 Open Access Article
Open Access ArticleBiobased vitrimers: towards sustainability and circularity
Alberto
Mariani
a and
Giulio
Malucelli
 *b
*b
aDepartment of Chemical, Physical, Mathematical and Natural Sciences, University of Sassari, Via Vienna 2, 07100 Sassari, Italy
bDepartment of Applied Science and Technology, Politecnico di Torino, Viale Teresa Michel 5, 15121 Alessandria, Italy. E-mail: giulio.malucelli@polito.it
First published on 6th January 2025
Abstract
In polymer science and technology, the distinction between thermoplastic and thermosetting materials has always been sharp, clear, and well-documented: indeed, the former can theoretically be reprocessed a potentially infinite number of times by heating, forming, and subsequent cooling. This cannot be done in the case of thermosetting polymers due to the presence of cross-links that covalently bind the macromolecular chains, giving rise to insoluble and infusible polymeric networks. In 2011, the discovery of vitrimers revolutionized the classification mentioned above, demonstrating the possibility of using new materials that consist of covalent adaptable networks (CANs): this way, they can change their topology through thermally-activated bond-exchange reactions. Recently, the increasing attention directed at green systems and circular economies has pushed the scientific community toward the synthesis and characterization of biobased materials, including vitrimers. Indeed, these latter represent a practical and reliable answer to the demanding issue of the eco sustainability of both materials and related technologies. The main advantage of using biobased vitrimers relies on their limited environmental impact as compared with the traditional systems deriving from fossil sources. Furthermore, biobased vitrimers exploit the same chemistries and plants already optimized for their fossil-based counterparts. The present work aims to review the current use of biobased vitrimers for advanced applications, highlighting their importance for designing novel, green, and sustainable materials that perfectly match the up-to-date circular economy concept.
Introduction
At present, plastics are in everyday life. According to recent statistics published by PlasticsEurope Organization in 2022,1 world plastic production reached 400.3 Mt (of which 58.7 Mt were produced in Europe), involving 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 companies, more than 1.5 million employees and with remarkable economic consistency (trade balance of 9.2 billion euros and turnover beyond 400 billion euros). The high versatility of plastic materials has always accounted for their massive use in different commercial sectors, including civil engineering, transportation, packaging, energy storage, and biomedicine, among others.2,3
150 companies, more than 1.5 million employees and with remarkable economic consistency (trade balance of 9.2 billion euros and turnover beyond 400 billion euros). The high versatility of plastic materials has always accounted for their massive use in different commercial sectors, including civil engineering, transportation, packaging, energy storage, and biomedicine, among others.2,3
However, because of their long lifetime, plastics at the end of their life may constitute environmentally pollutant wastes if not properly managed. This issue, together with the fluctuations in oil prices, is pushing both academic and industrial communities toward seeking efficient strategies for reducing polymer wastes and/or designing an economical way to either upcycle or recycle them, hence fulfilling the current approach driven by the circular economy.4,5
However, at present, conventional efforts (Fig. 1) account for the recycling of less than 10 wt% of plastics. In comparison, over 90 wt% of plastics are incinerated or landfill-confined.6
 | ||
| Fig. 1 Conventional recycling efforts. Reprinted from ref. 6 under CC-BY License. | ||
Thermosetting polymers are known for their high mechanical strength, heat resistance, and dimensional stability: all these features derive from the highly crosslinked structure that makes them suitable for a wide range of applications. However, this same crosslinking makes them difficult to recycle or reprocess.7
Conversely, thermoplastics, which possess a linear (sometimes branched) non-crosslinked structure, offer easy reprocessing and recyclability. They can be melted and reshaped multiple times without compromising their properties. In contrast, this structural difference makes thermoplastic polymers less chemically resistant and mechanically weaker at high temperatures with respect to thermosetting counterparts. Polymers made of covalent adaptable networks (CANs), often referred to as vitrimers, are emerging as a new class of materials that bridge the gap between thermosets and thermoplastics, offering advantageous properties of both classes.8 They achieve this by incorporating dynamic covalent bonds within their structure, allowing for reprocessing and reshaping beyond a certain temperature while maintaining the strength and stability characteristics of thermosets below this temperature. These features account for their ease of recycling at the end of their life (Fig. 2).9
 | ||
| Fig. 2 Closed-loop circularity provided by dynamic covalent chemistries leads to increased retention of plastics in the production and consumption cycle. Reprinted from ref. 6 under CC-BY License. | ||
The origins of vitrimers can be traced back to the pioneering work of Montarnal et al.,10 who demonstrated the concept using polyester epoxy resins that incorporated a zinc transesterification catalyst. When exposed to heat, these materials activate the interchain transesterification reactions, allowing the polymer network to dynamically rearrange and reflow under an applied stress.
At variance to conventional thermoplastics, which exhibit a sharp melting transition, vitrimers’ viscosity follows an Arrhenius relationship with temperature, in a fashion that is similar to the silica-based glasses.
This unique property stems from the fact that the flow of vitrimers is governed by the rate of the reversible bond exchange reactions in the melt state, rather than the typical viscoelastic response of thermoplastics.
The ability to reshape, self-weld, and reprocess vitrimers without compromising their structural integrity has sparked a surge of research in this field, with a focus on exploring various crosslink exchange chemistries, catalyst systems, and network structures.
Dynamic covalent bonds are a typical feature of vitrimers, offering a unique blend of stability and flexibility that traditional polymer networks lack. These reversible chemical bonds enable the restructuring of the polymer matrix in response to environmental stimuli, such as temperature and pressure changes, without the need for drastic alterations in the polymer's overall chemical composition. This dynamic behavior is primarily engineered through specific chemical reactions, allowing vitrimers to respond to mechanical and thermal stimuli adaptively. This adaptability enhances the material's lifespan and opens avenues for innovative applications in fields ranging from automotive to biomedical engineering.
It must be highlighted that the definition of vitrimer is currently a topic of active discussion in scientific literature, particularly concerning whether the term should be limited to associative systems or if it should also include dissociative counterparts.
In fact, dynamic rearrangements can exploit either dissociative or associative distinctive exchange pathways (Fig. 3); the former uses the exchange of dynamic bonds even in such highly crosslinked networks as in thermosets. The lowering in the crosslinking density is strictly related to the loss in network connectivity that takes place during the step-by-step breakage and reformation of chemical bonds.11 At variance, the bond exchanges occurring in associative dynamic CANs allow for a modest variation of the crosslinking density during the breakage and reformation of the chemical bonds, hence resulting in negligible changes in the macromolecular structure of the polymer networks.12
 | ||
| Fig. 3 Dissociative (A) and associative (B) exchange in dynamic covalent adaptive networks. Reprinted with permission from ref. 13. Copyright Elsevier, 2020. | ||
Despite these characteristics, the key aspect of all these materials is the existence of exchangeable covalent bonds in their organic structures, allowing them to change shape significantly with or without altering the overall level of crosslinking. This ability to dynamically rearrange their network structure through reversible bond exchange reactions sets vitrimers apart from traditional thermosetting polymers, which are typically static and resistant to reshaping or reprocessing. The dispute about vitrimer definition arises from varied understandings of the basic mechanism that allows these dynamic covalent networks to possess their distinctive characteristics. A key input to this debate was offered by Leibler,14 who suggested a categorization of CANs, identifying vitrimers as a distinct subclass. In particular, he differentiated between associative vitrimers, characterized by bond exchanges through associative mechanisms that do not involve the breaking of the main chain covalent bonds, and dissociative CANs, which – at variance – involve bond exchanges through the breaking of the main chain linkages that breaks a covalent bond. In this view, the designation vitrimer ought to be limited to associative systems that display a glassy structure at low temperatures and engage in bond exchanges at higher temperatures. Therefore, dissociative CANs should be categorized within a wider classification.
The discussion is, therefore, not resolved with just one definition.15,16 Additional investigation is needed to create a comprehensive understanding and classification system that captures the intricacies of these materials and to stimulate IUPAC to provide the scientific community with a universally accepted definition of vitrimer.
At variance to conventional thermoplastics, which exhibit a sharp melting transition, vitrimers’ viscosity follows an Arrhenius relationship with temperature, in a fashion that is similar to silica-based glasses.
This unique property stems from the fact that the flow of vitrimers is governed by the rate of the reversible bond exchange reactions in the melt state, rather than the typical viscoelastic response of thermoplastics.
The ability to reshape, self-weld, and reprocess vitrimers without compromising their structural integrity has sparked a surge of research in this field, with a focus on exploring various crosslink exchange chemistries, catalyst systems, and network structures.
Dynamic covalent bonds are a typical feature of vitrimers, offering a unique blend of stability and flexibility that traditional polymer networks lack. These reversible chemical bonds enable the restructuring of the polymer matrix in response to environmental stimuli, such as temperature and pressure changes, without the need for drastic alterations in the polymer's overall chemical composition. This dynamic behavior is primarily engineered through specific chemical reactions, allowing vitrimers to respond to mechanical and thermal stimuli adaptively. This adaptability enhances the material's lifespan and opens avenues for innovative applications in fields ranging from automotive to biomedical engineering.
The capability of dynamic covalent bonds to facilitate reconfiguration brings forth significant implications for materials design and sustainability. In fact, by enabling efficient recycling processes and adaptability, vitrimers are closely aligned with the principles of green chemistry, sustainability, and circular economy.17,18
In the last five years, further efforts toward sustainability and circularity have been carried out through the design, synthesis, and characterization of biobased vitrimers;19–21 these are materials that derive from biosources (i.e., bio-based monomers/chemicals) and exploit the same chemistries involved in the formation of non-biobased dynamic CANs.
Compared to the latter, biobased vitrimers exhibit peculiar characteristics that are presented in Table 1.
| Feature | Bio-based vitrimers | Fossil-based vitrimers |
|---|---|---|
| Raw materials22,23 | Derived from renewable resources (e.g., starch, cellulose, lignin, vegetable oils, sugars, agricultural waste). | Derived from non-renewable petroleum-based resources. |
| Sustainability21,24,25 | Reduced dependence on fossil fuels, lower carbon footprint, potential for biodegradability and compostability, use of waste materials. | Dependence on fossil fuels, higher carbon footprint, typically non-biodegradable, contributes to plastic waste accumulation. |
| Environmental impact22,24 | Lower greenhouse gas emissions during production and end-of-life management, reduced pollution from waste, potential for closed-loop recycling. | Higher greenhouse gas emissions, reliance on environmentally damaging extraction and refining processes, contribution to microplastics pollution. |
| Toxicity25 | Potential for lower toxicity, use of less harmful monomers, and reduction of reliance on toxic chemicals in production. | Potential for higher toxicity, reliance on hazardous chemicals in synthesis, possible release of harmful substances upon degradation. |
| Biodegradability/end-of-life21 | Many bio-based vitrimers offer the potential for biodegradation, composting, chemical recycling, and easier reuse options. | Typically non-biodegradable, leading to accumulation in the environment and requiring energy-intensive processes such as incineration or land filling. |
| Material properties25 | Performance can vary depending on the specific bio-based material. Some bio-based vitrimers can match, or even surpass, the performance of fossil-based ones. There may be challenges related to moisture, stability, etc. | Generally well-defined and optimized mechanical and thermal properties, consistency and reliability are generally well established, and therefore these polymers are widely used in many applications |
| Synthesis/processing21,26 | Synthesis processes may require optimization for scalability, potential for bio-based solvents and catalysts. Processing may need new equipment and specific processing parameters. | Synthesis processes are generally well established, are often based on bulk or solution processes, but require toxic catalysts and/or solvent. |
| Cost24 | Costs can vary significantly depending on the specific biomass source, production process, and desired performance. Costs may include biomass pre-treatment, purification, and monomer synthesis. | Costs are generally dependent on the price of crude oil, but are normally relatively low. |
| Scalability22 | Scaling-up production processes are still challenging and require investments. | Well-established production processes, easier to scale up but often at the expense of a high carbon footprint. |
It is noteworthy that bio-based vitrimers fully match the current needs for sustainability (and, in particular, for the fulfillment of the demanding Sustainable Development Goals)27 and circularity. The growing academic interest in biobased vitrimers is well documented in the significantly increasing number of papers published in the last few years (Fig. 4) on this topic.
 | ||
| Fig. 4 Number of publications (from 2017 to 2024) in peer-reviewed journals dealing with “biobased vitrimers” (data collected from the Web of Science™ database, accessed on Oct 27, 2024). | ||
In this context, the present work is aimed at reviewing the most recent literature on biobased vitrimers, highlighting the chemistry behind their design, their potential for advanced applications, and their importance within the up-to-date sustainability concept.
The chemistries behind vitrimers
Transition temperatures in vitrimers
Vitrimers exhibit two distinct types of transition temperatures: the traditional glass transition temperature, Tg, and the topology-freezing transition temperature, Tv.While Tg marks the shift from a glassy to rubbery state based on molecular motion, Tv represents the point where the network topology rearranges due to faster exchange reactions, transitioning from a viscoelastic solid to a viscoelastic liquid state, making it a unique feature of vitrimers.
In particular, at Tv, the viscosity of vitrimers reaches 1012 Pa s, i.e., the threshold between a viscoelastic solid and a viscoelastic liquid state,12 and the reversibility of dynamic bonds is initiated, thereby facilitating the polymer network's capability to undergo structural modifications (Fig. 5). Above Tv, the viscosity follows the Arrhenius temperature dependence, which is dictated by the kinetics associated with the reversible cleavage and reformation of bonds.
 | ||
| Fig. 5 Temperature dependence of viscosity in vitrimers. (A) When Tv > Tg, the viscosity follows an Arrhenius law near Tv. (B) When Tg > Tv, first, the viscosity follows WLF law near Tg and, then, Arrhenius law at sufficiently higher temperatures. Reprinted with permission from ref. 28. Copyright Elsevier, 2019. | ||
Even if Tv is generally higher than Tg, in some cases, bond reversibility can happen beneath Tg. Both Tg and Tv can be tuned by varying such parameters as crosslinking density, monomer architecture, catalysts, and activation energy of the bonds. An increase in crosslinking density, together with the utilization of more rigid monomers, generally leads to enhanced material strength.28
Main functional groups and reactions involved in vitrimerization
As shown in Fig. 6, a temperature variation influences the equilibrium between the formation and the dissociation of the Diels–Alder adducts, allowing the polymer network to be dynamically reconfigured and reprocessed through reversible bond cleavage and reformation.30
 | ||
| Fig. 6 Reversibility of the Diels–Alder reaction in a vitrimerization process. Reprinted with permission from ref. 28. Copyright Elsevier, 2019. | ||
Debnath et al.32 reported a polyester vitrimer that can be thermally reprocessed at 100 °C by using Sn(Oct)2 as a catalyst or at 150 °C without any catalyst, thus making recycling and reusability easier (Fig. 7).
 | ||
| Fig. 7 (A) Transesterification of model compounds and synthesis of poly(hydroxyethyl methacrylate)-diethyl malonate (PHEMA-DEM-x) vitrimers; (B) schematic showing the exchange of the ester bond under thermal conditions. Legend: PHEMA = poly(hydroxyethyl methacrylate); DEM = diethyl malonate; AIBN = α,α-azoisobutyronitrile. Reprinted with permission from ref. 32. Copyright American Chemical Society, 2020. | ||
 | ||
| Fig. 8 Dynamic covalent bonds in disulfide-based vitrimers. Reprinted with permission from ref. 28. Copyright Elsevier, 2019. | ||
Recently, Luo et al.34 proposed a dual dynamic covalent bond epoxy vitrimer made of disulfide bonds and imine bonds showing improved mechanical properties as compared with the corresponding non-vitrimerized epoxy polymer. The main benefit of employing disulfide bonds in this framework is their capacity to promote stress relaxation at lower activation energies. This property is fundamental for applications wherein materials are subjected to dynamic loads, as it facilitates improved energy dissipation and mitigates the likelihood of material failure under stress. Moreover, the dual-dynamic covalent bond architecture not only improves the mechanical behavior but also preserves commendable solvent resistance, elevated breakdown strength, and advantageous dielectric characteristics, which are indispensable for a myriad of industrial applications.
 | ||
| Fig. 9 Self-healing of a polymer matrix by boronic ester dynamic covalent bonds. Reprinted with permission from ref. 35. Copyright American Chemical Society, 2015. | ||
The characteristic feature of boronic esters relies on their capacity to establish reversible covalent bonds. This reversibility is mostly attributable to the electron-deficient characteristics of the boron atom, which facilitates its participation in dynamic interactions with nucleophiles, including alcohols and water. The bond between the boron atom and the alkoxy moiety undergoes cleavage and reformation under specific conditions, thereby allowing for the use of boronic esters in vitrimeric formulations. The reactivity of boronic esters is influenced by various parameters such as pH and temperature. In particular, at low pH, they may undergo hydrolysis, resulting in the formation of the corresponding alcohol and boronic acid. In contrast, under basic or neutral conditions, the boronic ester may exhibit stability, thereby facilitating controlled release and reformation of the covalent linkage.35
Zhang et al.36 proposed a new class of boronic diesters with improved performance in vitrimerization. In particular, they found that the hydrolytic stability and dynamic covalent chemistry of the nitrogen-coordinating cyclic boronic diester (NCB) linkages are greatly improved as compared with that of conventional boronic esters. In particular, the former can tolerate some water due to their hydrophobic NCB linkages, which, in turn, depends on the intramolecular N → B coordination. However, dynamic exchange reactions of NCB linkages can occur without catalysts upon heating, thus allowing their vitrimers to be reprocessed and recycled while maintaining mechanical properties similar to pristine materials.
Iminic and amidic groups
Imine bonds, created by the reaction of an amine with a carbonyl compound, are dynamic covalent bonds that can reversibly exchange, providing vitrimers with distinctive properties. The formation of imines from amines and aldehydes is a reversible reaction governed by thermodynamic control.37Geng et al.38 prepared bio-based imine vitrimeric films from vanillin and an amine cross-linker. The resulting materials exhibited self-healing ability, hot-reprocessing properties, and chemical recyclability under acid hydrolysis. They found that Tg increased as the cross-linking degree increased, and films exhibited thermal stability up to at least 300 °C.
However, the versatility of imine linkages in tailoring the properties of vitrimers goes well beyond the thermal and mechanical properties. Indeed, imine linkages also affect the mechanical properties, the chemical stability, and the response of these materials to their environment. By tailoring the components involved in the chemistry of the imine bonds, it is possible to prepare vitrimers that respond to specific stimuli, such as pH or temperature changes, thus obtaining materials that can be applied in many different sectors.26
Liang et al.39 introduced a novel class of poly(amide–imine)vitrimers, which combined the benefits of both amide and imine groups. They found enhanced mechanical properties, thermal stability, and creep resistance as compared with the materials bearing imine functionalities only. Besides, since a biobased curing agent (i.e., trimethyl citrate) was used, the new vitrimer was more eco-friendly.
Analogously, Petazzoni and co-workers40 proposed a series of new bio-based polyamide–polyamine vitrimers obtained from tris(2-aminoethyl)amine and epoxidized methyl oleate, a material that can be easily prepared from renewable resources (Fig. 10). The integration of free amine functionalities within the network facilitated the transamidation exchange with the crosslinking amide functionalities; this reaction, when suitably catalyzed, confers complete reprocessability to the material. The synthesized materials were thermally stable up to 300 °C and exhibited a glass transition temperature value between 7 and 21 °C.
 | ||
| Fig. 10 Polymerization (a) and transamidation exchange reactions (b) in bio-based polyamide–polyamine vitrimers. Reprinted from ref. 40 under CC-BY License. | ||
Considering that amidic groups can create strong hydrogen bonds, which are important for producing a polymer network with high mechanical properties, these functionalities were introduced in vitrimers. In particular, each amide group can be engaged in several hydrogen bonds with nearby polymer chains, resulting in a more connected network. This bonding not only improves the mechanical properties but also increases thermal resistance, as more energy is needed to break these bonds.
It was found that the mechanical characteristics of epoxy vitrimers may be optimized through the modulation of the crosslinking density.42 A high crosslinking density generally promotes increased stiffness and strength, whereas a low crosslinking density can improve flexibility and impact resistance. Epoxy vitrimers generally exhibit enhanced thermal stability with respect to traditional thermosetting polymers, thus preserving mechanical properties under high thermal stresses, which is essential for applications that require high thermal resistance. The ability of epoxy vitrimers to undergo stress relaxation through bond exchange reactions helps dissipate stress concentrations, which can prevent crack initiation and propagation, thereby enhancing the overall mechanical performance. Furthermore, epoxy vitrimers exhibit significant potential for recyclability and reprocessability due to their dynamic covalent bonding. This characteristic accounts for their reshaping and repairing, thus addressing a major limitation of traditional thermosetting polymers.
Recent applications of biobased vitrimers
Bio-based vitrimers combine the adaptable properties of dynamic covalent networks with the sustainability of bio-derived building blocks, leading to a wide range of potential applications.22 Existing research primarily explores their use in sustainable adhesives, self-healing coatings, and easily recyclable packaging.23,43Looking forward, their properties also make them attractive for composite materials, smart materials, and advanced biomedical applications.24,25 The specific studies detailed below offer a glimpse into the current progress, highlighting the versatility and potential of bio-based vitrimers across diverse fields.
Wu and co-workers43 designed fully biobased, multifunctional vitrimers by reacting epoxidized soybean oil (ESO) with natural glycyrrhizic acid (GL) (Fig. 11). Thanks to transesterification-induced topological network rearrangements, the so-obtained materials exhibited interesting welding/self-healing (Fig. 12), and shape memory (Fig. 13) features, suggesting their big potential as recyclable and repairable adhesives.
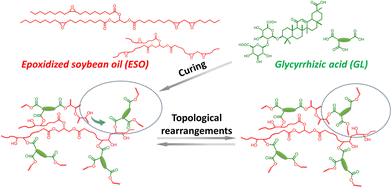 | ||
| Fig. 11 Scheme of epoxidized soybean oil (ESO) and glycyrrhizic acid (GL) chemical structures, curing reaction, and topological rearrangements of ESO/GL networks. Reprinted with permission from ref. 43. Copyright American Chemical Society, 2020. | ||
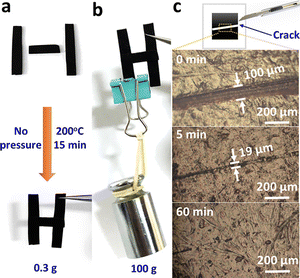 | ||
| Fig. 12 Welding test of ESO/GL networks (a) and (b). (c) Optical microscope picture of the crack reparation of ESO/GL vitrimeric networks (T = 200 °C). Reprinted with permission from ref. 43. Copyright American Chemical Society, 2020. | ||
 | ||
| Fig. 13 Consecutive shape memory cycles for ESO/GL networks (a) as assessed by dynamic-mechanical tests. Pictures of temporary shape-changing and permanent shape-changing of ESO/GL networks (b): (I) deformation using a tweezer at temperatures above Tg for 10 s. The temporary shape was fixed after cooling down to room temperature. (II) Fixation of the twisted shape by heating above malleability temperature (Tmall) for 10 min. The permanent deformation was achieved by cooling down to room temperature. (III) It was possible to remold the permanent shape into another temporary shape by heating the vitrimer at T > Tg for 10 s and subsequently cooling down to room temperature. (IV) The permanent shape was recovered by heating the sample above Tg for 10 s. Reprinted with permission from ref. 43. Copyright American Chemical Society, 2020. | ||
Zhao and co-workers44 exploited dual dynamic exchange reactions (i.e., simultaneous disulfide metathesis and transesterification) for obtaining ESO-derived vitrimers crosslinked with 4,4′-dithiodibutyric acid and embedding hydroxyl-functionalized silica nanoparticles (Fig. 14). These latter accounted for increased mechanical properties, with 113 and 150% increases, respectively, in elongation at break and tensile strength compared to the unfilled vitrimeric counterpart. Besides, the vitrimeric nanocomposites showed excellent self-healing and welding features, regardless of the possibility of being easily reprocessed and recycled (Fig. 15).
 | ||
| Fig. 14 Chemical structures of epoxidized soybean oil (ESO), 4,4′-dithiodibutyric acid (DA), 1,5,7-triazabicyclo[4,4,0]dec-5-ene (TBD, catalyst), hydroxyl-functionalized silica, and vitrimeric nanocomposite (ESO/SiO2 DPNs). Reprinted with permission from ref. 44. Copyright American Chemical Society, 2021. | ||
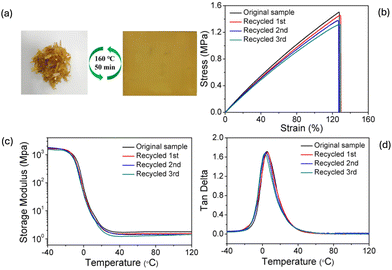 | ||
| Fig. 15 (a) Digital photographs showing the physical reprocessing of vitrimeric nanocomposite containing 3 wt% of silica. (b) Stress–strain curves and (c) and (d) dynamic-mechanical curves of the original and physically recycled vitrimeric nanocomposite. Reprinted with permission from ref. 44. Copyright American Chemical Society, 2021. | ||
In a further research effort, Zych et al.45 synthesized a diboronic ester dithiol dynamic cross-linker, suitable for coupling with epoxidized soybean oil acrylate through a thiol–acrylate coupling (Fig. 16).
 | ||
| Fig. 16 Schematic representation of the chemical synthesis of the epoxidized soybean oil acrylate/diboronic ester dithiol vitrimer via thiol–acrylate coupling and a picture of a transparent vitrimer film (about 0.5 mm thickness) obtained through compression molding (10 min at 120 °C under 5 tons of pressure). Reprinted with permission from ref. 45. Copyright American Chemical Society, 2021. | ||
The so-obtained vitrimer exhibited remarkable reprocessability, biodegradability, easy recyclability (it was possible to regenerate the vitrimer through reversible hydrolysis in ethanol and subsequent evaporation of the solvent), and self-healing capability at room temperature (because of its low Tg – about 10 °C, and the quick boronic ester exchange reaction, Fig. 17), envisaging its potential use as a self-mending coating.
 | ||
| Fig. 17 Epoxidized soybean oil acrylate/diboronic ester dithiol vitrimers: evolution of a scratched sample (A) and its cross sections (B) as a function of the healing time (self-healing is carried out at room temperature; a schematic representation of the self-healing process (C, side view)). Reprinted with permission from ref. 45. Copyright American Chemical Society, 2021. | ||
Moreno and co-workers46 exploited a one-pot, catalyst-free, and thermally activated “click” addition of softwood kraft lignin (28 to 50 wt% loading) to poly(ethylene glycol)divinyl ether (Fig. 18), obtaining different lignin-derived vitrimers. These latter showed outstanding features as recoverable adhesives for different substrates (namely, wood and aluminum), with high values of shear strength (up to 6.0 MPa for the vitrimer with the highest lignin content). Furthermore, the adhesion performance was preserved beyond 90% after two rebonding processes, demonstrating the vitrimers’ recyclability.
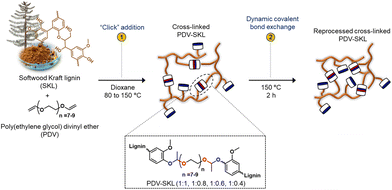 | ||
| Fig. 18 Scheme of the preparation of lignin-based vitrimers (PDV-SKL) and their recovery through catalyst-free dynamic acetal exchange reactions. Reprinted from ref. 46 under CC-BY-4.0 License. | ||
Adjaoud and co-workers47 synthesized a degradable isosorbide-based polybenzoxazine vitrimer through consecutive solvent-free Fischer esterification and Mannich-like ring-closure reactions. In particular, D-isosorbide underwent esterification with phloretic acid, and subsequently reacted with mono-ethanolamine and/or furfurylamine and paraformaldehyde. The reaction resulted in the formation of di-telechelic benzoxazine-terminated isosorbide monomers bearing aliphatic hydroxyl groups and/or furan rings connected to the nitrogen atom (Fig. 19). Finally, these monomers underwent a curing process at 150 °C (for 1 h), followed by a second curing at 170 °C (1 h), and by a post-curing at 190 °C (30 min), carried out for furan-containing compounds only.
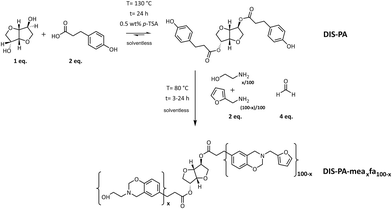 | ||
| Fig. 19 Synthesis of the di-telechelic benzoxazine-terminated isosorbide monomer (DIS-PA-meaxfa100−x). Legend: DIS-PA = D-isosorbide esterified with phloretic acid; mea = mono-ethanolamine: fa = furfurylamine. Reprinted from ref. 47 under CC-BY-4.0 License. | ||
The so-obtained vitrimers exhibited high Tg values (within 143 and 193 °C, as determined by the mono-ethanolamine/furfurylamine content), degradability in mild aqueous environments, self-healing ability (exploiting the fast dynamic exchanges induced by transesterification reactions catalyzed by the tertiary amine from the benzoxazine ring-opening polymerization) and recyclability (Fig. 20).
 | ||
| Fig. 20 Mechanical and chemical recycling of the isosorbide-based polybenzoxazine vitrimer (A); retention of the storage modulus of isosorbide-based polybenzoxazine vitrimer as a function of the number of reprocessing cycles (B). Adapted from ref. 47 under CC-BY-4.0 License. | ||
In a further research effort, Zhong et al.48 synthesized catalyst-free, fully biobased epoxy vitrimers made of a ferulic acid-based hyperbranched epoxy resin combined with a ferulic acid-derived epoxy resin and citric acid.
In this way, it was possible to obtain shape-memory and reprocessable networks, thanks to the hyperbranched structure bearing –OH and ester groups, which assisted the dynamic transesterification reactions and the formation of reversible crosslinks.
Furthermore, compared to the ferulic acid-derived epoxy system, 10 phr of the ferulic acid-based hyperbranched epoxy resin were enough to significantly increase tensile strength and fracture toughness by about 44 and 134%, respectively. Finally, the so-derived vitrimers accounted for easy chemical recyclability, as they could be depolymerized in NaOH and subsequently employed for preparing new epoxy vitrimers, thus demonstrating the possibility of using a closed-loop recycling approach.
Another sector where biobased vitrimers find interesting applications refers to the design of vitrimeric elastomers. In this context, Tong and co-workers49 exploited oxidized starch (57% carboxyl content) as a biomacromolecular crosslinking agent for epoxidized natural rubber. Thanks to the formation of β-hydroxyl ester bonds between the two components, 30 phr of oxidized starch were enough to achieve high elastic recovery and elongation at break (about 90 and 1100%, respectively), and significantly high shape fixed and shape recovery ratios (around 99.5 and 95.6%, respectively). Besides, the activation energy of the transesterification process (as low as about 80 kJ mol−1) accounted for the possibility of reprocessing the vitrimer through thermo-activation: the loss in the mechanical features was below 12% even after two consecutive thermally activated reprocessing steps. Finally, the proposed vitrimer exhibited biodegradability using α-amylase for degrading the polymer network.
Dynamic covalent imine bonds were exploited for the design of easily processable, recyclable, and self-healable biobased epoxy vitrimers.50 To this aim, vanillin was reacted with bisaminomethyl cyclohexane at 60 °C for three hours, and the obtained product was employed as an imine curing agent for diglycidyl ether of bisphenol F. Because of the difficult processing of vanillin-based imine curing agent, 4-methyl-1,3-cyclohexendiamine was employed as a co-curing agent (Fig. 21). The so-derived vitrimers exhibited enhanced mechanical features (with flexural strength and flexural moduli up to 122 and 2587 MPa, respectively), increased Tg values (up to 110 °C), recyclability, and self-healing features (Fig. 22).
 | ||
| Fig. 21 Schematic of the synthesis of biobased epoxy vitrimers. Legend: HDTA = 4-methyl-1,3-cyclohexendiamine; VIBCA = vanillin-based imine curing agent. Reprinted with permission from ref. 49. Copyright American Chemical Society, 2023. | ||
 | ||
| Fig. 22 Schematic of the synthesis of biobased polyimide vitrimers. Legend: HCCP = hexachlorocyclotriphosphazene; HVP = hexasubstituted cyclotriphosphazene; DMF = dimethyldiamide. Reprinted with permission from ref. 51. Copyright Elsevier, 2022. | ||
Pursuing this research, the same group52 synthesized a vanillin-imine-containing hardener for diglycidyl ether of bisphenol-F: to this aim, vanillin was reacted with xylene diamine. The resulting vitrimer showed high recyclability due to the reversible imine linkages present in the network structure; besides, the recovery of flexural properties approached 100% even after three reprocessing runs. Interestingly, the vitrimer showed an outstanding degradation resistance in various solvents while completely dissolving in an acidic solution (HCl, 1 M, operating at 50 °C for 12 hours). All these findings suggested the use of the proposed vitrimers as recoverable adhesives or recyclable polymer matrices in fiber-reinforced composites.
Monteserin and co-workers53 synthesized an epoxy vitrimer obtained through the reaction of an epoxidized linseed oil resin and a vanillin-derived Schiff base as a dynamic hardener, using different epoxy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hardener ratios. The presence of dynamic imine bonds accounted for the reprocessability of the obtained vitrimers at 190–210 °C. Furthermore, the vitrimers exhibited good solubility in acidic solutions of methanol, ethanol, dimethylformamide, and dichloromethane at room temperature and atmospheric pressure.
hardener ratios. The presence of dynamic imine bonds accounted for the reprocessability of the obtained vitrimers at 190–210 °C. Furthermore, the vitrimers exhibited good solubility in acidic solutions of methanol, ethanol, dimethylformamide, and dichloromethane at room temperature and atmospheric pressure.
Yang and co-workers51 synthesized multifunctional polyimide vitrimers through the reaction of vanillin with hexachlorocyclotriphosphazene and the subsequent curing with alkyl or alkoxyl diamines (Fig. 22). The resulting vitrimers showed easy reprocessability under hot-pressing conditions; this finding was ascribed to transamination or imine metathesis reactions taking place at the processing temperatures (Fig. 23). Besides, the vitrimers showed excellent flame retardant features, with V-0 rating in vertical flame spread tests. Finally, despite a very good solvent resistance in ethanol, water, alkaline medium, and natural solution brine, the polyimide vitrimers were able to undergo hydrolysis in acidic conditions, hence demonstrating easy chemical recyclability toward the obtainment of the hexasubstituted cyclotriphosphazene monomer.
 | ||
| Fig. 23 Reprocessability of polyimide vitrimers and proposed reactions occurring during reprocessing: (a) transimination; (b) imine metathesis. Reprinted with permission from ref. 51. Copyright Elsevier, 2022. | ||
Wang and co-workers54 were among the first to propose vanillin-derived epoxy vitrimers reinforced with carbon fibers. To this aim, a vanillin-based epoxy monomer, synthesized on purpose, was reacted with four different diamines (namely, 4,4′-diaminodiphenylmethane, diethylenetriamine, isophoronediamine, and polyetheramine) in the presence of two layers of plain weave carbon fiber cloth. All the vitrimeric composites were chemically recyclable (under mild conditions, i.e., in an acidic solution and operating at room temperature), with the only exception of the one cured with 4,4′-diaminodiphenylmethane. Interestingly, both the surface morphology and structure of the carbon fibers were not affected by the proposed chemical recycling process.
Similarly, Tang and co-workers55 designed an interesting vitrimer derived from a guaiacol-based epoxy resin cured with 4-aminophenyl disulfide (which bears dynamic covalent S–S bonds).
The obtained vitrimer showed a very good reprocessability at 200 °C, with relaxation times as short as 27.5 s. Furthermore, the vitrimer was successfully employed as the matrix of carbon fiber-reinforced composites: the epoxy matrix could be dissolved in a 50/50 N,N-dimethylformamide/2-mercaptoethanol mixture without any change in the carbon fibers before and after the recycling process.
Pursuing this research, Zamani et al.56 proposed a fast curing vanillin-based Schiff base polyimide network as the vitrimeric polymer matrix of carbon fiber-reinforced composites. The latter, obtained through compression molding, showed easy reprocessability at 180 °C and 4 bar, outstanding fire retardance (achieving a V0 rating in vertical flame spread tests), and high modulus (45 GPa) and tensile strength (427 MPa), which slightly reduced at 41.3 GPa and 418 MPa after remanufacturing, respectively.
Zhang et al.57 succeeded in synthesizing biobased, fully recyclable, self-healing, and shape memory vitrimers derived from castor oil and bearing dynamic pyrazole–urea bonds (Fig. 24).
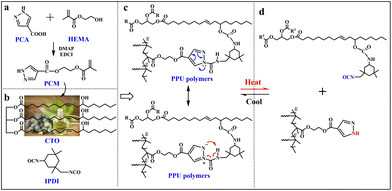 | ||
| Fig. 24 (a) Synthesis route for pyrazole ester (PCM). (b) Chemical structures of castor oil (CTO) and isophorone diisocyanate (IPDI). (c) Destabilized pyrazole–urea bonds: intramolecular electron transfer diminishes resonance effect and intramolecular 1,4-hydrogen transfer. (d) Thermal reversibility of pyrazole–urea bonds. Legend: PCA = 4-pyrazolecarboxylic acid; HEMA = hydroxyethyl methacrylate; PPU = poly(urethane-urea)vitrimer. Reprinted with permission from ref. 57. Copyright Elsevier, 2022. | ||
The presence of dynamic pyrazole–urea bonds and hydrogen bonds in the vitrimer accounted for outstanding remendability, malleability, recyclability, and shape memory features without a significant worsening in the mechanical behavior of the material (Fig. 25). Finally, because of the presence of numerous hydrogen bonds in its structure, the high adhesion strength (about 1.6 MPa), and the possibility of quickly re-bonding the vitrimer at least 30 times (through heating and cooling cycles), suggested its use as a recoverable adhesive.
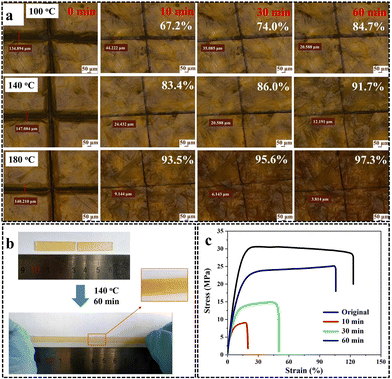 | ||
| Fig. 25 (a) Thermal repairing of poly(urethane-urea)vitrimer (biobased content: 46.3%) at different times and temperatures. (b) The welded vitrimer was able to stretch 100% without significantly rupturing. (c) Mechanical properties of the original and healed samples. Reprinted with permission from ref. 57. Copyright Elsevier, 2022. | ||
Xiao and co-workers58 synthesized two tung oil-based compounds (Fig. 26), differing in the content of carboxylic acid and anhydride, and employed them as curing agents for diglycidyl ether of bisphenol A. Compared to the epoxy resin cured with a commercial anhydride (namely, methyl nadic anhydride), the so-obtained vitrimers showed enhanced mechanical properties (specifically regarding tensile and bending strength, elongation at break and impact resistance) and ease of recyclability and malleability, thanks to the high content of reversible ester bonds and secondary –OH groups that assist the transesterification reactions during the recycling process. In particular, it is worth noticing that the remolding process of the biobased vitrimers could be performed without employing a catalyst, and the tensile strength of the remolded samples achieved almost 100% of the pristine counterparts.
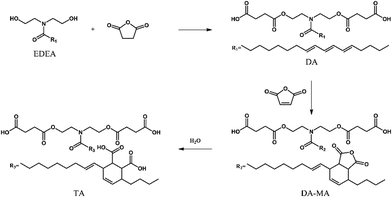 | ||
| Fig. 26 Schematic of the synthesis of tung oil-based compounds as curing agents for diglycidyl ether of bisphenol A. Legend: EDEA = eleostearic diethanolamide; DA = tung oil-based diacid; TA = tung oil-based tetracid; DA-MA = adduct of DA and maleic anhydride. Reprinted with permission from ref. 58. Copyright American Chemical Society, 2022. | ||
Li et al.59 selected castor oil, cysteamine, and vanillin as building blocks to design fully biobased polyimine vitrimers; for this purpose, an imination (namely, thiol–ene photo-click) reaction between cysteamine-functionalized castor oil and divanillin was exploited (Fig. 27). The prepared vitrimers exhibited remarkable adhesion (lap-shear strength of about 6.1 MPa on 304 stainless steel), good thermal stability, high self-healing features (within 30 min at 80 °C), recyclability, and even antibacterial capability (with antibacterial rates against S. aureus and E. coli beyond 90%). All these findings were ascribed to the peculiar structure of the vitrimers, including aromatic rings, flexible chain segments, and imine linkages. These characteristics suggested their use in the field of high-performance and recoverable adhesives.
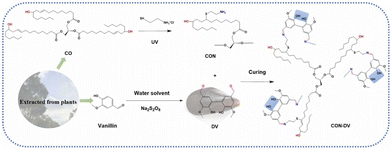 | ||
| Fig. 27 Schematic of the synthesis of polyimine vitrimers. Legend: CO = castor oil; CON = castor oil polyamine; DV = divanillin; CON-DV = castor oil and vanillin-based polyimine; UV = ultra-violet curing. Reprinted with permission from ref. 59. Copyright Elsevier, 2023. | ||
Pursuing this research, Stouten and co-workers60 exploited emulsion polymerization for synthesizing polymethacrylates based on 2-(methacryloyloxy)ethyl vanillin and 2-octyl acrylate, hence obtaining a waterborne latex (31% solid content, 49 nm average particle size), which was subsequently crosslinked with a multifunctional amine (Fig. 28). For comparison purposes, a similar system was prepared via solution polymerization. Even after three reprocessing cycles, the obtained vitrimers exhibited good reprocessability, with a negligible impact on their thermal stability and mechanical behavior. In addition, the specific interactions of imine bonds with water accounted for self-healing features, envisaging the potential of the vitrimers as a reliable replacement for fossil-based waterborne latexes.
 | ||
| Fig. 28 (a) Reaction scheme for the synthesis of water-borne vanillin-derived copolymers via solution polymerization and emulsion polymerization. (b) Reaction scheme for the production of vitrimers via cross-linking with tris(2-aminoethyl)amine (TREN) to obtain reversible imine bonds. Legend: MEV = 2-(methacryloyloxy)ethyl vanillin; 2OA = 2-octyl acrylate; DMF = dimethyl formamide; AIBN = azobisisobutyronitrile; POEGMA = poly(oligo(ethylene glycol)methyl ether methacrylate); AMPA = 2,2′-azobis[2-methylpropionamidine]dihydrochloride. Reprinted from ref. 60 under CC-BY License. | ||
Ren et al.61 succeeded in preparing vitrimeric castor oil-based polyurethanes based on dynamic covalent boronic ester crosslinking and boron–nitrogen (B–N) coordination. The synthesis was carried out using a two-step solvent- and catalyst-free approach (Fig. 29). The increase in boronic ester content accounted for enhanced stiffness, ductility, and mechanical strength; besides, the occurrence of supramolecular interactions due to the B–N coordination determined network topology rearrangements at high temperatures, providing polyurethane vitrimers with remarkable self-healing features and reprocessability.
 | ||
| Fig. 29 Schematic of the synthesis of castor oil-based polyurethane vitrimers. Legend: CO = castor oil; THF = tetrahydrofurane; HMDI = hexamethylene diisocyanate; DPA = N,N-dimethyl-N′,N′-di(2-hydroxypropyl)-1,3-propanediamine; HDB = 2,2′-(1,4-phenylene)-bis[4-(4-hydroxybutyl)-1,3,2-dioxaborolane]. Reprinted with permission from ref. 61. Copyright Elsevier, 2023. | ||
Zhang and co-workers62 synthesized castor oil-based epoxy vitrimers, employing epoxidized methacrylated castor oil (epoxy prepolymer), glycerol methacrylate (reactive diluent), and itaconic acid and 4,4′-dithiodiphenylamine (curing agents). Thanks to the presence of dynamic ester bonds and disulfide bonds, the vitrimers exhibited remodeling and reprocessing features (through hot pressing at 190 °C for 4 h), self-healing ability (achieving about 95% healing efficiency after thermal treatment at 180 °C for 4 h), and chemical degradability.
Salaeh et al.63 exploited the incorporation of a small amount (between 4 and 6 phr) of biobased polyols (namely glycerol or xylitol) in elastomers derived on epoxidized natural rubber and crosslinked with 3 phr of dimer fatty acid (Fig. 30). The concurrent presence of the β-hydroxy ester and hydroxyl groups allowed for the formation of a thermally rearrangeable network via transesterification exchange reactions (Fig. 31). Moreover, the vitrimeric elastomers exhibited good weldability, reprocessability, and self-healing features, hence indicating their potential as novel eco-friendly rubber formulations.
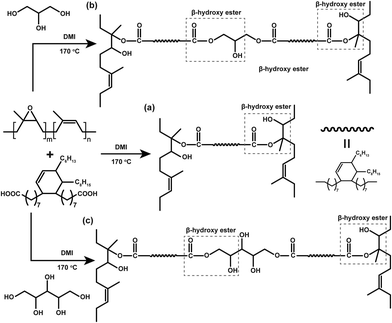 | ||
| Fig. 30 Plausible reaction scheme in (a) diacid crosslinking of epoxidized natural rubber, and (b) and (c) diacid cross-linking of epoxidized natural rubber in the presence of glycerol and xylitol, respectively. Legend: DMI = 1,2-dimethylimidazole. Reprinted with permission from ref. 63. Copyright Elsevier, 2023. | ||
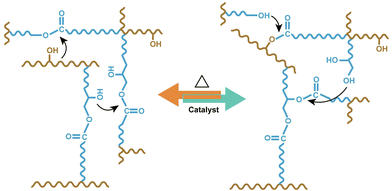 | ||
| Fig. 31 Scheme of transesterification exchange reaction of epoxidized natural rubber vitrimer. Reprinted with permission from ref. 63. Copyright Elsevier, 2023. | ||
A fully biobased epoxy cardanol glycidyl ether cured with citric acid (Fig. 32) was recently proposed by Liu et al.64 as a multifunctional vitrimer that showed self-healing, shape memory, and recyclability features. These findings were ascribed to the presence of hydroxyl groups in citric acid that gave rise to the formation of several hydrogen bonds, responsible for the catalyst-free topological rearrangement of the vitrimeric networks.
 | ||
| Fig. 32 (A) Scheme of the synthesis of the epoxy cardanol glycidyl ether and (B) scheme of the curing reaction of the synthesized epoxy cardanol glycidyl ether–citric acid vitrimers. Legend: TBAB = tetrabutylammonium bromide. Reprinted from ref. 64 under CC-BY-NC-ND 4.0 License. | ||
An up-to-date investigation area involves synthesizing bio-based epoxy vitrimers and elastomers, showing acceptable mechanical strength and thermal stability while maintaining their reprocessability and recyclability.
In this context, a novel fully bio-based epoxy vitrimer was synthesized utilizing commercially available glycerol triglycidyl ether and an imine-containing hardener derived from vanillin and 4-aminophenol (Gte-VA).65 The resulting vitrimer exhibited a Young's modulus of 1.6 GPa and tensile strength of 62 MPa, much like the conventional epoxy resins one might expect. Moreover, Gte-VA displayed exceptional reprocessability, recyclability, and UV-blocking properties, which were attributed to the dynamic imine bonds. The material exhibited remarkable tensile strength (449 MPa) and Young's modulus (12.9 GPa), which allowed its use as a matrix for carbon fiber-reinforced composites. Finally, the carbon fibers could be recovered after the resin degradation in an amine solution, and the recycled materials could be used to prepare composites with similar mechanical properties, showcasing a convenient path to fully recycling carbon fiber composites.
Liu et al. developed fully bio-based elastomeric vitrimers utilizing epoxidized natural rubber and dynamic imine bonds.24 Specifically, a dynamic cross-linker was prepared from vanillin and 1,10-decanediamine, which are both bio-sourced. The resulting elastomers exhibited excellent tensile strength (ca. 19 MPa) and an elongation at break beyond 880%, together with remarkable elastic recovery post-stretching and quick stress relaxation at high temperatures, which made them thermally reprocessable and recyclable. The simultaneous presence of benzene rings and active imine bonds accounted for the use of these vitrimers as reliable, eco-friendly substitutes for standard vulcanized elastomers.
Zhang et al.23 recently investigated the application of lignin, a byproduct from the paper sector, as a strengthening bio-filler for the design of advanced vitrimeric systems. To this aim, lignin was mixed with carboxylated nitrile rubber (i.e., butadiene–styrene–vinylpyridine rubber), and their interaction was improved through the creation of Zn–ligand coordination bonds, leading to good dispersion of lignin in the rubber matrix and enhanced interfacial interactions. The occurrence of exchange reactions through C–N transalkylation of the quaternized pyridine groups allowed the resulting composites to exhibit vitrimer-like characteristics, confirmed by excellent reprocessability at elevated temperatures.
In a further research effort, Qiu et al.66 implemented exchangeable β-hydroxyl ester bonds between carbon black and natural rubber into a commercial formulation, hence resulting in CANs showing good mechanical strength and reprocessability.
Zhang et al.67 succeeded in synthesizing spiro diacetal building blocks derived from bio-based benzaldehydes (namely, syringaldehyde, vanillin, and ethylvanillin) and erythritol, which were subsequently cured by employing bio-based epoxy soybean oil as a crosslinker. The reaction scheme is presented in Fig. 33.
 | ||
| Fig. 33 Synthetic route of fully bio-based CANs. Legend: DMI = dimethyl imidazole; p-TSA = p-toluenesulfonic acid. Reprinted with permission from ref. 67. Copyright American Chemical Society, 2023. | ||
The recyclability and reprocessability of the bio-based CANs were verified by hot-pressing small pieces of vitrimers at 170 °C for 1 h: flat and homogeneous polymer films were obtained, displaying only a slight decrease in the mechanical behavior (Fig. 34).
 | ||
| Fig. 34 (a) Images of the biobased CANs, before and after reprocessing at 170 °C and 10 MPa for 1 h; (b) stress–strain curves of the biobased CANs before and after three cycles of reprocessing. Reprinted with permission from ref. 67. Copyright American Chemical Society, 2023. | ||
Very recently, Gosecki and co-workers68 succeeded in synthesizing polyester-based covalent adaptable networks from birch suberin, which was extracted by hydrolyzing the outer bark of the tree under alkaline conditions. The addition of a low molecular weight polyol (for providing an excess of hydroxyl groups) to the suberin resin allowed for its polymerization (through transesterification) in the presence of different catalysts. Among these latter, only dibutyltin dilaurate ensured the formation of vitrimeric structures that could be remolded at 190 °C under 3 MPa pressure applied for 30 min. The resulting vitrimers, exhibiting a Tg of about −20 °C and a tensile modulus beyond 1 MPa, were hydrolytically stable under alkaline and acidic mild conditions. Furthermore, they were suggested for the design of elastomeric composites.
Very interestingly, Solle et al.69 designed and produced stimuli-responsive microstructures through UV-induced nanoimprint lithography. In particular, they synthetized a fully bio-based dynamic thiol–ene photopolymer through the radical-mediated addition of a trifunctional eugenol-based thiol crosslinker on an allylated linseed oil. Bond exchange reactions between –OH and ester groups within the obtained network were promoted by a biobased eugenol phosphate ester, employed as a transesterification catalyst. Additionally, pure eugenol was incorporated as a reactive diluent to increase the number of –OH groups and hasten the bond exchange reactions. Upon exposure to UV radiation, the vitrimers exhibited quite high stress relaxations when thermally treated at 160 °C for about 1 h (activation energy about 61 kJ mol−1). Besides, the fully cured imprints fabricated by nanoimprinting lithography were reshapeable, envisaging the versatility and suitability of the designed vitrimers for microfluidic devices.
Techno-economic analysis on biobased vitrimers
Evaluating bio-based vitrimers requires a comprehensive techno-economic analysis to ascertain their practical viability and market potential. This entails a meticulous examination of production costs, performance parameters, life cycle impacts, and the environmental advantages, juxtaposed with conventional fossil-fuel-based alternatives. While the aim of bio-based vitrimers aligns with sustainability and circularity principles, their economic feasibility is essential for gaining market competitiveness.Conventional polymers depend fundamentally on petroleum-based raw materials, whose prices are strictly connected with the fluctuations of the oil market and geopolitical uncertainties. In contrast, bio-based vitrimers utilize renewable materials such as starch, cellulose, lignin, vegetable oils, sugars, and agricultural byproducts. The cost of these biomass resources can vary, influenced by factors such as availability, seasonal shifts, location, and the difficulties related to their extraction and processing. Although certain biomass sources, such as agricultural wastes, are plentiful and inexpensive, others, like refined vegetable oils, may come at a higher price. This initial cost difference can frequently be offset by the later decrease in greenhouse gas emissions during the product's life cycle. Nevertheless, for the process to remain economically viable, it is essential that bio-based raw materials do not rival the food supply, highlighting the importance of utilizing agricultural waste or purpose-grown non-food crops. Studies on life cycle assessment (LCA) show that the cost of bio-based raw materials can vary widely based on their origin and processing techniques. Generally, these costs resemble those of polymers derived from petroleum, especially when factoring in waste management costs.23
Moving forward, synthesis and production costs must be considered. The synthesis of bio-based vitrimers involves chemical reactions such as polymerization, transesterification, imine exchange, or boronic ester bond formation. These are affected by the complexity of the reactions, the need for catalysts, the use of solvents, and the temperature and pressure conditions, among others. Production costs, which encompass labor, energy, equipment, and quality control, are also heavily influenced by the scalability of processes. To reduce these costs, research efforts are focused on optimizing the processes by substituting toxic catalysts with bio-based counterparts, using green solvents, or performing reactions in bulk or water.21 Studies indicate that more efficient, less energy-intensive processes can significantly lower synthesis and production costs. “Green chemistry” approaches and enzymatic catalysis are vital in this scenario.24
The practical utility of bio-based vitrimers hinges on achieving comparable or superior performance characteristics with conventional polymers. Important aspects for comparison encompass mechanical characteristics such as tensile strength and elongation at breaking point, thermal attributes like the glass transition temperature, along with long-term performance factors, including resistance to weathering, chemical substances, and biological influences. To justify increased costs, these materials need to provide benefits like enhanced durability, mechanical strength, and additional specific features like self-healing abilities. Comparative studies are, therefore, essential for substantiating these performance claims.
The economic and environmental advantages of bio-based vitrimers are significantly augmented by their potential for waste management and end-of-life strategies. Unlike most of their petroleum-based counterparts, bio-based vitrimers generally offer the possibility of biodegradability, composting, chemical recycling, or reuse. These processes reduce the environmental impact and can recover valuable resources from the materials. The costs of waste management are also generally lower for bio-based vitrimers due to their inherent material properties. A Life Cycle Assessment is therefore crucial in determining the overall impact by including costs related to waste disposal and recovery strategies.70–72
The transition to bio-based vitrimers also introduces substantial environmental and societal benefits. Employing renewable resources and low-impact methods can significantly lower greenhouse gas emissions, aiding in the fight against climate change. Biodegradability and the reduction in waste generation further contribute to lowering pollution. Furthermore, the expansion of bio-based technologies creates new job opportunities and fosters the circular economy, which is a significant driver in the current push for the transition to sustainable materials. Although such advantages are challenging to quantify directly, they are essential in justifying the use of bio-based vitrimers. Therefore, studies that quantify these benefits, such as LCA's that quantify carbon footprints, are also vital.22
The success of bio-based vitrimers is predicated on capitalizing on a growing market for sustainable materials, leveraging technological innovation, and obtaining support via favorable government incentives. They play a crucial part in a circular economy, which encourages the use of renewable resources. However, significant challenges exist. Scaling production from the laboratory to industrial levels is complex and requires significant investment and process optimization. To compete with petroleum-derived polymers, production costs must be lowered, and bio-based vitrimers should perform similarly to or better than conventional polymers. Establishing appropriate quality standards will facilitate the acceptance of these novel materials into the market.
In summary, even if a techno-economic analysis of bio-based vitrimers is a complex endeavor because of the multitude of interacting variables, these materials offer enormous promise for a more sustainable and circular polymer industry. By tackling the issues of expenses, efficiency, and scalability, while utilizing technological advancements and circular economy concepts, these materials will be equipped to compete successfully in the market, thereby significantly aiding in the pursuit of a more sustainable future.
Conclusions
The study of vitrimers, particularly those derived from bio-based sources, has ushered in a transformative era in the field of polymer science. This advancement is prominently reflected in several essential aspects, including mechanical efficacy, thermal stability, and the augmented potential for reshapeability and recyclability. Through the deliberate incorporation of dynamic covalent bonds within the molecular architecture of vitrimers, researchers have successfully developed materials that not only ensure good mechanical properties but also exceptional intrinsic self-healing capabilities, consequently prolonging their operational lifespan and utility. Moreover, vitrimers possess the capacity for facile reprocessing, presenting considerable benefits in the domains of material reclamation and reuse. The research underscores the remarkable adaptability of biobased vitrimers, unveiling a vast spectrum of prospective applications across diverse sectors. This adaptability emphasizes their potential as sustainable alternatives to conventional thermosetting polymers, which often present significant obstacles concerning environmental sustainability and recyclability. Indeed, traditional thermosetting polymers are frequently characterized by excessive rigidity and an inability to be reshaped post-curing, resulting in considerable waste and ecological issues. In contrast, vitrimers offer an innovative remedy that aligns with modern sustainability objectives. Furthermore, the capacity to meticulously tune crosslinking density—coupled with the utilization of biobased catalysts—has significantly improved the mechanical properties of these materials. This precise adjustment capability facilitates the optimization of material performance tailored to particular applications, ensuring that biobased vitrimers can fulfill the specifications of stringent environments. As research progresses, the potential of biobased vitrimers to transform the polymer industry becomes increasingly apparent, paving the way for more sustainable and ecologically responsible material solutions. The exceptional versatility exhibited by biobased vitrimers signifies a highly optimistic outlook across a diverse array of industrial domains, especially as the focus on performance properties and sustainability becomes increasingly pronounced. Considering the escalating global demand for eco-friendly materials, the ongoing advancements in the domain of vitrimers constitute a critical innovation in the pursuit of polymer solutions that are not only ecologically sound but also demonstrate superior efficiency and functionality. By promoting the recycling and reusability of substances, biobased vitrimers play a vital role in mitigating waste generation and preserving essential resources.While the discussion around biobased vitrimers highlights their potential for sustainability and circularity in polymer science and technology, it is essential to consider the limitations and challenges associated with their development and application. The production processes for biobased vitrimers may still rely on significant energy inputs and resource consumption, which could negate some of the environmental benefits they claim to offer. Furthermore, the scalability of biobased vitrimer production remains uncertain; transitioning from laboratory-scale synthesis to industrial-scale manufacturing presents numerous technical and economic hurdles that must be addressed. Furthermore, although biobased vitrimers are designed to mitigate the recycling challenges posed by conventional plastics, the presence of dynamic covalent bonds may introduce complexities in material behavior, raising concerns regarding their long-term stability and mechanical properties. The procurement of raw materials from biomass presents sustainability dilemmas, including alterations in land use and competition with agricultural and food supply chains. Consequently, while the enthusiasm for biobased materials is on the rise, it is imperative to adopt a balanced viewpoint, encompassing thorough life-cycle assessments to ascertain that they genuinely promote sustainability rather than merely redistributing environmental burdens.
Author contributions
A. M. and G. M. conceived the review work and equally drafted, reviewed, and edited the manuscript.Data availability
No primary research results, software, or code have been included, and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financed by the European Union – NextGenerationEU (National Sustainable Mobility Center CN00000023, Italian Ministry of University and Research Decree n. 1033 – 17/06/2022, Spoke 11 – Innovative Materials & Lightweighting). The opinions expressed are those of the authors only and should not be considered as representative of the European Union or the European Commission's official position. Neither the European Union nor the European Commission can be held responsible for them.References
- Plastics – the fast Facts 2023, Plastics Europe, https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/.
- A. Kapoor, M. Raghunathan, B. Lal, P. Kumar, N. Srivastava, G. L. Devnani and D. B. Pal, Chemosphere, 2024, 364, 143279 CrossRef.
- S. Mushtaq, F. Jamil, A. Inayat, C. Ghenai and A. Shanableh, Curr. Opin. Green Sustainable Chem., 2024, 49, 100950 CrossRef.
- H. Sardon and Z.-C. Li, Polym. Chem., 2020, 11, 4828–4829 RSC.
- B. von Vacano, H. Mangold, G. W. M. Vandermeulen, G. Battagliarin, M. Hofmann, J. Bean and A. Künkel, Angew. Chem., Int. Ed., 2022, 135(12), e202210823 CrossRef.
- T. Yan, A. H. Balzer, K. M. Herbert, T. H. Epps and L. T. J. Korley, Chem. Sci., 2023, 14, 5243–5265 RSC.
- Y. Liu, Z. Yu, B. Wang, L. Pengyun, J. Zhu and S. Ma, Green Chem., 2022, 24, 5691–5708 RSC.
- J. Zheng, Z. M. Png, S. H. Ng, G. X. Tham, E. Ye, S. S. Goh, X. J. Loh and Z. Li, Mater. Today, 2021, 51, 586–625 CrossRef.
- S. Wang, B. Li, J. Zheng, N. E. B. Surat’man, J. Wu, N. Wang, X. Xu, J. Zhu, X. J. Loh and Z. Li, ACS Mater. Lett., 2023, 5, 608–628 CrossRef.
- D. Montarnal, M. Capelot, F. Tournilhac and L. Leibler, Science, 2011, 334, 965–968 CrossRef PubMed.
- P. Chakma and D. Konkolewicz, Angew. Chem., Int. Ed., 2019, 131, 9784–9797 CrossRef.
- W. Denissen, J. M. Winne and F. E. Du Prez, Chem. Sci., 2016, 7, 30–38 RSC.
- B. Krishnakumar, R. V. S. P. Sanka, W. H. Binder, V. Parthasarthy, S. Rana and N. Karak, Chem. Eng. J., 2020, 385, 123820 CrossRef.
- L. Leibler, Mater. Horiz., 2019, 6, 430–436 Search PubMed.
- D. B. Tiz, F. A. Vicente, A. Kroflič and B. Likozar, ACS Sustainable Chem. Eng., 2023, 11, 13836–13867 CrossRef.
- A. Jourdain, R. Asbai, O. Anaya, M. M. Chehimi, E. Drockenmuller and D. Montarnal, Macromolecules, 2020, 53, 1884–1900 CrossRef.
- S. Tripathi, S. H. and S. Bose, SPE Polym., 2024, 5(1), 95–111 CrossRef.
- S. Sadashiv Rege, I. Dey, R. Sen Gupta, M. Ajnas N, R. Jose, A. Misra, K. Manna, K. Samanta and S. Bose, Eur. Polym. J., 2024, 220, 113451 CrossRef.
- S. Kamarulzaman, Z. M. Png, E. Q. Lim, I. Z. S. Lim, Z. Li and S. S. Goh, Chem, 2023, 9, 2771–2816 Search PubMed.
- D. B. Tiz, F. A. Vicente, A. Kroflič and B. Likoza, ACS Sustainable Chem. Eng., 2023, 11, 13836–13867 CrossRef.
- X.-L. Zhao, P. Tian, Y.-D. Li and J.-B. Zeng, Green Chem., 2022, 24, 4363–4387 RSC.
- M. A. Lucherelli, A. Duval and L. Avérous, Prog. Polym. Sci., 2022, 127, 101515 CrossRef.
- G. Zhang, C. Tian, H. Chu, J. Liu, B. Guo and L. Zhang, J. Mater. Chem. A, 2023, 11, 25356–25367 RSC.
- Y. Liu, M.-L. Wu, Y.-D. Li, L.-Y. Li and J.-B. Zeng, ACS Sustainable Chem. Eng., 2023, 11, 17190–17198 CrossRef.
- L. Wang, Y. Liu, Y. Wei, W. Zeng, Z. Cui and A. Du, Eur. Polym. J., 2023, 193, 112101 CrossRef.
- J. Zheng, Z. M. Png, X. C. N. Quek, X. J. Loh and Z. Li, Green Chem., 2023, 25, 8903–8934 RSC.
- C. Allen, G. Metternicht and T. Wiedmann, Sustain. Sci., 2018, 13, 1453–1467 CrossRef.
- Y. Jin, Z. Lei, P. Taynton, S. Huang and W. Zhang, Matter, 2019, 1, 1456–1493 CrossRef.
- J. S. Park, T. Darlington, A. F. Starr, K. Takahashi, J. Riendeau and H. Thomas Hahn, Compos. Sci. Technol., 2010, 70, 2154–2159 CrossRef.
- X. Chen, Science, 2002, 295, 1698–1702 CrossRef PubMed.
- M. Capelot, M. M. Unterlass, F. Tournilhac and L. Leibler, ACS Macro Lett., 2012, 1, 789–792 CrossRef PubMed.
- S. Debnath, S. Kaushal and U. Ojha, ACS Appl. Polym. Mater., 2020, 2, 1006–1013 CrossRef.
- P. Fanlo, A. Ruiz de Luzuriaga, G. Albizu, M. Ximenis, A. Rekondo, H. J. Grande and H. Sardon, RSC Appl. Polym., 2024, 2, 826 RSC.
- C. Luo, W. Wang, W. Yang, X. Liu, J. Lin, L. Zhang and S. He, ACS Sustainable Chem. Eng., 2023, 11, 14591–14600 CrossRef.
- J. J. Cash, T. Kubo, A. P. Bapat and B. S. Sumerlin, Macromolecules, 2015, 48, 2098–2106 CrossRef.
- X. Zhang, S. Wang, Z. Jiang, Y. Li and X. Jing, J. Am. Chem. Soc., 2020, 142, 21852–21860 CrossRef PubMed.
- M. E. Belowich and J. F. Stoddart, Chem. Soc. Rev., 2012, 41, 2003 RSC.
- H. Geng, Y. Wang, Q. Yu, S. Gu, Y. Zhou, W. Xu, X. Zhang and D. Ye, ACS Sustainable Chem. Eng., 2018, 6, 15463–15470 CrossRef.
- K. Liang, G. Zhang, J. Zhao, L. Shi, J. Cheng and J. Zhang, ACS Sustainable Chem. Eng., 2021, 9, 5673–5683 CrossRef.
- L. Pettazzoni, F. Leonelli, A. Martinelli, L. M. Migneco, S. Alfano, D. Di Luca, L. Celio and V. Di Lisio, J. Appl. Polym. Sci., 2022, 139, 52408 CrossRef.
- J. Li, B. Ju and S. Zhang, Ind. Crops Prod., 2023, 205, 117466 CrossRef.
- T. Telatin, S. De la Flor, À. Serra and X. Montané, Polym. Test., 2024, 135, 108465 CrossRef.
- J. Wu, X. Yu, H. Zhang, J. Guo, J. Hu and M.-H. Li, ACS Sustainable Chem. Eng., 2020, 8, 6479–6487 CrossRef.
- S. Zhao, D. Wang and T. P. Russell, ACS Sustainable Chem. Eng., 2021, 9, 11091–11099 CrossRef.
- A. Zych, J. Tellers, L. Bertolacci, L. Ceseracciu, L. Marini, G. Mancini and A. Athanassiou, ACS Appl. Polym. Mater., 2021, 3, 1135–1144 CrossRef.
- A. Moreno, M. Morsali and M. H. Sipponen, ACS Appl. Mater. Interfaces, 2021, 13, 57952–57961 CrossRef.
- A. Adjaoud, L. Puchot and P. Verge, ACS Sustainable Chem. Eng., 2021, 10, 594–602 CrossRef.
- L. Zhong, Y. Hao, J. Zhang, F. Wei, T. Li, M. Miao and D. Zhang, Macromolecules, 2022, 55, 595–607 CrossRef.
- H. Tong, Y. Chen, Y. Weng and S. Zhang, ACS Sustainable Chem. Eng., 2022, 10, 7942–7953 CrossRef.
- M. A. Rashid, S. Zhu, Q. Jiang, Y. Wei and W. Liu, ACS Appl. Polym. Mater., 2023, 5, 279–289 CrossRef.
- X. Yang, Y. Ke, Q. Chen, L. Shen, J. Xue, R. L. Quirino, Z. Yan, Y. Luo and C. Zhang, J. Cleaner Prod., 2022, 333, 130043 CrossRef.
- M. A. Rashid, M. Mian, Y. Wei and W. Liu, Mater. Today Commun., 2023, 35, 106178 CrossRef.
- C. Monteserin, M. Blanco, N. Uranga, J. Sanchez, J. M. Laza, J. L. Vilas and E. Aranzabe, Polymer, 2023, 285, 126339 CrossRef.
- Y. Wang, B. Jin, D. Ye and Z. Liu, Eur. Polym. J., 2022, 162, 110927 CrossRef.
- S. Tang, H. Lin, K. Dong, J. Zhang and C. Zhao, Polym. Degrad. Stab., 2023, 210, 110298 CrossRef.
- P. Zamani, O. Zabihi, M. Ahmadi, M. Zamani, M. Jalal Zohuriaan-Mehr, T. Kannangara, P. Joseph and M. Naebe, Composites, Part A, 2024, 179, 108016 CrossRef.
- J. Zhang, C. Zhang, Q. Shang, Y. Hu, F. Song, P. Jia, G. Zhu, J. Huang, C. Liu, L. Hu and Y. Zhou, Eur. Polym. J., 2022, 169, 111133 CrossRef.
- L. Xiao, W. Li, Z. Liu, K. Zhang, S. Li, Y. Wang, J. Chen, J. Huang and X. Nie, ACS Sustainable Chem. Eng., 2022, 10, 9829–9840 CrossRef.
- P. Li, J. Zhang, J. Ma, C.-A. Xu, X. Liang, T. Yuan, Y. Hu and Z. Yang, Ind. Crops Prod., 2023, 204, 117288 CrossRef.
- J. Stouten, M. K. N. de Roy and K. V. Bernaerts, Mater. Today Sustain., 2023, 22, 100396 Search PubMed.
- S. Ren, Z. Li, W. Zhou, J. Zhu, Y. Zhao, C. Liu, H. Fang and Y. Ding, Ind. Crops Prod., 2023, 206, 117738 CrossRef.
- Y. Zhang, S. Zhang, M. Zhai, B. Wei, B. Lyu and L. Liu, ACS Appl. Polym. Mater., 2024, 6, 8399–8408 CrossRef.
- S. Salaeh, B. Thongnuanchan, Y. Bueraheng, A. Das, N. H. M. Kaus and S. Wießner, Eur. Polym. J., 2023, 198, 112422 CrossRef.
- H.-H. Liu, Y. Ma, Y.-H. Zhou and G.-D. Feng, ACS Omega, 2024, 9, 36497–36508 Search PubMed.
- Y.-Y. Liu, G.-L. Liu, Y.-D. Li, Y. Weng and J.-B. Zeng, ACS Sustainable Chem. Eng., 2021, 9, 4638–4647 CrossRef.
- M. Qiu, S. Wu, S. Fang, Z. Tang and B. Guo, J. Mater. Chem. A, 2018, 6, 13607–13612 RSC.
- W. Zhang, F. Gao, X. Chen, L. Shen, Y. Chen and Y. Lin, ACS Sustainable Chem. Eng., 2023, 11(7), 3065–3073 CrossRef.
- M. Gosecki, M. Urbaniak, C. Makarewicz and M. Gosecka, ACS Sustainable Chem. Eng., 2024, 12, 3841–3850 CrossRef.
- B. Sölle, D. Reisinger, S. Heupl, A. Jelinek, S. Schlögl and E. Rossegger, React. Funct. Polym., 2024, 202, 105972 CrossRef.
- I. Bianchi, L. Greco, C. Mignanelli, M. Simoncini and A. Vita, Procedia CIRP, 2024, 122, 1059–1064 CrossRef.
- P. Zamani, O. Zabihi, M. Ahmadi, M. Zamani, M. Jalal Zohuriaan-Mehr, T. Kannangara, P. Joseph and M. Naebe, Composites, Part A, 2024, 179, 108016 CrossRef.
- A. Enayati-Gerdroodbar, A. Khayati, A. Zolfagharian, M. Salami-Kalajahi and M. Bodaghi, Smart Mater. Methods, 2024, 1, 1–43 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |


