon resonance. Such changes underscore the relevance of the structural state of water for applications in nanomedicine and stability of nanoparticle-based systems.22 Structural changes in liquid water around 43 °C have also been investigated regarding solvent parameters like hydrogen-bond donor acidity, hydrogen-bond acceptor basicity, polarizability, and dipolarity. Catalan et al. demonstrated the changes in basicity, polarizability, and dipolarity with discontinuities at 43 °C and 45 °C.23 This transformation is marked by a decrease in dipolarity and acidity, and an increase in basicity and polarizability, suggesting that water becomes less hydrogen-bonded and more loosely structured at higher temperatures. An iso-scattering point is confirmed by the two spectral components in Raman spectra in the range of 15 to 45 °C. This represents a thermal equilibrium between more extensive hydrogen-bonded low-density water and less extensive hydrogen-bonded high-density water.24 The effect of pH on the temperature of the anomalous behavior of water in terms of its changing structure has also been investigated.25 Notably, our recent investigation on the aqueous systems of ertugliflozin L-pyroglutamic acid revealed that the hydration number drops below zero at temperatures above 55 °C, indicating a net release of water from the solvation shell, an observation that underscores a bilinear, dichotomous behavior of water in the 50 ± 10 °C range. This crossover reflects a structural transition from strongly hydrogen-bonded, low-density water below the threshold to weakly bound, high-density configurations above it. Such behavior, consistent with a two-state model, further corroborates the existence of a discrete thermal transition in the hydrogen-bonding network of water, critically influencing solvation dynamics and molecular packing.26 These observations suggest a major structural change in liquid water in this particular temperature range, which, however, is not as straightforward as detecting phase changes. Some attempts have been made to fill this gap and explain water structure modification using different additives and analysis techniques. Spectroscopic and thermodynamic analyses of pure water and aqueous systems revealed three structural components of water with varying hydrogen bond strengths;27 the number of weaker and stronger hydrogen bonds changes readily with increasing temperature, whereas the concentration of a third component is much more resistant to the temperature changes in the range of 20–65 °C.28–31 It is found that the optical and structural characteristics of metal nanoparticles change above the crossover temperature (50–60 °C), which can be studied by analyzing their longitudinal surface plasmon resonances.19 Furthermore, the structural modifications in water caused by temperature affect biological macromolecules such as proteins. Therefore, the study of the pseudo-phase transition of liquid water has immense potential for applications in nanomedicine, bio-imaging, and tumor targeting. Considering these prodigious potentials, it is essential to unveil this dichotomous behavior of liquid water. Table S1 (ESI†) compares the previously reported transitions or anomalies in aqueous systems with the present work.
In this study, we aimed at examining the effect of this structural change thoroughly with an emphasis on the pseudo phase transition observed in the 40–60 °C range using four different additives, α-cyclodextrin (α-CD), urea, poly(vinyl alcohol) (PVA), and the inclusion complex of β-cyclodextrin (β-CD) and an ionic liquid, 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim]PF6), and studied their binary mixtures with water using dynamic light scattering (DLS) for particle size analysis and density measurements. This study also attempted to investigate the cause of the structural change in water by employing statistical and mathematical analyses, i.e., PCA and 2DCoS, respectively, of the NIR spectra of the aforementioned binary mixtures of water and additives. The term “dichotomous behavior” is used in the present research to describe the temperature-induced structural duality observed in water when perturbed by various solutes. Specifically, it refers to the emergence of two distinct regimes in the behavior of the hydrogen-bonding network of water as the temperature increases from ambient to moderately elevated levels (approximately 40 to 60 °C). Below this temperature range, water tends to maintain a more ordered, tetrahedral hydrogen-bonded structure, often associated with its “structured” or “ice-like” nature. Above this range, however, water exhibits a notable transition toward a more disordered and dynamic network, often described as “less structured”. This dichotomous behavior is not a sharp phase transition in the classical thermodynamic sense but rather a continuous, yet abrupt, structural reorganization, often referred to as a pseudo-phase transition. It is made evident by distinct shifts in various physicochemical properties (e.g., density, dynamic light scattering intensity, NIR spectral features) and is further supported by multivariate analyses (PCA and 2D correlation spectroscopy). Recognizing this bifurcation in behavior is crucial for understanding the role of water in solvation dynamics, molecular interactions, and the design of aqueous systems in chemistry and biology. A wide range of experimental methods, such as femtosecond mid-infrared spectroscopy, pump-probe 2D IR, far-infrared, high-pressure X-ray scattering, Raman spectroscopy, neutron diffraction, differential and titration calorimetry, NMR, optical Kerr effect, terahertz (THz) and high-pressure THz spectroscopy, as well as combined THz-Raman and optical pump-THz probe techniques, can also be utilized to explore this dichotomous behavior. These approaches enable a detailed investigation into hydration dynamics, rearrangement of hydrogen bonding networks upon solvation, properties of bulk and tetrahedrally coordinated water, and retardation in hydration bond dynamics in the vicinity of ions or in clathrate-like environments. Additionally, theoretical frameworks such as ab initio calculations, classical molecular dynamics, Monte Carlo simulations, QM/MM approaches, and density functional theory play a crucial role in modeling these phenomena at the molecular level.32–39
While previous studies have revealed structural anomalies in water near 43 °C and 60 °C, linked to changes in dipolarity, hydrogen bonding, and dielectric behavior, this work uncovers a new anomalous behavior of water occurring between 40 and 60 °C, evidenced through integrated spectroscopic and thermodynamic analyses. Unlike earlier investigations that focused on pure water or simple aqueous systems, this work highlights how specific additives (e.g., α-CD, urea, PVA, and β-CD + [C4mim]PF6) modulate this anomaly, revealing a tuneable structural transformation and turning point behavior in the interaction profile of water. By employing advanced tools, such as 2DCoS of NIR spectra, PCA, and DLS, it has been demonstrated that a complex, multi-component restructuring of the hydrogen-bonding network directly impacts the macroscopic properties of water. This multi-technique, multi-additive approach provides a more nuanced and application-relevant understanding of the structural dichotomy of water, with important implications for material design, biological systems, and pharmaceutical processes where water is the dominant solvent.
2. Methods and instrumentation
α-CD, β-CD, and [C4mim]PF6 were purchased from Sigma-Aldrich. PVA and urea were purchased from Merck. All aqueous solutions were prepared using ultrapure water (specific conductance = 0.055 μS cm−1), supplied by a BOECO Pure system (Model BOE 8082060, Germany). Binary mixtures were gravimetrically prepared using a high-precision analytical balance (Unilab UB-110, accuracy ±0.0001 g). After mixing, the samples were sonicated for 30 minutes using a LU-2 ultrasonic cleaner (Labnics Equipment) to ensure uniform dispersion. Dynamic light scattering was employed to assess the particle size distribution of the mixtures using a Zetasizer Nano ZS90 (ZEN3690, Malvern Instruments Ltd., UK). A 632.8 nm He–Ne laser served as the light source, and measurements were recorded at a 90° scattering angle. The scattering data were processed to determine the hydrodynamic diameters. A square glass cell with a circular aperture was used. The instrument was validated using a polystyrene latex standard for calibration, and the stability and reproducibility of the measurements were confirmed. The accuracy of the hydrodynamic diameter determined by DLS measurements is ±2%. All DLS measurements were conducted at temperature-controlled conditions using Peltier elements, and samples were equilibrated at each target temperature for 30 minutes prior to data collection. Density measurements were performed on an Anton Paar vibrating tube densitometer (Model DMA 4500), which utilizes the oscillating U-tube method. A 3.0 mL sample was loaded into a U-shaped borosilicate tube, whose resonant frequency, altered by sample density, was measured and converted into density values. The method provides a precision of ±0.00005 g cm−3 and repeatability of ±0.00001 g cm−3. The performance of the instrument was validated using deionized water and calibration against certified liquid density standards. Specific gravities were then calculated based on the measured solution and pure water densities at each temperature. Near-infrared spectra were acquired using a Frontier FT-IR/NIR spectrophotometer (PerkinElmer, USA) in absorbance mode. Each spectrum was averaged over 20 scans in the range of 4000–12000 cm−1 with a spectral resolution of 4.0 cm−1. The validation of the instrument was confirmed using a certified polystyrene film. The NIR samples were analyzed using a high-sensitivity, heatable liquid sampling cell fitted with CaF2 windows (Specac Model GS20522, 0.1 mm path length), which was maintained using polytetrafluoroethylene spacers. Temperature control was achieved through an electric heating jacket (Specac Model GS20730), with temperature stabilization supported by circulating chilled water around the jacket. The PCA was employed to reduce the dimensionality of the NIR spectral dataset and to extract the most significant variations associated with temperature changes in the binary mixtures. Pre-processed absorption spectra in the range of 4000–6000 cm−1 were mean-centered prior to PCA. The first few principal components (typically PC1 and PC2) were analyzed to interpret the temperature-induced changes in hydrogen bonding environments. Loading plots and scores plots were examined to identify the temperature range showing maximal spectral variance, corresponding to potential pseudo-phase transitions. The 2DCoS was performed to elucidate the sequential changes in spectral features under the influence of temperature. The generalized 2D correlation maps were generated using the Noda algorithm, with temperature as the perturbation variable. The spectra were uniformly spaced over the 20–70 °C range and smoothed using a Savitzky–Golay filter (3rd order polynomial, 7-point window). The resulting correlation maps enabled the identification of correlated and sequential spectral events, particularly within the –OH and combination band regions (∼4000–6000 cm−1). 2D correlation spectra revealed co-variations in hydrogen-bonded species and indicated the order of thermal events, distinguishing between progressive shifts and abrupt changes. PCA and 2DCoS analysis of the NIR spectra were performed using MATLAB R2016a (The MathWorks Inc.).3. Results and discussion
Since the binary mixtures of additives (α-CD, β-CD, [C4mim]PF6, PVA, and urea) with water are hydrogen bonded throughout, the association between the additives, additives and water, and water itself may be expected. Thus, the sizes of the species in the binary mixtures were first measured using DLS at several temperatures (cf. Fig. S1, ESI†). The data for the hydrodynamic diameters of the clusters formed in 2.0 mM α-CD, aqueous PVA, and 2.0 M urea are shown in Tables S2−S4.† The measurements of mixtures containing α-CD, PVA, and urea show a sudden decrease in particle size from the 200 to 700 nm range to a narrower 100 to 200 nm range, accompanied by a sharp increase in the intensity of scattered light and polydispersity index of the distribution above 40 °C. For the β-CD-[C4mim]PF6 inclusion complex, this change was observed at a higher temperature (60 °C), as shown in Fig. 1, indicating that the thermal stability of the complex itself eclipsed the change in the molecular environment of bulk water. The data for the hydrodynamic diameter of the inclusion complex clusters are shown in Table S5.† | ||
| Fig. 1 Size distribution of the β-CD-[C4mim]PF6 inclusion complex in water at different temperatures. | ||
From the results obtained from DLS measurements, it may be assumed that the physical properties of these systems are susceptible to change at higher temperatures (40−60 °C) due to the sharp change in size.
The specific gravity calculations based on the densities of aqueous α-CD revealed a subtle change at or above 40 °C, consistent with the particle size analysis. The specific gravity was found to steadily decline as the temperature increased from 20 °C, followed by a sharp drop at 40 or 50 °C, and then a steady decline yet again, as shown in Fig. 2. However, temperature-dependent density measurements of several concentrations of aqueous α-CD (cf. Fig. S2, ESI†) showed no particular atypical trend. The specific gravity of other aqueous solutions of hydrogen-bonded species, such as urea (cf. Fig. S3, ESI†), yielded mixed results, suggesting that aggregation of the additives may have affected the specific gravity.
The size and specific gravity results demonstrate the effect of structural changes in this temperature region, i.e., the weakening/breaking of hydrogen bonds to discourage association. This finding supports the shift of the structure from an ice-like to a vapor-like phase.
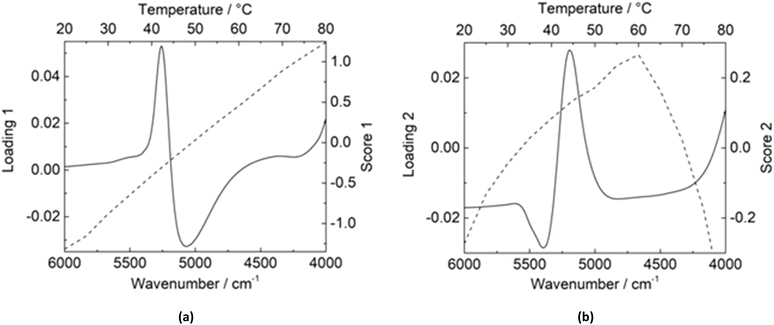
All three binary mixtures exhibit hydrogen bonding, and it may be assumed that the change at 40 °C may be a result of a change in the hydrogen bonding in the liquid mixtures. To support this claim, NIR spectroscopic measurements were performed (cf. Fig. S4, ESI†), and the data were further analyzed to obtain some interesting results. Fig. 3 shows the results of PCA of the NIR spectra of 2.0 mM α-CD.
The first principal component (PC1) accounted for 93.54% of the total spectral variance and exhibited a linear increase in score, which represents the overall changes in wavenumber with temperature. The loading indicated an increasing concentration of weaker hydrogen bonds (positive peak at 5260 cm−1) and a decreasing concentration of stronger ones (negative peak at 5058 cm−1). The second principal component (PC2) accounted for 6.16%. For PC2, a maximum score at 60 °C indicates a second, less significant change occurring at this temperature, related to the change in the moderately strong hydrogen bonds (positive peak at 5190 cm−1).
2DCoS analysis of the NIR data for 2.0 mM α-CD was performed in separate temperature regions. The synchronous spectra, i.e., the overall similarity or coincidental trends between two separate intensity variations measured at different temperatures, exhibit a strong autopeak at 5260 cm−1, which indicates the local reorientational motions of functional groups (cf. Fig. S5, ESI†). This indicates an alteration, like bending and asymmetric stretching, resulting in intensity change to a great extent with increasing temperature; however, the autopeak is visibly weaker for the higher temperature region, suggesting that the overall extent of spectral intensity variation is significantly higher between 20 and 40 °C than between 40 and 60 °C. NIR spectra were recorded to observe this phenomenon (cf. Fig. S6, ESI†). The disparity was even more pronounced for 0.5 M urea, with a strong autopeak for 20–40 °C and weak cross-peaks overall (cf. Fig. S7, ESI†). The synchronous spectra for both binary mixtures are shown in Fig. 4.
 | ||
| Fig. 4 3D representation of the synchronous spectra from 2DCoS analysis using NIR data for 2.0 mM α-CD (top) and 0.5 M urea (bottom) in different temperature ranges. | ||
This spectral variation can be attributed to a decrease in the concentration of strong hydrogen bonds and a corresponding increase in weaker hydrogen bonds in the system, as seen in PC1. The change was even less above 60 °C, and it may be concluded that the majority of the change occurs below 60 °C. This contradicts the assumption from the DLS and specific gravity results that the change is abrupt. Instead, we may now assume that the structural changes are built up as the temperature increases, and at a threshold temperature range, the transition occurs.
The results of the DLS and specific gravity measurements indicate a clear structural change occurring in this temperature range, and the spectroscopic analysis revealed the buildup of this change. The sharp changes in the parameters within a narrow temperature range led to the assumption that a pseudo-phase transition occurs between 40 and 60 °C, where the structure of water is ice-like, i.e., more extensively hydrogen-bonded and low density at sub-40 °C temperatures and vapor-like, i.e., less extensively hydrogen-bonded and high density at high temperatures.
Collectively, the spectroscopic and thermophysical analyses point to a distinct threshold temperature range of 40–60 °C, across which water exhibits a dichotomous structural behavior rather than a continuous or merely anomalous change. Below this range, the spectral features and density data suggest a dominance of a low-density, extensively hydrogen-bonded structure, resembling that of supercooled or ice-like water. Above the threshold, a sharp shift toward a high-density, less hydrogen-bonded structure, more vapor-like, becomes evident. This structural duality is not a gradual transformation but rather a clear bifurcation, with a sharp crossover occurring near the midpoint of the threshold range, typically between 40−45 °C, 45–50 °C, 50−55 °C, or 55−60 °C, depending on the solute and interaction environment. The PCA loading profiles and 2DCoS synchronous spectra reveal a pronounced buildup of spectral variation below the threshold, followed by relative spectral stabilization above it, which supports the hypothesis of a pseudo-phase transition. Therefore, the combined evidence strongly supports the view that water does not exhibit merely progressive or anomalous behavior in this temperature regime, but rather a dichotomous change in structure, characterized by a short, well-defined threshold zone where the transition occurs. The pseudo-phase transition region may experience expansion or contraction depending on pressure, and future research may be directed at unveiling this phenomenon.
4. Simply anomalous, progressive change, or dichotomous behavior of water?
The behavior of water in the 40–60 °C temperature range could be considered “anomalous” or interpreted as a continuous, progressive structural transition. However, our investigation provides compelling evidence that the changes observed in this temperature window represent a dichotomous behavior rather than a simple anomaly or gradual shift. Traditionally, anomalous behavior in water refers to deviations from classical liquid behavior, such as the density maximum at 4 °C or the increase in compressibility with temperature. These phenomena are typically smooth and continuous, and are well-explained by models involving the gradual weakening of hydrogen bonds. However, in the present study, temperature-dependent particle size analysis and physicochemical measurements of water in binary mixtures with diverse additives (α-CD, urea, PVA, and β-CD-[C4mim]PF6) reveal an abrupt reorganization of structure around 50 °C. This change is not additive-dependent, indicating an intrinsic reconfiguration of the water network itself.The DLS data show a clear bifurcation in particle size across this range, with distinct clusters below and above 50 °C, supporting a two-state model rather than a continuum of configurations. The findings from the DLS study of this work are significantly complemented by the molecular-level insights provided in the referenced work on CD hydration.40 Molecular dynamics simulations reveal that water molecules confined within the CD cavity are energetically frustrated due to a loss of hydrogen bonding and increased orientational freedom, making their expulsion into bulk water thermodynamically favourable. This expulsion process aligns well with the structural transitions captured in the DLS measurements of this study, where a marked shift in particle size and hydration behavior is observed across the 40–60 °C range. Such changes likely reflect the release of cavity-confined water and reorganization of the hydration shell, underscoring a cooperative restructuring event. These combined observations lend strong support to our interpretation of a dichotomous structural behavior in water, driven in part by solute-induced perturbations in the hydrogen-bonding network of water. Similarly, specific gravity exhibits a noticeable inflection point that correlates with the spectral transitions observed in NIR spectra. The application of PCA and 2DCoS further substantiates this dichotomy. 2D correlation spectra display significant shifts in band assignments across the 40–60 °C window, with clear differences in spectral features below and above 50 °C. A parabolic shape in the second principal component (PC2) is often observed due to the residual curvature from linear spectral changes, as it captures minor yet structured deviations.41 However, this study goes beyond a simple statistical observation by integrating multiple orthogonal techniques, including 2DCoS, DLS, and thermodynamic analysis, to confirm that the change is not merely a statistical maximum but reflects a true physicochemical transformation in the structure of water within the 40–60 °C range. The dichotomous behavior reported is not solely based on the second principal component of PCA, but is supported by the iso-scattering points in DLS measurements, indicating structural reorganization at the nanoscale. Additionally, this is supported by consistent turning points across NIR spectroscopic signatures and their 2D correlation maps, implying coordinated molecular-level rearrangement and systematic shifts in response to additive inclusion, such as α-CD, urea, and [C4mim]PF6, highlighting an interplay between the water structure and solute-specific interactions. Unlike prior works, which are limited to pure water, the findings of this study show that the position and character of the turning point depend on the interactions of additives with water, suggesting an underlying structural duality or dichotomy in the hydrogen-bonded network of water that becomes more or less prominent depending on the applied perturbation. This behavior is not trivial, nor is it simply the expected response of a second virial coefficient or an artifact of PCA curvature. Instead, it demonstrates a robust, tuneable structural shift in water, consistent with the broader literature reporting thermal anomalies in the dielectric constant, compressibility, and density in this range.
The complexity introduced by solutes in interpreting hydrogen-bonding dynamics in aqueous systems is an important issue in this study. Indeed, the presence of additives introduces competing hydrogen-bonding interactions between water molecules and solute functional groups, and among water molecules themselves, which cannot be trivially deconvoluted. However, this study does not aim to isolate only water–water interactions but rather to explore how the hydrogen-bonding environment of water as a whole is perturbed by solutes across a specific temperature window (40–60 °C). A consistent structural and dynamic transition has been observed in this investigation. While Segtnan et al. effectively applied 2DCoS and PCA to analyse pure water spectra, the approach of this study extends such chemometric tools to complex aqueous systems.41 The inherent challenges are acknowledged, but the concerted spectral, DLS, and PCA evidence from this study still supports the existence of a thermally induced dichotomous shift in the hydrogen-bonding behavior of water, modulated by solute-induced constraints and cavity effects. These findings contribute complementary insight into the broader understanding of aqueous structural transitions under perturbation. Importantly, the features that do not evolve smoothly but rather indicate a discrete transition in dominant structural motifs, likely reflecting a shift from more tetrahedral, low-density water structures to distorted, high-density arrangements, confirm the dichotomy of water at this particular temperature window. Therefore, the evidence does not support a merely anomalous or progressively changing behavior. Instead, the collective data point to a pseudo-phase-transition-like turnover between two structurally distinct states of water, qualifying this phenomenon as dichotomous. This recognition is critical for understanding the behavior of water in chemical, biological, and nanotechnological contexts where such discrete structural changes may influence the reaction kinetics, solvation dynamics, or biomolecular stability. After all, the integration of spectroscopic, thermodynamic, and scattering data across diverse solute systems provides a cohesive and compelling narrative for a distinct, thermally driven structural bifurcation in water. Rather than representing a smooth anomaly or simple additive effect, the observed behavior reflects an intrinsic, cooperative reorganization of the hydrogen-bonding network, indicative of a dichotomous transition. This insight not only enriches the fundamental understanding of water structure but also has significant implications for aqueous-phase processes in chemical, biological, and material systems.
Understanding the dichotomous behavior of water in the 40–60 °C range opens up transformative opportunities across multiple disciplines. In biological systems, this temperature window overlaps with many physiological and pathological processes. The sharp structural transition of water, shifting from a more extensively hydrogen-bonded low-density state to a less structured, high-density state, can influence protein folding, enzyme activity, and biomolecular interactions, which are all highly sensitive to the hydration environment.42–44 Consequently, this behavior is highly relevant to fields like biophysics, bioimaging, and nanomedicine, where temperature-controlled behavior of water affects molecular function and transport.19,45,46 For instance, the design of thermoresponsive drug delivery systems or tumour-targeting nanomaterials can benefit from exploiting the pseudo-phase transition behavior of water near hyperthermic treatment temperatures (∼43–45 °C).47 This special characteristic of water at this temperature range provides a mechanistic link to water structure modification that may be useful in fields like cryobiology, pharmaceutical formulation, or materials design. In materials science, this phenomenon could be applied to create phase-sensitive smart hydrogels, soft actuators, or self-assembling nanostructures that rely on the responsive structural change of water to temperature stimuli.48–50 It may also play a role in the thermal performance of nanofluids, where water serves as the medium and its structural dynamics influence the energy transfer mechanisms.51 Furthermore, insights into the pseudo-phase transition provide a basis for enhancing spectroscopic sensors or plasmonic materials, especially where water–nanoparticle interactions are temperature dependent.52 From a theoretical and fundamental science viewpoint, this study supports the idea that water cannot be represented by a single homogeneous phase but rather by coexisting structural motifs—such as low-density and high-density liquid water—that interconvert at specific thresholds.53–55 This invites further investigation using molecular dynamics simulations, quantum mechanical models, and advanced time-resolved spectroscopic techniques to uncover the kinetics and energetics behind this transition. Future work may also explore the effects of pressure, pH, and solute identity on shifting or modulating this threshold, thereby tailoring the structure of water for specific applications.18,56,57 Ultimately, redefining water not as a passive medium but as an active, tuneable participant in chemical and biological processes could reshape how aqueous systems are studied and engineered.
5. Conclusion
This study shows evidence of two different features of water – one below 40 °C and one above 60 °C – with a turnover point somewhere in between, depending on the conditions. The dichotomous behavior of water at this temperature range in the presence of four distinct additives, such as α-CD, urea, PVA, and the inclusion complex of β-CD and an ionic liquid, [C4mim]PF6, is shown for the first time based on the changes in particle size, specific gravity, principal component analysis, and 2D correlation spectroscopic analysis. These additives vary significantly in chemical structure and interactions, yet all exhibit a common temperature-dependent restructuring of water, indicating that this phenomenon is inherent to water itself rather than specific to any solute. The spectral and physical property changes display a nonlinear, threshold-dependent characteristic that cannot be reconciled with a simple anomalous or progressive temperature effect. Instead, the findings support the existence of a pseudo-phase transition, demarcating two structurally distinct states of water with a narrow intermediate region acting as a switch point. This dichotomous nature may be attributed to a shift in the relative populations of low- and high-density water motifs, aligning with current theories on the dual structural components of liquid water. Given the relevance of this temperature window to biological and material systems, these findings offer new perspectives on the role of water as an active, tuneable medium and provide a foundation for the rational design of temperature-responsive aqueous systems in fields ranging from biophysics to nanotechnology. Future investigations into the pressure dependence, time-resolved dynamics, and molecular-level origin of this behavior will be instrumental in deepening our understanding of this subtle but fundamental feature of water. However, this particular phenomenon remains unexplained. It does not have the definitive physicochemical markers of a phase transition as observed in supercooled water, yet derivative studies of physicochemical properties and spectroscopic studies show evidence of dichotomous behavior that cannot be ignored. The temperature range identified coincides with conditions relevant to cellular processes and soft matter systems, underscoring the importance of this structural transition in functional environments. Testable hypotheses, such as pressure-temperature coupling and molecular probe validation, can be proposed from this finding. High-pressure NIR and DLS studies can be conducted to test whether applying hydrostatic pressure shifts the observed transition temperature, thereby confirming the pressure dependence of the structural duality of water and distinguishing it from mere thermal perturbation. Also, solvatochromic or hydrogen-bond-sensitive fluorescent probes can be introduced into the aqueous systems studied here to detect real-time microenvironmental changes across the critical temperature window, providing molecular-level insight into the restructuring process. Future work in these directions will be crucial for elucidating the mechanistic origin and broader applicability of this phenomenon. While the dichotomous behavior lacks the definitive discontinuities of a classical phase transition (as seen in supercooled water), the convergent evidence from multiple analytical techniques clarifies that a fundamental reorganization occurs, one that demands closer scrutiny and could redefine how we understand the behavior of water under thermal stress.Data availability
Data supporting this article have been included as part of the ESI.† Additional data are available upon request.Author contributions
Amiya Atahar: conducting experiments, data interpretation, analysis, writing – original draft preparation; Noushaba Nusrat Mafy: data interpretation, analysis, writing, editing; Mohammad Hossain: data interpretation, analysis, writing, reviewing and editing; Md. Abu Bin Hasan Susan: conceptualization, writing – reviewing and editing, supervision, funding acquisition, resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial assistance provided by the DU-UGC research grant, Bangladesh, for supporting this themed research. Additional funding from the Bose Centre for Advanced Study and Research in Natural Sciences is also sincerely appreciated.References
- K. Stokely, M. G. Mazza, H. Eugene Stanley and G. Franzese, Effect of hydrogen bond cooperativity on the behavior of water, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1301–1306 CrossRef CAS PubMed.
- C. A. Angell and V. Rodgers, Near infrared spectra and the disrupted network model of normal and supercooled water, J. Chem. Phys., 1983, 80, 6245–6252 CrossRef.
- T. D. Kühne and R. Z. Khaliullin, Electronic signature of the instantaneous asymmetry in the first coordination shell of liquid water, Nat. Commun., 2013, 4, 1–7 Search PubMed.
- R. Ludwig, Water: from clusters to the bulk, Angew. Chem., Int. Ed., 2001, 40, 1808–1827 CrossRef CAS PubMed.
- Y. Zubavicus and M. Grunze, New insights into the structure of water with ultrafast probes, Science, 2004, 304, 974–976 CrossRef CAS PubMed.
- P. Gallo, K. Amann-Winkel, C. A. Angell, M. A. Anisimov, F. Caupin, C. Chakravarty, E. Lascaris, T. Loerting, A. Z. Panagiotopoulos, J. Russo, J. A. Sellberg, H. E. Stanley, H. Tanaka, C. Vega, L. Xu and L. G. M. Pettersson, Water: a tale of two liquids, Chem. Rev., 2016, 116, 7463–7500 CrossRef CAS PubMed.
- A. Priyadarshini, A. Biswas, D. Chakraborty and B. S. Mallik, Structural and thermophysical anomalies of liquid water: a tale of molecules in the instantaneous low- and high-density regions, J. Phys. Chem. B, 2020, 124, 1071–1081 CrossRef CAS PubMed.
- H. E. Stanley and J. Teixeira, Interpretation of the unusual behavior of H2O and D2O at low temperatures: tests of a percolation model, J. Chem. Phys., 1980, 73, 3404–3422 CrossRef CAS.
- L. Han, Y. Sun, W. Cai and X. Shao, Seeking the structure of water from the combination of bending and stretching vibrations in near infrared spectra, J. Near Infrared Spectrosc., 2023, 31, 204–210 CrossRef CAS.
- M. Wang, Y. Sun, C. Duan, W. Cai and X. Shao, Investigating the water structures in reverse micelles by temperature-dependent near infrared spectroscopy combined with independent component analysis, J. Near Infrared Spectrosc., 2022, 30, 154–159 CrossRef CAS.
- Y. Kato, J. Munćan, R. Tsenkova, D. Kojić, M. Yasui, J.-Y. Fan and J.-Y. Han, Aquaphotomics reveals subtle differences between natural mineral, processed and aged water using temperature perturbation near-infrared spectroscopy, Appl. Sci., 2021, 11, 9337 CrossRef CAS.
- T. Begušić and G. A. Blake, Two-dimensional infrared-Raman spectroscopy as a probe of water's tetrahedrality, Nat. Commun., 2023, 14, 1950 CrossRef PubMed.
- I. Noda and Y. Ozaki, Two-Dimensional Correlation Spectroscopy – Applications in Vibrational and Optical Spectroscopy, 2005 Search PubMed.
- V. H. Segtnan, T. Isaksson and Y. Ozaki, Studies on the structure of water using spectroscopy and principal component analysis, Anal. Chem., 2001, 73, 3153–3161 CrossRef CAS PubMed.
- B. Czarnik-Matusewicz and S. Pilorz, Study of the temperature-dependent near-infrared spectra of water by two-dimensional correlation spectroscopy and principal components analysis, Vib. Spectrosc., 2006, 40, 235–245 CrossRef CAS.
- C. Huang, K. T. Wikfeldt, T. Tokushima, D. Nordlund, Y. Harada, U. Bergmann, M. Niebuhr, T. M. Weiss, Y. Horikawa, M. Leetmaa, M. P. Ljungberg, O. Takahashi, A. Lenz, L. Ojamäe, A. P. Lyubartsev, S. Shin, L. G. M. Pettersson and A. Nilsson, The inhomogeneous structure of water at ambient conditions, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15214–15218 CrossRef CAS PubMed.
- M. C. Symons, Water structure and hydration, Philos. Trans. R. Soc. London, B, 1975, 272, 13–28 CAS.
- M. Hossain, N. Chowdhury, A. Atahar and M. A. B. H. Susan, Water structure modification by D-(+)-glucose at different concentrations and temperatures-effect of mutarotation, RSC Adv., 2023, 13, 19195–19206 RSC.
- L. M. Maestro, M. I. Marqués, E. Camarillo, D. Jaque, J. García Solé, J. A. Gonzalo, F. Jaque, J. C. Del Valle, F. Mallamace and H. E. Stanley, On the existence of two states in liquid water: impact on biological and nanoscopic systems, Int. J. Nanotechnol., 2016, 13, 667–677 CrossRef CAS.
- J. C. Del Valle, C. Aragó, M. I. Marqués and J. A. Gonzalo, Paraelectric response of water in the range 0–100 °C, Ferroelectrics, 2014, 466, 166–180 CrossRef CAS.
- J. C. Del Valle, E. Camarillo, L. M. Maestro, J. A. Gonzalo, C. Aragó, M. Marqués, D. Jaque, G. Lifante, J. G. Solé, K. Santacruz-Gómez, R. C. Carrillo-Torres and F. Jaque, Dielectric anomalous response of water at 60 °C, Philos. Mag., 2015, 95, 683–690 CrossRef CAS.
- J. C. Del Valle, E. Camarillo, L. Martinez Maestro, J. A. Gonzalo, C. Aragó, M. Marqués, D. Jaque, G. Lifante, J. G. Solé and K. Santacruz-Gómez, Dielectric anomalous response of water at 60 °C, Philos. Mag., 2015, 95, 683–690 CrossRef CAS.
- J. Catalán and J. A. Gonzalo, Liquid water changes its structure at 43 °C, Chem. Phys. Lett., 2017, 679, 86–89 CrossRef.
- H. Okajima, M. Ando and H. O. Hamaguchi, Formation of ‘nano-Ice’ and density maximum anomaly of water, Bull. Chem. Soc. Jpn., 2018, 91, 991–997 CrossRef CAS.
- L. Labrador-Páez, C. Mingoes, F. Jaque, P. Haro-González, H. Bazin, J. M. Zwier, D. Jaque and N. Hildebrandt, pH dependence of water anomaly temperature investigated by Eu(III) cryptate luminescence, Anal. Bioanal. Chem., 2020, 412, 73–80 CrossRef PubMed.
- M. Hossain and M. A. B. H. Susan, Revealing water structure modification by a sodium-glucose cotransporter-2 inhibitor type antidiabetic drug with D-(+)-glucose in aqueous media, Phys. Chem. Chem. Phys., 2025, 27, 10399–10412 RSC.
- A. Atahar, N. N. Mafy, M. M. Rahman, M. Y. A. Mollah and M. A. B. H. Susan, Aggregation of urea in water: dynamic light scattering analyses, J. Mol. Liq., 2019, 294, 111612 CrossRef CAS.
- T. Afrin, N. N. Mafy, M. M. Rahman, M. Y. A. Mollah and M. A. B. H. Susan, Temperature perturbed water structure modification by D(-)-fructose at different concentrations, RSC Adv., 2014, 4, 50906–50913 RSC.
- N. N. Mafy, T. Afrin, M. M. Rahman, M. Y. A. Mollah and M. A. B. H. Susan, Effect of temperature perturbation on hydrogen bonding in aqueous solutions of different urea concentrations, RSC Adv., 2015, 5, 59263–59272 RSC.
- M. T. I. Mredha, C. K. Roy, M. M. Rahman, M. Y. A. Mollah and M. A. B. H. Susan, An electrochemical approach to study water-D(-)fructose interactions, Electrochim. Acta, 2013, 97, 231–237 CrossRef CAS.
- S. Mallick and M. Murugesan, Hydrogen bond dynamics in aqueous malonamide system: an experimental and theoretical approach, J. Mol. Liq., 2020, 301, 112446 CrossRef CAS.
- F. Böhm, G. Schwaab and M. Havenith, Mapping hydration water around alcohol chains by THz calorimetry, Angew. Chem., Int. Ed., 2017, 56, 9981–9985 CrossRef PubMed.
- H. Vondracek, S. Imoto, L. Knake, G. Schwaab, D. Marx and M. Havenith, Hydrogen-bonding in liquid water at multikilobar pressures, J. Phys. Chem. B, 2019, 123, 7748–7753 CrossRef CAS PubMed.
- G. Schwaab, F. Sebastiani and M. Havenith, Ion hydration and ion pairing as probed by THz spectroscopy, Angew. Chem., Int. Ed., 2019, 58, 3000–3013 CrossRef CAS PubMed.
- H. Vondracek, S. Alfarano, C. Hoberg, I. Kolling, F. Novelli, F. Sebastiani, J.-B. Brubach, P. Roy, G. Schwaab and M. Havenith, Urea's match in the hydrogen-bond network? A high pressure THz study, Biophys. Chem., 2019, 254, 106240 CrossRef CAS PubMed.
- F. Sebastiani, T. A. Bender, S. Pezzotti, W.-L. Li, G. Schwaab, R. G. Bergman, K. N. Raymond, F. D. Toste, T. Head-Gordon and M. Havenith, An isolated water droplet in the aqueous solution of a supramolecular tetrahedral cage, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 32954–32961 CrossRef CAS PubMed.
- F. Novelli, B. Guchhait and M. Havenith, Towards intense THz spectroscopy on water: characterization of optical rectification by GaP, OH1, and DSTMS at OPA wavelengths, Materials, 2020, 13, 1311 CrossRef CAS PubMed.
- F. Sebastiani, C. Y. Ma, S. Funke, A. Bäumer, D. Decka, C. Hoberg, A. Esser, H. Forbert, G. Schwaab and D. Marx, Probing local electrostatics of glycine in aqueous solution by THz spectroscopy, Angew. Chem., Int. Ed., 2021, 60, 3768–3772 CrossRef CAS PubMed.
- C. Millon, J. Schmidt, S. Ramos, E. P. Van Dam, A. Buchmann, C. Saraceno and F. Novelli, Temperature-independent non-linear terahertz transmission by liquid water, AIP Adv., 2022, 12, 115319–115325 CrossRef.
- A. A. Sandilya, U. Natarajan and M. H. Priya, Molecular view into the cyclodextrin cavity: structure and hydration, ACS Omega, 2020, 5, 25655–25667 CrossRef CAS PubMed.
- V. H. Segtnan, Š. Šašić, T. Isaksson and Y. Ozaki, Studies on the structure of water using two-dimensional near-infrared correlation spectroscopy and principal component analysis, Anal. Chem., 2001, 73, 3153–3161 CrossRef CAS PubMed.
- M. Chaplin, Do we underestimate the importance of water in cell biology?, Nat. Rev. Mol. Cell Biol., 2006, 7, 861–866 CrossRef CAS PubMed.
- P. Gallo, K. Amann-Winkel, C. A. Angell, M. A. Anisimov, F. Caupin, C. Chakravarty, E. Lascaris, T. Loerting, A. Z. Panagiotopoulos and J. Russo, Water: a tale of two liquids, Chem. Rev., 2016, 116, 7463–7500 CrossRef CAS PubMed.
- F. Mallamace, C. Corsaro, M. Broccio, C. Branca, N. González-Segredo, J. Spooren, S.-H. Chen and H. E. Stanley, NMR evidence of a sharp change in a measure of local order in deeply supercooled confined water, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 12725–12729 CrossRef CAS PubMed.
- J. C. Del Valle, C. Aragó, M. I. Marqués and J. A. Gonzalo, Paraelectric response of water in the range 0–100 °C, Ferroelectrics, 2014, 466, 166–180 CrossRef CAS.
- L. Labrador-Páez, C. Mingoes, F. Jaque, P. Haro-González, H. Bazin, J. M. Zwier, D. Jaque and N. Hildebrandt, pH dependence of water anomaly temperature investigated by Eu(III) cryptate luminescence, Anal. Bioanal. Chem., 2020, 412, 73–80 CrossRef PubMed.
- D. Jaque, L. M. Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. M. Rodríguez and J. G. Solé, Nanoparticles for photothermal therapies, Nanoscale, 2014, 6, 9494–9530 RSC.
- J. Kopeček and J. Yang, Hydrogels as smart biomaterials, Polym. Int., 2007, 56, 1078–1098 CrossRef.
- B. Jeong, Y. H. Bae, D. S. Lee and S. W. Kim, Biodegradable block copolymers as injectable drug-delivery systems, Nature, 1997, 388, 860–862 CrossRef CAS PubMed.
- M. T. I. Mredha, C. K. Roy, M. M. Rahman, M. Y. A. Mollah and M. A. B. H. Susan, An electrochemical approach to study water–D(−) fructose interactions, Electrochim. Acta, 2013, 97, 231–237 CrossRef CAS.
- A. Koomer, Formulation of NPDDS for gene delivery, in Drug Delivery Nanoparticles Formulation and Characterization, 2016, vol. 191, p. 51 Search PubMed.
- L. Martinez Maestro, J. C. del Valle, E. Camarillo, J. A. Gonzalo, C. Aragó, M. Marqués, D. Jaque, G. Lifante, J. García Solé and K. Santacruz-Gómez, Dielectric anomalous response of water at 60 °C, Philos. Mag., 2015, 95, 6983–6990 Search PubMed.
- C. Huang, K. T. Wikfeldt, T. Tokushima, D. Nordlund, Y. Harada, U. Bergmann, M. Niebuhr, T. M. Weiss, Y. Horikawa and M. Leetmaa, The inhomogeneous structure of water at ambient conditions, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15214–15218 CrossRef CAS PubMed.
- H. E. Stanley and J. Teixeira, Interpretation of the unusual behavior of H2O and D2O at low temperatures: tests of a percolation model, J. Chem. Phys., 1980, 73, 3404–3422 CrossRef CAS.
- J. Catalán and J. A. Gonzalo, Liquid water changes its structure at 43 °C, Chem. Phys. Lett., 2017, 679, 86–89 CrossRef.
- N. N. Mafy, T. Afrin, M. M. Rahman, M. Y. A. Mollah and M. A. B. H. Susan, Effect of temperature perturbation on hydrogen bonding in aqueous solutions of different urea concentrations, RSC Adv., 2015, 5, 59263–59272 RSC.
- A. Priyadarshini, A. Biswas, D. Chakraborty and B. S. Mallik, Structural and thermophysical anomalies of liquid water: a tale of molecules in the instantaneous low-and high-density regions, J. Phys. Chem. B, 2020, 124, 1071–1081 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: DLS data, density data, specific gravity data, and synchronous spectra of α-CD and urea. See DOI: https://doi.org/10.1039/d5ra03224e |
| This journal is © The Royal Society of Chemistry 2025 |