A supramolecular assembly-based strategy towards the generation and amplification of photon up-conversion and circularly polarized luminescence
Alisha
Sengupta
,
Gargee
Roy
,
Aakash Ravikant
Likhar
and
Deepak
Asthana
 *
*
Department of Chemistry, Ashoka University, Sonipat, Haryana 131029, India. E-mail: deepak.asthana@ashoka.edu.in
First published on 9th November 2023
Abstract
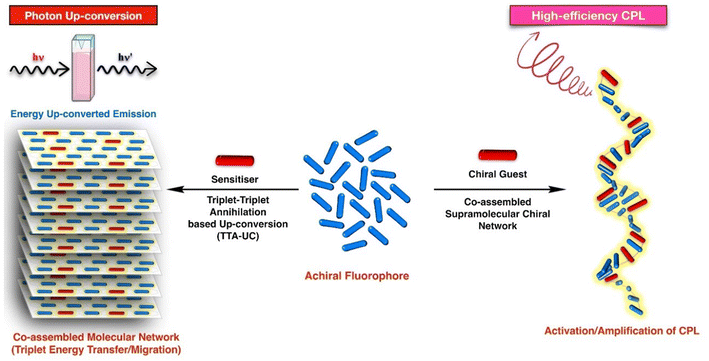
For the molecular properties in which energy transfer/migration is determinantal, such as triplet–triplet annihilation-based photon up-conversion (TTAUC), the overall performance is largely affected by the intermolecular distance and relative molecular orientations. In such scenarios, tools that may steer the intermolecular interactions and provide control over molecular organisation in the bulk, become most valuable. Often these non-covalent interactions, found predominantly in supramolecular assemblies, enable pre-programming of the molecular network in the assembled structures. In other words, by employing supramolecular chemistry principles, an arrangement where molecular units are arranged in a desired fashion, very much like a Lego toy, could be achieved. This leads to enhanced energy transfer from one molecule to other. In recent past, chiral luminescent systems have attracted huge attention for producing circularly polarized luminescence (CPL). In such systems, chirality is a necessary requirement. Chirality induction/transfer through supramolecular interactions has been known for a long time. It was realized recently that it may help in the generation and amplification of CPL signals as well. In this review article we have discussed the applicability of self-/co-assembly processes for achieving maximum TTA-UC and CPL in various molecular systems.
1. Introduction
Supramolecular systems, found ubiquitously in nature, are networks of molecules in which many molecular units are joined together to form nano-architectures with distinctive sizes and shapes. Glues that keep molecular building blocks held within the structure are weak noncovalent intermolecular interactions like hydrogen bonding, π–π stacking, dipolar interactions, van der Waals forces, hydrophobicity, etc. Despite being much weaker than any regular covalent bonds, such interactions control the realms of supramolecular chemistry. Molecules could be designed to fulfil any functional requirement, for example, they could be tuned to function as a molecular recognition unit, a receptor, an ion transporter etc., and for this reason, supramolecular chemistry has been described as “the designed chemistry of the intermolecular bond”.1 These forces are determinantal in many natural processes including photosynthesis, and are responsible for specific structures of proteins and DNA double helix formation.2 In some cases, the structural features of monomer building units are overwhelmingly restrictive and require a great amount of flexibility to establish intermolecular interactions. Weak noncovalent interactions discussed above can be effective over a significantly long range of distances and orientations. For example, hydrogen bonds (H-bonds) can vary from 2.5 Å to 3.0 Å in length and from 90° to 180° in angle.3 A new class of porous supramolecular systems, also known as hydrogen-bonded organic frameworks (HOFs), is one such example where hydrogen bonding and π–π stacking interactions play crucial roles.4,5The unique feature that supramolecular networks have to offer is the retention of individual molecules’ identity in the assembled network. This implies that specific applications where the molecular arrangement in the bulk is a prerequisite, for example, light harvesting, molecular recognition, drug delivery systems, molecular motors, molecular devices, etc., self-assembly could be of great importance.6–20 A large number of examples are available in the literature where supramolecular interactions have been used to control the molecular organisation in the bulk.21,22 An interesting collection of reviews discussing the progress in various fields of supramolecular chemistry was published in 2017 to celebrate the fiftieth anniversary of C. J. Pedersen's landmark work on metal ion–crown ether complexes.23
The supramolecular approach provides a unique way to modify some of the most desirable features of a molecular system, including excited state properties.24–27 Supramolecular platforms have been widely used to widen the range over which energy could be transferred.28 Inspiration comes directly from a natural light harvesting process in which long range energy transfer plays a key role. As we know that Dexter and Förster types of energy transfer processes have an intermolecular distance dependency, the molecular arrangement becomes a deciding factor.29 For the Dexter-type energy transfer to take place, donor (D) and acceptor (A) molecules must be within 1 nm of distance, whereas, the Förster-type energy transfer can take place at much longer distances (≤10 nm). This implies that the efficiency of energy transfer could be fine-tuned to reach the maximum amount by controlling the molecular organization in the bulk. In fact, a supramolecular self-/co-assembly tool has been frequently used to modulate the overall energy transfers and/or to manipulate the optical properties of the material.30
During the self-assembly process, the intermolecular distances reduce significantly which has a huge impact on the energy transfer processes. A judicious design of building blocks may lead to the formation of a desired molecular network allowing the manipulation of energy transfers in the bulk. This has found huge application in designing triplet–triplet annihilation-based photon up-conversion (TTA-UC).31–37 A prerequisite for this to happen is a specific arrangement of a chromophore that may allow the Dexter-type energy transfer.38
Self-assembly has been extremely useful in the fields of photonics where a bottom-up approach has been used to obtain photonic crystals or to create nanoparticle arrays for a variety of optical applications, including low molecular weight materials for white light emission, circularly polarised luminescence, etc.39–49 As the scope of supramolecular systems and their application is quite wide, in this review we will stay focused on the application of supramolecular interactions to create a self-assembled network of luminescent nano-architectures and its impact on the TTA-UC and CPL (Scheme 1).
 | ||
| Scheme 1 A general scheme showing various ways in which supramolecular chemistry and non-covalent interactions could be applied to fine tune the optical properties in the bulk. | ||
2. Supramolecular self-assembly and photon up-conversion
Photon up-conversion is the process in which the radiated energy contains photons that are higher in energy than the incident ones. This is in contrasts to the commonly observed phenomena, as during the fluorescence process some of the energy is lost in non-radiative processes and emitted light is always stokes-shifted.50 Photon up-conversion finds numerous applications in the fields of photovoltaics and photocatalysis.51,52 Upconverting visible light to the ultraviolet (UV) range or near infrared (NIR) light to the visible region would increase the absorbance of any light-based devices and therefore can significantly improve their efficiency.53–55 Up-conversion finds great applicability in biological processes as well. Bioimaging and photodynamic therapy (PDT) are two important areas where up-conversion could be hugely advantageous.56–61 It is known that various mechanisms including nonlinear optical effects, such as the second harmonic or sum-frequency generation, the two- (or multi-) photon absorption process, etc., may lead to anti-Stokes shifted emission of light.62,63 Among the available photon up-conversion methods, TTA-UC represents a special case as it makes possible to upconvert low intensity light (≤mW cm−2).35,64In general, TTA-UC occurs through a bimolecular process in systems comprised of a donor and an acceptor chromophore. Scheme 2 represents the mechanistic profile of the TTA-UC process. The entire process could be divided in five steps: (1) absorption of incident light by a donor, (2) formation of long-lived donor triplets, (3) a donor to acceptor triplet–triplet energy transfer (TTET), (4) formation of acceptor excited singlet via triplet–triplet annihilation (TTA), and (5) emission of energy upconverted light.
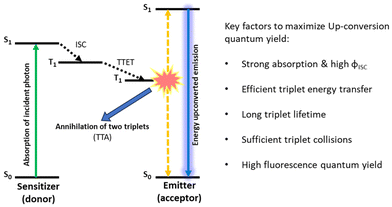 | ||
| Scheme 2 Schematic diagram depicting steps involved in TTA-UC, and the various structural features of the donor and acceptor that have a huge impact on the overall up-conversion quantum yield. | ||
Upon irradiation of suitable light, a donor, which is normally a heavy metal containing a porphyrin dye, gets excited to a singlet state which after passing through an intersystem crossing (ISC) step acquires a triplet state. Having an acceptor whose triplet energy level is only slightly lower than that of the donor in its proximity, a triplet energy transfer (TET) takes place. This donor to acceptor TET generates acceptor triplets, which ultimately annihilate and produce acceptor singlets. The final emission takes place from this high energy excited singlet. TET (step-3) and TTA (step-4) are distance dependent events and largely responsible for the up-conversion quantum yield (ΦUC). Another difficulty that is faced with TTA-UC is the interference from dissolved oxygen which might kill the triplets before TTA can take place. Achieving TTA-UC under ambient conditions has been a challenge.
Self-assembled networks provide an ideal platform where energy transfer processes could be controlled. A review article focusing on up-conversion in metal organic frameworks, covalent organic frameworks and gels could be found elsewhere.65 In the following sections we will see how the self-assembly process can effectively circumvent both energy transfer and triplet quenching issues (vide supra) that limit the applicability of TTA-UC in practical life.
2.1 Enhancement of the TET and TTA to improve ΦUC
The overall quantum yield (QY) of the TTA-UC process is given by following equation:| ΦUC = ½fΦISCΦTETΦTTAΦFl. | (1) |
As annihilation of two triplets generates one upconverted photon, a factor of ½ is introduced in QY calculations. Factor f represents the statistical probability of singlet formation, ΦISC and ΦFl are the efficiencies of triplet formation in the donor and the fluorescence QY of the acceptor, respectively. The remaining two quantities in eqn (1), ΦTET and ΦTTA, depend on the intermolecular distances in the system. These two factors could be maximized to reach a value close to unity thereby increasing the ΦUC.
A TET occurs through a Dexter-type energy transfer which involves electron exchange between the two participating molecules. An intermolecular distance ≤1 nm is a prerequisite for such energy transfers. Strategies to bring donor molecules nearer to the acceptor has been thoroughly investigated. In following subsections, we will explore the three major supramolecular chemistry-based strategies that have been applied to achieve efficient photon UC. The formation of host–guest-based energy donor and acceptor supramolecular complexes, the supramolecular co-assembly of donor and acceptor molecules, and the entrapment of the donor molecules in the self-assembled networks of acceptor systems have been shown to be quite successful in improving the UC efficiencies.
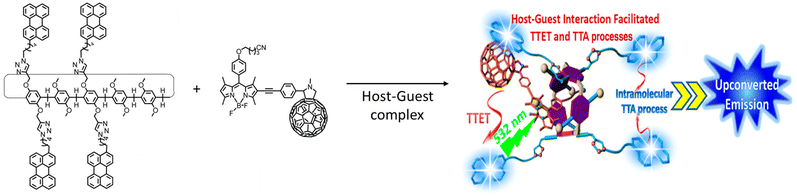 | ||
| Fig. 1 A pillar[5]arene-based host–guest complex containing perylene acceptors and a BODIPY sensitizer to exhibit TTA-UC in solution. Adopted with permission from ref. 71. Copyright 2016, American Chemical Society. | ||
Utilizing the host–guest chemistry of cyclodextrin (CD), W. Xu et al. prepared 9,10-diphenylanthracene (DPA) derivatives that self-assemble in aqueous media.72 Two types of derivatives were designed, one in which two γ-CD units are joined through a bridging DPA unit, and the second in which DPA is a tethered to γ-CD unit (Fig. 2). Using a Ru(II)–coumarin compound as a sensitizer and the DPA-bridged system as an emitter for TTA-UC, the authors obtained an up-conversion quantum yield in deaerated water of up to 6.87%.
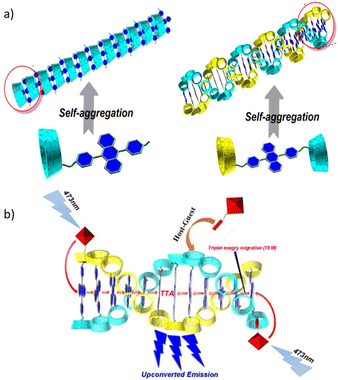 | ||
| Fig. 2 (a) Supramolecular self-assembled structure of fluorescent emitter (DPA-γ-CD) molecules in water and (b) sensitizer guest molecules hosted in CD cavities, leading to upconverted emission through the TTA-UC mechanism. Adopted with permission from ref. 72. Copyright 2018, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Another interesting report on host–guest interaction-based up-conversion was published by Wasielewski, Stoddart and co-workers.73 The group prepared a host–guest complex comprised of a fluorescent tetracationic host system (ExTzBox4+) in which a 5,15-diphenylporphyrin (DPP) sensitizer guest gets included (Fig. 3). The prepared supramolecular complex exhibited upconverted blue fluorescence when irradiated with Q bands of DPP (575 and 628 nm light). The obtained up-conversion quantum yields were up to 8.1% and thresholds values were as low as 4.8 mW cm−2. This report demonstrated the generalization of the host–guest strategy towards designing new photon up-conversion materials for specific applications.
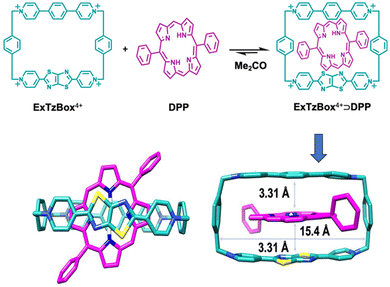 | ||
| Fig. 3 A host–guest complex for triplet fusion-based photon upconversion. Adopted with permission from ref. 73. Copyright 2020, American Chemical Society. | ||
T. Ogawa et al. functionalized 9,10-diphenylanthracene (DPA) to form a low molecular weight gelator (LMWG) by introducing alkyl chains that are connected viaL-glutamate linkers (Fig. 4).34 In the self-assembled structure of an acceptor and donor Pt(II)-octaethylporphyrin (PtOEP) mixture, donor molecules get dispersed and remain confined in the lipophilic regions. Phosphorescence measurements showed nearly 100% energy transfer from the donor to acceptor.
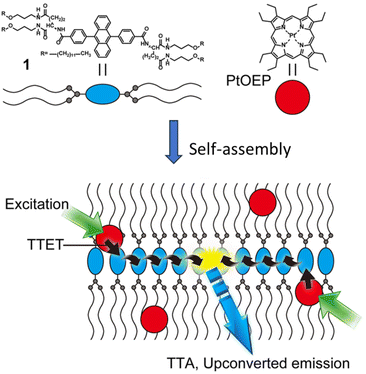 | ||
| Fig. 4 A self-assembled network of acceptor [10 mM] and entrapped donor molecules [10 μM], leading to extremely efficient TET and TTA processes enhancing the ΦUC up to 30%. Adopted with permission from ref. 34. Copyright 2015 Springer Nature. | ||
As we know that once acceptor triplets are produced, they must meet a second such acceptor molecule to allow TTA. Generally, this is achieved by diffusion in the system; however, the diffusion mechanism has some limitations as the risks of quenching are increased. In an appropriately arranged acceptor network, triplet energy can migrate without any need for molecular diffusion and therefore much improved TTA can be expected. The authors indeed reported an overall up-conversion QY of 30%. It should be noted that theoretical maximum of ΦUC is 50%. This report clearly signifies the important role of supramolecular chemistry in enhancing the energy transfer.
It is noteworthy that the commonly available acceptor chromophores are from the polyaromatic family having minimal solubility in water. To construct a self-assembled network, these are often conjugated with long alkyl tails, which makes them further hydrophobic. D. Asthana et al. reported a lipid-based supramolecular system in which the acceptor arranges itself along the bilayer structure.74 Lipid structures also helps in solubilizing the donor (PtOEP) which being hydrophobic in nature will have a tendency to get precipitated in water. A mixture of a cationic lipid–anionic acceptor (DPA-SO3−) complex and a PtOEP donor gets easily dispersed in water and exhibits TTA-UC under mild irradiation conditions (Ith = 25 mW cm−2). A lipid-assisted supramolecular self-assembly resulted in an ambient condition photon up-conversion and a long triplet lifetime (∼8 ms) in aqueous media. However, the up-conversion emission intensity from the aerated sample was found to be unstable under longer irradiation times.
In a smart design adopted by R. Haruki et al., a DPA-based acceptor was designed to have hydrophilic oligo(ethylene glycol) chains along with the alkyl chains to provide a self-assembled network in water medium (Fig. 5a).75 Due to their hydrophobicity, PtOEP molecules remain in the alkyl tail regions. This supramolecular network displays the most desirable properties, stable TTA-UC under ambient conditions. In an aqueous medium, oligo(ethylene glycol) chains orient outwards and remain exposed to water molecules, whereas the inner part forms a very lipophilic layer.
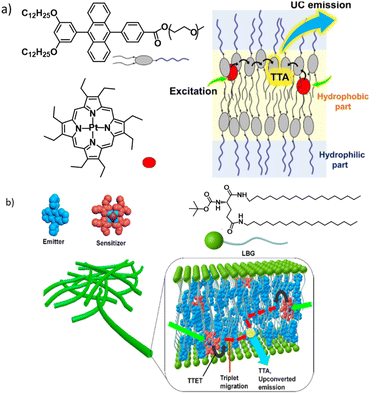 | ||
| Fig. 5 (a) A DPA-based acceptor self-assembled network showing TTA-UC in water under ambient conditions. Adopted with permission from ref. 75. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Scheme showing the inclusion of sensitizer and acceptor chromophores in an extended fibrous network of the gel. Adopted with permission from ref. 37. Copyright 2015, American Chemical Society. | ||
The strategy of converting an acceptor into a gelator has certain advantages such as the formation of acceptor arrays that may facilitate triplet energy migration, which can provide protection from solvent-based triplet quenching, etc.; however, it requires additional synthesis steps to be performed. It would be much more practical if TTA-UC could be achieved using a commonly available gelator that confines both the donor and acceptor. P. Duan et al. used a known gelator, N,N′-bis(octadecyl)-L-boc-glutamic diamide (LBG), to entrap DPA and PtOEP in their fibrous network.37 A ternary mixture containing LBG, DPA and PtOEP was heated in dimethylformamide to form a clear solution. Upon cooling the entire volume forms a pink colour gel. This gel system (Fig. 5b and 6a, b) showed surprising stable photon up-conversion under air saturated conditions and exhibited a threshold (Ith) value of 1.48 mW cm−2, which is slightly lower than the solar irradiance. By performing a control experiment, the authors further clarified that the solvophobic interactions which force the UC chromophore pair into the nonpolar inner regions of a gel matrix are the key here. When the same LBG/PtOEP/DPA mixture was used to form a gel in a nonpolar solvent CCl4, no UC was observed under similar conditions to the DMF gel. This difference might arise from the UC pair being dissolved and spread in the bulk CCl4 solvent environment.
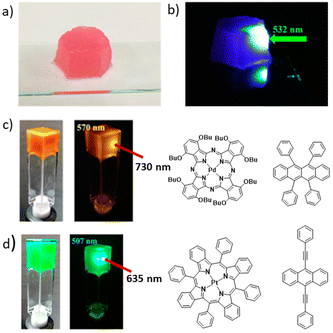 | ||
| Fig. 6 (a) DPA + PtOEP in an LBG gel matrix under normal light and (b) when irradiated with a 532 nm laser. (c and d) Generalization of the method with UC in LBG/rubrene/PdPc(OBu)8 and LBG/BPEA/PtTPBP gels, respectively. Adopted with permission from ref. 37. Copyright 2015, American Chemical Society. | ||
The concept of applying a gelator molecule to achieve a nano-matrix assisted TTA-UC was demonstrated to be a general strategy. To be of some practical importance, it should be possible to apply with different UC pairs. The authors prepared air-saturated DMF gels of UC pairs PdPc(OBu)8/rubrene and PtTPBP/BPEA, and showed the successful UC from NIR (730 nm) to yellow (570 nm) and from red (635 nm) to cyan (507 nm), respectively (Fig. 6c and d). These experiments clearly indicated the universality of this approach.
So far, we have discussed that how the confinement of a sensitizer in the supramolecular interactions led to the molecular assembly of acceptor chromophores or confinement of the sensitizer and acceptor chromophore in the supramolecular network of a gelator, leading to the generation of TTA-based photon up-conversion with high quantum yields and, in some cases, even in the presence of air. While the dispersion of a sensitizer (donor) in the self-assembled network of an acceptor seems extremely helpful, chances of the segregation of donor molecules remain a possibility, which might affect the overall performance of the TTA-UC system.
Co-assembly of the donor and acceptor should be able to overcome this issue. A properly functionalized donor and acceptor might form a supramolecular network in which both units are included homogeneously. P. Duan et al. reported a TTA-UC system comprised of a cyano-substituted distyrylbenzene acceptor and Pd(ii) mesoporphyrin IX donor systems (Fig. 7).76 The acceptor alone showed an aggregation induced enhancement in fluorescence intensity. Interestingly, this co-assembled network exhibited temperature-dependent turn on/off UC. When heated to form a solution, the UC was lost, whereas upon cooling back to the gel state, UC was reattained.
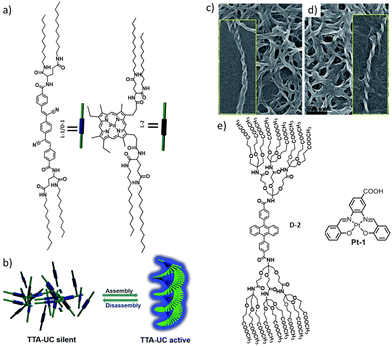 | ||
| Fig. 7 (a and b) Co-assembly of functionalized donor and acceptor units, leading to an aggregation-induced enhancement of fluorescence. Influence of the chiral glutamic acid linker is seen from the TEM image that showed the formation of helical nano structures in both acceptor (c) and donor gel samples (d). Adopted with permission from ref. 76. Copyright 2017, RSC. (e) Chemical structures of the DPA-based dendritic acceptor molecule and the Schiff-base-based Pt–acceptor system. Adopted with permission from ref. 77. Copyright 2020, Elsevier Ltd. | ||
Confinement of the donor or acceptor in host cavities require a size match with the guest structure. This might be a restrictive condition in generalising the host–guest interaction-based upconverting materials. Q. Guo et al. suggested the applications of a dendritic network of acceptor units and the utilization of various spaces in the three-dimensional dendrimer network to accommodate sensitizer molecules.77 An investigation in methanol and a methanol/water mixture revealed a huge difference in up-conversion yields. It was found that addition of water triggered the formation of self-assembled nanoparticles ranging up to 250 nm in size. Sensitizer molecules get confined in those cavities increase the ΦUC from 1.4% in methanol to 10.2% in the methanol/water medium.
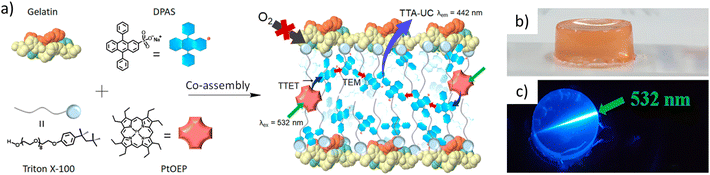 | ||
| Fig. 8 (a) Scheme showing the formation of a UC pair–surfactant–gelatin co-assembly in water and (b and c) a co-assembled hydrogel in normal light and upon irradiation with a 5432 nm laser. Adopted with permission from ref. 85. Copyright 2018, American Chemical Society. | ||
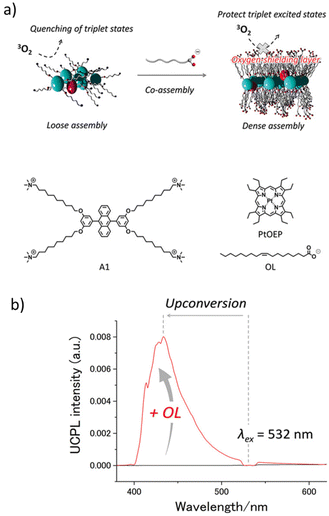
In a slightly different approach, H. Kouno et al. modified the DPA acceptor to have ten carbon long alkyl chains and quaternary ammonium groups on both sides, giving it an amphiphilic characteristic.32 A tetracarboxylic acid analogue of PtOEP was synthesized to overcome the insolubility issue. A modified donor and acceptor when dissolved in water leads to the formation of a co-assembled network. Up-conversion measurements performed in aerated water showed stable UC signals. Furthermore, extending the co-assembly approach to a slightly different type of supramolecular network, H. Kouno et al. investigated the effect of supramolecular crowding by introducing a fatty acid anion in the system.86 DPA functionalized with four quaternary ammonium groups present at the end part of an alkyl tail was prepared (Fig. 9a). As expected, the functionalized acceptor forms a self-assembled network in water, this supramolecular assembly failed to exhibit UC in aerated water (Fig. 9b). Sodium oleate was then introduced into the acceptor and PtOEP mixture, and a co-assembled network was developed in water. Addition of sodium oleate reduces the electrostatic repulsion between the ammonium groups and results in a much tighter supramolecular network formation, which is now capable of blocking the oxygen. This co-assembled network exhibited a remarkably high up-conversion quantum yield in deaerated water of 19.8%. Up-conversion measurement performed in an air saturated water medium gave a ΦUC of up to 14.4%.
 | ||
| Fig. 9 (a) Chemical structure of the functionalized DPA acceptor and scheme showing a co-assembled network that blocks the oxygen. (b) Plots showing the successful UC emission from the oleate–acceptor–PtOEP assembly and no UC from acceptor–PtOEP assembly in water. Adopted with permission from ref. 86. Copyright 2019, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Y. Kawashima et al. prepared a bola-type amphiphile acceptor and mixed it with decanoate.87 A sensitizer mixed with an acceptor and decanoate forms a co-assembled network in water. Upon excitation with 445 nm light, an upconverted emission at 390 nm was observed. In order to calculate the oxygen barrier efficiency of this ternary mixture, the UC efficiencies of aerated and deaerated samples were compared and they were found to be nearly 80%.
It is evident that the energy transfer processes are most efficient when there is a well organised molecular network of the participating chromophores. In this context, metal organic frameworks (MOFs) present a very suitable platform. J. Park et al. synthesized a water stable Zr-MOF of 4,4′-(9,10-anthracenediyl)dibenzoic acid (DCDPA) and demonstrated the successful TTA-UC using a Pd(II)-meso-tetrakis(4-carboxyphenyl)porphyrin (Pd-TCPP) sensitizer (Fig. 10).88 During the MOF synthesis, varying amounts of a sensitizer were taken with different concentration ratios of the sensitizer and annihilator. Sensitizer molecules get included in the defect coordination sites of Zr clusters. Transmission electron microscopy images revealed the formation of an octahedral structure of around 55 nm. Up-conversion measurement in an aqueous medium showed an up-conversion efficiency of 1.28% under illumination of 2.4 mW cm−2 intensity light. The prepared MOF was found to be photostable when irradiated with a laser power intensity of up to 100 mW cm−2, and also no cytotoxicity in HeLa cells was observed.
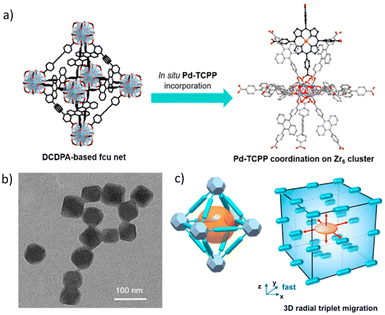 | ||
| Fig. 10 (a) Schematic diagram showing the preparation of a MOF containing an annihilator DCDPA and a sensitizer Pd-TCPP. (b) TEM image showing octahedral MOF structures. (c) Schematic design showing the three-dimensional triplet energy migration paths n of the MOF. Adopted with permission from ref. 88. Copyright 2018, American Chemical Society. | ||
Photon up-conversion under ambient conditions and in aqueous media broadens the overall applicability of these systems in biological processes such as bio-imaging and PDT.
3. Supramolecular self-/co-assembly and CPL
Word light refers to the visible part of electromagnetic (EM) radiation, which is a tiny part of the spectrum that extends from very short wavelength γ-rays to long wavelength radio waves. Human eyes are sensitive to only the visible part of it and cannot detect the remaining electromagnetic radiation. EM radiation contains two components, electric and magnetic fields, which oscillates in a mutually perpendicular direction along the wave propagation axis. Unpolarized light has electric field vectors oscillating in all possible planes perpendicular to the wave propagation direction. Light is said to be polarized if by passing the light through a polarizer, the random oscillations are filtered out.89,90 Depending on the electric field orientation in the polarized light, it could be categorised into three types: linearly polarized, circularly polarized, and elliptically polarized light.Curiosity about the polarized light started with the initial studies carried out by Christiaan Huygens and Erasmus Bartholinus (1669). In 1809, E. L. Malus explained the phenomenon of double refraction from the crystals of spar producing double images using the word “polarization”. During 1811–1817, a French physicist Jean Baptiste Biot discovered the rotation of the plane of polarization upon passing the polarized light through a properly cut quartz. Materials capable of rotating the plane of polarized light are called optically active. Optical activity is displayed by chiral systems.
Chirality is the property of a system which illustrates that the structure and its mirror image are non-superimposable. Chiral objects are found ubiquitously in nature and in many cases are endowed with characteristic chiroptical properties.91 There are several chiroptical methods that are used to characterize the stereochemical and electronic properties of chiral molecules. Standard measurements performed to investigate chiroptical properties are circular dichroism (CD) and optical rotatory dispersion. Being an absorption-based method, the CD spectrum contains information about the ground state chiroptical properties of any chiral system. CD spectroscopy is based on the differential absorption of left- and right circularly polarised light, and has become a widely used technique.89,92–95 Circularly polarized luminescence (CPL) is the phenomenon that produces polarized emission light (Scheme 3). CPL measurements hold information about the excited electronic states of chiral systems. Like CD spectroscopy, CPL measures the difference in left- and right-circularly polarised emissions from the system.96–98 With the introduction of commercially available spectrometers for CPL measurements, the field of CPL has seen a huge surge in research activity. This has resulted in a large number of publications reporting CPL-active systems, a themed collection on this topic recently published in Journal of Materials Chemistry C (Royal Society of Chemistry, 2023) is an example of that.
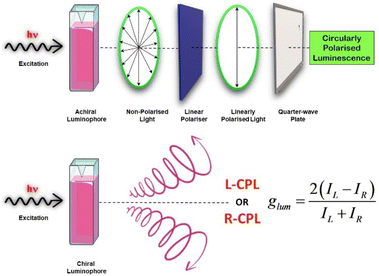 | ||
| Scheme 3 Diagram showing the procedure to obtain circularly polarised light using a physical method and the emission of circularly polarized light from a chiral CPL-active fluorophore. | ||
CPL materials find varied applications in different domains namely chiral opto-electronics, biological fields like chemo-sensors, light emitting transistors, CPL switches, 3D-displays, etc.99–109 CPL has also been applied to amplify the chirality in molecular/supramolecular systems.110–115 One of the criteria used to describe CPL-active systems is the luminescence dissymmetry factor (glum) which may lie between +2 and −2, positive indicating left-handed circularly polarised light and negative indicating right-handed circularly polarised light.
| glum = 2(IL − IR)/(IL + IR) | (2) |
Organic molecules usually exhibit smaller glum compared to inorganic compounds. However, as organic molecules could be easily modified to meet specific requirements, they represent vast opportunities of creating efficient and more benign CPL-active materials.116,117 Various strategies have been opted to produce new CPL-active molecules and/or to amplify the CPL signal of existing materials.118–121
A supramolecular chemistry-based approach has been particularly helpful at this. In the coming section, we will discuss some of the representative examples where supramolecular engineering has resulted in induced CPL activity or caused amplification in polarized luminescence and glum values.
3.1 CPL from supramolecular assemblies of the achiral chromophore: chirality transfer vs. chiral energy transfer (C-FRET)
Induction or amplification of chirality is the process in which doping an achiral system with a small amount of a chiral analogue creates chiral assemblies in the bulk. This is known as the “majority rule” or “sergeants and soldiers” principle.122–128 This approach has been widely applied in the field of helical polymers, supramolecular polymers, etc.127,129–135 In the context of CPL, induction of chirality through the majority rule could be extremely helpful as the preparation of chiral luminescent systems would not be a requirement. Simple doping with a small amount of a chiral analogue would make the entire system CPL active.In some cases, chirality could be acquired upon supramolecular self/co-assembly formation.136,137 Such systems allow the use of an achiral chromophore for chiroptical applications. P. Duan, M. Liu and co-workers prepared benzene-1,3,5-tricarboxamide benzoic acid (BTABA), a symmetric molecule that forms a gel in a DMF/H2O mixture.138 When this gel is heated to form a solution and then vortexed, chiral assemblies are formed which are then used as a chiral seed (Fig. 11). A fluorescent dye was then added to this chiral assembly. CPL measurement using this dye doped chiral assembly exhibited a glum value of ±0.0435 (at 465 nm).
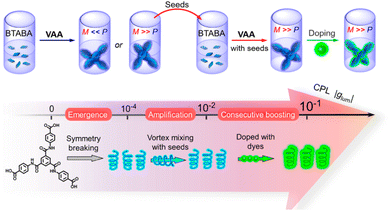 | ||
| Fig. 11 Scheme showing chiral aggregate formation from an achiral molecular system and then utilizing it as a chiral seed for further chiral supramolecular network formation (top). The scheme below shows the emergence of supramolecular chirality, leading to a CPL signal when doped with a fluorescent dye. Adopted with permission from ref. 138. Copyright 2020, American Chemical Society. | ||
The authors further extended this approach to accommodate photon upconverting materials inside the chiral assemblies obtained from BTABA to realize CPL from upconverted emission.139 A 9,10-diphenylanthracene derivative as a TTA-UC acceptor and a Pt(II) mesoporphyrin IX (PtMIX) donor were mixed with BTABA to form a co-assembled network. When irradiated with 532 nm light, an upconverted emission at 434 nm was observed. CD measurements confirmed the supramolecular chirality transfer from the BTABA gel to the acceptor chromophore. Upon excitation with a 532 nm laser, CPL activity from the upconverted emission (at 445 nm) with a glum value of ±0.014 was successfully recorded.
DNA is a naturally occurring chiral system and has been used to attach fluorophore units to achieve CPL. A synthetically less demanding route could be to use DNA nanostructures as a template to incorporate fluorescent dyes for CPL. Q. Jiang et al. applied a carbazole-based bicyanine dye to obtain a DNA–cyanine composite.140 When bound to DNA, the dye gets confined in a restricted environment and therefore the fluorescence intensity gets increased by a noticeable amount. Their study showed that a decrease of GC contents in a duplex DNA–biscyanine assembly resulted in an increase of fluorescence intensity and CPL emission. This observation was attributed to the preferential binding of the biscyanine dye to AT-rich dsDNA. Also, longer DNA duplex templates exhibited stronger CD signals and CPL activities. B.-C. Kim et al. demonstrated CPL activity in a liquid crystalline mesophase obtained from the assembly of achiral molecules.141 The authors employed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of a rod-shaped molecule (blended with a small amount of pyrromethene dye) and a bent-core host system. In the mixed state, dye molecules follow a mesogen ordering pattern. Utilization of the chiral spaces in the liquid crystalline host eliminates the need for multi-step chemical functionalization of the fluorophore to introduce a chiral centre in it.
1 mixture of a rod-shaped molecule (blended with a small amount of pyrromethene dye) and a bent-core host system. In the mixed state, dye molecules follow a mesogen ordering pattern. Utilization of the chiral spaces in the liquid crystalline host eliminates the need for multi-step chemical functionalization of the fluorophore to introduce a chiral centre in it.
Host–guest chemistry has also been proven to be an important tool to realize CPL from an achiral fluorophore. T. Li et al. used a sulfonate group containing naphthalene-based dye that binds with a chiral macrocycle (Fig. 12) through hydrogen bonding.142 This molecular host–guest complex exhibited induced CD and CPL. However, the observed dissymmetry factor was rather low (0.0005).
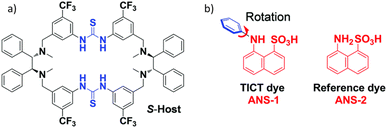 | ||
| Fig. 12 Chemical structures of the chiral host (a), and the achiral fluorescent guest (b). Adopted with permission from ref. 142. Copyright 20210, Royal Society of Chemistry. | ||
K. Q. Le et al. reported CPL from an achiral dye displaying strong dissymmetry between left- and right polarised light (glum > 0.1) using chiral metal nanoarchitectures.143 The authors demonstrated when achiral dye molecules were placed close to the chiral nanoparticles, through near-field interactions with chiral plasmons, an enhancement in photoluminescence takes place. For their study, the group fabricated a 50 nm thick two-dimensional chiral nanostructure of Au on a glass substrate using the electron beam lithography technique. A thin film of IR125 dye/polyvinyl alcohol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was deposited over the chiral nanostructures.
1) was deposited over the chiral nanostructures.
It appears that in systems in which one component is chiral and the other is achiral, chirality induction is essential to exhibit CPL. An interesting study performed by K. Yang et al. ruled this out by demonstrating the energy transfer of circularly polarized light to an achiral acceptor that finally emits circularly polarized light.144 The authors designed two modes of investigations to show that chirality transfer between the donor and acceptor dyes is not required. A CPL-active helical polyacetylene copolymer containing pyrene units was employed as an energy donor. Films of only the donor, only the acceptor and a blend film containing a mixture of the donor and acceptor were prepared by using a poly(vinyl butyral) polymer matrix (Fig. 13a and c). By putting individual chiral/achiral fluorophore films together in a side-by-side fashion, double layer films were prepared. Double layer films were investigated in two modes, once putting an achiral film side nearer to the excitation source and then reversing the film to put a chiral fluorophore film closer to the excitation light source (Fig. 13b). When subjected to CPL measurements, both types of films, the double layer and the blend layers showed CPL signals corresponding to the emission of an achiral fluorophore through energy transfer processes. An interesting phenomenon was observed when observation modes for the double layer sample was changed. Changing the mode resulted in a change from the left- to right-handed CPL output and vice versa.
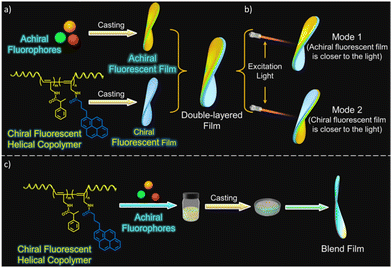 | ||
| Fig. 13 Schematic diagrams showing the formation of double layer film (a), two different modes of investigation with double layer film (b), and formation of blend films (c). Adopted with permission from ref. 144. Copyright 2023, American Chemical Society. | ||
Another very recent work from Z.-W. Luo et al. demonstrated a nonradiative chiral energy transfer from the chiral donor to achiral energy acceptor fluorophore exhibiting bright CPL.145 The authors synthesized a tri-cholesteryl-based emissive polymer, poly(4,4′,4′′-tricholesterylformate-oxytetraphenylethylene-methyl) acrylic acid ester, that exhibited a quantum yield of 21% in the solid state (Fig. 14a). Successful CPL with a glum value of +0.18 was observed when the polymer content in the plasticizer was above 20%. When Nile red dye (an achiral acceptor fluorophore) was mixed with a CPL-active polymer–plasticizer mixture, excitation at 360 nm resulted in narrower emission bands from the polymer unit with a concomitant increase in the emission band corresponding to Nile red (NR) dye, indicating an FRET-based energy transfer from the donor to acceptor.
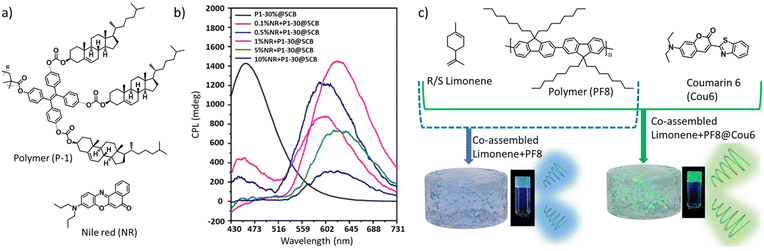 | ||
| Fig. 14 (a) Chemical structures of the emissive polymer (P-1) and achiral dye (Nile red). (b) CPL activity from mixtures containing varying amounts of the NR dye and 30% polymer mixed in a plasticizer (5CB). Adopted with permission from ref. 145. Copyright 2023, American Chemical Society. (c) C-FRET from a co-assembled ternary mixture of an achiral dye polymer (PF8), coumarin 6 (Cou6) and R- (or S)-limonene, leading to enhanced glum. Adopted with permission from ref. 146. Copyright 2023, Royal Society of Chemistry. | ||
CPL measurements revealed the appearance of a new CPL peak at around 600 nm corresponding to NR (Fig. 14b). A composition containing 1% NR when excited with 360 nm light initiates an energy transfer from the donor to acceptor, causing circularly polarized emission at 625 nm with a glum value of +0.20. The same mixture when was excited at 470 nm, a weak CPL signal was observed. This implies that NR dyes acquired some chirality through induction from the chiral polymer present in the mixture; however, the glum value was about five-time smaller.
A recent report, published by Y. Bao et al., represents a beautiful example of co-assembly-based tuning of the CPL wavelength and glum value in a ternary mixture, comprised of two achiral fluorophores and a nonfluorescent chiral component.146 A mixture of poly(9,9-di-n-octylfluorene), coumarin-6 and R/S-limonene displayed a chiral energy transfer (C-FRET)-based CPL with improved glum value (Fig. 14c). A co-assembly-supported FRET led to a green colour CPL from the originally blue CPL light.
The chiral energy transfer between donor–acceptor fluorophore could be maximized in a system in which electric and magnetic transition dipoles are coupled. J. Wade et al. investigated the impact of such dipolar coupling on the overall CPL by preparing an FRET system comprised of a chiral superhelicene (acceptor) and an achiral polymer matrix (energy donor). CD measurement revealed the formation of a chiral phase that displayed an approximately six-time stronger chiroptical signal than the helicine alone. Their study found an unprecedented enhancement in CPL (∼500-fold amplification).147
3.2 Self-assembly/crystallization-driven amplification of CPL
With the increasing interest towards the fabrication of optical devices based on CPL, various strategies have been explored to make achiral systems CPL active (vide supra). A straightforward way is to perform synthetic modification to convert an achiral chromophore into a chiral one. Another approach, rather a simple and less challenging way of obtaining CPL is chirality transfer, which has been proven to be an effective method to generate circularly polarized light. Chirality sometimes could also be achieved through a self-assembly process in which hierarchical helical architectures are formed.136,148–163 Attainment of chirality in an achiral molecular network through supramolecular interactions has been called a “soft approach” of generating chiral structures.150 The main driving force behind the chiral organization in the bulk could be twisting of ligands or metals with specific binding positions. S.-Y. Yu et al. prepared a luminescent chiral supramolecular Au ring that self-assembled from Au2 monomers.164Chiroptical properties are directly correlated to crystal space groups. Though it is not very common to observe and difficult to predict, it is possible to crystallize achiral molecules into chiral space groups. This has been well described by L. Caswell et al. as “optical activity can be created from nothing”.165 J. Zhao et al. demonstrated the CPL from an achiral organic–inorganic hybrid complex of manganese.166 The authors employed potassium(dibenzo-18-crown-6) as a cation to form [K(dibenzo-18-crown-6)]2MnX4 cocrystals where X is a halide. Single crystal X-ray diffraction studies revealed a non-centrosymmetric space group, monoclinic Cc. Microarea optical activity tests showed a successful exhibition of CPL from the single crystals and in some cases from the powder sample as well.
Taking advantage of the Pfeiffer effect,167 G. Park et al. demonstrated an amplified circularly polarized phosphorescence in a square planar Pt(II) complex.168 In the co-assembled structure containing a small fraction of the chiral version of the Pt complex (Fig. 15a), they observed a two orders of magnitude increase in the glum value with respect to the chiral complex alone.
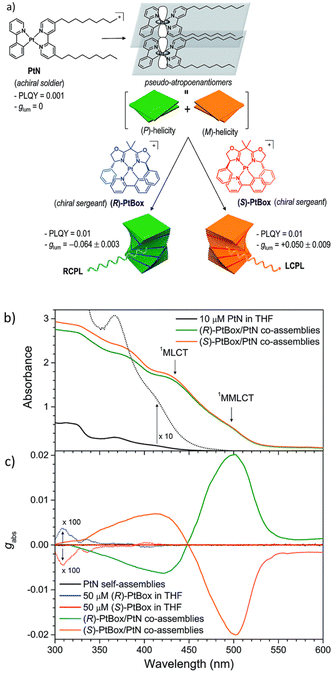 | ||
| Fig. 15 (a) Schematic diagram showing the co-assembled structure of chiral and achiral Pt complexes. (b) and (c) Absorption spectra and the ECD spectra, respectively. Adopted with permission from ref. 168. Copyright 2018, Royal Society of Chemistry. | ||
It is noteworthy that the achiral Pt complex referred to as a soldier, forming a helical self-assembled network even in the absence of the chiral Pt complex (sergeant). Helical stacking is driven by the presence of nonyl chains. However, no CPL activity could be observed from the self-assembled network, probably due to the presence of equal amounts of right- and left-handed stacked structures. Addition of a small amount of the chiral Pt complex (sergeant) resulted in an increase of the enantiomeric excess of the one type of helicity in the self-assembled structures. In co-assembled structures, a metal–metal interaction (MMLCT) was observed in the form of a weak absorption band at around 500 nm (Fig. 15b). The electronic CD spectra (ECD) showed the formation of a P- or M-type helical supramolecular network depending on the chirality of the added sergeant (Fig. 15c). This work represents an ideal example of applying supramolecular self-assembly tactics to generate and modulate symmetry-controlled properties such as CPL.
4. Conclusions
Supramolecular chemistry is governed by the weak noncovalent reversible intermolecular forces. When in action, these distance and direction dependent interactions, such as intermolecular hydrogen bonds, dictate the molecular arrangement in the assembled structures. Chemical engineering of the building block/supramolecular synthon has led to the formation of some extraordinarily large molecular structures with excellent precisions. For example, utilization of the interaction between the pyridyl ligand and metal ions such as Pd(II) or Pt(II) has resulted in the generation of some very interesting molecular boxes/cages.169–171 This approach has resulted in the development of a vast number of supramolecular assemblies that find diverse applications in a broad range of fields including encapsulation, drug delivery, molecular recognition, ion transport, artificial photosynthesis, photocatalysis, self-healing systems, investigation of photo-reactions in confined spaces, etc.170,172–175 Recently, the self-assembly of peptides and proteins and DNA nanotechnology have attracted huge attention as they allow the formation of highly ordered nanostructures with nearly absolute control over the shape and geometry.2,176–179 DNA origami-based nanotechnology has been predicted to have potential impact in various research fields, especially in biomedical applications.180–182The might of the supramolecular-self-assembly approach, a simple yet extremely powerful method, has already been proven in the fields of biology and soft materials. Herein, we have reviewed its huge potential in designing materials for emerging applications such as photon up-conversion and circularly polarized luminescence. Molecular self-/co-assembly (vide supra) has direct influence on the following inter-molecular processes that play crucial roles in TTA-UC:
1. Triplet–triplet energy transfer is enhanced in a properly positioned donor–acceptor network.
2. The formed acceptor triplets have greater chances of annihilation (TTA) in such closely placed acceptor arrays.
3. Triplet energy migration becomes a dominant energy transfer route making the entire process more efficient.
4. The self-assembled network may provide a shield from the quenchers present in the solvent/environment.
5. The supramolecular assembly may help in achieving a much longer triplet lifetime.
In the case of CPL, the supramolecular self-/co-assembly may be applied in the following three ways to generate or amplify the CPL response:
1. An achiral fluorophore co-assembled with a non-luminescent chiral matrix.
2. An achiral fluorophore self-assembled to form a chiral supramolecular network.
3. A chiral fluorophore self-assembled network.
In other words, application of the supramolecular strategy may allow even an achiral fluorophore to exhibit CPL. A co-assembled network of a judiciously chosen fluorophore pair may lead to energy down-converted CPL (via FRET). As supramolecular interactions are sensitive to external stimuli such as pH, temperature, mechanical disturbances, etc., stimulus responsive CPL can also be designed. Additionally, CPL in an energy up-converted (TTA-UC) system could also be achieved.
To conclude, this strategy holds tremendous potential towards designing future photon up-conversion and circularly polarized luminescent materials. Photon up-conversion materials that may work under illumination of sunlight would have an immense impact on the current solar energy-based techniques.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
AS, GR and ARL are thankful to Ashoka University for their doctoral research fellowship. DA is thankful to the Axis Bank Grant for funding the photon up-conversion project.References
- J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1988, 27, 89–112 CrossRef.
- Q. Luo, C. Hou, Y. Bai, R. Wang and J. Liu, Chem. Rev., 2016, 116, 13571–13632 CrossRef CAS PubMed.
- J. W. Steed and J. L. Atwood, Supramolecular chemistry, Wiley-Blackwell, John Wiley & Sons, Inc., Hoboken, 3rd edn, 2021 Search PubMed.
- J.-H. Deng, J. Luo, Y.-L. Mao, S. Lai, Y.-N. Gong, D.-C. Zhong and T.-B. Lu, Sci. Adv., 2020, 6, eaax9976 CrossRef CAS PubMed.
- P. Li, M. R. Ryder and J. F. Stoddart, Acc. Mater. Res., 2020, 1, 77–87 CrossRef CAS.
- L. Zhang, X. Zhong, E. Pavlica, S. Li, A. Klekachev, G. Bratina, T. W. Ebbesen, E. Orgiu and P. Samorì, Nat. Nanotechnol., 2016, 11, 900–906 CrossRef CAS PubMed.
- V. Allain, C. Bourgaux and P. Couvreur, Nucleic Acids Res., 2011, 40, 1891–1903 CrossRef PubMed.
- D. M. Bassani, L. Jonusauskaite, A. Lavie-Cambot, N. D. McClenaghan, J.-L. Pozzo, D. Ray and G. Vives, Coord. Chem. Rev., 2010, 254, 2429–2445 CrossRef CAS.
- S. Mohnani and D. Bonifazi, Coord. Chem. Rev., 2010, 254, 2342–2362 CrossRef CAS.
- A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002–8018 CrossRef CAS PubMed.
- A. J. Savyasachi, O. Kotova, S. Shanmugaraju, S. J. Bradberry, G. M. Ó'Máille and T. Gunnlaugsson, Chem, 2017, 3, 764–811 CAS.
- T. Keijer, T. Bouwens, J. Hessels and J. N. H. Reek, Chem. Sci., 2020, 12, 50–70 RSC.
- X. Ma and Y. Zhao, Chem. Rev., 2015, 115, 7794–7839 CrossRef CAS PubMed.
- Q. Zhang, D.-H. Qu, H. Tian and B. L. Feringa, Matter, 2020, 3, 355–370 CrossRef.
- A. Ciesielski and P. Samorì, Nanoscale, 2011, 3, 1397 RSC.
- I. V. Kolesnichenko and E. V. Anslyn, Chem. Soc. Rev., 2017, 46, 2385–2390 RSC.
- A. P. Silva, H. NimaláGunaratne, P. MarkáLynch, E. Glenn and K. SamankumaraáSandanayake, Chem. Soc. Rev., 1992, 21, 187–195 RSC.
- F. J. Hoeben, P. Jonkheijm, E. Meijer and A. P. Schenning, Chem. Rev., 2005, 105, 1491–1546 CrossRef CAS PubMed.
- X. Cao, A. Gao, J.-T. Hou and T. Yi, Coord. Chem. Rev., 2021, 434, 213792 CrossRef CAS.
- X. Dou, N. Mehwish, C. Zhao, J. Liu, C. Xing and C. Feng, Acc. Chem. Res., 2020, 53, 852–862 CrossRef CAS PubMed.
- E. Busseron, Y. Ruff, E. Moulin and N. Giuseppone, Nanoscale, 2013, 5, 7098–7140 RSC.
- S. I. Stupp and L. C. Palmer, Chem. Mater., 2014, 26, 507–518 CrossRef CAS.
- D. B. Amabilino and P. A. Gale, Chem. Soc. Rev., 2017, 46, 2376–2377 RSC.
- C. Mongin, C.-K. Liang, B. Bibal and D. M. Bassani, Pure Appl. Chem., 2017, 89, 269–277 CrossRef CAS.
- R. Caballero, M. Barrejon, J. Cerda, J. Arago, S. Seetharaman, P. de la Cruz, E. Orti, F. D'Souza and F. Langa, J. Am. Chem. Soc., 2021, 143, 11199–11208 CrossRef CAS PubMed.
- B. Ferrer, G. Rogez, A. Credi, R. Ballardini, M. T. Gandolfi, V. Balzani, Y. Liu, H.-R. Tseng and J. F. Stoddart, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 18411–18416 CrossRef CAS PubMed.
- K. Kreger, H.-W. Schmidt and R. Hildner, Electron. Struct., 2021, 3, 023001 CrossRef CAS.
- B. Wittmann, F. A. Wenzel, S. Wiesneth, A. T. Haedler, M. Drechsler, K. Kreger, J. Köhler, E. W. Meijer, H.-W. Schmidt and R. Hildner, J. Am. Chem. Soc., 2020, 142, 8323–8330 CrossRef CAS PubMed.
- Applied Nanophotonics, ed. H. V. Demir and S. V. Gaponenko, Cambridge University Press, Cambridge, 2018, pp. 210–226, DOI:10.1017/9781316535868.008.
- Z. Huang and X. Ma, Cell Rep. Phys. Sci., 2020, 1, 100167 CrossRef CAS.
- N. Yanai and N. Kimizuka, Chem. Commun., 2016, 52, 5354–5370 RSC.
- H. Kouno, T. Ogawa, S. Amemori, P. Mahato, N. Yanai and N. Kimizuka, Chem. Sci., 2016, 7, 5224–5229 RSC.
- P. Bharmoria, N. Yanai and N. Kimizuka, Gels, 2019, 5, 18 CrossRef CAS PubMed.
- T. Ogawa, N. Yanai, A. Monguzzi and N. Kimizuka, Sci. Rep., 2015, 5, 10882 CrossRef CAS PubMed.
- A. Monguzzi, R. Tubino, S. Hoseinkhani, M. Campione and F. Meinardi, Phys. Chem. Chem. Phys., 2012, 14, 4322–4332 RSC.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- P. Duan, N. Yanai, H. Nagatomi and N. Kimizuka, J. Am. Chem. Soc., 2015, 137, 1887–1894 CrossRef CAS PubMed.
- S. S. Skourtis, C. Liu, P. Antoniou, A. M. Virshup and D. N. Beratan, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 8115–8120 CrossRef CAS PubMed.
- V. K. Praveen, C. Ranjith and N. Armaroli, Angew. Chem., Int. Ed., 2014, 53, 365–368 CrossRef CAS PubMed.
- Q. Zhao, Y. Chen, S.-H. Li and Y. Liu, Chem. Commun., 2018, 54, 200–203 RSC.
- R. Abbel, C. Grenier, M. J. Pouderoijen, J. W. Stouwdam, P. E. Leclere, R. P. Sijbesma, E. W. Meijer and A. P. Schenning, J. Am. Chem. Soc., 2009, 131, 833–843 CrossRef CAS PubMed.
- G. Sun, J. Pan, Y. Wu, Y. Liu, W. Chen, Z. Zhang and J. Su, ACS Appl. Mater. Interfaces, 2020, 12, 10875–10882 CrossRef CAS PubMed.
- T. Minami, Bull. Chem. Soc. Jpn., 2021, 94, 24–33 CrossRef CAS.
- K. T. Wong and D. Bassani, NPG Asia Mater., 2014, 6, e116 CrossRef CAS.
- F. Song, Z. Zhao, Z. Liu, J. W. Lam and B. Z. Tang, J. Mater. Chem. C, 2020, 8, 3284–3301 RSC.
- Z. Cai, Z. Li, S. Ravaine, M. He, Y. Song, Y. Yin, H. Zheng, J. Teng and A. Zhang, Chem. Soc. Rev., 2021, 50, 5898–5951 RSC.
- Z. Wang, C. L. C. Chan, T. H. Zhao, R. M. Parker and S. Vignolini, Adv. Opt. Mater., 2021, 9, 2100519 CrossRef CAS.
- M. Grzelczak, J. Vermant, E. M. Furst and L. M. Liz-Marzán, ACS Nano, 2010, 4, 3591–3605 CrossRef CAS PubMed.
- W. Wei, F. Bai and H. Fan, Angew. Chem., Int. Ed., 2019, 58, 11956–11966 CrossRef CAS PubMed.
- J. R. Lakowicz, Principles of fluorescence spectroscopy, Springer, 2006 Search PubMed.
- B. S. Richards, D. Hudry, D. Busko, A. Turshatov and I. A. Howard, Chem. Rev., 2021, 121, 9165–9195 CrossRef CAS PubMed.
- D. Beery, T. W. Schmidt and K. Hanson, ACS Appl. Mater. Interfaces, 2021, 13, 32601–32605 CrossRef CAS PubMed.
- A. J. Carrod, V. Gray and K. Börjesson, Energy Environ. Sci., 2022, 15, 4982–5016 RSC.
- J. C. Goldschmidt and S. Fischer, Adv. Opt. Mater., 2015, 3, 510–535 CrossRef CAS.
- J. Pedrini and A. Monguzzi, J. Photonics Energy, 2017, 8, 022005 Search PubMed.
- J. Zhang, R. Zhang, K. Liu, Y. Li, X. Wang, X. Xie, X. Jiao and B. Tang, Chem. Commun., 2021, 57, 8320–8323 RSC.
- L. Zeng, L. Huang, J. Han and G. Han, Acc. Chem. Res., 2022, 55, 2604–2615 CrossRef CAS PubMed.
- L. Huang and G. Han, Small Methods, 2018, 2, 1700370 CrossRef PubMed.
- S. H. Askes, A. Bahreman and S. Bonnet, Angew. Chem., 2014, 126, 1047–1051 CrossRef.
- L. Huang, Y. Zhao, H. Zhang, K. Huang, J. Yang and G. Han, Angew. Chem., 2017, 129, 14592–14596 CrossRef.
- T. Schloemer, P. Narayanan, Q. Zhou, E. Belliveau, M. Seitz and D. N. Congreve, ACS Nano, 2023, 17, 3259–3288 CrossRef CAS PubMed.
- F. Auzel, Chem. Rev., 2004, 104, 139–174 CrossRef CAS PubMed.
- N. Yanai and N. Kimizuka, Angew. Chem., Int. Ed., 2020, 59, 10252–10264 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- C. Healy, L. Hermanspahn and P. E. Kruger, Coord. Chem. Rev., 2021, 432, 213756 CrossRef CAS.
- X. Ma and Y. Zhao, Chem. Rev., 2015, 115, 7794–7839 CrossRef CAS PubMed.
- R. Pinalli, A. Pedrini and E. Dalcanale, Chem. Soc. Rev., 2018, 47, 7006–7026 RSC.
- M. J. Webber and R. Langer, Chem. Soc. Rev., 2017, 46, 6600–6620 RSC.
- G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS PubMed.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- C. Fan, W. Wu, J. J. Chruma, J. Zhao and C. Yang, J. Am. Chem. Soc., 2016, 138, 15405–15412 CrossRef CAS PubMed.
- W. Xu, W. Liang, W. Wu, C. Fan, M. Rao, D. Su, Z. Zhong and C. Yang, Chem. – Eur. J., 2018, 24, 16677–16685 CrossRef CAS PubMed.
- I. Roy, A. Garci, Y. Beldjoudi, R. M. Young, D. J. Pe, M. T. Nguyen, P. J. Das, M. R. Wasielewski and J. F. Stoddart, J. Am. Chem. Soc., 2020, 142, 16600–16609 CrossRef CAS PubMed.
- D. Asthana, S. Hisamitsu, M.-A. Morikawa, P. Duan, T. Nakashima, T. Kawai, N. Yanai and N. Kimizuka, Org. Mater., 2019, 1, 043–049 CrossRef CAS.
- R. Haruki, H. Kouno, M. Hosoyamada, T. Ogawa, N. Yanai and N. Kimizuka, Chem. – Asian J., 2019, 14, 1723–1728 CrossRef CAS PubMed.
- P. Duan, D. Asthana, T. Nakashima, T. Kawai, N. Yanai and N. Kimizuka, Faraday Discuss., 2017, 196, 305–316 RSC.
- Q. Guo, G. Li, M. Rao, L. Wei, F. Gao, W. Wu, Q. Yang, G. Cheng and C. Yang, Dyes Pigm., 2020, 182, 108643 CrossRef CAS.
- M. Hosoyamada, N. Yanai, T. Ogawa and N. Kimizuka, Chem. – Eur. J., 2016, 22, 2060–2067 CrossRef CAS PubMed.
- A. Monguzzi, M. Frigoli, C. Larpent, R. Tubino and F. Meinardi, Adv. Funct. Mater., 2012, 22, 139–143 CrossRef CAS.
- A. Monguzzi, S. M. Borisov, J. Pedrini, I. Klimant, M. Salvalaggio, P. Biagini, F. Melchiorre, C. Lelii and F. Meinardi, Adv. Funct. Mater., 2015, 25, 5617–5624 CrossRef CAS.
- N. Harada, Y. Sasaki, M. Hosoyamada, N. Kimizuka and N. Yanai, Angew. Chem., Int. Ed., 2021, 60, 142–147 CrossRef CAS PubMed.
- S. P. Hill and K. Hanson, J. Am. Chem. Soc., 2017, 139, 10988–10991 CrossRef CAS PubMed.
- T. Dilbeck, S. P. Hill and K. Hanson, J. Mater. Chem. A, 2017, 5, 11652–11660 RSC.
- J. Pedrini, A. Monguzzi and F. Meinardi, Phys. Chem. Chem. Phys., 2018, 20, 9745–9750 RSC.
- P. Bharmoria, S. Hisamitsu, H. Nagatomi, T. Ogawa, M.-A. Morikawa, N. Yanai and N. Kimizuka, J. Am. Chem. Soc., 2018, 140, 10848–10855 CrossRef CAS PubMed.
- H. Kouno, Y. Sasaki, N. Yanai and N. Kimizuka, Chem. – Eur. J., 2019, 25, 6124–6130 CrossRef CAS PubMed.
- Y. Kawashima, H. Kouno, K. Orihashi, K. Nishimura, N. Yanai and N. Kimizuka, Mol. Syst. Des. Eng., 2020, 5, 792–796 RSC.
- J. Park, M. Xu, F. Li and H.-C. Zhou, J. Am. Chem. Soc., 2018, 140, 5493–5499 CrossRef CAS PubMed.
- B. Nordén, A. Rodger and T. Dafforn, Linear dichroism and circular dichroism: a textbook on polarized-light spectroscopy, Royal Society of Chemistry, 2019 Search PubMed.
- Introduction to Polarized Light, https://www.microscopyu.com/techniques/polarized-light/introduction-to-polarized-light.
- P. C. Mondal, D. Asthana, R. K. Parashar and S. Jadhav, Mater. Adv., 2021, 2, 7620–7637 RSC.
- A. Rodger and B. Nordén, Circular dichroism and linear dichroism, Oxford University Press, USA, 1997 Search PubMed.
- N. J. Greenfield, Nat. Protoc., 2006, 1, 2876–2890 CrossRef CAS PubMed.
- A. J. Miles, R. W. Janes and B. A. Wallace, Chem. Soc. Rev., 2021, 50, 8400–8413 RSC.
- A. Rodger and D. Marshall, Biochemist, 2021, 43, 58–64 CrossRef CAS.
- J. Kumar, T. Nakashima and T. Kawai, J. Phys. Chem. Lett., 2015, 6, 3445–3452 CrossRef CAS PubMed.
- F. S. Richardson and J. P. Riehl, Chem. Rev., 1977, 77, 773–792 CrossRef CAS.
- J. P. Riehl and F. S. Richardson, Chem. Rev., 1986, 86, 1–16 CrossRef CAS.
- J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef.
- L. Yang, J. Huang, M. Qin, X. Ma, X. Dou and C. Feng, Nanoscale, 2020, 12, 6233–6238 RSC.
- T. He, C. Ren, Y. Luo, Q. Wang, J. Li, X. Lin, C. Ye, W. Hu and J. Zhang, Chem. Sci., 2019, 10, 4163–4168 RSC.
- S. Furumi, Chem. Rec., 2010, 10, 394–408 CrossRef CAS PubMed.
- A. Montali, C. Bastiaansen, P. Smith and C. Weder, Nature, 1998, 392, 261–264 CrossRef CAS.
- B. L. Feringa and R. A. Van Delden, Angew. Chem., Int. Ed., 1999, 38, 3418–3438 CrossRef PubMed.
- S. Suzuki, in Circularly Polarized Luminescence of Isolated Small Organic Molecules, ed. T. Mori, Springer Singapore, Singapore, 2020, pp. 309–325, DOI:10.1007/978-981-15-2309-0_14.
- S. C. J. Meskers, ChemPhotoChem, 2022, 6, e202100154 CrossRef CAS.
- Y. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao and Z. Tang, Matter, 2022, 5, 837–875 CrossRef CAS.
- Y. Dai, J. Chen, C. Zhao, L. Feng and X. Qu, Angew. Chem., Int. Ed., 2022, 61, e202211822 CrossRef CAS PubMed.
- Y. Liu and P. Xing, Adv. Mater., 2023, 2300968 CrossRef PubMed.
- N. P. M. Huck, W. F. Jager, B. de Lange and B. L. Feringa, Science, 1996, 273, 1686–1688 CrossRef CAS.
- J. S. Kang, N. Kim, T. Kim, M. Seo and B.-S. Kim, Macromol. Rapid Commun., 2022, 43, 2100649 CrossRef CAS PubMed.
- J. S. Kang, S. Kang, J.-M. Suh, S. M. Park, D. K. Yoon, M. H. Lim, W. Y. Kim and M. Seo, J. Am. Chem. Soc., 2022, 144, 2657–2666 CrossRef CAS PubMed.
- C. Meinert, S. V. Hoffmann, P. Cassam-Chenaï, A. C. Evans, C. Giri, L. Nahon and U. J. Meierhenrich, Angew. Chem., Int. Ed., 2014, 53, 210–214 CrossRef CAS PubMed.
- P. K. Hashim and N. Tamaoki, ChemPhotoChem, 2019, 3, 347–355 CrossRef CAS.
- J. S. Kang, S. Kang, J.-M. Suh, S. M. Park, D. K. Yoon, M. H. Lim, W. Y. Kim and M. Seo, J. Am. Chem. Soc., 2022, 144, 2657–2666 CrossRef CAS PubMed.
- Y. Deng, M. Wang, Y. Zhuang, S. Liu, W. Huang and Q. Zhao, Light: Sci. Appl., 2021, 10, 76 CrossRef CAS PubMed.
- E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Chem. – Eur. J., 2015, 21, 13488–13500 CrossRef PubMed.
- Y. Sang, J. Han, T. Zhao, P. Duan and M. Liu, Adv. Mater., 2020, 32, 1900110 CrossRef CAS PubMed.
- F. Song, Z. Zhao, Z. Liu, J. W. Y. Lam and B. Z. Tang, J. Mater. Chem. C, 2020, 8, 3284–3301 RSC.
- J. Zhao and P. Xing, ChemPhotoChem, 2022, 6, e202100124 CrossRef CAS.
- Q. Li, J. Zhang, Y. Wang, G. Zhang, W. Qi, S. You, R. Su and Z. He, Nano Lett., 2021, 21, 6406–6415 CrossRef CAS PubMed.
- A. R. A. Palmans, J. A. J. M. Vekemans, E. E. Havinga and E. W. Meijer, Angew. Chem., Int. Ed. Engl., 1997, 36, 2648–2651 CrossRef CAS.
- M. M. Green, N. C. Peterson, T. Sato, A. Teramoto, R. Cook and S. Lifson, Science, 1995, 268, 1860–1866 CrossRef CAS PubMed.
- A. R. A. Palmans and E. W. Meijer, Angew. Chem., Int. Ed., 2007, 46, 8948–8968 CrossRef CAS PubMed.
- M. M. J. Smulders, A. P. H. J. Schenning and E. W. Meijer, J. Am. Chem. Soc., 2008, 130, 606–611 CrossRef CAS PubMed.
- R. Eelkema and B. L. Feringa, Org. Biomol. Chem., 2006, 4, 3729–3745 RSC.
- M. A. Mateos-Timoneda, M. Crego-Calama and D. N. Reinhoudt, Chem. Soc. Rev., 2004, 33, 363–372 RSC.
- J. J. L. M. Cornelissen, A. E. Rowan, R. J. M. Nolte and N. A. J. M. Sommerdijk, Chem. Rev., 2001, 101, 4039–4070 CrossRef CAS PubMed.
- L. J. Prins, J. Huskens, F. de Jong, P. Timmerman and D. N. Reinhoudt, Nature, 1999, 398, 498–502 CrossRef CAS.
- D. J. van Dijken, J. M. Beierle, M. C. A. Stuart, W. Szymański, W. R. Browne and B. L. Feringa, Angew. Chem., Int. Ed., 2014, 53, 5073–5077 CrossRef CAS PubMed.
- P. Duan, H. Cao, L. Zhang and M. Liu, Soft Matter, 2014, 10, 5428–5448 RSC.
- L. Zhang, L. Qin, X. Wang, H. Cao and M. Liu, Adv. Mater., 2014, 26, 6959–6964 CrossRef CAS PubMed.
- S. Huang, H. Yu and Q. Li, Adv. Sci., 2021, 8, 2002132 CrossRef PubMed.
- A. E. Rowan and R. J. M. Nolte, Angew. Chem., Int. Ed., 1998, 37, 63–68 CrossRef CAS.
- D. K. Smith, Chem. Soc. Rev., 2009, 38, 684–694 RSC.
- M. Liu, L. Zhang and T. Wang, Chem. Rev., 2015, 115, 7304–7397 CrossRef CAS PubMed.
- C. Liu, J.-C. Yang, J. W. Y. Lam, H.-T. Feng and B. Z. Tang, Chem. Sci., 2022, 13, 611–632 RSC.
- Y. Sang, D. Yang, Z. Shen, P. Duan and M. Liu, J. Phys. Chem. C, 2020, 124, 17274–17281 CrossRef CAS.
- M. Zhou, Y. Sang, X. Jin, S. Chen, J. Guo, P. Duan and M. Liu, ACS Nano, 2021, 15, 2753–2761 CrossRef CAS PubMed.
- Q. Jiang, X. Xu, P.-A. Yin, K. Ma, Y. Zhen, P. Duan, Q. Peng, W.-Q. Chen and B. Ding, J. Am. Chem. Soc., 2019, 141, 9490–9494 CrossRef CAS PubMed.
- B.-C. Kim, H.-J. Choi, J.-J. Lee, F. Araoka and S.-W. Choi, Adv. Funct. Mater., 2019, 29, 1903246 CrossRef.
- T. Li, H. Guo, Y. Wang, G. Ouyang, Q.-Q. Wang and M. Liu, Chem. Commun., 2021, 57, 13554–13557 RSC.
- K. Q. Le, S. Hashiyada, M. Kondo and H. Okamoto, J. Phys. Chem. C, 2018, 122, 24924–24932 CrossRef CAS.
- K. Yang, S. Ma, Y. Wu, B. Zhao and J. Deng, Chem. Mater., 2023, 35, 1273–1282 CrossRef CAS.
- Z.-W. Luo, A. Huang, H.-Y. Luo, J.-K. Chen, J. Huang and H.-L. Xie, Macromolecules, 2023, 56, 2700–2708 CrossRef CAS.
- Y. Bao, G. Zhang, N. Wang, M. Pan and W. Zhang, J. Mater. Chem. C, 2023, 11, 2475–2479 RSC.
- J. Wade, J. R. Brandt, D. Reger, F. Zinna, K. Y. Amsharov, N. Jux, D. L. Andrews and M. J. Fuchter, Angew. Chem., Int. Ed., 2021, 60, 222–227 CrossRef CAS PubMed.
- K. S. Park, Z. Xue, B. B. Patel, H. An, J. J. Kwok, P. Kafle, Q. Chen, D. Shukla and Y. Diao, Nat. Commun., 2022, 13, 2738 CrossRef CAS PubMed.
- J. Zhang, J. Hao, Y. Wei, F. Xiao, P. Yin and L. Wang, J. Am. Chem. Soc., 2010, 132, 14–15 CrossRef CAS PubMed.
- L.-J. Chen, H.-B. Yang and M. Shionoya, Chem. Soc. Rev., 2017, 46, 2555–2576 RSC.
- H. Cao, Q. Yuan, X. Zhu, Y.-P. Zhao and M. Liu, Langmuir, 2012, 28, 15410–15417 CrossRef CAS PubMed.
- B. Chang, X. Li and T. Sun, Eur. Polym. J., 2019, 118, 365–381 CrossRef CAS.
- H. Song, H. Zhu, Z. Huang, Y. Zhang, W. Zhao, J. Liu, Q. Chen, C. Yin, L. Xing, Z. Peng, P. Liao, Y. Wang, Y. Wang and K. Wu, ACS Nano, 2019, 13, 7202–7208 CrossRef CAS PubMed.
- Z. Cheng and M. R. Jones, Nat. Commun., 2022, 13, 4207 CrossRef CAS PubMed.
- S. Kikkawa, M. Okayasu, H. Hikawa and I. Azumaya, Cryst. Growth Des., 2021, 21, 1148–1158 CrossRef CAS.
- W. Shang, X. Zhu, T. Liang, C. Du, L. Hu, T. Li and M. Liu, Angew. Chem., Int. Ed., 2020, 59, 12811–12816 CrossRef CAS PubMed.
- W. Yang, X. Chai, L. Chi, X. Liu, Y. Cao, R. Lu, Y. Jiang, X. Tang, H. Fuchs and T. Li, Chem. – Eur. J., 1999, 5, 1144–1149 CrossRef CAS.
- Anuradha, D. D. La, M. Al Kobaisi and S. V. Bhosale, Sci. Rep., 2015, 5, 15652 CrossRef CAS PubMed.
- S. Pakhomov, R. P. Hammer, B. K. Mishra and B. N. Thomas, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3040–3042 CrossRef PubMed.
- E. Yashima, N. Ousaka, D. Taura, K. Shimomura, T. Ikai and K. Maeda, Chem. Rev., 2016, 116, 13752–13990 CrossRef CAS PubMed.
- E. R. Zubarev, M. U. Pralle, E. D. Sone and S. I. Stupp, J. Am. Chem. Soc., 2001, 123, 4105–4106 CrossRef CAS PubMed.
- D. Asthana, J. Shukla, S. Dana, V. Rani, M. R. Ajayakumar, K. Rawat, K. Mandal, P. Yadav, S. Ghosh and P. Mukhopadhyay, Chem. Commun., 2015, 51, 15237–15240 RSC.
- G. Zhang, X. Cheng, Y. Wang and W. Zhang, Aggregate, 2023, 4, e262 CrossRef CAS.
- S.-Y. Yu, Z.-X. Zhang, E. C.-C. Cheng, Y.-Z. Li, V. W.-W. Yam, H.-P. Huang and R. Zhang, J. Am. Chem. Soc., 2005, 127, 17994–17995 CrossRef CAS PubMed.
- L. Caswell, M. A. Garcia-Garibay, J. R. Scheffer and J. Trotter, J. Chem. Educ., 1993, 70, 785 CrossRef CAS.
- J. Zhao, T. Zhang, X.-Y. Dong, M.-E. Sun, C. Zhang, X. Li, Y. S. Zhao and S.-Q. Zang, J. Am. Chem. Soc., 2019, 141, 15755–15760 CrossRef CAS PubMed.
- S. Kirschner and K. R. Magnell, in Werner Centennial, American chemical society, 1967, vol. 62, ch. 24, pp. 366–377 Search PubMed.
- G. Park, H. Kim, H. Yang, K. R. Park, I. Song, J. H. Oh, C. Kim and Y. You, Chem. Sci., 2019, 10, 1294–1301 RSC.
- S. Sato, T. Murase and M. Fujita, in Supramol. Chem, 2012, DOI:10.1002/9780470661345.smc078.
- M. Han, D. M. Engelhard and G. H. Clever, Chem. Soc. Rev., 2014, 43, 1848–1860 RSC.
- R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed.
- F. Hof, S. L. Craig, C. Nuckolls and J. Rebek, Jr., Angew. Chem., Int. Ed., 2002, 41, 1488–1508 CrossRef CAS PubMed.
- A. B. Grommet, L. M. Lee and R. Klajn, Acc. Chem. Res., 2020, 53, 2600–2610 CrossRef CAS PubMed.
- A. B. Grommet, M. Feller and R. Klajn, Nat. Nanotechnol., 2020, 15, 256–271 CrossRef CAS PubMed.
- R. Ham, C. J. Nielsen, S. Pullen and J. N. H. Reek, Chem. Rev., 2023, 123, 5225–5261 CrossRef CAS PubMed.
- F. Zhang, J. Nangreave, Y. Liu and H. Yan, J. Am. Chem. Soc., 2014, 136, 11198–11211 CrossRef CAS PubMed.
- N. C. Seeman and H. F. Sleiman, Nat. Rev. Mater., 2017, 3, 17068 CrossRef.
- F. Hong, F. Zhang, Y. Liu and H. Yan, Chem. Rev., 2017, 117, 12584–12640 CrossRef CAS PubMed.
- Q. Li, Y. Wang, G. Zhang, R. Su and W. Qi, Chem. Soc. Rev., 2023, 52, 1549–1590 RSC.
- L. Li, S. Nie, T. Du, J. Zhao and X. Chen, MedComm – Biomaterials and Applications, 2023, vol. 2, p. e37 Search PubMed.
- M. Endo, Y. Yang and H. Sugiyama, Biomater. Sci., 2013, 1, 347–360 RSC.
- P. Zhan, A. Peil, Q. Jiang, D. Wang, S. Mousavi, Q. Xiong, Q. Shen, Y. Shang, B. Ding, C. Lin, Y. Ke and N. Liu, Chem. Rev., 2023, 123, 3976–4050 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |