The effects of denitrification with sludge alkaline fermentation liquid and thermal hydrolysis liquid as carbon sources
Jian Suna,
Mei Suna,
Liang Guo*abc,
Yangguo Zhaoa,
Mengchun Gaoa and
Zonglian She*a
aCollege of Environmental Science and Engineering, Ocean University of China, Qingdao, 266100, China. E-mail: geletu@ouc.edu.cn; Fax: +86-532-66782810; Tel: +86-532-66781020
bKey Laboratory of Marine Environmental and Ecology, Ministry of Education, Ocean University of China, Qingdao, 266100, China
cShandong Provincial Key Laboratory of Marine Environment and Geological Engineering, Qingdao, 266100, China
First published on 19th July 2016
Abstract
Nitrate removal using the sludge alkaline fermentation liquid and thermal hydrolysis liquid as external carbon sources was investigated in this study. The nitrate removal efficiency with sludge fermentation liquid was higher than that with sludge thermal hydrolysis liquid, and the specific denitrifying rates were 7.94 g per kg MLVSS per h (MLVSS is Mixed Liquor Volatile Suspended Solids) and 1.16 g per kg MLVSS per h, respectively. The utilization of dissolved organic matters (DOM) during the denitrification process was analyzed by three-dimensional excitation-emission matrix (EEM) fluorescence spectroscopy with fluorescence regional integration (FRI) analysis. The EEM fluorescence intensity of region I (tyrosine-like protein) and IV (soluble microbial by-product) all decreased with two kinds of sludge carbon sources. Furthermore, the consumption of soluble chemical oxygen demand (SCOD), volatile fatty acids (VFAs), protein and carbohydrate by the denitrifier during the denitrification process were also analyzed.
1. Introduction
In the wastewater biological nitrogen removal process, the traditional heterotrophic denitrification process requires a series of steps from nitrate to nitrogen gas. And denitrification is the key removal mechanisms for transforming nitrate to gaseous forms of nitrogen. In addition, the amount of organic compounds in influential wastewater often is not sufficient for efficient denitrification. In such cases, specific available commercial compounds (“conventional” external carbon sources), such as methanol, ethanol, acetic acid, and sodium acetate, are usually added to improve the biological nutrient removal performance.1 It was reported that methanol has to be oxidized to its corresponding VFAs before denitrifier utilization, which leads to lower denitrifying rates.2,3 Furthermore, these commercial external carbon sources are expensive and increases operational cost. So it is important to find an economical carbon sources.Various industrial by-products or waste materials have recently received more attention as “alternative”, cost-effective external carbon sources for denitrification. It was reported that with waste sludge fermentation liquid as carbon source, the removal of nitrate were enhanced more markedly than that with glucose; if inexpensive and renewable sludge fermentation liquid was used instead of traditional organic carbon as feedstock for biological nitrate removal process, the operation cost could be greatly reduced.4 The methods how to extract and utilize the sludge internal carbon source efficiently are critical and have attracted more and more attention. The VFAs have been proven to be more direct carbon sources to enhance biological nitrogen removal comparing to the traditional extra organics.5 The alkaline fermentation liquid showed better performance for denitrification and higher specific endogenous denitrification rate.6 The sludge fermentation liquid is rich in VFAs, which can be obtained from anaerobic acidification and subsequently consumed for denitrification in many anaerobic–anoxic coupled processes.7–9
There are not only VFAs but also protein and carbohydrate in the sludge fermentation liquid, which also can be served as external carbon sources. Until recently, there was little information on the transformation and consumption of protein, carbohydrate and other organic compounds during denitrification process using sludge external carbon sources. So the objective of this study was to investigate the utilization of the sludge fermentation liquid and the hydrolysis liquid as carbon sources on the nitrate removal. The changes of COD, protein and carbohydrate, as well as the EEM fluorescence characters with FRI during the denitrification process was also analyzed.
2. Material and method
2.1 Seeding sludge and synthetic wastewater
Two internal recycle SBRs, each with working volume of 2 L, were used to investigate the effect of the carbon sources on nitrate removal. The seeding sludge and sludge carbon sources were taken from the secondary sedimentation tank of the Tuandao municipal waste water treatment plant in Qingdao, China. Prior to use, sludge was sieved by grid size 2.0 mm to remove coarse matter and stored in 4 °C refrigerator. The characteristics of waste sludge are given in Table 1.| TCOD (mg L−1) | SCOD (mg L−1) | Protein (mg L−1) | Carbohydrate (mg L−1) | TSS (g L−1) | VSS (g L−1) | pH |
|---|---|---|---|---|---|---|
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620 |
2857 | 43.41 | 28.08 | 10.85 | 7.69 | 6.50 |
The synthetic wastewater fed into the two SBRs contained (per liter): 432.9 mg KNO3 (60 mg NO3−–N), 52.6 mg KH2PO4, 100 mg NaHCO3. The sludge fermentation liquid and the sludge thermal hydrolysis liquid were added in two SBRs as denitrification carbon source, and the influent COD was about 600 mg L−1, respectively. Additionally, 1 mL trace-element was added per liter of synthetic wastewater. Each liter of trace-element was composed of 30 g MgSO4, 17.2 g FeSO4·7H2O, 7.5 g CaCl2·2H2O, 0.5 g MnCl2, 0.01 g NiCl2·6H2O, 0.05 g H3BO3, 6.105 g ZnSO4·7H2O, 0.22 g CuSO4·5H2O, 0.5 g CoCl2·6H2O.
2.2 Experimental procedure
The SBRs were automatically operated with time controllers regulating all peristaltic pumps and stirrers. Each SBR was operated on the three cycles per day, each cycle consisted 7 min feeding, 7 h anoxic, 43 min settling, 2 min decanting and 8 min idle periods. During the 7 min feeding period, 1.0 L of synthetic wastewater was pumped into the reactor.Sealed conical flasks (250 mL) were used in the batch experiments to investigate denitrifying rate. Denitrifying sludge was inoculated in sealed conical flask. The concentrations of denitrifying sludge was 1250 ± 300 mg VSS per L. Afterwards, fermentation liquid and hydrolyzed liquid were added into the reactors respectively. Then nitrogen gas was immediately blown into the reactors to establish an anoxic environment.
2.3 Characteristics of the sludge alkaline fermentation liquid and thermal hydrolysis liquid
The sludge alkaline fermentation liquid was obtained by fermenting in the incubator at pH 10.0 and 35 °C for 12 h. The sludge thermal hydrolysis liquid got by hydrolyzing at 100 °C for 1 h. Then the mixture was centrifuged at 4800 rpm for 20 min, and the supernatant was used as carbon source and stored at 4 °C before used. The characteristics of the sludge fermentation liquid and the hydrolysis liquid are given in Table 2.| Parameters | TCOD | SCOD | VFAs | Protein | Carbohydrate | NO3−–N | NO2−–N | NH4+–N |
|---|---|---|---|---|---|---|---|---|
| Fermentation | 2909 | 2670 | 819 | 877 | 430 | 19.48 | 0.04 | 203 |
| Hydrolysis | 3648 | 3576 | — | 1791 | 1076 | 17.88 | 0.31 | 254 |
2.4 Analytical methods
The FRI technique was adopted for EEM spectral data analysis.14 EEM peaks were divided into five regions,16 including simple aromatic proteins such as tyrosine and tryptophan (regions I and II), fulvic acid-like substances (region III), related to soluble microbial by-product-like materials (region IV), humic acid-like organics (region V).
The FRI technique was developed to integrate the area beneath EEM spectra. The volume beneath region “i”of the EEM was ϕi. The normalized ex/em area volumes (ϕi,n, ϕT,n) can be calculated with eqn (1) and (2).
 | (1) |
| ϕT,n = ∑ϕi,n | (2) |
In which Δλex is the excitation wavelength interval (taken as 5 nm), Δλem is the emission wavelength interval (taken as 5 nm), and I(λexλem) is the fluorescence intensity (au) at each excitation-emission wavelength pair. MFi is multiplication factor, equal to the inverse of the fractional projected excitation-emission area.
The percent fluorescence response (Pi,n, %) were calculated as eqn (3).
 | (3) |
3. Discussion and results
3.1 Comparison of nitrogen removal performance with different sludge carbon sources
The profile of NO3−–N, NH4+–N and NO2−–N using sludge alkaline fermentation liquid and the sludge thermal hydrolysis liquid as external carbon sources are shown in Fig. 1. The denitrification process was divided into three stages. At stage I (day 1–14), the nitrate removal efficiency with the sludge fermentation liquid and the sludge thermal hydrolysis liquid carbon sources were 10.14% and 7.32%, respectively. Accumulated nitrite concentration of effluent were 3–6 mg L−1 (sludge fermentation liquid) and 3–5 mg L−1 (sludge thermal hydrolysis liquid). It was indicated that the denitrification bacteria didn't accommodate to the sludge carbon sources, and the nitrate was partly converted to nitrite. | ||
| Fig. 1 The variations of NO3−–N, NO2−–N and NH4+–N with different sludge carbon sources ((a–c): with the sludge fermentation liquid; (d–f): with the sludge thermal hydrolysis liquid). | ||
At stage II (day 15–40), nitrate removal efficiencies were higher than that of stage I, which were 60.65% and 30.23%, respectively. It implied that the denitrification bacteria adapted to the sludge carbon sources gradually, and the denitrification efficiency of sludge fermentation liquid was better than that of the sludge thermal hydrolysis liquid. Using sludge fermentation liquid as the carbon source resulted in high nitrate removal efficiency. The incomplete denitrifiers was also dominant in the denitrifying bacterial communities during the denitrification process at stage II. The nitrite concentration of effluent were 6–10 mg L−1 (sludge fermentation liquid) and 3–8 mg L−1 (sludge thermal hydrolysis liquid), respectively. The insufficient carbon leaded to nitrite accumulated and incomplete denitrification.17
At stage III (day 41–52), the removal efficiencies of nitrate reached up to 98.07% (sludge fermentation liquid) and 90.18% (sludge thermal hydrolysis liquid), respectively. At the same time, there was little nitrite accumulation, and the effluent of nitrite declined to 0.4 mg L−1 (sludge fermentation liquid) and 1.0 mg L−1 (sludge thermal hydrolysis liquid). It was implied that the denitrifiers could accommodate the sludge carbon sources after 40 d cultivation, and the denitrification efficiency of the sludge fermentation liquid was better than that of the sludge thermal hydrolysis liquid. Anything else, the specific denitrifying rates with the sludge fermentation liquid and the sludge thermal hydrolysis liquid were 7.94 g per kg MLVSS per h and 1.16 g per kg MLVSS per h, respectively. The nitrate removal efficiency of sludge alkaline fermentation liquid was faster than that of sludge thermal hydrolysis liquid. The sludge fermentation liquid could perform more efficiency as external carbon source for nitrate removal than the sludge thermal hydrolysis liquid. There was a large amount of large molecular organics, such as carbohydrates and protein in the sludge thermal hydrolysis liquid. The higher denitrification rate is provided by the most readily biodegradable COD and the slowly biodegradable COD needs to be hydrolysed prior to denitrification.18 VFAs were the main products in the fermentation system.19 When the sludge fermentation liquid used as the external carbon source, the nutrient removal efficiency could be improved obviously.20,21
It was also found that the concentration of NH4+–N decreased about 10 mg L−1 with the sludge alkaline fermentation liquid during the denitrification process. Using the sludge fermentation liquid as carbon source, biodegradable organic matters had been transformed to VFAs which could be easily used by denitrifiers, and the NH4+–N was consumed to synthesise microbial cells during the process. Oppositely, the NH4+–N concentration increased about 11.6 mg L−1 with the sludge thermal hydrolysis liquid. It was reported that waste activated sludge (WAS) acidification could be integrated with denitrification in sludge fermentors.22 The nitritation effluent recycled into the fermentor for denitrification, leading to the stimulation of denitrification and methanogenesis fermentation.23,24 When using the sludge thermal hydrolysis liquid as the carbon source, anaerobic fermentation and denitrification process were co-existence, some organic matters such as protein could be further hydrolysed before being used as carbon source, which reduced to the enhancement of NH4+–N.
3.2 Organic matters consumption during denitrification process
The COD, protein and carbohydrates concentration profiles with the sludge thermal hydrolysis liquid and the sludge fermentation liquid as the external carbon sources are shown in Fig. 2. The COD, protein and carbohydrates were consumed gradually during the denitrification process. When using the sludge thermal hydrolysis liquid as the carbon source, the COD, protein and carbohydrates consumed 126.43, 64.19 and 16.31 mg L−1 at stage I, consumed 197.97, 102.44 and 26.05 mg L−1 at stage II, consumed 224.86, 151.72 and 58.07 mg L−1 at stage III, respectively. For the sludge fermentation liquid, the COD, protein and carbohydrates consumed 137.50, 17.74 and 3.37 mg L−1 at stage I, consumed 243.01, 66.04 and 9.76 mg L−1 at stage II, consumed 289.13, 96.60 and 21.86 mg L−1 at stage III, respectively. It could be found that the consumption of protein and carbohydrates with the sludge thermal hydrolysis liquid was higher than that of the sludge fermentation liquid. As the sludge fermentation liquid contained a mass of VFAs, which were small molecular compounds and could be used more easily than the protein and carbohydrates by the denitrifers.1 So the denitrifers utilized VFAs prior, and then used the protein and carbohydrates. The protein and carbohydrates were the main composition of the sludge thermal hydrolysis liquid, which could be further hydrolysed at first and then used by the denitrifers. | ||
| Fig. 2 The COD, protein and carbohydrate concentration profiles with different sludge carbon source ((a–c): with the sludge fermentation liquid; (e–f): with the sludge thermal hydrolysis liquid). | ||
Fig. 3a illustrates that the composition of VFAs were acetic, propionic, butyric, and valeric acids. Acetic acid, which accountd for 90% of VFAs, was the dominant VFAs.25,26 Detectable SCFAs (Short Chain Fatty Acids) produced in sludge alkaline fermentation liquid because of the carbohydrates and proteins anaerobic bacterical metabolism, and acetic acid was the main product in the fermentation system.19 Fig. 3b shows the variations of the VFAs with the sludge alkaline fermentation liquid as the carbon source. Denitrifiers preferred acetic acid, followed by butyric acid and then propionic acid, the valeric acid was consumed only after the aforementioned VFA species became limiting.27 In this study, the consumption of VFAs was 60–80 mg L−1 at stage III, and the utilization rate of acetic acid achieved to 80%. The results illustrated that denitrifers preferred to use acetic acid in sludge fermentation liquid than other VFAs.
 | ||
| Fig. 3 The composition of influent VFAs (a) and changes of VFAs concentration during denitrification (b) with the sludge alkaline fermentation liquid as carbon source. | ||
3.3 Fluorescence EEM spectra and FRI technique assessment
To gain a better understanding of the organic compounds utilization during denitrification process, the DOM was analyzed by EEM spectroscopy. Spectroscopy method is a powerful tool in environmental analyses to study the structure of the complex organic compounds.28 The EEM fluorescence spectra were usually used to investigate the fluorescent characteristics of organic compounds, such as protein, humic and fulvic acids.29,30 Compared with available approaches, EEM fluorescence spectroscopy gives more fluorescent information with high sensitivity and component selectivity, and is a method for quantifying and characterizing the utilization of the organic compounds for the denitrification process. In order to better understand the EEM fluorescence characteristics, the FRI technique was used to quantitative assess the changes of five Ex/Em regions (Fig. 6).During stage I, the denitrifers didn't adapt to the sludge carbon sources, so the changes of fluorescence intensity in the five regions was little. During stage II, the Pi,n decreased from 10.29–13.26% to 7.05–10.60% (regions I), and decreased from 60.85–70.29% to 54.62–60.07% (regions IV) with the sludge fermentation liquid as carbon source, respectively. When using the sludge thermal hydrolysis liquid as carbon source, the Pi,n of regions I, II and IV decreased from 8.42–10.61%, 5.35–11.4%, 61.8–68.01% to 5.61–9.15%, 2.80–8.51%, and 53.22–59.57%, respectively (Fig. 6). The Pi,n of other regions all increased.
The denitrifers began to adapt sludge carbon sources at stage III, and the organic compounds were consumed during the denitrification process. When using the sludge fermentation liquid as external carbon source, the fluorescence intensity of regions I and IV decreased from 8428–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 366 (au) and 39
366 (au) and 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 108–49
108–49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 952 (au) to 3642–7001 (au) and 16
952 (au) to 3642–7001 (au) and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 656–25
656–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 599 (au), respectively (Fig. 4). Correspondingly, the Pi,n of regions I and IV decreased from 9–12% and 50–60% to 5–9% and 35–40%, respectively (Fig. 6a and b). However, for using the sludge thermal hydrolysis liquid as carbon source, the fluorescence intensity of regions I, II and IV decreased significantly from 5174–7579 (au), 5473–8711 (au) and 18
599 (au), respectively (Fig. 4). Correspondingly, the Pi,n of regions I and IV decreased from 9–12% and 50–60% to 5–9% and 35–40%, respectively (Fig. 6a and b). However, for using the sludge thermal hydrolysis liquid as carbon source, the fluorescence intensity of regions I, II and IV decreased significantly from 5174–7579 (au), 5473–8711 (au) and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353–39
353–39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 516 (au) to 1942–2584 (au), 2058–3283 (au) and 8618–12
516 (au) to 1942–2584 (au), 2058–3283 (au) and 8618–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 486 (au), respectively (Fig. 5). The Pi,n of regions I, II and IV decreased from 7–15%, 9–13% and 40–50% to 5–8%, 5–8% and 30–40%, respectively (Fig. 6c and d). It was implied that the tyrosine-like protein (region I) and soluble microbial by-product (region IV) consumed markedly with the sludge fermentation liquid. For using the sludge thermal hydrolysis liquid, the tryptophan-like protein (region II) was also consumed markedly. It was indicated that the tyrosine-like protein, the tryptophan-like protein and the soluble microbial by-product could all be used by denitrifier bacteria. In this study, the consumption of fulvic acid-like substances (region III) and humic acid-like organics (region V) was little which were non-biodegradable for denitrifier. The consumption of tyrosine-like protein and tryptophan-like protein with the sludge thermal hydrolysis liquid were higher than that of the sludge fermentation liquid. On the contrary, the soluble microbial by-product with the sludge fermentation liquid as the carbon source consumed more than that of the sludge thermal hydrolysis liquid. There were VFAs in the sludge fermentation liquid, which could be easily used by the denitrifier than protein and carbohydrates. Therefore, the sludge fermentation liquid could perform more efficiency as external carbon source for nitrate removal than the sludge thermal hydrolysis liquid.
486 (au), respectively (Fig. 5). The Pi,n of regions I, II and IV decreased from 7–15%, 9–13% and 40–50% to 5–8%, 5–8% and 30–40%, respectively (Fig. 6c and d). It was implied that the tyrosine-like protein (region I) and soluble microbial by-product (region IV) consumed markedly with the sludge fermentation liquid. For using the sludge thermal hydrolysis liquid, the tryptophan-like protein (region II) was also consumed markedly. It was indicated that the tyrosine-like protein, the tryptophan-like protein and the soluble microbial by-product could all be used by denitrifier bacteria. In this study, the consumption of fulvic acid-like substances (region III) and humic acid-like organics (region V) was little which were non-biodegradable for denitrifier. The consumption of tyrosine-like protein and tryptophan-like protein with the sludge thermal hydrolysis liquid were higher than that of the sludge fermentation liquid. On the contrary, the soluble microbial by-product with the sludge fermentation liquid as the carbon source consumed more than that of the sludge thermal hydrolysis liquid. There were VFAs in the sludge fermentation liquid, which could be easily used by the denitrifier than protein and carbohydrates. Therefore, the sludge fermentation liquid could perform more efficiency as external carbon source for nitrate removal than the sludge thermal hydrolysis liquid.
 | ||
| Fig. 4 EEM spectra with the sludge fermentation liquid used as carbon source ((a): stage I; (b): stage II; (c): stage III). | ||
 | ||
| Fig. 5 EEM spectra with the sludge thermal hydrolysis liquid used as carbon source ((a): stage I; (b): stage II; (c): stage III). | ||
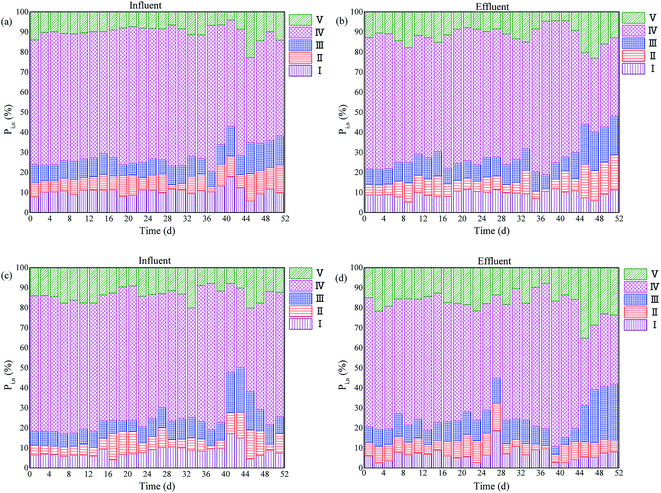 | ||
| Fig. 6 Distribution of FRI in the two SBRs with different sludge carbon sources (a and b): with the sludge fermentation liquid; (c and d): with the sludge thermal hydrolysis liquid). | ||
4. Conclusion
High nitrate removal efficiency of 95% and 90% was achieved using sludge fermentation liquid and thermal hydrolysis liquid as denitrification carbon sources. The sludge fermentation liquid could perform efficiency for nitrate removal and the specific denitrifying rate of sludge fermentation liquid (7.94 g per kg MLVSS per h) was higher than that of sludge thermal hydrolysis liquid (1.16 g per kg MLVSS per h). By EEM fluorescence spectra, non-biodegradable matters of humic acid-like organics were not be consumed; the tyrosine-like protein and soluble microbial by-product were mainly used by denitrifiers with sludge fermentation liquid, and the tryptophan-like protein could also be used by denitrifiers with sludge thermal hydrolysis liquid.Acknowledgements
The study was supported by the Natural Science Foundation of China (Grant Number: 51208481); the Natural Science Foundation of Shandong (Grant Number: ZR2010EQ028).References
- F. Bu, X. Hu, L. Xie and Q. Zhou, J. Zhejiang Univ., Sci., B, 2015, 16, 304–316 CrossRef CAS PubMed.
- C. Li, J. Cao, H. Ren, Y. Li and S. Tang, Process Biochem., 2015, 50, 447–455 CrossRef CAS.
- Y. Xu, J. Environ. Sci., 1996, 8, 257–268 CAS.
- X. Wang, Y. Zhang, T. Zhang, J. Zhou and M. Chen, Chem. Eng. J., 2016, 283, 167–174 CrossRef CAS.
- S. Cao, S. Wang, Y. Peng, C. Wu, R. Du, L. Gong and B. Ma, Bioresour. Technol., 2013, 149, 570–574 CrossRef CAS PubMed.
- X. Zheng, Y. Chen and C. Liu, Biotechnol. Bioeng., 2010, 18, 478–485 CAS.
- P. Elefsiniotis, D. G. Wareham and M. O. Smith, Water Environ. Res., 2005, 77, 366–371 CrossRef CAS PubMed.
- X. M. Li, J. W. Zhao, D. B. Wang and Q. Yang, Chemosphere, 2016, 144, 160–167 CrossRef CAS PubMed.
- J. W. Zhao, D. B. Wang, X. M. Li, Q. Yang, H. B. Chen, Y. Zhong and G. M. Zeng, Water Res., 2015, 78, 111–120 CrossRef CAS PubMed.
- APHA (American Public Health Association), Standard methods for the examination of water and wastewater, APHA, Washigton, DC, 20th edn, 1998 Search PubMed.
- M. DuBois, K. A. Gilles, J. K. Hamilton, P. A. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- O. H. Lowry, N. J. Rosebrough, A. L. Farr and R. J. Randall, J. Biol. Chem., 1951, 193, 265–275 CAS.
- L. Guo, J. Zhao, Z. She, M. Lu and Y. Zong, Bioresour. Technol., 2012, 117, 368–372 CrossRef CAS PubMed.
- W. Chen, P. Westerhoff, J. A. Leenheer and K. Booksh, Environ. Sci. Technol., 2003, 37, 5701–5710 CrossRef CAS PubMed.
- M. Bahram, R. Bro, C. Stedmon and A. Afkhami, J. Chemom., 2006, 20, 99–105 CrossRef CAS.
- L. Guo, M. Lu, Q. Li, J. Zhang and Z. She, Int. J. Hydrogen Energy, 2015, 40, 197–208 CrossRef CAS.
- S. Ge, Y. Peng, S. Wang, C. Lu, X. Cao and Y. Zhu, Bioresour. Technol., 2012, 114, 137–143 CrossRef CAS PubMed.
- Y. Fernández-Nava, E. Maranon, J. Soons and L. Castrillon, J. Hazard. Mater., 2010, 173, 682–688 CrossRef PubMed.
- H. Yuan, Y. Chen, H. Zhang, S. Hang, Q. Zhou and G. Gu, Environ. Sci. Technol., 2006, 40, 2025–2029 CrossRef CAS PubMed.
- X. Li, H. Chen, L. Hu, L. Yu, Y. Chen and G. Gu, Environ. Sci. Technol., 2011, 45, 1834–1839 CrossRef CAS PubMed.
- L. Pang, J. Ni, X. Tang and Q. Chen, Chem. Eng. J., 2015, 259, 911–917 CrossRef CAS.
- B. Wang, S. Wang, B. Li, C. Peng and Y. Peng, Biomass Bioenergy, 2014, 67, 460–465 CrossRef CAS.
- L. Zhang, S. Zhang, S. Wang, C. Wu, Y. Chen, Y. Wang and Y. Peng, Chemosphere, 2013, 91, 635–640 CrossRef CAS PubMed.
- L. Xie, J. Chen, R. Wang and Q. Zhou, J. Biosci. Bioeng., 2012, 113, 759–764 CrossRef CAS PubMed.
- P. Elefsiniotis, D. G. Wareham and W. K. Oldham, Environ. Sci. Technol., 1996, 30, 1508–1514 CrossRef CAS.
- J. Z. Li, D. S. Mavinic and H. G. Kelly, J. Environ. Eng., 2004, 130, 397–407 CrossRef CAS.
- P. Elefsiniotis, D. G. Wareham and D. Wareham, Enzyme Microb. Technol., 2007, 41, 92–97 CrossRef CAS.
- K. Wang, W. Li, X. Gong, Y. Li, C. Wu and N. Ren, Int. Biodeterior. Biodegrad., 2013, 85, 617–623 CrossRef CAS.
- F. C. Marhuenda-Egea, E. Martinez-Sabater, J. Jorda, R. Moral, M. A. Bustamante, C. Paredes and M. D. Perez-Murcia, Chemosphere, 2007, 68, 301–309 CrossRef CAS PubMed.
- W. Tian, L. Li, F. Liu, Z. Zhang, G. Yu, Q. Shen and B. Shen, Bioresour. Technol., 2012, 110, 330–337 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |
