N,N-Diethyl-3-methylbenzamide (DEET) acts as a metal–organic framework synthesis solvent with phase-directing capabilities†
Ryan A.
Dodson
 a,
Andre P.
Kalenak
a,
Andre P.
Kalenak
 a,
Derek R.
Du Bois
a,
Sasha L.
Gill-Ljunghammer
a and
Adam J.
Matzger
a,
Derek R.
Du Bois
a,
Sasha L.
Gill-Ljunghammer
a and
Adam J.
Matzger
 *ab
*ab
aDepartment of Chemistry, University of Michigan, 930 North University Avenue, Ann Arbor, Michigan 48109-1055, USA. E-mail: matzger@umich.edu
bMacromolecular Science and Engineering Program, University of Michigan, 930 North University Avenue, Ann Arbor, Michigan 48109-1055, USA
First published on 23rd July 2020
Abstract
Metal–organic frameworks (MOFs) are generally synthesized in toxic formamide solvents. Greener solvents would lower production barriers and facilitate applications such as drug delivery. N,N-Diethyl-3-methylbenzamide (DEET), the most widely used insect repellent, is shown to serve this role. Furthermore, DEET-loaded MOFs can be leveraged in controlled-release insect repellent formulations.
Metal–organic frameworks (MOFs) are a class of crystalline coordination polymers composed of metal ions or clusters bound by organic linker molecules. The often-high porosity and surface area of MOFs make these materials attractive for applications such as gas storage/separation, catalysis, and drug delivery. MOFs are generally synthesized solvothermally in formamide solvents such as N,N-dimethylformamide (DMF) and N,N-diethylformamide (DEF), or more rarely in water and/or alcohols. It is well-known in the field that choice of solvent is an important parameter for MOF synthesis, as two otherwise identical synthetic procedures, differentiated by synthesis solvent only, can yield unique materials with correspondingly disparate properties.
N,N-Diethyl-3-methylbenzamide, commonly known as DEET, is a potent insect repellent with an excellent history of efficacy and safety.1 Notably, the structure of DEET is similar to DEF, except with the presence of a 3-methylbenzyl group rather than a hydrogen on its amide carbon (Fig. 1). Despite its structural similarity to this widely used formamide solvent, few reports exist of researchers utilizing DEET for applications outside of its insect repellency.‡ Though comparable in cost to DMF, DEET has a much better safety record versus formamide solvents, which have well-documented hepatotoxicity.2,3 This toxicity is important to consider because complete removal of synthesis solvents from MOFs can be difficult to achieve. This is especially concerning for MOFs intended to be used in applications involving food contact or drug delivery, where residual toxic solvents can pose a health risk to consumers. Use of DEET as a synthesis solvent (for either materials synthesis or chemical synthesis) is currently unexplored.§
The success of DEET as an insect repellent arises in part from its slow evaporation rate, which permits a longer duration of protection than more-volatile repellents. Because of the importance of volatility control, there have been many attempts to develop controlled-release formulations of DEET to further improve its longevity. Development of these formulations began in the 1980s, resulting in a polymer-based formulation commercialized by 3M.4 Subsequently, a variety of other controlled-release formulations have been developed. Recently, a MOF–fabric composite, created by incubation of fabric in a DMF-based MOF synthesis solution, was developed and tested for DEET release.5 This system achieved extended release by virtue of its higher DEET capacity, and showed quicker evaporation rates relative to the unmodified fabrics. In this study we (a) demonstrate efficient MOF synthesis in DEET and (b) show the potential of DEET-synthesized MOFs to extend the DEET release profile relative to the neat liquid via vapor pressure suppression.
The utility of DEET as a MOF synthesis solvent was critically assessed by screening a series of prototypical MOFs. Because of the large variety of systems attempted, synthetic conditions were not optimized, with representative synthetic parameters such as concentration, linker:metal ratio, and temperature left as described in previous synthetic protocols; therefore the results reported here represent a worst case scenario for the generality of DEET in MOF synthesis. Synthesis of MOF-56 was found to proceed when the synthesis solvent was changed from DEF to DEET, giving material with comparable cubic morphology (see ESI† for morphological characterization data) and a powder X-ray diffraction (PXRD) pattern in excellent accord with that computed from the MOF-5 crystal structure (Fig. 2). Furthermore, the BET surface area of the MOF-5 obtained from DEET was comparable to that of the DEF-synthesized material (∼3300 m2 g−1), and no residual DEET was observed in the MOF post-activation via solution 1H-NMR (see ESI† for details). As in the case of MOF-5, the syntheses of the Zn4O-based MOFs UMCM-1 (Zn, terephthalate (bdc), and 1,3,5-tris(4-carboxyphenyl)benzene (btb)),7 UMCM-9 (Zn, 2,6-naphthalenedicarboxylate (ndc), and 4,4′-biphenyldicarboxylate (bpdc)),8 and MOF-177 (Zn and btb)9 were also successful in DEET. Three other prototypical MOF systems – HKUST-1 (Cu and trimesate (btc)),10 MIL-53(Al) (Al and bdc),11 and MOF-519 (Al and btb)12 – were also successfully synthesized in DEET (Fig. 3), all with no apparent decrease in bulk sample crystallinity relative to standard synthetic protocols.
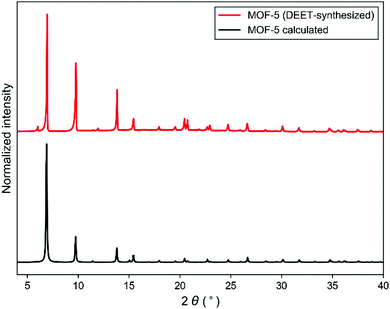 | ||
| Fig. 2 PXRD patterns of DEET-synthesized MOF-5 (above) and calculated PXRD pattern for MOF-5 (below). | ||
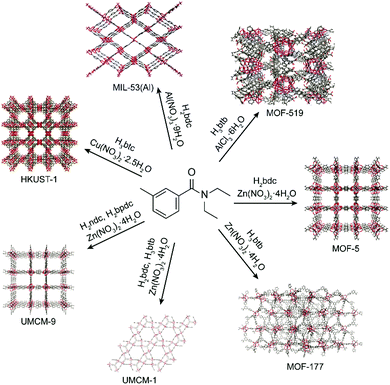 | ||
| Fig. 3 Metal–organic frameworks successfully synthesized in DEET along with their precursor metal salts and linker acids. | ||
Attempted syntheses which were not observed to yield crystalline products with conditions that were successful when using DMF include the Zn-based MOFs FJI-1 and ZIF-8, and the Zr-based MOFs UiO-66, DUT-52, and UiO-67. It is likely that further optimization of synthetic conditions (temperature, concentration, cosolvent) will afford these materials in DEET. Several of the attempted MOF syntheses in DEET yielded new crystalline phases. In particular, this was found when attempting to synthesize IRMOF-3 (Zn and 2-aminoterephthalate (NH2bdc)), Zn-HKUST-1 (Zn and btc), and Cu-MOF-2 (Cu and bdc) (PXRD patterns of the resultant products as well as those of the targeted MOFs are given in the ESI†). Zn/btc yields crystals large enough for single crystal X-ray diffraction. The structure of this phase is shown in Fig. 4. Of particular note is its rare Zn2(RCO2)3 cluster, distinct from the commonly seen M2(RCO2)4 paddlewheel cluster. The M2(RCO2)3 cluster in this structure achieves charge balance with one axial NO3− per cluster, while the opposite axial site on each cluster is bound by a DEET molecule. The ability of DEET to yield a novel MOF with such a seldom seen cluster demonstrates its phase-directing ability.
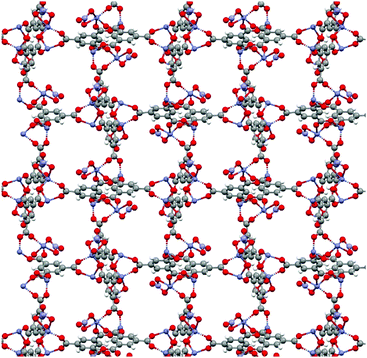 | ||
| Fig. 4 Crystal structure of the novel Zn/btc phase with coordinated solvent omitted for clarity. Viewed along the a-axis. | ||
MOF syntheses generally utilize formamide solvents both because of their ability to solubilize a broad range of metal salts and carboxylic acid linkers and because formamide decomposition generates alkylamine species that increase the solution pH; this slowly increasing basicity facilitates controlled deprotonation of linker molecules and thus reversible MOF growth. Although DEET is an amide solvent, its decomposition pathways are expected to be distinct from formamide solvents such as DMF and DEF.13 The latter solvents can exude CO yielding alkylamine through a thermal decomposition pathway; no such path is evident in DEET, although hydrolysis might provide diethylamine and ultimately raise solution pH.14
In contrast with previously reported HKUST-1/fabric composite materials,5 MOF-5 was found to behave exceptionally well at reducing the effective vapor pressure of DEET. In particular, relative to the extrapolated vapor pressure of DEET at 37 °C (1.2 mPa, this work), DEET loaded in MOF-5 has an equilibrium vapor pressure of 0.11 mPa at 37 °C, corresponding to 9.1% of the bulk vapor pressure as determined by measurements between 125 and 200 °C using the Knudsen effusion method (Fig. 5, see ESI† for full details on these measurements). This volatility suppression is substantial. Because the evaporation rate of a liquid empirically scales linearly with its vapor pressure,15 we can project that this vapor pressure reduction would lead to a ∼11× longer evaporation time. However, these calculations reflect the behavior of the DEET/MOF composite in a dry N2 environment, which is not representative of climates in which mosquitoes represent a public health concern. In the presence of moisture and/or liquid water, MOF-5 is well-known to undergo structural degradation and eventual hydrolysis, which has been leveraged previously to increase drug release rates.16 Thus, these evaporation rate estimates represent a lower limit scenario for DEET release, with real-world release rates likely falling between these results and those of the bulk liquid.
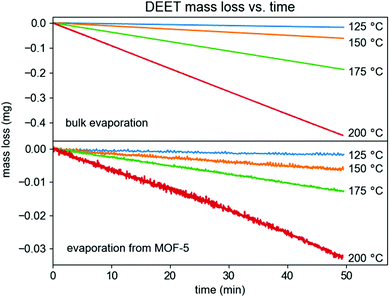 | ||
| Fig. 5 Mass loss versus time for bulk liquid DEET (above) and for DEET loaded in MOF-5 (below) in a Knudsen effusion cell. | ||
Beyond its exceptional ability to lower the vapor pressure of guest molecules, there are several other properties of MOF-5 that could be valuable in a controlled-release formulation. One such property that could be exploited in topical formulations is UV absorption. For example, MOF-5 strongly absorbs UV radiation up to ∼310 nm, with red-shifting of the absorption edge to ∼325 nm upon exposure to water.17 This would allow MOF-5-based topical formulations to block a significant portion of harmful UV-B radiation (290–320 nm). Another benefit of this MOF/DEET system is the simplicity of its creation: the controlled-release composite can be used directly after MOF synthesis. Choice of MOF will allow further tuning of release rate, moisture sensitivity, and UV-absorption profile offering a number of pathways for optimizing application of MOF-based topical formulations offering environment protection to the wearer.
DEET is an inexpensive, versatile, and low-toxicity MOF synthesis solvent. As MOFs find increasingly widespread use at the industrial scale,18,19 it is imperative from an industrial health and safety perspective to have routes for synthesizing MOFs using the least toxic solvents possible. Because many MOF syntheses require amide solvents, DEET has the potential to fill this role as a cheap, readily available, and safer synthesis solvent. Furthermore, the utility of MOFs to lower the effective vapor pressure in insect repellent formulations represents a promising new application for these materials.
The authors acknowledge the support of the United States Department of Energy (DE-SC0004888). SEM work was done at the Robert B. Mitchell Electron Microbeam Analysis Lab, part of the University of Michigan's Department of Earth & Environmental Sciences.
Conflicts of interest
There are no conflicts to declare.Notes and references
- T. M. Katz, J. H. Miller and A. A. Hebert, J. Am. Acad. Dermatol., 2008, 58, 865–871 CrossRef PubMed.
- G. L. Kennedy and R. D. Short, CRC Crit. Rev. Toxicol., 1986, 17, 129–182 CrossRef CAS PubMed.
- V. Scailteur and R. R. Lauwerys, Toxicology, 1987, 43, 231–238 CrossRef CAS PubMed.
- L. C. Rutledge, R. K. Gupta, Z. A. Mehr, M. D. Buescher and W. G. Reifenrath, J. Am. Mosq. Control Assoc., 1996, 12, 39–44 CAS.
- H. E. Emam and R. M. Abdelhameed, J. Porous Mater., 2017, 24, 1175–1185 CrossRef CAS.
- H. Li, M. Eddaoudi, M. O’Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- K. Koh, A. G. Wong-Foy and A. J. Matzger, Angew. Chem. Int. Ed., 2008, 47, 677–680 CrossRef CAS PubMed.
- K. Koh, J. D. Van Oosterhout, S. Roy, A. G. Wong-Foy and A. J. Matzger, Chem. Sci., 2012, 3, 2429 RSC.
- H. Furukawa, M. A. Miller and O. M. Yaghi, J. Mater. Chem., 2007, 17, 3197–3204 RSC.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- W. P. Mounfield and K. S. Walton, J. Colloid Interface Sci., 2015, 447, 33–39 CrossRef CAS.
- F. Gándara, H. Furukawa, S. Lee and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 5271–5274 CrossRef.
- S. S. Kaye, A. Dailly, O. M. Yaghi and J. R. Long, J. Am. Chem. Soc., 2007, 129, 14176–14177 CrossRef CAS PubMed.
- P. Calza, C. Medana, E. Raso, V. Giancotti and C. Minero, Sci. Total Environ., 2011, 409, 3894–3901 CAS.
- J. E. Woodrow, J. N. Seiber and C. Dary, J. Agric. Food Chem., 2001, 49, 3841–3846 CrossRef CAS PubMed.
- K. Suresh and A. J. Matzger, Angew. Chem. Int. Ed., 2019, 58, 16790–16794 CrossRef CAS PubMed.
- N. A. Rodríguez, R. Parra, E. San Román and M. A. Grela, J. Mater. Sci., 2020, 55, 6588–6597 CrossRef.
- M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch and M. R. Hill, Chem. Soc. Rev., 2017, 46, 3453–3480 RSC.
- J. Ren, X. Dyosiba, N. M. Musyoka, H. W. Langmi, M. Mathe and S. Liao, Coord. Chem. Rev., 2017, 352, 187–219 CrossRef CAS.
- J. J. Windheuser, J. L. Haslam, L. Caldwell and R. D. Shaffer, J. Pharm. Sci., 1982, 71, 1211–1213 CrossRef CAS.
- M. L. Di Lorenzo and A. Longo, Thermochim. Acta, 2019, 677, 180–185 CrossRef CAS.
- J. R. Holsten and N. E. Neely, US Pat., 5207803, 1993 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials syntheses and characterization. CCDC 1995462. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc02741c |
| ‡ DEET has also been reported or proposed as an additive to increase the skin permeability of drugs,20 a polymer plasticizer,21 and a carrier for dyes and flame retardants.22 |
| § As of 22 January 2020, a SciFinder reaction substructure search with DEET included as a reagent yielded two hits, both of which included DEET during reactions for the purpose of physically embedding it in the final product. A Reaxys search yielded no instances of DEET being used as a solvent in any reaction. |
| This journal is © The Royal Society of Chemistry 2020 |