Open Access Article
Open Access ArticleFirst steps towards a stable neon compound: observation and bonding analysis of [B12(CN)11Ne]−†
Martin
Mayer‡
 a,
Markus
Rohdenburg‡
a,
Markus
Rohdenburg‡
 b,
Valentin
van Lessen
c,
Marc C.
Nierstenhöfer
c,
Edoardo
Aprà
b,
Valentin
van Lessen
c,
Marc C.
Nierstenhöfer
c,
Edoardo
Aprà
 d,
Simon
Grabowsky
d,
Simon
Grabowsky
 e,
Knut R.
Asmis
e,
Knut R.
Asmis
 a,
Carsten
Jenne
a,
Carsten
Jenne
 *c and
Jonas
Warneke
*c and
Jonas
Warneke
 *a
*a
aWilhelm-Ostwald-Institut für Physikalische und Theoretische Chemie, Universität Leipzig, 04103 Leipzig, Germany. E-mail: jonas.warneke@uni-leipzig.de
bInstitut für Angewandte und Physikalische Chemie, Universität Bremen, Fachbereich 2-Biologie/Chemie, Leobener Str. 5, D-28359 Bremen, Germany
cAnorganische Chemie, Fakultät für Mathematik und Naturwissenschaften, Bergische Universität Wuppertal, 42119 Wuppertal, Germany. E-mail: carsten.jenne@uni-wuppertal.de
dEnvironmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA 99352, USA
eUniversity of Bern, Department of Chemistry and Biochemistry, Freiestrasse 3, 3012 Bern, Switzerland
First published on 10th March 2020
Abstract
Noble gas (Ng) containing molecular anions are much scarcer than Ng containing cations. No neon containing anion has been reported so far. Here, the experimental observation of the molecular anion [B12(CN)11Ne]− and a theoretical analysis of the boron–neon bond is reported.
Binding to noble gases – the most unreactive elements – has fascinated chemists since their discovery.1 Today, a diverse Xe chemistry is established2 and several stable Kr compounds are known.3 Extreme pressures can force even He to form chemical bonds, (Na2He is stable at pressures >100 MPa),4 but evidence for stable compounds containing the light noble gases at ambient or lower pressures are extremely rare. The neutral compound HArF has been shown to exist in a cryogenic matrix.5 Some other interactions, which exceed the strength of van-der-Waals attractions, have been detected between the atoms of cryogenic noble gas matrices and embedded molecules.6 Stable Ne and He compounds are unknown at ambient pressure conditions. On the other hand, isolated cations in the gas phase, containing small noble gases, are long known.7 Therefore, it may be assumed that ionic compounds formed with small noble gas containing molecular ions, may have a chance to exist.
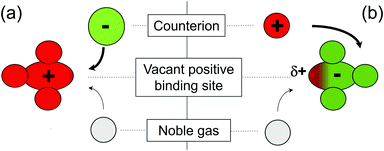
Noble gases have a negative electron affinity and do therefore not exist as an atomic anion.8 They are also unable to accept electrons provided by nucleophiles. The only way to form a localized, directed bond with a noble gas is by abstracting electron density from the noble gas atom. Therefore, spontaneous binding of noble gases is the sole privilege of the strongest electrophiles, which are usually cationic. Complex polyatomic cations containing Ar have been discovered.9 Schröder and Roithová detected a Ne-containing molecular dication by the endothermic substitution of F˙ in SiF32+˙ with Ne.10 Although the strongest bonds with noble gas atoms have been found for cations, they bear an intrinsic problem for the formation of stable compounds in the condensed phase: a cation needs to be paired with an anion. A negatively charged ion always competes with the noble gas for the highly electrophilic binding site in the cation (see Fig. 1a). The noble gas atom will be replaced by the anion due to its stronger nucleophilic character. In contrast, a noble gas containing anion may be a more promising ionic building block for condensed phase material:11 A carefully chosen countercation should not compete with a noble gas for the electrophilic binding site, enabling the formation of stable ion pairs (Fig. 1b). Unfortunately, most anions do not possess a sufficiently strong electrophilic binding site to form a stable noble gas bond.
Chemical intuition may suggest a consistent trend in the periodic table row so that He is the most unreactive element. However, theoretical investigations predict that binding of Ne to a reactive molecule can be substantially less favorable than binding of He.12–14 In contrast to He, Ne contains occupied p-orbitals which results in higher orbital repulsion during formation of a σ-bond between Ne and a reactive atom. Often, this disadvantage overcompensates the higher polarizability of Ne compared to He. Therefore, with respect to chemical bonding, Ne may actually be ranked as the stubbornest element in the periodic table. Molecular anions containing He and Ne have not been observed experimentally so far. While some anionic species such as [FHeX]− (X = O, S, Se) have been theoretically predicted to be metastable, the equivalent Ne anions were shown to be unbound.11,15 In particular for Ne, the above-mentioned concept (molecular noble gas anions as building blocks for salts) remains purely hypothetical because no Ne containing anions have been observed or even predicted so far.
The presence of a strongly electrophilic binding site within an anion represents a critical key aspect of the discussed concept. We have recently reported on the exceptional reactivity of the [B12(CN)11]− anion,16 a fragment of the highly stable closo-dodecaborate dianion [B12(CN)12]2−.16,17 The monoanion [B12(CN)11]−, although being overall negatively charged, possesses a free boron atom with a significant positive partial charge which behaves as a strong electrophile. [B12(CN)11]− even binds Ar spontaneously at room temperature. Although spontaneous Ne-binding was not observed, we believe that [B12(CN)11]− is the most promising candidate for the observation of a bound Ne-containing anion, which may exist at lower temperatures. Here, we communicate the first steps towards the generation of an ionic compound based on a Ne-containing molecular anion:
(1) We report an improved synthetic procedure based on a photochemical approach, which enables a room-temperature and metal-free synthesis of [B12(CN)12]2−. An easy and cost-efficient synthesis of the precursor constitutes the baseline for potential applications of the [B12(CN)11]− anions.
(2) We experimentally probe the binding properties of [B12(CN)11]− towards the small noble gases He and Ne and perform a complementary theoretical bonding analysis of the B-Ne bond.
In our first study on [B12(CN)11]−, its precursor [B12(CN)12]2− was generated only as a side product in a reaction mixture16 by irradiation of an aqueous solution of K2[B12I12] and KCN with a 150 W medium pressure mercury lamp. Very recently, a new synthesis procedure was published enabling generation of [B12(CN)12]2− based on Pd-catalyzed cross coupling in a high temperature microwave reaction on a small scale.18 The advanced synthesis procedure of the percyano-closo-dodecaborate anion reported here is based on a photochemical reaction, which exchanges iodine substituents in [B12I12]2− with CN groups. This approach was mentioned first by Trofimenko in 1965, but yielded only products with a maximum of 8–9 CN groups.19 Our improved procedure consists of the use of Na+ as a counter cation instead of K+ and reducing the amount of the solvent water, which produces a soluble fraction and a white precipitate. The soluble fraction contains [B12(CN)12−x(OH)x]2− (x = 0–3) ions, while the precipitate consists of an approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of [B12(CN)12]2− and [B12(CN)11I]2−. From the latter product single crystals of the solid solution of [B12(CN)12]2− and [B12(CN)11I]2− as [PPh4]+ salt could be obtained (Fig. 2). For details on synthesis and characterization see ESI.†
1 mixture of [B12(CN)12]2− and [B12(CN)11I]2−. From the latter product single crystals of the solid solution of [B12(CN)12]2− and [B12(CN)11I]2− as [PPh4]+ salt could be obtained (Fig. 2). For details on synthesis and characterization see ESI.†
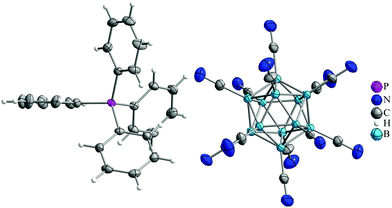 | ||
Fig. 2 Part of the crystal structure of [PPh4]2[B12(CN)12]. The disordered iodine atoms and the second [PPh4]+ cation were omitted for clarity. Thermal ellipsoids are drawn at 50% probability and hydrogen atoms are shown with arbitrary radii. The averaged B–C (1.541 Å, cf. 1.541 Å in ref. 18 and 1.538 Å on the B3LYP/def2-QZVPP level) and C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond lengths (1.130 Å) in the free [B12(CN)12]2− anion are in accord with those in the very recently published Cu-complex (CH3CN)3Cu[μ-B12(CN)12]Cu-(CH3CN)3 (1.134 and 1.549 Å)18 and quantum chemically predicted values using the B3LYP/def2-QZVPP method (1.150 and 1.538 Å). N bond lengths (1.130 Å) in the free [B12(CN)12]2− anion are in accord with those in the very recently published Cu-complex (CH3CN)3Cu[μ-B12(CN)12]Cu-(CH3CN)3 (1.134 and 1.549 Å)18 and quantum chemically predicted values using the B3LYP/def2-QZVPP method (1.150 and 1.538 Å). | ||
For a first orientation, we performed high-level computational investigations to obtain a reasonable estimate of the binding energy of He and Ne to [B12(CN)11]−. Details of the calculations can be found in the ESI.† Based on dispersion-corrected B3LYP/def2-QZVPP and on an SCS-MP2 basis-set limit approach, 0 K attachment enthalpies of around 0 to −2 kJ mol−1 for He and −6 to −9 kJ mol−1 for Ne binding are found. This means that the existence of [B12(CN)11Ne]− should in principle be possible.
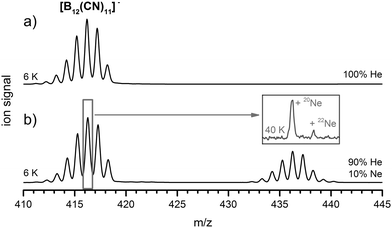
Observation of spontaneous binding of a reagent to a reactive ion in the gas phase requires several preconditions, even if the bond formation is energetically favorable. Energy from the collision event is redistributed within the internal degrees of freedom of the molecular ion, making the ion “hot”. Cooling by collisions with cold background gases can stabilize the product, however, very weak bonds do often not survive the necessary timeframe for collisional cooling. Here, we used an ion trap cryogenically cooled to ∼6 K.20 The internal temperature of captured ions under these conditions is typically estimated to be slightly higher which can be rationalized by absorption of infrared blackbody radiation, radiofrequency heating and inefficient energy transfer.21 Generation and isolation of [B12(CN)11]− was performed following previously described procedures.16 He gas was introduced as a buffer gas for cooling of the ions. Even at the lowest temperatures amenable in our experiments, spontaneous He binding to this ion was not observed (Fig. 3a). However, when we mixed the He gas with 10% of Ne, a signal 20 mass units higher than [B12(CN)11]− was observed, see Fig. 3b. The ion trap temperature was heated in steps of 5 K. The additional signal was clearly visible up to an ion trap temperature of 45 K. At around 50 K, the intensity dropped below a signal to noise ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using our standard experimental settings (see ESI†). At 40 K ion trap temperature, we isolated all ions with m/z 416 (width of one mass unit, marked grey in Fig. 3b). These ions of the composition [10B211B10(CN)11]− lead to the ions [10B211B10(CN)1120Ne]− and [10B211B10(CN)1122Ne]− after reaction with Ne gas. The two product signals, which reflect the natural isotopic distribution of Ne (inset in Fig. 3b), evidence that the observed signal indeed corresponds to the Ne-bound product. This constitutes the first observation of a thermodynamically bound Ne containing anion.
1 using our standard experimental settings (see ESI†). At 40 K ion trap temperature, we isolated all ions with m/z 416 (width of one mass unit, marked grey in Fig. 3b). These ions of the composition [10B211B10(CN)11]− lead to the ions [10B211B10(CN)1120Ne]− and [10B211B10(CN)1122Ne]− after reaction with Ne gas. The two product signals, which reflect the natural isotopic distribution of Ne (inset in Fig. 3b), evidence that the observed signal indeed corresponds to the Ne-bound product. This constitutes the first observation of a thermodynamically bound Ne containing anion.
Remarkably, calculated attachment enthalpies and experimental results show consistently that Ne-binding is substantially preferred over He-binding in the case of [B12(CN)11]−. As discussed above, this is in contrast to many theoretical investigations on other highly reactive binding sites which have shown that the dative bond to Ne is weaker than to He.12–14 Thus, other interactions than the direct Ne–B bond may be of critical relevance. Electrostatic and dispersion forces play a significant role for the binding properties of the electrophilic anions of type [B12X11]−.16 The positive binding site lies in a “crater” of negatively charged substituents. Compared with He, the better polarizability of Ne results in stronger for dispersion interactions. This hypothesis is corroborated by a complementary bonding analysis (Table 1). The B–Ne bond (2.09 Å) is predicted to be significantly longer than the B–He bond (1.59 Å) and even longer than the B–Ar bond with 1.98 Å.16 Natural population analysis (NPA)22 predicts a positive charge of +0.1e for the bound Ne, while the atomic charge of the bound He is more than doubled (+0.2e). This indicates that He has provided more electron density to the positive boron in the anion than Ne, although He is less polarizable. A Quantum Theory of Atoms in Molecules (QTAIM)23 analysis shows that the electron density ρ and the energy density H at the bond critical point (bcp) are smaller in the case of the B–Ne bond compared to the B–He bond. Also, the delocalization index δ, which is often discussed as a measure of covalent bond order, is significantly lower in the case of Ne. This indicates consistently that the direct interaction is indeed weaker for B–Ne than for B–He. It follows that the stronger binding of Ne must have another origin.
| Ng | He | Ne | |
|---|---|---|---|
| Ng attachment enthalpy (0 K), kJ mol−1 | −1.3 | −8.9 | |
| Bond length, Å | 1.59 | 2.09 | |
| ρ bcp, e Å−3 | 0.27 | 0.16 | |
| H bcp/ρbcp, Ha e−1 | −0.45 | −0.22 | |
| δ | 0.20 | 0.14 | |
| Q NPA bound Ng, e | +0.22 | +0.11 | |
| Energy components (EDA) | Orbital interaction, kJ mol−1 | −75.0 | −33.0 |
| Electrostatics, kJ mol−1 | −23.3 | −17.1 | |
| Pauli repulsion, kJ mol−1 | +93.4 | +44.8 | |
| Dispersion, kJ mol−1 | −5.7 | −8.0 | |
| Total interaction energy, kJ mol−1 | −10.6 | −13.2 | |
We performed an energy decomposition analysis (EDA)24 to partition the interactions between the two binding partners [B12(CN)11]− and Ne/He into orbital interactions, electrostatics, Pauli repulsion and dispersion forces, see Table 1. The total interaction energy calculated by this method is higher for Ne than for He. We note that this quantity is not directly comparable to the previously discussed 0 K attachment enthalpies, because (i) optimization of the separated binding partners is not part of the EDA and (ii) different base functions are used for the calculation (see details in the ESI†). All contributions with the exception of dispersion are significantly lower in their absolute number for Ne than for He. In particular, the contribution of the orbital interaction, which can be associated with the directed B–Ne bond, is smaller. Note that also the Pauli repulsion is smaller due to the large bond length, which significantly contributes to the higher interaction energy with Ne.
The upper temperature limit of the [B12(CN)11Ne]− detection in our instrument (50 K) is remarkable, because it lies significantly above the Ne condensation temperature of 25 K.25 We note that so-called low temperature “tagging” of ions with small noble gas atoms is a common process used in spectroscopy26 and was observed in similar instrument for Ne and some cations at roughly 10 K.26 In contrast, to the best of our knowledge, Ne tagging to anions has not been reported. This underlines that the observed binding of Ne is not a low temperature condensation effect but the result of a significant chemical interaction.
To study the influence of a countercation, a Na+ cation was positioned at several different locations on the surface of the [B12(CN)11Ne]− anion. Substitution of the Ne atom is not favorable, since the Na+ cation has no binding tendency towards the positive boron site, which binds the Ne atom. Instead the energetically lowest lying configuration was found by positioning the Na+ cation close to the most negatively charged surface of the anion, which lies on the backside of the Ne binding site, see ESI.† The counterion further polarizes the anion and attracts its electron density. This increases the positive charge of the reactive boron atom and therefore the calculated 0 K attachment enthalpy of Ne by nearly 30%. Further information on the energy of different Na+ position isomers, changes in QTAIM and NPA parameters in comparison to the free anion can be found in the ESI.† We conclude that in contrast to noble gas binding cations, the presence of a counterion strengthens the boron–neon bond and does not lead to dissociation confirming a recent theoretical study.27
The practical realization of condensed phase salts like Na[B12(CN)11Ne] still bears significant challenges: no synthesis procedures exists to generate the key anion [B12(CN)11]− outside a mass spectrometer. However, molecular ion deposition methods have recently shown significant progress in the generation of condensed phase material layers on surfaces.28 Very recently new experimental setups were introduced which enable high frequency switching between anion and cation deposition.29 This allows salt formation from combined gaseous cations and anions. This method may be combined with an ultrahigh vacuum approach allowing deposition on a cryogenically cooled target in the presence of an introduced Ne background atmosphere. Then, the formation of the mentioned type of salts may be possible.
M. C. N. acknowledges a Kekulé fellowship from the Fonds der Chemischen Industrie and J. W. is grateful to the Volkswagen foundation for a Freigeist Fellowship. A portion of this research (E. A.) was performed in the Environmental Molecular Sciences Laboratory (EMSL), a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research and located at the Pacific Northwest National Laboratory (PNNL). K. R. A. acknowledges instrumental support from the Fritz-Haber-Institute of the Max-Planck-Society.
Conflicts of interest
There are no conflicts to declare.Notes and references
- D. S. Brock, G. J. Schrobilgen and B. Žemva, in Comprehensive Inorganic Chemistry II. From Elements to Applications, ed. J. Reedijk and K. R. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 755–822 Search PubMed.
- (a) J. Haner and G. J. Schrobilgen, Chem. Rev., 2015, 115, 1255–1295 CrossRef CAS PubMed; (b) K. Edel, J. S. A. Ishibashi, S.-Y. Liu and H. F. Bettinger, Angew. Chem., Int. Ed., 2019, 58, 4061–4064 CrossRef CAS PubMed.
- J. Lehmann, Coord. Chem. Rev., 2002, 233-234, 1–39 CrossRef CAS.
- X. Dong, A. R. Oganov, A. F. Goncharov, E. Stavrou, S. Lobanov, G. Saleh, G.-R. Qian, Q. Zhu, C. Gatti, V. L. Deringer, R. Dronskowski, X.-F. Zhou, V. B. Prakapenka, Z. Konôpková, I. A. Popov, A. I. Boldyrev and H.-T. Wang, Nat. Chem., 2017, 9, 440–445 CrossRef CAS PubMed.
- L. Khriachtchev, M. Pettersson, N. Runeberg, J. Lundell and M. Rasanen, Nature, 2000, 406, 874–876 CrossRef CAS PubMed.
- Some examples are (a) X. Wang, L. Andrews, F. Brosi and S. Riedel, Chem. – Eur. J., 2013, 19, 1397–1409 CrossRef CAS PubMed; (b) Q. Zhang, M. Chen, M. Zhou, D. M. Andrada and G. Frenking, J. Phys. Chem. A, 2015, 119, 2543–2552 CrossRef CAS PubMed; (c) D. Cappelletti, A. Bartocci, F. Grandinetti, S. Falcinelli, L. Belpassi, F. Tarantelli and F. Pirani, Chem. – Eur. J., 2015, 21, 6234–6240 CrossRef CAS PubMed.
- J. Roithová, Pure Appl. Chem., 2011, 83, 1499–1506 Search PubMed.
- R. E. Dickerson, H. B. Gray and G. P. Haight, Chemical Principles, Benjamin/Cummings Publishing Company, Inc., Menlo Park, 1979 Search PubMed.
- J. Jin, W. Li, Y. Liu, G. Wang and M. Zhou, Chem. Sci., 2017, 8, 6594–6600 RSC.
- J. Roithová and D. Schröder, Angew. Chem., Int. Ed., 2009, 48, 8788–8790 CrossRef PubMed.
- T.-H. Li, C.-H. Mou, H.-R. Chen and W.-P. Hu, J. Am. Chem. Soc., 2005, 127, 9241–9245 CrossRef PubMed.
- F. Grandinetti, Nat. Chem., 2013, 5, 438 CrossRef CAS PubMed.
- G. Frenking, W. Koch, F. Reichel and D. Cremer, J. Am. Chem. Soc., 1990, 112, 4240–4256 CrossRef CAS.
- W. Grochala, Found. Chem., 2018, 20, 191–207 CrossRef CAS.
- S. Borocci, N. Bronzolino and F. Grandinetti, Chem. Phys. Lett., 2008, 458, 48–53 CrossRef CAS.
- M. Mayer, V. van Lessen, M. Rohdenburg, G.-L. Hou, Z. Yang, R. M. Exner, E. Aprà, V. A. Azov, S. Grabowsky, S. S. Xantheas, K. R. Asmis, X.-B. Wang, C. Jenne and J. Warneke, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 8167–8172 CrossRef CAS PubMed.
- H. Zhao, J. Zhou and P. Jena, Angew. Chem., Int. Ed., 2016, 55, 3704–3708 CrossRef CAS PubMed.
- A. A. Kamin and M. A. Juhasz, Inorg. Chem., 2020, 59, 189–192 CrossRef CAS.
- (a) S. Trofimenko and H. N. Cripps, J. Am. Chem. Soc., 1965, 87, 653–654 CrossRef CAS; (b) S. Trofimenko, J. Am. Chem. Soc., 1966, 88, 1899–1904 CrossRef CAS.
- N. Heine and K. R. Asmis, Int. Rev. Phys. Chem., 2015, 34, 1–34 Search PubMed.
- N. Heine, Vibrational Spectroscopy of Gaseous Hydrogen-Bonded Clusters: On the Role of Isomer-Specicity and Anharmonicity, Dissertation, Leipzig University, 2014 Search PubMed.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS.
- (a) R. F. W. Bader, Atoms in Molecules. A Quantum Theory, Clarendon Press, Oxford, 2003, vol. 22 Search PubMed; (b) R. F. W. Bader and M. E. Stephens, J. Am. Chem. Soc., 1975, 97, 7391–7399 CrossRef CAS.
- F. M. Bickelhaupt and E. J. Baerends, in Reviews in Computational Chemistry, ed. K. B. Lipkowitz and D. B. Boyd, John Wiley & Sons, Inc, Hoboken, NJ, USA, 2000, vol. 15, pp. 1–86 Search PubMed.
- D. R. Lide, CRC Handbook of Chemistry and Physics. A Ready-Reference Book of Chemical and Physical Data, CRC Press, Boca Raton, 81st edn, 2000 Search PubMed.
- A. M. Burow, T. Wende, M. Sierka, R. Włodarczyk, J. Sauer, P. Claes, L. Jiang, G. Meijer, P. Lievens and K. R. Asmis, Phys. Chem. Chem. Phys., 2011, 13, 19393–19400 RSC.
- M. Joshi and T. K. Ghanty, Chem. Commun., 2019, 55, 14379–14382 RSC.
- (a) J. Warneke, M. E. McBriarty, S. L. Riechers, S. China, M. H. Engelhard, E. Aprà, R. P. Young, N. M. Washton, C. Jenne, G. E. Johnson and J. Laskin, Nat. Commun., 2018, 9, 1889 CrossRef PubMed; (b) J. Laskin, G. E. Johnson, J. Warneke and V. Prabhakaran, Angew. Chem., Int. Ed., 2018, 57, 16270–16284 CrossRef CAS PubMed.
- P. Su, H. Hu, J. Warneke, M. E. Belov, G. A. Anderson and J. Laskin, Anal. Chem., 2019, 91, 5904–5912 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1979144. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc01423k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |