Nature of the Pd–CNT interaction in Pd nanoparticles dispersed on multi-walled carbon nanotubes and its implications in hydrogen storage properties
Tapas Dasa,
Seemita Banerjee*a,
Kinshuk Dasguptab,
J. B. Joshic and
V. Sudarsana
aChemistry Division, Bhabha Atomic Research Centre, Mumbai 400085, India. E-mail: seemita@barc.gov.in; Fax: +91-22-25505151; Tel: +91-22-25595093
bRare Earths Development Section, Materials Group, Bhabha Atomic Research Centre, Mumbai 400085, India
cHomi Bhabha National Institute, Anushaktinagar, Mumbai 400094, India
First published on 30th April 2015
Abstract
Oleyl amine stabilised Pd nanoparticles have been prepared by reverse micro-emulsion method and supported on multi walled CNTs. TEM studies have confirmed that Pd nanoparticles, having sizes in the range of 3–5 nm, are well dispersed on the CNTs. Based on 13C MAS NMR and TG-DTA studies it is inferred that the Pd nanoparticles interact with CNT support to form sp3 carbon species, which get effectively dispersed on the CNTs. Such finely dispersed Pd nanoparticles facilitate the spillover of hydrogen to the CNT support and improve the hydrogen storage capacity.
1 Introduction
In the recent decades, studies on nanoparticles (1–100 nm in diameter) of coinage metals, especially those of gold, platinum, palladium etc., have increased rapidly because of their interesting physical, chemical, optical and electronic properties. The high surface-area-to-volume ratio, combined with significantly different density of states for nanomaterials, is responsible for the variation in their properties compared to their bulk forms. Palladium nanoparticles are widely used as catalysts in the hydrogenation of olefins,1 oxidation of alcohols,2 electrochemical reactions in fuel cells,3 carbon–carbon bond formation,4 selective hydrogenation,5 etc. Apart from that, Pd nanoparticles also have tremendous potential in the field of hydrogen storage6–10 and sensing applications.11 Our earlier studies have indicated that, depending upon the dispersion method of Pd on CNT supports, the hydrogen storage capacity changes significantly.12 Pd nanoparticles can be prepared by a variety of methods, which include chemical reduction in presence of coordinating solvents, gas evaporation, electrochemical reduction, UV photo activation, sol gel processes, reduction using microwave plasma, hydrothermal, microemulsion, laser pyrolysis, sputtering etc.13 In chemical methods, the nanoparticles obtained after nucleation and growth are stabilized with suitable ligands, which forms a protective layer at the surface of nano particles (NPs), thereby providing electrostatic protection against agglomeration. Though metal nanoparticles of various sizes can be synthesized using chemical methods in presence of different stabilising ligands, preparation of nanoparticles with uniform size distribution and its efficient dispersion over supports like CNTs is still a major challenge.Reverse micelle or reverse micro emulsion method is widely used for the preparation of different noble metal nanoparticles with controlled size and morphology.14–18 In this route, metal nanoparticles are synthesized in organic medium using a less expensive water soluble metal precursor. In general, micro emulsion is an isotropic, thermodynamically stable system, where the small water droplets are dispersed in organic phase. In micro emulsions, water soluble metal precursor is extracted from the aqueous solution to the organic medium by solvent extraction method using a phase transfer catalyst. Subsequently, the metal precursor is subjected to reduction in organic phase by adding different reducing agents like, sodium borohydride (NaBH4), hexadecanediol (HDD), hydrazine or tetrabutyl ammonium borohydride (TBAB). The phase transfer catalyst also stabilises Pd nanoparticles after reduction by forming a protective coating, which prevents the coagulation of as prepared nanoparticles. Uniform, monodispersed and monophasic nanostructures can be obtained by controlling the micro-emulsion parameters such as amounts of solvents, surfactant, as well as water to oil (W/O) ratio.
In the present study, reverse micelle route has been adopted for preparation of Pd nanoparticles and its dispersion on carbon nanotubes. Dispersion of Pd nanoparticles on multi walled carbon nanotubes has been achieved in organic phase (toluene), using hydrazine as reducing agent and oleyl amine as phase transfer catalyst. Such Pd dispersed CNT samples were characterized using Transmission Electron Microscopy (TEM), solid state Nuclear Magnetic Resonance (NMR), X-Ray Diffraction (XRD) techniques to understand the nature of interaction between CNTs and Pd nanoparticles. Hydrogen storage properties have been evaluated for different concentrations of Pd dispersed CNTs and the results have been compared with Pd doped CNTs prepared by polyol and conventional wet impregnation methods. To the best of authors' knowledge, this is the first time that such a detailed study is being carried out on CNT samples dispersed with very fine Pd nanoparticles using reverse micro-emulsion route.
2 Experimental
2.1 Preparation of Pd dispersed CNTs
Preparation of Pd doped CNTs involves three steps which are briefly described below.1. Preparation of CNT's by chemical vapor deposition.
2. Activation of carbon nanotubes.
3. Dispersion of Pd on CNTs by reverse micelle route.
The carbon nanotubes used in the present study were prepared by chemical vapor deposition technique. In a typical synthesis method Fe dispersed over carbon black was used as supported catalyst and acetylene diluted with nitrogen was used as carbon source. The reaction was carried out in a fluidized bed reactor at 700 °C.19 As prepared carbon nanotubes were purified by treating with hydrochloric acid for two hours. Finally the samples were thoroughly rinsed with deionized water to remove traces of acid. The carbon nanotubes prepared by this method are hydrophobic in nature and they were activated by treatment with nitric acid under refluxing conditions for about 12 hours at 110 °C. The activated CNTs were dried in an oven at 80 °C.
For dispersion of 5% Pd nanoparticles on CNT (CNT-Pd5), 0.085 g of PdCl2 in water, 25 ml ethanol and 1 ml oleyl amine were mixed thoroughly using magnetic stirrer for 5 minutes. Around 25 ml of toluene was added to that and stirring was continued for another 30 minutes. Here ethanol acts as an intermediate solvent, which is miscible with water and increases the contact between oleyl amine and metal ion. Then the whole mixture was transferred to a separating funnel and subjected to vigorous shaking. Then the top part, which is brown in color containing PdCl2 in toluene, was separated from the bottom part, which is water–ethanol mixture deprived of PdCl2. The organic phase containing PdCl2 was mixed with 1 g of activated carbon nanotubes (ACNT) and continuously stirred for 3 hours for homogeneous mixing. In situ reduction of PdCl2 on CNT support was done by slow addition of dilute hydrazine solution under vigorous stirring. The color change of the solution from brown to black indicates complete reduction of Pd. The product was filtered, washed with distilled water and ethanol and dried in an oven at 85 °C. Two more samples containing 1 wt% Pd (CNT-Pd1) and 10 wt% Pd (CNT-Pd10) were also prepared by the same method and subjected to hydrogen absorption study.
2.2 Characterization
The Pd doped CNTs were characterized by X-ray diffraction (XRD) (INEL France make, EQUINOX) using Cr Kα radiation (λ = 2.29 Å). The morphology and diameter of multi-walled carbon nanotubes (MW-CNTs) and Pd nanoparticles were determined using a Transmission Electron Microscope (Jeol 2000 Fx). Elemental compositions of Pd dispersed MW-CNTs were determined by EDX (Oxford instrument, Model: INCAE350) technique. 13C Magic Angle Spinning Nuclear Magnetic Resonance (MAS NMR) experiments were performed using an AVANCE 400 MHz NMR machine with a 13C basic frequency of 100.57 MHz. A single pulse experiment with pulse duration of 3.5 μs and a relaxation delay time of 10 s were used for recording 13C MAS NMR spectra. The samples were packed in 4 mm zirconia rotors and subjected to a spinning speed of 10 kHz. All 13C MAS NMR chemical shift values are expressed with respect to tetramethyl silane (TMS).Thermogravimetric analysis (TGA) was performed on the samples using a thermobalance (Setaram France) in air atmosphere. The samples were heated upto 700 °C at a heating rate of 5 °C min−1. The weight loss and heat change as a function of time and temperature were recorded.
2.3 Hydrogen adsorption measurements
The H2 adsorption isotherms were recorded at different temperatures by volumetric method using IMI analyzer (Hiden Isochema, UK). Known weight of the sample was loaded in the sample holder and sealed using a metallic gasket. Before recording hydrogen adsorption isotherm, helium pycnometric measurement was done to correct for the sample volume. Prior to recording adsorption isotherms, the samples were degassed under turbo vacuum at a temperature of 80 °C and at a heating rate of 5 °C min−1. The isothermal measurements were done at different temperatures upto a hydrogen pressure of 50 atm.3 Results and discussion
3.1 TEM analysis
Fig. 1(a) shows the low resolution image of oleyl amine stabilised Pd nanoparticles dispersed on CNTs. Multi-walled carbon nanotubes having diameter in the range of 20–30 nm can be clearly seen in the images. No Pd nanoparticles could be seen in the low resolution image (Fig. 1(a)) probably due to its low concentration combined with very fine dispersion in CNT matrix. However, high resolution image recorded from the sample (Fig. 1(b)) shows the presence of finely dispersed nearly spherical Pd nanoparticles with size in the range of 3–5 nm. Significantly different electron densities between Pd nanoparticles and CNTs give better contrast in the TEM images. Selected Area Electron Diffraction (SAED) pattern from a representative region from the sample is shown as an inset of Fig. 1(b). Only concentric rings, rather than spots, are seen in the SAED images confirming the polycrystalline nature of Pd nanoparticles. The diffraction rings correspond to (110), (200) and (220) lattice planes of Pd nanoparticles with face centered cubic structure (Fig. 1(b)). TEM images taken at higher resolution (Fig. 1(c) and (d)) shows multiple graphitic cylinders nesting within each other, which is characteristic of multi-walled carbon nanotubes.3.2 Nuclear magnetic resonance analysis
Fig. 2 shows the 13C MAS NMR spectra of as prepared CNT sample before and after activation, along with Pd incorporated CNT. The patterns are noisy with poor signal to noise ratio for all the samples and this has been attributed to the conducting nature the sample and associated decrease in the extent of radio-frequency penetration (absorption) during NMR experiments. As prepared CNT sample gave a broad peak around 124 ppm with a line width (FWHM) of ∼64 ppm, which is characteristic of sp2 carbon (Fig. 2(a)). The broad peak arises due to chemical shift anisotropy existing around C atoms in the CNT sample, brought about by the lateral overlapping of 2pz orbital of carbon atoms in the ring structure. Once the CNTs are activated, functional groups, like –COOH and –OH, get attached on the surface, forming bonds with the unpaired 2pz orbital of carbon. This results in decrease of chemical shift value as well as chemical shift anisotropy. Observed decrease in chemical shift value (105 ppm) and the line width (41 ppm) of 13C MAS NMR peak corresponding to activated CNT sample, shown in Fig. 2(b), further confirm this. With incorporation of Pd, 13C MAS NMR peak becomes broader (line width ∼73 ppm) and the chemical shift changes to 125 ppm, which corresponds to the chemical shift value of as prepared CNT sample (Fig. 2(c)). An additional peak around 30 ppm is also observed in the 13C NMR spectrum as can be seen from Fig. 2(c). This peak can be either due to the presence of oleyl amine ligand stabilized on the surface of the nanoparticles (which is unlikely as the content of stabilizing ligands are very small) or due to the formation of sp3 carbon from CNTs. To confirm this 13C{1H}cross polarization magic angle spinning experiments (13C{1H}-CPMAS) were performed on the sample and the obtained pattern was compared with 13C MAS NMR experiments. It is observed that the NMR line shapes are identical in both 13C{1H}-CPMAS and 13C MAS NMR experiments, confirming that the peak at 30 ppm is not due to C–H linkages of oleyl amine ligand. Hence the peak at 30 ppm is attributed to the sp3 carbon species formed from CNTs due to the interaction of finely dispersed Pd nanoparticles with the surface of CNTs. Hence from NMR studies it is established that Pd species strongly interact with CNTs, leading to the formation of sp3 carbon and the same along with Pd get finely dispersed in the CNT matrix. To further understand the interaction of Pd with CNTs, XRD, thermo gravimetry and hydrogen absorption studies were carried out on the samples which are described below. | ||
| Fig. 2 13C MAS NMR spectra of (a) carbon nanotube (CNT), (b) activated carbon nanotube (ACNT) and (c) 5 wt% Pd dispersed CNT (CNT-Pd5). | ||
3.3 XRD and EDX characterization of Pd doped CNTs
Fig. 3 shows the powder XRD patterns of Pd nanoparticles dispersed CNT samples. The XRD patterns consist of peaks due to carbon nanotube and Pd nanoparticles. The peaks at 2θ values 39.34°, 65.71° and 68.66° correspond to (200), (100) and (101) reflections from CNT (JCPDS Card no. 010646). The peaks at 2θ values of 61.29°, 72.06° and 112.67° corresponds to (111), (200) and (220) planes of nano crystalline Pd metal (JCPDS Card no. 7440053). With increase in Pd concentration, the intensity of the peaks corresponding to Pd nanoparticles increases systematically. It is confirmed from the XRD patterns that Pd nanoparticles crystallize in face centered cubic structure with a lattice parameter a = 3.89 Å. Minor changes in the intensities of diffraction peaks corresponding to Pd-CNT sample (CNT-Pd10) has been attributed to the orientation and or aspect ratio changes taking place with CNTs due to interaction of CNTs with Pd nanoparticles. The percentage of Pd doping in the samples were determined using energy dispersive X-ray analysis (EDX). From the intensity of peaks corresponding to C and Pd (Fig. 4), the relative percentage of C and Pd has been calculated and found to be close to the expected value.3.4 Thermo gravimetric analysis
Thermo gravimetric analysis (TGA) and differential thermal analysis curves corresponding to carbon nanotubes, activated carbon nanotubes and Pd doped carbon nanotubes are shown in Fig. 5(a)–(e). From the curves it is seen that pristine CNT is stable up to 463 °C in air, after which it reacts with oxygen to produce CO2. The combustion peak temperature is found to be 575 °C. Almost 100% combustion occurs in the case of pristine MW-CNT, which confirms the absence of metal catalyst impurities in the sample. The initial weight loss up to 463 °C is due to the presence of certain amount of amorphous carbon. The combustion peak temperature for ACNT remains same as that of pristine CNT. However, combustion starts at a relatively lower temperature than that of the pristine CNT, due to the incorporation of oxygen functionalities and creation of defects during activation. It is worth mentioning here that activation of CNTs always does not lead to reduction in their thermal stability, as reported earlier.20,21 This has been explained based on the formation of hydrogen bonds between the surface carboxylate species present on the activated nanotubes. For TG curves corresponding to Pd dispersed carbon nanotubes, there are two major weight losses, which are coupled with heat changes, as seen in the DTA patterns. For CNT-Pd1, the initial weight loss at 273 °C is due to the decomposition of oleyl amine. It was used as the surfactant in reverse micelle synthesis route and it also stabilizes the Pd nanoparticles. The second weight loss is due to the combustion of CNTs, forming CO2. The combustion peak temperature is found to be ∼537 °C, which is lower than that of the pristine CNT. With increase in concentration of Pd, there is a steady decrease in the combustion peak temperatures of CNT's. The value for CNT-Pd5 and CNT-Pd10 are found to be 458 and 438 °C, respectively. The decrease in the weight loss temperature is due to the catalyzing effect of Pd nanoparticles for the oxidation of CNTs to CO2. This also confirms the close proximity (effective mixing) of Pd nanoparticles with CNT and plausible interaction between them. These results further support the inferences drawn from the NMR studies. The width of CNT combustion peak increases with increase in Pd concentration as can be seen from Fig. 5(c)–(e). This is because, due to dispersion of Pd on CNT surface, the local inhomogenity increases, which gets reflected in the combustion peak width. Hence from the NMR and TG results it is confirmed that, Pd nanoparticles interact with CNTs resulting in the formation of sp3 carbon species. It is now worthwhile to understand, how such structural modifications are affecting the hydrogen storage capacity. This aspect is described in the following section.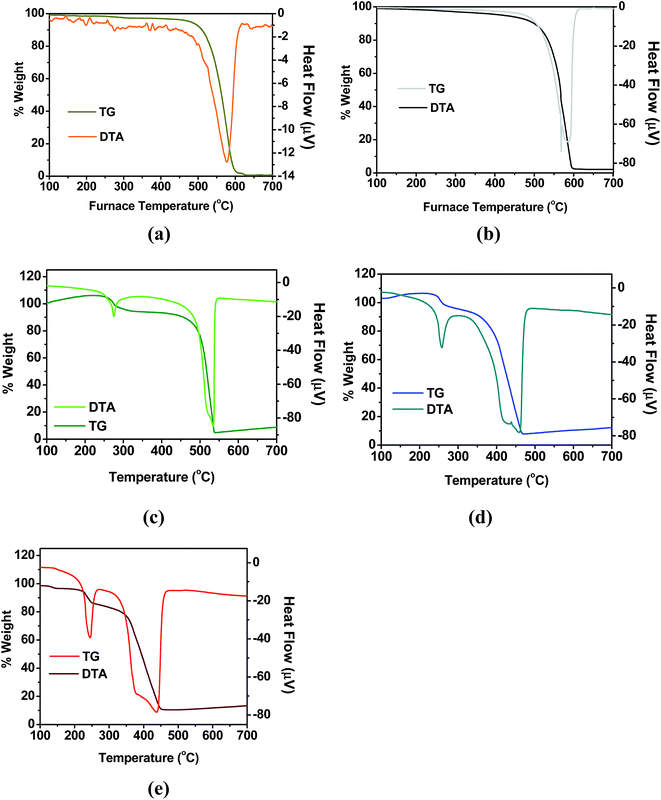 | ||
| Fig. 5 TG-DTA analysis curves for (a) pristine CNT, (b) activated CNT, (c) CNT-Pd1, (d) CNT-Pd5 and (e) CNT-Pd10. | ||
3.5 Hydrogen storage properties of Pd dispersed CNT
Our earlier studies we have revealed that the hydrogen storage capacity of Pd dispersed CNTs strongly depends on the degree of dispersion of Pd nanoparticles.12 In the present preparation method, Pd nanoparticles get very effectively dispersed on carbon nanotubes as seen from the TEM images. Hence it is expected that such sample should exhibit increased hydrogen storage capacity. The hydrogen adsorption isotherms obtained by volumetric method at 123, 223 and 303 K are shown in Fig. 6(a)–(c). CNT-Pd1 sample shows hydrogen storage capacity of 0.91, 0.45 and 0.24 wt% at 123, 223 and 303 K, respectively. For the CNT-Pd5 sample, maximum hydrogen storage capacities are found to be 0.49, 0.81 and 1.16 wt% at 303, 223 and 123 K, respectively. The hydrogen storage capacity of CNT-Pd10 was found to be 1.25, 1.05 and 0.64 wt% at 123, 223 and 303 K, respectively. The increase in storage capacity with decrease in temperature is quite expected, as at low temperatures, due to lowering of kinetic energy of hydrogen molecules, more hydrogen can be stored in CNT. The hydrogen storage capacity increases with pressure and after certain pressures the values remains same due to saturation. From the interpolation of the data, hydrogen storage capacities at higher pressure also can be found out. | ||
| Fig. 6 Hydrogen storage in Pd doped MWCNT at different temperatures (a) CNT-Pd1 (b) CNT-Pd5 (c) CNT-Pd10. | ||
It is obvious that the hydrogen storage capacity increases in the order CNT-Pd1 < CNT-Pd5 < CNT-Pd10, which means with increase in Pd concentration, the hydrogen storage capacity increases. Generally, in such Pd doped samples, hydrogen storage takes place through a process called spill-over effect. The spillover efficiency is the excess amount of hydrogen, the sample can absorb, due to spillover of hydrogen towards CNT in Pd-CNT composite. To understand the observed variation in hydrogen storage capacity, it is necessary to know how Pd concentration affects the spillover efficiency. When Pd is dispersed on CNT, apart from the physisorption of hydrogen in CNT, chemisorption of hydrogen in Pd also occurs, leading to the formation of H atoms. The H atoms spillover/move to CNT support as shown schematically in Fig. 7. The purified CNT can absorb 0.056 wt% of hydrogen at room temperature and 50 atm. pressure, and in the same condition Pd can absorb 0.68 wt% of hydrogen. So if we consider physical mixing between Pd and CNT and assume no spillover, the calculated hydrogen storage capacities for CNT-Pd1, CNT-Pd5 and CNT-Pd10 are 0.06, 0.09 and 0.12 wt%, respectively at room temperature. The observed hydrogen storage capacity is found to be greater than that. The increase in the extent of hydrogen absorption by Pd-CNT sample is due to the spill over mechanism brought about by the close proximity of Pd and CNT. The spillover efficiency of CNT-Pd1, CNT-Pd5 and CNT-Pd10 can be determined from the differences between the experimental and the calculated hydrogen storage capacities and are found to be 0.18, 0.41 and 0.52, respectively. It can be seen that the spillover efficiency increases appreciably from CNT-Pd1 to CNT-Pd5 due to higher surface coverage by the Pd nanoparticles. Unlike this for CNT-Pd10, even though the Pd concentration is high, the increase in spillover efficiency is only marginal. This is because in CNT-Pd10, agglomeration of Pd nanoparticles will be relatively more compared to 5% Pd doped sample (CNT-Pd5). Agglomeration reduces the active site for hydrogen absorption, leading to only marginal increase in the spill over efficiency.
It is also important to make out how the method of dispersion of Pd nanoparticles on CNTs helps in spillover process. For this we have considered 5% Pd doped CNT prepared by three different methods. We have already reported the hydrogen storage capacity of 5% Pd doped CNT prepared by the conventional wet impregnation and polyol method. The hydrogen storage capacities of all the samples at different temperatures were presented in Table 1. It can be seen for 5% Pd dispersed samples that the hydrogen storage capacity increases in the following order: Pd dispersed by wet impregnation route < Pd dispersed by polyol method < Pd dispersed by reverse micelle route. Since in all the cases, same amount of Pd nanoparticles (5%) are dispersed on CNTs, observed increase in the hydrogen storage capacity can be explained only based on the degree of dispersion of Pd nanoparticles on CNT. In Pd-CNT sample prepared by the reverse micelle route, Pd dispersion is much more effective compared to other two methods and hence leads to improved hydrogen storage capacities.
| Preparation method | Wet impregnation | Polyol method | Reverse micelle route | Reverse micelle route | Reverse micelle route |
|---|---|---|---|---|---|
| Pd% | 5% | 5% | 5% | 1% | 10% |
| Temp (K) | Hydrogen storage capacity (wt%) | ||||
| 123 | 0.4 | 0.8 | 1.15 | 0.9 | 1.25 |
| 223 | 0.20 | 0.6 | 0.81 | 0.45 | 1.05 |
| 303 | 0.09 | 0.45 | 0.50 | 0.24 | 0.64 |
4 Conclusions
Pd nanoparticles prepared with oleyl amine as stabilising ligands can be very finely dispersed on CNTs and such materials show improved hydrogen absorption characteristics compared to bare CNTs and Pd doped CNTs prepared by conventional wet impregnation method. Based on 13C MAS NMR and TG-DTA studies it is concluded that Pd nanoparticles strongly interacts with CNTs forming sp3 carbon, and Pd nanoparticles get finely dispersed with the CNTs. Improved hydrogen storage capacity of Pd containing samples is explained based on the spillover mechanism, the efficiency of which strongly depends on Pd concentration and extent of interaction of Pd with CNT structure.Acknowledgements
The authors are thankful to Dr V. K. Jain, Head, Chemistry Division, Bhabha Atomic Research Centre for his encouragements and productive discussions during the work.References
- Y. Niu, L. K. Yeung and R. M. Crooks, J. Am. Chem. Soc., 2001, 123, 6840–6846 CrossRef CAS.
- B. Karimi, A. Zamani, S. Abedi and J. H. Clark, Green Chem., 2009, 11, 109–119 RSC.
- S. Cheong, J. D. Watt and R. D. Tilley, Nanoscale, 2010, 2(10), 2045–2053 RSC.
- D. Astruc, Inorg. Chem., 2007, 46(6), 1884–1894 CrossRef CAS PubMed.
- S. Kidambi and M. L. Bruening, Chem. Mater., 2005, 17, 301–307 CrossRef CAS.
- S. Horinouchi, Y. Yamanoi, T. Yonezawa, T. Mouri and H. Nishihara, Langmuir, 2006, 22(4), 1880–1884 CrossRef CAS PubMed.
- H. Kobayashi, M. Yamauchi, R. Ikeda and H. Kitagawa, Chem. Commun., 2009, 4806–4808 RSC.
- Y. Suttiwat, P. Rangsunvigit, B. Kitiyanan, M. Williams, P. Ndungu, M. V. Lototskyy, A. Nechaev, V. Linkov and S. Kulprathipanja, Int. J. Hydrogen Energy, 2009, 34, 6669–6675 CrossRef PubMed.
- S. W. Hwang, S. Rather, M. Naik, C. S. Soo and K. S. Nahm, J. Alloys Compd., 2009, 480(2), L20–L24 CrossRef CAS PubMed.
- S. Rather, R. Zacharia, S. W. Hwang, M. Naik and K. S. Nahm, Chem. Phys. Lett., 2007, 441, 261–267 CrossRef CAS PubMed.
- J. Zou, B. Zdyrko, I. Luzinov, C. L. Rastona and K. S. Iyer, Chem. Commun., 2012, 48, 1033–1035 RSC.
- S. Banerjee, K. Dasgupta, A. Kumar, P. Ruz, B. Vishwanadh, J. B. Joshi and V. Sudarsan, Int. J. Hydrogen Energy, 2015, 40(8), 3268–3276 CrossRef CAS PubMed.
- J. Cookson, Platinum Met. Rev., 2012, 56(2), 83–98 CrossRef.
- J. Yang, E. Sargent, S. Kelley and J. Y. Ying, Nat. Mater., 2009, 8, 683–689 CrossRef CAS PubMed.
- J. Eastoe, M. J. Hollamby and L. Hudson, Adv. Colloid Interface Sci., 2006, 5–15, 128–130 Search PubMed.
- E. M. Egorova and A. A. Revina, Colloids Surf., A, 2000, 168, 87–96 CrossRef CAS.
- S. K. Kim, C. Kim, J. H. Lee, J. Kim, H. Lee and S. H. Moon, J. Catal., 2013, 306, 146–154 CrossRef CAS PubMed.
- L. Zhang, L. Wang, Z. Jiang and Z. Xie, Nanoscale Res. Lett., 2012, 7, 312 CrossRef PubMed.
- K. Dasgupta, J. B. Joshi, H. Singh and S. Banerjee, AIChE J., 2014, 60(8), 2882–2892 CrossRef CAS PubMed.
- Y.-C. Hsieh, Y.-C. Chou, C.-P. Lin, T.-F. Hsieh and C.-M. Shu, Aerosol Air Qual. Res., 2010, 10, 212–218 CAS.
- B. Scheibe, E. Borowiak-Palen and R. J. Kalenczuk, Mater. Charact., 2010, 61, 185–191 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |




