Open Access Article
Open Access ArticleMedicinal chemistry inspired by ginger: exploring the chemical space around 6-gingerol
Sara Hassan Hassan Ahmed†
 ab,
Tímea Gonda†
ab,
Tímea Gonda† a and
Attila Hunyadi
a and
Attila Hunyadi *ac
*ac
aInstitute of Pharmacognosy, Interdisciplinary Excellence Centre, University of Szeged, Eötvös str. 6, H-6720 Szeged, Hungary. E-mail: hunyadi.a@pharmacognosy.hu; Tel: +3662546456
bFaculty of Pharmacy, University of Khartoum, 1996, Khartoum, Sudan
cInterdisciplinary Centre for Natural Products, University of Szeged, Eötvös str. 6, H-6720 Szeged, Hungary
First published on 4th August 2021
Abstract
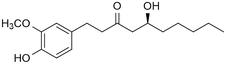
Ginger (Zingiber officinale Roscoe) has been used as a spice and as a traditional remedy since ancient times, especially in traditional Chinese medicine. It has been applied as a treatment for many diseases either alone or in combination with other remedies. Many studies were conducted on ginger and its constituents and a wide array of bioactivities were reported, e.g., antioxidant, anti-inflammatory, antiemetic, and anticancer activity. Most of these had been correlated to gingerols and shogaols, the most abundant secondary metabolites in ginger. This inspired several research groups to explore the biomedical value of the chemical space around these compounds, and many of their synthetic or semi-synthetic analogues have been prepared and studied for various bioactivities. Thanks to this, many valuable structure activity relationships have been revealed for such compounds. Herein, we provide a brief summary on the synthetic derivatization efforts that had so far been implemented on 6-gingerol, the main constituent of fresh ginger. This review covers 160 natural, semisynthetic, or synthetic 6-gingerol derivatives and their reported bioactivities.
Introduction
Ginger, Zingiber officinale Roscoe (Zingiberaceae) is widely renowned and has been historically used for culinary and medicinal purposes.1,2 The roots of this plant have been used as a herbal remedy for the prevention and/or treatment of nausea and vomiting, diarrhea, abdominal discomfort, headache, rheumatism and respiratory illnesses (e.g. common cold),2 and have also been studied for a wide range of bioactivities including anti-inflammatory, antioxidant,3 antimicrobial,4 antidiabetic,5 antihypertensive, cardioprotective,6 neuroprotective,7 anti-obesity,8 anti-migraine,9 and anticancer effects.2,10 Most recently, ginger gained a significant public attention when it was also suggested as a natural home remedy that may help against COVID-19,11–13 and even though at this time this is by no means an evidence-based use,14 the anti-inflammatory effect of ginger may hold some promise in the relief of respiratory symptoms connected to the SARS-CoV-2 infection.15A wide array of bioactive compounds have been identified in ginger such as phenolic compounds and terpenes; these have recently been reviewed.2 Among these constituents, the so-called gingerols are present in by far the most significant amount in ginger (23–25%), and this is accompanied by relatively lower levels of other, related compounds such as shogaols and paradols.10,16,17 The main compound in the gingerol series, 6-gingerol (Fig. 1) is partially responsible for the strong pungent taste of ginger. This compound has been correlated with many bioactivities of ginger, and it is present in much higher amounts in fresh ginger roots compared to the dried roots because drying converts it into 6-shogaol through a water elimination.16,18
Much research has been devoted to the biomedical value of 6-gingerol and its semisynthetic derivatives, and several clinical trials had been performed using ginger extract and its constituents, and some of these are still in progress. The high interest in ginger is shown well by the fact that searching the term “ginger” in Scopus gives over a thousand hits only for the year 2020. There are many recent reviews on ginger constituents and their potential therapeutic applications.2,10,16,18,19 However, to the best of our knowledge, currently no reviews are available on the semi- and total-synthetic efforts to explore the biomedical value of the chemical space around 6-gingerol, i.e. the medicinal chemistry inspired by this compound. Therefore, the aim of this paper is to provide such a coverage with a hope that it may draw a roadmap for further possible structural manipulations of 6-gingerol towards new promising lead compounds based on this simple but versatile bioactive natural product.
Review scope and coverage
The information included in this review is based on papers collected from a search in four online databases: PubMed, Scopus, Embase and SciFinder, with no time limits, while limiting the language to English and the search domain to title, abstract and keywords. The search strategy included different combinations of the following keywords: (“Ginger” OR “gingerol”) AND (“semisynthetic derivative(s)” OR “synthetic derivative(s)” OR “semisynthetic analog(s)” OR “synthetic analog(s)” OR “semisynthetic analogue(s)” OR “synthetic analogue(s)” OR “analogs” OR “derivatives” OR “semisynthetic” OR “synthetic” OR “synth*”). The wild-card term “*” was used to increase the sensitivity of the search. The search in SciFinder database was conducted as a sub-structure search using 6-gingerol structure to access publications reporting related synthetic or semisynthetic work. The hits from the four databases together with the Cochrane Library returned no review papers in the subject of the current review.This paper aims to provide an as complete as possible coverage of reports dealing with semisynthetic derivatives prepared from 6-gingerol regardless of its origin (i.e. isolated from ginger roots or prepared by total synthesis). Some diarylheptanoids are included if they were not the focus of the referred publication, e.g. if they are mentioned as compounds synthesized together with other gingerol derivatives. The same applies for derivatives of 8- and 10-gingerol, shogaol, zingerone, and other ginger constituents.
When presenting chemical structures in the following sections, the therapeutic aim was taken as the primary organizing principle. In the descriptive text, semi- and total-synthetic gingerol analogues are presented after a brief overview on the current knowledge about ginger and ginger constituents, particularly 6-gingerol, in relation to the targeted bioactivities.
Anticancer analogues
A growing body of research suggests the possibility for the use of ginger in cancer prevention and treatment. Ginger extract was claimed to have promising activity against various types of cancer (e.g. oral squamous cell carcinoma,20 chronic myeloid leukemia,21 lung cancer22 etc). It was also studied for reducing chemotherapy-induced nausea and vomiting.23–25A number of biochemical pathways were implied in the possible anticancer activity of ginger and its constituents.16,26 6-Gingerol was reported to induce cell cycle arrest and exert anti-invasive and apoptosis promoting effects through acting on multiple signaling pathways in different types of cancer cell lines.27–29 This seems to be at least partially connected to the antioxidant–prooxidant properties of 6-gingerol. This compound was reported to induce reactive oxygen species (ROS) generation leading to DNA damage in cancer cells.18 It is also of interest that gingerol was found to have antioxidant and chemopreventive activity through modulating nuclear factor erythroid 2-related factor 2 (Nrf2);30,31 this transcription factor is considered as a master switch in cellular antioxidant defense and redox signaling, and has implications as a potential antitumor target.32–34 Unsurprisingly, the anticancer effect of gingerol appears to be the result of a multitarget action. According to a most recent review on this subject, transcription factors (NF-κB, activator protein-1; AP-1), β-catenin, mitogen activated protein kinases (MAPK), growth factor receptors (EGFR, VEGFR) and pro-inflammatory mediators (COX-2, TNFα) were reported to contribute to the anticancer activity exerted by this compound.35
A series of clinical trials were conducted to evaluate the possible efficacy of a 50% aqueous ethanol extract of ginger roots (normalized to 5% of total gingerols) in preventing colorectal cancer (CRC). Results from a pilot, randomized controlled trial in patients with high risk of developing colorectal cancer suggested that this extract may increase apoptosis and differentiation and reduce proliferation of normal-appearing colon mucosa cells.36 Increased eicosanoid, and mainly prostaglandin E2 (PGE2) level is a marker of early stages of CRC development. Consumption of ginger root extract was found to decrease cycloxygenase-1 (COX-1) expression and consequentially lower PGE2 levels in people with increased risk of developing colorectal cancer (CRC) but not in participants with normal risk,37 and similar results were found in a phase II clinical study on the PGE2 levels in the colon mucosa of healthy people at normal risk for developing CRC.38 The PGE2-decreasing activity was later not confirmed in volunteers with increased CRC risk, which certainly does not rule out chemopreventive action through other mechanisms.39
The apparent antitumor potential of ginger inspired several research groups to take 6-gingerol as a lead compound aiming at various bioactivities with a special emphasis on cancer. Based on our literature survey, 160 compounds have been synthesized, some of which are naturally present in ginger; these compounds are discussed hereinafter.
Gingerol derivatives with antiproliferative and/or cytotoxic activity
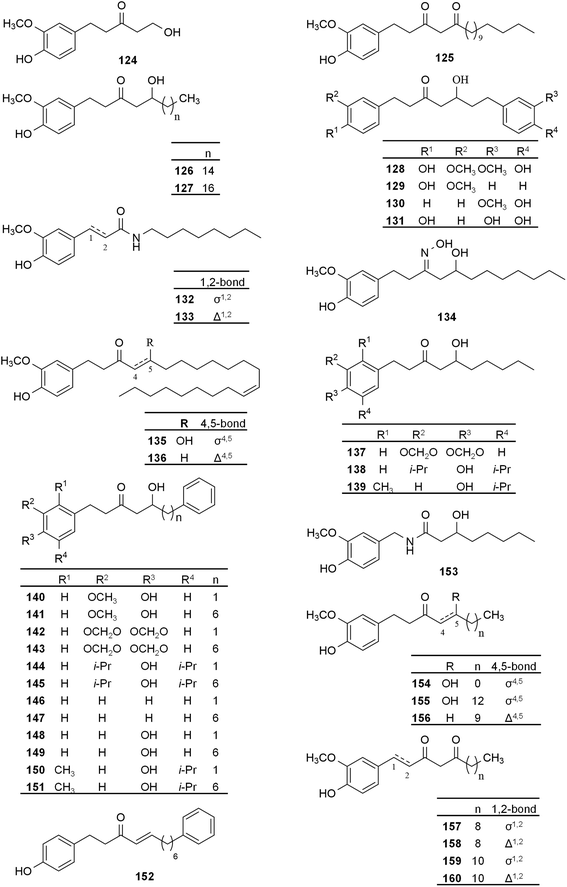
De Lima Silva et al. recently reported the synthesis of 6-gingerol derivatives, its O-propargyl ether (1) and several compounds where this moiety was transformed to a 1,2,3-triazol linker to a second phenolic ring (2–8). The compounds were tested on colon adenocarcinoma (HCT-116) and breast metastatic adenocarcinoma (MCF-7) cell lines. The propargyl substitution (1) did not influence the effect of 6-gingerol on cell viability, however, most of the triazole derivatives exerted somewhat increased activity that was the strongest in case of the meta-bromine-substituted compound 8. This was still moderate, i.e. ca. 87% and 71% inhibition in MCF-7 and HCT-116 cell lines, respectively, at 50 μM concentration, while these values were 37% and 36% for 6-gingerol. The cells were much more sensitive to the positive control doxorubicin (IC50 = 0.5 and 1.9 μM against HCT-116 and MCF-7 cells, respectively).40Another study introduced different changes in the skeleton of 6-gingerol (compounds 9–14, 17 i.e. 6-shogaol, 19, and 22–24), with the aim of understanding the structure activity relationships (SAR) concerning cytotoxicity of these compounds against MCF-7 breast cancer cells. Among these compounds, only a 4-allyloxy derivative (10) showed higher inhibitory activity against MCF-7 cells (IC50 = 21 μM) as compared to 6-gingerol (IC50 = 30.3 μM). While these values seem to indicate moderate activity, the same experimental setup resulted in unusually high IC50 values for the positive controls doxorubicin and 5-fluorouracil (IC50 = 120 μM and 158.5 μM, respectively, after 72 h incubation), which may suggest the involvement of an unknown resistance mechanism in the cells line used. Through comparing different substitution patterns, the authors claimed that the aromatic ring and a free hydroxyl group on the aliphatic side chain are important for the activity against breast cancer. It was also noted that the length of the alkyl side chain is optimal for this activity. Dehydrated products of 6-gingerol and its analogue 11 (compounds 17 and 14, respectively) showed lower inhibitory activity compared to 6-gingerol. Further, a surprising opposite activity was found for the dimerization product of 6-gingerol (24) that exerted a concentration-dependent increase in cell viability.41
Another semi-synthetic effort yielded compound 25 that was cytotoxic on triple negative breast cancer (TNBC) cell line MDA-MB-231 (IC50 = 22.9 μM after 48 h incubation), whereas 6-gingerol was technically inactive (IC50 = 404.5 μM). The mechanism of action was postulated to be the induction of early autophagy, in addition to a significant increase in ROS levels leading to caspase-independent cellular death at later periods of the incubation. When comparing the cytotoxicity of 25 on MDA-MB-231 to that on a non-tumorigenic epithelial cell line MCF-10A, a mild selectivity was found (IC50 = 26.13 and 40.46 μM, respectively, after 24 h incubation). Further, compound 25 also inhibited migration and invasion of TNBC cells, caused cell cycle arrest at the G1-phase, and promoted apoptosis.42,43
An interesting hybrid molecule of 6-gingerol and acetylsalicylic acid (46) was synthesized by Zhu et al. with an aim to combine the chemo-preventive and gastroprotective effect of the former with the anti-inflammatory activity of the latter, and to simultaneously counteract the well-known gastric irritative action of Aspirin. In vitro, compound 46 showed superior activity as compared to the two compounds alone or in combination, and it exerted protective effect against acute gastric ulceration in mice, suggesting that the hybrid could potentially be used as a multitarget chemo-preventive agent against gastrointestinal malignancies.44
Gingerol derivatives with LTA4H inhibitory activity
Leukotriene A4 hydrolase (LTA4H), a bifunctional metalloenzyme with aminopeptidase and epoxide hydrolase activities, plays an important role in chronic inflammation associated with carcinogenesis.45,46 Badria et al. reported the synthesis and LTA4H inhibitory activity of natural and semisynthetic gingerol derivatives (9–17, and 19–24). Compound 11, a prenylated gingerol derivative, demonstrated the strongest activity against both functions of the enzyme (aminopeptidase: IC50 = 3.0 μM, epoxide hydrolase: IC50 = 7.3 μM) followed by methylshogaol (16; aminopeptidase: IC50 = 4.9 μM, epoxide hydrolase: IC50 = 11.3 μM).47 Concerning the cytotoxicity of these compounds against HCT-116 colorectal cancer cells, compound 16 was the most effective (IC50 = 1.5 μM), much more potent than the positive controls used in the study (betastin and 4-(4-benzylphenyl)-thiazol-2-amine (4BSA) with IC50 = 42.5 and 30.5 μM, respectively). 6-Shogaol (17) itself was less potent (IC50 = 12.9 μM) than compound 16, i.e. the ortho-dimethoxy group in the aromatic ring increased the cytotoxic activity by nearly an order of magnitude. The same increase in efficacy was, however, not observed when 6-gingerol was similarly methylated to compound 19 (IC50 = 76.5 μM), which highlights the importance of the enone group in the side chain for this bioactivity. The compounds showed no toxicity to normal cells, and several of them had high selectivity towards HCT-116 cells as compared to normal TIG-1 cells: the selectivity index was over 52 in case of the most potent compound 16.47Gingerol derivatives with antioxidant and/or HDAC inhibitory activity
In the last two decades, histone deacetylase (HDAC) enzymes have been emerging as anticancer targets.48 In 2019, A combination of tucidinostatin, an HDAC enzyme inhibitor, and exemestane, an aromatase enzyme inhibitor was studied in a randomized, double-blind, placebo-controlled, phase III trial on postmenopausal patients with advanced hormone receptor-positive breast cancer, and encouraging results were reported.49 A link between the HDAC inhibitory activity and ROS levels had been highlighted. A suggested mechanism for HDAC inhibitors in cancer is through increasing intracellular ROS levels and downregulating antioxidant pathways resulting in increased level of DNA damage,50 and the ability to repair this damage is impaired in cancer cells.51 In connection to this, it is of interest that many plant-originated phenolic antioxidants have been reported as HDAC inhibitors.52–54The anticancer activity of natural and semi-synthetic 6-gingerol derivatives (9, 17, 18 and 26–29, (3R,5S)-30 and 31–39), was studied through assessment of their HDAC enzyme inhibition and antioxidant activity by Kunboonma et al. All these compounds showed HDAC inhibitory activity in the micromolar concentration range, and compound 29 was the most active among all (IC50 = 42 μM). Compound 18, a demethylated 6-shogaol derivative was the most potent semi-synthetic compound (IC50 = 45 μM; compared to 61 μM for gingerol), a role of the catechol moiety was suggested for the increase in activity as compared with that of 6-shogaol itself. In case of compounds 31–39, the oxime orientation did not influence the bioactivity. When testing the compounds by the DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) scavenging assay, most of the investigated derivatives (except compounds 28, (3R,5S)-30, and 38), showed higher antioxidant activity compared to 6-gingerol (IC50 = 81 μM) but lower than the applied positive control, gallic acid (IC50 = 37 μM), and 35 and 38 were the most active (IC50 = 42 μM). Among the tested compounds, 29 (IC50 = 58 μM) was reported to exert the highest antioxidant activity. Furthermore, based on in silico docking studies it was suggested that compounds 17, 18, and 29 may serve as promising anti-HDAC leads with different isoform selectivity. Nevertheless, the reported activities were still moderate and the compounds would require further structural optimization to exert pharmacologically relevant HDAC inhibitory effect.55
Influence of the length and structure of the side chain on the antioxidant properties of 6-gingerol was studied on its analogues 17 and 40–45. Four experimental models were used including DPPH scavenging, ferric reducing antioxidant power (FRAP), DNA strand breakage inhibition and human red blood cell haemolysis protection. Regarding DPPH scavenging activity, shogaols were found the most effective, followed by gingerols, while dehydrogingerols and dehydroshogaols were the least effective; therefore, the C4–5 double bond may have a role in boosting the activity. Increasing the side chain length had no remarkable effect on the DPPH scavenging activity, however in case of FRAP measurements it had a negative effect on the potency. Increasing the side chain length significantly decreased the DNA strand breakage ability, while enhanced the anti-haemolysis activity. Thus, it was concluded that the antioxidant activity largely depends on the side chain.56
Altogether, concerning the antitumor potential of gingerol derivatives, the currently known compounds are not very cytotoxic, still, they seem to have antitumor potential due to their abilities to interfere with several pathways relevant to antitumor drug discovery. This concerns mainly the chemo-preventive potential of the semi-synthetic derivatives, similarly to the inspiring compound 6-gingerol itself. Nevertheless, the potent and selective cytotoxic activity of compound 16 against colon cancer cells suggests that related analogues may also be developed with a potential to fight the already developed disease.
Anti-inflammatory gingerol analogues
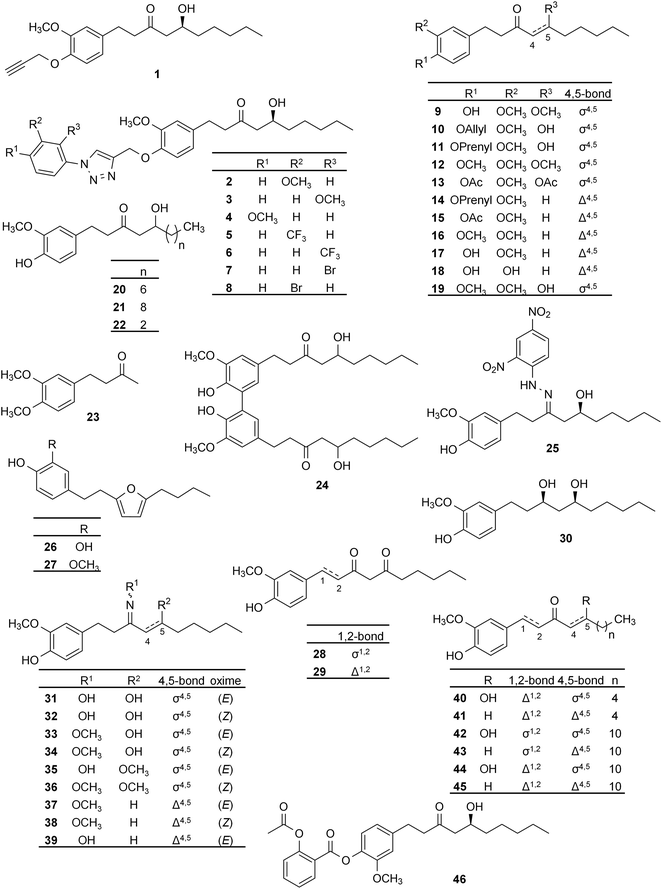
The anti-inflammatory effect of ginger is among its most deeply studied medicinal properties. The effects of ginger were studied against many inflammatory conditions in human subjects, including knee osteoarthritis57,58 joint pain,59 and obesity as a risk for cardiovascular complications.60 The anti-inflammatory effect of ginger supplementation was studied on subjects who are free of any inflammatory conditions and at varying degree of exercise levels (NCT03219463). After ginger consumption a drop in the levels of inflammatory biomarkers tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6) was reported. Therefore, it had been concluded that ginger may serve as a potential adjuvant to prevent diabetes, heart diseases and other chronic disorders connected to inflammation. Ginger consumption was also reported to reduce inflammatory mediators C-reactive protein (CRP) and interleukin-1β (IL-1β) in patients with rheumatoid arthritis (RA),61 and 6-gingerol was reported to inhibit sepsis development through interfering with pro-inflammatory cytokines' secretion and attenuating pyroptosis in macrophages,62 i.e., an inflammatory-type programmed cell death.63These findings on ginger inspired the synthesis of several 6-gingerol analogues aiming to develop new anti-inflammatory agents. A set of natural and semi-synthetic ginger constituents, i.e., compounds 17, 20, 21, 42 (Fig. 2), and compounds 47–58 (Fig. 3) were studied for their cyclooxygenase-2 (COX-2) inhibitory activity in intact A549 cells that are known to express this enzyme. Compound 56 was found to be the most active (IC50 = 1.4 μM) among these compounds, followed by compounds 17, 58, 47, 8-paradol (21), 10-gingerol (57), and 49 that was still active with an IC50 value of 5.5 μM. All other compounds showed moderate activity, and 6-gingerol itself did not reach 50% inhibition at up to 50 μM. Concerning SAR, an aromatic group substituted with a free hydroxyl group at C3 or C4 was found important for a potent COX-2 inhibition: compound 53 with the hydroxyl group at C2 of the aromatic ring and compound 50 with methoxy-substituents at both C3 and C4 showed only moderate activity (IC50 > 50 μM). Also, importance of the length of the alkyl chain was highlighted, and a 14-C length was suggested as the optimum. A significant increase in the activity was observed with a hydroxyl group on the alkyl chain, while replacement of the carbonyl group with a hydroxyl group had no remarkable effect on the potency of compounds, as in, e.g., compound 54 (IC50 = 12.5 μM) compared with compound 20, i.e., 8-gingerol (IC50 = 10 μM). Nevertheless, reduction of the carbonyl group to a methylene boosted the activity as in compound 56 (IC50 = 1.4 μM).64
 | ||
| Fig. 2 Semi-synthetic gingerol derivatives prepared and directly or indirectly evaluated for their in vitro antitumor potential. | ||
 | ||
| Fig. 3 Semi-synthetic gingerol derivatives prepared and evaluated for their in vitro anti-inflammatory activity. | ||
Anti-inflammatory activity of two racemic gingerol derivatives, a stable metabolite of 6-gingerol (58), and another derivative named Capsarol (49), joining some molecular properties of gingerol and capsaicin, were evaluated as possible anti-inflammatory agents by Aktan et al. The compounds were tested for their effect on inducible nitric oxide synthase (iNOS) and found to suppress NO production in murine macrophages through a partial inhibition of the enzyme and by simultaneously decreasing iNOS expression through its NF-κβ-mediated transcriptional regulation.65
As seen from the above examples, the anti-inflammatory activity of 6-gingerol analogues seems to be encouraging to further studies that may have a chance for the development of a suitable anti-inflammatory drug candidate in the future.
Antimicrobial gingerol analogues
The potential antibacterial activity of ginger was also assessed. Ginger extract showed activity against dental caries-causing bacteria; Streptococcus mutans and S. sanguinis with minimum inhibitory concentration (MIC) values of 20 and 300 μg ml−1, respectively, and it was suggested that this activity may be attributed to gingerols.66 In another, in vitro and in vivo study on the effect of ginger on S. mutans bacteria, ginger extract inhibited bacterial growth with MIC = 256 μg ml; differences in MIC values might be attributed to different experimental conditions and/or extraction method. Glucan synthesis, bacterial adhesion, and biofilm formation were also reduced in vitro, and a reduction of caries development was observed in ginger-treated rats versus the control group.67Components of ginger were investigated for anti-virulence and antibiofilm activities against a fluconazole resistant Candida albicans strain. It was reported that 6-gingerol, 8-gingerol and 6-shogaol effectively inhibited biofilm formation. Furthermore, 6-gingerol and 6-shogaol also reduced virulence of the fungus.68
Gingerol analogues including 6-gingerol and 6-shogaol were also studied for their antibacterial activity against a range of multi-drug resistant bacteria. Plasmid conjugal transfer property was also assessed. It was concluded that the investigated compounds are valuable antibacterial agents with an ability to reverse horizontal antibiotic resistance spread in bacteria.69
In combination with tea polyphenols, 6-gingerol was also reported to maintain the quality of shrimp paste during storage, and the reduction of bacterial growth was a suggested mechanism for this effect.70
6-Gingerol was found to reduce virulence and biofilm formation of Pseudomonas aeruginosa via the inhibition of quorum sensing (QS),71 i.e., a mechanism of bacterial cell to cell communication that is of crucial importance in controlling their colony-wide functions and plays an important role in their virulence.72 In further research into the bioactivities of ginger constituents, the antibacterial activity of 6-gingerol, 6-shogaol (compound 17, Fig. 2), zingerone (59) and two new synthetic analogues, namely 6-azashogaol (61) and an isoxazole derivative of 6-gingerol (62) was assessed (Fig. 4). The antibacterial activity of these compounds was investigated by assessing their growth inhibitory activity on Pseudomonas aeruginosa and Chromobacterium violaceum bacterial strains, and by measuring the concentration of pyocyanin pigment produced by P. aeruginosa.73 The expression of this pigment is controlled by QS.72 Compound 61 was reported to have the highest activity with the lowest MIC against both strains and 90% reduction of pyocyanin pigment produced by P. aeruginosa, while zingerone (59) was the least active. The authors highlighted that low molecular weight compounds with long side chain are needed for anti-QS activity which may explain the low activity of zingerone (only 4 carbons long side chain). It was also observed that the presence of an amide linker enhances the activity, as evidenced by the activity of compound 61, while the isoxazoline linker had only minor influence on the activity.73
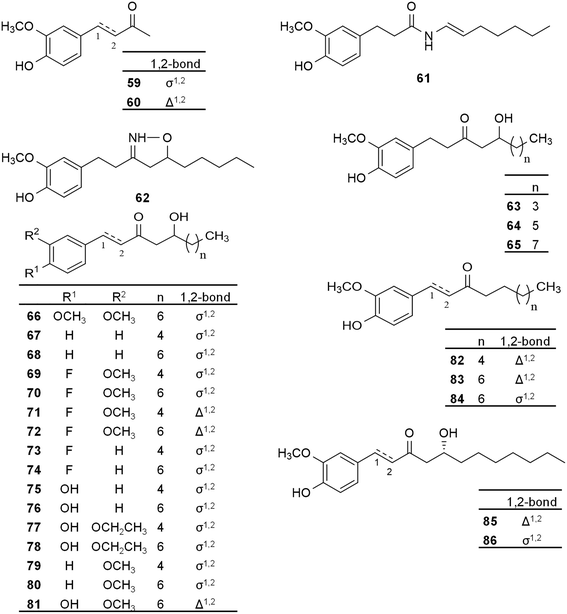 | ||
| Fig. 4 Semi-synthetic gingerol derivatives prepared and evaluated for their in vitro antibacterial activity. | ||
Investigating the same pathway of QS and the possibility of discovering new antibacterial gingerol derivatives, Choi et al. reported the synthesis of 6-gingerol and further derivatives (19–22, 40, Fig. 2; 47, Fig. 3; 59, 60, and 63–86, Fig. 4), some of them naturally present in ginger root. The compounds were tested for their binding to LasR, a transcriptional regulator protein playing a major role in the processes of QS in P. aeruginosa and biofilm formation, which in turn confers virulence to the bacteria and poses a health problem especially to immunocompromised patients. Bacterial inhibition was also monitored. It was concluded that (R)-8-gingerol (86) and its C1–C2 unsaturated analogue (85) were the most promising, and the importance of stereochemistry regarding the activity against P. aeruginosa was emphasized. At 10 μM concentration, compound 85 decreased bacterial biofilm thickness from 34.5 μm (negative control, DMSO) to 10.3 μm, which was 13.9 μm in case of compound 86 and 17 μm for the naturally occurring (S)-8-gingerol (compound 20), even though bacterial growth inhibition was not observed for either compound even at 100 μM concentration. The authors emphasized the importance of rotational rigidity between the head section and the carbonyl group for LasR-binding affinity and for inhibition of biofilm formation as evidenced by the higher activity of compound 85 as compared to that of 86. This was also supported by in silico docking. SAR evaluation showed that an increase of the alkyl chain length up to 12 carbons led to an increase of activity, which may explain why 8-gingerol analogues (compounds 47, 66, 68, 70, 72, 74, 76, 78, 80, 81, 82, 85 and 86) were found to be more active than 6-gingerol derivatives (compounds 19, 40, 67, 69, 71, 73, 75, 77, and 79). Also, it was reported that a hydrogen bond acceptor is needed at position C4 on the aromatic ring for a higher potency. In addition, the presence of a hydroxyl group substituent on the alkyl chain has been correlated with higher antibacterial activity.4
A recent study, published in 2019, reported the application of 6-gingerol and two of its derivatives (85, 86) to reduce biofouling in reverse osmosis water treatment systems by disrupting the QS processes of P. aeruginosa. The study aimed to propose effective but harmless solution to membrane biofouling as the biocides in use may pose toxicity problems. In accordance with the above-mentioned results reported by Choi et al., compound 85 was the most effective in inhibiting biofilm formation followed by compound 86 and then 6-gingerol (38%, 35%, and 22% reduction in biofilm formation, respectively), while only 4% inhibition was observed for sodium hypochlorite (NaOCl) used as a positive control in this study (only 10 μM NaOCl concentration was used in the study for the purpose of comparison, while its MIC is ranging between 33.6–40.3 mM). These results were confirmed by the reduction in QS-responsive gene expression. However, bacterial growth was not affected by the compounds investigated.74
Anti-platelet gingerol analogues
The anti-platelet activity of ginger was also investigated, and ginger constituents like 6-gingerol and 6-shogaol were reported to exert potent antiplatelet activity.75,76Shih et al. reported the synthesis of 6-gingerol and a group of 45 derivatives that are either naturally present in ginger root or new synthetic analogues (compounds 17, 20, 21, 29, 40, and 41, Fig. 2; 47, 48, and 51, Fig. 3; 63–65, 81, 82, and 83, Fig. 4; and 87–116, Fig. 5), and testing these compounds for anti-platelet aggregation activity. It was demonstrated that at 10 μg ml−1 concentration most of the compounds exert an over 90% inhibition of platelet aggregation induced by 100 μM of arachidonic acid. Compounds of the paradol series (47, 51 and 103–107) were the most active, and 6-paradol (51) showed the highest activity (IC50 = 0.070 μg ml−1 compared to 1 μg ml−1 for 6-gingerol). A decrease in the activity was observed with the introduction of a double bond (as in shogaols, or dehydroparadols) or a hydroxyl group (as in gingerols) into the paradol side chain, however, increasing the alkyl side chain length increased the activity (e.g. the dehydroparadol compound 110 showed an IC50 value of 0.160 μg ml−1). The epoxide derivatives (111–116) showed a lower potency compared to n-paradols (IC50 = 0.96–2.38 μg ml−1). On the other hand, it was reported that the compounds showed negligible activity against platelet aggregation induced by platelet activating factor (PAF) or thrombin (Thr), suggesting that compound 51 is a selective inhibitor.77
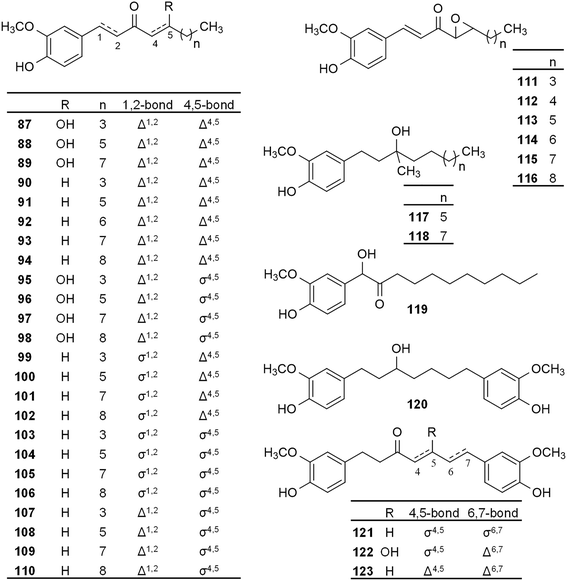 | ||
| Fig. 5 Semi-synthetic gingerol derivatives prepared and evaluated for their in vitro anti-platelet activity. | ||
Koo et al. studied the effect of 6-gingerol and its synthetic analogues (20, Fig. 2; 54, 55, Fig. 3; and 117–119, Fig. 5) on the arachidonic acid-induced platelet serotonin release and aggregation, and found lower platelet aggregation inhibitory activity for all compounds (ICmax = 10–25 μM) as compared to acetylsalicylic acid (ICmax = 6 μM). However, the compounds acted in a similar dose range as Aspirin when tested on arachidonic acid-induced platelet serotonin release. To examine the underlying mechanism, COX-inhibitory activity of these compounds was assessed. Compounds 55, 20, and 54 exerted similarly potent inhibitory activity (IC50 = 1.2, 1.5, and 3.3 μM, respectively) as the positive control indomethacin (IC50 = 0.76 μM), unlike 6-gingerol (IC50 = 50 μM). It is worth mentioning that the COX inhibitory activity of the compounds correlated with their hydrophobicity, with compound 55 being the most active and the most hydrophobic at the same time.78
The anti-platelet and COX-1 inhibitory activity were also studied for another set of gingerol derivatives (17, 20, 21, 30, 42, Fig. 2; and 47–49, 51, 54–58, Fig. 3; 65, Fig. 4; and 120–123, Fig. 5). 8-Paradol (47) was reported as the most effective anti-platelet agent among the investigated analogues (75% inhibition at 2 μM while 6-gingerol exerted 3.4% inhibition at the same concentration). In case of compounds 47, 49 and 58 the COX-1 inhibitory activity was also assessed through monitoring the amount of the pro-aggregatory product thromboxane A2. Compound 47 also exerted the highest activity and it was more potent than aspirin under the conditions described in the study (IC50 = 4 μM vs. 20 μM). Investigations into the SAR revealed that presence of the carbonyl function at C3 is important for activity, any other substituents on the alkyl chain interfere with the COX-1 inhibitory activity. This was evidenced through comparing the activity of compound 47 with that of 49 (IC50 = 20 μM). No correlation between the molecular hydrophobicity and anti-platelet aggregation activity was found in this case.79
The above-mentioned in vitro results seem to be promising and may also point towards cardiovascular protective drug development. Nevertheless, further research would be needed to evaluate the in vivo efficacy and safety of gingerol analogues to assess their potential as anti-platelet lead or candidate drugs.
Miscellaneous bioactivities
Several other bioactivities were reported for ginger and its constituents, e.g. smooth muscle relaxant, painkiller, antiemetic, and anti-obesity activity. Altogether, these reports suggest a versatile pharmacology to these natural products. Anti-emetic activity of 6-gingerol was also confirmed in a phase II randomized double-blind placebo-controlled study in cancer patients treated with highly emetogenic chemotherapeutic agents.25 A recent study on rats revealed that the underlying mechanism of action is the modulation of serotonin levels.80 Although the effect of ginger in nausea and vomiting prevention and treatment was thoroughly studied and showed promising results, to the best of our knowledge no related studies were dedicated for semisynthetic derivatives of 6-gingerol.Gingerol analogues with activity on muscles' Ca2+ homeostasis
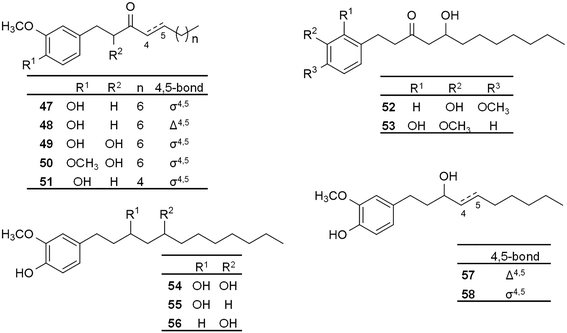
In 1988, 6-gingerol and compounds 20–22, 28, 30, 42 (Fig. 2), and 124–131 (Fig. 6) were investigated for their effect on spontaneous Ca2+ spikes and contraction of portal veins isolated from mice. Most of the tested compounds suppressed spontaneous contractions; however, the most promising activity was found for 6-gingerol and derivative 129. Interestingly, compound 20 (8-gingerol) also inhibited spontaneous contractions but without affecting Ca2+ spikes. It was suggested that the inhibition of spontaneous contraction is due to Ca2+ spike suppression in case of 6-gingerol, while 8-gingerol acts through a different mechanism of action. SAR evaluation revealed that the optimal alkyl chain length for higher potency is ten carbons (i.e., the chain length of 6-gingerol), and the reduction of carbonyl group on C3 of the alkyl side chain or oxidation of the 5-hydroxyl group decreases smooth muscle relaxant activity.81Based on its stimulating activity on cardiac sarcoplasmic reticulum (CSR) Ca2+ ATPase, 6-gingerol was reported as a potent cardiotonic agent by Kobayashi et al., and it was also found active on skeletal muscles.82 Based on a streptozotocin-treated diabetic mouse model, this bioactivity also confers 6-gingerol a beneficial activity in diabetes-related diastolic dysfunction.83 When studying this bioactivity for 6-, 8-, and 10-gingerol and their derivatives (20, 21; Fig. 2, and 132–134; Fig. 6), all the tested compounds were found to increase the SR-ATPase activity in a concentration-dependent manner. Therefore, it was postulated that they may play a role in Ca2+ – pumping from the cytoplasm to the SR lumen causing skeletal muscle relaxation, and both the hydrocarbon chain and the o-methoxyphenol parts were postulated as necessary for the activity.84
Pain management: TRPV and TRPA modulation
Ginger has a proven painkiller activity operating via multiple mechanisms: COX and lipoxygenase (LOX) enzymes inhibition, inhibition of NF-kβ, or acting as vanilloid receptor agonist.85 The transient receptor potential (TRP) cation channel subfamily V, member 1 (TRPV1), and subfamily A, member 1 (TRPA1) are known for their integral role in pain and neurogenic inflammation. Recent studies revealed that TRP channels are crucial in other physiological and pathological conditions, like cancer, cardiac health, and renal physiology. However, their precise function in vivo is still yet to be revealed.86–88In 2007, inspired by the activity of the oleyl moiety on TRPV1 receptor,89 the synthesis of related gingerol and shogaol analogues (oleylgingerol; 135, and oleylshogaol; 136) was reported. When testing TRPV1 activating effect of 6-gingerol and its derivatives (17, 20, 21; Fig. 2, 48; Fig. 3, 101; Fig. 5, 135 and 136; Fig. 6), all compounds showed higher activity (EC50 = 0.26–4.17 μM) than 6-gingerol (EC50 = 4.55 μM) but lower than the positive control capsaicin (EC50 = 0.082 μM). Oleylgingerol was the most active, while oleylshogaol the least active of them, suggesting that the 5-hydroxyl group has a significant role in activating the TRPV1 channel.90
To explore the chemical space around 6-gingerol concerning its potential for vanilloid receptor modulation, Morera et al. reported the synthesis of racemic 6-gingerol analogues (17; Fig. 1, 67, 75; Fig. 4 and 137–152; Fig. 6) and their biological evaluation on TRPV1 and TRPA1 channels.91 With regard to the activity on TRPV1, compound 141 exerted the highest potency and selectivity among all (EC50 = 0.11 μM, compared to 3.3 μM for 6-gingerol), whereas compounds 138, 144, and 145, each containing two isopropyl groups in ortho position to the phenolic hydroxyl group, were found to be inactive. This indicates that steric hindrance around the phenolic hydroxyl function negatively affects the activity. Further studies into the SAR showed importance of the phenolic hydroxyl group and noted an increase in activity with the increase in molecular lipophilicity. The free hydroxyl group on the side chain was found to be of less importance as proved by the efficacy of 6-shogaol (17) and its analogue (152) on TRPV1 channels. On the contrary, activity on TRPA1 channels was the highest in case of compound 139, with favoured branched alkyl substituents at the ortho position to the phenolic hydroxyl group. It is noteworthy that compounds 17, 141 and 151 acted as selective TRPV1 agonists, while compounds 143, 144, 146, and 147 were selective TRPA1 antagonists.91
Antidiabetic and antihepatotoxic activities
Results from a randomized clinical trial on the possible antidiabetic potential of ginger consumption suggested a decrease in blood glucose and cholesterol levels.92Moreover, synergistic effect of 6-gingerol and quercetin was investigated in streptozotocin-induced type 2 diabetes and poloxamer P-407 induced hyperlipidaemia on rats. The combination treatment was found to exert remarkable antidiabetic and beneficial cardiac effects, and the synergism was suggested to occur through the modulation of serotonergic system.93
In metabolic syndrome, the prophylactic effect of 6-gingerol and its synthetic analogue aza-6-gingerol (153) was investigated on high fat diet-fed type 2 diabetic mice. Both compounds caused a reduction of lipogenic genes, which was postulated to occur through downregulation of sterol regulatory element-binding protein 1c (SREBP-1c), a protein that is responsible for regulating the transcription of many lipogenic genes. Compound 153 was found more effective than 6-gingerol in enhancing metabolism and reducing the extent of lipogenesis. It was suggested that compound 153 might possess potential therapeutic value to reduce the risk of obesity-associated diseases, however, further studies are needed to uncover the underlying mechanism of action.94
Liu et al. reported the repressive activity of 6-gingerol on nutritional steatohepatitis induced in mice. The protective effect was postulated to occur due to regulation of key genes related to oxidative stress, inflammation and fibrogenesis.95 Antihepatotoxic effects of gingerols and shogaols on carbon tetrachloride- and galactosamine-induced cytotoxicity in primary cultured rat hepatocytes were also studied. Compounds 17, 20–22, 28–30, 42, 43 (Fig. 2), 48 (Fig. 3), 59 (Fig. 4), 100–102 (Fig. 5), 124, 126, and 154–160 (Fig. 6) were evaluated, and both gingerols and shogaols were found to exert anti-hepatotoxic actions, with gingerols being superior in this regard. Studies into the SAR revealed importance of the side chain length with the highest activity achieved with (7) and (8)-congeners. Reduction of the carbonyl group on the side chain, oxidation of the 5-hydroxyl group, or introduction of a double bond into the side chain were found to decrease the activity of gingerols.96
Conclusions
6-Gingerol, the main constituent of Zingiber officinale (Zingiberaceae) has been extensively studied in the last century for its potential anti-inflammatory, antioxidant, antimicrobial and anticancer effects. Several clinical studies have been performed and some are currently in progress to confirm these observations. The beneficial activities of ginger inspired several research groups to explore the chemical space around 6-gingerol: chemical modifications have led to numerous derivatives interfering with multiple cellular pathways leading to improved anticancer, anti-inflammatory, antimicrobial anti-platelet, and/or various other bioactivities. Some of these derivatives seem to show serious potential to drug discovery, concerning their sub-micromolar efficacy as anti-inflammatory and/or antiplatelet agents, or vanilloid receptor agonist. Nevertheless, in vivo studies are missing for many of these compounds so that their efficacy and safety profile could be better evaluated, and to the best of our knowledge none of the semi-synthetic derivatives of gingerol reached a clinical trial until now. Therefore, there is still much to learn on the true drug discovery potential that can be attributed to the chemical space surrounding this relatively simple, but versatile natural product, 6-gingerol.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Support from the National Research, Development and Innovation Office, Hungary (NKFIH; K-134704), and the Ministry of Human Capacities, Hungary grant 20391-3/2018/FEKUSTRAT is acknowledged.References
- Y. a. Han, C. w. Song, W. s. Koh, G. h. Yon, Y. s. Kim, S. y. Ryu, H. j. Kwon and K. h. Lee, Phytother. Res., 2013, 27, 1200–1205 CrossRef CAS PubMed.
- Q. Q. Mao, X. Y. Xu, S. Y. Cao, R. Y. Gan, H. Corke, T. Beta and H. B. Li, Foods, 2019, 8, 185 CrossRef CAS PubMed.
- S. H. Nile and P. Se Won, Ind. Crops Prod., 2015, 70, 238–244 CrossRef CAS.
- H. Choi, S. Y. Ham, E. Cha, Y. Shin, H. S. Kim, J. K. Bang, S. H. Son, H. D. Park and Y. Byun, J. Med. Chem., 2017, 60, 9821–9837 CrossRef CAS PubMed.
- C. K. Wei, Y. H. Tsai, M. Korinek, P. H. Hung, M. El-Shazly, Y. B. Cheng, Y. C. Wu, T. J. Hsieh and F. R. Chang, Int. J. Mol. Sci., 2017, 18, 168 CrossRef.
- A. J. Akinyemi, G. R. Thome, V. M. Morsch, N. Stefanello, J. F. Goularte, A. Belló-Klein, G. Oboh and M. R. C. Schetinger, J. Funct. Foods, 2015, 17, 792–801 CrossRef CAS.
- S. C. Ho, K. S. Chang and C. C. Lin, Food Chem., 2013, 141, 3183–3191 CrossRef CAS.
- S. Suk, G. T. Kwon, E. Lee, W. J. Jang, H. Yang, J. H. Kim, N. R. Thimmegowda, M. Y. Chung, J. Y. Kwon, S. Yang, J. K. Kim, J. H. Y. Park and K. W. Lee, Mol. Nutr. Food Res., 2017, 61 DOI:10.1002/mnfr.201700139.
- L. B. Martins, A. Rodrigues, D. F. Rodrigues, L. C. Dos Santos, A. L. Teixeira and A. V. M. Ferreira, Cephalalgia, 2019, 39, 68–76 CrossRef.
- M. Jalali, M. Mahmoodi, S. P. Moosavian, R. Jalali, G. Ferns, A. Mosallanezhad, M. H. Imanieh and Z. Mosallanezhad, Phytother. Res., 2020, 1723–1733 CrossRef CAS.
- O. E. Orisakwe, C. N. Orish and E. O. Nwanaforo, Scientific African, 2020, e00620, DOI:10.1016/j.sciaf.2020.e00620.
- D. Sen and P. Debnath, J. Biomol. Struct. Dyn., 2020, 1–22 CrossRef.
- S. Kumar, P. Kashyap, S. Chowdhury, S. Kumar, A. Panwar and A. Kumar, Phytomedicine, 2020, 153317, DOI:10.1016/j.phymed.2020.153317.
- WHO, https://www.who.int/southeastasia/outbreaks-and-emergencies/novel-coronavirus-2019/fact-or-fiction, accessed 18.12 2020.
- D. Silveira, J. M. Prieto-Garcia, F. Boylan, O. Estrada, Y. M. Fonseca-Bazzo, C. M. Jamal, P. O. Magalhães, E. O. Pereira, M. Tomczyk and M. Heinrich, Front. Pharmacol., 2020, 11, 581840 CrossRef CAS.
- M. F. Mahomoodally, M. Z. Aumeeruddy, K. R. R. Rengasamy, S. Roshan, S. Hammad, J. Pandohee, X. Hu and G. Zengin, Semin. Cancer Biol., 2021, 69, 140–149 CrossRef CAS.
- M.-J. Ko, H.-H. Nam and M.-S. Chung, Food Chem., 2019, 270, 149–155 CrossRef CAS.
- R. M. T. de Lima, A. C. Dos Reis, A. P. M. de Menezes, J. V. O. Santos, J. Filho, J. R. O. Ferreira, M. de Alencar, A. da Mata, I. N. Khan, A. Islam, S. J. Uddin, E. S. Ali, M. T. Islam, S. Tripathi, S. K. Mishra, M. S. Mubarak and A. A. C. Melo-Cavalcante, Phytother. Res., 2018, 32, 1885–1907 CrossRef CAS PubMed.
- S. Wang, C. Zhang, G. Yang and Y. Yang, Nat. Prod. Commun., 2014, 9, 1027–1030 CAS.
- A. Dehghani Nazhvani, N. Sarafraz, F. Askari, F. Heidari and M. Razmkhah, Asian Pac. J. Cancer Prev., 2020, 21, 479–484 CrossRef PubMed.
- P. Mega Tiber, S. Kocyigit Sevinc, O. Kilinc and O. Orun, Gene, 2019, 692, 217–222 CrossRef CAS.
- N. Kaewtunjai, R. Wongpoomchai, A. Imsumran, W. Pompimon, A. Athipornchai, A. Suksamrarn, T. R. Lee and W. Tuntiwechapikul, ACS Omega, 2018, 3, 18572–18581 CrossRef CAS.
- M. Crichton, S. Marshall, W. Marx, A. L. McCarthy and E. Isenring, J. Acad. Nutr. Diet., 2019, 119, 2055–2068 CrossRef PubMed.
- A. Uthaipaisanwong and S. Oranratanaphan, Support. Care Cancer, 2019, 3831–3838 Search PubMed.
- J. Konmun, K. Danwilai, N. Ngamphaiboon, B. Sripanidkulchai, A. Sookprasert and S. Subongkot, Med. Oncol., 2017, 34, 69 CrossRef CAS PubMed.
- A. Almatroudi, M. A. Alsahli, F. Alrumaihi, K. S. Allemailem and A. H. Rahmani, Curr. Pharm. Biotechnol., 2019, 20, 5–16 CAS.
- C. Chatupheeraphat, C. Nantasenamat, K. Deesrisak, S. Roytrakul, U. Anurathapan and D. Tanyong, EXCLI J., 2020, 19, 582–595 Search PubMed.
- N. Sp, D. Y. Kang, J. M. Lee, S. W. Bae and K. J. Jang, Int. J. Mol. Sci., 2021, 22, 4660 CrossRef CAS PubMed.
- J. Czarnik-Kwasniak, K. Kwasniak, P. Kwasek, E. Swierzowska, A. Strojewska and J. Tabarkiewicz, Nutrients, 2019, 12, 96 CrossRef.
- Y. Sun, J. Ren and F. Wang, J. Biochem. Mol. Toxicol., 2021, 35, e22689 CAS.
- C. J. Weng and G. C. Yen, Cancer Treat. Rev., 2012, 38, 76–87 CrossRef CAS PubMed.
- C. Tonelli, I. I. C. Chio and D. A. Tuveson, Antioxid. Redox Signaling, 2018, 29, 1727–1745 CrossRef CAS.
- M. G. van der Wijst, R. Brown and M. G. Rots, Biochim. Biophys. Acta, 2014, 1846, 494–509 CAS.
- S. Vomund, A. Schäfer, M. J. Parnham, B. Brüne and A. von Knethen, Int. J. Mol. Sci., 2017, 18, 2772 CrossRef.
- S. Nafees, M. Zafaryab, S. H. Mehdi, B. Zia, M. A. Rizvi and M. A. Khan, Anti-Cancer Agents Med. Chem., 2021, 21, 428–432 CrossRef CAS.
- J. Citronberg, R. Bostick, T. Ahearn, D. K. Turgeon, M. T. Ruffin, Z. Djuric, A. Sen, D. E. Brenner and S. M. Zick, Cancer Prev. Res., 2013, 6, 271–281 CrossRef.
- Y. Jiang, D. K. Turgeon, B. D. Wright, E. Sidahmed, M. T. Ruffin, D. E. Brenner, A. Sen and S. M. Zick, Eur. J. Cancer Prev., 2013, 22, 455–460 CrossRef CAS.
- S. M. Zick, D. K. Turgeon, S. K. Vareed, M. T. Ruffin, A. J. Litzinger, B. D. Wright, S. Alrawi, D. P. Normolle, Z. Djuric and D. E. Brenner, Cancer Prev. Res., 2011, 4, 1929–1937 CrossRef.
- S. M. Zick, D. K. Turgeon, J. Ren, M. T. Ruffin, B. D. Wright, A. Sen, Z. Djuric and D. E. Brenner, Mol. Carcinog., 2015, 54, 908–915 CrossRef CAS.
- W. C. de Lima Silva, R. Conti, L. C. de Almeida, P. A. B. Morais, K. B. Borges, V. L. Junior, L. V. Costa-Lotufo and W. de Souza Borges, Curr. Top. Med. Chem., 2020, 20, 161–169 CrossRef PubMed.
- A. S. Ibrahim, M. A. Sobh, H. M. Eid, A. Salem, H. H. Elbelasi, M. H. El-Naggar, F. M. AbdelBar, H. Sheashaa, M. A. Sobh and F. A. Badria, Tumor Biol., 2014, 35, 9941–9948 CrossRef CAS.
- L. Luna-Dulcey, J. A. da Silva and M. R. Cominetti, Anti-Cancer Drugs, 2020, 31, 35–43 CrossRef CAS.
- L. Luna-Dulcey, R. Tomasin, M. A. Naves, J. A. da Silva and M. R. Cominetti, Oncotarget, 2018, 9, 30787–30804 CrossRef.
- Y. Zhu, F. Wang, Y. Zhao, P. Wang and S. Sang, Sci. Rep., 2017, 7, 40119 CrossRef CAS PubMed.
- X. Chen, S. Wang, N. Wu and C. S. Yang, Curr. Cancer Drug Targets, 2004, 4, 267–283 CrossRef CAS.
- N. Oi, H. Yamamoto, A. Langfald, R. Bai, M. H. Lee, A. M. Bode and Z. Dong, Carcinogenesis, 2017, 38, 728–737 CrossRef CAS.
- M. H. El-Naggar, A. Mira, F. M. Abdel Bar, K. Shimizu, M. M. Amer and F. A. Badria, Bioorg. Med. Chem., 2017, 25, 1277–1285 CrossRef CAS PubMed.
- J. E. Bolden, M. J. Peart and R. W. Johnstone, Nat. Rev. Drug Discovery, 2006, 5, 769–784 CrossRef CAS PubMed.
- Z. Jiang, W. Li, X. Hu, Q. Zhang, T. Sun, S. Cui, S. Wang, Q. Ouyang, Y. Yin, C. Geng, Z. Tong, Y. Cheng, Y. Pan, Y. Sun, H. Wang, T. Ouyang, K. Gu, J. Feng, X. Wang, S. Wang, T. Liu, J. Gao, M. Cristofanilli, Z. Ning and X. Lu, Lancet Oncol., 2019, 20, 806–815 CrossRef CAS PubMed.
- C. Robert and F. V. Rassool, Adv. Cancer Res., 2012, 116, 87–129 CrossRef CAS PubMed.
- J.-H. Lee, M. L. Choy, L. Ngo, S. S. Foster and P. A. Marks, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14639–14644 CrossRef CAS PubMed.
- I. A. M. Groh, C. Chen, C. Lüske, A. T. Cartus and M. Esselen, J. Nutr. Metab., 2013, 2013, 821082 Search PubMed.
- A. K. Kiss, in Handbook of Nutrition, Diet, and Epigenetics, ed. V. Patel and V. Preedy, Springer International Publishing, Cham, 2017, DOI: DOI:10.1007/978-3-319-31143-2_105-1, pp. 1–21.
- L. W. Evans and B. S. Ferguson, Nutrients, 2018, 10, 1120 CrossRef.
- P. Kumboonma, T. Senawong, S. Saenglee, C. Yenjai and C. Phaosiri, Med. Chem. Res., 2017, 26, 650–661 CrossRef CAS.
- D.-L. Lu, X.-Z. Li, F. Dai, Y.-F. Kang, Y. Li, M.-M. Ma, X.-R. Ren, G.-W. Du, X.-L. Jin and B. Zhou, Food Chem., 2014, 165, 191–197 CrossRef CAS PubMed.
- M. Rondanelli, A. Riva, P. Allegrini, M. A. Faliva, M. Naso, G. Peroni, M. Nichetti, C. Gasparri, D. Spadaccini, G. Iannello, V. Infantino, T. Fazia, L. Bernardinelli and S. Perna, J. Pain Res., 2020, 13, 761–770 CrossRef CAS.
- R. D. Altman and K. C. Marcussen, Arthritis Rheum., 2001, 44, 2531–2538 CrossRef CAS.
- D. C. Nieman, R. A. Shanely, B. Luo, D. Dew, M. P. Meaney and W. Sha, Nutr. J., 2013, 12, 154 CrossRef.
- S. Atashak, M. Peeri, M. A. Azarbayjani, S. R. Stannard and M. M. Haghighi, J. Sports Sci. Med., 2011, 10, 685–691 Search PubMed.
- N. Aryaeian, M. Mahmoudi, F. Shahram, S. Poursani, F. Jamshidi and H. Tavakoli, `Med J. Islam Repub. Iran., 2019, 33, 154 Search PubMed.
- F. L. Zhang, B. W. Zhou, Z. Z. Yan, J. Zhao, B. C. Zhao, W. F. Liu, C. Li and K. X. Liu, Cytokine, 2020, 125, 154854 CrossRef CAS.
- T. Bergsbaken, S. L. Fink and B. T. Cookson, Nat. Rev. Microbiol., 2009, 7, 99–109 CrossRef CAS.
- E. Tjendraputra, V. H. Tran, D. Liu-Brennan, B. D. Roufogalis and C. C. Duke, Bioorg. Chem., 2001, 29, 156–163 CrossRef CAS PubMed.
- F. Aktan, S. Henness, V. H. Tran, C. C. Duke, B. D. Roufogalis and A. J. Ammit, Planta Med., 2006, 72, 727–734 CrossRef CAS PubMed.
- A. Azizi, S. Aghayan, S. Zaker, M. Shakeri, N. Entezari and S. Lawaf, Int. J. Dent., 2015, 2015, 489842 Search PubMed.
- S. Hasan, M. Danishuddin and A. U. Khan, BMC Microbiol., 2015, 15, 1 CrossRef.
- J. H. Lee, Y. G. Kim, P. Choi, J. Ham, J. G. Park and J. Lee, Front. Cell. Infect. Microbiol., 2018, 8, 299 CrossRef.
- B. O. Oyedemi, E. M. Kotsia, P. D. Stapleton and S. Gibbons, J. Ethnopharmacol., 2019, 245, 111871 CrossRef CAS.
- L. Cai, S. Liu, L. Sun, Y. Wang, H. Ji and J. Li, Front. microbiol., 2015, 6, 981 Search PubMed.
- H. S. Kim, S. H. Lee, Y. Byun and H. D. Park, Sci. Rep., 2015, 5, 8656 CrossRef CAS PubMed.
- S. T. Rutherford and B. L. Bassler, Cold Spring Harbor Perspect. Med., 2012, 2, a012427 Search PubMed.
- N. V. Kumar, P. S. Murthy, J. R. Manjunatha and B. K. Bettadaiah, Food Chem., 2014, 159, 451–457 CrossRef.
- S.-Y. Ham, H.-S. Kim, Y. Jang, P.-F. Sun, J.-H. Park, J. Lee, Y. Byun and H.-D. Park, Fuel, 2019, 250, 79–87 CrossRef CAS.
- G. E. Hirsch, P. R. N. Viecili, A. S. de Almeida, S. Nascimento, F. G. Porto, J. Otero, A. Schmidt, B. da Silva, M. M. Parisi and J. Z. Klafke, Curr. Pharm. Des., 2017, 23, 1228–1246 CrossRef CAS.
- Y. R. Liao, Y. L. Leu, Y. Y. Chan, P. C. Kuo and T. S. Wu, Molecules, 2012, 17, 8928–8937 CrossRef CAS.
- H. C. Shih, C. Y. Chern, P. C. Kuo, Y. C. Wu, Y. Y. Chan, Y. R. Liao, C. M. Teng and T. S. Wu, Int. J. Mol. Sci., 2014, 15, 3926–3951 CrossRef CAS PubMed.
- K. L. Koo, A. J. Ammit, V. H. Tran, C. C. Duke and B. D. Roufogalis, Thromb. Res., 2001, 103, 387–397 CrossRef CAS PubMed.
- E. Nurtjahja-Tjendraputra, A. J. Ammit, B. D. Roufogalis, V. H. Tran and C. C. Duke, Thromb. Res., 2003, 111, 259–265 CrossRef CAS.
- Q. Cheng and X. Feng, Drug Des., Dev. Ther., 2020, 14, 4085–4099 CrossRef CAS.
- I. Kimura, L. R. Pancho, T. Shiori and M. Kimura, Jpn. J. Pharmacol., 1988, 48, 257–262 CrossRef CAS.
- M. Kobayashi, N. Shoji and Y. Ohizumi, Biochim. Biophys. Acta, Biomembr., 1987, 903, 96–102 CrossRef CAS.
- I. Namekata, S. Hamaguchi, Y. Wakasugi, M. Ohhara, Y. Hirota and H. Tanaka, Eur. J. Pharmacol., 2013, 706, 48–55 CrossRef CAS PubMed.
- Y. Ohizumi, S. Sasaki, K. Shibusawa, K. Ishikawa and F. Iketomo, Biol. Pharm. Bull., 1996, 19, 1377–1379 CrossRef CAS.
- M. Rondanelli, F. Fossari, V. Vecchio and C. Gasparri, Phytother. Res., 2020, 34, 2843–2856 CrossRef PubMed.
- E. S. Fernandes, M. A. Fernandes and J. E. Keeble, Br. J. Pharmacol., 2012, 166, 510–521 CrossRef CAS.
- M. M. Moran, M. A. McAlexander, T. Bíró and A. Szallasi, Nat. Rev. Drug Discovery, 2011, 10, 601–620 CrossRef CAS.
- A. Samanta, T. E. T. Hughes and V. Y. Moiseenkova-Bell, Subcell. Biochem., 2018, 87, 141–165 CAS.
- A. Morita, Y. Iwasaki, K. Kobata, T. Iida, T. Higashi, K. Oda, A. Suzuki, M. Narukawa, S. Sasakuma, H. Yokogoshi, S. Yazawa, M. Tominaga and T. Watanabe, Life Sci., 2006, 79, 2303–2310 CrossRef CAS PubMed.
- A. Morita, Y. Iwasaki, K. Kobata, H. Yokogoshi and T. Watanabe, Biosci., Biotechnol., Biochem., 2007, 71, 2304–2307 CrossRef CAS.
- E. Morera, L. De Petrocellis, L. Morera, A. S. Moriello, M. Nalli, V. Di Marzo and G. Ortar, Bioorg. Med. Chem. Lett., 2012, 22, 1674–1677 CrossRef CAS.
- G. C. N. Carvalho, J. C. G. Lira-Neto, M. F. M. d. Araújo, R. W. J. F. d. Freitas, M. L. Zanetti and M. M. C. Damasceno, Rev. Lat.-Am. Enferm., 2020, 28, e3369 CrossRef.
- Y. Shao, Y. Yu, C. Li, J. Yu, R. Zong and C. Pei, RSC Adv., 2016, 6, 12235–12242 RSC.
- M. Okamoto, H. Irii, Y. Tahara, H. Ishii, A. Hirao, H. Udagawa, M. Hiramoto, K. Yasuda, A. Takanishi, S. Shibata and I. Shimizu, J. Med. Chem., 2011, 54, 6295–6304 CrossRef CAS.
- T.-F. Tzeng, S.-S. Liou and I. M. Liu, RSC Adv., 2014, 4, 61427–61436 RSC.
- H. Hikino, Y. Kiso, N. Kato, Y. Hamada, T. Shioiri, R. Aiyama, H. Itokawa, F. Kiuchi and U. Sankawa, J. Ethnopharmacol., 1985, 14, 31–39 CrossRef CAS.
Footnote |
| † Equal contribution from the first two authors. |
| This journal is © The Royal Society of Chemistry 2021 |