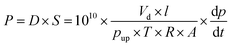Pristine and thermally-rearranged gas separation membranes from novel o-hydroxyl-functionalized spirobifluorene-based polyimides†
Xiaohua
Ma
,
Octavio
Salinas
,
Eric
Litwiller
and
Ingo
Pinnau
*
Advanced Membranes and Porous Materials Center, Physical Sciences and Engineering Division, King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia. E-mail: ingo.pinnau@kaust.edu.sa; Fax: +966 02 80821328
First published on 22nd September 2014
Abstract
A novel o-hydroxyl-functionalized spirobifluorene-based diamine monomer, 2,2′-dihydroxyl-9,9′-spirobifluorene-3,3′-diamine (HSBF), was successfully prepared by a universal synthetic method. Two o-hydroxyl-containing polyimides, denoted as 6FDA-HSBF and SPDA-HSBF, were synthesized and characterized. The BET surface areas of 6FDA-HSBF and SPDA-HSBF are 70 and 464 m2 g−1, respectively. To date, SPDA-HSBF exhibits the highest CO2 permeability (568 Barrer) among all hydroxyl-containing polyimides. The HSBF-based polyimides exhibited higher CO2/CH4 selectivity than their spirobifluorene (SBF) analogues (42 for 6FDA-HSBF vs. 27 for 6FDA-SBF) due to an increase in their diffusivity selectivity. Polybenzoxazole (PBO) membranes obtained from HSBF-based polyimide precursors by thermal rearrangement showed enhanced permeability but at the cost of significantly decreased selectivity.
Introduction
Membrane-based gas separation is a well-established industrial technology with great potential for a wide variety of large-scale industrial applications, such as natural gas sweetening, hydrogen recovery, on-site nitrogen generation, and CO2 separation from flue gas.1–3 An ideal membrane is characterized by both high permeability and selectivity. Generally, there is a trade-off in the performance of polymeric membranes, often referred to as the Robeson upper bound,4,5 in which materials with higher permeability exhibit lower selectivity and vice versa. However, rational molecular design can provide an efficient way to create novel polymeric membranes with superior gas transport properties. One strategy is to introduce micropores (<20 Å) or ultra-micropores (<7 Å) into polymers to form intrinsically microporous polymers (PIMs). The prototype glassy PIM, linear chain poly(1-trimethyl-silyl-1-propyne) (PTMSP), first reported in 1983, is still one of the highest permeable materials to date due to inefficient chain packing resulting from its highly rigid main chain and a bulky trimethylsilyl side group.6,7 PTMSP and other di-substituted polyacetylenes exhibit exceptionally high gas and organic vapor permeability. Furthermore, PTMSP shows the highest mixed C3+ hydrocarbon/CH4 and C3+ hydrocarbon/H2 selectivity of all known polymers.7–9 In 2004, a new class of spirobisindane-based ladder PIMs was reported by Mckeown and Budd.10–12 These rigid glassy polymers consist of contorted spiro-centers linked with fused dioxane rings that prevent efficient polymer chain packing. Many ladder-type PIMs are solution processable from a variety of solvents (e.g. chloroform, THF, etc.) and, therefore, can be readily processed into membranes. Ladder PIMs show promising gas and vapor separation performance,13–16 and extensive investigations have been applied to PIM-1 and its analogues, including a spirobifluorene (SBF) ladder PIM.17–31 Recently, significant advances have been achieved with the development of AB-type triptycene- (TPIM-1 and TPIM-2)32 and Tröger-based33 ladder polymers that significantly outperform all polymers listed on the 2008 Robeson upper bound for O2/N2, H2/N2 and H2/CH4 separation.The concept of introducing intrinsic micropores into glassy polymers has also been extended to polyimides (PI). Ghanem et al.34,35 and Rogan et al.36 reported spirobisindane dianhydride-based PIM-PIs, which exhibit significantly improved permeability among all known polyimides. For example, PIM-PI-8 showed a CO2 permeability of 3700 Barrer combined with a moderate pure-gas CO2/CH4 selectivity of 13.35 PIM-PIs based on triptycene diamines have shown higher selectivity but at the cost of lower permeability; e.g. 6FDA-2,6-diaminotriptycene (DATRI) showed a CO2 permeability of 189 Barrer and a CO2/CH4 selectivity of 30.5.37,38 Recently, Ghanem et al. and Swaidan et al.39,40 reported a series of PIM-PIs made from 9,10-bridgehead-substituted triptycene dianhydrides, which significantly surpassed the 2008 upper bound for several gas pairs due to their strong size-sieving ultramicroporous structures. A rigid ethanoanthracene dianhydride-based polyimide (PIM-EA-TB) reported by Rogan et al. also gave excellent gas permeation results.41
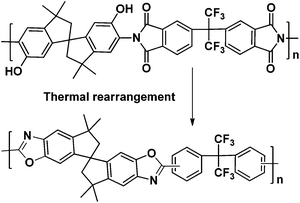
Another efficient way to generate micropores is to form polybenzoxazoles (PBO) from o-hydroxyl-containing polyimide precursors by thermal rearrangement (TR), typically in the range of 400 to 450 °C.42–44 The resulting PBOs exhibit significant increase in gas permeability as well as enhanced chemical and thermal stability. For example, the PBO of 6FDA-bisAPAF polyimide shows a CO2 permeability of 1600 Barrer, and a CO2/CH4 selectivity of 45, which lies well above the 2008 Robeson upper bound. The TR method has also successfully been applied to a variety of other polymer precursors, such as polyetherimides,45 polypyrrolones,46etc. However, the development of a larger variety of TR-generated PBOs has been restricted due to the availability of only a few commercially available o-hydroxyl diamines, such as 3,3-dihydroxy-4,4-diaminobiphenyl (HAB),47 2,2-bis(3-amino-4-hydroxy-phenyl) hexafluoropropane (APAF), 4,6-diamino resorcinol,48 and 2,4-diaminophenol.49
Recently, a newly developed o-hydroxyl functionalized spirobisindane-based diamine was introduced by Ma et al.50 and Li et al.51 and used for the first TR-based PBOs from PIM-PIs (Scheme 1). A several-fold increase of gas permeability was observed with a drop in selectivity. Similar results were also observed by Shamsipur et al. using T-PIM-PI-OH-1 as the PBO precursor.49 The effect of precursor microporosity on TR PBO performance still needs further investigation.
In this study, a general method for synthesis of o-hydroxyl-functionalized aromatic diamine monomers is reported. For example, the synthesis of a novel monomer, 2,2′-dihydroxyl-9,9′-spirobifluorene-2,2′-diamine (HSBF), is shown in Scheme 2. Two ortho-hydroxyl containing polyimides were made by one-pot polycondensation reaction with two dianhydrides, 6FDA and SPDA, respectively. Furthermore, the polyimides were heat-treated to produce PBO membranes by thermal re-arrangement.
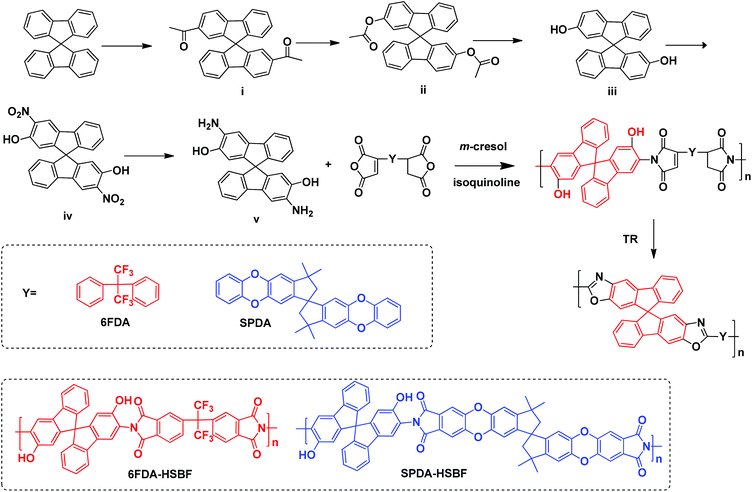 | ||
| Scheme 2 Synthetic route of the HSBF monomer (v), HSBF-based polyimides, and their thermally-rearranged polybenzoxazoles. | ||
Experimental
Materials
4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA, 99%) was obtained from Sigma-Aldrich and purified by sublimation before use. 9,9-Spirobifluorene, m-cresol, acetic chloride, aluminum trichloride, palladium on carbon (10%), dichloromethane, tetrahydrofuran, petrol oil ether, m-chloroperoxybenzoic acid (m-cpba), potassium carbonate, sodium hydroxide, ethyl acetate, acetic acid, nitric acid and N,N′-dimethylformamide were obtained from Sigma-Aldrich and used as received. Spirobisindane-based dianhydride (SPDA) was synthesized according to a previously reported method and dried at 120 °C under vacuum overnight prior to use.35 Polyimides without OH group, 6FDA-SBF and SPDA-SBF, were prepared as described in our previous report.52Characterization and methods
1H NMR and 13C NMR spectra of the newly synthesized monomer and polymers were recorded with a Bruker AVANCE-III spectrometer at a frequency of 400 MHz in either deuterated chloroform or deuterated dimethylsulfone with tetramethylsilane as an internal standard and recorded in ppm. Elemental analysis was carried out using a Perkin-Elmer 2400 elemental analyzer. High-resolution mass spectroscopy (HRMS) was conducted on a Thermo LC/MS system with LTQ Orbitrap Velos detectors. Fourier transform infrared spectra (FT-IR) of the polyimides and their TR-based PBOs were acquired using a Varian 670-IR FT-IR spectrometer. Molecular weights (Mn) and molecular weight distribution (PDI) of the polymers were obtained by gel permeation chromatography (Agilent GPC 1200) with polystyrene as an external standard. X-ray scattering was conducted on a Bruker D8 Advance diffractometer with a scanning rate of 0.5° min−1 from 6 to 50°. Thermal gravimetric analysis (TGA) of the polymers was carried out using a TGA Q5000 under a N2 atmosphere from room temperature to 800 °C at a heating rate of 3 °C min−1. The BET surface areas of the polymers and their thermally-rearranged membranes were measured by N2 sorption at 77 K (Micrometrics ASAP 2020); each of the samples was degassed at 150 °C for 12 h before testing.Synthesis
PI membrane fabrication
The polymers were dissolved in THF (2–3% wt/v) and then filtered using 1.0 μm PTFE filter cartridges. The solution was carefully transferred into a stainless steel ring supported by a leveled glass plate; thereafter, the solvent was slowly evaporated in an oven at 35 °C. After 2 days, the essentially dry membranes (∼80 to 100 μm thick) were soaked in a mixture of n-hexane–dichloromethane (80/20) for 24 h, air-dried for 24 h and then dried at 120 °C for 24 h under high vacuum. Finally, the membranes were heated to 250 °C under a N2 atmosphere in a furnace for 2 h to remove any remaining solvent.PBO membrane formation
The pristine 6FDA-HSBF and SPDA-HSBF films were heated from room temperature to the target temperature at 3 °C min−1 under a N2 atmosphere. The final heating conditions for the two polyimides were set at: (i) 420 °C and soaked 4 h for 6FDA-HSBF and (ii) 450 °C and soaked 2 h for SPDA-HSBF.Gas permeation measurements
The gas permeability of the membranes was determined using the constant-volume/variable-pressure method. The membranes were degassed in the permeation system on both sides under high vacuum at 35 °C for at least 24 h. The increase in permeate pressure with time was measured by a MKS Baratron transducer. The permeability of all gases was measured using an upstream pressure of 2 bar at 35 °C and was determined bywhere P is the permeability (Barrer) – 1 Barrer = 10−10 cm3(STP) cm cm−2 s−1 cmHg−1, pup is the upstream pressure (cmHg), dp/dt is the steady-state permeate-side pressure increase (cmHg s−1), Vd is the calibrated permeate volume (cm3), l is the membrane thickness (cm), A is the effective membrane area (cm2), T is the operating temperature (K), and R is the gas constant (0.278 cm3 cmHg cm−3(STP) K−1). The apparent diffusion coefficient D (cm2 s−1) of the polymer membrane was calculated by D = l2/6θ, where l is the membrane thickness and θ is the time lag of the permeability measurement. The solubility coefficient S (cm3(STP) cm−3 cmHg−1) was obtained from the relationship S = P/D.
Results and discussion
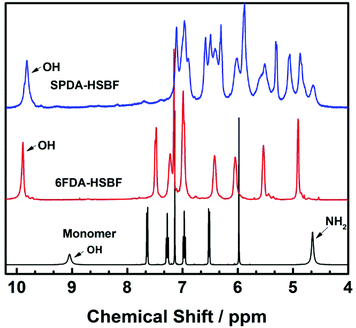
The novel diamine, HSBF, was synthesized from 9,9′-spirobifluorene via a five-step procedure. Firstly, the 9,9′-spirobifluorene was converted to 2,2′-diacetyl-9,9′-spirobifluorene (i) via Friedel–Crafts reaction in the presence of aluminum trichloride as a Lewis acid catalyst. The ketone intermediate (i) was then selectively converted to the 2,2′-diacetoxy-9,9′-spirobifluorene (ii) via Baeyer–Villiger oxidation reaction, as previously reported.53 By hydrolysis under basic conditions, the 2,2′-dihydroxyl-9,9′-spirobifluorene (iii) was obtained. It was then further reacted with dilute nitric acid to give the dinitrol intermediate, similar to the previously reported 6,6′-dihydroxyl-spirobisindane.50 The presence of electron donating OH groups enhanced the activity of both nearby carbon atoms, giving the dinitrol substitution with both the symmetric and asymmetric counterparts (positions 1 and 3 in the 9,9′-spirobifluorene structure). The symmetric dinitrol intermediate (iv) was then reduced to the monomer 3,3′-diamino-2,2′-dihydroxyl-9,9′-spirobifluorene (v), which was identified and confirmed by 1H and 13C NMR, HRMS, and elemental analysis.This synthetic procedure provides a general method for the formation of o-hydroxyl functionalized diamines directly from aromatic compounds. Two novel hydroxyl (HSBF) containing polyimides were obtained by cycloimidization condensation reaction with two different dianhydrides (6FDA and SPDA), using a one-step high temperature azeotropic method. Their proton NMR (shown in Fig. 1) clearly indicates the presence of hydroxyl groups with a chemical shift from 9.5 to 10 ppm, similar to previously reported hydroxyl-based polyimides.54
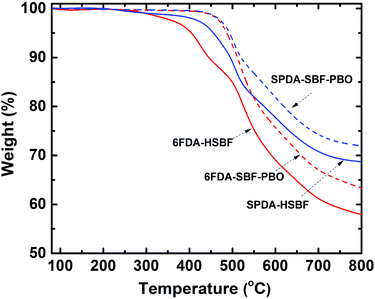
HSBF-based polyimides exhibit very good solubility in a variety of conventional solvents such as NMP, m-cresol, DMF, DMSO and THF, etc. They can readily be cast into transparent membranes. Similar to most aromatic polyimides, HSBF-based polymers exhibit good thermal stability with no weight loss below 380 °C (Fig. 2).
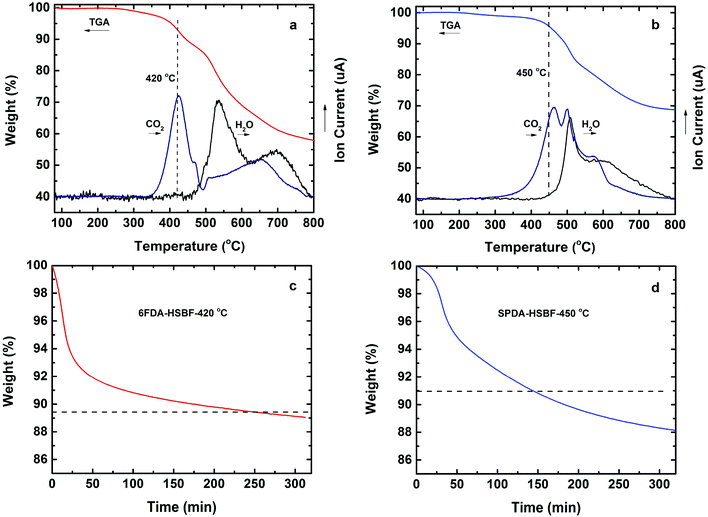
As previously reported, o-hydroxyl containing polyimides can be converted to polybenzoxazoles by thermal rearrangement.42 The online mass spectroscopy following the TGA during heating of HSBF-based polyimides is shown in Fig. 3. It is interesting to note that the two polymers exhibited quite different thermal rearrangement behavior. When 6FDA was selected as dianhydride, the evolution of CO2 (corresponding to the TR-induced PBO formation) started at 380 °C and reached a maximum at 420 °C (Fig. 3a). The isothermal TGA of 6FDA-HSBF at 420 °C (Fig. 3c) indicates that the TR process was complete after 4 hours as identified by reaching the theoretical weight loss value of 11.2% due to CO2 loss (100% formation of PBO). On the other hand, when the more bulky SPDA was chosen as dianhydride, the evolution of CO2 started around 400 °C, about 20 °C higher than 6FDA-HSBF. Furthermore, there is evidence of bimodality during the CO2 evolving process, accompanied with H2O loss immediately generated around 460 °C (Fig. 3b), corresponding to the decomposition of the polymer main chain.51 The isothermal TGA of SPDA-HSBF at 450 °C (Fig. 3d) indicates that after 2 hours, the TR was complete by reaching the theoretical weight loss of 9%.
The PBO formation of the polymers by the TR process was further confirmed by FT-IR, as shown in Fig. S1.† The hydroxyl stretching bond (between 3200 to 3600 cm−1) and imide group (around 1780 cm−1) disappeared during PBO formation, while the other two characteristic peaks of PBO (1555 cm−1 and 1064 cm−1) emerged.42 Similar to all previously reported TR polymers, they exhibited very good chemical stability, e.g., they were insoluble in any conventional solvent (Table S1†). In addition, the PBOs also exhibited better thermal stability (Fig. 2) than their polyimide precursors with no thermal decomposition observed below 450 °C (Table 1).
| Polymers | M n × 10−4 (g mol−1) | PDI | T d (°C) | S BET (m2 g−1) |
|---|---|---|---|---|
| 6FDA-HSBF | 2.89 | 2.41 | 380 | 70 |
| SPDA-HSBF | 4.24 | 2.30 | 400 | 464 |
| 6FDA-SBF-PBO | — | — | 450 | 417 |
| SPDA-SBF-PBO | — | — | 450 | 480 |
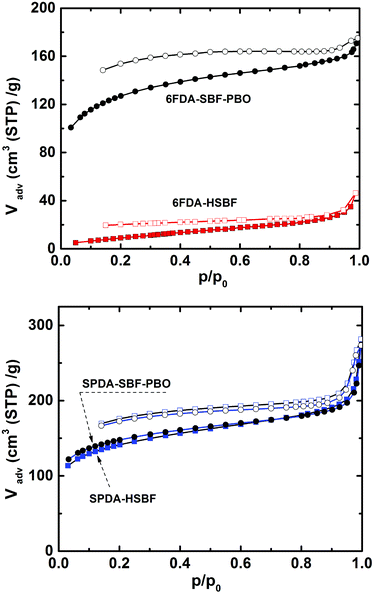
The porosity of the polymers before and after PBO formation was evaluated by nitrogen adsorption/desorption, as shown in Fig. 4. The 6FDA-based polyimide (6FDA-HSBF) exhibits a relatively low BET surface area of 70 m2 g−1, whereas the SPDA-based polyimide (SPDA-HSBF) has a surface area of 464 m2 g−1, which is among the highest for hydroxyl-containing polyimides to date. After PBO formation, there is an expected increase of the surface area for the two polymers. In the case of the low surface area 6FDA-HSBF precursor, the corresponding 6FDA-SBF-PBO exhibited a 6-fold enhancement in surface area (from 70 to 417 m2 g−1). On the other hand, the relatively high surface area precursor SPDA-HSBF yielded only a small increase in BET surface area of less than 5% for SPDA-SBF-PBO (from 464 to 480 m2 g−1). A previously reported microporous spirobisindane-based polyimide, PIM-6FDA-OH, also showed a relatively small increase in surface area for the PBO counterpart (6FDA-SP-PBO) from 368 to 466 m2 g−1 (Scheme 1).51
The significantly different structural changes induced by the TR process were also identified by wide-angle X-ray scattering results, as shown in Fig. S2.† The X-ray peaks give some information about the prevalent chain center-to-center distance. Similar to previously reported TR results, the main halo peak of 6FDA-HSBF (d-spacing = 5.59 Å) moved to a lower angle for the 6FDA-SBF-PBO (d-spacing = 5.75 Å); moreover, an additional halo emerged at an even smaller angle corresponding to the d-spacing of 7.8 Å. However, in the case of SPDA-HSBF and SPDA-SBF-PBO, the X-ray scattering signals were almost identical before and after TR, which agrees qualitatively well with the trends in the BET surface area results discussed above.
Gas permeation properties
To evaluate the effect of hydroxyl functionalization on the gas transport properties of the polyimide precursors, as well as their effect on the performance of the resulting PBOs, pure-gas permeabilities of H2, N2, O2, CH4 and CO2 were obtained by the constant-volume/variable-pressure method and the results are shown in Table 2. The permeability and selectivity of the non-hydroxyl-based polymers, that is, 6FDA-SBF and SPDA-SBF, have previously been reported.52 Introduction of a spirobifluorene “kink” gives rise to improved permeability compared with conventional hydroxyl-containing polyimides. For example, 6FDA-HAB, 6FDA-BisAPAF and HPEI, exhibited CO2 permeabilities of only 2.9, 17 and 8.6 Barrer,45,55,56 respectively, whereas the polyimides based on HSBF had significantly higher CO2 permeabilities (6FDA-HSBF = 100 Barrer; SPDA-HSBF = 568 Barrer). It is noteworthy that the CO2 permeability of SFDA-HSBF is among the highest of all previously reported OH-containing polyimides. The introduction of OH groups in the polyimides resulted in significantly increased selectivity, that is, the CO2/CH4 selectivity of 6FDA-SBF increased by 80% from 27.3 to 41.2 for 6FDA-HSBF. In the case of SPDA-SBF and SPDA-HSBF, the CO2/CH4 selectivity increased from 14.9 to 19.6 (Table 2).| Polymers | Permeability (Barrer)a | Selectivity (α) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H2 | N2 | O2 | CH4 | CO2 | H2/N2 | O2/N2 | CH4/N2 | CO2/N2 | CO2/CH4 | |
| a 1 Barrer = 10−10 cm3(STP) cm cm−2 s−1 cm Hg−1 or 7.5 × 10−18 m3(STP) m m−2 s−1 Pa−1. b Ref. 52. c PBO formation was carried out at 420 °C for 4 h. d PBO formation was carried out at 450 °C for 2 h. e Ref. 51. | ||||||||||
| 6FDA-SBFb | 234 | 7.8 | 35.1 | 6.4 | 182 | 30.0 | 4.5 | 0.8 | 23.3 | 27.3 |
| 6FDA-HSBF | 162 | 3.8 | 19.3 | 2.4 | 100 | 42.5 | 5.1 | 0.6 | 26.3 | 41.7 |
| 6FDA-SBF-PBOc | 985 | 55 | 215 | 56 | 1160 | 17.9 | 3.9 | 1.0 | 21.1 | 20.7 |
| SPDA-SBFb | 501 | 28.6 | 111 | 41.1 | 614 | 17.5 | 3.9 | 1.4 | 21.5 | 14.9 |
| SPDA-HSBF | 519 | 24 | 98 | 29 | 568 | 21.6 | 4.1 | 1.2 | 23.7 | 19.6 |
| SPDA-SBF-PBOd | 775 | 61.6 | 225 | 84.8 | 1280 | 12.5 | 3.7 | 1.4 | 20.8 | 15.1 |
| 6FDA-SP-PBOe | 429 | 30 | 120 | 34 | 675 | 14.3 | 4.2 | 1.1 | 22.0 | 20 |
It is interesting to note that hydroxyl- or PBO modification of the polyimides resulted in only minor differences in CO2/N2 selectivity (ranging from 21 to 26). The effect of the hydroxyl group on the gas permeability was strongly dependent on the BET surface area of the polymers. The low BET surface area 6FDA-HSBF membrane showed a 45% decrease in CO2 permeability from 182 Barrer (6FDA-SBF) to 100 Barrer. On the other hand, the effect of the OH group on the permeability of the relatively high BET surface area SPDA-HSBF was much less pronounced, e.g., PCO2 for SPDA-SBF and SPDA-HSBF were 614 and 568, respectively, a drop of only 8%.
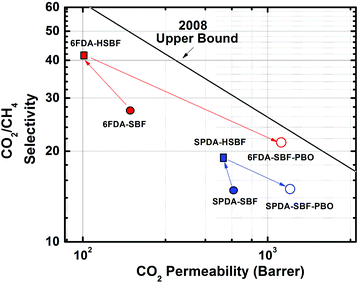
When the HSBF-based polyimide membranes were heat-treated to form PBOs, the permeabilities increased whereas the selectivities decreased, as expected based on previous reports. For 6FDA-SBF-PBO, a 12-fold increase in CO2 permeability was observed relative to that of the 6FDA-HSBF precursor. This relative increase in CO2 permeability is lower than that of low permeability polymers, such as 6FDA-BisAPAF or 6FDA-HAB, which showed ∼30- to 100-fold improvement in CO2 permeability after PBO formation.42,57 The CO2/CH4 selectivity of 6FDA-SBF-PBO dropped to about 50% of that of 6FDA-HSBF. This significant decrease in selectivity had previously been reported for 6FDA-bisAPAF- and 6FDA-HAB-based PBO membranes.44,57 The relatively high BET surface area o-OH-functionalized polyimide precursor (SFDA-HSBF) showed a much smaller relative increase in permeability of the resulting PBO; e.g. the CO2 permeability of SPDA-SBF-PBO increased only about 2-fold, from 568 to 1279 Barrer coupled with a drop in CO2/CH4 selectivity from 19.6 to 15.1. The overall performance of the o-OH-functionalized polyimide precursors and thermally-treated PBO membranes for CO2/CH4 separation is shown in Fig. 5. The HBSF-based polyimide precursor membranes and their counterpart PBO analogues show good CO2/CH4 separation properties, close to the 2008 Robeson upper bound.
The selectivity determined from pure-gas permeation measurements (αX/Y) involves contributions from the solubility selectivity (SX/SY) and diffusion selectivity (DX/DY). To better understand the role of hydroxyl groups in increasing CO2/CH4 selectivity, diffusion coefficients (D) and solubility coefficients (S) of SBF-PIs, HSBF-PIs and HSBF-based PBO membranes are summarized in Table 3. Although the o-OH-containing HSBF-based polyimides have a smaller BET surface area, they exhibit about the same solubility (S) for CO2 and CH4 compared to the non-hydroxyl SBF-based polymers. The lower permeability of o-OH-functionalized polyimides was mainly caused by a decrease in the diffusion coefficients (D); however, concurrently, it results in a tighter, more size-sieving structure, which leads to an increase in the diffusion selectivity αD.
| Polymers | D (10−8 cm2 s−1) | S (10−2 cm3 cm−3 cmHg−1) | α D | α S | ||||
|---|---|---|---|---|---|---|---|---|
| N2 | CH4 | CO2 | N2 | CH4 | CO2 | CO2/CH4 | CO2/CH4 | |
| a D was determined by the time-lag method. b S was deduced from the equation P = D × S. | ||||||||
| 6FDA-SBF | 5.2 | 1.2 | 7.2 | 1.5 | 5.3 | 25 | 6.0 | 4.7 |
| 6FDA-HSBF | 2.57 | 0.46 | 3.71 | 1.48 | 5.17 | 26.8 | 8.1 | 5.2 |
| 6FDA-SBF-PBO | 14.7 | 3.91 | 22.6 | 3.75 | 14.4 | 51.1 | 5.8 | 3.6 |
| SPDA-SBF | 15 | 4.76 | 19.6 | 1.9 | 8.64 | 31.2 | 4.1 | 3.6 |
| SPDA-HSBF | 11.0 | 3.19 | 17.5 | 2.16 | 9.27 | 32.4 | 5.5 | 3.5 |
| SPDA-SBF-PBO | 16.2 | 5.16 | 21.7 | 3.79 | 16.4 | 58.9 | 4.2 | 3.6 |
When the o-OH polyimides were converted to PBO membranes, the low surface area 6FDA-HSBF membrane exhibited a significant increase in the diffusion coefficients (e.g. DCO2 increased 6-fold, from 3.71 × 10−8 cm2 s−1 to 22.6 × 10−8 cm2 s−1) as well as solubility coefficient from 26.8 × 10−2 to 51.1 × 10−2 cm3 cm−3 cmHg−1. However, for the high BET surface area SPDA-HSBF membrane, only a small increase in the diffusion coefficient of CO2 (from 17.5 × 10−8 cm2 s−1 to 21.7 × 10−8 cm2 s−1) and solubility coefficient (from 32.4 × 10−2 to 58.9 × 10−2 cm3 cm−3 cmHg−1) was observed.
Conclusions
A spirobifluorene-based o-dihydroxyl-functionalized diamine (HSBF) was synthesized and two novel hydroxyl-containing polyimides were obtained by reaction with two dianhyrides (6FDA and SPDA). Both polyimides (6FDA-HSBF and SPDA-HSBF) exhibited good solubility in organic solvents and had high thermal stability. The BET surface area of the polyimides depended strongly on the choice of the dianhydride (6FDA-HSBF = 70 m2 g−1 and SPDA-HSBF = 464 m2 g−1). Hydroxyl-functionalization (HSBF) resulted in polyimides with higher selectivity compared to their non-functionalized analogues (SBF) due to an increase in their diffusivity selectivity. PBOs formed by thermal treatment of HSBF-based polyimides showed higher gas permeability but at the expense of significantly reduced selectivity. This study suggests that the higher the microporosity of the pristine hydroxyl-containing polyimide, the smaller is the effect on the structural changes occurring during PBO formation by thermal rearrangement.Acknowledgements
The authors acknowledge KAUST funding for Professor Ingo Pinnau.Notes and references
- P. Bernardo, E. Drioli and G. Golemme, Ind. Eng. Chem. Res., 2009, 48, 4638–4663 CrossRef CAS.
- R. W. Baker and K. Lokhandwala, Ind. Eng. Chem. Res., 2008, 47, 2109–2121 CrossRef CAS.
- R. W. Baker, Ind. Eng. Chem. Res., 2002, 41, 1393–1411 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390–400 CrossRef CAS PubMed.
- L. M. Robeson, J. Membr. Sci., 1991, 62, 165–185 CrossRef CAS.
- T. Masuda, E. Isobe, T. Higashimura and K. Takada, J. Am. Chem. Soc., 1983, 105, 7473–7474 CrossRef CAS.
- I. Pinnau and L. G. Toy, J. Membr. Sci., 1996, 116, 199–209 CrossRef CAS.
- I. Pinnau, C. G. Casillas, A. Morisato and B. D. Freeman, J. Polym. Sci., Part B: Polym. Phys., 1997, 35, 1483–1490 CrossRef CAS.
- K. Nagai, T. Masuda, T. Nakagawa, B. D. Freeman and I. Pinnau, Prog. Polym. Sci., 2001, 26, 721–798 CrossRef CAS.
- P. M. Budd, E. S. Elabas, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib, C. E. Tattershall and D. Wang, Adv. Mater., 2004, 16, 456–460 CrossRef CAS.
- P. M. Budd, B. S. Ghanem, S. Makhseed, N. B. McKeown, K. J. Msayib and C. E. Tattershall, Chem. Commun., 2004, 230–231 RSC.
- N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675–683 RSC.
- P. Budd, K. Msayib, C. Tattershall, B. Ghanem, K. Reynolds, N. McKeown and D. Fritsch, J. Membr. Sci., 2005, 251, 263–269 CrossRef CAS PubMed.
- P. Budd, N. McKeown, B. Ghanem, K. Msayib, D. Fritsch, L. Starannikova, N. Belov, O. Sanfirova, Y. Yampolskii and V. Shantarovich, J. Membr. Sci., 2008, 325, 851–860 CrossRef CAS PubMed.
- S. Thomas, I. Pinnau, N. Du and M. D. Guiver, J. Membr. Sci., 2009, 333, 125–131 CrossRef CAS PubMed.
- S. Thomas, I. Pinnau, N. Y. Du and M. D. Guiver, J. Membr. Sci., 2009, 338, 1–4 CrossRef CAS PubMed.
- N. Du, G. P. Robertson, J. Song, I. Pinnau, S. Thomas and M. D. Guiver, Macromolecules, 2008, 41, 9656–9662 CrossRef CAS.
- N. Du, J. Song, G. P. Robertson, I. Pinnau and M. D. Guiver, Macromol. Rapid Commun., 2008, 29, 783–788 CrossRef CAS.
- N. Du, G. P. Robertson, I. Pinnau and M. D. Guiver, Macromolecules, 2009, 42, 6023–6030 CrossRef CAS.
- N. Du, G. P. Robertson, J. Song, I. Pinnau and M. D. Guiver, Macromolecules, 2009, 42, 6038–6043 CrossRef CAS.
- N. Du, G. P. Robertson, I. Pinnau and M. D. Guiver, Macromolecules, 2010, 43, 8580–8587 CrossRef CAS.
- N. Du, H. B. Park, G. P. Robertson, M. M. Dal-Cin, T. Visser, L. Scoles and M. D. Guiver, Nat. Mater., 2011, 10, 372–375 CrossRef CAS PubMed.
- N. Du, H. B. Park, M. M. Dal-Cin and M. D. Guiver, Energy Environ. Sci., 2012, 5, 7306–7322 CAS.
- C. R. Mason, L. Maynard-Atem, N. M. Al-Harbi, P. M. Budd, P. Bernardo, F. Bazzarelli, G. Clarizia and J. C. Jansen, Macromolecules, 2011, 44, 6471–6479 CrossRef CAS.
- C. R. Mason, L. Maynard-Atem, K. W. Heard, B. Satilmis, P. M. Budd, K. Friess, M. Lanc, P. Bernardo, G. Clarizia and J. C. Jansen, Macromolecules, 2014, 47, 1021–1029 CrossRef CAS PubMed.
- B. S. Ghanem, N. B. McKeown, P. M. Budd and D. Fritsch, Macromolecules, 2008, 41, 1640–1646 CrossRef CAS.
- M. Carta, K. J. Msayib, P. M. Budd and N. B. McKeown, Org. Lett., 2008, 10, 2641–2643 CrossRef CAS PubMed.
- M. Carta, K. J. Msayib and N. B. McKeown, Tetrahedron Lett., 2009, 50, 5954–5957 CrossRef CAS PubMed.
- R. Short, M. Carta, C. G. Bezzu, D. Fritsch, B. M. Kariuki and N. B. McKeown, Chem. Commun., 2011, 47, 6822–6824 RSC.
- C. G. Bezzu, M. Carta, A. Tonkins, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Adv. Mater., 2012, 24, 5930–5934 CrossRef CAS PubMed.
- M. Carta, R. Malpass-Evans, M. Croad, Y. Rogan, J. C. Jansen, P. Bernardo, F. Bazzarelli and N. B. McKeown, Science, 2013, 339, 303–307 CrossRef CAS PubMed.
- B. S. Ghanem, R. Swaidan, X. Ma, E. Litwiller and I. Pinnau, Adv. Mater., 2014 DOI:10.1002/adma.201401328.
- M. Carta, M. Croad, R. Malpass-Evans, J. C. Jansen, P. Bernardo, G. Clarizia, K. Friess, M. Lanc and N. B. McKeown, Adv. Mater., 2014, 26, 3526–3531 CrossRef CAS PubMed.
- B. S. Ghanem, N. B. McKeown, P. M. Budd, J. D. Selbie and D. Fritsch, Adv. Mater., 2008, 20, 2766–2770 CrossRef CAS PubMed.
- B. S. Ghanem, N. B. McKeown, P. M. Budd, N. M. Al-Harbi, D. Fritsch, K. Heinrich, L. Starannikova, A. Tokarev and Y. Yampolskii, Macromolecules, 2009, 42, 7881–7888 CrossRef CAS.
- Y. Rogan, L. Starannikova, V. Ryzhikh, Y. Yampolskii, P. Bernardo, F. Bazzarelli, J. C. Jansen and N. B. McKeown, Polym. Chem., 2013, 4, 3813 RSC.
- Y. J. Cho and H. B. Park, Macromol. Rapid Commun., 2011, 32, 579–586 CrossRef CAS PubMed.
- S. A. Sydlik, Z. H. Chen and T. M. Swager, Macromolecules, 2011, 44, 976–980 CrossRef CAS.
- B. S. Ghanem, R. Swaidan, E. Litwiller and I. Pinnau, Adv. Mater., 2014, 22, 3688–3692 CrossRef PubMed.
- R. Swaidan, M. Al-Saeedi, B. Ghanem, E. Litwiller and I. Pinnau, Macromolecules, 2014, 47, 5104–5114 CrossRef CAS.
- Y. Rogan, R. Malpass-Evans, M. Carta, M. Lee, J. C. Jansen, P. Bernardo, G. Clarizia, E. Tocci, K. Friess, M. Lanc and N. B. McKeown, J. Mater. Chem. A, 2014, 2, 4874–4877 CAS.
- H. B. Park, C. H. Jung, Y. M. Lee, A. J. Hill, S. J. Pas, S. T. Mudie, E. Van Wagner, B. D. Freeman and D. J. Cookson, Science, 2007, 318, 254–258 CrossRef CAS PubMed.
- H. B. Park, S. H. Han, C. H. Jung, Y. M. Lee and A. J. Hill, J. Membr. Sci., 2010, 359, 11–24 CrossRef CAS PubMed.
- R. Guo, D. F. Sanders, Z. P. Smith, B. D. Freeman, D. R. Paul and J. E. McGrath, J. Mater. Chem. A, 2013, 1, 262–272 CAS.
- M. Calle and Y. M. Lee, Macromolecules, 2011, 44, 1156–1165 CrossRef CAS.
- S. H. Han, J. E. Lee, K. J. Lee, H. B. Park and Y. M. Lee, J. Membr. Sci., 2010, 357, 143–151 CrossRef CAS PubMed.
- Z. P. Smith, D. F. Sanders, C. P. Ribeiro, R. Guo, B. D. Freeman, D. R. Paul, J. E. McGrath and S. Swinnea, J. Membr. Sci., 2012, 415–416, 558–567 CrossRef CAS PubMed.
- T. M. Moy and J. E. Mcgrath, J. Polym. Sci., Part A: Polym. Chem., 1994, 32, 1903–1908 CrossRef CAS.
- H. Shamsipur, B. A. Dawood, P. M. Budd, P. Bernardo, G. Clarizia and J. C. Jansen, Macromolecules, 2014, 47, 5595–5606 CrossRef CAS.
- X. Ma, R. Swaidan, Y. Belmabkhout, Y. Zhu, E. Litwiller, M. Jouiad, I. Pinnau and Y. Han, Macromolecules, 2012, 45, 3841–3849 CrossRef CAS.
- S. Li, H. J. Jo, S. H. Han, C. H. Park, S. Kim, P. M. Budd and Y. M. Lee, J. Membr. Sci., 2013, 434, 137–147 CrossRef CAS PubMed.
- X. Ma, O. Salinas, E. Litwiller and I. Pinnau, Macromolecules, 2013, 46, 9618–9624 CrossRef CAS.
- F. Thiemann, T. Piehler, D. Haase, W. Saak and A. Lutzen, Eur. J. Org. Chem., 2005, 1991–2001 CrossRef CAS.
- M. Calle and Y. M. Lee, Macromolecules, 2011, 44, 1156–1165 CrossRef CAS.
- R. Guo, D. F. Sanders, Z. P. Smith, B. D. Freeman, D. R. Paul and J. E. McGrath, J. Mater. Chem. A, 2013, 1, 6063–6072 CAS.
- C. H. Jung and Y. M. Lee, Macromol. Res., 2008, 16, 555–560 CrossRef CAS.
- S. H. Han, N. Misdan, S. Kim, C. M. Doherty, A. J. Hill and Y. M. Lee, Macromolecules, 2010, 43, 7657–7667 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Solubility, FT-IR, and wide-angle X-ray scattering of the polymers before and after TR. See DOI: 10.1039/c4py01221f |
| This journal is © The Royal Society of Chemistry 2014 |