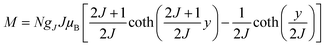Open Access Article
Open Access ArticleSynthesis, optical, electronic and magnetic studies of air-stable chiral Cu(II) chlorides†
Ali
Azmy
a,
Nivarthana W. Y. A. Y.
Mudiyanselage
b,
Kamal E. S.
Nassar
a,
Mike
Pham
a,
Nourdine
Zibouche
 c,
Manh-Huong
Phan
c,
Manh-Huong
Phan
 b and
Ioannis
Spanopoulos
b and
Ioannis
Spanopoulos
 *a
*a
aDepartment of Chemistry, University of South Florida, Tampa, Florida 33620, USA. E-mail: spanopoulos@usf.edu
bDepartment of Physics, University of South Florida, Tampa, Florida 33620, USA
cDepartment of Chemistry, University of Lancaster, Lancaster, LA1 4YW, UK
First published on 13th September 2024
Abstract
Chiral magnetic metal halide semiconductors (MHSs) have recently emerged as a unique platform of hybrid materials for state-of-the-art applications, where both chiroptical and magnetic properties are essential. Motivated by the scarcity of MHS compounds that feature the latter traits and the absence of relevant structure–property relationships, we present here the synthesis and the optical, electronic, and magnetic properties of two new polar 0D, Cu(II) based materials, namely (S-/R-THBTD)2CuCl6, where THBTD = 4,5,6,7-tetrahydro-benzothiazole-2,6-diamine. Both enantiomers exhibit direct and indirect optical bandgap characteristics based on UV-vis and density functional theory (DFT) calculations, while circular dichroism (CD) measurements confirmed their chiral character. Magnetometry measurements revealed a notable transition from a paramagnetic to a ferromagnetic-like state at around 4 K, with a low-temperature saturation magnetization value of up to 9.86 emu g−1, which is among the highest reported for chiral magnetic MHSs. Notably, (S-/R-THBTD)2CuCl6 maintain their structural integrity and magnetic and optical properties (based on UV-vis studies) after one year of air exposure, a record stability performance among chiral magnetic MHSs. This work proves that air-stable MHSs with competitive chiroptical and magnetic properties can be acquired by properly selecting suitable templating agents, paving the way for further materials development.
Introduction
Chiral materials are the basis of chiral photonics and are exploited in various applications, including quantum communication,1 spin-based transistors,2 spin valves,3 magnetic random-access memory (MRAM),4 and photonic artificial synapses (PAS).5 Their performance is ascribed to a unique combination of attributes, including circular dichroism (CD), circularly polarized photoluminescence (CPL), nonlinear optical (NLO) properties, and the bulk photovoltaic effect (BPVE).6–8 Combining chirality and magnetism within the same material gives access to more intricate applications, such as spin–orbit torque memory devices9 and magneto-optical modulators.10 Therefore, the development of chiral magnetic semiconductor materials is greatly sought after.To this end, hybrid (organic–inorganic) metal halide semiconductors (MHSs) represent a versatile solution for the aforementioned demanding applications. Evidently, by means of molecular and crystal engineering, it is possible not only to introduce chirality and magnetism within the same crystal structure but also to fine-tune the corresponding optoelectronic and magnetic properties. Furthermore, MHSs feature a plethora of traits, such as long spin lifetimes,7 long diffusion lengths,11 high carrier mobility,12 strong spin–orbit coupling (SOC),13 and large Rashba/Dresselhaus (R/D) splitting,14,15 which are difficult to be encountered altogether in other classes of fully organic or inorganic semiconductors.
Incorporating chirality into MHSs can generally be achieved by utilizing chiral organic molecules as counter cations and structure-directing agents, giving rise to non-centrosymmetric structural motifs.16 Moreover, their hybrid nature allows the use of transition-metal ions, such as Cr(II), Cu(II), Mn(II), Fe(II), Co(II), and Ni(II), thus promoting the acquisition of chiral, polar ferromagnetic or ferroelectric MHSs.17–19
Despite the exquisite properties of chiral magnetic transition metal halides, there are a limited number of materials reported, evaluating both their magnetic and chiro-optical properties. In particular, in 2020 Sun et al. reported two copper-based metal halides, namely (R-MPEA)2CuCl4 and (S-MPEA)2CuCl4 (R-/S-MPEA = R-/S-(+)-β-methylphenethylammonium), revealing strong magneto-chiral anisotropy,20 Xue et al. reported a new layered chiral iron double perovskite with magnetic ordering with the formula (R-MPA)4AgFeCl8, where R-MPA is R-(+)-β-methylphenethylammonium21 and Panda et al. reported two manganese halides based on 1,4-diazabicyclo[2.2.2]octane (DABCO) with the formula (H2DABCO)MnX4·4H2O (X = Cl and Br) that crystallized in the P212121 space group and exhibited the magnetocaloric effect at low temperatures.22 In 2021, Taniguchi et al. evaluated the magnetoelectric effect in (R-/S-MPA)2CuCl4,17 while in 2022, they developed two chiral manganese chloride enantiomers [R-/S-MPA]2[MnCl4(H2O)] featuring weak ferromagnetic properties.23 Ai et al. synthesized a 2D Cr2+-based compound, namely ([DFCBA]2CrCl4], (DFCBA = 3,3-difluor-ocyclobutyslammonium), which exhibited both ferroelectricity and ferromagnetism,24 while Lu et al. shed light on the chiroptical and magnetic properties of (R-/S-MBA)3Ru2Br9 (MBA = methylbenzylammonium).18
On the road to device assembly and commercialization,25 the long-term environmental stability of chiral magnetic MHSs is crucial and must be evaluated. Although there are multiple reports on the air stability of chiral lead-free MHSs,26 there is almost a complete absence of information on the air stability of chiral magnetic MHSs.
Motivated by the above concerns, we report here the synthesis and chiro-optical and magnetic properties of two new 0D Cu(II) chloride compounds, namely (S-/R-THBTD)2CuCl6 (THBTD = 4,5,6,7-tetrahydro-benzothiazole-2,6-diamine), which have been air stable for a year so far. Both compounds crystallize in the triclinic space group P1, diffuse reflectance CD (DRCD) verified the chiral nature of the two structures, while magnetic studies revealed a transition from the paramagnetic to a ferromagnetic-like state at ∼4 K, with a low-temperature saturation magnetization value of up to 9.86 emu g−1, among the highest reported for chiral magnetic MHSs.
Results and discussion
Synthetic aspects and structural characterization
Green block crystals of (S-/R-THBTD)2CuCl6 (R or S) were acquired through the reaction of copper(II) chloride with the chiral ligand in a hot HCl solution. When the concentration of the organic linker varies from 0.25 M to 0.1 M, the size of the crystals can be tuned from 30 μm to 0.3 cm (Fig. S1†). Scanning electron microscopy (SEM) studies revealed the formation of well-faceted block crystals, while energy dispersive spectroscopy (EDS) measurements confirmed the atomic ratio between Cu and Cl to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, consistent with the proposed formula (Fig. S2†).
6, consistent with the proposed formula (Fig. S2†).
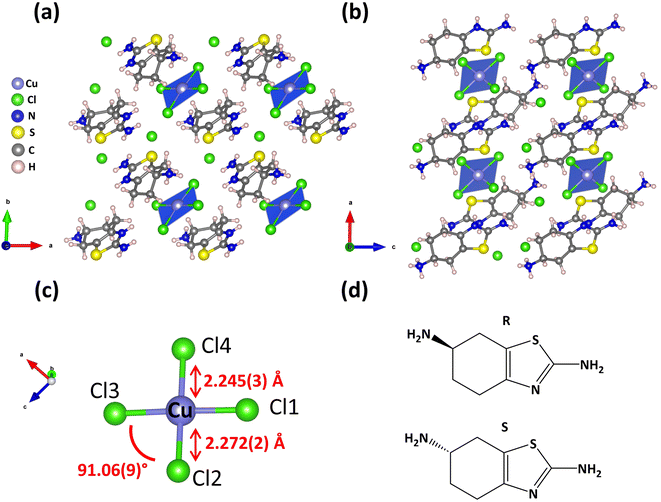
Single crystal XRD studies demonstrate that both enantiomers are isostructural, crystalizing in the polar triclinic space group P1 (Table 1). The structure consists of isolated square-planar [CuCl4]2− moieties separated and charge-balanced by two Cl− and two double protonated THBTD ligands (Fig. 1a and b). Under current synthetic conditions only two of the three amine groups are protonated, the aliphatic primary amine (R–NH3) and the aromatic secondary amine (–NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ). The corresponding chiral organic cations feature an antiparallel brick-wall configuration and are eclipsed along the c-axis, lying at a distance of 3.2 and 3.6 Å along the c and b-axes, respectively.
). The corresponding chiral organic cations feature an antiparallel brick-wall configuration and are eclipsed along the c-axis, lying at a distance of 3.2 and 3.6 Å along the c and b-axes, respectively.
| Compound | (R-THBTD)2CuCl6 at 296 K | (S-THBTD)2CuCl6 at 296 K | (S-THBTD)2CuCl6 at 100 K | (S-THBTD)2CuCl6 at 400 K |
|---|---|---|---|---|
| a R = Σ‖Fo| − |Fc‖/Σ|Fo|, wR = {Σ[w(|Fo|2 − |Fc|2)2]/Σ[w(|Fo|4)]}1/2 and w = 1/[σ2(Fo2) + (0.0247P)2 + 0.3469P], where P = (Fo2 + 2Fc2)/3. | ||||
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | P1 | P1 | P1 | P1 |
| Unit cell dimensions | a = 7.9289(3) Å, α = 80.228(2)° | a = 7.9425(8) Å, α = 80.3380(10)° | a = 7.9251(6) Å, α = 81.219(2)° | a = 7.9476(10) Å, α = 79.779(7)° |
| b = 8.7451(3) Å, β = 88.9030(10)° | b = 8.6593(8) Å, β = 88.8210(10)° | b = 8.5677(6) Å, β = 88.198(2)° | b = 8.8059(12) Å, β = 89.351(7)° | |
| c = 8.8198(3) Å, γ = 87.605(2)° | c = 8.8291(9) Å, γ = 87.3220(10)° | c = 8.7377(7) Å, γ = 86.916(2)° | c = 8.8600(11) Å, γ = 88.008(7)° | |
| Volume | 602.11(4) Å3 | 597.92(10) Å3 | 585.32(8) Å3 | 609.86(14) Å3 |
| Z | 1 | 1 | 1 | 1 |
| Independent reflections | 4308 [Rint = 0.0258] | 4339 [Rint = 0.0217] | 3970 [Rint = 0.0362] | 4245 [Rint = 0.0445] |
| Completeness to θ = 25.242° | 99.2% | 99.9% | 98.3% | 99.3% |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 4308/3/265 | 4339/3/265 | 3970/3/265 | 4245/3/265 |
| Goodness-of-fit | 1.014 | 1.070 | 1.055 | 1.056 |
| Final R indices [I > 2σ(I)] | R obs = 0.0290, wRobs = 0.0754 | R obs = 0.0217, wRobs = 0.0625 | R obs = 0.0240, wRobs = 0.0615 | R obs = 0.0339, wRobs = 0.0954 |
| R indices [all data] | R all = 0.0327, wRall = 0.0797 | R all = 0.0227, wRall = 0.0633 | R all = 0.0241, wRall = 0.0616 | R all = 0.0432, wRall = 0.1005 |
| Largest diff. peak and hole | 1.125 and −0.291 e·Å−3 | 0.380 and −0.256 e·Å−3 | 0.301 and −0.463 e·Å−3 | 0.479 and −0.345 e·Å−3 |
Having a closer look at the R analog, we observe that the corresponding Cu–Cl bond length ranges between 2.245(3) and 2.272(2) Å (Table S3† and Fig. 1c). It is pointed out that these Cu(II)–Cl bond lengths are consistent with previously reported 0D Cu(II) chlorides, such as (R-/S-MBA)2CuCl4 with values ranging from 2.234 to 2.262 Å, (R-/S-NEA)2CuCl4 (NEA = α-naphthylethylamine) featuring bond lengths spanning from 2.2380 to 2.2592 Å and (R-/S-)-(FE)2CuCl4 (FE![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) (4-fluorophenyl)ethylamine) exhibiting Cu–Cl values varying from 2.240 to 2.267 Å, albeit at 100 K.27–30 There is a miniscule asymmetric distortion in [CuCl4]2− deriving from the deviation of Cl–Cu–Cl angles from the ideal value of 90° for square planar moieties. Two of the corresponding bond angles lie slightly below the latter value (89.02° and 89.97°), while the other two angles lie above it (90.25° and 91.06°) (Table S4†).
(4-fluorophenyl)ethylamine) exhibiting Cu–Cl values varying from 2.240 to 2.267 Å, albeit at 100 K.27–30 There is a miniscule asymmetric distortion in [CuCl4]2− deriving from the deviation of Cl–Cu–Cl angles from the ideal value of 90° for square planar moieties. Two of the corresponding bond angles lie slightly below the latter value (89.02° and 89.97°), while the other two angles lie above it (90.25° and 91.06°) (Table S4†).
Interestingly, the coordination environment of Cu(II) in (S-/R-THBTD)2CuCl6 is quite uncommon for 0D Cu(II) halides, featuring usually a tetrahedral geometry, as in the case of (R/S)-(MBA)2CuCl4.31 Apparently, for (R-THBTD)2CuCl6 in particular, the number and magnitude of hydrogen bonds in-plane strongly impose a square planar coordination geometry. There are multiple interactions among the hydrogen atoms of the three amine groups of the ligand, the aliphatic primary amine (R–NH3), the aromatic primary amine (R–NH2) and the aromatic secondary amine (–NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), and the Cl atoms of the [CuCl4]2− moieties. The corresponding bond distances (N–H⋯Cl) feature values spanning from 2.39 Å to 2.71 Å, signaling moderate to strong hydrogen bonding interactions (Fig. S3†). On the other hand, the out-of-plane hydrogen bonding interactions are much weaker, spanning from 3.14 to 3.37 Å, thus impacting to a much smaller extent the coordination geometry of Cu(II) ions.
), and the Cl atoms of the [CuCl4]2− moieties. The corresponding bond distances (N–H⋯Cl) feature values spanning from 2.39 Å to 2.71 Å, signaling moderate to strong hydrogen bonding interactions (Fig. S3†). On the other hand, the out-of-plane hydrogen bonding interactions are much weaker, spanning from 3.14 to 3.37 Å, thus impacting to a much smaller extent the coordination geometry of Cu(II) ions.
The absence of symmetry elements dictates a triclinic P1 space group, which prompted us to explore whether there are underlying structural phase transitions that would lift the non-centrosymmetric nature.32 Evidently, variable-temperature XRD studies at 100 K and 400 K revealed that the structure maintains its polar nature, as (S-THBTD)2CuCl6 crystallizes in the P1 space group in both cases (Table 1). This performance is quite uncommon for hybrid metal halide materials, which feature multiple phase transitions within the examined temperature range (100 K–400 K).33 We observed only a gradual increase in the unit cell volume due to thermal expansion (from 585.32(8) Å3 at 100 K to 609.86(14) Å3 at 400 K).
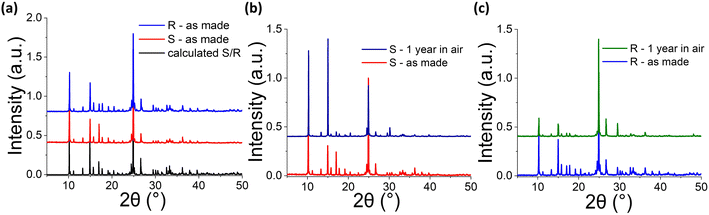
In-house powder X-ray diffraction (PXRD) studies confirmed the uniform phase purity of the as-made crystals since experimental PXRD patterns and calculated ones from single crystal XRD studies are identical. Furthermore, (R-/S-THBTD)2CuCl6 have maintained their structural integrity in air for a year so far. After 12 months of air exposure, the experimental PXRD patterns of the air-treated materials are identical to those of the freshly made ones (Fig. 2 and S4†). There is no appearance of additional, unpredicted from the crystal structure diffraction peaks, indicative of structure degradation. Single crystal XRD studies of the 1 year air-treated (R-THBTD)2CuCl6 analog (Tables S17–21†) corroborate the above results. A comparison of the PXRD patterns for the 1 year air-treated (R-THBTD)2CuCl6 compound with the calculated pattern derived from the corresponding solved single-crystal structure reveals no changes (Fig. S5†), confirming phase purity. It is pointed out that this is record air stability performance for chiral magnetic MHSs.30,34 We ascribe this to the 0D nature of the structure, where the [CuCl4]2− moieties are shielded from incoming H2O molecules due to the presence of the bulky, hydrophobic THBTD counter cations. In general, hybrid Cu halides exhibit improved air stability as compared to fully inorganic ones due to the presence of the organic part of the structure. For example, Rb2CuCl3 degrades within a day in moist air,35 while (R/S-MBA)2CuX4 (X = Cl, Br) are air-stable for 1 month.30 Degradation products include the formation of hydrates (e.g., Rb2CuCl4·2H2O). Therefore, any strategy aiming at enhancing the hydrophobic nature of the material will improve air/moisture stability.
Thermogravimetric analysis (TGA) revealed that (R-/S-THBTD)2CuCl6 are thermally stable up to 181 °C. There are two decomposition steps at ∼181 °C and ∼293 °C (Fig. S6a†). The first weight loss (∼35%) corresponds to the decomposition of the organic part of the structure and HCl, while the second step (∼65%) is ascribed to the decomposition of the inorganic metal halide.
The corresponding thermal stability behavior is consistent with other reported Cu(II) metal halides.22,36,37 Moreover, differential scanning calorimetry (DSC) measurements support the absence of phase transitions in the 25–180 °C range, further corroborating the XRD studies (Fig. S6b†).
DFT studies
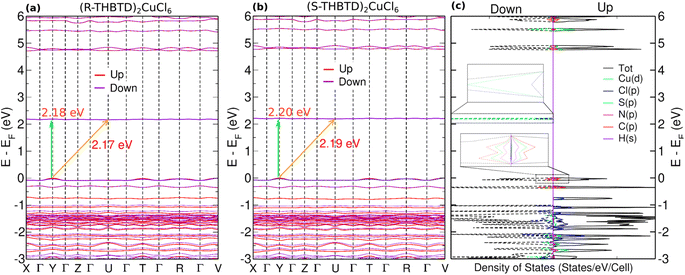
In order to elucidate the electronic structure of the new compounds, first-principles DFT calculations were performed (the details of the calculations are given in the ESI†). The optimized structural parameters are in good agreement with the corresponding experimental values. Specifically, for both R/S systems, the lattice parameters a are slightly elongated with values of 0.08%/0.12%, whereas the b and c parameters are shortened by −2.2%/−1.5% and −0.3%/−0.4%, respectively. Similarly, the angles α are enlarged by 0.7%/1.3%, whereas the angles β and γ are contracted by −1.3%/−1.3% and −1.1%/−0.6%, for R/S systems, respectively. The calculated Cu–Cl2/Cu–Cl4 bond lengths, as shown in Fig. 1c, are found to be 2.303 Å/2.293 Å, respectively, whereas the corresponding angle is found to be 91.38°.The systems are shown to exhibit ferromagnetic-like characteristics at low temperatures based on magnetometry studies. We have calculated the electronic band structures for (R-/S-THBTD)2CuCl6, in the ferromagnetic state, as plotted in Fig. 3a and b. The highest valence band (VBM) for each system is slightly dispersed, whereas the lowest conduction band (CBM) is entirely flat, given the 0D nature of these systems. Both materials exhibit an indirect character for the fundamental band gap with values of 2.17 and 2.19 eV for (R-THBTD)2CuCl6 and (S-THBTD)2CuCl6, respectively. The corresponding band gap values feature a small difference of 0.02 eV and are in good agreement with the measured optical band gaps of the two materials, as discussed below. The direct transitions of the (R-/S-THBTD)2CuCl6 materials are found at the Y points of the Brillouin zone with values of 2.18 and 2.20 eV, for the R and S analogs, respectively, as shown in Fig. 3. Direct bandgap values lie at slightly higher energy than the indirect ones, closely matching the experimental trend from Tauc plot analysis (see the next section). It is pointed out that direct transitions from the highest valence band to the lowest conduction band may also occur in different regions of the Brillouin zone due to the flatness of the bands. The density of states (DOS) plotted in Fig. 3c for the (S-THBTD)2CuCl6 system exhibits a sharp peak structure due to the zero dimensionality of the system. The partial density of states (PDOS) (and the band structures) show that both the spin-up and spin-down states contribute to the VBM, which is mainly dominated by the hybridization of the p orbitals of the C, N, and S atoms composing the organic molecule, whereas only the spin-down states contribute to the CBM that is primarily composed of the d and p orbitals of Cu and Cl elements.
Optical properties
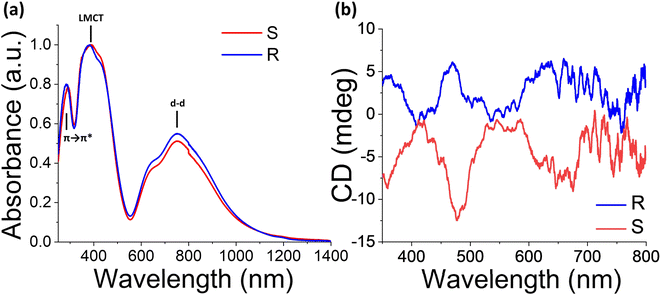
Utilization of the Kubelka–Munk equation (see the ESI†) allowed the determination of the optical absorbance from UV-vis diffuse reflectance studies (Fig. S7†). The absorption spectra of (R-/S-THBTD)2CuCl6 display three major peaks, a narrow one at ∼285 nm which is associated with the π → π* transition of THBTD (Fig. S8†), a broad one at ∼390 nm which is attributed to the Cl to metal charge transfer (LMCT) and a broader vis-NIR at ∼752 nm which can be attributed to Laporte forbidden Cu(II) d–d transitions (Fig. 4a).28,31,38,39 Tauc plot analysis reveals both direct and indirect bandgaps associated with CT transitions. Apparently, for the indirect transition (Fig. S9c and d†), the corresponding bandgap values are 2.21 and 2.18 eV for the S and R analogs, while for the direct transition, the determined values are 2.53 and 2.50 eV for the S and R analogs, respectively (Fig. S9a and b†). The latter trend is consistent with the DFT band structure calculations. Prominently, both enantiomers show only miniscule changes in their optical absorption spectra after 1 year of air exposure, showcasing the robustness of the optical properties (Fig. S10†). The corresponding differences can be explained by examining closely the single crystal structure of the 1 year air-treated (R-/S-THBTD)2CuCl6 sample to the freshly prepared one. Apparently, the only noticeable difference is a slight elongation of the Cu–Cl bond lengths for the air-treated sample (see Table S22†), expanding from 2.247(2) and 2.272(2) Å to 2.2531(14) and 2.2761(17) Å for the fresh and air treated ones, respectively. This difference is potentially responsible for the minuscule shift in the corresponding LMCT peak in the UV-vis spectrum (Fig. S10a†) to higher energy values (from 2.88 eV to 2.93 eV), derived from the reduction in the Cu–Cl orbital overlap due to the bond elongation. In the same regard, the decrease in the relative intensity for the d–d transitions (1.65 eV) for the air-treated samples compared to the fresh ones is also ascribed to the increase in the distance of the Cl ligand from the metal center, thus inducing a decrease in the d–d transition probability.Diffuse reflectance CD (DRCD) measurements on the as-made powder samples of (S-/R-THBTD)2CuCl6 reveal mirrored CD signals, confirming the non-centrosymmetric nature of the materials. The CD spectra of the two enantiomers feature multiple peaks at ∼660, 470, and 360 nm, which are correlated to the CT and d–d transitions of the absorbance spectra. Completely different CD spectra for the hybrid semiconductors versus the chiral molecules S-THBTD and R-THBTD indicate that the corresponding CD signals do not derive from the states of the chiral organic cations.40 For determining the anisotropy factor (gCD), the following equation was utilized:
Magnetic properties
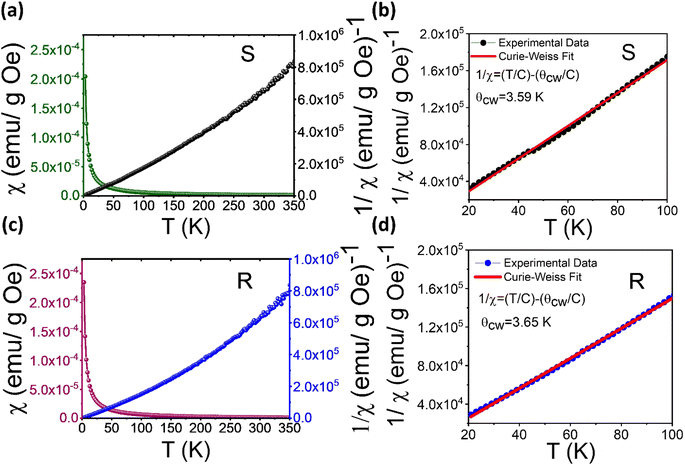
To characterize the magnetic properties of the samples, we evaluated the temperature-dependent magnetic susceptibility (χ). In Fig. 5a and c, the observed χ increases with decreasing temperature under a 0.1 T static magnetic field, as thermal fluctuations become less potent. It is worth noticing that χ increases sharply at temperatures below 6 K. This might imply that the material undergoes a transition from the paramagnetic (PM) to a ferromagnetic (FM)-like state below 6 K. The Curie–Weiss equation (shown in Fig. 5b and d), capturing the magnetic susceptibility of a material in the paramagnetic region, was utilized to fit the experimental data for the temperature-dependent inverse susceptibility (Fig. 5a and c). As shown in Fig. 5b and d, the fitting focused on the temperature range of 20 K to 100 K, revealing a linear behavior of χ with respect to change in temperature. It is noticed here that we have found a negligible effect of diamagnetic correction from ligands present in the samples on the fit of the temperature-dependent susceptibility data.The Curie–Weiss temperatures (θCW) serve as approximate indicators of the strength of magnetic correlations between magnetic ions, with higher θCW values suggesting stronger magnetic correlations. The positive sign of θCW implies FM interactions, while its negative sign refers to antiferromagnetic (AFM) interactions at T < θCW. The θCW values obtained from the fitting results (Fig. 5b and d) for (S-THBTD)2CuCl6 and (R-THBTD)2CuCl6 are 3.59 K and 3.65 K, respectively. The positive values of θCW seem to suggest the existence of FM-like couplings at T < θCW. From the obtained fit parameters (Curie constant values, C = 5.62 × 10−4 emu K Oe−1 g−1 for (S-THBTD)2CuCl6 and C= 6.44 × 10−4 emu K Oe−1 g−1 for (R-THBTD)2CuCl6), the effective magnetic moment (μeff) values are also calculated to be 1.67 μB and 1.78 μB for (S-THBTD)2CuCl6 and (R-THBTD)2CuCl6, respectively, using the relationship: 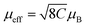 in cgs, where μB is the Bohr magneton.
in cgs, where μB is the Bohr magneton.
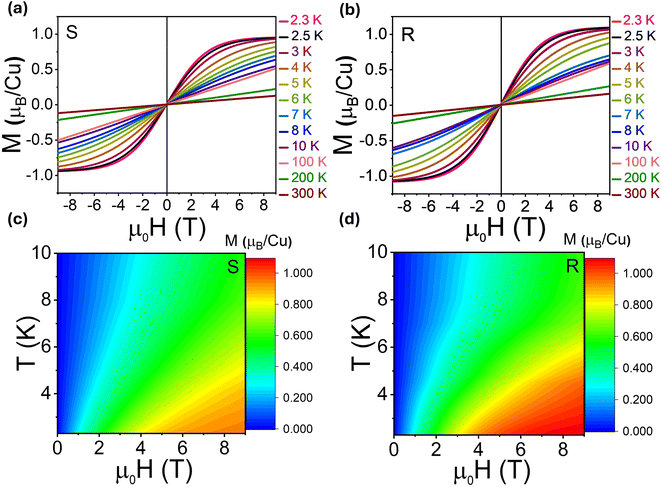
We note that while the χ−1(T) curve may not distinctly deviate from linearity due to the onset of FM-like ordering at low temperatures, the combination of the χ−1(T) fit and field-dependent magnetization data suggests a deviation from purely paramagnetic behavior. Fig. 6a and b and 7a and b display the magnetic field-dependent magnetization (M–H) curves of the two samples at different temperatures. Notably, the M−H loops for temperatures below 6 K exhibit a subtle alteration in shape, deviating from a typical paramagnetic behavior observed at higher temperatures for both samples (Fig. 6a and b). A similar behavior has recently been reported for (R)-(FE)2CuCl4-(1D).27 This suggests that as the applied external magnetic field increases, magnetic moments become more aligned with the magnetic field, giving rise to the total magnetic moment for both samples. A saturation trend in magnetization with respect to the magnetic field observed at low temperatures (<4 K) is of particular interest, as it might give hints at the occurrence of the PM–FM transition and the existence of structurally driven spin chirality in the presently investigated MHSs.
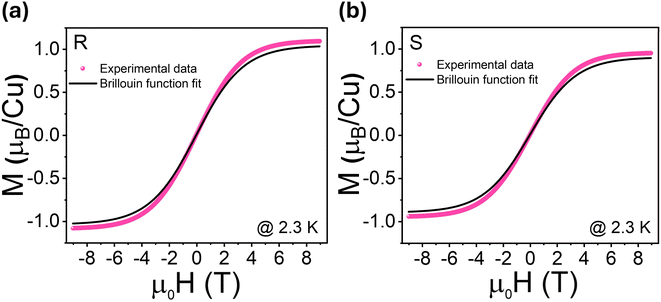 | ||
| Fig. 6 The M−H data taken at 2.3 K for (a) (R-THBTD)2CuCl6 and (b) (S-THBTD)2CuCl6. The solid line (black) represents the Brillouin function for S = 1/2 (g = 2.0 and T = 2.3 K). | ||
Following the method adopted in previous studies,42,43 we have treated this weak ferromagnetism akin to ideal paramagnetism to estimate the saturation magnetization of our samples. Thus, the magnetic response at low temperatures (e.g., M−H at 2.3 K) can be modeled using the Brillouin function within the context of equations describing ferromagnetic ordering:
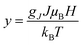 ;
;
H = Happ + Hmol (Happ is the applied magnetic field and Hmol is the Weiss molecular magnetic field); 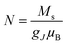 , where Ms is the saturation magnetization; gJ is the Lande’ g-factor. It is evident in Fig. 6 that for both samples, the experimental data diverge from the Brillouin function fit anticipated for S = 1/2, indicating non-paramagnetic behavior at low temperatures (e.g., 2.3 K). The experimentally determined magnetic moment exceeds that derived from the Brillouin function fit, suggesting FM-like behavior in the samples at low temperatures. A similar phenomenon has been observed in cyclic dimer Ln2L2 complexes constructed from (3-pyridinylmethoxy)phenyl-substituted nitronyl nitroxide ligands, where the magnetic moment value derived from the Brillouin function fit exceeds the experimental value, suggesting the presence of antiferromagnetic-like behavior.43
, where Ms is the saturation magnetization; gJ is the Lande’ g-factor. It is evident in Fig. 6 that for both samples, the experimental data diverge from the Brillouin function fit anticipated for S = 1/2, indicating non-paramagnetic behavior at low temperatures (e.g., 2.3 K). The experimentally determined magnetic moment exceeds that derived from the Brillouin function fit, suggesting FM-like behavior in the samples at low temperatures. A similar phenomenon has been observed in cyclic dimer Ln2L2 complexes constructed from (3-pyridinylmethoxy)phenyl-substituted nitronyl nitroxide ligands, where the magnetic moment value derived from the Brillouin function fit exceeds the experimental value, suggesting the presence of antiferromagnetic-like behavior.43
The low-temperature FM-like behavior of (S-THBTD)2CuCl6 and (R-THBTD)2CuCl6 has been further confirmed by the observation of a small magnetic hysteresis in the M−H curve at low temperature (e.g., 2.5 K), as shown in Fig. S12.† We have determined the coercive field (Hc) values from M−H measurements at temperatures below 6 K for (S-THBTD)2CuCl6. Our findings indicate a decrease in Hc from 2.4 mT at 2.5 K to 2.2 mT at 4 K, approaching 0 mT at 5 K, considering the uncertainty of the PPMS measurement. This observed trend in Hc(T) strengthens our assertion that the material displays FM-like ordering at low temperatures.
From the M−H data, we also find that (R-THBTD)2CuCl6 possesses a greater magnetic moment (≈9.86 emu g−1) (at μ0H = 9 T and at 2.3 K) as compared to (S-THBTD)2CuCl6 (≈8.57 emu g−1) (at μ0H = 9 T and at 2.3 K). This is in good agreement with that obtained from the μeff calculations. These values correlate well to other Cu(II) based MHSs, such as (3ampy)CuCl4 with 17.17 emu g−1,44 (R-/S-MPEA)2CuCl4 with 12.5 emu g−1,20 and (R-/S-)-(FE)2CuCl4 with 9.33 emu g−1.27
To capture the interplay of magnetization, magnetic field and temperature, two-dimensional (2D) surface plots are displayed in Fig. 7c and d. Cool colors denote lower magnetic moment values, while warm colors signify higher values. It can be seen that at the measured lowest temperature (2.3 K), the magnetic field at which the magnetization changes its slope is around 4 T for (S-THBTD)2CuCl6 (Fig. 7c), while it is considerably smaller, around 3 T, for (R-THBTD)2CuCl6 (Fig. 7d). This might suggest some correlation between the structure and magnetism in the MHS systems. Nevertheless, further studies are needed to confirm this in a concrete way.
To demonstrate that 1 year of air exposure had no impact on the magnetic properties of (R-THBTD)2CuCl6, magnetic field-dependent magnetization (M−H) studies were performed, revealing no change for the air-treated sample compared to the fresh one (Fig. S13† and 7b). The corresponding magnetic moment for the 1 year air-treated sample is 9.67 emu g−1 (at μ0H = 9 T and 2.3 K), which correlates pretty well to the fresh sample's value of 9.86 emu g−1 (at μ0H = 9 T and 2.3 K), validating the integrity of the magnetic properties.
Conclusions
Considering the importance of developing chiral magnetic MHSs and elucidating the underlying structure–property relationships, we synthesized two new polar 0D materials, namely (S-/R-THBTD)2CuCl6, and characterized their properties. Cu(II) cations feature an uncommon characteristic for MHSs, square planar geometry, imposed by multiple hydrogen bonds in-plane, while the structure maintains its non-centrosymmetric nature from 100 to 400 K based on single crystal XRD studies. Both analogs have retained their structural integrity and magnetic and optical properties for a year so far, based on XRD, magnetometry and UV-vis studies, a record performance among chiral magnetic MHSs. DRCD measurements confirmed the chiral character of the two enantiomers, while magnetization studies revealed a saturation magnetization value of up to 9.86 emu g−1 at low temperatures (e.g., 2.3 K), among the highest reported for chiral magnetic MHSs. Our work demonstrates the versatility and immense potential of MHSs for demanding applications, where chirality, magnetism and stability are interwoven.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the donors of the ACS Petroleum Research Fund under Doctoral New Investigator Grant 65721-DNI5. The authors thank the Chemical Purification Analysis and Screening (CPAS) core facility and Dr Laurent Calcul for access to the JASCO Model J-1500 Circular Dichroism Optical Rotatory Dispersion (CD/ORD) spectrometer. We also thank the X-ray core facility and Dr Lukasz Wojtas. The authors acknowledge the use of the High-End Computing (HEC) facility at Lancaster University. M.-H. P. acknowledges the support of the US Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering under Award No. DE-FG02-07ER46438.References
- N. Gisin and R. Thew, Quantum communication, Nat. Photonics, 2007, 1, 165–171 CrossRef CAS.
- Y. Yang, R. C. da Costa, M. J. Fuchter and A. J. Campbell, Circularly polarized light detection by a chiral organic semiconductor transistor, Nat. Photonics, 2013, 7, 634–638 CrossRef CAS.
- T. Liu, X. Wang, H. Wang, G. Shi, F. Gao, H. Feng, H. Deng, L. Hu, E. Lochner, P. Schlottmann, S. von Molnár, Y. Li, J. Zhao and P. Xiong, Linear and Nonlinear Two-Terminal Spin-Valve Effect from Chirality-Induced Spin Selectivity, ACS Nano, 2020, 14, 15983–15991 CrossRef CAS PubMed.
- O. B. Dor, S. Yochelis, S. P. Mathew, R. Naaman and Y. Paltiel, A chiral-based magnetic memory device without a permanent magnet, Nat. Commun., 2013, 4, 2256 CrossRef PubMed.
- Q. Liu, Q. Wei, H. Ren, L. Zhou, Y. Zhou, P. Wang, C. Wang, J. Yin and M. Li, Circular polarization-resolved ultraviolet photonic artificial synapse based on chiral perovskite, Nat. Commun., 2023, 14, 7179 CrossRef CAS PubMed.
- J. Ma, H. Wang and D. Li, Recent Progress of Chiral Perovskites: Materials, Synthesis, and Properties, Adv. Mater., 2021, 33, 2008785 CrossRef CAS PubMed.
- S. Ma, J. Ahn and J. Moon, Chiral Perovskites for Next-Generation Photonics: From Chirality Transfer to Chiroptical Activity, Adv. Mater., 2021, 33, 2005760 CrossRef CAS PubMed.
- P.-J. Huang, K. Taniguchi and H. Miyasaka, Bulk Photovoltaic Effect in a Pair of Chiral–Polar Layered Perovskite-Type Lead Iodides Altered by Chirality of Organic Cations, J. Am. Chem. Soc., 2019, 141, 14520–14523 CrossRef CAS PubMed.
- K. Wang, L. Qian, S.-C. Ying and G. Xiao, Spin–orbit torque switching of chiral magnetization across a synthetic antiferromagnet, Commun. Phys., 2021, 4, 10 CrossRef CAS.
- F. Fan, D. Zhao, Z. Tan, Y. Ji, J. Cheng and S. Chang, Magnetically Induced Terahertz Birefringence and Chirality Manipulation in Transverse-Magnetized Metasurface, Adv. Opt. Mater., 2021, 9, 2101097 CrossRef CAS.
- Q. Dong, Y. Fang, Y. Shao, P. Mulligan, J. Qiu, L. Cao and J. Huang, Electron–hole diffusion lengths >175 μm in solution-grown CH3NH3PbI3 single crystals, Science, 2015, 347, 967–970 CrossRef CAS PubMed.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- C. Zhang, D. Sun, C. X. Sheng, Y. X. Zhai, K. Mielczarek, A. Zakhidov and Z. V. Vardeny, Magnetic field effects in hybrid perovskite devices, Nat. Phys., 2015, 11, 427–434 Search PubMed.
- M. Isarov, L. Z. Tan, M. I. Bodnarchuk, M. V. Kovalenko, A. M. Rappe and E. Lifshitz, Rashba Effect in a Single Colloidal CsPbBr3 Perovskite Nanocrystal Detected by Magneto-Optical Measurements, Nano Lett., 2017, 17, 5020–5026 CrossRef CAS PubMed.
- M. Kepenekian and J. Even, Rashba and Dresselhaus Couplings in Halide Perovskites: Accomplishments and Opportunities for Spintronics and Spin–Orbitronics, J. Phys. Chem. Lett., 2017, 8, 3362–3370 CrossRef CAS PubMed.
- T. H. Moon, S.-J. Oh and K. M. Ok, [((R)-C8H12N)4][Bi2Br10] and [((S)-C8H12N)4][Bi2Br10]: Chiral Hybrid Bismuth Bromides Templated by Chiral Organic Cations, ACS Omega, 2018, 3, 17895–17903 CrossRef CAS PubMed.
- K. Taniguchi, M. Nishio, N. Abe, P.-J. Huang, S. Kimura, T.-h. Arima and H. Miyasaka, Magneto-Electric Directional Anisotropy in Polar Soft Ferromagnets of Two-Dimensional Organic–Inorganic Hybrid Perovskites, Angew. Chem., Int. Ed., 2021, 60, 14350–14354 CrossRef CAS PubMed.
- H. Lu, T. He, H. Wu, F. Qi, H. Wang, B. Sun, T. Shao, T. Qiao, H.-L. Zhang, D. Sun, Y. Chen, Z. Tang and G. Long, Chiral Ruthenium Halide Semiconductor with Strong Antiferromagnetic Coupling, Adv. Funct. Mater., 2024, 34, 2308862 CrossRef CAS.
- L. Mao, J. Chen, P. Vishnoi and A. K. Cheetham, The Renaissance of Functional Hybrid Transition-Metal Halides, Acc. Mater. Res., 2022, 3, 439–448 CrossRef CAS.
- B. Sun, X.-F. Liu, X.-Y. Li, Y. Zhang, X. Shao, D. Yang and H.-L. Zhang, Two-Dimensional Perovskite Chiral Ferromagnets, Chem. Mater., 2020, 32, 8914–8920 CrossRef CAS.
- J. Xue, Z. Wang, A. Comstock, Z. Wang, H. H. Y. Sung, I. D. Williams, D. Sun, J. Liu and H. Lu, Chemical Control of Magnetic Ordering in Hybrid Fe–Cl Layered Double Perovskites, Chem. Mater., 2022, 34, 2813–2823 CrossRef CAS.
- D. P. Panda, D. Swain and A. Sundaresan, Photophysical and Magnetic Properties in Zero-Dimensional (H2DABCO)MX4·nH2O (M = Mn and Cu; X = Cl and Br; n = 0, 1, and 4), J. Phys. Chem. C, 2022, 126, 13291–13299 CrossRef CAS.
- K. Taniguchi, P.-J. Huang, S. Kimura and H. Miyasaka, Chiral weak ferromagnets formed in one-dimensional organic–inorganic hybrid manganese chloride hydrates, Dalton Trans., 2022, 51, 17030–17034 RSC.
- Y. Ai, R. Sun, W.-Q. Liao, X.-J. Song, Y.-Y. Tang, B.-W. Wang, Z.-M. Wang, S. Gao and R.-G. Xiong, Unprecedented Ferroelectricity and Ferromagnetism in a Cr2+-Based Two-Dimensional Hybrid Perovskite, Angew. Chem., Int. Ed., 2022, 61, e202206034 CrossRef CAS PubMed.
- S.-P. Feng, Y. Cheng, H.-L. Yip, Y. Zhong, P. W. K. Fong, G. Li, A. Ng, C. Chen, L. A. Castriotta, F. Matteocci, L. Vesce, D. Saranin, A. D. Carlo, P. Wang, J. Wei Ho, Y. Hou, F. Lin, A. G. Aberle, Z. Song, Y. Yan, X. Chen, Y. Yang, A. A. Syed, I. Ahmad, T. Leung, Y. Wang, J. Lin, A. M. C. Ng, Y. Li, F. Ebadi, W. Tress, G. Richardson, C. Ge, H. Hu, M. Karimipour, F. Baumann, K. Tabah, C. Pereyra, S. R. Raga, H. Xie, M. Lira-Cantu, M. V. Khenkin, I. Visoly-Fisher, E. A. Katz, Y. Vaynzof, R. Vidal, G. Yu, H. Lin, S. Weng, S. Wang and A. B. Djurišić, Roadmap on commercialization of metal halide perovskite photovoltaics, J. Phys.: Mater., 2023, 6, 032501 Search PubMed.
- Z. Song, B. Yu, G. Liu, L. Meng and Y. Dang, Chiral Hybrid Copper(I) Iodide Single Crystals Enable Highly Selective Ultraviolet-Pumped Circularly Polarized Luminescence Applications, J. Phys. Chem. Lett., 2022, 13, 2567–2575 CrossRef CAS PubMed.
- H. Zheng, R. Zhang, X. Wu, Q. Zhang, Z. Wu, W. P. D. Wong, J. Chen, Q.-H. Xu and K. P. Loh, Strain-Driven Solid–Solid Crystal Conversion in Chiral Hybrid Pseudo-Perovskites with Paramagnetic-to-Ferromagnetic Transition, J. Am. Chem. Soc., 2023, 145, 3569–3576 CrossRef CAS PubMed.
- R. Das, D. Swain, A. Mahata, D. Prajapat, S. K. Upadhyay, S. Saikia, V. R. Reddy, F. De Angelis and D. D. Sarma, Family of Chiral Ferroelectric Compounds with Widely Tunable Band Gaps, Chem. Mater., 2024, 36, 1891–1898 CrossRef CAS.
- B. Li, Y. Yu, M. Xin, J. Xu, T. Zhao, H. Kang, G. Xing, P. Zhao, T. Zhang and S. Jiang, Second-order nonlinear optical properties of copper-based hybrid organic–inorganic perovskites induced by chiral amines, Nanoscale, 2023, 15, 1595–1601 RSC.
- Y. Lu, Q. Wang, R. He, F. Zhou, X. Yang, D. Wang, H. Cao, W. He, F. Pan, Z. Yang and C. Song, Highly Efficient Spin-Filtering Transport in Chiral Hybrid Copper Halides, Angew. Chem., Int. Ed., 2021, 60, 23578–23583 CrossRef CAS PubMed.
- J. Hao, H. Lu, L. Mao, X. Chen, M. C. Beard and J. L. Blackburn, Direct Detection of Circularly Polarized Light Using Chiral Copper Chloride–Carbon Nanotube Heterostructures, ACS Nano, 2021, 15, 7608–7617 CrossRef CAS PubMed.
- Y. Xie, R. Song, A. Singh, M. K. Jana, V. Blum and D. B. Mitzi, Kinetically Controlled Structural Transitions in Layered Halide-Based Perovskites: An Approach to Modulate Spin Splitting, J. Am. Chem. Soc., 2022, 144, 15223–15235 CrossRef CAS PubMed.
- S. A. Cuthriell, C. D. Malliakas, M. G. Kanatzidis and R. D. Schaller, Cyclic versus Linear Alkylammonium Cations: Preventing Phase Transitions at Operational Temperatures in 2D Perovskites, J. Am. Chem. Soc., 2023, 145, 11710–11716 CrossRef CAS PubMed.
- T. M. McWhorter, Z. Zhang, T. D. Creason, L. Thomas, M.-H. Du and B. Saparov, (C7H11N2)2MBr4 (M = Cu, Zn): X-Ray Sensitive 0D Hybrid Metal Halides with Tunable Broadband Emission, Eur. J. Inorg. Chem., 2022, e202100954 CrossRef CAS.
- T. D. Creason, A. Yangui, R. Roccanova, A. Strom, M.-H. Du and B. Saparov, Rb2CuX3 (X = Cl, Br): 1D All-Inorganic Copper Halides with Ultrabright Blue Emission and Up-Conversion Photoluminescence, Adv. Opt. Mater., 2020, 8, 1901338 CrossRef CAS.
- C. Han, A. J. Bradford, J. A. McNulty, W. Zhang, P. S. Halasyamani, A. M. Z. Slawin, F. D. Morrison, S. L. Lee and P. Lightfoot, Polarity and Ferromagnetism in Two-Dimensional Hybrid Copper Perovskites with Chlorinated Aromatic Spacers, Chem. Mater., 2022, 34, 2458–2467 CrossRef CAS PubMed.
- C. Han, A. J. Bradford, A. M. Z. Slawin, B. E. Bode, E. Fusco, S. L. Lee, C. C. Tang and P. Lightfoot, Structural Features in Some Layered Hybrid Copper Chloride Perovskites: ACuCl4 or A2CuCl4, Inorg. Chem., 2021, 60, 11014–11024 CrossRef CAS PubMed.
- R. Das, M. Hossain, A. Mahata, D. Swain, F. De Angelis, P. K. Santra and D. D. Sarma, Unique Chiro-optical Properties of the Weakly-2D (R-/S-MBA)2CuBr4 Hybrid Material, ACS Mater. Lett., 2023, 5, 1556–1564 CrossRef CAS.
- D. Cortecchia, H. A. Dewi, J. Yin, A. Bruno, S. Chen, T. Baikie, P. P. Boix, M. Grätzel, S. Mhaisalkar, C. Soci and N. Mathews, Lead-Free MA2CuClxBr4−x Hybrid Perovskites, Inorg. Chem., 2016, 55, 1044–1052 CrossRef CAS PubMed.
- A. Azmy, D. M. Konovalova, L. Lepore, A. Fyffe, D. Kim, L. Wojtas, Q. Tu, M. T. Trinh, N. Zibouche and I. Spanopoulos, Synthesis and Optical Properties of One Year Air-Stable Chiral Sb(III) Halide Semiconductors, Inorg. Chem., 2023, 62, 20142–20152 CrossRef CAS PubMed.
- Z. Guo, J. Li, J. Liang, C. Wang, X. Zhu and T. He, Regulating Optical Activity and Anisotropic Second-Harmonic Generation in Zero-Dimensional Hybrid Copper Halides, Nano Lett., 2022, 22, 846–852 CrossRef CAS PubMed.
- N. Pathak, S. K. Gupta, C. L. Prajapat, S. K. Sharma, P. S. Ghosh, B. Kanrar, P. K. Pujari and R. M. Kadam, Defect induced ferromagnetism in MgO and its exceptional enhancement upon thermal annealing: a case of transformation of various defect states, Phys. Chem. Chem. Phys., 2017, 19, 11975–11989 RSC.
- M. Zhu, Y. Li, L. Jia, L. Zhang and W. Zhang, Syntheses, crystal structures, and magnetic properties of cyclic dimer Ln2L2 complexes constructed from (3-pyridinylmethoxy)phenyl-substituted nitronyl nitroxide ligands, RSC Adv., 2017, 7, 36895–36901 RSC.
- B. Sun, Z. Yan, Y. Cao, S. Ding, R. Li, B. Ma, X.-Y. Li, H. Yang, W. Yin, Y. Zhang, Q. Wang, X. Shao, D. Yang, D. Xue and H.-L. Zhang, Intrinsic Ferromagnetic Semiconductors with High Saturation Magnetization from Hybrid Perovskites, Adv. Mater., 2023, 35, 2303945 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, synthetic details, additional supplementary figures and tables related to material characterization, X-ray diffraction measurements, thermogravimetric analysis, SEM, EDS, and magnetometry studies. CCDC 2329807–2329810 and 2365208. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ta03010a |
| This journal is © The Royal Society of Chemistry 2024 |