Open Access Article
Open Access ArticleAll-in-one mitochondria-targeted NIR-II fluorophores for cancer therapy and imaging†
Yujia
Zheng‡
ab,
Qianqian
Li‡
ab,
Jing
Wu
a,
Ziyi
Luo
a,
Wenyi
Zhou
a,
Anguo
Li
a,
Yanling
Chen
a,
Tuerxunayi
Rouzi
a,
Tian
Tian
b,
Hui
Zhou
 ad,
Xiaodong
Zeng
ad,
Xiaodong
Zeng
 ad,
Yang
Li
ad,
Xiaoding
Cheng
ad,
Yongchang
Wei
c,
Zixin
Deng
a,
Fuling
Zhou
*a and
Xuechuan
Hong
ad,
Yang
Li
ad,
Xiaoding
Cheng
ad,
Yongchang
Wei
c,
Zixin
Deng
a,
Fuling
Zhou
*a and
Xuechuan
Hong
 *abd
*abd
aDepartment of Hematology, Zhongnan Hospital of Wuhan University, State Key Laboratory of Virology, Wuhan University School of Pharmaceutical Sciences, Wuhan 430071, China. E-mail: Xhy78@whu.edu.cn; zhoufuling@whu.edu.cn
bCollege of Science, Innovation Center for Traditional Tibetan Medicine Modernization and Quality Control, Tibet University, Lhasa, 850000, China
cDepartment of Radiation Oncology, Zhongnan Hospital of Wuhan University, Wuhan, 430071, China
dShenzhen Institute of Wuhan University, Shenzhen 518057, China
First published on 27th November 2020
Abstract
Small-molecule subcellular organelle-targeting theranostic probes are crucial for early disease diagnosis and treatment. The imaging window of these molecules is mainly focused on the visible and near-infrared region (below ∼900 nm) which limits the tissue penetration depth and therapeutic effects. Herein, a novel NIR-II small-molecule probe H4–PEG-Glu with a thiopyrylium cation was synthesized. H4–PEG-Glu not only can quickly and effectively image mitochondria in acute myeloid leukemia (AML) cells, and induce G0/G1 phase arrest by the intrinsic mitochondrial apoptosis pathway w/o irradiation, but also exhibit moderate cytotoxicity against AML cancer cells in a dose dependent-manner without laser irradiation. The THP-1 cells treated with H4–PEG-Glu upon NIR laser irradiation showed enhanced chemo- and photothermal therapy (CPTT) with 93.07% ± 6.43 apoptosis by Annexin V staining. Meanwhile, H4–PEG-Glu displayed high synergistic CPTT effects in vivo, as well as specific NIR-II tumor imaging in AML patient derived PDX mouse models for the first time. Our work lays down a solid foundation for designing small-molecule NIR-II mitochondria-selective theranostic probes.
Introduction
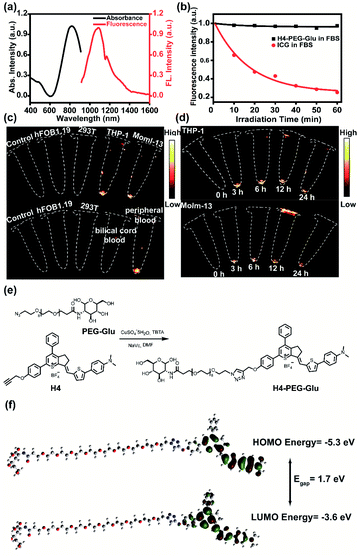
Mitochondria, as an important type of subcellular organelles, plays an essential role in energy production and cell survival.1 Mitochondria regulates many cellular behaviors such as cellular signaling, differentiation, growth, apoptosis, metabolism and death.2 Because of the importance of mitochondria in cellular regulation and death, a number of small-molecule mitochondria-targeted theranostic probes, including rhodamine, triphenyl phosphonium (TPP), F16, dequalinium (DQA), and guanidine have been developed.3 Furthermore, when introducing 2-deoxy-D-glucose into theranostic probes, it is possible to enhance their potency against drug-resistant tumors in one delivery system.4 It is worth noting that an F16 derivative 5BMF was firstly accomplished with an IC50 value of ∼50 nM against H2228 cells and in vivo fluorescence imaging at ∼550 nm.5 However, small-molecule subcellular organelle-targeting theranostic probes are still in their infancy. The imaging window of these molecules is mainly focused on the visible and near-infrared region (below ∼900 nm) which still limits the tissue penetration depth and therapeutic effects. Acute Myeloid Leukemia (AML) is a heterogeneous and complex hematological malignancy that affects ∼1 million people each year globally.6 Various treatments such as molecularly targeted chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT) have been extensively applied in clinic treatment.7 However, the 5 year survival rate for patients 20 years older with AML is still quite gloomy (∼25%), and the treatment of AML remains one of the most formidable challenges in clinic research. Recent developments in cancer therapy were mostly focused on solid tumor models, and very few small-molecule probes have been exploited for the diagnosis and treatment of AML owing to the circulation of cancer cells in the immune system.8 Thus, it is imperative to pursue a novel therapeutic strategy for AML.In recent years, fluorescence imaging in the second near-infrared window (NIR-II, 1000–1700 nm) has emerged as a novel technique, and been widely used in tumor imaging, image-guided surgery and therapy with deep tissue penetration and subcellular resolution.9 To date, several types of NIR-II inorganic materials,10 macromolecules11 and small-molecule fluorophores,12 such as quantum dots,13 ultrafine gold nanoclusters,14 single-walled carbon nanotubes,15 rare earth nanoparticles,16 and CH1055,17 have been reported. Of note, organic dyes FD-1080,18HQL2,19HL3 (ref. 20) and 2TT-oC26B21 were employed for NIR-IIb (1500–1700 nm) lymphatic drainage, the whole body, cerebral vasculature and vascular imaging with unparalleled tissue-imaging depths, near-zero auto-fluorescence and negligible scattering. Among them, a few of the inorganic fluorophores had potential safety concerns in clinic translation because of high uptakes and long-term retention in the immune system.22 Currently, small-molecule NIR-II fluorophores are mainly focused on donor–acceptor–donor (D–A–D) or polymethine structures.23 However, no research based on small-molecule mitochondria-targeted NIR-II imaging technology has been reported for in vivo AML imaging, and anti-AML chemo- and photothermal therapy.24 Herein, NIR-II thiopyrylium fluorophores H4–PEG2K and H4–PEG-Glu were synthesized based on our previous reports.25 Then, anti-cancer cell activity, the selective mitochondria-targeting ability and photothermal properties of NIR-II probes H4–PEG2K and H4–PEG-Glu against THP-1 and Molm-13 cells were investigated. In vitro cell studies of H4–PEG-Glu showed high affinity and moderate cytotoxicity against THP-1 and Molm-13 cells. Upon 808 nm laser irradiation, H4–PEG-Glu produced hyperthermia and showed enhanced cellular cytotoxicity toward AML cells, exhibiting synergistic CPTT effects. An enhanced cytotoxicity with 93.07% ± 6.43 and 89.44% ± 2.84 apoptosis of THP-1 and Molm-13 cells was observed by Annexin V staining through flow cytometry. It was further demonstrated that H4–PEG-Glu induced G0/G1 phase arrest w/o irradiation and showed super mitochondria-targeted bioimaging in live cancer cells. Subsequently, AML patient derived PDX mouse models were, for the first time, adopted for NIR-II imaging and image-guided CPPT. In addition, a high level of accumulation was also observed in the bone marrow of the AML models. The leukemia stem cell (LSC) population CD34+ decreased sharply under irradiation in comparison to the other groups, manifesting high synergistic CPTT effects of H4–PEG-Glu toward AML in vivo. In a proof-of-concept study, we have developed a small-molecule NIR-II mitochondria-selective theranostic agent for the real-time imaging and therapy of AML in AML patient derived PDX mouse models, which could be a powerful biomedical research tool for detection, imaging and image-guided therapy of AML with a good safety profile, subcellular resolution and deep tissue penetration.
Results and discussion
Lipophilic or delocalized cations (LDC) play a crucial role in designing mitochondria-specific delivery of imaging and therapeutic agents. LDC can distribute electrophoretically into the mitochondrial matrix in response to the electric potential between the inner and the outer mitochondrial membrane. Therefore, simple molecular cations with synergetic mitochondria targeted chemo- and photothermal therapy may be capable of exerting all-in-one theranostic anti-tumor activity against AML. Thus, we utilized a thiopyrylium cation as an NIR acceptor to construct a novel mitochondria-targeted NIR-II chemo- and photothermal therapeutic dye H4–PEG-Glu. First, we designed and synthesized an unsymmetrical fluorophore H4 through a condensation reaction between the key intermediate thiopyrylium tetrafluoroborate 1 and aldehyde 2 in acetic anhydride at 75 °C for 2 h under microwave irradiation in 52% yield (see ESI†).25 To further explore the biological application, H4 was further conjugated with PEG-Gluvia a Cu-catalyzed click reaction to afford H4–PEG-Glu in anhydrous DMF in 52.5% yield (Fig. 1e). The highest occupied molecular orbital (HOMO, −5.30 eV) and the lowest unoccupied molecular orbital (LUMO, −3.60 eV) of H4–PEG-Glu were obtained by DFT calculations with the B3LYP exchange functional employing 6-31G(d) basis sets (Fig. 1f). The Egap (1.7 eV) of H4–PEG-Glu was close to that of CH1055 (1.5 eV) with a typical NIR-II optical Egap based on previous results.17H4–PEG-Glu was further purified using HPLC and all compounds were characterized by 1H-NMR, 13C-NMR, HRMS and MALDI-TOF-MS (Fig. S1–S6†).H4–PEG-Glu exhibited excellent solubility in common organic solvents and high aqueous solubility. The UV-vis-NIR absorption and NIR-II fluorescence emission spectra of H4–PEG-Glu in CH2Cl2 were investigated under 808 nm excitation. As shown in Fig. 1a, the absorption peak was at ∼810 nm, while the fluorescence emission peak was at ∼1085 nm and a shoulder peak was at ∼1200 nm. The NIR-II quantum yield (QY) of H4–PEG-Glu in fetal bovine serum (FBS) was measured to be ∼1.3% under 808 nm laser excitation using IR-26 dye as a reference (QY = 0.5%) (Fig. S7†). H4–PEG-Glu showed spherical shapes with an average particle size of ∼96 nm and hydrodynamic radius of ∼120 nm, as determined from transmission electron microscopy (TEM) and dynamic light scattering (DLS), respectively (Fig. S8†). H4–PEG-Glu displayed negligible decay in FBS under continuous 808 nm laser irradiation at a power density of 180 mW cm−2 for 60 min (50 ms exposure time), while indocyanine green (ICG) exhibited a sharp decrease in fluorescence intensity under the same conditions, indicating its high resistance to photo-bleaching for NIR-II biomedical application (Fig. 1b). Additionally, the cellular uptake behavior and imaging ability of ALM cancer cells were also investigated. As depicted in Fig. 1c and d, the human myeloid leukemia mononuclear cells THP-1 and Molm-13 exhibited high affinity and strong intracellular NIR-II fluorescence whereas negligible binding was observed in 293T and hFOB1.19 cells treated with H4–PEG-Glu (20 μM) (Fig. 1c). The THP-1 and Molm-13 cell lines can be clearly visualized after 3 h of incubation, and fluorescence signals reach a maximum at 6 h, while H4–PEG2K has demonstrated almost no targeting ability without the glucose moiety (Fig. 1d, Fig. S12a and b†). To further illustrate the specificity toward AML cancer cells, we further studied the cellular uptake behavior in peripheral blood donated from AML patients and umbilical cord blood collected from healthy people (Fig. 1c). We were delighted to find out that the peripheral blood samples had bright NIR-II fluorescence instead of umbilical cord blood, indicating that H4–PEG-Glu has an AML targeting and accumulation capability and is a promising candidate for targeted NIR-II imaging.
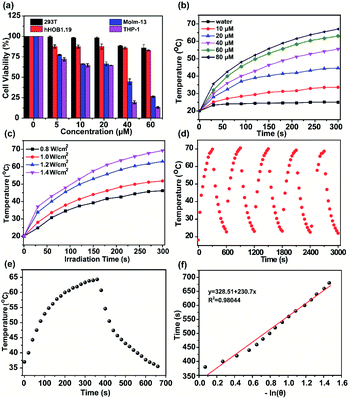
The cell viabilities of H4–PEG-Glu were investigated by CCK-8 assays with 293T, hFOB1.19, THP-1 and Molm-13 cell lines with different concentrations within 24 h without laser irradiation. As shown in Fig. 2a, no obvious cytotoxicity of H4–PEG-Glu was observed even at higher concentrations up to 60 μM, suggesting H4–PEG-Glu had excellent biocompatibility toward 293T and hFOB1.19 normal cells (Fig. 2a). In contrast, H4–PEG-Glu suppressed proliferation of AML cells (THP-1 and Molm-13) in a concentration-dependent manner with IC50 values of 23.97 ± 3.24 and 29.66 ± 1.09 μM, respectively, indicating a specific cytotoxic effect against AML cells without laser irradiation (Fig. 2a). Thereafter, we evaluated the photothermal performance of H4–PEG-Glu. The temperature of H4–PEG-Glu in serum at various concentrations (0 μM, 10 μM, 20 μM, 40 μM, 60 μM, 80 μM) was recorded under 808 nm laser irradiation at a power density of 1.2 W cm−2 for 5 min. Rapid photothermal effects occurred on laser irradiation even at the dose of 40 μM (Fig. 2b). The temperature increased significantly (up to 63 °C) at the dose of 60 μM by varying the laser power density (1.2 and 1.4 W cm−2) (Fig. 2c). The photothermal stability of H4–PEG-Glu (60 μM) in serum was further assessed by continuously monitoring the temperature variation under 808 nm laser irradiation (1.4 W cm−2) for 5 min, and it was naturally cooled to room temperature for five heating/cooling cycles. As shown in Fig. 2d, no obvious decline of temperature was observed in its photothermal conversion performance. The photothermal conversion efficiency of H4–PEG-Glu was ∼11.6% according to the reported methods (Fig. 2e and f).26 No significant amount of ROS in vitro was observed in any group of H4–PEG-Glu under 808 nm laser irradiation (Fig. S9†). As a consequence, the good photostability and excellent photothermal performance of H4–PEG-Glu make it an ideal NIR-II probe for in vivo fluorescence image-guided photothermal therapy.
Subsequently, the photothermal ablation of THP-1 and Molm-13 cells induced by H4–PEG-Glu was conducted under 808 nm NIR laser irradiation (1.2 W cm−2). After NIR laser treatment, the cells were observed with a bright-field microscope. As shown in Fig. 3a, H4–PEG-Glu (20 μM) treated THP-1 or Molm-13 cells had an enhanced chemo- and photothermal cytotoxicity under NIR irradiation compared to that under PBS, 808 nm laser or H4–PEG-Glu (20 μM) alone. The cell viability of THP-1 and Molm-13 cells was also quantitatively analyzed by the Trypan Blue dye exclusion test. As shown in Fig. 3b, approximately 39.57% ± 5.87 of THP-1 cells and 42.9% ± 5.58 of Molm-13 cells were reduced by H4–PEG-Glu (20 μM) induced synergistic therapy. The apoptosis of THP-1 and Molm-13 cells was also studied by Annexin V staining through flow cytometry. THP-1 and Molm-13 cells treated with H4–PEG-Glu under 808 nm laser irradiation showed an enhanced cytotoxicity with 93.07% ± 6.43 and 89.44% ± 2.84 apoptosis, respectively. The results were in good agreement with the aforementioned cell viability (Fig. 3c and d). All these results demonstrated that the NIR-II probe H4–PEG-Glu exhibited effective chemo- and photothermal synergistic treatment (CPTT) toward ALM cancer cells (THP-1 and Molm-13) in vitro.
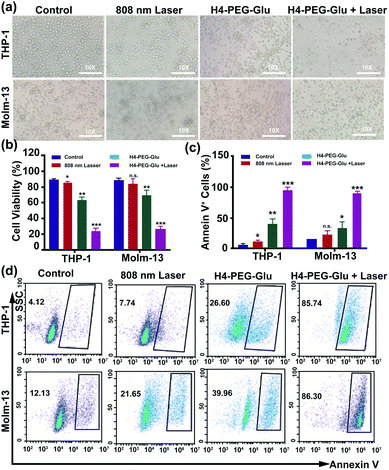 | ||
| Fig. 3 (a) The bright-field microscopy images of THP-1 and Molm-13 cells treated with PBS, 808 nm laser, H4–PEG-Glu (20 μM), and H4–PEG-Glu (20 μM) under 808 nm laser irradiation (1.2 W cm−2, 5 min). (b) The cell viability of THP-1 and Molm-13 by the Trypan Blue Exclusion test according to the bright-field microscopy images. (c) Proportions of Annexin V+ THP-1 cells and Annexin V+ Molm-13 cells calculated from the cell apoptosis in Fig. 3d. (d) Flow cytometry analysis of cell apoptosis of THP-1 and Molm-13 cells treated with PBS, 808 nm laser, H4–PEG-Glu (20 μM), and H4–PEG-Glu (20 μM) under 808 nm laser irradiation (1.2 W cm−2, 5 min). *p < 0.05; **p < 0.01; ***p < 0.001; ns, not significant. | ||
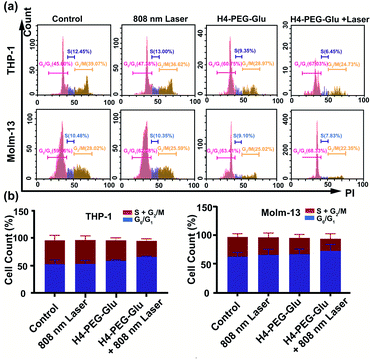
To further explore the mechanism of toxicity and apoptosis of H4–PEG-Glu, the cell cycle progression of the above cancer cells was analyzed by fluorescence-activated cell sorting (FACS) analysis. THP-1 or Molm-13 cells in the G0/G1 phase and G2/M phase have unreplicated diploid (2n) DNA content and replicated ploid (4n) DNA, implying the different procedures of DNA replication. As shown in Fig. 4a and b, THP-1 cells treated with H4–PEG-Glu under 808 nm laser irradiation exhibited an obviously increased percentage in the G0/G1 phase (53.64% ± 7.46 to 66.68% ± 0.78) and a decreased percentage in the S phase and G2/M phase (42.24% ± 9.40 to 28.61% ± 3.55), demonstrating that H4–PEG-Glu inhibited DNA replication due to inducing cell cycle arrest in the G0/G1 phase. A similar apoptosis pathway was also observed in Molm-13 cells.
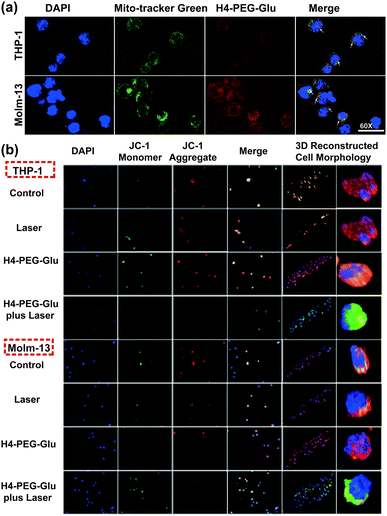
Mitochondria is critically important in energy production and apoptosis.27 To further assess the targeting ability of H4–PEG-Glu to mitochondria and its efficiency in mitochondria-targeted cancer therapy, THP-1 and Molm-13 cells were incubated with H4–PEG-Glu (20 μM) for 24 h followed by staining with Mito-tracker Green (5 μM), a commercial available kit used to label the mitochondria. The intrinsic fluorescence of H4–PEG-Glu (red color) was observed to be completely overlapped with Mito-tracker (Green) (Fig. 5a, Fig. S10†), implying its excellent targeting ability to mitochondria. The feedback of mitochondrial membrane potential (MMP) reflected different cellular status and has been used for assessing the therapeutic effects.28 Thus, MMP levels of THP-1 and Molm-13 cells in each group were detected using a commercial fluorescent probe JC-1. The fluorescence images of each group have been shown in Fig. 5b. According to Fig. 5b, the red fluorescence signal from THP-1 cancer cells was bright while the green fluorescence signal was weak before H4–PEG-Glu or laser treatment, demonstrating that the MMP level was normal. With the progress of the treatment process, the green fluorescence signal intensified and red fluorescence signal gradually vanished, illustrating the decrease of the MMP level, and the transformation of JC-1 from J-aggregates into monomers. Upon treatment with H4–PEG-Glu under 808 nm laser irradiation, the red fluorescence signals of THP-1 cells vanished, suggesting that the therapeutic effects of THP-1 cancer cells were greatly enhanced. The same phenomenon was also detected in Molm-13 cancer cells. All these results demonstrated that H4–PEG-Glu is a novel therapeutic probe with NIR-II fluorescence and mitochondrial-targeting properties.
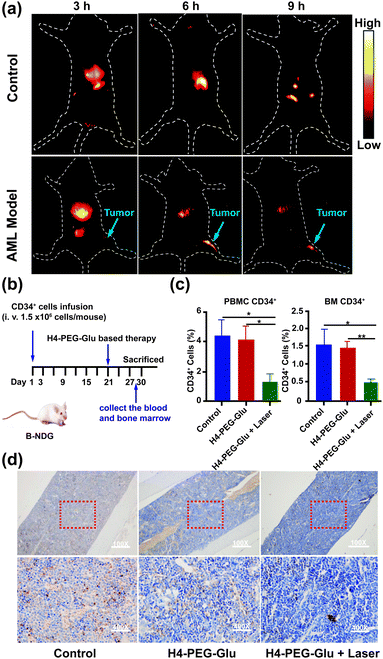
Inspired by the intriguing chemo- and photothermal performance of H4–PEG-Gluin vitro, subsequently, we envisioned that H4–PEG-Glu can achieve great NIR-II image-guided therapeutic effects in vivo. Thus, the NIR-II fluorescence imaging performance of H4–PEG-Glu was firstly evaluated in vivo using AML patient derived PDX mouse models. H4–PEG-Glu (200 μL, 1 μg μL−1 in PBS) was first administered into AML patient derived PDX mouse models (n = 3) through the tail vein. As shown in Fig. 6a, the fluorescence signal was gradually enhanced at the bone marrow (blue arrow in Fig. 6a) of AML patient derived PDX mouse models, and reached a maximum at 6 h post injection (808 nm excitation, 90 mW cm−2, 1000 LP and 100 ms). In contrast, no obvious signals were detected in bone marrow at all-time points i.v. post-injection of H4–PEG2K in the AML patient derived PDX mouse models or in the normal B-NDG mice (n = 3), demonstrating that H4–PEG-Glu exhibited specific targeting ability to AML cancer cells in bone marrow (Fig. S12c†). Thereafter, AML patient derived PDX mouse models (n = 4 group, ∼20 g each) were treated with PBS, H4–PEG-Glu (200 μg), and H4–PEG-Glu (200 μg) under 808 nm laser irradiation (1.2 W cm−2, 5 min), respectively. No obvious body weight loss was observed for all groups during the CPPT process in a week. Then they were all sacrificed to collect the peripheral blood mononuclear cells (PBMC) and bone marrow (BM) to analyze the expression of AML stem cell marker CD34. The immunohistochemical analysis of bone marrow (BM) was further conducted. The results showed that CD34 expressed in H4–PEG-Glu under the 808 nm laser irradiation group (the right images in Fig. 6d) was obviously lower than that in the control group (left images in Fig. 6d) and in the H4–PEG-Glu group (the middle images in Fig. 6d). The CD34+ cells in all three groups were also quantified by flow cytometry. The percentage of CD34+ cells in PBMC and bone marrow with H4–PEG-Glu under 808 nm laser irradiation was significantly lower than that of the other two groups (Fig. 6c). To assess the hepatotoxicity of H4–PEG-Glu, H4–PEG-Glu (200 μg) and PBS were injected in ICR female mice (n = 3 per group, ∼20 g each), respectively. The blood was collected at 7 days post-injection to analyze the biochemistry index (ALT, AST, CREA, BUN). The concentration in the experiment group was similar to that in the PBS group, indicating that H4–PEG-Glu has no obvious toxicity to the kidneys and liver (Fig. S11†). All the above results demonstrated that H4–PEG-Glu is a potential small-molecule NIR-II probe for clinical translation in AML cancer theranostics.
Experimental section
Methods and materials are provided in the ESI. All animal experiments were approved by the Chinese Regulations for the Administration of Affairs Concerning Experimental Animals and Institutional Animal Care and Use Committee (IACUC) of Wuhan University. The study has been approved by the Institutional Ethical Committee of Zhongnan Hospital of Wuhan University, China (No. 2018278), and performed in accordance with the ethical standards as laid down in the 1964 Helsinki declaration and its later amendments or comparable ethical standards.Conclusions
In conclusion, we have successfully designed and synthesized a novel mitochondria-targeted NIR-II fluorophore H4–PEG-Glu with a new thiopyrylium skeleton for AML CPTT. H4–PEG-Glu showed excellent water solubility, high photo-stability and biocompatibility, and superior targeting affinity toward AML cancer cells (THP-1 and Molm-13) and peripheral blood donated from AML patients. H4–PEG-Glu not only can quickly and effectively image mitochondria in acute myeloid leukemia (AML) cells, and induce G0/G1 phase arrest by the intrinsic mitochondrial apoptosis pathway w/o irradiation, but also exhibited moderate cytotoxicity against AML cancer cells in a dose dependent-manner without laser irradiation. THP-1 and Molm-13 cells treated with H4–PEG-Glu upon NIR laser irradiation showed enhanced CPTT with 93.07% ± 6.43 and 89.44% ± 2.84 apoptosis by Annexin V staining. AML patient derived PDX mouse models were finally used for the first time for in vivo mitochondria-targeted NIR-II Imaging of acute myeloid leukemia and image-guided CPTT. The percentage of CD34+ cells in PBMC and bone marrow was significantly reduced after H4–PEG-Glu treatment and laser irradiation. To the best of our knowledge, this is the first NIR-II small-molecule probe for mitochondrial-targeted chemo- and photothermal synergistic therapy for AML cancer. It is hoped that this novel NIR-II cation fluorophore may provide a practical strategy to develop small-molecule NIR-II fluorophores for non-solid tumor therapy with a deeper penetration depth and higher resolution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Key R&D Program of China (2020YFA0908800), NSFC (81773674, 81770179), Shenzhen Science and Technology Research Grant (JCYJ20190808152019182), Hubei Province Scientific and Technical Innovation Key Project (2020BAB058), the Applied Basic Research Program of Wuhan Municipal Bureau of Science and Technology (2019020701011429), the Local Development Funds of Science and Technology Department of Tibet (XZ202001YD0028C), Health Commission of Hubei Province Scientific Research Project (WJ2019M177, WJ2019M178, WJ2019H008), the Fundamental Research Funds for the Central Universities (ZNJC201931).Notes and references
- (a) D. Jaque, L. Martínez Maestro, B. Del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. Martín Rodríguez and J. García Solé, Nanoscale, 2014, 6, 9494–9530 RSC; (b) H. He, J. Wang, H. Wang, N. Zhou, D. Yang, D. R. Green and B. Xu, J. Am. Chem. Soc., 2018, 140, 1215–1218 CrossRef CAS PubMed; (c) Y. Liu, J. Zhou, L. Wang, X. Hu, X. Liu, M. Liu, Z. Cao, D. Shangguan and W. Tan, J. Am. Chem. Soc., 2016, 138, 12368–12374 CrossRef CAS PubMed; (d) R. M. Shallis, R. Wang, A. Davidoff, X. Ma and A. M. Zeidan, Blood Rev., 2019, 36, 70–87 CrossRef PubMed.
- H. S. Jung, J.-H. Lee, K. Kim, S. Koo, P. Verwilst, J. L. Sessler, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2017, 139, 9972–9978 CrossRef CAS PubMed.
- (a) R. Guo, J. Yin, Y. Ma, Q. Wang and W. Lin, J. Mater. Chem. B, 2018, 6, 2894–2900 RSC; (b) Y. Koide, Y. Urano, S. Kenmoku, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2007, 129, 10324–10325 CrossRef CAS PubMed; (c) M. Sibrian- Vazquez, I. V. Nesterova, T. J. Jensen and M. G. H. Vicente, Bioconjugate Chem., 2008, 19, 705–713 CrossRef CAS PubMed; (d) J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef CAS PubMed.
- (a) G. Cheng, J. Zielonka, B. P. Dranka, D. McAllister, A. C. Mackinnon, J. Joseph and B. Kalyanaraman, Cancer Res., 2012, 72, 2634–2644 CrossRef CAS PubMed; (b) J. C. Wang, Z. Jiang, L. P. Xiang, Y. F. Li, M. R. Ou, X. Yang, J. W. Shao, Y. S. Lu, L. F. Lin, J. Z. Chen, Y. Dai and L. Jia, Sci. Rep., 2014, 4, 5006 CrossRef PubMed; (c) B. Kalyanaraman, G. Cheng, M. Hardy, O. Ouari, M. Lopez, J. Joseph, J. Zielonka and M. B. Dwinell, Redox Biol., 2018, 14, 316–327 CrossRef CAS PubMed.
- H. Chen, J. Wang, X. Feng, M. Zhu, S. Hoffmann, A. Hsu, K. Qian, D. Huang, F. Zhao, W. Liu, H. Zhang and Z. Cheng, Chem. Sci., 2019, 10, 7946–7951 RSC.
- (a) H. Dohner, D. J. Weisdorf and C. D. Bloomfield, N. Engl. J. Med., 2015, 373, 1136–1152 CrossRef PubMed; (b) J. N. Saultz and R. Garzon, J. Clin. Med., 2016, 5, 33 CrossRef PubMed; (c) B. M. Barth, E. I. Altinoğlu, S. S. Shanmugavelandy, J. M. Kaiser, D. Crespo-Gonzalez, N. A. Divittore, C. Mcgovern, T. M. Goff, N. R. Keasey, J. H. Adair, T. P. Loughran, D. F. Claxton and M. Kester, ACS Nano, 2011, 5, 5325–5337 CrossRef CAS PubMed.
- (a) P. Bose, P. Vachhani and J. E. Cortes, Curr. Treat. Options Oncol., 2017, 18, 17 CrossRef PubMed; (b) K. D. Miller, L. Nogueira, A. B. Mariotto, J. H. Rowland, K. R. Yabroff, C. M. Alfano, A. Jemal, J. L. Kramer and R. L. Siegel, Ca-Cancer J. Clin., 2019, 69, 363–385 CrossRef PubMed.
- (a) W. Wang, G. Liang, W. Zhang, D. Xing and X. Hu, Chem. Mater., 2018, 30, 3486–3498 CrossRef CAS; (b) Y. Chen, Z. Li, H. Wang, Y. Wang, H. Han, Q. Jin and J. Ji, ACS Appl. Mater. Interfaces, 2016, 8, 6852–6858 CrossRef CAS PubMed; (c) Y. Zhang, C. Y. Ang, M. Li, S. Y. Tan, Q. Qu and Y. Zhao, ACS Appl. Mater. Interfaces, 2016, 8, 6869–6879 CrossRef CAS PubMed.
- (a) S. Kunjachan, J. Ehling, G. Storm, F. Kiessling and T. Lammers, Chem. Rev., 2015, 115, 10907–10937 CrossRef CAS PubMed; (b) G. Hong, J. C. Lee, J. T. Robinson, U. Raaz, L. Xie, N. F. Huang, J. P. Cooke and H. Dai, Nat. Med., 2012, 18, 1841–1846 CrossRef CAS PubMed; (c) G. Hong, S. Diao, J. Chang, A. L. Antaris, C. Chen, B. Zhang, S. Zhao, D. N. Atochin, P. L. Huang, K. I. Andreasson, C. J. Kuo and H. Dai, Nat. Photonics, 2014, 8, 723 CrossRef CAS PubMed; (d) B. Li, L. Lu, M. Zhao, Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2018, 57, 7483 CrossRef CAS PubMed; (e) C. Qu, Y. Xiao, H. Zhou, B. Ding, A. Li, J. Lin, X. Zeng, H. Chen, K. Qian, X. Zhang, W. Fang, J. Wu, Z. Deng, Z. Cheng and X. Hong, Adv. Opt. Mater., 2019, 7, 1900229 CrossRef PubMed.
- (a) D. Kim, N. Lee, Y. I. Park and T. Hyeon, Bioconjugate Chem., 2017, 28, 115–123 CrossRef CAS PubMed; (b) G. Hong, S. Diao, A. L. Antaris and H. Dai, Chem. Rev., 2015, 115, 10816–10906 CrossRef CAS PubMed; (c) H. Zhang, Z.-H. Chen, X. Liu and F. Zhang, Nano Res., 2020, 13, 1795–1809 CrossRef CAS.
- (a) K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570–6597 RSC; (b) Y. Liu, J. Liu, D. Chen, X. Wang, Z. Liu, H. Liu, L. Jiang, C. Wu and Y. Zou, Macromolecules, 2019, 52, 5735–5740 CrossRef CAS; (c) B. Guo, J. Chen, N. Chen, M. Wu, K. Li, C. Liu and B. Liu, Adv. Mater., 2019, 31, 1808355 CrossRef PubMed; (d) Z. Zhang, X. Fang, Z. Liu, H. Liu, D. Chen, S. He, X. Zhang and C. Wu, Angew. Chem., Int. Ed., 2020, 59, 3691–3698 CrossRef CAS PubMed; (e) H. Zhou, S. Li, X. Zeng, M. Zhang, L. Tang, Q. Li, D. Chen, X. Meng and X. Hong, Chin. Chem. Lett., 2020, 31, 1382–1386 CrossRef CAS.
- (a) S. He, J. Song, J. Qu and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC; (b) J. Li, Y. Liu, Y. Xu, L. Li, Y. Sun and W. Huang, Coord. Chem. Rev., 2020, 415, 213318 CrossRef CAS; (c) B. Li, M. Zhao and F. Zhang, ACS Mater. Lett., 2020, 2, 905–917 CrossRef CAS; (d) C. Sun, B. Li, M. Zhao, S. Wang, Z. Lei, L. Lu, H. Zhang, L. Feng, C. Dou, D. Yin, H. Xu, Y. Cheng and F. Zhang, J. Am. Chem. Soc., 2019, 141, 19221–19225 CrossRef CAS PubMed; (e) Q. Wang, Y. Dai, J. Xu, J. Cai, X. Niu, L. Zhang, R. Chen, Q. Shen, W. Huang and Q. Fan, Adv. Funct. Mater., 2019, 29, 1901480 CrossRef.
- (a) Y. Du, B. Xu, T. Fu, M. Cai, F. Li, Y. Zhang and Q. Wang, J. Am. Chem. Soc., 2010, 132, 1470–1471 CrossRef CAS PubMed; (b) J. Lin, Q. Li, X. Hong and Y. Xiao, Sci. China: Chem., 2020, 63, 766–770 CrossRef CAS; (c) C. Li, Y. Zhang, M. Wang, Y. Zhang, G. Chen, L. Li, D. Wu and Q. Wang, Biomaterials, 2014, 35, 393–400 CrossRef CAS PubMed; (d) C. Li, W. Li, H. Liu, Y. Zhang, G. Chen, Z. Li and Q. Wang, Angew. Chem., Int. Ed., 2020, 132, 253–258 CrossRef; (e) M. Zhang, J. Yue, R. Cui, Z. Ma, H. Wan, Y. Zhou, Y. Kuang, Y. Zhong, D. W. Pang and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6590 CrossRef CAS PubMed.
- H. Liu, G. Hong, Z. Luo, J. Chen, J. Chang, M. Gong, H. He, J. Yang, X. Yuan, L. Li, X. Mu, J. Wang, W. Mi, J. Luo, J. Xie and X.-D. Zhang, Adv. Mater., 2019, 31, 1901015 CrossRef CAS PubMed.
- (a) K. Welsher, Z. Liu, S. P. Sherlock, J. T. Robinson, Z. Chen, D. Daranciang and H. Dai, Nat. Nanotechnol., 2009, 4, 773–780 CrossRef CAS PubMed; (b) S. Diao, G. Hong, J. T. Robinson, L. Jiao, A. L. Antaris, J. Z. Wu, C. L. Choi and H. Dai, J. Am. Chem. Soc., 2012, 134, 16971 CrossRef CAS PubMed; (c) C. Liang, S. Diao, C. Wang, H. Gong, T. Liu, G. Hong, X. Shi, H. Dai and Z. Liu, Adv. Mater., 2014, 26, 5646 CrossRef CAS PubMed; (d) J. D. Harvey, P. V. Jena, H. A. Baker, G. H. Zerze, R. M. Williams, T. V. Galassi, D. Roxbury, J. Mittal and D. A. Heller, Nat. Biomed. Eng., 2017, 1, 0041 CrossRef CAS PubMed.
- (a) Y. Zhong, Z. Ma, S. Zhu, J. Yue, M. Zhang, A. L. Antaris, J. Yuan, R. Cui, H. Wan, Y. Zhou, W. Wang, N. F. Huang, J. Luo, Z. Hu and H. Dai, Nat. Commun., 2017, 8, 737 CrossRef PubMed; (b) P. Wang, Y. Fan, L. Lu, L. Liu, L. Fan, M. Zhao, Y. Xie, C. Xu and F. Zhang, Nat. Commun., 2018, 9, 2898 CrossRef PubMed; (c) Y. Fan and F. Zhang, Adv. Opt. Mater., 2019, 7, 1801417 CrossRef; (d) H. Zhou, Y. Xiao and X. Hong, Chin. Chem. Lett., 2018, 29, 1425–1428 CrossRef CAS.
- (a) A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS PubMed; (b) A. L. Antaris, H. Chen, S. Diao, Z. Ma, Z. Zhang, S. Zhu, J. Wang, A. X. Lozano, Q. Fan, L. Chew, M. Zhu, K. Cheng, X. Hong, H. Dai and Z. Cheng, Nat. Commun., 2017, 8, 15269 CrossRef CAS PubMed; (c) H. Zhou, X. Zeng, A. Li, W. Zhou, L. Tang, W. Hu, Q. Fan, X. Meng, H. Deng, L. Duan, Y. Li, Z. Deng, X. Hong and Y. Xiao, Nat. Commun., 2020, 11, 6183 CrossRef CAS PubMed.
- (a) B. Li, Z. Lei and F. Zhang, Angew. Chem., Int. Ed., 2018, 57, 7483–7487 CrossRef CAS PubMed; (b) J. Yang and X. Hong, Sci. China: Chem., 2019, 62, 7–8 CrossRef CAS.
- Q. Li, Q. Ding, Y. Li, X. Zeng, Y. Liu, S. Lu, H. Zhou, X. Wang, J. Wu, X. Meng, Z. Deng and Y. Xiao, Chem. Commun., 2020, 56, 3289 RSC.
- Y. Li, Y. Liu, Q. Li, X. Zeng, T. Tian, W. Zhou, Y. Cui, X. Wang, X. Cheng, Q. Ding, X. Wang, J. Wu, H. Deng, Y. Li, X. Meng, Z. Deng, X. Hong and Y. Xiao, Chem. Sci., 2020, 11, 2621–2626 RSC.
- Y. Li, Z. Cai, S. Liu, H. Zhang, S. T. H. Wong, J. W. Y. Lam, R. T. K. Kwok, J. Qian and B. Z. Tang, Nat. Commun., 2020, 11, 1255 CrossRef CAS PubMed.
- L. Szablewski, Biochim. Biophys. Acta, 2013, 1835, 164–169 CAS.
- (a) H. Zhang, Y. Fan, P. Pei, C. Sun, L. Lu and F. Zhang, Angew. Chem., Int. Ed., 2019, 58, 10153–10157 CrossRef CAS PubMed; (b) B. Li, M. Zhao, L. Feng, C. Dou, S. Ding, G. Zhou, L. Lu, H. Zhang, F. Chen, X. Li, G. Li, S. Zhao, C. Jiang, Y. Wang, D. Zhao, Y. Cheng and F. Zhang, Nat. Commun., 2020, 11, 3102 CrossRef CAS PubMed; (c) H. Ma, C. Liu, Z. Hu, P. Yu, X. Zhu, R. Ma, Z. Sun, C.-H. Zhang, H. Sun, S. Zhu and Y. Liang, Chem. Mater., 2020, 32, 2061–2069 CrossRef CAS; (d) Q. Yang, Z. Hu, S. Zhu, R. Ma, H. Ma, Z. Ma, H. Wan, T. Zhu, Z. Jiang, W. Liu, L. Jiao, H. Sun, Y. Liang and H. Dai, J. Am. Chem. Soc., 2018, 140, 1715–1724 CrossRef CAS PubMed.
- (a) Y.-X. Zhu, H.-R. Jia, G. Gao, G.-Y. Pan, Y.-W. Jiang, P. Li, N. Li, C. Zhou, C. She, N. W. Ulrich, Z. Chen and F.-G. Wu, Biomaterials, 2020, 232, 119668 CrossRef CAS PubMed; (b) G.-Y. Pan, H.-R. Jia, Y.-X. Zhu, R.-H. Wang, F.-G. Wu and Z. Chen, ACS Biomater. Sci. Eng., 2017, 3, 3596–3606 CrossRef CAS PubMed.
- (a) B. Ding, Y. Xiao, H. Zhou, X. Zhang, C. Qu, F. Xu, Z. Deng, Z. Cheng and X. Hong, J. Med. Chem., 2019, 62, 2049–2059 CrossRef CAS PubMed; (b) X. Hong, H. Zhou, Y. Xiao, B. Ding, J. Duan, CN Pat., 109369633B, 2018.
- X. Yang, D. Wang, Y. Shi, J. Zou, Q. Zhao, Q. Zhang, W. Huang, J. Shao, X. Xie and X. Dong, ACS Appl. Mater. Interfaces, 2018, 10, 12431–12440 CrossRef CAS PubMed.
- X.-S. Hou, H.-S. Wang, B. P. Mugaka, G.-J. Yang and Y. Ding, Biomater. Sci., 2018, 6, 2786–2797 RSC.
- X. Li, M. Tian, G. Zhang, R. Zhang, R. Feng, L. Guo, X. Yu, N. Zhao and X. He, Anal. Chem., 2017, 89, 3335–3344 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc04727a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |