DOI:
10.1039/C5RA07669B
(Paper)
RSC Adv., 2015,
5, 43990-44002
Synthesis of imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8-diones via a rearrangement of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7-diones in the reaction with isatins†
Received
27th April 2015
, Accepted 8th May 2015
First published on 8th May 2015
Abstract
An aldol condensation/skeletal rearrangement protocol for the synthesis of 1,3-dialkyl-7-(2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-diones in good to high yields via a one-pot reaction of 1,3-dialkyl-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-diones and 1H-indole-2,3-diones (isatins) or through the generation and rearrangement of 1,3-dialkyl-6-(2-oxo-1,2-dihydro-3H-indol-3-ylidene)-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-diones has been developed.
Introduction
Nitrogen- and sulfur-containing fused heterocycles have a broad range of biological activities and are attractive compounds in medicinal chemistry. Therefore, new strategies for the synthesis of such heterocyclic compounds and study of their practically valuable properties represent a challenging task for organic chemists.1
For instance, the synthesis of thiazolo[3,2-b]- or thiazolo[2,3-c]-1,2,4-triazines has attracted interest due to their antidepressant,2 anti-HIV, anticancer,3 antibacterial and antifungal activities.4 The most common method reported in the literature for the synthesis of thiazolotriazines involves the reactions of triazinethiones with various α,β-bifunctional compounds, such as α-halogenoketones, α-halogeno-aldehydes, α-halogenoacids, α,β-dihalogenoalkanes, chloro-acetonitrile and others.5 The mode of cyclization to thiazolo[3,2-b]-1,2,4-triazine or thiazolo[2,3-c]-1,2,4-triazine has been governed by the stability of the transition state, which is affected by the substituents in the triazine cycle and usually only one isomer is formed in each case.5,6 On the one hand, unique regioselectivity is an advantage of the reactions of triazinethiones with α,β-bifunctional compounds. But, on the other hand, alternative methods for the synthesis of another isomer should be developed.4a,7 Until now, the rearrangements of thiazolo[3,2-b]-1,2,4-triazine and thiazolo-[2,3-c]-1,2,4-triazine into each other have not been observed.
In general, the rearrangements and transformations of heterocycles in new heterocyclic structures are a nontrivial method for their preparation and have been rarely used for their target synthesis. Nevertheless, rearrangements and transformations are perspective approaches to the heterocycles that are inaccessible by other synthetic methods.8
5,7-Dialkyl-3-thioxoperhydroimidazo[4,5-e]-1,2,4-triazin-6-ones (thiones) react with halogenoacetic acids to give only imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine derivatives (see e.g. compound 1 in Scheme 1).5e,6e Recently, we have found that aldol condensation of compound 1 with 3,5-di-tert-butyl-1,2-benzoquinone in acetic acid led to two isomeric derivatives of imidazothiazolo[3,2-b]triazine 2 and imidazothiazolo[2,3-c]-triazine 3, and the former was irreversibly converted into the latter upon reflux in acetic acid (Scheme 1).9 In addition, we have studied the condensation of compound 1 with 1H-indole-2,3-dione (isatin) derivatives in acetic acid or in methanol in the presence of potassium hydroxide. In the latter case, in the 1H NMR spectra of the reaction products 4, the proton signals of the minor isomeric products 5 were also observed. One of the compounds 5 (R = allyl) was isolated and characterized (Scheme 1).10 We have considered the rearrangement observed as potentially interesting for the preparation of new heterocyclic compounds. Taking into account the various biological activities of oxoindolinylidenethiazolidinones,11 herein, we report a strategy for the synthesis of oxoindolinylidene derivatives of imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine via a rearrangement of corresponding readily available imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines.
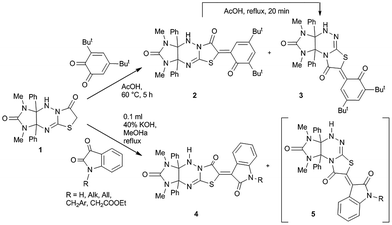 |
| | Scheme 1 Background of this work. | |
Results and discussion
General method for the preparation of starting compounds 6a,b from thioxoimidazotriazines 7a,b and bromoacetic acid has been previously reported5e and is depicted in Scheme 2. Imidazotriazines 7a,b were synthesized by cyclization of 4,5-dihydroxyimidazolidin-2-ones 8a,b with thiosemicarbazide.12
 |
| | Scheme 2 Synthesis of starting 6a, b. | |
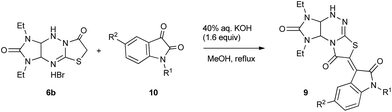
We aimed on designing an aldol condensation/skeletal rearrangement protocol for the synthesis of oxoindolinylidene derivatives of imidazothiazolo[2,3-c]triazines 9 via one-pot reaction of imidazothiazolo[3,2-b]triazines 6 and isatins 10 or through generation and rearrangement of oxoindolinylidene derivatives 11 (Scheme 3).
 |
| | Scheme 3 Synthesis of 9 and 11. | |
We started by examining the reaction between 1,3-dimethyl derivative 6a and isatin 10a to optimize the reaction conditions, and the representative results are summarized in Table 1. The type of catalyst was examined using acetic acid or 40% aqueous potassium hydroxide. The reaction of compounds 6a and 10a led to product 11a both in acetic acid and in methanol in the presence of KOH, but only in the presence of KOH the formation of isomeric derivative 9a was observed (entries 1–4). Subsequently, we screened the amount of KOH (entries 3–6) and found that 1.07 equivalent of potassium hydroxide was enough to obtain compound 11a in good yield (entry 3). To prepare isomeric product 9a in high yield, 1.6 equivalent of potassium hydroxide was enough (entry 6). In the 1H NMR spectrum of a filtrate concentrated to dryness after isolation of compound 9a, the signals for the protons of decomposition products were observed when using 1.6 equivalent of KOH; so we have not increased the amount of catalyst any more. Further optimization was done by varying the reaction temperature, and it was found that refluxing in methanol gave the best result (entries 6, 7). Finally, it was established that the best yields of compounds 9a and 11a were achieved for 120 and 30 min, respectively (entries 3, 6, 8–12).
Table 1 Optimization of the reaction conditionsa

|
| Entry |
Catalyst (equiv.) |
Temp. (°C) |
Time (min) |
Yieldb of 11a (%) |
Yieldb of 9a (%) |
| Reaction conditions: heating the mixture of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine 6a (2.0 mmol), and isatin 10a (2 mmol) either in acetic acid (15 ml) or in methanol (15 ml) with 40% aqueous KOH for 20–150 min. Isolated yield. |
| 1 |
AcOH (as solvent) |
Reflux |
120 |
17 |
0 |
| 2 |
AcOH (as solvent) |
65 |
120 |
48 |
0 |
| 3 |
KOH (1.07) |
Reflux |
120 |
57 |
0 |
| 4 |
KOH (1.24) |
Reflux |
120 |
46 |
18 |
| 5 |
KOH (1.5) |
Reflux |
120 |
0 |
51 |
| 6 |
KOH (1.6) |
Reflux |
120 |
0 |
71 |
| 7 |
KOH (1.6) |
40 |
120 |
0 |
28 |
| 8 |
KOH (1.07) |
Reflux |
150 |
56 |
0 |
| 9 |
KOH (1.07) |
Reflux |
90 |
26 |
0 |
| 10 |
KOH (1.6) |
Reflux |
45 |
0 |
73 |
| 11 |
KOH (1.6) |
Reflux |
30 |
0 |
74 |
| 12 |
KOH (1.6) |
Reflux |
20 |
0 |
65 |
With the optimized conditions in hand, we then investigated the substrate scope for this reaction. First, reactivity of different isatins 10 was studied in the condensation with compound 6a under the optimal conditions for the synthesis of products 9 (Table 2). It was found that in addition to model substrate 10a, various N-alkyl derivatives 10b–e and N-phenylethyl isatin 10f reacted efficiently with compound 6a to afford the desired products 9a–f in good to high yields (entries 1–6). N-Allyl and N-propargyl isatins 10g,h were also applicable to this aldol condensation/skeletal rearrangement one-pot reaction, and the target products 9g,h were obtained in 80 and 66% yields, respectively (entries 7, 8). Isatin 10i bearing methyl substituent at the nitrogen atom and bromine atom at the 5-position was an effective substrate for this transformation, and the corresponding derivative 9i was synthesized in 92% yield. The reaction of methyl ester of dioxoindolylpropanoic acid 10j proceeded under the same conditions, affording the product with ester group 9j in 71% yield. When ethyl ester of 2-(2,3-dioxo-1H-indol-1-yl)acetic acid 10k was used in this reaction, no desired product was obtained. Due to the partial reesterification of ethyl ester, a mixture of methyl and ethyl esters of corresponding acid 9 was obtained (see ESI†).
Table 2 Synthesis of 9 via a reaction of 6a with 10a
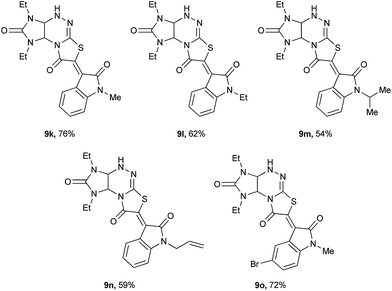
Next, 1,3-diethyl derivative 6b was subjected to reaction with isatins 10 under the same conditions, and the results are shown in Table 3. Compound 6b was a suitable substrate to react with isatins 10b–d,g,i, giving the corresponding derivatives 9 bearing alkyl (9k–m), allyl (9n) substituents at the nitrogen atom and bromine atom at the 5-position of indole fragment (9o) in 54–76% yields.
Table 3 Synthesis of 9 via a reaction of 6b with 10a
|
| Reaction conditions: refluxing the mixture of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine 6b (2.0 mmol), isatins 10 (2 mmol), and 0.32 ml of 40% aqueous KOH (3.2 mmol) in methanol for 30 min. |
 |
Then various isatins 10 underwent condensation with compound 6a under the optimal conditions for the synthesis of products 11. It was found that unsubstituted isatin 10a as well as isatins bearing either alkyl(arylalkyl) (10b–d,f,m) or functional substituent at the nitrogen atom (10g,h,j–l,n,o) and bromine atom at the 5-position (10i) could react with compound 6a to produce the desired products 11a–n in moderate to high yields (Table 4). The reaction was found to be tolerant to ethyl esters of dioxoindolylacetic or propanoic acids 10k,l as well as ester of benzoic acid 10o and generated the target products in good yields (entries 10, 11, 14).
Table 4 Synthesis of 11 via a reaction of 6a with 10a
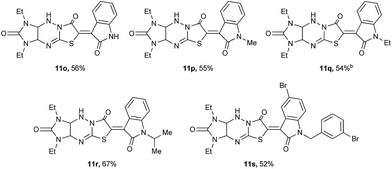
1,3-Diethyl derivative 6b was also studied in the reaction with isatins 10 under the same conditions, and the results are shown in Table 5. The desired N-unsubstituted (11o), N-alkyl (11p-r), and brominated at the 5-position of indole fragment N-arylalkyl (11s) products were synthesized in 52–67% yields. It was found that the condensation of compounds 6a,b with 1-ethylisatin 10c led to intermediates 12 along with the target compounds 11c,q for 2 h (Scheme 4). Similar products were obtained by the reaction of thiazolidin-4-ones with isatins in ethanol with diethylamine as catalyst13 or “on water” without catalyst.14 To obtain the products 11c,q in better yields, the interaction of compounds 6a,b and 10c was carried out for 2.5 h (Tables 4 and 5).
Table 5 Synthesis of 11 via a reaction of 6b with 10a
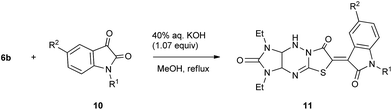
|
| Reaction conditions: refluxing the mixture of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine 6b (2.0 mmol), isatins 10 (2 mmol), and 0.215 ml of 40% aqueous KOH (2.14 mmol) in methanol for 2 h. The reaction time was 2.5 h. |
 |
 |
| | Scheme 4 Synthesis of intermediates 12. | |
Further, imidazothiazolo[3,2-b]triazine derivatives 11 underwent rearrangement into isomeric compounds 9 (Table 6); 0.6 equivalent of KOH were used instead of neutralized hydrobromide, which was unavailable. All the studied compounds 11 were converted to isomers 9 in 88–94% yields. As 1,3-dimethylderivatives 11 (entries 1–9) could be employed to give the corresponding isomers 9, the 1,3-diethylderivatives 11 (entries 10–12) could be used as well. N-Unsubstituted (11a,o), N-alkyl(arylalkyl)- (11b–e,j,p,r), N-allylsubstituted (11f) in indole fragment compounds underwent successfully rearrangement into target products 9. When ethyl ester 11j was used as substrate under the same conditions, however, the mixture of ethyl and methyl esters 9 was obtained again. Therefore, ethyl ester 11j underwent rearrangement and reesterification with methanol using 1 equivalent of KOH.
Table 6 Synthesis of 9 via a rearrangement of 11a

|
| Entry |
11 R1 |
R2 |
Productb (R1) |
Yieldc (%) |
| Reaction conditions: refluxing the mixture of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine derivative 11 (2.0 mmol), and 0.12 ml of 40% aqueous KOH (1.2 mmol) in methanol for 20 min. For the synthesis of 9r,s, 0.20 ml of 40% aqueous KOH (2.0 mmol) was used. Isolated yield. |
| 1 |
11a H |
Me |
9a |
89 |
| 2 |
11b Me |
Me |
9b |
92 |
| 3 |
11c Et |
Me |
9c |
91 |
| 4 |
11d Pri |
Me |
9d |
93 |
| 5 |
11e Ph(CH2)2 |
Me |
9f |
94 |
| 6 |
11f CH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 CH2 |
Me |
9g |
89 |
| 7 |
11h CH(Me)COOMe |
Me |
9j |
94 |
| 8 |
11l CH2C6H4Cl-4 |
Me |
9p |
88 |
| 9 |
11j CH2COOEt |
Me |
9r (CH2COOMe)b |
37 |
| 9s (CH2COOK)b |
5 |
| 10 |
11o H |
Et |
9q |
92 |
| 11 |
11p Me |
Et |
9k |
91 |
| 12 |
11r Pri |
Et |
9m |
90 |
Partial hydrolysis of ethyl ester also took place. The yields of corresponding methyl ester 9r and potassium salt of acid 9s were 37 and 5%, respectively (entry 9).
All the compounds were characterized by IR, NMR and HRMS analytical methods. The signals were assigned using highly sensitive NOESY and HMBC methods. All reactions are diastereoselective and provide the products 9 and 11 as Z-isomers. 1H NMR spectra of compounds 9a–s and 11a–s displayed a strong downfield shift of the indole H-4′ proton signals (8.75–8.95 and 8.79–9.06 ppm, respectively), which is characteristic of proximity of the carbonyl group C(8)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or C(7)
O or C(7)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Besides, compounds 9j, 11i and 11k with additional chiral carbon atom in indole moiety are obtained as a mixture of two diastereomers (1′′R*,3aS*,9aR*- and 1′′S*,3aS*,9aR*-isomers) and so some signals in the 1H and 13C NMR spectra of these products are double. The homogeneity of compounds 9b,d,e,n and 11b,c was confirmed by powder X-ray diffraction. Results of the analysis of the experimental powder diffraction patterns of the compounds 9b,d,e,n and 11b,c show that the investigated samples were single-phase.
O. Besides, compounds 9j, 11i and 11k with additional chiral carbon atom in indole moiety are obtained as a mixture of two diastereomers (1′′R*,3aS*,9aR*- and 1′′S*,3aS*,9aR*-isomers) and so some signals in the 1H and 13C NMR spectra of these products are double. The homogeneity of compounds 9b,d,e,n and 11b,c was confirmed by powder X-ray diffraction. Results of the analysis of the experimental powder diffraction patterns of the compounds 9b,d,e,n and 11b,c show that the investigated samples were single-phase.
Many successful cascade sequences initiated by Knoevenagel condensation9,15 have been reported; Knoevenagel condensation/intramolecular aldol cyclization,16 Knoevenagel condensation/hetero-Diels–Alder reactions17 and Knoevenagel condensation/Michael addition/cyclization reactions are among them.18 A similar sequence that includes Knoevenagel condensation/skeletal rearrangement could be possible for the formation of imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine derivatives 9 from the starting 6a,b and 10. To get insight into the details of the reaction pathway, we performed the rearrangement of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7-diones (both hydrobromides 6a,b and bases 13a,b)5e into imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8-diones 14a,b (Scheme 5). After heating compounds 13a,b with 0.6 equivalent of KOH or hydrobromides 6a,b with 1.6 equivalent of KOH for 45 to 60 min, the target isomers 14a,b were prepared in high yields. The structures of 13a and 14b (the latter as its solvate with methanol) were unambiguously elucidated by X-ray diffraction (Fig. 1 and 2).
 |
| | Scheme 5 Synthesis of 14a,b. | |
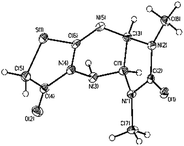 |
| | Fig. 1 General views of 13a in representation of atoms via thermal ellipsoids (at 50% probability level). | |
 |
| | Fig. 2 General views of 14b in representation of atoms via thermal ellipsoids (at 50% probability level). | |
Meanwhile, one more controlled reaction was carried out to explore a plausible pathway for the formation of imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine derivatives 9. When isatins 10 underwent condensation with imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine 14a under reflux in methanol with 0.07 equiv. of 40% KOH, target compounds 9 were also formed (Scheme 6).
 |
| | Scheme 6 Reaction of 14a with 10. | |
Based on the experimental results, at least two reaction pathways may be suggested. First, Knoevenagel type condensation of 13 (6) with isatins 10 may forego rearrangement of derivatives 11 formed into isomers 9. Second, compounds 13 (6) can initially undergo rearrangement into isomers 14 followed by Knoevenagel type condensation of the latter with isatins 10. Quantum chemical study of the reaction mechanism and investigation of biological activity of the products 9 and 11 are in progress.
Conclusions
We have developed an aldol condensation/skeletal rearrangement protocol for the synthesis of 7-ylideneimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine derivatives 9 in good to high yields via one-pot reaction of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines 6a,b and isatins 10 or through generation and rearrangement of 6-ylideneimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazines 11. Other sequence of the reactions including rearrangement of imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7-diones 6a,b into imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8-diones 14 and Knoevenagel type condensation of the latter with isatins may be also used for two-step conversion into derivatives 9.
Experimental
General methods
All the reagents were purchased from Acros organics and used without further purification. Melting points were determined in open glass capillaries on a Gallenkamp (Sanyo) melting point apparatus. The 1H NMR and 13C NMR spectra were recorded on Bruker AM300 (300.13 MHz and 75.5 MHz, respectively) and Bruker AV600 (150.90 MHz (13C)) spectrometers using DMSO-d6 as solvent. Chemical shifts (δ) are given in ppm from TMS as internal standard. The NOESY and 1H–13C HMBC experiments were carried out on Bruker AV600 spectrometer. Infrared (IR) spectra were recorded on a Bruker ALPHA instrument in KBr pellets. High resolution mass spectra (HRMS) were measured on a Bruker micrOTOF II instrument using electrospray ionization (ESI).
General procedure for the synthesis of compounds 9a–j
Procedure 1. To a stirred suspension of compound 6a (644 mg, 2.0 mmol) and isatin 10a (294 mg, 2.0 mmol) in refluxing methanol (15 ml), 0.32 ml of 40% aqueous KOH (3.2 mmol) was added. The resulting mixture was refluxed with stirring for 30 min. After cooling, the precipitate was filtered off and washed with water. The resulting solid product was purified by boiling in methanol (15 ml) or chloroform (15 ml) to give 9a (548 mg, 74%).
(Z)-1,3-Dimethyl-7-(2-oxo-1,2-dihydro-3H-indol-3-ylide-ne)-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9a). Orange solid, mp 328–330 °C (decomp). Yield: 548 mg (74%); IR (KBr): vmax/cm−1 3268, 3210, 2924, 1711, 1691, 1646, 1614, 1463, 1402, 1333, 1317, 1241, 1193, 1087, 1020, 1003, 846, 827, 752; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.67 (s, 3H), 2.93 (s, 3H), 4.82 (d, J = 5.7 Hz, 1H), 5.68 (d, J = 5.7 Hz, 1H), 6.95 (d, J = 7.7 Hz, 1H), 7.06 (t, J = 7.7 Hz, 1H), 7.33 (t, J = 7.7 Hz, 1H), 8.03 (s, 1H), 8.76 (d, J = 7.9 Hz, 1H), 11.14 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.9, 31.3, 63.8, 65.7, 110.1, 120.3, 121.6, 123.0, 127.2, 130.8, 132.1, 136.9, 142.4, 159.0, 164.1, 168.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H14N6O3S 371.0921, found 371.0925.
(Z)-1,3-Dimethyl-7-(1-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9b). Red solid, mp 290–291 °C (decomp). Yield: 510 mg (66%); IR (KBr): vmax/cm−1 3437, 3297, 2966, 2930, 1720, 1708, 1672, 1644, 1610, 1469, 1384, 1349, 1323, 1058, 1023, 802, 754; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.67 (s, 3H), 2.93 (s, 3H), 3.26 (s, 3H), 4.83 (d, J = 5.4 Hz, 1H), 5.68 (d, J = 5.4 Hz, 1H), 7.09–7.14 (m, 2H), 7.42 (t, J = 7.5 Hz, 1H), 8.05 (s, 1H), 8.78 (d, J = 7.9 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.4, 28.0, 31.5, 63.9, 65.9, 109.1, 119.7, 122.4, 127.1, 131.0, 131.8, 133.1, 136.8, 143.6, 159.1, 164.1, 167.1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H16N6O3S 385.1077, found 385.1079.
(Z)-7-(1-Ethyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-dimethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9c). Orange solid, mp 282–284 °C (decomp). Yield: 640 mg (80%); IR (KBr): vmax/cm−1 3309, 2972, 1701, 1676, 1643, 1607, 1465, 1421, 1372, 1309, 1281, 1260, 1085, 784, 749; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.19 (t, J = 7.0 Hz, 3H), 2.67 (s, 3H), 2.93 (s, 3H), 3.80–3.87 (q, J = 7.0 Hz, 2H), 4.83 (d, J = 5.7 Hz, 1H), 5.68 (d, J = 5.7 Hz, 1H), 7.09–7.19 (m, 2H), 7.41 (t, J = 7.7 Hz, 1H), 8.05 (s, 1H), 8.80 (d, J = 7.7 Hz, 1H); 13C NMR (151 MHz, DMSO-d6): δ (ppm) 13.1, 28.4, 31.9, 35.0, 64.3, 66.3, 109.5, 120.2, 122.58, 122.61, 127.7, 131.3, 133.5, 137.2, 142.9, 159.5, 164.5, 167.2; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H18N6O3S 399.1234, found 399.1231.
(Z)-1,3-Dimethyl-7-[1-(2-propyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo-[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9d). Orange solid, mp 298–299 °C (decomp). Yield: 773 mg (94%); IR (KBr) vmax/cm−1 3433, 3279, 2971, 2925, 1727, 1680, 1647, 1606, 1465, 1339, 1315, 1246, 1197, 1023, 838, 754; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.46 (d, J = 6.4 Hz, 6H), 2.67 (s, 3H), 2.92 (s, 3H), 4.56–4.65 (m, 1H), 4.83 (d, J = 4.9 Hz, 1H), 5.69 (d, J = 5.6 Hz, 1H), 7.11 (t, J = 7.5 Hz, 1H), 7.29 (d, J = 7.8 Hz, 1H), 7.40 (t, J = 7.4 Hz, 1H), 8.04 (s, 1H), 8.85 (d, J = 7.6 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 19.2, 27.9, 31.3, 44.1, 63.7, 65.7, 109.9, 119.9, 121.8, 122.3, 127.3, 130.7, 132.8, 136.8, 142.1, 159.0, 164.0, 166.7; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H20N6O3S 413.1390, found 413.1392.
(Z)-7-(1-Buthyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-dimethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9e). Orange solid, mp 258–260 °C (decomp). Yield: 741 mg (87%); IR (KBr): vmax/cm−1 3435, 3308, 2956, 2931, 1708, 1677, 1643, 1607, 1466, 1365, 1347, 1310, 1282, 1194, 1085, 784, 750; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.89 (t, J = 7.2 Hz, 3H), 1.26–1.33 (m, 2H), 1.56–1.64 (m, 2H), 2.67 (s, 3H), 2.92 (s, 3H), 3.80 (t, J = 6.7 Hz, 2H), 4.82 (d, J = 5.6 Hz, 1H), 5.68 (d, J = 5.7 Hz, 1H), 7.09–7.18 (m, 2H), 7.41 (t, J = 7.6 Hz, 1H), 8.05 (s, 1H), 8.80 (d, J = 7.6 Hz, 1H); 13C NMR (151 MHz, DMSO-d6): δ (ppm) 13.6, 19.6, 28.0, 29.2, 31.5, 40.0, 63.9, 65.8, 109.2, 119.8, 122.0, 122.2, 127.3, 130.9, 133.2, 136.8, 142.8, 159.1, 164.1, 167.1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H22N6O3S 427.1547, found 427.1550.
(Z)-1,3-Dimethyl-7-(2-oxo-1-phenethyl-1,2-dihydro-3H-indol-3-ylidene)-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo- [2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9f). Orange solid, mp 290–292 °C (decomp). Yield: 826 mg (87%); IR (KBr): vmax/cm−1 3269, 2922, 1718, 1684, 1646, 1607, 1467, 1384, 1365, 1336, 1318, 1248, 1177, 1024, 750; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.67 (s, 3H), 2.92–2.95 (m, 5H), 4.03 (t, J = 7.3 Hz, 2H), 4.82 (d, J = 5.5 Hz, 1H), 5.67 (d, J = 5.9 Hz, 1H), 7.08–7.25 (m, 7H), 7.38 (t, J = 7.7 Hz, 1H), 8.05 (s, 1H), 8.80 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.9, 31.4, 33.0, 41.2, 63.8, 65.8, 109.2, 119.6, 121.8, 122.1, 126.4, 127.1, 128.3, 128.8, 130.8, 133.0, 136.6, 138.1, 142.5, 159.0, 164.0, 166.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H22N6O3S 475.1547, found 475.1540.
(Z)-7-(1-Allyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-dimethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9g). Orange solid, mp 287–289 °C (decomp). Yield: 655 mg (80%); IR (KBr): vmax/cm−1 3306, 2981, 1702, 1680, 1640, 1607, 1465, 1380, 1363, 1349, 1192, 1090, 1023, 784, 752; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.67 (s, 3H), 2.93 (s, 3H), 4.45 (br s, 2H), 4.83 (d, J = 5.1 Hz, 1H), 5.11–5.19 (m, 2H), 5.69 (d, J = 5.5 Hz, 1H), 5.84–5.93 (m, 1H), 7.07–7.17 (m, 2H), 7.41 (t, J = 7.7 Hz, 1H), 8.07 (s, 1H), 8.83 (d, J = 7.7 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.9, 31.5, 41.9, 63.9, 65.8, 109.5, 117.1, 119.7, 122.3, 127.2, 127.5, 130.8, 130.9, 131.7, 136.7, 142.5, 159.1, 164.0, 166.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H18N6O3S 411.1234, found 411.1237.
(Z)-1,3-Dimethyl-7-[2-oxo-1-(prop-2-yn-1-yl)-1,2-dihydro-3H-indol-3-ylidene]-1,3a,4,9a-tetrahydroimidazo[4,5-e]thia-zolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9h). Orange solid, mp 303–305 °C (decomp). Yield: 539 mg (66%); IR (KBr): vmax/cm−1 3435, 3300, 2969, 2929, 2120, 1718, 1703, 1687, 1638, 1608, 1468, 1417, 1361, 1349, 1330, 1235, 1191, 1025, 808, 753; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.69 (s, 3H), 2.95 (s, 3H), 3.23 (s, 1H), 4.69 (s, 2H), 4.85 (d, J = 5.7 Hz, 1H), 5.71 (d, J = 5.7 Hz, 1H), 7.16–7.22 (m, 2H), 7.47 (t, J = 7.7 Hz, 1H), 8.03 (s, 1H), 8.84 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.9, 29.1, 31.4, 63.8, 65.8, 74.6, 77.7, 109.5, 119.8, 121.4, 122.7, 127.2, 130.7, 134.1, 136.3, 141.5, 159.0, 163.9, 166.4; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H16N6O3S 409.1077, found 409.1073.
(Z)-7-(5-Bromo-1-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-dimethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]-thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9i). Dark-red solid, mp 269–271 °C (decomp). Yield: 850 mg (92%); IR (KBr): vmax/cm−1 3435, 3282, 2930, 1690 (br), 1639, 1606, 1479, 1465, 1366, 1333, 1273, 1191, 1140, 1083, 1059, 1025, 808, 755; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.68 (s, 3H), 2.94 (s, 3H), 3.26 (s, 3H), 4.85 (d, J = 5.1 Hz, 1H), 5.70 (d, J = 5.6 Hz, 1H), 7.11 (d, J = 8.3 Hz, 1H), 7.60 (d, J = 8.3 Hz, 1H), 8.14 (s, 1H), 8.93 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.3, 27.8, 31.3, 63.7, 65.8, 110.7, 113.9, 120.5, 121.2, 129.0, 132.7, 135.0, 136.1, 142.4, 158.9, 163.8, 166.7; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H15BrN6O3S 463.0182, found 463.0171.
(R*)-Methyl 2-((Z)-3-((3aS*,9aR*)-1,3-dimethyl-2,8-dioxo-1,2,3,3a,4,9a-hexahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazin-7(8H)-ylidene)-2-oxo-1,2-dihydro-3H-indol-1-yl)propanoate and (S*)-methyl 2-((Z)-3-((3aS*,9aR*)-1,3-dimethyl-2,8-dioxo-1,2,3,3a,4,9a-hexahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazin-7(8H)-ylidene)-2-oxo-1,2-dihydro-3H-indol-1-yl)-propanoate (9j). Orange solid, mp 284–286 °C (decomp). Yield: 648 mg (71%); IR (KBr): vmax/cm−1 3278, 2952, 2923, 1738, 1723, 1699, 1685, 1644, 1608, 1467, 1394, 1377, 1318, 1231, 1196, 1087, 784, 755, 747; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.58 (d, J = 7.0 Hz, 3H), 2.67 (s, 3H), 2.93 (s, 3H), 3.65 (s, 3H), 4.83 (d, J = 5.7 Hz, 1H), 5.32 (q, J = 7.0 Hz, 1H), 5.70 (d, J = 5.7 Hz, 1H), 7.10–7.18 (m, 2H), 7.41 (t, J = 7.6 Hz, 1H), 8.08 (s, 1H), 8.86 (d, J = 7.7 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 14.2, 27.9, 31.3, 48.9, 52.5, 63.66, 63.73, 65.7, 109.2, 119.8, 121.3, 122.4, 127.3, 130.7, 134.0, 136.3, 141.5, 158.9, 163.8, 166.7, 170.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H20N6O5S 457.1289, found 457.1280.
(Z)-1,3-Diethyl-7-(1-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9k). Orange solid, mp 265–267 °C (decomp). Yield: 626 mg (76%); IR (KBr): vmax/cm−1 3294, 2971, 2933, 1708, 1668, 1640, 1609, 1469, 1380, 1348, 1327, 1228, 1054, 753; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.07 (t, J = 6.7 Hz, 3H), 1.15 (t, J = 6.6 Hz, 3H), 3.27–3.36 (m, 3H), 3.28 (s, 3H), 3.49–3.56 (m, 1H), 4.93 (d, J = 4.8 Hz, 1H), 5.77 (d, J = 5.5 Hz, 1H), 7.10–7.16 (m, 2H), 7.42 (t, J = 7.3 Hz, 1H), 7.91 (s, 1H), 8.78 (d, J = 7.7 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.8, 13.1, 26.3, 34.9, 38.0, 61.7, 63.6, 109.0, 119.6, 122.2, 122.3, 126.9, 130.9, 132.8, 136.5, 143.5, 158.0, 164.0, 167.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H20N6O3S 413.1390, found 413.1383.
(Z)-1,3-Diethyl-7-(1-ethyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9l). Orange solid, mp 255–256 °C (decomp). Yield: 529 mg (62%); IR (KBr): vmax/cm−1 3278, 2973, 2935, 1721, 1684, 1642, 1608, 1468, 1368, 1342, 1326, 1232, 1082, 1053, 752; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.04 (t, J = 6.6 Hz, 3H), 1.13 (t, J = 6.7 Hz, 3H), 1.19 (t, J = 7.4 Hz, 3H), 3.06–3.10 (m, 1H), 3.27–3.34 (m, 2H), 3.48–3.54 (m, 1H), 3.82–3.86 (q, J = 7.4 Hz, 2H), 4.90 (d, J = 4.5 Hz, 1H), 5.76 (d, J = 5.6 Hz, 1H), 7.14 (t, J = 6.9 Hz, 1H), 7.18 (t, J = 7.2 Hz, 1H), 7.41 (t, J = 7.5 Hz, 1H), 8.04 (s, 1H), 8.79 (d, J = 7.5 Hz, 1H); 13C NMR (151 MHz, DMSO-d6): δ (ppm) 14.6, 14.7, 15.0, 36.4, 36.8, 39.9, 63.5, 65.5, 111.0, 121.6, 124.0, 124.1, 129.1, 132.8, 134.8, 138.4, 144.3, 159.9, 166.0, 168.6; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C20H22N6O3SNa 449.1366, found 449.1363.
(Z)-1,3-Diethyl-7-[1-(2-propyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo-[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9m). Orange solid, mp 257–259 °C (decomp). Yield: 476 mg (54%); IR (KBr): vmax/cm−1 3280, 2979, 2937, 1722, 1682, 1637, 1605, 1464, 1353, 1344, 1325, 1236, 1196, 1086, 1027, 753; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.04 (t, J = 6.9 Hz, 3H), 1.12 (t, J = 6.9 Hz, 3H), 1.45 (d, J = 6.7 Hz, 6H), 3.03–3.10 (m, 1H), 3.24–3.31 (m, 2H), 3.46–3.53 (m, 1H), 4.56–4.62 (m, 1H), 4.90 (d, J = 5.1 Hz, 1H), 5.75 (d, J = 5.6 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 7.29 (d, J = 7.8 Hz, 1H), 7.40 (t, J = 7.5 Hz, 1H), 8.01 (s, 1H), 8.84 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.7, 13.0, 19.2, 34.9, 38.0, 44.1, 61.6, 63.5, 109.9, 119.8, 121.9, 122.3, 127.2, 130.8, 132.7, 136.6, 142.1, 157.9, 164.0, 166.8; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H24N6O3S 441.1703, found 441.1695.
(Z)-7-(1-Allyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-diethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9n). Orange solid, mp 255–257 °C (decomp). Yield: 517 mg (59%); IR (KBr): vmax/cm−1 3426, 3280, 2969, 2932, 2919, 1721, 1686, 1640, 1610, 1468, 1362, 1344, 1325, 1229, 1191, 1088, 753; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.04 (t, J = 6.9 Hz, 3H), 1.14 (t, J = 6.7 Hz, 3H), 3.04–3.11 (m, 1H), 3.25–3.32 (m, 2H), 3.47–3.54 (m, 1H), 4.45 (br s, 2H), 4.91 (d, J = 5.5 Hz, 1H), 5.11–5.19 (m, 2H), 5.76 (d, J = 5.7 Hz, 1H), 5.83–5.91 (m, 1H), 7.06–7.17 (m, 2H), 7.40 (t, J = 7.7 Hz, 1H), 8.04 (s, 1H), 8.81 (d, J = 7.8 Hz, 1H); 13C NMR (151 MHz, DMSO-d6): δ (ppm) 13.3, 13.6, 35.4, 38.5, 42.4, 62.2, 64.1, 110.0, 117.6, 120.2, 122.4, 122.8, 127.6, 131.3, 132.2, 133.7, 136.9, 143.0, 158.5, 164.5, 167.3; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H22N6O3S 439.1547, found 439.1541.
(Z)-7-(5-Bromo-1-methyl-2-oxo-1,2-dihydro-3H-indol-3-yli-dene)-1,3-diethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9o). Red solid, mp 285–287 °C (decomp). Yield: 708 mg (72%); IR (KBr): vmax/cm−1 3300, 2972, 2933, 1723, 1687, 1634, 1604, 1464, 1415, 1366, 1333, 1224, 1187, 1081, 1049, 908, 814, 752; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.07 (t, J = 6.9 Hz, 3H), 1.15 (t, J = 6.9 Hz, 3H), 3.07–3.14 (m, 1H), 3.27 (s, 3H), 3.27–3.37 (m, 2H), 3.50–3.57 (m, 1H), 4.95 (d, J = 4.8 Hz, 1H), 5.78 (d, J = 5.8 Hz, 1H), 7.10 (d, J = 8.2 Hz, 1H), 7.59 (d, J = 8.2 Hz, 1H), 7.99 (s, 1H), 8.95 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.8, 13.2, 26.5, 35.0, 38.2, 61.9, 63.9, 111.0, 114.0, 120.8, 121.4, 129.1, 132.9, 135.1, 136.1, 142.7, 158.0, 164.0, 166.8; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H19BrN6O3S 491.0501, found 491.0498.
General procedure for the synthesis of compounds 11a–n
To a stirred suspension of compound 6a (644 mg, 2.0 mmol) and isatin 10a (294 mg, 2.0 mmol) in refluxing methanol (15 ml), 0.215 ml of 40% aqueous KOH (2.14 mmol) was added. The resulting mixture was refluxed with stirring for 2 h. After cooling, the precipitate was filtered off and washed with water. The resulting solid product was purified by boiling in methanol (15 ml) or chloroform (15 ml) to give 11a (421 mg, 57%).
(Z)-1,3-Dimethyl-6-(2-oxo-1,2-dihydro-3H-indol-3-ylide-ne)-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11a). Light brown solid, mp 224–226 °C (decomp). Yield: 421 mg (57%); IR (KBr): vmax/cm−1 3430, 3176, 3063, 2932, 1697, 1635, 1482, 1461, 1399, 1346, 1306, 1265, 1132, 1081, 1016, 789, 750; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.61 (s, 3H), 2.79 (s, 3H), 4.80 (d, J = 5.9 Hz, 1H), 4.91 (d, J = 5.9 Hz, 1H), 6.95 (d, J = 7.6 Hz, 1H), 6.96 (s, 1H), 7.07 (t, J = 7.8 Hz, 1H), 7.37 (t, J = 7.7 Hz, 1H), 8.79 (d, J = 7.9 Hz, 1H), 11.18 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.9, 27.8, 65.1, 66.1, 110.3, 120.0, 121.9, 125.5, 127.6, 129.0, 131.8, 143.1, 150.2, 158.7, 160.5, 168.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C16H14N6O3S 371.0921, found 371.0918.
(Z)-1,3-Dimethyl-6-(1-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11b). Orange solid, mp 297–299 °C (decomp). Yield: 579 mg (75%); IR (KBr): vmax/cm−1 3435, 3195, 3005, 2970, 2935, 1720, 1689, 1644, 1610, 1590, 1486, 1471, 1452, 1377, 1348, 1266, 1244, 1138, 1113, 1069, 1016, 878, 783, 744; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.65 (s, 3H), 2.82 (s, 3H), 3.25 (s, 3H), 4.80 (d, J = 5.7 Hz, 1H), 4.92 (d, J = 5.7 Hz, 1H), 6.93 (s, 1H), 7.08–7.14 (m, 2H), 7.44 (t, J = 6.6 Hz, 1H), 8.81 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.2, 26.9, 27.8, 65.1, 66.1, 109.1, 119.3, 122.4, 125.8, 127.3, 131.2, 131.7, 144.1, 150.0, 158.7, 160.3, 166.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H16N6O3S 385.1077, found 385.1069.
(Z)-6-(1-Ethyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-dimethyl-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11c). The product was isolated via the general procedure but the reaction time was 2.5 h. Orange solid, mp 269–271 °C (decomp). Yield: 469 mg (59%); IR (KBr): vmax/cm−1 3433, 3218, 3002, 2917, 1723, 1688, 1639, 1607, 1466, 1376, 1349, 1248, 1120, 1071, 1015, 879, 745; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.18 (t, J = 6.9 Hz, 3H), 2.61 (s, 3H), 2.80 (s, 3H), 3.78–3.85 (q, J = 6.9 Hz, 2H), 4.80 (dd, J = 2.2, 5.9 Hz, 1H), 4.92 (d, J = 5.9 Hz, 1H), 6.98 (d, J = 2.2 Hz, 1H), 7.10–7.20 (m, 2H), 7.45 (t, J = 7.7 Hz, 1H), 8.84 (d, J = 7.7 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.6, 26.9, 27.8, 34.5, 65.2, 66.1, 109.1, 119.5, 122.3, 124.6, 127.6, 129.7, 131.7, 143.0, 150.0, 158.7, 160.4, 166.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H18N6O3S 399.1234, found 399.1234.
(Z)-1,3-Dimethyl-6-[1-(2-propyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo-[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11d). Orange solid, mp 236–237 °C (decomp). Yield: 577 mg (70%); IR (KBr): vmax/cm−1 3431, 3271, 2974, 2937, 1726, 1702, 1681, 1638, 1605, 1461, 1364, 1313, 1135, 1259, 1079, 1009, 871, 787, 756; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.44 (d, J = 6.6 Hz, 6H), 2.61 (s, 3H), 2.79 (s, 3H), 4.55–4.61 (m, 1H), 4.80 (d, J = 5.1 Hz, 1H), 4.91 (d, J = 5.7 Hz, 1H), 6.99 (s, 1H), 7.12 (t, J = 7.5 Hz, 1H), 7.28 (d, J = 7.9 Hz, 1H), 7.43 (t, J = 7.5 Hz, 1H), 8.88 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 19.09, 19.13, 26.9, 27.8, 44.2, 65.1, 66.0, 110.1, 119.6, 122.0, 124.7, 127.7, 129.6, 131.6, 142.8, 150.1, 158.6, 160.4, 166.7; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H20N6O3S 413.1390; found 413.1382.
(Z)-1,3-Dimethyl-6-(2-oxo-1-phenethyl-1,2-dihydro-3H-indol-3-ylidene)-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo-[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11e). Orange solid, mp 244–246 °C (decomp). Yield: 778 mg (82%); IR (KBr): vmax/cm−1 3435, 3239, 2948, 2930, 2897, 1738, 1682, 1626, 1607, 1466, 1349, 1250, 1126, 1012, 873, 778, 750; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.62 (s, 3H), 2.80 (s, 3H), 2.92 (t, J = 7.3 Hz, 2H), 4.00 (t, J = 7.3 Hz, 2H), 4.80 (d, J = 5.9 Hz, 1H), 4.92 (d, J = 5.9 Hz, 1H), 6.96 (s, 1H), 7.08–7.28 (m, 7H), 7.41 (t, J = 7.7 Hz, 1H), 8.82 (d, J = 7.8 Hz, 1H); 13C NMR (151 MHz, DMSO-d6): δ (ppm) 27.0, 27.9, 33.0, 41.2, 65.2, 66.1, 109.4, 119.3, 122.4, 124.5, 126.5, 127.5, 128.4, 128.8, 129.8, 131.8, 138.1, 143.3, 150.0, 158.7, 160.4, 166.9; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C24H22N6O3SNa 497.1366, found 497.1359.
(Z)-6-(1-Allyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-dimethyl-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11f). Orange solid, mp 238–240 °C (decomp). Yield: 549 mg (67%); IR (KBr): vmax/cm−1 3435, 3217, 2929, 1723, 1686, 1638, 1607, 1466, 1377, 1351, 1247, 1119, 1076, 1015, 876, 750; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.63 (s, 3H), 2.81 (s, 3H), 4.40 (d, J = 3.8 Hz, 2H), 4.81 (d, J = 5.9 Hz, 1H), 4.93 (d, J = 5.9 Hz, 1H), 5.11–5.18 (m, 2H), 5.80–5.91 (m, 1H), 6.99 (s, 1H), 7.03–7.13 (m, 2H), 7.40 (t, J = 7.7 Hz, 1H), 8.82 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.9, 27.8, 41.9, 65.2, 66.1, 109.6, 117.2, 119.4, 122.4, 124.3, 126.2, 127.5, 130.1, 131.6, 143.1, 149.9, 158.7, 160.3, 166.7; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H18N6O3S 411.1234, found 411.1226.
(Z)-1,3-Dimethyl-6-[2-oxo-1-(prop-2-yn-1-yl)-1,2-dihydro-3H-indol-3-ylidene]-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11g). Orange solid, mp 238–240 °C (decomp). Yield: 547 mg (67%); IR (KBr): vmax/cm−1 3436, 3222, 2928, 2126, 1690, 1635, 1608, 1466, 1362, 1245, 1125, 1081, 1014, 875, 750; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.61 (s, 3H), 2.80 (s, 3H), 3.32 (s, 1H), 4.67 (br s, 2H), 4.81 (d, J = 5.9 Hz, 1H), 4.93 (d, J = 5.9 Hz, 1H), 6.99 (br s, 1H), 7.16–7.25 (m, 2H), 7.50 (t, J = 7.7 Hz, 1H), 8.87 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.0, 27.9, 29.1, 65.2, 66.0, 74.7, 77.5, 109.6, 119.5, 122.8, 123.9, 127.5, 130.8, 131.6, 142.0, 149.8, 158.7, 160.2, 166.2; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H16N6O3S 409.1077, found 409.1075.
(Z)-6-(5-Bromo-1-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-dimethyl-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11h). Light orange solid, mp 272–273 °C (decomp). Yield: 704 mg (76%); IR (KBr): vmax/cm−1 3178, 2931, 1729, 1688, 1645, 1607, 1466, 1366, 1320, 1267, 1144, 1126, 1078, 1063, 1016, 849, 798, 787; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.63 (s, 3H), 2.81 (s, 3H), 3.22 (s, 3H), 4.82 (d, J = 5.7 Hz, 1H), 4.95 (d, J = 5.7 Hz, 1H), 7.05 (s, 1H), 7.08 (d, J = 8.3 Hz, 1H), 7.62 (d, J = 8.3 Hz, 1H), 8.96 (1H, s); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.4, 26.9, 27.8, 65.2, 66.0, 110.9, 114.1, 120.9, 123.1, 129.4, 131.7, 133.6, 143.1, 149.6, 158.6, 160.2, 166.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C17H15BrN6O3S 463.0182, found 463.0192.
(R*)-Methyl 2-((Z)-3-((3aS*,9aR*)-1,3-dimethyl-2,7-dioxo-1,2,3,3a,9,9a-hexahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazin-6(7H)-ylidene)-2-oxo-1,2-dihydro-3H-indolyl-1-)propanoate and (S*)-methyl 2-((Z)-3-((3aS*,9aR*)-1,3-dimethyl-2,7-dioxo-1,2,3,3a,9,9a-hexahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazin-6(7H)-ylidene)-2-oxo-1,2-dihydro-3H-indolyl-1-) propanoate (11i). Light orange solid, mp 252–254 °C (decomp). Yield: 776 mg (85%); IR (KBr): vmax/cm−1 3231, 2954, 1751, 1737, 1722, 1696, 1640, 1607, 1463, 1370, 1264, 1252, 1075, 1015, 877, 745; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.57 (d, J = 7.1 Hz, 3H), 2.61 (s, 3H), 2.79 (s, 3H), 3.64, 3.65 (both s, in total 3H), 4.81 (dd, J = 2.0, 5.8 Hz, 1H), 4.92 (d, J = 5.8 Hz, 1H), 5.27–5.34 (q, J = 7.1 Hz, 1H), 7.00 (br s, 1H), 7.11–7.19 (m, 2H), 7.45 (t, J = 7.7 Hz, 1H), 8.90 (d, J = 7.9 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 14.17, 14.21, 26.9, 27.8, 49.0, 52.5, 52.6, 65.2, 66.0, 109.5, 119.6, 122.6, 123.9, 127.8, 130.8, 131.7, 142.2, 149.7, 158.6, 160.2, 166.7, 170.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H20N6O5S 457.1289, found 457.1281.
(Z)-Ethyl 2-(3-(1,3-dimethyl-2,7-dioxo-1,2,3,3a,9,9a-hexahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazin-6(7H)-ylidene)-2-oxo-1,2-dihydro-3H-indolyl-1-)acetate (11j). Orange solid, mp 275–276 °C (decomp). Yield: 685 mg (75%); IR (KBr): vmax/cm−1 3432, 3277, 2931, 1742, 1699, 1638, 1609, 1469, 1374, 1349, 1233, 1128, 1013, 876, 760; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.20 (t, J = 7.0 Hz, 3H), 2.63 (s, 3H), 2.80 (s, 3H), 4.12–4.19 (q, J = 7.0 Hz, 2H), 4.69 (s, 2H), 4.81 (d, J = 5.8 Hz, 1H), 4.92 (d, J = 5.8 Hz, 1H), 6.98 (s, 1H), 7.12–7.17 (m, 2H), 7.42 (t, J = 7.6 Hz, 1H), 8.84 (d, J = 7.6 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 14.0, 27.0, 27.8, 41.4, 61.4, 65.3, 66.1, 109.4, 119.4, 122.8, 123.9, 127.5, 130.7, 131.7, 143.0, 149.8, 158.7, 160.3, 167.2, 167.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H20N6O5S 457.1289, found 457.1280.
(R*)-Ethyl 2-((Z)-3-((3aS*,9aR*)-1,3-dimethyl-2,7-dioxo-1,2,3,3a,9,9a-hexahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazin-6(7H)-ylidene)-2-oxo-1,2-dihydro-3H-indolyl-1-)propanoate and (S*)-ethyl 2-((Z)-3-((3aS*,9aR*)-1,3-dimethyl-2,7-dioxo-1,2,3,3a,9,9a-hexahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazin-6(7H)-ylidene)-2-oxo-1,2-dihydro-3H-indolyl-1-)propanoate (11k). Orange solid, mp 250–252 °C (decomp). Yield: 508 mg (54%); IR (KBr): vmax/cm−1 3197, 2963, 2941, 1751, 1726, 1699, 1640, 1605, 1464, 1368, 1352, 1253, 1179, 1074, 1017, 874, 786, 744; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.11 (t, J = 7.0 Hz, 3H), 1.57 (d, J = 6.3 Hz, 3H), 2.61 (s, 3H), 2.79 (s, 3H), 4.07–4.18 (m, 2H), 4.81 (dd, J = 2.2, 5.9 Hz, 1H), 4.92 (d, J = 5.9 Hz, 1H), 5.24–5.31 (q, J = 6.3 Hz, 1H), 6.99 (t, J = 2.9 Hz, 1H), 7.11–7.19 (m, 2H), 7.45 (t, J = 7.8 Hz, 1H), 8.89 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 13.9, 14.17, 14.20, 26.9, 27.8, 49.1, 61.3, 65.2, 66.0, 109.5, 119.6, 122.6, 123.9, 127.7, 130.7, 131.6, 142.2, 149.76, 149.80, 158.6, 160.3, 166.7, 169.4; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H22N6O5S 471.1445, found 471.1440.
(Z)-6-[1-(4-Chlorobenzyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1,3-dimethyl-3,3a,9,9a-tetrahydroimidazo[4,5-e]-thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11l). Orange solid, mp 245–247 °C (decomp). Yield: 545 mg (55%); IR (KBr): vmax/cm−1 3287, 2929, 1714, 1686, 1639, 1608, 1465, 1380, 1360, 1260, 1187, 1131, 1084, 1008, 756; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.61 (s, 3H), 2.80 (s, 3H), 4.81 (dd, J = 2.0, 5.9 Hz, 1H), 4.93 (d, J = 5.9 Hz, 1H), 5.03 (s, 2H), 7.00 (d, J = 2.0 Hz, 1H), 7.05–7.16 (m, 2H), 7.32–7.41 (m, 5H), 8.86 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.9, 27.8, 42.4, 65.2, 66.1, 109.6, 119.6, 122.7, 124.2, 127.6, 128.7, 129.1, 130.6, 131.6, 132.2, 134.9, 142.8, 149.9, 158.7, 160.3, 167.2; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H19ClN6O3S 495.1001, found 495.0997.
(Z)-6-(1-Hydroxymethyl-2-oxo-1,2-dihydro-3H-indol-3-yli-dene)-1,3-dimethyl-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11m). Orange solid, mp 254–256 °C (decomp). Yield: 552 mg (69%); IR (KBr): vmax/cm−1 3369, 3246, 2963, 1706, 1687, 1638, 1607, 1464, 1365, 1262, 1138, 1062, 1038, 878, 751; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.62 (s, 3H), 2.80 (s, 3H), 4.80 (dd, J = 2.2, 5.9 Hz, 1H), 4.92 (d, J = 5.9 Hz, 1H), 5.18 (d, J = 7.1 Hz, 2H), 6.44 (t, J = 7.1 Hz, 1H), 6.97 (d, J = 2.2 Hz, 1H), 7.16 (t, J = 7.7 Hz, 1H), 7.24 (d, J = 7.7 Hz, 1H), 7.47 (t, J = 7.7 Hz, 1H), 8.86 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 26.9, 27.9, 62.8, 65.1, 66.1, 110.1, 119.4, 122.7, 124.5, 127.5, 130.1, 131.6, 142.8, 149.9, 158.7, 160.3, 166.8; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C17H16N6O4SNa 423.0846, found 423.0847.
(Z)-(3-(1,3-Dimethyl-2,7-dioxo-1,2,3,3a,9,9a-hexahydro-imidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazin-6(7H)-ylidene)-2-oxo-1,2-dihydro-3H-indolyl-1-)methyl benzoate (11n). Orange solid, mp 262–264 °C (decomp). Yield: 621 mg (57%); IR (KBr): vmax/cm−1 3261, 2965, 2912, 1714, 1694, 1637, 1609, 1468, 1365, 1263, 1132, 1080, 1063, 1009, 950, 878, 767; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.62 (s, 3H), 2.80 (s, 3H), 4.82 (dd, J = 2.3, 5.9 Hz, 1H), 4.93 (d, J = 5.9 Hz, 1H), 6.10 (dd, J = 1.2, 13.7 Hz, 2H), 7.01 (d, J = 2.3 Hz, 1H), 7.22 (t, J = 7.6 Hz, 1H), 7.42 (d, J = 7.9 Hz, 1H), 7.48–7.53 (m, 3H), 7.66 (t, J = 7.4 Hz, 1H), 7.94 (d, J = 7.5 Hz, 2H), 8.91 (d, J = 7.9 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.1, 28.0, 64.2, 65.4, 66.2, 110.1, 119.7, 123.5, 127.8, 128.8, 128.9, 129.0, 129.49, 129.54, 132.0, 134.0, 141.8, 149.8, 158.9, 160.4, 165.1, 167.3; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H20N6O5S 505.1289, found 505.1290.
(Z)-1,3-Diethyl-6-(2-oxo-1,2-dihydro-3H-indol-3-ylidene)-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11o). Red-orange solid, mp 285–287 °C (decomp). Yield: 446 mg (56%); IR (KBr): vmax/cm−1 3250, 2976, 2944, 1706, 1642, 1618, 1459, 1381, 1334, 1244, 1077, 752; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.97 (t, J = 6.9 Hz, 3H), 1.15 (t, J = 7.1 Hz, 3H), 3.11–3.18 (m, 3H), 3.32–3.39 (m, 1H), 4.92–4.98 (m, 2H), 6.93–6.96 (m, 2H), 7.06 (t, J = 7.6 Hz, 1H), 7.37 (t, J = 7.6 Hz, 1H), 8.79 (d, J = 7.8 Hz, 1H), 11.18 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.8, 13.4, 34.4, 35.1, 63.1, 64.3, 110.3, 120.0, 121.9, 125.5, 127.6, 128.9, 131.8, 143.2, 150.2, 157.7, 160.5, 168.5; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C18H18N6O3SNa 421.1053, found 421.1052.
(Z)-1,3-Diethyl-6-(1-methyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11p). Orange solid, mp 240–241 °C (decomp). Yield: 453 mg (55%); IR (KBr): vmax/cm−1 3434, 3253, 2972, 2938, 1697, 1642, 1610, 1469, 1380, 1357, 1247, 1070, 877, 753; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.97 (t, J = 6.9 Hz, 3H), 1.15 (t, J = 7.1 Hz, 3H), 3.12–3.18 (m, 3H), 3.23 (s, 3H), 3.33–3.40 (m, 1H), 4.96 (m, 2H), 6.93 (s, 1H), 7.09–7.15 (m, 2H), 7.44 (t, J = 7.7 Hz, 1H), 8.80 (d, J = 7.7 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.8, 13.5, 26.3, 34.5, 35.1, 63.1, 64.3, 109.1, 119.3, 122.5, 124.7, 127.4, 129.6, 131.8, 144.2, 150.1, 157.8, 160.4, 166.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H20N6O3S 413.1390, found 413.1383.
(Z)-1,3-Diethyl-6-(1-ethyl-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11q). The product was isolated via the general procedure but the reaction time was 2.5 h. Orange solid, mp 257–259 °C (decomp). Yield: 461 mg (54%); IR (KBr): vmax/cm−1 3435, 3229, 2975, 2937, 1699, 1689, 1637, 1610, 1468, 1426, 1373, 1356, 1245, 1072, 874, 747; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.97 (t, J = 7.0 Hz, 3H), 1.13–1.21 (m, 6H), 3.09–3.18 (m, 3H), 3.33–3.39 (m, 1H), 3.79–3.86 (q, J = 7.0 Hz, 2H), 4.96 (m, 2H), 6.96 (br s, 1H), 7.11 (t, J = 7.6 Hz, 1H), 7.20 (d, J = 7.9 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 8.84 (d, J = 7.8 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.6, 12.8, 13.4, 34.4, 34.5, 35.1, 63.1, 64.3, 109.1, 119.5, 122.3, 124.6, 127.6, 129.7, 131.8, 143.1, 150.0, 157.7, 160.4, 166.6; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H22N6O3S 427.1547, found 427.1538.
(Z)-1,3-Diethyl-6-[1-(2-propyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo-[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11r). Orange solid, mp 257–259 °C (decomp). Yield: 590 mg (67%); IR (KBr): vmax/cm−1 3433, 3220, 2978, 2940, 1720, 1694, 1642, 1607, 1465, 1381, 1357, 1316, 1246, 1128, 1075, 1047, 871, 745; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.99 (t, J = 7.0 Hz, 3H), 1.17 (t, J = 7.2 Hz, 3H), 1.46 (d, J = 6.9 Hz, 6H), 3.10–3.19 (m, 3H), 3.32–3.41 (m, 1H), 4.54–4.63 (m, 1H), 4.96 (m, 2H), 6.92 (br s, 1H), 7.12 (t, J = 7.7 Hz, 1H), 7.28 (d, J = 7.9 Hz, 1H), 7.44 (t, J = 7.7 Hz, 1H), 8.89 (d, J = 7.9 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.9, 13.4, 19.1, 19.2, 34.4, 35.1, 44.2, 63.1, 64.4, 110.1, 119.6, 122.1, 124.9, 127.7, 129.6, 131.7, 142.8, 150.1, 157.7, 160.4, 166.7; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H24N6O3S 441.1703, found 441.1702.
(Z)-6-[5-Bromo-1-(3-bromobenzyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene]-1,3-diethyl-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (11s). Orange solid, mp 222–224 °C (decomp). Yield: 672 mg (52%); IR (KBr): vmax/cm−1 3435, 3200, 2972, 2933, 1719, 1691, 1643, 1606, 1462, 1430, 1375, 1352, 1244, 1082, 889, 772; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.99 (t, J = 7.0 Hz, 3H), 1.17 (t, J = 7.1 Hz, 3H), 3.11–3.20 (m, 3H), 3.33–3.42 (m, 1H), 4.96–5.05 (m, 4H), 7.05–7.08 (m, 2H), 7.28–7.30 (m, 2H), 7.49 (m, 1H), 7.56 (s, 1H), 7.61 (d, J = 8.5 Hz, 1H), 9.06 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.7, 13.4, 34.5, 35.0, 42.5, 63.3, 64.2, 111.4, 114.5, 121.3, 121.9, 123.0, 126.2, 129.7, 129.9, 130.5, 130.8, 132.6, 133.6, 138.4, 141.8, 149.5, 157.6, 160.3, 166.8; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C25H22Br2N6O3SNa 666.9733, found 666.9730.
6-(1-Ethyl-3-hydroxy-2-oxo-1,2-dihydro-3H-indol-3-yl)-1,3-dimethyl-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (12c). The product was obtained via the general procedure for the preparation of compounds 11 from starting 6a and 10c for 2 h. Beige crystals, mp 257–259 °C (decomp). Yield: 350 mg (42%); IR (KBr): vmax/cm−1 3432, 3209, 2972, 2937, 2879, 1724, 1698, 1674, 1638, 1613, 1489, 1469, 1450, 1376, 1261, 1247, 1133, 1113, 1082, 1010, 789, 757; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.16 (t, J = 7.0 Hz, 3H), 1.97 (s, 3H), 2.75 (s, 3H), 3.63–3.73 (m, 2H), 4.44 (d, J = 5.6 Hz, 1H), 4.64 (d, J = 5.6 Hz, 1H), 4.80 (s, 1H), 6.51 (s, 1H), 6.85 (t, J = 7.5 Hz, 1H), 6.94 (s, 1H), 7.00 (d, J = 7.9 Hz, 1H), 7.34 (t, J = 7.7 Hz, 1H), 7.44 (d, J = 7.3 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.0, 26.6, 26.9, 34.2, 52.9, 64.9, 65.5, 74.2, 108.5, 122.2, 123.7, 126.4, 130.2, 142.8, 150.6, 158.2, 164.2, 174.0; HRMS (ESI-TOF) m/z: [M + Na]+ calcd for C18H20N6O4SNa 439.1159, found 439.1152.After isolation of 12c, orange solid was precipitated from the filtrate for one day. Filtration and recrystallization from methanol gave 88 mg (11%) of compound 11c.
1,3-Diethyl-6-(1-ethyl-3-hydroxy-2-oxo-1,2-dihydro-3H-indol-3-yl)-3,3a,9,9a-tetrahydroimidazo[4,5-e]thiazolo[3,2-b]-1,2,4-triazine-2,7(1H,6H)-dione (12q). The product was obtained via the general procedure for the preparation of compounds 11 from starting 6b and 10c for 2 h. Beige crystals, mp 223–225 °C (decomp). Yield: 169 mg (19%); IR (KBr): vmax/cm−1 3254, 2973, 2935, 2874, 1731, 1711, 1685, 1634, 1614, 1468, 1378, 1246, 1094, 1036, 775, 755; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.48 (t, J = 6.9 Hz, 3H), 1.10–1.18 (m, 6H), 2.34–2.44 (m, 1H), 2.62–2.71 (m, 1H), 3.08–3.17 (m, 1H), 3.23–3.35 (m, 1H), 3.59–3.77 (m, 2H), 4.57 (d, J = 5.6 Hz, 1H), 4.71 (d, J = 5.6 Hz, 1H), 4.83 (s, 1H), 6.46 (s, 1H), 6.86 (t, J = 7.5 Hz, 1H), 6.92 (s, 1H), 6.99 (d, J = 7.8 Hz, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.47 (d, J = 7.3 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.0, 13.4, 34.1, 34.3, 52.8, 62.5, 63.7, 74.0, 108.6, 122.1, 124.0, 126.5, 130.2, 142.9, 150.6, 157.3, 164.2, 174.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H24N6O4S 445.1653, found 445.1646.After isolation of 12q, orange solid was precipitated from the filtrate for one day. Filtration and recrystallization from methanol gave 239 mg (28%) of compound 11q.
General procedure for the synthesis of compounds 9 via a rearrangement of compounds 11
Procedure 2. To a stirred suspension of compound 11a (741 mg, 2.0 mmol) in refluxing methanol (15 ml), 0.12 ml of 40% aqueous KOH (1.2 mmol) was added. The resulting mixture was refluxed with stirring for 20 min. After cooling, the precipitate was filtered off and washed with water to give 9a (659 mg, 89%).Compounds 9b–d,f,g,j,k,m,p were obtained via the general procedure in 92% (707 mg), 91% (725 mg), 93% (767 mg), 94% (892 mg), 89% (731 mg), 94% (858 mg), 91% (751 mg), 90% (793 mg), 88% (871 mg), respectively.
Compound 9p is too insoluble to record a good 13C NMR spectrum.
(Z)-7-(1-(4-Chlorobenzyl)-2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3-dimethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9p). Orange solid, mp 296–298 °C (decomp). Yield: 871 mg (88%); IR (KBr): vmax/cm−1 3308, 2946, 1701, 1676, 1643, 1608, 1465, 1383, 1364, 1308, 1279, 1091, 1014, 783, 752; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.67 (s, 3H), 2.93 (s, 3H), 4.84 (d, J = 4.7 Hz, 1H), 5.04 (br s, 2H), 5.70 (d, J = 5.1 Hz, 1H), 7.05–7.15 (m, 2H), 7.32–7.40 (m, 5H), 8.07 (s, 1H), 8.82 (d, J = 7.7 Hz, 1H); HRMS (ESI-TOF) m/z: [M + H]+ calcd for C23H19ClN6O3S 495.1001, found 495.0996.
(Z)-1,3-Diethyl-7-(2-oxo-1,2-dihydro-3H-indol-3-ylidene)-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (9q). Orange solid, mp 325–327 °C (decomp). Yield: 733 mg (92%); IR (KBr): vmax/cm−1 3294, 3204, 3181, 2971, 1704, 1687, 1637, 1614, 1463, 1328, 1232, 1086, 1004, 778, 749; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.04 (t, J = 6.7 Hz, 3H), 1.13 (t, J = 6.5 Hz, 3H), 3.04–3.13 (m, 1H), 3.31–3.54 (m, 3H), 4.90 (d, J = 5.3 Hz, 1H), 5.76 (d, J = 5.2 Hz, 1H), 6.96 (d, J = 7.8 Hz, 1H), 7.07 (t, J = 7.6 Hz, 1H), 7.34 (t, J = 7.6 Hz, 1H), 7.99 (s, 1H), 8.75 (d, J = 7.7 Hz, 1H), 11.14 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.7, 13.0, 34.9, 37.9, 61.6, 63.6, 110.2, 120.3, 121.7, 123.1, 127.2, 129.0, 130.9, 136.7, 142.5, 158.0, 164.2, 168.5; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H18N6O3S 399.1234, found 399.1228.
Synthesis of compounds 9r,s via a rearrangement of 11j
To a stirred suspension of compound 11j (913 mg, 2.0 mmol) in refluxing methanol (15 ml), 0.20 ml of 40% aqueous KOH (2.0 mmol) was added. The resulting mixture was refluxed with stirring for 20 min. After cooling, the precipitate was filtered off and washed with water to give 9r (327 mg, 37%). After isolation of 9r, orange solid was precipitated from the water–methanol filtrate for one day. Filtration and washing with methanol gave compound 9s (47 mg, 5%).
(Z)-Methyl 2-(3-(1,3-dimethyl-2,8-dioxo-1,2,3,3a,4,9a-hexahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazin-7(8H)-ylidene)-2-oxo-1,2-dihydro-3H-indolyl-1-)acetate (9r). Orange solid, mp 275–277 °C (decomp). Yield: 327 mg (37%); IR (KBr): vmax/cm−1 3308, 3271, 2953, 2928, 1741, 1723, 1700, 1682, 1644, 1610, 1468, 1391, 1359, 1232, 1190, 1091, 1020, 783, 750; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.67 (s, 3H), 2.93 (s, 3H), 3.70 (s, 3H), 4.74 (s, 2H), 4.83 (d, J = 5.3 Hz, 1H), 5.69 (d, J = 5.8 Hz, 1H), 7.14–7.18 (m, 2H), 7.40 (t, J = 7.7 Hz, 1H), 8.10 (s, 1H), 8.83 (d, J = 7.9 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.9, 31.3, 41.2, 52.3, 63.7, 65.8, 109.2, 119.6, 121.3, 122.5, 127.1, 130.7, 134.0, 136.3, 142.3, 159.0, 163.9, 167.2, 168.2; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C19H18N6O5S 443.1132, found 443.1121.
Potassium (Z)-2-(3-(1,3-dimethyl-2,8-dioxo-1,2,3,3a,4,9a-hexahydroimidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazin-7(8H)-ylidene)-2-oxo-1,2-dihydro-3H-indolyl-1-)acetate (9s). Orange solid, mp 287–289 °C (decomp). Yield: 47 mg (5%); IR (KBr): vmax/cm−1 3432, 3271, 2928, 1719, 1684, 1676, 1645, 1609, 1468, 1397, 1381, 1361, 1340, 1235, 1194, 1087, 1037, 1021, 753; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.66 (s, 3H), 2.92 (s, 3H), 3.97–4.05 (m, 2H), 4.80 (d, J = 4.5 Hz, 1H), 5.68 (d, J = 5.2 Hz, 1H), 6.88 (d, J = 7.5 Hz, 1H), 7.04 (t, J = 7.3 Hz, 1H), 7.33 (t, J = 7.3 Hz, 1H), 8.11 (s, 1H), 8.75 (d, J = 7.5 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.8, 31.3, 44.5, 63.6, 65.6, 109.5, 119.4, 121.4, 122.9, 126.6, 130.5, 131.6, 136.7, 144.4, 158.9, 164.0, 166.7, 167.8; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C18H16N6O5S 429.0976, found 429.0963; [M + K]+ calcd for C18H16N6O5SK 467.0534, found 467.0524.
General procedure for the synthesis of compounds 14 via a rearrangement of compounds 6 (or 13)
To a stirred suspension of compound 6a (644 mg, 2.0 mmol) or 13a (483 mg, 2.0 mmol) in refluxing methanol (15 ml), 0.32 ml (3.2 mmol) or 0.12 ml (1.2 mmol), respectively, of 40% aqueous KOH was added. The resulting mixture was refluxed with stirring for 1 h. After cooling and filtration from cloudiness, the filtrate was left overnight. The separated precipitate was filtered off and washed with water to give 14a (449 mg, 93%).
1,3-Dimethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo-[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (14a). Off-white crystals, mp 226–228 °C. Yield: 449 mg (93%); IR (KBr): vmax/cm−1 3318, 2935, 1710, 1638, 1474, 1448, 1379, 1313, 1290, 1012, 786; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.60 (s, 3H), 2.81 (s, 3H), 3.96 (d, J = 16.7 Hz, 1H), 4.07 (d, J = 16.7 Hz, 1H), 4.70 (d, J = 6.2 Hz, 1H), 5.49 (d, J = 6.2 Hz, 1H), 7.47 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 27.6, 30.9, 31.3, 64.3, 66.0, 138.9, 158.9, 170.9; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C8H11N5O2S 242.0706, found 242.0704.
1,3-Diethyl-1,3a,4,9a-tetrahydroimidazo[4,5-e]thiazolo-[2,3-c]-1,2,4-triazine-2,8(3H,7H)-dione (14b). Off-white crystals, mp 171–173 °C. Yield: 522 mg (97%); IR (KBr): vmax/cm−1 3385, 3163, 2977, 1723, 1640, 1606, 1520, 1470, 1424, 1308, 1229, 1089, 836, 771; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 0.98 (t, J = 7.0 Hz, 3H), 1.05 (t, J = 7.0 Hz, 3H), 2.98–3.24 (m, 3H), 3.34–3.45 (m, 1H), 3.95 (d, J = 16.7 Hz, 1H), 4.08 (d, J = 16.7 Hz, 1H), 4.75 (d, J = 5.0 Hz, 1H), 5.55 (d, J = 6.2 Hz, 1H), 7.44 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 12.7, 13.1, 31.3, 34.6, 37.4, 62.0, 63.7, 138.7, 157.9, 171.0; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C10H15N5O2S 270.1019, found 270.1018.
General procedure for the synthesis of compounds 9 via a condensation of starting 14 and 10
To a stirred suspension of imidazo[4,5-e]thiazolo[2,3-c]-1,2,4-triazine 14a (483 mg, 2.0 mmol) and isatin 10b (322 mg, 2.0 mmol) in refluxing methanol (15 ml), 0.014 ml of 40% aqueous KOH (0.14 mmol) was added. The resulting mixture was refluxed with stirring for 30 min. After cooling, the precipitate was filtered off, washed with water and to give 9b (646 mg, 84%).
Compound 9g was obtained from starting 14a and 10g via the general procedure in 85% (698 mg) yield.
Crystallographic data
Crystals of 13a (C8H11N5O2S, M = 241.28) are monoclinic, space group P21/n, at 100 K: a = 6.8510(5), b = 7.7360(6), c = 19.3150(14) Å, β = 95.218(2)°, V = 1019.44(13) Å3, Z = 4 (Z′ = 1), dcalc = 1.572 g cm−3, μ(MoKα) = 3.11 cm−1, F(000) = 504. Crystals of 14b (C11H19N5O3S, M = 301.37) are monoclinic, space group C2/c, at 100 K: a = 26.710(4), b = 7.5095(10), c = 14.0757(19) Å, β = 96.942(2)°, V = 2802.6(7) Å3, Z = 8 (Z′ = 2), dcalc = 1.429 g cm−3, μ(MoKα) = 2.47 cm−1, F(000) = 1280. Intensities of 8078 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 581 reflections were measured for 13a and 14b with a Bruker APEX2 CCD diffractometer [λ(MoKα) = 0.71072 Å, ω-scans, 2θ < 58°], and 2712 and 3653 independent reflections [Rint = 0.0334 and 0.0426], respectively, were used in further refinement. The structures were solved by direct method and refined by the full-matrix least-squares technique against F2 in the anisotropic–isotropic approximation. The hydrogen atoms of NH groups and that of OH group of the solvent methanol molecule in 14b were found in difference Fourier synthesis, the positions of other hydrogen atoms were calculated, and all of them were refined in the isotropic approximation within the riding model. For 13a, the refinement converged to wR2 = 0.0975 and GOF = 1.005 for all the independent reflections (R1 = 0.0367 was calculated against F for 2192 observed reflections with I > 2σ(I)). For 14b, the refinement converged to wR2 = 0.1166 and GOF = 1.005 for all the independent reflections (R1 = 0.0381 was calculated against F for 3251 observed reflections with I > 2σ(I)). All calculations were performed using SHELXTL PLUS 5.0 (ESI†).19
581 reflections were measured for 13a and 14b with a Bruker APEX2 CCD diffractometer [λ(MoKα) = 0.71072 Å, ω-scans, 2θ < 58°], and 2712 and 3653 independent reflections [Rint = 0.0334 and 0.0426], respectively, were used in further refinement. The structures were solved by direct method and refined by the full-matrix least-squares technique against F2 in the anisotropic–isotropic approximation. The hydrogen atoms of NH groups and that of OH group of the solvent methanol molecule in 14b were found in difference Fourier synthesis, the positions of other hydrogen atoms were calculated, and all of them were refined in the isotropic approximation within the riding model. For 13a, the refinement converged to wR2 = 0.0975 and GOF = 1.005 for all the independent reflections (R1 = 0.0367 was calculated against F for 2192 observed reflections with I > 2σ(I)). For 14b, the refinement converged to wR2 = 0.1166 and GOF = 1.005 for all the independent reflections (R1 = 0.0381 was calculated against F for 3251 observed reflections with I > 2σ(I)). All calculations were performed using SHELXTL PLUS 5.0 (ESI†).19
Powder diffraction data
High-quality experimental powder X-ray diffraction data for compounds 9b,d,e,n,11c,b were obtained with a PANalytical EMPYREAN diffractometer (fine-focus sealed tube, Cu Kα1 radiation (λ = 1.5406 Å), Johanson's Hybrid Ge{111} monochromator for the primary beam, Bragg-Brentano geometry) using a position-sensitive detector PIXcel1D. The patterns were scanned in reflection mode, θ/2θ continuously scanned over the angular range 5° to 60° (2θ) with a step 0.013° (2θ) and counting time of 1000 s per step. Preferred orientation effects were reduced by grinding. Alignment and calibration were checked using Al2O3 (SRM676). Diffraction data were collected at room temperature (296 K).
The extraction of peak position for indexing was performed with Pawley method. Patterns indexing were carried out by means of the program Ito or TREOR. Unit cell parameters were refined by least-squares fitting of Bragg's equation to the position of the diffraction lines. All calculations for the refinement of the diffraction patterns and refine the unit cell parameters were performed using complex programs available in PC software “High Score Plus” supplied by PANalytical EMPYREAN (Version: 3.0.t (3.0.5), Date 30-01-2012. Produced by: PANalytical B. V. Amelo, The Netherland).
The experimental powder XRD data and cell parameters obtained for compounds 9b,d,e,n,11c,b are deposited at the PDF-base of International Centre for Diffraction Data (ICDD).
Results of the analysis of the experimental powder diffraction pattern of the compounds 9b,d,e,n,11c,b show that the investigated samples were single-phase. Space group, unit cell parameters and characteristics of the investigated verification phases shown in Table 7. Figures giving powder diffraction patterns for the products are in ESI.†
Table 7 Space groups, unit cell parameters and characteristics of the investigated verification phases of compounds 9b,d,e,n,11c,b
| Compound |
9b |
9d |
9e |
9n |
11b |
11c |
| Sp. gr, Z |
P21/c 1, Z = 4 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2 , Z = 2 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 4 , Z = 4 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 4 , Z = 4 |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 4 , Z = 4 |
P21/m, Z = 4 |
| a, (Å) |
15.603(2) |
4.492(8) |
13.373(2) |
7.638(1) |
10.275(2) |
7.164(1) |
| b, (Å) |
26.769(5) |
14.27(2) |
11.724(2) |
14.360(2) |
25.970(4) |
10.787(2) |
| c, (Å) |
4.3094(8) |
13.97(2) |
14.623(2) |
19.700(3) |
6.864(1) |
23.390(4) |
| α, (°) |
90 |
82.465(9) |
78.57(6) |
93.656(5) |
93.43(1) |
90 |
| β, (°) |
93.281(3) |
88.576(3) |
94.779(2) |
90.918(2) |
105.308(3) |
92.340(3) |
| γ, (°) |
90 |
89.482(3) |
80.003(3) |
77.517(2) |
89.194(3) |
90 |
| V, (Å3) |
1796.92 |
887.64 |
2196.52 |
2105.59 |
1763.42 |
1806.02 |
| Number of reflections |
56 |
87 |
105 |
108 |
70 |
83 |
| Snyder's FOM |
24.7299 |
19.4289 |
9.0048 |
13.1699 |
10.7239 |
14.4113 |
Acknowledgements
The authors thank Dr Yuri A. Strelenko and Dr Paul A. Belyakov (1D and 2D NMR), Alexander S. Kulikov for provision of isatin derivatives.
Notes and references
-
(a) Chemistry of Heterocyclic Compounds, ed. G. P. Ellis, Wiley, New York, 2008, vol. 47 Search PubMed;
(b) Advances in Heterocyclic Chemistry, ed. A. R. Katritzky, Elsevier, London, 2008, vol. 96 Search PubMed.
- D. L. Trepanier and P. E. Krieger, US Pat., 3 641 019, 1968.
-
(a) R. M. Abdel-Rahman, M. Seada, M. Fawzy and I. El-Baz, Pharmazie, 1994, 49, 729 CAS;
(b) R. M. Abdel-Rahman, M. Seada, M. Fawzy and I. El-Baz, Boll. Chim. Farm., 1994, 133, 381 CAS.
-
(a) K. S. Dhaka, H. S. Chaudhary, K. S. Sharma and H. K. Pujari, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1976, 14, 541 Search PubMed;
(b) S. B. Levy, M. N. Alekshun, B. L. Podlogar, K. Ohemeng, A. K. Verma, T. Warchol and B. Bhatia, US Pat., 0 229 065, 2003.
-
(a) D. L. Trepanier and P. E. Krieger, J. Heterocycl. Chem., 1971, 621 CrossRef CAS PubMed;
(b) V. J. Rany, Liebigs Ann. Chem., 1988, 11, 1089 Search PubMed;
(c) M. M. Heravi, M. Rahimizadeh, E. Iravani and M. Ghassemzadeh, Phosphorus, Sulfur Silicon Relat. Elem., 2003, 178, 797 CrossRef CAS PubMed;
(d) G. A. Gazieva and A. N. Kravchenko, Russ. Chem. Rev., 2012, 81, 494 CrossRef CAS PubMed;
(e) G. A. Gazieva, P. A. Poluboyarov, Y. V. Nelyubina, M. I. Struchkova and A. N. Kravchenko, Chem. Heterocyclic Compd., 2012, 48, 1382 (Khim. Geterotsikl. Soedin., 2012, 1483) CrossRef CAS PubMed.
-
(a) A. Singh, K. S. Dhaka, H. S. Chaudhary and H. K. Pujari, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1977, 15, 46 CAS;
(b) S. Bala, M. L. Sachdeva, R. N. Handa and H. K. Pujari, Heterocycles, 1980, 14, 149 CrossRef CAS;
(c) M. M. Heravi, K. Aghapoor, M. A. Nooshabadi and M. M. Mojtahedi, Monatsh. Chem., 1997, 128, 1143 CrossRef CAS;
(d) R. M. Abdel-Rahman, M. S. T. Makki, T. E. Ali and M. A. Ibrahim, Eur. J. Chem., 2010, 1, 236 CrossRef CAS PubMed;
(e) S. V. Vasilevskii, P. A. Belyakov, G. A. Gazieva, Y. V. Nelyubina, N. G. Kolotyrkina and A. N. Kravchenko, Mendeleev Commun., 2010, 20, 47 CrossRef CAS PubMed.
- J. Mohan and A. Kumar, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2002, 41, 2364 Search PubMed.
-
(a) M. I. Pleshchev, V. Y. Petukhova, V. V. Kuznetsov, D. V. Khakimov, T. S. Pivina, M. I. Struchkova, Y. V. Nelyubina and N. N. Makhova, Mendeleev Commun., 2013, 23, 34 CrossRef CAS PubMed;
(b) J. C. Orejarena Pacheco and T. Opatz, J. Org. Chem., 2014, 79, 5182 CrossRef CAS PubMed;
(c) M. Ueda, Y. Ito, Y. Ichii, M. Kakiuchi, H. Shono and O. Miyata, Chem.–Eur. J., 2014, 20, 6763 CrossRef CAS PubMed.
- A. N. Kravchenko, G. A. Gazieva, S. V. Vasilevskii and Y. V. Nelyubina, Mendeleev Commun., 2014, 24, 119 CrossRef CAS PubMed.
- G. A. Gazieva, E. A. Shishkova, L. B. Kulikova, N. G. Kolotyrkina, N. V. Sigay and A. N. Kravchenko, J. Heterocycl. Chem., 2014, 51, 921 CrossRef CAS PubMed.
-
(a) G. A. Gazieva and A. N. Izmest'ev, Chem. Heterocyclic
Compd., 2015, 50, 1515 (Khim. Geterotsikl. Soedin., 2014, 1649) CAS;
(b) F. Erben, D. Michalik, H. Feist, D. Kleeblatt, M. Hein, A. Matin, J. Iqbal and P. Langer, RSC Adv., 2014, 4, 10879 RSC.
- A. S. Sigachev, A. N. Kravchenko, P. A. Belyakov, O. V. Lebedev and N. N. Makhova, Russ. Chem. Bull., Int. Ed., 2006, 55, 865 (Izv. AN. Ser. Khim., 2006, 836) CrossRef CAS.
- S. Minyan, S. M. Ramsh, V. N. Plotkin and S. Y. Solov'eva, Russ. J. Gen. Chem., 2011, 81, 1886 (Zhurnal Obshchei Khimii, 2011, 81, 1549) CrossRef.
-
(a) P. B. Thakur and H. M. Meshram, RSC Adv., 2014, 4, 5343 RSC;
(b) S. Paladhi, M. Bhati, D. Panda and J. Dash, J. Org. Chem., 2014, 79, 1473 CrossRef CAS PubMed.
-
(a) L. F. Tietze and N. Rackelmann, in Multicomponent Reactions, ed. J. Zhu and H. Bienayme, Wiley-VCH, Weinheim, 2005, p. 121 Search PubMed;
(b) A. Kumar and R. A. Maurya, Tetrahedron, 2007, 63, 1946 CrossRef CAS PubMed;
(c) D. B. Ramachary and M. Kishor, J. Org. Chem., 2007, 72, 5056 CrossRef CAS PubMed;
(d) M. Vilches-Herrera, I. Knepper, N. de Souza, A. Villinger, V. Y. Sosnovskikh and V. O. Iaroshenko, ACS Comb. Sci., 2012, 14, 434 CrossRef CAS PubMed;
(e) M. Li, X.-L. Lv, L.-R. Wen and Z.-Q. Hu, Org. Lett., 2013, 15, 1262 CrossRef CAS PubMed;
(f) V. Jeyachandran, R. R. Kumar, M. A. Ali and T. S. Choon, Bioorg. Med. Chem. Lett., 2013, 23, 2101 CrossRef CAS PubMed;
(g) M. Xia and R.-Z. Ma, J. Heterocycl. Chem., 2014, 51, 539 CrossRef CAS PubMed.
- M. Kim, Y. Jung and I. Kim, J. Org. Chem., 2013, 78, 10395 CrossRef CAS PubMed.
-
(a) I. Kim, S. G. Kim, J. Choi and G. H. Lee, Tetrahedron, 2008, 64, 664 CrossRef CAS PubMed;
(b) K. C. Majumdar, A. Taher and R. K. Nandi, Tetrahedron, 2012, 68, 5693 CrossRef CAS PubMed.
-
(a) R. G. Redkin, L. A. Shemchuk, V. P. Chernykh, O. V. Shishkin and S. V. Shishkina, Tetrahedron, 2007, 63, 11444 CrossRef CAS PubMed;
(b) Y. M. Litvinov, V. Y. Mortikov and A. M. Shestopalov, J. Comb. Chem., 2008, 10, 741 CrossRef CAS PubMed;
(c) M. N. Elinson, A. S. Dorofeev, F. M. Miloserdov and G. I. Nikishin, Mol. Diversity, 2009, 13, 47 CrossRef CAS PubMed;
(d) G. S. Hari and Y. R. Lee, Synthesis, 2010, 453 CAS;
(e) R. Ghahremanzadeh, F. Fereshtehnejad, Z. Yesaei, T. Amanpour and A. Bazgir, J. Heterocycl. Chem., 2010, 47, 967 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH
CH
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH
CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2
CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or C(7)
O or C(7)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Besides, compounds 9j, 11i and 11k with additional chiral carbon atom in indole moiety are obtained as a mixture of two diastereomers (1′′R*,3aS*,9aR*- and 1′′S*,3aS*,9aR*-isomers) and so some signals in the 1H and 13C NMR spectra of these products are double. The homogeneity of compounds 9b,d,e,n and 11b,c was confirmed by powder X-ray diffraction. Results of the analysis of the experimental powder diffraction patterns of the compounds 9b,d,e,n and 11b,c show that the investigated samples were single-phase.
O. Besides, compounds 9j, 11i and 11k with additional chiral carbon atom in indole moiety are obtained as a mixture of two diastereomers (1′′R*,3aS*,9aR*- and 1′′S*,3aS*,9aR*-isomers) and so some signals in the 1H and 13C NMR spectra of these products are double. The homogeneity of compounds 9b,d,e,n and 11b,c was confirmed by powder X-ray diffraction. Results of the analysis of the experimental powder diffraction patterns of the compounds 9b,d,e,n and 11b,c show that the investigated samples were single-phase.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 581 reflections were measured for 13a and 14b with a Bruker APEX2 CCD diffractometer [λ(MoKα) = 0.71072 Å, ω-scans, 2θ < 58°], and 2712 and 3653 independent reflections [Rint = 0.0334 and 0.0426], respectively, were used in further refinement. The structures were solved by direct method and refined by the full-matrix least-squares technique against F2 in the anisotropic–isotropic approximation. The hydrogen atoms of NH groups and that of OH group of the solvent methanol molecule in 14b were found in difference Fourier synthesis, the positions of other hydrogen atoms were calculated, and all of them were refined in the isotropic approximation within the riding model. For 13a, the refinement converged to wR2 = 0.0975 and GOF = 1.005 for all the independent reflections (R1 = 0.0367 was calculated against F for 2192 observed reflections with I > 2σ(I)). For 14b, the refinement converged to wR2 = 0.1166 and GOF = 1.005 for all the independent reflections (R1 = 0.0381 was calculated against F for 3251 observed reflections with I > 2σ(I)). All calculations were performed using SHELXTL PLUS 5.0 (ESI†).19
581 reflections were measured for 13a and 14b with a Bruker APEX2 CCD diffractometer [λ(MoKα) = 0.71072 Å, ω-scans, 2θ < 58°], and 2712 and 3653 independent reflections [Rint = 0.0334 and 0.0426], respectively, were used in further refinement. The structures were solved by direct method and refined by the full-matrix least-squares technique against F2 in the anisotropic–isotropic approximation. The hydrogen atoms of NH groups and that of OH group of the solvent methanol molecule in 14b were found in difference Fourier synthesis, the positions of other hydrogen atoms were calculated, and all of them were refined in the isotropic approximation within the riding model. For 13a, the refinement converged to wR2 = 0.0975 and GOF = 1.005 for all the independent reflections (R1 = 0.0367 was calculated against F for 2192 observed reflections with I > 2σ(I)). For 14b, the refinement converged to wR2 = 0.1166 and GOF = 1.005 for all the independent reflections (R1 = 0.0381 was calculated against F for 3251 observed reflections with I > 2σ(I)). All calculations were performed using SHELXTL PLUS 5.0 (ESI†).19
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 2
, Z = 2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 4
, Z = 4![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 4
, Z = 4![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , Z = 4
, Z = 4












