Open Access Article
Open Access ArticleGraphitic carbon nitride (g-C3N4) as an emerging photocatalyst for sustainable environmental applications: a comprehensive review
Dhavalkumar
Bhanderi
 ,
Pratikkumar
Lakhani
,
Pratikkumar
Lakhani
 and
Chetan K.
Modi
and
Chetan K.
Modi
 *
*
Applied Chemistry Department, Faculty of Technology & Engineering, The Maharaja Sayajirao University of Baroda, Vadodara 390001, Gujarat, India. E-mail: chetanmodi-appchem@msubaroda.ac.in; Tel: +91-265-2434188
First published on 4th December 2023
Abstract
Graphitic carbon nitride (g-C3N4) stands as a prominent and sustainable photocatalyst, offering a transformative solution to pressing environmental and energy challenges. This review article provides a comprehensive examination of g-C3N4, spanning its synthesis methods, structural properties, photocatalytic mechanisms, and diverse applications. By delving into various synthesis techniques and their respective merits, we reveal recent breakthroughs that underscore the material's growing significance. Unveiling the critical structural attributes governing photocatalytic performance, including bandgap, surface area, and porosity, we explore the impact of doping and modification on enhancing its capabilities. In elucidating the photocatalytic mechanisms, we showcase how g-C3N4 facilitates crucial processes like water splitting, pollutant degradation, and solar-driven carbon dioxide reduction, emphasizing its unique selectivity and efficiency. Through concrete examples and case studies, we highlight its versatility in applications ranging from water purification to hydrogen production and air quality enhancement, underscoring the environmental and economic benefits that come with its adoption. Challenges such as quantum efficiency and charge carrier recombination are addressed, alongside a forward-looking perspective on emerging trends and innovations. Ultimately, this review positions g-C3N4 as a sustainable game-changer in the realm of environmental and energy technologies, offering a promising path towards a more sustainable future.
Sustainability spotlightGraphitic carbon nitride (g-C3N4) is emerging as a promising catalyst in the realm of sustainable environmental applications. Its unique properties, including efficient light absorption and electron–hole pair generation, make it a key player in addressing pressing environmental challenges. By utilizing g-C3N4 as a photocatalyst, we are taking a significant step towards a greener and cleaner future. This sustainable innovation paves the way for eco-friendly solutions in wastewater treatment, air purification, and renewable energy production, marking a brighter path towards a more sustainable planet. |
1. Introduction
In an era characterized by escalating environmental challenges and the unrelenting pursuit of sustainable solutions, photocatalysis has emerged as a transformative technology with the potential to revolutionize our approach to pressing global issues.1–3 The ability to harness sunlight and convert it into a powerful driving force for a myriad of chemical reactions holds the promise of addressing some of the most urgent concerns facing society today.4–7 Among the plethora of photocatalytic materials, graphitic carbon nitride (g-C3N4) has risen to prominence, captivating the imagination of researchers and practitioners alike. Its unique combination of properties, including excellent stability, ease of synthesis, and a suitable bandgap, positions it as an ideal candidate for sustainable applications, ranging from water purification and renewable energy production to air quality improvement and carbon management.8–12The imperative for sustainable solutions in the face of mounting environmental challenges is undeniable. Water scarcity, pollution, and the depletion of fossil fuels have compelled humanity to explore novel technologies that mitigate these issues while minimizing environmental harm.13–16 Photocatalysis, as an eco-friendly and energy-efficient process, has emerged as a ray of hope.17,18 By exploiting semiconductor materials that can absorb solar radiation and catalyze chemical reactions, photocatalysis has the potential to transform pollutants into harmless byproducts, produce clean energy from sunlight, and address the daunting issue of global warming through carbon capture and utilization.19–22
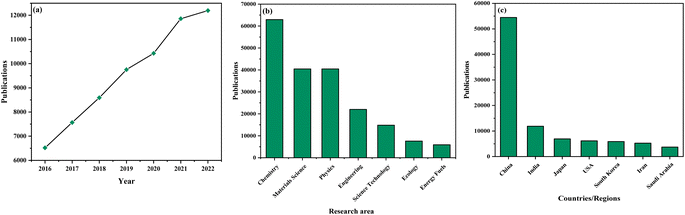
The utilization of graphitic carbon nitride (g-C3N4) as a photocatalyst has garnered substantial attention within the scientific community, where a concerted effort is being made to decipher the distinctive qualities that render these catalysts truly exceptional. A thorough examination of literature resources accessible through prominent databases unveils a notable surge in publications pertaining to the application of graphitic carbon nitride (g-C3N4) as a photocatalyst in recent times, indicative of a remarkable growth trajectory (as illustrated in Fig. 1(a)). This escalating trend emphatically underscores the escalating significance of graphitic carbon nitride (g-C3N4) as a photocatalyst in the context of addressing intricate chemical transformations, sustainability-related challenges, and the formulation of environmentally friendly and more efficacious processes. Researchers are increasingly captivated by the extensive potential and the diverse array of applications offered by graphitic carbon nitride (g-C3N4) as a photocatalyst, thereby reflecting the field's profound importance in shaping the future landscape of chemistry and sustainable technologies.23–28
Research in this field spans a broad spectrum of prominent scientific disciplines, with a central focus on areas including chemistry, materials science, physics, engineering, science technology, ecology, and energy fuels (as depicted in Fig. 1(b)). These research endeavors predominantly revolve around foundational and fundamental inquiries, representing a fundamental exploration into the core principles and mechanisms that govern the functionality of graphitic carbon nitride (g-C3N4) as a photocatalyst. This interdisciplinary approach emphasizes the wide-ranging and profound implications associated with graphitic carbon nitride (g-C3N4) as a photocatalyst, as it extends its influence into diverse scientific domains, ultimately accentuating its pivotal role in advancing our comprehension of chemical processes, sustainability practices, and the evolution of innovative technologies.29–34
The global landscape of publications in this field presents an intriguing picture, as depicted in Fig. 1(c). Notably, China emerges as the epicenter of active research on graphitic carbon nitride (g-C3N4) as a photocatalyst, underlining the nation's significant contributions to the advancements in this particular domain. Following closely are India, Japan, and a host of other nations, all actively engaged in research pursuits pertaining to graphitic carbon nitride (g-C3N4) as a photocatalyst. This worldwide distribution underscores the widespread acknowledgment of the paramount importance of graphitic carbon nitride (g-C3N4) as a photocatalyst across diverse regions on a global scale. Furthermore, it mirrors the collaborative and international character intrinsic to scientific research, with researchers hailing from various nations collectively striving to expand the frontiers of knowledge and devise sustainable solutions in the realm of photocatalysis.
While various photocatalysts have demonstrated promising capabilities, the distinctive properties of g-C3N4 have thrust it into the limelight. Its structure, comprised of carbon and nitrogen atoms arranged in a two-dimensional conjugated framework, not only imparts exceptional chemical stability but also affords a favorable electronic band structure, making it well-suited for photochemical applications.35–38 The synthesis of g-C3N4 has been refined through diverse methods, offering researchers the flexibility to tailor its properties for specific applications. These methods encompass thermal polymerization, chemical vapor deposition, and solvothermal processes, among others, each with its own advantages and limitations.39–45
Intriguingly, g-C3N4's remarkable photocatalytic performance extends beyond the realm of traditional semiconductor photocatalysts. Its capacity to generate electron–hole pairs upon exposure to light, facilitate charge separation, and participate in redox reactions has opened up new avenues for sustainable applications.46–48 Whether it be the degradation of organic pollutants in water, the production of clean hydrogen fuel via water splitting, the removal of noxious gases from the atmosphere, or the conversion of carbon dioxide into value-added chemicals, g-C3N4's versatility and efficiency have illuminated the path toward a more sustainable future.27,49–53
This comprehensive review endeavors to shed light on the multifaceted attributes of g-C3N4 as a photocatalyst, exploring its synthesis techniques, structural properties, photocatalytic mechanisms, and diverse applications. It is our aspiration that by delving into the intricacies of g-C3N4 photocatalysis, we can provide a roadmap for academic and industrial researchers to harness the immense potential of this material in advancing sustainability, catalyzing environmental stewardship, and paving the way for a cleaner, greener, and more sustainable world.
2. Synthesis methods
The synthesis of graphitic carbon nitride (g-C3N4) has undergone significant development over the years, offering researchers a versatile toolkit to tailor its properties for specific photocatalytic applications. The following sections explore several key synthesis methods, each with its own advantages and limitations.2.1 Thermal polymerization
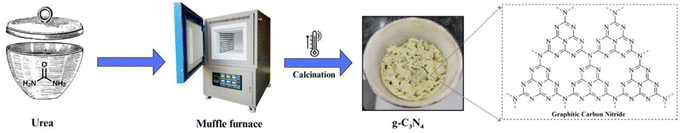
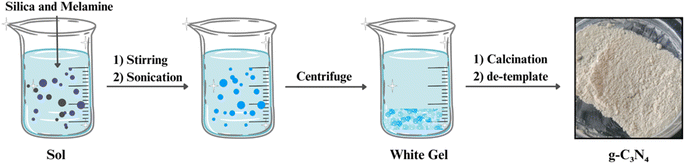
One of the most widely employed methods for g-C3N4 synthesis is thermal polymerization of low-cost precursors, such as melamine, urea, cyanamide, dicyanamide, thiourea, cyanuric acid etc. This process typically involves the heating of these precursors at moderate temperatures (around 500–600 °C) under inert gas atmospheres (Fig. 2). The thermal polymerization route generates a layered g-C3N4 structure with a high surface area, making it suitable for various photocatalytic applications. The method's simplicity and cost-effectiveness have contributed to its popularity.54–562.2 Chemical vapor deposition (CVD)
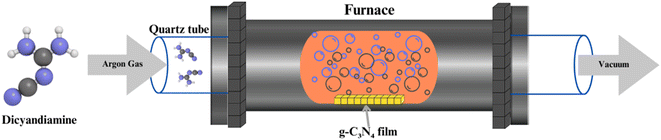
CVD represents an alternative approach to g-C3N4 synthesis, offering precise control over the material's thickness and morphology. In CVD, volatile precursors, such as cyanamide, are introduced into a high-temperature reactor, where they decompose and deposit as g-C3N4 on substrates (shown in Fig. 3). This method allows for the growth of thin films and nanostructures, enabling applications in photovoltaics and optoelectronics. However, CVD may require more specialized equipment and is often associated with higher production costs.57–602.3 Solvothermal and hydrothermal methods
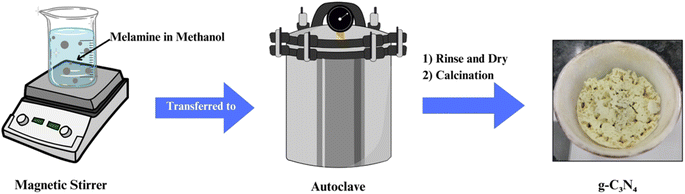
Solvothermal and hydrothermal routes involve the reaction of precursors in high-pressure, high-temperature aqueous or organic solvents (Fig. 4). These methods offer control over the morphology and structure of g-C3N4 by adjusting reaction conditions. Solvent selection plays a crucial role in influencing the final product's properties. Hydrothermal synthesis is particularly effective in producing hierarchical g-C3N4 structures with enhanced photocatalytic activity. These methods are advantageous for tailoring g-C3N4 for specific applications and have gained prominence in recent years.61–652.4 Template-assisted synthesis
Template-assisted synthesis involves the use of templates, such as mesoporous silica or carbonaceous materials, to guide the formation of g-C3N4 with specific structures (Fig. 5). By using templates with desired pore sizes and shapes, researchers can control the surface area and porosity of g-C3N4, which are critical factors affecting its photocatalytic performance. This approach enables the creation of g-C3N4 materials with finely tuned properties for applications like pollutant removal and solar energy conversion.66–702.5 Doping and co-doping strategies
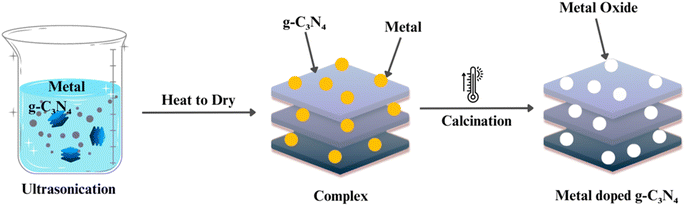
To further enhance the photocatalytic activity of g-C3N4, doping and co-doping with other elements, such as sulfur, boron, and metals, have been explored (as shown in Fig. 6). Doping introduces impurities into the g-C3N4 lattice, modifying its electronic structure and creating additional active sites for photocatalytic reactions. Co-doping involves the simultaneous incorporation of two or more elements to achieve synergistic effects. These strategies play a pivotal role in improving g-C3N4's efficiency and selectivity in various photocatalytic processes.71–75The synthesis of graphitic carbon nitride (g-C3N4) has advanced significantly, providing a diverse set of methods with distinct advantages and limitations. Thermal polymerization, involving low-cost precursors like melamine, offers simplicity and cost-effectiveness, resulting in a layered g-C3N4 structure suitable for various applications. Chemical vapor deposition (CVD) allows precise control over thickness and morphology but may entail higher production costs and specialized equipment. Solvothermal and hydrothermal methods, utilizing high-pressure and high-temperature conditions, provide morphology control and have gained popularity for tailoring g-C3N4 properties. Template-assisted synthesis uses templates to guide specific structures, influencing surface area and porosity critical for photocatalytic performance. Doping and co-doping strategies enhance photocatalytic activity by modifying the electronic structure.
Comparing these methods reveals trade-offs in terms of cost, complexity, and yield. Thermal polymerization is cost-effective and straightforward but might lack precision. CVD offers control but at higher costs. Solvothermal and hydrothermal methods provide control over morphology but involve specialized conditions. Template-assisted synthesis allows tailored structures but might be more intricate. Doping strategies enhance performance but add complexity. The choice depends on desired properties and applications, influencing cost-effectiveness and efficiency.24
In summary, the choice of synthesis methods for g-C3N4 is influenced by the desired properties and intended applications. Researchers and engineers can select from these diverse synthesis techniques to tailor g-C3N4 materials that meet the specific demands of sustainable environmental applications, from water purification to renewable energy production.
3. Structural properties
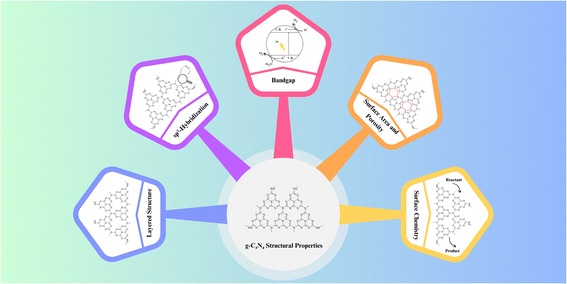
Graphitic carbon nitride (g-C3N4) exhibits a unique two-dimensional structure composed of carbon and nitrogen atoms, which imparts distinctive structural properties crucial for its photocatalytic performance.76 Understanding these properties is essential for tailoring g-C3N4 materials to specific applications and optimizing their efficiency. The key structural attributes are provided in Fig. 7.3.1 Layered structure
At its core, g-C3N4 consists of stacked layers of carbon and nitrogen atoms arranged in a planar, hexagonal lattice. This layered structure resembles that of graphite, giving rise to its name, “graphitic”. Each layer is composed of tri-s-triazine (C3N3) units, and the layers are held together by weak van der Waals forces. This layered configuration provides a large surface area for potential reactant adsorption and photocatalytic reactions, making g-C3N4 an attractive material for various applications.363.2 sp2-hybridization
The carbon atoms within the g-C3N4 lattice adopt sp2-hybridization, resulting in trigonal planar geometry. This sp2-hybridized carbon configuration is responsible for the formation of delocalized π-bonds, contributing to the material's excellent electrical conductivity and optical properties. This electron-rich network facilitates charge carrier mobility and separation, which are essential for efficient photocatalysis.343.3 Bandgap
The electronic band structure of g-C3N4 plays a pivotal role in its photocatalytic activity. It exhibits a moderate bandgap typically around 2.7 to 2.8 eV, making it responsive to visible light. Photons with energy equal to or greater than the bandgap can excite electrons from the valence band to the conduction band, initiating the photocatalytic process. The bandgap value allows g-C3N4 to harness a substantial portion of the solar spectrum, rendering it effective for solar-driven applications.313.4 Surface area and porosity
The layer-by-layer structure of g-C3N4 results in a high surface area, providing ample sites for reactant adsorption and subsequent photocatalytic reactions. The interlayer spacing between g-C3N4 layers can be tuned to create mesopores and micropores, further enhancing its surface area and porosity. These structural features facilitate efficient mass transport and reactant accessibility, promoting photocatalytic efficiency.73.5 Surface chemistry
The surface of g-C3N4 can be modified through functionalization, which introduces various functional groups such as amino, hydroxyl, and carboxyl groups. These modifications can influence the material's surface charge, hydrophilicity, and chemical reactivity, thus tailoring its suitability for specific photocatalytic applications. Surface functionalization also enables the attachment of co-catalysts, enhancing charge separation and overall photocatalytic performance.31Moving to structural properties, g-C3N4's layered structure, sp2-hybridization, moderate bandgap, high surface area, and porosity are key features. The layered structure provides a large surface area for reactions, while sp2-hybridization contributes to electrical conductivity. The moderate bandgap (2.7–2.8 eV) enables responsiveness to visible light, crucial for solar-driven applications. High surface area and porosity, achieved through the layer-by-layer structure and tuned interlayer spacing, facilitate efficient mass transport and reactant accessibility. Surface chemistry, modified through functionalization, further tailors properties for specific applications.24 Understanding these structural properties allows researchers to design and engineer g-C3N4 materials with optimized characteristics for diverse photocatalytic applications. By tailoring the layer spacing, bandgap, and surface chemistry, g-C3N4 can be fine-tuned to address specific environmental and energy challenges, contributing to a sustainable and cleaner future.
4. Photocatalytic mechanism
The remarkable photocatalytic activity of graphitic carbon nitride (g-C3N4) stems from its unique structural and electronic properties, which enable it to initiate and accelerate photochemical reactions under illumination. Understanding the underlying photocatalytic mechanisms is pivotal for harnessing the full potential of g-C3N4 in sustainable environmental and energy applications. The primary steps involved in the photocatalytic mechanism of g-C3N4 are depicted in Scheme 1.77–79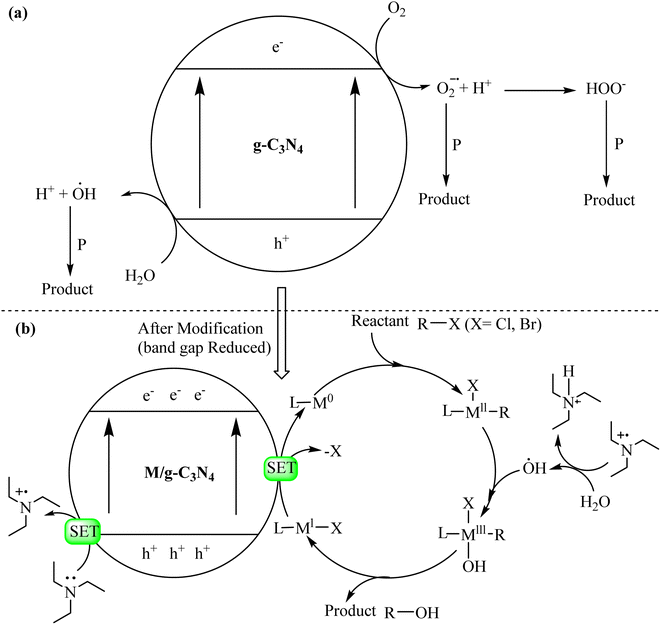 | ||
| Scheme 1 General mechanisms of (a) g-C3N4 catalyzed reactions and (b) metal/g-C3N4-catalyzed visible light driven reactions. | ||
The modification of g-C3N4 is important for photocatalytic applications. The interface formed can reduce the recombination of photoinduced charge and improve the absorptivity of visible light. Meanwhile, the high redox ability of electrons and holes is maintained. Therefore, design and synthesis of different types of g-C3N4-based heterojunction photocatalysts has important research value for achieving efficient photodegradation of pollutants and improving the increasingly serious environmental pollution. In recent years, photocatalysts for type II-heterojunctions, g-C3N4-based p–n heterojunctions and Z-scheme heterojunctions have been developed rapidly and have shown great potential advantages in various photocatalytic systems. By constructing different types of heterostructures, the photocatalytic performance of g-C3N4 has been greatly improved.80
4.1 Light absorption and electron–hole generation
When g-C3N4 is exposed to photons with energy equal to or greater than its bandgap (typically around 2.7 to 2.8 eV), electrons in the valence band are excited to the conduction band, creating electron–hole pairs. This initial step is the foundation of photocatalysis, as it generates highly reactive species that can drive subsequent reactions.4.2 Charge separation
Once electron–hole pairs are generated, the electrons and holes become mobile within the g-C3N4 lattice. Efficient charge separation is crucial to prevent recombination of electrons and holes, which would diminish photocatalytic efficiency. The unique layered structure of g-C3N4, with its sp2-hybridized carbon atoms, facilitates rapid charge separation, allowing electrons to move freely through the lattice.4.3 Redox reactions
The separated electrons and holes are poised to participate in redox reactions on the g-C3N4 surface. Electrons in the conduction band are capable of reducing various species, while the holes in the valence band can oxidize substances. For instance, in the context of water purification, electrons can reduce water molecules to form hydroxyl radicals (˙OH), which are highly reactive and effective at degrading organic pollutants.4.4 Reaction with adsorbates
Adsorption of target molecules or pollutants onto the g-C3N4 surface is a critical prerequisite for their degradation. The large surface area and active sites provided by g-C3N4 facilitate the adsorption of organic molecules, pollutants, or gases. Once adsorbed, these species can directly interact with the photogenerated electrons or holes, initiating degradation or transformation reactions.4.5 Oxygen activation (for oxygen-dependent reactions)
In reactions involving oxygen, g-C3N4 can also activate molecular oxygen (O2) to form reactive oxygen species (ROS) like superoxide radicals (˙O2−) and singlet oxygen (1O2). These ROS further enhance the photocatalytic activity by participating in oxidation reactions and promoting the breakdown of organic pollutants.4.6 Surface states and co-catalysts
Surface states on g-C3N4, as well as co-catalysts like metal nanoparticles or semiconductors, can further enhance charge separation and facilitate specific reaction pathways. Co-catalysts, for example, can serve as electron sinks or sources, improving overall photocatalytic performance.The specific photocatalytic mechanisms can vary depending on the target reaction and the environment in which g-C3N4 is employed. Whether it's water splitting, pollutant degradation, or carbon dioxide reduction, g-C3N4's ability to generate and effectively utilize electron–hole pairs makes it a versatile and efficient photocatalyst for a wide range of sustainable applications. By elucidating these fundamental photocatalytic mechanisms, researchers can design and optimize g-C3N4-based photocatalysts to achieve higher efficiency, selectivity, and stability, ultimately contributing to the advancement of sustainable technologies and environmental preservation.81–83
5. Applications
Graphitic carbon nitride (g-C3N4) has emerged as a versatile and eco-friendly photocatalyst with a remarkable ability to address pressing environmental and energy challenges. Its wide range of applications spans various domains, each contributing to the advancement of sustainability. Here, we delve into the diverse and transformative applications of g-C3N4, elucidating its role in:5.1 Various organic transformations
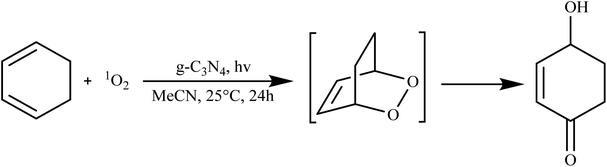
g-C3N4 has showcased its prowess in catalyzing various organic transformations, including the synthesis of organic chemicals, pharmaceuticals, and fine chemicals. Its unique surface chemistry and catalytic activity enable selective reactions, reducing the need for harsh reaction conditions and hazardous reagents. For instance, g-C3N4 has been employed in the synthesis of diverse organic compounds, such as benzene derivatives, imines, and quinolines, showcasing its potential in green and sustainable chemistry.84In their study, Quadrelli et al. found that when g-C3N4 is oxidized, it exhibits excellent catalytic capabilities for generating singlet oxygen (1O2) under photochemical conditions. This particular form of graphitic carbon nitride, produced through the bulk and hard template pyrolysis of melamine, is considered a highly promising material for fostering environmentally-friendly chemical reactions. The oxidized g-C3N4 catalyst, referred to as catalyst, demonstrated a range of performance levels, from satisfactory to outstanding, in the Diels–Alder cycloaddition reaction with in situ generated 1O2, particularly in the case of 1,3-cyclohexadiene and with alkenes during ene reactions. Additionally, it displayed notable oxidative capabilities when interacting with aromatic olefins, as illustrated in Scheme 2.85
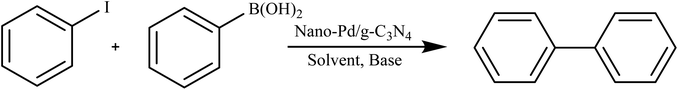
Sarkar et al. conducted a study on the utilization of a nano-Pd/gC3N4 composite as a catalyst for the microwave-assisted Suzuki cross-coupling of various aryl halides with different arylboronic acids. The reactions took place in an aqueous medium under aerobic conditions. The catalyst proved to be exceptionally efficient, with impressive turnover frequencies (TOFs) of approximately 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per hour across a range of substituted reactants. This work exemplifies an innovative and environmentally friendly approach to catalysis, combining the advantages of reusability, water as a solvent, and microwave irradiation as an alternative heating method, as depicted in Scheme 3.86
000 per hour across a range of substituted reactants. This work exemplifies an innovative and environmentally friendly approach to catalysis, combining the advantages of reusability, water as a solvent, and microwave irradiation as an alternative heating method, as depicted in Scheme 3.86
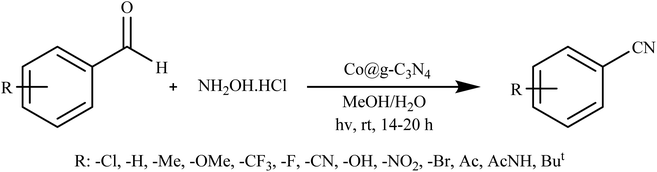
Rai et al. introduced an innovative and environmentally friendly process using visible light to efficiently convert aldehydes into nitriles. This method employs Co@g-C3N4 as a photocatalyst in an aqueous environment, operating under normal ambient conditions, as illustrated in Scheme 4. Key attributes of this designed photocatalyst include its selectivity, stability, and reusability across multiple reactions. Its heterogeneous nature, combined with its effectiveness under ambient conditions, makes it a highly advantageous alternative for potential application in various industries.87
Yao and colleagues introduced an inventive technique for producing oxygen-doped carbon nitride material (OCN) through a hydrothermal–calcination method. The OCN material exhibited outstanding performance as a stable photocatalyst, displaying excellent efficiency and recyclability. This catalyst facilitated the creation of 3-arylquinoxalin-2(1H)-ones by catalytically breaking down aryl diazonium salts under blue light exposure, all accomplished without the utilization of metals or external additives, as illustrated in Scheme 5. This approach not only provides a novel, environmentally friendly, and easily recyclable pathway but also signifies a sustainable synthetic strategy for generating these valuable structural entities.88
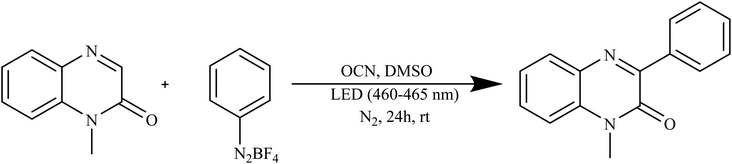 | ||
| Scheme 5 Oxygen doped carbon nitride used as a catalyst for the synthesis of 3-arylquinoxalin-2(1H)-ones. | ||
The case study illustrates various catalytic systems (Table 1) such as oxidized g-C3N4, nano-Pd/g-C3N4 composites, Co@g-C3N4, and oxygen-doped graphitic carbon nitride (OCN) which displayed remarkable performance in diverse organic transformation reactions. These innovative catalysts showcase assorted strengths, including high efficiency, recyclability, and environmental friendliness, providing a promising array of options for sustainable and eco-friendly chemical synthesis across various industries.
In the realm of organic transformations, g-C3N4 emerges as a formidable ally, ushering in a new era of sustainable chemistry. Its ability to facilitate selective reactions under mild conditions reduces the environmental footprint of chemical synthesis. From the creation of valuable pharmaceutical intermediates to the sustainable production of fine chemicals, g-C3N4 showcases its versatility and eco-friendliness. As the world seeks greener and more sustainable pathways in the realm of chemical synthesis, g-C3N4 stands as a beacon of hope, embodying the promise of harnessing solar-driven catalysis to meet the demands of a rapidly changing world, where sustainability and efficiency are paramount.
5.2 Hydrogen production through water splitting
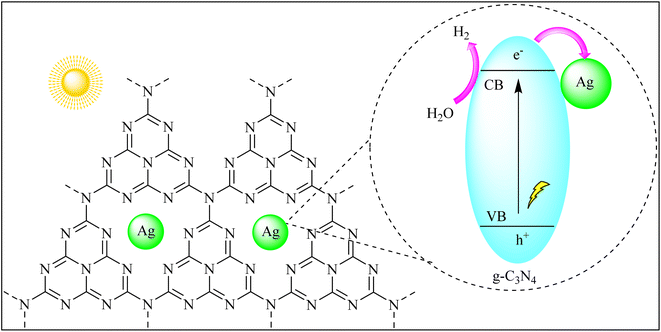
The efficient utilization of solar energy to split water into hydrogen and oxygen holds the key to clean and renewable hydrogen production. g-C3N4's bandgap aligns well with the solar spectrum, allowing it to harness sunlight for photocatalytic water splitting. By absorbing photons and generating electron–hole pairs, g-C3N4 initiates the water splitting reaction, producing clean hydrogen fuel, a promising step toward a hydrogen-based economy.Han et al. created g-C3N4 composites with silver (Ag) using two methods: precipitation–calcination (DeCNexAg) and annealing (ZeCNexAg). These Ag-based composites exhibited enhanced photocatalytic activity under visible light. The best hydrogen production was achieved with DeCNexAg containing 5% Ag2CO3, producing 4.6 times more hydrogen than ZeCNe5% Ag. This enhanced performance was attributed to the presence of metallic Ag and additional active sites on the DeCNe5% Ag surface, aiding in charge separation and transfer. The study also highlighted the role of the local surface plasmon resonance (LSPR) effect and the close interaction between Ag and g-C3N4 in improving hydrogen production through water splitting as shown in Fig. 8.89
Samaniego-Benitez et al. created a g-C3N4/NiS hybrid photocatalyst through a combination of thermal decomposition and hydrothermal techniques. This hybrid material exhibited a remarkable hydrogen production yield of 1230 mmol H2/h, which was significantly higher than that achieved by g-C3N4 and TiO2 photocatalysts. The kinetic constant for this hybrid material was measured at 307.1 h−1. This enhanced photocatalytic activity can be attributed to the reduced rate of electron–hole recombination, which results from the mixed phases found in the hybrid structure of the photocatalyst, as depicted in Fig. 9(a).90
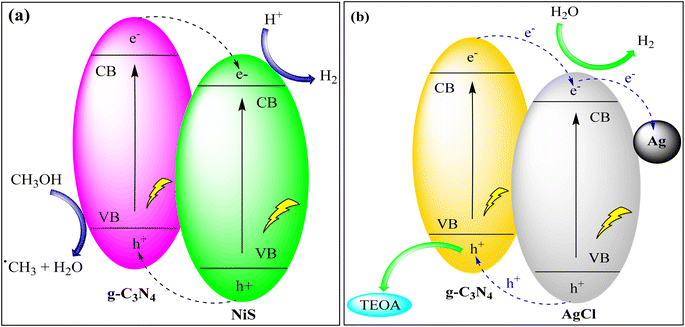 | ||
| Fig. 9 (a) Hydrogen production via g-C3N4/NiS photocatalyst under UV light irradiation and (b) photocatalytic hydrogen production via the Ag@g-C3N4/AgCl heterostructure. | ||
Li et al. used g-C3N4 and Ag/AgBr NPs as the carrier and co-catalyst to create g-C3N4/Ag/AgBr heterojunctions through solvothermal technology. They analyzed the samples' characteristics and studied their hydrogen evolution catalytic activity through water splitting. The catalyst exhibited the best performance with low overvoltage, a shallow Tafel slope, and high catalytic activity, even without a Pt co-catalyst. This heterojunction photocatalyst demonstrated excellent photostability and recyclability. The addition of Ag/AgBr NPs to two-dimensional g-C3N4 nanosheets enhanced their photo-response and photocatalytic activity as depicted in Fig. 9(b).91
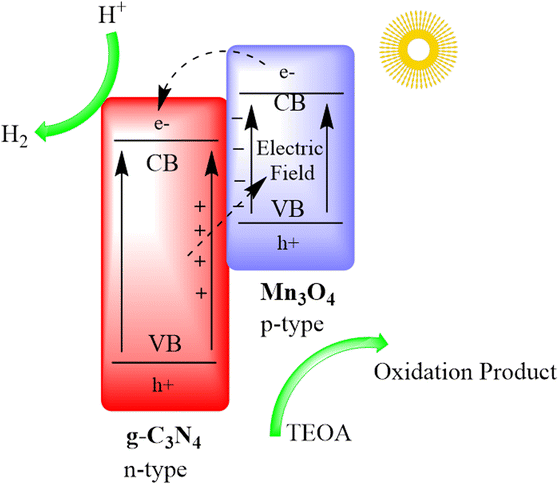
Zhu and co-authors devised a distinctive Mn3O4/g-C3N4 p–n heterojunction through an in situ growth method. This heterojunction notably enhances the light absorption capacity of g-C3N4. The resulting photocatalyst exhibits remarkable efficiency in the hydrogen evolution reaction (HER), achieving a rate of approximately 2700 μmol g−1 h−1 for wavelengths beyond 420 nm and maintaining stability over 15 hours of continuous H2 production. The Mn3O4/g-C3N4 system also displays elevated external quantum efficiencies (EQEs) at various wavelengths. Additionally, it adeptly catalyzes both H2 and O2 evolution under simulated sunlight and accomplishes overall water splitting to generate H2 and O2 products in a stoichiometric molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This research introduces a straightforward method for crafting high-performance g-C3N4-based heterostructure materials for efficient photocatalytic overall water splitting, as depicted in Fig. 10.92
1. This research introduces a straightforward method for crafting high-performance g-C3N4-based heterostructure materials for efficient photocatalytic overall water splitting, as depicted in Fig. 10.92
The case study highlights diverse approaches to enhancing photocatalytic hydrogen production using g-C3N4 composites (Table 2). While Han et al.'s Ag-based composites leverage metallic Ag and active sites for improved charge separation, Samaniego-Benitez et al.'s g-C3N4/NiS hybrid focuses on minimizing electron–hole recombination through mixed phases. Li et al.'s g-C3N4/Ag/AgBr heterojunction demonstrates the effectiveness of incorporating co-catalysts for superior catalytic activity and stability, while Zhu et al.'s Mn3O4/g-C3N4 p–n heterojunction introduces a unique strategy with high efficiency and overall water splitting capabilities under simulated sunlight. These studies collectively underscore the versatility and innovation in tailoring g-C3N4-based photocatalysts for enhanced hydrogen evolution.
| Entry | Catalyst | Conditions | H2 productivity/μmol g−1 h−1 | Reference material H2 productivity/μmol g−1 h−1 | Enhancement relative to conventional g-C3N4 | Ref. |
|---|---|---|---|---|---|---|
| 1 | Ag/g-C3N4 | 300 W Xe lamp (λ > 420 nm) | 153.33 | 33.49 | 4.6 | 89 |
| 2 | NiS/g-C3N4 | UV pen ray Hg lamp (254 nm, 4.4 mW cm−2) | 307.5 | 277.5 | 1.10 | 90 |
| 3 | g-C3N4-Ag/AgBr | 300 W Xe lamp | 47.84 | NA | NA | 91 |
| 4 | Mn3O4/g-C3N4 | 300 W Xe lamp (λ > 420 nm) | 2700 | NA | NA | 92 |
In the quest for clean and renewable energy sources, g-C3N4 emerges as a key player in the vital endeavor of hydrogen production through water splitting. Its unique ability to harvest solar energy and catalyze the conversion of water into hydrogen represents a sustainable solution with far-reaching implications. As we look toward a future fueled by clean energy, g-C3N4 offers a promising pathway, contributing not only to the mitigation of climate change but also to the establishment of a hydrogen-based economy. With the absorption of each photon and splitting of each water molecule, g-C3N4 takes us one step closer to a world powered by abundant, eco-friendly hydrogen fuel, ushering in a new era of energy sustainability.
5.3 Water purification and treatment
One of the most critical applications of g-C3N4 is in water purification and treatment. Its photocatalytic prowess enables the degradation of organic pollutants and the removal of toxic heavy metals from contaminated water sources. Specific examples include the degradation of dyes, pharmaceuticals, and pesticides, as well as the removal of heavy metals like chromium and lead. By providing a sustainable and efficient means of water remediation, g-C3N4 contributes to safer and cleaner water resources.93Ding et al. successfully synthesized a nanocomposite of magnetic graphene oxide (mGO) and graphitic carbon nitride (g-C3N4), denoted as mGCN, for the photoreduction of U(VI) in wastewater using visible LED light irradiation. mGCN exhibited high photocatalytic activity, reusability, and selectivity for U(VI) reduction. The photoreduction process achieved a remarkable U(VI) extraction capacity of 2880.6 mg g−1. Mechanistic studies revealed that U(VI) was converted to metastudtite during the photoreduction process. This research contributes to the advancement of photocatalytic methods for addressing uranium wastewater treatment, as illustrated in Fig. 11.94
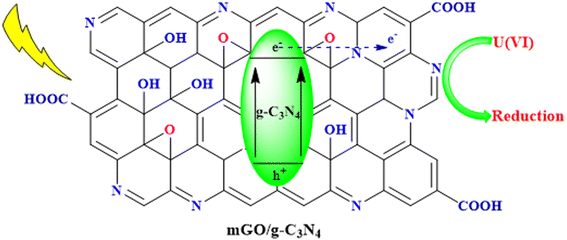 | ||
| Fig. 11 Photocatalytic reduction of U(VI) in wastewater using the mGO/g-C3N4 nanocomposite under visible LED light irradiation. | ||
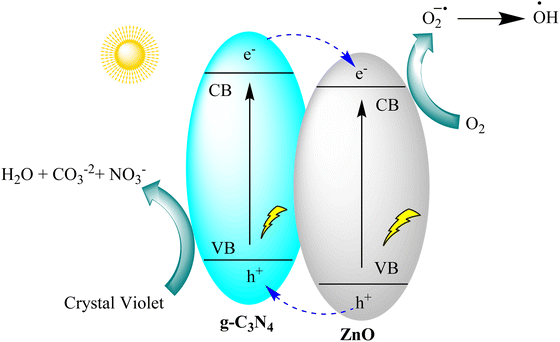
Gnana Prakash et al. developed g-C3N4 and g-C3N4/ZnO nanocomposite photocatalysts for crystal violet degradation using melamine pyrolysis and hydrothermal methods. The nanocomposite showed superior photocatalytic efficiency (97%) compared to pristine ZnO and g-C3N4, with a 1.4 times higher rate constant. It exhibited excellent stability through multiple recycling tests. Hole (h+) species were found to be the primary drivers of crystal violet degradation. The enhanced performance of the g-C3N4/ZnO nanocomposite is attributed to improved visible light absorption and the formation of a heterojunction between g-C3N4 and ZnO, promoting efficient charge carrier separation, as depicted in Fig. 12.95
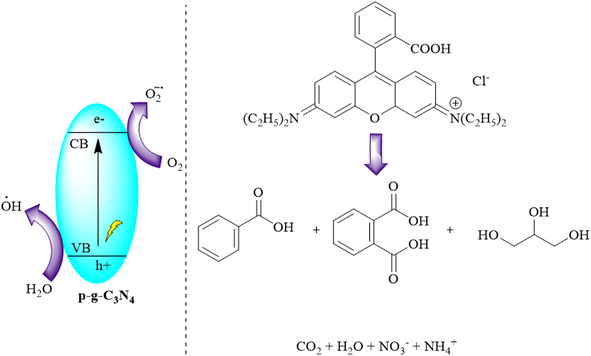
Kumar and colleagues synthesized graphitic carbon nitride (g-C3N4) by reducing melamine using a pyrolysis method. They then improved its characteristics to produce porous g-C3N4 (p-g-C3N4) through chemical protonation. The photocatalytic performances of both materials were assessed by decomposing organic dyes under sunlight. The protonation process induced a blue-shift in the optical absorption edge of g-C3N4. The study demonstrated that g-C3N4 exhibited superior photocatalytic efficiency compared to its pristine counterpart. p-g-C3N4 showed significantly enhanced degradation efficiency for various dyes under sunlight, attributed to an increased surface area that exposed more active sites for dye degradation, as depicted in Fig. 13.96
Comparing the three studies (Table 3), Ding et al.'s mGCN nanocomposite stands out for its effective photoreduction of U(VI) in wastewater, showcasing high activity, reusability, and selectivity with a remarkable extraction capacity. Gnana Prakash et al.'s g-C3N4/ZnO nanocomposite demonstrates superior photocatalytic efficiency in crystal violet degradation, attributed to enhanced visible light absorption and efficient charge carrier separation in the heterojunction structure. Kumar et al.'s work focuses on porous g-C3N4, highlighting its superior photocatalytic efficiency for dye degradation under sunlight due to increased surface area and exposure of active sites. These studies collectively underscore the versatility of g-C3N4-based photocatalysts for diverse applications in environmental remediation.
| Entry | Catalyst | Organic dye | Conditions | Removal efficiency (%) | Reference material efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| 1 | mGO/g-C3N4 | NA | 5 mg catalyst, methanol, 30 mL 20 mg L−1 U(VI) solution, 8 W LED lamp | 96 | NA | 94 |
| 2 | g-C3N4/ZnO | Crystal violet | 25 mg of catalyst and 25 mL of 20 ppm crystal violet solution, solar irradiation | 97 | 88 | 95 |
| 3 | p-g-C3N4 | Crystal violet | 25 mg catalyst, 25 mL (20 mg L−1) of an aqueous solution, sunlight irradiation | 98 | 92 | 96 |
| Methylene blue | 99 | 83 | ||||
| Rhodamine-B | 93 | 32 |
In the realm of water purification and treatment, g-C3N4 stands as a beacon of hope, offering a sustainable and efficient solution to the pressing challenges of water pollution. Its ability to harness the power of sunlight to degrade organic pollutants and remove toxic heavy metals from contaminated water sources represents a transformative approach to safeguarding one of our most precious resources. As we confront the ever-growing concerns of water quality and scarcity, g-C3N4 paves the way for cleaner and safer water supplies. With each photocatalytic reaction, it exemplifies the potential for science and innovation to address the critical issue of water pollution, ensuring that future generations will inherit a world with access to clean and potable water. In the intersection of environmental stewardship and technological advancement, g-C3N4's role in water purification and treatment is not merely a solution; it is a promise of a more sustainable and water-secure future.
5.4 Air purification and pollutant removal
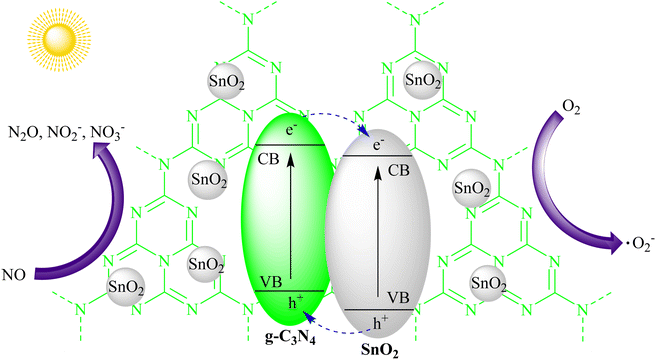
The degradation of volatile organic compounds (VOCs) and the removal of noxious gases from the atmosphere are essential for improving air quality. g-C3N4's photocatalytic activity extends to air purification applications, where it effectively degrades VOCs like formaldehyde, toluene, and xylene. Additionally, it can facilitate the conversion of harmful gases like nitrogen oxides (NOx) into less harmful compounds. The utilization of g-C3N4 in air purification technologies helps combat air pollution and promote healthier living environments.Zhou and their team successfully incorporated SnO2 quantum dots into graphitic carbon nitride (g-C3N4) via a simple synthesis method. This led to the development of photocatalysts that generated minimal NO2 and achieved a 32% NO removal rate when exposed to visible light for 30 minutes. The attachment of SnO2 quantum dots not only improved the photocatalysts' ability to reduce NO2 but also enhanced the transfer of photogenerated carriers, improving NO removal. This study provided an accessible way to combine a wide bandgap semiconductor (SnO2) with strong photo-oxidation capabilities and layered g-C3N4, which responds well to visible light for NO removal. Future research is needed to eliminate NO2 byproducts in the photocatalytic NO removal process, as shown in Fig. 14.97
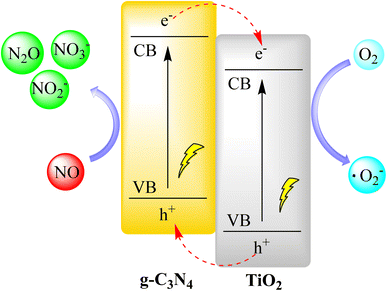
Huang et al. created a g-C3N4/TiO2 composite coating for removing NOx in ambient air. They applied it directly to roads using TiO2 hydrosol, eliminating the need for dispersants or binders. The coating had strong adhesion, easy regeneration, and light-induced superhydrophilicity, making it suitable for long-term use. It achieved daily NO and NOx reductions of 59.0% and 27.8%, respectively, and higher rates during morning and afternoon hours with weak sunlight. This was due to the coating's broad visible light absorption, critical for pollution reduction. Outdoor results showed that NOx removal depended on solar irradiation and pollutant concentration, with the latter being more important due to reaction kinetics. In conclusion, the g-C3N4/TiO2 composite and spraying method offer a simple and effective way to create photocatalytic pavement for air purification as shown in Fig. 15.98,99
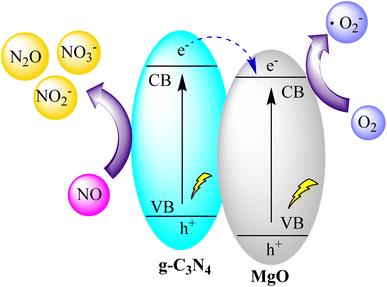
You and collaborators synthesized MgO@g-C3N4 heterojunctions through a one-step pyrolysis process involving commercial MgO and urea, leading to a significant enhancement in photocatalytic efficiency. The 3% MgO@g-C3N4 sample exhibited the highest efficiency in NO photodegradation at 75.4%. However, beyond 3% MgO, the efficiency experienced a decline. The heterojunction structure, resulting from the combination of MgO and g-C3N4, played a crucial role in improving appearance quantum efficiency and prolonging electron recombination, ultimately contributing to superior NO photodegradation. The composite also demonstrated favorable conversion of NO to NO2 and other by-products. Notably, the 3% MgO@g-C3N4 maintained high reusability after five cycles, with only a modest 7.1% decrease in efficiency. Characterization techniques confirmed the presence of MgO and its impact on optical properties, suggesting scalability for future applications. Overall, the study provides valuable insights into optimizing MgO@g-C3N4 photocatalysis, as illustrated in Fig. 16.100
Comparing the three studies (Table 4), Zhou et al.'s incorporation of SnO2 quantum dots into g-C3N4 showcases a simple method for developing photocatalysts with a 32% NO removal rate under visible light. This approach effectively combines the strong photo-oxidation capabilities of SnO2 with the layered structure of g-C3N4, demonstrating potential for NO reduction. Huang et al.'s g-C3N4/TiO2 composite coating directly applied to roads offers a practical and efficient solution for NOx removal from ambient air, achieving daily reductions and demonstrating adaptability to varying sunlight conditions. You and collaborators' synthesis of MgO@g-C3N4 heterojunctions provides valuable insights into optimizing photocatalysis for NO degradation, emphasizing the importance of the composite structure for enhanced efficiency and reusability. These studies collectively contribute to the development of effective and accessible photocatalytic methods for NO and NOx removal in different environmental contexts.
| Entry | Catalyst | Conditions | NO removal efficiency (%) | Reference material efficiency (%) | Ref. |
|---|---|---|---|---|---|
| 1 | SnO2 quantum dots on g-C3N4 | 0.4 g catalyst, NO concentration 600 ppb, 150 W tungsten halogen lamp (λ > 420 nm) | 32 | 19 | 97 |
| 2 | g-C3N4/TiO2 | 5 L Teflon air bag (232-05, Tedlar®) for 45 min with a height of 1.0–1.5 m from the ground by using a sampling pump with a flow rate of 0.1 L min−1 | 38.9 | 23.5 (TiO2) | 98 |
| 3 | MgO@g-C3N4 | 0.2 g catalyst, NO concentration 500 ppb, 10 mL water, 80 °C, 300 W xenon lamp | 75.4 | 62.8 | 100 |
In the realm of air purification and the removal of noxious pollutants, g-C3N4 emerges as a powerful agent of change, working tirelessly to enhance the quality of the air we breathe. Its remarkable photocatalytic abilities, adept at degrading volatile organic compounds (VOCs) and transforming harmful gases, have the potential to combat air pollution and improve the health of our environments. As the world grapples with the consequences of urbanization and industrialization, g-C3N4 offers a glimmer of hope for cleaner and healthier air. With each chemical bond broken and each pollutant neutralized, it embodies the promise of harnessing sustainable technologies to restore the purity of our atmosphere. In the ongoing battle against air pollution, g-C3N4's role is not merely that of a catalyst; it is a symbol of our collective commitment to cleaner, more breathable air, ensuring a future where all can thrive in an environment free from harmful pollutants.
5.5 Solar-driven carbon dioxide reduction
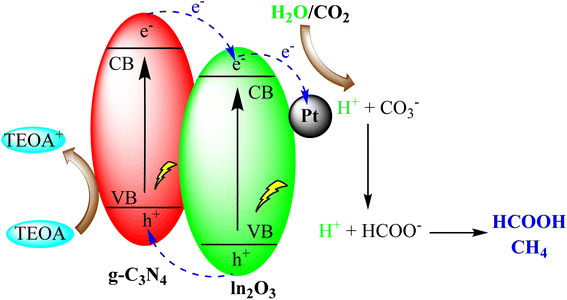
Addressing the challenge of increasing carbon dioxide (CO2) levels in the atmosphere necessitates innovative approaches for its conversion into valuable products. g-C3N4 has demonstrated promise in the solar-driven reduction of CO2 into hydrocarbons and other valuable chemicals. This application holds immense potential for both mitigating climate change and producing sustainable feedstocks for various industries.Li et al. created a versatile Pt/In2O3/g-C3N4 catalyst using precise assembly techniques. This catalyst efficiently produced formic acid (HCOOH) during visible-light-driven CO2 reduction by synergistically promoting hydrogen generation, CO2 activation, and minimizing electron–hole recombination. It also had a long operational life due to stability and prevented Pt leaching. This work offers a promising method to design multifunctional catalysts for high-yield, room-temperature, and normal-pressure photocatalytic CO2 reduction as depicted in Fig. 17.101
Khan et al. used a CuO/g-C3N4 photocatalyst to produce methanol via photoelectrochemical reduction of CO2. The CuO/g-C3N4 photocathode outperformed others, showing a tenfold increase in the photocurrent response. It had high incident photon-to-current efficiency and significantly increased methanol production. The photocathode also had improved faradaic and quantum efficiency compared to other configurations. The study discussed the CO2-to-methanol conversion mechanism in detail, as shown in Fig. 18.102
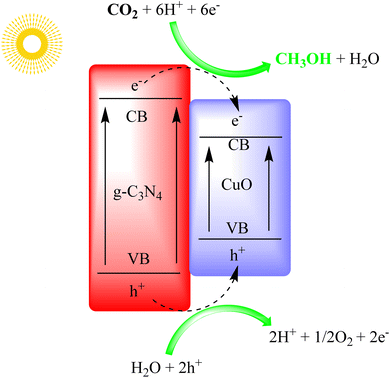 | ||
| Fig. 18 g-C3N4/CuO heterostructure for photo electrocatalytic CO2 reduction under visible light irradiation. | ||
Yu et al. successfully created a Z-scheme binary composite photocatalytic system by combining g-C3N4 and ZnO through a simple calcination process, using affordable precursors, urea, and zinc nitrate hexahydrate. This composite exhibited improved light absorption in the visible spectrum compared to pure g-C3N4, thanks to the introduction of defect states within the g-C3N4 due to the interference of ZnO crystallization. Notably, the g-C3N4/ZnO photocatalyst displayed a 2.3-fold increase in its ability to catalyze the conversion of CO2 into CH3OH while maintaining selectivity, as shown in Fig. 19. This indicates the promising potential of this composite for efficient CO2 reduction applications.103
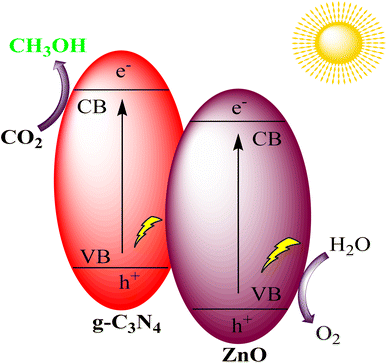 | ||
| Fig. 19 g-C3N4/ZnO heterostructure for photocatalytic CO2 reduction under visible light irradiation. | ||
Pant and co-authors presented a straightforward approach for crafting a conjugated organic–inorganic hybrid photocatalyst (TiO2/g-C3N4) through sol–gel synthesis followed by conventional pyrolysis. The heterostructure formed by TiO2 and g-C3N4 efficiently facilitated the separation of electrons and holes, as evidenced by their respective band edge positions. This TiO2 and g-C3N4 heterostructure exhibited a type II heterojunction, renowned for promoting enhanced charge separation and mitigating charge recombination. The augmented charge separation contributed to an increased photoreduction of CO2, yielding valuable methanol as the photo-reduced product. The composite catalysts demonstrated a higher methanol yield (31.7 μmol g−1) compared to the individual components. The study underscores the crucial role of charge separation in a straightforward heterostructure catalyst, leading to heightened product formation through CO2 reduction under a visible light source, as depicted in Fig. 20.104
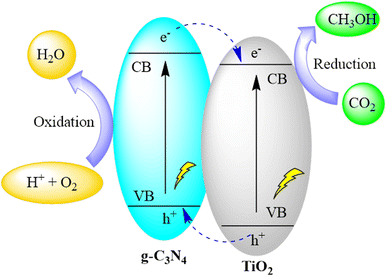 | ||
| Fig. 20 g-C3N4/TiO2 heterostructure for photocatalytic CO2 reduction under visible light irradiation. | ||
Li et al.'s versatile Pt/In2O3/g-C3N4 catalyst stands out for its ability to efficiently produce formic acid during visible-light-driven CO2 reduction. The catalyst's multifunctionality, promoting hydrogen generation, CO2 activation, and minimizing electron–hole recombination, demonstrates promise for high-yield, room-temperature, and normal-pressure photocatalytic CO2 reduction. Khan et al.'s CuO/g-C3N4 photocathode excels in producing methanol via photoelectrochemical reduction of CO2, showcasing a tenfold increase in the photocurrent response and improved faradaic and quantum efficiency. Yu et al.'s Z-scheme g-C3N4/ZnO composite offers enhanced light absorption and a 2.3-fold increase in catalyzing CO2 conversion to CH3OH, highlighting its potential for efficient CO2 reduction. Pant and co-authors' TiO2/g-C3N4 heterostructure demonstrates the importance of charge separation, yielding higher methanol production compared to individual components. These studies collectively contribute valuable insights into designing efficient photocatalysts for diverse applications in CO2 reduction (Table 5).
| Entry | Catalyst | Conditions | Productivity | Reference material | Ref. |
|---|---|---|---|---|---|
| 1 | Pt/In2O3/g-C3N4 | 20 mg catalyst, 1 atm CO2, 10 mL H2O, 1 mL TEOA, 35 °C, 4 h, 3 W LED (420 nm) | HCOOH – 63.1, CH4 – 7.907 (μmol g−1 h−1) | NA | 101 |
| 2 | CuO/g-C3N4 | (Nafion/CuO/g-C3N4), NaHCO3 (0.1 M), bias potential illumination | 25.1 (μmol L−1 cm−2) | 5.92 (μmol L−1 cm−2) | 102 |
| 3 | ZnO/g-C3N4 | 100 mg sample in 10 mL deionized water, 80 °C, 0.12 g NaHCO3, 0.25 mL 4 M HCl, 300 W Xe arc lamp | 0.60 (μmol g−1 h−1) | 0.26 (μmol g−1 h−1) | 103 |
| 4 | TiO2/g-C3N4 | 0.2 g of catalyst, high purity CO2, 0.2 g of catalyst, 200 mL of 0.1 M NaOH, 250 W halogen lamp | 31.7 (μmol g−1) | 14.1 (μmol g−1) | 104 |
In the ambitious endeavor to combat climate change and harness solar energy, g-C3N4 emerges as a beacon of innovation and sustainability in the realm of solar-driven carbon dioxide reduction. Its capacity to convert the ubiquitous greenhouse gas, carbon dioxide, into valuable hydrocarbons and chemicals represents a profound stride towards a carbon-neutral future. As we confront the urgent need to reduce carbon emissions and transition to renewable energy sources, g-C3N4 embodies the promise of a cleaner, more sustainable world. With the absorption of each photon and transformation of each carbon dioxide molecule, it not only mitigates climate change but also offers a glimpse into the boundless possibilities of harnessing solar energy for a greener tomorrow. In the intersection of environmental preservation and renewable energy, g-C3N4's role in solar-driven carbon dioxide reduction is a testament to our commitment to a more sustainable, carbon-conscious, and brighter future.
5.6 Solar fuel cells
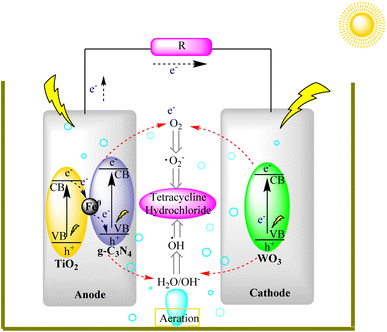
Solar fuel cells represent a vital component of the renewable energy landscape. g-C3N4's ability to generate hydrogen and other energy carriers through photocatalysis aligns with the development of solar fuel cells. These cells can store and utilize solar energy for power generation and transportation, reducing reliance on fossil fuels and minimizing greenhouse gas emissions.Liu et al. developed a visible-light-driven photocatalytic fuel cell (PFC) that efficiently degrades tetracycline hydrochloride (TC) and generates electricity in a single reactor. The PFC used paired stainless-steel mesh electrodes loaded with specific catalysts under different conditions, with and without visible light. With visible light, TC removal was highly effective (97.3% in 90 minutes), with a cell voltage of 0.98 V and 24 W m−2 power density. Simultaneous removal of chemical oxygen demand (COD) and total organic carbon (TOC) was notable, driven by the anodic g-C3N4/Fe0 (1%)/TiO2 component. Without light, performance dropped significantly, but the PFC continued to operate as a self-driven system, degrading pollutants. The PFC maintained its performance over multiple cycles with light, offering potential for wastewater treatment. In the dark, it still generated electricity but at reduced capacity. This research shows promise for efficient pollutant removal and energy recovery in wastewater treatment, as shown in Fig. 21.105,106
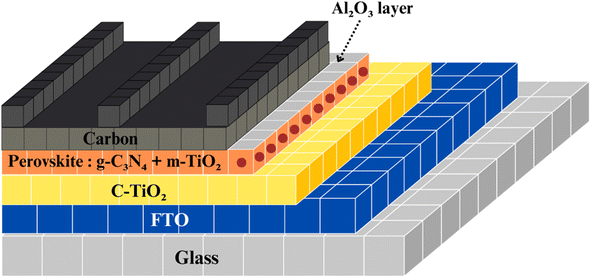
Zhang et al. significantly enhanced the performance of carbon-based perovskite solar cells without a hole-transporting material (HTM). They achieved a power conversion efficiency (PCE) above 14% by introducing g-C3N4 doping and an Al2O3 insulating layer, as depicted in Fig. 22. g-C3N4 improved the perovskite film quality, and with just 0.5 wt% g-C3N4 doping, the PCE increased to 12.85%. The Al2O3 insulating layer enhanced open-circuit voltage (Voc) and further increased PCE to 14.34% by reducing recombination at the ETM/perovskite interface. The resulting device demonstrated good stability, offering potential for cost-effective, high-performance perovskite solar cells without a HTM, which could have a significant impact on the market.107,108
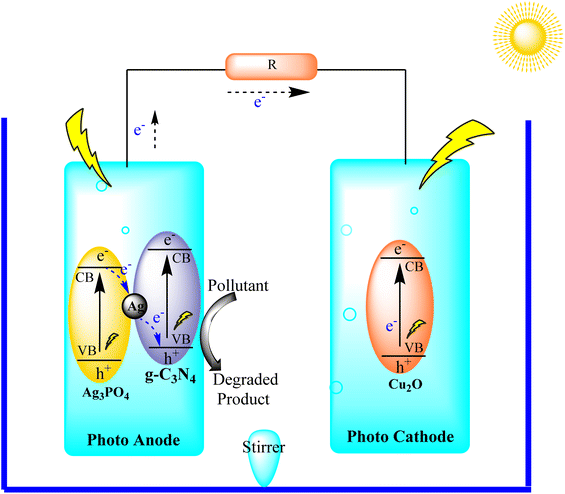
Wang and collaborators synthesized a Z-scheme photocatalyst, Ag3PO4@g-C3N4, which served as the photoanode in a photoelectro-Fenton (PFC) system alongside Cu2O as the photocathode, as illustrated in Fig. 23. The Z-scheme structure was deliberately designed to ensure effective separation of charge carriers, thereby enhancing the overall PFC performance. Furthermore, the catalyst exhibited a high redox ability, actively participating in the degradation of organic pollutants. The electricity production of the PFC system was observed to increase with N2 aeration, while O2 purging proved beneficial for the photocatalytic degradation of organic pollutants. The study highlights the efficacy of the Z-scheme photocatalyst in achieving efficient charge separation and contributing to the effective degradation of organic pollutants in a PFC system.109
Liu et al.'s visible-light-driven photocatalytic fuel cell (PFC) efficiently degrades tetracycline hydrochloride (TC) and generates electricity, demonstrating a promising approach for simultaneous wastewater treatment and energy recovery. The PFC, operating under visible light, achieves high TC removal, and COD, and TOC reduction, showcasing its potential for sustainable wastewater treatment with electricity generation. Zhang et al.'s work on carbon-based perovskite solar cells with g-C3N4 doping and an Al2O3 insulating layer enhances power conversion efficiency (PCE) to over 14%, offering a cost-effective and stable solution for high-performance solar cells without a hole-transporting material (HTM). Finally, Wang et al.'s Z-scheme Ag3PO4@g-C3N4 photocatalyst in a photoelectro-Fenton (PFC) system demonstrates efficient charge separation and active participation in organic pollutant degradation, emphasizing its potential for environmentally friendly energy production and wastewater treatment. These studies collectively showcase the versatile applications of g-C3N4 in advancing sustainable technologies (Table 6).
| Entry | System | Conditions | Current density | Fuels | Ref. |
|---|---|---|---|---|---|
| 1 | Ag3PO4@g-C3N4 | Photocatalytic fuel cell (50 mg of photocatalyst, 10 mg L−1 berberine chloride, 50 W Xe lamp) | 2.02 mA cm−2 | Berberine chloride | 109 |
| 2 | FTO/c-TiO2/m-TiO2/perovskite: g-C3N4/carbon | FTO/c-TiO2/m-TiO2/perovskite: g-C3N4/carbon device | (Perovskite) 21.5 mA cm−2 (0.5 wt% g-C3N4/perovskite) 24.0 mA cm−2 | NA | 107 |
| 3 | g-C3N4/FeO/TiO2 | 0.05 g of photocatalyst, 100 mL of TC (10 mg L−1), Na2SO4 (0.1 mol L−1), 300 W Xe lamp | 6.06 μW cm−2 | Rhodamine-B | 105 |
| 0.067 μW cm−2 | Methylene blue | ||||
| Tetracycline |
As we strive for a future powered by clean and renewable energy, g-C3N4 emerges as a pivotal player in the realm of solar fuel cells. Its remarkable ability to harness sunlight and catalyze the conversion of solar energy into clean fuels holds the key to reducing our reliance on fossil fuels and mitigating climate change. With each electron it liberates and each hydrogen molecule it generates, g-C3N4 not only powers our aspirations for a sustainable energy landscape but also illuminates the path toward a greener, more promising future. In the synergy of advanced technology and environmental responsibility, g-C3N4's role in solar fuel cells is a testament to our commitment to a world where clean energy sources drive our progress, ushering in an era of energy sustainability and environmental preservation.
Each of these applications of g-C3N4 as a photocatalyst embodies a sustainable approach to addressing environmental and energy challenges. Specific case studies and examples underscore the material's effectiveness in various contexts. Furthermore, the environmental benefits, such as reduced energy consumption and lower pollutant emissions, coupled with potential economic advantages, position g-C3N4 as a pivotal catalyst for a cleaner, more sustainable future.
6. Challenges and future prospecs
Graphitic carbon nitride (g-C3N4) undoubtedly exhibits exceptional photocatalytic capabilities, yet several challenges warrant attention as we aim to harness its full potential. Foremost among these challenges is the need to enhance its quantum efficiency—the efficiency with which it utilizes incoming photons. Researchers are actively working to minimize electron–hole pair recombination rates and boost the overall photocatalytic conversion efficiency. Additionally, ensuring photostability over extended usage periods is critical. Factors such as photo-corrosion and aggregation can impact g-C3N4's performance, necessitating the development of stable g-C3N4 materials and protective coatings. Achieving high selectivity in complex reaction mixtures remains a formidable challenge, even though g-C3N4 exhibits remarkable selectivity in many reactions. Fine-tuning the material's surface properties and optimizing reaction conditions continue to be areas of investigation. Finally, scaling up g-C3N4 production from laboratory-scale to practical, large-scale applications presents logistical challenges, requiring cost-effective manufacturing methods and seamless integration into the existing infrastructure.The future prospects for g-C3N4 photocatalysis are exceptionally promising, driven by ongoing research and innovative strategies. The integration of advanced co-catalysts, such as metal nanoparticles or semiconductor materials, holds the potential to significantly enhance charge separation and overall photocatalytic performance. The tailoring of g-C3N4 materials with specific structural and electronic properties optimized for various applications is another exciting avenue of exploration, allowing for custom-designed photocatalysts. These developments pave the way for tailored solutions to environmental and energy challenges. Moreover, the integration of g-C3N4 photocatalysis with solar cell technologies in tandem or integrated systems offers the possibility of continuous and efficient energy conversion. The exploration of g-C3N4's application in environmental remediation, such as addressing emerging pollutants like pharmaceuticals and microplastics in water and air, remains an area of interest. Additionally, the concept of artificial photosynthesis systems, where g-C3N4 plays a pivotal role in replicating natural photosynthesis for sustainable fuel production and carbon capture, holds immense promise. As the world continues to prioritize environmental sustainability and clean energy, g-C3N4 remains poised at the forefront of these advancements, representing a cornerstone in the journey toward a greener and more sustainable future.
7. Conclusion
In this comprehensive review, we have delved into the multifaceted world of graphitic carbon nitride (g-C3N4) as a promising photocatalyst, exploring its diverse synthesis methods, unique structural properties, intricate photocatalytic mechanisms, and transformative applications in sustainability. We've navigated through its pivotal role in various domains, from organic transformations and hydrogen production through water splitting to water purification, air purification, solar-driven carbon dioxide reduction, and solar fuel cells. Throughout our journey, we've highlighted the environmental and economic benefits of g-C3N4 in these applications. As we conclude, it's evident that g-C3N4's innovative potential holds the promise of a cleaner, more sustainable future, making it a beacon of hope in the intersection of science and environmental stewardship.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
One of the contributing authors (CKM) wishes to extend heartfelt gratitude to the fellow authors of this review article. Their unwavering enthusiasm, dedication, and relentless efforts were instrumental in turning this comprehensive review article into the final shape.References
- P. Li, J. A. Terrett and J. R. Zbieg, Visible-Light Photocatalysis as an Enabling Technology for Drug Discovery: A Paradigm Shift for Chemical Reactivity, ACS Med. Chem. Lett., 2020, 11, 2120–2130 CrossRef CAS PubMed.
- A. Xie, et al., Photocatalytic Technologies for Transformation and Degradation of Microplastics in the Environment: Current Achievements and Future Prospects, Catalysts, 2023, 13, 846 CrossRef CAS.
- A. Galushchinskiy, R. González-Gómez, K. McCarthy, P. Farràs and A. Savateev, Progress in Development of Photocatalytic Processes for Synthesis of Fuels and Organic Compounds under Outdoor Solar Light, Energy Fuel., 2022, 36, 4625–4639 CrossRef CAS PubMed.
- B. Ran, et al., Photocatalytic Antimicrobials: Principles, Design Strategies, and Applications, Chem. Rev., 2023, 123(22), 12371–12430 CrossRef CAS.
- L. Buglioni, F. Raymenants, A. Slattery, S. D. A. Zondag and T. Noël, Technological Innovations in Photochemistry for Organic Synthesis: Flow Chemistry, High-Throughput Experimentation, Scale-up, and Photoelectrochemistry, Chem. Rev., 2022, 122, 2752–2906 CrossRef CAS PubMed.
- J. Wen, J. Xie, X. Chen and X. Li, A review on g-C 3 N 4 -based photocatalysts, Appl. Surf. Sci., 2017, 391, 72–123 CrossRef CAS.
- M. Ismael and Y. Wu, A mini-review on the synthesis and structural modification of g-C3N4-based materials, and their applications in solar energy conversion and environmental remediation, Sustainable Energy Fuels, 2019, 3, 2907–2925 RSC.
- P. Wang, Q. Zhao, W. Xiao and J. Chen, Recent advances in visible-light photoredox-catalyzed nitrogen radical cyclization, Green Synth. Catal., 2020, 1, 42–51 CrossRef.
- P. P. Singh and V. Srivastava, Recent advances in visible-light graphitic carbon nitride (g-C3N4) photocatalysts for chemical transformations, RSC Adv., 2022, 12, 18245–18265 RSC.
- B. Tiwari and S. Ram, Biogenic Synthesis of Graphitic Carbon Nitride for Photocatalytic Degradation of Organic Dyes, ACS Omega, 2019, 4, 10263–10272 CrossRef CAS.
- X. Wang, et al., A metal-free polymeric photocatalyst for hydrogen production from water under visible light, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- M. A. Qamar, et al., Synthesis and applications of graphitic carbon nitride (g-C3N4) based membranes for wastewater treatment: a critical review, Heliyon, 2023, 9, e12685 CrossRef CAS.
- P. Lakhani and C. K. Modi, Asymmetric Hydrogenation using Covalently Immobilized Ru-BINOL-AP@MSNs Catalyst, New J. Chem., 2023, 47, 8767–8775 RSC.
- P. Lakhani and C. K. Modi, Spick-and-span protocol for designing of silica-supported enantioselective organocatalyst for the asymmetric aldol reaction, Mol. Catal., 2022, 525, 112359 CrossRef CAS.
- P. Lakhani, et al., DFT stimulation and experimental insights of chiral Cu(ii)-salen scaffold within the pocket of MWW-zeolite and its catalytic study, Phys. Chem. Chem. Phys., 2023, 25, 14374–14386 RSC.
- P. Lakhani, S. Kane, H. Srivastava, U. K. Goutam and C. K. Modi, Sustainable approach for the synthesis of chiral β-aminoketones using an encapsulated chiral Zn(ii)–salen complex, RSC Sustainability, 2023, 1, 1773–1782 RSC.
- V. Hasija, et al., Advanced activation of persulfate by polymeric g-C 3 N 4 based photocatalysts for environmental remediation: a review, J. Hazard. Mater., 2021, 413, 125324 CrossRef CAS PubMed.
- A. Kumar, et al., Frontier nanoarchitectonics of graphitic carbon nitride based plasmonic photocatalysts and photoelectrocatalysts for energy, environment and organic reactions, Mater. Chem. Front., 2023, 7, 1197–1247 RSC.
- G. Q. Zhao, J. Zou, J. Hu, X. Long and F. P. Jiao, A critical review on graphitic carbon nitride (g-C3N4)-based composites for environmental remediation, Sep. Purif. Technol., 2021, 279, 119769 CrossRef CAS.
- N. Kumar, et al., Graphitic carbon nitride (g–C3N4)–assisted materials for the detection and remediation of hazardous gases and VOCs, Environ. Res., 2023, 231, 116149 CrossRef CAS.
- Y. Ding, et al., Emerging heterostructured C3N4 photocatalysts for photocatalytic environmental pollutant elimination and sterilization, Inorg. Chem. Front., 2023, 10, 3756–3780 RSC.
- Y. Luo, et al., g-C3N4-based photocatalysts for organic pollutant removal: a critical review, Carbon Research, 2023, 2(14), 1–24 Search PubMed.
- A. Akhundi, A. Zaker Moshfegh, A. Habibi-Yangjeh and M. Sillanpää, Simultaneous Dual-Functional Photocatalysis by g-C3N4-Based Nanostructures, ACS ES&T Engg, 2022, 2, 564–585 Search PubMed.
- X. L. Song, L. Chen, L. J. Gao, J. T. Ren and Z. Y. Yuan, Engineering g-C3N4 based materials for advanced photocatalysis: recent advances, Green Energy Environ., 2022 DOI:10.1016/j.gee.2022.12.005.
- A. Alaghmandfard and K. Ghandi, A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing, Nanomaterials, 2022, 12, 294 CrossRef CAS.
- F. K. Butt, S. Ullah, J. Ahmad, S. U. Rehman and Z. Tariq, Graphitic Carbon Nitride/Metal Oxides Nanocomposites and Their Applications in Engineering, Compos. Mater. Appl. Eng. Biomed. Food Sci., 2020, pp. 231–265 Search PubMed.
- X. Liu, et al., Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants, Crit. Rev. Environ. Sci. Technol., 2021, 51, 751–790 CrossRef CAS.
- P. Lakhani and C. K. Modi, Shaping enantiochemistry: recent advances in enantioselective reactions via heterogeneous chiral catalysis, Mol. Catal., 2023, 548, 113429 CrossRef CAS.
- X. Yang, L. Zhao, S. Wang, J. Li and B. Chi, Recent progress of g-C3N4 applied in solar cells, J. Mater., 2021, 7, 728–741 Search PubMed.
- Y. Chen and X. Bai, A review on quantum dots modified g-C3N4-based photocatalysts with improved photocatalytic activity, Catalysts, 2020, 10, 142 CrossRef CAS.
- N. S. N. Hasnan, M. A. Mohamed and Z. A. Mohd Hir, Surface Physicochemistry Modification and Structural Nanoarchitectures of g-C3N4 for Wastewater Remediation and Solar Fuel Generation, Adv. Mater. Technol., 2022, 7, 2100993 CrossRef CAS.
- S. Dolai, S. K. Bhunia, P. Kluson, P. Stavarek and A. Pittermannova, Solvent-Assisted Synthesis of Supramolecular-Assembled Graphitic Carbon Nitride for Visible Light Induced Hydrogen Evolution – A Review, ChemCatChem, 2022, 14, e202101299 CrossRef CAS.
- J. Wang and S. Wang, A critical review on graphitic carbon nitride (g-C3N4)-based materials: preparation, modification and environmental application, Coord. Chem. Rev., 2022, 453, 214338 CrossRef CAS.
- A. Dandia, et al., Structure couture and appraisal of catalytic activity of carbon nitride (g-C3N4) based materials towards sustainability, Curr. Res. Green Sustainable Chem., 2020, 3, 100039 CrossRef.
- V. Srivastava, P. K. Singh and P. P. Singh, Recent advances of visible-light photocatalysis in the functionalization of organic compounds, J. Photochem. Photobiol., C, 2022, 50, 100488 CrossRef CAS.
- S. Kumar, S. Karthikeyan and A. F. Lee, g-C3N4-based nanomaterials for visible light-driven photocatalysis, Catalysts, 2018, 8, 74 CrossRef.
- A. Thomas, et al., Graphitic carbon nitride materials: variation of structure and morphology and their use as metal-free catalysts, J. Mater. Chem., 2008, 18, 4893–4908 RSC.
- J. Liu, et al., Metal-free efficient photocatalyst forstable visible water splitting via atwo-electron pathway, Science, 2015, 347, 970–974 CrossRef CAS PubMed.
- K. Maślana, R. J. Kaleńczuk, B. Zielińska and E. Mijowska, Synthesis and characterization of nitrogen-doped carbon nanotubes derived from g-C3N4, Materials, 2020, 13, 1349 CrossRef PubMed.
- L. Wang, et al., Graphitic carbon nitride-based photocatalytic materials: preparation strategy and application, ACS Sustain. Chem. Eng., 2020, 8, 16048–16085 CrossRef CAS.
- A. Hayat, et al., State of the art advancement in rational design of g-C3N4 photocatalyst for efficient solar fuel transformation, environmental decontamination and future perspectives, Int. J. Hydrogen Energy, 2022, 47, 10837–10867 CrossRef CAS.
- C. K. Bandoh, E. S. Agorku and F. K. Ampong, Graphitic Carbon Nitride Based Composites as Advanced Photocatalysts, from Synthesis to Application: A Review, Res. Rev.: J. Mater. Sci., 2022, 10, 1–17 Search PubMed.
- W. Iqbal, et al., Controllable synthesis of graphitic carbon nitride nanomaterials for solar energy conversion and environmental remediation: the road travelled and the way forward, Catal. Sci. Technol., 2018, 8, 4576–4599 RSC.
- H. Ghafuri, Z. Tajik, N. Ghanbari and P. Hanifehnejad, Preparation and characterization of graphitic carbon nitride-supported l-arginine as a highly efficient and recyclable catalyst for the one-pot synthesis of condensation reactions, Sci. Rep., 2021, 11, 19792 CrossRef CAS.
- L. Wang, C. Wang, X. Hu, H. Xue and H. Pang, Metal/Graphitic Carbon Nitride Composites: Synthesis, Structures, and Applications, Chem.–Asian J., 2016, 11, 3305–3328 CrossRef CAS PubMed.
- W. Shang, et al., Synergistic Redox Reaction for Value-Added Organic Transformation via Dual-Functional Photocatalytic Systems, ACS Catal., 2021, 11, 4613–4632 CrossRef CAS.
- J. Vasiljević, I. Jerman and B. Simončič, Graphitic carbon nitride as a new sustainable photocatalyst for textile functionalization, Polymers, 2021, 13, 2568 CrossRef.
- A. Behera, A. K. Kar and R. Srivastava, Challenges and prospects in the selective photoreduction of CO2to C1 and C2 products with nanostructured materials: a review, Mater. Horiz., 2022, 9, 607–639 RSC.
- M. Ismael, Hydrogen production via water splitting over graphitic carbon nitride (g-C3N4)-based photocatalysis, Process Syst. Eng. a Smooth Energy Transit., 2022, 8, pp. 1–39 Search PubMed.
- J. C. Wang, et al., Mn-Doped g-C3N4 Nanoribbon for Efficient Visible-Light Photocatalytic Water Splitting Coupling with Methylene Blue Degradation, ACS Sustain. Chem. Eng., 2018, 6, 8754–8761 CrossRef CAS.
- Y. Che, et al., Plasmonic ternary hybrid photocatalyst based on polymeric g-C3N4 towards visible light hydrogen generation, Sci. Rep., 2020, 10, 721 CrossRef CAS.
- S. Qin, et al., Real-Time Adsorption and Photodegradation Investigation of Dye Removal on g-C3N4 Surface by Attenuated Total Reflectance Induced Evanescent Spectroscopy, J. Phys. Chem. C, 2021, 125, 4027–4040 CrossRef CAS.
- A. Modwi, A. Albadri and K. K. Taha, High Malachite Green Dye Removal by ZrO2-g-C3N4 (ZOCN) Meso-sorbent: Characteristics and Adsorption Mechanism, Diamond Relat. Mater., 2023, 132, 109698 CrossRef CAS.
- H. B. Fang, Y. Luo, Y. Z. Zheng, W. Ma and X. Tao, Facile Large-Scale Synthesis of Urea-Derived Porous Graphitic Carbon Nitride with Extraordinary Visible-Light Spectrum Photodegradation, Ind. Eng. Chem. Res., 2016, 55, 4506–4514 CrossRef CAS.
- A. Kharlamov, M. Bondarenko, G. Kharlamova and N. Gubareni, Features of the synthesis of carbon nitride oxide (g-C3N4)O at urea pyrolysis, Diamond Relat. Mater., 2016, 66, 16–22 CrossRef CAS.
- A. Hayat, et al., Graphitic carbon nitride (g–C3N4)–based semiconductor as a beneficial candidate in photocatalysis diversity, Int. J. Hydrogen Energy, 2022, 47, 5142–5191 CrossRef CAS.
- B. Yuan, et al., Physical vapor deposition of graphitic carbon nitride (g-C3N4) films on biomass substrate: optoelectronic performance evaluation and life cycle assessment, Adv. Compos. Hybrid Mater., 2022, 5, 813–822 CrossRef CAS.
- M. I. Chebanenko, et al., Chemical and structural changes of g-C3N4 through oxidative physical vapor deposition, Appl. Surf. Sci., 2022, 600, 154079 CrossRef CAS.
- E. B. Chubenko, N. G. Kovalchuk, I. V. Komissarov and V. E. Borisenko, Chemical Vapor Deposition of 2D Crystallized g-C3N4 Layered Films, J. Phys. Chem. C, 2022, 126, 4710–4714 CrossRef CAS.
- R. M. Yadav, et al., Facile synthesis of highly fluorescent free-standing films comprising graphitic carbon nitride (g-C3N4) nanolayers, New J. Chem., 2020, 44, 2644–2651 RSC.
- A. Raza, H. Shen, A. A. Haidry and S. Cui, Hydrothermal synthesis of Fe3O4/TiO2/g-C3N4: advanced photocatalytic application, Appl. Surf. Sci., 2019, 488, 887–895 CrossRef CAS.
- A. R. Kuldeep, R. S. Dhabbe and K. M. Garadkar, Development of g-C3N4-TiO2 visible active hybrid photocatalyst for the photodegradation of methyl orange, Res. Chem. Intermed., 2021, 47, 5155–5174 CrossRef CAS.
- F. Idrees, R. Dillert, D. Bahnemann, F. K. Butt and M. Tahir, In-situ synthesis of Nb2O5/g-C3N4 heterostructures as highly efficient photocatalysts for molecular h2 evolution under solar illumination, Catalysts, 2019, 9, 169 CrossRef.
- L. Kunhikrishnan, K. Vishal and S. Palaniyappan, Mechanical and Thermal Characterization on Synthesized Silane-Treated Graphitic Carbon Nitride (g-C3N4) Reinforced 3D Printed Poly (Lactic Acid) Composite, J. Inorg. Organomet. Polym. Mater., 2023, 33, 1234–1245 CrossRef CAS.
- S. Wu, et al., A simple synthesis route of sodium-doped g-C3N4 nanotubes with enhanced photocatalytic performance, J. Photochem. Photobiol., A, 2021, 406, 112999 CrossRef CAS.
- J. Xi, et al., Preparation of high porosity biochar materials by template method: a review, Environ. Sci. Pollut. Res., 2020, 27, 20675–20684 CrossRef CAS.
- K. Li, et al., Template-Assisted Surface Hydrophilicity of Graphitic Carbon Nitride for Enhanced Photocatalytic H2Evolution, ACS Appl. Energy Mater., 2021, 4, 12965–12973 CrossRef CAS.
- F. T. Li, et al., Precipitation synthesis of mesoporous photoactive Al2O3 for constructing g-C3N4-based heterojunctions with enhanced photocatalytic activity, Ind. Eng. Chem. Res., 2014, 53, 19540–19549 CrossRef CAS.
- M. Hao, et al., In-situ hard template synthesis of mesoporous carbon/graphite carbon nitride (C/CN-T-x) composites with high photocatalytic activities under visible light irridation, Solid State Sci., 2020, 109, 106428 CrossRef CAS.
- J. Liu, et al., Controlled synthesis of ordered mesoporous g-C3N4 with a confined space effect on its photocatalytic activity, Mater. Sci. Semicond. Process., 2016, 46, 59–68 CrossRef CAS.
- C. T. Yang, et al., A novel heterojunction photocatalyst, Bi2SiO5/g-C3N4: synthesis, characterization, photocatalytic activity, and mechanism, RSC Adv., 2016, 6, 40664–40675 RSC.
- T. S. Bui, P. Bansal, B. K. Lee, T. Mahvelati-Shamsabadi and T. Soltani, Facile fabrication of novel Ba-doped g-C3N4 photocatalyst with remarkably enhanced photocatalytic activity towards tetracycline elimination under visible-light irradiation, Appl. Surf. Sci., 2020, 506, 144184 CrossRef CAS.
- H. Starukh and P. Praus, Doping of graphitic carbon nitride with non-metal elements and its applications in photocatalysis, Catalysts, 2020, 10, 1–38 CrossRef.
- M. A. Qamar, M. Javed, S. Shahid and M. Sher, Fabrication of g-C3N4/transition metal (Fe, Co, Ni, Mn and Cr)-doped ZnO ternary composites: excellent visible light active photocatalysts for the degradation of organic pollutants from wastewater, Mater. Res. Bull., 2022, 147, 111630 CrossRef CAS.
- H. Jiang, Y. Li, D. Wang, X. Hong and B. Liang, Recent Advances in Heteroatom Doped Graphitic Carbon Nitride (g-C3N4) and g-C3N4/Metal Oxide Composite Photocatalysts, Curr. Org. Chem., 2020, 24, 673–693 CrossRef CAS.
- L. Tan, et al., Novel two-dimensional crystalline carbon nitrides beyond g-C3N4: structure and applications, J. Mater. Chem. A, 2021, 9, 17–33 RSC.
- Y. Rangraz, M. M. Heravi and A. Elhampour, Recent Advances on Heteroatom-Doped Porous Carbon/Metal Materials: Fascinating Heterogeneous Catalysts for Organic Transformations, Chem. Rec., 2021, 21, 1985–2073 CrossRef CAS PubMed.
- P. P. Singh, Nitride (g-C3N4) photocatalysts for chemical transformations, RSC Adv., 2022, 12, 18245–18265 RSC.
- P. K. Singh, S. R. Bhardiya, A. Asati, V. K. Rai and M. Singh, Cu/Cu2O@g-C3N4: Recyclable Photocatalyst under Visible Light to Access 2-Aryl-/benzimidazoles/benzothiazoles in Water, ChemistrySelect, 2020, 5, 14270–14275 CrossRef CAS.
- Y. Wu, Y. Wang and M. Li, Progress in Photocatalysis of g-C 3 N 4 and its Modified Compounds, E3S Web Conf., 2021, 114, 1–6 Search PubMed.
- J. Xiao, et al., Heterogeneous Photocatalytic Organic Transformation Reactions Using Conjugated Polymers-Based Materials, ACS Catal., 2020, 10, 12256–12283 CrossRef CAS.
- N. Rajalakshmi, D. Barathi, S. Meyvel and P. Sathya, S-scheme Ag2CrO4/g-C3N4 photocatalyst for effective degradation of organic pollutants under visible light, Inorg. Chem. Commun., 2021, 132, 108849 CrossRef CAS.
- D. Bhanderi, P. Lakhani, A. Sharma, S. Soni and C. K. Modi, Efficient Visible Light Active Photocatalyst: Magnesium Oxide Doped Graphitic Carbon Nitride for Knoevenagel Condensation Reaction, ACS Appl. Eng. Mater., 2023, 10, 2752–2764 CrossRef.
- B. A. Maru, et al., Fe@g-C3N4: an effective photocatalyst for Baeyer-Villiger oxidation under visible light condition, New J. Chem., 2023, 47, 9797–9805 RSC.
- I. Camussi, et al., G-C3N4 - Singlet Oxygen Made Easy for Organic Synthesis: Scope and Limitations, ACS Sustain. Chem. Eng., 2019, 7, 8176–8182 CrossRef CAS.
- S. Sharma, R. Nazir, S. Pande and B. R. Sarkar, Microwave-Assisted Efficient Suzuki-Miyaura Cross-Coupling Reactions in Water Catalyzed by Nano-Pd/gC3N4Composite, ChemistrySelect, 2017, 2, 8745–8750 CrossRef CAS.
- F. Verma, et al., Visible light-induced direct conversion of aldehydes into nitriles in aqueous medium using Co@g-C3N4 as photocatalyst, Catal. Commun., 2019, 119, 76–81 CrossRef CAS.
- H. Gao, et al., Synthesis of 3-Arylquinoxalin-2(1H)-ones under Visible-Light Irradiation with Oxygen-Doped Carbon Nitride as Heterogenous Photocatalyst, Eur. J. Org. Chem., 2023, 26, e202300252 CrossRef CAS.
- S. Zhao, et al., Ag2CO3-derived Ag/g-C3N4 composite with enhanced visible-light photocatalytic activity for hydrogen production from water splitting, Int. J. Hydrogen Energy, 2020, 45, 20851–20858 CrossRef CAS.
- J. E. Samaniego-Benitez, K. Jimenez-Rangel, L. Lartundo-Rojas, A. García-García and A. Mantilla, Enhanced photocatalytic H2 production over g-C3N4/NiS hybrid photocatalyst, Mater. Lett., 2021, 290, 129476 CrossRef CAS.
- W. Li, et al., Hydrogen evolution by catalyzing water splitting on two-dimensional g-C3N4-Ag/AgBr heterostructure, Appl. Surf. Sci., 2019, 494, 275–284 CrossRef CAS.
- Y. Li, et al., Journal of Colloid and Interface Science In situ Synthesis of a Novel Mn3O4/g-C3N4 p–n Heterostructure Photocatalyst for Water Splitting, J. Colloid Interface Sci., 2020, 586, 778–784 CrossRef.
- W. S. Chai, et al., A review on conventional and novel materials towards heavy metal adsorption in wastewater treatment application, J. Cleaner Prod., 2021, 296, 126589 CrossRef CAS.
- Y. Shi, et al., Evaluation of self-cleaning performance of the modified g-C3N4 and GO based PVDF membrane toward oil-in-water separation under visible-light, Chemosphere, 2019, 230, 40–50 CrossRef CAS.
- R. Manimozhi, M. Mathankumar and A. P. G. Prakash, Synthesis of g-C3N4/ZnO heterostructure photocatalyst for enhanced visible degradation of organic dye, Optik, 2021, 229, 165548 CrossRef CAS.
- S. Kumaravel, M. Manoharan and Y. Haldorai, Enhanced visible-light degradation of organic dyes via porous g-C 3 N 4, Phosphorus, Sulfur Silicon Relat. Elem., 2022, 197, 200–208 CrossRef CAS.
- Y. Zou, et al., SnO2 quantum dots anchored on g-C3N4 for enhanced visible-light photocatalytic removal of NO and toxic NO2 inhibition, Appl. Surf. Sci., 2019, 496, 143630 CrossRef CAS.
- I. Papailias, et al., Selective removal of organic and inorganic air pollutants by adjusting the g-C3N4/TiO2 ratio, Catal. Today, 2021, 361, 37–42 CrossRef CAS.
- Y. Huang, et al., g-C3N4/TiO2 Composite Film in the Fabrication of a Photocatalytic Air-Purifying Pavements, Sol. RRL, 2020, 4, 2000170 CrossRef CAS.
- M.-T. Pham, D. P. H. Tran, X.-T. Bui and S.-J. You, Rapid Fabrication of MgO@ g-C3N4 Heterojunction for Photocatalytic Nitric Oxide degradation under Visible Light, Beilstein Arch., 2022, 30, 1141–1154 Search PubMed.
- J. He, P. Lv, J. Zhu and H. Li, Selective CO2 reduction to HCOOH on a Pt/In2O3/g-C3N4 multifunctional visible-photocatalyst, RSC Adv., 2020, 10, 22460–22467 RSC.
- X. X. Jiang, et al., Tailoring the properties of g-C3N4 with CuO for enhanced photoelectrocatalytic CO2 reduction to methanol, J. CO2 Util., 2020, 40, 101222 CrossRef CAS.
- W. Yu, D. Xu and T. Peng, Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism, J. Mater. Chem. A, 2015, 3, 19936–19947 RSC.
- S. Singh, A. Modak and K. Kishore, CO2 Reduction to Methanol Using a Conjugated Organic – Inorganic Hybrid - TiO2 – C3N4 Nano - assembly, Transactions of the Indian National Academy of Engineering, 2021, 6, 395–404 CrossRef.
- K. Rabé, L. Liu, N. A. Nahyoon, Y. Zhang and A. M. Idris, Visible-light photocatalytic fuel cell with Z-scheme g-C3N4/Fe0/TiO2 anode and WO3 cathode efficiently degrades berberine chloride and stably generates electricity, Sep. Purif. Technol., 2019, 212, 774–782 CrossRef.
- K. Rabé, L. Liu and N. A. Nahyoon, Electricity generation in fuel cell with light and without light and decomposition of tetracycline hydrochloride using g-C3N4/Fe0(1%)/TiO2 anode and WO3 cathode, Chemosphere, 2020, 243, 125425 CrossRef PubMed.
- Z. L. Yang, et al., High-performance g-C3N4 added carbon-based perovskite solar cells insulated by Al2O3 layer, Sol. Energy, 2019, 193, 859–865 CrossRef CAS.
- J. Yang, Y. Ma, J. Yang, W. Liu and X. Li, Recent Advances in g-C3N4 for the Application of Perovskite Solar Cells, Nanomaterials, 2022, 12, 1–14 Search PubMed.
- H. Yu, et al., Novel application of a Z-scheme photocatalyst of Ag3PO4@g-C3N4 for photocatalytic fuel cells, J. Environ. Manage., 2020, 254, 109738 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |