HEPES-involved hydrothermal synthesis of Fe3O4 nanoparticles and their biological application†
Hui Li,
Zhong Lu,
Gang Cheng,
Kaifeng Rong,
Fengxi Chen and
Rong Chen*
School of Chemistry and Environmental Engineering, Key Laboratory for Green Chemical Process of Ministry of Education, Wuhan Institute of Technology, Wuhan, 430073, P. R. China. E-mail: rchenhku@hotmail.com; Fax: +86 2787195680; Tel: +86 13659815698
First published on 11th December 2014
Abstract
A simple one-step hydrothermal route was developed for the synthesis of magnetite (Fe3O4) nanoparticles in HEPES solution, using FeCl2 as the Fe(II) precursor. It was found that HEPES played an important role in the fabrication of Fe3O4 nanoparticles, where HEPES could act as a weak antioxidant to prevent the complete oxidation of Fe(II) to Fe(III). The possible formation mechanism of HEPES-coated Fe3O4 nanomaterials was also discussed. In the reaction system, the influence of inorganic anions on the morphologies of Fe3O4 nanostructures was also investigated. The as-synthesized spherical Fe3O4 nanoparticles with a concentration of 5–100 μg mL−1 exhibited nontoxicity towards HUVEC normal cells, indicating their potential application in biology. Combining the preparation of Ag and Au nanoparticles in HEPES solution, Ag/Fe3O4 and Au/Fe3O4 nanocomposites were successfully synthesized by a two-step method, which showed excellent antibacterial properties against S. aureus. Furthermore, the Ag/Fe3O4 and Au/Fe3O4 nanocomposites could be recycled and reused due to their superparamagnetic properties and retained their antibacterial activities.
1. Introduction
In the past decade, magnetite iron oxide nanostructures including Fe3O4 have attracted increasing attention in the biomedical field, due to their biocompatibility, higher stability in physiological conditions, and low cytotoxicity, which endows them with potential applications in targeted drug delivery,1–3 magnetic resonance imaging (MRI),4–7 multimodal and ultrasensitive detection of cancer cells.8,9 Therefore, much effort has been applied to the development of synthetic strategies for hydrophilic and biocompatible Fe3O4 nanoparticles. To date, various synthetic methods, such as co-precipitation,10 sol–gel processing,11 microemulsion technique,12 solvothermal synthesis,13,14 sonochemical approach15 and thermal decomposition of organic iron precursor,16–19 have been applied to the synthesis of magnetite nanoparticles. Among them, variations in the molar ratio of Fe3+ and Fe2+ often lead to rather complicated changes in the crystalline structures of iron oxides,20 while the products synthesized by a direct thermal decomposition route are generally soluble in organic solvents, which limits their applications in biomedical fields. As a matter of fact, it was a big challenge to maintain the coexistence of Fe2+ ions and Fe3+ ions with a fixed proportion (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and prevent them from being reoxidized in the synthesis of Fe3O4 nanomaterials. Although purgation and protection with nitrogen10,18 or argon16,17 has been widely used. It still suffers from drawbacks such as high cost and complicated control processes. Therefore, it is of significance to explore a novel efficient, facile, and economic way to fabricate Fe3O4 nanostructures.
2) and prevent them from being reoxidized in the synthesis of Fe3O4 nanomaterials. Although purgation and protection with nitrogen10,18 or argon16,17 has been widely used. It still suffers from drawbacks such as high cost and complicated control processes. Therefore, it is of significance to explore a novel efficient, facile, and economic way to fabricate Fe3O4 nanostructures.
HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid) solution, as a convenient and environmental friendly buffer solution, is widely used in analytical, inorganic and biological studies.21,22 Recently, HEPES solution was also used as an ideal reaction medium and precursor to prepare inorganic nanomaterials, including silver, gold, platinum metal nanoparticles and transition metal oxide (Co3O4, Mn3O4, and ZnO, etc.) nanomaterials with tunable morphology and size.23–28
In our previous work, α-Fe2O3 nanostructures with controllable size and shape were successfully prepared in HEPES buffer solution,29 in which HEPES acted as a stabilizer to prevent nanoparticles from aggregation. Inspired by the big challenge and previous work, we synthesized Fe3O4 nanomaterials from FeCl2 precursor in HEPES buffer solution, without employing either purgation or protection procedure. The possible formation mechanism of Fe3O4 nanomaterials and the influence of inorganic anions on the morphologies of the Fe3O4 nanostructures were discussed. To the best of our knowledge, there are a few reports relevant to synthesis of Fe3O4 nanoparticles from a single Fe(II) precursor.30,31 Besides, the fabrication of Fe3O4 nanostructures in HEPES buffer solution is also seldom reported. The magnetic properties and cytotoxicity of Fe3O4 nanoparticles towards HUVEC normal cells was also evaluated. Moreover, Au/Fe3O4 and Ag/Fe3O4 nanocomposites were also prepared in HEPES buffer solution and their enhanced antibacterial activities of Ag/Fe3O4 and Au/Fe3O4 against Staphylococcus aureus (S. aureus) were investigated. By utilizing superparamagnetic property of Fe3O4 nanospheres, the recyclability and stability of Au/Fe3O4 and Ag/Fe3O4 nanocomposites for the antibacterial test were also evaluated.
2. Experimental section
2.1 Chemicals
2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid (HEPES) was purchased from Sigma-Aldrich Chemical Company. Iron(II) chloride hydrate (FeCl2·2H2O), sodium nitrate (NaNO3), sodium sulfate (Na2SO4), sodium bromide (NaBr), potassium iodide (NaI), sodium formate (HCOONa), and sodium acetate anhydrous (CH3COONa) were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China). All chemicals were directly used without further purification. The bacteria Staphylococcus aureus (S. aureus ATCC 9118) was provided by College of Life Science, Huazhong Normal University (Wuhan, China). The Luria-Bertani (LB) medium was used for for the growth of aerobic bacteria. The human umbilical vein endothelial cells (HUVEC) were supplied by College of Life Science, Wuhan University (Wuhan, China).2.2 Synthesis
In a typical procedure, 15 mL FeCl2 aqueous solution (200 mmol L−1) was added into 15 mL HEPES aqueous solution (200 mmol L−1, pH = 7.4) at room temperature. The mixed solution was stirred vigorously and transferred into a stainless steel autoclave with a Teflon liner. The autoclave was sealed and maintained at 150 °C for 12 h. After cooling down to room temperature, the black solid product was collected by centrifugation and washed with deionized water and alcohol for five times. The sample was finally dried in a vacuum desiccator at 80 °C for 12 h for further characterization. Other samples were also prepared under identical conditions by varying molar ratios of HEPES/FeCl2, pH value of HEPES solution, reaction time, and inorganic additive. The detailed experimental parameters are listed in Table 1.| No. | Fe(II) precursor | Molar ratioa | Time/h | T/°C | pHb | Additive | Phase | Morphology |
|---|---|---|---|---|---|---|---|---|
| a HEPES/FeCl2 molar ratio.b pH value of HEPES solution. | ||||||||
| S1 | FeCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | None | Fe3O4 | Polyhedron |
| S2 | FeCl2 | 0 | 12 | 150 | 7 | None | α-Fe2O3 | Polydispersed particles |
| S3 | FeCl2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | None | Fe3O4 | Irregular polyhedron |
| S4 | FeCl2 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | None | Fe3O4 | Congeries |
| S5 | FeCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 5.2 | None | α-Fe2O3 | Polydispersed particles |
| S6 | FeCl2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 10.9 | None | Fe3O4 | Nanoparticles |
| S7 | FeSO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | None | Fe3O4 | Nanorods |
| S8 | FeCl2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | NaNO3 | Fe3O4 | Nanoparticles |
| S9 | FeCl2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | NaSO4 | Fe3O4 | Polyhedral |
| S10 | FeCl2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | NaBr | Fe3O4 | Polyhedral |
| S11 | FeCl2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | NaI | Fe3O4 | Nanoparticles |
| S12 | FeCl2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | HCOONa | Fe3O4 | Polyhedra |
| S13 | FeCl2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 | 150 | 7.4 | CH3COONa | Fe3O4 | Spherical |
For Au/Fe3O4 nanocomposites, 50 mg Fe3O4 nanoparticles (S15) was added to 10 mL HEPES buffer solution (50 mmol L−1, pH 7.4) with vigorous stirring. Then, 1 mL HAuCl4 solution (10 mmol L−1) was introduced into the mixture and stirred vigorously for 2 h in the dark. The Au/Fe3O4 nanocomposites were collected by centrifugation and washed three times with distilled water and absolute ethanol. For Ag/Fe3O4 nanocomposites, 50 mg Fe3O4 nanoparticles (S15) was added to 10 mL HEPES buffer solution (50 mmol L−1, pH 7.4) with vigorous stirring. Then, 1 mL AgNO3 solution (10 mmol L−1) was introduced into the mixture. After that, the reaction system was irradiated by UV light for 2 h under vigorous stirring. Finally, Ag/Fe3O4 nanocomposites were collected by centrifugation and washed three times with distilled water and absolute ethanol. The final Au/Fe3O4 and Ag/Fe3O4 products were stored in a desiccator for characterization and antibacterial activity studies.
2.3 Characterization
Power X-ray diffraction (XRD) was carried out on a Bruker AXS D8 Discover (Cu Kα = 1.5406 Å). The scanning rate is 1° min−1 in the 2θ range from 10 to 80 degrees. The scanning electron microscopy (SEM) images were performed on a S4800 field emission scanning electron microscope (FESEM, Hitachi, Japan) at an accelerating voltage of 5 kV. The transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM) images and selected area electron diffraction (SAED) patterns were recorded on a Philips Tecnai G2 20 electron microscope at an accelerating voltage of 200 kV. TEM samples were prepared by dispersing some solid product into ethanol and then sonicating for approximately 30 seconds. A few drops of the suspension were deposited on copper grids, which were then put into the desiccators for drying. The energy-dispersive X-ray (EDX) analysis was performed on an Oxford Instruments INCA with a scanning range from 0 to 20 keV. The Raman spectra were measured at room temperature under the backscattering geometric configuration using WITec-Alpha confocal micro-Raman system. The excitation light was the 514.5 nm line of an Ar+ laser with 30 mW output power. The magnetic measurements were recorded on a SQUID magnetometer at 298 K, Quantum Design MPMS.2.4 In vitro antibacterial activity and cytotoxicty assessment
The antibacterial activities of Ag/Fe3O4 and Au/Fe3O4 were tested using the antibacterial drop test against a gram positive bacterium (S. aureus). Suspension culture of the model organism S. aureus was cultured in Luria-Bertani (LB) medium at 37 °C for 24 h. The bacterial suspension was then washed and diluted to 5 × 105 CFU mL−1. 0.1 g Ag/Fe3O4 or Au/Fe3O4 nanocomposites was added into LB medium (10 mL) containing 5 × 105 CFU mL−1 S. aureus and then incubated at 37 °C on a rotary shaker for 18 h. Subsequently, aliquots (10 μL each) of diluted bacterial solution were spread over LB agar plates. After 24 h incubation at 37 °C, the number of bacterial colonies of surviving S. aureus was counted. The antibacterial activities of the recycled Ag/Fe3O4 or Au/Fe3O4 nanocomposites were also evaluated by same procedure. Additionally, 10 μL 5 × 105 CFU mL−1 of live S. aureus without treatment with Ag/Fe3O4 or Au/Fe3O4 nanocomposites was spread onto the LB agar plate and the number of colonies was counted as a control. The cytotoxicity study was carried out through the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay toward HUVEC cells.3. Results and discussion
3.1 Characterization of the polyhedron Fe3O4 nanoparticles
Fig. 1a shows the XRD pattern of the product prepared by hydrothermal method from FeCl2 precursor in HEPES buffer solution with HEPES/FeCl2 molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S1). Typically, all the diffraction peaks in Fig. 1a could be readily indexed to cubic phase Fe3O4, which is in good agreement with the standard data of Fe3O4 (JCPDS no. 01-1111). No other impurity peak was detected in this pattern, indicative of high purity of the product. Taking into account of both magnetite (Fe3O4) and maghemite (γ-Fe2O3) have a similar XRD pattern,32 Raman spectroscopy was further used to distinguish the crystal phase of the product. The Raman spectroscopy of sample S1 was shown in Fig. 1b, in which a characteristic intense absorption peak at 665 cm−1 was observed, corresponding to typical A1g model of Fe3O4,33–35 confirming the formation of Fe3O4 by employing a HEPES-involved hydrothermal method.
1 (S1). Typically, all the diffraction peaks in Fig. 1a could be readily indexed to cubic phase Fe3O4, which is in good agreement with the standard data of Fe3O4 (JCPDS no. 01-1111). No other impurity peak was detected in this pattern, indicative of high purity of the product. Taking into account of both magnetite (Fe3O4) and maghemite (γ-Fe2O3) have a similar XRD pattern,32 Raman spectroscopy was further used to distinguish the crystal phase of the product. The Raman spectroscopy of sample S1 was shown in Fig. 1b, in which a characteristic intense absorption peak at 665 cm−1 was observed, corresponding to typical A1g model of Fe3O4,33–35 confirming the formation of Fe3O4 by employing a HEPES-involved hydrothermal method.
 | ||
Fig. 1 XRD pattern (a), Raman spectroscopy (b) and SEM images (c and d) synthesized by hydrothermal process at 150 °C for 12 h with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of HEPES/FeCl2 mole ration. 1 of HEPES/FeCl2 mole ration. | ||
SEM images (Fig. 1c and d) reveal that a large quantity of Fe3O4 nanoparticles was obtained in HEPES solution by hydrothermal method. It was observed that these nanoparticles were well-dispersed and had polyhedron morphology. The diameters of the Fe3O4 nanoparticles varied from 50 to 100 nm, and the average diameters estimated from SEM images observation was about 70 nm. EDX spectrum was also used to analyze the elemental compositions of the as-synthesized Fe3O4 nanoparticles. It illustrates that the sample is composed of Fe and O, which is in good agreement with the XRD result (Fig. S1, ESI†).
The morphology and structure of Fe3O4 nanoparticles (S1) was further characterized by TEM, HRTEM images, and SAED patterns. TEM images of Fig. 2a and b demonstrate that these nanoparticles are well-dispersed and have diameters of 50 to 100 nm. SAED pattern of sample S1 (Fig. 2c) displays several diffraction rings, which is the sum of diffraction patterns of different Fe3O4 nanoparticles. Each diffraction ring could be well indexed to the corresponding crystal planes of magnetite, indicative of good crystallinity of Fe3O4 nanoparticles. HRTEM image of a single Fe3O4 nanoparticle shown in Fig. 2d reveals clear lattice fringes, which also illustrates the highly crystalline nature of the as-synthesized Fe3O4 nanoparticles. A corresponding SAED pattern (inset of Fig. 2d) of a single nanoparticle was also examined, and the unique pattern of diffraction spots confirms a single crystalline structure of Fe3O4 nanoparticles.
 | ||
| Fig. 2 Typical TEM images (a and b), ED pattern (c) and HRTEM image (d) of sample S1. Inset of (d) is a SAED pattern of the selected single nanoparticle. | ||
3.2 Function of HEPES
In the previous work, it was reported that HEPES could be used as a reducing agent in the fabrication of silver, gold and palladium nanoparticles,23–25 as well as capping agent in the formation of metal oxide nanomaterials.29 In the present reaction system, Fe2+ ions were not reduced to metallic iron in the HEPES solution, but partially were oxidized to Fe(III). However, when HEPES buffer solution was replaced by deionized water under identical conditions (S2), no Fe3O4 nanoparticles were prepared in the aqueous solution. Interestingly, the XRD pattern of the sample obtained in deionized water (S2) was in good agreement with the standard data of hexagonal α-Fe2O3 (JCPDS no. 84-0307) (Fig. 3a), indicating that the product was composed of α-Fe2O3. Fig. 3b shows the Raman spectroscopy of as-prepared product (S2), in which several typical modes of α-Fe2O3 including Eg(1), Eg(2), Eg(3), and A1g(2) are observed, corresponding to five characteristics bands at 608, 496, 407, 243 and 223 cm−1, respectively. It further demonstrates that the as-synthesized product (S2) belongs to pure α-Fe2O3 phase. | ||
| Fig. 3 XRD pattern (a), Raman spectroscopy (b) and SEM images (c and d) of the as-synthesized α-Fe2O3 particles (S2) in aqueous solution. | ||
SEM images (Fig. 3c and d) reveal that α-Fe2O3 products have irregular nanoparticles morphology and a broad size distribution. The results indicate that HEPES played an important role in the fabrication of Fe3O4 nanoparticles. It was proposed that HEPES could act as a weak antioxidant to prevent the complete oxidation of Fe(II) to Fe(III) during the hydrothermal process. On the other hand, in this HEPES-involved system, –OH groups, SO32− groups and –N– could absorb on certain crystal planes or chelate with Fe atoms as a competing agent to form steric hindrance, which could also prevent partial Fe2+ ions from being oxidization.36 Besides, HEPES molecules adsorbed on the surface of Fe3O4 crystals could further influence the crystal growth. The adorable HEPES molecules on the surface of Fe3O4 as verified by FTIR spectrum of Fe3O4. Before FTIR measurement, the Fe3O4 nanoparticles (S1) were washed with absolute ethanol and deioned water for several times to remove remaining HEPES molecules. Compared with the FTIR spectrum of pure HEPES, the peaks at 3394, 2356, 2330, 1645 and 1062 cm−1 in the IR spectrum of Fe3O4 nanoparticles (S1) could be assignable to corresponding stretching vibrations of HEPES molecules, indicating of the existence of HEPES molecules on the surface of Fe3O4 nanoparticles (Fig. S2, ESI†). The strong absorption bands at 572 cm−1 are assigned to Fe–O stretching vibration modes, being one of the characteristic adsorption bands of Fe3O4.37 The disappearance of peak at 1180 cm−1 assigning to the SO32− vibration of HEPES might be due to the formation of O–Fe band. It suggests that the surface of the as-synthesized Fe3O4 nanoparticles were covered with HEPES molecules, which could alter the resistance of each facet, resulting in an isotropic growth of Fe3O4 seeds.
However, the excessive HEPES molecules adsorbed on the surface of Fe3O4 nanoparticles has a significant influence on the disparity and particle size of Fe3O4 nanocrystals. When the HEPES/FeCl2 molar ratio was increased to 2, irregular polyhedron Fe3O4 nanostructures were obtained (S3), as shown in Fig. S3a (ESI†). These irregular Fe3O4 nanoparticles were aggregated and the diameters varied from 40 to 500 nm, having a broad size distribution. When the HEPES/FeCl2 molar ratio was further increased to 5 (S4), the congeries of Fe3O4 nanoparticles with the average diameter of ∼300 nm were obtained (Fig. S3b, ESI†). The results illustrate that the diameter of Fe3O4 nanoparticles increased with the increase of HEPES/FeCl2 molar ratio, which might be due to the excessive HEPES molecules absorbed on the surface of Fe3O4 nanoparticles.
Noticeably, the antioxidant capability of HEPES could be tuned by changing pH value of the reaction system, which has an influence on the fabrication of Fe3O4 nanoparticles. When the pH value of HEPES solution was 5.2 (S5), only hematite α-Fe2O3 nanoparticles (JCPDS no. 84-0307) with a broad size distribution from 0.1 to 1 μm were obtained (Fig. S4a and b, ESI†). However, when the pH value of HEPES solution was adjusted to 10.9, uniform Fe3O4 nanoparticles (S6) with an average size of 40 nm were fabricated (Fig. S4c and d, ESI†), which is smaller than that of the product prepared in HEPES solution with pH value of 7.4. The results indicate that pH value of HEPES solution not only could influence the oxidation state of final products but also the control particle size of Fe3O4. It was proposed that the acidic solution could accelerate the oxidation of Fe2+ to Fe3+ ions at low pH value.38 While at high pH value, free OH− is beneficial for the formation of Fe3O4 products, because the alkalescent HEPES solution could not only control the concentration of OH−, but also alter the concentration of Fe2+ ions via playing the role of complex agents.
3.3 Possible formation process
On the basis of the experimental results, it was proposed that the formation process of Fe3O4 nanoparticles involved the following steps. In the HEPES-involved reaction system, the weak alkali environment could provide small amount of OH− ions. Fe(HEPES)m(OH)2 nanoclusters were formed through the coordination of Fe2+ ions with OH− and HEPES molecules. Then, part of Fe (HEPES)m(OH)2 would react with the oxygen in the air and solution, gradually being oxidized to generate Fe3+ ions. Once Fe2+ and Fe3+ were coexisted with OH− in the reaction system, Fe3O4 nanocrystals will be quickly formed. At the same time, free HEPES molecules could act as an antioxidant and a capping agent in the formation of Fe3O4 nanoparticles. The morphologies and sizes of Fe3O4 nanocrystals could be tailored by changing the amounts of HEPES and the pH value of the reaction system. As a weak reductant, excessive HEPES molecules could prevent the fully oxidation of Fe2+ to Fe3+. On the other hand, HEPES presents different forms under different pH value, leading to different combination of HEPES molecule and Fe3O4 nuclei. The possible reaction processes in aqueous solution can be summarized in equations below (1)–(3):| Fe2+ + m HEPES + 2OH− → Fe(HEPES)m(OH)2 | (1) |
| 4 Fe(HEPES)m(OH)2 + O2 + 2H2O → 4Fe3+ + 12OH− + 4m HEPES | (2) |
| Fe2+ + 2Fe3+ + 8OH− → Fe3O4 + 4H2O | (3) |
3.4 Influence of inorganic anions
Interestingly, it was found that Fe3O4 nanorods were fabricated when FeSO4 was used as iron precursor, insteading of FeCl2. Fig. 4a shows the low and high-magnification SEM images of Fe3O4 product prepared from FeSO4 (S7), revealing that buddles of Fe3O4 nanorods were obtained. TEM image (Fig. 4b) shows that the nanorods are about 20–50 nm in diameter and 150–400 nm in length. SAED pattern of Fe3O4 nanorods (inset of Fig. 4b) displays several diffraction rings, which is the sum of the diffraction pattern of different individual nanorods, indicative of good crystallinity of the as-synthesized Fe3O4 nanorods. The results indicate that the morphologies of Fe3O4 nanostructures could be altered by using different iron precursors containing different inorganic anions, which plays an important role in the controlled synthesis of Fe3O4 nanocrystals.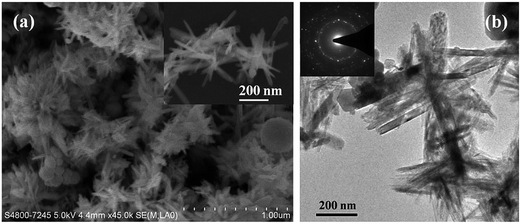 | ||
| Fig. 4 SEM (a) and TEM (b) images of the as-synthesized Fe3O4 nanorods by using FeSO4 as the iron precursor (S7). Inset of Fig. 4(b) is the SAED pattern of sample S7. | ||
To further systematically investigate the effects of inorganic anions on the morphologies of Fe3O4 nanocrystals, controlled experiments were carried out under the identical conditions by involving different inorganic salts, including NaNO3, Na2SO4, NaBr, NaI, HCOONa and CH3COONa (Table 1). As shown in Fig. 5a, uniform Fe3O4 nanoparticles with an average diameter of about 100 nm were obtained in the presence of NaNO3 (S8). HRTEM image (Fig. 5b) of a single nanoparticle reveals the well-resolved interplanar d-spacing of 0.30 nm, which corresponds to the (220) lattice planes. The corresponding SAED pattern (inset of Fig. 5b) shows a unique pattern of diffraction spots, confirming its single crystal structure. Fig. 5c and d show SEM images of the products obtained with the addition of Na2SO4 (S9). It reveals that the product is mainly composed of polyhedral Fe3O4 nanocrystals with a diameter ranging 100 to 800 nm. When NaBr was involved in the synthesis, relatively uniform polyhedral Fe3O4 nanocrystals with an average diameter of 100 nm were obtained, which shows a narrow size distribution (S10, Fig. 5e and f). Meanwhile, SAED pattern of a single nanocrystal (insert of Fig. 5f) demonstrates that the Fe3O4 nanocrystal is a single crystal. In the presence of NaI, it was observed that aggregated Fe3O4 nanoparticles with a broad size distribution were obtained (S11, Fig. 5g and h). When HCOONa was introduced to the reaction system, uniform polyhedral Fe3O4 nanocrystals with an average diameter of 100 nm were formed (S12, Fig. 5i and j). SAED pattern of sample S12 reveals several diffraction rings, indicative of well-crystalline nature. Importantly, when CH3COONa was instead of HCOONa in this synthesis, the product was mainly composed of uniform spherical Fe3O4 nanoparticles with an average diameter of 50 nm (S13, Fig. 5k and l). TEM image clearly reveals the spherical morphology of sample S13. The clear diffraction spots in SAED pattern of a single nanoparticle could readily be indexed to (111) and (220), which confirmed that the nanoparticle was well-crystallized. HRTEM image (inset of Fig. 5l) reveals a clear lattice fringe with d-spacing of 0.49 nm, corresponding to the interplanar d-spacing of (111).
Based on the experiment results, it was concluded that the morphologies of the Fe3O4 products were closely related to the anions involved in the solution. Generally speaking, it has a strong tendency to agglomerate during the formation of magnetic nanoparticles in the liquid phase. In this synthesis, it was proposed that the inorganic anions could act as a promoter for electrostatic stabilization, which can prevent the agglomeration of nanocrystals during the nucleation and growth. Similar results were also reported by Cao et al. and Deng et al. in the synthesis of Fe3O4 microspheres when NaCl and CH3COONa were used.39,40
3.5 Magnetic and in vitro cytotoxicity properties of Fe3O4 nanoparticles
The magnetic properties of the as-synthesized Fe3O4 nanoparticles (S1, S3, and S13) were evaluated by using a superconducting quantum interference device (SQUID) magnetometer at room temperature. Fig. 6a shows the magnetic hysteresis loop of different Fe3O4 nanoparticles, and the measured magnetic saturation values (Ms) are 75.0, 80.7, and 86.6 emu g−1 for S1, S13 and S3, respectively. Compared with samples S1 and S3, high saturation magnetization was detected when the particle size increased. It was in good agreement with the previous reports that the magnetization of small particles decreases as the particle size decreases.41 On the other hand, it was also reported that magnetic oxidation could cause a decrease in saturation magnetization.42 In this work, a higher mole ratio of HEPES/FeCl2 resulted in the coating of more HEPES molecules on the surface of Fe3O4 nanoparticles (S3), which could prohibit its oxidization, thus reading to the increase of Ms. In addition, compared with many organics-coated Fe3O4 nanocrystals synthesized by conventional aqueous solution routes,43–45 it was found the Fe3O4 nanocrystals prepared in this work generally showed a better magnetization, which implies the potential of current approach toward synthesis advantages of highly crystallized, uniform, and magnetized iron oxide nanoparticles.The good magnetic property endows Fe3O4 nanoparticles with potential biological application. Hence, the cytotoxicity of the as-synthesized Fe3O4 nanoparticles (S13) towards HUVEC normal cells was investigated by MTT assay. Fig. 6b shows the viability of HUVEC cells after 24 h incubation with different concentrations of Fe3O4 nanoparticles (S13). It was obviously observed that the spherical Fe3O4 nanoparticles (S13) exhibited a negligible cytotoxic profile even at the concentration of 100 μg mL−1. It illustrates that the Fe3O4 nanoparticles displayed none cytotoxicity towards the growth of HUVEC normal cells, indicative of good biocompatibility and promising biological applications.
3.6 Noble metal (Au, Ag)/Fe3O4 nanocomposites
To explore the biological application of Fe3O4 nanoparticles, Ag/Fe3O4 and Au/Fe3O4 nanocomposites prepared via a two-step method. The morphology and structure of obtained Ag/Fe3O4 and Au/Fe3O4 nanocomposites were characterized by TEM images. Fig. 7a and b show TEM images of the as-prepared Ag/Fe3O4 nanocomposites, which reveals that large quantities of silver nanoparticles were deposited on the surface of Fe3O4 nanocrystals. The diameters of Ag nanoparticles varied from 20 to 40 nm. EDX analysis of Ag/Fe3O4 nanocomposites confirms its elemental compositions of the prepared composites, which illustrates that the composite is composed of Fe, O, and Ag, indicative of the formation of Ag/Fe3O4 composites. Employing a similar route, Au/Fe3O4 nanocomposites were also successfully prepared. The amount of Ag and Au nanoparticles in the composites is 3.49% (Atomic%) and 1.69% (Atomic%), respectively. As shown in Fig. 7d and e, Au nanoparticles with diameters of 10–20 nm were deposited on the surface of Fe3O4 nanocrystals. EDX spectrum shown in Fig. 7f also confirms that the elemental composition of Au, Fe, and O in the Au/Fe3O4 nanocomposites.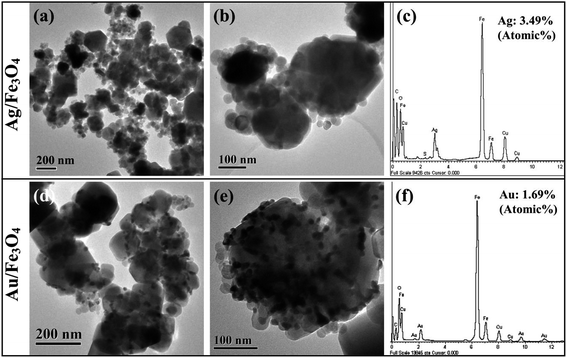 | ||
| Fig. 7 TEM images and EDX spectrum of Ag/Fe3O4 (a–c) and Au/Fe3O4 (d–f) nanocomposites obtained in HEPES buffer solution. | ||
The magnetic properties of the as-prepared Au/Fe3O4 and Ag/Fe3O4 nanocomposites were also investigated. As depicted in Fig. 8a, the magnetic hysteresis measurements of Ag/Fe3O4 and Au/Fe3O4 nanocomposites was carried out in applied magnetic field at 300 K. It was found that the coercively force of these two nanocomposites was almost negligible at 300 K, indicative of the superparamagnetic property of Ag/Fe3O4 and Au/Fe3O4 nanocomposites at room temperature. The saturation magnetization (Ms) of Ag/Fe3O4 and Au/Fe3O4 nanocomposites was about 73 and 67 emu g−1, respectively, illustrating a little decrease compared with that of pure Fe3O4 nanocrystals (S13, 80 emu g−1). The decrease in magnetization was not only due to mass decreasing of Fe3O4 nanocrystals resulted from the involving of silver and gold nanoparticle but also the diamagnetic contribution of the Au and Ag nanoparticles coating on the surface of Fe3O4 nanocrystals.46 However, Ag/Fe3O4 and Au/Fe3O4 nanocomposites still remained strong magnetization, which could have potential applications in bacterial disinfect, protein separation, photothermal therapeutics, catalysis, biological sensing, and so on. For example, it displayed ideal recycle properties in the antibacterial test by utilizing its strong magnetization, as illustrated in Fig. 8b.
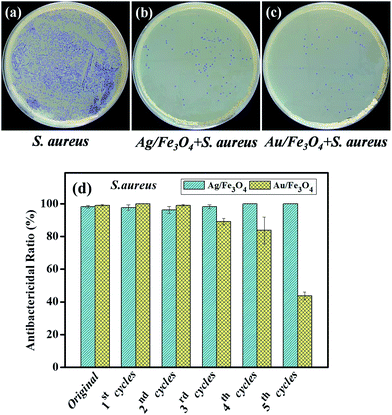
3.7 Antibacterial activities of Ag/Fe3O4 and Au/Fe3O4 nanocomposites
It is well known that many metal nanoparticles exhibited intrinsic antibacterial properties, as well as pose potential toxicity to human cells.47–49 Hence, to develop safe antibiotics, modification and isolation of metal nanoparticles is of great significance. In this work, the antibacterial activities against S. aureus of Fe3O4, Ag/Fe3O4 and Au/Fe3O4 were investigated by colony counting method. As shown in Fig. S5 (ESI†), no inhibition zone was observed in the disc cultured with S. aureus, indicating that bare Fe3O4 nanoparticles exhibited no antibacterial activity towards S. aureus. Fig. 9a–c show the bacterial growth in LB agar plates containing Ag/Fe3O4 and Au/Fe3O4 nanocomposites, respectively. It was observed the CFU numbers decreased dramatically with addition of Ag/Fe3O4 and Au/Fe3O4 nanocomposites into the S. aureus suspension, which indicates that Ag/Fe3O4 and Au/Fe3O4 nanocomposites possess excellent antibacterial properties. Furthermore, Ag/Fe3O4 and Au/Fe3O4 nanocomposites can be easily recycled due to their superparamagnetism property. The antibacterial properties of recycled Ag/Fe3O4 and Au/Fe3O4 nanocomposites were evaluated by measuring their antibacterial rate. Fig. 9d shows the antibacterial rates of recycled Ag/Fe3O4 and Au/Fe3O4 nanocomposites against S. aureus at different recycling times. It was observed that Ag/Fe3O4 nanocomposites remained excellent antibacterial property after five recycling times. The bacterial inhibition rates against S. aureus of Au/Fe3O4 nanocomposites shows a slightly decrease in the fourth cycle and obviously reduce to 40% after five recycling.4. Conclusions
A facile and environment-friendly hydrothermal route has been employed to synthesize magnetite (Fe3O4) nanoparticles in HEPES solution. HEPES molecules played an important role in the fabrication of Fe3O4 nanoparticles. It was proposed that HEPES acted as a weak antioxidant to prevent from the complete oxidation of Fe(II) to Fe(III) in the formation of Fe3O4 nanocrystals. On the other hand, HEPES was also considered as a capping agent adsorbing on the surface of Fe3O4 nanoparticle to mediate the crystal growth and aggregation. The possible mechanism for the formation of Fe3O4 nanomaterials in HEPES solution was discussed. The influence of inorganic anions on the morphologies of Fe3O4 nanocrystals was also studied. The synthesized Fe3O4 nanoparticles exhibited nontoxicity towards HUVEC normal cells. Furthermore, Ag/Fe3O4 and Au/Fe3O4 nanocomposites were successfully synthesized and showed excellent antibacterial ability against S. aureus. Meanwhile, the nanocomposite could be easily separated from solution by utilizing their superparamagnetic properties. Furthermore, Ag/Fe3O4 and Au/Fe3O4 nanocomposites still remained good antibacterial activities in the recycling usage. This work not only provides a novel strategy to prepare magnetite nanoparticles, but also indicates the potential biological applications of magnetic nanoparticles.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21371139), Key Program of Wuhan Science and Technology Bureau (201260523183) and High-Tech Industry Technology Innovation Team Training Program of Wuhan Science and Technology Bureau (2014070504020243).References
- J. Kim, J. E. Lee, S. H. Lee, J. H. Yu, J. H. Lee, T. G. Park and T. Hyeon, Adv. Mater., 2008, 20, 478–483 CrossRef CAS.
- J. H. Maeng, D.-H. Lee, K. H. Jung, Y.-H. Bae, I.-S. Park, S. Jeong, Y.-S. Jeon, C.-K. Shim, W. Kim, J. Kim, J. Lee, Y.-M. Lee, J.-H. Kim, W.-H. Kim and S.-S. Hong, Biomaterials, 2010, 31, 4995–5006 CrossRef CAS PubMed.
- J. E. Lee, N. Lee, H. Kim, J. Kim, S. H. Choi, J. H. Kim, T. Kim, I. C. Song, S. P. Park, W. K. Moon and T. Hyeon, J. Am. Chem. Soc., 2009, 132, 552–557 CrossRef PubMed.
- J. Zhu, Y. Lu, Y. Li, J. Jiang, L. Cheng, Z. Liu, L. Guo, Y. Pan and H. Gu, Nanoscale, 2014, 6, 199–202 RSC.
- X. Shi, S. H. Wang, S. D. Swanson, S. Ge, Z. Cao, M. E. Van Antwerp, K. J. Landmark and J. R. Baker, Adv. Mater., 2008, 20, 1671–1678 CrossRef CAS.
- L. Xiao, J. Li, D. F. Brougham, E. K. Fox, N. Feliu, A. Bushmelev, A. Schmidt, N. Mertens, F. Kiessling, M. Valldor, B. Fadeel and S. Mathur, ACS Nano, 2011, 5, 6315–6324 CrossRef CAS PubMed.
- N. Lee and T. Hyeon, Chem. Soc. Rev., 2012, 41, 2575–2589 RSC.
- J. Liu, W. Zhang, H. Zhang, Z. Yang, T. Li, B. Wang, X. Huo, R. Wang and H. Chen, Chem. Commun., 2013, 49, 4938–4940 RSC.
- L. Zhang, W.-F. Dong and H.-B. Sun, Nanoscale, 2013, 5, 7664–7684 RSC.
- Y. S. Kang, S. Risbud, J. F. Rabolt and P. Stroeve, Chem. Mater., 1996, 8, 2209–2211 CrossRef CAS.
- Y. Lu, Y. Yin, B. T. Mayers and Y. Xia, Nano Lett., 2002, 2, 183–186 CrossRef CAS.
- Z. H. Zhou, J. Wang, X. Liu and H. S. O. Chan, J. Mater. Chem., 2001, 11, 1704–1709 RSC.
- J. Lu, X. Jiao, D. Chen and W. Li, J. Phys. Chem. C, 2009, 113, 4012–4017 CAS.
- A. Yan, X. Liu, G. Qiu, N. Zhang, R. Shi, R. Yi, M. Tang and R. Che, Solid State Commun., 2007, 144, 315–318 CrossRef CAS PubMed.
- R. V. Kumar, Y. Koltypin, X. N. Xu, Y. Yeshurun, A. Gedanken and I. Felner, J. Appl. Phys., 2001, 89, 6324–6328 CrossRef CAS PubMed.
- T. Hyeon, S. S. Lee, J. Park, Y. Chung and H. B. Na, J. Am. Chem. Soc., 2001, 123, 12798–12801 CrossRef CAS PubMed.
- J. Rockenberger, E. C. Scher and A. P. Alivisatos, J. Am. Chem. Soc., 1999, 121, 11595–11596 CrossRef CAS.
- S. Sun, H. Zeng, D. B. Robinson, S. Raoux, P. M. Rice, S. X. Wang and G. Li, J. Am. Chem. Soc., 2003, 126, 273–279 CrossRef PubMed.
- F.-h. Lin, H.-H. Peng, Y.-H. Yang and R.-a. Doong, J. Nanopart. Res., 2013, 15, 1–13 Search PubMed.
- E. Tronc, P. Belleville, J. P. Jolivet and J. Livage, Langmuir, 1992, 8, 313–319 CrossRef CAS.
- N. E. Good, G. D. Winget, W. Winter, T. N. Connolly, S. Izawa and R. M. M. Singh, Biochemistry, 1966, 5, 467–477 CrossRef CAS.
- Q. Yu, A. Kandegedara, Y. Xu and D. B. Rorabacher, Anal. Biochem., 1997, 253, 50–56 CrossRef CAS PubMed.
- M.-H. So, C.-M. Ho, R. Chen and C.-M. Che, Chem.–Asian J., 2010, 5, 1322–1331 CAS.
- R. Chen, J. Wu, H. Li, G. Cheng, Z. Lu and C.-M. Che, Rare Met., 2010, 29, 180–186 CrossRef CAS PubMed.
- R. W.-Y. Sun, R. Chen, N. P.-Y. Chung, C.-M. Ho, C.-L. S. Lin and C.-M. Che, Chem. Commun., 2005, 5059–5061 RSC.
- J. Xie, J. Y. Lee and D. I. C. Wang, Chem. Mater., 2007, 19, 2823–2830 CrossRef CAS.
- H. Li, Z. Lu, J. Wu, H. Yu, X. Yu and R. Chen, Mater. Lett., 2010, 64, 1939–1942 CrossRef CAS PubMed.
- Q. Li, E.-t. Liu, Z. Lu, H. Yang and R. Chen, Mater. Lett., 2014, 130, 115–119 CrossRef CAS PubMed.
- H. Li, Z. Lu, Q. Li, M.-H. So, C.-M. Che and R. Chen, Chem.–Asian J., 2011, 6, 2320–2331 CrossRef CAS PubMed.
- X. Zhang, X. Xu, T. Li, M. Lin, X. Lin, H. Zhang, H. Sun and B. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 14552–14561 CAS.
- T. Wang, L. Zhang, H. Wang, W. Yang, Y. Fu, W. Zhou, W. Yu, K. Xiang, Z. Su, S. Dai and L. Chai, ACS Appl. Mater. Interfaces, 2013, 5, 12449–12459 CAS.
- N. Pinna, S. Grancharov, P. Beato, P. Bonville, M. Antonietti and M. Niederberger, Chem. Mater., 2005, 17, 3044–3049 CrossRef CAS.
- D. Bersani, P. P. Lottici and A. Montenero, J. Raman Spectrosc., 1999, 30, 355–360 CrossRef CAS.
- D. L. A. de Faria, S. Venâncio Silva and M. T. de Oliveira, J. Raman Spectrosc., 1997, 28, 873–878 CrossRef CAS.
- J. Zhang, H. Ji, Y. Wei, Y. Wang and N. Wu, J. Phys. Chem. C, 2008, 112, 10688–10691 CAS.
- R. Nakon and C. Krishnamoorthy, Science, 1983, 221, 749–750 CAS.
- H. Tang, B. J. Kwon, J. Kim and J.-Y. Park, J. Phys. Chem. C, 2010, 114, 21366–21370 CAS.
- X. Yang and N. D. Chasteen, Biochem. J., 1999, 338, 615–618 CrossRef CAS.
- S.-W. Cao and Y.-J. Zhu, Nanoscale Res. Lett., 2011, 6, 1–7 CrossRef PubMed.
- H. Deng, X. Li, Q. Peng, X. Wang, J. Chen and Y. Li, Angew. Chem., Int. Ed., 2005, 44, 2782–2785 CrossRef CAS PubMed.
- S.-W. Cao, Y.-J. Zhu and J. Chang, New J. Chem., 2008, 32, 1526–1530 RSC.
- R. L. Rebodos and P. J. Vikesland, Langmuir, 2010, 26, 16745–16753 CrossRef CAS PubMed.
- K. Tao, H. Dou and K. Sun, Chem. Mater., 2006, 18, 5273–5278 CrossRef CAS.
- X. Liang, X. Wang, J. Zhuang, Y. Chen, D. Wang and Y. Li, Adv. Funct. Mater., 2006, 16, 1805–1813 CrossRef CAS.
- J. Sun, S. Zhou, P. Hou, Y. Yang, J. Weng, X. Li and M. Li, J. Biomed. Mater. Res., Part A, 2007, 80A, 333–341 CrossRef CAS PubMed.
- Y. Zhai, J. Zhai, Y. Wang, S. Guo, W. Ren and S. Dong, J. Phys. Chem. C, 2009, 113, 7009–7014 CAS.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- N. Khlebtsov and L. Dykman, Chem. Soc. Rev., 2011, 40, 1647–1671 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra12536c |
| This journal is © The Royal Society of Chemistry 2015 |