Open Access Article
Open Access ArticleTargeting of the mitochondrion by dinuclear thiolato-bridged arene ruthenium complexes in cancer cells and in the apicomplexan parasite Neospora caninum
Afonso P.
Basto†
a,
Nicoleta
Anghel†
a,
Riccardo
Rubbiani†
b,
Joachim
Müller†
a,
David
Stibal
c,
Federico
Giannini
d,
Georg
Süss-Fink
c,
Vreni
Balmer
a,
Gilles
Gasser
 *e,
Julien
Furrer
*e,
Julien
Furrer
 *d and
Andrew
Hemphill
*d and
Andrew
Hemphill
 *a
*a
aInstitute of Parasitology, Vetsuisse Faculty, University of Bern, Länggassstrasse 122, CH-3012 Berne, Switzerland. E-mail: andrew.hemphill@vetsuisse.unibe.ch; Web: http://www.ipa.vetsuisse.unibe.ch/ueber_uns/personen/prof_dr_hemphill_andrew/index_ger.html Fax: +41-31-6312477; Tel: +41-31-6312384
bDepartment of Chemistry, University of Zurich, Winterthurerstrasse 190, CH-8057 Zurich, Switzerland
cInstitut de Chimie, Université de Neuchâtel, Avenue de Bellevaux 51, CH-2000 Neuchâtel, Switzerland
dDepartment of Chemistry and Biochemistry, Freiestrasse 3, CH-3012 Bern, Switzerland. E-mail: julien.furrer@dcb.unibe.ch; Web: https://furrer.dcb.unibe.ch Tel: +41 631 43 83
eChimie ParisTech, PSL University, Laboratory for Inorganic Chemical Biology, F-75005 Paris, France. E-mail: gilles.gasser@chimieparistech.psl.eu; Web: http://www.gassergroup.com Tel: +33 1 44 27 56 02
First published on 8th January 2019
Abstract
A library of 18 dinuclear-thiolato bridged arene ruthenium complexes, some of which with demonstrated activity against cancer cells, was screened for activity against a transgenic Neospora caninum strain that constitutively expresses beta-galactosidase. Initial assessments were done at concentrations of 2500, 250, 25 and 2.5 nM, and 5 compounds were further evaluated with regard to their half maximal proliferation-inhibiting concentration (IC50). Among those, [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-CH3)3]Cl (1), [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-But)3]Cl (2) and [(η6-p-MeC6H4Pri)2Ru2(μ2-SCH2C6H4-p-But)2(μ2-SC6H4-p-OH)]BF4 (9) inhibited N. caninum proliferation with low C50 values of 15, 5 and 1 nM, respectively, while [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-OH)3]Cl (3) and [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-mco)3]Cl (5, mco = 4-methylcoumarinyl) were less active (IC50 = 280 and 108 nM, respectively). These compounds did not affect human foreskin fibroblast (HFF) host cells at dosages of 5 μM and above, but impaired proliferation of the human ovarian carcinoma cell line A2780 (IC50 values of 130 nM (1), 30 nM (2), 530 nM (3), 7730 nM (5), 130 nM (9)). A2780 cancer cells were treated with complexes 1, 2, and 5, and biodistribution analysis using inductively coupled plasma mass spectrometry (ICP-MS) showed that most of the drugs accumulated in the mitochondrial fractions. Transmission electron microscopy showed that the parasite mitochondrion is the primary target also in N. caninum tachyzoites, but these compounds, when applied at 200 nM for 15 days in vitro, did not act parasiticidal. Complexes 1, 2 and 9 applied orally at 2 and 10 mg kg−1 day−1 during 5 days in a neosporosis mouse model did not reduce parasite load and did not limit parasite dissemination to the central nervous system. In accordance with these results, ICP-MS carried out on different organs of mice orally administrated with complexes 1 and 9, demonstrated that the drugs were readily absorbed, and after 3 and 48 h, were mainly detected in liver and kidney, but were largely absent from the brain. Thus, dinuclear thiolato-bridged arene ruthenium complexes exhibit interesting activities against N. caninum in vitro, but further modifications of these promising molecules are required to improve their bioavailability and pharmacokinetic properties in order to exert a pronounced and selective effect against N. caninum in vivo.
Significance to metallomicsRuthenium(II) complexes have attracted attention because of their favorable antiparasitic properties. Many different structural scaffolds are being investigated but their mode of action and targets remain largely unknown. Here, we report on the antiparasitic properties of dinuclear-thiolato bridged arene ruthenium complexes against N. caninum. We show that their activity and uptake can be correlated with their lipophilicity and we demonstrate their ability to selectively target the mitochondria of the parasite. In vivo experiments also reveal that the compounds accumulated in liver and kidneys, but were absent from the brain. However, the compounds did not act as parasiticidal. |
Introduction
Cancer cells and apicomplexan parasites such as Neospora caninum and Toxoplasma gondii share a number of distinct biological features.1 They both live and multiply within a host organism, and they do not immediately kill their hosts, but rather scavenge metabolites and nutrients to satisfy their own needs.2,3 Intracellular apicomplexans have evolved distinct mechanisms to interfere with the cell death machinery of their host cell, by affecting distinct signalling pathways.4 Similar to the rapidly replicating stages (tachyzoites) of these apicomplexans, cancer cells exhibit a seemingly unlimited proliferative potential. Tissue cyst-forming stages of these parasites (bradyzoites) and benign tumours survive within their hosts for extended periods of time, mostly without causing extensive pathology, and they achieve this goal by (i) disseminating into immune compromised tissues and (ii) exhibiting immunomodulatory properties that skew host immunity to their own favour.5In contrast to the research on novel anti-cancer compounds, drug discovery in parasitology is a neglected field.6 While novel drugs have been developed against the classical neglected diseases such as malaria,7 trypanosomiasis8–10 and leishmaniasis,11,12 novel options for the treatment of protozoan diseases that affect farm animals are largely missing.13 Due to the intrinsic similarities between parasites and cancer cells, a reasonable starting point for the discovery of novel anti-parasitic drug candidates is to examine compounds that are being developed against cancer. While metal-based drugs are well-established as anti-cancer agents,14,15 the currently used drugs against protozoan parasites are organic compounds, and do not contain any metal atoms. However, based on different kinetic, geometric and electronic properties, metal complexes and organometallic complexes in particular can undergo reactions that are different from organic agents that could be used against parasites,16–18 as exemplified by the antimalarial drug candidate Ferroquine.19 The application of platinum anti-tumour agents clearly demonstrates that metal agents can be developed into drugs with a high impact in therapy.20 Potentially, the same could be done for targeting protozoan parasites.
Dinuclear thiolato-bridged arene ruthenium complexes [(η6-p-MeC6H4Pri)2Ru2(μ2-SR)3]+ and [(η6-p-MeC6H4Pri)2Ru2(μ2-SR1)(μ2-SR2)2]+ were shown to exhibit high toxicity against several types of cancer cells in vitro (IC50 values as low as 30 nM).21–25In vivo, one of these complexes, [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-But)3]Cl (2, DiRu-1), prolonged significantly the survival rate of mice experimentally inoculated with tumour cells.26,27 Some other mononuclear ruthenium complexes were shown to exhibit promising in vitro activities against T. gondii and N. caninum,28,29 and we have recently reported on the activity of a panel of dinuclear thiolato-bridged arene ruthenium complexes against T. gondii tachyzoites.30 Some of these compounds efficiently inhibited host cell invasion, and had an IC50 in the low nanomolar range, low host cell toxicity, and exposure of parasites to these compounds led to distinct alterations in the parasite mitochondrion.30
Neospora caninum is an apicomplexan, which belongs to the family Sarcocystidae. The parasite is distributed worldwide and infects a broad range of animals including cattle, sheep, goats, deer, horses and dogs.31Neospora caninum is the causative agent of neosporosis, a serious disease associated with abortion, stillbirth and maternal infertility in cattle and neurological disorders in dogs. Dogs and other canids represent the definitive hosts of N. caninum, where sexual development takes place. Due to its close resemblance to T. gondii, which is of high medial as well as veterinary medical importance, N. caninum was recurrently misdiagnosed as such until Dubey and colleagues succeeded in isolating the parasite in the late 1980ies.32 In contrast to T. gondii, N. caninum has never been shown to be transmitted from animals to humans.31 The prevalence of N. caninum varies considerably in different regions, however, the annual economic impact of neosporosis, based on data of 10 countries, was calculated to be around 1.28 billion US dollars.33
Vaccination and chemotherapy have been identified as economically promising options for control of neosporosis, provided suitable targets and effective reagents can be made available.34,35 Although considerable efforts have been undertaken to develop vaccines,31,36 success with recombinant vaccines has been limited,37–39 and only live vaccines using attenuated N. caninum strains have shown promising efficacy so far.40 As an alternative approach, treatment with anti-parasitic drugs could be envisaged, carried out either during pregnancy or right after birth, with the goal to eliminate the parasite before it can cause extensive damage to the next generation.
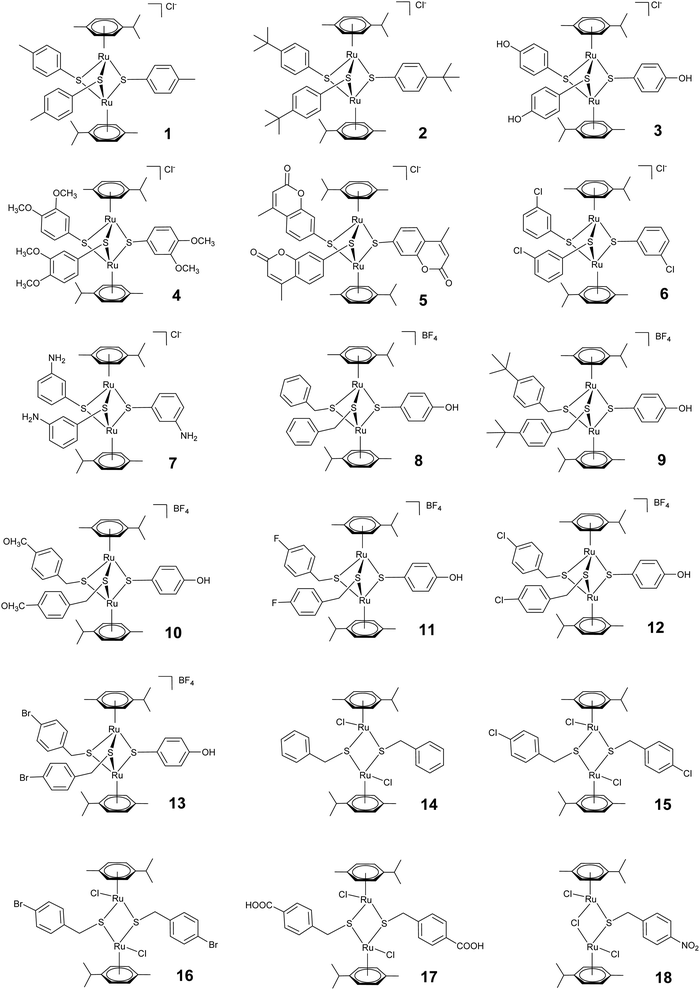
We here comparatively assessed the effects of a series of 18 thiolato-bridged dinuclear arene ruthenium complexes, consisting of 7 cationic trithiolato complexes [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-R)3]Cl (1–7), 6 cationic mixed trithiolato complexes [(η6-p-MeC6H4Pri)2Ru2(μ2-SCH2C6H4-R)2(μ2-SC6H4-R)]BF4 (8–13), 4 neutral dithiolato complexes [(η6-p-MeC6H4Pri)2Ru2(μ2-SCH2C6H4-p-R)2Cl2] (14–17), and one neutral monothiolato complex [(η6-p-MeC6H4Pri)2Ru2Cl2(μ-Cl)(μ2-SCH2C6H4-p-NO2)] (18) in the human ovarian cancer cell line A2780 and in tachyzoites of the intracellular apicomplexan N. caninum in vitro, and confirm that the mitochondrion is the primary target in both. In addition, we report on the limited efficacy of [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-CH3)3]Cl (1), [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-But)3]Cl (2) and [(η6-p-MeC6H4Pri)2Ru2(μ2-SCH2C6H4-p-But)2(μ2-SC6H4-p-OH)]BF4 (9) in an experimental neosporosis mouse model, and studied the biodistribution of complex 1 in different organs of mice.
Materials and methods
Chemicals and synthesis of ruthenium complexes.
All reagents for chemical synthesis were commercially available and were used as received. Complexes 1–18 and their in vitro efficacy against T. gondii tachyzoites were previously reported.30 All complexes were isolated as chloride or tetrafluoroborate salts, were stable orange to red solids, and were dried in vacuum. The analytical data matched those previously reported in the literature.21–23,41Culture of mammalian cells and parasite cultures
If not stated otherwise, all tissue culture media were purchased from Gibco-BRL (Zurich, Switzerland), and biochemical reagents were from Sigma (St. Louis, MO). Human foreskin fibroblasts (HFF) and Vero cells (green monkey kidney epithelial cells) were maintained in RPMI-medium containing 10% fetal calf serum (FCS) (Gibco-BRL, Zürich, Switzerland) and antibiotics as described earlier.37 The human ovarian carcinoma A2780 cell line was cultured in RPMI 1640 medium (Gibco) supplemented with 10% FCS, 100 U mL−1 penicillin, 100 μg mL−1 streptomycin at 37 °C and 6% CO2. N. caninum beta-gal (transgenic N. caninum Nc-1) expressing the beta-galactosidase gene from E. coli,42 and the N. caninum Spain7 isolate (Nc-Spain7) were maintained in Vero cells, and were isolated and separated from their host cells as described.43In vitro assessment of drug efficacy against N. caninum tachyzoites
To study the effects of compounds against N. caninum-beta-Gal tachyzoites in vitro, 0.5 mM stock solutions of the complexes were prepared in water, sterile filtered, and stored at 4 °C. The stability of the complexes in water solutions and in mixtures water/DMSO has been previously established.24,44,45 Drug assays were performed using HFF as host cells.29 In short, 5 × 103 HFF per well were grown to confluence in a 96 well plate in phenol-red free culture medium at 37 °C with 5% CO2. Cultures were infected with freshly isolated N. caninum beta-gal tachyzoites (1 × 103 per well) and drugs were added at the time point of infection. Initial assessments were done by exposing parasite cultures to 2500 nM, 250 nM, 25 nM or 2.5 nM of each compound for a period of three days, or water was added as a control. For IC50 determinations, 5 selected complexes (1–3, 5 and 9; see Table 1) were added at concentrations ranging between 0 and 2000 nM. After three days at 37 °C/5% CO2, plates were centrifuged at 500 × g, medium was removed, and cell cultures were exposed to PBS containing 0.05% Triton-X-100. After addition of 10 μL of 5 mM chlorophenol red-β-D-galactopyranoside (CPRG; Roche Diagnostics, Rotkreuz, Switzerland) dissolved in PBS, the absorption shift was measured at 570 nm wavelength at various time points on a VersaMax multiplate reader (Bucher Biotec, Basel, Switzerland). The activity, measured as the release of chlorophenol red over time, was proportional to the number of live parasites down to 50 per well as determined in pilot assays. IC50 values were calculated after the logit-log-transformation of relative growth and subsequent regression analysis by the corresponding software tool contained in the Excel software package (Microsoft, Seattle, WA).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values, Hammett constants σP, and efficacies of dinuclear thiolato-bridged arene ruthenium complexes against N. caninum tachyzoites, and for comparative purposes, of T. gondii tachyzoites, human foreskin fibroblasts (HFF) and the human ovarian carcinoma cell line A2780
P values, Hammett constants σP, and efficacies of dinuclear thiolato-bridged arene ruthenium complexes against N. caninum tachyzoites, and for comparative purposes, of T. gondii tachyzoites, human foreskin fibroblasts (HFF) and the human ovarian carcinoma cell line A2780
| Complexa | N. caninum beta-gal IC50 (nM) | T. gondii beta-gal IC50b (nM) | HFF IC50b (μM) | A2780 IC50c (nM) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (RSH)c P (RSH)c |
σ P(R)c,d |
|---|---|---|---|---|---|---|
| a Chloride salts of complexes 1–3 and 5 or tetrafluoroborate salts of complex 9 were used for all experiments. b Data from ref. 30. c Data from ref. 21–23. d For complex 9, two Hammett constants σP are given, one for each group R1 and R2. | ||||||
| 1 | 15 ± 1 | 34 ± 4 | 800 | 130 ± 10 | 2.98 ± 0.28 | −0.17 |
| 2 | 5 ± 1 | 62 ± 10 | >1000 | 30 ± 10 | 4.21 ± 0.29 | −0.20 |
| 3 | 280 ± 50 | 540 ± 60 | ND | 530 ± 20 | 1.68 ± 0.29 | −0.37 |
| 5 | 108 ± 19 | 120 ± 20 | ND | 7730 ± 1500 | 2.83 ± 0.42 | ND |
| 9 | 1 ± 0.3 | 1.2 ± 0.5 | 5 | 130 ± 3 | 3.51 ± 0.32 | −0.20; −0.37 |
For long term treatment assays, N. caninum infected HFF grown in T25 culture flasks were exposed to 250 nM of complexes 1, 2 or 9 for a period of 15 days, with medium changes and addition of fresh compounds every 3 days. At the end, the cultures were washed with medium and were further maintained in medium devoid of drugs. Regrowth of parasites was monitored on a daily basis by light microscopy.
Measurements of cytotoxicity
Cytotoxicity assays on non-infected confluent HFF were performed by Alamarblue assay as previously described.30 The assessments of selected compounds on A2780 cells have been described earlier.21–23,41Transmission electron microscopy (TEM)
HFF (5 × 104 per inoculum) cultured in T25 tissue culture flasks for 24 h were infected with 105N. caninum beta-gal tachyzoites, and 200 nM of complexes 1, 2 or 9 were added at 24 h post-infection. After 6, 24 and 48 h, cells were harvested using a cell scraper, and they were placed into the primary fixation solution (2.5% glutaraldehyde in 100 mM sodium cacodylate buffer pH 7.3) for 2 h. Specimens were then washed 2 times in cacodylate buffer and were post-fixed in 2% OsO4 in cacodylate buffer for 2 h, followed by washing in water, pre-staining in saturated uranyle acetate solution, and step wise dehydration in ethanol. They were then embedded in Epon 812-resin, and processed for TEM as described.30,46 Specimens were viewed on a Phillips CM12 transmission electron microscope operating at 60 kV.Studies on drug uptake in human carcinoma A2780 cells
A2780 cells were maintained in RPMI 1640 medium (Gibco) supplemented with 10% fetal calf serum (FCS, Gibco) 100 U mL−1 penicillin, 100 μg mL−1 streptomycin at 37 °C and 6% CO2. Seven days prior to treatments, 1 × 106 cells were seeded in 175 cm2 cell culture flask and grown until 80% of confluency, and were incubated with the target complexes (previously dissolved in 0.1% DMSO at a concentration of 1.0 μM for 4 h). Working concentration and incubation time were chosen in order to avoid extended cell mass lost due to the high cytotoxicity of the complexes and Ru amount that could afford a significant determination. After this, the cultures were washed in PBS, trypsinized, and after centrifugation (500 g for 5 min at 4 °C) and re-suspension in PBS, the pellet was collected. Mitochondria were isolated using a mitochondria isolation kit (Cat. Nr.: MITISO2, Sigma Aldrich) following the producer's instructions. Briefly, the collected pellets were re-dissolved in 1.5 mL of extraction buffer (delivered with the kit) and were incubated for 15 min on ice. The samples were homogenized with a pre-chilled dounce homogenizer (7 mL, tight pestle A, 30 strokes) and centrifuged at 600g for 10 min at 4 °C. The supernatant was transferred in a fresh tube and centrifuged at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min at 4 °C. The obtained pellets represented mitochondrial fractions. Nuclei of A2780 cells were obtained following an established procedure with minor modifications.47 All the fractions were isolated from the same cellular sample as the mitochondrial fraction for direct comparative purposes. After homogenization, the pellet obtained was redissolved in 2 mL of a sucrose solution (0.25 M sucrose, 10 mM MgCl2) and layered with 2 mL of a second hypertonic sucrose solution (0.35 M sucrose, 0.5 mM MgCl2). The suspension was centrifuged at 1450g and 4 °C for 5 min. The pellet was re-suspended in 3 mL of the second sucrose solution and centrifuged at 1450g and 4 °C for 5 min to obtain the pure nuclear extract. All the steps of the isolation procedure were monitored by phase contrast microscopy. The supernatant phases discarded during the isolation of nuclei and mitochondria procedures were collected and formed the “residual” fraction. An aliquot of crude lysate supernatant, nuclear, mitochondrial and residual fraction was each used for protein quantification using the Bradford method.48 The isolated samples were then lyophilized on an Alpha 2–4 LD plus (CHRIST) and stored at 4 °C. The distribution of ruthenium in different subcellular fractions was quantified by inductively coupled plasma mass spectrometry (ICP-MS) as described below.
000g for 10 min at 4 °C. The obtained pellets represented mitochondrial fractions. Nuclei of A2780 cells were obtained following an established procedure with minor modifications.47 All the fractions were isolated from the same cellular sample as the mitochondrial fraction for direct comparative purposes. After homogenization, the pellet obtained was redissolved in 2 mL of a sucrose solution (0.25 M sucrose, 10 mM MgCl2) and layered with 2 mL of a second hypertonic sucrose solution (0.35 M sucrose, 0.5 mM MgCl2). The suspension was centrifuged at 1450g and 4 °C for 5 min. The pellet was re-suspended in 3 mL of the second sucrose solution and centrifuged at 1450g and 4 °C for 5 min to obtain the pure nuclear extract. All the steps of the isolation procedure were monitored by phase contrast microscopy. The supernatant phases discarded during the isolation of nuclei and mitochondria procedures were collected and formed the “residual” fraction. An aliquot of crude lysate supernatant, nuclear, mitochondrial and residual fraction was each used for protein quantification using the Bradford method.48 The isolated samples were then lyophilized on an Alpha 2–4 LD plus (CHRIST) and stored at 4 °C. The distribution of ruthenium in different subcellular fractions was quantified by inductively coupled plasma mass spectrometry (ICP-MS) as described below.
Inductively coupled plasma mass spectrometry (ICP-MS)
ICP-MS measurements were performed on an Agilent QQQ 8800 Triple quad ICP-MS spectrometer (Agilent Technologies) with an ASX200 autosampler (Agilent Technologies), equipped with standard nickel cones and a “micro-mist” quartz nebulizer fed with 0.3 mL min−1 analytic flow (as a 2% HNO3 aqueous solution). Ruthenium was measured against a Ru single element standard (Merck 170347) and verified by a control (Agilent5188-6524 PA Tuning 2). Ruthenium content of the samples was determined by means of a 7-step serial dilution in the range between 0 and 100 ppb in Ru (R > 0.99) with a background equivalent concentration of BEC: 3.3 ppt and a detection limit of DL: 5.4 ppt. The isotope Ru99 (12.76% abundance) 101Ru (17.06% abundance) was evaluated in “no-gas” mode and He-gas mode. Spiking the samples with 1% methanol (to account for eventual carbon content from the biological samples) resulted in equivalent values within error ranges. A solution of Indium (500 ppb) and Tungsten (500 ppb) was used as internal standard. The results are expressed as ng Ru per mg protein (correction due to the different mass of the observed cellular compartments or organs), as mean ± standard deviation error of different independent experiments.Treatment of N. caninum-infected mice with complexes 1, 2 and 9
Infection and treatment experiments were approved by the Animal Welfare Committee of the Canton of Bern under the license BE115/14. All animals were handled in strict accordance with practices made to minimize suffering. Balb/c mice each, which were purchased from Charles River Laboratories (Sulzfeld, Germany) and maintained under conventional day/night cycle housing conditions with water and food ad libitum. The mice were used for the experiment after two weeks of acclimatization. Mice were separated into experimental groups of 6 mice each, and were infected subcutaneously with 105 cell culture-derived tachyzoites of the Nc-Spain7 strain.43 Prior to the start of treatments, complexes were emulsified in corn oil by vortexing and ultrasound bath at 37 °C. At 48 h post infection, treatments were initiated. For each compound, one group received 2 mg of complexes 1, 2 or 9 per kg bodyweight per day for 5 consecutive days, the other group was given 10 mg of complexes 1, 2 or 9 per kg bodyweight per day, and a third group received corn oil only (placebo). All treatments were applied in 100 μL by oral gavage. Following infection, all mice were closely monitored for clinical signs by using a standardized score sheet. Mice were inspected daily with regard to their coat condition (ruffled coat = score 1; starry stiff coat = score 2), weight loss (10% loss = score 1; 15–20% weight loss = score 4), and behaviour (hunched appearance, walking in circles, head tilt, apathy and ataxia, all = score 1). Animals were euthanized when the score exceeded 3 points. The experiment was terminated latest at 21 days post-infection. Lungs and brain were collected to determine parasite loads by real-time PCR as previously described,43 and cerebral and lung parasite burdens were compared between groups by the non-parametric Kruskal–Wallis test.Uptake and biodistribution of complex 1 in mice
Complex 1 was emulsified in corn oil by vortexing and ultrasound bath at 37 °C. Six Balb/c mice received the drug by oral gavage of 100 μL at a dose of 10 mg kg−1 of body weight and two control mice received corn oil only. One control and three treated mice were euthanized at 3 h and at 48 h post-administration. Whole blood, liver, kidney, lungs and brain were collected, lyophilized and the weights of the lyophilized samples were determined. The distribution of ruthenium in the different organs was quantified by ICP-MS as described above.Results
In vitro activities of arene ruthenium complexes against N. caninum tachyzoites
A set of 18 ruthenium complexes (see Fig. 1) were screened for their potential to inhibit N. caninum tachyzoite proliferation in HFF monolayers. The symmetrical Ru(II) trithiolato complexes [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-CH3)3]Cl (1), [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-But)3]Cl (2), [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-OH)3]Cl (3) and [(η6-p-MeC6H4Pri)2Ru2(μ2-SC6H4-p-mco)3]Cl (5, mco = 4-methylcoumarinyl), bearing one type of thiol ligand21,23 and the “mixed” Ru(II) trithiolato complex [(η6-p-MeC6H4Pri)2Ru2(μ2-SCH2C6H4-p-But)2(μ2-SC6H4-p-OH)]BF4 (9), bearing two different thiol ligands22 inhibited the proliferation of N. caninum tachyzoites in vitro with IC50 values between 1 and 280 nM, complex 9 being the most efficient (1 ± 0.3 nM) and complex 3 (280 ± 50 nM) the least active (Table 1). The trithiolato complexes 4, 6 and 7, the mixed complexes 8 and 10–13, the dithiolato complexes 14–17, and the monothiolato complex 18 had no measurable anti-parasitic activity or were toxic for HFF already at concentrations of 250 nM or 2500 nM. Overall, these compounds exhibited similar activities against N. caninum as for T. gondii tachyzoites, with the exception of complex 2, which was 10 times more active against N. caninum than against T. gondii (Table 1). The activity of the compounds against N. caninum parallels to a certain extent the results previously found against several cancer cell lines: the IC50 values of 7 were two orders of magnitude larger than that of the other complexes, and the mono- and dithiolato complexes were found to be only moderately cytotoxic against cancer cell lines (IC50 values between 0.2 and 2.5 μM).In contrast, HFF used here as host cells for intracellular proliferation of N. caninum tachyzoites, appeared largely unaffected at several thousand-fold higher concentrations [selective toxicity index: >50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for complex 1, >160
000 for complex 1, >160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for complex 2, and >5000 for complex 9 (Table 1)]. These compounds did not act parasiticidal: long-term drug treatments of infected HFF with complexes 1, 2 and 9 at 250 nM during a period of up to 15 days did not eliminate all parasites in a culture, since regrowth of tachyzoites was observed 5–10 days after releasing drug pressure in all three instances.
000 for complex 2, and >5000 for complex 9 (Table 1)]. These compounds did not act parasiticidal: long-term drug treatments of infected HFF with complexes 1, 2 and 9 at 250 nM during a period of up to 15 days did not eliminate all parasites in a culture, since regrowth of tachyzoites was observed 5–10 days after releasing drug pressure in all three instances.
Inductively coupled plasma mass spectrometry (ICP-MS) shows that ruthenium complexes target the mitochondrion in A2780 cancer cells
The presence of the non-physiological metal ruthenium in dinuclear thiolato-bridged arene ruthenium complexes allows for the use of ICP-MS to detect and quantify the uptake of the drug candidates into cells and different subcellular fractions.49,50 Complexes 1, 2 and 5 were chosen for these studies, 1 and 2 being active and selective, and complex 5 representing a less active compound. Subcellular fractionation was carried out using treated A2780 cancer cells. Strikingly, already after a relative short incubation time of 4 h, the large majority of the ruthenium taken up by these cells was found to accumulate in the mitochondrial fraction (ca. 90, 97 and 71% for complexes 1, 2 and 5, respectively) (see Fig. 2). Also, the total values for ruthenium were much higher in fractions originating from complex 2 treated cells compared to the others, indicating that complex 2 is taken up much more efficiently, which perfectly parallels with the respective lipophilicity of the three complexes (Table 1 & Fig. 2).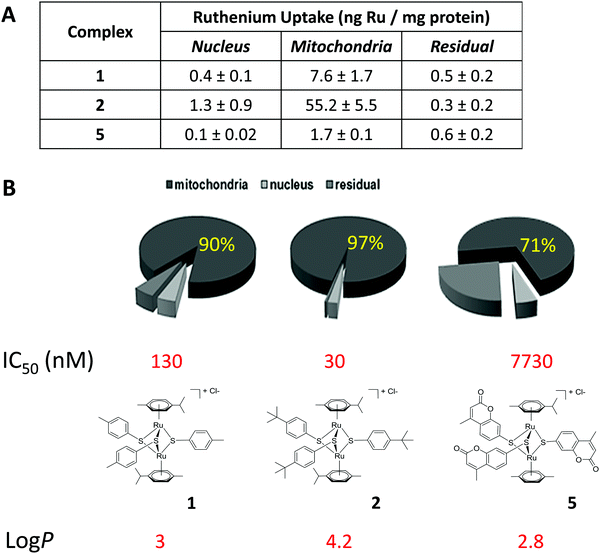 | ||
| Fig. 2 Total uptake (A) and relative cellular biodistribution (B) of ruthenium in A2780 ovarian carcinoma cells treated for 4 h with 1 μM complex 1, 2 and 5. | ||
Transmission electron microscopy (TEM) identifies the mitochondrion as the primary target of ruthenium complexes in N. caninum tachyzoites
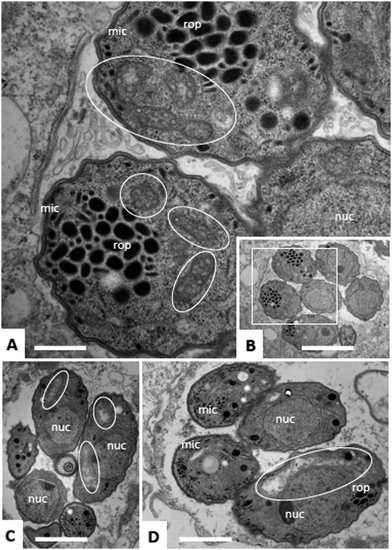
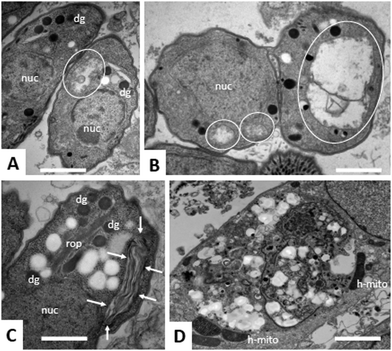
Due to the lack of reliable subcellular fractionation procedures for N. caninum, the effects of ruthenium complexes in this parasite were studied by TEM (Fig. 3 and 4). In cultures maintained in the absence of ruthenium complexes, tachyzoites were located intracellularly and proliferated within a parasitophorous vacuole (PV), surrounded by a distinct PV membrane. Tachyzoites exhibited their typical structural features such as rhoptries, dense granules and micronemes, and the parasite mitochondria filled with a structured electron dense matrix and cristae could be readily identified (Fig. 3A and B). In drug-treated cultures, changes evolved rapidly, and were most pronounced within the mitochondrial matrix, which appeared less electron dense in some parasites already after 6 h of treatment with complexes 1 and 2 (Fig. 3C and D) and also complex 9 (Fig. 4A). The other organelles appeared non-affected. After 24 h, as exemplified here for complex 9, the changes became more evident, and the mitochondrion with its characteristic cristae and electron dense matrix was replaced by large vacuoles, which were either seemingly empty (Fig. 4B), or filled with tightly spaced membrane stacks resembling autophagosomes (Fig. 4C). However, the outer membrane of the mitochondria was still intact, and parasites maintained their overall shape and structural features. From 72 h onwards (Fig. 4D), the majority of parasitophorous vacuoles contained largely lysed or distorted parasites, with only few of the typical organelles still recognizable (Fig. 4D). These changes were similar upon treatments with all three complexes 1, 2 and 9. However, the host cell mitochondria were still structurally intact and associated with the PV membrane (Fig. 4D).Effects of ruthenium complexes 1, 2 and 9 in the neosporosis mouse model
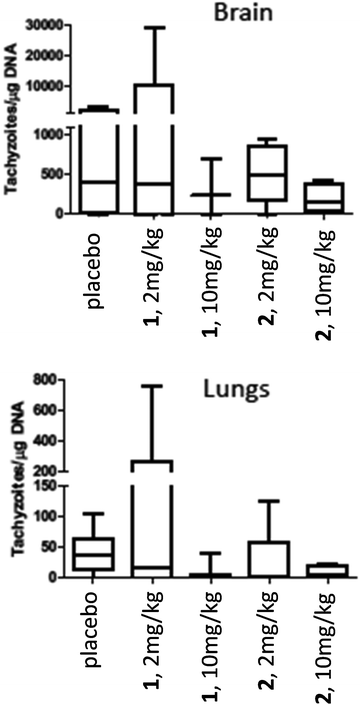
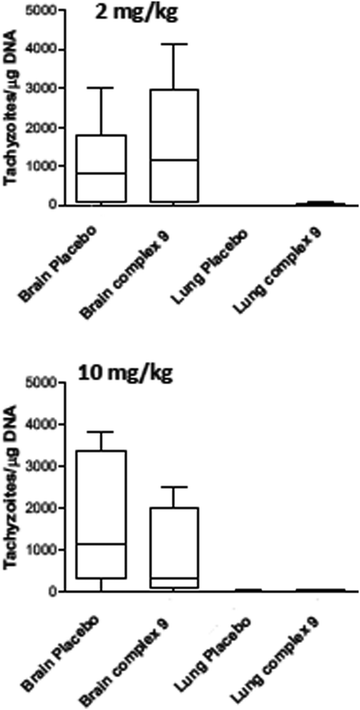
For assessment of in vivo anti-parasitic effects of these ruthenium complexes, initial studies were carried out on complexes 1 and 2. Balb/c mice were infected subcutaneously with 105 NcSpain-7 tachyzoites, a dose that does not induce severe clinical signs in non-pregnant mice,43 and after 48 h, daily treatments by gavage (either 2 mg kg−1 or 10 mg kg−1 of drugs emulsified in corn oil for 5 days) were initiated. Parasite loads in lungs and brain were determined by real-time PCR at 21 dpi (Fig. 5). In the placebo group (infected, but treated with corn oil only), two mice showed symptoms compatible to neosporosis, such as ruffled coat and body tilting, but they did not have to be euthanized as the clinical score was below the threshold. In the group treated with complexes 1 at 2 mg kg−1 day−1, one mouse exhibited a ruffled coat and survived, whereas in the group treated with 10 mg kg−1 days−1, 2 out of 6 mice had to be euthanized on days 14 and 17 dpi. In the group treated with complex 2, one mouse treated with 2 mg kg−1 exhibited clinical signs and survived until the end of the experiment, while 3 out of 6 mice did in the group treated with 10 mg kg−1 complex 2 for 5 days. All the remaining mice survived until 21 dpi. No statistically significant effects of drug treatments on parasite load could be detected in lungs and in the CNS in any of the drug treated groups. However, the higher rate of fatalities in the drug treated groups compared to the placebo group indicates that adverse side effects could play a role (Fig. 5).Due to the low IC50 of complex 9 (1 ± 0.3 nM), this compound was also evaluated in vivo in 2 independent experiments (Fig. 6). First, mice were treated with 2 mg kg−1, and in a second experiment with 10 mg kg−1 for 5 days. In placebo groups (infected, but treated with corn oil only), one mouse each showed symptoms compatible to neosporosis, but they did not have to be euthanized. None of the mice in the group treated with 2 mg kg−1, and one of the mice treated with 10 mg kg−1, died during this experiment. Determination of the parasite load in lungs and CNS by real time PCR revealed that the average parasite levels in the two organs of drug treated mice were lower compared to the placebo group. However, these values were not statistically significant (Fig. 6). Overall, the ruthenium complexes developed herein exhibited only limited in vivo efficacy in the neosporosis mouse model.
Biodistribution of ruthenium complex 1 indicates low drug levels in the CNS
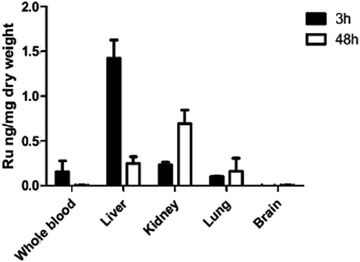
Since in vivo studies in mice consistently resulted in high level brain infection comparable to the placebo treatments, we performed a biodistribution study. ICP-MS biodistribution analysis was performed in mice that had received a single dose of 10 mg kg−1 of complex 1 by oral gavage. Ruthenium was identified in blood, liver, kidney and lungs at levels above those detected for a placebo-treated mouse, demonstrating the drug absorption after oral administration (Fig. 7). The majority of the ruthenium was detected in the liver at 3 h and in the kidney at 48 h post-administration, but no drug was detected in the brain. These results are in agreement with those previously reported for complex 2 (also known as diruthenium-1 or DiRu-1) which had been intraperitoneally injected into mice.26Discussion
The plethora of compounds that have been developed against cancer has paved the way for numerous new and innovative approaches to combat parasitic diseases that affect not only humans, but also animals. There has been much recent interest in the development of therapeutic transition metal-based complexes in part fuelled by the clinical success of the platinum(II) anticancer drug, cisplatin. Yet, known platinum drugs are limited by their high toxicity, severe side-effects, and incidences of drug resistance. Organometallic ruthenium-arene complexes have risen to prominence as a pharmacophore due to the success of other ruthenium drug candidates in clinical trials.51 Substantial activities of arene ruthenium complexes have been reported in infectious diseases caused by bacteria52,53 against protozoan parasites including T. gondii29,30 and Plasmodium falciparum,54,55 and against helminths such as Schistosoma mansoni56,57 and Echinococcus multilocularis.28 In addition, a series of ruthenium–clotrimazole (ctz) complexes displayed high and specific in vitro activity against Leishmania major and Trypanosoma cruzi.58 Other metal complexes containing Ag, Pt, Pd and Au, already in the market for cancer therapy, treatments of bacterial infections or as anti-inflammatory agents, have been proposed for the treatment of trypanosomatid-induced infections.10 A recent review by Gasser et al. provides an overview of metal complexes against tropical neglected diseases.59We here report on the in vitro and in vivo activities of 18 dinuclear thiolato-bridged arene ruthenium complexes against the apicomplexan parasite N. caninum. N. caninum causes substantial losses in the bovine dairy and meat industry, and no vaccine and no drugs are currently on the market to limit these losses.31 Of the 18 complexes investigated herein, three were identified that exhibited IC50 values between 1 and 62 nM against N. caninum tachyzoites, and IC50 values between 30 and 530 nM against the ovarian cancer cell line A2780.21–23,41 The mono- and dithiolato complexes 13–18 had been also found to be only moderately cytotoxic in vitro against cancer cell lines (IC50 values between 0.2 and 2.5 μM),25,60,61 reflecting their propensity to readily hydrolyse and their reactivity towards biomolecules compared to their trithiolato counterparts.25,45
As indicated in Table 1 for the ovarian carcinoma cell line A2780, the activity of the complexes against N. caninum largely mirrors the findings obtained earlier in several cancer cells,21–23,41 and in the closely related apicomplexan parasite Toxoplasma gondii.30 Generally, the in vitro activity of anticancer and antiparasitic drugs can be related in part to their lipophilic character. The resulting hydrophobicity may contribute to an increased uptake of the compound by the cells or the parasites, thereby enhancing the antiproliferative activity. We have previously evidenced a correlation between lipophilicity and cytotoxic activity for these types of dinuclear arene ruthenium complexes.21–23,25 Complexes with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P parameters up to the value 4 show a steady decrease of their IC50 values, which increase again for very lipophilic complexes with log
P parameters up to the value 4 show a steady decrease of their IC50 values, which increase again for very lipophilic complexes with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values above 4.5, the tendency being the same for all cell lines investigated.21–23,25 The optimum lipophilicity for these complexes was found to be for log
P values above 4.5, the tendency being the same for all cell lines investigated.21–23,25 The optimum lipophilicity for these complexes was found to be for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values in the range between 3.5 and 4.0, whereas complexes with log
P values in the range between 3.5 and 4.0, whereas complexes with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values lower than 3.5 systematically exhibited a steady increase of their IC50 values, presumably due to an insufficient cellular uptake, since the less lipophilic compounds have more difficulties to cross cell membranes or are more rapidly excreted by exocytosis.
P values lower than 3.5 systematically exhibited a steady increase of their IC50 values, presumably due to an insufficient cellular uptake, since the less lipophilic compounds have more difficulties to cross cell membranes or are more rapidly excreted by exocytosis.
A similar tendency can be found in this study against N. caninum and previously against T. gondii.30 Complexes with log P values in the range between 3.0 and 4.0 (1, 2 and 9) showed the highest cytotoxicities, whereas complexes with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values lower than 3.0 (3–8 and 10–13) belong to the less active ones of the series. If the Hammett constants σP are included, our results obtained on cancer cells show that the complexes having Hammett constants in the range −0.2 < σP < 0 and log
P values lower than 3.0 (3–8 and 10–13) belong to the less active ones of the series. If the Hammett constants σP are included, our results obtained on cancer cells show that the complexes having Hammett constants in the range −0.2 < σP < 0 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values above 3.0 have the lowest IC50 values.21–23,25 This tendency seems to be also valid against N. caninum and T. gondii, as shown in Table 1.
P values above 3.0 have the lowest IC50 values.21–23,25 This tendency seems to be also valid against N. caninum and T. gondii, as shown in Table 1.
In both cancer cells and the apicomplexan parasite N. caninum, the mitochondrion represents the major target. This was shown by ICP-MS in drug treated A2870 cells and by performing TEM of drug-treated infected HFF. The disruption of mitochondria has been investigated as a potential novel chemotherapeutic mechanism for cancer treatment, because it circumvents upstream apoptotic pathways that may be mutated or lacking in cancer cells.62 Moreover, cancer cells have higher mitochondrial membrane potentials, rendering them more susceptible to mitochondrial perturbations than non-immortalized cells.63 On the basis of these factors, numerous mitochondria-targeting agents have been developed in order to disrupt the mitochondrial membrane potential and further permeabilise the mitochondrial outer membrane. It has been reported that some ruthenium(II) complexes can induce mitochondria – mediated apoptosis in cancer cells.64
In N. caninum, ICP-MS could not be carried out due to the lack of protocols for obtaining pure mitochondrial fractions, but TEM clearly indicated that treatments with ruthenium complexes 1, 2 and 9 are characterized by severe ultrastructural alterations in the mitochondrial matrix and cristae early on, indicating that also in these cells the mitochondrion is the primary target. Compounds that affect the mitochondrial integrity in apicomplexan parasites have been identified earlier. For instance, low nanomolar concentrations of the naphtoquinone buparvaquone inhibited N. caninum proliferation in vitro at concentrations similar to ruthenium complexes, and buparvaquone also impaired vertical transmission in the pregnant neosporosis mouse model, but did not affect CNS infection in dams.13 Another interesting class are endochin-like quinolones (ELQs), which target the cytochrome bc1 complex of the mitochondrial electron transport chain in protozoan parasites.65 Cytochrome bc1 facilitates the transfer of electrons from ubiquinol to cytochrome c and contains both oxidative (Qo) sites and reductive (Qi) catalytic sites. The anti-malarial drug atovaquone, also used for the treatment of toxoplasmosis, and buparvaquone, are classical Qo site inhibitors.66 More recently, novel endochin analogues with a diphenylether side chain at the third position, and various substitutions at position 5, 6 or 7 of the quinolone-ring were synthesized.67,68 One of these compounds, ELQ-400,69 targets both Qo and Qi sites of cytochrome bc1 of S. cerevisiae and potentially other organisms.70 ELQ-400 was one of the compounds of outstanding activity against N. caninum (IC50 2 nM) that was identified during a screening of the open-source MMV (Medicines for Malaria Venture) Pathogen Box.71 One of the ELQs with excellent efficacy against T. gondii is ELQ-316,65 exhibiting in vitro IC50 values below 1 nM, and in vivo affecting not only tachyzoites, but also tissue cysts.67
In vivo studies on complexes 1, 2 and 9 were carried out using a standardized non-pregnant Balb/c mouse model for the assessment of acute and cerebral N. caninum infection. In this model, mice normally experience mild to medium clinical signs during the acute phase of infection (day 1–10 post infection), then immunity takes over and parasites are largely controlled, with the exception of the brain, which is an immune-privileged site.43,72 This is reflected by the fact that the parasite load in the lung tissues is clearly lower compared to the brain in all experiments. For all three complexes, the results indicated that they did not significantly impact on the parasite load, neither in the lungs nor in the brain. In addition, several mice from the treated groups had to be euthanized during prior to termination of the experiment on day 21 p.i.
A possible explanation for the observed failure of these complexes to exert activity in mice could be found, as exemplified for complex 1 in this study, in the respective biodistribution pattern after oral gavage, which was investigated by ICP-MS, showing that this compound is well absorbed and detectable in whole blood after 3 h, but eliminated from blood after 48 h. Instead, ruthenium was detected in the liver, lungs and kidneys after 48 h, but at no point in the brain tissue. Similar findings were obtained earlier when complex 2 was injected intraperitoneally into mice.26 These findings are important as they suggest that these compounds may be ineffective against cerebral parasitic stages found in cyst-forming coccidia such as Neospora and Toxoplasma.
Conclusion
In conclusion, this study demonstrated that dinuclear thiolato-bridged arene ruthenium complexes target the mitochondrion in cancer cells as well as in the proliferative stages of apicomplexan parasites such as N. caninum. For future development of this class of compounds, it will be of interest to investigate which parts of the mitochondria (i.e. DNA, membrane, etc.), or potentially also other targets mediate the efficacy. The lipophilicity as well as the ligands undoubtedly play an important role for future design of dinuclear thiolato-bridged arene ruthenium complexes and conjugates. Additional studies, including a broader panel of parasites, as well as new dinuclear thiolato-bridged arene ruthenium conjugates bearing known anti-parasitic drugs such as atovaquone and buparvaquone, are needed to define precisely the antiparasitic effectiveness and potential of these ruthenium complexes as well as their molecular targets and modes of action. Overall, our work opens new avenues in the field of parasitology, as it clearly demonstrates, using metallomics studies, the importance of targeting mitochondria in parasites.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was financially supported by the Swiss National Science Foundation (SNSF professorships PP00P2_133568 and PP00P2_157545 [to G. G.], SNSF grants 310030_165782 [to A. H.] and CRSII5_173718 [to J. F., A. H., and G. G.]), the University of Bern (UniBe-ID; to J. F. and A. H.), the University of Zurich (to G. G.), the Stiftung für wissenschaftliche Forschung of the University of Zurich (to G. G.), the UBS Promedica Stiftung (to R. R. and G. G.), the Forschungskredit of the University of Zurich (to R. R.), and the Novartis Jubilee Foundation (to R. R. and G. G.). This work has received support under the program Investissements d’Avenir, launched by the French government and implemented by the ANR, with the reference ANR-10-IDEX-0001-02 PSL (to G. G.). Many thanks are addressed to David Sibley (Washington University, St. Louis, MO, USA) and Sabrina Sonda (University of Zürich) for providing us with N. caninum beta-gal tachyzoites for screening purposes, and to Luis-Miguel Ortega-Mora for the N. caninum NcSpain-7 isolate.References
- M. Q. Klinkert and V. Heussler, The use of anticancer drugs in antiparasitic chemotherapy, Mini-Rev. Med. Chem., 2006, 6, 131–143 CrossRef CAS PubMed.
- S. J. Nolan, J. D. Romano, T. Luechtefeld and I. Coppens, Neospora caninum Recruits Host Cell Structures to Its Parasitophorous Vacuole and Salvages Lipids from Organelles, Eukaryotic Cell, 2015, 14, 454–473 CrossRef CAS PubMed.
- J. D. Romano, C. de Beaumont, J. A. Carrasco, K. Ehrenman, P. M. Bavoil and I. Coppens, A novel co-infection model with Toxoplasma and Chlamydia trachomatis highlights the importance of host cell manipulation for nutrient scavenging, Cell. Microbiol., 2013, 15, 619–646 CrossRef CAS PubMed.
- C. G. K. Lüder, R. R. Stanway, M. Chaussepied, G. Langsley and V. T. Heussler, Intracellular survival of apicomplexan parasites and host cell modification, Int. J. Parasitol., 2009, 39, 163–173 CrossRef PubMed.
- E. A. Innes, P. M. Bartley, S. W. Maley, S. E. Wright and D. Buxton, Comparative host–parasite relationships in ovine toxoplasmosis and bovine neosporosis and strategies for vaccination, Vaccine, 2007, 25, 5495–5503 CrossRef CAS PubMed.
- K. T. Andrews, G. Fisher and T. S. Skinner-Adams, Drug repurposing and human parasitic protozoan diseases, Int. J. Parasitol., 2014, 4, 95–111 Search PubMed.
- B. R. Moore and T. M. E. Davis, Pharmacotherapy for the prevention of malaria in pregnant women: currently available drugs and challenges, Expert Opin. Pharmacother., 2018, 1–18 Search PubMed.
- M. Berninger, I. Schmidt, A. Ponte-Sucre and U. Holzgrabe, Novel lead compounds in pre-clinical development against African sleeping sickness, MedChemComm, 2017, 8, 1872–1890 RSC.
- P. A. Sales Junior, I. Molina, S. M. Fonseca Murta, A. Sánchez-Montalvá, F. Salvador, R. Corrêa-Oliveira and C. M. Carneiro, Experimental and Clinical Treatment of Chagas Disease: A Review, Am. J. Trop. Med. Hyg., 2017, 97, 1289–1303 CrossRef PubMed.
- J. Colotti, A. Fiorillo and A. Ilari, Metal- and metalloid-containing drugs for the treatment of trypanosomatid diseases, Front. Biosci., 2018, 23, 954–966 CrossRef.
- L. M. Alcântara, T. C. S. Ferreira, F. R. Gadelha and D. C. Miguel, Challenges in drug discovery targeting TriTryp diseases with an emphasis on leishmaniasis, Int. J. Parasitol., 2018, 8, 430–439 Search PubMed.
- S. Kapil, P. K. Singh and O. Silakari, An update on small molecule strategies targeting leishmaniasis, Eur. J. Med. Chem., 2018, 157, 339–367 CrossRef CAS PubMed.
- J. Muller, A. Aguado-Martinez, V. Manser, H. N. Wong, R. K. Haynes and A. Hemphill, Repurposing of antiparasitic drugs: the hydroxy-naphthoquinone buparvaquone inhibits vertical transmission in the pregnant neosporosis mouse model, Vet. Res., 2016, 47, 32 CrossRef PubMed.
- N. P. E. Barry and P. J. Sadler, Exploration of the medical periodic table: towards new targets, Chem. Commun., 2013, 49, 5106–5131 RSC.
- E. Alessio, Bioinorganic Medicinal Chemistry, VCH-Verlag, Weinheim, 2011 Search PubMed.
- G. Gasser, I. Ott and N. Metzler-Nolte, Organometallic Anticancer Compounds, J. Med. Chem., 2011, 54, 3–25 CrossRef CAS PubMed.
- G. Gasser and N. Metzler-Nolte, The potential of organometallic complexes in medicinal chemistry, Curr. Opin. Chem. Biol., 2012, 16, 84–91 CrossRef CAS PubMed.
- M. Patra and G. Gasser, The medicinal chemistry of ferrocene and its derivatives, Nat. Rev. Chem., 2017, 1, 0066 CrossRef CAS.
- F. Dubar, C. Slomianny, J. Khalife, D. Dive, H. Kalamou, Y. Guérardel, P. Grellier and C. Biot, The Ferroquine Antimalarial Conundrum: Redox Activation and Reinvasion Inhibition, Angew. Chem., 2013, 52, 7690–7693 CrossRef CAS.
- T. C. Johnstone, K. Suntharalingam and S. J. Lippard, The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs, Chem. Rev., 2016, 116, 3436–3486 CrossRef CAS PubMed.
- F. Giannini, J. Furrer, A. F. Ibao, G. Suss-Fink, B. Therrien, O. Zava, M. Baquie, P. J. Dyson and P. Stepnicka, Highly cytotoxic trithiophenolatodiruthenium complexes of the type (eta(6)-p-MeC6H4Pr (i))(2)Ru-2(SC6H4-p-X)(3) (+): synthesis, molecular structure, electrochemistry, cytotoxicity, and glutathione oxidation potential, J. Biol. Inorg. Chem., 2012, 17, 951–960 CrossRef CAS PubMed.
- F. Giannini, J. Furrer, G. Süss-Fink, C. M. Clavel and P. J. Dyson, Synthesis, characterization and in vitro anticancer activity of highly cytotoxic trithiolato diruthenium complexes of the type [(η6-p-MeC6H4iPr)2Ru2(μ2-SR1)2(μ2-SR2)]+ containing different thiolato bridges, J. Organomet. Chem., 2013, 744, 41–48 CrossRef CAS.
- F. Giannini, L. E. H. Paul, J. Furrer, B. Therrien and G. Suss-Fink, Highly cytotoxic diruthenium trithiolato complexes of the type (eta(6)-p-MeC6H4Pri)(2)Ru-2(mu(2)-SR)(3) (+): synthesis, characterization, molecular structure and in vitro anticancer activity, New J. Chem., 2013, 37, 3503–3511 RSC.
- F. Giannini, L. Geiser, L. E. H. Paul, T. Roder, B. Therrien, G. Suss-Fink and J. Furrer, Tuning the in vitro cell cytotoxicity of dinuclear arene ruthenium trithiolato complexes: influence of the arene ligand, J. Organomet. Chem., 2015, 783, 40–45 CrossRef CAS.
- J. Furrer and G. Suss-Fink, Thiolato-bridged dinuclear arene ruthenium complexes and their potential as anticancer drugs, Coord. Chem. Rev., 2016, 309, 36–50 CrossRef CAS.
- P. Tomšík, D. Muthná, M. Řezáčová, S. Mičuda, J. Ćmielová, M. Hroch, R. Endlicher, Z. Červinková, E. Rudolf, S. Hann, D. Stíbal, B. Therrien and G. Süss-Fink, [(p-MeC6H4Pri)2Ru2(SC6H4-p-But)3]Cl (diruthenium-1), a dinuclear arene ruthenium compound with very high anticancer activity: an in vitro and in vivo study, J. Organomet. Chem., 2015, 782, 42–51 CrossRef.
- A. Koceva-Chyła, K. Matczak, M. P. Hikisz, M. K. Durka, M. K. Kochel, G. Süss-Fink, J. Furrer and K. Kowalski, Insights into the in vitro Anticancer Effects of Diruthenium-1, ChemMedChem, 2016, 11, 2171–2187 CrossRef.
- T. Kuster, N. Lense, F. Barna, A. Hemphill, M. K. Kindermann, J. W. Heinicke and C. A. Vock, A New Promising Application for Highly Cytotoxic Metal Compounds: eta(6)-Areneruthenium(II) Phosphite Complexes for the Treatment of Alveolar Echinococcosis, J. Med. Chem., 2012, 55, 4178–4188 CrossRef CAS PubMed.
- F. Barna, K. Debache, C. A. Vock, T. Kuster and A. Hemphill, In Vitro Effects of Novel Ruthenium Complexes in Neospora caninum and Toxoplasma gondii Tachyzoites, Antimicrob. Agents Chemother., 2013, 57, 5747–5754 CrossRef CAS PubMed.
- A. P. Basto, J. Muller, R. Rubbiani, D. Stibal, F. Giannini, G. Suss-Fink, V. Balmer, A. Hemphill, G. Gasser and J. Furrer, Characterization of the Activities of Dinuclear Thiolato-Bridged Arene Ruthenium Complexes against Toxoplasma gondii, Antimicrob. Agents Chemother., 2017, 61, e01031-17 CrossRef PubMed.
- J. P. Dubey, A. Hemphill, R. Calero-Bernal and G. Schares, Neosporosis in animals, CRC Press, Taylor & Francis, Boca Raton, FL, USA, 2017 Search PubMed.
- J. P. Dubey, A. L. Hattel, D. S. Lindsay and M. J. Topper, Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission, J. Am. Vet. Med. Assoc., 1988, 193, 1259–1263 CAS.
- M. P. Reichel, M. Alejandra Ayanegui-Alcérreca, L. F. P. Gondim and J. T. Ellis, What is the global economic impact of Neospora caninum in cattle – The billion dollar question, Int. J. Parasitol., 2013, 43, 133–142 CrossRef PubMed.
- B. Häsler, G. Regula, K. D. C. Stärk, H. Sager, B. Gottstein and M. Reist, Financial analysis of various strategies for the control of Neospora caninum in dairy cattle in Switzerland, Prev. Vet. Med., 2006, 77, 230–253 CrossRef PubMed.
- B. Häsler, K. D. C. Stärk, H. Sager, B. Gottstein and M. Reist, Simulating the impact of four control strategies on the population dynamics of Neospora caninum infection in Swiss dairy cattle, Prev. Vet. Med., 2006, 77, 254–283 CrossRef PubMed.
- A. Hemphill, A. Aguado-Martinez and J. Muller, Approaches for the vaccination and treatment of Neospora caninum infections in mice and ruminant models, Parasitology, 2016, 143, 245–259 CrossRef PubMed.
- A. Aguado-Martinez, A. P. Basto, J. Muller, V. Balmer, V. Manser, A. Leitao and A. Hemphill, N-terminal fusion of a toll-like receptor 2-ligand to a Neospora caninum chimeric antigen efficiently modifies the properties of the specific immune response, Parasitology, 2016, 143, 606–616 CrossRef CAS.
- P. Horcajo, J. Regidor-Cerrillo, A. Aguado-Martinez, A. Hemphill and L. M. Ortega-Mora, Vaccines for bovine neosporosis: current status and key aspects for development, Parasite Immunol., 2016, 38, 709–723 CrossRef CAS.
- A. Aguado-Martinez, A. P. Basto, A. Leitao and A. Hemphill, Neospora caninum in non-pregnant and pregnant mouse models: cross-talk between infection and immunity, Int. J. Parasitol., 2017, 47, 723–735 CrossRef CAS PubMed.
- M. P. Reichel, D. P. Moore, A. Hemphill, L. M. Ortega-Mora, J. P. Dubey and J. T. Ellis, A live vaccine against Neospora caninum abortions in cattle, Vaccine, 2015, 33, 1299–1301 CrossRef CAS PubMed.
- D. Stíbal, B. Therrien, G. Süss-Fink, P. Nowak-Sliwinska, P. J. Dyson, E. Čermáková, M. Řezáčová and P. Tomšík, Chlorambucil conjugates of dinuclear p-cymene ruthenium trithiolato complexes: synthesis, characterization and cytotoxicity study in vitro and in vivo, J. Biol. Inorg. Chem., 2016, 21, 443–452 CrossRef PubMed.
- D. C. McFadden, F. Seeber and J. C. Boothroyd, Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro, Antimicrob. Agents Chemother., 1997, 41, 1849–1853 CrossRef CAS.
- D. Arranz-Solis, A. Aguado-Martinez, J. Muller, J. Regidor-Cerrillo, L. M. Ortega-Mora and A. Hemphill, Dose-dependent effects of experimental infection with the virulent Neospora caninum Nc-Spain7 isolate in a pregnant mouse model, Vet. Parasitol., 2015, 211, 133–140 CrossRef PubMed.
- F. Giannini, G. Süss-Fink and J. Furrer, Efficient Oxidation of Cysteine and Glutathione Catalyzed by a Dinuclear Areneruthenium Trithiolato Anticancer Complex, Inorg. Chem., 2011, 50, 10552–10554 CrossRef CAS PubMed.
- D. Stibal, L. Geiser, G. Suss-Fink and J. Furrer, Hydrolytic behaviour of mono- and dithiolato-bridged dinuclear arene ruthenium complexes and their interactions with biological ligands, RSC Adv., 2016, 6, 38332–38341 RSC.
- J. Müller, V. Manser and A. Hemphill, In vitro treatment of Besnoitia besnoiti with the naphto-quinone buparvaquone results in marked inhibition of tachyzoite proliferation, mitochondrial alterations and rapid adaptation of tachyzoites to increased drug concentrations, Parasitology, 2018, 1–9 CrossRef PubMed.
- A. Naik, R. Rubbiani, G. Gasser and B. Spingler, Visible-Light-Induced Annihilation of Tumor Cells with Platinum–Porphyrin Conjugates, Angew. Chem., 2014, 53, 6938–6941 CrossRef CAS PubMed.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- A. Frei, R. Rubbiani, S. Tubafard, O. Blacque, P. Anstaett, A. Felgentrager, T. Maisch, L. Spiccia and G. Gasser, Synthesis, Characterization, and Biological Evaluation of New Ru(II) Polypyridyl Photosensitizers for Photodynamic Therapy, J. Med. Chem., 2014, 57, 7280–7292 CrossRef CAS PubMed.
- S. Nikolic, L. Rangasamy, N. Gligorijevic, S. Arandelovic, S. Radulovic, G. Gasser and S. Grguric-Sipka, Synthesis, characterization and biological evaluation of novel Ru(II)-arene complexes containing intercalating ligands, J. Inorg. Biochem., 2016, 160, 156–165 CrossRef CAS PubMed.
- M. V. Babak and W. H. Ang, Multinuclear Organometallic Ruthenium-Arene Complexes for Cancer Therapy, Met. Ions Life Sci., 2018, 18, 171–198 CAS.
- F. Li, J. G. Collins and F. R. Keene, Ruthenium complexes as antimicrobial agents, Chem. Soc. Rev., 2015, 44, 2529–2542 RSC.
- A. I. Ramos, T. M. Braga and S. S. Braga, Ru(II)-Based Antimicrobials: Looking Beyond Organic Drugs, Mini-Rev. Med. Chem., 2012, 12, 227–235 CrossRef CAS.
- C. S. K. Rajapakse, A. Martínez, B. Naoulou, A. A. Jarzecki, L. Suárez, C. Deregnaucourt, V. Sinou, J. Schrével, E. Musi, G. Ambrosini, G. K. Schwartz and R. A. Sánchez-Delgado, Synthesis, Characterization, and in vitro Antimalarial and Antitumor Activity of New Ruthenium(II) Complexes of Chloroquine, Inorg. Chem., 2009, 48, 1122–1131 CrossRef CAS.
- T. S. Macedo, L. Colina-Vegas, M. Da PaixÃO, M. Navarro, B. C. Barreto, P. C. M. Oliveira, S. G. Macambira, M. Machado, M. PrudÊNcio, S. D'Alessandro, N. Basilico, D. R. M. Moreira, A. A. Batista and M. B. P. Soares, Chloroquine-containing organoruthenium complexes are fast-acting multistage antimalarial agents, Parasitology, 2016, 143, 1543–1556 CrossRef CAS PubMed.
- J. Hess, J. Keiser and G. Gasser, Toward organometallic antischistosomal drug candidates, Future Med. Chem., 2015, 7, 821–830 CrossRef CAS PubMed.
- J. Kljun, A. J. Scott, T. Lanišnik Rižner, J. Keiser and I. Turel, Synthesis and Biological Evaluation of Organoruthenium Complexes with Azole Antifungal Agents. First Crystal Structure of a Tioconazole Metal Complex, Organometallics, 2014, 33, 1594–1601 CrossRef CAS.
- A. Martínez, T. Carreon, E. Iniguez, A. Anzellotti, A. Sánchez, M. Tyan, A. Sattler, L. Herrera, R. A. Maldonado and R. A. Sánchez-Delgado, Searching for New Chemotherapies for Tropical Diseases: Ruthenium–Clotrimazole Complexes Display High in Vitro Activity against Leishmania major and Trypanosoma cruzi and Low Toxicity toward Normal Mammalian Cells, J. Med. Chem., 2012, 55, 3867–3877 CrossRef PubMed.
- Y. Ong, S. Roy, P. Andrews and G. Gasser, Metal Complexes against Tropical Neglected Diseases, Chem. Rev., 2018 DOI:10.1021/acs.chemrev.8b00338.
- A.-F. Ibao, M. Gras, B. Therrien, G. Süss-Fink, O. Zava and P. J. Dyson, Thiolato-Bridged Arene–Ruthenium Complexes: Synthesis, Molecular Structure, Reactivity, and Anticancer Activity of the Dinuclear Complexes [(arene)2Ru2(SR)2Cl2], Eur. J. Inorg. Chem., 2012, 1531–1535 CrossRef CAS.
- D. Stíbal, B. Therrien, F. Giannini, L. E. H. Paul, J. Furrer and G. Süss-Fink, Monothiolato-Bridged Dinuclear Arene Ruthenium Complexes: The Missing Link in the Reaction of Arene Ruthenium Dichloride Dimers with Thiols, Eur. J. Inorg. Chem., 2014, 5925–5931 CrossRef.
- L. F. Yousif, K. M. Stewart and S. O. Kelley, Targeting Mitochondria with Organelle-Specific Compounds: Strategies and Applications, ChemBioChem, 2009, 10, 1939–1950 CrossRef CAS PubMed.
- L. B. Chen, Mitochondrial Membrane Potential in Living Cells, Annu. Rev. Cell Biol., 1988, 4, 155–181 CrossRef CAS PubMed.
- S. P. Mulcahy, K. Gründler, C. Frias, L. Wagner, A. Prokop and E. Meggers, Discovery of a strongly apoptotic ruthenium complex through combinatorial coordination chemistry, Dalton Trans., 2010, 39, 8177–8182 RSC.
- P. H. Alday and J. S. Doggett, Drugs in development for toxoplasmosis: advances, challenges, and current status, Drug Des. Delivery, 2017, 11, 273–293 CAS.
- H. J. Painter, J. M. Morrisey, M. W. Mather and A. B. Vaidya, Specific role of mitochondrial electron transport in blood-stage Plasmodium falciparum, Nature, 2007, 446, 88 CrossRef CAS PubMed.
- J. S. Doggett, A. Nilsen, I. Forquer, K. W. Wegmann, L. Jones-Brando, R. H. Yolken, C. Bordón, S. A. Charman, K. Katneni, T. Schultz, J. N. Burrows, D. J. Hinrichs, B. Meunier, V. B. Carruthers and M. K. Riscoe, Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15936–15941 CrossRef CAS PubMed.
- A. Nilsen, G. P. Miley, I. P. Forquer, M. W. Mather, K. Katneni, Y. Li, S. Pou, A. M. Pershing, A. M. Stickles, E. Ryan, J. X. Kelly, J. S. Doggett, K. L. White, D. J. Hinrichs, R. W. Winter, S. A. Charman, L. N. Zakharov, I. Bathurst, J. N. Burrows, A. B. Vaidya and M. K. Riscoe, Discovery, Synthesis, and Optimization of Antimalarial 4(1H)-Quinolone-3-Diarylethers, J. Med. Chem., 2014, 57, 3818–3834 CrossRef CAS PubMed.
- A. M. Stickles, L.-M. Ting, J. M. Morrisey, Y. Li, M. W. Mather, E. Meermeier, A. M. Pershing, I. P. Forquer, G. P. Miley, S. Pou, R. W. Winter, D. J. Hinrichs, J. X. Kelly, K. Kim, A. B. Vaidya, M. K. Riscoe and A. Nilsen, Inhibition of Cytochrome bc1 as a Strategy for Single-Dose, Multi-Stage Antimalarial Therapy, Am. J. Trop. Med. Hyg., 2015, 92, 1195–1201 CrossRef CAS PubMed.
- Z. Song, B. I. Iorga, P. Mounkoro, N. Fisher and B. Meunier, The antimalarial compound ELQ-400 is an unusual inhibitor of the bc1 complex, targeting both Qo and Qi sites, FEBS Lett., 2018, 592, 1346–1356 CrossRef CAS PubMed.
- J. Muller, A. Aguado, B. Laleu, V. Balmer, D. Ritler and A. Hemphill, In vitro screening of the open source Pathogen Box identifies novel compounds with profound activities against Neospora caninum, Int. J. Parasitol., 2017, 47, 801–809 CrossRef CAS PubMed.
- F. Alaeddine, N. Keller, A. Leepin and A. Hemphill, Reduced infection and protection from clinical signs of cerebral neosporosis in C57BL/6 mice vaccinated with recombinant microneme antigen NcMIC1, J. Parasitol., 2005, 91, 657–665 CrossRef PubMed.
Footnote |
| † These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2019 |