 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Remanufacturing of perovskite solar cells
Karen
Valadez-Villalobos
a and
Matthew L.
Davies
 ab
ab
aSPECIFIC IKC, Materials Science and Engineering, Faculty of Science and Engineering, Swansea University, Swansea, UK. E-mail: k.valadezvillalobos@swansea.ac.uk; m.l.davies@swansea.ac.uk
bSchool of Chemistry and Physics, University of KwaZulu-Natal, Durban, South Africa
First published on 22nd May 2024
Abstract
The rapid evolution of perovskite solar cells (PSCs) has positioned this technology as a promising candidate in the global transition towards sustainable energy sources. As the renewable energy sector continues to gain momentum, driven by global initiatives aimed at achieving net-zero emissions, the integration of circular economy principles into the production of PSCs has become increasingly significant. In recent years, a growing body of research has been dedicated to exploring various strategies for recovering and reusing the components of perovskite photovoltaic devices. This review offers a comprehensive analysis of the current state of remanufacturing processes as they apply to PSCs, encompassing material recovery, the essential capturing and recycling of lead, and device refurbishment. Moreover, we review the available information pertaining to the environmental impact of reported remanufacturing strategies, solvent management, the introduction of greener solvents, exempt and design compatibility, all aimed at further enhancing the sustainability profile of PSCs remanufacturing.
Sustainability spotlightIntegrating circular economy principles into PV manufacturing is paramount for ensuring long-term sustainability. Perovskite solar cells (PSCs) are an emerging photovoltaic energy technology that hold great promise for the development of a low-cost, low-embodied energy and efficient solar technology. This work details the advance in remanufacturing approaches for PSCs with the potential to significantly improve the sustainability of this emerging technology. Strategies for recovering and reusing all device components, including lead, while minimising environmental impact are detailed. This aligns with the UN's Sustainable Development Goals, particularly Goal 7 (Affordable and Clean Energy) and Goal 12 (Responsible Consumption and Production), by promoting renewable energy adoption and sustainable manufacturing practices. |
1. Introduction
As the impacts of climate change continue to intensify, nations and organisations have joined in the goal to achieve net-zero carbon emissions by 2050, seeking to limit the rise in global temperature to 1.5 °C above pre-industrial levels. A critical component of this effort is the replacement of fossil fuels with renewable energy sources. The International Energy Agency (IEA) predicts that these sources will contribute almost 90% of the global power capacity growth over the next decade.1 Photovoltaic (PV) installations have experienced a remarkable growth in recent years, witnessing a twenty-sixfold increase between 2010 and 2022. As of the close of 2022, the global cumulative PV installed capacity reached 1047 GW and is expected to surpass 5400 GW by 2030.2 As demand increases and deployment expands in the pursuit of achieving net-zero, ensuring the sustainable production, utilisation, and end-of-life (EoL) management of PV modules becomes imperative. A report by the International Renewable Energy Agency (IRENA), states that recycling or repurposing silicon PV panels at the end of their lifetime can unlock an estimated 78 million tonnes of raw materials and valuable components by 2050, with a recovered material value exceeding USD 15 billion if fully injected into the economy.3 At present, the design of PV panels and the insufficient and inadequate infrastructure for recycling and remanufacturing are significant barriers to the efficient recovery of valuable components.4–6 Consequently, landfilling, stockpiling or downcycling of recovered materials is the most common EoL scenario. To overcome these challenges, it is essential to integrate circular economy principles into the design and production of PV technology, minimising waste and maximising the recovery of valuable materials throughout the entire life cycle of the devices, from raw material extraction to EoL disposal.7,8 Emerging PV technologies, such as perovskite solar cells (PSCs), offer an attractive alternative to silicon PV with promising balance between performance and low cost due to the use of inexpensive materials and facile solution-based methods of device production. Since their introduction in 2009, there has been remarkable progress in the power conversion efficiency (PCE) of PSCs, currently exceeding 26% in laboratory-scale devices and 33% in tandem configurations.9 Despite their performance and fabrication advantages, the commercial feasibility of PSCs remains uncertain due to concerns about the toxicity of the lead present in the absorber material and the short device lifetime linked to material instability. Given the potential adverse effects on human health and the environment, it is essential these issues are addressed prior to large-scale deployment. Integrating circular economy principles from the early stages of this emerging technology can help deliver sustainable and safe production, mitigate lead use and ensure effective EoL management.10 This will help PSCs become a competitive technology to help achieve the rapid deployment of renewables needed to mitigate climate change.Refurbishing strategies could help prolong components and device lifespan. Material recycling has been estimated to reduce over 70% of both primary energy consumption and carbon footprint associated with the lifecycle of a PSCs, improving the energy payback time (EPBT). Tian et al. conducted a study where the fabrication process of 1 m2 solar modules was modelled using data derived from reported laboratory scale devices.11 Life cycle assessment (LCA) was conducted contrasting the EoL scenarios of landfilling against recycling. A significant reduction of the EPBT was found for the recycled EoL scenario where the FTO substrate is reused, with EPBTs as low as 0.09 years for a SnO2/perovskite/spiro-MeOTAD/Cu module.11 Shorter EPBT and improved sustainability introduced by material recovery and remanufacturing strategies could, to some degree, alleviate the constraint of short device lifetimes in PSC technology and help facilitate commercialisation.
While there is no current access to industrial-scale production data for perovskite modules, remanufacturing strategies based on laboratory-scale devices can provide valuable insights into potential viability, issues and impacts associated with recovery processes. These strategies can help inform the design of devices and production methods at this early, pre-commercial stage, avoiding issues of ‘linear lock-in’ faced by existing commercial technologies.
Recent LCA of lab-scale PSCs has identified transparent conductive oxide (TCO) substrates, gold contacts and the energy-intensive annealing processes required to deposit charge-transfer semiconductor materials films, as the main contributors to production costs and embodied carbon of the device.11–14 These findings, along with the concerns linked to the device toxicity, have led to the development of remanufacturing methods focused on reusing the most expensive and impactful components of the device as well as aiming for the recovery and reuse of lead to reduce potential impact on health and the environment.
This review highlights a body of research that departs from the traditional emphasis on performance enhancement and underlines the importance of environmental sustainability in the progress of PSCs. The reviewed key areas include the development of methodologies for the recovery and reuse of substrates and transport layers in PSCs, and the capturing and recycling of lead. Additionally, the review examines studies that explore device designs with great potential for remanufacturing and delve into aspects such as the impact of materials and solvent use, all within the context of developing an effective and sustainable remanufacturing strategy for PSCs.
2. Substrate recovery
Cost analyses conducted on various projections from laboratory-scale fabrication methods of PSCs suggest that TCO substrates account for 40–60% of the total device cost, making recovery and reuse of TCOs an area of significant interest.13–15 In addition to the cost of the TCO substrate, the semiconductor oxide films deposited onto the TCO used as charge-selective/scaffold materials (TiO2, NiOX, SnO2, Al2O3, and ZrO2) typically require high-temperature annealing processes. Carneiro et al. found that, for mesoscopic TiO2-based perovskite devices, the high temperature required for the production of the mesoporous TiO2 layer (typically annealed at 500 °C for 30 minutes) accounted for 74% of the energy consumption for the production of the PSC module.16 Developing efficient methods for recovering both TCO substrates and semiconductor oxide films deposited on top of the TCO would have a significant impact in the cost and embodied energy of devices that incorporate recovered components on PSC production.Several methods have been proposed for recovering and reusing TCO substrates (Fig. 1). These methods include selective dissolution of device components using organic solvents such as dimethylformamide (DMF), chlorobenzene, and toluene, where the removal of a 20 nm dense TiO2 layer deposited from a sol–gel solution was achieved by immersing in DMF for an extended time (at least 4 minutes)15 as well as single-step substrate recovery methods using DMF on ETL-free devices.18 Alternatives to organic solvents have been reported in the work of Augustine et al., where ITO substrates were successfully recovered and reused in inverted devices where PEDOT was used as an ETL,17 and more recently in the work by O'Hara et al., where a 4 M ammonium iodide solution was demonstrated to be effective for the dissolution of perovskite and the recovery of FTO substrates.21
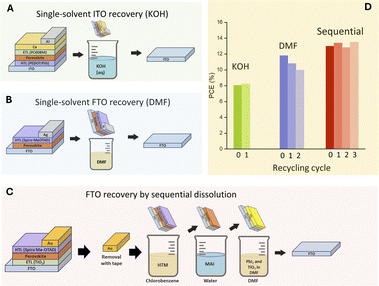 | ||
| Fig. 1 Schematic of single-solvent TCO-substrate recovery method using (A) an alkaline solution or (B) dimethilformamide. (C) Multi-solvent TCO recovery methods. (D) Performance of the devices fabricated with recycled TCO substrates as reported by Augustine et al.,17 Huang et al.18 and Binek et al.15 | ||
The recovery of TCO substrates in combination with charge transport layers has been documented for different device architectures. Feng et al. reported a method employing alkylamines for the recovery of TCO/NiOX from inverted devices and demonstrated that the substrate could be reused without any loss in performance.19,20
The recovery and reuse of TCO substrates with compact TiO2 (TCO/c-TiO2) and with mesoporous TiO2 (TCO/mp-TiO2) has been reported with variable degrees of success.22–25Fig. 2 summarizes the methodologies reported for the recovery and reuse of TCO/TiO2 substrates and the results achieved with recycled substrates. In general, compact TiO2 layers have proven to be robust enough for recovery and reuse. Kim et al. reported the reuse of the compact TiO2 for up to 10 cycles without loss in the device performance.26
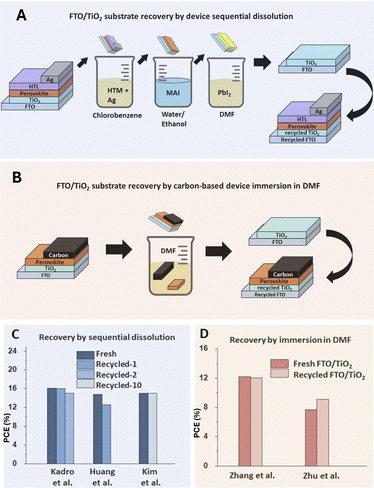 | ||
| Fig. 2 (A) Sequential dissolution of perovskite device components by a multi-step, multi-solvent method. (B) Single-solvent method for the recovery and reuse of TCO/TiO2 substrates. Efficiencies reported for (C) sequential dissolution22,23,26 and (D) single-solvent recovery of TCO/TiO2.24,25 | ||
The recovery of mesoporous TiO2 has proved more challenging due to the solubility of TiO2 nanoparticles in DMF, the organic solvent most commonly used in the recovery process, as well as subtle morphology changes in the mesoporous structure caused by multiple cleaning cycles.22 The works by Kadro et al. and Huang et al. found a slight decrease in the performance of recycled TCO/mp-TiO2 substrates.22,23
3. Recovery of lead
The toxicity of lead-based PSCs has raised concerns regarding the feasibility of widespread deployment of the technology. The presence of lead in air, soil, and water can cause serious health problems and significant disruption to valuable ecosystems. Inorganic lead compounds have been classified as probable carcinogens, and short-term or low-level exposure to lead is linked to detrimental health effects such as developmental issues, mental decline, blood pressure issues, and kidney problems.27–31 Several studies have addressed the possible environmental consequences of lead release from PSCs and have found that, while the risk to the environment and human health is low, it is still non-negligible.32–35 Alternatives to lead-based perovskites have been widely studied, but so far, their performance and stability are still behind those of optimised lead halide perovskites.36,37 Tin halide perovskites are one of the most promising alternatives to replace lead-based materials, however, the toxicity of these materials under certain conditions, particularly in aquatic environments, is still a topic of ongoing research. Furthermore, the global availability and material security challenges of tin could potentially set back the development of tin-based devices.38,39 Given the current state of research in PSCs, it is likely that optimised devices will contain lead, and their commercialisation will require careful consideration of containment strategies and recovery protocols.Moody et al. employed microwave digestion to assess the lead content in perovskite films, finding concentrations of 344 mg kg−1 in films on glass substrates and 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 mg kg−1 in films on PET substrates.40 In the EU, the RoHS directive is the primary regulation for hazardous substances, setting a limit of 1000 mg of lead per kg of homogeneous material. Currently, due to climate change-related policies, PV modules are exempt from RoHS compliance, and it is unclear how these limits would be enforced if exemptions were lifted. The definition of ‘homogeneous material’ is “a substance or group of substances that cannot be mechanically separated” which does not clearly state whether the threshold applies to the entire PV module or only to the perovskite absorber layer.41,42 If the entire device is considered as homogeneous material, devices with glass substrates would likely comply with RoHS limits based on Moody et al.'s estimations. However, compliance might be compromised if the standard applies to individual layers within the device. This uncertainty could prompt regulatory authorities to revisit and refine standards to ensure their effectiveness amidst evolving environmental concerns and the varied lead content in devices entering the market.
400 mg kg−1 in films on PET substrates.40 In the EU, the RoHS directive is the primary regulation for hazardous substances, setting a limit of 1000 mg of lead per kg of homogeneous material. Currently, due to climate change-related policies, PV modules are exempt from RoHS compliance, and it is unclear how these limits would be enforced if exemptions were lifted. The definition of ‘homogeneous material’ is “a substance or group of substances that cannot be mechanically separated” which does not clearly state whether the threshold applies to the entire PV module or only to the perovskite absorber layer.41,42 If the entire device is considered as homogeneous material, devices with glass substrates would likely comply with RoHS limits based on Moody et al.'s estimations. However, compliance might be compromised if the standard applies to individual layers within the device. This uncertainty could prompt regulatory authorities to revisit and refine standards to ensure their effectiveness amidst evolving environmental concerns and the varied lead content in devices entering the market.
In the US, hazardous waste regulation is governed by the Resource Conservation and Recovery Act (RCRA), which focuses on leaching potential determined by a protocol simulating landfill conditions. Under RCRA, leached lead concentrations are limited to 5 mg L−1. Moody et al. followed the RCRA ‘toxicity characteristic leaching procedure’ and estimated the leaching potential of lead from perovskite films on glass substrates to be >14 mg L−1.40 Thus, perovskite waste would be classified as hazardous waste and require appropriate disposal. Implementing re-use strategies could reduce the risk of lead pollution and be a cost-effective alternative to high disposal costs associated with hazardous waste requirements.
Some of the studies that have focussed on the recovery of substrates have also addressed the capturing and recovering of lead,15,24,43 either to avoid lead pollution or for reuse in remanufacturing of new perovskite films (Table 1). With the aim of maximising capture and effective reuse, the strategies so far reported span in situ recovery, electrochemical methods, dissolution/precipitation, adsorption and ionic exchange (Fig. 3).
| Method | Reference | Lead source | Pb retrieval | Pb recovery | Device performance | |
|---|---|---|---|---|---|---|
| Fresh PbI2 | Recycled PbI2 | |||||
| Electrochemical methods | Wang et al.47 | Devices in molten LiCl–KCI | >99.8% | 98% | — | — |
| Poll et al.48 | Perovskite films dissolved in a deep eutectic solvent | 98.7–99.8% | 99.80% | — | — | |
| Ionic exchange | Park et al.54 | Planar TiO2 devices submerged in DMF | 99.99% | 99.97% | 16.70% | 16.50% |
| Hong et al.55 | Pb(NO3)2 aqueous solutions | — | — | 19.30% | 19% | |
| Ren et al.56 | Pbl2 (aq) from degraded devices | 88% | 96.50% | 21% | 21% | |
| Chen et al.43 | Pbl2 from mini modules submerged in DMF | 99.60% | 99.20% | 21% | 20.50% | |
| Solvent extraction/recrystallisation | Zhang et al.24 | Pbl2 from carbon-based devices submerged in DM | 99.90% | 95.70% | 12.20% | 11.40% |
| Feng et al.20 | Inverted devices submerged in butylamine | — | 98.90% | 17% | 17% | |
| Binek et al.15 | TiO2-devices submerged in DMF | — | — | — | 13.50% | |
| Schmidt et al.60 | Synthetic solution (TiO2, spiro-OMeTAD and Pb12, MAI in water) | 91.30% | — | — | — | |
| Wang et al.61 | Full device in bleaching solution | 99.90% | 100% | 21% | 21% | |
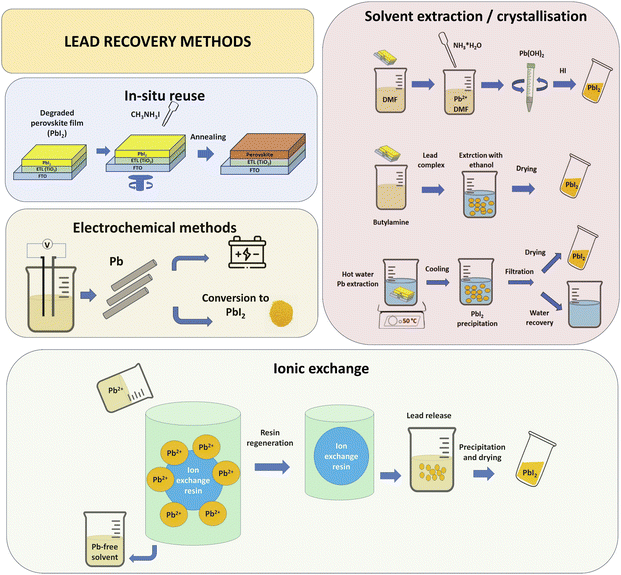 | ||
| Fig. 3 Schematic of PbI2 recovery through different methods: in situ reuse, electrochemical methods, solvent extraction and crystallisation, and adsorption and ionic exchange. | ||
3.1 In situ recovery of lead
Exposure to moisture can result in the dissolution and/or volatilization of the organic species in lead halide perovskite materials, leaving behind PbI2 and rendering devices unsuitable for PV operation. One direct method for reusing degraded devices involves in situ conversion techniques. The evaporated metal electrodes and hole-transporting material (HTM) are removed using selective solvents, leaving the perovskite film exposed. This film may have partially or fully converted into PbI2, making it available for restoration treatment with a methylammonium iodide (MAI) solution44,45 or methylamine gas.46 Chhillar et al. demonstrated that spin coating of a MAI solution followed by annealing at 100 °C on partially degraded MAPbI3 films can effectively restore the film optical properties.44 Xu et al. conducted a similar study where degraded perovskite films were thermally treated at 300 °C to achieve full conversion of partially degraded perovskite films into PbI2.45 A MAI solution was subsequently spin coated on the PbI2 films and annealed to achieve crystallisation of the perovskite film. Devices fabricated with refurbished perovskite films exhibited a higher PCE than devices with fresh films. The superior performance of refurbished films was attributed to an improved crystallinity promoted by the annealing at 300 °C of PbI2 during the restoration process.45 The in situ approach for reuse of lead eliminates the need to dissolve the lead iodide. This method can significantly reduce the use of toxic solvents, the risk of lead leakage through contaminated effluents, and enables direct reutilization of the TCO substrate and charge-selective semiconductor films deposited on the TCO substrate. However, introducing an annealing step of 300 °C to restore the perovskite film adds an energy-intensive process to the remanufacturing procedure. This step might not be entirely advantageous, considering that the deposition of fresh PbI2 films typically occurs at temperatures of around 80 °C.Although in situ restoration of the perovskite layer offers an exciting prospect scientifically and offers practical advantages over other forms of lead recovery and reuse, the economic and environmental ‘cost’ of the solvent used to remove the upper layers to expose the perovskite film needs to be quantified to determine if this approach is ultimately worthwhile. Moreover, the energy required for redeposition of the expensive HTM and re-evaporating the gold, could render this method ineffective in enhancing the device's sustainability through remanufacturing. In contrast, the in situ restoration approach may be more advantageous for HTM-free devices or alternative architectures aligned with direct refurbishing. Such a scenario could offer a more cost-effective and energy-efficient solution, as explored in more detail in Section 5.
3.2 Electrochemical methods for the recovery of Pb and I2
Wang et al. reported an electrochemical method where PbI2 is separated from perovskite modules by dissolution in molten lithium chloride-potassium chloride (LiCl–KCl) at 450 °C with subsequent in situ electrochemical deposition of Pb and I2.47 This resulted in a lead recovery efficiency of 99.7%. Poll et al., proposed a method where perovskite is dissolved in a mix of choline chloride (ChCl) and ethylene glycol (EG), which is shown to easily dissolve MAPbI3, FAPbI3 and MAPbI3−xClx at a capacity of recovery of 1 m2 per solvent litre.48 After dissolution, lead is electrodeposited directly from the solvent with a recovery efficiency of 99.8%.46While electrochemical methods report high recovery efficiency and provide a high purity of recovered materials, dissolution/precipitation methods, adsorption and ionic exchange are more likely to be adopted due to their compatibility with large scale applications and lower costs.49,50
3.3 Adsorption composites and ionic exchange
Due to its versatility in design, ease of implementation, and cost-effectiveness, adsorption is a commonly employed technique for removing heavy metals from contaminated water sources. The process involves the attachment of heavy metal ions onto the surface of a solid adsorbent material, which can then be separated from the solution. The adsorbent material can be of various types, including activated carbon, zeolites, and various natural and synthetic polymers.51–53For successful recovery and capture of lead from EoL PSC devices, adsorption composites should be cost-effective, reusable, and scalable, while also enabling the efficient retrieval and reuse of the high purity lead halides. Park et al. synthesized Fe-decorated hydroxyapatite (HAP/Fe) for this purpose and achieved a high recycling yield of 99.97% from a PbI2 solution in DMF.54 The solvent treated with the HAP/Fe absorption composites resulted in final concentrations varying between 0 and 6 ppb, which is in compliance with EPA regulations, where an upper limit of 15 ppb is set for Pb emissions. Although the purity of the recovered lead compound is not specified, devices fabricated using the reused PbI2 exhibited similar performance to devices prepared with as-purchased PbI2, with an average efficiency of 16%.54
Hong et al. reported the synthesis and application of an adsorbent based on whitlockite, an abundant mineral, and compared it to the performance HAP.55 An absorption capacity of 2339 mg g−1 was obtained for the whitlockite, 1.68 times higher than the value found for HAP in the same study. This improvement was ascribed to the difference in adsorption mechanisms (Fig. 4) where whitlockite has a strong interaction with Pb2+ ions and rapid protonation in an aqueous solution, vs. the ion exchange mechanisms in HAP, which is limited by formation of a passivated Pb2+ surface layer.55 Although this material shows promising potential, further studies are necessary to evaluate the efficacy of whitlockite in the organic solvents typically employed for the dissolution of perovskite.
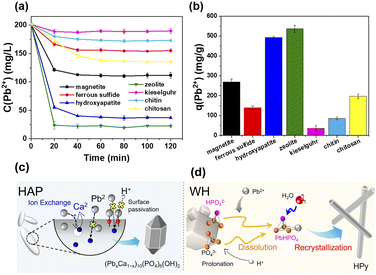 | ||
| Fig. 4 (a and b) Performance of different adsorption composites for Pb2+ retrieval from aqueous solutions (reproduced with permission from ref. 56. Copyright 2021, American Chemical Society). Pb2+ adsorption mechanisms of (c) hydroxyapatite and (d) withlockite (reproduced from ref. 55, available under a Creative Commons CC BY licence). | ||
Adsorption composites have proven to be an efficient method for recovering lead cations from aqueous solutions. However, their non-reusability poses a significant challenge. The synthesis of HAP/Fe composites requires a high energy input due to their high annealing temperature, exceeding 400 °C. Moreover, extracting absorbed lead cations involves acidic dissolution of the composite, making the material unfit for reuse and rendering the process relatively energy intensive.
Recovery systems that can be regenerated and reused are preferred because of their potential cost-effective nature. Ren et al. studied zeolite to stabilize the lead by enhancing the dissolution of PbI2 in an aqueous solution.56 The adsorption capacity of zeolite was compared with that of other minerals and commercial adsorbents. Lead adsorption from super saturated solutions was proven successful after observing the precipitation of zeolite powder leaving a transparent solution. In this method, the concentrated iodide solution left after the adsorption of lead could potentially react with lead ions desorbed from zeolite to form PbI2 which could be reused in the fabrication of devices.56
Chen et al. reported recovery of lead iodide from small perovskite modules (25 cm2 active area) via a carboxylic acid cation-exchange resin.43 This method was demonstrated effective with a lead concentration twenty times larger than that reported for the purification with Fe/HAP, potentially reducing the volume of solvent required in the recovery process. 99.2% recycling efficiency was achieved and the estimated purity of the PbI2 was of 99.9%. Devices fabricated with recycled PbI2 had an average efficiency of 20.5%, comparable with the 21% average efficiency of devices prepared with as-purchased 99.99% purity commercial PbI2.43
This study also addresses the presence of a small amount of PbO in degraded perovskite devices, as previous studies on perovskite degradation mechanisms have demonstrated.57–59 In this method, PbO particles can be filtered from the DMF effluent and then dissolved in HNO3 for recycling.43
Overall, the recovery of materials using ion exchange columns offers the potential for resin and solvent reuse, along with improved outcomes relative to other reported composites.
3.4 PbI2 recovery through solvent extraction and crystallisation
Recovery of lead through solvent extraction and precipitation involves the immersion of the perovskite film in DMF, amines or aqueous solutions that allow the dissolution of the perovskite components. In this method, the lead is subsequently recovered by vacuum evaporation of the solvent, precipitation, or separation of the components through physical methods such as centrifugation. This route has shown high recovery efficiency for lead, typically >95%. However, recrystallization of the PbI2 is often required to achieve adequate purity for reuse in perovskite synthesis.Zhang et al. developed a method for recovering lead from carbon-based perovskites through dissolution in DMF.24 The lead ions are then recovered through precipitation using NH3·H2O (eqn (1)) and subsequent reaction with HI to obtain PbI2 (eqn (2)), with a recovery efficiency of 95.7%.
| Pb2+ + 2NH3·H2O ⇄ Pb(OH)2↓ + 2NH4+ | (1) |
| Pb(OH)2 + 2HI ⇄ PbI2 + 2H2O | (2) |
The recycled PbI2 results in a device with a champion efficiency of 11.36%, comparable to 12.17% for the champion device fabricated with commercial PbI2.24
Feng et al. reported a reversible liquefaction of perovskite by interaction with butylamine.20 Amines can be used as an effective solvent for the successful liquefaction of methylammonium lead iodide, thus allowing for a rapid and efficient separation of the perovskite layer from other functional layers in the full device stack.19 Using selective solvent extraction to separate the other elements of an inverted device and finally recrystallizing the PbI2 with a recovery efficiency of 98.9%, this study reports efficiencies of >17% for devices fabricated with recycled PbI2.
Single-solvent extraction methods have been shown to effectively recover lead with the added benefit of simpler implementation. Binek et al. demonstrated that immersion of PSCs devices in DMF can recover 600 mg of PbI2 (1.25 mM) from 70 dm2 of devices, which can then be concentrated through solvent vacuum evaporation.15 The resulting 1 ml solution of 1.25 M can be used to produce 2 dm2 of perovskite films. However, due to impurities present in the recovered DMF solution, recrystallization of the lead iodide in water is ultimately required before reuse of PbI2 to achieve performances similar to fresh PbI2.15
Schmidt et al. propose an alternative method, free of organic solvents, using hot water for the extraction of lead.60 By taking advantage of the PbI2 increased solubility in hot water, a lead extraction efficiency of 91 ± 3% was achieved. PbI2 is recovered at ambient temperature, while the remaining components obtained from the device dissolution remain soluble and do not interfere as contaminants in the precipitation process. The recovery efficiency was 92.6% for one cycle and 100% for two cycles with a final PbI2 purity of 95.9%.60
Wang et al. reported a novel one-step recovery of all the device elements using a bleacher solution composed of methylamine and a non-polar solvent (THF).61 This approach facilitated the retrieval of the substrate and ETL, FTO/SnO2, and the gold contacts as solid precipitates. The perovskite and spiro-MeOTAD, were recovered as two separate liquid phases: the THF-dissolved spiro-MeOTAD and the liquefied perovskite and were reused for the fabrication of new devices. As a result, this one-step method allowed for the recovery and direct reuse of all the functional layers present in a device with efficiencies of over 20%, similar to device prepared with pristine materials.61
3.5 Encapsulation for lead capture
In addition to EoL strategies for lead recovery, encapsulation with effective lead capture is imperative for deploying lead-safe commercial perovskite modules.62 Various effective approaches have been explored, including self-healing polymer-based encapsulation63 and the integration of lead-absorbing chemical agents. The integration of cation exchange resins (CERs), as demonstrated by Chen et al., has successfully reduced lead leakage to 7 ppb, below the safe level (15 ppb) of drinking water according to the US Federal 40 CFR 141 regulation.64 Another promising option is self-healing Pb-absorbent ionogels, as documented by Xiao et al., which achieved lead leakage levels as low as <1 ppb from damaged perovskite modules after simulated hail impact tests followed by 24 hours of water soaking.65 Despite its significance for future commercial modules, remanufacturing methods have not yet broadly addressed the challenge of encapsulation for recovery and remanufacturing of devices. Chen et al. showcased material recovery through delamination of epoxy-encapsulated devices following thermal treatment at 250 °C for 2 minutes,43 while Bogachuk et al. employed mechanically assisted thermal delamination at 120–140 °C for modules encapsulated with thermoplastic olefins (TPO) and polyisobutylene (PIB)-based edge seal.66 These examples underscore the varying energy requirements and methodologies associated with delamination, contingent upon the sealing methods and materials used. More research is required to identify materials and methods that are not only effective for encapsulation and lead capture but also energy-efficient in application, constituted by abundant and inexpensive materials and are conducive with EoL processes.4. Recovery of gold, spiro-MeOTAD and organic halides
4.1 Recovery of gold
The recovery and reuse of other components in PSCs, beyond substrates and lead ions, has not been extensively studied to date. The gold contact, along with TCO substrates, are considered the most expensive and environmentally impactful components present in PSCs. Depending on the configuration studied, the deposition of the gold counter electrode may contribute 53–65% of the carbon footprint and between 45–90% of the embedded material cost, owing to both the value of the material and the energy requirements of thermal evaporation used for its deposition.13,16,67Although the recovery of gold from perovskite devices is a straightforward procedure, direct reuse of the recovered gold foil is challenging. The shape of the material recovered is not suitable to use as an alternative to gold pellets/wire, and melting the foil recovered before reusing it in a new evaporation is an energy-intensive process and not an economically viable option for large scale application.61 It is very likely that low-cost, abundant materials, such as carbon,68–70 will substitute gold as electrode for large-scale production of perovskite devices. However, the advantageous properties of gold, such as its stability and high conductivity, could still be used in alternative device configurations that enable material reuse, increasing the feasibility of the material application in PSCs.
Li et al. (Fig. 5a and b) developed a printable stack with Ni/Au bilayer which forms a NiO/Au electrode of interconnected Au network embedded in oxidized Ni.71 After optimization of the device and infiltration parameters, this template could potentially reach comparable performance with triple-mesoscopic carbon-PSCs, with the added advantage of charge collection using the NiO/Au layer which functions both as an HTM and a conductive electrode. In this study, the optimised device reached a PCE of >10% and it was demonstrated it could be reused by rinsing with DMF followed by reinfiltrating, with negligible loss on efficiency.71 Using a different approach, Yang et al. (Fig. 5c and d) reported a method where gold is introduced as a removable nanoporous film obtained from the dealloying of a commercial Au35Ag65 with HNO3.72 The film was then recovered and reused in fresh devices up to 12 times with no significant loss in device performance.
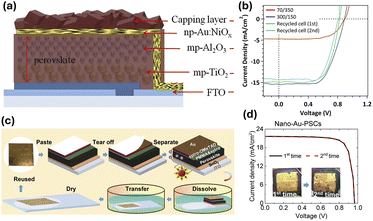 | ||
| Fig. 5 Alternative designs allowing for gold reuse. (a) Au/NiOX is used as HTM/counter electrode in a recyclable all inorganic fully printable stack. (b) JV curves of fresh vs. recycled devices with architecture (Reproduced with permission from ref. 71. Copyright 2017, American Chemical Society). (c) Process for removal and reuse of nano-Au film, (d) performance of devices fabricated with recycled Au film (Reproduced from ref. 72, available under a Creative Commons CC BY licence). | ||
4.2 Recovery and reuse of spiro-MeOTAD
While the multi-solvent sequential dissolution method has proved effective in separating the components of PSCs, there has been limited research on the recovery and reuse of spiro-MeOTAD, PCBM, organic halides and other organic compounds and additives used as dopants or in small amounts in perovskite devices. Spiro-MeOTAD is the most commonly used HTM in high-performance PSCs. Although it has been demonstrated that it can be separated from perovskite films using selective dissolution with chlorobenzene,15,22,23,26 there has been a lack of subsequent research addressing the recovery and potential reuse of spiro-MeOTAD following this separation method.In the work by Wang et al. where perovskite and spiro-MeOTAD are recovered with a single bleacher solution and separated in different liquid phases, the recovery and successful reuse of spiro-MeOTAD dissolved in THF was demonstrated without loss in performance.61 Additionally, this work points to evidence in the morphology of the recycled HTM that suggests the dopants might be recovered/retained alongside the recycled spiro-MeOTAD due to the formation of a chemical bond between the dopants and the HTM.61,73 Further research is needed to assess the quality of the spiro-MeOTAD upon recovery from degraded devices, and its ability to perform as a high-quality HTM upon reuse. It has been suggested spiro-MeOTAD may undergo degradation when in contact with perovskite materials due to ionic migration.74,75 This degradation can result in irreversible changes to the properties of spiro-MeOTAD, which could impact its ability to function as a HTM in future perovskite devices.75,76 This highlights the importance of research into remanufacturing of aged devices to ensure developed strategies are suitable to future EoL management.
4.3 Organic halides
While the recovery of lead ions remains a primary concern, other ions present in perovskite materials can also influence the environmental sustainability of PSCs. Alberola Borras et al. conducted a LCA of multiple cation/anion PSCs and found that the utilization of the FA (formamidinium) cation contributes to higher life cycle greenhouse gas (GHG) emissions.77 Furthermore, a study by Hutter et al. investigated the impact of perovskite materials on plant exposure and found a correlation between plant growth inhibition and the accumulation of iodine.78 Conversely, the use of bromide salts resulted in reduced toxicity levels. The iodine content is influenced by the concentration of both lead iodide and organic iodides (MAI, FAI, CsI) present in the precursor solutions. These findings emphasize the importance of developing recovery methods that extend beyond the existing techniques focused solely on the recovery of lead ions. Comprehensive methods are needed to enable the capture and retrieval of other elements within the perovskite devices with potential toxic effects.5. Compatibility of device design with remanufacturing
Triple-mesoscopic carbon-PSCs69,70 offer the advantages of inexpensive and scalable fabrication methods and the omission of the expensive spiro-MeOTAD transport layer.In remanufacturing methods requiring the removal of layers atop the perovskite, the absence of spiro-MeOTAD presents an advantage, as the carbon layer is comparatively inexpensive. Bogachuk et al.66 recently reported a remanufacturing technique for encapsulated triple-mesoscopic carbon-PSCs with a 1.5 cm2 active area, and assessed potential carbon footprints for 1 m2 remanufactured modules. The proposed method involves thermally-assisted mechanical separation and acetone immersion to remove sealant and encapsulant, while methylamine and ethanol baths are used to remove the carbon layer and the degraded perovskite. A fresh carbon layer is then applied onto the recovered FTO/TiO2/ZrO2 stack, followed by re-infiltration of perovskite. Remanufactured devices using the recovered stack showed a performance of up to 88% of those fabricated with fresh stacks. The authors concentrated on the global warming potential (GWP), which is expressed in kilograms of CO2-equivalent per kilowatt-peak (kWp) of generated power. They considered this metric the most direct link between the energy generation system and its associated greenhouse gas emissions. The CO2-equivalent emissions produced during the processing of each module component, as obtained from the life cycle assessment (LCA), were correlated with the total energy expected to be produced during the lifespan of the PV module. A 33% reduction in GWP was estimated for optimised remanufactured modules, and a decrease of the “break-even” lifetime from 16 years to 10.7 years.66
Remanufacturing techniques involving the removal and redeposition of films pose several challenges. There is no clear method for reintroducing dissolved materials in the remanufacturing of new devices, and the redeposition of the removed layers requires energy expenditure. Some methods reported for the remanufacturing of printable devices allow for the recovery of the full stack by regenerating the perovskite through chemical post-treatment, solvent extraction and re-infiltration of the absorber material, or direct restoration of the perovskite by heat treatment in the case of fully inorganic perovskite materials.46,79,80
Hong et al. have demonstrated crystal reconstruction induced by treatment with methylamine gas for triple-mesoscopic carbon-PSCs.46 After experiencing a reduction in the power conversion efficiency (PCE) to 40% of its initial value due to perovskite degradation, the application of methylamine gas as a post-treatment successfully restored the PCE to 91% of its original value, following two cycles of photodegradation and recovery (Fig. 6).46
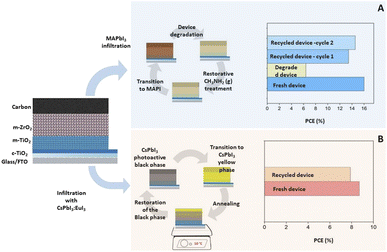 | ||
| Fig. 6 Remanufacturing methodologies for triple-mesoscopic carbon-PSCs device architectures applying: (A) a methylamine gas restoration treatment (Reproduced with permission from ref. 46. Copyright 2017, John Wiley and Sons Inc.) and (B) restoration of all-inorganic CsPbI3 perovskite through thermal treatment (Adapted from ref. 80, available under a Creative Commons CC BY licence). | ||
Using the same device configuration with an all-inorganic perovskite composition, Valastro et al. demonstrated the remarkable recovery of a degraded fully printable device through a heat treatment at 350 °C.80 Initially, they enhanced the performance of all-inorganic CsPbI3 PSCs by introducing europium doping, increasing the efficiency from 1.4% to 9.2%. CsPbI3 perovskite is known to undergo a phase transition, shifting from its photoactive black orthorhombic phase to a non-photoactive yellow phase, resulting in a decline in device performance. However, this material does not release any volatile species, which can be advantageous for device stability. Valastro et al. applied a heat treatment at 350 °C for two minutes to the entire device, successfully reversing the transition to the black phase. This process effectively restored the device's performance to 90% of its initial efficiency.80 The ability to rejuvenate these devices without the need for solvents offers a promising approach for sustainable and cost-effective device maintenance. While an all-inorganic perovskite material without organic components offers a compelling option for solar cell fabrication, it is essential to address concerns regarding the availability of materials. As the industry progresses, it becomes imperative to reduce the reliance on scarce resources and critical raw materials. Notably, europium, being a highly rare material, and cesium and rubidium, listed among the top 35 critical minerals in the U.S. supply chain, raise significant concerns.81 Developing alternative materials or recycling strategies is crucial to ensure long-term viability and environmental responsibility in solar cell manufacturing.80
Ku et al. reported an alternative to printable triple-mesoscopic carbon PSCs by incorporating a mesoporous Ni layer as the p-type contact.79 In this configuration, the perovskite was infiltrated into the mp-TiO2/Al2O3/mp-Ni stack through a two-step process, resulting in a PCE of 13.6%. Following the degradation and a decrease in PCE in unencapsulated devices, dissolution of the degraded perovskite by immersion in DMF followed by re-infiltration of the perovskite material enabled a remarkable recovery of 90% of their initial PCE (Fig. 7).79
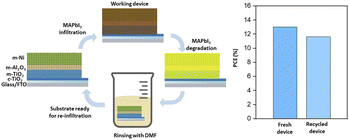 | ||
| Fig. 7 Remanufacturing methodology for a fully printable device architectures with a mesoporous nickel counter electrode (adapted from ref. 79 with permission of the Royal Society of Chemistry). | ||
In terms of compatibility with remanufacturing strategies, all-back-contact perovskites provide an interesting device design (Fig. 8). In this configuration, both electron and hole selective contacts are on one side of the device, offering advantages towards novel ways of perovskite passivation, encapsulation and, most relevant to this work, it offers the possibility of refurbishing by a simple process of dissolution and redeposition of the perovskite layer, with full recovery of the substrate with both charge selective contacts.82–85 This technology is, however, in its infancy, efficiencies are not optimized, and the mechanisms of charge transfer are not fully understood.
 | ||
| Fig. 8 All-back-contact (ABC) perovskite architecture (Reproduced from ref. 83, available under a Creative Commons CC-BY licence.). | ||
6. Sustainability and impact of the recovery processes
Since the goal of the remanufacturing process is the reduction of cost and the increased sustainability of the device, a trade-off exists between the effective remanufacturing and the impact linked to the solvents, materials and energy invested in the recovery. Solvent processing of perovskite device offers a great advantage over other technologies, but it also implies that the impact to human health and environment of the solvent used at an industrial scale should be addressed.The use of DMF, the most common solvent reported in the fabrication of perovskite devices due to its ability to dissolve lead iodide and low volatility, has been linked with liver damage and reproductive health issues.86,87 There is a current effort to introduce greener solvents in the fabrication of perovskite devices and remanufacturing processes are required to evolve in the same direction, towards increasing the recycling of solvents and towards the reduction of energy embedded into the process.11,88–90
There is currently a gap for studies on the potential reuse of solvents at large scale, but evaluation of the current methods reported for lab-scale devices has been addressed in a few studies. In the study by Rodriguez Garcia et al., the evaluation of the environmental impact of substrate recovery methods points at solvents use as the cause of major impact.91 According to their results, the choice of multi-solvent and high toxicity solvents recovery strategies were the most impactful.91
Kim et al. recently reported the utilization of recycled chlorobenzene and DMF in the production of new devices.92 These solvents were initially used to remove spiro-MeOTAD and perovskite from devices, and later subjected to purification using hematite nanoparticles to eliminate lead ions. It was observed that, upon following sequential dissolution and avoiding the contamination of DMF with spiro-MeOTAD, the remaining impurities had no measurable impact on device performance, achieving a 24% efficiency, similar to devices prepared with pristine solvents.92 Nonetheless, further investigation is still necessary to evaluate the long-term stability of perovskite and HTM films prepared with these recycled solvents, considering potential impurity effects.
While recycling strategies could mitigate the impact of toxic solvent use, the risks of exposure and spillage and its potential effect on health and environment are still present. Consequently, it is essential to shift towards non-organic or non-toxic organic solvents to enhance the feasibility and sustainability of remanufacturing methods.
7. Conclusions
As demonstrated by the various methods reviewed in this study, remanufacturing strategies hold the potential to address critical concerns related to the lifetime viability and toxicity of perovskite-based devices. The recovery of key components such as lead, TCO substrates, and transporting layers offer significant potential reductions in production costs and waste generation. Additionally, the adoption of diverse methods for lead capture, recovery, and reuse can effectively mitigate environmental impacts and toxicity, thereby alleviating major challenges facing the viability of perovskite-based PV technology.However, to fully optimise the efficiency and sustainability of remanufacturing strategies for perovskite devices, a more integrated approach is necessary. Key considerations for advancing research in this area include:
Prioritising adequate device configurations: research on remanufacturing would benefit from focusing on perovskite device configurations that align with the most cost-effective and energy-efficient refurbishing methods, potentially, even if these devices are not the highest efficiency configurations. This is particularly pertinent for triple-mesoscopic carbon perovskite solar cells, where there is the possible potential for the perovskite to be refurbished in situ or to be removed and replaced without the removal of contact layers. This would provide significant economic and environmental benefit if viable. For long term feasibility, it is essential to select configurations that are compatible with remanufacturing processes that can be carried out at scale, and this likely means avoiding processes that require sequential dissolution. Wherever possible the use of materials robust enough to withstand multiple life cycles without needing redeposition through costly and energy-intensive methods is crucial. Alternatives like carbon-based devices, hole transport-free designs, or all-inorganic configurations using abundant materials could offer greater durability and reusability after the perovskite absorber degrades.
Addressing real life conditions: current remanufacturing methodologies often overlook real-life operational conditions, predominantly focusing on fresh or recently manufactured lab-scale devices. This approach neglects critical factors such as encapsulation, chemical changes upon material degradation, and other operational stressors, which can severely compromise the effectiveness of these methodologies in practical scenarios. It is therefore imperative to develop remanufacturing methodologies that are tailored to the realistic end-of-life conditions of aged modules, considering potential degradation and changes at the materials and interfaces. Without addressing these issues, the methodologies may prove to be ineffective in real-world applications.
Optimising methodologies ensuring payback and sustainability: when proposing methods for the remanufacturing, recovery, and reuse of materials, it's crucial to adopt a holistic approach that considers the subsequent implications on cost, environmental impact, and energy expenditure throughout the device's lifecycle. The criteria for integrating optimal steps should extend beyond mere effectiveness to include sufficient payback and sustainability assurance. Early research into the reuse of materials from perovskite solar cells included the use of toxic solvents or complex and energy-intensive processes. Although useful as a proof of concept at this stage research in this field has matured enough to warrant a shift away from such approaches. Utilising life cycle assessment (LCA) and similar methodologies can ensure that each step, whether it involves solvents, heat treatment, or other processes, is carefully evaluated with the benefit of such processes quantified.
When applied together, these approaches could contribute to meaningful and sustained innovation in perovskite solar cells, bringing us closer to a sustainable and robust PV technology with the potential of setting a precedent in terms of technology design for circular economy.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was made possible by support from UKRI and the EU Horizon Europe Framework Programme (101122277), the Engineering and Physical Science Research Council (EP/S001336/1) and through the funding of the SPECIFIC Innovation and Knowledge Centre by EPSRC (EP/N020863/1) and the Welsh Government (CRISP22-301). MLD is also grateful for EPSRC funding EP/W019167/1.References
- International Energy Agency, Renewables 2022 Analysis and Forecast to 2027, 2023 Search PubMed.
- IRENA, World Energy Transitions Outlook 2023: 1.5 °C Pathway, Volume 1, International Renewable Energy Agency, Abu Dhabi, 2023 Search PubMed.
- S. Weckend, A. Wade and G. Heath, End of Life Management: Solar Photovoltaic Panels, 2016 Search PubMed.
- P. Majewski, W. Al-shammari, M. Dudley, J. Jit, S.-H. Lee, K. Myoung-Kug and K. Sung-Jim, Energy Policy, 2021, 149, 112062 CrossRef.
- D. Lin, Z. Liu, X. Li, Z. Cao and R. Xiong, Clean Energy, 2023, 7, 532–546 CrossRef.
- G. A. Heath, T. J. Silverman, M. Kempe, M. Deceglie, D. Ravikumar, T. Remo, H. Cui, P. Sinha, C. Libby, S. Shaw, K. Komoto, K. Wambach, E. Butler, T. Barnes and A. Wade, Nat. Energy, 2020, 5, 502–510 CrossRef CAS.
- R. Deng, N. L. Chang, Z. Ouyang and C. M. Chong, Renewable Sustainable Energy Rev., 2019, 109, 532–550 CrossRef CAS.
- R. Deng, Y. Zhuo and Y. Shen, Resour., Conserv. Recycl., 2022, 187, 106612 CrossRef.
- NREL, Best Research Cell Efficiency Chart, https://www.nrel.gov/pv/cell-efficiency.html, Last accessed: May 28, 2024.
- R. G. Charles, A. Doolin, R. García-Rodríguez, K. V. Villalobos and M. L. Davies, Energy Environ. Sci., 2023, 16, 3711–3733 RSC.
- X. Tian, S. D. Stranks and F. You, Nat. Sustain., 2021, 4, 821–829 CrossRef.
- I. Celik, Z. Song, A. J. Cimaroli, Y. Yan, M. J. Heben and D. Apul, Sol. Energy Mater. Sol. Cells, 2016, 156, 157–169 CrossRef CAS.
- J. Gong, S. B. Darling and F. You, Energy Environ. Sci., 2015, 8, 1953–1968 RSC.
- J. Zhang, X. Gao, Y. Deng, B. Li and C. Yuan, ChemSusChem, 2015, 8, 3882–3891 CrossRef CAS PubMed.
- A. Binek, M. L. Petrus, N. Huber, H. Bristow, Y. Hu, T. Bein and P. Docampo, ACS Appl. Mater. Interfaces, 2016, 8, 12881–12886 CrossRef CAS PubMed.
- A. L. Carneiro, A. A. Martins, V. C. M. Duarte, T. M. Mata and L. Andrade, Energy Rep., 2022, 8, 475–481 CrossRef.
- B. Augustine, K. Remes, G. S. Lorite, J. Varghese and T. Fabritius, Sol. Energy Mater. Sol. Cells, 2019, 194, 74–82 CrossRef CAS.
- L. Huang, Z. Hu, J. Xu, X. Sun, Y. Du, J. Ni, H. Cai, J. Li and J. Zhang, Sol. Energy Mater. Sol. Cells, 2016, 152, 118–124 CrossRef CAS.
- X. Feng, S. Wang, Q. Guo, Y. Zhu, J. Xiu, L. Huang, Z. Tang and Z. He, J. Phys. Chem. Lett., 2021, 12, 4735–4741 CrossRef CAS PubMed.
- X. Feng, Q. Guo, J. Xiu, Z. Ying, K. W. Ng, L. Huang, S. Wang, H. Pan, Z. Tang and Z. He, Cell Rep. Phys. Sci., 2021, 2, 100341 CrossRef CAS.
- T. O'Hara, A. Ravilla, E. McCalmont, B. Carlson, J. Kellar, Z. Song and I. Celik, MRS Adv., 2023, 8, 296–301 CrossRef.
- L. Huang, J. Xu, X. Sun, R. Xu, Y. Du, J. Ni, H. Cai, J. Li, Z. Hu and J. Zhang, ACS Sustainable Chem. Eng., 2017, 5, 3261–3269 CrossRef CAS.
- J. M. Kadro, N. Pellet, F. Giordano, A. Ulianov, O. Müntener, J. Maier, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2016, 9, 3172–3179 RSC.
- S. Zhang, L. Shen, M. Huang, Y. Yu, L. Lei, J. Shao, Q. Zhao, Z. Wu, J. Wang and S. Yang, ACS Sustainable Chem. Eng., 2018, 6, 7558–7564 CrossRef CAS.
- W. Zhu, W. Chai, D. Chen, H. Xi, D. Chen, J. Chang, J. Zhang, C. Zhang and Y. Hao, ACS Appl. Mater. Interfaces, 2020, 12, 4549–4557 CrossRef CAS PubMed.
- B. J. Kim, D. H. Kim, S. L. Kwon, S. Y. Park, Z. Li, K. Zhu and H. S. Jung, Nat. Commun., 2016, 7, 11735 CrossRef CAS PubMed.
- H. W. Mielke and P. L. Reagan, Environ. Health Perspect., 1998, 106(Suppl 1), 217–229 CrossRef CAS PubMed.
- S. Tong, Y. E. von Schirnding and T. Prapamontol, Bull. W. H. O., 2000, 78, 1068–1077 CAS.
- E. K. Silbergeld, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2003, 533, 121–133 CrossRef CAS PubMed.
- K. Koller, T. Brown, A. Spurgeon and L. Levy, Environ. Health Perspect., 2004, 112, 987–994 CrossRef CAS PubMed.
- D. A. Gidlow, Occup. Med., 2015, 65, 348–356 CrossRef CAS PubMed.
- M. Ren, X. Qian, Y. Chen, T. Wang and Y. Zhao, J. Hazard. Mater., 2022, 426, 127848 CrossRef CAS PubMed.
- B. Hailegnaw, S. Kirmayer, E. Edri, G. Hodes and D. Cahen, J. Phys. Chem. Lett., 2015, 6, 1543–1547 CrossRef CAS PubMed.
- I. R. Benmessaoud, A.-L. Mahul-Mellier, E. Horváth, B. Maco, M. Spina, H. A. Lashuel and L. Forró, Toxicol. Res., 2016, 5, 407–419 CrossRef CAS PubMed.
- J. Li, H.-L. Cao, W.-B. Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Nat. Commun., 2020, 11, 310 CrossRef CAS PubMed.
- X. Jiang, H. Li, Q. Zhou, Q. Wei, M. Wei, L. Jiang, Z. Wang, Z. Peng, F. Wang, Z. Zang, K. Xu, Y. Hou, S. Teale, W. Zhou, R. Si, X. Gao, E. H. Sargent and Z. Ning, J. Am. Chem. Soc., 2021, 143, 10970–10976 CrossRef CAS PubMed.
- B.-B. Yu, Z. Chen, Y. Zhu, Y. Wang, B. Han, G. Chen, X. Zhang, Z. Du and Z. He, Adv. Mater., 2021, 33, 2102055 CrossRef CAS PubMed.
- A. Babayigit, D. Duy Thanh, A. Ethirajan, J. Manca, M. Muller, H.-G. Boyen and B. Conings, Sci. Rep., 2016, 6, 18721 CrossRef CAS PubMed.
- L. Serrano-Lujan, N. Espinosa, T. T. Larsen-Olsen, J. Abad, A. Urbina and F. C. Krebs, Adv. Energy Mater., 2015, 5, 1501119 CrossRef.
- N. Moody, S. Sesena, D. W. deQuilettes, B. D. Dou, R. Swartwout, J. T. Buchman, A. Johnson, U. Eze, R. Brenes, M. Johnston, C. L. Haynes, V. Bulović and M. G. Bawendi, Joule, 2020, 4, 970–974 CrossRef.
- F. Li, S. Shaw, C. Libby, N. Preciado, B. Bicer and G. Tamizhmani, Waste Manage., 2024, 174, 646–665 CrossRef CAS PubMed.
- Directive 2011/65/EU of the European Parliament and of the Council of 8 June 2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment, 2024, http://data.europa.eu/eli/dir/2011/65/2024-02-01.
- B. Chen, C. Fei, S. Chen, H. Gu, X. Xiao and J. Huang, Nat. Commun., 2021, 12, 5859 CrossRef CAS PubMed.
- P. Chhillar, B. P. Dhamaniya, V. Dutta and S. K. Pathak, ACS Omega, 2019, 4, 11880–11887 CrossRef CAS PubMed.
- J. Xu, Z. Hu, L. Huang, X. Huang, X. Jia, J. Zhang, J. Zhang and Y. Zhu, Prog. Photovolt.: Res. Appl., 2017, 25, 1022–1033 CrossRef CAS.
- L. Hong, Y. Hu, A. Mei, Y. Sheng, P. Jiang, C. Tian, Y. Rong and H. Han, Adv. Funct. Mater., 2017, 27, 1703060 CrossRef.
- H. Wang, X. Chen, X. Li, J. Qu, H. Xie, S. Gao, D. Wang and H. Yin, Chem. Eng. J., 2022, 447, 137498 CrossRef CAS.
- C. G. Poll, G. W. Nelson, D. M. Pickup, A. V. Chadwick, D. J. Riley and D. J. Payne, Green Chem., 2016, 18, 2946–2955 RSC.
- S. Tan, D. J. Payne, J. P. Hallett and G. H. Kelsall, Curr. Opin. Electrochem., 2019, 16, 83–89 CrossRef CAS.
- N. A. A. Qasem, R. H. Mohammed and D. U. Lawal, npj Clean Water, 2021, 4, 1–15 CrossRef.
- R. Arora, Mater. Today: Proc., 2019, 18, 4745–4750 CAS.
- W. S. Chai, J. Y. Cheun, P. S. Kumar, M. Mubashir, Z. Majeed, F. Banat, S.-H. Ho and P. L. Show, J. Cleaner Prod., 2021, 296, 126589 CrossRef CAS.
- D. Lakherwal, Int. J. Environ. Sci. Dev., 2014, 4, 41–48 Search PubMed.
- S. Y. Park, J.-S. Park, B. J. Kim, H. Lee, A. Walsh, K. Zhu, D. H. Kim and H. S. Jung, Nat. Sustain., 2020, 3, 1044–1051 CrossRef.
- J. S. Hong, H. J. Kim, C. H. Sohn, O. Y. Gong, J. H. Choi, K. H. Cho, G. S. Han, K. T. Nam and H. S. Jung, Energy Environ. Mater., 2022, 6, e12374 CrossRef.
- M. Ren, Y. Miao, T. Zhang, Z. Qin, Y. Chen, N. Wei, X. Qian, T. Wang and Y. Zhao, ACS Sustainable Chem. Eng., 2021, 9, 16519–16525 CrossRef CAS.
- S. Kundu and T. L. Kelly, EcoMat, 2020, 2, e12025 CrossRef CAS.
- X. Tang, M. Brandl, B. May, I. Levchuk, Y. Hou, M. Richter, H. Chen, S. Chen, S. Kahmann, A. Osvet, F. Maier, H.-P. Steinrück, R. Hock, G. J. Matt and C. J. Brabec, J. Mater. Chem. A, 2016, 4, 15896–15903 RSC.
- W. Huang, J. S. Manser, P. V. Kamat and S. Ptasinska, Chem. Mater., 2016, 28, 303–311 CrossRef CAS.
- F. Schmidt, M. Amrein, S. Hedwig, M. Kober-Czerny, A. Paracchino, V. Holappa, R. Suhonen, A. Schäffer, E. C. Constable, H. J. Snaith and M. Lenz, J. Hazard. Mater., 2023, 447, 130829 CrossRef CAS PubMed.
- K. Wang, T. Ye, X. Huang, Y. Hou, J. Yoon, D. Yang, X. Hu, X. Jiang, C. Wu, G. Zhou and S. Priya, Matter, 2021, 4, 2522–2541 CrossRef CAS.
- Y. Yin, L. Yang, X. Zhang and J. Zhang, J. Mater. Chem. A, 2023, 11, 25825–25848 RSC.
- Y. Jiang, L. Qiu, E. J. Juarez-Perez, L. K. Ono, Z. Hu, Z. Liu, Z. Wu, L. Meng, Q. Wang and Y. Qi, Nat. Energy, 2019, 4, 585–593 CrossRef CAS.
- S. Chen, Y. Deng, H. Gu, S. Xu, S. Wang, Z. Yu, V. Blum and J. Huang, Nat. Energy, 2020, 5, 1003–1011 CrossRef.
- X. Xiao, M. Wang, S. Chen, Y. Zhang, H. Gu, Y. Deng, G. Yang, C. Fei, B. Chen, Y. Lin, M. D. Dickey and J. Huang, Sci. Adv., 2021, 7, eabi8249 CrossRef CAS PubMed.
- D. Bogachuk, P. van der Windt, L. Wagner, D. Martineau, S. Narbey, A. Verma, J. Lim, S. Zouhair, M. Kohlstädt, A. Hinsch, S. D. Stranks, U. Würfel and S. W. Glunz, ACS Sustainable Resour. Manage., 2024, 1, 417–426 CrossRef CAS PubMed.
- J. Zhang, X. Gao, Y. Deng, Y. Zha and C. Yuan, Sol. Energy Mater. Sol. Cells, 2017, 166, 9–17 CrossRef CAS.
- D. Beynon, E. Parvazian, K. Hooper, J. Mcgettrick, R. Patidar, T. Dunlop, Z. Wei, P. Davies, R. G. Rodriguez, M. Carnie, M. Davies and T. Watson, Adv. Mater., 2023, 35, 2208561 CrossRef CAS PubMed.
- S. M. P. Meroni, C. Worsley, D. Raptis and T. M. Watson, Energies, 2021, 14, 386 CrossRef CAS.
- C. Worsley, D. Raptis, S. M. P. Meroni, R. Patidar, A. Pockett, T. Dunlop, S. J. Potts, R. Bolton, C. M. E. Charbonneau, M. Carnie, E. Jewell and T. Watson, Mater. Adv., 2022, 3, 1125–1138 RSC.
- M.-H. Li, Y.-S. Yang, K.-C. Wang, Y.-H. Chiang, P.-S. Shen, W.-C. Lai, T.-F. Guo and P. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 41845–41854 CrossRef CAS PubMed.
- F. Yang, J. Liu, Z. Lu, P. Dai, T. Nakamura, S. Wang, L. Chen, A. Wakamiya and K. Matsuda, Adv. Sci., 2020, 7, 1902474 CrossRef CAS PubMed.
- F. Lamberti, T. Gatti, E. Cescon, R. Sorrentino, A. Rizzo, E. Menna, G. Meneghesso, M. Meneghetti, A. Petrozza and L. Franco, Chem, 2019, 5, 1806–1817 CAS.
- E. Kasparavicius, M. Franckevičius, V. Malinauskiene, K. Genevičius, V. Getautis and T. Malinauskas, ACS Appl. Energy Mater., 2021, 4, 13696–13705 CrossRef CAS PubMed.
- S. Kim, S. Bae, S.-W. Lee, K. Cho, K. D. Lee, H. Kim, S. Park, G. Kwon, S.-W. Ahn, H.-M. Lee, Y. Kang, H.-S. Lee and D. Kim, Sci. Rep., 2017, 7, 1200 CrossRef PubMed.
- S. Ito, S. Kanaya, H. Nishino, T. Umeyama and H. Imahori, Photonics, 2015, 2, 1043–1053 CrossRef CAS.
- J.-A. Alberola-Borràs, R. Vidal and I. Mora-Seró, Sustainable Energy Fuels, 2018, 2, 1600–1609 RSC.
- E. M. Hutter, R. Sangster, C. Testerink, B. Ehrler and C. M. M. Gommers, iScience, 2022, 25, 103583 CrossRef CAS PubMed.
- Z. Ku, X. Xia, H. Shen, N. H. Tiep and H. J. Fan, Nanoscale, 2015, 7, 13363–13368 RSC.
- S. Valastro, E. Smecca, C. Bongiorno, C. Spampinato, G. Mannino, S. Biagi, I. Deretzis, F. Giannazzo, A. K. Jena, T. Miyasaka, A. La Magna and A. Alberti, Sol. RRL, 2022, 6, 2200267 CrossRef CAS.
- S. A. Khalifa, S. Spatari, A. T. Fafarman and J. B. Baxter, ACS Sustainable Chem. Eng., 2020, 8, 16537–16548 CrossRef CAS.
- Q. Hou, D. Bacal, A. N. Jumabekov, W. Li, Z. Wang, X. Lin, S. H. Ng, B. Tan, Q. Bao, A. S. R. Chesman, Y.-B. Cheng and U. Bach, Nano Energy, 2018, 50, 710–716 CrossRef CAS.
- K. J. Prince, M. Nardone, S. P. Dunfield, G. Teeter, M. Mirzokarimov, E. L. Warren, D. T. Moore, J. J. Berry, C. A. Wolden and L. M. Wheeler, Cell Rep. Phys. Sci., 2021, 2, 100363 CrossRef CAS.
- T. Ma, Q. Song, D. Tadaki, M. Niwano and A. Hirano-Iwata, ACS Appl. Energy Mater., 2018, 1, 970–975 CrossRef CAS.
- H. M. Mirletz, S. Ovaitt, A. Gaulding, S. Sridhar and T. Barnes, in 2022 IEEE 49th Photovoltaics Specialists Conference (PVSC), IEEE, Philadelphia, PA, USA, 2022, pp. 0299–0301 Search PubMed.
- T. H. Kim and S. G. Kim, Saf. Health Work, 2011, 2, 97–104 CrossRef CAS PubMed.
- M.-J. Li and T. Zeng, Chem.-Biol. Interact., 2019, 298, 129–136 CrossRef CAS PubMed.
- R. Vidal, J.-A. Alberola-Borràs, S. N. Habisreutinger, J.-L. Gimeno-Molina, D. T. Moore, T. H. Schloemer, I. Mora-Seró, J. J. Berry and J. M. Luther, Nat Sustainability, 2021, 4, 277–285 CrossRef.
- A. J. Doolin, R. G. Charles, C. S. P. De Castro, R. G. Rodriguez, E. V. Péan, R. Patidar, T. Dunlop, C. Charbonneau, T. Watson and M. L. Davies, Green Chem., 2021, 23, 2471–2486 RSC.
- G. Rodriguez-Garcia, E. Aydin, S. De Wolf, B. Carlson, J. Kellar and I. Celik, ACS Sustainable Chem. Eng., 2021, 9, 15239–15248 CrossRef CAS.
- G. Rodriguez-Garcia, E. Aydin, S. De Wolf, B. Carlson, J. Kellar and I. Celik, ACS Sustainable Chem. Eng., 2021, 9, 15239–15248 CrossRef CAS.
- H. J. Kim, O. Y. Gong, Y. J. Kim, G. W. Yoon, G. S. Han, H. Shin and H. S. Jung, ACS Energy Lett., 2023, 8, 4330–4337 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |
