Medicinal effects of Peruvian maca (Lepidium meyenii): a review
Natália
da Silva Leitão Peres
a,
Letícia
Cabrera Parra Bortoluzzi
a,
Leila Larisa
Medeiros Marques
a,
Maysa
Formigoni
b,
Renata Hernandez Barros
Fuchs
c,
Adriana Aparecida
Droval
d and
Flávia Aparecida
Reitz Cardoso
 *e
*e
aDepartment of Food Engineering, Federal University of Technology – Paraná (UTFPR), Campo Mourão, Paraná 87301-005, Brazil. E-mail: natalia.utfpr@hotmail.com; leticia_cabrera@outlook.com; leilamarques@utfpr.edu.br
bPost-Graduation Program of Food Science, State University of Maringá (UEM), Maringá, Paraná 87020-900, Brazil. E-mail: mayformigoni@live.com
cPost-Graduation Program of Food Technology, Federal University of Technology – Paraná (UTFPR), Campo Mourão, Paraná 87301-005, Brazil. E-mail: renata@utfpr.edu.br
dDepartment of Food Engineering, Federal Technological University of Paraná, Campo Mourao, Paraná 87301-005, Brazil. E-mail: adrianadroval@utfpr.edu.br
eDepartment of Mathematics, Federal University of Technology – Paraná (UTFPR), Campo Mourão, Paraná 87301-005, Brazil. E-mail: reitz@utfpr.edu.br; Tel: +55(44)999307070
First published on 17th January 2020
Abstract
Peruvian maca (Lepidium meyenii) is a root native to the Andean region, cultivated for at least 2000 years. Maca is rich in fiber, a large number of essential amino acids, fatty acids, and other nutrients, including vitamin C, copper, iron, and calcium. Besides these essential nutrients, this root contains bioactive compounds responsible for benefits to the human body, which has caused a considerable increase in its consumption in the last 20 years worldwide. This review documents the Peruvian maca composition and the recent findings regarding the medicinal effects of this root in sexual dysfunction regulation, neuroprotective effects, action in memory enhancement, antidepressant, antioxidant, anti-cancer, and anti-inflammatory activities, and skin protection.
1. Introduction
Over the last two decades, the interest and demand for maca have grown worldwide through an aggressive marketing promotion of the plant. This interest has established maca as one of the flagship products of Peru, being sold as powder, pills, capsules, flour, liquor, and extracts and is available from a variety of retail outlets such as health food stores and smoothie shops. Native to the Andean region, Peruvian maca (Lepidium meyenii) is rich in fiber and nutrients, including vitamin C, copper, and iron. Besides these essential nutrients, this root contains bioactive compounds responsible for providing benefits to the people who look for a healthy diet.Maca root has been cultivated for at least 2000 years1 and belongs to the Brassicaceae family; it grows in high altitude regions characterized by rocky formations, intense sunlight, strong winds, and extreme weather conditions, unsuitable for the growth of many other species.1–5
The indigenous population traditionally consume maca; it has a unique flavor and an aroma similar to caramel.6 The hypocotyls are consumed cooked or stored dried, and can be used for juices, soups, and extracts, and for enriching other foods with their powder.7,8 Maca root has different colors (Fig. 1) that are responsible for positively influencing its pharmacological and biological action. Yellow maca corresponds to about 60% of all maca hypocotyls harvested in Peru. It is the most widely used and researched form among all maca products. Its properties increase energy, improve concentration, and balance hormones. Red maca accounts for about 25% of the annual harvest and is the sweetest and highest in phytochemical levels among maca powders with all litter colors.9 It is known as the most effective type for women because of its hormonal balance effects and its action on bone health. The black maca is the rarest of all colors, accounting for about 15% of the annual harvest. Studies have shown that it is the most effective form for men, especially for muscle gain, endurance, mental focus, and libido.9
Gonzales et al.10 suggested that black maca may cause an increase in the sperm count compared to the use of yellow maca. In turn, the red maca is effective against benign prostatic hyperplasia. Black, yellow, and red macas showed effects on depression.
Clément et al.11 suggested that cultivation conditions and color types may affect their metabolites, also influencing biological activities. In 1961, the first secondary metabolites were determined, reporting the presence of alkaloids, glycosides, tannins, and saponins,8,9,12 and later, also the macamides and the macaenes. The antifungal action of a maca extract could be related to the presence of imidazole alkaloids because they are chemically similar to antifungal or imidazole antifungal azoles such as miconazole.11 Glucosinolates and their degradation products, known for their fungicidal attributes, bactericidal properties, and nematicidal activity, have recently attracted great interest and intense research for their chemoprotective action against some cancers.7–9,11,12
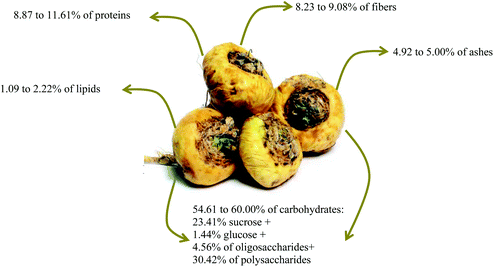
The Peruvian maca has a high nutritional value similar to cereal grains and a better composition compared to other hypocotyls, such as potatoes, carrots, and turnips. When fresh, this root has 80% water content, leaving a small portion for the other nutrients. Hence, a study of the dry matter reveals that this root is rich in protein (8.87–11.6%), with a small lipid portion (1.09–2.22%), in addition to 8.23–9.08% fiber, 4.9–5.0% ash, and 54.61 to 60.00% carbohydrates, where 23.41% is sucrose, 1.55% glucose, 4.56% oligosaccharides, and 30.42% polysaccharides (Fig. 2). The latter is the most studied by Dini et al.13
2. Peruvian maca medicinal effects
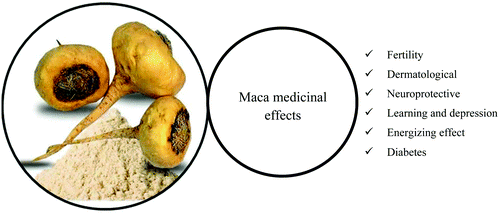
Many root-based (hypocotyl) food supplements of different colors of powdered maca are available on the world market due to the possible effect on the treatment of a wide range of diseases and medical conditions (Fig. 3).9 Sexual dysfunction regulation,14 neuroprotective effects,15,16 memory enhancement, antidepressant,14,17 antioxidant, anti-cancer, and anti-inflammatory effects,18 and skin protection19 (Table 1) are the most common effects of maca. The root also presents a large number of essential amino acids, fatty acids, and mineral content, in particular, iron, calcium, and copper.13,20,21| Medicinal effects | Reports | |
|---|---|---|
| Neuroprotective | Improvement of cognitive function in stroke patients and age-related animals and humans, reduction in oxidative stress, anti-inflammatory action, regulation of transcription factors and protein inhibition | 16, 22 and 24–26 |
| Dermatological | Prevention and improvement of the damage to skin caused by UV radiation and acceleration of wound healing at high altitudes | 19 and 27 |
| Diabetes | Reduction in glucose levels and lower levels of lipid oxidation inhibiting the oxidative damage in the liver, a significant increase in the insulin values, and an increase in the glutathione content | 28–30 |
| Learning | Improved spatial learning and memory deficits and passive avoidance learning and memory deficits | 17 and 33 |
| Fertility | Improvement in sexual desire, volume of ejaculation, sperm concentration and total sperm count | 35 and 41–43 |
| Energizing | Providing a physical improvement in athletes and everyone. Higher levels of resistance and enzyme superoxide dismutase, and lower levels of catalase, lactate dehydrogenase and lipid peroxidation | 46 and 47 |
| Anti-fatigue | Improvement of the enzymatic activity of glutathione peroxidase and creatine kinase and, helping in delaying the onset of symptoms of fatigue. Increasing the antioxidant capacity and accelerating the conversion of energy in ATP. Decreasing the level of nitrogen urea. Increasing the level of glycogen (dose-dependent effect). | 49, 50 and 53 |
| Insecticide | Possible insecticidal effect for dengue mosquito control | 52 |
| Antioxidant | Intermediate antioxidant effect, wherein antioxidant activity depends on the extract concentration and microenvironment in which the compound is located. Antioxidant activity capable of inhibiting the formation of oxidation products | 55 and 58 |
2.1. Peruvian maca neuroprotective effect
Recent work shows that natural compounds are capable of improving cognitive function in stroke patients. Current data show that a diet rich in phytochemical foods can improve deficits in cognitive function of older animals and humans.A methanolic extract of Peruvian maca, re-extracted with n-pentane (98%), was used to observe its effect in neuroprotective cells.16 Regarding the in vivo study, adult rats that suffered from stroke received the pentane extract at 3, 10, and 30 mg kg−1 doses per injection daily. The results showed significant differences among treatments, and the 3 mg kg−1 dose was the only dose that showed significant neuroprotective effects. The 10 and 30 mg kg−1 doses showed an increase in brain damage, revealing toxicity with high concentrations of the extract. Concerning the in vitro study, scampi neuronal cells were tested using the following final concentrations of the pentane extract: 0.1, 0.3, 1.3, 10, and 30 μg mL−1. The cells also received 20 μL of hydrogen peroxide (0.001 mol L−1) as a neurotoxic agent that causes oxidative stress, responsible for several neurodegenerative diseases. Microscopic evaluations demonstrated a significant reduction in oxidative stress in the cells treated with the pentane extract. The cells treated with the 30 μg mL−1 dose exhibited dendritic cellular forms and higher cell density compared with those treated with the 3 μg mL−1 dose. The reduction in oxidative stress through the Peruvian maca pentane extract suggests potential neuroprotective properties.16
Nguyen et al.22 demonstrated the neuroprotective activity of maca extracts in vitro in neurons subjected to oxidative stress with H2O2. The neuroprotective effect of these substances is historically attributed to their antioxidant action. However, nowadays, scientists have known that antioxidant action is not solely responsible for the ability to prevent or reverse neuronal dysfunction and cognitive deficits. Recent studies point to several other potential mechanisms, such as anti-inflammatory action, regulation of transcription factors, and protein inhibition.23 Macamides present in maca extracts are believed to be the active compounds responsible for the neuroprotective effect through inhibition of FAAH.24 FAAH inhibits anandamide endocannabinoid degradation that is involved in cell proliferation of neural progenitor cells, involving CB1 and CB2 receptors in this process.25 Reactive oxygen (ROS) and nitrogen (RNS) species (in the forms of superoxide, hydroxyl radical, peroxyl, H2O2, and peroxynitrite) are implicated in the etiology of degenerative disorders due to excessive production and release of excitatory neurotransmitters. Maca could indirectly prevent the formation of ROS/RNS by a modulatory mechanism of neurotransmitter release, and thus help protect cells from pathological changes. Also, an inflammatory marker, cytokine IL-6, at a severe level was reduced in maca users, and this was associated with a better quality of life, which may indicate an anti-inflammatory maca property.26
2.2. Peruvian maca dermatological effect
Despite the small number of studies on the effects of Peruvian maca on dermatology, Gonzales-Castaneda et al.19 employed aqueous extracts (heated and not heated) prepared from the yellow maca. Heated and unheated aqueous extracts of maca (40 mL per circle) were applied in circles on the back of male rats to compare the effects of different extracts under UVA, UVB, and UVC radiation. The treated areas received 0.05 mg of the unheated hypocotyl powdered extract or aqueous extract of freeze-dried maca. The three doses of aqueous extract were 0.13, 0.65 and 1.3 mg mL−1, respectively. The animals received different types of radiation once a week, for 15 minutes, over 3 weeks. The treatment with the heated maca extract and spray dried maca extract can prevent skin damage induced by UV radiation. The treatment with different concentrations of maca prevents the damage caused by UVA, UVB, and UVC radiation, avoiding the epidermis hyperproliferation, as observed in the control group that received radiation without treatment. The administration of maca before the exposure can prevent skin damage, improving the harms caused by radiation.19Also, in 2017, Nuñez et al.27 studied red litter for healing skin wounds at sea level and high altitudes in adult male rats. Previous studies suggest that there is a delay in the healing process at high altitudes, mainly due to changes in the inflammatory phase. In this sense, the objective of the study was to determine the effect of high altitude on tissue repair and the effect of topical administration of a dry extract by red litter spray (RM) on tissue repair with male mice at sea level and high altitudes. Lesions were inflicted through a 10 mm diameter excisional wound on the dorsal surface of the skin. RM treatment accelerated wound closure, decreased the level of epidermal hyperplasia, and decreased the number of inflammatory cells at the wound site. The authors mentioned that high-altitude RM has a positive effect on wound healing by decreasing the number of neutrophils and increasing the number of macrophages after day 7, a fact not observed at sea level.
2.3. Peruvian maca and diabetes
Studies by Cisneros et al. and Rodrigo et al.28,29 evaluated twenty-four rats randomly assigned to groups to study the influence of yellow Peruvian maca on diabetes. The animals received 4 and 6 g day−1 of yellow Peruvian maca flour and were compared to a control group induced with diabetes and drug-treated. The intake of 6 g day−1 produced a reduction in glucose levels between 35 and 57.8%, and the 4 g day−1 dose showed a decrease of 20–34.4%. The liver is the organ commonly used as a marker of antioxidant defenses in the body. Regarding the lipid peroxidation, the 4 and 6 g day−1 doses of maca inhibited the oxidative damage compared with the drug-treated control group.28,29Rodrigo et al.29 also observed a significant increase in the insulin values in diabetic animals compared with the control group treated with drugs.
Another study divided 100 diabetic rats and 10 healthy rats in 11 groups of 10 animals each. They observed the antioxidant effect of the maca extract based on the following groups: healthy control group, diabetic control group, and black maca, yellow maca, and purple maca groups. The maca extracts administered to the animals were prepared with methanol. The oral administration of the extracts reduces the levels of glucose in the plasma of rats with diabetes and recovers glucose levels to normal; the different colors of Peruvian maca showed no significant differences in the results. The rats fed with maca for 60 days showed lower levels of lipid oxidation, indicated by the decrease of substances reactive to thiobarbituric acid and protein carbonyls of the liver. The black maca showed better results than the other two types regarding lipid oxidation. The levels of protein carbonyls were also reduced, highlighting the animals of the yellow maca group. The purple maca extracts increased the activity of antioxidant enzymes both in blood cells and liver cells in diabetic rats. The animals that were brought in contact with the purple maca showed an increase in the glutathione content. Glutathione is a substance with an essential antioxidant function that prevents damage to certain cellular compounds in the diabetic rat's plasma model. Black and yellow maca extracts did not affect the glutathione content in the plasma of diabetic rats.30
To study the hypoglycemic activity in male rats spray-dried Peruvian black maca was used.31 The animals were randomly divided into two groups: diabetics not fed with maca and diabetics fed with maca. The maca was administered in rats at 2 g per kg of body weight for 14 days, which is comparable to the human consumption in the Peruvian Andes. On the fifth day of treatment, the animals that were not treated showed an increase of 136.1% in glycemia, against 75.1% in those treated with maca. The glycemic peak in those not fed with maca occurred on day 9, and on day 14 in those fed with maca, meaning that the treatment delayed the glycemic peak. The rats with diabetes fed with maca maintained a glycemic degree below 300 mg mL−1 in the highest peak, an increase of 107.0% compared to the glycemic level from day 1. In contrast, rats without maca supplementation had a 272.4% increase in the highest peak, more than double of those that received maca in their diet.31
Gonzales et al.32 investigated the effect of two Peruvian plant extracts on the sperm count and blood glucose in diabetic streptozotocin rats. Normal or diabetic rats were divided into several groups. They received a vehicle, black litter (Lepidium meyenii), yacon (Smallanthus sonchifolius), or three mixtures of black/yacon litter extracts (90/10, 50/50 and 10/90%). The groups were treated for 7 days and evaluated for blood glucose, daily sperm production (DSP), the sperm count in the epididymis and vas deferens, polyphenol content, and antioxidant activity. Black maca (BM), yacon, and mixed extracts reduced the glucose levels in diabetic rats. Non-diabetic patients treated with BM and yacon had higher DSP than those treated with the vehicle. Diabetic rats treated with BM, yacon, and the maca/yacon mixture had increased DSP and sperm count in the vas deferens and epididymis compared to non-diabetic rats. Since yacon has a 3.05 times higher polyphenol content than maca, it was associated with higher antioxidant activity. The combination of two extracts improved glycemic levels and male reproductive function in diabetic rats. Streptozotocin increased 1.43 times the liver weight, which was reversed with the evaluated plant extracts. The authors concluded that streptozotocin-induced diabetes resulted in reduced sperm count and liver damage, probably due to the action of black maca, yacon, and the black maca and yacon mixture.
2.4. Peruvian maca on learning
Two tests were performed to evaluate latent learning in finding water and forced swimming. Ovariectomized rats were divided into three groups: the control group, treated with 1 g kg−1 of yellow maca, one group treated with 1 g kg−1 of red maca, and another treated with 1 g kg−1 of black maca. For the task of finding water, 80 animals made up four groups, and each group was divided again into two groups: trained and untrained animals for this assignment. For the forced swim test, 40 mice were divided into four groups, as already mentioned. The animals treated with Peruvian maca showed better test results. The best effect in the latent learning test in the task of finding water was observed for black maca, which reduced latency throughout the test. In the forced swim test, the three types of maca reduced the time of immobility, showing no difference between them.17A study investigated the effects of different doses of aqueous and hydroalcoholic maca extracts on scopolamine-induced learning and memory deficits (1 mg kg−1) in rats for 35 days. The maca improved spatial learning, memory deficits and passive avoidance learning. The results indicated that scopolamine increases AChE activity in the rat brain up to 1.5-fold. Maca extracts reduced AChE activity in the brain by 45% compared to the group receiving scopolamine alone.33
More recently, ethanol-treated rats took longer to find the hidden platform than the control during the Morris water maze test and escape acquisition test. Incidentally, the black maca reversed the effect of ethanol. The black maca improved the deleterious effect of ethanol during the probe test.34
In summary, contrary evidence suggests that a black maca improves learning and memory. The effect of black maca on learning and memory may be due to the presence of polyphenolic compounds.17 Previous studies have shown no toxic effects during short and long term administration.17
2.5. Peruvian maca and fertility
The indigenous population believed that maca is able to increase vitality and treat infertility, accentuated by residents of high altitude regions.1,3 These beliefs, previously popular, have been analyzed in several studies, where some have confirmed and others have not confirmed its efficacy in sexual performance and increased fertility.12,26,35,36 Since then, the products derived from maca are recommended for enhancing fertility, and as aphrodisiacs.9,37 Sexual difficulties are more prevalent among men, and sexual desire may be affected by factors such a depression, stress,38 anxiety,39 and testosterone concentration.40Gonzales et al.10 conducted a 12-week, randomized, double-blind, placebo-controlled, parallel study that compared active treatment with different doses of the gelatinized maca and with a placebo. The study aimed to demonstrate whether the effect of maca on the subjective reporting of sexual desire was caused by the effect on mood or serum testosterone levels. Men aged 21 to 56 years received maca in one of two doses: 1500 mg or 3000 mg or placebo. The study evaluated the groups at 4, 8, and 12 weeks of treatment. An improvement in sexual desire was observed with maca since 8 weeks of treatment. Serum testosterone and estradiol levels were not different in men treated with maca and those treated with a placebo. Regression analysis showed that maca has an independent effect on sexual desire at 8 and 12 weeks of treatment. This effect is not due to changes in depression or anxiety or serum testosterone and estradiol levels.
The increased volume of ejaculation in men due to the consumption of maca may be influenced by an increase in sexual desire,41 which is in contrast to the result in animals.42 However, tests performed in horses treated with 20 g day−1 of maca over 60 days show that the dietary supplementation improved the sperm concentration. The total sperm count was almost two times higher at the end than at the beginning of the trial and stabilized the semen quality during refrigerated storage at 5 °C.43 The sperm increase was also observed in rats at 4340 m above sea level, where the spermatogenic disorder is induced by high altitudes.44
2.6. Peruvian maca and energizing effect
Maca is famous for its energizing effect.45 In sports, it is necessary to improve physical performance and optimize oxygen consumption, benefits that a few licit substances provide. To check the effectiveness of maca in these circumstances, a soccer team from Cusco, a city located at 3400 meters above sea level, ingested 1500 mg per day for 60 days.46 Tests were conducted to determine the highest speed, oxygen consumption, and performance before and after the ingestion. The physical performance of the athletes increased by 10.3% on average, and the maximum value for the increase in oxygen consumption was 33.6%. Based on the results, it is believed that maca provides a physical improvement not only in athletes but everyone.46When administering an aqueous extract of yellow maca to newly weaned male rats,47 a favorable response of the organism to a stressful and physically exhausting situation was observed. In four groups, the rats were managed with different amounts of maca (control: 0.4 mg maca g−1, 0.8 mg g−1, and 1.2 mg g−1), and subjected to the forced swim test after 30 days of treatment. After the test, scientists studied the average activity of the enzymes superoxide dismutase (SOD), catalase (CAT), and lactate dehydrogenase (LDH), as an indicator of the oxidative process. The lipid peroxidation (TBARS) was measured. The rats that consumed a more significant amount of maca showed higher levels of resistance and SOD, and lower levels of CAT, LDH, and TBARS, proving its benefits to the organism and corresponding to its role as an adaptogen.47
3. Peruvian maca and its polysaccharides
Hot water extraction is the most frequently used method to study the composition of polysaccharides or starches in Peruvian maca.48–51 The alcoholic extraction was also used in some cases.52 After the extraction of polysaccharides or starches, a deproteinization process, where the polysaccharides, most often, precipitate with cold ethanol48,49,51 or sodium borate is followed.50For analysis, the crude polysaccharides are eluted for purification in some chromatography methods. Gel chromatography was the method chosen to determine the molecular weight.48,49,51 One can use gas chromatography,49 gas chromatography with flame ionization detection,48,51 and gas–liquid chromatography.52Table 2 shows the percentage of Peruvian maca polysaccharides.49,50,52,53
Combined with the monosaccharide composition of Peruvian maca, it is found that the galactose was in greater quantity (47.95%), followed by arabinose (35.25%) and D-glucuronic acid (27.34%). The rest of the monosaccharides are rhamnose, glucose, and mannose.48 The methylation analysis of polysaccharides revealed that 39.81% galactose/galacturonic acid, 32.86% glucose, 12.67% mannose, 10.74% arabinose, and 0.95% rhamnose were generated.49 The alkaline fractions are highlighted by the high content of mannose (24.8%) and galactose (21.8%).52 The differences in the characteristics of the polysaccharides can probably be attributed to several conditions of extraction and different tissues of the same plant.48 Years of development of seeds and different culture conditions can lead to a considerable divergence that can still be expanded by different pre-treatments such as temperature changes, column chromatography, and analytical methods.49 The polysaccharides found in Peruvian maca are homogeneous heteropolysaccharides.48,51 The molecular weights varied from 9.83 kDa to 793.5 kDa, and because of this highest number (793.5 kDa), some authors considered the Peruvian maca polysaccharides as heterogeneous.49 Therefore, it is suggested that Peruvian maca polysaccharides have six forms of connection, are linear, and have a triple helix conformation.51
Comparing the starches of three types of maca (yellow, purple, and black), it is found that the swelling power of the yellow kind was higher, indicating less resistance. The extension and hydrolysis rate among the three starches followed the order: black, yellow, and purple maca, a fact that can be attributed to differences in their crystallinity. The order of the gelatinization temperature was yellow > purple > black. The gelatinization enthalpy values among the three starches of maca were similar. Higher hardness was found in the following order: black (34.3 g), purple (25.0 g), and yellow maca (21.1 g). The adhesiveness of the starches ranged from −105 to −245 g and followed the order: black, yellow, and purple maca. The starch from maca is easy to gelatinize, and has a high degree of swelling and solubility in water, high bonding viscosity, low shear strength, and a high tendency to retrograde.50
In a study, the effect of enzymes on Peruvian maca during the storage period did not vary.54 The maca is a plant that has no starch degradation by the action of amylolytic enzymes during storage, although there are enzymes capable of merging the starch as a substrate. This study suggests that after harvesting, the hypocotyls of maca enter a latent period before senescence because they are not in an environment suitable for growing.
3.1. Polysaccharides and lipid-soluble extract from Peruvian maca with the anti-fatigue effect
For analysis of the impact of polysaccharides in the anti-fatigue effect, Tang et al.49 used doses of 25 mg kg−1, 50 mg kg−1, and 100 mg kg−1 in treating mice for 30 consecutive days. The authors concluded that the average speed of mice treated with medium and high doses of Peruvian maca polysaccharides showed higher performance with the anti-fatigue effect. The polysaccharides of Peruvian maca can effectively improve the enzymatic activity of glutathione peroxidase and creatine kinase and, therefore, help delay the onset of symptoms of fatigue, increasing the antioxidant capacity and accelerating the conversion of energy in ATP. Furthermore, the level of nitrogen urea decreased significantly in the mice treated with polysaccharides; this compound is the end product of liver metabolism and is closely related to fatigue. The polysaccharides in Peruvian maca could effectively improve the endogenous antioxidant activity and reduce the levels of metabolic products in the serum of mice. The minimum sufficient amount is equivalent to the recommended daily dose of 7 g of human litter powder.Li et al.53 investigated how fat-soluble extract may contribute to the anti-fatigue effect in mice. These were treated with doses ranging from 20 mg kg−1 to 100 mg kg−1 of MS1 (xylose, arabinose, galactose, and glucose), and MS2 (arabinose, galactose, and glucose), separately, for 30 consecutive days, with glucose as the central unit of the monosaccharide for both segments. The animals that had been treated with MS2 showed better results at the time of swimming than those treated with MS1. The animals treated with MS2 at higher doses showed a decrease of approximately 41.8% in their swim time, indicating significant differences between those treated with MS1 and MS2. The animals treated with low doses of MS showed no significant differences from the group that did not receive any treatment, which demonstrates that the supplementation of maca with polysaccharides may not affect the activity of LDH. The glycogen content of all animals except those treated with low doses of MS1 was significantly higher than those that received no treatment. Those treated with high doses of MS2 were approximately 60.8% greater than the animals without treatment. The anti-fatigue activity with treatment with polysaccharides of Peruvian maca contributed mainly to the increase in the levels of glycogen. The duration of swimming among the mice was prolonged significantly depending on the dose; the animals treated with higher doses presented better results. Therefore, the rats treated with polysaccharides of Peruvian maca showed a decrease of the activity of lactic dehydrogenase, which indicates a low concentration of lactic acid in the serum showing a potential anti-fatigue activity.51,53
3.2. Polysaccharides of Peruvian maca and their insecticidal effect on dengue mosquitoes
Paredes52 conducted a study to analyze the effects of Peruvian maca polysaccharides as an insecticide on Aedes aegypti mosquitoes (dengue mosquito). To this end, an aqueous ethanol extract was prepared using maca flour, at 200 g of the sample in 750 ml of the solvent. The analysis was performed in a final volume of 200 mL, containing 20 larvae in different concentrations of the extract: 0.12, 0.25, 0.60, 0.85 and 1.00%. The results suggest that extracts of Peruvian maca may have an insecticidal effect for dengue mosquito control; however, further experiments are needed to confirm this possibility.4. Peruvian maca and its antioxidant effect
Like many other plants, Peruvian maca contains several antioxidant compounds. Quantities of these substances vary according to the soil composition, litter ecotype, harvest time, drying process, and extraction method. Despite differences in quantity, litter contains substantial amounts of several compost antioxidants. These are especially phenols, glucosinolates, alkamides, and polysaccharides. They have various functions in metabolism and antioxidant effects in scientific research. By in vitro studies, its antioxidant effects are mainly established using various methods, such as measurements of the ferric reducing antioxidant potential, hydroxyl radical scavenging capacity, lipid peroxidation inhibiting capacity, 2,2-diphenyl-1-picrylhydrazyl, and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid radical scavenging abilities of bioactive compounds.52–59Inoue et al.56 studied the antioxidant effect of the yellow and black Peruvian maca from an aqueous extract dissolved in methanol. Both demonstrated a low antioxidant capacity, but the yellow maca extract was more efficient than the black maca extract.
In a study of the antioxidant effect of Peruvian maca, an aqueous extract of maca was used dissolved in ethanol at different concentrations.54 The antioxidant activity of Peruvian maca was also evaluated in vitro through the deoxyribose protection against hydroxyl radicals. Depending on the dose, the maca reacted with DPPH, as the extracts with greater concentration showed higher activity to capture free radicals. The results of the peroxyl tests showed that maca decreased the formation of this compound. The maca (1 to 3 mg mL−1) protected deoxyribose against hydroxyl radicals ranging from 57 to 74%, respectively. In the same study, the authors also assessed whether the maca could maintain the intracellular levels of ATP in macrophages exposed to oxidative stress conditions. They found that treatment with maca (1 mg mL−1) increased the production of ATP.
Three extracts of Peruvian maca with different solvents (diethyl ether, ethanol, and water) at concentrations of 1, 10, 25, 50, and 100 μg mL−1 were prepared. All the samples were activated when reacting with DPPH, showing a higher free radical capture of the ethyl ether extract at concentrations of 50 and 100 μg mL−1. The data show that different extracts from Peruvian maca leaves may have an antioxidant effect, and it is worth mentioning that the antioxidant activity depends on the extract concentration.55
Hypertriglyceridemic rats were fed with high levels of sucrose and 1% (p/p) of Peruvian maca for two weeks, to evaluate the antioxidant activity of this root. The diet of the mice with maca led to a significant decrease in plasma cholesterol, the levels of total plasma triacylglycerols, and the content of triacylglycerols in their livers. Maca did not influence the level of triacylglycerols in muscle tissue significantly. There was a significant improvement in glucose tolerance after administration of 3 g of glucose per kg of body weight. There was also a notable reduction in the levels of blood glucose. The lipid peroxidation markers in blood and the liver were not significantly affected by the administration of maca. Therefore, the Peruvian maca could be used as a dietary supplement in the treatment of chronic diseases, and also in their prevention.59
Another study analyzed the antioxidant activity of Peruvian maca by adding 2.5 g of flour in 50 ml soybean oil and observing the oxidation products formed by its heating. The results suggested that the plant showed an antioxidant activity capable of inhibiting the formation of oxidation products, in addition to stabilizing antioxidant species.58
Troya-Santos et al.31 employed two agents for analysis of the antioxidant activity: ABTS˙+ and DPPH. To this end, spray-dried black Peruvian maca hypocotyls boiled in water were used. The boiling time varied in three ways: baking for 30 minutes (maca A), baking for 45 minutes (maca B), and baking for 60 minutes (maca C). The three aqueous extracts showed antioxidant capacity, highlighting the one cooked for 60 minutes.
Several factors can change the antioxidant activity of Peruvian maca, including variations in its composition of mono- and polysaccharides, molecular weight, and string type.48,60 It is essential to highlight that the maca antioxidant activity may be related to the concentration of the extract and the microenvironment in which the compound is located. They both may interact with each other, producing inhibitory effects.55
So the Peruvian maca can be used as a natural antioxidant agent,48 and can also help in maintaining a balance between oxidants and antioxidants.57
5. Conclusion
The Peruvian maca is highlighted in the literature in the treatment of several diseases and dysfunctions. However, there is a lack of studies in humans and the application of maca in food products to evaluate its potential pharmacological functions. The different colors of root litter were investigated in these studies in dietary supplements, ethanolic, methanolic, and aqueous extracts, and others. However, extrinsic and intrinsic factors related to cultivation and the form of extraction can diversify its pharmacological function due to different bioactive groups obtained. Based on this, many techniques can be explored to use extracts with high potential, such as modern techniques of supercritical extraction, ultrasound, and enzyme-assistance.Conflicts of interest
There are no conflicts to declare.References
- J. León, The “Maca” (Lepidium meyenii), a little known food plant of Peru, Econ. Bot., 1964, 18, 122–127 CrossRef.
- J. Brinckmann and E. Smith, Maca culture of the Junin Plateau, J. Altern. Complementary Med., 2004, 10, 426–430 CrossRef PubMed.
- G. F. Gonzales, C. Gonzales and C. Gonzales-Castañeda, Lepidium meyenii (Maca): A Plant from the Highlands of Peru – from Tradition to Science, Complementary Med. Res., 2009, 16, 373–380 CrossRef PubMed.
- K. Valentová, J. Frcek and J. Ulrichová, Yacon (Smallanthus sonchifolius) and Maca (Lepidium meyenii), traditional Andean crops as new functional foods on the European market, Chem. Listy, 1997, 95, 594–601 Search PubMed.
- Y. Wang, Y. Wang, B. McNeil and L. M. Harvey, Maca: An Andean crop with multi-pharmacological functions, Food Res. Int., 2007, 40, 783–792 CrossRef.
- J. Zhao, B. Avula, M. Chan, C. Clément, M. Kreuzer and I. A. Khan, Metabolomic Differentiation of Maca (Lepidium meyenii) Accessions Cultivated under Different Conditions Using NMR and Chemometric Analysis, Planta Med., 2011, 78, 90–101 CrossRef PubMed.
- A. F. G. Cicero, E. Bandieri and R. Arletti, Lepidium meyenii Walp. improves sexual behaviour in male rats independently from its action on spontaneous locomotor activity, J. Ethnopharmacol., 2001, 75, 225–229 CrossRef PubMed.
- C. F. Quiros, A. Epperson, J. Hu and M. Holle, Physiological Studies and Determination of Chromosome Number in Maca, Econ. Bot., 1996, 50, 216–223 CrossRef.
- M. J. Balick and R. Lee, Maca: from traditional food crop to energy and libido stimulant, Altern. Ther., 2002, 8, 96–98 Search PubMed.
- G. F. Gonzales, J. Nieto, J. Rubio and M. Gasco, Effect of Black maca (Lepidium meyenii) on one spermatogenic cycle in rats, Andrologia, 2006, 38, 166–172 CrossRef PubMed.
- C. Clément, D. Diaz, I. Manrique, B. Avula, I. A. Khan, D. D. Ponce Aguirre, C. Kunz, A. C. Mayer and M. Kreuzer, Secondary Metabolites in Maca as Affected by Hypocotyl Color, Cultivation History, and Site, Agron. J., 2010, 102, 431–439 CrossRef.
- G. F. Gonzales, A. Cordova, K. Vega, A. Chung, A. Villena and C. Gonez, Effect of Lepidium meyenii (Maca), a root with aphrodisiac and fertility-enhancing properties, on serum reproductive hormone levels in adult healthy men, Int. J. Endocrinol., 2003, 176, 163–168 Search PubMed.
- A. Dini, G. Migliuolo, L. Rastrelli, P. Saturnino and O. Schettino, Chemical composition of Lepidium meyenii, Food Chem., 1994, 49, 347–349 CrossRef.
- N. A. Brooks, G. Wilcox, K. Z. Walker, J. F. Ashton, M. B. Cox and L. Stojanovska, Beneficial effects of Lepidium meyenii, (Maca) on psychological symptoms and measures of sexual dysfunction in postmenopausal women are not related to estrogen or androgen content, Menopause, 2008, 15, 1157–1162 CrossRef.
- A. Alquraini, D. Waggas, M. Böhlke, T. Maher and A. Pino-Figueroa, Neuroprotective effects of Lepidium meyenii, (Maca) and macamides against amyloid-beta (25-35) induced toxicity in B-35 neuroblastoma cells, FASEB J., 2014, 28 Search PubMed , 657.13.
- A. Pino-Figueroa, D. Nguyen and T. J. Maher, Neuroprotective effects of Lepidium meyenii (Maca), Ann. N. Y. Acad. Sci., 2010, 1199, 77–85 CrossRef.
- J. Rubio, M. Caldas, S. Dávila, M. Gasco and G. F. Gonzales, Effect of three different cultivars of Lepidium meyenii (Maca) on learning and depression in ovariectomized mice, BMC Complementary Altern. Med., 2006, 6, 1–7 CrossRef.
- N. Bai, K. He, M. Roller, C.-S. Lai, L. Bai and M.-H. Pan, Flavonolignans and Other Constituents from Lepidium meyenii with Activities in Anti-inflammation and Human Cancer Cell Lines, J. Agric. Food Chem., 2015, 63, 2458–2463 CrossRef.
- C. Gonzales-Castaneda and G. F. Gonzales, Hypocotyls of Lepidium meyenii (maca), a plant of the Peruvian highlands, prevent ultraviolet A-, B-, and C-induced skin damage in rats, Photodermatol., Photoimmunol. Photomed., 2007, 24, 24–31 CrossRef.
- Y. Long-jiang and J. Wen-wen, Study on the Nutritional Components and the Anti-fatigue Effects of Dry Powder of Maca (Lepidium meyenii), Food Sci., 2004, 25, 164–166 Search PubMed.
- K. Valentová, D. Buckiová, V. Kren, J. Peknicová, J. Ulrichová and V. Simánek, The in vitro biological activity of Lepidium meyenii extracts, Cell Biol. Toxicol., 2006, 22, 91–99 CrossRef.
- D. Nguyen, A. Pino-Figueroa and T. J. Maher, In vitro evaluation of the neuroprotective effects of Lepidium meyenii (maca) in crayfish neuronal and rat neuroblastoma cell lines, FASEB J., 2009, 23, 77–85 CrossRef.
- C. Rendeiro, J. D. Guerreiro, C. M. Williams and J. P. Spencer, Flavonoids as modulators of memory and learning: molecular interactions resulting in behavioural effects, Proc. Nutr. Soc., 2012, 71, 246–262 CrossRef.
- H. Wu, C. J. Kelley, A. Pino-Figueroa, H. D. Vu and T. J. Maher, Macamides and their synthetic analogs: evaluation of in vitro FAAH inhibition, Bioorg. Med. Chem., 2013, 21, 5188–5197 CrossRef.
- A. Cohen-Yeshurun, D. Willner, V. Trembovler, A. Alexandrovich, R. Mechoulam, E. Shohami and R. R. Leker, N-arachidonoyl-L-serine (AraS) possesses proneurogenic properties in vitro and in vivo after traumatic brain injury, J. Cereb. Blood Flow Metab., 2013, 33, 1242–1250 CrossRef.
- G. F. Gonzales, S. Miranda, J. Nieto, G. Fernández, S. Yucra, J. Rubio, P. Yi and M. Gasco, Red maca (Lepidium meyenii) reduced prostate size in rats, Reprod. Biol. Endocrinol., 2005, 3, 1–16 CrossRef.
- D. Nuñez, P. Olavegoya, G. F. Gonzales and C. Gonzales-Castaneda, Red Maca (Lepidium meyenii), a Plant from the Peruvian Highlands, Promotes Skin Wound Healing at Sea Level and at High Altitude in Adult Male Mice, High Alt. Med. Biol., 2017, 18, 372–383 CrossRef.
- R. Cisneros, R. Oré, I. Arnao and S. Suárez, Relación de glutatión reducido/oxidado (GSH/GSSG) en ratas diabéticas tratadas con maca (Lepidium meyenii walp), An. Fac. Med., 2011, 72, 107–111 CrossRef.
- M. E. Rodrigo, R. Valdivieso, S. Suárez, R. Oriondo and R. Oré, Disminucion del daño oxidativo y efecto hipoglicemiante de la maca (Lepidium meyenii Walp) en ratas con diabetes inducida por streptozotocina, An. Fac. Med., 2011, 72, 7–11 CrossRef.
- C. Qiu, T. Zhu, L. Lan, Q. Zeng and Z. Du, Analysis of Maceaene and Macamide Contents of Petroleum Ether Extract of Black, Yellow, and Purple Lepidium Meyenii (Maca) and Their Antioxidant Effect on Diabetes Mellitus Rat Model, Braz. Arch. Biol. Technol., 2016, 59, 1–9 Search PubMed.
- J. Troya-Santos, N. Ale-Borja and S. Suárez-Cunza, Capacidad Antioxidante in vitro y Efecto Hipoglucemiante de la Maca Negra (Lepidium meyenii) Preparada Tradicionalmente, Rev. Soc. Quim. Peru, 2017, 83, 40–51 CAS.
- G. F. Gonzales, C. Gonzales-Castaneda and M. Gasco, A mixture of extracts from Peruvian plants (black maca and yacon) improves sperm count and reduced glycemia in mice with streptozotocin-induced diabetes, Toxicol. Mech. Methods, 2013, 23, 509–518 CrossRef CAS.
- J. Rubio, H. Dang, M. Gong, X. Liu, S. L. Chen and G. F. Gonzales, Aqueous and hydroalcoholic extracts of Black Maca (Lepidium meyenii) improve scopolamine-induced memory impairment in mice, Food Chem. Toxicol., 2007, 45, 1882–1890 CrossRef CAS PubMed.
- J. Rubio, W. Qiong, X. Liu, Z. Jiang, H. Dang, S. L. Chen and G. F. Gonzales, Aqueous Extract of Black Maca (Lepidium meyenii) on Memory Impairment Induced by Ovariectomy in Mice, Evid. Based Complement. Alternat. Med., 2011, 253958, 14 Search PubMed.
- G. F. Gonzales, A. Córdova, K. Vega, A. Chung, A. Villena, C. Góñez and S. Castillo, Effect of Lepidium meyenii (MACA) on sexual desire and its absent relationship with serum testosterone levels in adult healthy men, J. Androl., 2002, 34, 367–372 CrossRef CAS.
- B. L. Zheng, K. He, C. H. Kim, L. Rogers, Y. Shao, Z. Y. Huang, Y. Lu, S. J. Yan, L. C. Qien and Q. Y. Zheng, Effect of a lipidic extract from Lepidium meyenii on sexual behavior in mice and rats, Urology, 2000, 55, 598–602 CrossRef CAS.
- K. Valentová and J. Ulrichová, Smallanthus Sonchifolius and Lepidium Meyenii – Prospective Andean Crops for the Prevention of Chronic Diseases, Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub., 2003, 147, 119–130 CrossRef.
- V. Kumar, P. N. Singh and S. Bhattacharya, Anti-stress activity of Indian Hypericum perforatum L., Indian J. Exp. Biol., 2001, 39, 344–349 CAS.
- D. L. Rowland, J. R. Heiman, B. A. Gladue, J. P. Hatch, C. H. Doering and S. J. Weiler, Endocrine, psychological and genital response to sexual arousal in men, Psychoneuroendocrinology, 1987, 12, 149–158 CrossRef CAS.
- Y. Ohta, K. Yoshida, S. Kamiya, N. Kawate, M. Takahashi, T. Inaba, S. Hatoya, H. Morii, K. Takahashi, M. Ito, H. Ogawa and H. Tamada, Feeding hydroalcoholic extract powder of Lepidium meyenii (maca) increases serum testosterone concentration and enhances steroidogenic ability of Leydig cells in male rats, Andrologia, 2016, 48, 347–354 CrossRef CAS PubMed.
- I. Melnikovova, T. Fait, M. Kolarova, E. C. Fernandez and L. Milella, Effect of Lepidium meyenii Walp. on Semen Parameters and Serum Hormone Levels in Healthy Adult Men: A Double-Blind, Randomized, Placebo-Controlled Pilot Study, J. Evidence-Based Complementary Altern. Med., 2015, 2015, 6 Search PubMed.
- C. Clément, J. Kneubühler, A. Urwyler, U. Witschi and M. Kreuzer, Effect of maca supplementation on bovine sperm quantity and quality followed over two spermatogenic cycles, Theriogenology, 2010, 74, 173–183 CrossRef.
- C. D. Prete, S. Tafuri, F. Ciani, M. P. Pasolini, F. Ciotola, S. Albarella, D. Carotenuto, V. Peretti and N. Cocchia, Influences of dietary supplementation with Lepidium meyenii (Maca) on stallion sperm production and on preservation of sperm quality during storage at 5 °C, Andrology, 2018, 6, 351–361 CrossRef.
- G. F. Gonzales, M. Gasco, A. Cordova, A. Chung, J. Rubio and L. Villegas, Effect of Lepidium meyenii (Maca) on spermatogenesis in male rats acutely exposed to high altitude (4340 m), Int. J. Endocrinol., 2004, 180, 87–95 CAS.
- M. Hermann, J. Heller and J. Engels, Andean roots and tubers: Ahipa, arracacha, maca and yacon, 1997 Search PubMed.
- G. Ronceros, W. Ramos, F. Garmendia, J. Arroyo and J. Gutiérrez, Eficacia de la maca fresca (Lepidium meyenii walp) en el incremento del rendimiento físico de deportistas en altura, An. Fac. Med., 2005, 66, 269–273 CrossRef.
- S. Suárez, R. Oré, I. Arnao, L. Rojas and J. Trabucco, Aqueous Lepidium meyenii Walp (maca) extract and its role as an adaptogen, in an endurance animal model, An. Fac. Med., 2009, 70, 181–185 CrossRef.
- S. Li, L. Hao, Q. Kang, Y. Cui, H. Jiang, X. Liu and J. Lu, Purification, characterization and biological activities of a polysaccharide from Lepidium meyenii leaves, Int. J. Biol. Macromol., 2017, 103, 1302–1310 CrossRef CAS PubMed.
- W. Tang, L. Jin, L. Xie, J. Huang, N. Wang, B. Chu, X. Dai, Y. Liu, R. Wang and Y. Zhang, Structural Characterization and Antifatigue Effect In Vivo of Maca (Lepidium meyenii Walp) Polysaccharide, J. Food Sci., 2017, 82, 757–764 CrossRef CAS.
- L. Zhang, G. Li, S. Wang, W. Yao and F. Zhu, Physicochemical properties of maca starch, Food Chem., 2017, 218, 56–63 CrossRef CAS PubMed.
- M. Zhang, W. Wu, Y. Ren, X. Li, Y. Tang, T. Min, F. Lai and H. Wu, Structural Characterization of a Novel Polysaccharide from Lepidium meyenii (Maca) and Analysis of Its Regulatory Function in Macrophage Polarization in Vitro, J. Agric. Food Chem., 2017, 65, 1146–1157 CrossRef CAS PubMed.
- L. L. R. Paredes, Dissertação apresentada ao Programa de PósGraduação em Bioquímica da Universidade Federal do Paraná, como requisito para obtenção do grau de Mestre em Ciências-Bioquímica, Universidade Federal do Paraná, 2009 Search PubMed.
- J. Li, Q. Sun, Q. Meng, L. Wang, W. Xiong and L. Zhang, Anti-fatigue activity of polysaccharide fractions from Lepidium meyenii Walp. (maca), Int. J. Biol. Macromol., 2017, 95, 1305–1311 CrossRef CAS.
- G. G. Rondan-Sanabria, B. Valcarcel-Yamani and F. Finardi-Filho, Effects on starch and amylolytic enzymes during Lepidium meyenii Walpers root storage, Food Chem., 2012, 134, 1461–1467 CrossRef CAS.
- R. Cuentas, L. De la Cruz, G. Hernández, I. Mateo and C. Castañeda, Evaluación del efecto antioxidante de hojas de Lepidium peruvianum Chacón, “maca”, Horiz. Med., 2008, 8, 45–55 Search PubMed.
- N. Inoue, C. Farfan and G. F. Gonzales, Effect of butanolic fraction of yellow and black maca (Lepidium meyenii) on the sperm count of adult mice, Andrology, 2016, 48, 915–921 CrossRef CAS.
- M. Sandoval, N. N. Okuhama, F. M. Angeles, V. V. Melchor, L. A. Condezo, J. Lao and M. J. S. Miller, Antioxidant activity of the cruciferous vegetable Maca (Lepidium meyenii), Food Chem., 2001, 79, 207–213 CrossRef.
- D. R. Soares, Trabalho de Conclusão de Curso (Graduação), Universidade Tecnológica Federal do Paraná, 2015 Search PubMed.
- R. Vecera, J. Orolin, N. Skottova, L. Kazdova, O. Oliyarnik, J. Ulrichova and V. Simanek, The influence of maca (Lepidium meyenii) on antioxidant status, lipid and glucose metabolism in rat, Plant Foods Hum. Nutr., 2007, 62, 59–63 CrossRef.
- S. Zha, Q. Zhao, J. Chen, L. Wang, G. Zhang, H. Zhang and B. Zhao, Extraction, purification and antioxidant activities of the polysaccharides from maca (Lepidium meyenii), Carbohydr. Polym., 2014, 111, 584–587 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |