Advancements in MOF-based resistive gas sensors: synthesis methods and applications for toxic gas detection†
Linh Ho Thuy Nguyen
ab,
Ali Mirzaei
c,
Jin-Young Kim
de,
Thang Bach Phan
 bf,
Lam Dai Tran
g,
Kevin C.-W. Wu
bf,
Lam Dai Tran
g,
Kevin C.-W. Wu
 hij,
Hyoun Woo Kim
*k,
Sang Sub Kim
hij,
Hyoun Woo Kim
*k,
Sang Sub Kim
 *e and
Tan Le Hoang Doan
*e and
Tan Le Hoang Doan
 *bf
*bf
aFaculty of Pharmacy, University of Health Sciences, Ho Chi Minh City 70000, Vietnam
bVietnam National University, Ho Chi Minh City 70000, Vietnam. E-mail: dlhtan@inomar.edu.vn
cDepartment of Materials Science and Engineering, Shiraz University of Technology, Shiraz 71557-13876, Iran
dThe Research Institute of Industrial Science, Hanyang University, Seoul, 04763, Republic of Korea
eDepartment of Materials Science and Engineering, Inha University, Incheon 22212, Republic of Korea. E-mail: sangsub@inha.ac.kr
fCenter for Innovative Materials and Architectures (INOMAR), Ho Chi Minh City 70000, Vietnam
gInstitute of Materials Science, Vietnam Academy of Science and Technology, Hanoi 11300, Vietnam
hMolecular Science and Technology Program, Taiwan International Graduate Program, Academia Sinica, Taipei 11529, Taiwan
iDepartment of Chemical Engineering and Materials Science, Yuan Ze University, Chung-Li, Taoyuan 32003, Taiwan
jDepartment of Chemical Engineering, National Taiwan University, Taipei 10617, Taiwan
kDivision of Materials Science and Engineering, Hanyang University, Seoul 04763, Republic of Korea. E-mail: hyounwoo@hanyang.ac.kr
First published on 20th March 2025
Abstract
Gas sensors are essential tools for safeguarding public health and safety because they allow the detection of hazardous gases. To advance gas-sensing technologies, novel sensing materials with distinct properties are needed. Metal–organic frameworks (MOFs) hold great potential because of their extensive surface areas, high porosity, unique chemical properties, and capabilities for preconcentration and molecular sieving. These attributes make MOFs highly suitable for designing and creating innovative resistive gas sensors. This review article examines resistive gas sensors made from pristine, doped, decorated, and composite MOFs. The first part of the review focuses on the synthesis strategies of MOFs, while the second part discusses MOF-based resistive gas sensors that operate based on changes in resistance.
Linh Ho Thuy Nguyen obtained her BS (2013) and MSc (2017) degrees from the University of Science, Vietnam National University, Ho Chi Minh City (VNU-HCM). She later earned her PhD from the same institution in 2023 and is now a lecturer at the Faculty of Pharmacy, University of Health Sciences, VNU-HCM. Her research interests include the synthesis, functionalization, and applications of metal–organic frameworks (MOFs). |
Ali Mirzaei received his PhD degree in Materials Science and Engineering from Shiraz University in 2016. He was a visiting student at Messina University, Italy, in 2015 and from 2016 to 2018, he was accepted as a postdoctoral fellow at Hanyang University in Seoul. He is the author or co-author of more than 130 peer-reviewed papers. Currently, he is an assistant professor of materials science and engineering at Shiraz University of Technology, Iran. He is interested in the synthesis and characterization of nanocomposites for gas sensing applications. |
Jin-Young Kim received his B.S. degree from Inha University, Republic of Korea, in 2017. He is currently working as an integrated course candidate at Inha University, Republic of Korea. He has been working on 1-dimensional oxide nanowires and 2-dimensional transition metal dichalcogenide gas sensors. |
Bach Thang Phan is an Assoc. Professor at Center for Innovative Materials and Architectures (INOMAR), a member of Vietnam National University in Ho Chi Minh city. He received his PhD degree from Sungkyunkwan University, Korea, in 2009. His research interests include the synthesis and applications of nanostructural materials for memristor, thermoelectric conversion, drug delivery, biosensors and gas sensors. |
Lam Dai Tran is currently the Director of the Institute for Tropical Technology, Vietnam Academy of Science and Technology (VAST). He graduated with a bachelor's degree in Solid and Semiconductor Chemistry from the Faculty of Chemistry, Belarusian State University, in 1994; and he received his PhD degree in Surfaces–Intersurfaces Chemistry from the University of Paris VII Denis Diderot (France) in 2003. He was appointed as Associate Professor of Chemistry in 2009 and as Professor of Chemistry in 2018. Currently, the main research directions of Prof. Tran are inorganic chemistry, nanomaterials and biomaterials. |
Kevin C.-W. Wu got his PhD degree at The University of Tokyo in 2005. After that, he was a post-doc at Waseda Univ. with Prof. Prof. Kazuyuki Kuroda in Japan and at Iowa State Univ. with Prof. Victor Lin in USA from 2005 to 2008. He is currently a professor at the Dept. Chem. Eng., National Taiwan University (NTU), Taiwan. He has published more than 160 international journal papers with a total citation over 7400 times (h index 46). In 2018, he was selected as a Humboldt scholar from Humboldt Foundation, Germany, and received an outstanding research award from the Ministry of Science and Technology (MOST), Taiwan. He is serving as an editor for three SCI journals (Advanced Powder Technology, Scientific Reports and Journal of the Taiwan Institute of Chemical Engineers) and is an editorial board member for two prestigious international journals (ACS Sustainable Chemistry & Engineering (ACS) and Applied Catalysis B: Environmental (Elsevier)). His research interests include the design and synthesis of functional nanoporous materials for sustainable chemistry and engineering applications including biomass conversion, membrane separation, biomedicine and energy devices. |
Hyoun Woo Kim joined the Division of Materials Science and Engineering at Hanyang University as a full professor in 2011. He received his B.S. and M. S. degrees from Seoul National University and his PhD degree from Massachusetts Institute of Technology (MIT) in electronic materials in 1986, 1988, and 1994, respectively. He was a senior researcher in Samsung Electronics Co., Ltd. from 1994 to 2000. He has been a professor of materials science and engineering at Inha University from 2000 to 2010. He was a visiting professor at the Department of Chemistry of Michigan State University, in 2009. His research interests include gas sensors. |
Sang Sub Kim joined the Department of Materials Science and Engineering, Inha University, in 2007 as a full professor. He received his B.S. degree from Seoul National University and his M.S and PhD degrees from Pohang University of Science and Technology (POSTECH) in Material Science and Engineering in 1987, 1990, and 1994, respectively. He was a visiting researcher at the National Research in Inorganic Materials (currently NIMS), Japan, for 2 years each in 1995 and in 2000. In 2006, he was a visiting professor at the Department of Chemistry, University of Alberta, Canada. In 2010, he also served as a cooperative professor at Nagaoka University of Technology, Japan. His research interests include the synthesis and applications of nanomaterials such as nanowires and nanofibers, functional thin films, and surface and interfacial characterization, for application in gas sensors. |
Tan Le Hoang Doan received his B.S. degree in honor program from the University of Science, Viet Nam National University – Ho Chi Minh City in 2009 and his PhD degree in Molecular and Nanoarchitecture program from Viet Nam National University – Ho Chi Minh City in 2017. He was a postdoctoral researcher at Kyoto University, Japan, in 2018. He is currently working as a senior researcher at Center for Innovative Materials and Architectures (INOMAR), Viet Nam National University – Ho Chi Minh City. His research interests include the design, synthesis, and applications of porous materials. |
1. Introduction to metal–organic frameworks (MOFs)
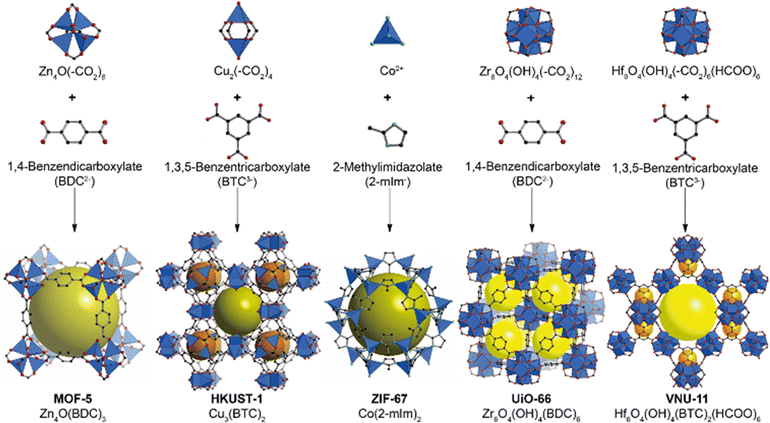
MOFs are defined by their three-dimensional porous networks, in which inorganic clusters are linked to organic linkers via coordination bonds. The flexibility and diversity in the design of these metal clusters and linkers enable the formation of a wide range of frameworks with varying structures, stability, properties, surface areas, and pore sizes (Fig. 1).1–3 These exceptional properties of MOFs have enabled their use in a wide range of applications, including clean energy development, greenhouse gas capture (e.g., CO2), selective molecular separation from contaminated mixtures, and heterogeneous catalysis in gas or liquid phases.4–7 Additionally, MOFs with pre-designed structures provide advantages over traditional porous materials and have been assessed for gas-sensing applications in environmental fields.8–11 MOFs exhibit a range of intriguing properties due to their unique structure and composition.12,13 Their porous structures have large surface areas, which makes them ideal for gas adsorption,14 storage,15 and sensing applications.16,17 MOFs can be designed with tuneable properties by adjusting their pore sizes, surface functionalities, and chemical reactivity through the selection of specific precursors during synthesis. This tunability also extends to their stability, which provides versatility for applications ranging from catalysis to gas separation.18–21Furthermore, the modular nature of MOFs facilitates the incorporation of various functional groups, which allows for customization for specific applications such as drug delivery in biomedical fields.22–26 Due to their high porosity and capacity for reversible structural changes, MOFs are promising candidates for sensing and controlled release systems.27–29 Their unique electronic and magnetic properties further extend their utility to fields such as electronics and magnetism.30,31 Consequently, the diverse and tunable properties of MOFs drive extensive research and innovation, establishing them as a cornerstone in materials science and engineering.
Gas sensors are essential for enhancing safety and awareness by detecting toxic and dangerous gases.32 While traditional gas sensing technologies are effective, there is an ongoing need for novel sensing materials that offer improved performance. Due to their porosity and distinctive chemical features, MOFs hold great promise for gas-sensing applications.33,34 MOFs' high surface areas and significant porosity enhance gas adsorption, which leads to measurable changes in electrical resistance. These sensors also benefit from the chemical tunability of MOFs, which allows for customization to detect specific gases by modification of their metal nodes and linkers. Advances in synthesis methods, such as solvothermal, microwave irradiation, and mechanochemical methods, have enabled the creation of MOFs with tailored properties for enhanced gas-sensing performance.35 The integration of MOFs into resistive sensors has expanded their applications to fields including industrial safety, environmental monitoring, and healthcare diagnostics.36 This review explores advancements in MOF-based resistive gas sensors, focussing on synthesis strategies and their performance in detecting various gases.
2. Synthesis of MOFs
MOF synthesis involves combining inorganic ions or clusters with organic bridges under controlled conditions to form a three-dimensional porous network. This process requires meticulous control over several critical parameters to tailor the structural and functional properties of the resulting MOFs.4,37,38 The choice of the metal precursor and organic ligand is fundamental, as it dictates the composition and properties of the MOF.39–41 The solvent and solvothermal conditions, including temperature and pressure, affect the thermodynamics and kinetics of the reaction, which alters crystal growth and morphology.42–45 Furthermore, maintaining optimal pH levels is essential to control the stability of metal ions and ligand deprotonation.46 The reaction temperature, duration, and concentration of reactants are crucial in determining the rate of MOF formation and the properties of the final product.47,48 Templates or modulator agents can influence crystal growth, while mechanochemical synthesis parameters, such as milling time and speed, affect energy input and MOF formation.49–51 Careful consideration and control of these parameters enable the fine-tuning of MOF synthesis, ensuring reproducibility and customization of diverse applications. Moreover, various synthesis methods offer distinct advantages and disadvantages, influencing the final properties of the material (Table 1).| Synthesis technique | Advantages | Disadvantages |
|---|---|---|
| Hydrothermal synthesis | – Controlled conditions result in well-defined MOFs | – Requires high-temperature and pressure equipment |
| – Enhanced crystallinity and purity | – Limited scalability | |
| – Versatile for various MOF compositions | ||
| Solvothermal synthesis | – Tunable synthesis conditions in organic solvents | – Solvent dependence and potential toxicity |
| – Flexible reaction parameters | – Longer reaction times compared to hydrothermal | |
| – Suitable for scalable production | ||
| Microwave synthesis | – Rapid heating reduces reaction times | – Equipment limitations for large-scale production |
| – Improved reproducibility | – Limited control over local temperature variations | |
| – Energy-efficient compared to conventional methods | ||
| Ionothermal synthesis | – Enhanced stability of MOFs | – Limited availability and cost of ionic liquids |
| – Unique properties due to ionic liquid medium | – Limited scalability | |
| – Potential for direct integration in devices | ||
| Pyrolysis synthesis | – Simple and energy-efficient | – Potential for MOF decomposition at high temperatures |
| – No need for solvents | – Limited control over morphology | |
| – Applicable under harsh conditions | ||
| Mechanochemical synthesis | – Green, solvent-free synthesis | – Limited control over reaction selectivity |
| – Short reaction times | – Potential for contamination from milling equipment | |
| – Scalable and energy-efficient | ||
| Electrochemical synthesis | – Direct integration onto conductive substrates | – Limited control over MOF size and morphology |
| – Accurate control over MOF properties | – Requires specific electrode materials and setups | |
| – Applicable in electrocatalysis and energy storage | ||
| Template-directed synthesis | – Controlled morphology and properties | – Template removal can be challenging |
| – Tailored MOFs for specific applications | – Limited versatility with some template systems | |
| – Enhanced reproducibility |
Commonly used synthesis techniques include hydrothermal, solvothermal, microwave, ionothermal, pyrolysis, mechanochemical, electrochemical, and template-directed synthesis (Fig. 2). Solvothermal and mechanochemical synthesis methods are among the most promising routes for industrial-scale production of MOFs. Solvothermal synthesis offers tunable reaction conditions and is already used for large-scale applications, while mechanochemical synthesis is a green, solvent-free approach with excellent scalability and energy efficiency. Microwave-assisted synthesis also shows potential due to its rapid reaction times and reproducibility, though the equipment limitations may affect large-scale implementation. The selection of a synthesis technique depends on the desired MOF features, scalability, and the intended application.42,52 Researchers are continually exploring innovative approaches to enhance the efficiency, reproducibility, and sustainability of MOF synthesis techniques.53,54
 | ||
| Fig. 2 Representative synthesis methods for MOFs, highlighting different approaches for assembling metal ions (black dots) and organic linkers (blue lines) into highly porous structures. | ||
2.1. Hydrothermal synthesis
Hydrothermal synthesis is widely used for preparing MOFs, providing precise control over the formation of well-defined crystalline structures. In this method, metal salts and organic linkers are combined in a high-temperature/high-pressure aqueous solution, typically within an autoclave. The hydrothermal environment promotes the dissolution of reactants, accelerates their interaction, and fosters the nucleation and growth of MOFs.55 Controlled conditions, including temperature, pressure, and synthesis time, play important roles in determining the final size, morphology, and properties of the resulting MOFs.56 In addition, hydrothermal synthesis is advantageous for producing MOFs with enhanced crystallinity and purity, which improves structural integrity and performance in various applications. This method is also valuable for generating MOFs with tailored functionalities, making it a versatile tool for MOF synthesis for gas sorption,57 catalysis,58 and drug delivery.522.2. Solvothermal synthesis
Solvothermal synthesis is a prominent technique for MOF fabrication and it utilizes organic solvents under controlled temperature and pressure conditions. In this method, organic ligands and metal salts react within closed systems filled with an organic solvent rather than water.59 Moreover, the choice of solvent is vital as it affects the solubility of the reactants and the resulting MOF characteristics. The solvothermal conditions enable fine-tuning of the reaction kinetics, which allows the production of MOFs with tailored structures, sizes, and functionalities.60 In addition, using organic solvents for solvothermal synthesis offers flexibility, which enables researchers to manipulate the reaction parameters and obtain the desired properties.61 This method is valuable for developing MOFs with enhanced porosity, surface area, and stability, making them well-suited for gas storage and sensing,62,63 catalysis,64,65 and separation processes.66,67The versatility and controllability of solvothermal synthesis contribute to its widespread adoption for synthesizing MOFs with diverse compositions and properties.2.3. Microwave synthesis
Microwave synthesis is an efficient and fast method for producing MOFs. This technique involves exposing the precursors to microwave radiation, which rapidly heats the reaction mixture.68 The localized and uniform heating provided by microwaves accelerates the reaction kinetics, which results in shorter synthesis times compared to conventional methods. Microwave synthesis offers several advantages, including improved reproducibility, enhanced purity, and high yields.69 The controlled heating also minimizes side reactions, which leads to MOFs with well-defined structures.70,71 This method is advantageous due to its energy efficiency and scalability, which makes it an attractive option for MOF production.72 In addition, microwave synthesis is effective in tailoring the size, morphology, and porosity of MOFs, which enhances their versatility for applications such as gas adsorption,73,74 drug delivery,75 and sensing.76,77 Microwave synthesis of MOFs offers rapid crystallization and energy efficiency, often yielding materials with comparable or even enhanced crystallinity compared to that achieved with conventional methods. However, the resulting morphology can be varied and sometimes finer or irregular particles form due to rapid nucleation and growth. While there may be trade-offs in porosity or defect formation, careful optimization of reaction parameters can mitigate these issues, ensuring high-quality MOFs within a significantly reduced synthesis time.78 The combination of speed, efficiency, and tunability makes microwave synthesis a valuable tool for the production of advanced MOF materials.2.4. Ionothermal synthesis
Ionothermal synthesis is a distinctive method for producing MOFs utilizing ionic liquids as reaction solvents and templating agents. Metal salts and organic ligands react within the ionic liquid medium, where the exceptional properties of ionic liquids—such as low volatility and high thermal stability—are important in defining the characteristics of the resulting MOFs.79 The ionothermal conditions provide an alternative to conventional solvents, which results in MOFs with enhanced stability and unique structural features. Ionic liquids serve as both the reaction medium and template, which affect crystal growth and morphology.80–82 Moreover, the tailored properties of MOFs synthesized via ionothermal routes make them attractive for gas adsorption, catalysis, and environmental applications.83,84 This synthesis technique highlights the significance of the reaction environment in shaping the final characteristics of MOFs, providing a valuable route for the synthesis of advanced MOFs with tailored features.2.5. Pyrolysis synthesis
Pyrolysis synthesis offers a distinctive and unconventional route for producing MOFs, where metal–organic complexes are subjected to high temperatures in the absence of oxygen, which leads to the thermal decomposition of the precursors and the subsequent formation of MOFs.85 Pyrolysis induces a controlled and rapid thermal transformation that forms MOFs with unique structures and properties. The absence of oxygen prevents both oxidation and combustion reactions and preserves the MOF framework. Pyrolysis synthesis is advantageous due to its simplicity and efficiency, often producing MOFs with enhanced thermal stability and well-defined crystalline structures.86 This method is particularly useful in applications where traditional solution-based approaches may be problematic.87,88 Pyrolysis is an efficient strategy for the scalable or controlled synthesis of MOFs, as it can leverage high temperatures without compromising the integrity of the MOF structure.2.6. Mechanochemical synthesis
Mechanochemical synthesis is an innovative and sustainable method for producing MOFs. This technique uses mechanical energy, usually generated by ball milling, to induce chemical reactions between solid-state reactants, including metal salts and organic linkers. The grinding action in mechanochemical synthesis helps break chemical bonds and promotes the intimate mixing of precursors, which leads to the formation of MOFs. This method offers several advantages, including reduced reaction times, increased yields, and the elimination of solvents, which makes it environmentally friendly.89,90 The simplicity of this approach enables straightforward scalability and enhances energy efficiency. In addition, mechanochemical synthesis allows for control over particle size and morphology, which can help tailor the characteristics of the MOFs. The green and sustainable attributes of this method, coupled with its ability to produce MOFs with unique features, position it as a promising method for efficient and environmentally friendly MOF fabrication.912.7. Electrochemical synthesis
Electrochemical synthesis is a powerful and versatile approach to synthesize MOFs. Metal ions and organic linkers undergo electrochemical reactions within a conductive solution or on an electrode surface. Metal ions are reduced at the cathode and subsequently react with organic linkers to form MOFs.92 The applied electrochemical potential serves as a driving force for the formation of MOFs, which enables control over the growth and deposition of the framework. Furthermore, this method offers advantages, such as tunable synthesis conditions, which facilitate the manipulation of MOF properties such as size, morphology, and crystallinity.93,94 In addition, electrochemical synthesis allows for the direct integration of MOFs onto conductive substrates, which enables their use in electrocatalysis,95–97 sensing,98 gas and liquid separation,99 and energy storage devices.100 The ability to tailor the MOF properties through electrochemical control, combined with its eco-friendly characteristics and potential for direct integration into electronic devices, makes electrochemical synthesis a promising approach for advancing MOF applications in various technological fields.2.8. Template-directed synthesis
Template-directed synthesis is a strategic and controlled method that can be used for producing MOFs, where pre-existing templates or sacrificial agents guide the formation of the desired crystalline structures. This innovative approach allows researchers to manipulate the nucleation and growth of MOFs and target specific morphologies and properties. The chosen template (often an auxiliary molecule or framework) serves as a mold that imparts its characteristics onto the growing MOF structure.101 The template is removed after synthesis, leaving behind a thoroughly tailored MOF. This technique is especially valuable for applications that demand exact control of MOF features such as pore size and functionality.102–105 Furthermore, template-directed synthesis enables the engineering of MOFs with targeted properties and enhances their utility in various areas, including gas separation, catalysis, and drug delivery. This is done by ensuring the reproducibility and customization of these materials for specific applications.106–109The synthesis of MOFs for resistive gas sensors is a critical process that impacts the performance of the sensors directly. Several advanced synthesis techniques have been developed to create MOFs with tailored properties, increasing their suitability for gas sensing applications.110 In addition, each method can be fine-tuned to produce MOFs with specific surface areas, porosity, and chemical functionalities, which are essential for achieving high sensitivity and selectivity in resistive gas sensors.111 These tailored MOFs are subsequently incorporated into sensor devices, where their ability to adsorb gas molecules alters the electrical resistance and enables effective gas detection.112
3. Functionalization of MOFs
The functionalization of MOFs is a vital aspect that enhances their versatility and tailors their properties for certain applications. MOF functionalization involves the introduction of additional chemical groups or functionalities to the MOFs, affecting surface chemistry, porosity, and reactivity. The MOF functionalization can introduce minor structural changes, but well-controlled approaches such as post-synthetic modification (PSM), metal site functionalization, and surface grafting help preserve their integrity and porosity. PSM allows the incorporation of functional groups without disrupting the framework, while metal site functionalization enhances reactivity, and surface grafting prevents pore blockage while maintaining a high surface area. Careful selection of functional groups and optimization of synthesis conditions minimize any reduction in porosity, ensuring MOFs remain effective for gas detection applications.113,114 The following section reviews several common strategies that are used for functionalizing MOFs.3.1. Post-synthetic modification (PSM)
PSM involves introducing functional groups or ligands into existing MOFs after their synthesis. Nguyen et al. reported a novel Zr-MOF, MOF-700, for methane storage.115 The group employed post-synthesis metalation with various copper salts to produce an isoreticular series of MOFs with enhanced volumetric methane storage capacities. As a result, MOF-701 showed a 50% enhancement compared to the parent MOF-700 (Fig. 3).116 Moreover, amine-functionalization on ZIF-11 could be performed after the synthesis using 2-aminobenzimidazole, which improved the stability in aqueous media and generated fluorescent nanoparticles, highlighting its possible application as a marker material for detecting copper ions.117 In other words, PSM allows precise control of the type and amount of functionalization while minimizing the impact on the overall structure and improving the MOF activity.118 | ||
| Fig. 3 The enhancement of methane uptake in MOFs through PSM. The graph presents CH4 adsorption capacities at 80 bar and 298 K, comparing the initial MOF-700 structure with its modified derivatives MOF-701, MOF-702, and MOF-703. The structural representations show the transformation of MOF-700, where PSM introduces additional functional groups (highlighted in orange), potentially enhancing gas adsorption properties.115 Reproduced from ref. 115 with permission from The American Chemical Society, copyright 2022. | ||
3.2. Functionalization of metal sites
Tailoring the metal sites within an MOF structure by replacing or modifying metal ions can endow the material with unique reactivity or selectivity. This strategy is particularly advantageous for catalytic applications.119–122 Moreover, through the sulfation of an Hf-MOF, sulfate groups were attached to an Hf-oxo cluster, which then acted as an effective heterogeneous catalyst for heterocyclization in benzoxazole preparation (Fig. 4).119 In addition, modulator-mediated functionalization on the metal cluster can provide a versatile platform to improve the separation capability of MOF membranes, which is exemplified by producing mixed-matrix membranes with high CO2 separation yields.120 Also, Eu3+ ions were introduced to produce a luminescent Eu3+@Ni-MOF composite with high sensitivity and selectivity to aspartic acid, highlighting its potential as a sensitive luminescent probe for detecting aspartic acid.121 | ||
| Fig. 4 Assembly of metal building units and organic linkers (a) forms the three-dimensional MOF, called VNU-11 (b), which features two distinct pores. The spn net (c) is shown for the MOF and its sulfate-functionalized structure (d). The spheres represent the cages. Reproduced with permission.119 Reproduced from ref. 119 with permission from The Royal Society of Chemistry, copyright 2017. | ||
3.3. Incorporation of functional linkers
During MOF synthesis, selecting specific linkers with desired functionalities allows for the creation of a functionalized MOF from the outset. This approach integrates functional groups into the framework during its formation.123–125 An examination of the adsorption mechanism of indole on Zr-MOFs (specifically Zr-BDC-NH2 and Zr-BDC-NO2) showed that linker-functionalized Zr-MOFs exhibit enhanced indole adsorption capacities via their hydrogen and π-hydrogen bonds (Fig. 5).123 The presence of functional groups can induce splitting of the π orbital of the linker. In addition, the specific functional groups can cause a half-metal-to-insulator transition in the ferromagnetic configuration of the host material.125 | ||
| Fig. 5 The adsorption of indole on functionalized Zr-MOFs. The top left diagram: The porous structure of Zr-MOF, emphasizing its capacity to accommodate indole molecules. The bar chart on the top right: The indole loading capacities of amino-functionalized (Zr-BDC-NH2) and nitro-functionalized (Zr-BDC-NO2) MOFs across various concentrations, showing a consistent increase in adsorption with higher indole concentrations and slight performance differences between the two functional groups. The bottom panels: molecular-level visualization of the adsorption process, highlighting the interactions and distribution of indole within the framework pores, demonstrating the critical role of functional groups in optimizing adsorption efficiency. Reproduced with permission.123 Reproduced from ref. 123 with permission from Elsevier, copyright 2017. | ||
3.4. Coordinative unsaturation
Certain MOFs feature unsaturated metal sites, which enable the addition of ligands and functional groups. This method can also enhance the binding affinity for specific molecules or facilitate catalytic processes.126–128 Furthermore, defect-engineered MOFs (called UiO-66-NH2-X) were synthesized with controlled structural defects induced by an acetic acid modulator. This revealed a switch-on effect for photocatalysis with a volcano-type trend in the H2 generation rate, where Pt@UiO-66-NH2-100 showed the highest activity (Fig. 6).128 | ||
| Fig. 6 The photocatalytic water splitting mechanism using a defect-engineered Zr-based Zr-MOF. The left diagram: The role of defect sites in the MOF structure, which enhance light absorption and facilitate the separation of water molecules (H2O) into hydrogen gas (H2) under sunlight irradiation. The right schematic: The atomic-level configuration of the defect sites, showcasing their contribution to the improved catalytic activity and hydrogen production efficiency.128 Reproduced from ref. 128 with permission from Wiley, copyright 2019. | ||
3.5. Surface functionalization
Modifying the external surface of MOFs can improve their interaction with specific molecules. Common surface functionalization methods include grafting, chemical derivatization, or the introduction of reactive groups.117,129–131 The tobacco mosaic virus (TMV) has been utilized as a robust protein template to control the morphology of MOFs. This approach resulted in rod-like TMV@MOF core–shell structures (C–S) with tunable diameters (Fig. 7). The synthetic conditions affected its stability and allowed the chemical modification of the virus particle beneath the MOF shell, which indicated mass transportation inside the MOF shell.117 Moreover, a fluorescent organosilica coating named ZIF-8@BPMO was applied to nano ZIF-8 to enhance bio-interactions and reduce flocculation.131 | ||
| Fig. 7 The synthesis of a composite material combining TMV with ZIF-8. The process involves using TMV as a biological template, where Zn2+ ions and 2-methylimidazole (HMIM) coordinate to form the ZIF-8 shell around the TMV core. The resulting TMV@MOF structure integrates the biological features of TMV with the porous and protective properties of ZIF-8, offering potential applications in catalysis, biosensing, and drug delivery. Reproduced from ref. 132 with permission from Wiley, copyright 2016. | ||
3.6. Encapsulation of functional molecules
Incorporating functional molecules or nanoparticles into the pores of MOFs can provide additional functionalities, such as guest molecule adsorption or controlled release.133,134 Homogeneous porous ZIFs, with particle sizes below 100 nm, serve as effective platforms for chemosensors for fluorine ion detection (Fig. 8).133 | ||
| Fig. 8 ZIF-8 and ZIF-11 as effective platforms of curcumin-based on/off fluorescent chemosensors for fluorine ion detection. Reproduced from ref. 129 with permission from Elsevier, copyright 2021. | ||
Owing to the fascinating nature and properties of MOFs, they are widely used in different applications such as storage and conversion applications,45 water oxidation,48 electrocatalysis,17,97 solar energy,135 photocalatysts,136 and bio applications.137 However, the main focus of this review paper is application of MOFs in gas sensing devices and so in the next parts we have delved more into this application.
4. Toxic gases and resistive gas sensors
Air pollution is a significant issue in most countries which is caused by the rapid growth of different industries, transport sections, mineral extraction, and more. Air pollution results from the existence of toxic gases, heavy metals, and particulate matter (PM) in the air. The main toxic gases in the air are CO, NOx, SO2, O3, and volatile organic compounds (VOCs).138,139 On the other hand, other toxic gases (e.g., NH3 and H2S), explosive gases (e.g., H2 and CH4), and greenhouse gases (e.g., CO2) may also exist in the surrounding environment, agricultural areas, and mines.140 It is well known that air pollution has significant adverse effects on human health.141 Specifically, it may cause cough, headache, asthma, cardiovascular diseases, diabetes, and high rates of hospitalization. In addition, air pollution can negatively affect early human life via cardiovascular, respiratory, mental, and other perinatal disorders and increase infant mortality.142,143 Furthermore, air pollution was reported to cause cognitive dysfunction and the development of neurodegenerative phenomena underlying both Alzheimer's and Parkinson's disease.144 Besides, it tends to affect happiness and life satisfaction.145 Today, most people breathe polluted air with high concentrations of pollutants,146 and more than 4.2 million premature deaths have been attributed to air pollution in 2016 alone.147The olfactory system is very sensitive and complex (Fig. 9). It comprises receptor cells with ∼900 olfactory receptor genes.148 On the other hand, with such a complex system, many toxic gases are only sensible at high concentrations. In addition, some gases are odorless, colorless, and tasteless, making them undetectable. Some diseases, such as Alzheimer's disease, can lead to olfactory dysfunction.149
 | ||
| Fig. 9 Schematic illustration of the human olfactory system.149 Reproduced from ref. 149 with permission from MDPI, copyright 2018. | ||
Standardized techniques for gas monitoring, such as gas and ion chromatography, enable high sensitivity to trace amounts of gases. Unfortunately, they tend to be complicated, expensive, bulky, and off-line.150 A sensor is a sensitive device that generates an electrical signal from a physical or chemical input stimulus. Gas sensors are chemical sensors where a specific property, such as electrical resistance, optical properties, or capacitance, changes upon exposure to target gases.151 Gas sensors come in various types, including catalytic,152 gasochromic,153 optical,154 electrochemical,155 capacitive,156,157 transistor-type,158 mass sensitive,159 and resistive.160 Resistive gas sensors are the simplest and cheapest sensing devices. In addition, they are most widely applied for the detection of gases due to their simple operation, small size, real-time operability, fast dynamics, and high stability.33,161,162 Resistive gas sensors are manufactured by depositing a sensing layer on insulating substrates, like Al2O3, equipped with interdigitated electrodes and micro-heated at the back of the substrate (Fig. 10(a)–(c)).163
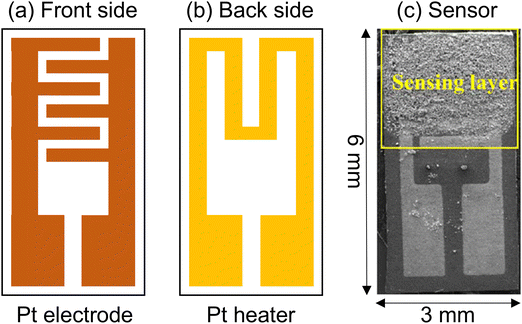 | ||
| Fig. 10 Schematic of the (a) front side and (b) backside of a resistive gas sensor and (c) optical photograph of the final sensor. | ||
These gas sensors are primarily constructed using semiconductor metal oxides such as SnO2164 and ZnO.165 However, despite their many advantages, they have significant limitations, such as high sensing temperatures, humidity interference, and poor selectivity.166 Therefore, novel sensing materials or a combination of novel and traditional sensing materials are needed to develop improved resistive sensors with superior capabilities such as better selectivity. MOFs, with their porous structures, provide highly specific surface areas (SSAs) and abundant functional sites like unsaturated metal sites, which are favorable for selective binding and sensing of gases. Accordingly, they are promising gas-sensing materials167 for resistive gas sensors as well as for quartz crystal microbalances,168 capacitive,169 and other gas sensors. The following sections discuss MOF-based resistive gas sensors.
4.1. MOFs as resistive gas sensors: an introduction
MOFs with unique features, such as very high SSA, abundant porosity, exceptional adsorption affinity, and outstanding surface chemistry, are promising materials for gas sensors,170–172 supercapacitors, batteries, water purification, and catalysts.173 For gas-sensing applications, their excellent tunability and chemical selectivity make them ideal for selective sensors.174 One of the mechanisms contributing to their selectivity is molecular sieving, where smaller molecules can be adsorbed through the MOF apertures, while larger molecules are excluded and cannot pass through.174–176 Furthermore, they can be used as a template to synthesize metal oxides, where the final product after annealing features a morphology and structure similar to that of the parent MOF, porous and with a high SSA.177–179More specifically, MOF-based gas sensors offer several advantages for sensing applications: (i) the highly porous MOFs provide a large specific surface area (SSA) and numerous gas adsorption sites. Additionally, they contain unsaturated metal sites and functional groups that enhance gas adsorption. (ii) The porosity, geometry, and physicochemical features of MOFs can be tailored to improve selectivity for targeting gases.179 In particular, in MOF-based gas sensors, achieving high selectivity often involves tailoring the material's properties to preferentially adsorb or interact with a desired gas, which can lead to a trade-off in which the sensitivity to other gases is reduced. In this regard, by altering the pore size structure or surface chemistry of a MOF, the selectivity to a particular gas may be enhanced, while its sensitivity to other gases may be decreased. For example, H2 gas has a small kinetic diameter; by tailoring the pore size of the MOF in the range comparable with the kinetic diameter of H2 gas, the resultant MOF gas sensor can not only show high response to H2 gas, but at the same time can also show reduced response to other gases, leading to enhanced selectivity to H2 gas.180,181
In resistive MOFs, resistance changes occur because of the adsorption of and interaction with the target gas. Furthermore, the redox reactions between gases and active metal sites in MOFs modulate the resistance. Moreover, the resistance of MOFs is influenced by structural changes resulting from gas adsorption. However, the limited electrical conductivity of most MOFs and their relatively low chemical stability impede their application in pristine form.182,183 In fact, MOFs often show poor conductivity because the metal cations in MOFs cannot migrate, and inert organic ligands attached to metal cations do not facilitate good electron flow.184,185
4.2. MOF-based resistive gas sensors
Nguyen et al. synthesized a novel Fe-MOF or VNU-15 with a high SSA (735 m2 g−1) and a pore size of 10 Å to detect acetone (Fig. 11(a)).27 At 50 °C, it exhibited a response of 1.7 to 10 ppm acetone (Fig. 11(b)–(d)). Based on the gas chromatography analysis, the appearance of CO2 peaks upon exposure to acetone confirmed the oxidation of acetone at the sensing temperature. Acetone oxidation was facilitated at 50 °C by metal cations in VNU-15. In addition, Fe2+/Fe3+ ions acted as catalytic sites to generate oxygen species, which accelerated the oxidation of –CH3 in acetone. Because the formation energy of acetone (366 kJ mol−1) was lower than that of CO, C6H6, and C7H8 gases (all >370 kJ mol−1), it was readily dissociated at the sensor surface and generated a higher signal than other gases.
 | ||
| Fig. 11 (a) Schematic of the VNU-15 sensor. (b) Dynamic sensing graphs to acetone at various temperatures. (c) Response vs. temperature. (d) Response and recovery times to various concentrations of acetone at 50 °C.27 Reproduced from ref. 27 with permission from Elsevier, copyright 2023. | ||
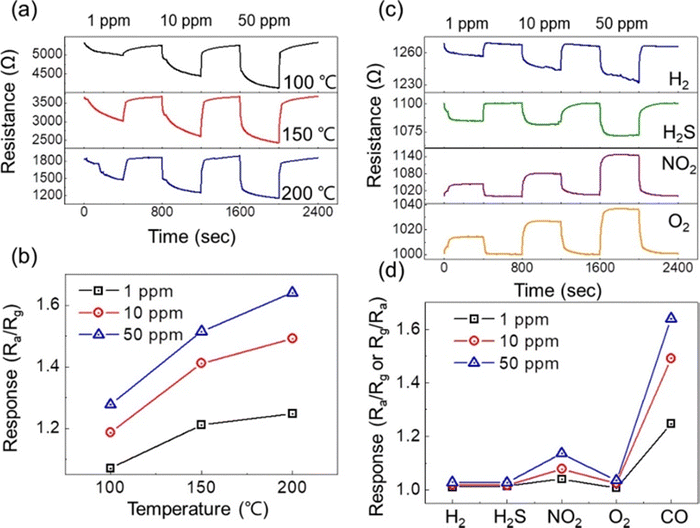
In another study, Co-MOF-I and II were prepared for H2 gas sensing with a very high SSA of 2100 and 2420 m2 g−1, respectively. The response of the Co-MOF-II sensor was higher than that of the other sensor at all temperatures (Fig. 12(a)). In addition, it exhibited higher selectivity to CO than to the interfering gases (Fig. 12(b)). In addition to a higher SSA, the Co-MOF-II sensor featured a larger pore volume (1.27 cm3 g−1) and smaller pore size (23.2 Å) than the other sensor (1.10 cm3 g−1 and 27.6 Å, respectively). In particular, smaller pores led to easier interaction between the open Co sites and small H2 molecules. Moreover, the size of H2 gas molecules was smaller than that of other gases, which led to a better response and selectivity to H2 gas. Furthermore, the excellent response was related to the higher Co(II)/Co concentration in the sensor, where the higher amount of the open Co sites in the Co-MOF-II sensor increased the probability of attaching the H2 molecules at the sensor surface.16
 | ||
| Fig. 12 (a) Comparison of the H2 gas responses of Co-MOF-I and II sensors. (b) Selectivity histograms of Co-MOF-I and II sensors at 200 °C.16 Reproduced from ref. 16 with permission from Elsevier, copyright 2020. | ||
CO is a particularly hazardous gas because it is also colorless, odorless, and tasteless. Inhalation of CO primarily affects the cardiovascular and nervous systems.187 When CO enters the body, it attaches to hemoglobin (Hb), generating COHb that considerably decreases the oxygen-carrying ability of blood. Hence, death is likely even upon exposure to relatively small amounts of CO gas.188 To address this problem, Ni-MOFs (Ni-VNU-74-I and II) were synthesized for CO sensing. At 200 °C, the Ni-VNU-74-II sensor showed a response of ∼1.65 to CO gas (50 ppm) (Fig. 13(a)–(d)). The better performance is mainly due to a higher SSA (2350 m2 g−1) and smaller pore size (23.6 Å) of the Ni-VNU-74-II sensor compared to the other sensor (2020 m2 g−1 and 27.3 Å). The interaction between CO and Ni2+ ions resulted in the effective adsorption of CO at the sensor surface. In addition, FTIR spectroscopy revealed signals at ∼2200 and 2085 cm−1, which were assigned to the Ni2+–CO bonds and the sub-carbonyls of Ni, respectively. These findings confirm the strong interaction between CO gas and Ni2+ ions.29
4.2.2.1. MOF/carbon-based composite gas sensors. Due to the low conductivity of MOFs, composites with materials of higher conductivity, such as graphene, conducting polymers (CPs), and metal oxides, are used to enhance MOF performance.189 Graphene, which consists of a single layer of carbon atoms arranged in a nanosheet-like morphology, is one such material. It has a high SSA, high stability, mechanical flexibility, and high conductivity owing to the high carrier mobility and low electrical noise.190,191 However, it has no bandgap and is hydrophobic, which makes it challenging to use for gas sensing. Reduced graphene oxide (rGO) has many defects, high conductivity, and high hydrophilicity, and it can be used in place of graphene for sensing studies.192,193 Therefore, a combination of rGO with high conductivity and MOFs can enhance the sensing properties of the resulting nanocomposite. In one study, the NiO-MOF and NiO-MOF/rGO nanocomposites were hydrothermally prepared at 120 °C. The SSA of NiO-MOF was 810 m2 g−1, and it increased to 872 m2 g−1 after incorporating rGO due to the 2D morphology and high SSA of rGO. The composite sensor responded more strongly to CO than to NH3 and SO2 gases. In addition, the response was higher than that of the pristine NiO-MOF sensor—mainly because of a higher SSA and the formation of Ni-MOF/rGO heterojunctions. The comparable kinetic diameter of CO to the aperture size of NiO-MOF, the high conductivity of rGO, the porous nature of Ni-MOF, and the formation of Ni-MOF/rGO heterojunctions all contribute to the enhanced sensing capabilities.194 Another study developed a flexible Cu(INA)2/rGO composite for NH3 gas sensing at room temperature, using a combination of MOF and reduced graphene oxide. NH3 gas readily diffused into and adsorbed on Cu(INA)2 due to the porous nature of the sensing material. In addition, the sensor's high SSA provided adsorption sites for NH3 gas. The adsorption ability of Cu(INA)2 and the high conductance of rGO improved the response to NH3 gas.195
In another study involving MOF composites with graphene derivatives, a graphene acid (GA)/UiO-66-NH2 composite was synthesized by covalently bonding GA with the UiO-66-NH2 MOF through amide bonds. With the hierarchical pores, the composite showed a high SSA of 598 m2 g−1, a pore volume of 1.09 cm3 g−1, and good conductivity because of the presence of GA and good stability. Both GA and UiO-66-NH2 sensors showed low responses to CO2 gas. Although pristine GA is very conductive due to delocalized π-electrons, it did not have any favorable sites for CO2 adsorption. Pristine UiO-66-NH2, however, has sufficient interaction sites for CO2 gas adsorption, but its resistance is very high. However, the GA/UiO-66-NH2 composite offered a good and rapid response to CO2 gas at 200 °C due to synergistic effects between the two materials. Raman analysis indicates that CO2 only weakly interacts with CO–NH above 200 °C. In composite sensors, the MOF controlled the space between the GA layers and led to the creation of adsorption sites at the functional groups at the GA–MOF interface.196
An interesting application of gas sensors is the monitoring of the freshness of fruit,197 where the emission of certain gases is used as an indicator of fruit quality.198 On the other hand, for these applications, flexible gas sensors, which retain their performance upon stretching, tilting, and bending,199 are preferred over rigid sensors because they can be easily attached to fruit skin. Ethylene is a hormone that facilitates the growth and maturation of plants. It is released during the growth and decay of fruit. Generally, fruit rapidly ripens and decays because of the autocatalytic action of the accumulated ethylene.200,201 For these reasons, an ethylene sensor was fabricated to detect fruit freshness and spoilage of kiwi that uses a single-walled carbon nanotube (SWCNT)/Pd NPs/Cu-MOF-74 (SPM) nanocomposite. The nanocomposite was prepared using a self-assembly technique. Flexibility was achieved by depositing the sensor onto a flexible PET substrate. The SSA (13 m2 g−1) of the SWCNTs increased to 92 m2 g−1 for the composite sensor. In other words, the composite sensor provided many adsorption sites for ethylene gas. Moreover, the water contact angle (WCA) of the SPM composite was 34.87°, significantly higher than that of Cu-MOF-74 (24.30°), which confirms the effectiveness of SWCNTs in enhancing the hydrophobicity of the sensor. In addition, the SPM sensor was highly resistant to humidity above 40–70%. The sensor exhibited a response of 34.2% to 100 ppb C2H4. C2H4 was easily oxidized on Pd NPs because of the catalytic effect of Pd. The released electrons migrated to the sensor surface, which decreased resistance. Cu-MOF-74 contained Cu2+ ions, which formed a covalent bond with C2H4 via π–π stacking interactions. In addition, the bonds between Cu-MOF-74 and C2H4 expanded, and the MOF was distorted into a stable pseudo-octahedral shape because of the Jahn–Teller effect (Fig. 14).
 | ||
| Fig. 14 Schematic illustration of the Cu-MOF-74 structure showing Jahn–Teller distortion.202 Reproduced from ref. 202 with permission from Elsevier, copyright 2024. | ||
The sensor performance to monitor the ripeness and decomposition of kiwi was assessed by attaching the sensor to a kiwi at room temperature and 75% relative humidity (Fig. 15a). The sensor generated various signals that could be related to the ripeness and decomposition of kiwi (Fig. 15(a)–(c)).202
 | ||
| Fig. 15 (a) Flexible sensors attached to a kiwi fruit. (b) Response and change in resistance of the sensor during 13 days. (c) Optical images of the kiwi after various time intervals.202 Reproduced from ref. 202 with permission from Elsevier, copyright 2024. | ||
SO2 is a significant factor in air pollution, which can adversely affect human health and lead to respiratory issues and mortality.203 Hence, flexible SO2 gas sensors were manufactured based on Ni-MOF/–OH-SWCNTs and Ni-MOF/–OH-MWCNTs. The sensor with SWNTs performed better for SO2 gas and could detect 0.5 ppm SO2. The sensing signal was attributed to a high SSA, good conductivity, and the formation of heterojunctions between Ni-MOF and SWCNTs.204
4.2.2.2. MOF/CP-based composite gas sensors. Conducting polymers (CPs) have electrical conductivity, and therefore, can be used for sensing applications. Polyaniline (PANI) is a CP with unusually high electrical conductance,205 and like rGO, it can be composited with MOFs to further increase the electrical conductivity and the overall sensing performance of the MOF-PANI composite.206 Hence, a composite sensor consisting of SiO2-coated Cu-MOF, graphene and PANI was prepared. Cu-MOF and graphene in the composite were connected via PANI. The sensor showed a high SSA of 760 m2 g−1 and a micropore volume of 0.35 cm3 g−1. In other words, it could offer a high SSA to gas molecules. The NH3 gas could diffuse into the deeper regions of the sensor because of the many pores in the sensing material. As a result, NH3 gas was detected at levels as low as 0.6 ppm with relatively fast dynamics. The enhanced response to NH3 gas was due to the high sensitivity of PANI to NH3. PANI donates one proton to NH3, forming NH4+ ions, which increase the sensor resistance. In addition, significant resistance modulation occurred because of the creation of heterojunctions between Cu-MOF/PANI and rGO, which contributes to the sensing signal.207
Like most MOFs, the UiO-66-NH2 MOF has high resistance, which hinders its usage in sensing studies and hence conductive polymers such as poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS), which features high conductivity,208 can be added to address this problem. Therefore, a UiO-66-NH2/PEDOT:PSS composite was synthesized for toluene sensing using a hydrothermal reaction. It exhibited a response of 1.42 to toluene (10 ppm) at room temperature. Operation at room temperature can significantly lower the energy consumption of the sensing device.
However, the response time of the sensor was long (340 s) due to low kinetics at room temperature. Furthermore, the high SSA of the UiO-66-NH2 MOF and the conductivity of the PEDOT:PSS polymer affected the output of the sensor. Moreover, at the contact areas, electrons migrated from PEDOT:PSS to UiO-66-NH2 to equilibrate the Fermi levels, which resulted in the generation of potential barriers. In the presence of toluene, electrons were released to the sensor surface through a reaction with chemisorbed oxygen species, which lowered both the potential barrier height and electrical resistance.209
In another study on CP/MOF composite sensors, a Cu3(HHTP)2 MOF/PVA composite mixed with an ionic liquid (IL) was synthesized to enhance conductivity for H2S sensing. The composite was hydrophilic due to the presence of –OH groups in each ionic liquid molecule. In an acidic H2S gas atmosphere, the molecules of H2S reacted with oxygen ions on the PVA, ionic liquid, and Cu-MOF, which led to the release of electrons. Hence, a flow of charged ions and protons became possible. In addition, the highly conjugated linker also improved the conductivity of the composite sensor. Moreover, Cu-MOF had 1.55 nm pores, which exceeded the size of the H2S molecule (0.36 nm). Therefore, H2S gas easily diffused within the MOF, resulting in high sensitivity and selectivity to this gas.210
A composite gas sensor using MOF-5 microparticles/chitosan (CS) was fabricated for H2S gas sensing, and a glycerol ionic liquid was added to enhance the conductivity of the sensor. MOF-5 nanoparticles dispersed within the CS-IL matrix enhanced the transport of H2S through the membrane because of the porosity of MOF-5. The sensor exhibited a 91% response to H2S gas (100 ppm) in just 8 seconds. The enhanced capability was attributed to the high SSA and the porous MOF, along with the excellent conductivity of the sensor as a result of the CS-IL matrix.210
4.2.2.3. MOF/metal oxide-based composite gas sensors. ZIFs are MOFs with a 3D mesh structure consisting of tetrahedral MN4 (M = metal cation) and tetrahedral clusters, which are connected via imidazolate linkers with high porosity and a high internal SSA. Both ZIF-8 and ZIF-71 are widely used for sensing applications. These materials have pore sizes of 3.4 Å and 4.8 Å, respectively. Accordingly, ZIF-8 is typically used to detect small molecules, such as H2, while ZIF-71 is employed to sense large molecules, such as ethanol.211 Drobeck et al.180 coated ZnO nanowires (NWs) with a thin layer of ZIF-8 using a hydrothermal route at 100 °C/24 hours for selective H2 sensing. At 300 °C, the ZIF-8-coated sensor showed a stronger response to H2 gas than to C7H8 and C6H6 gases because of its molecular sieving effect. In fact, these gases were unable to pass this layer and reach the ZnO surface because the average pore size of ZIF-8 (3.4 Å) was below that of C7H8 (5.92 Å) and C6H6 (5.27 Å) gases.
On the other hand, H2 gas with a smaller molecular size (2.89 Å) than the aperture size of ZIF-8 easily diffused through and reached the surface of ZnO. The response of the ZIF-8 coated sensor was slightly lower than that of the pristine ZnO because of the presence of ZIF-8 (with lower conductivity) and the prevention of adsorption of all H2 gas molecules on the ZnO NWs. In other words, ZIF-based gas sensors can be tuned to respond to a specific gas.
Since the stability of MOFs is generally low, a composite of MOFs with metal oxides may increase the overall stability of the resulting sensor and increase the gas-sensitive output. For this reason, an In2O3@MIL-68(In) composite was synthesized hydrothermally at 80, 100, and 120 °C (Fig. 16). A sensor prepared hydrothermally at 100 °C showed a response of 18 to 100 ppm HCHO gas, which was six times higher than that of pristine In2O3. The excellent formaldehyde sensing performance was attributed to the low bond-dissociation energy. The bond energy of formaldehyde is 368 kJ mol−1, whereas the bond energies of ethanol, methanol, methane, and acetone are all higher than 400 kJ mol−1. Therefore, formaldehyde decomposed quickly at the sensing temperature and released more electrons than other gases. Furthermore, the sea urchin shape morphology and the presence of MIL-68(In) on In2O3 enhanced the sensor's response.212 The detection of formaldehyde is essential as exposure to it can cause allergies, asthma, pulmonary damage, and cancer from contaminated foods, water, and air.213
 | ||
| Fig. 16 (a) and (b) TEM and (c) HRTEM views of In2O3@MIL-68 (In) prepared at 100 °C under hydrothermal conditions. (d)–(g) TEM-EDS elemental mapping analysis.212 Reproduced from ref. 212 with permission from Elsevier, copyright 2023. | ||
NO2 is a hazardous gas emitted mainly from the combustion of fossil fuels. Exposure to this gas causes coughing and respiratory problems, such as asthma.214 In addition, the intensity of a COVID-19 infection is increased in the presence of NO2 gas.207,215 Shin et al.216 coated a titanium-based MOF on SnO2 NWs to enable the selective detection of NO2. In a dry atmosphere at 200 °C, the responses of the pristine and Ti-MOF-coated SnO2 sensors to 2 ppm NO2 were 5.4 and 4.3, respectively. Under humid conditions, however, the response of the coated sensor was higher (Fig. 17(a)–(d)). The water contact angles (WCAs) of the pristine and Ti-MOF-coated sensors were 10° and 123°, respectively (Fig. 17(e)), indicating the hydrophobicity of the Ti-MOF coating, which is highly beneficial for gas-sensing in a humid atmosphere.
 | ||
| Fig. 17 Dynamic sensing curves of (a) pristine SnO2 NW and (b) Ti-MOF-coated SnO2 NW sensors to various levels of RH. (c) Comparison of dynamic sensing responses to various levels of RH. (d) Corresponding calibration graphs. (e) WCA values of the gas sensors.216 Reproduced from ref. 216 with permission from Elsevier, copyright 2023. | ||
 | ||
| Fig. 18 Schematic illustration of the H2 gas sensing mechanism of Zn-BDC-NH2 in (a) air and (b) H2 atmospheres, and (c) change of the thickness of the EDL of Zn-BDC-NH2 in air and H2 atmospheres.220 Reproduced from ref. 220 with permission from Elsevier, copyright 2022. | ||
The presence of open metal sites of Zn provided additional adsorption sites for H2 gas. H2 gas with a small molecular size of 0.289 nm could quickly diffuse into all regions of the sensing layer, which resulted in better sensor response to H2 relative to interfering gases. The functionalization of –NH2 enhanced the number of active sites for H2, which improved the adsorption capability of H2.
Moreover, the reaction of H2 gas with Zn4O(CO2)6 helped to decrease the sensor resistance. H2 gas was chemisorbed at the “Zn” and “O” sites. Upon adsorption on “O” sites, O–H bonds were formed through strong hybridization between the H-s and O-p orbitals. Moreover, the delocalization of electrons between the Zn and O–H bonds facilitated the surface metallization of Zn, with a significant change in conductance.220
Few studies have explored the incorporation of noble metals on MOFs for gas sensing. In this context, sulfone-functionalized Zr-MOF (Zr–BPDC–SO2) and Pd-loaded Zr–BPDC–SO2 composites were hydrothermally prepared at 120 °C/24 hours. Zr–BPDC–SO2 exhibited a stronger response to ethanol, while Pd/Zr–BPDC–SO2 showed better sensing with respect to H2 gas. At 150 °C, the Zr–BPDC–SO2 gas sensor manifested a response of 1.45 to 100 ppm ethanol. Also, the response of the Pd/Zr–BPDC–SO2 sensor was ∼2 to 10 ppm H2 gas at 150 °C. The SSAs of the two sensors were 1360 and 530 m2 g−1, respectively. In addition, the pore sizes of Zr–BPDC–SO2 and Pd/Zr–BPDC–SO2 were 12 and 6 Å, respectively. Therefore, following Pd-loading, some pores were closed, which caused a decrease in both SSA and pore size and a lower response to ethanol compared to the Zr–BPDC–SO2 sensor. Fig. 19(a) and (b) show the reaction of the Zr–BPDC–SO2 and Pd/Zr–BPDC–SO2 gas sensors to ethanol and H2 gases, respectively. Furthermore, Fig. 19(c) shows the variation of the hole accumulation layer (HAL) of gas sensors in air, ethanol, and H2 gas. In terms of H2 gas sensing, the catalytic effect of Pd plays an important role. First, H2 gas molecules adsorb easily on Pd and dissociate into atomic species. Under the spillover effect, they move to the neighboring sensing material and then readily react with adsorbed oxygen. Thus, a strong response to H2 gas was observed. In addition, PdHx, formed in an H2 gas atmosphere, exhibits different resistance compared to metallic Pd due to Pd's ability to adsorb H2 gas, which substantially modulates its resistance.130
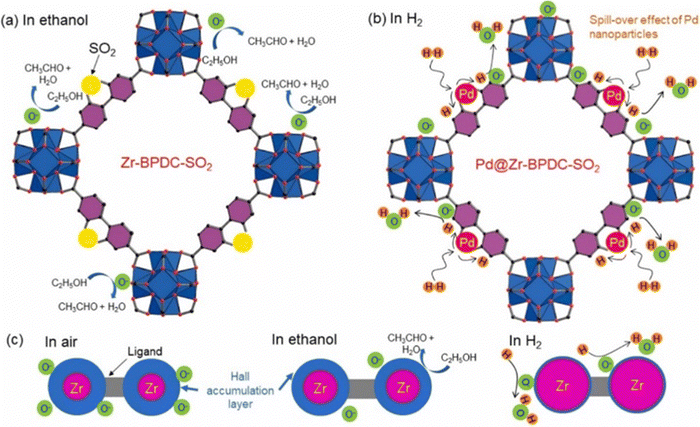 | ||
| Fig. 19 Schematic illustrating the gas sensing mechanism of (a) Zr–BPDC–SO2 and (b) Pd/Zr–BPDC–SO2 gas sensors to ethanol and H2, respectively. (c) Modulation of HAL in air, ethanol, and H2 atmospheres.130 Reproduced from ref. 130 with permission from Elsevier, copyright 2021. | ||
NH3 is a hazardous and corrosive gas that can irritate the eyes, nose, and lungs and damage the immune system.221 In air, it produces aerosols that exhibit a temperature-reducing effect and negatively affect the global greenhouse balance.222 Moreover, it is considered a medical biomarker for kidney disorders.223 An Hf-BTC MOF was synthesized and functionalized via the SO4 group to boost the sensing response to NH3 (Fig. 20).
 | ||
| Fig. 20 Schematic illustration of the synthesis and functionalization of Hf-BTC. (a) The Hf6O4(OH)4(CO2)12 cluster as the secondary building unit (SBU). (b) 1,3,5-Benzenetricarboxylic acid (H3BTC) as the organic linker. (c) Formation of the Hf-BTC MOF via coordination between Hf clusters and BTC linkers. (d) Sulfation of Hf-BTC using H2SO4 to obtain Hf-BTC-SO4 for ammonia (NH3) sensor.222 Reproduced from ref. 222 with permission from Elsevier, copyright 2022. | ||
The Hf-BTC MOF showed a very high SSA of 1100 m2 g−1 and an average pore size of 15 Å that promoted sensing reactions through the rapid diffusion of gases. At 100 °C, the sensor exhibited a response of 2.1 to 10 ppm NH3. In addition to the high SSA, the excellent crystallinity of Hf-BTC-SO4 allowed NH3 to be adsorbed on the sensor through strong host/guest interactions. The SO4 groups, which were Brønsted acid sites, interacted with the Lewis acid sites on the Hf6 cluster. This led to a strong interaction with NH3 and improved the response to NH3. In addition, the good selectivity to NH3 was due to the interactions of N atoms in NH3 with metal sites on the MOF. The kinetic diameter of NH3 (0.26 nm) was also smaller than that of other gases, which enabled easy penetration through the pores inside the sensing layer.222
Table 2 compares the gas sensing properties of various MOF-based gas sensors. The main criteria for comparing sensing performance are response, sensing temperature, selectivity, and response and recovery times. Obviously, lower sensing temperature is a beneficial factor for gas sensors as it leads to reduced power consumption. Also, higher sensing response is better for practical application. Selectivity to a particular gas is important for practical application to avoid the false alarms. Faster dynamics in terms of response and recovery times is also a beneficial factor for real applications. Specially, for highly toxic gases such as NO2 and CO, swift response time is highly important from a safety point of view. Overall, MOF-based gas sensors have been successfully used for the detection of various gases. The sensing temperature is often low, compared with metal oxide gas sensors. However, response and recovery times are usually longer than those of metal oxide-based gas sensors, mainly due to working at low temperatures. Also, generally detection limits are down to 1 ppm, which is higher than those of metal oxide gas sensors, which are generally in the ppb range. Also, by properly adjusting pore sizes and the use of different strategies such as functionalization, MOF gas sensors often can have better selectivity relative to metal oxide gas sensors.
| Sensing material | Specific surface areas (m2 g−1) | T (°C) | Gas | Conc. (ppm) | Response (Ra/Rg) or (Rg/Ra) or [(ΔR/Ra) × 100] | Response/recovery time (s/s) | Detection limit (ppm) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fe-MOF | 735 | 50 | Acetone | 10 | 1.7 | 64/78 | 1 | 27 |
| Co-MOF-II | 2420 | 200 | H2 | 50 | 101.4% | —/— | 1 | 16 |
| Ni-VNU-74-II | 2020 | 200 | CO | 50 | 1.65 | —/— | 1 | 29 |
| NiO-MOF/rGO | 872 | 25 | CO | 25 | ∼0.9% | 30/75 | 25 | 194 |
| Cu(INA)2/rGO | Not mentioned | 25 | NH3 | 50 | ∼4.651% | —/— | 50 | 195 |
| GA/UiO-66-NH2 | 598 | 200 | CO2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
8.6% | 18.5/18.7 | — | 196 |
| SWCNT/Pd NPs/Cu-MOF-74 | 92 | 25 | C2H4 | 0.1 | 34.2% | 200/50 | 0.1 | 202 |
| SiO2-coated Cu-MOF/graphene/PANI | 756 | 25 | NH3 | 1 | — | 30/180 | 0.6 | 207 |
| UiO-66-NH2 MOF | 876 | 25 | C7H8 | 10 | 1.42 | 340/— | 1 | 209 |
| Cu3(HHTP)2 MOF/PVA | 279.997 | 25 | H2S | 100 | 91% | 8/— | 1 | 210 |
| ZIF-8 coated ZnO NWs | 1760 | 300 | H2 | 50 | 1.44 | —/— | 10 | 180 |
| In2O3@MIL-68 | 133.48 | 300 | HCHO | 100 | 18 | 28/43 | 5 | 212 |
| Ti-MOF-coated SnO2 NWs | Not mentioned | 200 | NO2 | 2 | 4.3 | 672/843 | 2 | 216 |
| Zr–BPDC–SO2 | 1360 | 150 | C2H5OH | 100 | 1.383 | —/— | 10 | 130 |
| Pd/Zr–BPDC–SO2 | 530 | 150 | H2 | 100 | 1.947 | —/— | 10 | 130 |
| Hf-BTC-SO4 | 1100 | 100 | NH3 | 10 | 2.1 | 72/138 | 1 | 222 |
5. Degradation and long-term stability of MOF gas sensors
Despite their numerous advantages, the practical deployment of MOFs is often limited by their stability and degradation under operational conditions. Generally, three types of degradation mechanisms have been identified for MOFs:(i) Hydrolytic degradation: exposure to moisture can lead to the hydrolysis of metal–ligand bonds in MOFs, resulting in structural collapse. For instance, the Zr6O4(OH)4 secondary building unit in some MOFs undergoes significant changes in chemical and thermal stability upon incorporation of different organic linkers, affecting their resistance to hydrolysis.224
(ii) Thermal degradation: elevated temperatures can weaken the coordination bonds within MOFs, leading to decomposition. The thermal stability of a MOF is influenced by factors such as the strength of metal–ligand bonds and the robustness of its framework.225
(iii) Chemical degradation: exposure to acidic or basic environments can disrupt the coordination bonds in MOFs. For example, carboxylate-based MOFs with high-valent metal ions exhibit remarkable robustness in acidic water but show moderate resistance in alkaline environments. The degradation in basic solutions is attributed to the high affinity between hydroxide ions and high-valency metal ions.225
Also, factors influencing the long-term stability of MOFs are as follows:225
(i) Framework hydrophobicity: hydrophobic frameworks can repel water molecules, reducing the likelihood of hydrolytic degradation. This property is particularly beneficial for MOFs intended for application in humid or aqueous environments.
(ii) Metal–ligand bond strength: stronger coordination bonds between metal ions and ligands enhance the resilience of MOFs to environmental stressors. Hence, MOFs constructed with inert metal ions and robust, hydrophobic linkers demonstrate increased resistance to hydrolysis.
In the case of MOFs for gas sensing applications, the main stability issues are related to temperature and humidity. In particular, low thermal stability at the sensing temperature causes changes in the phase and composition of the MOF sensor, affecting the overall sensing performance. In this regard, some strategies such as chemical treatment after synthesis to increase the strength of the framework, composite formation and the use of appropriate ligands can increase the stability of MOFs. Integrating MOFs with other materials with high thermal stability can enhance their mechanical strength and thermal stability. Also, applying protective coatings to MOFs can shield them from moisture, contaminants, and other environmental factors that may cause degradation. Advances in MOF coatings have led to the development of composites that exhibit improved stability and functionality. These coatings not only protect the MOF structures but also enhance their applicability in various fields, including gas sensing.226,227
Also, humid air generally has a negative effect on the performance of almost all MOFs. In fact, in humid air, water molecules are adsorbed on the surface of MOFs, decreasing the number of adsorption sites and leading to a decrease of sensing response. However, the use of hydrophobic MOFs can somewhat overcome this negative humidity effect.216 Contaminants can occupy or block the MOF pores, resulting in a decrease of the effective surface area and sensing performance.
6. Conclusion and outlooks
This study explored the synthesis methods for preparing MOFs and their applications as resistive gas sensors. MOFs were synthesized via different routes, including hydrothermal/solvothermal, microwave, ionothermal, pyrolysis, mechanochemical, electrochemical, and template-directed synthesis methods. Scalability and industrial feasibility remain key challenges for MOF-based sensors due to high synthesis costs, complex fabrication, and energy-intensive processes. To address these issues, alternative synthesis methods such as mechanochemical, microwave-assisted, and electrochemical approaches have been explored for faster and more cost-effective production. Additionally, integrating MOFs with scalable substrates and developing continuous-flow synthesis methods can enhance their industrial applicability. Furthermore, the functionalization of MOFs is a crucial factor that enhances their versatility and tailors their properties for specific applications. The functionalization of MOFs was achieved through several strategies, including post-synthetic modification, incorporation of functional linkers, metal site functionalization, coordinative unsaturation, encapsulation of functional molecules, and surface functionalization. Highly porous MOFs with a high SSA are promising materials for gas-sensing applications. In addition, with tuneable pore sizes, they can act as molecular sieves for the selective detection of target gases. However they generally show low conductivity, which limits their application in pristine form.Because stability is a critical factor in gas sensors, both chemical and physical durability play a significant role in maintaining performance over extended use. Chemically, the incorporation of stable metal clusters (e.g., Zr-, Ti-, and Hf-based MOFs) enhances resistance to hydrolysis and oxidation, while the addition of carbon materials and conductive polymers as well as doping with metal or redox-active species to facilitate charge transport improves moisture resistance and electrical conductivity, reducing framework degradation. Physically, these composites exhibit superior structural integrity compared to pristine MOFs, as carbon materials help prevent framework collapse during repeated gas adsorption/desorption cycles, and polymer matrices enhance mechanical robustness and adhesion to substrates.
Different materials with higher conductivity than MOFs, like carbon materials, metal oxides, and conductive polymers, were added to MOFs to increase the overall conductance and sensing performance of the resulting composite. Also, in the composite form, MOFs can act as molecular sieves, preventing the adsorption of undesired gases or humidity on the surface of the sensing layer. For example, coating metal oxides with MOFs can reduce the interference caused by humidity and enhance the selectivity of the sensor.
We also explained the gas sensing performance of various MOF-based gas sensors, demonstrating the fact that MOFs have high potential to be used as gas sensing devices. However, to further boost their performance, they can be integrated with metal oxides, metal sulfides, and MXenes. In particular, MXenes with high conductivity, tunable properties, and high surface area are the most promising materials to be integrated with MOFs to significantly increase the sensing performance at low temperatures. With high potential of working at low temperatures down to room temperature along with relatively good flexibility, MOFs can be used for the realization of flexible/wearable gas sensing devices. However, when sensing temperature is high, by use of different strategies such as UV illumination or operation of the sensor in self-heating mode the sensing temperature and power consumption can be significantly decreased. Operation in self-heating mode means applying an external voltage directly to the sensor electrodes, which induces heat internally within the sensing material via the Joule heating effect.
Author contributions
Linh Ho Thuy Nguyen: conceptualization; writing – review & editing. Ali Mirzaei: conceptualization; writing – review & editing. Jin-Young Kim: writing – review & editing. Thang Bach Phan: writing – review & editing. Lam Dai Tran: writing – review & editing. Kevin C.-W. Wu: writing – review & editing. Hyoun Woo Kim: supervision. Sang Sub Kim: review & supervision. Tan Hoang Le Doan: writing – review & editing; supervision.Data availability
We would like to confirm that no primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Vietnam National University, Ho Chi Minh City, under grant number TX2025-50-01. In addition, this research was supported by the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (RS-2024-00412249).References
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, The Chemistry and Applications of Metal-Organic Frameworks, Science, 2013, 341(6149), 1230444 CrossRef PubMed.
- H. Wang, X. Dong, J. Lin, S. J. Teat, S. Jensen, J. Cure, E. V. Alexandrov, Q. Xia, K. Tan, Q. Wang, D. H. Olson, D. M. Proserpio, Y. J. Chabal, T. Thonhauser, J. Sun, Y. Han and J. Li, Topologically guided tuning of Zr-MOF pore structures for highly selective separation of C6 alkane isomers, Nat. Commun., 2018, 9(1), 1745 CrossRef PubMed.
- Y. Yao, X. Zhao, G. Chang, X. Yang and B. Chen, Hierarchically Porous Metal–Organic Frameworks: Synthetic Strategies and Applications, Small Struct., 2023, 4(1), 2200187 CrossRef CAS.
- G. Lin, B. Zeng, J. Li, Z. Wang, S. Wang, T. Hu and L. Zhang, A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism, Chem. Eng. J., 2023, 460, 141710 CrossRef CAS.
- N. H. Ly, N. B. Nguyen, H. N. Tran, T. T. H. Hoang, S.-W. Joo, Y. Vasseghian, H. Kamyab, S. Chelliapan and J. J. Klemeš, Metal-organic framework nanopesticide carrier for accurate pesticide delivery and decrement of groundwater pollution, J. Cleaner Prod., 2023, 402, 136809 CrossRef CAS.
- Y. Ye, Y. Xie, Y. Shi, L. Gong, J. Phipps, A. M. Al-Enizi, A. Nafady, B. Chen and S. Ma, A Microporous Metal-Organic Framework with Unique Aromatic Pore Surfaces for High Performance C2H6/C2H4 Separation, Angew. Chem., Int. Ed., 2023, 62(21), e202302564 CrossRef CAS PubMed.
- Z. Zhu, H. Tsai, S. T. Parker, J.-H. Lee, Y. Yabuuchi, H. Z. H. Jiang, Y. Wang, S. Xiong, A. C. Forse, B. Dinakar, A. Huang, C. Dun, P. J. Milner, A. Smith, P. Guimarães Martins, K. R. Meihaus, J. J. Urban, J. A. Reimer, J. B. Neaton and J. R. Long, High-Capacity, Cooperative CO2 Capture in a Diamine-Appended Metal–Organic Framework through a Combined Chemisorptive and Physisorptive Mechanism, J. Am. Chem. Soc., 2024, 146(9), 6072–6083 CrossRef CAS PubMed.
- B. Jie, H. Lin, Y. Zhai, J. Ye, D. Zhang, Y. Xie, X. Zhang and Y. Yang, Mechanism, design and application of fluorescent recognition based on metal organic frameworks in pollutant detection, Chem. Eng. J., 2023, 454, 139931 Search PubMed.
- Z. Xu, Z. Wu, S. Huang, K. Ye, Y. Jiang, J. Liu, J. Liu, X. Lu and B. Li, A metal-organic framework-based immunomodulatory nanoplatform for anti-atherosclerosis treatment, J. Controlled Release, 2023, 354, 615–625 CrossRef CAS PubMed.
- B. Q. G. Le and T. L. H. Doan, Trend in biodegradable porous nanomaterials for anticancer drug delivery, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2023, 15(4), e1874 CAS.
- H. Hu and Y. Wang, Recent advances in metal–organic frameworks as emerging platforms for immunoassays, TrAC, Trends Anal. Chem., 2024, 171, 117520 CrossRef CAS.
- M. H. Teplensky, M. Fantham, P. Li, T. C. Wang, J. P. Mehta, L. J. Young, P. Z. Moghadam, J. T. Hupp, O. K. Farha, C. F. Kaminski and D. Fairen-Jimenez, Temperature Treatment of Highly Porous Zirconium-Containing Metal–Organic Frameworks Extends Drug Delivery Release, J. Am. Chem. Soc., 2017, 139(22), 7522–7532 CrossRef CAS PubMed.
- Y. Shi, Z. Wang, Z. Li, H. Wang, D. Xiong, J. Qiu, X. Tian, G. Feng and J. Wang, Anchoring LiCl in the Nanopores of Metal–Organic Frameworks for Ultra-High Uptake and Selective Separation of Ammonia, Angew. Chem., Int. Ed., 2022, 61(47), e202212032 CrossRef CAS PubMed.
- J. Wang, J. Liu, H. Wang, M. Zhou, G. Ke, L. Zhang, J. Wu, Z. Gao and D. Lu, A comprehensive transformer-based approach for high-accuracy gas adsorption predictions in metal-organic frameworks, Nat. Commun., 2024, 15(1), 1904 CrossRef CAS PubMed.
- A. R. Yuvaraj, A. Jayarama, D. Sharma, S. S. Nagarkar, S. P. Duttagupta and R. Pinto, Role of metal-organic framework in hydrogen gas storage: A critical review, Int. J. Hydrogen Energy, 2024, 59, 1434–1458 Search PubMed.
- D.-K. Nguyen, J.-H. Lee, T. L.-H. Doan, T.-B. Nguyen, S. Park, S. S. Kim and B. T. Phan, H2 gas sensing of Co-incorporated metal-organic frameworks, Appl. Surf. Sci., 2020, 523, 146487 CrossRef CAS.
- K.-K. Liu, Z. Meng, Y. Fang and H.-L. Jiang, Conductive MOFs for electrocatalysis and electrochemical sensor, eScience, 2023, 3(6), 100133 CrossRef.
- H. Li, K. Wang, Y. Sun, C. T. Lollar, J. Li and H.-C. Zhou, Recent advances in gas storage and separation using metal–organic frameworks, Mater. Today, 2018, 21(2), 108–121 Search PubMed.
- M.-H. D. Dang, T. T. T. Nguyen, B. Q. G. Le, L. H. T. Nguyen, N. X. D. Mai, M. V. Nguyen, P. H. Tran and T. L. H. Doan, An effective combination of reusable Pd@MOF catalyst and deep eutectic solvents for high-performance C–C coupling reaction, J. Ind. Eng. Chem., 2022, 111, 111–120 Search PubMed.
- L. H. T. Nguyen, T. T. T. Nguyen, P. H. Tran, Y. Kawazoe, H. M. Le and T. L. H. Doan, Zr and Hf-metal-organic frameworks: Efficient and recyclable heterogeneous catalysts for the synthesis of 2-arylbenzoxazole via ring open pathway acylation reaction, J. Catal., 2019, 374, 110–117 Search PubMed.
- Y. Wang, W. Chen, J. Fu and Y. Liu, Efficient air water harvesting of TpPa-1 COFs@LiCl composite driven by solar energy, eScience, 2023, 3(4), 100154 CrossRef.
- S. Bazzazan, K. Moeinabadi-Bidgoli, Z. A. Lalami, S. Bazzazan, M. Mehrarya, F. E. Yeganeh, F. Hejabi, I. Akbarzadeh, H. Noorbazargan, M. Jahanbakhshi, N. Hossein-khannazer and E. Mostafavi, Engineered UIO-66 metal-organic framework for delivery of curcumin against breast cancer cells: An in vitro evaluation, J. Drug Delivery Sci. Technol., 2023, 79, 104009 CrossRef CAS.
- M. Pourmadadi, S. Ostovar, M. M. Eshaghi, M. Rajabzadeh-Khosroshahi, S. Safakhah, S. Ghotekar, A. Rahdar and A. M. Díez-Pascual, Nanoscale metallic-organic frameworks as an advanced tool for medical applications: Challenges and recent progress, Appl. Organomet. Chem., 2023, 37(3), e6982 Search PubMed.
- L. H. T. Nguyen, Y. Thi Dang, T. T. T. Nguyen, B. Q. G. Le, N. X. D. Mai, H. V. Nguyen, M.-T. Le, T. B. Phan and T. L. H. Doan, Pore engineering of biomolecule-based metal–organic framework nanocarriers for improving loading and release of paclitaxel, New J. Chem., 2022, 46(14), 6630–6635 RSC.
- S. Wei, R. Hou, Q. Zhu, I. Shakir, Z. Fang, X. Duan and Y. Xu, Hybrid materials based on covalent organic frameworks for photocatalysis, InfoMat, 2025, e12646 Search PubMed.
- J. Zhao, Z. Chen, S. Liu, P. Li, S. Yu, D. Ling and F. Li, Nano-bio interactions between 2D nanomaterials and mononuclear phagocyte system cells, BMEMat, 2024, 2(2), e12066 CrossRef CAS.
- L. H. Thuy Nguyen, S. T. Navale, D. H. Yang, H. T. T. Nguyen, T. B. Phan, J.-Y. Kim, A. Mirzaei, T. L. H. Doan, S. S. Kim and H. W. Kim, Fe-based metal-organic framework as a chemiresistive sensor for low-temperature monitoring of acetone gas, Sens. Actuators, B, 2023, 388, 133799 CrossRef CAS.
- M. Zhan, C. Ge, S. Hussain, A. S. Alkorbi, R. Alsaiari, N. A. Alhemiary, G. Qiao and G. Liu, Enhanced NO2 gas-sensing performance by core-shell SnO2/ZIF-8 nanospheres, Chemosphere, 2022, 291, 132842 CrossRef CAS PubMed.
- D.-K. Nguyen, J.-H. Lee, T.-B. Nguyen, T. Le Hoang Doan, B. T. Phan, A. Mirzaei, H. W. Kim and S. S. Kim, Realization of selective CO detection by Ni-incorporated metal-organic frameworks, Sens. Actuators, B, 2020, 315, 128110 Search PubMed.
- J. Abdi, N. M. Mahmoodi, M. Vossoughi and I. Alemzadeh, Synthesis of magnetic metal-organic framework nanocomposite (ZIF-8@SiO2@MnFe2O4) as a novel adsorbent for selective dye removal from multicomponent systems, Microporous Mesoporous Mater., 2019, 273, 177–188 Search PubMed.
- A. K. Singh, N. Jaiswal, R. K. Gautam and I. Tiwari, Development of g-C3N4/Cu-DTO MOF nanocomposite based electrochemical sensor towards sensitive determination of an endocrine disruptor BPSIP, J. Electroanal. Chem., 2021, 887, 115170 CrossRef CAS.
- Z. Xiao, L. B. Kong, S. Ruan, X. Li, S. Yu, X. Li, Y. Jiang, Z. Yao, S. Ye, C. Wang, T. Zhang, K. Zhou and S. Li, Recent development in nanocarbon materials for gas sensor applications, Sens. Actuators, B, 2018, 274, 235–267 CrossRef CAS.
- M.-S. Yao, W.-H. Li and G. Xu, Metal–organic frameworks and their derivatives for electrically-transduced gas sensors, Coord. Chem. Rev., 2021, 426, 213479 CrossRef CAS.
- N. Garg, A. Deep and A. L. Sharma, Metal-organic frameworks based nanostructure platforms for chemo-resistive sensing of gases, Coord. Chem. Rev., 2021, 445, 214073 Search PubMed.
- F. Dai, X. Cui, Y. Luo, D. Zhang, N. Li, Y. Huang and Y. Peng, Ultrathin MOF nanosheet-based resistive sensors for highly sensitive detection of methanol, Chem. Commun., 2022, 58(82), 11543–11546 RSC.
- I. Strauss, A. Mundstock, M. Treger, K. Lange, S. Hwang, C. Chmelik, P. Rusch, N. C. Bigall, T. Pichler, H. Shiozawa and J. Caro, Metal–Organic Framework Co-MOF-74-Based Host–Guest Composites for Resistive Gas Sensing, ACS Appl. Mater. Interfaces, 2019, 11(15), 14175–14181 Search PubMed.
- X. Shi, Y. Shan, M. Du and H. Pang, Synthesis and application of metal-organic framework films, Coord. Chem. Rev., 2021, 444, 214060 Search PubMed.
- X. Zhang, J. Wang, X.-X. Dong and Y.-K. Lv, Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in environment, Chemosphere, 2020, 242, 125144 CrossRef CAS PubMed.
- V. Bon, I. Senkovska, M. S. Weiss and S. Kaskel, Tailoring of network dimensionality and porosity adjustment in Zr- and Hf-based MOFs, CrystEngComm, 2013, 15(45), 9572–9577 Search PubMed.
- T. L. H. Doan, H. L. Nguyen, H. Q. Pham, N.-N. Pham-Tran, T. N. Le and K. E. Cordova, Tailoring the Optical Absorption of Water-Stable ZrIV- and HfIV-Based Metal–Organic Framework Photocatalysts, Chem. – Asian J., 2015, 10(12), 2660–2668 CrossRef CAS PubMed.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle Iii, M. Bosch and H.-C. Zhou, Tuning the structure and function of metal–organic frameworks via linker design, Chem. Soc. Rev., 2014, 43(16), 5561–5593 Search PubMed.
- C. McKinstry, R. J. Cathcart, E. J. Cussen, A. J. Fletcher, S. V. Patwardhan and J. Sefcik, Scalable continuous solvothermal synthesis of metal organic framework (MOF-5) crystals, Chem. Eng. J., 2016, 285, 718–725 Search PubMed.
- A. A. Bhoite, K. V. Patil, R. S. Redekar, P. S. Patil, V. A. Sawant and N. L. Tarwal, Solvothermal synthesis of binder free Ni-MOF thin films for supercapacitor electrodes, J. Solid State Chem., 2023, 326, 124192 CrossRef CAS.
- Y. Ban, Y. Li, X. Liu, Y. Peng and W. Yang, Solvothermal synthesis of mixed-ligand metal–organic framework ZIF-78 with controllable size and morphology, Microporous Mesoporous Mater., 2013, 173, 29–36 CrossRef CAS.
- Z. Meng, Z. Qiu, Y. Shi, S. Wang, G. Zhang, Y. Pi and H. Pang, Micro/nano metal–organic frameworks meet energy chemistry: A review of materials synthesis and applications, eScience, 2023, 3(2), 100092 Search PubMed.
- L. Huelsenbeck, H. Luo, P. Verma, J. Dane, R. Ho, E. Beyer, H. Hall, G. M. Geise and G. Giri, Generalized Approach for Rapid Aqueous MOF Synthesis by Controlling Solution pH, Cryst. Growth Des., 2020, 20(10), 6787–6795 Search PubMed.
- W. Xuan, R. Ramachandran, C. Zhao and F. Wang, Influence of synthesis temperature on cobalt metal-organic framework (Co-MOF) formation and its electrochemical performance towards supercapacitor electrodes, J. Solid State Electrochem., 2018, 22(12), 3873–3881 CrossRef CAS.
- M. Huang, C. Cao, L. Liu, W. Wei, Q.-L. Zhu and Z. Huang, Controlled synthesis of MOF-derived hollow and yolk–shell nanocages for improved water oxidation and selective ethylene glycol reformation, eScience, 2023, 3(5), 100118 CrossRef.
- I. Akpinar, X. Wang, K. Fahy, F. Sha, S. Yang, T. W. Kwon, P. J. Das, T. Islamoglu, O. K. Farha and J. F. Stoddart, Biomimetic Mineralization of Large Enzymes Utilizing a Stable Zirconium-Based Metal-Organic Frameworks, J. Am. Chem. Soc., 2024, 146(8), 5108–5117 CrossRef CAS PubMed.
- H. Wang, Y. Ren, X. Feng and H. Jiang, Ultrathin 2D Porphyrin-Based Zr-MOF Nanosheets as Heterogeneous Catalysts for Styrene Epoxidation and Benzylic C-H Oxidation, ChemCatChem, 2024, 16(15), e202400119 Search PubMed.
- T. E. Webber, W.-G. Liu, S. P. Desai, C. C. Lu, D. G. Truhlar and R. L. Penn, Role of a Modulator in the Synthesis of Phase-Pure NU-1000, ACS Appl. Mater. Interfaces, 2017, 9(45), 39342–39346 CrossRef CAS PubMed.
- T. T. Thu Nguyen, L. H. Thuy Nguyen, N. X. Dat Mai, H. K. Thi Ta, T. L. Thuong Nguyen, U.-C. Nguyen Le, B. T. Phan, N. N. Doan and T. Le Hoang Doan, Mild and Large-scale Synthesis of Nanoscale Metal-Organic Framework Used as a Potential Adenine-based Drug Nanocarrier, J. Drug Delivery Sci. Technol., 2020, 102135 Search PubMed.
- W. Y. Siew, N. H. H. Abu Bakar, M. Abu Bakar and A. Zainal Abidin, Influence of various Cu/Fe ratios on the surface properties of green synthesized Cu-Fe-BTC and it's relation to methylene blue adsorption, J. Hazard. Mater., 2021, 416, 125846 Search PubMed.
- R. K. A. Amali, H. N. Lim, I. Ibrahim, Z. Zainal and S. A. A. Ahmad, Energy-efficient green synthesis of metal-organic frameworks for effective electrochemical nitrite sensing, Appl. Mater. Today, 2023, 33, 101871 Search PubMed.
- R. Udaya Rajesh, T. Mathew, H. Kumar, A. Singhal and L. Thomas, Metal-organic frameworks: Recent advances in synthesis strategies and applications, Inorg. Chem. Commun., 2024, 162, 112223 Search PubMed.
- F. Sun, H. Guo, W. Zhang, T. Li, X. Lu, L. Zhao, D. Zhou and Q. Xia, Catalytic epoxidation of limonene and air with high performance by zirconium cobalt bimetallic MOF lamellar materials, Mol. Catal., 2024, 557, 113958 CrossRef CAS.
- H. R. Abid, A. Hanif, A. Keshavarz, J. Shang and S. Iglauer, C. H. 4 CO2, and H2 Adsorption Performance of the Metal–Organic Framework HKUST-1 by Modified Synthesis Strategies, Energy Fuels, 2023, 37(10), 7260–7267 CrossRef CAS.
- Y. R. Rosyara, A. Muthurasu, K. Chhetri, I. Pathak, T. H. Ko, P. C. Lohani, D. Acharya, T. Kim, D. Lee and H. Y. Kim, Highly Porous Metal–Organic Framework Entrapped by Cobalt Telluride–Manganese Telluride as an Efficient Bifunctional Electrocatalyst, ACS Appl. Mater. Interfaces, 2024, 16(8), 10238–10250 CrossRef CAS PubMed.
- Y. Xie, T. Zhang, B. Wang and W. Wang, The Application of Metal-Organic Frameworks in Water Treatment and Their Large-Scale Preparation: A Review, Materials, 2024, 17(9), 1972 CrossRef CAS PubMed.
- D. Sud and G. Kaur, A comprehensive review on synthetic approaches for metal-organic frameworks: From traditional solvothermal to greener protocols, Polyhedron, 2021, 193, 114897 CrossRef CAS.
- H. Wang, Y. Dai, Y. Wang and L. Yin, One-pot solvothermal synthesis of Cu–Fe-MOF for efficiently activating peroxymonosulfate to degrade organic pollutants in water:Effect of electron shuttle, Chemosphere, 2024, 352, 141333 CrossRef CAS PubMed.
- P. Kukkar, D. Kukkar, H. Sammi, K. Singh, M. Rawat, P. Singh, S. Basu and K.-H. Kim, A facile means for the improvement of sensing properties of metal-organic frameworks through control on the key synthesis variables, Sens. Actuators, B, 2018, 271, 157–163 CrossRef CAS.
- Y. Li, D.-G. Cai, Z.-H. Zhu, H. Xu, T.-F. Zheng, J.-L. Chen, S.-J. Liu and H.-R. Wen, Solvothermal synthesis and device fabrication of a Eu3+-based metal–organic framework as a turn-on and blue-shift fluorescence sensor toward Cr3+, Al3+ and Ga3+, Dalton Trans., 2023, 52(13), 4167–4175 RSC.
- T. L. H. Doan, T. Q. Dao, H. N. Tran, P. H. Tran and T. N. Le, An efficient combination of Zr-MOF and microwave irradiation in catalytic Lewis acid Friedel–Crafts benzoylation, Dalton Trans., 2016, 45(18), 7875–7880 RSC.
- T. V. Tran, H. T. N. Le, H. Q. Ha, X. N. T. Duong, L. H. T. Nguyen, T. L. H. Doan, H. L. Nguyen and T. Truong, A five coordination Cu(ii) cluster-based MOF and its application in the synthesis of pharmaceuticals via sp3 C–H/N–H oxidative coupling, Catal. Sci. Technol., 2017, 7(16), 3453–3458 RSC.
- D. Pedrero, C. Edo, F. Fernández-Piñas, R. Rosal and S. Aguado, Efficient removal of nanoplastics from water using mesoporous metal organic frameworks, Sep. Purif. Technol., 2024, 333, 125816 CrossRef CAS.
- Z. Gao, B. Li, Z. Li, T. Yu, S. Wang, Q. Fang, S. Qiu and M. Xue, Free-Standing Metal–Organic Framework Membranes Made by Solvent-Free Space-Confined Conversion for Efficient H2/CO2 Separation, ACS Appl. Mater. Interfaces, 2023, 15(15), 19241–19249 CrossRef CAS PubMed.
- L. H. T. Nguyen, T. T. T. Nguyen, Y. T. Dang, P. H. Tran and T. Le Hoang Doan, Microwave-assisted synthesis as an efficient method to enhance the catalytic activity of Zr-based metal organic framework UiO-66 in a heterocyclization reaction, Asian J. Org. Chem., 2019, 8(12), 2276–2281 CrossRef CAS.
- L. H. T. Nguyen, H. T. T. Nguyen, B. Q. G. Le, M.-H. D. Dang, T. T. T. Nguyen, N. X. D. Mai and T. L. H. Doan, Microwave-assisted solvothermal synthesis of defective zirconium-organic framework as a recyclable nano-adsorbent with superior adsorption capacity for efficient removal of toxic organic dyes, Colloid Interface Sci. Commun., 2022, 46, 100511 CrossRef CAS.
- Y. Dang, H. Hoang, H. Dong, K.-B. Bui, L. Nguyen, T. Phan, Y. Kawazoe and T. Doan, Microwave-assisted Synthesis of Nano Hf- and Zr-based Metal-Organic Frameworks for Enhancement of Curcumin Adsorption, Microporous Mesoporous Mater., 2020, 298, 110064 CrossRef CAS.
- L. H. T. Nguyen, T. T. T. Nguyen, M.-H. D. Dang, P. H. Tran and T. L. H. Doan, Heterocyclic reaction inducted by Brønsted–Lewis dual acidic Hf-MOF under microwave irradiation, Mol. Catal., 2021, 499, 111291 CrossRef CAS.
- M. Wen, N. Sun, L. Jiao, S. Q. Zang and H. L. Jiang, Microwave-Assisted Rapid Synthesis of MOF-Based Single-Atom Ni Catalyst for CO2 Electroreduction at Ampere-Level Current, Angew. Chem., 2024, 136(10), e202318338 CrossRef.
- X. Wu, Z. Bao, B. Yuan, J. Wang, Y. Sun, H. Luo and S. Deng, Microwave synthesis and characterization of MOF-74 (M= Ni, Mg) for gas separation, Microporous Mesoporous Mater., 2013, 180, 114–122 CrossRef CAS.
- C. Carrillo-Carrión, J. Farrando-Perez, L. L. Daemen, Y. Q. Cheng, A. J. Ramirez-Cuesta and J. Silvestre-Albero, Zr-Porphyrin Metal–Organic Framework as nanoreactor for boosting the formation of hydrogen clathrates, Angew. Chem., 2024, 136(6), e202315280 CrossRef.
- T. T. T. Nguyen, B. Q. G. Le, V. T. H. Nguyen, J. H. Lee, N. X. D. Mai, L. H. T. Nguyen and T. L. H. Doan, Microwave-Assisted Synthesis of Porous Biomolecule-Incorporated Metal-Organic Frameworks as Efficient Nanocarriers for Anti-Cancer Drugs, ChemistrySelect, 2023, 8(25), e202301543 CrossRef CAS.
- L. N. Appelhans, L. Hughes, B. McKenzie, M. Rodriguez, J. Griego, J. Briscoe, M. Moorman, E. Frederick and J. B. Wright, Facile microwave synthesis of zirconium metal-organic framework thin films on gold and silicon and application to sensor functionalization, Microporous Mesoporous Mater., 2021, 323, 111133 CrossRef CAS.
- P. Tapangpan, K. Panyarat, C. Chankaew, K. Grudpan and A. Rujiwatra, Terbium metal organic framework: microwave synthesis and selective sensing of nitrite, Inorg. Chem. Commun., 2020, 111, 107627 CrossRef CAS.
- I. Thomas-Hillman, A. Laybourn, C. Dodds and S. W. Kingman, Realising the environmental benefits of metal–organic frameworks: recent advances in microwave synthesis, J. Mater. Chem. A, 2018, 6(25), 11564–11581 RSC.
- T. J. Azbell, T. A. Pitt, M. M. Bollmeyer, C. Cong, K. M. Lancaster and P. J. Milner, Ionothermal Synthesis of Metal-Organic Frameworks Using Low-Melting Metal Salt Precursors, Angew. Chem., Int. Ed., 2023, 62(17), e202218252 CrossRef CAS PubMed.
- Q.-Y. Liu, Y.-L. Wang, N. Zhang, Y.-L. Jiang, J.-J. Wei and F. Luo, Spontaneous resolution in the ionothermal synthesis of homochiral Zn (II) metal–organic frameworks with (10, 3)-a topology constructed from achiral 5-sulfoisophthalate, Cryst. Growth Des., 2011, 11(9), 3717–3720 CrossRef CAS.
- K. Lu, A. Liebman Pelaez, L.-C. Wu, Y. Cao, C.-H. Zhu and H. Fu, Ionothermal synthesis of five keggin-type polyoxometalate-based metal–organic frameworks, Inorg. Chem., 2019, 58(3), 1794–1805 CrossRef CAS PubMed.
- M. Teixeira, R. A. Maia, L. Karmazin, B. Louis and S. A. Baudron, Ionothermal synthesis of calcium-based metal–organic frameworks in a deep eutectic solvent, CrystEngComm, 2022, 24(3), 601–608 RSC.
- P. Li, F.-F. Cheng, W.-W. Xiong and Q. Zhang, New synthetic strategies to prepare metal–organic frameworks, Inorg. Chem. Front., 2018, 5(11), 2693–2708 RSC.
- W. Li, W. Xiao, Q. Luo, J. Yan, G. Zhang, L. Chen and J. Sun, Ionic liquids promoted synthesis, enhanced functions, and expanded applications of porous organic frameworks, Coord. Chem. Rev., 2023, 493, 215304 CrossRef CAS.
- B. Y. Xia, Y. Yan, N. Li, H. B. Wu, X. W. D. Lou and X. Wang, A metal–organic framework-derived bifunctional oxygen electrocatalyst, Nat. Energy, 2016, 1(1), 1–8 Search PubMed.
- T. Huang, Y. Chen and J.-M. Lee, Two-dimensional cobalt/N-doped carbon hybrid structure derived from metal–organic frameworks as efficient electrocatalysts for hydrogen evolution, ACS Sustainable Chem. Eng., 2017, 5(7), 5646–5650 Search PubMed.
- J. Li, Q. Wu, X. Wang, B. Wang and T. Liu, Metal-organic framework-derived Co/CoO nanoparticles with tunable particle size for strong low-frequency microwave absorption in the S and C bands, J. Colloid Interface Sci., 2022, 628, 10–21 Search PubMed.
- W. Wang, X. Xu, W. Zhou and Z. Shao, Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting, Adv. Sci., 2017, 4(4), 1600371 Search PubMed.
- S. Głowniak, B. Szczęśniak, J. Choma and M. Jaroniec, Mechanochemistry: Toward green synthesis of metal–organic frameworks, Mater. Today, 2021, 46, 109–124 CrossRef.
- A. Metawea, R. Soto, M. Khraisheh, G. Walker and A. B. Albadarin, Application of Raman and chemometrics to the mechanochemical synthesis of 4, 4-bipyridine/cobalt based MOF using twin screw extrusion, Powder Technol., 2024, 437, 119558 CrossRef CAS.
- D. Chen, J. Zhao, P. Zhang and S. Dai, Mechanochemical synthesis of metal–organic frameworks, Polyhedron, 2019, 162, 59–64 Search PubMed.
- H. Ren and T. Wei, Electrochemical synthesis methods of metal-organic frameworks and their environmental analysis applications: a review, ChemElectroChem, 2022, 9(13), e202200196 Search PubMed.
- L. Wu, M. Zhao, Q. Ye, X. Xin, K. Zhang, Y. Chen and Z. Wang, Electrochemical synthesis of ultra-flexible PES/HKUST-1 composite membranes for wastewater treatment, Microporous Mesoporous Mater., 2024, 366, 112917 CrossRef CAS.
- Y. Liu, Y. Wei, M. Liu, Y. Bai, X. Wang, S. Shang, J. Chen and Y. Liu, Electrochemical Synthesis of Large Area Two-Dimensional Metal–Organic Framework Films on Copper Anodes, Angew. Chem., Int. Ed., 2021, 60(6), 2887–2891 CrossRef CAS PubMed.
- C. Lu, T. Ben, S. Xu and S. Qiu, Electrochemical synthesis of a microporous conductive polymer based on a metal–organic framework thin film, Angew. Chem., 2014, 126(25), 6572–6576 CrossRef.
- S. Jabarian and A. Ghaffarinejad, Electrochemical synthesis of NiBTC metal organic framework thin layer on nickel foam: an efficient electrocatalyst for the hydrogen evolution reaction, J. Inorg. Organomet. Polym. Mater., 2019, 29, 1565–1574 CrossRef CAS.
- Z. Ye, Y. Jiang, L. Li, F. Wu and R. Chen, MOF-related electrocatalysts for sulfur reduction/evolution reactions: Composition modulation, structure design, and mechanism research, eScience, 2023, 3(5), 100107 CrossRef.
- A. Asghar, N. Iqbal, T. Noor, B. M. Kariuki, L. Kidwell and T. L. Easun, Efficient electrochemical synthesis of a manganese-based metal–organic framework for H 2 and CO 2 uptake, Green Chem., 2021, 23(3), 1220–1227 RSC.
- S. Zhou, O. Shekhah, J. Jia, J. Czaban-Jóźwiak, P. M. Bhatt, A. Ramírez, J. Gascon and M. Eddaoudi, Electrochemical synthesis of continuous metal–organic framework membranes for separation of hydrocarbons, Nat. Energy, 2021, 6(9), 882–891 CrossRef CAS.
- F. Zhang, T. Zhang, X. Zou, X. Liang, G. Zhu and F. Qu, Electrochemical synthesis of metal organic framework films with proton conductive property, Solid State Ionics, 2017, 301, 125–132 CrossRef CAS.
- H. Yuan, J. Cui, N. Li, M. Li, X. Yu, W. Fan, A. Karmakar, J. Dong, S. J. Pennycook and H. Cai, On-chip template-directed conversion of metal hydroxides to metal–organic framework films with enhanced adhesion, ACS Appl. Mater. Interfaces, 2020, 12(32), 36715–36722 CrossRef CAS PubMed.
- W. Liu, Y. Yang, X. Yang, Y.-L. Peng, P. Cheng, Z. Zhang and Y. Chen, Template-directed fabrication of highly efficient metal–organic framework photocatalysts, ACS Appl. Mater. Interfaces, 2021, 13(49), 58619–58629 CrossRef CAS PubMed.
- Y. Yang, D. Cong and S. Hao, Template-Directed Ordered Mesoporous Silica@ Palladium-Containing Zinc Metal–Organic Framework Composites as Highly Efficient Suzuki Coupling Catalysts, ChemCatChem, 2016, 8(5), 900–905 CrossRef CAS.
- H. Huang, J.-R. Li, K. Wang, T. Han, M. Tong, L. Li, Y. Xie, Q. Yang, D. Liu and C. Zhong, An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks, Nat. Commun., 2015, 6(1), 8847 Search PubMed.
- L. Meng, B. Yu and Y. Qin, Templated interfacial synthesis of metal-organic framework (MOF) nano-and micro-structures with precisely controlled shapes and sizes, Commun. Chem., 2021, 4(1), 82 CrossRef CAS PubMed.
- R. Fan, N. Kang, Y. Li and L. Gao, A template-directed synthesis of metal–organic framework (MOF-74) ultrathin nanosheets for oxygen reduction electrocatalysis, RSC Adv., 2021, 11(16), 9353–9360 RSC.
- X. Yang, T. Liang, J. Sun, M. J. Zaworotko, Y. Chen, P. Cheng and Z. Zhang, Template-Directed Synthesis of Photocatalyst-Encapsulating Metal–Organic Frameworks with Boosted Photocatalytic Activity, ACS Catal., 2019, 9(8), 7486–7493 CrossRef CAS.
- B. Ding, J. Wang, Z. Chang, G. Xu, X. Hao, L. Shen, H. Dou and X. Zhang, Self-Sacrificial Template-Directed Synthesis of Metal–Organic Framework-Derived Porous Carbon for Energy-Storage Devices, ChemElectroChem, 2016, 3(4), 668–674 CrossRef CAS.
- S. Li, M. Dharmarwardana, R. P. Welch, Y. Ren, C. M. Thompson, R. A. Smaldone and J. J. Gassensmith, Template-directed synthesis of porous and protective core–shell bionanoparticles, Angew. Chem., Int. Ed., 2016, 55(36), 10691–10696 CrossRef CAS PubMed.
- M. Kim, M. Pander and H. R. Moon, Advancing Metal–Organic Framework Designs for Room-Temperature Chemiresistive Gas Sensors, ACS Appl. Electron. Mater., 2024, 6(5), 3024–3038 CrossRef CAS.
- R. Zhang, L. Lu, Y. Chang and M. Liu, Gas sensing based on metal-organic frameworks: Concepts, functions, and developments, J. Hazard. Mater., 2022, 429, 128321 CrossRef CAS PubMed.
- X. Peng, X. Wu, M. Zhang and H. Yuan, Metal–organic framework coated devices for gas sensing, ACS Sen., 2023, 8(7), 2471–2492 CrossRef CAS PubMed.
- J. Hao, X. Xu, H. Fei, L. Li and B. Yan, Functionalization of metal–organic frameworks for photoactive materials, Adv. Mater., 2018, 30(17), 1705634 CrossRef PubMed.
- S. A. A. Razavi and A. Morsali, Linker functionalized metal-organic frameworks, Coord. Chem. Rev., 2019, 399, 213023 CrossRef.
- B. T. Nguyen, H. L. Nguyen, T. C. Nguyen, K. E. Cordova and H. Furukawa, High Methanol Uptake Capacity in Two New Series of Metal–Organic Frameworks: Promising Materials for Adsorption-Driven Heat Pump Applications, Chem. Mater., 2016, 28(17), 6243–6249 CrossRef CAS.
- H. L. Nguyen, R. Matheu, C. S. Diercks, T. L. Doan, B. T. Nguyen and K. E. Cordova, Postsynthetic metalation of a new metal–organic framework to improve methane working storage capacity, ACS Mater. Lett., 2022, 4(11), 2375–2380 CrossRef CAS.
- T. M. T. Le, N. T. T. Nguyen, N. M. Q. Tran, Q. T. Pham, T. M. Le, L. D. Nguyen, P. H. Tran, T. L. H. Doan and L. H. T. Nguyen, Post-synthesis of curcumin-embedded zeolitic imidazole framework for copper ions detection, J. Porous Mater., 2024, 1–14 Search PubMed.
- S. Mandal, S. Natarajan, P. Mani and A. Pankajakshan, Post-synthetic modification of metal–organic frameworks toward applications, Adv. Funct. Mater., 2021, 31(4), 2006291 CrossRef CAS.
- L. H. Nguyen, H. L. Nguyen, T. L. Doan and P. H. Tran, A new superacid hafnium-based metal–organic framework as a highly active heterogeneous catalyst for the synthesis of benzoxazoles under solvent-free conditions, Catal. Sci. Technol., 2017, 7(19), 4346–4350 RSC.
- R. Thür, N. Van Velthoven, V. Lemmens, M. Bastin, S. Smolders, D. De Vos and I. F. Vankelecom, Modulator-mediated functionalization of MOF-808 as a platform tool to create high-performance mixed-matrix membranes, ACS Appl. Mater. Interfaces, 2019, 11(47), 44792–44801 CrossRef PubMed.
- Y. Liu, Y.-K. Lu, B. Zhang, L. Hou and Y.-Y. Wang, Post-synthetic functionalization of Ni-MOF by Eu3+ ions: luminescent probe for aspartic acid and magnetic property, Inorg. Chem., 2020, 59(11), 7531–7538 CrossRef CAS PubMed.
- Y. T. Dang, M.-H. D. Dang, N. X. D. Mai, L. H. T. Nguyen, T. B. Phan, H. V. Le and T. L. H. Doan, Room temperature synthesis of biocompatible nano Zn-MOF for the rapid and selective adsorption of curcumin, J. Sci.:Adv. Mater. Devices, 2020, 5(4), 560–565 CAS.
- L. H. T. Nguyen, M.-T. Le Nguyen, T. N. Ha, H. T. Hoang, N. K. Pham, Y. Kawazoe, D. Nguyen-Manh and T. L. H. Doan, Engineering functionalized Zr-MOFs as facile removal of indole: Experimental studies and first-principles modeling, J. Sci.: Adv. Mater. Devices, 2024, 9(1), 100661 CAS.
- D. E. Vanpoucke, Linker functionalization in MIL-47 (V)-R metal–organic frameworks: understanding the electronic structure, J. Phys. Chem. C, 2017, 121(14), 8014–8022 CrossRef CAS.
- M.-H. D. Dang, L. H. T. Nguyen, T. T. T. Nguyen, N. X. D. Mai, P. H. Tran and T. L. H. Doan, Using sulfate-functionalized Hf-based metal–organic frameworks as a heterogeneous catalyst for solvent-free synthesis of pyrimido [1, 2-a] benzimidazoles via one-pot three-component reaction, J. Ind. Eng. Chem., 2021, 103, 340–347 CrossRef.
- S. Dissegna, K. Epp, W. R. Heinz, G. Kieslich and R. A. Fischer, Defective metal-organic frameworks, Adv. Mater., 2018, 30(37), 1704501 CrossRef PubMed.
- W. Xiang, Y. Zhang, Y. Chen, C.-J. Liu and X. Tu, Synthesis, characterization and application of defective metal–organic frameworks: current status and perspectives, J. Mater. Chem. A, 2020, 8(41), 21526–21546 RSC.
- X. Ma, L. Wang, Q. Zhang and H. L. Jiang, Switching on the photocatalysis of metal–organic frameworks by engineering structural defects, Angew. Chem., Int. Ed., 2019, 58(35), 12175–12179 CrossRef CAS PubMed.
- D. T. Nguyen, L. D. T. Nguyen, Q. T. Pham, T. M. Le, B. Q. G. Le, N. X. D. Mai, T. L. H. Doan and L. H. T. Nguyen, Zeolitic imidazolate frameworks as an efficient platform for potential curcumin-based on/off fluorescent chemosensor, Microporous Mesoporous Mater., 2021, 327, 111445 CrossRef CAS.
- J.-H. Lee, T. T. T. Nguyen, L. H. T. Nguyen, T. B. Phan, S. S. Kim and T. L. H. Doan, Functionalization of zirconium-based metal–organic frameworks for gas sensing applications, J. Hazard. Mater., 2021, 403, 124104 CrossRef CAS PubMed.
- T. T. T. Nguyen, B. Q. G. Le, M.-H. D. Dang, B. T. Phan, N. X. D. Mai and T. L. H. Doan, Facile synthesis of novel fluorescent organosilica-coated MOF nanoparticles for fast curcumin adsorption, Microporous Mesoporous Mater., 2022, 338, 111944 CrossRef CAS.
- S. Li, M. Dharmarwardana, R. Welch, Y. Ren, C. Thompson, R. Smaldone and J. Gassensmith, Template-Directed Synthesis of Porous and Protective Core-Shell Bionanoparticles, Angew. Chem., 2016, 128, 10849–10854 CrossRef.
- S. Wang, W. Morris, Y. Liu, C. M. McGuirk, Y. Zhou, J. T. Hupp, O. K. Farha and C. A. Mirkin, Surface-specific functionalization of nanoscale metal–organic frameworks, Angew. Chem., Int. Ed., 2015, 54(49), 14738–14742 CrossRef CAS PubMed.
- S. Darvishi, S. Sadjadi, E. Monflier, A. Heydari and M. M. Heravi, Sulfonic acid-functionalized k-carrageenan/Cr-based metal-organic framework: An efficient and recyclable catalyst for fructose conversion to 5-hydroxymethylfurfural, Int. J. Biol. Macromol., 2024, 264, 130555 CrossRef CAS PubMed.
- Y. Fang, Y. Ma, M. Zheng, P. Yang, A. M. Asiri and X. Wang, Metal–organic frameworks for solar energy conversion by photoredox catalysis, Coord. Chem. Rev., 2018, 373, 83–115 CrossRef CAS.
- M. S. Khan, Y. Li, D.-S. Li, J. Qiu, X. Xu and H. Y. Yang, A review of metal–organic framework (MOF) materials as an effective photocatalyst for degradation of organic pollutants, Nanoscale Adv., 2023, 5(23), 6318–6348 RSC.
- M. Hefayathullah, S. Singh, V. Ganesan and G. Maduraiveeran, Metal-organic frameworks for biomedical applications: A review, Adv. Colloid Interface Sci., 2024, 331, 103210 CrossRef CAS PubMed.
- S.-J. Fan, J. Heinrich, M. S. Bloom, T.-Y. Zhao, T.-X. Shi, W.-R. Feng, Y. Sun, J.-C. Shen, Z.-C. Yang and B.-Y. Yang, Ambient air pollution and depression: a systematic review with meta-analysis up to 2019, Sci. Total Environ., 2020, 701, 134721 CrossRef CAS PubMed.
- D. A. Glencross, T.-R. Ho, N. Camina, C. M. Hawrylowicz and P. E. Pfeffer, Air pollution and its effects on the immune system, Free Radicals Biol. Med., 2020, 151, 56–68 CrossRef CAS PubMed.
- K. Wetchakun, T. Samerjai, N. Tamaekong, C. Liewhiran, C. Siriwong, V. Kruefu, A. Wisitsoraat, A. Tuantranont and S. Phanichphant, Semiconducting metal oxides as sensors for environmentally hazardous gases, Sens. Actuators, B, 2011, 160(1), 580–591 CrossRef CAS.
- P. Wu, Q. Guo, Y. Zhao, M. Bian, S. Cao, J. J. Zhang and X. Duan, Emerging concern on air pollution and health: Trade-off between air pollution exposure and physical activity, Eco-Environ. Health, 2024, 3(2), 202–207 CrossRef PubMed.
- M. Kampa and E. Castanas, Human health effects of air pollution, Environ. Pollut., 2008, 151(2), 362–367 CrossRef CAS PubMed.
- I. Manisalidis, E. Stavropoulou, A. Stavropoulos and E. Bezirtzoglou, Environmental and health impacts of air pollution: a review, Front. Public Health, 2020, 8, 14 CrossRef PubMed.
- R. M. Babadjouni, D. M. Hodis, R. Radwanski, R. Durazo, A. Patel, Q. Liu and W. J. Mack, Clinical effects of air pollution on the central nervous system; a review, J. Clin. Neurosci., 2017, 43, 16–24 Search PubMed.
- J. G. Lu, Air pollution: A systematic review of its psychological, economic, and social effects, Curr. Opin. Psychol., 2020, 32, 52–65 CrossRef PubMed.
- C. Li, X. Xia, Y. Lin and H. Wen, Polluted cognition: The effect of air pollution on online purchasing behavior, J. Retail. Consum. Serv., 2024, 79, 103839 CrossRef.
- K. Liu, H. Zhang, Y. Bo, Y. Chen, P. Zhang, C. Huang, Z. Yu and Z. Gao, Ambient air pollution and Children's health: An umbrella review, Atmos. Pollut. Res., 2024, 102108 Search PubMed.
- A. Mirzaei, H. R. Ansari, M. Shahbaz, J.-Y. Kim, H. W. Kim and S. S. Kim, Metal oxide semiconductor nanostructure gas sensors with different morphologies, Chemosensors, 2022, 10(7), 289 CrossRef CAS.
- A. M. Kotecha, A. D. Corrêa, K. M. Fisher and J. V. Rushworth, Olfactory dysfunction as a global biomarker for sniffing out Alzheimer's disease: a meta-analysis, Biosensors, 2018, 8(2), 41 Search PubMed.
- A. Mirzaei, S. S. Kim and H. W. Kim, Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances, J. Hazard. Mater., 2018, 357, 314–331 Search PubMed.
- A. Nasri, M. Petrissans, V. Fierro and A. Celzard, Gas sensing based on organic composite materials: Review of sensor types, progresses and challenges, Mater. Sci. Semicond. Process., 2021, 128, 105744 CrossRef CAS.
- L. Hao, W. Ruijie, L. Hua-Bin, H. Li-Ying, Y. Wen-Jing, H. Zhong-Qiu, S.-R. Fan and W. Yi, A novel catalytic-type gas sensor based on alumina ceramic substrates loaded with catalysts and printed electrodes, Chin. J. Anal. Chem., 2021, 49(11), 93–101 Search PubMed.
- A. Mirzaei, J.-H. Kim, H. W. Kim and S. S. Kim, Gasochromic WO3 nanostructures for the detection of hydrogen gas: An overview, Appl. Sci., 2019, 9(9), 1775 CrossRef CAS.
- A. H. Ismail and Y. Sulaiman, Review on the utilisation of sensing materials for intrinsic optical NH3 gas sensors, Synth. Met., 2021, 280, 116860 Search PubMed.
- G. Korotcenkov, S. D. Han and J. R. Stetter, Review of electrochemical hydrogen sensors, Chem. Rev., 2009, 109(3), 1402–1433 Search PubMed.
- Z. Zhai, X. Zhang, X. Hao, B. Niu and C. Li, Metal–organic frameworks materials for capacitive gas sensors, Adv. Mater. Technol., 2021, 6(10), 2100127 CrossRef CAS.
- A. Singh, N. Chowdhury, S. C. Roy and B. Bhowmik, Review of thin film transistor gas sensors: Comparison with resistive and capacitive sensors, J. Electron. Mater., 2022, 51(5), 1974–2003 CrossRef CAS.
- E. H. Kwon, M. Kim, C. Y. Lee, M. Kim and Y. D. Park, Metal–organic-framework-decorated carbon nanofibers with enhanced gas sensitivity when incorporated into an organic semiconductor-based gas sensor, ACS Appl. Mater. Interfaces, 2022, 14(8), 10637–10647 Search PubMed.
- S. Fanget, S. Hentz, P. Puget, J. Arcamone, M. Matheron, E. Colinet, P. Andreucci, L. Duraffourg, E. Myers and M. Roukes, Gas sensors based on gravimetric detection—A review, Sens. Actuators, B, 2011, 160(1), 804–821 Search PubMed.
- Z. Li, Z. Yao, A. A. Haidry, T. Plecenik, L. Xie, L. Sun and Q. Fatima, Resistive-type hydrogen gas sensor based on TiO2: A review, Int. J. Hydrogen Energy, 2018, 43(45), 21114–21132 CrossRef CAS.
- S. M. Majhi, A. Mirzaei, H. W. Kim, S. S. Kim and T. W. Kim, Recent advances in energy-saving chemiresistive gas sensors: A review, Nano Energy, 2021, 79, 105369 CrossRef CAS PubMed.
- A. Mirzaei, S. Leonardi and G. Neri, Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review, Ceram. Int., 2016, 42(14), 15119–15141 CrossRef CAS.
- V. Amiri, H. Roshan, A. Mirzaei, G. Neri and A. I. Ayesh, Nanostructured metal oxide-based acetone gas sensors: A review, Sensors, 2020, 20(11), 3096 CrossRef CAS PubMed.
- Y. Masuda, Recent advances in SnO2 nanostructure based gas sensors, Sens. Actuators, B, 2022, 364, 131876 Search PubMed.
- M. A. Franco, P. P. Conti, R. S. Andre and D. S. Correa, A review on chemiresistive ZnO gas sensors, Sens. Actuators Rep., 2022, 4, 100100 CrossRef.
- M. Wusiman and F. Taghipour, Methods and mechanisms of gas sensor selectivity, Crit. Rev. Solid State Mater. Sci., 2022, 47(3), 416–435 CrossRef CAS.
- X. Huang, Z. Gong and Y. Lv, Advances in metal-organic frameworks-based gas sensors for hazardous substances, TrAC, Trends Anal. Chem., 2022, 153, 116644 Search PubMed.
- S. Chen, X. Duan, C. Liu, S. Liu, P. Li, D. Su, X. Sun, Y. Guo, W. Chen and Z. Wang, La-Ce-MOF nanocomposite coated quartz crystal microbalance gas sensor for the detection of amine gases and formaldehyde, J. Hazard. Mater., 2024, 467, 133672 Search PubMed.
- S. G. Surya, S. Bhanoth, S. M. Majhi, Y. D. More, V. M. Teja and K. N. Chappanda, A silver nanoparticle-anchored UiO-66 (Zr) metal–organic framework (MOF)-based capacitive H 2 S gas sensor, CrystEngComm, 2019, 21(47), 7303–7312 RSC.
- H. Sohrabi, S. Ghasemzadeh, Z. Ghoreishi, M. R. Majidi, Y. Yoon, N. Dizge and A. Khataee, Metal-organic frameworks (MOF)-based sensors for detection of toxic gases: A review of current status and future prospects, Mater. Chem. Phys., 2023, 299, 127512 Search PubMed.
- Y. M. Jo, Y. K. Jo, J. H. Lee, H. W. Jang, I. S. Hwang and D. J. Yoo, MOF-based chemiresistive gas sensors: toward new functionalities, Adv. Mater., 2023, 35(43), 2206842 CrossRef CAS PubMed.
- S. M. Majhi, A. Ali, P. Rai, Y. E. Greish, A. Alzamly, S. G. Surya, N. Qamhieh and S. T. Mahmoud, Metal–organic frameworks for advanced transducer based gas sensors: review and perspectives, Nanoscale Adv., 2022, 4(3), 697–732 Search PubMed.
- X. Zhang, S. Zhang, Y. Tang, X. Huang and H. Pang, Recent advances and challenges of metal–organic framework/graphene-based composites, Composites, Part B, 2022, 230, 109532 Search PubMed.
- L. J. Small, M. E. Schindelholz and T. M. Nenoff, Hold on tight: MOF-based irreversible gas sensors, Ind. Eng. Chem. Res., 2021, 60(21), 7998–8006 CrossRef CAS.
- L. Chen, X. Zhang, X. Cheng, Z. Xie, Q. Kuang and L. Zheng, The function of metal–organic frameworks in the application of MOF-based composites, Nanoscale Adv., 2020, 2(7), 2628–2647 Search PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Metal–organic framework materials as chemical sensors, Chem. Rev., 2012, 112(2), 1105–1125 Search PubMed.
- X.-F. Wang, X.-Z. Song, K.-M. Sun, L. Cheng and W. Ma, MOFs-derived porous nanomaterials for gas sensing, Polyhedron, 2018, 152, 155–163 CrossRef CAS.
- H. Wei, H. Zhang, B. Song, K. Yuan, H. Xiao, Y. Cao and Q. Cao, Metal–Organic Framework (MOF) derivatives as promising chemiresistive gas sensing materials: A Review, Int. J. Environ. Res. Public Health, 2023, 20(5), 4388 Search PubMed.
- H. Yuan, N. Li, W. Fan, H. Cai and D. Zhao, Metal-organic framework based gas sensors, Adv. Sci., 2022, 9(6), 2104374 Search PubMed.
- M. Drobek, J.-H. Kim, M. Bechelany, C. Vallicari, A. Julbe and S. S. Kim, MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity, ACS Appl. Mater. Interfaces, 2016, 8(13), 8323–8328 CrossRef CAS PubMed.
- M.-S. Yao, L.-A. Cao, Y.-X. Tang, G.-E. Wang, R.-H. Liu, P. N. Kumar, G.-D. Wu, W.-H. Deng, W.-J. Hong and G. Xu, Gas transport regulation in a MO/MOF interface for enhanced selective gas detection, J. Mater. Chem. A, 2019, 7(31), 18397–18403 RSC.
- Z. Wang, L. Zhu, J. Wang, R. Zhuang, P. Mu, J. Wang and W. Yan, Advances in functional guest materials for resistive gas sensors, RSC Adv., 2022, 12(38), 24614–24632 Search PubMed.
- X. Zhuang, S. Zhang, Y. Tang, F. Yu, Z. Li and H. Pang, Recent progress of MOF/MXene-based composites: synthesis, functionality and application, Coord. Chem. Rev., 2023, 490, 215208 Search PubMed.
- H. Shiozawa, B. C. Bayer, H. Peterlik, J. C. Meyer, W. Lang and T. Pichler, Doping of metal–organic frameworks towards resistive sensing, Sci. Rep., 2017, 7(1), 2439 Search PubMed.
- H.-Y. Li, S.-N. Zhao, S.-Q. Zang and J. Li, Functional metal–organic frameworks as effective sensors of gases and volatile compounds, Chem. Soc. Rev., 2020, 49(17), 6364–6401 RSC.
- C. Arul, K. Moulaee, N. Donato, D. Iannazzo, N. Lavanya, G. Neri and C. Sekar, Temperature modulated Cu-MOF based gas sensor with dual selectivity to acetone and NO2 at low operating temperatures, Sens. Actuators, B, 2021, 329, 129053 CrossRef CAS.
- W.-T. Koo, H.-J. Cho, D.-H. Kim, Y. H. Kim, H. Shin, R. M. Penner and I.-D. Kim, Chemiresistive hydrogen sensors: fundamentals, recent advances, and challenges, ACS Nano, 2020, 14(11), 14284–14322 Search PubMed.
- J. Baeyens, H. Zhang, J. Nie, L. Appels, R. Dewil, R. Ansart and Y. Deng, Reviewing the potential of bio-hydrogen production by fermentation, Renewable Sustainable Energy Rev., 2020, 131, 110023 CrossRef CAS.
- Q. Zhang, H. Yang, T. Zhou, X. Chen, W. Li and H. Pang, Metal–organic frameworks and their composites for environmental applications, Adv. Sci., 2022, 9(32), 2204141 CrossRef CAS PubMed.
- M. A. Deshmukh, M. D. Shirsat, A. Ramanaviciene and A. Ramanavicius, Composites based on conducting polymers and carbon nanomaterials for heavy metal ion sensing, Crit. Rev. Anal. Chem., 2018, 48(4), 293–304 CrossRef CAS PubMed.
- A. Mirzaei, S. P. Bharath, J.-Y. Kim, K. K. Pawar, H. W. Kim and S. S. Kim, N-Doped Graphene and its derivatives as resistive gas sensors: An overview, Chemosensors, 2023, 11(6), 334 Search PubMed.
- C. Wang, Y. Wang, Z. Yang and N. Hu, Review of recent progress on graphene-based composite gas sensors, Ceram. Int., 2021, 47(12), 16367–16384 Search PubMed.
- S. M. Majhi, A. Mirzaei, H. W. Kim and S. S. Kim, Reduced graphene oxide (rGO)-loaded metal-oxide nanofiber gas sensors: An overview, Sensors, 2021, 21(4), 1352 CrossRef CAS PubMed.
- M. S. More, G. A. Bodkhe, N. N. Ingle, F. Singh, M.-L. Tsai, M. Kim and M. D. Shirsat, Metal-organic framework (MOF)/reduced graphene oxide (rGO) composite for high performance CO sensor, Solid-State Electron., 2023, 204, 108638 Search PubMed.
- C. Gao, T. Wang, X. Wang and A. NH3, Gas Sensor Based on Flexible Copper (II) isonicotinate MOF/Reduced Graphene Oxide Composite Modified Interdigital Electrode, Int. J. Electrochem. Sci., 2022, 17(7), 220763 Search PubMed.
- K. Jayaramulu, M. E. DMello, K. Kesavan, A. Schneemann, M. Otyepka, S. Kment, C. Narayana, S. B. Kalidindi, R. S. Varma and R. Zboril, A multifunctional covalently linked graphene–MOF hybrid as an effective chemiresistive gas sensor, J. Mater. Chem. A, 2021, 9(32), 17434–17441 RSC.
- Y. Yuan, J. Chen, K. Polat and A. Alhudhaif, An innovative approach to detecting the freshness of fruits and vegetables through the integration of convolutional neural networks and bidirectional long short-term memory network, Curr. Res. Food Sci., 2024, 8, 100723 Search PubMed.
- Q. Zhao, Z. Duan, Z. Yuan, X. Li, W. Si, B. Liu, Y. Zhang, Y. Jiang and H. Tai, High performance ethylene sensor based on palladium-loaded tin oxide: Application in fruit quality detection, Chin. Chem. Lett., 2020, 31(8), 2045–2049 CrossRef CAS.
- H. R. Ansari, A. Mirzaei, H. Shokrollahi, R. Kumar, J.-Y. Kim, H. W. Kim, M. Kumar and S. S. Kim, Flexible/wearable resistive gas sensors based on 2D materials, J. Mater. Chem. C, 2023, 11(20), 6528–6549 Search PubMed.
- S. Li, X. Hu, S. Chen, X. Wang, H. Shang, Y. Zhou, J. Dai, L. Xiao, W. Qin and Y. Liu, Synthesis of γ-cyclodextrin metal-organic framework as ethylene absorber for improving postharvest quality of kiwi fruit, Food Hydrocolloids, 2023, 136, 108294 Search PubMed.
- J. Chai, B. Liao, R. Li and Z. Liu, Changes in taste and volatile compounds and ethylene production determined the eating window of ‘Xuxiang’and ‘Cuixiang’kiwifruit cultivars, Postharvest Biol. Technol., 2022, 194, 112093 Search PubMed.
- H. Yan, J. Wang, N. Shi, Y. Han, S. Zhang and G. Zhao, A flexible and wearable chemiresistive ethylene gas sensor modified with PdNPs-SWCNTs@ Cu-MOF-74 nanocomposite: a targeted strategy for the dynamic monitoring of fruit freshness, Chem. Eng. J., 2024, 488, 151142 Search PubMed.
- P. Orellano, J. Reynoso and N. Quaranta, Short-term exposure to sulphur dioxide (SO2) and all-cause and respiratory mortality: A systematic review and meta-analysis, Environ. Int., 2021, 150, 106434 CrossRef CAS PubMed.
- N. Ingle, P. Sayyad, M. Deshmukh, G. Bodkhe, M. Mahadik, T. Al-Gahouari, S. Shirsat and M. D. Shirsat, A chemiresistive gas sensor for sensitive detection of SO 2 employing Ni-MOF modified–OH-SWNTs and–OH-MWNTs, Appl. Phys. A: Mater. Sci. Process., 2021, 127, 1–10 CrossRef.
- I. Fratoddi, I. Venditti, C. Cametti and M. V. Russo, Chemiresistive polyaniline-based gas sensors: A mini review, Sens. Actuators, B, 2015, 220, 534–548 Search PubMed.
- V. Kumar, A. Mirzaei, M. Bonyani, K.-H. Kim, H. W. Kim and S. S. Kim, Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia, TrAC, Trends Anal. Chem., 2020, 129, 115938 Search PubMed.
- S. K. Bhardwaj, G. C. Mohanta, A. L. Sharma, K.-H. Kim and A. Deep, A three-phase copper MOF-graphene-polyaniline composite for effective sensing of ammonia, Anal. Chim. Acta, 2018, 1043, 89–97 CrossRef CAS PubMed.
- L. V. Kayser and D. J. Lipomi, Stretchable conductive polymers and composites based on PEDOT and PEDOT:PSS, Adv. Mater., 2019, 31(10), 1806133 Search PubMed.
- G. Garg, N. Garg, A. Deep and D. Soni, Zr-MOF and PEDOT:PSS composite sensor for chemoresistive sensing of toluene at room temperature, J. Alloys Compd., 2023, 956, 170309 CrossRef CAS.
- A. Ali, H. H. AlTakroori, Y. E. Greish, A. Alzamly, L. A. Siddig, N. Qamhieh and S. T. Mahmoud, Flexible Cu3 (HHTP) 2 MOF membranes for gas sensing application at room temperature, Nanomaterials, 2022, 12(6), 913 CrossRef CAS PubMed.
- B. Huang, Y. Li and W. Zeng, Application of metal-organic framework-based composites for gas sensing and effects of synthesis strategies on gas-sensitive performance, Chemosensors, 2021, 9(8), 226 CrossRef CAS.
- D. Huang, P. Yong and S. Shen, MOF-decorated sea urchin-like In2O3 gas sensor with higher gas sensitivity to formaldehyde, J. Solid State Chem., 2023, 328, 124336 Search PubMed.
- L. Fappiano, F. Carriera, A. Iannone, I. Notardonato and P. Avino, A review on recent sensing methods for determining formaldehyde in agri-food chain: A comparison with the conventional analytical approaches, Foods, 2022, 11(9), 1351 CrossRef CAS PubMed.
- H. Salonen, T. Salthammer and L. Morawska, Human exposure to NO2 in school and office indoor environments, Environ. Int., 2019, 130, 104887 Search PubMed.
- Y. Ogen, Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality, Sci. Total Environ., 2020, 726, 138605 CrossRef CAS PubMed.
- K. Y. Shin, L. H. T. Nguyen, H. L. Nguyen, A. Mirzaei, V. N. H. Tran, N. X. D. Mai, N. Q. Tran, W. Oum, E. B. Kim and H. M. Kim, Titanium-based metal-organic-framework-coated SnO2 nanowires with enhanced NO2 gas sensing capability in humid environment, Sens. Actuators, B, 2023, 394, 134425 CrossRef CAS.
- J.-H. Kim, A. Mirzaei, H. W. Kim and S. S. Kim, Combination of Pd loading and electron beam irradiation for superior hydrogen sensing of electrospun ZnO nanofibers, Sens. Actuators, B, 2019, 284, 628–637 Search PubMed.
- D. H. Yang, T. T. T. Nguyen, S. T. Navale, L. H. T. Nguyen, Y. T. Dang, N. X. D. Mai, T. B. Phan, J.-Y. Kim, T. L. H. Doan and S. S. Kim, Novel amine-functionalized zinc-based metal-organic framework for low-temperature chemiresistive hydrogen sensing, Sens. Actuators, B, 2022, 368, 132120 Search PubMed.
- P. Goswami and G. Gupta, Recent progress of flexible NO2 and NH3 gas sensors based on transition metal dichalcogenides for room temperature sensing. Materials Today, Chemistry, 2022, 23, 100726 Search PubMed.
- D. Kwak, Y. Lei and R. Maric, Ammonia gas sensors: A comprehensive review, Talanta, 2019, 204, 713–730 CrossRef CAS PubMed.
- H.-Y. Li, C.-S. Lee, D. H. Kim and J.-H. Lee, Flexible room-temperature NH3 sensor for ultrasensitive, selective, and humidity-independent gas detection, ACS Appl. Mater. Interfaces, 2018, 10(33), 27858–27867 CrossRef CAS PubMed.
- M.-H. D. Dang, S. T. Navale, D. H. Yang, J.-Y. Kim, L. H. T. Nguyen, N. X. D. Mai, T. B. Phan, H. W. Kim, T. L. H. Doan and S. S. Kim, Sulfate-functionalized hafnium-organic frameworks as a highly effective chemiresistive sensor for low-temperature detection of hazardous NH3 gas, Sens. Actuators, B, 2022, 367, 132094 CrossRef CAS.
- J. A. Guzman, Carbon monoxide poisoning, Crit. Care Clin., 2012, 28(4), 537–548 CrossRef PubMed.
- J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-G. Huang and K. S. Walton, Stability and degradation mechanisms of metal–organic frameworks containing the Zr6O4(OH)4 secondary building unit, J. Mater. Chem. A, 2013, 1(18), 5642–5650 RSC.
- L. P. L. Mosca, A. B. Gapan, R. A. Angeles and E. C. R. Lopez, Adv. Future Trends Eng. Proc., 2023, 56(1), 146 Search PubMed.
- E. Namratha, T. Ghosh, S. Manabolu, A. Reddy, R. R. Gangavarapu and K. Suresh, Design and synthesis of M-TCPP@PVP (M = Ni, Zn) MOF composites for enhanced ammonia gas sensing and supercapacitor applications, J. Mater. Sci.: Mater. Electron., 2024, 35 Search PubMed.
- J. Meng, X. Liu, C. Niu, Q. Pang, J. Li, F. Liu, Z. Liu and L. Mai, Advances in metal–organic framework coatings: versatile synthesis and broad applications, Chem. Soc. Rev., 2020, 49(10), 3142–3186 RSC.
Footnote |
| † Congratulations to Vietnam National University Ho Chi Minh City on its 30th Anniversary (1995–2025). |
| This journal is © The Royal Society of Chemistry 2025 |