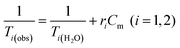Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Di- and tri-component spinel ferrite nanocubes: synthesis and their comparative characterization for theranostic applications†
Niccolò
Silvestri
 a,
Helena
Gavilán
a,
Pablo
Guardia
ab,
Rosaria
Brescia
a,
Helena
Gavilán
a,
Pablo
Guardia
ab,
Rosaria
Brescia
 a,
Soraia
Fernandes‡
a,
Anna Cristina S.
Samia
a,
Soraia
Fernandes‡
a,
Anna Cristina S.
Samia
 c,
Francisco J.
Teran
c,
Francisco J.
Teran
 de and
Teresa
Pellegrino
de and
Teresa
Pellegrino
 *a
*a
aIstituto Italiano di Tecnologia, Via Morego 30, 16163 Genova, Italy. E-mail: Teresa.Pellegrino@iit.it
bIREC-Catalonia Institute for Energy Research, Jardins de les Dones de Negre 1, Sant Adria de Besos, 08930 Barcelona, Spain
cDepartment of Chemistry, Case Western Reserve University, 10900 Euclid Avenue, Cleveland, OH 44106, USA
diMdea Nanociencia, Campus Universitario de Cantoblanco, 28049 Madrid, Spain
eNanobiotecnología (iMdea-Nanociencia), Unidad Asociada al Centro Nacional de Biotecnología (CSIC), 28049 Madrid, Spain
First published on 3rd August 2021
Abstract
Spinel ferrite nanocubes (NCs), consisting of pure iron oxide or mixed ferrites, are largely acknowledged for their outstanding performance in magnetic hyperthermia treatment (MHT) or magnetic resonance imaging (MRI) applications while their magnetic particle imaging (MPI) properties, particularly for this peculiar shape different from the conventional spherical nanoparticles (NPs), are relatively less investigated. In this work, we report on a non-hydrolytic synthesis approach to prepare mixed transition metal ferrite NCs. A series of NCs of mixed zinc-cobalt-ferrite were prepared and their magnetic theranostic properties were compared to those of cobalt ferrite or zinc ferrite NCs of similar sizes. For each of the nanomaterials, the synthesis parameters were adjusted to obtain NCs in the size range from 8 up to 15 nm. The chemical and structural nature of the different NCs was correlated to their magnetic properties. In particular, to evaluate magnetic losses, we compared the data obtained from calorimetric measurements to the data measured by dynamic magnetic hysteresis obtained under alternating magnetic field (AMF) excitation. Cobalt-ferrite and zinc-cobalt ferrite NCs showed high specific adsorption rate (SAR) values in aqueous solutions but their heating ability was drastically suppressed once in viscous media even for NCs as small as 12 nm. On the other hand, non-stoichiometric zinc-ferrite NCs showed significant but lower SAR values than the other ferrites, but these zinc-ferrite NCs preserved almost unaltered their heating trend in viscous environments. Also, the presence of zinc in the crystal lattice of zinc–cobalt ferrite NCs showed increased contrast enhancement for MRI with the highest T2 relaxation time and in the MPI signal with the best point spread function and signal-to-noise ratio in comparison to the analogue cobalt-ferrite NC. Among the different compositions investigated, non-stoichiometric zinc-ferrite NCs can be considered the most promising material as a multifunctional theranostic platform for MHT, MPI and MRI regardless of the media viscosity in which they will be applied, while ensuring the best biocompatibility with respect to the cobalt ferrite NCs.
Introduction
Iron oxide nanocubes (IONCs) are promising materials in the biomedical field.1–4 A clear example are magnetosomes,5 which are naturally produced liposome coated nanocrystals (30–120 nm) of magnetite with a cubic/cuboid shape that are often organized in chain structures. Their high chemical and crystal purity and narrow size distribution, positively affect their T2-relaxivity (as negative MRI contrast agents6), MPI performance7 and SAR value for MHT.1,8 Perhaps one of the main drawbacks is their bacteria derivation, which limits their use in human applications due to possible immune-response reactions. On the other hand, engineered IONCs that are chemically synthesized using thermal decomposition methods display similar qualities and excellent magnetic performance similar to that of magnetosomes.9 Moreover, the synthetic route offers the possibility of fine tuning the size in a large range (from 14 to 80 nm) and the composition of the NCs. Ferrites (such as IONPs) of the general formula MFe2O4, display a spinel structure (both normal and inverse) where M could be any of the divalent transition metal ions (Fe2+, Co2+, Zn2+, Mn2+, etc.). The spinel ferrite structure corresponds to a face-centered cubic arrangement of oxygen atoms, with M2+ and Fe3+ occupying tetrahedral (A) sites and Fe3+ occupying also octahedral (B) sites. For instance, stoichiometric zinc-ferrite (Zn-ferrite) has a spinel structure with Zn2+ ions occupying the A sites and Fe3+ ions occupying the B sites.10,11 The doping of iron oxide (magnetite) ferrites with other metal ions (as Co2+, Zn2+, Mn2+) offers an alternative way to improve the physical properties of magnetic ferrite NPs and thus their usefulness in bioapplications.12 For instance, starting from Fe3O4 (magnetite), Fe2+ cations can be partially substituted by divalent cations such as Co2+ or Zn2+ leading to Zn-ferrite and cobalt-ferrite (Co-ferrite) NPs.10,11,13 Co-ferrite has gained attention because of its strong magneto-crystalline anisotropy, moderate high saturation magnetization, and high coercivity.14–16 For example, Co-ferrite NCs synthesized by non-hydrolytic methods were proven to have higher SAR values than IONCs prepared in aqueous media: indeed, by finely tuning the NC size (from 15 to 27 nm) and the cobalt stoichiometry (from x = 0.1–0.7 in CoxFe3−xO4) it was found that the cobalt content governs the heating properties of the final nanocubes.16,17 However, increasing the viscosity of the dispersion media reduces SAR values due to restriction of the Brownian magnetic relaxation.18 Moreover, in an in vivo preclinical study on Co-ferrite NCs at the primary tumor site, the authors have shown how it is possible to exploit the intrinsic toxicity of cobalt ions, slowly released upon NC degradation in combination with their very mild hyperthermia and their unique NC alignment feature under MHT as multifunctional platforms to completely suppress tumor growth.19,20 Alternatively, the Zn-ferrite composition may be used as a more biocompatible formulation than Co-ferrite. The Food and Drug Administration (FDA) sets the reference daily intake (RDI) doses for Fe and Zn at 18 and 15 mg day−1 much higher than that of Co ions (5–8 μg day−1).21 Spherical non-stoichiometric Zn-ferrite NPs meaning ZnxFe3−xO4 with x < 1, hereafter referred to with the general name of Zn-ferrite NPs/NCs, have been extensively studied as contrast agents in MRI.22–26 Despite their poor size and shape distributions, good relaxivity values and improved detection sensitivity were achieved.25,27 Noh et al. reported an interesting work on 18 nm Zn0.4Fe2.6O4 NCs exhibiting high Ms (165 Am2 kg−1) and a maximum SAR of 1860 W g−1 under AMF excitation at a field frequency of 500 kHz and amplitude of 37.4 kA m−1.17 Moreover, these NCs showed good performance as MPI tracers.7 The clear improvement of these nanocube-shaped NPs over the previously reported spherical NPs is partially ascribed to a higher crystallinity and partially to the less spin canting effects occurring at the surface of the NCs with respect to the spherical NPs.28 Beyond this work, some studies reported double ion substitution in spherical shaped IONPs.29–32 For instance the mixed nanoparticle formulation Co1−xZnxFe2O4 could improve the chemical stability, corrosion resistivity, magneto–crystalline anisotropy, and magnetostriction and magneto–optical properties.33–35 Ben Ali et al. observed a significant decrease in coercivity from 2000 Oe to 170 Oe with increasing zinc substitution in Co1−xZnxFe2O4 NPs,36 which was attributed to the reduction of the magneto-crystalline anisotropy.37They also reported a Ms of about 61 Am2 kg−1 for CoFe2O4 NPs of 11.7 nm (versus 81 Am2 kg−1 for bulk CoFe2O4),38 while the introduction of zinc ions brought an increase in saturation magnetization up to 75 Am2 kg−1 for Co0.7Zn0.3Fe2O4.36 This result is also supported by the research findings of other groups.39,40 The goal of this study is to synthesize high quality cubic shaped NPs with tunable Co and Zn stoichiometry using a non-hydrolytic synthetic approach. Starting with high quality Co-ferrite NCs and by partially substituting some of the cobalt by zinc ions, high quality zinc-cobalt mixed ferrite NCs were obtained. By exploiting a combination of experimental parameters such as Schlenk line pressure during the degassing step, volume of the reaction flask, and nitrogen flow rate, size control of Co-ferrite NCs smaller than 15 nm and up to 8 nm was reported; a size range that minimizes inter-particle interactions while maintaining high crystallinity. By changing the metal precursor mixture under similar reaction conditions, zinc-cobalt mixed ferrite NC samples were also obtained and compared to the Co-ferrite and Zn-ferrite NCs for their performance in MHT, MRI, and MPI to evaluate composition-dependent features. Finally, the cytotoxicity of Co and Zn-ferrite NCs was also evaluated using in vitro cell viability assays.
Results and discussion
Synthesis of cobalt ferrite NCs: control over size and shape
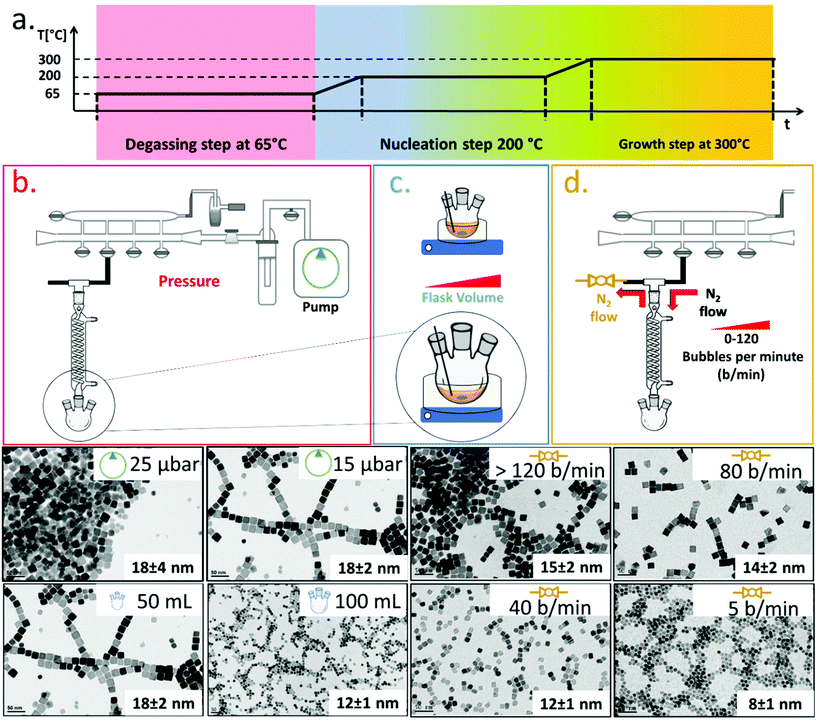
To synthesize ferrite NCs, the starting protocol used here refers to a previous published procedure by our group41 consisting of three-steps: (1) a degassing step at 65 °C under reduced pressure that ensures dissolution of the reagents and guaranteed oxygen and water-free atmosphere conditions; (2) a nanocrystal nucleation step at 200 °C under nitrogen gas; and (3) a growth step at 300 °C under a nitrogen gas atmosphere. We started with the protocol to obtain Co-ferrite NCs of 18 nm in cube edge.16 For this synthesis, 0.5 mmol Co(II) acetylacetonate (Co (acac)2), 1 mmol Fe(III) acetylacetonate (Fe (acac)3), 6 mmol decanoic acid (DA), 12 ml of squalane (SQ) and 13 mL of dibenzylether (DBE) were mixed and the three-step heat profile was applied (Fig. 1a). First, the effect of the Schlenk line pressure during the degassing step was evaluated. For a standard degassing step at 65 °C a usual 25–50 μbar is achieved leading to NCs of 18 ± 4 nm average edge size, as measured by bright field transmission electron microscopy (BF-TEM). On the other hand, by achieving a better vacuum with a more powerful vacuum pump, we reached a 15 μbar pressure and a narrower NC size distribution was obtained corresponding to 18 ± 2 nm (Fig. 1a). Likely, the presence of oxygen or moisture during the reaction may interfere with the growth of the NCs and therefore a more efficient degassing step is required for the control of the size distribution. Next, having fixed the final degassing pressure at 15 μbar, the second parameter that was changed was the reaction flask volume. Indeed, heat distribution in the reaction mixture plays a key role on the NC nucleation and the growth step, and to burst the nucleation process, a homogeneous and fast heat transfer from the heating mantle to the reaction solution should be guaranteed. Two syntheses were run in parallel in which the reaction mixture volume was kept constant (25 mL) at the same composition as described above but this same amount was placed either in a flask of 50 mL, as usually done, or choosing a flask with a bigger volume capacity of 100 mL. When comparing the product of the two syntheses, Co-ferrite NCs of 12 ± 1 nm were obtained upon using the flask of 100 mL capacity, instead of the 18 ± 2 nm NCs usually obtained when using the 50 mL flask (Fig. 1b). Likely, a flask with larger volume capacity maximizes the surface contact between the solution and the outer heating mantle source; hence, the heat distribution and the temperature remain more controlled during the reaction synthesis process. This experimental set-up seems to facilitate the nucleation as smaller NCs were obtained.As a third parameter, we have considered the flow rate of the nitrogen gas that is used to purge the reaction flask during the NC synthesis. Usually, the nitrogen pressure at the inlet of the Schlenk line is set at 2 bar and during the nucleation and growth steps does not change but, so far, the nitrogen flow rate has not been properly controlled. The nitrogen flow into the reaction flask can be changed by controlling the nitrogen bubbles per minute (b per min) observed in the oil bubbler, positioned at the outlet of the flask (Fig. 1). In this set of reactions, having set the degassing pressure to 15 μbar and the volume flask at 100 mL, by drastically decreasing the nitrogen flow rate from 120 b per min to 5–10 b per min, it was possible to reduce the size of the Co-ferrite NCs from 15 nm to 8 nm (Fig. 1c). Also, by choosing intermediate nitrogen flow rates, at 80 or 40 b per min, NCs with a mean size of 14 ± 2 nm and 12 ± 1 nm were obtained, respectively. It is known that in this synthesis, one of the two solvents, dibenzylether (DBE), is not inert but it decomposes at 295 °C and especially in the presence of carboxylic acid, it generates volatile by-products (benzaldehyde, benzyl-alcohol and toluene), with benzaldehyde having an important contribution to the cubic shape control.1,42 Here, it is likely that at a low nitrogen flow rate (5–10 b per min), the purging of these by-product species is slower than that occurring upon exposure to nitrogen flow at 120 b per min, and their longer persistence in the reaction mixture favours the nucleation of the nanocrystals and helps the cubic shape formation during the growth step.
Synthesis of mixed ferrite NCs: control over the composition
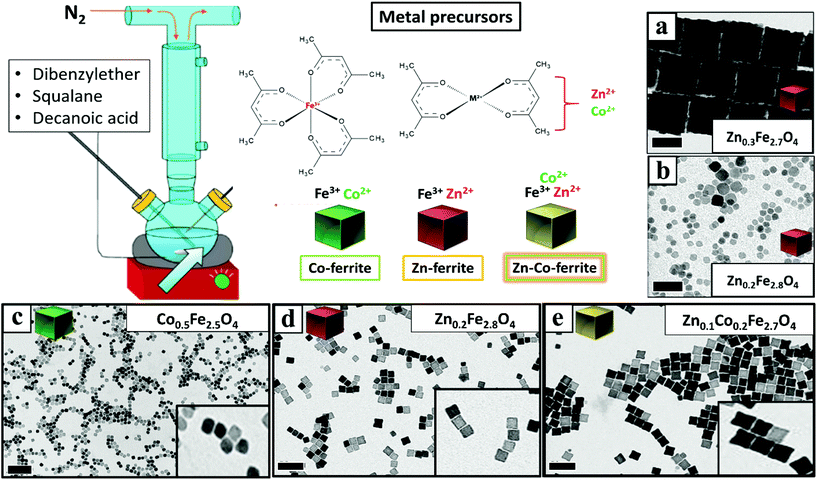
Once having set the above crucial parameters for the synthesis of NCs smaller than 15 nm (degassing final pressure 15 μbar, 100 mL flask and nitrogen flow at 40 b per min), we aimed at changing the composition of the NCs from that of Co-ferrite to others such as that of Zn-ferrites. First, the complete substitution of the Co precursor with the Zn precursor led to NCs with a marked increase in size, passing from 12 nm for the Co-ferrite to 65 nm for that of Zn-ferrite (Fig. 2a and Fig. S1†). This is likely attributed to the slower decomposition rate of the Zn precursor in comparison to the cobalt precursor, in the reaction mixture.43 Indeed, when looking at the decomposition temperature measured by thermogravimetric analysis of Zn(acac)2, it was found that it is higher (300 °C) than that of Co(acac)2 (250 °C).44,45To reduce the Zn-ferrite NC size, the amount of surfactant (DA) in the synthesis was increased from 6 to 7 mmol (corresponding to a surfactant to metal Fe + Zn molar ratio of 4.7) while keeping the rest of the precursors as in the first synthesis attempt for Zn-ferrite (1 mmol Fe (acac)2, 0.5 mmol Zn(acac)2, 13 mL of DBE and 12 mL of SQ). As expected, the increase in surfactant promotes nucleation and the presence of more nuclei consumes more precursors leading to smaller and well-defined NCs of 12 ± 1 nm in cube edge and with a stoichiometry of Zn0.2Fe2.8O4 (Fig. 2d).46 Moreover, in the attempt to increase the Zn content in the NCs, the Zn precursor was doubled (1 rather than 0.5 mmol as in the synthesis of Fig. 2d while maintaining the same reaction conditions), but the final Zn stoichiometry remained at 0.2 even if the NCs obtained were very similar in terms of cube-edge size (ca. 12 nm, data not shown). It is worth noting that when the same synthesis was repeated by adding an even higher DA surfactant amount (8 mmol), smaller NPs (9 ± 3 nm) were obtained but being too small, their cubic shape was not well-defined (Fig. 2b). These first set of results suggest that the decomposition kinetics of the Zn and Co precursors are rather different, hence, the synthesis parameters for the synthesis of Zn–Co-ferrite NCs needed to be re-adjusted.
To this aim, we set the same synthesis parameters used for the synthesis of Co-ferrite NCs, but both Zn(II) and Co(II) acetylacetonate precursors were added. For the mixed ferrites the overall amount of cobalt metal ions (0.5 mmol) was replaced with the sum of Zn + Co metal precursors (0.25 mmol Zn and 0.25 mmol Co) while the iron precursor amount (1 mmol) was kept constant. Also in this case the DA surfactant (6 mmol) to metal precursor (iron + cobalt + zinc) molar ratio was kept to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Under these conditions NCs of 15 ± 1 nm with a well-defined shape and with a mixed composition (Zn0.1Co0.2Fe2.7O4) were obtained (Fig. 2e and Fig. S1†). It should also be noted that the Zn stoichiometry obtained is well below 1 typical of pure Zn-ferrite (ZnFe2O4) and is much lower than that of Co.
1. Under these conditions NCs of 15 ± 1 nm with a well-defined shape and with a mixed composition (Zn0.1Co0.2Fe2.7O4) were obtained (Fig. 2e and Fig. S1†). It should also be noted that the Zn stoichiometry obtained is well below 1 typical of pure Zn-ferrite (ZnFe2O4) and is much lower than that of Co.
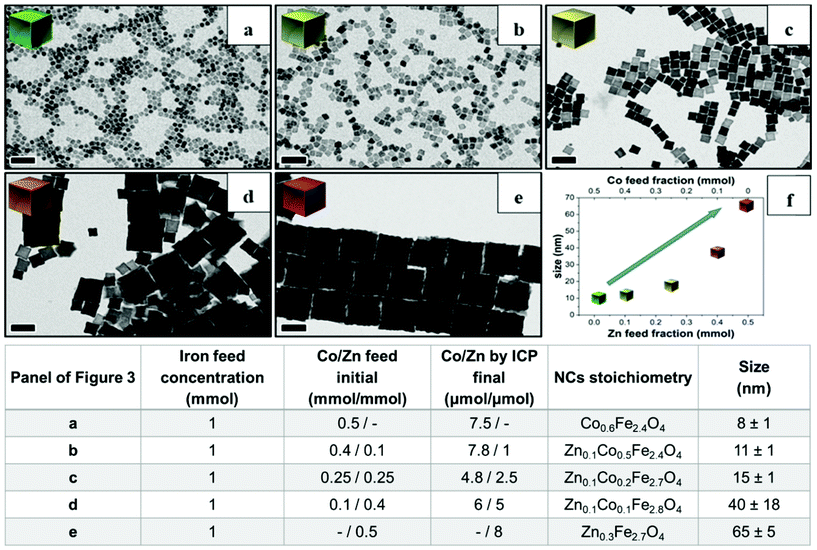
Next, to change the relative Zn to Co stoichiometry, a set of experiments were performed by changing the starting amounts of Zn(acac)2 and Co(acac)2 in the feeding reaction pot, while consistently keeping the amount of Fe(acac)3 (1 mmol), the DA amount (6 mmol) and the rest of the reaction parameters constant. Zn-Co-ferrite NCs at different stoichiometries could be obtained, however, the size of the NCs was markedly affected, and NCs in the range from 8 to 65 nm were obtained, depending on the precursor amounts (Fig. 3 and Fig. S2†). At an increased amount of Zn precursors in the feeding pot, the size of the NCs tended to increase, as the Zn precursors hindered the nucleation while promoting crystal growth. When using an equimolar amount of Zn and Co (a Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn feeding ratio 1
Zn feeding ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.25
1, 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 mmol), the amount of Co was double that of Zn in the final NCs as measured from ICP-OES elemental analysis. The same trend was found for other mixed compositions : when starting with Co
0.25 mmol), the amount of Co was double that of Zn in the final NCs as measured from ICP-OES elemental analysis. The same trend was found for other mixed compositions : when starting with Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn feeding ratio of 0.4
Zn feeding ratio of 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 or 0.1
0.1 or 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 mmol, the Co
0.4 mmol, the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratio in the NCs was 7.8
Zn ratio in the NCs was 7.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 6
1 or 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 μmol, respectively.2 Therefore, in all the investigated mixed Co–Zn-ferrite NCs, the amount of Zn in the final NCs was always lower than that of Co. These data again confirm that the decomposition of the Co precursor is certainly faster than that of the Zn precursor, thus favouring the incorporation of more Co than Zn into the growing mixed ferrite NCs.44,45
5 μmol, respectively.2 Therefore, in all the investigated mixed Co–Zn-ferrite NCs, the amount of Zn in the final NCs was always lower than that of Co. These data again confirm that the decomposition of the Co precursor is certainly faster than that of the Zn precursor, thus favouring the incorporation of more Co than Zn into the growing mixed ferrite NCs.44,45
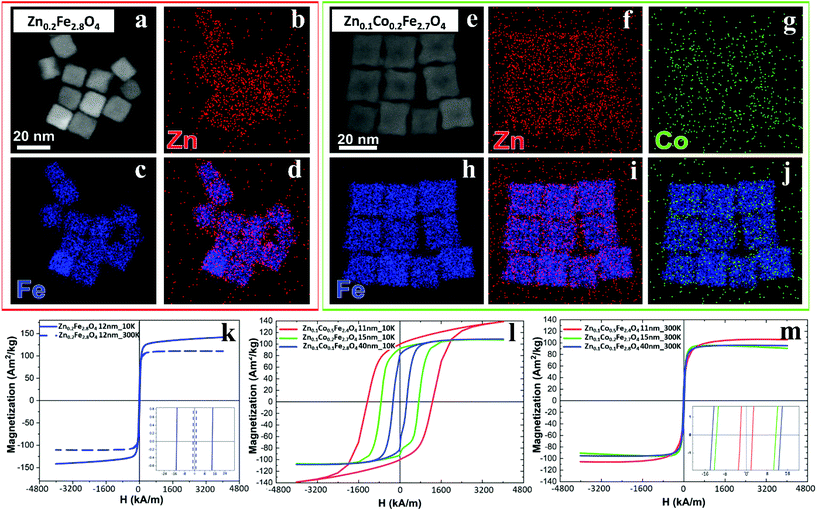
The cation concentration for the Zn-ferrite (Zn0.2Fe2.8O4) and Zn-Co-ferrite (Zn0.1Co0.2Fe2.7O4) NCs were analyzed by energy-dispersive X-ray spectroscopy (EDS) combined with scanning TEM mode (STEM-EDS) (Fig. 4a–j and S3†). For both samples, the average cation concentration in the NCs measured by EDS matched very well the composition measured via elemental analysis using ICP (Table S1†).
Next, X-ray diffraction (XRD) analysis indicates that the positions and relative intensities of all diffraction peaks matched very well to the standard diffraction patterns for inverse spinel Fe3O4 powder (Fig. S4†). Moreover, high resolution TEM (HRTEM) analyses of individual NCs confirmed the expected {100} faceting of the NCs and match the phase evaluated from XRD analysis, with no significant changes in lattice parameters along the NCs’ volume (Fig. S5†).
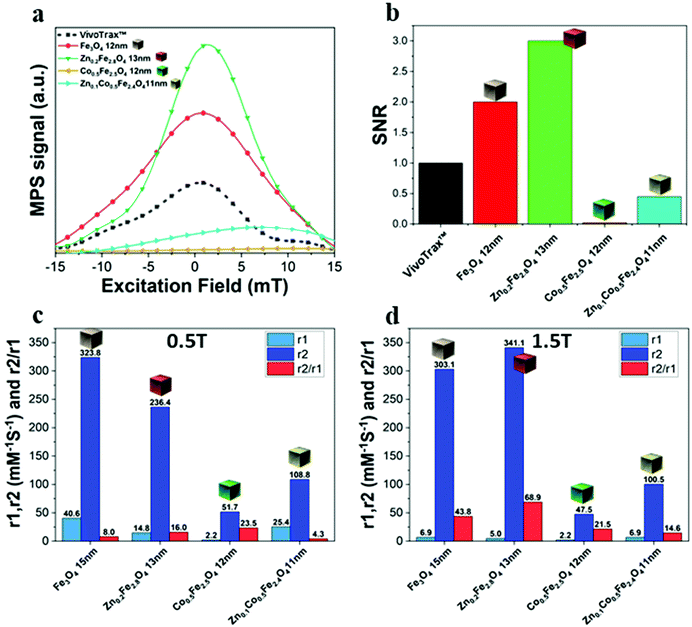
The same samples were characterized to investigate their magnetic properties, which was obtained by recording the hysteresis loops with finite coercive fields (Hc) and the temperature dependent magnetization curves (Fig. 4k–m). The 12 ± 1 nm Zn-ferrite NC (Zn0.2Fe2.8O4) sample showed a significantly low coercive field and high saturation magnetization at both 300 K and 10 K, being superparamagnetic at 300 K, in accordance with the literature.7,47 The inclusion of cobalt ions into the Zn-Co-ferrite NC of the 11 nm sample (Zn0.1Co0.5Fe2.4O4) also showed superparamagnetic behaviour at 300 K, but the Hc drastically increased at 10 K (1600 kA m−1), so other magnetic structures may have affected the overall magnetic features. In general, such a behaviour is ascribed to the formation of core–shell structures,16,48 which may lead to the hypothesis that the NCs present an iron-rich core and a Zn/Co-rich shell ferrite structure. This hypothesis however is not fully supported by STEM-EDS elemental mapping of the cation distribution over the NCs (Fig. 4a–j), which does not show a clear enrichment in Zn and Co at the surface of the NCs. The Zn/Co surface enrichment may however be of a few percent compared to the overall content and therefore too low to be assessed by this technique (see Table S1†). Moreover, in the STEM-EDS maps the Co signal does not appear clearly localized within the NCs (Fig. 4g), while the Zn signal is more intense within the NCs. This is also due to the fact that the highest intensity peak of Co (Kα, centered at 6.93 keV) could not be used for the maps because of its partial overlap with the Fe Kβ peak (centered at 7.06 keV, see Fig. S3†).
Characterization of ferrite NCs for magnetic hyperthermia, MRI, and MPI applications
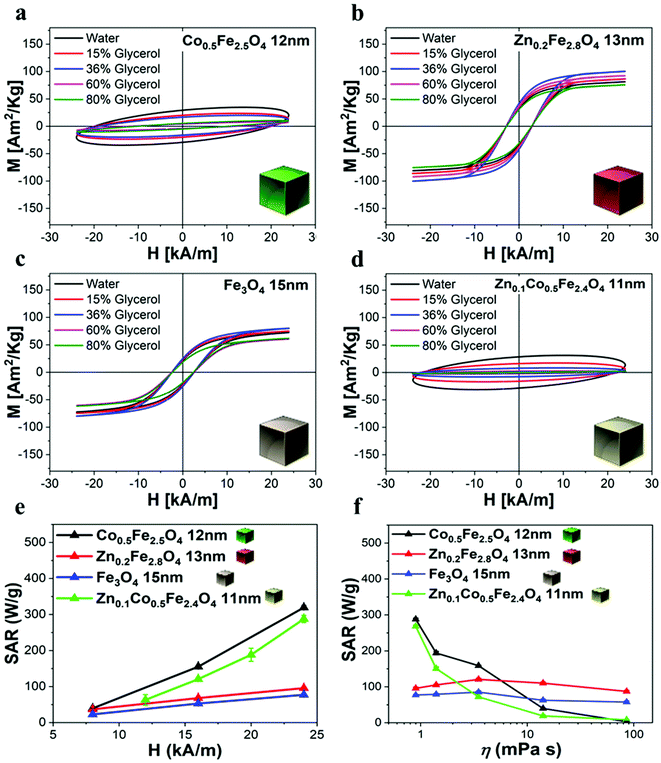
To evaluate their MHT, MRI, and MPI performance, the as-synthesized ferrite NCs were transferred from chloroform to water by using an amphiphilic polymer, poly(maleic anhydride-alt-1-octadecene) (PMAO), and following a well-established polymer coating procedure (see Experimental section).49 The Zn-ferrite NCs and mixed Zn-Co-ferrite samples’ properties were compared to those of Co-ferrite NCs (sample in Fig. 2) by choosing a set of samples that had a very similar TEM NCs size (ca. 15 nm). The polymer coating protocol provided NCs well-dispersed in water with a monomodal hydrodynamic peak as measured by dynamic light scattering (DLS), with a polydispersity index (PDI) below 0.15 for all the samples as an indication of their good dispersion in aqueous media (Table S2†). Indeed, the hydrodynamic intensity peaks for Co-ferrite, Zn-ferrite and Zn-Co-ferrite NCs were centered at 32 ± 12 nm (PDI 0.14), 57 ± 20 nm (PDI 0.12) and 36 ± 14 nm (PDI 0.13), respectively (Table S2†), indicating that the NCs have larger hydrodynamic sizes (Dh) than the corresponding TEM core size, which is in accordance with being coated with the hydrated polymer shell in water, but still they have quite a small size and PDI below 0.2 indicating the presence of individually coated NCs in solution.It is worth noting that for all the aqueous samples, the SAR data obtained by AC magnetometry measurements are fully in accordance with the ones obtained from calorimetric measurements when using similar sample volumes and field conditions (Table 1 and Fig. S7†). The field conditions of the calorimetry and AC magnetometry equipment were close enough in values but not identical according to the instrument available (100 kHz and 16 kA m−1 for the AC magnetometer and 100 kHz and 20 kA m−1 for the calorimetric device).
| Sample | SAR magnetic measurements (W g−1) f: 100 kHz, H: 16 kA m−1 | SAR calorimetric measurements (W g−1) f: 100 kHz, H: 20 kA m−1 |
|---|---|---|
| Fe3O4 NCs, 15 nm | 53 ± 1 | 69 ± 14 |
| Co0.5Fe2.5O4 NCs, 12 nm | 155 ± 2 | 151 ± 9 |
| Zn0.2Fe2.8O4 NCs, 13 nm | 68 ± 2 | 93 ± 7 |
| Zn0.1Co0.5Fe2.4O4 NCs, 11 nm | 121 ± 2 | 188 ± 18 |
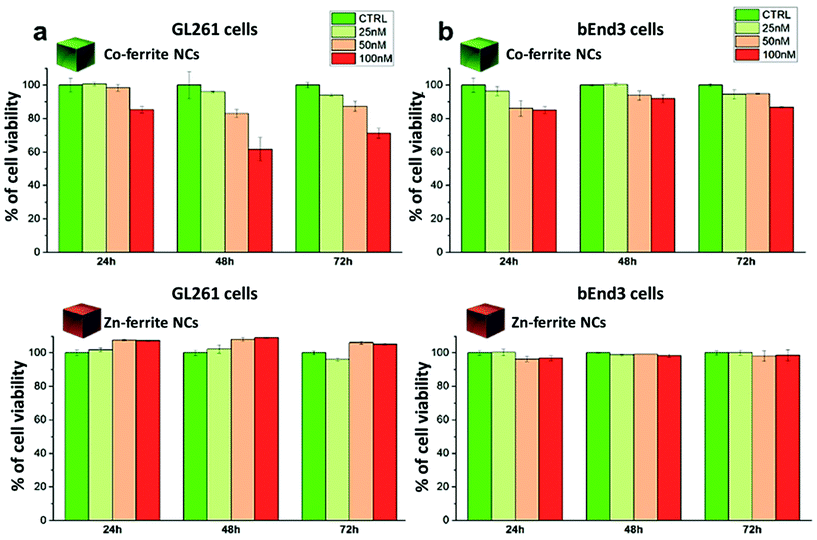
For the Co-ferrite NCs, at 100 nM and after 72 h, the cell morphology looked altered, with membrane blabbing and clear signs of cell suffering. This is likely due not only to the intrinsic toxicity of the Co leaching from the Co-ferrite NCs but, also, to the NCs’ aggregation on their surface: some evident black spots were present on the cells and on the culture dish. On the other hand, the cells exposed to Zn-ferrite NCs under the same incubation conditions were in a healthy state showing a regular cell morphology although some Zn-ferrite NCs were also present as tiny aggregates on the cells.
Conclusions
We have reported here the synthesis conditions to obtain ferrite nanocubes with a narrow size distribution and well-defined cubic shape below 15 nm and up to 8 nm cube edge. In particular, the effect of pressure during the degassing step, the temperature distribution during the nucleation and growth step, and the nitrogen flow bubbling rate were the parameters that were studied and have allowed to obtain such synthesis control on Co-ferrite NCs (Fig. 1). Moreover, the complete or partial substitution of the cobalt precursor with the zinc precursor has enabled to achieve Zn-ferrite or Zn-Co-ferrite NCs of high quality and in the same size range. In addition, the effect of Zn and Co feed metal precursor ratio on the composition of the resulting Zn-Co-ferrite NCs was also discussed (Fig. 2 and 3). The further wide range of magnetic characterization on the ferrite NCs has enabled us to compare their heating performance for MHT and their potential to be used as contrast agents for MRI and MPI applications (Fig. 4–6). For MHT, Co0.5Fe2.5O4 (12 nm) and Zn0.1Co0.5Fe2.4O4 (11 nm) NCs, in water, showed high SAR values (300 W g−1), but at low frequency (100 kHz and 24 kA m−1), when the media viscosity is increased, their heating ability is markedly suppressed (Fig. 5). Zn0.2Fe2.8O4 NCs with a size of 12 nm showed a high Ms value (about 120 Am2 kg−1 at 300 K) and superparamagnetic behaviours at 300 K; they displayed the best SAR performance among all the samples investigated especially in viscous media, achieving SAR values of 480 W g−1 at 300 kHz and 24 kA m−1 in water. Indeed, this Zn-ferrite sample showed a viscosity-independent SAR behaviour, and it does not lose its heating ability even in an 81% solution of glycerol and at a viscosity similar to those of the tumour environment, making these NCs very promising for in vivo MHT applications (Fig. 5). Furthermore, this sample showed the best performance among the different ferrite compositions investigated for acting as an MRI contrast agent with a r2 of 341 mM−1 s−1 at 1200 kA m−1 (1.5 T) and, also, the first candidate as a promising MPI tracer with the best point spread function and signal-to-noise ratio, that was 3-fold higher than the one recorded for VivoTrax™. It is noteworthy to mention that the Co-ferrite NC (12 nm) sample showed the lowest signal for both MRI (47.5 mM−1 s−1 at 1200 kA m−1) and MPI (almost no signal) measurements (Fig. 6). However, the partial substitution of the Co with Zn ions increased the NC performance, in particular Zn0.1Co0.5Fe2.4O4 (11 nm) NC samples had an SNR ratio 5-fold higher than that of Co0.5Fe2.5O4 (12 nm) as shown in Fig. 6b and r2 value 2-fold higher (100 mM−1 s−1 at 1200 kA m−1) than that of the Co-ferrite NCs as shown in Fig. 6d, making it useful for MPI and MRI applications. Finally, the biocompatibility of the synthesized Zn-ferrite NCs was shown in vitro on healthy brain endothelial murine cells (bEnd3) and brain tumour murine cells (GL261), confirming the suitability of such materials to be simultaneously employed as a heating mediator in MHT and as an imaging agent in MRI and MPI applications (Fig. 7). The proposed synthetic routes lead to metal doping of ferrites with tuneable magnetic properties suitable for biomedical applications.Materials
Iron(III) acetylacetonate (Fe(acac)3, 99%) and decanoic acid (DA, 99%) were purchased from Acros. Cobalt(II) acetylacetonate (Co (acac)2, 99%), zinc(II) acetylacetonate hydrate (Zn (acac)2, 99%), poly maleic anhydride-alt-1-octadecene (PMAO), sucrose, agarose, dibenzylether (DBE, 98%), acetone (99.5%), chloroform (99.8%) and isopropanol (99.8%) were purchased from Sigma Aldrich. Squalane (SQ, 98%) was purchased from Alfa Aesar. Milli-Q water filtered through 0.22 μm pore size hydrophilic filters (18.2 MΩ cm) was supplied by a Milli-Q® Integral Water Purification System, and VivoTrax™ (Resovist®) licensed for distribution in the USA for pre-clinical MPI studies, was purchased from Magnetic Insight (Alameda, CA, USA). All chemicals were used as received. bEnd3 and GL 261 cell lines were purchased from ATCC, and Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), penicillin streptomycin (PS), pyruvate, non-essential amino acids (100×)(MEM) and glutamine were purchased from Sigma Aldrich.Synthesis of Fe3O4 nanocubes
Fe3O4 nanocubes (IONCs) were synthesized by thermal decomposition following an established procedure, with suitable modifications.1 The protocol relies on the synthesis of particles of 15 nm in size. In a 100 mL three-neck flask connected to a standard Schlenk line, 1.5 mmol (529 mg) Fe(acac)3 and 7 mmol (1205 mg) decanoic acid were dissolved in a mixture of 12 mL of squalane and 13 mL of benzyl ether. The resulting deep red solution was degassed at 65 °C reaching 15–20 μbar vacuum after 1.5 h. After switching to a N2 flow, the temperature of the mixture was increased to 200 °C at a rate of 5 °C min−1 and kept at 200 °C for 1.5 h. After, the reaction temperature was increased to 300 °C at a rate of 7.5 °C min−1 keeping the mixture under reflux for 1 h. The nitrogen flow rate was fixed to intermediate flow (40–80 bubbles per min). The flask was then cooled down to room temperature under an inert atmosphere. The black colloidal solution was washed 2 times with an excess amount of acetone (45 mL) and centrifuged at 4500 rpm for 20 min to collect the particle as a black precipitate. The final particles were dispersed in chloroform (8 mL) for further experiments.Synthesis of CoxFe3−xO4 nanocubes
Cobalt ferrite NCs (Co0.5Fe2.5O4) were synthesized by thermal decomposition following an established procedure, with suitable modifications.16 The protocol relies on the synthesis of particles of 12 nm in size with 0.5 Co stoichiometry. In a 100 mL three-neck flask connected to a standard Schlenk line, 0.5 mmol (120 mg) Co(acac)2, 1 mmol (353 mg) Fe(acac)3 and 6 mmol (1033 mg) decanoic acid were dissolved in a mixture of 12 mL of squalane and 13 mL of benzyl ether. The resulting deep red solution was degassed at 65 °C for 2 h reaching 16–20 μbar vacuum after 2 h. After switching to a N2 flow, the temperature of the mixture was increased to 200 °C at a rate of 5 °C min−1 and kept at 200 °C for 1.5 h. After, the reaction temperature was increased to 300 °C at a rate of 7.5 °C min−1 keeping the mixture under reflux for 1 h. The nitrogen flow rate was fixed to intermediate flow (5–120 bubbles per min). The flask was then cooled down to room temperature under an inert atmosphere. The black colloidal solution was washed 2 times with an excess amount of acetone (45 mL) and centrifuged at 4500 rpm for 20 min to collect the particle as a black precipitate. The final particles were dispersed in chloroform (8 mL) for further experiments.Synthesis of ZnxFe3−xO4 nanocubes
Zinc ferrite NCs (ZnxFe3−xO4 NCs) were synthesized by thermal decomposition. The protocol here reported relies on the synthesis of particles of 12 nm in size and with 0.2 Zn stoichiometry. In a 100 mL three-neck flask connected to a standard Schlenk line, 0.5 mmol (131 mg) Zn(acac)2, 1 mmol (353 mg) Fe(acac)3 and 7 mmol (1033 mg) decanoic acid were dissolved in a mixture of 12 mL of squalane and 13 mL of benzyl ether. The solution was degassed at 65 °C for 1.5 h reaching 16–20 μbar vacuum after 1.5 h. After switching to a N2 flow, the temperature of the mixture was increased to 200 °C at a rate of 5 °C min−1 and was then kept at 200 °C for 2 h. The reaction temperature was increased to 300 °C at a rate of 7.5 °C min−1 keeping the mixture refluxing for 1 h. The nitrogen flow rate was fixed to intermediate flow (80 bubbles per min). The flask was left to room temperature to cooled down under an inert atmosphere. The black colloidal solution was washed 2 times with an excess amount of acetone (45 mL) and centrifuged at 4500 rpm for 20 min to collect the particles as a black precipitate. The final particles were dispersed in chloroform (8 mL) for further experiments.Syntheses of ZnxCoyFe3-(x+y)O4 ferrite nanocubes
Zinc–cobalt NCs (Zn0.1Co0.2Fe2.7O4 NCs) of 15 nm were synthesized using the here detailed protocol: in a 100 mL three-neck flask connected to a standard Schlenk line, 0.25 mmol (63 mg) Zn(acac)2, 0.25 mmol (62 mg) Co(acac)2, 1 mmol (353 mg) Fe(acac)3 and 6 mmol (1033 mg) decanoic acid were dissolved in a mixture of 12 mL of squalane and 13 mL of benzyl ether. The solution was degassed at 65 °C for 1.5 h reaching 16–20 μbar vacuum after 1.5 h. After switching to a N2 flow, the temperature of the mixture was increased to 200 °C at a rate of 5 °C min−1 kept at 200 °C for 2 h. The reaction temperature was increased to 300 °C at a rate of 7.5 °C min−1 keeping the mixture refluxing for 1 h. The nitrogen flow rate was fixed to intermediate flow (80 bubbles per min). The flask was than cooled down to RT under an inert atmosphere. The black colloidal solution was washed 2 times with an excess amount of acetone (45 mL) and centrifuged at 4500 rpm for 20 min to collect the particles as a black precipitate. The final particles were dispersed in chloroform (8 mL) for further experiments.Phase transfer of the NCs in water with poly(maleic anhydride-alt-1-octadecene)
The synthesized, hydrophobic NCs dispersed in chloroform were transferred to water, using poly(maleic anhydride-alt-1-octadecene) (PMAO), following a protocol developed by our group with suitable modifications.49 NCs of 15 nm in size at a concentration of about 400 nM (were diluted with an excess of chloroform (70 mL) and sonicated for 5 minutes. A certain amount of PMAO polymer solution in chloroform (137 mM, concentration referring to the monomer units) was added, with a ratio of 500 molecules of monomer unit per nm2 of the NC surface. The solvent was evaporated using a Rotavapor (140 rpm, 50 °C) following three steps of evaporation under reduced pressure: 800 mbar for 30 minutes, 700 mbar for 30 minutes and finally 600 mbar until complete solvent evaporation was achieved. The dried sample was then soaked in 20 mL of borate buffer and sonicated at 65 °C for 1 h until complete solubilization was verified. The water soluble NCs were concentrated by an ultrafiltration system. The sample volume was reduced to nearly 3 mL under continuous shaking at 150 rpm for 15 min at RT. Subsequently, the sample was loaded on top of a sucrose gradient (2 mL of 20%, 3 mL of 40% and 3 mL of 60% in an ultra-centrifugal tube, from top to bottom) and centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 45 min. The excess of polymer (visible under an UV lamp) found at the top of the sucrose gradient at 20% was removed, by collecting it with a syringe. Instead, the NCs, usually found in the 40% to 60% fraction of the sucrose gradient, were collected and further washed with pure water (Milli-Q) three times to remove the excess of sucrose and finally concentrated to a final volume of about 4 mL in water.
000 rpm for 45 min. The excess of polymer (visible under an UV lamp) found at the top of the sucrose gradient at 20% was removed, by collecting it with a syringe. Instead, the NCs, usually found in the 40% to 60% fraction of the sucrose gradient, were collected and further washed with pure water (Milli-Q) three times to remove the excess of sucrose and finally concentrated to a final volume of about 4 mL in water.
Poly(ethylene glycol) functionalization
The functionalization of the polymeric shell of NCs with diamine–PEG (NH2–PEG–NH2) (MW 2000) was carried out following a procedure developed in our group, with minor modifications.62 A solution of Co-ferrite NCs of 0.1 μM was mixed with the same volume of 0.125 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and a diamine–PEG (NH2–PEG–NH2) 0.1 mM solution in pure water. The amount of PEG was kept as 1000-fold excess compared to Co-ferrite NC molar concentration. The mixture was vigorously shaken for one minute and left under gentle shaking at 150 rpm for 2 h at RT. Finally, the solution was diluted 3 times to quench the reaction and washed in pure water using centrifugal filters. The final sample was collected in few microliters of water.Elemental analysis
Inductively coupled plasma optical emission spectroscopy (ICP-OES) analysis was performed on an iCAP 6000 spectrometer (Thermo Scientific) to quantify the concentration of the elements of interest. Briefly, 25 μL samples were digested in 2.5 mL of aqua regia (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 ratio is 3
HNO3 ratio is 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)) overnight and diluted with 25 mL of Milli-Q water prior to the measurement.
1 (v/v)) overnight and diluted with 25 mL of Milli-Q water prior to the measurement.
Transmission electron microscopy
Overview transmission electron microscopy (TEM) analyses were carried out on a JEOL JEM-1011 instrument, using an acceleration voltage of 100 kV. The sample preparation was conducted by drop-casting a droplet of the sample solution onto a 400 mesh ultra-thin carbon-coated copper grid with a subsequent removal of the solvent by evaporation at RT. For high-resolution TEM and compositional analyses, an image-CS-corrected JEOL JEM-2200FS, operated at 200 kV, was used. Energy-dispersive X-ray spectroscopy (EDS) analyses were carried out using a Bruker XFlash 5060 silicon-drift detector. For these analyses, a small volume of each sample was drop-cast onto an ultrathin carbon/holey carbon-coated Au grid. STEM-EDS elemental maps shown here are obtained by integration of the Lα peak for Zn, Kα peak for Fe and Kβ peak for Co in the raw spectra. Oxygen was not mapped nor quantified by STEM-EDS.X-Ray diffraction analysis
XRD patterns were recorded on a Malvern PANalytical Empyrean diffractometer machine. The X-ray source is operated at 40 kV and 150 mA. The diffractometer was equipped with a Cu source and a Göbel mirror to obtain a parallel beam as well as to suppress Cu Kβ radiation (1.392 Å). The 2-theta/omega scan geometry was used to acquire the data. The samples were prepared by drop casting concentrated NC solutions onto a zero background silicon substrate. The PDXL software of Rigaku was used for phase identification.Magnetic characterization
Magnetic measurements were performed on a superconducting quantum interference device (SQUID) from Quantum Design Inc. Hysteresis loops were recorded within the magnetic field of ±4800 kA m−1 at 5 K and 298 K. Samples were prepared by dropping 50 μL of solution (ca. 1 g L−1) on a Teflon tape. Magnetization curves were normalized by the metal oxide mass obtained by ICP.AC magnetic measurements
The inductive magnetic characterization was carried out with a home-made AC magnetometer developed by Advanced Magnetic Instrumentation Unit at iMdea Nanociencia. AC hysteresis loops were recorded at different field intensities (8–16–24 kA m−1) and frequencies (100–300 kHz). Each AC hysteresis loop measurement is obtained as the average of three repetition of AC magnetization curves under given field conditions, leading to an averaged and standard deviation of the magnetic area values, among other magnetic parameters related to the acquired AC hysteresis loop. The experiments were performed by using a 40 μL volume of the different ferrite NC colloidal samples at a concentration of 2 g of metal elements (Co + Fe or Zn + Fe or Zn + Co + Fe, for Co-ferrite, Zn-ferrite and Zn-Co-ferrite respectively) per liter, when changing media viscosity by adding glycerol at 0%–15%–36%–60%–80% (v/v) fraction in a water volume. The AC magnetization signal was normalized to the mass of magnetic elements present in the studied magnetic colloid.Calorimetric measurements of the specific absorption rate
A commercially available magnetic nano-heating device (DM100 Series, nanoScale Biomagnetics) was used to evaluate the heating performance (SAR values) under quasi-adiabatic conditions. The water soluble NCs were exposed to an alternating magnetic field (from 12 up to 32 kA m−1) at 2 different frequencies (105, 300 kHz). All measurements were performed using 50 μL of sample in water at a total metal concentration of 2 g L−1 (Co + Fe or Zn + Fe or Zn + Co + Fe, for Co-ferrite, Zn-ferrite and Zn-Co-ferrite, respectively) and the SAR values were normalized using the metal mass concentration determined by elemental analysis. SAR values were calculated according to the following equation: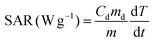 | (1) |
Relaxivity measurements
Water solutions of NCs containing different magnetic material concentrations ranging from 0.001 to 1 mM (0.001–0.005–0.01–0.05–0.1–0.2–0.4–0.6–0.8–1 mM) were prepared. The longitudinal (T1) and transverse (T2) relaxation times were measured at 40 °C using a Minispec spectrometer (Bruker, Germany) mq 20–0.5 T (400 kA m−1) and mq 60–1.5 T (1200 kA m−1). The T1 relaxation profile was obtained using an inversion-recovery sequence, with 20 data points and 4 acquisitions for each measurement. The T2 relaxation time was measured using a Carr–Purcell–Meiboom–Gill (CPMG) spin-echo pulse sequence with 200 data points with an interecho time of 0.5 ms. The relaxivities ri (i = 1, 2) were determined by the following equation:where Cm is the concentration of magnetic ions. The values are reproducible within 5% deviation.
MPI relaxometry
MPI analyses were performed in a custom made MPI relaxometer device.7 Representative samples of ferrite NCs were analyzed as suspensions in chloroform or water of 500 μL at a fixed iron concentration of 1.0 mgFe mL−1; the samples were sonicated before performing each MPI relaxometry measurement. All MPI studies were conducted under a magnetic excitation field strength and frequency of 16 kA m−1 and 16.8 kHz, respectively. The MPI signals generated were normalized to the MPI signal of a commercial MPI tracer, VivoTrax™ (Resorvist® licensed for distribution in USA through Magnetic Insight for pre-clinical MPI studies) (500 μL, 1.0 mgFe mL−1).Cell culture
Mouse cerebral endothelial cells (bEnd3) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin streptomycin (PS), 1% pyruvate, 1% non-essential amino acids (100×)(MEM) and 1% glutamine at 37 °C, 5% CO2 and 95% humidity. Cells were split every 3–4 days, before reaching 100% confluence. Mouse glioblastoma cells (GL261) were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% penicillin streptomycin (PS) and 1% glutamine at 37 °C, 5% CO2 and 95% humidity. Cells were split every 2–3 days, before reaching 100% confluence.NC cytotoxicity studies
Viability of the bEnd3 and GL261 cells upon incubation with NCs were tested with a dead/live assay by flow cytometry. Briefly, cells at a concentration of 105 cells per well were seeded in a 24 multi-well plate and allowed to adhere for 24 h. The day after, Zn0.2Fe2.8O4 NCs (13 ± 1 nm) or Co0.5Fe2.5O4 NCs (12 ± 2 nm), diluted in fresh medium (at 100 nM, 50 nM and 25 nM NC concentration corresponding to a NC dose of 0.6, 0.3 and 0.15 mg mL−1 respectively), were added to the cells and incubated for additional 24 h, 48 h or 72 h at 37 °C. At each time point of interest, the cells were detached from the wells, washed several times in PBS and stained with a dead cell dye, ethidium homodimer-1 (EthD-1), following the manufacture's protocol and then analysed by flow cytometry (FACSariaIII -BD-bioscience device). Briefly, a staining solution of 2 μL of dye (EthD-1) in 1 mL of phosphate buffered saline (PBS) was prepared. Upon removal of media, cells in each well were incubated with 500 μL of the staining solution for 20 min at RT. Next, cells were rinsed twice with PBS solution and then collected in 200 μL of PBS for the analysis with FACS to count the dead cells. Cells with compromised cellular membranes allowed the dye to enter inside the cell, thus identifying as dead cells. In contrast, non-stained cells corresponded to viable cells. Phase contrast imaging of the cells treated with the different NCs were performed using an optical microscope (Motic AE31, 20× magnification) confirming the results obtained by FACS. The two samples (Zn0.2Fe2.8O4 NCs and Co0.5Fe2.5O4 NCs) were sterilized before the use; briefly, they were placed in open vials inside a closed biological fume hood and exposed to three cycle of UV light (20 minutes each).Author contributions
T.P. and N.S. conceived and designed the study. N.S. produced and characterized the materials. N.S. and H.G. performed the calorimetric measurements. P.G performed the SQUID magnetic characterization and the XRD analysis of the samples. R.B. performed the high resolution TEM and the EDS analysis. A.C.S performed the MPI relaxivity measurements. N.S. and F.J.T performed the AC magnetic measurements. N.S. and S.F. performed in vitro experiments. T.P., N.S. and H.G. wrote the paper with contributions obtained from all authors.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was partially funded by the European Research Council (starting grant ICARO, Contract No. 678109 and ERC proof of concept Hypercube, Contract No. 899661) and partially by the AIRC project (contract no. 14527). COST action TD1402 “RADIOMAG” is acknowledged. F.J. Teran acknowledges support from the ‘Severo Ochoa’ Programme for Centres of Excellence in R&D (MINECO, Grant SEV-2016-0686)”, and funding from Comunidad de Madrid (NANOMAGCOST, P2018/NMT-4321). We thank Simone Nitti for providing iron oxide nanocubes. The Advanced Magnetic Instrumentation Unit (IMDEA Nanociencia) is acknowledged for technical support during AC magnetometry measurements. The MPI measurement experimental work was supported by an NSF-CAREER Grant (DMR-1253358) from the Solid State and Materials Chemistry Program. P. G. acknowledges financial support from the Spanish government (MICIU) through the RTI2018-102006-J-I00 project. P.G and T.G acknowledge financial support form the Catalan Agency of Competitiveness (ACCIO) through the TecnioSpring+MarieCurie action TECSPR16-1-0082.References
- P. Guardia, A. Riedinger, S. Nitti, G. Pugliese, S. Marras, A. Genovese, M. E. Materia, C. Lefevre, L. Manna and T. Pellegrino, J. Mater. Chem. B, 2014, 2, 4426 RSC.
- Q. Song and Z. J. Zhang, J. Am. Chem. Soc., 2012, 134, 10182–10190 CrossRef CAS PubMed.
- A. C. Anselmo and S. Mitragotri, AAPS J., 2015, 17, 1041–1054 CrossRef CAS.
- M. Allione, B. Torre, A. Casu, A. Falqui, P. Piacenza, R. Di Corato, T. Pellegrino and A. Diaspro, J. Appl. Phys., 2011, 110, 064907 CrossRef.
- C. Martinez-boubeta, K. Simeonidis, A. Makridis, M. Angelakeris, D. Serantes, D. Baldomir, Z. Saghi and P. A. Midgley, Sci. Rep., 2013, 3(1), 1–8 Search PubMed.
- M. E. Materia, P. Guardia, A. Sathya, M. Pernia Leal, R. Marotta, R. Di Corato and T. Pellegrino, Langmuir, 2015, 31, 808–816 CrossRef CAS.
- L. M. Bauer, S. F. Situ, M. A. Griswold and A. C. S. Samia, Nanoscale, 2016, 8, 12162–12169 RSC.
- E. A. Périgo, G. Hemery, O. Sandre, D. Ortega, E. Garaio, F. Plazaola and F. J. Teran, Appl. Phys. Rev., 2015, 2 Search PubMed.
- P. Guardia, R. Di Corato, L. Lartigue, C. Wilhelm, A. Espinosa, M. Garcia-Hernandez, F. Gazeau, L. Manna and T. Pellegrino, ACS Nano, 2012, 6, 3080–3091 CrossRef CAS PubMed.
- A. Cristina and S. Samia, Prog. Nat. Sci.: Mater. Int., 2016, 26, 440–448 CrossRef.
- D. Carta, M. F. Casula, A. Falqui, D. Loche, G. Mountjoy, C. Sangregorio and A. Corrias, J. Phys. Chem. C, 2009, 113, 8606–8615 CrossRef CAS.
- Y. Xu, J. Sherwood, Y. Qin, R. A. Holler and Y. Bao, Nanoscale, 2015, 7, 12641–12649 RSC.
- Y. Yang, X. Liu, Y. Yang, W. Xiao, Z. Li, D. Xue, F. Li and J. Ding, J. Mater. Chem. C, 2013, 1, 2875–2885 RSC.
- A. E. Berkowitz and W. J. Schuele, J. Appl. Phys., 1959, 30, S134–S135 CrossRef.
- J.-G. Lee, J. Y. Park, Y.-J. Oh and C. S. Kim, J. Appl. Phys., 1998, 84, 2801 CrossRef CAS.
- A. Sathya, P. Guardia, R. Brescia, N. Silvestri, G. Pugliese, S. Nitti, L. Manna and T. Pellegrino, Chem. Mater., 2016, 28(6), 1769–1780 CrossRef CAS.
- S. H. Noh, W. Na, J. T. Jang, J. H. Lee, E. J. Lee, S. H. Moon, Y. Lim, J. S. Shin and J. Cheon, Nano Lett., 2012, 12, 3716–3721 CrossRef CAS PubMed.
- D. Cabrera, A. Lak, T. Yoshida, M. E. Materia, D. Ortega, F. Ludwig, P. Guardia, A. Sathya, T. Pellegrino and F. J. Teran, Nanoscale, 2017, 9, 5094–5101 RSC.
- L. Horev-Azaria, G. Baldi, D. Beno, D. Bonacchi, U. Golla-Schindler, J. C. Kirkpatrick, S. Kolle, R. Landsiedel, O. Maimon, P. N. Marche, J. Ponti, R. Romano, F. Rossi, D. Sommer, C. Uboldi, R. E. Unger, C. Villiers and R. Korenstein, Part. Fibre Toxicol., 2013, 10, 32 CrossRef CAS PubMed.
- P. B. Balakrishnan, N. Silvestri, T. Fernandez-Cabada, F. Marinaro, S. Fernandes, S. Fiorito, M. Miscuglio, D. Serantes, S. Ruta, K. Livesey, O. Hovorka, R. Chantrell and T. Pellegrino, Adv. Mater., 2020, 32, 2003712 CrossRef CAS PubMed.
- S. B. Goldhaber, Regul. Toxicol. Pharmacol., 2003, 38, 232–242 CrossRef CAS PubMed.
- S. Ammar, N. Jouini, F. Fiévet, Z. Beji, L. Smiri, P. Moliné, M. Danot and J.-M. Grenèche, J. Phys.: Condens. Matter, 2006, 18, 9055–9069 CrossRef CAS.
- C. N. Chinnasamy, A. Narayanasamy, N. Ponpandian, K. Chattopadhyay, H. Guerault and J. M. Greneche, J. Phys.: Condens. Matter, 2000, 12(35), 7795–7805 CrossRef CAS.
- M. G. Naseri, E. B. Saion, M. Hashim, A. H. Shaari and H. A. Ahangar, Solid State Commun., 2011, 151, 1031–1035 CrossRef CAS.
- C. Bárcena, A. K. Sra, G. S. Chaubey, C. Khemtong, J. P. Liu and J. Gao, Chem. Commun., 2008, 2224 RSC.
- Z. P. Chen, W. Q. Fang, B. Zhang and H. G. Yang, J. Alloys Compd., 2013, 550, 348–352 CrossRef CAS.
- J. Wan, X. Jiang, H. Li and K. Chen, J. Mater. Chem., 2012, 22, 13500 RSC.
- G. Salazar-Alvarez, J. Qin, V. Šepelák, I. Bergmann, M. Vasilakaki, K. N. Trohidou, J. D. Ardisson, W. A. A. Macedo, M. Mikhaylova, M. Muhammed, M. D. Baró and J. Nogués, J. Am. Chem. Soc., 2008, 130, 13234–13239 CrossRef CAS PubMed.
- Y. Köseoğlu, A. Baykal, F. Gözüak and H. Kavas, Polyhedron, 2009, 28, 2887–2892 CrossRef.
- R. Arulmurugan, B. Jeyadevan, G. Vaidyanathan and S. Sendhilnathan, J. Magn. Magn. Mater., 2005, 288, 470–477 CrossRef CAS.
- Y. Köseoğlu, F. Alan, M. Tan, R. Yilgin and M. Öztürk, Ceram. Int., 2012, 38, 3625–3634 CrossRef.
- J. A. Paulsen, A. P. Ring, C. C. H. Lo, J. E. Snyder and D. C. Jiles, J. Appl. Phys., 2005, 97, 044502 CrossRef.
- G. Vaidyanathan and S. Sendhilnathan, Physica B: Condens. Matter, 2008, 403, 2157–2167 CrossRef CAS.
- N. Sanpo, C. C. Berndt, C. Wen and J. Wang, Acta Biomater., 2013, 9, 5830–5837 CrossRef CAS PubMed.
- M. Gharibshahian, M. S. Nourbakhsh and O. Mirzaee, J. Sol-Gel Sci. Technol., 2018, 85, 684–692 CrossRef CAS.
- M. Ben Ali, K. El Maalam, H. El Moussaoui, O. Mounkachi, M. Hamedoun, R. Masrour, E. K. Hlil and A. Benyoussef, J. Magn. Magn. Mater., 2016, 398, 20–25 CrossRef CAS.
- S. SINGHAL, S. JAUHAR, K. CHANDRA and S. BANSAL, Bull. Mater. Sci., 2013, 36, 107–114 CrossRef CAS.
- R. P. Moyet, Y. Cardona, P. Vargas, J. Silva and O. N. C. Uwakweh, Mater. Charact., 2010, 61, 1317–1325 CrossRef CAS.
- T. Wang, Z.-Z. Luan, J.-Y. Ge, L. Liu, D. Wu, Z.-P. Lv, J.-L. Zuo and S. Sun, Phys. Chem. Chem. Phys., 2018, 20, 17245–17252 RSC.
- V. Mameli, A. Musinu, A. Ardu, G. Ennas, D. Peddis, D. Niznansky, C. Sangregorio, C. Innocenti, N. T. K. Thanh and C. Cannas, Nanoscale, 2016, 8, 10124–10137 RSC.
- A. Sathya, P. Guardia, R. Brescia, N. Silvestri, G. Pugliese, S. Nitti, L. Manna and T. Pellegrino, Chem. Mater., 2016, 28, 1769–1780 CrossRef CAS.
- K. E. Gilbert and J. J. Gajewski, J. Org. Chem., 1982, 47, 4899–4902 CrossRef CAS.
- A. Lak, T. Kahmann, S. J. Schaper, J. Obel, F. Ludwig, P. Müller-Buschbaum and J. Lipfert, ACS Appl. Mater. Interfaces, 2019, 12(1), 217–226 CrossRef.
- Y. Q. Ma and Y. Y. Pang, Chin. J. Polym. Sci., 2015, 33, 772–782 CrossRef CAS.
- A. T. Najafabadi, A. A. Khodadadi, M. J. Parnian and Y. Mortazavi, Appl. Catal., A, 2016, 511, 31–46 CrossRef.
- Y. Bao, W. An, C. H. Turner and K. M. Krishnan, Langmuir, 2010, 26, 478–483 CrossRef CAS PubMed.
- A. Pardo, S. Yáñez, Y. Piñeiro, R. Iglesias-Rey, A. Al-Modlej, S. Barbosa, J. Rivas and P. Taboada, ACS Appl. Mater. Interfaces, 2020, 12, 9017–9031 CrossRef CAS PubMed.
- A. López-Ortega, M. Estrader, G. Salazar-Alvarez, S. Estradé, I. V. Golosovsky, R. K. Dumas, D. J. Keavney, M. Vasilakaki, K. N. Trohidou, J. Sort, F. Peiró, S. Suriñach, M. D. Baró and J. Nogués, Nanoscale, 2012, 4, 5138 RSC.
- R. Di Corato, A. Quarta, P. Piacenza, A. Ragusa, A. Figuerola, R. Buonsanti, R. Cingolani, L. Manna and T. Pellegrino, J. Mater. Chem., 2008, 18, 1991 RSC.
- B. Mehdaoui, J. Carrey, M. Stadler, A. Cornejo, C. Nayral, F. Delpech, B. Chaudret and M. Respaud, Appl. Phys. Lett., 2012, 100(5), 052403 CrossRef.
- R. Hergt and S. Dutz, J. Magn. Magn. Mater., 2007, 311, 187–192 CrossRef CAS.
- B. Gleich and J. Weizenecker, Nature, 2005, 435, 1214–1217 CrossRef CAS PubMed.
- G. Song, M. Kenney, Y. S. Chen, X. Zheng, Y. Deng, Z. Chen, S. X. Wang, S. S. Gambhir, H. Dai and J. Rao, Nat. Biomed. Eng., 2020, 4, 325–334 CrossRef CAS PubMed.
- Q. Wang, X. Ma, H. Liao, Z. Liang, F. Li, J. Tian and D. Ling, ACS Nano, 2020, 14(2), 2053–2062 CrossRef CAS PubMed.
- Y. Du, X. Liu, Q. Liang, X. J. Liang and J. Tian, Nano Lett., 2019, 19, 3618–3626 CrossRef CAS.
- H. Arami, R. M. Ferguson, A. P. Khandhar and K. M. Krishnan, Med. Phys., 2013, 40, 071904 CrossRef PubMed.
- E. C. Abenojar, S. Wickramasinghe, J. Bas-Concepcion and A. C. S. Samia, Prog. Nat. Sci.: Mater. Int., 2016, 26, 440–448 CrossRef CAS.
- T. E. Torres, E. Lima, M. P. Calatayud, B. Sanz, A. Ibarra, R. Fernández-Pacheco, A. Mayoral, C. Marquina, M. R. Ibarra and G. F. Goya, Sci. Rep., 2019, 9, 1–11 CAS.
- Y. W. Jun, Y. M. Huh, J. S. Choi, J. H. Lee, H. T. Song, S. Kim, S. Yoon, K. S. Kim, J. S. Shin, J. S. Suh and J. Cheon, J. Am. Chem. Soc., 2005, 127, 5732–5733 CrossRef CAS PubMed.
- Q. L. Vuong, J. F. Berret, J. Fresnais, Y. Gossuin and O. Sandre, Adv. Healthcare Mater., 2012, 1, 502–512 CrossRef CAS PubMed.
- H. Duan, M. Kuang, X. Wang, Y. A. Wang, H. Mao and S. Nie, J. Phys. Chem. C, 2008, 112(22), 8127–8131 CrossRef CAS.
- R. A. Sperling, T. Pellegrino, J. K. Li, W. H. Chang and W. J. Parak, Adv. Funct. Mater., 2006, 16, 943–948 CrossRef CAS.
- E. W. Newcomb and D. Zagzag, in CNS Cancer, Humana Press, Totowa, NJ, 2009, pp. 227–241 Search PubMed.
- G. Li, M. J. Simon, L. M. Cancel, Z.-D. Shi, X. Ji, J. M. Tarbell, B. Morrison and B. M. Fu, Ann. Biomed. Eng., 2010, 38, 2499–2511 CrossRef.
- N. L. Martínez-Rodríguez, S. Tavárez and Z. I. González-Sánchez, Toxicol. In Vitro, 2019, 57, 54–61 CrossRef PubMed.
- H. A. Alhadlaq, M. J. Akhtar and M. Ahamed, Cell Biosci., 2015, 5, 55 CrossRef.
- M. Rivero, M. Marín-Barba, L. Gutiérrez, E. Lozano-Velasco, G. N. Wheeler, J. Sánchez-Marcos, A. Muñoz-Bonilla, C. J. Morris and A. Ruiz, J. Nanopart. Res., 2019, 21(8), 1–19 CrossRef CAS.
- Q. Saquib, A. A. Al-Khedhairy, J. Ahmad, M. A. Siddiqui, S. Dwivedi, S. T. Khan and J. Musarrat, DOI:10.1016/j.taap.2013.09.001.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1nr01044a |
| ‡ Current affiliation: International Clinical Research Center (FNUSA-ICRC), St. Anne's University Hospital, Brno, Czech Republic. |
| This journal is © The Royal Society of Chemistry 2021 |