Binder-free V2O5/CNT paper electrode for high rate performance zinc ion battery†
Bosi
Yin
ab,
Siwen
Zhang
ab,
Ke
Ke
a,
Ting
Xiong
b,
Yinming
Wang
b,
Boon Kiang David
Lim
 b,
Wee Siang Vincent
Lee
*b,
Zhenbo
Wang
*a and
Junmin
Xue
b,
Wee Siang Vincent
Lee
*b,
Zhenbo
Wang
*a and
Junmin
Xue
 *b
*b
aMIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage, School of Chemistry and Chemical Engineering, Harbin Institute of Technology, No. 92 West-Da Zhi Street, Harbin 150001, China. E-mail: wangzhb@hit.edu.cn
bDepartment of Materials Science and Engineering, National University of Singapore, 117576 Singapore, Singapore
First published on 2nd October 2019
Abstract
Organic compounds, such as polyvinylidene fluoride (PVDF), have been widely used as a binder in battery electrode preparations. While such an approach does not have a significant impact on the performance of the batteries that utilize low valence ions, such as the Li ion battery (LIB), the diffusion of high valence ions (such as Zn2+) will be severely impaired. This will be especially pronounced if the polymeric binder contains highly electronegative atoms, such as fluorine. The high charge density ions, such as Zn2+, tend to adsorb onto these electronegative atoms, thus the mobility of these ions across the material is inevitably affected. As such, it becomes highly necessary to consider the binder-free electrode architecture when designing a high rate performing and cycling-stable zinc ion battery (ZIB) cathode. Herein, this work demonstrates an improved Zn ion battery by adopting a freestanding electrode. The obtained V2O5/CNT paper electrode delivers a specific capacity of 312 mA h g−1, while achieving a respectable 75% retention in capacity after increasing the current density by 10-fold. Furthermore, excellent cycling stability is recorded with 81% capacity retention after 2000 cycles at 1.0 A g−1. Thus, this work clearly demonstrated that the freestanding electrode is a promising approach for high valence ion batteries.
1. Introduction
Energy storage devices, such as lithium ion batteries (LIBs), have become an essential part that contributes toward technological advancements in modern society.1–6 In this technological revolution, energy storage devices are not only expected to last longer, they are also expected to be fully charged within a short time period. Such a shifting paradigm indicates that the rate performances of these energy storage devices are gradually capturing increasing attention and growing scrutiny.7–12 As one of the most widely studied materials in LIB, V2O5 has gained considerable attention due to its ease of fabrication, high capacity, earth abundance, and low cost.13–16 While V2O5 has generally shown promising electrochemical performance as an LIB electrode, V2O5 possesses low ionic conductivity, which inevitably leads to a poor rate performance and less-than-ideal cycling stability. Such a challenge is easily mitigated with the incorporation of carbonaceous materials to enhance the overall conductivity of the composite. Even though improvements can be observed via the V2O5/carbon composite, these materials are usually in powder form and require the addition of foreign additives, such as polymeric binders, during electrode preparation.17–20 This addition of an organic binder not only complicates the electrode preparation process, but also clogs the transportation channels, which can severely affect the rate capability and cycling stability. However, it should be noted that in the case of LIBs, such channel blockages are not very pronounced due to the easy diffusion of the low valence Li+ ions. Such a situation would be further aggravated when these diffusing ions have higher charge densities than Li+ and hence, this consideration must be revitalized for alternative high valence ion batteries.With the recent elevated interest in V2O5 as a zinc ion battery (ZIB) cathode, the magnitude of this sluggish diffusion across the material with an organic binder is greatly increased. Even though the Zn2+ diameter is close to that of Li+, it possesses a higher charge density that can pose a polarizing effect on the material during the diffusion process. Due to the high charge density, Zn2+ tends to be adsorbed to the C–F bonds in the polarizing polymeric binder. This consequently reduces the mobility of Zn2+ and ultimately leads to a poor rate performance and large initial irreversible capacity loss. Thus, it becomes highly necessary to avoid the use of polymeric binders to ensure minimal disruption to the mobility of Zn2+ during the charging/discharging process. In order to achieve this, interest in a freestanding electrode should be renewed, especially for high valence ion batteries such as ZIB. A typical strategy is to introduce a carbonaceous scaffold that can offer structural integrity for the metal oxides, while simultaneously contributing to the overall conductivity of the electrode. As such, one of the favorite carbon monoliths is graphene paper. However, due to its layer-by-layer arrangement, the extent of electrolyte infiltration becomes a challenge as the electrolyte can only enter the material in the planar direction. In order to address such a limitation, carbon nanotube (CNT) paper would hence become an appealing scaffold material for V2O5 due to its random stacking arrangement, which offers better electrolyte infiltration from all directions.
Herein, this work reports a significant electrochemical performance enhancement (especially the rate performance and cycling stability) by adopting a freestanding V2O5/CNT composite paper (VCP) as the ZIB cathode. As a polymer binder is not used in the electrode preparation, the V2O5/CNT paper delivers a specific capacity of 312 mA h g−1, while achieving a respectable 75% retention in capacity after increasing the current density by 10-fold. It is interesting to note that its counterpart with an organic binder demonstrated a poor rate performance of 36% at the same current density. Furthermore, our binder-free composite papers exhibit superior cycling stability, as compared to the counterpart with an organic binder. This work demonstrates a significant progress of the binder-free electrode in achieving excellent electrochemical performance and its potential for high valence ion batteries.
2. Experimental
In a typical synthesis procedure, the V2O5/CNT paper electrode was fabricated via a vacuum filtration route. The CNTs were purchased from time/nano and the V2O5 powder was purchased from Sigma. First, 1 g Triton X-100 was added to 100 mL of deionized water. V2O5 powder and CNTs were subsequently dispersed under magnetic ultrasonication for 1 h in air with a mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Then, the as-obtained solution was filtered through the membrane filter. After the film was dried, it was easily peeled off from the filtration paper. The mass loading was about 7 mg cm−2.
3. Then, the as-obtained solution was filtered through the membrane filter. After the film was dried, it was easily peeled off from the filtration paper. The mass loading was about 7 mg cm−2.
Morphology and structure characterization
The powder XRD pattern was measured using a powder diffractometer (Bruker D8 Advanced Diffractometer System) with a Cu Kα (1.5418 Å) source. The component content was determined using thermogravimetric analysis (TGA, DMSE SDTQ600). The morphology and size of the samples were determined using scanning electron microscopy (SEM, ZEISS SEM Supra 40 (5 kV)) and energy dispersive X-ray spectroscopy (EDS). The micro-structure was observed using a three-dimensional white light interference surface topography instrument. Transmission electron microscopy (TEM) was done on a JEOL-3010 (300 kV acceleration voltage) microscope. TEM samples were prepared by dripping the sample solutions onto a copper grid. The surface composition was analyzed by XPS using a Kratos Analytical Axis UltraDLD UHV spectrometer with a monochromatized Al Kα X-ray source (1486.6 eV), scanning a spot size of 700 μm × 300 μm.Electrochemical measurements
All electrochemical tests were performed at room temperature. CV and galvanostatic charge/discharge measurements were conducted using an electrochemical system (Bio-logic VMP 3). Zinc foil and filter paper were used as the anode and separator, respectively, and 1 M ZnSO4 was employed as the electrolyte. A CR2025-type coin cell was assembled under ambient conditions to evaluate the electrochemical performance. Three different working electrodes were prepared. The V2O5 electrode was prepared by mixing the purchased V2O5 powder with carbon black and polyvinylidene fluoride in a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with N-methyl-2-pyrrolidone. The mixture was hand-ground for at least 20 min to obtain a slurry. The slurry was later coated onto carbon paper, which served as a current collector, and then heated at 80 °C overnight for further use. The CNT/V2O5 mixed powder was obtained in the same way. The ratio of CNT/V2O5 was 7
1 with N-methyl-2-pyrrolidone. The mixture was hand-ground for at least 20 min to obtain a slurry. The slurry was later coated onto carbon paper, which served as a current collector, and then heated at 80 °C overnight for further use. The CNT/V2O5 mixed powder was obtained in the same way. The ratio of CNT/V2O5 was 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Then, the powder was mixed by ultrasonic dispersing technology in DI water. After drying at 80 °C in the atmosphere, the mixed powder was collected and used as the electrochemical active substance. Then, it was mixed with carbon black and polyvinylidene fluoride in a ratio of 7
3. Then, the powder was mixed by ultrasonic dispersing technology in DI water. After drying at 80 °C in the atmosphere, the mixed powder was collected and used as the electrochemical active substance. Then, it was mixed with carbon black and polyvinylidene fluoride in a ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The V2O5/CNT paper electrode was directly used as the working electrode. For both CV and charge/discharge of the full cell test, the measurement voltage was controlled in the range of 0.2–1.7 V for the aqueous electrolyte test. The current densities of 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0 A g−1 were used for the charge/discharge measurement. The capacity, energy density, and power density were calculated based on the mass of the active materials from the cathode.
1. The V2O5/CNT paper electrode was directly used as the working electrode. For both CV and charge/discharge of the full cell test, the measurement voltage was controlled in the range of 0.2–1.7 V for the aqueous electrolyte test. The current densities of 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0 and 10.0 A g−1 were used for the charge/discharge measurement. The capacity, energy density, and power density were calculated based on the mass of the active materials from the cathode.
3. Results and discussion
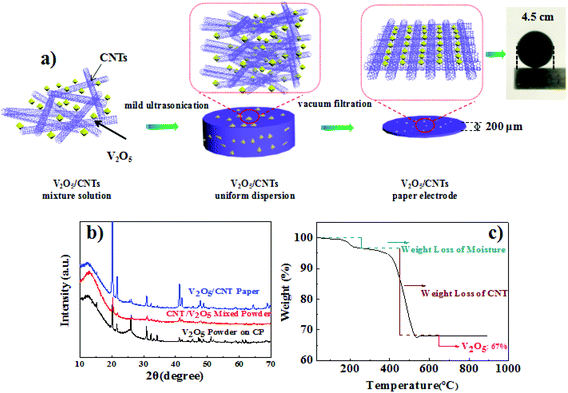
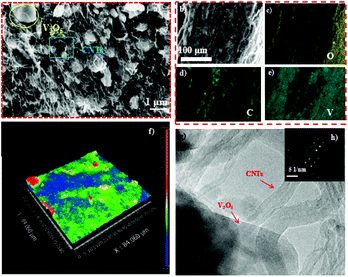
A facile vacuum filtration method was employed to fabricate the V2O5/CNT paper (VCP) electrode, as presented in Fig. 1a. Two more samples pasted on carbon paper were prepared as control: (1) V2O5 powder (VP) mixed with carbon black and binder and (2) CNT/V2O5 mixture powder (CVMP) mixed with carbon black and binder. Fig. 1b presented the X-ray diffraction (XRD) patterns of these three different electrodes. All Bragg peaks of the XRD patterns of the three samples showed the shcherbinaite reflections, which belonged to an orthorhombic structure of V2O5 with space group Pmmm (indexed the standard PDF card 41-1426).21 To determine the amount ratio of V2O5 to CNT in VCP, thermogravimetric analysis was performed under ambient conditions. As shown in Fig. 1c, two pronounced degradation steps were identified. The first degradation step at 100 °C represented the evaporation of the water molecules in VCP, and the second step corresponded to the decomposition of the CNTs. Based on the TGA result, the weight percentage of the electrochemical active material V2O5 in VCP was ca. 67%.Fig. 2a presents a typical scanning electron microscopy (SEM) image of the VCP. Under the SEM observation, the VCP electrode exhibited a 3-dimensional porous structure that comprised a continuous carbon nanotube network and V2O5 particles. Fig. 2(b–e) show the cross-sectional image of VCP from energy dispersive X-ray spectra (EDX). The thickness of the paper electrode was about 200 μm and the EDX results indicated a uniform distribution of V and C elements across the entire cross-section, which confirmed an even distribution of V2O5 in the CNT conductive network. Fig. 2f is an image of the three-dimensional white light interference surface topography. The appearance of a large number of blue areas confirmed the existence of 3D channels, which were beneficial to the zinc ion transportation. Transmission electron microscopy (TEM) was employed to further study the morphology of the paper electrode. Based on Fig. 2g, the presence of the CNTs and V2O5 particles further confirmed the successful preparation of a freestanding V2O5/CNT paper. The CNT and single-crystalline V2O5 (according to the SAED pattern in Fig. 2h) were entangled together to form a continuous electron conducting network.
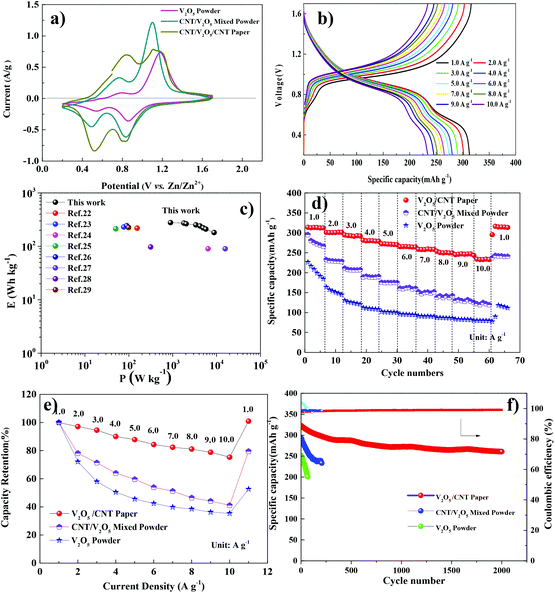
To compare the electrode performance with/without a binder, electrochemical testing was conducted for the pristine V2O5 powder (VP), the CNT/V2O5 mixed powder (CVMP) and freestanding V2O5/CNT composite paper (VCP). The CV curves of these three electrodes are presented in Fig. 3a. Under the same scan rate of 0.2 mV s−1, the integrated area of the CV curves for VCP was larger than those of the two control electrodes, suggesting a larger capacity. The cyclic voltammetry (CV) curves of the VCP electrode conducted from 0.2 mV s−1 to 1 mV s−1 were recorded and are presented in Fig. S1a.† Two reduction peaks (around 0.4–0.5 V and 0.8–0.9 V) and two oxidation peaks (around 0.9–1.0 V and 1.2–1.3 V) were observed, which were caused by the zinc ion intercalation/deintercalation during the discharge/charge processes. The galvanostatic charge/discharge (GCD) measurements of VCP were performed in the range of 0.2–1.7 V (as shown in Fig. 3b). An average specific discharge capacity of 312 mA h g−1 was recorded at a current density of 1 A g−1. Even at a higher current density of 10 A g−1, a good specific capacity of 232 mA h g−1 was achieved. The areal and volumetric capacities at various current densities are also shown in Fig. S2a.† An areal capacity of 2.2 mA h cm−2 and a volumetric capacity of 109.2 mA h cm−3 can be obtained at a current density of 1 A g−1. The corresponding energy/power density is presented in Fig. 3c and the areal/volumetric energy/power density are also given in Fig. S2b.† The VCP electrode exhibited an energy density of 891 W h kg−1 at a power density of 278 W kg−1 (Fig. 3c). A maximum power density of 8871 W kg−1 could be reached. The excellent rate capability was further reflected in the Ragone plots by comparison with some recent reports (see ESI Table 1†).22–29 The rate performances of the VP, CVMP and VCP are shown in Fig. 3d. The VCP exhibited superior rate capability with average specific discharge capacities of 312, 303, 281, 253 and 235 mA h g−1 at current densities of 1, 2, 4, 8 and 10 A g−1, respectively. Recently, some studies also demonstrated that structural water could function as a charge screening media in the redox reactions. The H2O-solvated Zn2+ possesses a largely reduced effective charge and thus, reduced electrostatic interactions with the V2O5 framework, effectively promoting its diffusion.24 Based on Fig. 3d, the capacity retention of these three electrodes were calculated and is shown in Fig. 3e. For the VCP electrode, the capacity retention is 75% (at 10 A g−1) of the initial value (at 1 A g−1), while only 42% retention was calculated for CVP and a poor 36% retention for VP. When the current density was decreased from 10 A g−1 to 1 A g−1, the capacity of VCP was fully recovered, which was superior to the 52% and 79% capacity recovery for VP and CVMP, respectively. It was believed that the capacity loss in VP and CVMP was due to the presence of the organic binder, which generated a strong electrostatic attraction with the highly positively charged zinc ions, and thus these electrostatically “glued” zinc ions could not be completely extracted. The capacity fading of electrodes with the polymeric binder could be clearly seen by comparing the discharge capacity of the second and third cycles (Fig. S3†). On the other hand, the capacity loss of the VCP electrode was negligible. To demonstrate the high reversibility of VCP, all three electrodes were cycled at a low current density of 1.0 A g−1 to evaluate the long-term cycling stability (as shown in Fig. 3f). After 2000 cycles, the VCP electrode retained 81% of its initial capacity with ca. 99% coulombic efficiency for all the cycles, suggesting an excellent cycling life. However, the other two powder electrodes demonstrated 80% capacity retention under 250 cycles. The structure and morphology of the V2O5/CNT paper after a long-term cycling process was also an important factor toward the stable electrochemical properties of the Zn ion batteries. After 2000 cycles, compared with the original XRD result (Fig. S4a†), similar peaks were revealed without an obvious change. The morphology of the V2O5 blocks in the CNT network could still be clearly distinguished in Fig. S4b.†
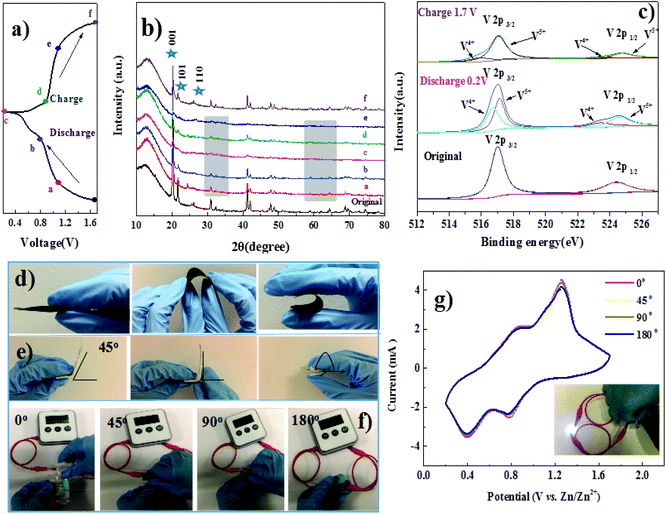
An ex situ XRD technique was employed to explore the structural changes of VCP during the discharge/charge process (Fig. 4a). When the electrode was discharged to a lower voltage, the intensity of the characteristic peaks became weaker, which was ascribed to the intercalation of Zn2+ ions from the layered structure and resulted in lattice distortions. It could be seen that the location of the diffraction peaks corresponding to the (001), (101), and (110) lattice planes were consistent with those in the initial sample, indicating that the sample was still a layered structure of V2O5 after the charge–discharge process.30 The valence state changes of vanadium are also presented in Fig. 4b. When discharged to 0.2 V, the V 2p3/2 peaks were split into two peaks. The reduction in vanadium happened along with the insertion of Zn2+. Then, the vanadium was further oxidized back to its original state during the charging process.31 The SEM images under different potentials are shown in Fig. S5a to S5f.† The morphology of the V2O5/CNT paper remained the same after the discharging–charging process. In order to investigate the mechanical property of our freestanding film electrode, we have performed a simple mechanical fatigue test, as shown in Fig. S6.† After 100 bends, the freestanding film remained intact without flaking. Ultimately, we assembled a flexible zinc ion battery using VCP as the cathode, zinc foil as the anode, filter membrane as the separator and 1 M ZnSO4 aqueous solution as the electrolyte (Fig. 4d and e). The digital watch and white LED were powered by two charged zinc batteries connected in series (as shown in Fig. 4f and inset of Fig. 4g). The unchanged shape of the CV curves under different bending angles also verified the stable performance of this flexible device (Fig. 4g). This paper electrode will open up new opportunities for advanced energy storage systems taking advantage of large mass-loading, flexibility, and long cycling ability.
4. Conclusions
A binder-free, freestanding V2O5/CNT electrode was successfully prepared as a high rate performance and high cycling-stable zinc ion battery cathode. This work demonstrated a strategy that is beneficial to realize the high-performance multivalent metal battery (such as ZIB), by using a freestanding and binder-free electrode. Because an organic binder such as a polymer additive was not used in the electrode preparation, the V2O5/CNT paper delivered a specific capacity of 312 mA h g−1 while achieving a respectable 75% retention in capacity after increasing the current density by 10-fold. Finally, the flexible device based on the paper electrode easily powered the digital watch and white LED even at different bending states. These achievements cast light on the design of more advanced, binder-free, flexible cathode materials for rechargeable zinc ion batteries.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Singapore MOE Tier 2 grant MOE-2018-T2-1-149, China Scholarship Council (201806120308), the National Natural Science Foundation of China (Grant No. 21273058, 21673064, 51802059 and 21503059), China postdoctoral science foundation (Grant No. 2017M621285 and 2018T110292), Fundamental Research Funds for the Central Universities (Grant No. HIT. NSRIF. 2019040 and 2019041).References
- P. Grey and J. M. Tarascon, Nat. Mater., 2017, 16, 45–56 CrossRef PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- P. K. Nayak, L. Yang, W. Brehm and P. Adelhelm, Angew. Chem., Int. Ed., 2018, 57, 102–120 CrossRef CAS PubMed.
- W. N. Xu, K. N. Zhao, W. C. Huo, Y. Z. Wang, G. Yao, X. Gu, H. W. Cheng, L. Q. Mai, C. G. Hu and X. D. Wang, Nano Energy, 2019, 62, 275–281 CrossRef CAS.
- S. T. Lian, C. L. Sun, W. N. Xu, W. C. Huo, Y. Z. Luo, K. N. Zhao, G. Yao, W. W. Xu, Y. X. Zhang, Z. Li, K. S. Yu, H. B. Zhao, H. W. Cheng, J. J. Zhang and L. Q. Mai, Nano Energy, 2019, 62, 79–84 CrossRef CAS.
- B. S. Yin, S. W. Zhang, K. Ke and Z. B. Wang, Chem. Eng. J., 2019, 375, 122056 CrossRef CAS.
- T. Z. Hou, X. Chen, H. J. Peng, J. Q. Huang, B. Q. Li, Q. Zhang and B. Li, Small, 2016, 12, 3283–3291 CrossRef CAS PubMed.
- X. Liu, J. Q. Huang, Q. Zhang and L. Mai, Adv. Mater., 2017, 29, 1601759 CrossRef PubMed.
- R. Fang, S. Zhao, Z. Sun, D. W. Wang, H. M. Cheng and F. Li, Adv. Mater., 2017, 29, 1606823 CrossRef PubMed.
- Z. W. Seh, Y. Sun, Q. Zhang and Y. Cui, Chem. Soc. Rev., 2016, 45, 5605–5634 RSC.
- R. Van Noorden, Nature, 2013, 498, 416 CrossRef CAS PubMed.
- S. W. Zhang, B. S. Yin, X. X. Liu, D. M. Gu, H. Gong and Z. B. Wang, Nano Energy, 2019, 59, 41–49 CrossRef CAS.
- F. W. Ming, H. F. Liang, Y. J. Lei, S. Kandambeth, M. Eddaoudi and H. N. Alshareef, ACS Energy Lett., 2018, 3, 2602–2609 CrossRef CAS.
- P. Hu, M. Y. Yan, T. Zhu, X. P. Wang, X. J. Wei, J. T. Li, L. Zhou, Z. H. Li, L. N. Chen and L. Q. Mai, ACS Appl. Mater. Interfaces, 2017, 9, 42717–42722 CrossRef CAS PubMed.
- Y. K. Li, Z. M. Huang, P. K. Kalambate, Y. Zhong, Z. M. Huang, M. L. Xie, Y. Shen and Y. H. Huang, Nano Energy, 2019, 60, 752–759 CrossRef CAS.
- D. M. Xu, H. W. Wang, F. Y. Li, Z. C. Guan, R. Wang, B. B. He, Y. S. Gong and X. L. Hu, Adv. Mater. Interfaces, 2019, 6, 1801506 CrossRef.
- E. Madej, M. Espig, R. R. Baumann, W. Schuhmann and E. La Mantia, J. Power Sources, 2014, 261, 356–362 CrossRef CAS.
- L. N. Chen, M. Y. Yan, Z. W. Mei and L. Q. Mai, J. Inorg. Mater., 2017, 32, 225 CrossRef.
- D. Kundu, B. D. Adams, V. Duffort, S. H. Vajargah and L. F. Nazar, Nat. Energy, 2016, 1, 16119 CrossRef CAS.
- X. Hong, B. Zhang, E. Murphy, J. Zou and F. Kim, J. Power Sources, 2017, 343, 60–66 CrossRef CAS.
- K. Dewangan, N. N. Sinha, P. G. Chavan, P. K. Sharma, A. C. Pandey, M. A. More, D. S. Joag, N. Munichandraiah and N. S. Gajbhiye, Nanoscale, 2012, 4, 645–651 RSC.
- H. G. Qin, L. L. Chen, L. M. Wang, X. Chen and Z. H. Yang, Electrochim. Acta, 2019, 306, 307–316 CrossRef CAS.
- Y. C. Ding, Y. Q. Peng, W. Y. Chen, Y. J. Niu, S. G. Wu, X. X. Zhang and L. H. Hu, Appl. Surf. Sci., 2019, 493, 368–374 CrossRef CAS.
- M. Y. Yan, P. He, Y. Chen, S. Y. Wang, Q. L. Wei, K. N. Zhao, X. Xu, Q. Y. An, Y. Shuang, Y. Y. Shao, K. l. T. Mueller, L. Q. Mai, J. Liu and J. H. Yang, Adv. Mater., 2018, 30, 1703725 CrossRef PubMed.
- C. Xia, J. Guo, Y. J. Lei, H. F. Liang, C. Zhao and H. N. Alshareef, Adv. Mater., 2018, 30, 1705580 CrossRef PubMed.
- D. M. Xu, H. W. Wang, F. Y. Li, Z. C. Guan, R. Wang, B. B. He, Y. S. Gong and X. L. Hu, Adv. Mater. Interfaces, 2019, 6, 1801506 CrossRef.
- V. Soundharrajan, B. Sambandam, S. J. Kim, M. H. Alfaruqi, D. Y. Putro, J. Jo, S. Kim, V. Mathew, Y. K. Sun and J. Kim, Nano Lett., 2018, 18, 2402–2410 CrossRef CAS PubMed.
- W. Li, K. L. Wang, S. J. Cheng and K. Jiang, Energy Storage Mater., 2018, 15, 14–21 CrossRef.
- T. Y. Wei, Q. Li, G. Z. Yang and C. X. Wang, J. Mater. Chem. A, 2018, 6, 20402–20410 RSC.
- F. Liu, Z. X. Chen, G. Z. Fang, Z. Q. Wang, Y. S. Cai, B. Y. Tang, J. Zhou and S. Q. Liang, Nano-Micro Lett., 2019, 11, 25 CrossRef CAS.
- J. Zhou, L. T. Shan, Z. X. Wu, X. Guo, G. Z. Fang and S. Q. Liang, Chem. Commun., 2018, 54, 4457–4460 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nr07458a |
| This journal is © The Royal Society of Chemistry 2019 |