Open Access Article
Open Access ArticleUnlocking the potential of platinum drugs: organelle-targeted small-molecule platinum complexes for improved anticancer performance
Zhiqin
Deng
 abc,
Shu
Chen
ab,
Gongyuan
Liu
ab and
Guangyu
Zhu
abc,
Shu
Chen
ab,
Gongyuan
Liu
ab and
Guangyu
Zhu
 *ab
*ab
aDepartment of Chemistry, City University of Hong Kong, Hong Kong SAR, P. R. China. E-mail: guangzhu@cityu.edu.hk
bCity University of Hong Kong Shenzhen Research Institute, Shenzhen 518057, P. R. China
cSchool of Medicine, Chongqing University, Chongqing 400030, P. R. China
First published on 3rd October 2023
Abstract
Platinum-based drugs have revolutionized cancer chemotherapy; however, their therapeutic efficacy has been limited by severe side effects and drug resistance. Recently, approaches that target specific organelles in cancer cells have emerged as attractive alternatives to overcome these challenges. Many studies have validated these strategies and highlighted that organelle-targeted platinum complexes demonstrate increased anticancer activity, the ability to overcome drug resistance, novel molecular mechanisms, or even lower toxicity. This review provides a brief summary of various organelle-targeting strategies that promote the accumulation of platinum complexes in certain intracellular areas, such as the nucleus, mitochondria, endoplasmic reticulum (ER), and lysosomes. Moreover, the mechanisms through which these strategies improve anticancer performance, overcome drug resistance, and alter the action mode of conventional platinum drugs are discussed. By providing an extensive account of platinum complexes targeting different organelles, this review aims to assist researchers in understanding the design principles, identifying potential targets, and fostering innovative ideas for the development of platinum complexes.
Introduction
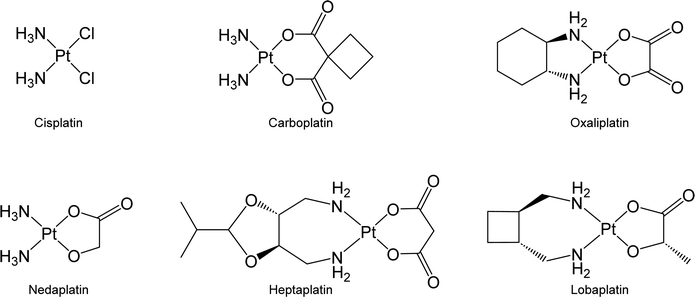
Chemotherapy is the predominant therapeutic modality for most types of malignant cancer, and its efficacy significantly affects patient outcomes.1,2 Among the agents commonly used in chemotherapy, platinum-based anticancer drugs have emerged as a cornerstone in cancer treatment.3 The administration of platinum-based agents has notably improved the therapeutic prognosis of cancer patients. For instance, the overall 5 year relative survival rate of patients with testicular cancer has increased to 90% due to the administration of cisplatin-based chemotherapy.4 Inspired by the success of cisplatin, great efforts have been devoted to developing new platinum drugs. Carboplatin and oxaliplatin, which are second and third-generation Pt-based drugs, respectively, have received global approval for clinical use. Additionally, nedaplatin, heptaplatin, and lobaplatin, three other Pt-based drugs, are being exclusively used in Japan, South Korea, and China, respectively (Fig. 1).3,5,6Upon entering cancer cells, platinum drugs undergo aquation, which triggers their ability to initiate cytotoxic effects against biomolecules, mainly DNA, resulting in cell death.7 Although platinum drugs exhibit high efficacy, their potential is curtailed by dose-limiting toxicity and the development of drug resistance in malignant cells.8,9 Conventional Pt drugs lack selectivity toward cancer cells. Thus, they may simultaneously harm both cancer and normal cells and result in various side effects, such as nephrotoxicity and neurotoxicity.10 Cancer cells can develop resistance to platinum drugs through mechanisms such as the overexpression of transporter proteins or biomolecules that sequester platinum drugs and decrease their effective concentration; meanwhile, cancer cells may repair DNA that has been damaged by platinum drugs to avoid the activation of cell death signals.11,12 The identification of strategies to overcome platinum resistance is crucial, particularly in recurrent cases where drug resistance impedes the use of platinum-based drugs in cancer treatment.13
To improve anticancer activity while overcoming drug resistance, recent studies have focused on developing organelle-targeted platinum complexes that can accumulate in subcellular regions and initiate cell death by damaging specific organelles. These organelle-targeted platinum complexes not only demonstrate increased anticancer activities and improved capacities to counteract drug resistance by strengthening interactions between platinum complexes and target biomolecules (e.g., DNA) but also possess the potential to minimize side effects. This is because certain organelles, such as the lysosomes, are more susceptible to anticancer agents in cancer cells than in normal cells.14 Additionally, emerging evidence suggests that concentrating platinum complexes in different organelles may trigger diverse cell death modes beyond apoptosis, thereby providing valuable insights into the action mechanisms of platinum drugs. The applications of nanomaterials to deliver platinum complexes to different organelles and other metal-based organelles-targeted anticancer complexes have been extensively reviewed.15–19 In this account, we examine different strategies for obtaining organelle-targeted small-molecule platinum complexes with a focus on their anticancer performance, ability to overcome platinum resistance, and mechanisms of action.
Nucleus-targeted Pt complexes
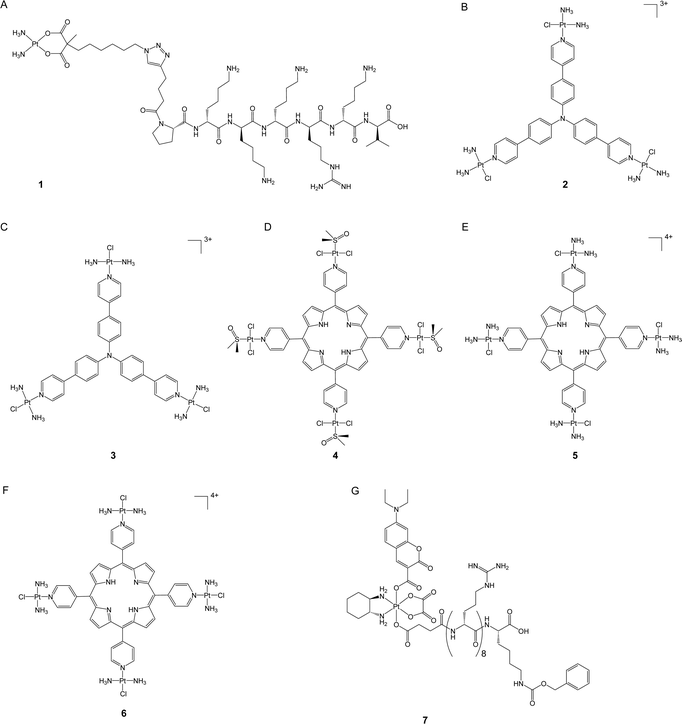
DNA is widely recognized as the primary target of clinically used platinum drugs. The amount of platinum drugs that bind to DNA, however, is relatively low, with less than 10% of the drugs ultimately binding to DNA and damaging it.20 Improving the nuclear accumulation ability of platinum-based anticancer complexes may enhance their efficacy by increasing their amount in genomic DNA, which, in turn, intensifies DNA damage and potentially overcomes platinum resistance.The nuclear envelope is a double membrane that encloses the nucleus and provides a strong obstruction against the entry of different molecules, including many anticancer drugs. Despite this challenge, specific molecules can still pass through the nuclear envelope via nuclear pore complexes (NPCs). Consequently, a proven method for creating effective nuclear-targeting anticancer complexes is by conjugating the toxic component with nuclear localization sequences (NLS), which are short peptides that act as protein tags and are recognized by the importins of the nuclear membrane for nuclear translocation. These peptides have been widely used to transport various cargoes into the nucleus.21,22 In a recent study, Mieszawska et al. used click chemistry to introduce an NLS sequence (PKKKRKV) to the carboxylate ligand of a carboplatin-like Pt(II) complex (1, Fig. 2A).23 The authors assessed the nuclear accumulation of the NLS peptide by conjugating it with fluorescein. The labeled peptide demonstrated nuclear localization after incubation for 72 hours, validating the potential of NLS sequences in delivering small-molecule cargoes. In addition, the authors evaluated the cytotoxicity of complex 1 in various cancer cell lines. The complex demonstrated increased anticancer activity and was noted to be up to twice as potent as carboplatin in platinum-resistant ovarian carcinoma cells (e.g., CP70, SKOV3, and OV-90 cells). This study highlighted the feasibility of promoting the nuclear accumulation of platinum drugs as a potent means to enhance their anticancer efficacy and overcome drug resistance.
In addition to NLS, previous studies have demonstrated that certain triphenylamine derivatives possess excellent nuclear-targeting abilities.24 Mao's group synthesized a new class of photodynamic therapy (PDT) agents by conjugating Pt(II) complexes with triphenylamine at either the para- or meta-position of pyridines.25,26 The synthetic Pt(II) complexes enabled efficient binding with DNA through intercalation and generated an enormous amount of reactive oxygen species (ROS) upon blue light irradiation to prompt DNA damage and trigger apoptosis. Notably, the para-position-coordinated Pt(II) complexes (2 and 3, Fig. 2B and C) showed excellent nuclear accumulation in tested cancer cells when compared to their meta-position-coordinated counterparts; the latter complexes were majorly localized in the cytoplasm. As a consequence, the para-position-coordinated Pt(II) complexes exhibited higher DNA damage ability and up to 99.7- and 270-fold increased photocytotoxicity when compared to the meta-position-coordinated Pt(II) complexes and cisplatin, respectively. In vitro assays revealed that complex 2 displayed better cell-killing efficacy against drug-resistant A549cisR cancer cells compared to cisplatin. The resistance factor (RF, defined as the IC50 in resistant cells/the IC50 in sensitive cells) of complex 2 was 0.42 in A549/A549cisR cell lines, whereas cisplatin had an RF of 3.5. Complex 2 also presented in vivo anticancer activity comparable with cisplatin but with negligible toxicity. These findings emphasize the significance of nucleus targeting in the development of platinum-based PDT agents.
Spingler and co-workers developed a novel class of tetraplatinated porphyrins (4–6, Fig. 2D–F) by conjugating cisplatin-like Pt(II) complexes with the peripheral nitrogen of porphyrins.27 These synthetic compounds effectively localized in the nucleus of HeLa cells, exhibiting over a 30-fold increase in nucleus accumulation level compared with cisplatin in the same cell line. The representative tetraplatinated porphyrin complex 6 was shown to bind to DNA via intercalation; however, it failed to induce DNA damage, potentially limiting its potency as a DNA-damaging agent in the context of conventional chemotherapy. Nonetheless, upon irradiation with 420 nm blue light, complex 6 effectively triggered DNA cleavage and revealed its potential as a PDT agent instead. These complexes exhibited low dark cytotoxicity in HeLa, A2780, and CP70 cells. In contrast, upon exposure to blue light-irradiation at 420 nm, their cytotoxicity increased up to 5260-fold compared to that in the dark, which is 4157 and 325 times higher than that of cisplatin and “cisplatin + porphyrin PDT agent,” respectively, suggesting the advantage of nuclear targeting strategy for both chemotherapy and PDT. Importantly, complexes 4–6 demonstrated an impressive ability to overcome platinum resistance in A2780/CP70 cells, with RF values ranging from 0.56 to 1.0, while that for cisplatin was as high as 7.3. This study highlights a promising approach to enhancing the anticancer activity of both PDT agents and platinum complexes by utilizing the platinum moiety to reinforce PDT agents’ affinity with DNA.
Pt(IV) prodrugs have gained great attention in addition to conventional Pt(II) anticancer complexes.28 Pt(IV) complexes bear two additional axial ligands that can be further modified to generate compounds with additional functionality, biological activities, and even controllable activation properties.29 In the presence of internal stimuli such as reducing agents or external stimuli such as light, Pt(IV) prodrugs can be activated to Pt(II) counterparts to kill cancer cells.7 Recently, our group developed a nucleolus-targeted and photoactivatable Pt(IV) prodrug, coumaplatin (7, Fig. 2G), by conjugating the axial ligands of oxaliplatin-based Pt(IV) complexes with a coumarin-based photocage and an NLS (R8K).30 For oxaliplatin, only 21.5% of Pt was detected in the nucleus region, whereas 68.2% of coumaplatin was effectively accumulated in the nucleolus region but remained non-toxic in the absence of light. Upon blue light irradiation, coumaplatin was reduced to oxaliplatin and displayed more than 62-fold increased cytotoxicity compared to that in the dark in cancer cells. Photoactivated coumaplatin showed more than twice the DNA binding efficiency of oxaliplatin, up to a 96-fold enhancement in phototoxicity, and a greater ability to overcome platinum resistance. The RF values of coumaplatin were 1.3 and 0.6 in A2780/A780cisR and A549/A549cisR cells, while those of oxaliplatin were 5.1 and 2.8, respectively. Importantly, coumaplatin's dramatically increased ability to accumulate in the nucleolus enabled it to exhibit a mode of action distinct from that of oxaliplatin. While oxaliplatin mainly killed cancer cells by inducing apoptosis, photoactivated coumaplatin predominantly initiated senescence in cancer cells and exhibited an improved ability to trigger immunogenetic cell death (ICD). These findings suggest that accumulating clinical Pt(II) drugs in target areas in cells may not only strengthen their anticancer effects but also shift their mechanisms of action.
Mitochondria-targeted Pt complexes
The resistance of cancer cells to Pt drugs is largely attributable to their ability to repair nuclear DNA damage.31 Recent studies have suggested that targeting organelles other than the nucleus, such as the mitochondria, represents a promising strategy to overcome this problem. Mitochondria play critical roles in cellular respiration and ATP synthesis, and emerging evidence suggests that they can be selectively targeted for cancer therapy.32 Mitochondrial DNA (mtDNA) presents a unique vulnerability to platinum-containing compounds, as it lacks nucleotide excision repair and protective histones.33 The disruption of mitochondrial metabolic processes by platinum complexes has been shown to induce cell death by impairing ROS homeostasis, redox-regulation homeostasis, and the tricarboxylic acid cycle. Therefore, mitochondrial targeting of platinum complexes may represent a promising strategy to overcome platinum resistance.Mitochondria are enclosed by a double-layered membrane structure that consists of an outer mitochondrial membrane (OMM) with pores and an inner mitochondrial membrane (IMM) that is tightly packed and hydrophobic. Due to the electrochemical gradient created through the electron transport chain, the IMM maintains a significantly higher membrane potential in the mitochondria (150–180 mV) compared to the plasma membrane (60–90 mV).34 Consequently, positively charged molecules have a higher likelihood of translocating into the mitochondria.35 Various mitochondria-targeting compounds have been developed, including peptides, triphenylphosphonium (TPP)-based targeting groups, and nanoparticle-based cargo systems. These targeting molecules, utilized in conjugation with platinum complexes, constitute a promising strategy for efficiently delivering such complexes to the mitochondria.
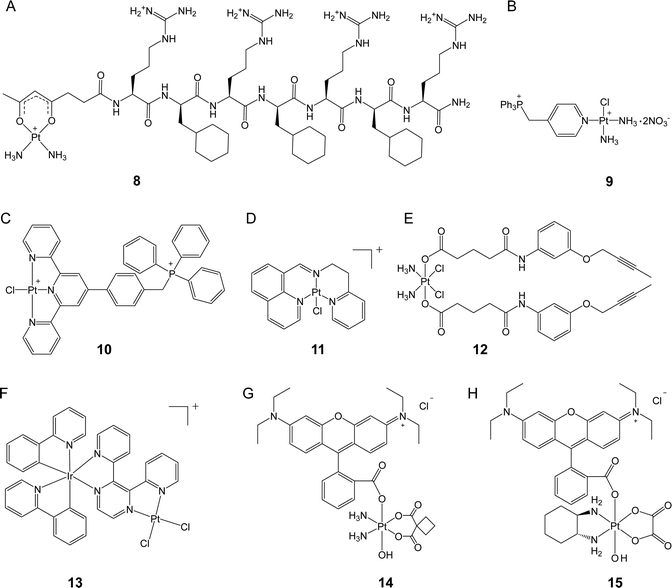
Kelley, Lippard, and co-workers conjugated a mitochondria-targeting peptide with the leaving group of Pt(II) complex to obtain a novel complex (8, Fig. 3A) that can accumulate efficiently in the mitochondria of cancer cells.36 Unlike cisplatin, 8 did not induce nuclear DNA damage, resulting in no cell cycle arrest. Instead, this complex was capable of inducing mtDNA damage and launching mitochondrial damage signaling pathways, which triggered apoptosis. Although 8 was less cytotoxic than cisplatin (IC50 7.5 vs. 0.6 μM) in A2780 cells due to its slower ligand exchange rate, 8 exhibited favorable activity towards both platinum-sensitive and platinum-resistant cancer cells with an RF value of 0.7, while that of cisplatin was as high as 8.7, confirming that targeted delivery of platinum complexes to the mitochondria can be an effective approach to overcome drug resistance. These results also highlight the potential of targeting the mitochondria for the development of new platinum anticancer complexes.
Pyriplatin is a monofunctional platinum(II) complex that differs from cisplatin and oxaliplatin in its anticancer spectrum and mode of action.37 Wang, Guo, and colleagues developed a class of mitochondria-targeting platinum complexes derived from pyriplatin by adding a TPP group to its pyridine ligand.38 Compared with cisplatin, the ortho-substituted complex (9, Fig. 3B) exhibited 11.6 higher accumulation levels in the mitochondria and demonstrated superior anticancer activity both in vitro and in vivo. Mechanistically, real-time polymerase chain reaction (PCR) assay indicated that 9 induced 2 times higher levels of mitochondrial DNA lesion than cisplatin. Considering the significantly higher mitochondrial accumulation level and greatly enhanced cytotoxicity of 9 over cisplatin, DNA damage-independent pathways might also be involved. Further investigation showed that 9 disrupted mitochondrial oxidative phosphorylation and glycolysis and caused cytochrome c release, leading to apoptosis. These findings suggest that targeting the mitochondria with platinum complexes has the potential to interfere with mitochondrial energy metabolism and represents a promising strategy for overcoming drug resistance towards conventional platinum drugs. In a recent study, another mitochondria-targeted platinum(II) complex was reported by the same group.39 The synthetic complex (10, Fig. 3C) demonstrated comparable cellular and nuclear accumulation levels to those of cisplatin. However, complex 10 induced almost no DNA damage after incubation for 24 h. Instead, the complex exhibited 2.6 times higher mitochondrial accumulation levels than cisplatin and effectively disrupted the mitochondrial structure. A mechanism study revealed that complex 10 simultaneously inhibited mitochondrial respiration and glycolysis by down-regulating thioredoxin reductase (TrxR) and triggering non-caspase-3-mediated non-apoptotic cell death. Consequently, complex 10 reduced platinum resistance in the A549cisR cell line, with an RF value of 2.4, which is lower than that of cisplatin (RF = 4.1). This study highlights the potential of utilizing platinum complexes as a means to target redox homeostasis in order to enhance anticancer efficacy.
Liang, Liu, Huang, and colleagues constructed a monofunctional platinum complex (11, Fig. 3D) that effectively targets the mitochondria.40 Complex 11 entered cancer cells via a non-endocytic, energy-dependent transport pathway and, due to its lipophilic and cationic structure, over 30% of complexes were found to accumulate in the mitochondria. This complex was able to prompt mitochondrial dysfunction, such as ATP depletion and loss of mitochondrial membrane potential. While conventional platinum drugs initiate apoptosis to eliminate cancer cells, complex 11 activated both caspase-dependent apoptosis and autophagy, demonstrating its potential to overcome drug resistance. In vitro experiments revealed that complex 11 exhibited up to ten-fold increase in cytotoxicity compared to cisplatin and effectively overcame platinum resistance in A549cisR cells. The RF value of 11 was 1.23, whereas that of cisplatin was 7.12. Furthermore, in a mouse model, complex 11 was found to inhibit tumor volume by 53.1%, which was higher than that from cisplatin (44.3%). Additionally, less body weight loss was observed in complex 11-treated mice than in cisplatin-treated ones, indicating that 11 has the potential as a promising candidate for further investigation and development.
Hu, Chen, Meng, and their colleagues designed a mitochondria-targeted Pt(IV) prodrug (12, Fig. 3E) by utilizing the core functional group of epidermal growth factor receptor (EGFR) and Bruton's tyrosine kinase (BTK) inhibitors as the axial ligands in a cisplatin-based Pt(IV) complex.41 Their investigation revealed that, compared to cisplatin, complex 12 exhibited a 42-fold higher level of accumulation in the mitochondria, up to 22-fold increased anticancer activity, and a stronger ability to overcome drug resistance (RF = 0.6 vs. RF = 4.3). Although most of the complexes were found to accumulate in the mitochondria, intense nuclear DNA damage was also induced by complex 12. Markers of nuclear DNA damage, such as γ-H2AX, and related proapoptotic signals, such as Bax, PARP, and p53, showed significantly increased levels in the cells treated with 12. However, the extent to which mitochondrial damage contributes to the observed effects remains unclear and warrants further investigation.
Chao et al. devised a binuclear Ir(III)–Pt(II) complex (Ir–Pt) that utilized an Ir(III) complex to serve as both a cell penetration unit and a mitochondria-targeting moiety.42 The Ir–Pt complex (13, Fig. 3F) possessed the ability to enter cancer cells via both passive diffusion and energy-dependent active transport, which resulted in cellular uptake 8.2-fold higher than that of cisplatin. Approximately 80% of complex 13 accumulated in the mitochondria, resulting in efficient mitochondrial DNA damage. Further studies revealed that complex 13 promoted mitochondrial ROS generation and disrupted cellular metabolism by switching from glucose oxidation to glycolysis and suppressing basal and maximal respiration, leading to necrotic rather than apoptotic cell death. In comparison to cisplatin, complex 13 exhibited 5.8 and 32.1 times higher cytotoxicities in A549 and platinum-resistant A549R cells, respectively, underscoring its superior anticancer activity especially against cancer cells that are refractory to platinum drugs.
Rhodamine B (RhB) is a widely recognized fluorescent agent that targets the mitochondria. Our research group recently discovered that RhB could function as a photocatalyst in the conversion of platinum(IV) complexes into platinum(II) forms, albeit with very low efficiency.43 By using RhB as a photoswitch to be conjugated at the axial position of platinum(IV) complexes, we obtained rhodaplatins (14 and 15, Fig. 3G) that were able to achieve a photoreduction rate that was up to 4.8 × 104 times higher than the ‘RhB + platinum(IV) ’ platform. Moreover, the rhodaplatins inherit the mitochondria-targeting property of RhB and can be further converted to clinical Pt(II) drugs (i.e., carboplatin and oxaliplatin) upon exposure to visible light. The released Pt(II) drugs triggered mtDNA damage and incited apoptosis mediated by mitochondrial damage. In contrast to the parent Pt(II) drugs, complex 15 demonstrated a photocytotoxicity that was more than 10.7 times higher and was able to effectively overcome drug resistance, as evidenced by a RF value as low as 0.8. Our findings suggest that the photoactivatable platinum(IV) complexes hold promise in the mitigation of platinum resistance in cancer cells through specific targeting to the mitochondria.
ER-targeted Pt complexes
Owing to the DNA-targeting nature of platinum complexes, many researchers have focused on nucleus- and mitochondria-targeted platinum complexes. The potential of platinum complexes in targeting the ER has only been recently explored. The ER is the largest organelle involved in the synthesis and assembly of various proteins that are overexpressed in cancer cells. Therefore, targeting this organelle with platinum complexes may offer a promising avenue for cancer therapy. Platinum complexes exhibit high binding affinity toward multiple proteins,44,45 which suggests that ER-targeted Pt complexes may interact with ER-resident proteins and disrupt their function, thereby inducing cell death. Recent examples indicate that ER-targeted metal complexes can induce ER stress, resulting in cell death.46 Notably, the ER stress caused by metal complexes is often associated with ICD, which may stimulate the immune response system and enhance the anticancer efficacy of metal complexes.47Che's group developed a luminescent platinum(II) complex coordinated with N-heterocyclic carbene ligands (16, Fig. 4A).48 In contrast to many platinum(II) complexes that predominantly target DNA, this synthetic platinum(II) complex had a significantly higher affinity towards proteins, such as bovine serum albumin (BSA), rather than DNA. Intracellular fluorescence analysis revealed that the majority of these complexes displayed predominant localization in the ER region of cancer cells, leading to the generation of a vast amount of reactive oxygen species (ROS) that activate intense ER stress and caspase-mediated pathways. This process ultimately induced apoptosis. Compared to cisplatin, this complex exhibited 5.2- to 60-fold increased cytotoxicity towards several cancer cell lines and did not have cross-resistance with cisplatin. This study demonstrates that the ER is a valuable target for platinum complexes to improve anticancer performance.
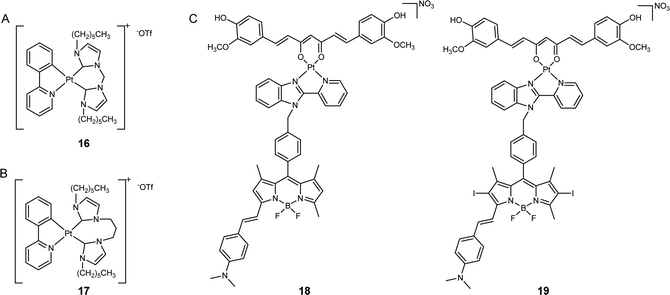 | ||
| Fig. 4 ER-targeted platinum anticancer complexes. The chemical structures of (A) complex 16; (B) PlatinER (17); (C) curcumin- and BODIPY-based photoactivatable Pt(II) complexes (18 and 19). | ||
Ang, Babak, and co-workers synthesized a cyclometalated platinum(II) complex, PlatinER (17, Fig. 4B), which localized in the ER.49 This platinum complex showed low affinity towards DNA, indicating that DNA is not its major target. Instead, complex 17 could effectively trigger ROS generation and ER stress to eliminate cancer cells. Compared with cisplatin, PlatinER presented 2.67 times higher cytotoxicity in CT26 murine colorectal carcinoma cell line. Intriguingly, 17 acted as an inducer of ICD by generating ROS localized within the ER and activating the unfolded protein response (UPR). While conventional ICD inducers trigger the exposure of calreticulin (CRT) to mark the cells as targets for recognition and to be attacked by immune cells, 17 also activated heat shock protein 90 (HSP90) to amplify this ICD signal. In turn, 17-treated cancer cells were found to be phagocytosed by macrophages at 2.8-fold higher magnitudes than cisplatin-treated cells, highlighting the benefits of targeting the ER to initiate immunogenic cell death.
Chakravarty's group developed a type of photoactivatable Pt(II) complexes by chelating Pt(II) center with curcumin- and BODIPY-based ligands (18 and 19, Fig. 4C).50 These complexes have been found to accumulate selectively in the ER but remained low cytotoxicity in the dark. Upon irradiation with visible light, the complexes elicited the production of two types of reactive oxygen species (ROS): hydroxyl radicals from the curcumin moiety and singlet oxygen from the BODIPY moiety. This led to the induction of severe ER stress, calcium imbalance, and mitochondrial dysfunction, ultimately resulting in apoptosis. In vitro studies demonstrated that when compared to the BODIPY PDT ligand, the photocytotoxicity of 18 and 19 increased up to 15.3-fold, indicating that the conjugation with platinum complex has the potential to improve the anticancer performance of PDT agents.
Lysosome-targeted Pt complexes
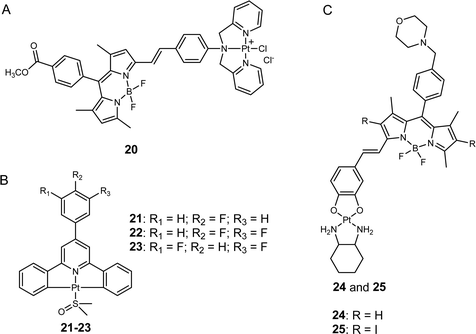
Lysosomes are essential intracellular organelles that harbor various hydrolytic enzymes to facilitate the degradation and recycling of cellular components.51 Moreover, lysosomes help maintain cellular homeostasis through autophagy.52 Although platinum-based anticancer drugs traditionally do not target lysosomes, recent studies have demonstrated that anticancer compounds targeting to the lysosomes of cancer cells disrupt their function,53 providing a promising avenue for inducing cancer cell death and overcoming platinum drug resistance. Notably, lysosomes play a critical role in the metabolism and proliferation of cancer cells,54 and certain signaling channels (e.g., zinc and calcium ion-permeable channels) are upregulated in the lysosomes of cancer cells but not in those of normal cells. This phenomenon can be exploited to selectively eliminate cancer cells while leaving normal cells intact, thereby mitigating the adverse effects of anticancer agents.55He, Guo, Liu, and co-workers developed a photosensitive platinum(II) complex, Pt-BDPA (20, Fig. 5A), that entered cancer cells through endocytosis and then became trapped in the lysosomes.56 Pt-BDPA was of low toxicity in the dark. However, under light irradiation, this complex generated ROS, inducing glutathione depletion and promoting lysosomal escape. Subsequently, the escaped platinum complexes could translocate into the nucleus and trigger DNA damage. As a result, the cytotoxicity of 20 increased more than 13-fold upon light irradiation. Compared with cisplatin, complex 20 showed 1.7–2.9 times higher photocytotoxicity in various cancer cell lines, and 8.6-fold lower dark cytotoxicity towards normal cells, suggesting the potential of this strategy to reduce the side effects of platinum complexes. In addition, the lysosome-escape property may also bring Pt-BDPA a greater potential to overcome drug resistance, as the lysosome sequestration of platinum drugs has been regarded as an important contributor to platinum resistance in cancer cells.57
Tian, Zhang, and colleagues designed a type of C^N^C ligand-cyclometalated Pt(II) complexes that can be excited through a four-photon absorption manner for cancer theragnostics (21–23, Fig. 5B).58 Compared to conventional Pt(II) drugs, these synthetic complexes showed improved selectivity against cancer cells over normal cells, as evidenced by the weak fluorescence of 22 in human embryo liver fibroblast (HELF) normal cells and the intense fluorescence observed in three different cancer cell lines (HeLa, A549, and HepG2). Further fluorescence analysis revealed that the lead complex (22) effectively localized in the lysosome of HeLa cells, potentially due to its high affinity for lecithin on the lysosomal membrane. In vivo experiments demonstrated that treatment with 22 reduced tumor volume by approximately 45%, whereas cisplatin only produced a decrease of less than 30%. Additionally, the body weights of mice treated with 19 were comparable to those treated with PBS, while a significant body weight decrease was observed in the cisplatin-treated group. These results suggest that 22 has higher anticancer activity but lower toxicity than cisplatin in vivo, making it a promising candidate for cancer theragnostic applications.
Chakravarty et al. synthesized a new class of mono-styryl BODIPY-platinum(II) conjugates that were selectivity localized in the lysosomes (24 and 25, Fig. 5C).59 Upon irradiation with red light, the synthetic complexes generated a large amount of ROS in cancer cells. Although the authors also suggested that these complexes might release the active platinum(II) moiety through aquation to damage DNA, further evidence is still needed to support his hypothesis. Through the assessment of intracellular fluorescence levels of platinum(II) conjugates, the authors observed that these complexes were taken up more readily by cancer cells than by normal cells, possibly due to the elevated presence of lysosomes in cancerous cells.57 Consequently, the photocytotoxicity of complexes 24 and 25 was found to be 7.4- to 35.2-fold higher in cancer cell lines compared to normal ones, respectively. These findings reinforce the notion that targeting lysosomes could represent a promising strategy to minimize toxicity to normal tissues by platinum complexes.
Conclusions and perspectives
Platinum-based drugs have been widely used in cancer treatment for several decades. However, their clinical efficacy often has been limited by the development of drug resistance. A promising strategy to overcome this challenge is to target specific organelles with platinum-based anticancer complexes. This review provides a brief summary of the strategies currently used to obtain organelle-targeted platinum complexes and highlights their potential to address the limitations associated with conventional platinum drugs. These advanced organelle-targeted complexes have demonstrated improved anticancer activity, the potential to overcome drug resistance, and even enhanced cancer cell selectivity compared with conventional platinum drugs. Thus, they constitute a promising approach to address the limitations of existing platinum drugs and promote the development of more effective metal-based anticancer agents.Significant progress has been made in designing platinum complexes that can specifically target the nucleus and mitochondria. However, the development of platinum complexes that can effectively target other organelles, such as the ER and lysosomes, requires the establishment of additional strategies. Moreover, since these platinum complexes are intended to be activated within specific organelles, it is crucial to ensure their stability. Creating complexes that maintain their stability until reaching the intended target could substantially improve their efficacy as anticancer agents.
Although the promising anticancer effects of organelle-targeted platinum complexes have been demonstrated in vitro, few studies have explored their anticancer performance in vivo. As these compounds have been recently developed, their pharmacokinetics remain to be clarified, and their more detailed mechanisms of action need more investigation. These complexes can target certain organelles; however, their tumor accumulation levels in vivo may not be optimal, and many lack the specificity required for cancer-selective treatment. For instance, several positively charged platinum complexes have exhibited exceptional mitochondria-targeting abilities in vitro. During in vivo administration, however, the positive charge may lead to nonspecific interactions with serum proteins, consequently reducing the efficiency of tumor targeting.60 These limitations may hinder their further exploration, particularly in animal models.
To fully harness the potential of organelle-targeted platinum agents, future research may focus on enhancing their selectivity against cancer cells over normal cells. One promising approach is to develop organelle-targeted complexes that can be controllably activated, which have shown superior anticancer performance compared to conventional platinum complexes. By employing light, ultrasound, X-ray, or other stimuli to selectively activate these complexes in cancerous regions, the anticancer effects can be improved while minimizing toxicity towards unstimulated normal tissues. Furthermore, numerous studies have highlighted the promising potential of nanomaterial- or antibody-based drug delivery systems. Utilizing these delivery vehicles to transport organelle-targeted platinum complexes could address the limitations of platinum drugs for further applications in vivo. Combining controllable activation and organelle targeting in suitable drug delivery systems may fully unlock the potential of platinum complexes and propel new-generation platinum complexes into preclinical or even clinical studies. Furthermore, certain complexes exhibit molecular mechanisms that differ from those of conventional platinum drugs and thus result in novel cell death modes and immune system activation. Consequently, future research may focus on clarifying the underlying molecular mechanisms of these complexes. This endeavor can provide a deeper understanding of the action mechanisms of these platinum cancer-fighting agents, reveal new anticancer targets, and potentially enhance their anticancer efficacy. Furthermore, with recent advancements, immunotherapy has emerged as an excellent treatment option for certain cancer types,61 and platinum drugs have been found to enhance patients' responses to immunotherapy.62 Complexes that target specific organelles, such as the ER or lysosomes, have exhibited the ability to effectively activate the immune system.63,64 Thus, the development of more potent organelle-targeted platinum complexes with enhanced immune response activation capabilities and the clarification of the underlying mechanisms are promising directions for future exploration.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (grant no. 22077108 and 22277103), the Hong Kong Research Grants Council (grant no. CityU 11307419, 11303320, 11302221, and 11313222), and the Science Technology and Innovation Committee of Shenzhen Municipality (JCYJ20210324120004011) for funding support.References
- B. A. Chabner and T. G. Roberts, Chemotherapy and the war on cancer, Nat. Rev. Cancer, 2005, 5(1), 65–72 CrossRef CAS PubMed.
- V. T. DeVita and E. Chu, A history of cancer chemotherapy, Cancer Res., 2008, 68(21), 8643–8653 CrossRef CAS PubMed.
- L. Kelland, The resurgence of platinum-based cancer chemotherapy, Nat. Rev. Cancer, 2007, 7(8), 573–584 CrossRef CAS PubMed.
- C. Bucher-Johannessen, C. M. Page, T. B. Haugen, M. W. Wojewodzic, S. D. Fosså, T. Grotmol, H. S. Haugnes and T. B. Rounge, Cisplatin treatment of testicular cancer patients introduces long-term changes in the epigenome, Clin. Epigenetics, 2019, 11, 1–13 CrossRef PubMed.
- B. Desoize and C. Madoulet, Particular aspects of platinum compounds used at present in cancer treatment, Crit. Rev. Oncol. Hemat., 2002, 42(3), 317–325 CrossRef PubMed.
- S. Rottenberg, C. Disler and P. Perego, The rediscovery of platinum-based cancer therapy, Nat. Rev. Cancer, 2021, 21(1), 37–50 CrossRef CAS PubMed.
- T. C. Johnstone, K. Suntharalingam and S. J. Lippard, The next generation of platinum drugs: targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs, Chem. Rev., 2016, 116(5), 3436–3486 CrossRef CAS PubMed.
- V. Brabec and J. Kasparkova, Molecular aspects of resistance to antitumor platinum drugs, Drug Resist. Update., 2002, 5(3–4), 147–161 CrossRef CAS PubMed.
- J. T. Hartmann and H.-P. Lipp, Toxicity of platinum compounds, Expert Opin. Pharmacother., 2003, 4(6), 889–901 CrossRef CAS PubMed.
- R. Oun, Y. E. Moussa and N. J. Wheate, The side effects of platinum-based chemotherapy drugs: a review for chemists, Dalton Trans., 2018, 47(19), 6645–6653 RSC.
- R. P. Wernyj and P. J. Morin, Molecular mechanisms of platinum resistance: still searching for the Achilles’ heel, Drug Resist. Update., 2004, 7(4–5), 227–232 CrossRef CAS PubMed.
- M. Ohmichi, J. Hayakawa, K. Tasaka, H. Kurachi and Y. Murata, Mechanisms of platinum drug resistance, Trends Pharmacol. Sci., 2005, 26(3), 113–116 CrossRef CAS PubMed.
- B. Mansoori, A. Mohammadi, S. Davudian, S. Shirjang and B. Baradaran, The different mechanisms of cancer drug resistance: a brief review, Adv. Pharm. Bull., 2017, 7(3), 339 CrossRef CAS PubMed.
- S. Hämälistö and M. Jäättelä, Lysosomes in cancer—living on the edge (of the cell), Curr. Opin. Cell Biol., 2016, 39, 69–76 CrossRef PubMed.
- P. Gao, W. Pan, N. Li and B. Tang, Boosting cancer therapy with organelle-targeted nanomaterials, ACS Appl. Mater. Interfaces., 2019, 11(30), 26529–26558 CrossRef CAS PubMed.
- X. Ma, N. Gong, L. Zhong, J. Sun and X.-J. Liang, Future of nanotherapeutics: Targeting the cellular sub-organelles, Biomaterials, 2016, 97, 10–21 CrossRef CAS PubMed.
- W. Zhen, S. An, S. Wang, W. Hu, Y. Li, X. Jiang and J. Li, Precise subcellular organelle targeting for boosting endogenous-stimuli-mediated tumor therapy, Adv. Mater., 2021, 33(51), 2101572 CrossRef CAS PubMed.
- K. Qiu, Y. Chen, T. W. Rees, L. Ji and H. Chao, Organelle-targeting metal complexes: From molecular design to bio-applications, Coord. Chem. Rev., 2019, 378, 66–86 CrossRef CAS.
- R. G. Kenny and C. J. Marmion, Toward multi-targeted platinum and ruthenium drugs—a new paradigm in cancer drug treatment regimens?, Chem. Rev., 2019, 119(2), 1058–1137 CrossRef CAS PubMed.
- M. A. Fuertes, C. Alonso and J. M. Pérez, Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance, Chem. Rev., 2003, 103(3), 645–662 CrossRef CAS PubMed.
- R. Cartier and R. Reszka, Utilization of synthetic peptides containing nuclear localization signals for nonviral gene transfer systems, Gene Ther., 2002, 9(3), 157–167 CrossRef CAS PubMed.
- D. Drescher, T. Büchner, P. Schrade, H. Traub, S. Werner, P. Guttmann, S. Bachmann and J. Kneipp, Influence of Nuclear Localization Sequences on the Intracellular Fate of Gold Nanoparticles, ACS Nano, 2021, 15(9), 14838–14849 CrossRef CAS PubMed.
- M. T. Wlodarczyk, S. A. Dragulska, O. Camacho-Vanegas, P. R. Dottino, A. A. Jarzecki, J. A. Martignetti and A. J. Mieszawska, Platinum(II) complex-nuclear localization sequence peptide hybrid for overcoming platinum resistance in cancer therapy, ACS Biomater. Sci. Eng., 2018, 4(2), 463–467 CrossRef CAS PubMed.
- B. Dumat, G. Bordeau, E. Faurel-Paul, F. Mahuteau-Betzer, N. Saettel, G. Metge, C. Fiorini-Debuisschert, F. Charra and M.-P. Teulade-Fichou, DNA switches on the two-photon efficiency of an ultrabright triphenylamine fluorescent probe specific of AT regions, J. Am. Chem. Soc., 2013, 135(34), 12697–12706 CrossRef CAS PubMed.
- Y. F. Zhong, H. Zhang, W. T. Liu, X. H. Zheng, Y. W. Zhou, Q. Cao, Y. Shen, Y. Zhao, P. Z. Qin, L. N. Ji and Z.-W. Mao, A platinum(II)-based photosensitive tripod as an effective photodynamic anticancer agent through DNA damage, Chem. – Eur. J., 2017, 23(65), 16442–16446 CrossRef CAS PubMed.
- Y.-F. Zhong, H. Zhang, G. Mu, W.-T. Liu, Q. Cao, C.-P. Tan, L.-N. Ji and Z.-W. Mao, Nucleus-localized platinum(II)–triphenylamine complexes as potent photodynamic anticancer agents, Inorg. Chem. Front., 2019, 6(10), 2817–2823 RSC.
- A. Naik, R. Rubbiani, G. Gasser and B. Spingler, Visible-light-induced annihilation of tumor cells with platinum-porphyrin conjugates, Angew. Chem., Int. Ed., 2014, 53(27), 6938–6941 CrossRef CAS PubMed.
- D. Gibson, Platinum(IV) anticancer prodrugs - hypotheses and facts, Dalton Trans., 2016, 45(33), 12983–12991 RSC.
- Z. Xu, Z. Wang, Z. Deng and G. Zhu, Recent advances in the synthesis, stability, and activation of platinum(IV) anticancer prodrugs, Coord. Chem. Rev., 2021, 442, 213991 CrossRef CAS.
- Z. Deng, N. Wang, Y. Liu, Z. Xu, Z. Wang, T.-C. Lau and G. Zhu, A photocaged, water-oxidizing, and nucleolus-targeted Pt(IV) complex with a distinct anticancer mechanism, J. Am. Chem. Soc., 2020, 142(17), 7803–7812 CrossRef CAS PubMed.
- L. P. Martin, T. C. Hamilton and R. J. Schilder, Platinum resistance: the role of DNA repair pathways, Clin. Cancer Res., 2008, 14(5), 1291–1295 CrossRef CAS PubMed.
- K. Vasan, M. Werner and N. S. Chandel, Mitochondrial metabolism as a target for cancer therapy, Cell Metab., 2020, 32(3), 341–352 CrossRef CAS PubMed.
- E. F. Fang, M. Scheibye-Knudsen, K. F. Chua, M. P. Mattson, D. L. Croteau and V. A. Bohr, Nuclear DNA damage signalling to mitochondria in ageing, Nat. Rev. Mol. Cell Bio., 2016, 17(5), 308–321 CrossRef CAS PubMed.
- S. W. Perry, J. P. Norman, J. Barbieri, E. B. Brown and H. A. Gelbard, Mitochondrial membrane potential probes and the proton gradient: a practical usage guide, Biotechniques, 2011, 50(2), 98–115 CrossRef CAS PubMed.
- S. Kim, H. Y. Nam, J. Lee and J. Seo, Mitochondrion-targeting peptides and peptidomimetics: recent progress and design principles, Biochem, 2019, 59(3), 270–284 CrossRef PubMed.
- S. P. Wisnovsky, J. J. Wilson, R. J. Radford, M. P. Pereira, M. R. Chan, R. R. Laposa, S. J. Lippard and S. O. Kelley, Targeting mitochondrial DNA with a platinum-based anticancer agent, Chem. Biol., 2013, 20(11), 1323–1328 CrossRef CAS PubMed.
- K. S. Lovejoy, M. Serova, I. Bieche, S. Emami, M. D'Incalci, M. Broggini, E. Erba, C. Gespach, E. Cvitkovic and S. Faivre, Spectrum of cellular responses to pyriplatin, a monofunctional cationic antineoplastic platinum(II) compound, in human cancer cells, Mol. Cancer Ther., 2011, 10(9), 1709–1719 CrossRef CAS PubMed.
- Z. Zhu, Z. Wang, C. Zhang, Y. Wang, H. Zhang, Z. Gan, Z. Guo and X. Wang, Mitochondrion-targeted platinum complexes suppressing lung cancer through multiple pathways involving energy metabolism, Chem. Sci., 2019, 10(10), 3089–3095 RSC.
- K. Wang, C. Zhu, Y. He, Z. Zhang, W. Zhou, N. Muhammad, Y. Guo, X. Wang and Z. Guo, Restraining cancer cells by dual metabolic inhibition with a mitochondrion-targeted platinum(II) complex, Angew. Chem., Int. Ed., 2019, 58(14), 4638–4643 CrossRef CAS PubMed.
- F.-Y. Wang, X.-M. Tang, X. Wang, K.-B. Huang, H.-W. Feng, Z.-F. Chen, Y.-N. Liu and H. Liang, Mitochondria-targeted platinum(II) complexes induce apoptosis-dependent autophagic cell death mediated by ER-stress in A549 cancer cells, Eur. J. Med. Chem., 2018, 155, 639–650 CrossRef CAS PubMed.
- B. Fang, X. Chen, X. Zhou, X. Hu, Y. Luo, Z. Xu, C.-H. Zhou, J.-P. Meng, Z.-Z. Chen and C. Hu, Highly potent platinum(IV) complexes with multiple-bond ligands targeting mitochondria to overcome cisplatin resistance, Eur. J. Med. Chem., 2023, 250, 115235 CrossRef CAS PubMed.
- C. Ouyang, L. Chen, T. W. Rees, Y. Chen, J. Liu, L. Ji, J. Long and H. Chao, A mitochondria-targeting hetero-binuclear Ir(III)-Pt(II) complex induces necrosis in cisplatin-resistant tumor cells, Chem. Commun., 2018, 54(49), 6268–6271 RSC.
- Z. Deng, C. Li, S. Chen, Q. Zhou, Z. Xu, Z. Wang, H. Yao, H. Hirao and G. Zhu, An intramolecular photoswitch can significantly promote photoactivation of Pt(IV) prodrugs, Chem. Sci., 2021, 12(19), 6536–6542 RSC.
- A. Casini, C. Gabbiani, G. Mastrobuoni, L. Messori, G. Moneti and G. Pieraccini, Exploring metallodrug-protein interactions by ESI mass spectrometry: The reaction of anticancer platinum drugs with horse heart cytochrome c, ChemMedChem, 2006, 1(4), 413–417 CrossRef CAS PubMed.
- R. M. Cunningham and V. J. DeRose, Platinum binds proteins in the endoplasmic reticulum of S. cerevisiae and induces endoplasmic reticulum stress, ACS Chem. Biol., 2017, 12(11), 2737–2745 CrossRef CAS PubMed.
- A. P. King and J. J. Wilson, Endoplasmic reticulum stress: an arising target for metal-based anticancer agents, Chem. Soc. Rev., 2020, 49(22), 8113–8136 RSC.
- C. Huang, T. Li, J. Liang, H. Huang, P. Zhang and S. Banerjee, Recent advances in endoplasmic reticulum targeting metal complexes, Coord. Chem. Rev., 2020, 408, 213178 CrossRef CAS.
- T. Zou, C.-N. Lok, Y. M. E. Fung and C.-M. Che, Luminescent organoplatinum(II) complexes containing bis(N-heterocyclic carbene) ligands selectively target the endoplasmic reticulum and induce potent photo-toxicity, Chem. Commun., 2013, 49(47), 5423–5425 RSC.
- M. J. R. Tham, M. V. Babak and W. H. Ang, PlatinER: A highly potent anticancer platinum(II) complex that induces endoplasmic reticulum stress driven immunogenic cell death, Angew. Chem., Int. Ed., 2020, 59(43), 19070–19078 CrossRef CAS PubMed.
- A. Upadhyay, P. Kundu, V. Ramu, P. Kondaiah and A. R. Chakravarty, BODIPY-tagged platinum(II) curcumin complexes for endoplasmic reticulum-targeted red light PDT, lnorg. Chem., 2022, 61(3), 1335–1348 CrossRef CAS PubMed.
- R. E. Lawrence and R. Zoncu, The lysosome as a cellular centre for signalling, metabolism and quality control, Nat. Cell Biol., 2019, 21(2), 133–142 CrossRef CAS PubMed.
- W. W.-Y. Yim and N. Mizushima, Lysosome biology in autophagy, Cell Discovery, 2020, 6(1), 6 CrossRef CAS PubMed.
- C. G. Towers and A. Thorburn, Targeting the lysosome for cancer therapy, Cancer Discov., 2017, 7(11), 1218–1220 CrossRef PubMed.
- R. M. Perera, S. Stoykova, B. N. Nicolay, K. N. Ross, J. Fitamant, M. Boukhali, J. Lengrand, V. Deshpande, M. K. Selig and C. R. Ferrone, Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism, Nature, 2015, 524(7565), 361–365 CrossRef CAS PubMed.
- W. Du, M. Gu, M. Hu, P. Pinchi, W. Chen, M. Ryan, T. Nold, A. Bannaga and H. Xu, Lysosomal Zn2+ release triggers rapid, mitochondria-mediated, non-apoptotic cell death in metastatic melanoma, Cell Rep., 2021, 37(3), 109848 CrossRef CAS PubMed.
- X. Xue, C. Qian, H. Fang, H. K. Liu, H. Yuan, Z. Guo, Y. Bai and W. He, Photoactivated lysosomal escape of a monofunctional PtII complex Pt-BDPA for nucleus access, Angew. Chem., Int. Ed., 2019, 58(36), 12661–12666 CrossRef CAS PubMed.
- B. Zhitomirsky and Y. G. Assaraf, Lysosomes as mediators of drug resistance in cancer, Drug Resist. Update., 2016, 24, 23–33 CrossRef PubMed.
- Q. Zhang, S. Wang, Y. Zhu, C. Zhang, H. Cao, W. Ma, X. Tian, J. Wu, H. Zhou and Y. Tian, Functional platinum(II) complexes with four-photon absorption activity, lysosome specificity, and precise cancer therapy, lnorg. Chem., 2021, 60(4), 2362–2371 CrossRef CAS PubMed.
- V. Ramu, P. Kundu, A. Upadhyay, P. Kondaiah and A. R. Chakravarty, Lysosome specific platinum(II) catecholates with photoactive BODIPY for imaging and photodynamic therapy in near-IR light, Eur. J. Inorg. Chem., 2021,(9), 831–839 CrossRef CAS.
- M. Jeena, S. Kim, S. Jin and J.-H. Ryu, Recent progress in mitochondria-targeted drug and drug-free agents for cancer therapy, Cancers, 2019, 12(1), 4 CrossRef PubMed.
- S. Tan, D. Li and X. Zhu, Cancer immunotherapy: Pros, cons and beyond, Biomed. Pharmacother., 2020, 124, 109821 CrossRef PubMed.
- A. M. Cook, W. J. Lesterhuis, A. K. Nowak and R. A. Lake, Chemotherapy and immunotherapy: mapping the road ahead, Curr. Opin. Immunol., 2016, 39, 23–29 CrossRef CAS PubMed.
- S. E. Bettigole and L. H. Glimcher, Endoplasmic reticulum stress in immunity, Annu. Rev. Immunol., 2015, 33, 107–138 CrossRef CAS PubMed.
- T. Iulianna, N. Kuldeep and F. Eric, The Achilles’ heel of cancer: targeting tumors via lysosome-induced immunogenic cell death, Cell Death Dis., 2022, 13(5), 509 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |