Economic, energy and carbon footprint assessment of integrated forward osmosis membrane bioreactor (FOMBR) process in urban wastewater treatment†
Nur Hafizah
Ab Hamid
 a,
Simon
Smart
a,
Simon
Smart
 a,
David K.
Wang
a,
David K.
Wang
 b,
Kaniel Wei Jun
Koh
ac,
Kalvin Jiak Chern
Ng
ac and
Liu
Ye
b,
Kaniel Wei Jun
Koh
ac,
Kalvin Jiak Chern
Ng
ac and
Liu
Ye
 *a
*a
aSchool of Chemical Engineering, The University of Queensland, St Lucia, Brisbane, QLD 4072, Australia. E-mail: l.ye@uq.edu.au; Fax: +61 7 3365 4199; Tel: +61 7 3365 4152
bSchool of Chemical and Biomolecular Engineering, The University of Sydney, Sydney, NSW 2006, Australia
cNgee Ann Polytechnic, 599489, Singapore
First published on 19th November 2019
Abstract
The application of forward osmosis (FO) membrane-based technology in urban wastewater treatment has received increased attention, however, its techno-economic feasibility and sustainability have not been fully demonstrated. In this study, the feasibility of FO application in urban wastewater treatment was assessed in terms of economic performance, energy consumption and greenhouse gas (GHG) emissions benchmarked against microfiltration (MF). Three different scenarios of wastewater treatment and water reclamation were proposed: (A) forward osmosis aerobic membrane bioreactor (FOAeMBR); (B) FOAeMBR integrated with reverse osmosis (RO); (C) forward osmosis anaerobic membrane bioreactor (FOAnMBR) integrated with partial nitrification/anammox (PN/AMOX) process. In this study, the wastewater treatment and reclamation costs by using FO in scenarios A and B were more expensive than MF by $0.16 per m3 and $0.75 per m3 respectively due to the larger surface area of FO membrane required. In scenario C, the wastewater treatment cost of using FO ($1.11 per m3) was equivalent to MF. This was due to the good rejection performance of FO and its ability to concentrate wastewater, hence, resulting in a higher efficiency of (PN/AMOX) in comparison to MF. In addition, the application of FO in scenario C generated total GHG emissions to be as low as 0.93 kg CO2 equivalent m−3, which was 1.5 and 4.1 times lower than scenarios A and B respectively. The minimal net energy consumption and low carbon footprint of FO application in scenario C suggests this integration will likely be a feasible membrane-based technology for the next generation of wastewater treatment.
Water impactThis study systematically explores the potential applications of forward osmosis (FO) membrane based technology in urban wastewater treatment and water reclamation for their techno-economic feasibility and sustainability. The integrated FO membrane bioreactor with partial nitrification/anammox process shows a relatively lower treatment cost and a minimal net energy consumption, suggesting this integration will likely be a feasible technology for the next generation of wastewater treatment. |
1. Introduction
In the past one hundred years, the conventional activated sludge (CAS) configuration has been widely applied for urban wastewater treatment with an energy demand between 0.2 to 0.8 kW h m−3 and a carbon footprint of 0.13 to 0.69 kg CO2 equivalent m−3 (kg CO2 – e m−3).1,2 However, with increasingly more stringent water quality requirements, membrane-based technologies are at the forefront to address this challenge. Membrane-based technologies are robust processes that provide a barrier to a broad spectrum of contaminants and contribute to the production of higher quality effluent which can be used for water reclamation purpose.3 However, their energy consumption and carbon footprint are typically higher than the CAS configuration. To date, the application of membrane based technology can result in a high energy demand (0.8–1.2 kW h m−3) which also contributes to a higher carbon footprint (0.6–2.4 kg CO2 – e m−3) in comparison to CAS.2 In the wastewater treatment field, the porous hydraulic pressure membrane-based technology such as microfiltration (MF) or ultrafiltration (UF), is normally applied in a membrane bioreactor (MBR), followed by further purification with reverse osmosis (RO) or nanofiltration (NF).4,5 The MBR is operated either under aerobic–anoxic (AeMBR) or anaerobic (AnMBR) conditions to remove contaminants from the wastewater system.6 Whilst AeMBR undergoes a nitrification–denitrification process to remove contaminants, the anaerobic process converts the carbon based compounds in wastewater to methane (CH4) gas as the by-product.6,7 With the low concentration of carbon/nitrogen (C/N) ratio after AnMBR treatment, Dai (2015) suggested implementing partial nitrification/anammox (PN/AMOX) to remove the nitrogen based compounds in the AnMBR permeate.8 Approximately half of the ammonium (NH4+) ions that present in the wastewater were firstly oxidized via the PN process forming nitrite (NO2−) ions, which then could be used by AMOX bacteria as electron acceptors to react with the residual NH4+ to form nitrogen (N2) gas.9 The AnMBR + PN/AMOX integrated system provides a promising future to produce high quality effluent, with the potential of lowering the energy consumption.2 In comparison to the conventional nitrification–denitrification, PN/AMOX process lowers the oxygen demand by 60%, eliminates the requirement of organic carbon source and produce 90% lesser sludge.9–11 However, this integration has not been successfully applied in the mainstream MBR technology of wastewater treatment due to the low concentration of NH4+.10,12 This is due to MF and UF membranes having poor rejection performance of carbon and nitrogen based compounds.13 Hence, wastewater could not be concentrated for an efficient nitrogen removal via the PN/AMOX pathway.13 Aside from that, compact fouling formation on the membrane surface and inside the membrane pores limits the broader applications of hydraulic pressure membrane-based technology in wastewater treatment.8Due to the limitations of the current membrane-based technologies, forward osmosis (FO) becomes an attractive solution.14 It was reported that FO has a comparable rejection performance with RO and is feasible in concentrating wastewater.15,16 In addition, it also has a lower propensity to foul and the fouling layer that is formed is relatively loose.15,16 Most of the FO studies in wastewater treatment applied sodium chloride (NaCl) or seawater as the draw solution (DS). This is due to the fact that seawater is easily accessible and has a high enough osmotic pressure to enable relatively high water fluxes. To date, several studies have been conducted to evaluate the potential of integrating the wastewater treatment plant with a seawater desalination plant to optimise the production cost of water reclamation.17–21 The FO + RO integration was reported to reduce the energy consumption of seawater desalination from 2.5–4.0 kW h m−3 to 1.3–1.5 kW h m−3.21 Therefore, this integration provides the potential to offset the desalination treatment cost.20–22 However, based on the previous studies, FO + RO integration only becomes economically viable when the FO water flux performance ≥15 L m−2 h−1 (LMH) and membrane cost ≤$50 per m2.17,23
Although previous studies have shown that FO was economically viable for application in water reclamation, most of the studies only optimised FO technology from the desalination point of view.17,18 Apart from that, the effect of FO application on economics of wastewater treatment has not yet been comprehensively addressed by the literature. Previous studies have shown tertiary wastewater effluent or wastewater retentate were used for the FO filtration process.17,18,20,21 This configuration served as the pre-treatment for desalination, and hence, did not reflect the actual application of FO in wastewater treatment.17,18,20,21 Linares's study compared the economics of FO application in wastewater treatment and seawater desalination for water reclamation, however, the study was limited to one wastewater treatment design only.19 In addition, there are limited studies which evaluate the energy consumption19,21 and none of these has critically assessed the carbon footprint associated with both the direct and indirect emissions of a wastewater treatment which incorporate FO into the process schema.
Therefore, this study aims to explore the feasibility of FO application in wastewater treatment in terms of techno-economics, energy consumption and carbon footprint from different process design perspectives. In this study, three wastewater treatment scenarios, namely conventional MBR, MBR + RO and AnMBR + PN/AMOX, were proposed, and benchmarked against MF in each case to elucidate FO performance in wastewater treatment. The conventional MBR is the current existing membrane technology application in the wastewater treatment while MBR + RO is widely applied for water reclamation.5 On the other hand, the AnMBR + PN/AMOX is a new generation of wastewater treatment design with the potential of minimising the environmental and economic costs.24 Thus, this study also aims to evaluate the potential of an integrated FO anaerobic membrane bioreactor (FOAnMBR) with PN/AMOX system, for the first time, as a next generation wastewater treatment technology.
2. Methodology
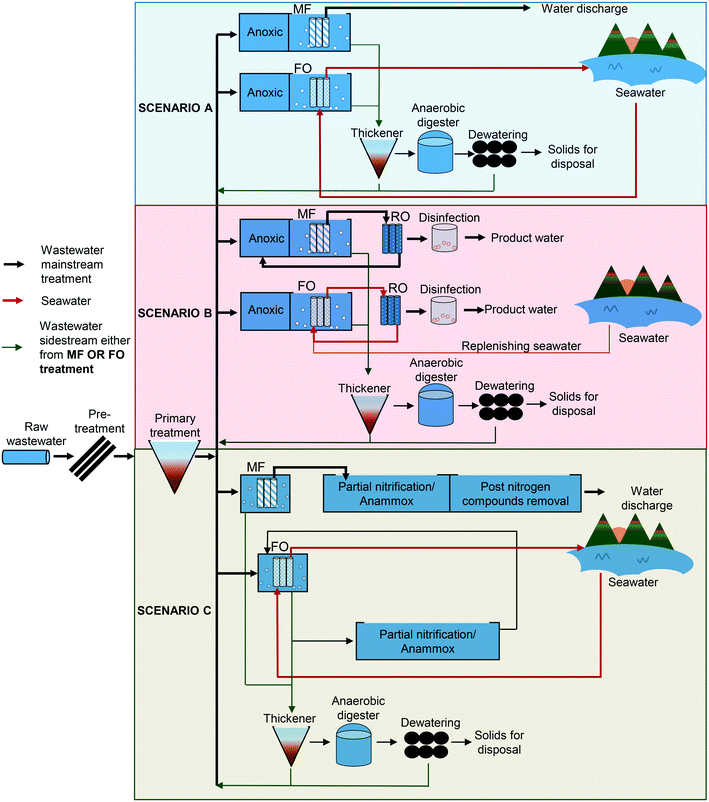
2.1 Scenario design
In this study, three scenarios of urban wastewater treatment were designed. The main difference for each scenario was the operation of the membrane filtration system. For each scenario, FO was compared to MF, in which, MF served as the benchmark for the wastewater treatment. The schematic illustrations of each scenario are shown in Fig. 1. In scenario A, a MBR was operated under aerobic–anoxic conditions to remove the contaminants via the conventional pathway of nitrification–denitrification. It was expected that the effluent of the MBR meets the guidelines for environmental discharge. Scenario B is the advancement of scenario A, where a RO filtration unit was added to further purify the MBR effluent. The product water of the RO filtration was expected to meet the quality standards for the purpose of water reclamation. For FO + RO integration, the treated water was entirely from the wastewater source. The seawater was circulated in a closed-loop which periodically need to be replenished with fresh seawater to maintain the desired salinity. In scenario C, a different design of wastewater treatment was proposed; here the MBR was operated under anaerobic conditions, where most of the carbon compounds were converted into methane (CH4) and carbon dioxide (CO2) gases. After that, the nitrogen compounds in the wastewater were removed via the PN/AMOX process. The pathways for removing carbon and nitrogen based compounds in each scenario are illustrated in Fig. 2.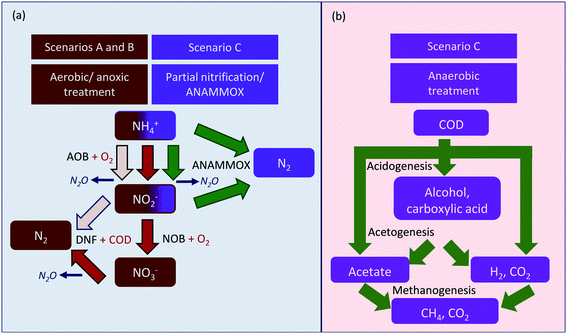 | ||
| Fig. 2 Nitrogen and carbon based compounds conversion via (a) conventional nitrification–denitrification and PN/AMOX pathway; (b) anaerobic pathway. | ||
In scenario C, as the MF membrane is poor in rejecting the nitrogen based compounds, a denitrifying moving bed biofilm reactor (MBBR) and an aeration zone for post nitrogen compounds removal were required to ensure the effluent discharge meets the quality guidelines.24 The other main units: pre-treatment, primary treatment (primary settler), and sludge handling (thickener, digester, belt press) were designed to be similar for all scenarios. The wastewater treatment plant was expected to be able to treat 15 megalitres per day (MLD) of urban wastewater to mimic a medium sized wastewater treatment plant. The characteristics of the wastewater influent which formed the basis for design are listed in Table A1 (ESI†).
2.2 Mass balance calculation
To-date, no commercial tool or software has been developed to simulate FO, hybrid systems of FO + RO or FO + low pressure RO (FO + LPRO) system, hence, spreadsheets for each model were generated and the assumptions applied in the calculations will be further described.19 Apart from that, reverse osmosis system analysis (ROSA, The Dow Chemical Company) software was used for the mass balance calculation for the RO system of FO + RO integration in scenario B. The parameters applied in the ROSA simulation are as listed in Table 2, whilst the estimated output from ROSA are listed in Table 1. As for scenario C, the anaerobic process was simplified to acidogenesis and methanogenesis pathways only. The carbon source in the wastewater was assumed to be fully degradable. For the membrane filtration system, the water flux performance and recovery were assumed to be constant throughout the operation and the parameters are listed in Table 1. Apart from that, it was assumed the effects of the draw solution (DS) dilution and reverse salt diffusion were insignificant to affect the water flux and overall performance of FOMBR. The parameters and other assumptions applied in the mass balance calculations are attached in the ESI† (appendix A).| Scenario | Types of membrane | Water flux performance (LMH) | Applied hydraulic pressure (bar) | Water recovery (%) | Ref. |
|---|---|---|---|---|---|
| a Value was selected based on the range reported by literatures. b Output from ROSA. | |||||
| A, B, and C | MF | 50 | 2 | 95 | 25, 26 |
| A, B and C | FO | 15a | n.a. | 80 | 16, 23 |
| B | RO (in MF-RO integration) | 17.6 | 8.2 | 75 | 4, 5 |
| RO (in FO-RO integration) | 16.98b | 41.49b | 50 | ROSA | |
| Parameters | Values | Assumptions/comments |
|---|---|---|
| Seawater composition | — | It was assumed seawater contained 35 g L−1 of total dissolved solids (TDS) |
| Seawater inflow | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 m3 per day 990 m3 per day |
— |
| Wastewater inflow | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 m3 per day 990 m3 per day |
— |
| Permeate flow | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 m3 per day 990 m3 per day |
— |
| Element | SW30HXR-440i | — |
| Number of elements | 900 | — |
2.3 Capital (CAPEX) and operating (OPEX) expenditures calculation
The capital (CAPEX) and operating (OPEX) expenditure calculations were conducted based on the following assumptions/parameters:i. All calculations are present values with the discount rate of 8.5% and 30 years of the project lifetime and the calculations are in $AUD.
ii. The CAPEX for each of the unit was identified based on the unit sizing and further normalization was executed based on values reported by Plumlee (2014) and Solley (2015).24,27
iii. Labour cost, land, maintenance, insurance and other taxes are not included in the calculations. Hence, the values calculated and presented are pre-tax values.
iv. The equations applied to calculate the energy and cost for the pumps (membrane filtration), CAPEX, annualized CAPEX, OPEX and water cost were referred to Choi's work (2015).17
v. Net present value (NPV) was calculated based on the following equation:
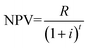 | (1) |
Other parameters and assumptions applied in CAPEX and OPEX calculations are attached in the ESI† (appendix A), whilst the membrane costs are listed in Table 3.
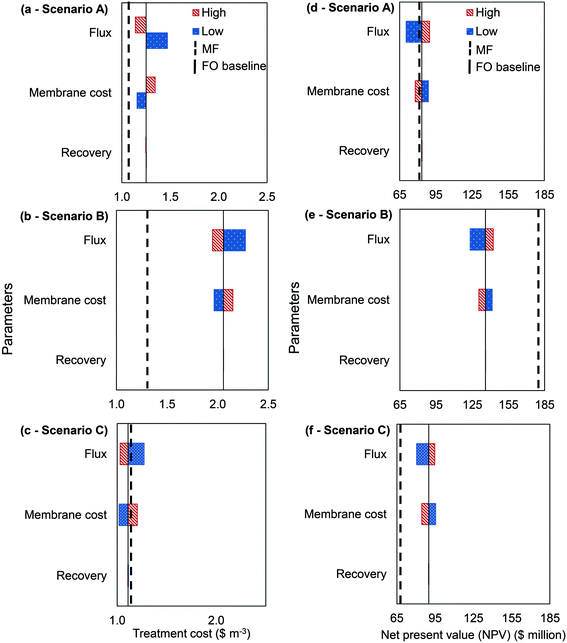
A sensitivity analysis was conducted to further evaluate the feasibility of FO application in the urban wastewater treatment. This analysis was evaluated in terms of treatment cost and net present value (NPV). Previous studies listed several key parameters that determined the potential of full-scale FO application in water and wastewater treatment industries, which were: (i) water flux performance (ii) water recovery and (iii) membrane cost.17,18 Thus, a sensitivity analysis was conducted on these key parameters by plotting several tornado graphs. Water flux performance was analysed by adjusting the baseline value (15 LMH) to be higher or lower by a factor of 2. The higher value of water flux performance (around 30 LMH) was reported to be achievable with the current commercialised membranes, however it requires higher concentration of draw solution.23 As for the membrane cost, this parameter was varied by ±$30 per m2. The reason was to evaluate the economic feasibility of FO application when the membrane cost drops to that of MF. On the other hand, the recovery parameter was varied by ±10% as FO has the potential to achieve 90% of water recovery.29
2.4 Carbon footprint calculation
The greenhouse gas (GHG) emissions from all three scenarios were estimated from direct and indirect emissions. Direct emissions which were mainly contributed by biological process were calculated using the guidelines from 2006 IPCC Guidelines for National Greenhouse Gas Inventories and other literatures.30–32| CH4 emission(kg per day) = BO × MCF × (TOW − S) − R | (2) |
| (a) Direct emissions factor | |||||
|---|---|---|---|---|---|
| Treatment | Scenarios | Factor | Values | Unit | Ref. |
| Aerobic–anoxic | A and B | MCF of CH4 emission | 0.32 | — | 32 |
| Ratio of N2O–N emit/N influent | 0.4 | % (of N influent) | 30, 31 | ||
| Anaerobic + partial nitrification/anammox | C | MCF of CH4 emission | 0.34 | — | 32 |
| Ratio of N2O–N emit/N influent | 2.3 | % (of N influent) | 30, 31 | ||
| (b) Indirect emissions factor | |||||
|---|---|---|---|---|---|
| Treatment | Scenarios | Factor | Values | Unit | Ref. |
| Aerobic–anoxic, anaerobic + partial nitrification/anammox | A, B and C | Carbon emission factor from electricity generation | 0.845 | kg CO2 per kW h | 33 |
| (c) Normalised emissions value of CH4, N2O and CO2 to CO2 – equivalent | |||||
|---|---|---|---|---|---|
| Conversions to CO2 – equivalent | CH4 | N2O | CO2 | Ref. | |
| kg (CH4, N2O, CO2 respectively)/kg CO2 – equivalent | 25 | 298 | 1 | 34 | |
A sensitivity analysis was conducted on the direct emissions of the GHG. The emissions of CH4 and N2O gases are dependent on the management, operation and design of the wastewater treatment plant.30,31 For CH4 emissions, these normally occur from settling basins or other air and wastewater gas pockets for the aerobic treatment.30 For anaerobic treatment, the CH4 emission is dependent on the efficiency of the CH4 capture.30 A sensitivity analysis was conducted by evaluating the GHG emissions when the plant was operated sub-optimally. For CH4 emission, literature reported the MCF values varied in the range of 0–0.4 and 0–1.0 in aerobic–anoxic and anaerobic operation respectively.30 As for N2O emission, the ratio of N2O–N emitted/N influent varied in the range of 0.003–2.6% (of N influent) for aerobic–anoxic treatment and 0.4–6.6% (of N influent) for PN/AMOX treatment.31,35
3. Results and discussion
3.1 Economic analysis and evaluation
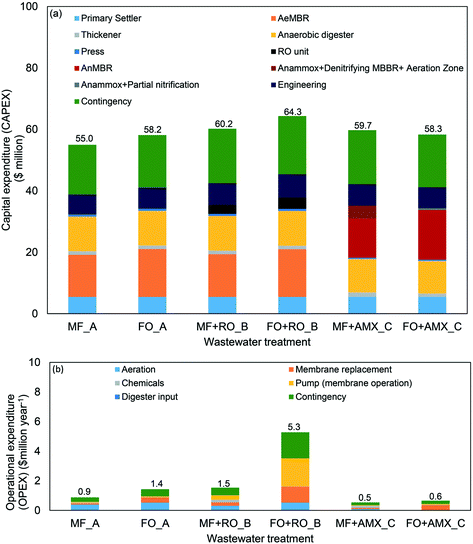 | ||
| Fig. 3 (a) Capital expenditure (CAPEX); (b) operational expenditure (OPEX) of each scenarios. *AMX represents PN/AMOX. | ||
3.1.1.1 Scenario A. For scenario A, Fig. 3(a and b) shows that CAPEX and OPEX of FO were higher than MF by $3.2 million and $0.5 million per year respectively. However, FO application had higher NPV than MF by $1.8 million due its higher rejection performance (Fig. 4(d)), therefore producing higher quality effluent.13,36 In general, MF can only reject 50% of carbon based compounds and has a very poor rejection performance of nitrogen based compounds.13 By contrast, FO has the ability to reject 99% of carbon based compounds and >90% NH4+ in the membrane bioreactor configuration.36 The good rejection performance of FO eliminates the requirement of an additional carbon source to enhance the nitrification–denitrification process.13 Based on Fig. 3(a and b), the bioreactor (25–27% of CAPEX) and anaerobic digester (19–21% of CAPEX) significantly affect CAPEX, meanwhile, aeration (36–44% of OPEX) and membrane replacement (10–24% of OPEX) significantly affect OPEX. In both applications, aeration was required for air scouring in controlling membrane fouling and also for nutrient removal.6
3.1.1.2 Scenario B. Significant increment of CAPEX and OPEX could be observed when the wastewater treatment system was upgraded from scenario A to scenario B with the addition of a RO system. The upgrading improved the effluent quality to meet the criteria for water reclamation purpose. Based on Fig. 3, CAPEX and OPEX of FO in scenario B were $4.1 million and $3.7 million per year respectively higher than the MF application. The installation of the RO into the treatment plant contributed 5–6% of the overall CAPEX (Fig. 3(a)). As for OPEX, membrane replacement (15–21% of OPEX) is still one of the dominant contributors, and the installation of the RO into the system led to the increment of the energy consumption. The specific energy consumed in the MF + RO system was only 0.4 kW h m−3 whilst the specific energy for the FO + RO system was 2.9 kW h m−3. The high specific energy requirement for FO + RO was due to the utilisation of the high osmotic pressure of seawater as the draw solution for the FO system, hence, higher hydraulic pressure for the RO was required.19
3.1.1.3 Scenario C. In scenario C, the integration of FO + PN/AMOX was observed to be more cost effective in comparison to MF + PN/AMOX integration. Although the OPEX of FO in this scenario was $0.1 million per year higher than MF, the overall CAPEX of FO was calculated to be $1.4 million lower. To-date, the full scale application of MF + PN/AMOX in the mainstream of the wastewater treatment process has not been successfully implemented, due to the low concentration of NH4+ and high C/N ratio.10,12 However, in order to ensure that the economic evaluation for both the membrane-based technologies were comparable, the PN/AMOX was proposed to be operated in the mainstream for the MF system.24 Based on Fig. 3(a), the integration of PN/AMOX (including a post nitrogen compound removal unit) with MF (7% of CAPEX) was more expensive in comparison to the FO (1% of CAPEX). Whilst carbon based compounds were converted to CH4 in the AnMBR, the nitrogen based compounds were not yet removed from the wastewater system. As MF membrane could not reject nitrogen based compounds at all, the permeate was then further treated in PN/AMOX.13 However, the concentration of nitrogen in the MF permeate was not high enough to achieve an efficient nitrogen based compounds removal. Ali and Okabe (2015)10 reported anammox is suitable for NH4+-rich wastewater streams, with the concentration ≥500 mg NH4+–N L−1 while the mainstream only contains <70 mg NH4+–N L−1.37 As a consequence, a post nitrogen based compounds removal unit is required to ensure the quality of the discharged water was within the limit specified by National Water Quality Management Strategy: Australian Guidelines for Sewerage Systems – Effluent Management.10,24,38 In comparison, with the good rejection performance of NH4+ by FO membrane, NH4+ can be concentrated up to approximately five to eightfold at a water recovery of 90%.36,39 The increased NH4+ loading will greatly increase the potential of the stable and successful operation of the PN/AMOX process. The requirement of additional units explains the reason of higher CAPEX of MF + PN/AMOX integration in comparison to the FO. The membrane replacement cost remained as the dominant factor of OPEX for the FO system as the FO membranes are more expensive than the MF membranes. The chemicals and aeration costs were more significant in the MF and this was due to the additional unit of post nitrogen compounds removal (denitrification MBBR and aeration zone).
For scenario A, the wastewater treatment cost using the MF system was calculated to be $1.09 per m3. By implementing the baseline parameters (water flux = 15 LMH, water recovery = 80%, membrane cost = $50 per m2) for FO operation, the cost of the treatment was $1.25 per m3. However, further improvement of the membrane characteristics will enable FO application in wastewater treatment to be cost-competitive with MF. Based on Fig. 4(a), water flux performance was the main factor affecting the economic feasibility of the FO application, followed by membrane cost and recovery performance. In achieving an equivalent treatment cost as MF, the FO membrane needed to achieve at least 80% water recovery, 30 LMH water flux, and $20 per m2 of membrane cost. Similar water flux threshold value (30 LMH) was suggested by Blandin (2015) while conducting the economic evaluation on FO + RO integration from the perspective of the desalination process.22
Although wastewater treatment cost using FO was more expensive than MF, the calculated NPV of FO was higher (Fig. 4(d)). The NPV of MF was $81.5 million, meanwhile, FO recorded NPV of $83.4 million by using baseline parameters. Further improvement of the FO characteristics to the suggested parameters will increase the NPV of FO to be $92.6 million. The higher NPV was mainly contributed by the high rejection performance of the contaminants, leading to a more efficient removal of nutrients in the bioreactor and a higher quality effluent discharge. The higher NPV of FO in scenario A suggests the potential of its application in the wastewater treatment.
In scenario B, the hybrid technology of FO + RO could not outcompete the performance of the MF + RO irrespective of the chosen FO membrane characteristics. The improvement of membrane parameters only reduced the water reclamation cost to be $1.90 per m3 while MF + RO cost was $1.30 per m3 (Fig. 4(b)). On top of that, the NPV of MF + RO was $44.3 million higher than FO + RO which is economically compelling (Fig. 4(e)). The optimisation of this scenario was limited due to unavailability of commercialised software for the FO + RO or FO + LPRO, hence, the optimum flow of DS required could not be identified.19 The development of this software will further improve the economic feasibility of the FO + RO application.
The result of this study was not consistent with the previous studies conducted from the desalination process point of view.17,22 The FO + RO setup from this scenario should be compared to the conventional seawater reverse osmosis (SWRO) treatment. Of note is the lower specific energy requirement for this hybrid system to produce product water in comparison to the SWRO process. SWRO consumes 2.5 kW h m−3 to 4 kW h m−3 of specific energy, meanwhile the proposed FO + LPRO required 1.3 kW h m−3 to 1.5 kW h m−3.21 In this study, the specific energy required by the RO system to re-concentrate the diluted seawater was 2.88 kW m−3, due to the concentration of the RO feed (i.e. essentially seawater) more closely matching the input for a SWRO process, rather than the FO + LPRO process. To achieve a lower energy consumption as FO + LPRO in this study, seawater need to be diluted by a factor of 2.5.23 This dilution would substantially lower the water flux performance of FO in filtering the wastewater, hence, rendering the process uncompetitive due to poor water fluxes.
As for scenario C, the application of FO had a better potential than MF. The treatment cost for the FO system was equivalent to MF when the baseline parameters of FO were implemented (Fig. 4(c)). This further verifies the feasibility of the FO application in the wastewater treatment, especially when this membrane-based technology was proposed to be integrated with the PN/AMOX. Based on the sensitivity analysis conducted, FOAnMBR + PN/AMOX application will be able to outcompete the treatment cost of MF application in scenario A, when the water flux performance of FO improves to be at least 20 LMH. Further improvement of FO membrane characteristics to the suggested values (membrane cost = $20 per m2; water recovery = 90%; water flux = 30 LMH) reduces the treatment cost of the FOAnMBR + PN/AMOX ($0.98 per m3) to be even cheaper than MF application in scenario A ($1.09 per m3). In addition, FO application in this scenario had a higher NPV ($90 million) than MF ($67.6 million) even when the baseline parameters were implemented. Further improvement of the FO performance resulted in an NPV of $153 million. Based on the sensitivity analysis conducted, it can be concluded that the application of FO integrated with the PN/AMOX process has a promising future in lowering the current cost of the urban wastewater treatment.
3.2 Carbon footprint and energy balance analysis
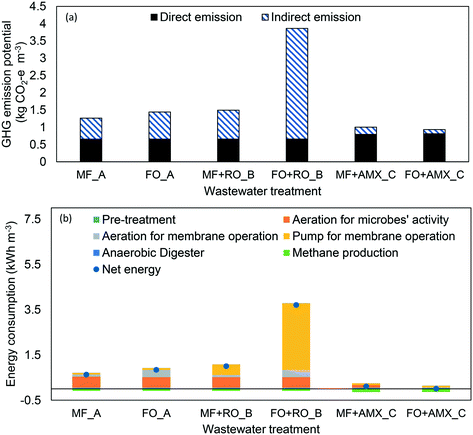 | ||
| Fig. 5 (a) Greenhouse gas emission potential for each wastewater treatment design; (b) breakdown of energy consumption by each unit of each wastewater treatment design. *AMX represents PN/AMOX. | ||
As for the indirect emission, the greenhouse gas emissions follow the order of: FOAeMBR + RO (scenario B) (3.20 kg CO2 – e m−3) > MFAeMBR + RO (scenario B) (0.84 kg CO2 – e m−3) > FOAeMBR (scenario A) (0.78 kg CO2 – e m−3) > MFAeMBR (scenario A) (0.61 kg CO2 – e m−3) > MF + PN/AMOX (scenario C) (0.21 kg CO2 – e m−3) > FO + PN/AMOX (scenario C) (0.12 kg CO2 – e m−3). For FOAeMBR + RO application in scenario B, 80% of the energy consumed was due to the high pressure pump in the RO system. The energy required for RO operation in MF + RO was 8 times lower than FO + RO. The other significant energy requirement was contributed by the aeration required for the microorganisms' activity in scenarios A and B, which was around 0.5 kW h m−3. In scenario C, the MF application required less aeration (0.1 kW h m−3) in comparison to scenario A and B, whilst the aeration requirement was less significant for the FO application (0.005 kW h m−3). Apart from the microorganisms' activity, aeration is also required for fouling control of the membrane operation.40 The FO application in scenarios A and B consumed 0.32 kW h m−3 for aeration, while MF required 0.09 kW h m−3 and this remarkable difference was contributed by larger FO membrane area required to treat the similar capacity of wastewater. No aeration was required in scenario C for fouling control as CH4 gas produced will be recycled back and used as the sparging gas. Based on Fig. 5(b), the integration of FOAnMBR + PN/AMOX can potentially provide a minimal energy balance for wastewater treatment.
3.3 Challenges and future applications of FO in urban wastewater treatment
Previous studies conducted on FO proposed the idea of integrating wastewater treatment with desalination. However, only a few of those studies properly articulate the economic feasibility of this integrated treatment from the perspective of wastewater utilities.18,23 From the desalination perspective, FO + RO integration provides the potential to reduce the treatment cost by lowering the energy consumption of desalination. It has been reported that FO was capable of reducing the energy consumption to be as low as 1.5 kW h m−3 from the range of 2.5–4.0 kW h m−3 of traditional seawater reverse osmosis desalination (SWRO).21 In this study, the application of FOAeMBR + RO operation consumed 2.9 kW h m−3 energy for the pumps operation and this was due to the assumption of minimal dilution of seawater to ensure constant water flux performance of FO. In achieving lower energy consumption (1.5 kW h m−3), seawater need to be diluted by a factor of 2.5, which would substantially lower the water flux performance of FO in filtering the wastewater, rendering the process uncompetitive due to poor water fluxes.23 Future studies should assess the impact of partial seawater dilution or effluent recycling on the FO water flux performance, energy consumption and economics of the operation.A significant challenge with integrating wastewater treatment with desalination is the reverse diffusion of salt into the membrane bioreactor. As the salinity of the wastewater in the MBR increased: (i) the water flux performance of the membrane is reduced, as the osmotic pressure gradient becomes lower and (ii) the performance of microorganisms in the MBR is lowered, resulting in the accumulation of contaminants in the wastewater system.41,42 One of the options in resolving this issue is by having a microfiltration (MF) or ultrafiltration (UF) membrane running together in the similar MBR system with FO.43,44 Alternatively, these hydraulic pressure membrane based systems can be run as a side-stream. As both MF and UF are porous membranes, the application of either one of them will allow small salt molecules to pass across the membrane, therefore reducing the salinity level in the MBR.43 Since the reverse salt diffusion was not critically assessed in this study, a baseline calculation was conducted to further evaluate the effect of MF installation to control the salinity in the FOMBR on the economics of the treatment (appendix C, ESI†). For this calculation, it was assumed the salinity in the MBR was controlled in the range of 0–3 g NaCl per L which is in accordance with the previous study.44 It is well-studied that under 3 g L−1 of salinity, the key functional bacteria involved in carbon and nitrogen removal can easily adapt in an elevated salinity environment and the nutrient efficiency will not be affected44,45 By assuming the reverse salt flux was constant throughout the operation at 10 g m−2 h−1, the MF membrane installation cost was only 5% of FO installation while the MF pump operation cost was only 7% of the FO pump operation. Based on the result of this calculation, it can be concluded that additional installation of MF to control the salinity in FOMBR will not significantly affect the economics of the treatment.
Although FO suffers reverse salt diffusion of the DS, this membrane-based technology application has a good rejection performance of contaminants. With the ability of rejecting 99% of carbon based compounds and >90% nitrogen based compounds, FO can theoretically concentrate wastewater for the application of anaerobic treatment and PN/AMOX processes in the mainstream.16,29,36 The current membrane systems that use hydraulic pressure have several operational limitations due to: (i) poor rejection performance of carbon and nitrogen based compounds by MF and UF, resulting in low concentration of organics and NH4+ present in the mainstream13 and (ii) severe fouling formation on the membrane if RO or NF are applied directly in the MBR to concentrate wastewater.46 To-date, PN/AMOX process only has been successfully applied on the side-stream due to higher NH4+ concentration after sludge digestion.10 Nevertheless, future applications of FO with PN/AMOX in treating the mainstream wastewater may reduce the aeration consumption in the wastewater treatment by 60%.10 The proposed integration of FOAnMBR with the PN/AMOX process will contribute to a promising energy-minimal future of wastewater treatment.
Aside from the treatment configuration and the economic evaluation of each scenario, the generation of GHG also need to be considered. Previous studies conducted on the GHG emissions reported carbon footprints ranging from 0.13–0.69 kg CO2 – e m−3 for CAS treatment and 0.60–2.40 kg CO2 – e m−3 when membrane-based technology was applied for water reclamation.2 The GHG emissions for MFAeMBR + RO in scenario B was 1.50 kg CO2 – e m−3 which lies within the range of values reported in the literature.2 Although the application of PN/AMOX in wastewater treatment contributes to a higher direct emission of GHG, this configuration had lower total emission of GHG than the conventional nitrification–denitrification treatment under an optimum treatment operation. For MFAnMBR + PN/AMOX, the indirect GHG emissions were primarily due to the energy consumption, specifically the aeration requirement for microorganism growth in the aeration zone of the post nitrogen removal unit.13,24,36
4. Conclusion
In this study, the aims of FO application in treating the wastewater were not only focused on producing high quality effluent, but also on achieving high energy efficiency by recovering useful resources from wastewater. However, the current performance of FO membrane (water flux = 15 LMH, water recovery = 80%, membrane cost = $50 per m2) is not economically competitive compared to MF application, specifically when the integration of FO + RO for water reclamation was implemented. This was due to the high energy consumption of the high pressure pump for RO in filtering diluted seawater, which resulted in a net energy consumption for the whole treatment operation of 3.71 kW h m−3. The net energy consumption in scenario A was 4 times lower than in scenario B, and a minimal energy balance of wastewater treatment was calculated to be achievable by integrating FO with PN/AMOX process in scenario C. The high energy consumption of FO in scenario B also generated higher GHG emissions, with 3.86 kg CO2 – e m−3, which was 3 times and 4 times higher than those of scenarios A and C, respectively.To improve the competitiveness of the FO membrane in wastewater treatment, a least a doubling of water flux performance (30 LMH) and a reduction of membrane cost to $20 per m2 are required to reduce the treatment cost to be as low as using the MF in scenario A. If the above improved FO membrane to be used in scenario C (FOAnMBR + PN/AMOX), it was expected that the wastewater treatment cost could be further reduced to $0.98 per m3, which is even cheaper than the conventional MFAeMBR in scenario A ($1.09 per m3). For future study, it is recommended that an optimisation analysis be conducted on the flow rate of seawater required, specifically in scenario B, to assess the impact of seawater dilution on the FO water flux performance, energy consumption and economics of the operation.
List of abbreviations
| ANAMMOX or AMOX | Anaerobic ammonium oxidation |
| CAPEX | Capital expenditure |
| CAS | Conventional activated sludge |
| DS | Draw solution |
| FO | Forward osmosis |
| FOAeMBR | Forward osmosis aerobic membrane bioreactor |
| FOAnMBR | Forward osmosis anaerobic membrane bioreactor |
| GHG | Greenhouse gases |
| LMH | L m−2 per hour |
| LPRO | Low pressure reverse osmosis |
| MBBR | Moving bed biofilm reactor |
| MF | Microfiltration |
| MFAeMBR | Microfiltration aerobic membrane biorector |
| MFAnMBR | Microfiltration anaerobic membrane bioreactor |
| NaCl | Sodium chloride |
| NPV | Net present value |
| PN | Partial nitrification |
| RO | Reverse osmosis |
| ROSA | Reverse osmosis system analysis |
| SWRO | Seawater reverse osmosis |
| OPEX | Operational expenditure |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the funding support through The University of Queensland's Academic Start-Up Funding and Australian Research Council Discovery Early Career Researcher Award DE150100393 awarded to Dr. Liu Ye. Ms Nur Hafizah Ab Hamid acknowledges the scholarship support from the University of Queensland (UQI scholarship) for her PhD study.References
- G. Venkatesh and H. Brattebø, Energy consumption, costs and environmental impacts for urban water cycle services: Case study of Oslo (Norway), Energy, 2011, 36, 792–800 CrossRef.
- N. Horstmeyer, M. Weißbach, K. Koch and J. E. Drewes, A novel concept to integrate energy recovery into potable water reuse treatment schemes, J. Water Reuse Desalin., 2018, 1–13 Search PubMed.
- S. Judd, in The MBR Book: Principles and Applications of Membrane Bioreactors in Water and Wastewater Treatment, Elsevier, UK, 1st edn, 2006 Search PubMed.
- C. Bartels, R. Franks and K. Andes, Operational Performance and Optimization of RO Wastewater Treatment Plants, Singapore Int. Water Week, Singapore, 2010, pp. 1–12 Search PubMed.
- C. R. Bartels, M. Wilf, K. Andes and J. Iong, Design considerations for wastewater treatment by reverse osmosis, Water Sci. Technol., 2005, 51, 473–482 CrossRef CAS PubMed.
- J. Radjenovi, M. Matosic, I. Mijatovic, M. Petrovic and D. Barcelo, Membrane Bioreactor (MBR) as an Advanced Wastewater Treatment Technology, Handb. Environ. Chem., 2008, 5, 37–101 Search PubMed.
- S. Chang, Anaerobic Membrane Bioreactors (AnMBR) for Wastewater Treatment, Adv. Chem. Eng. Sci., 2014, 4, 56–61 CrossRef.
- W. Dai, X. Xu, B. Liu and F. Yang, Toward energy-neutral wastewater treatment: A membrane combined process of anaerobic digestion and nitritation-anammox for biogas recovery and nitrogen removal, Chem. Eng. J., 2015, 279, 725–734 CrossRef CAS.
- B. Ma, S. Wang, S. Cao, Y. Miao, F. Jia, R. Du and Y. Peng, Biological nitrogen removal from sewage via anammox: Recent advances, Bioresour. Technol., 2016, 200, 981–990 CrossRef CAS PubMed.
- M. Ali and S. Okabe, Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues, Chemosphere, 2015, 141, 144–153 CrossRef CAS PubMed.
- B. Kartal, J. G. Kuenen and M. C. van Loosdrecht, Sewage Treatment with Anammox, Science, 2010, 328, 702–703 CrossRef CAS.
- M. Laureni, D. G. Weissbrodt, I. Szivak, O. Robin, J. L. Nielsen, E. Morgenroth and A. Joss, Activity and growth of anammox biomass on aerobically pre-treated municipal wastewater, Water Res., 2015, 80, 325–336 CrossRef CAS PubMed.
- G. Mezohegyi, M. R. Bilad and I. F. J. Vankelecom, Direct sewage up-concentration by submerged aerated and vibrated membranes, Bioresour. Technol., 2012, 118, 1–7 CrossRef CAS PubMed.
- A. Achilli, T. Y. Cath, E. A. Marchand and A. E. Childress, The forward osmosis membrane bioreactor: A low fouling alternative to MBR processes, Desalination, 2009, 238, 10–21 CrossRef.
- S. Lee, C. Boo, M. Elimelech and S. Hong, Comparison of fouling behavior in forward osmosis (FO) and reverse osmosis (RO), J. Membr. Sci., 2010, 365, 34–39 CrossRef CAS.
- X. Zhang, Z. Ning, D. K. Wang and J. C. Diniz da Costa, Processing municipal wastewaters by forward osmosis using CTA membrane, J. Membr. Sci., 2014, 468, 269–275 CrossRef CAS.
- Y. Choi, H. Cho, Y. Shin, Y. Jang and S. Lee, Economic evaluation of a hybrid desalination system combining forward and reverse osmosis, Membranes, 2015, 6, 1–19 CrossRef PubMed.
- A. Teusner, G. Blandin and P. Le-Clech, Augmenting water supply by combined desalination/water recycling methods: an economic assessment, Environ. Technol., 2017, 38, 257–265 CrossRef CAS PubMed.
- R. V. Linares, Z. Li, V. Yangali-Quintanilla, N. Ghaffour, G. Amy, T. Leiknes and J. S. Vrouwenvelder, Life cycle cost of a hybrid forward osmosis - low pressure reverse osmosis system for seawater desalination and wastewater recovery, Water Res., 2016, 88, 225–234 CrossRef.
- C. F. Wan and T. S. Chung, Techno-economic evaluation of various RO+PRO and RO+FO integrated processes, Appl. Energy, 2018, 212, 1038–1050 CrossRef CAS.
- V. Yangali-Quintanilla, Z. Li, R. Valladares, Q. Li and G. Amy, Indirect desalination of Red Sea water with forward osmosis and low pressure reverse osmosis for water reuse, Desalination, 2011, 280, 160–166 CrossRef CAS.
- G. Blandin, A. R. D. Verliefde, C. Y. Tang and P. Le-Clech, Opportunities to reach economic sustainability in forward osmosis-reverse osmosis hybrids for seawater desalination, Desalination, 2015, 363, 26–36 CrossRef CAS.
- G. Blandin, A. R. D. Verliefde, J. Comas, I. Rodriguez-Roda and P. Le-Clech, Efficiently Combining Water Reuse and Desalination through Forward Osmosis-Reverse Osmosis (FO-RO) Hybrids: A Critical Review, Membranes, 2016, 6, 37 CrossRef.
- D. Solley, S. Hu, C. Hertle, D. Batstone, T. Karastergiou-Hogan, Q. Rider and J. Keller, Identifying novel wastewater treatment options through optimal technology integration, Water Practice and Technology, 2015, 10, 496–504 CrossRef.
- S. Ripperger, W. Gosele, C. Alt and T. Loewe, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2013, pp. 1–38 Search PubMed.
- S. Shirazi and C.-J. Lin, in Sustainable Water Technologies, ed. D. H. Chen, Taylor & Francis Group, Boca Raton, 2nd edn, 2016, pp. 269–296 Search PubMed.
- M. H. Plumlee, B. D. Stanford, J. F. Debroux, D. C. Hopkins and S. A. Snyder, Costs of Advanced Treatment in Water Reclamation, Ozone: Sci. Eng., 2014, 36, 485–495 CrossRef CAS.
- A. Bick, L. Gillerman, Y. Manor and G. Oron, Economic assessment of an integrated membrane system for secondary effluent polishing for unrestricted reuse, Water, 2012, 4, 219–236 CrossRef CAS.
- A. J. Ansari, F. I. Hai, W. Guo, H. H. Ngo, W. E. Price and L. D. Nghiem, Factors governing the pre-concentration of wastewater using forward osmosis for subsequent resource recovery, Sci. Total Environ., 2016, 566, 559–566 CrossRef PubMed.
- M. R. J. Doorn, S. Towprayoon, S. M. Manso Vieira, W. Irving, C. Palmer, R. Pipatti and C. Wang, Wastewater Treatment and Discharge, 2006 IPCC Guidelines for National Greenhouse Gas Inventories, 2006, pp. 1–28 Search PubMed.
- Y. Law, L. Ye, Y. Pan and Z. Yuan, Nitrous oxide emissions from wastewater treatment processes, Philos. Trans. R. Soc., B, 2012, 367, 1265–1277 CrossRef CAS PubMed.
- A. Noyola, M. G. Paredes, L. P. Güereca, L. T. Molina and M. Zavala, Methane correction factors for estimating emissions from aerobic wastewater treatment facilities based on field data in Mexico and on literature review, Sci. Total Environ., 2018, 639, 84–91 CrossRef CAS PubMed.
- I. E. Agency, CO2 Emissions from Fuel Combustion 2017 - Highlights, 2017, vol. 1 Search PubMed.
- P. Forster, V. Ramaswamy, P. Artaxo, T. Berntsen, R. Betts, D. W. Fahey, J. Haywood, J. Lean, D. C. Lowe, G. Myhre, J. Nganga, R. Prinn, G. Raga, M. Schulz and R. Van Dorland, in Climate Change, ed. T. Nakajima and V. Ramanathan, Cambridge University Press, Cambridge, United Kingdom and New York, 2007, vol. 30, pp. 129–234 Search PubMed.
- V. Parravicini, K. Svardal and J. Krampe, Greenhouse Gas Emissions from Wastewater Treatment Plants, Energy Procedia, 2016, 97, 246–253 CrossRef CAS.
- N. C. Nguyen, S. S. Chen, H. Y. Yang and N. T. Hau, Application of forward osmosis on dewatering of high nutrient sludge, Bioresour. Technol., 2013, 132, 224–229 CrossRef CAS PubMed.
- T. L. G. Hendrickx, Y. Wang, C. Kampman, G. Zeeman, H. Temmink and C. J. N. Buisman, Autotrophic nitrogen removal from low strength waste water at low temperature, Water Res., 2012, 46, 2187–2193 CrossRef CAS PubMed.
- Agriculture and Resource Management Council of Australia and New Zealand and Australian; New Zealand Council Environment and Conservation, National Water Quality Management Strategy: Australian Guidelines for Sewerage Systems - Effluent Management, 1997.
- R. V. Linares, Z. Li, M. Abu Ghdaib, C.-H. Wei, G. Amy and J. S. Vrouwenvelder, Water harvesting from municipal wastewater via osmotic gradient: An evaluation of process performance, J. Membr. Sci., 2013, 447, 50–56 CrossRef.
- X. Wang, Y. Chen, B. Yuan, X. Li and Y. Ren, Impacts of sludge retention time on sludge characteristics and membrane fouling in a submerged osmotic membrane bioreactor, Bioresour. Technol., 2014, 161, 340–347 CrossRef CAS.
- W. C. L. Lay, Q. Zhang, J. Zhang, D. McDougald, C. Tang, Y. Liu and A. G. Fane, Study of integration of forward osmosis and biological process: Membrane performance under elevated salt environment, Desalination, 2011, 283, 123–130 CrossRef CAS.
- W. C. L. Lay, Y. Liu and A. G. Fane, Impacts of salinity on the performance of high retention membrane bioreactors for water reclamation: A review, Water Res., 2010, 44, 21–40 CrossRef CAS PubMed.
- G. Qiu, Y. M. Law, S. Das and Y. P. Ting, Direct and complete phosphorus recovery from municipal wastewater using a hybrid microfiltration-forward osmosis membrane bioreactor process with seawater brine as draw solution, Environ. Sci. Technol., 2015, 49, 6156–6163 CrossRef CAS PubMed.
- W. Zhu, X. Wang, Q. She, X. Li and Y. Ren, Osmotic membrane bioreactors assisted with microfiltration membrane for salinity control (MF-OMBR) operating at high sludge concentrations: Performance and implications, Chem. Eng. J., 2018, 337, 576–583 CrossRef CAS.
- A. Uygur and F. Kargi, Salt inhibition on biological nutrient removal from saline wastewater in a sequencing batch reactor, Enzyme Microb. Technol., 2004, 34, 313–318 CrossRef CAS.
- I. L. Alsvik and M. B. Hägg, Pressure retarded osmosis and forward osmosis membranes: Materials and methods, Polymers, 2013, 5, 303–327 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ew00608g |
| This journal is © The Royal Society of Chemistry 2020 |