Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sugaring-out strategy for counter-current chromatography isolation: podophyllotoxins and flavones from Dysosma versipellis as examples†
Lihong Zhang,
Yanyan Wang and
Shihua Wu *
*
Research Centre of Siyuan Natural Pharmacy and Biotoxicology, College of Life Sciences, Zhejiang University, Hangzhou 310058, China. E-mail: drwushihua@zju.edu.cn; Fax: +86-571-88206287; Tel: +86-571-88206287
First published on 15th February 2017
Abstract
Counter-current chromatography is an efficient and practical liquid–liquid chromatography technique for the separation and purification of complex mixtures such as natural product extracts and synthetic chemicals. However, counter-current chromatography is a challenging approach and requires special knowledge, especially for selection of the solvent system, which may be estimated as 90% of the entire amount of work. In this work, we introduced a sugaring-out strategy for optimizing two-phase solvent systems for counter-current chromatography by using sugars as additives. Thirteen podophyllotoxins and flavones in the extract of the Traditional Chinese Medicine Dysosma versipellis were selected as model compounds, and nine sugars, including sucrose, glucose, fructose, maltose, D-galactose, D-sorbose, mannose, rhamnose, and xylopyranose, were selected as modifiers added into the two-phase solvent systems. As a result, we found that almost all of the sugars used in this work could increase the values of the partition coefficients of almost all of targets in the two-phase hexane–ethyl acetate–methanol–water solvent systems. In addition, sugars with different chemical structures seemed to have different sugaring-out effects on the resolution and selective separation of some components of Dysosma versipellis, although they were able to increase the partition coefficients of several components. Therefore, it may be an alternative strategy to quickly obtain an optimal two-phase solvent system by adding some sugars into the selected two-phase solvent system. This method is very simple and efficient for the separation and purification of single or multiple targets from natural products.
1. Introduction
Natural products remain a major resource of new chemical entities and drug production1,2 due to their diverse structures and bioactivities.3 However, there are still several challenges in natural product research in spite of the many advantages to be achieved through the screening and purification of natural products. For example, a common challenge faced by analysts is how to rapidly and efficiently isolate more components from natural product resources that contain a large number of known and unknown entities.4,5Counter-current chromatography (CCC) is a useful method for rapid chromatographic purification employing highly efficient fractionation by a hybrid technique of liquid–liquid counter-current distribution and liquid chromatography, in conjunction with the use of centrifugal force.6 Since the method does not employ a solid support matrix, it thus eliminates complications such as irreversible adsorptive sample loss and deactivation, the tailing of solute peaks and contamination. Therefore, it has been successfully applied to analyse and separate various natural and synthetic products.7–11
Usually, the selection of an appropriate solvent system is the first step and also the most important step of a common CCC separation process, which may be estimated as 90% of the entire amount of work.6 Although there are several comprehensive two-phase solvent system families and relative solvent selection methods such as Ito's rules on Ito's solvents,6 Arizona solvent systems,12 GUESS-mix based TLC methods on the hexane–ethyl acetate–methanol–water (HEMWat) family,13 9 × 9 HEMWat map-based linear calculation methods14 and other experimental-based calculation methods that have been developed, the selection of an optimum two-phase solvent system still requires special knowledge and seems tedious as well as time-consuming. Recently, several studies indicated that some salting-out and salting-in methods using inorganic or organic salts15–17 along with room temperature ionic liquids18–20 as modifiers in some cases were considered to be efficient and alternative methods to optimize two-phase solvent systems.
In this work, we found that sugars can play a “sugaring-out” role, which is similar to the “salting-out” effect for the partitioning of several natural products in HEMWat two-phase solvent systems. The effects of several sugars on the partition coefficients of compounds were investigated by adding different types of sugars with different concentrations into the desired two-phase solvent system. To demonstrate the capacity of this sugaring-out method, 13 podophyllotoxins and flavones were selected as model compounds, which are major bioactive components of the extracts of Dysosma versipellis, a well-known Traditional Chinese Medicine possessing versatile biological activities.21–23 To the best of our knowledge, this is the first report of using the sugaring-out strategy and concentric CCC for the extraction and separation of multiple components from D. versipellis.
2. Materials and methods
2.1. Reagents and materials
Organic solvents for the chromatographic fractionation and separation, including hexane, ethyl acetate and methanol, were of analytical grade (Sinopharm Chemical Reagent Co., Shanghai, China). The methanol used for HPLC was of chromatographic grade (Merck, Darmstadt, Germany). The water was purified by a water purifier (18.2 MΩ cm, Wanjie Water Treatment Equipment, Hangzhou, China) and used for all solutions.The sugars used as additives were of analytical grade with greater than 98% purity. Sucrose, glucose, D-mannitol, xylopyranose and rhamnose were from the Sinopharm Shanghai Chemical Reagent Co., Ltd., China. Fructose, maltose, mannose were from the Shanghai Bodung Technology Co., Ltd., China. D-Galactose and D-sorbose were from the Hengxin Chemical Reagent Co. Ltd., China.
The dry roots of D. versipellis were bought from Bozhou Traditional Chinese Medicines Market (Bozhou, China). The species was identified by the Institute of Plant Science, College of Life Sciences, Zhejiang University, China. The sample used for CCC was prepared from the ethanol extract of the dry roots of D. versipellis by flash glass silica chromatography, which was similar to a previous report23 but with petroleum ether–ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) elution. The thirteen podophyllotoxins and flavones used for this work as standards were isolated and identified by electrospray ionization tandem mass spectrometry (ESI-MS/MS) and 1D and 2D nuclear magnetic resonance (NMR) spectra in our lab.5,23 Their chemical structures and physical properties are summarized in Fig. S1† and Table 1.
1, v/v) elution. The thirteen podophyllotoxins and flavones used for this work as standards were isolated and identified by electrospray ionization tandem mass spectrometry (ESI-MS/MS) and 1D and 2D nuclear magnetic resonance (NMR) spectra in our lab.5,23 Their chemical structures and physical properties are summarized in Fig. S1† and Table 1.
| No. | Compound name | Rt (min) | Molecular formula | M.W. (Da) | pKa | MR | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
|---|---|---|---|---|---|---|---|---|
| a The physical properties were calculated by ChemBioOffice Ultra 13.0, Cambridge Soft. | ||||||||
| 1 | 3,4-Dihydroxybenzoic acid | 9.98 | C7H6O4 | 154 | 3.90, 9.20, 13.91 | 35.72 | 0.81 | 1.06 |
| 2 | p-Hydroxybenzoic acid | 13.09 | C7H6O3 | 138 | 4.11, 9.69 | 33.90 | 1.2 | 1.56 |
| 3 | α-Peltatin | 27.08 | C21H20O8 | 400 | 8.01, 8.58 | 103.86 | 2.51 | 1.08 |
| 4 | Quercetin | 29.35 | C15H10O7 | 302 | 6.87, 7.75, 10.88, 12 54, 16.68 | 76.51 | 0.35 | 1.50 |
| 5 | Isopicropodophyllotoxone | 31.07 | C22H20O8 | 412 | — | 108.52 | 2.11 | 1.66 |
| 6 | β-Peltatin | 31.94 | C22H22O8 | 414 | 8.00 | 109.29 | 2.77 | 1.42 |
| 7 | Podophyllotoxin | 32.31 | C22H22O8 | 414 | 13.15 | 108.94 | 2.12 | 1.30 |
| 8 | Kaempferol | 33.42 | C15H10O6 | 286 | 6.88, 8.93, 12.03, 12.65 | 74.70 | 0.74 | 2.10 |
| 9 | Podophyllotoxone | 33.78 | C22H20O8 | 412 | — | 108.52 | 2.11 | 1.66 |
| 10 | Podoverine A | 37.64 | C21H20O7 | 384 | 6.78, 7.72, 12.39, 14.13 | 106.57 | 2.31 | 3.40 |
| 11 | Podoverine D | 41.89 | C36H28O13 | 668 | 5.79, 6.84, 7.80, 9.50, 10.10, 12.39 | 177.28 | 4.82 | 4.51 |
| 12 | Podoverine E | 44.58 | C37H30O13 | 682 | 5.78, 6.84, 7.80, 9.56, 11.85 | 182.71 | 5.09 | 5.09 |
| 13 | Podoverine H | 46.16 | C41H36O13 | 736 | 6.90, 7.12, 7.67, 8.94, 12.31, 14.07 | 202.54 | 6.67 | 19.35 |
2.2. Measurement of the partition coefficient (K)
The partition coefficients (K) for the target compounds were determined by high-performance liquid chromatography (HPLC) analysis according to a previous study.24 In brief, sample was added into the two-phase solvent systems including n-hexane, ethyl acetate, methanol and water or sugar aqueous solution with the desired volume ratio in a 2 mL test tube. After the equilibration was established, both the upper phase and lower phase were separated centrifugally and submitted to HPLC analysis. The partition coefficient (K) was the ratio of the HPLC peak areas of each compound in the upper phase and lower phase. The concentrations of sugar aqueous solutions were expressed as the percentage of the mass of the sugar in the solution (wt%). For example, a 20% sucrose aqueous solution is prepared by dissolving 25 g of sucrose into 100 mL (100 g) of water for analysis of the K values or 250 g of sucrose into 1000 mL (1000 g) of water for CCC separation. Similarly, the HEMWat 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 system with 20% sucrose means 4 volumes of hexane, 6 volumes of ethyl acetate, 4 volumes of methanol and 6 volumes of a 20% aqueous sucrose solution.
6 system with 20% sucrose means 4 volumes of hexane, 6 volumes of ethyl acetate, 4 volumes of methanol and 6 volumes of a 20% aqueous sucrose solution.
2.3. CCC separation procedure
Preparative CCC separation was performed on a new upright concentric CCC device as previously described.25 Briefly, the apparatus held three identical disc-shaped holders (diameter, 160 mm), and their revolution radius was 10 cm. Five circular grooves (3.3 mm width and 5 mm depth) were distributed evenly on the disc-shaped holder ranging from an inner diameter of 90.4 mm to an outer diameter of 145 mm. A long polytetrafluoroethylene (PTFE, 1.8 mm i.d. and 0.6 mm wall thickness) tube was fitted into the innermost circular groove in the left-handed direction from the rotation axis. Once it filled the space of one round of the circular groove, the PTFE tube was drawn into the nearby outer circular groove through the gap and wound in the same direction. When PTFE filled the space of the outermost groove, it was drawn into the near inner groove through the gap and wound in the same direction. Then, the PTFE tube was repeatedly wound in the same mode until the whole disc groove was filled to form a concentric coil (capacity, 80 mL; β, 0.5–0.75). The three same concentric coils distributed on three disc-shape holders were connected by the same PTFE tube making a total column volume of 260 mL. The revolution speed was regulated by a speed controller and ranged from 0 to 1500 rpm.According to the previous CCC separation process,24,26 two-phase solvents were prepared with hexane, ethyl acetate, methanol and water or sugar aqueous solution in the desired proportion and mixed thoroughly in a separatory funnel at room temperature. The two liquid phases were separated and put in a 37 kHz ultrasonic water bath (Elma Elmasonic P 120 H, Elma Schmidbauer GmbH, Germany) to remove air bubbles before use. The upper phase of the solvent system used as the stationary phase was first pumped with the desired flow ratio to completely fill the column. Then, the sample was injected through the injection valve, and the apparatus was rotated at the desired rotation speed (900 rpm), while the lower phase as the mobile phase was pumped into the CCC column from the head to the tail of the column at the desired flow rate. The injection mode was called “injection before equilibrium”.27 The effluent was monitored by a diode assay detector (DAD) and automatically collected in 20 mL test tubes with BSZ-100 fraction collectors. Peak fractions were collected according to the elution profile and analysed by HPLC detection.
For the separation of D. versipellis, 250 mg of sample was dissolved in 3 mL of the upper phase and the same volume of the lower phase of the solvent system for each injection. The elution flow speed was set at 3 mL min−1. The effluents were detected at 254 nm, and the effluent was collected in a test tube for 4 min. After CCC separation, the effluents were submitted to further solvent extraction or reversed phase C18 solid-extraction for removing the added sugars and obtaining the pure compounds. The obtained pure components were further identified by HPLC and electrospray ionization tandem mass spectrometry (ESI-MS/MS) analyses.
2.4. HPLC analysis of crude samples and CCC fractions
The high-performance liquid chromatography (HPLC) analyses were performed on an Agilent 1100 system including a G1379A degasser, a G1311A Quat Pump, a G1367A Wpals, a G1316A column oven, and a G1315B diode assay detector (DAD). The conditions for the analyses of D. versipellis were same as the previously described conditions.23 In short, the column was a reversed-phase Zorbax SB-C18 (250 mm length × 4.6 mm i.d., 5 mm) column. Methanol (A) and aqueous 0.1% trifluoroacetic acid (TFA) (B) were used as the mobile phase in the gradient mode as follows: 0–5 min, A from 10% to 30% and B from 90% to 70%; 5–35 min, A from 30% to 70% and B from 70% to 30%; and 35–45 min, A from 70% to 100% and B from 30% to 0%. The flow rate of the mobile phase was 0.8 mL min−1, and the effluents were monitored at 254 nm by a DAD detector. Other conditions included a 30 °C column temperature and a 10 μL injection volume.3. Results and discussion
3.1. Sugaring-out effects of sugars in HEMWat systems
As described above, the selection of a suitable solvent system with an appropriate partition coefficient (K) is very important for CCC separation. Usually, the K values of targets in the optimal solvent systems are described as an area, named “sweet spot”, where the K values of compounds are between 0.4 and 2.5.13 For the isolation of the target compounds, the suitable K values for high-speed CCC are 0.5 ≤ K ≤ 1.0.6 Meanwhile, for multiple targets, the range may even be extended to 0.25 < K < 16.28In the course of screening new solvent selection strategies for appropriate solvent systems, we noted that there are genuine “sweet” agents referred to as sugars, which contain many hydrophilic hydroxyl groups (–OH) and have strong hydrophilicity. We guessed that if sugars were added into a solvent system, they might more easily form stronger hydrogen bonds than the analytes with water, resulting in that the analytes distributed in the aqueous phase would be pushed into the organic phase, which was similar to previous salting-out principles.16,17,29 In addition, it has been known that sugars can also push the organic solvents (i.e., ethyl acetate) out of the aqueous phase,30 thus a new two-phase equilibrium was re-established when sugars were added into the system. As a result, the K values of the analytes were significantly increased.
Previous studies5,22,23,31 indicated that D. versipellis contained a large number of components ranging from hydrophilic podophyllotoxin glycosides to hydrophobic flavonoids. Thus, it was selected as a representative natural product extract to investigate the sugaring-out effects. After simple elution with petroleum ether–ethyl acetate (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
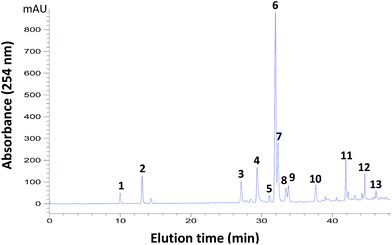
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) on flash silica chromatography,23 the yielded fraction of D. versipellis was used for this study. As shown in Fig. 1, the selected sample yielded a chromatogram in which 20 peaks can be identified.5,23 Among these components, 13 prominent components including 5 podophyllotoxins, 2 phenolic acids and 6 flavones were applied as model components for the evaluation of the sugaring-out effects of sugars on the partition coefficients and CCC separation. These components have very different structures (Fig. S1†) and physical properties (Table 1), thus they can be representative of natural products.
1, v/v) on flash silica chromatography,23 the yielded fraction of D. versipellis was used for this study. As shown in Fig. 1, the selected sample yielded a chromatogram in which 20 peaks can be identified.5,23 Among these components, 13 prominent components including 5 podophyllotoxins, 2 phenolic acids and 6 flavones were applied as model components for the evaluation of the sugaring-out effects of sugars on the partition coefficients and CCC separation. These components have very different structures (Fig. S1†) and physical properties (Table 1), thus they can be representative of natural products.
Sucrose is one of the most common sugars in the social life and scientific research. Typically, there is a well-known experimental technology called “sucrose gradient centrifugation”, which is a type of centrifugation that often uses sucrose to purify enveloped viruses and ribosomes as well as to separate cell organelles from crude cellular extracts. Thus, sucrose was first selected to investigate the sugaring-out effects. Recent work5,31 indicated that the HEMWat system with a volume ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 is suitable for the isolation of the podophyllotoxins and flavonoids from the extract of D. versipellis. Thus, this system was first selected as the two-phase solvent system.
6 is suitable for the isolation of the podophyllotoxins and flavonoids from the extract of D. versipellis. Thus, this system was first selected as the two-phase solvent system.
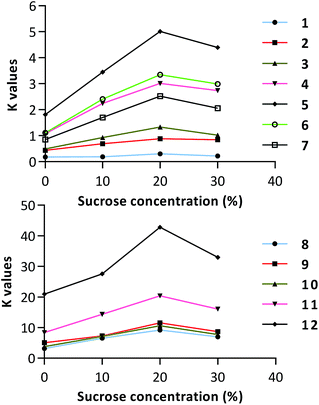
As expected, the partition coefficients of almost all of the investigated compounds showed dose-dependent increases after sucrose was added into the system (Table 2 and Fig. 2). The K values of most of the components in the system with the addition of 20% sucrose were almost double those in the system without additive. However, at extremely high sucrose concentrations (30%) (Fig. 2), there are some inverted effects on most of the selected compounds, which might due to the polarity changes of entire systems after the addition of too much sugars. The hydrophilic hydroxyl groups of a high dose of sugars could form more hydrogen bonds with other molecules including solvents, analytes, and even intermolecular sugars in addition to the sugaring-out effect by competing for hydrogen bonding with the analytes.
| Sucrose (w/v%) | K values | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
HEMWat 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
||||||||||||
| 0 | 0.18 | 0.44 | 0.49 | 1.08 | 1.81 | 1.11 | 0.85 | 3.17 | 5.02 | 3.80 | 8.36 | 20.91 |
| 10% | 0.19 | 0.69 | 0.93 | 2.24 | 3.45 | 2.41 | 1.70 | 6.53 | 7.29 | 7.09 | 14.30 | 27.51 |
| 20% | 0.30 | 0.88 | 1.33 | 3.01 | 5.01 | 3.35 | 2.52 | 9.20 | 11.51 | 10.59 | 20.36 | 42.82 |
| 30% | 0.22 | 0.84 | 1.02 | 2.74 | 4.39 | 2.99 | 2.06 | 6.97 | 8.70 | 7.73 | 16.07 | 32.93 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
HEMWat 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
||||||||||||
| 0 | 0.11 | 0.32 | 0.37 | 0.93 | 1.74 | 1.09 | 0.80 | 2.85 | 4.29 | 3.68 | 5.30 | 14.07 |
| 10% | 0.14 | 0.34 | 0.47 | 1.06 | 2.09 | 1.33 | 0.85 | 3.02 | 4.45 | 4.18 | 6.30 | 15.46 |
| 20% | 0.29 | 0.52 | 0.62 | 1.47 | 2.69 | 1.56 | 1.23 | 3.64 | 5.41 | 5.31 | 7.57 | 18.09 |
| 30% | 0.18 | 0.38 | 0.54 | 1.14 | 2.36 | 1.39 | 0.96 | 3.48 | 5.08 | 5.06 | 6.67 | 16.58 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||
HEMWat 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
||||||||||||
| 0 | 0.08 | 0.21 | 0.23 | 0.44 | 0.93 | 0.54 | 0.31 | 0.94 | 1.37 | 1.12 | 1.82 | 3.09 |
| 10% | 0.13 | 0.23 | 0.26 | 0.53 | 1.12 | 0.68 | 0.49 | 1.31 | 2.08 | 1.53 | 2.64 | 3.29 |
| 20% | 0.22 | 0.42 | 0.53 | 1.19 | 1.65 | 1.26 | 1.07 | 1.87 | 2.69 | 2.06 | 3.17 | 4.23 |
| 30% | 0.17 | 0.24 | 0.36 | 0.84 | 1.43 | 1.02 | 0.52 | 1.50 | 2.45 | 1.80 | 2.80 | 3.38 |
Interestingly, Table 2 and Fig. 2 also showed the trend that the K values of the analytes in the selected two-phase solvent systems were larger, and the sugaring-out effects seemed more remarkable. As shown in Fig. S2,† there is approximately a linear response of relative changes of the partition coefficients of the components. This could be explained by that the partition coefficient of a compound is closely correlated to its hydrophobicity,32 while the hydrophobicities of compounds play a crucial role in the sugaring-out effects of sugars. When a compound is very hydrophilic, the relative change of the K value will be inconspicuous. In this case, the sugaring-out effect of the sugar is limited, and selecting other solvent systems is also required. Compared with other methods to change the HEMW system compositions, the method is only a way of adjusting K. Therefore, the K value is still a golden criterion for the choice of a replaceable solvent system or sugaring-out strategy.
Furthermore, we measured the sugaring-out effects of sucrose in other HEMWat solvent systems. As shown in Table 2 and Fig. 3, although the K values of all of the compounds in the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 4.5
5 and 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 HEMWat systems were less than the K values in the 4
5.5 HEMWat systems were less than the K values in the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 system, the relative sugaring-out powers observed in these systems were in very close agreement with the above results in the 4
6 system, the relative sugaring-out powers observed in these systems were in very close agreement with the above results in the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 system. In addition, the relative changes of K values of each compound in the 5
6 system. In addition, the relative changes of K values of each compound in the 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 4.5
5 and 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 systems were smaller than the changes in the 4
5.5 systems were smaller than the changes in the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 system with larger K values, which further confirmed the trend shown in Fig. S2.† Therefore, sucrose could have potent sugaring-out effects in these two-phase solvent systems.
6 system with larger K values, which further confirmed the trend shown in Fig. S2.† Therefore, sucrose could have potent sugaring-out effects in these two-phase solvent systems.
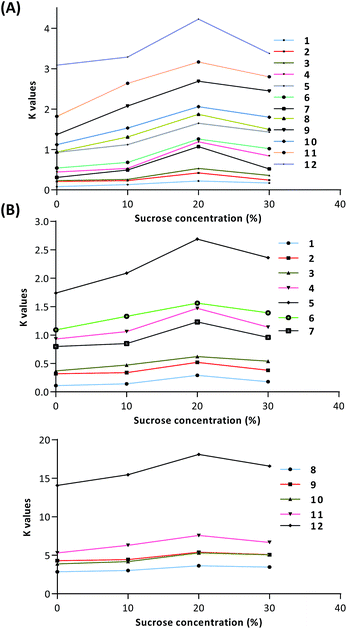 | ||
Fig. 3 Sugaring-out effects of sucrose on the partition coefficients of the selected compounds in HEMWat systems of (A) 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and (B) 4.5 5 and (B) 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 (v/v). 5.5 (v/v). | ||
Next, we further investigated the sugaring-out effect of glucose, one of the most widely used aldohexoses in living organisms. It is different from sucrose in that glucose has reactive aldehyde groups and has a lower tendency than other aldohexoses to non-specifically react with the amine groups of other molecules. As shown in Fig. S3,† glucose showed stronger sugaring-out effects on some compounds such as 5, 8, 9 and 11, which had bigger K values after adding glucose into the two-phase solvent systems. Thus, the role of glucose seemed different from that of sucrose.
The above results indicated that sugars are a type of efficient additives to increase the partitioning of solutes in two-phase solvent systems. Thus, several sugars were selected to systematically investigate the effects of sugars on the partitioning of multiple components in the 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 HEMWat system. As shown in Table S1, Fig. 4 and S4,† except for rhamnose, all sugars could increase the K values of almost all of the components with concentrations of 10% and 20%. In addition, the changes in the K values were related to the concentrations of the sugars. The effects of some sugars with different concentrations on the K values in the HEMWat 4.5
5.5 HEMWat system. As shown in Table S1, Fig. 4 and S4,† except for rhamnose, all sugars could increase the K values of almost all of the components with concentrations of 10% and 20%. In addition, the changes in the K values were related to the concentrations of the sugars. The effects of some sugars with different concentrations on the K values in the HEMWat 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 (v/v) system were different (Fig. S3 and S5†). Similar to the above results in the 4
5.5 (v/v) system were different (Fig. S3 and S5†). Similar to the above results in the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 HEMWat system, inverted effects were observed in higher concentrations of the most of the sugars (i.e., 30%), and they could not further increase the K values. However, we have not observed these inverted effects of maltose (Fig. S5B†), which showed strong sugaring-out effects for all of the tested concentrations (0–30%). It should be noted that the 30% sugar concentration is too high for some of the compounds. For example, when adding a 30% dose of galactose into the 4.5
6 HEMWat system, inverted effects were observed in higher concentrations of the most of the sugars (i.e., 30%), and they could not further increase the K values. However, we have not observed these inverted effects of maltose (Fig. S5B†), which showed strong sugaring-out effects for all of the tested concentrations (0–30%). It should be noted that the 30% sugar concentration is too high for some of the compounds. For example, when adding a 30% dose of galactose into the 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 HEMWat system, it could not be dissolved completely (Table S1†). Therefore, for CCC separations, the selection of an appropriate concentration of the sugar is also important.
5.5 HEMWat system, it could not be dissolved completely (Table S1†). Therefore, for CCC separations, the selection of an appropriate concentration of the sugar is also important.
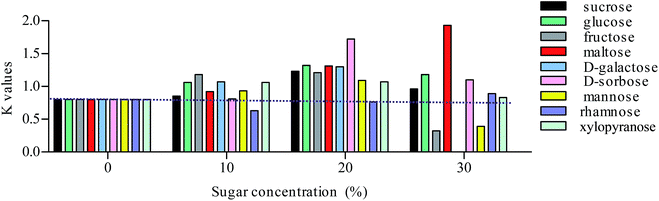 | ||
Fig. 4 Sugaring-out effects of different types of sugars on the partition coefficients of compound 7 in the 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 (v/v) HEMWat system. 5.5 (v/v) HEMWat system. | ||
The sugaring-out effects may be because sugars compete for hydrogen bonding with the selected analytes. As the sugar content increases and the possibility of the compounds forming hydrogen bonds with water decrease, analytes are “pushed” into the organic phase. Similar phenomena were previously observed in the analyses of gas/solution partition coefficients of volatile compounds,30,33 where the relative changes in the headspace concentrations of volatiles were linearly dependent on the concentrations of sucrose.33 Although similar trends were found in Fig. 2–4 and S4,† the influences of sugars on the analytes in the two-phase solvent systems were not related to previously reported effects30,33 of sugars on the volatiles in a pure water phase (gas/solution equilibrium) because the two-phase solvents systems were composed of multiple solvent components, i.e., HEMWat systems contained four components ranging from hydrophobic hexane to hydrophilic methanol.
A previous study30 indicated that sucrose could push the hydrophobic molecules such as ethyl acetate, methyl butanoate, and ethyl butanoate dose-dependently out of water ranging from a low dose (5–20%) to an extremely high sucrose concentration (60%). However, for hexanal, a similar sucrose dose-dependent increase of the gas/solution partition coefficients was just shown in a sucrose concentration range of 0–40%, while at an extremely high sucrose concentrations (60%), the trend was inverted. Especially for octanal, the trend was thoroughly inverted, and sucrose could not increase the gas/solution partition coefficient of octanal at any dose of sucrose (0–60%).
In this work, each liquid phase, whatever the upper organic phase and lower aqueous phase, contains more or less hydrophobic hexane/ethyl acetate and hydrophilic methanol/water.14,24,34 Therefore, when sugars were added into the HEMWat two-phase solvent systems, it was possible that the sugars not only exerted the sugaring-out effect for analytes but also could push some components of the solvent system away from the lower phase toward the upper phase, resulting in a new two-phase equilibrium and changes in the polarity of the entire solvent system.
As shown in Fig. S6A and Table S2,† the volume of the lower phase was reduced significantly when 10% or 20% sucrose was added into the HEMWat system, which implies changes in the polarities and components of the two phases of the newly equilibrated system. In addition, these changes were very similar to the changes in salting-out methods using NaCl (Fig. S6B and D†), which further confirmed a similar mechanism between the sugaring-out and salting-out methods, where both competed for hydrogen bonding with the analytes. However, at an extremely high sucrose concentration (30%), the volumes of the two phases of the system were not further changed (Fig. S6A and C†), while when using NaCl as a salting-out agent (Fig. S6B and D†), an inverted effect was not observed, implying that sugars play diverse roles besides the sugaring-out mechanism.
3.2. Validation of sugaring-out effects by CCC
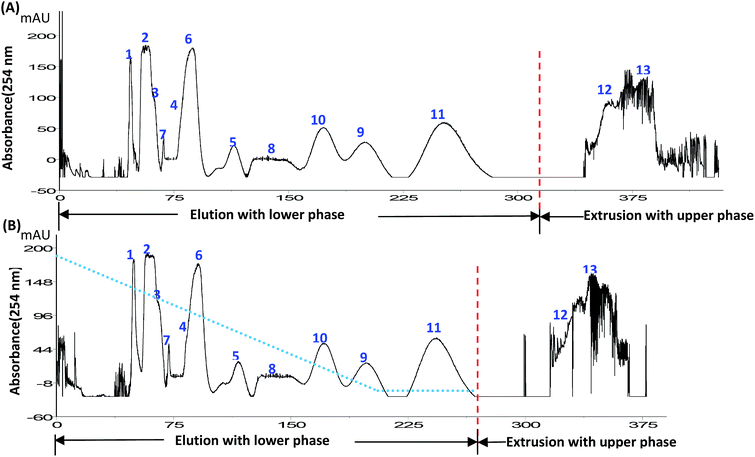
The above experiments showed that sugars can exert potent sugaring-out effects on the selected compounds in the HEMWat systems. Further CCC experiments also showed the same trends. As shown in Fig. 5, the elution time of the largest component was delayed significantly (Fig. 5B), which was in close agreement with the partition coefficient results (Fig. 2 and Table 2) that the K values of the most of the components increased when sucrose was added into the HEMWat 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
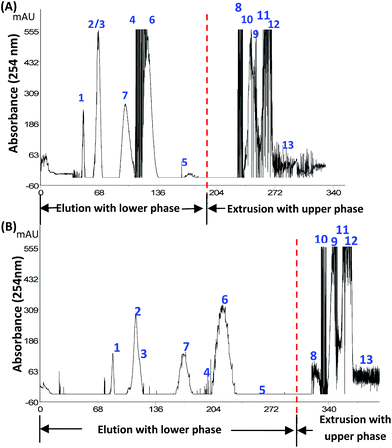
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 system (20% sucrose aqueous solution). Components 1–7 were well resolved, although the increasing K values by the addition of sugars resulted in that the peak of minor compound 5 became too broad and was not observable (Fig. 5B). The resolution of peaks 6 and 7 increased from 3.2 to 5.5.
6 system (20% sucrose aqueous solution). Components 1–7 were well resolved, although the increasing K values by the addition of sugars resulted in that the peak of minor compound 5 became too broad and was not observable (Fig. 5B). The resolution of peaks 6 and 7 increased from 3.2 to 5.5.
In addition, although there was a minor change in the viscosity of the aqueous phase,30 the settling time of the two-phase HEMWat solvent system was shortened, resulting in a remarkable increase of the retention of the stationary phase. With the addition of sucrose into the two-phase solvent system, a 64% retention of the stationary phase could be obtained, while the retention without sucrose was only 55.6%. Increases in the K values and the retention of stationary phase were both beneficial for CCC separation. Of course, for the compounds with large K values such as components 8–13, the K values were increased more sharply and thus retained in the column for a longer time until finally being extruded.
Furthermore, we checked if sucrose and glucose may play different roles in the separation of the D. versipellis extract. CCC experiments in Fig. 6 showed that glucose (20%) was better than sucrose (20%) in resolving components 9, 10 and 11, although some of the compounds were not well resolved in the HEMWat 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 system. In addition, similar to in the above CCC experiment (Fig. 5), the retention of the stationary phase after adding sucrose or glucose into the HEMWat 5
5 system. In addition, similar to in the above CCC experiment (Fig. 5), the retention of the stationary phase after adding sucrose or glucose into the HEMWat 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 system was still better than in the system without sugar. Using glucose (20%) as an additive, the elution time of several compounds such as 10, 11, and 12 were significantly delayed, which is closely in agreement with the K value analysis (Fig. S3†). Glucose was better than sucrose in increasing the partition coefficients of some components.
5 system was still better than in the system without sugar. Using glucose (20%) as an additive, the elution time of several compounds such as 10, 11, and 12 were significantly delayed, which is closely in agreement with the K value analysis (Fig. S3†). Glucose was better than sucrose in increasing the partition coefficients of some components.
In addition, further CCC experiments also confirmed the inverted effects of sugars as observed in Fig. 2–4 after adding high sugar concentrations (30%). In the HEMWat 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
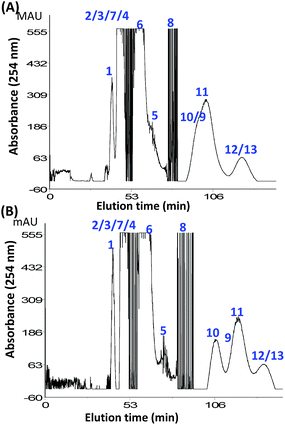
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 system with the addition of 30% sucrose (Fig. S7B†), the elution time of most of the components were less than in systems with the addition of 20% sugar (Fig. 7). Of course, some of the compounds, such as 9 and 11, could be well resolved even with the presence of inverted effects. This may be an interesting mechanism of “sugaring-in” with a high sugar dose, which requires further studies.
5.5 system with the addition of 30% sucrose (Fig. S7B†), the elution time of most of the components were less than in systems with the addition of 20% sugar (Fig. 7). Of course, some of the compounds, such as 9 and 11, could be well resolved even with the presence of inverted effects. This may be an interesting mechanism of “sugaring-in” with a high sugar dose, which requires further studies.
3.3. Preparative separation with the sugaring-out CCC method
As shown in Fig. 7, preparative CCC separation was performed using the HEMWat 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 (v/v) system with 20% sucrose as an additive. The elution times were significantly delayed, and the resolution of the peaks was significantly improved compared with the system without sucrose (Fig. S7A†). Almost all of the targeted components could be isolated and purified except for the extruded hydrophilic components 12 and 13 with 85% purity. It is interesting to find that with the addition of sucrose into the HEMWat 4
5.5 (v/v) system with 20% sucrose as an additive. The elution times were significantly delayed, and the resolution of the peaks was significantly improved compared with the system without sucrose (Fig. S7A†). Almost all of the targeted components could be isolated and purified except for the extruded hydrophilic components 12 and 13 with 85% purity. It is interesting to find that with the addition of sucrose into the HEMWat 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (v/v) (Fig. 5) and 4.5
6 (v/v) (Fig. 5) and 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 (v/v) systems (Fig. 7 and S7†), the noise of the chromatograms was greatly decreased, implying that sucrose might have another role in the CCC separation besides the sugaring-out effects.
5.5 (v/v) systems (Fig. 7 and S7†), the noise of the chromatograms was greatly decreased, implying that sucrose might have another role in the CCC separation besides the sugaring-out effects.
The above CCC experiments in Fig. 6 showed that glucose (20%) was better than sucrose (20%) in resolving components 9, 10 and 11. Since some of the compounds were not well resolved in the HEMWat 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 system, we then performed the CCC experiments with the addition of glucose in the HEMWat 4.5
5 system, we then performed the CCC experiments with the addition of glucose in the HEMWat 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5
5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5
4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 system, as shown in Fig. S8.† The results also confirmed that sucrose and glucose played different roles for the separation of the D. versipellis extract, and glucose was better than sucrose in increasing the partition coefficients of some components. Additionally, the inverted effects observed in Fig. 2–4 after adding high sugar concentrations (30%) were again confirmed.
5.5 system, as shown in Fig. S8.† The results also confirmed that sucrose and glucose played different roles for the separation of the D. versipellis extract, and glucose was better than sucrose in increasing the partition coefficients of some components. Additionally, the inverted effects observed in Fig. 2–4 after adding high sugar concentrations (30%) were again confirmed.
To save sugar consumption, a linear sucrose gradient CCC experiment was performed. As shown in Fig. 7B (sucrose-gradient) and S8C† (glucose-gradient), it can also resolve most of the components well but requires less solvents, sugars, and time. Gradient solvent elution is a simple and efficient elution mode. It is possible to save more sugars and achieve the same resolution if using multiple steps of a linear gradient or step-gradient elution.31,35,36
3.4. Recoveries of the components from the eluted fractions
As demonstrated above, sugars as additives could improve the partition coefficients and resolution of some components in the desired two-phase systems as well as help us obtain better separation with much easier solvent system selection. However, the added sugars also exist in the collected CCC fractions. Thus, the method cannot be applied for ELSD detection for the separation of samples that do not absorb UV. In addition, the sugaring-out strategy requires extra work to remove the added sugars after the CCC separation compared to the classical separation method.In this paper, we used two methods with the purpose of removing the sugars. The first is dry extraction by ethyl acetate for the components with low polarities. To be specific, we lyophilized the pure components directly obtained from the CCC fractions and then extracted them with ethyl acetate five times as completely as possible. The other method is reversed-phase liquid chromatography (RPLC) fractionation using a C18 column. Methanol (A) and water (B) were used as the mobile phase. At first, the sugar was eluted by water, and then, the pure compounds were eluted by methanol.
To evaluate the effects for removing the sugars, sensitive positive and negative ESI-MS analyses were performed. All purified compounds of CCC effluents before and after removing the sugars were analysed by ESI-MS. As shown in Fig. 8A and D, sucrose had a strong positive complex ion [sucrose + Na]+ at m/z 365 in the CCC fraction without removing the sugars. However, the ion was not detected after removing the sugars (Fig. 8B, C, E and F), which indicated that the sugar was completely removed. Fig. S9† shows the representative HPLC analyses of purified components by CCC separation with 20% sugar. If using similar on-line hyphenated CCC-LC techniques to desalt in salting-out methods,16,29 it might be easier to remove sugars from sugaring-out CCC fractions.
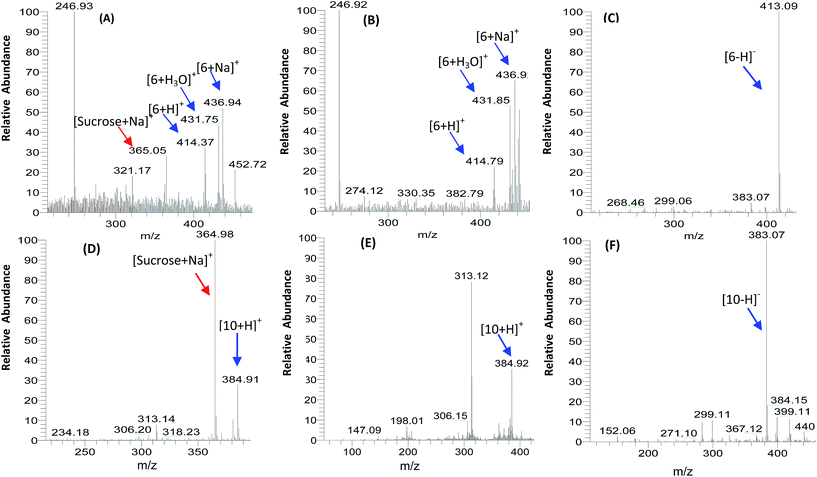 | ||
| Fig. 8 The representative ESI-MS profiles of CCC fractions (A and D) before removing sugars and (B, C, E, and F) after: (A–C) for compound 6 and (D–F) for compound 10. | ||
4. Conclusion
A successful CCC separation largely depends on correct solvent selection. In this work, we presented a sugaring-out strategy for the counter-current chromatographic separation of natural products by adding a dose of sugars into the two-phase solvent systems. Sugars are very common in nature and widely used in foods. They may help to quickly obtain a suitable solvent system. Indeed, if a two-phase solvent system is found to have favourable K values (“sweet spot”) after adding sugars, the system may be called a genuine “sweet solvent system” because sugars are sweet short-chain soluble carbohydrates. In contrast to the “salting-out” strategy using salts, the “sugaring-out” strategy using sugars is greener and more environmentally friendly, and it does not produce some of the complications of salty corrosion.It should be pointed that sugars can be classified into at least two types: aldoses containing aldehyde groups and ketoses containing ketone groups. The aldehyde group is a reactive group and can easily react with many compounds. Therefore, using aldoses such as glucose and maltose as additives should take into account the possible reactivity between the aldoses and targets, although sometimes some aldoses, i.e., glucose, showed different sugaring-out effects. In contrast, ketoses, i.e., sucrose, are very inert and can be suitable for comprehensive non-target and target separation.
In addition, the K value is still a golden criterion for solvent selection. If the hydrophobicity of a primary selected solvent system, i.e., the HEMWat 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 system, is too small, resulting in too small of K values of most of the compounds, the sugaring-out effects of sugars will be limited. In this case, changing the solvent system is essential.
5 system, is too small, resulting in too small of K values of most of the compounds, the sugaring-out effects of sugars will be limited. In this case, changing the solvent system is essential.
In general, the sugaring-out strategy using sugars as modifiers provides cheap and efficient methods for optimizing the solvent system and simplifying the CCC separation of single or multiple targets of natural products. Once a beginning solvent system is selected, the sugar (i.e., 5–20% sucrose aqueous solution) may play a key role in increasing the partition coefficients of targets and improve the CCC separation. Therefore, the sugaring-out strategy can be used as an alternative method for the rapid optimization of CCC solvent systems.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (grant no. 21272209 and 21672188) and Zhejiang Province (grant no. LY16B020004). Part of this research has been presented orally at the 9th international conference on counter-current chromatography CCC2016 held at Dominican University, Chicago, IL, USA in August 2016, which was successfully organized by Prof. J. B. Friesen and G. F. Pauli. We are also indebted to the responses and discussion with colleagues attending this biennial CCC meeting. We also thank at least three anonymous reviewers for their critical suggestions.References
- J. W. H. Li and J. C. Vederas, Science, 2009, 325, 161–165 CrossRef PubMed.
- M. S. Butler, A. A. B. Robertson and M. A. Cooper, Nat. Prod. Rep., 2014, 31, 1612–1661 RSC.
- B. B. Mishra and V. K. Tiwari, Eur. J. Med. Chem., 2011, 46, 4769–4807 CrossRef CAS PubMed.
- F. Bucar, A. Wube and M. Schmid, Nat. Prod. Rep., 2013, 30, 525–545 RSC.
- Z. Yang, Y. Wu and S. Wu, J. Chromatogr. A, 2016, 1431, 184–196 CrossRef CAS PubMed.
- Y. Ito, J. Chromatogr. A, 2005, 1065, 145–168 CrossRef CAS PubMed.
- S. Wu and J. Liang, Comb. Chem. High Throughput Screening, 2010, 13, 932–942 CrossRef CAS PubMed.
- J. B. Friesen, J. B. McAlpine, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2015, 78, 1765–1796 CrossRef CAS PubMed.
- I. A. Sutherland and D. Fisher, J. Chromatogr. A, 2009, 1216, 740–753 CrossRef CAS PubMed.
- A. Berthod, M. J. Ruiz-Angel and S. Carda-Broch, J. Chromatogr. A, 2009, 1216, 4206–4217 CrossRef CAS PubMed.
- G. F. Pauli, S. M. Pro and J. B. Friesen, J. Nat. Prod., 2008, 71, 1489–1508 CrossRef CAS PubMed.
- A. Berthod, M. Hassoun and M. J. Ruiz-Angel, Anal. Bioanal. Chem., 2005, 383, 327–340 CrossRef CAS PubMed.
- J. B. Friesen and G. F. Pauli, J. Liq. Chromatogr. Relat. Technol., 2005, 28, 2777–2806 CrossRef CAS.
- J. Liang, J. Meng, D. Wu, M. Guo and S. Wu, J. Chromatogr. A, 2015, 1400, 27–39 CrossRef CAS PubMed.
- Z. Yang, X. Hu and S. Wu, J. Sep. Sci., 2016, 39, 732–740 CrossRef CAS PubMed.
- M. Guo, J. Liang and S. Wu, J. Chromatogr. A, 2010, 1217, 5398–5406 CrossRef CAS PubMed.
- R. R. Romero-Gonzalez and R. Verpoorte, J. Chromatogr. A, 2009, 1216, 4245–4251 CrossRef CAS PubMed.
- F. Bezold, J. Goll and M. Minceva, J. Chromatogr. A, 2015, 1388, 126–132 CrossRef CAS PubMed.
- A. Berthod, M. J. Ruiz-Ángel and S. Carda-Broch, J. Chromatogr. A, 2008, 1184, 6–18 CrossRef CAS PubMed.
- A. Berthod and S. Carda-Broch, Anal. Bioanal. Chem., 2004, 380, 168–177 CrossRef CAS PubMed.
- J. V. Marques, K. W. Kim, C. Lee, M. A. Costa, G. D. May, J. A. Crow, L. B. Davin and N. G. Lewis, J. Biol. Chem., 2012, 288, 466–479 CrossRef PubMed.
- Y. Zhou, S.-Y. Jiang, L.-S. Ding, S.-W. Cheng, H.-X. Xu, P. P.-H. But and P.-C. Shaw, Chromatographia, 2008, 68, 781–789 CAS.
- Z. Yang, Y. Wu, H. Zhou, X. Cao, X. Jiang, K. Wang and S. Wu, RSC Adv., 2015, 5, 77553–77564 RSC.
- D. Wu, X. Jiang and S. Wu, J. Sep. Sci., 2010, 33, 67–73 CrossRef CAS PubMed.
- L. Zhang, Y. Wang, X. Guo and S. Wu, The 9th International Conference on Counter-current Chromatography held at Dominican University, Chicago, USA, 1–3 August 2016 Search PubMed.
- J. Meng, Z. Yang, J. L. Liang, M. Z. Guo and S. H. Wu, J. Chromatogr. A, 2014, 1327, 27–38 CrossRef CAS PubMed.
- D. Wu, X. Cao and S. Wu, J. Chromatogr. A, 2012, 1223, 53–63 CrossRef CAS PubMed.
- J. B. Friesent and G. F. Pauli, J. Agric. Food Chem., 2008, 56, 19–28 CrossRef PubMed.
- J. Liang, Z. Yang, X. Cao, B. Wu and S. Wu, J. Chromatogr. A, 2011, 1218, 6191–6199 CrossRef CAS PubMed.
- D. F. Nahon, M. Harrison and J. P. Roozen, J. Agric. Food Chem., 2000, 48, 1278–1284 CrossRef CAS PubMed.
- Z. Yang, X. Liu, K. Wang, X. Cao and S. Wu, J. Sep. Sci., 2013, 36, 1022–1028 CrossRef CAS PubMed.
- S. Carda-Broch and A. Berthod, J. Chromatogr. A, 2003, 995, 55–66 CrossRef CAS PubMed.
- E. N. Friel, R. S. T. Linforth and A. J. Taylor, Food Chem., 2000, 71, 309–317 CrossRef CAS.
- Y. B. Lu, A. Berthod, R. L. Hu, W. Y. Ma and Y. J. Pan, Anal. Chem., 2009, 81, 4048–4059 CrossRef CAS PubMed.
- S. Wu, D. Wu, J. Liang and A. Berthod, J. Sep. Sci., 2012, 35, 964–976 CrossRef CAS PubMed.
- S. Wu, J. Liang and A. Berthod, J. Chromatogr. A, 2013, 1274, 77–81 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra27838h |
| This journal is © The Royal Society of Chemistry 2017 |