Open Access Article
Open Access ArticleThe first water-soluble bowl complex: molecular recognition of acetate by the biomimetic tris(imidazole) Zn(II) system at pH 7.4†
Stéphanie
Rat
,
Jérôme
Gout
,
Olivia
Bistri
and
Olivia
Reinaud
*
Laboratoire de Chimie et de Biochimie pharmacologiques et toxicologiques, Université Paris Descartes, Sorbonne Paris Cité, CNRS UMR 8601, 45 rue des Saints Pères, 75006 Paris, France. E-mail: olivia.reinaud@parisdescartes.fr; Tel: +33 14286 2183
First published on 28th January 2015
Abstract
A supramolecular approach for modeling active sites of metallo-enzymes relies on the association of a metal ion bound to a tris(imidazole) core under the control of a cavity. One step further is the water-solubilization of the cavity-complex. Here, we describe the synthesis of a water-soluble bowl-ligand that has been successively achieved through an 11-step strategy from resorcinol. First insights into its coordination properties in water show that it readily binds Zn(II) at physiological pH and acts as a molecular receptor for the hydrophilic acetate guest ligand.
Metallo-enzymes are very sophisticated natural receptors able to catalyze highly selective reactions under very mild conditions and with high turnover numbers. Classical model complexes aim at reproducing the first coordination sphere of the metal ion.1 Poly-aza chelating ligands are thus employed to mimic the poly-histidine core often encountered in Zn, Cu and Fe enzymes. One of the key issues for modeling these metallo-enzyme active sites is to maintain free labile sites for an interaction with exogenous molecules (substrate/reactant) with the simultaneous control of the nuclearity of the metal complex.2 Pursuing this objective, we developed a supramolecular approach where a tris(imidazole) binding site is grafted onto the edge of a macrocycle, namely a calix[6]arene or a resorcin[4]arene.3,4 In these funnel- and bowl-shaped systems, the metal ion is in a 4- or 5-coordinate environment, displaying one or two labile sites, respectively. The role of the macrocyclic cavity is to protect the metal center against dimerization, while acting as a selective receptor for guest-ligands. A further step in the biomimetic quest is to obtain water-soluble systems. As a matter of fact, most of the biomimetic complexes aimed at reproducing a specific feature observed in enzymes are not water-soluble compounds. There are several reasons for this. First, in enzymes, the metal center is buried in the heart of the protein that defines a medium resembling more to an organic solvent than to water. Second, it is very difficult to control the reactivity of a metal center in water due to its propensity to form hydro/oxo species that readily undergo dimerization if not polymerization and precipitation. Third, the synthesis of a ligand allowing simultaneously coordination of an open-shell metal ion, protection against water, and water-solubilization is a difficult task, from both a design and synthetic point of view. Water as a solvent is also a highly competitive medium for molecular recognition due its polar character and hydrogen-bonding propensity.5,6
Our first success in the elaboration of a water-soluble supramolecular mimic of zinc enzyme active sites was obtained with a tris(imidazole)calix[6]arene-based ligand, WXim3 (Scheme 1).7 The latter was made water-soluble thanks to the introduction of three ammonium groups into the macrocycle, at the rim that is opposite to the coordination site, in order not to perturb it. It was found that the ligand alone was not capable of stabilizing a mono-nuclear complex, unless a primary amine guest was stabilizing the complex as a ternary adduct. This led to the first receptor capable of recognizing primary amines in pure water, near physiological pH. It was shown that interaction with the metal center, H-bonding at the small rim defining the second coordination sphere and hydrophobic effect were all at play for the efficiency of the molecular recognition process with the synergistic stabilization of Zn(II) in the tripodal tris(imidazole) core and coordination/embedment of the amino-guest.
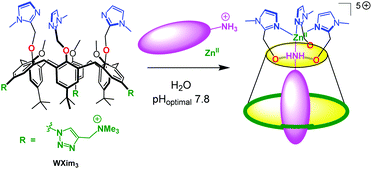 | ||
| Scheme 1 Schematized funnel complex based on the calix[6]arene ligand WXim3 and synergistic binding of Zn2+ and hydrophobic primary amines in water. | ||
In order to also develop water-soluble cavity-complexes with the bowl-shaped resorcinarene scaffold, we thought of introducing hydrophilic groups as “footed” substituents (R′, Scheme 2). The first goal is to obtain biomimetic complexes that are stable in water, the second is to obtain a molecular receptor efficient in water, and the third will be to explore the reactivity of these systems when the metal center is embedded in a hydrophobic cavity, with easy but controlled access to water/protons that are keys in many reactions. Here, we describe the first step, i.e. the synthesis of a water-soluble bowl ligand together with preliminary results relative to biological metal ion complexation and molecular recognition in water, as a proof of concept. We show that Zn(II) is readily bound to the tris(imidazole) core of the bowl ligand and that it can act as a receptor for acetate, two features that stand in contrast with the previously reported funnel system.
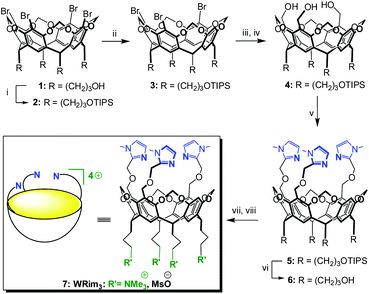 | ||
| Scheme 2 Synthesis of the tris-imidazole bowl ligand WRim3.† (i) TIPSCl, imidazole, DMF, r.t., 82%; (ii) n-BuLi (1 equiv.), THF, −78 °C, then MeOH −78 °C → r.t., 81%; (iii) n-BuLi (3.3 equiv.), THF, −78 °C, then ClCO2Me, −78 °C → r.t.; (iv) LiAlH4, THF, 0 °C → r.t., 74% over 2 steps; (v) 2-chloromethyl-1-methyl-1H-imidazole hydrochloride, NaH, DMF, 0 °C → r.t., 74%; (vi) TFA, THF–H2O, r.t., 91%; (vii) MsCl, DIPEA, DMF, 0 °C → r.t., (viii) NMe3, 40 °C, 85%. | ||
Results and discussion
Synthesis of the water-soluble WRim3 ligand
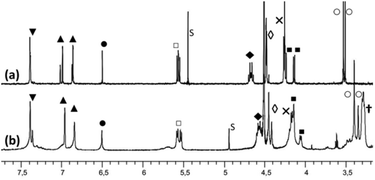
The propanol-based resorcin[4]arene 18 was first protected as a tris(isopropylsilyl) ether 2 (Scheme 2). Three imidazole units were then grafted at the large rim of the silyl-protected cavitand 2 following the synthetic strategy used for the organosoluble Rim3 ligand (Scheme 2, R = C5H11).9 First, compound 2 was treated with 1 equiv. of n-BuLi to yield the tris(bromo) derivative 3. After the lithium–bromide exchange of the three remaining brominated aromatic units and reaction with methylchloroformate, the corresponding triester derivative was obtained. The latter was subsequently reduced by lithium aluminium hydride, to yield triol 4. The reaction of 4 with sodium hydride and 2-chloromethyl-1-methyl-1H-imidazole hydrochloride afforded the tris(imidazole) ligand 5. Finally, the tris(isopropylsilyl) groups were removed by treatment with TFA to give compound 6, which was subsequently mesylated and treated by trimethylamine to give the water-soluble tris(imidazol) ligand WRim3. An overall yield of 30% was obtained starting from resorcinarene 1 (8 steps).†The water-soluble ligand WRim3 was characterized by HRMS ESI and 1H NMR spectroscopy in D2O.† Its 1H NMR spectrum in D2O (Fig. 1b) displays sharp resonances, which stands in contrast with the calixarene-based ligand WXim3,7 the spectrum of which displayed extremely broad resonances indicating conformational mobility and partial aggregation in water. With WRim3, the sharpness of the peaks indicates that there is no aggregation and that the rigid bowl has a well-defined conformation. The 1H NMR spectrum of WRim3 in D2O displays an overall signature presenting many similarities with the 1H NMR profile of the organosoluble Rim3 ligand (R′ = nC5H11-footed) in CD3CN (Fig. 1a). As for Rim3, WRim3 presents a symmetry plane passing through the central imidazole group. The aromatic protons located at the small rim of the macrocycle (annotated ▼ in Fig. 1) are distributed in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. The signals corresponding to the N-methyl protons of the imidazole groups (annotated ○ in Fig. 1) are distributed in two signals in a 2
1 ratio. The signals corresponding to the N-methyl protons of the imidazole groups (annotated ○ in Fig. 1) are distributed in two signals in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and imidazole protons (HIm, annotated ▲ in Fig. 1) are separated in two signals of equal intensity.
1 ratio and imidazole protons (HIm, annotated ▲ in Fig. 1) are separated in two signals of equal intensity.
The variation of the pD (Fig. S18†) of the solution allowed the identification of the protonation state of the ligand. 1H NMR spectra were recorded at various pD from 1.9 to 11.7 (by addition of diluted solutions of deuterated nitric acid or sodium hydroxide). The variation of the chemical shift of the imidazole groups as a function of the pD gave an estimation of their pKa values between 5 and 8. These values are slightly more basic than the pKa values of the three imidazolyl groups of the water-soluble calix[6]arene: pKa = 4.76, 4.89, 6.09.7
Zn(II) complexation in water
The addition of 1 equivalent of Zn(NO3)2 at pD = 8.1 decreased the pD of the solution down to 7.3 and strongly modified the recorded 1H NMR spectrum in D2O at 300 K. The six protons belonging to the imidazole nuclei (HIm, annotated ▲ in Fig. 2b) are shifted and distributed in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Their methyl substituents are downfield shifted and also split into two signals in a 2
1 ratio. Their methyl substituents are downfield shifted and also split into two signals in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. These observations attest to the coordination of the imidazole groups to Zn(II). Indeed, the same 1H NMR changes were observed for the Zn(II) complex obtained with the organo-soluble Rim3 ligand in an organic solvent (CD3CN).9 The WRim3Zn(II) complex was detectable from pD = 6.5 to 9.5. This result is different from the one obtained in the case of the water-soluble WXim3 ligand, for which the complexation of Zn(II) was conditioned by the presence of a hydrophobic guest-ligand, hence stabilizing the complex as a ternary adduct (Scheme 1). This is attributable to the higher rigidity of the resorcinarene macrocycle that better pre-organizes the tris(imidazole) core for Zn(II) coordination compared to the flexible calix[6]arene core.
1 ratio. These observations attest to the coordination of the imidazole groups to Zn(II). Indeed, the same 1H NMR changes were observed for the Zn(II) complex obtained with the organo-soluble Rim3 ligand in an organic solvent (CD3CN).9 The WRim3Zn(II) complex was detectable from pD = 6.5 to 9.5. This result is different from the one obtained in the case of the water-soluble WXim3 ligand, for which the complexation of Zn(II) was conditioned by the presence of a hydrophobic guest-ligand, hence stabilizing the complex as a ternary adduct (Scheme 1). This is attributable to the higher rigidity of the resorcinarene macrocycle that better pre-organizes the tris(imidazole) core for Zn(II) coordination compared to the flexible calix[6]arene core.
Acetate coordination in water
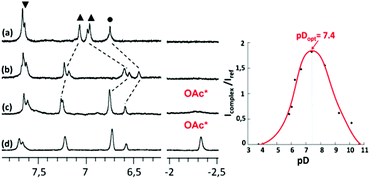
Interestingly, when one equivalent of Zn(OAc)2 was added to a solution of ligand WRim3 in H2O (Fig. 2c) and after adjusting the pH to 7.2 with sodium hydroxide, a different 1H NMR spectrum was obtained, compared to that obtained with Zn(NO3)2. Adding sodium acetate to a solution of the complex obtained with Zn(NO3)2 or adding Zn(NO3)2 to a solution containing WRim3 and acetate led to the same NMR profile. The imidazole protons are downfield shifted and a new broad signal integrated for 3 protons is observable in the high-field region at −2.34 ppm. This signal is attributed to the methyl group of the acetate anion coordinated inside the cavity (Scheme 3). The corresponding up-field shift value, Δδ = −4.2 ppm, is characteristic of an encapsulated guest experiencing the shielding effect of the aromatic units. The broadness of the signal is attributed to the exchange between free and bound acetate: cooling of the solution to 280 K (Fig. 2d) slowed down the exchange rate and sharpened drastically the peak in the high-field region.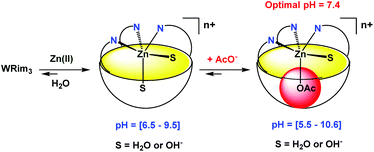 | ||
| Scheme 3 Schematic representation of the coordination of Zn(II) and acetate by ligand WRim3 in water. The variations of pH were obtained with nitric acid and sodium hydroxide. | ||
Optimal pH
The optimal pH for the formation of [WRim3Zn(OAc)]+ was determined by 1H NMR spectroscopy in D2O ([WRim3] = 2.5 mM) in the presence of one equivalent of Zn(OAc)2. By integrating the methyl signal of the encapsulated acetate anion (Icomplex) in comparison with a reference peak (Iref), the presence of the complex [WRim3Zn(OAc)]+ was quantified as a function of pD. The results displayed in Fig. 2 clearly show an optimum pD value for the formation of the acetato complex at pD 7.4. It is also important to note that [WRim3Zn(OAc)]+ was detectable from pD 5.5 to 10.6.† This pD window is wider than that observed in the absence of acetate, which emphasizes the stabilizing effect of the acetate guest on the WRim3Zn complex. The presence of an anionic guest inside the cavity stabilizes the ternary adduct toward the numerous competitive acid–base equilibria in water. An estimation of the binding constant corresponding to the equilibrium [WRim3Zn + AcO = WRim3ZnOAc] gave K′ = 600 ± 100 M−1 at pD = 7.4.The ability of this new bowl-shaped Zn(II) complex to coordinate acetate in pure water is quite remarkable. Indeed, whereas many receptors for acetate have been described in the literature, most of them working in organic solvents such as DMSO or MeCN.10 Those reported to bind acetate in pure water are rare and the corresponding binding constants are relatively moderate.10 A copper(II) tren-based complex equipped with three guanidinium pending arms was reported to bind acetate at pH 7.4 with a K′ value of 300 M−1.11 A di-copper(II) complex obtained with a tetra-aminoethyltetra-azacycloalcane binds acetate with K′ = 130 M−1 (unbuffered solution).12 A higher binding constant was obtained with a dicopper(II) cage complex at pH 7 (K′ = 5000 M−1).13,14 A ruthenium containing self-assembled helicate was shown to bind acetate into a poly-ammonium pocket at pD 6.6 (K′ = 270 M−1).15 Recently, a fluorescent probe for efficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β = 5.2) and selective acetate detection in 90% aqueous DMSO at pH 7.4 was reported. It is based on an anthracene unit to which two benzyimidazolium H-bond donors were appended, which bind acetate only when they are substituted by naphtenyl groups. This was attributed to the hydrophobic effect associated with the embedment of the acetate guest in between these aromatic groups.16 Interestingly, the bowl complex herein described makes use of both, a coordination link to the Zn(II) ion, and the hydrophobic effect due to the acetate hosting in the resorcinarene cavity.
β = 5.2) and selective acetate detection in 90% aqueous DMSO at pH 7.4 was reported. It is based on an anthracene unit to which two benzyimidazolium H-bond donors were appended, which bind acetate only when they are substituted by naphtenyl groups. This was attributed to the hydrophobic effect associated with the embedment of the acetate guest in between these aromatic groups.16 Interestingly, the bowl complex herein described makes use of both, a coordination link to the Zn(II) ion, and the hydrophobic effect due to the acetate hosting in the resorcinarene cavity.
Conclusion
The water-soluble version of a bowl-shaped ligand was efficiently synthesized within 8 steps starting from resorcinarene 1 (11 steps from resorcinol), with a good overall yield (30% from 1). First insights into its coordination properties in water show that it readily binds Zn(II) at physiological pH and millimolar concentration. Compared to its sister funnel ligand based on the calix[6]arene scaffold (WXim3, Scheme 1), the bowl ligand allows stronger coordination of Zn(II), which can be ascribed to a more rigid scaffold allowing better preorganization of the tris(imidazole) core. The complex also behaves as a molecular receptor able to coordinate the small hydrophilic acetate anion in its cavity, in pure water, at an optimal pH of 7.4, and K′ = 600 M−1. Upon acetate binding, the pH window at which the Zn(II) complex is detected is increased from [6.5–9.5] to [5.5–10.6], which denotes some cooperativity in the binding process. Hence, this study represents one step further in biomimetism: a biomimetic coordination core (three imidazole residues) is able to coordinate a biological metal ion, Zn(II), that can bind exogenous ligands in a hydrophobic cavity, in pure water, at physiological pH. The presence of two labile coordination sites in the cis-position relative to each other is favorable for hydrolytic metallo-enzyme mimicry, since the mechanism is known to proceed via a five-coordinate intermediate. This system opens new perspectives in both molecular recognition of small hydrophilic anions and reactivity. Further studies to evaluate the binding constants and selectivity toward various metal ions and anions are now underway, together with reactivity studies aimed at taking advantage of this hydrophobic metallated cavity and turning it into a molecular reactor.Acknowledgements
This project was supported by the CNRS (Institut de Chimie), the Ministère de l'Enseignement Supérieur et de la Recherche, the Agence Nationale pour la Recherche [Cavity-zyme(Cu) Project No. ANR-2010-BLAN-7141] and the COST Action “Supramolecular Chemistry in Water” (CM 1005).Notes and references
- (a) I. Bertini, I. H. B. Gray, E. I. Stiefel and J. S. Valentine, Biological Inorganic Chemistry, Structure and Reactivity, University Science Books, Sausalito, CA, 2007 Search PubMed; (b) S. J. Lippard and J. M. Berg, Principles of Bioinorganic Chemistry, University Science Books, Mill-Valley, CA, 1994 Search PubMed; (c) R. H. Holm, P. Kennepohl and E. I. Solomon, Chem. Rev., 1996, 96, 2239 CrossRef CAS.
- For a general review on metallated cavities, see: R. Gramage-Doria, D. Armspach and D. Matt, Coord. Chem. Rev., 2013, 257, 776 CrossRef CAS PubMed.
- J.-N. Rebilly and O. Reinaud, Supramol. Chem., 2014, 26, 454 CrossRef CAS.
- J.-N. Rebilly, B. Colasson, O. Bistri, D. Over and O. Reinaud, Chem. Soc. Rev., 2015, 44, 467 RSC.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648 RSC.
- O. Bistri and O. Reinaud, Org. Biomol. Chem, 2015, 10.1039/C4OB02511C, Advance Article (review).
- O. Bistri, B. Colasson and O. Reinaud, Chem. Sci., 2012, 3, 811 RSC.
- B. C. Gibb, R. G. Chapman and J. C. Sherman, J. Org. Chem., 1996, 61, 1505 CrossRef CAS.
- (a) A. Višnjevac, J. Gout, N. Ingert, O. Bistri and O. Reinaud, Org. Lett., 2010, 12, 2044 CrossRef PubMed; (b) J. Gout, J. S. Rat, O. Bistri and O. Reinaud, Eur. J. Inorg. Chem., 2014, 2819 CrossRef CAS.
- For recent highlights in anion receptor chemistry, see: P. A. Gale, N. Busschaert, C. J. E. Haynes, L. E. Karagiannidis and I. L. Kirby, Chem. Soc. Rev., 2014, 43, 205 RSC , and references therein cited for previous highlights.
- S. L. Tobey and E. V. Anslyn, J. Am. Chem. Soc., 2003, 125, 14807 CrossRef CAS PubMed.
- E. Asato, K. Ozutsumi, S.-I. Ishiguro, S. Kida and I. Murase, Inorg. Chim. Acta, 1990, 167, 189 CrossRef CAS.
- L. Fabbrizzi, A. Leone and A. Taglietti, Angew. Chem., Int. Ed., 2001, 40, 3066 CrossRef CAS.
- L. Fabbrizzi and A. Poggi, Chem. Soc. Rev., 2013, 42, 1681 RSC.
- C. Olivier, Z. Grote, E. Solari, R. Scopelliti and K. Severin, Chem. Commun., 2007, 4000 RSC.
- S. Kumar, P. Singh and S. Kumar, Tetrahedron Lett., 2012, 53, 2248 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, spectroscopic data and supplementary spectral data. See DOI: 10.1039/c4ob02514h |
| This journal is © The Royal Society of Chemistry 2015 |