Preparation of magnetic Ag/AgCl/CoFe2O4 composites with high photocatalytic and antibacterial ability
Yuanguo Xuab,
Teng Zhoua,
Shuquan Huanga,
Meng Xiec,
Hongping Lia,
Hui Xu*a,
Jiexiang Xiaa and
Huaming Li*a
aSchool of Chemistry and Chemical Engineering, Jiangsu University, 301 Xuefu Road, Zhenjiang, 212013, PR China. E-mail: lhm@ujs.edu.cn
bSchool of Energy and Power Engineering, Jiangsu University, 301 Xuefu Road, Zhenjiang, 212013, PR China
cSchool of Pharmacy, Jiangsu University, 301 Xuefu Road, Zhenjiang, 212013, PR China
First published on 20th April 2015
Abstract
Novel plasmonic photocatalysts, Ag/AgCl/CoFe2O4, were prepared via a two-step synthesis method. The obtained Ag/AgCl/CoFe2O4 composites were characterized using X-ray diffraction (XRD), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and ultraviolet-visible absorption spectroscopy (UV-vis). The magnetic properties of the samples were studied by vibrating sample magnetometer (VSM) analysis. Methyl orange (MO), bisphenol A (BPA) and ciprofloxacin (CIP) were used as target pollutants to investigate the degradation capability of Ag/AgCl/CoFe2O4. Results showed that the composite can degrade both colored and colorless pollutants, while Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite showed the highest photoactivity in the degradation of MO. It can degrade about 93.38% MO in 1.5 h. The reactive species scavenger results indicated that hydroxyl radicals (˙OH) were not the main photooxidant, while holes (h+) and superoxide anion radicals (˙O2−) played key roles in MO decoloration. Furthermore, the degraded solution of BPA was analyzed using high performance liquid chromatography (HPLC). The results showed that BPA was decomposed gradually. The composite was magnetically separated and investigated using three successive recycle experiments under visible light. The results exhibited that the photoactivity of Ag/AgCl/CoFe2O4 is stable. Besides, Ag/AgCl/CoFe2O4 also exhibited good antibacterial activity against Escherichia coli (E. coli). The method used to prepare the composite can be expanded and applied to synthesize other magnetically separable photocatalysts.
1) composite showed the highest photoactivity in the degradation of MO. It can degrade about 93.38% MO in 1.5 h. The reactive species scavenger results indicated that hydroxyl radicals (˙OH) were not the main photooxidant, while holes (h+) and superoxide anion radicals (˙O2−) played key roles in MO decoloration. Furthermore, the degraded solution of BPA was analyzed using high performance liquid chromatography (HPLC). The results showed that BPA was decomposed gradually. The composite was magnetically separated and investigated using three successive recycle experiments under visible light. The results exhibited that the photoactivity of Ag/AgCl/CoFe2O4 is stable. Besides, Ag/AgCl/CoFe2O4 also exhibited good antibacterial activity against Escherichia coli (E. coli). The method used to prepare the composite can be expanded and applied to synthesize other magnetically separable photocatalysts.
1 Introduction
Nowadays, with the development of modern industries, environmental problems have become even more serious and have attracted increasing attention. Water pollution is one of the serious issues among all of them, which requires significant attention because clean water is a necessity. Numerous water sources are polluted not only by hazardous chemicals but also by pathogenic microorganisms.1 Pathogenic bacteria in water cause diseases. Among the various solutions available, the semiconductor-based photocatalysis technique is an efficient and low-cost strategy for the treatment of water pollutants and disinfection.2 In recent years, visible-light response plasmonic photocatalysts have attracted extensive research attention due to the surface plasmon resonance (SPR) effect.3 In general, photocatalysts with surface plasmon resonance (SPR) effect have excellent absorption properties in the visible light region and can efficiently separate photogenerated electrons and holes, which is beneficial for photocatalytic degradation reactions. Besides, Ag related materials have excellent antibacterial ability.4,5 Plasmonic photocatalysts based on silver/silver halides (Ag/AgX, X = Cl, Br) have attracted widespread attention due to their extraordinarily high photoactivity. Numerous works have been reported for the preparation of Ag/AgX (X = Cl, Br). For example, Huang et al. fabricated a series of Ag@AgX (X = Cl, Br) plasmonic photocatalysts,6,7 which exhibited excellent photoactivity and stability under visible-light illumination. In the silver halide systems, AgX is the main photoactive species. However, pure AgX is unstable under sunlight due to its photosensitive property.6,8,9 Furthermore, when a certain amount of Ag(0) nanoparticles was reduced on the surface of AgX, the metallic Ag(0) could suppress the further decomposition of AgX. Therefore, Ag/AgX is a photo stable photocatalyst with high visible light photoactivity.10–16 Besides, Ag/AgX can also be dispersed on other materials to improve the photoactivity of the composites.17–19 Ag/AgX (Cl, Br) have become very important photocatalysts and they play an increasingly important role in solving water pollutant problems. Many relevant studies have also been reported with respect to their antibacterial activity (such as Ag/AgCl/W18O49, Ag/AgBr/TiO2, and Ag/AgX/CNTs). The Ag/AgX system shows very high antibacterial ability under light irradiation because it can efficiently generate reactive species to kill bacteria.1,5 However, Ag/AgX photocatalysts face difficulty during separation after photocatalytic reactions, which limits their application in practical fields. To solve this problem, Ag/AgX was combined with a magnetic material. Dai et al.20 prepared core–shell Fe3O4@SiO2 NPs first, and then fabricated an Ag–AgI/Fe3O4@SiO2 plasmonic photocatalyst. The composite showed an excellent photocatalytic activity and possessed the capability of being easily recovered by a magnet. Soon afterwards, An et al.21 constructed Fe3O4@SiO2 nanospheres through a polyol and sol–gel process, and then fabricated ferromagnetic Fe3O4@SiO2@AgCl:Ag plasmonic nanophotocatalysts. The composite exhibited an excellent performance in the decomposition of rhodamine B (RhB) under visible-light irradiation. Besides, this catalyst was separated easily by applying an external magnetic field. Tian et al.22 synthesized a core–shell structured γ-Fe2O3@SiO2@AgBr:Ag composite by a versatile multistep route. They fabricated the magnetic core first, and then coated a SiO2 interlayer. Subsequently, they deposited a AgBr shell. Finally, the AgBr was reduced to form Ag nanoparticles on its surface by light irradiation. This photocatalyst exhibited very high photocatalytic activity and good magnetic property. All these strategies have achieved great advantages in the development of magnetic plasmonic nanophotocatalysts. However, as we all know, either Fe3O4 or γ-Fe2O3 is not a stable crystal structure iron oxide. They both face the risk of conversion. The introduction of a SiO2 shell can solve this problem. However, the synthesis route could become cumbersome. Thus, the discovery of a stable magnetic material and facile synthesis route is vital.Spinel CoFe2O4 nanoparticles prepared by the sol–gel method have excellent chemical stability, remarkable mechanical hardness and excellent magnetic property; thus, they are a good option for the preparation of semiconductor–magnetic composites, and some relevant studies have been reported. Wang et al.23 synthesized magnetic photocatalyst Bi2WO6/CoFe2O4 composites by a two-step hydrothermal method. This photocatalyst retained the effective photoactivity of Bi2WO6 and was easily separated by a magnet. Ribeiro et al.24 fabricated TiO2/CoFe2O4 using a polymeric precursor method. The composite can degrade rhodamine B dye and atrazine pesticide under UV light irradiation and can be recovered by a magnet after the reaction. Phukan et al.25 prepared a CoFe2O4–ZnS magnetic composite. The composite showed a good photoactivity in the degradation of methyl orange under UV irradiation and was easily separated by a magnet. Besides, numerous other studies have been reported such as those involving CoFe2O4/ZnO,26 CoFe2O4/TiO2 (ref. 27) Pd(0)/SiO2–CoFe2O4,28 and GO/CoFe2O4.29 Briefly, CoFe2O4 is a promising magnetic material for the fabrication of magnetic photocatalysts.
Based on the analysis above, it can be suggested that combining Ag/AgX and CoFe2O4 is a good proposal because it could lead to the construction of a new type of magnetic plasmonic photocatalysts. In this study, CoFe2O4 was selected as the magnetic material to synthesize magnetic photocatalyst Ag/AgCl/CoFe2O4 composites by a facile solvothermal method. The as-prepared photocatalyst exhibited an excellent degradation ability for methyl orange (MO), bisphenol A (BPA) and ciprofloxacin (CIP). Furthermore, this photocatalyst has stable photoactivity and magnetic recoverable property. Besides, the composite showed good photocatalytic antibacterial ability against Escherichia coli (E. coli). This study may provide new insights in the fabrication of stable magnetic plasmonic photocatalysts by a facile method and expand the application of plasmonic photocatalysts in environmental remediation and water disinfection field.
2 Experimental
2.1 Materials
All the reagents were of analytical grade and were used without further purification.2.2 Preparation of photocatalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.5
1, 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 4
1, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were used to synthesize the Ag/AgCl/CoFe2O4 photocatalyst. Typically, AgNO3 and CoFe2O4 with the mass ratio of 3
1) were used to synthesize the Ag/AgCl/CoFe2O4 photocatalyst. Typically, AgNO3 and CoFe2O4 with the mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were used as the example for the synthesis procedure, and the obtained Ag/AgCl/CoFe2O4 is referred to as Ag/AgCl/CoFe2O4 (3
1 were used as the example for the synthesis procedure, and the obtained Ag/AgCl/CoFe2O4 is referred to as Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). First, 0.0666 g CoFe2O4 and 10 mL ethylene glycol solution of NaCl (0.0688 g) were mixed together and stirred for 30 min to obtain a homogeneous dispersion. Then, 7 mL AgNO3 EG solution (containing 0.2000 g AgNO3) was added to the abovementioned homogeneous dispersion and stirred for 30 min at room temperature. Subsequently, the resultant mixture was transferred to a Teflon-lined autoclave and heated at 120 °C for 24 h. The resultant product was centrifuged, washed and dried at 60 °C for 8 h. Similarly, other samples were obtained with the addition of the appropriate amount of CoFe2O4.
1). First, 0.0666 g CoFe2O4 and 10 mL ethylene glycol solution of NaCl (0.0688 g) were mixed together and stirred for 30 min to obtain a homogeneous dispersion. Then, 7 mL AgNO3 EG solution (containing 0.2000 g AgNO3) was added to the abovementioned homogeneous dispersion and stirred for 30 min at room temperature. Subsequently, the resultant mixture was transferred to a Teflon-lined autoclave and heated at 120 °C for 24 h. The resultant product was centrifuged, washed and dried at 60 °C for 8 h. Similarly, other samples were obtained with the addition of the appropriate amount of CoFe2O4.2.3 Characterization
The crystal phase of the samples was analyzed by X-ray diffraction (XRD) analysis using an AdvantXP4200 (American) in the 2θ range of 10–80°. Transmission electron microscopy (TEM) micrographs were obtained with a JEOL-JEM-2010 (JEOL, Japan) operating at 200 kV. UV-vis absorption spectra of liquid samples were obtained on a UV-vis spectrophotometer (UV-2450, Shimadzu Corporation, Japan). The UV-vis absorption spectra of solid samples (in the diffuse reflectance spectra mode) were measured in the solid state, and BaSO4 powder was used as the substrate. X-ray photoelectron spectroscopy (XPS) was performed on a PHI5300 with a monochromatic Mg Kα source to explore the elements on the surface. The magnetic properties of the composites were tested in a vibrating sample magnetometer (VSM) (Quantum Design Corporation, USA) with a maximum applied field of ±2 T. High performance liquid chromatography (HPLC) was used to analyze the degradation liquid. The HPLC setup was equipped with two Varian ProStar210 pumps, an Agilent TC-C (18) column, and a Varian ProStar325 UV-Vis Detector at 230 nm. A solution of methanol and H2O in the ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (v/v) was used as the mobile phase at the rate of 1 mL min−1. Then, 20 μL of the degraded solution was injected. The column oven temperature was 30 °C.
25 (v/v) was used as the mobile phase at the rate of 1 mL min−1. Then, 20 μL of the degraded solution was injected. The column oven temperature was 30 °C.
2.4 Photocatalytic activity
The application of the Ag/AgCl/CoFe2O4 composite in the degradation of the organic dye, methyl orange (MO), was investigated under visible-light irradiation at 30 °C. In a typical procedure, 0.0700 g of sample was dispersed in 70 mL of MO solution (10 mg L−1). Before the lamp (300 W Xe arc lamp, with a cut-off filter supply visible light with λ ≥ 400 nm) was turned on to start irradiation, the solution was stirred for 0.5 h in the dark to obtain adsorption/desorption equilibrium between the photocatalyst and the dye. The solution was sampled at 0.5 h intervals and was centrifuged, and then the abovementioned liquid was monitored by UV-vis spectroscopy at 463 nm. The degradation of CIP and BPA was similar to the abovementioned operation. The degraded solution was analyzed by UV-vis spectrophotometry and HPLC.2.5 Antibacterial activity
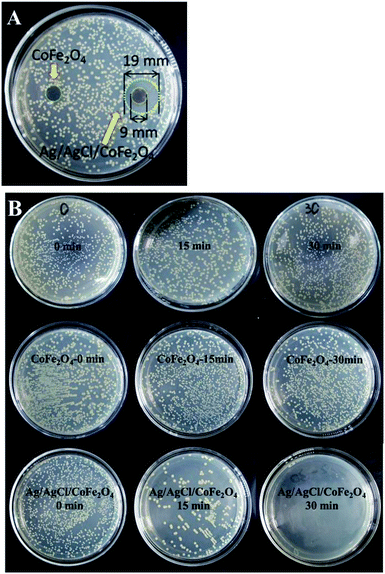
All the glassware used and the culture medium solution were sterilized by autoclaving at 121 °C for 20 min prior to use. All the experiments were performed under sterile conditions.The effect of a sample on bacterial growth in the absence of light was studied using the qualitative test, zone of inhibition (disc diffusion test). First, 0.02 mL of the prepared Escherichia coli (E. coli) bacteria suspension was dispersed on a nutrient agar medium. Then, about 9 mm diameter of the sample was dispersed on the nutrient agar medium. Finally, the culture dish was incubated at 37 °C for 24 h in the dark.
The photocatalytic antibacterial experiments were performed using the following typical method: 20 mg of the as-prepared sample was added into 20 mL culture medium solution. Then, a certain volume of the prepared Escherichia coli (E. coli) bacterial suspension was transferred into the mixture (the mixture concentration is 1/(2 × 104) that of the original E. coli concentration). The mixture was then magnetically stirred in the dark for 0.5 h; subsequently, the light was switched on to start irradiation. 0.02 mL of the solution was sampled at the time intervals of 0 min, 15 min and 30 min. Each solution was dispersed on the nutrient agar medium and incubated at 37 °C for 24 h in dark.
3 Results and discussion
3.1 XRD and TEM analysis
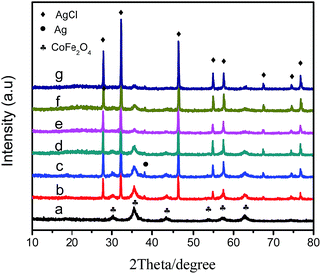
Fig. 1 shows the XRD patterns of the magnetic photocatalyst, Ag/AgCl/CoFe2O4, and pure CoFe2O4. As shown in Fig. 1a, the 2θ values at 30.1°, 35.2°, 43.0°, 53.4°, 56.9° and 62.6° (marked with “♣”) correspond to the (220), (311), (222), (400), (422), (511) and (440) crystalline planes of CoFe2O4 (JCPDS card no. 22-1086),30 respectively. As shown in Fig. 1b, seven distinct peaks at 32.1°, 46.2°, 54.7°, 57.4°, 67.4°, 74.4° and 76.6° (marked with “◆”) were observed, which correspond to the (200), (220), (311), (222), (400), (331) and (420) planes of AgCl crystal (JCPDS cards no. 31-1238) in Ag/AgCl/CoFe2O4 (0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The distinctive peak at 38.1° (marked with “●”) is ascribed to the (111) plane of metallic Ag (JCPDS cards no. 04-0783). Besides, no other diffraction peaks are found, which indicates that the composites are composed of Ag, AgCl and CoFe2O4. For further investigation of the different compositions of the Ag/AgCl/CoFe2O4 photocatalyst, Ag/AgCl/CoFe2O4 with different ratios were characterized and are shown in Fig. 1c–g. It can be seen that the intensity of the peaks for CoFe2O4 decreased gradually as the Ag/AgCl content increased, but the diffraction peaks of CoFe2O4 did not shift. This indicates that the added Ag/AgCl was present on the CoFe2O4 surface, but not incorporated into its lattice.
1). The distinctive peak at 38.1° (marked with “●”) is ascribed to the (111) plane of metallic Ag (JCPDS cards no. 04-0783). Besides, no other diffraction peaks are found, which indicates that the composites are composed of Ag, AgCl and CoFe2O4. For further investigation of the different compositions of the Ag/AgCl/CoFe2O4 photocatalyst, Ag/AgCl/CoFe2O4 with different ratios were characterized and are shown in Fig. 1c–g. It can be seen that the intensity of the peaks for CoFe2O4 decreased gradually as the Ag/AgCl content increased, but the diffraction peaks of CoFe2O4 did not shift. This indicates that the added Ag/AgCl was present on the CoFe2O4 surface, but not incorporated into its lattice.
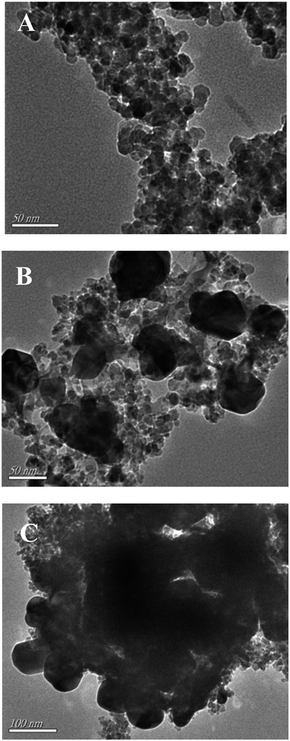
The morphology of pure CoFe2O4 and Ag/AgCl/CoFe2O4 was investigated by TEM and the results are shown below. Fig. 2A shows that CoFe2O4 is in the form of small particles, which stick together, and the average particle size is approximately 20 nm. Fig. 2B shows the TEM image of Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The image reveals several large particles (in the range of 50–100 nm) that are obviously different to that of CoFe2O4, which is ascribed to the introduction of Ag/AgCl. It can be seen that the Ag/AgCl particles and the CoFe2O4 particles stick together, which indicates that they undergo good combination because they did not separate after the ultrasonic process, which was carried out before the TEM analysis. The combination is beneficial to the magnetic separation of Ag/AgCl/CoFe2O4 from the contaminant solution. When the content of Ag/AgCl was increased (Ag/AgCl/CoFe2O4 (4
1). The image reveals several large particles (in the range of 50–100 nm) that are obviously different to that of CoFe2O4, which is ascribed to the introduction of Ag/AgCl. It can be seen that the Ag/AgCl particles and the CoFe2O4 particles stick together, which indicates that they undergo good combination because they did not separate after the ultrasonic process, which was carried out before the TEM analysis. The combination is beneficial to the magnetic separation of Ag/AgCl/CoFe2O4 from the contaminant solution. When the content of Ag/AgCl was increased (Ag/AgCl/CoFe2O4 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)), the number of large particles increased and they agglomerated together (as shown in Fig. 2C), which may not be beneficial to the photoactivity of the composite.
1)), the number of large particles increased and they agglomerated together (as shown in Fig. 2C), which may not be beneficial to the photoactivity of the composite.
3.2 XPS analysis
XPS was used to measure the valences of the surface elements and components of CoFe2O4 and Ag/AgCl/CoFe2O4. Fig. 3A shows the survey spectrum of CoFe2O4 and Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It is obvious that the Ag/AgCl/CoFe2O4 (3
1). It is obvious that the Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite exhibited Ag 3d and Cl 2p signals compared to that of CoFe2O4 (which possess O 1s, Co 2p and Fe 2p). Fig. 3B–E show the high-resolution XPS spectra of Co 2p, Fe 2p, Ag 3d and Cl 2p of the samples. As shown in Fig. 3B, there are two peaks in the Co 2p spectrum for CoFe2O4; the peak at 795.3 eV corresponds to Co 2p1/2, while the peak at about 780.0 eV is attributed to Co 2p3/2.31 When Ag/AgCl was introduced, both the peaks showed a little shift. The peak at 795.3 eV shifted to 795.9 eV, whereas the peak at 780.0 eV shifted to 780.5 eV. As shown in Fig. 3C, Fe 2p spectra possess two peaks at 711.0 eV (Fe 2p3/2) and 724.5 eV (Fe 2p1/2), which suggest the presence of Fe3+ cation.32 Furthermore, these two peaks shift after the deposition of Ag/AgCl (the peak at 711.0 eV shifts to 710.7 eV and the peak at 724.5 eV shifts to 724.0 eV). The change in the Co and Fe spectra indicate that the added Ag/AgCl combined with CoFe2O4, which affected the chemical condition of Co and Fe in the CoFe2O4 sample. Fig. 3D shows the high-resolution spectrum of Ag 3d. The two peaks around 366.4 eV and 372.4 eV are ascribed to the 3d5/2 and 3d3/2 of Ag, respectively. Here, both the peaks in Ag/AgCl/CoFe2O4 exhibited a shift of about 1 eV compared to pure Ag/AgCl (which was reported in our previous work Ag@AgCl).33 Moreover, for Cl spectrum, shown in Fig. 3E, the two peaks observed at the binding energy of about 196.7 eV and 198.3 eV, represent Cl 2p3/2 and Cl 2p1/2, respectively. In addition, a shift of about 1 eV occurred for the Cl 2p peak.33 In all, the shifts in Ag and Cl peaks reveal that the chemical environment in the Ag/AgCl/CoFe2O4 sample has been changed when compared to pure Ag/AgCl. The XPS results disclosed the information on the different types of elements in Ag/AgCl/CoFe2O4 as well as proved the interaction between Ag/AgCl and CoFe2O4. This suggests that Ag/AgCl and CoFe2O4 have been successfully combined instead of simple adsorption. Therefore, separation by an external magnet would be beneficial.
1) composite exhibited Ag 3d and Cl 2p signals compared to that of CoFe2O4 (which possess O 1s, Co 2p and Fe 2p). Fig. 3B–E show the high-resolution XPS spectra of Co 2p, Fe 2p, Ag 3d and Cl 2p of the samples. As shown in Fig. 3B, there are two peaks in the Co 2p spectrum for CoFe2O4; the peak at 795.3 eV corresponds to Co 2p1/2, while the peak at about 780.0 eV is attributed to Co 2p3/2.31 When Ag/AgCl was introduced, both the peaks showed a little shift. The peak at 795.3 eV shifted to 795.9 eV, whereas the peak at 780.0 eV shifted to 780.5 eV. As shown in Fig. 3C, Fe 2p spectra possess two peaks at 711.0 eV (Fe 2p3/2) and 724.5 eV (Fe 2p1/2), which suggest the presence of Fe3+ cation.32 Furthermore, these two peaks shift after the deposition of Ag/AgCl (the peak at 711.0 eV shifts to 710.7 eV and the peak at 724.5 eV shifts to 724.0 eV). The change in the Co and Fe spectra indicate that the added Ag/AgCl combined with CoFe2O4, which affected the chemical condition of Co and Fe in the CoFe2O4 sample. Fig. 3D shows the high-resolution spectrum of Ag 3d. The two peaks around 366.4 eV and 372.4 eV are ascribed to the 3d5/2 and 3d3/2 of Ag, respectively. Here, both the peaks in Ag/AgCl/CoFe2O4 exhibited a shift of about 1 eV compared to pure Ag/AgCl (which was reported in our previous work Ag@AgCl).33 Moreover, for Cl spectrum, shown in Fig. 3E, the two peaks observed at the binding energy of about 196.7 eV and 198.3 eV, represent Cl 2p3/2 and Cl 2p1/2, respectively. In addition, a shift of about 1 eV occurred for the Cl 2p peak.33 In all, the shifts in Ag and Cl peaks reveal that the chemical environment in the Ag/AgCl/CoFe2O4 sample has been changed when compared to pure Ag/AgCl. The XPS results disclosed the information on the different types of elements in Ag/AgCl/CoFe2O4 as well as proved the interaction between Ag/AgCl and CoFe2O4. This suggests that Ag/AgCl and CoFe2O4 have been successfully combined instead of simple adsorption. Therefore, separation by an external magnet would be beneficial.
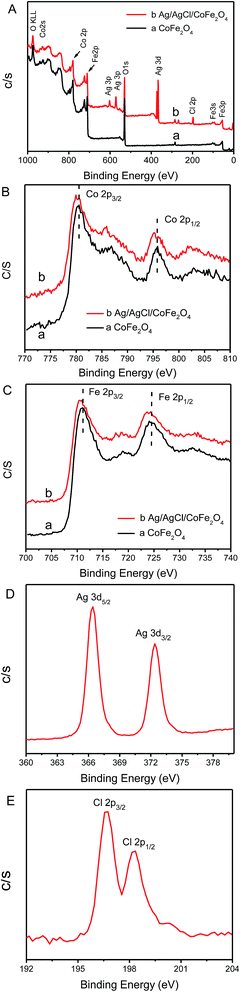 | ||
| Fig. 3 The survey spectra of CoFe2O4, Ag/AgCl/CoFe2O4 (A) and the high resolution XPS spectra of Co 2p (B), Fe 2p (C), Ag 3d (D) and Cl 2p (E). | ||
3.3 UV-vis analysis
The optical properties of the CoFe2O4 photocatalyst and Ag/AgCl/CoFe2O4 composite were investigated by UV-vis absorption spectroscopy (in the diffuse reflectance spectra (DRS) mode), and the results are shown in Fig. 4. As shown in Fig. 4a, the absorption intensity of pure CoFe2O4 is very high in both the UV and visible light region. This observation may be due to the black color of the CoFe2O4 material. When Ag/AgCl was introduced, the absorption intensity of the Ag/AgCl/CoFe2O4 composites decreased in the UV and visible light region with the increase of Ag/AgCl content (as shown in Fig. 4b–f). The higher content of Ag/AgCl leads to lighter colored composites. The phenomenon suggests that the introduced Ag/AgCl covers the surface of CoFe2O4.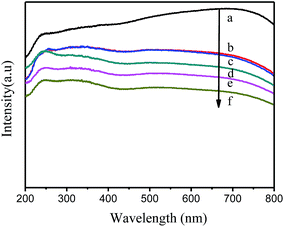 | ||
Fig. 4 UV-vis absorption spectra of (a) CoFe2O4, (b) Ag/AgCl/CoFe2O4 (0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (c) Ag/AgCl/CoFe2O4 (1 1), (c) Ag/AgCl/CoFe2O4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (d) Ag/AgCl/CoFe2O4 (2 1), (d) Ag/AgCl/CoFe2O4 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), (e) Ag/AgCl/CoFe2O4 (3 1), (e) Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and (f) Ag/AgCl/CoFe2O4 (4 1) and (f) Ag/AgCl/CoFe2O4 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
3.4 Magnetic performances
The magnetic property of composites is very useful for the recovery of magnetic photocatalysts in solution reactions. The magnetic property of CoFe2O4 and Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was measured using a vibrating sample magnetometer (VSM). The magnetization measurements were carried out at room temperature and the applied magnetic field was 20 kOe. As shown in Fig. 5, the detected magnetic saturation (Ms) values of CoFe2O4 and Ag/AgCl/CoFe2O4 (3
1) was measured using a vibrating sample magnetometer (VSM). The magnetization measurements were carried out at room temperature and the applied magnetic field was 20 kOe. As shown in Fig. 5, the detected magnetic saturation (Ms) values of CoFe2O4 and Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are 93.4 emu g−1 and 17.2 emu g−1, respectively. The coercivities of the two composites are nearly 1003.1 Oe and 351.1 Oe, and the remnant magnetizations (Mr) are approximately 26.8 emu g−1 and 2.2 emu g−1 for CoFe2O4 and Ag/AgCl/CoFe2O4 (3
1) are 93.4 emu g−1 and 17.2 emu g−1, respectively. The coercivities of the two composites are nearly 1003.1 Oe and 351.1 Oe, and the remnant magnetizations (Mr) are approximately 26.8 emu g−1 and 2.2 emu g−1 for CoFe2O4 and Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), respectively. Obviously, the abovementioned three properties of the Ag/AgCl/CoFe2O4 composite are lower than that of CoFe2O4. However, the magnetic property of CoFe2O4 in the composite was retained and can be used to separate the Ag/AgCl/CoFe2O4 (3
1), respectively. Obviously, the abovementioned three properties of the Ag/AgCl/CoFe2O4 composite are lower than that of CoFe2O4. However, the magnetic property of CoFe2O4 in the composite was retained and can be used to separate the Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) photocatalyst from a reaction solution. The inset photos in Fig. 5 display that Ag/AgCl/CoFe2O4 (3
1) photocatalyst from a reaction solution. The inset photos in Fig. 5 display that Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was dispersed in solution and it was separated by an external magnet.
1) was dispersed in solution and it was separated by an external magnet.
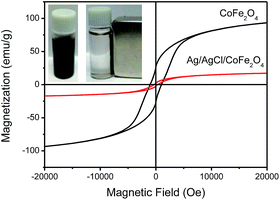 | ||
Fig. 5 Field-dependent magnetization curves of CoFe2O4 and Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The inset photos show Ag/AgCl/CoFe2O4 (3 1). The inset photos show Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) before and after magnetic separation. 1) before and after magnetic separation. | ||
3.5 Photocatalytic activity
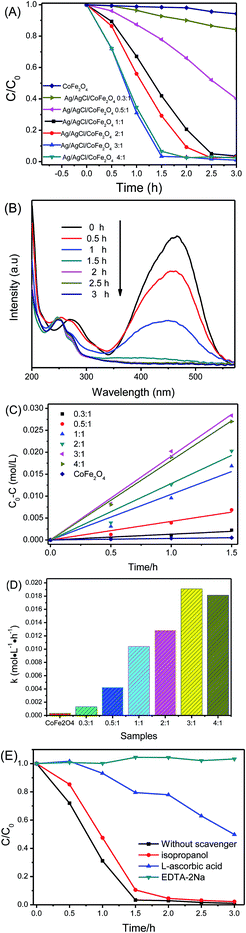
Fig. 6A illustrates the photocatalytic activity of CoFe2O4 and Ag/AgCl/CoFe2O4 (with different Ag/AgCl contents) for the degradation of MO in aqueous solution under visible-light irradiation. It is clear that the pure CoFe2O4 showed extremely low degradation efficiency for MO in 3 h. When the Ag/AgCl was combined with CoFe2O4, the degradation ability of the Ag/AgCl/CoFe2O4 photocatalyst increased with the increasing content of the Ag/AgCl. Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed the highest photoactivity. It can decompose about 93.38% of MO in 1.5 h and about 99.03% in 3 h. Fig. 6B shows the full UV-vis spectra of MO during the photodegradation. It is clear that the intensity of the absorption peak at about 463 nm decreased gradually. This means that MO was gradually photodecomposed by Ag/AgCl/CoFe2O4 (3
1) showed the highest photoactivity. It can decompose about 93.38% of MO in 1.5 h and about 99.03% in 3 h. Fig. 6B shows the full UV-vis spectra of MO during the photodegradation. It is clear that the intensity of the absorption peak at about 463 nm decreased gradually. This means that MO was gradually photodecomposed by Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under visible-light irradiation. When the Ag/AgCl content was further increased, the Ag/AgCl/CoFe2O4 (4
1) under visible-light irradiation. When the Ag/AgCl content was further increased, the Ag/AgCl/CoFe2O4 (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) degradation rate decreased compared to Ag/AgCl/CoFe2O4 (3
1) degradation rate decreased compared to Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This is probably because of excessive AgCl agglomeration (as shown in Fig. 2). The corresponding (C0 − C) plot has a good linearity (Fig. 6C), which indicates that the sunlight-driven photodegradation of MO solutions in the presence of the photocatalyst follows the zero-order kinetics. The degradation rate constant of MO is shown in Fig. 6D. The degradation rate constant of Ag/AgCl/CoFe2O4 (3
1). This is probably because of excessive AgCl agglomeration (as shown in Fig. 2). The corresponding (C0 − C) plot has a good linearity (Fig. 6C), which indicates that the sunlight-driven photodegradation of MO solutions in the presence of the photocatalyst follows the zero-order kinetics. The degradation rate constant of MO is shown in Fig. 6D. The degradation rate constant of Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is the highest. This indicates that it has the highest photoactivity, which is in good agreement with Fig. 6A.
1) is the highest. This indicates that it has the highest photoactivity, which is in good agreement with Fig. 6A.
It is known that a series of reactive species may be involved in the degradation process. To probe the underlying reactive species of Ag/AgCl/CoFe2O4 during the photocatalytic process, some scavengers were used to investigate them. In this study, isopropyl alcohol (IPA) was used as an ˙OH scavenger, L-ascorbic acid was used as an ˙O2− scavenger and disodium ethylenediaminetetraacetate (EDTA-2Na) was added as an h+ scavenger. As shown in Fig. 6E, the introduction of isopropanol had little effect on the degradation efficiency. This indicates that ˙OH was not the main dominant reactive species in this system. The degradation efficiency of MO was reduced significantly by the introduction of L-ascorbic acid (a quencher of ˙O2−) or EDTA-2Na (a quencher of h+), which suggests that ˙O2− and h+ played an important role in the photocatalytic degradation process. This is in good agreement with the reported studies.34,35
Moreover, the photocatalytic stability of Ag/AgCl/CoFe2O4 was investigated by three repeated MO degradation experiments. As shown in Fig. 7, the photoactivity of Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is still very high after three cycle experiments. The small decrease activity may be due to a loss of a small amount photocatalyst in the recycle experiments. Therefore, Ag/AgCl/CoFe2O4 can be used as an effective, stable magnetic recoverable photocatalyst for the degradation of organic compounds.
1) is still very high after three cycle experiments. The small decrease activity may be due to a loss of a small amount photocatalyst in the recycle experiments. Therefore, Ag/AgCl/CoFe2O4 can be used as an effective, stable magnetic recoverable photocatalyst for the degradation of organic compounds.
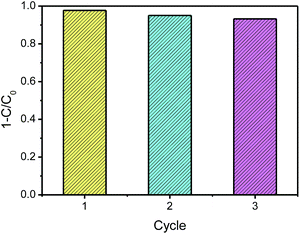 | ||
Fig. 7 The recycle experiments in the repeated MO degradation experiments with Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under visible-light irradiation. 1) under visible-light irradiation. | ||
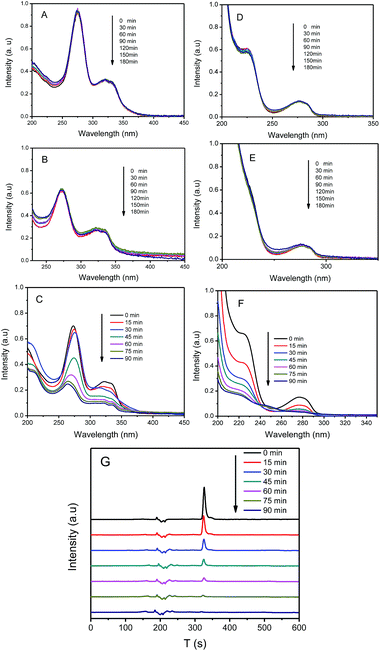
As can be seen from the abovementioned results, Ag/AgCl/CoFe2O4 can efficiently degrade the color pollutant MO. To exclude the dye-sensitized reaction, the colorless pollutants CIP and BPA were also used as contaminants to further evaluate the mineralization ability of the Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite. As shown in Fig. 8A and D, it is clear that CIP and BPA cannot be degraded under visible light irradiation without the existence of a photocatalyst. Their intensity showed a little decrease after CoFe2O4 was introduced, which may be ascribed to the fact that some pollutant was adsorbed by CoFe2O4 (as shown in Fig. 8B and E). However, the pure CoFe2O4 has no photoactivity for the degradation of CIP or BPA. The intensity of the pollutant did not decrease even after 3 h of irradiation in the presence of CoFe2O4. As shown in Fig. 8C, the two characteristic peaks of CIP decreased gradually. This suggests that CIP can be efficiently degraded in the presence of Ag/AgCl/CoFe2O4 (3
1) composite. As shown in Fig. 8A and D, it is clear that CIP and BPA cannot be degraded under visible light irradiation without the existence of a photocatalyst. Their intensity showed a little decrease after CoFe2O4 was introduced, which may be ascribed to the fact that some pollutant was adsorbed by CoFe2O4 (as shown in Fig. 8B and E). However, the pure CoFe2O4 has no photoactivity for the degradation of CIP or BPA. The intensity of the pollutant did not decrease even after 3 h of irradiation in the presence of CoFe2O4. As shown in Fig. 8C, the two characteristic peaks of CIP decreased gradually. This suggests that CIP can be efficiently degraded in the presence of Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under visible-light irradiation. Fig. 8F shows the UV-vis absorption spectra of the aqueous solutions of BPA at different periods. It is clear that the characteristic peak of BPA decreased with the reaction time. Within 90 min of reaction time, BPA was almost completely decomposed by the Ag/AgCl/CoFe2O4 (3
1) under visible-light irradiation. Fig. 8F shows the UV-vis absorption spectra of the aqueous solutions of BPA at different periods. It is clear that the characteristic peak of BPA decreased with the reaction time. Within 90 min of reaction time, BPA was almost completely decomposed by the Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) photocatalyst. The degraded BPA solution was also investigated using HPLC, and the results are shown in Fig. 8G. It is obvious that the characteristic peak of BPA decreased with the photoreaction process and almost disappeared in 90 min, which confirmed that BPA was indeed decomposed by Ag/AgCl/CoFe2O4 (3
1) photocatalyst. The degraded BPA solution was also investigated using HPLC, and the results are shown in Fig. 8G. It is obvious that the characteristic peak of BPA decreased with the photoreaction process and almost disappeared in 90 min, which confirmed that BPA was indeed decomposed by Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
3.6 Antibacterial activity
Fig. 9A shows the results of the antibacterial experiment of CoFe2O4 and Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the dark. It is clear that CoFe2O4 has no inhibition zone, while Ag/AgCl/CoFe2O4 (3
1) in the dark. It is clear that CoFe2O4 has no inhibition zone, while Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) has a clear inhibition zone of about 19 mm against E. coli. The results indicate that pure CoFe2O4 has no antibacterial ability, while Ag/AgCl/CoFe2O4 (3
1) has a clear inhibition zone of about 19 mm against E. coli. The results indicate that pure CoFe2O4 has no antibacterial ability, while Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) has a good antibacterial ability in the dark.36 Fig. 9B shows the results of the photocatalytic antibacterial experiments. Without the photocatalyst, no obvious decrease in the amount of E. coli was observed after irradiation for 30 min. This indicates that E. coli cannot be inhibited by light irradiation. In the presence of pure CoFe2O4, a large amount of E. coli was still alive after visible light irradiation for 30 min and no obvious decrease in amount was observed. This indicates that pure CoFe2O4 has no significant ability in killing E. coli. In the presence of Ag/AgCl/CoFe2O4 (3
1) has a good antibacterial ability in the dark.36 Fig. 9B shows the results of the photocatalytic antibacterial experiments. Without the photocatalyst, no obvious decrease in the amount of E. coli was observed after irradiation for 30 min. This indicates that E. coli cannot be inhibited by light irradiation. In the presence of pure CoFe2O4, a large amount of E. coli was still alive after visible light irradiation for 30 min and no obvious decrease in amount was observed. This indicates that pure CoFe2O4 has no significant ability in killing E. coli. In the presence of Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), numerous E. coli were still alive without light irradiation. However, when the system was irradiated by visible light for 15 min, more than half of the E. coli were killed. When the system was irradiated for 30 min, all the E. coli were killed. The results indicate that Ag/AgCl/CoFe2O4 can be activated by the visible light to efficiently kill E. coli. The results reveal that Ag/AgCl/CoFe2O4 possesses antibacterial ability with or without light.
1), numerous E. coli were still alive without light irradiation. However, when the system was irradiated by visible light for 15 min, more than half of the E. coli were killed. When the system was irradiated for 30 min, all the E. coli were killed. The results indicate that Ag/AgCl/CoFe2O4 can be activated by the visible light to efficiently kill E. coli. The results reveal that Ag/AgCl/CoFe2O4 possesses antibacterial ability with or without light.
Based on the abovementioned results, it can be concluded that Ag/AgCl/CoFe2O4 is an effective and stable magnetic photocatalyst, which can degrade colored and colorless pollutants as well as kill bacteria in water at the same time. Besides, its magnetic property is beneficial for its recovery after a reaction. Thus, Ag/AgCl/CoFe2O4 is a promising composite in practical fields for water processing.
4 Conclusions
A magnetically recoverable Ag/AgCl/CoFe2O4 photocatalyst has been successfully fabricated by a two-step method. The composite showed high degradation ability in the degradation of MO, CIP and BPA pollutants. HPLC results showed that BPA can be completely decomposed by Ag/AgCl/CoFe2O4 (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in 90 min. The combination of Ag/AgCl and CoFe2O4 is so strong that the composite can be easily recovered by a magnet after three cycle experiments, and it still maintains its high photoactivity. Besides, Ag/AgCl/CoFe2O4 showed high photocatalytic antibacterial ability against E. coli. This work provides a new strategy to synthesize magnetically recoverable photocatalysts.
1) in 90 min. The combination of Ag/AgCl and CoFe2O4 is so strong that the composite can be easily recovered by a magnet after three cycle experiments, and it still maintains its high photoactivity. Besides, Ag/AgCl/CoFe2O4 showed high photocatalytic antibacterial ability against E. coli. This work provides a new strategy to synthesize magnetically recoverable photocatalysts.
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China for Youths (no. 21407065), Natural Science Foundation of Jiangsu Province for Youths (BK20140533), China Postdoctoral Science Foundation (no. 2014M551520, 2014M560399), Jiangsu Postdoctoral Science Foundation (1401143C), and Jiangsu University Scientific Research Funding (no. 14JDG052).Notes and references
- H. X. Shi, G. S. Li, H. W. Sun, T. C. An, H. J. Zhao and P. K. Wong, Appl. Catal., B, 2014, 158–159, 301–307 CrossRef CAS PubMed.
- H. Tong, S. X. Ouyang, Y. P. Bi, N. Umezawa, M. Oshikiri and J. H. Ye, Adv. Mater., 2012, 24, 229–251 CrossRef CAS PubMed.
- W. B. Hou and S. B. Cronin, Adv. Funct. Mater., 2013, 23, 1612–1619 CrossRef CAS PubMed.
- Z. Z. Lou, Z. Y. Wang, B. B. Huang and Y. Dai, ChemCatChem, 2014, 6, 2456–2476 CrossRef CAS PubMed.
- J. G. McEvoy and Z. S. Zhang, J. Photochem. Photobiol., C, 2014, 19, 62–75 CrossRef PubMed.
- P. Wang, B. B. Huang, X. Y. Qin, X. Y. Zhang, Y. Dai, J. Y. Wei and M. H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931–7933 CrossRef CAS PubMed.
- P. Wang, B. B. Huang, X. Y. Zhang, X. Y. Qin, H. Jin, Y. Dai, Z. Wang, J. Y. Wei, J. Zhan, S. Wang, J. Wang and M. H. Whangbo, Chem.–Eur. J., 2009, 15, 1821–1824 CrossRef CAS PubMed.
- C. H. An, S. Peng and Y. G. Sun, Adv. Mater., 2010, 22, 2570–2574 CrossRef CAS PubMed.
- H. S. Lee, J. E. Kim, T. Y. Kim and K. S. Suh, J. Alloys Compd., 2015, 621, 378–382 CrossRef CAS PubMed.
- P. Wang, B. B. Huang, Q. Zhang, X. Y. Zhang, X. Y. Qin, Y. Dai, J. Zhan, J. Yu, H. Liu and Z. Z. Lou, Chem.–Eur. J., 2010, 16, 10042–10047 CrossRef CAS PubMed.
- P. Wang, B. B. Huang, Z. Z. Lou, X. Y. Zhang, X. Y. Qin, Y. Dai, Z. K. Zheng and X. N. Wang, Chem.–Eur. J., 2010, 16, 538–544 CrossRef CAS PubMed.
- H. G. Yu, L. L. Xu, P. Wang, X. F. Wang and J. G. Yu, Appl. Catal., B, 2014, 144, 75–82 CrossRef CAS PubMed.
- Y. P. Bi and J. H. Ye, Chem. Commun., 2009, 655, 6551–6553 RSC.
- Y. Y. Li and Y. Ding, J. Phys. Chem. C, 2010, 114, 3175–3179 CAS.
- Y. X. Tang, Z. L. Jiang, G. C. Xing, A. R. Li, P. D. Kanhere, Y. Y. Zhang, T. C. Sum, S. Z. Li, X. D. Chen, Z. L. Dong and Z. Chen, Adv. Funct. Mater., 2013, 23, 2932–2940 CrossRef CAS PubMed.
- R. F. Dong, B. Z. Tian, C. Y. Zeng, T. Y. Li, T. T. Wang and J. L. Zhang, J. Phys. Chem. C, 2013, 117, 213–220 CAS.
- Y. S. Xu and W. D. Zhang, ChemCatChem, 2013, 5, 2343–2351 CrossRef CAS PubMed.
- Y. G. Xu, H. Xu, H. M. Li, J. X. Xia, C. T. Liu and L. Liu, J. Alloys Compd., 2011, 509, 3286–3292 CrossRef CAS PubMed.
- W. S. Wang, H. Du, R. X. Wang, T. Wen and A. W. Xu, Nanoscale, 2013, 5, 3315–3321 RSC.
- J. F. Guo, B. W. Ma, A. Y. Yin, K. N. Fan and W. L. Dai, Appl. Catal., B, 2011, 101, 580–586 CrossRef CAS PubMed.
- C. H. An, X. J. Ming, J. Z. Wang and S. T. Wang, J. Mater. Chem., 2012, 22, 5171–5176 RSC.
- B. Z. Tian, T. T. Wang, R. F. Dong, S. Y. Bao, F. Yang and J. L. Zhang, Appl. Catal., B, 2014, 147, 22–28 CrossRef CAS PubMed.
- C. Y. Wang, L. Y. Zhu, C. Chang, Y. Fu and X. L. Chu, Catal. Commun., 2013, 37, 92–95 CrossRef CAS PubMed.
- H. A. J. L. Mourão, A. Malagutti and C. Ribeiro, Appl. Catal., A, 2010, 382, 284–292 CrossRef PubMed.
- K. K. Senapati, C. Borgohain and P. Phukan, Catal. Sci. Technol., 2012, 2, 2361–2366 CAS.
- P. Sathishkumar, N. Pugazhenthirana, R. V. Mangalaraja, A. M. Asiric and S. Anandan, J. Hazard. Mater., 2013, 252–253, 171–179 CrossRef CAS PubMed.
- P. Sathishkumar, R. V. Mangalaraja, S. Anandan and M. Ashokkumar, Chem. Eng. J., 2013, 220, 302–310 CrossRef CAS PubMed.
- S. Akbayraka, M. Kayab, M. Volkana and S. Özkar, Appl. Catal., B, 2014, 147, 387–393 CrossRef PubMed.
- L. J. Xu, W. Chu and L. Gan, Chem.–Eur. J., 2015, 263, 435–443 CAS.
- J. Deng, Y. S. Shao, N. Y. Gao, C. Q. Tan, S. Q. Zhou and X. H. Hu, J. Hazard. Mater., 2013, 262, 836–844 CrossRef CAS PubMed.
- N. W. Li, M. B. Zheng, X. F. Chang, G. B. Ji, H. L. Lu, L. P. Xue, L. J. Pan and J. M. Cao, J. Solid State Chem., 2011, 184, 953–958 CrossRef CAS PubMed.
- W. Y. Bian, Z. R. Yang, P. Strasser and R. Z. Yang, J. Power Sources, 2014, 250, 196–203 CrossRef CAS PubMed.
- Y. G. Xu, H. Xu, H. M. Li, J. Yan, J. X. Xia, S. Yin and Q. Zhang, Colloids Surf., A, 2013, 416, 80–85 CrossRef PubMed.
- J. X. Shu, Z. H. Wang, G. Q. Xia, Y. Y. Zheng, L. H. Yang and W. Zhang, Chem. Eng. J., 2014, 252, 374–381 CrossRef CAS PubMed.
- Y. H. Liang, S. L. Lin, L. Liu, J. S. Hu and W. Q. Cui, Appl. Catal., B, 2015, 164, 192–203 CrossRef CAS PubMed.
- S. G. Chen, Y. J. Guo, H. Q. Zhong, S. J. Chen, J. N. Li, Z. C. Ge and J. N. Tang, Chem. Eng. J., 2014, 256, 238–246 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |