TCM, brain function and drug space
Chunping
Tang
abc,
Yang
Ye
bc,
Yunjiang
Feng
ac and
Ronald J.
Quinn
*ac
aEskitis Institute for Drug Discovery, Griffith University, Brisbane, QLD 4111, Australia. E-mail: r.quinn@griffith.edu.au
bState Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
cEskitis-SIMM Joint Laboratory for Drug Discovery, Australia
First published on 19th November 2015
Abstract
Covering: up to 2015
Traditional Chinese medicine has played a significant role in the mainstream healthcare system in China for thousands of years. Here, we summarize 84 major compounds from 15 selected herbal medicines targeting neurodegenerative diseases. We present a perspective based on the analysis of physicochemical properties of these TCM compounds, and comparison with current drugs and candidates for the treatment of Parkinson's and Alzheimer's disease. The results demonstrate that traditional Chinese medicines contain compounds possessing physicochemical properties that have excellent overlap with developed western medicines.
1 Introduction
Traditional Chinese Medicine (TCM) has played a significant role in the mainstream healthcare system in China for thousands of years. It has its own theoretical basis and practical application. The comprehensive system of TCM features the use of large numbers of herbal medicines, which have been recorded scrupulously and precisely in a number of ancient texts. For example, the Compendium of Materia Medica, one of the most famous classics written in the Ming dynasty (∼1590 AD), recorded 1892 drugs with 1095 of them being herbal medicines (58%). Herbal medicines, as well as animal and mineral medicines, have also been documented in the Chinese Pharmacopeia since 1953, with the number increasing from 78 in the first version to 1146 in the 2005 version.Based on such accumulated knowledge, the chance to identify biologically active small molecules from TCMs is higher in comparison with random collection and screening. One of the success stories is the discovery of artemisinin (qinghaosu) from Artemisia annua as an effective anti-malaria therapy that has saved millions of lives around the world. Youyou Tu, “for her discoveries concerning a novel therapy against Malaria” (nobelprize.org), shared the Nobel Prize in Physiology or Medicine 2015 with two other scientists. She narrated the story about the discovery of artemisinin and its development into a drug in Nature Medicine.1 She said the medicinal herb Qinghao was one of the most frequently used herbs for the treatment of malaria after investigating more than 2000 Chinese herbal prescriptions. Artimisinin is “a true gift from old Chinese medicine”.1 Another representative example is depside salts from Salvia miltiorrhiza, a traditional herb known as “Danshen”. Depside salts is a mixture in which magnesium lithospermate B and its 5 analogs are the active components.2 China's State Food and Drug Administration (SFDA) granted a new drug license for this mixture and its injectable form in May 2005 for the treatment of angina and other cardiovascular diseases.
These two cases provide a glimpse of successful drugs derived from traditional medicines. Professor Zhibi Hu, a member of the Chinese Academy of Engineering, said in Nature Biotechnology, “This success means China's biopharmaceutical industry can develop innovative drugs by investigating the chemical ingredients of TCMs whose clinical effects have long been observed. Compared with developing new compounds from scratch, the approach is potentially more rapid and less expensive”.3
Although the successful stories have shown that therapeutic molecules can be derived from TCMs, the majority of the traditional medicines are still waiting to be studied. The chemicals responsible for the therapeutic effects, that have the potential to be leads, need thorough and in-depth investigations from multiple perspectives. Herein, we review the literature and classical Chinese medicinal books to search for commonly used herbal medicines applied to neurodegenerative diseases. Major and bioactive compounds identified from these herbs are summarized. Their physicochemical properties are analyzed and compared with anti-Parkinson's and anti-Alzheimer's disease drugs launched and in clinical trial in order to assess the drug potential.
2 TCM herbs targeting neurodegenerative diseases and the major bioactive compounds
Neurodegenerative disease is an umbrella term for a range of conditions that primarily affect the neurons in the human brain such as Parkinson's (PD), Alzheimer's (AD), and Huntington's disease (HD). The global prevalence of dementia in people aged over 60 years was estimated to be over 24 million in 2001 and is expected to double every 20 years.4 With the increase in the aging population, neurodegenerative diseases are becoming a major social and economic burden worldwide. There is an urgent need to develop more effective therapeutic strategies for neurodegenerative disease.In Traditional Chinese Medicine, there are no specific terms corresponding exactly to PD, AD or HD. However, many herbs are recorded to treat symptoms like tremor, muscle rigidity, forgetfulness, insomnia, and dementia. It is commonly believed that these symptoms are due to brain degeneration with aging, and therefore are directly related to neurodegenerative diseases.
Many TCM formulae have been used for hundreds or even thousands of years to treat these symptoms and continue to be used clinically. For example, Tianma Gouteng Decoction, a formula comprising 11 herbs, was firstly recorded in Xiaoer Weisheng Zongwei Lunfang, a medicinal book published in 1156. He et al. reported in 2010 that Tianma Gouteng Decoction significantly restrained the apoptosis of dopaminergic neurons in rats with Parkinson's disease and its mechanism may be explained by increasing expression of B-cell lymphoma 2 (Bcl-2) and restraining activation of Bax through anti-oxidative stress.5 The results suggested that the formula has potential in the treatment of Parkinson's disease.
2.1 TCM herbs targeting neurodegenerative diseases
Some herbal medicines are frequently used in these formulae, particularly as the monarch (principal) drug in a prescription. Hu et al. collected 132 prescriptions for treating senile dementia or AD. There were 150 herbs used in these prescriptions, and the top 10 most frequently used were Acori Tatarinowii Rhizoma, Chuanxiong Rhizoma, Astragali Radix, Polygoni Multiflori Radix, Ginseng Radix et Rhizoma, Salviae Miltiorrhizae Radix et Rhizoma, Polygalae Radix, Rehmanniae Radix Preparata, Poria, and Lycii Fructus.6 Su et al. found 157 herbs used in prescriptions for the treatment of relevant symptoms of neurodegenerative diseases. The top 8 of the most frequently used herbs were Paeoniae Radix Alba, Ramulus Uncariae Cum Uncis, Gastrodiae Rhizoma, Rehmanniae Radix Preparata, Glycyrrhizae Radix et Rhizoma, Angelicae Sinensis Radix, Chuanxiong Rhizoma, and Salviae Miltiorrhizae Radix et Rhizoma.7According to the literature and the formulae recorded in the medicinal classics, we selected 15 commonly used TCMs, which are frequently present in formulae to treat neurodegenerative diseases. The detailed information about the selected herbal medicines is shown in Table 1.
| No | Chinese name | Pinyin | English name | Original plant(s) |
|---|---|---|---|---|
| a All information is presented according to the Chinese Pharmacopoeia of the 2010 version. The herbs are denoted by the English name and Pinyin in the text, in order to be distinguished from the Latin names of the original plants. | ||||
| 1 |
|
Renshen | Ginseng Radix et Rhizoma | Panax ginseng C. A. Mey |
| 2 |
|
Dangshen | Codonopsis Radix | Codonopsis pilosula (Franch.) Nannf, Codonopsis pilosula Nannf. var. modesta (Nannf.) L. T. Shen; Codonopsis tangshen Oliv. |
| 3 |

|
Baishao | Paeoniae Radix Alba | Paeonia lactiflora Pall. |
| 4 |

|
Heshouwu | Polygoni Multiflori Radix | Polygonum multiflorum Thunb. |
| 5 |
|
Shudihuang | Rehmanniae Radix Praeparata | Rehmannia glutinosa Libosch. |
| 6 |
|
Tianma | Gastrodiae Rhizoma | Gastrodia elata Bl. |
| 7 |
|
Gouteng | Ramulus Uncariae Cum Uncis | Uncaria rhynchophylla (Miq.) Miq. ex Hail. Uncaria macrophylla Wall. Uncaria hirsute Havil. Uncaria sinensis (Oliv.) Havil. Uncaria sessilifructus Roxb. |
| 8 |

|
Chuanxiong | Chuanxiong Rhizoma | Ligusticum chuanxiong Hort. |
| 9 |
|
Danshen | Salviae Miltiorrhizae Radix et Rhizoma | Salvia miltiorrhiza Bge. |
| 10 |

|
Shichangpu | Acori Tatarinowii Rhizoma | Acorus tatarinowii Schott |
| 11 |

|
Yuanzhi | Polygalae Radix | Polygala tenuifolia Willd. Polygala sibirica L. |
| 12 |
|
Suanzaoren | Ziziphi Spinosae Semen | Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou |
| 13 |
|
Baiziren | Platycladi Semen | Platycladus orientalis (L.) Franco |
| 14 |
|
Gegen | Puerariae Lobatae Radix | Pueraria lobata (Willd.) Ohwi |
| 15 |

|
Doukou | Amomi Fructus Rotundus | Amomum kravanh Pierre ex Gagnep, Amomum compactum Soland ex Maton |
2.2 Major compounds from the selected TCM herbs
From the selected 15 herbal medicines, a variety of compounds have been reported. We list 84 compounds, which are either the major compounds existing in the plants or have relevant bioactivities to brain-related diseases including AD, PD.Ginseng Radix et Rhizoma (Renshen) is one of the most famous Chinese medicines and has been used for thousands of years with significant functions of strengthening the body resistance, consolidating the constitution, improving the cognitive functions, and prolonging life.
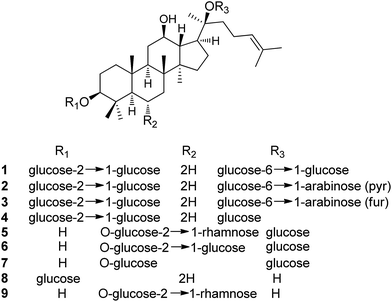
Ginsenosides, a series of dammarane or oleanane type triterpenoid glycosides, are believed to be the pharmacologically active ingredients in ginseng.8 The Ginseng Evaluation Program of the American Botanical Council recommended seven major components, Rb1, Rb2, Rc, Rd, Re, Rf and Rg1 (1–7), for quality control of Ginseng and its related species. In the Chinese Pharmacopeia, ginsenosides Rb1, Re, Rf and Rg1 are used as standard compounds for identification and quality control of the raw material.
The effects of ginseng extracts and ginsenosides are relevant to a variety of diseases. Ginseng was reported to have potentially positive effects on heart disease through its various properties including antioxidation, reduced platelet adhesion, vasomotor regulation, improving lipid profiles, and influencing various ion channels.9–11 Lü et al. reviewed the bioactive effects of ginsenosides on antioxidation, the vascular system, signal transduction pathways and interaction with receptors, and their therapeutic applications in animal models and humans as well as the pharmacokinetics and toxicity of ginsenosides.12
A number of publications reported the effects of ginsenosides on the central nervous system (CNS) and the peripheral nervous system by regulating various types of ion channels.13,14 Ginsenoside Rg3 regulates voltage-gated ion channels such as Ca2+, K+, and Na+ channels, and ligand-gated ion channels such as GABAA, 5-HT3, nicotinic acetylcholine, and N-methyl-D-aspartate (NMDA) receptors through interactions with various sites including channel blocker binding sites, toxin-binding sites, channel gating regions, and allosteric channel regulator binding sites with the respective ion channels or receptors.15 Rg1 can increase proliferation and differentiation of neural progenitor cells in dentate gyrus of the hippocampus of normal adult mice and the global ischemia model in gerbils.16 Ginsenoside Rh2 (8) was reported to reverse memory impairment caused by scopolamine in mice,17 to increase pituitary adenylate cyclase-activating polypeptide (PACAP), to activate a PACAP selective receptor (PAC1), and to attenuate amyloid β (Aβ)-induced toxicity.18 In a vascular dementia (VD) rat model, ginsenoside Rg2 (9) was able to improve neurological performance and memory ability of VD rats through mechanisms related to anti-apoptosis.19
Pharmacokinetic studies by oral administration of Ginseng extract or ginsenosides indicated that the major metabolites detectable in plasma or in urine involved several compounds such as ginsenosides K, F1, Rb1, Rb3, Rd, Rg2, Rg3, Rh1, Rh2, protopanaxadiol, and protopananatriol. Compound K, for example, is a major metabolite of the ginsenosides present in P. ginseng extracts.20
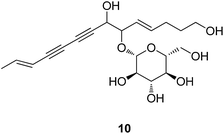
Codonopsis Radix (Dangshen) has been traditionally used as a tonic and a substitute of Ginseng although its therapeutic efficacy is much lower than Ginseng. Its chemical constituents do not contain the ginsenosides, but mainly carbenes, atractylenolides, phenylpropanoids, alkaloids, triterpenoids, and polysaccharides. Lobetyolin (10) exists in all the original plants, and is documented in the Chinese Pharmacopeia to be a standard compound for the identification of the raw material. It was reported that lobetyolin was able to trigger the transcriptional activity of NF-κB, which is related to neuro cell growth.21
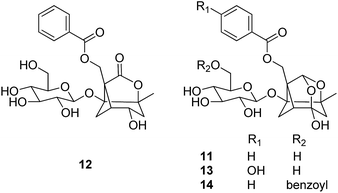
Monoterpene glucosides are an important class of bioactive compounds identified from Paeoniae Radix Alba (Baishao). Major compounds include paeoniflorin (11), albiflorin (12), oxypaeoniflorin (13), and benzoylpaeoniflorin (14).22 The content of paeoniflorin (11) is greater than 70% of the total glucoside extract, and is used as a chemical marker for the identification and content determination of the raw material in the Chinese Pharmacopeia.
Paeoniflorin showed neuroprotective effects against glutamate (Glu)-induced neurotoxicity in PC12 cells through amelioration of the reduction of cell viability, nuclear and mitochondrial apoptotic alteration, reactive oxygen species (ROS) accumulation, and Bcl-2/Bax ratio.23,24 Paeoniflorin could also protect PC12 cells against MPP+- or acid-induced injury associated with the upregulation of LC3-II protein.25 Wang et al. found that paeoniflorin could attenuate or restore the viability loss, apoptotic increase, and ROS production induced by Aβ25–35 in SH-SY5Y cells, and strikingly inhibit Aβ25–35-induced mitochondrial dysfunction.26
Paeoniflorin (11) showed extremely poor absorption and low bioavailability when orally administrated, but it was found to be transformed to a major metabolite paeonimetabolin I in high percentage by intestinal bacteria and absorbed rapidly from the gastrointestinal tract. A high concentration of paeonimetabolin I rather than paeoniflorin was detected in the rat plasma.27 Another investigation of oral administration to rats with the decoction of Baishao revealed that paeoniflorin was not absorbed per se, whereas its aglycone paeoniflorgenin was absorbed and circulating in the bloodstream.28
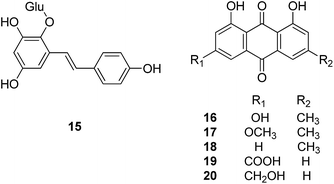
Stilbene glucosides, anthraquinones and phospholipids are three major classes of compounds isolated from Polygoni Multiflori Radix (Heshouwu). 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucopyranoside (15), the main stilbene glucoside present in the plant material, is used in the Chinese Pharmacopeia as quality control of the raw material, together with two anthraquinones, emodin (16) and physcion (17). Chrysophanol (18), rhein (19) and aloeemodin (20) are other major anthraquinones identified from this plant.
Compound 15 was reported to attenuate MPP+-induced apoptosis in PC12 cells by inhibiting ROS generation, modulating JNK activation, and improving mitochondrial function.29,30 It could also reduce LPS-induced microglia-derived release of proinflammatory factors such as TNF-α, IL-1β, and NO.31 The pharmacokinetic profile of 15 in rat plasma and tissues after oral administration showed that compound 15 was rapidly absorbed and widely distributed throughout the body, followed by quick elimination. The highest levels were detected in liver and lung whereas there was little in brain and testes, indicating 15 cannot readily penetrate the blood–brain and blood–testicle barriers.32
As one major class of compounds widely existing in many plants, anthraquinones were found to inhibit insulin, amyloid and tau aggregations, which are linked directly to a number of neurodegenerative syndromes.33–35
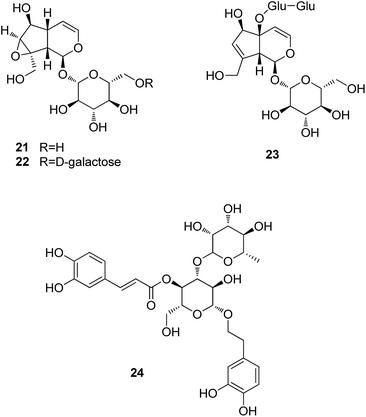
Glycosides are the most important constituents identified from Rehmanniae Radix Praeparata (Shudihuang), particularly iridoid glycosides. Catalpol (21), rehmannioside A (22), and rehmannioside D (23) are three major iridoid glycosides isolated from this plant. Verbascoside (24, also known as acteoside), the major phenolic glycoside, is used for the identification of the authentic material in the Chinese Pharmacopeia.
Pretreatment by catalpol (21) protected dopaminergic neurons against LPS-induced neurotoxicity,36 and attenuated the Aβ1–42-triggered neurotoxicity to neurons and inhibited glial activation.37 Catalpol also showed neuroprotective effect against MPP+-induced oxidative stress in mesencephalic neurons.38
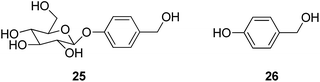
p-Hydroxybenzyl derivatives are the principle constituents identified from Gastrodiae Rhizoma (Tianma), represented by two major compounds, gastrodin (25) and gastrodigenin (p-hydroxybenzyl alcohol, 26).
Dai et al. reported that gastrodin significantly attenuated levels of neurotoxic proinflammatory mediators and proinflammatory cytokines by inhibition of the NF-κB signaling pathway and phosphorylation of MAPKs in LPS-stimulated microglial cells.39 Gastrodin also prevented glutamate-induced [Ca2+]i influx, blocked the activation of the calmodulin-dependent kinase II (CaMKII) and the apoptosis signaling-regulating kinase-1 (ASK-1), and inhibited phosphorylation of p38 mitogen-activated kinase (MAPK).40 Gastrodin showed neuroprotective effects in the subchronic MPTP mouse PD model by ameliorating bradykinesia and motor impairment in the pole and rotarod tests, respectively. Consistent with this finding, gastrodin prevented dopamine depletion and reduced reactive astrogliosis caused by MPTP in the substantiae nigrae and striatata of mice. Moreover, gastrodin was effective in preventing neuronal apoptosis by attenuating antioxidant and antiapoptotic activities in the brain.41
When gastrodin was administered via the femoral vein at a dose of 200 mg kg−1, its distribution in rat showed that levels of gastrodin declined rapidly after administration, and the entry of gastrodin into the brain was rapid. However, the ratios of AUCbrain/AUCplasma were not high. The AUC in the cerebellum was significantly higher than that in other brain regions such as CSF, frontal cortex, hippocampus, and thalamus. The concentrations of p-hydroxybenzyl alcohol, the main metabolite of gastrodin, were very low both in the CSF and plasma.42
Indole alkaloids, which are believed to be responsible for its therapeutic effects, are the most important type of constituents identified from Ramulus Uncariae Cum Uncis (Gouteng). Rhynchophylline (27) and isorhynchophylline (28) are two alkaloids present abundantly in all the original plants of the herbal material.
Xian et al. reported that rhynchophylline and isorhynchophylline significantly decreased Aβ25–35-induced cell death, intracellular calcium overload, and tau protein hyperphosphorylation in PC12 cells.43 These two compounds could also, concentration-dependently, attenuate LPS-induced production of pro-inflammatory cytokines such as TNF-α and IL-1β as well as NO in mouse N9 microglial cells through suppression of iNOS protein level, phosphorylation of ERK and p38 MAPKs, and degradation of IκBα.44,45
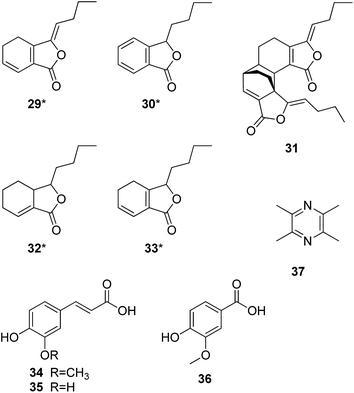
Chuanxiong Rhizoma (Chuanxiong) contains a high level of volatile oil, mainly lactone derivatives, including ligustilide (29†), butylphthalide (30), levistilide A (31), neocnidilide (32), and senkyunolide (33). The yield of ligustilide is more than 50%, but its structure is unstable. Levistilide A is therefore used in the Chinese Pharmacopeia as the chemical marker for the identification of the raw material. Phenolic acids are other major constituents responsible for its bioactivities. Ferulic acid (34), caffeic acid (35), and vanillic acid (36) are the major compounds of this type, and the level of ferulic acid in the raw material is used in the Chinese Pharmacopeia for its quality control. Ligustrazine (37) is the major alkaloid identified from the raw material.
Ligustilide was reported to exert neuroprotective effects against ischemia/reperfusion (I/R) injury by promoting EPO transcription via an ERK signalling pathway and inhibition of RTP801 expression.46 Ligustrazine and butylphthalide have been applied in practice in China to treat ischemic cerebrovascular diseases like cerebral arterial thrombosis. Butylphthalide showed effects on improving cognitive impairment and reducing amyloid-β proteins in a triple-transgenic AD mouse model.47 The long-term treatment with butylphthalide might prevent age-related neurodegenerative changes by modulation of the cholinergic system, cause reduction of phosphorylated tau and maintain structure and morphology of neurons.48 Phenolic acids are natural antioxidants with a wide range of therapeutic effects against various diseases including neurodegeneration. Ferulic acid was reported as a potent scavenger of ROS and reactive nitrogen species (RNS) and thereby reduced the chance of free radical attack on proteins and hence prevented their oxidative modification.49
Salviae Miltiorrhizae Radix et Rhizoma (Danshen) is one of the most famous Chinese traditional herbs with more than 1000 years of clinical application as a common hemorheologic agent to promote blood circulation, remove blood stasis, nourish the blood, and tranquilize the mind. Nowadays Danshen is mainly used for the treatment of cardiovascular and cerebrovascular diseases.50 Pharmaceutical investigations revealed multiple bioactivities of Danshen and its constituents such as antioxidative stress, antiplatelet aggregation, anti-inflammation, anti-cancer, and etc.51,52
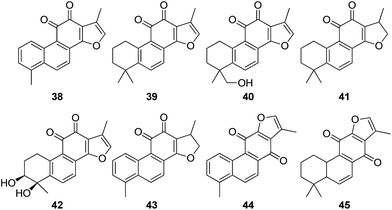
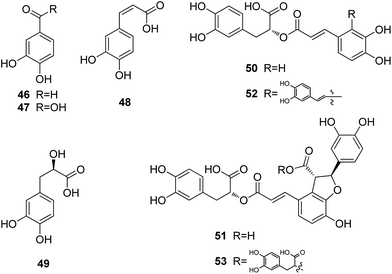
There are two groups of chemical constituents isolated from Danshen.53 One is the fat-soluble diterpene quinones, such as tanshinone I (38), tanshinone IIA (39), tanshinone IIB (40), cryptotanshinone (41), tanshindiol C (42), dihydrotanshinone I (43), isotanshinone I (44), and isotanshinone II (45). The other group is the water-soluble phenolic acids, including protocatechuic aldehyde (46), protocatechuic acid (47), caffeic acid (48), danshensu (49), rosmarinic acid (50), lithospermic acid (51), salviandic acid A (52), and salviandic acid B (53).
Han et al. reported in a review that the water soluble fraction of Salviae miltiorrhiza root extract, as well as water-soluble compounds, protocatechuic aldehyde (46), danshensu (49) and salvianolic acid B (53) have an ability to scavenge peroxides and are able to inhibit the expression of adhesion molecules in vascular endothelium and leukocytes. Moreover, lipophilic compounds from S. miltiorrhiza root extract also prevent the development of vascular damage. NADPH oxidase and platelet aggregation are inhibited by tanshinone IIA (52) and tanshinone IIB (53), respectively, and mast cell degranulation is hindered by cryptotanshinone (41) and 15,16-dihydrotanshinone I. Thus, the water-soluble and lipophilic compounds appear to improve the I/R-induced vascular damage multifactorially and synergistically.54
Depside salts from S. miltiorrhiza are an approved drug in China and its active components include magnesium lithospermate B and its analogues. A pharmacokinetic study of three major components, lithospermic acid B, rosmarinic acid, and lithospermic acid, showed that they are readily distributed to most tissues but cannot efficiently cross the blood–brain barrier. The elimination of the active components in blood is rapid and the major route of elimination of these compounds is excretion in the bile.2 Another metabolism and pharmacokinetic studies of lithospermic acid B on rat showed that lithospermic acid B was rapidly and extensively metabolized to its methylated metabolites, and these metabolites were detected in rat bile, plasma and feces samples after intravenous administration of lithospermic acid B.55
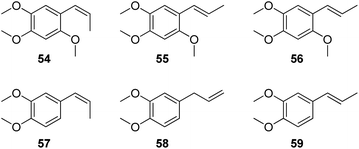
Volatile oil is believed to be the active principles of Acori Tatarinowii Rhizoma (Shichangpu). The major constituents identified from the volatile oil were β-asarone (54), α-asarone (55), γ-asarone (56), cis-methylisoeugenol (57), methyl eugenol (58), and trans-methylisoeugenol (59).56
β-Asarone (54) and α-asarone (55) were reported to be the chemical bases for its effects on sedation, convulsion, and depression. In asenescence-accelerated prone 8 (SAMP8) mice, which mimic many of the salient features of Alzheimer's disease, β-asarone prevented autophagy and synaptic loss by reducing ROCK expression, and improved cognitive function of the SAMP8 mice.57 Mo et al. reported that β-asarone could protect PC12 cells against OGD/R-induced injury partly due to attenuating beclin-1-dependent autophagy caused by decreasing [Ca2+]i and increasing MMP.58 α-Asarone exhibited antioxidant property against noise-stress-induced changes in different brain regions of model rats.59
Polygalae Radix (Yuanzhi) is rich in triterpenoid saponins, oligosaccharide esters, and xanthones. Taxifolin (60), 3,6′-disinapoyl sucrose (61), and polygalaxanthone III (62) are three compounds documented in the Chinese Pharmacopeia for identification and content determination of the original materials.
Chen et al. reported that oral administration of the crude saponins to rats could significantly improve learning and memory ability in the step-through test. The muscarinic receptor density and the activity of ChAT within rat brain were markedly enhanced, whereas the activity of AchE in rat brain was significantly inhibited.60 Hu et al. reported that 3,6′-disinapoyl sucrose could have neuroprotective effects and antidepressive activity in rats partially by increased expression of cyclic AMP response element (CRE)-binding protein (CREB) and its downstream target protein, brain-derived neurotrophic factor (BDNF). At concentrations above 30 μM, 3,6′-disinapoyl sucrose could promote the neuron cell viability and protect the glutamate and H2O2-induced toxicity in the human neuroblastoma (SH-SY5Y) cell line.61,62 Ikeya et al. pointed out that tenuifoliside B (63),63 one of the acylated oligosaccharides in the roots of P. tenuifolia, showed cerebral protective effect on potassium cyanide (KCN)-induced anoxia in mice, and also had an ameliorative effect on the scopolamine-induced impairment of performance in passive avoidance task in rats.64
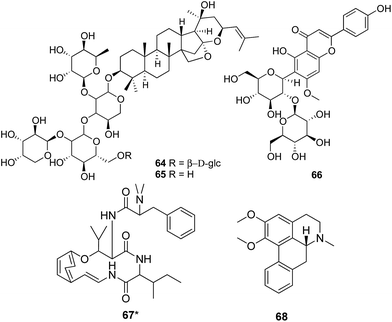
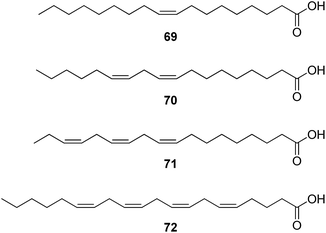
The chemical investigations of Ziziphi Spinosae Semen (Suanzaoren) revealed the presence of triterpenoid glycosides, flavonoid glycosides, alkaloids, and fatty oil. Jujubosides A (64) and B (65) are two major triterpenoids identified from the original plants and also documented in the Chinese Pharmacopeia as the chemical markers for the identification and content determination of the raw material. The flavonoid glycosides, spinosin (66) and its derivatives, are also used as chemical markers. The alkaloidal constituents include sanjoinine A (frangufoline, 67) and sanjoinine E (nuciferine, 68). More than 60% of the herbal material are fatty acids, of which 38.8% is oleic acid (69) and 37.1% is linoleic acid (70).65
Ziziphi Spinosae Semen has very unique effects on insomnia, which is directly associative with its sedative activity. The total triterpenoid glycoside,66 the total flavonoid glycoside,67 the total alkaloid,68 and the fatty oil fraction,69 all could significantly reduce spontaneous activity and increase sleeping time in mice. Jujuboside A was reported to have an inhibitory effect on the spontaneous activity in mice, and could regulate the transcription of Mark3 and Rpgrip1 in mouse hippocampus.70
Platycladi Semen (Baiziren) was reported to contain about 60% of fatty oil with 62.39% being unsaturated fatty acids.71 GC-MS analysis of the fatty oil further identified three major unsaturated fatty acids – linoleic acid (70), linolenic acid (71) and arachidonic acid (72).72
This herb was reported to ameliorate memory acquisition disorder induced by amygdala lesion in mice following oral administration.73 Linoleic and linolenic acid are essential for normal cellular function, and act as precursors for the synthesis of longer chained polyunsaturated fatty acids such as arachidonic acid. Neurodegenerative disorders such as PD and AD appear to exhibit membrane loss of polyunsaturated fatty acids. Supplementary of these essential fatty acids may help to delay their onset or reduce the insult to brain functions which these diseases elicit.74
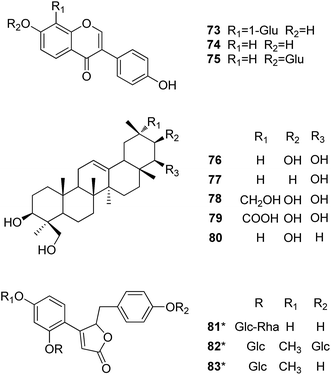
Puerariae Lobatae Radix (Gegen) is rich in isoflavones. Puerarin (73), daidzein (74), and daidzin (75) were identified in high yields from this material. Triterpenoids, another major constituent, are characterized by an oleanane type nucleus such as soyasapogenols A and B (76–77), and kudzusapogenols A–C (78–80). Puerosides A–C (81–83) represent dihydrochalcones isolated from this material.75
Puerarin (73) was reported to be a possible therapeutic agent in neurodegenerative diseases involving oxidative stress induced by ROS. Zhang et al. found that puerarin could inhibit oxidative-stress-induced apoptosis through down-regulation of Bax/Bcl-2 ratio.76 It can also protect differentiated PC12 cells from H2O2-induced apoptosis through activation of the PI3K/Akt signalling pathway.77 Wang et al. reported that puerarin protected PC12 cells against MPP+-induced neurotoxicity through the inhibition of the INK signalling pathways.78 In addition, pretreatment of primary hippocampal neurons with puerarin significantly reduced Aβ25–35-induced oxidative stress characterized by scavenging of ROS and inhibiting lipid peroxidation.79 Daidzein (74) was also reported to protect dopaminergic neurons against LPS-induced injury through inhibition of microglia activation and proinflammatory factors generation,80 and exhibit neurocytoprotective effects against 6-hydroxydopamine (6-OHDA)-induced cytotoxicity in nerve growth factor (NGF)-differentiated PC12 cells.81
Prasain et al. reported that puerarin was hydrolyzed to daidzein by bacterial enzymes in the large intestine and subsequently reduced to dihydrodaidzein and equol. The persistence of puerarin in blood and urine as the principal metabolic form during the period of 4–72 h after oral administration suggested that puerarin was rapidly absorbed from the intestine without metabolism. Its presence in organs such as the brain suggested that pueratin might enter tissues by specific transport pathways.82
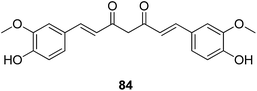
The investigations of the Amomum genus such as A. kravanh and A. compactum (Doukou) revealed the presence of diarylheptanoids, flavonoids, chalcones, and terpenoids.83 Curcumin (84) and its analogues diarylheptanoids are present in the family of Zingiberaceae in high yield. Ahmed and Gilani summarized the literature and found that the curcuminoid mixture, as well as the individual compounds including curcumin, bisdemethoxycurcumin, and demethoxycurcumin, shows an array of activities that can be helpful in ameliorating AD symptoms by acting on various target sites. These compounds were reported to be able to prevent the spread of plaque found in the brains of Alzheimer's patients, and are regarded as a potential treatment for Alzheimer's disease.84
The uptake and metabolism of curcumin has been reviewed.85 The known in vivo metabolism of curcumin and other curcuminoids comprises stepwise reduction of the olefinic heptanoid chain and conjugation of the parent compound and the reductive metabolites with glucuronic acid and sulfate.85
3 Physicochemical properties
Lipinski's rule of five, also known as the rule of five (Ro5), is a rule of thumb to evaluate drug-likeness and predict if a chemical compound has physico-chemical properties that would make it orally bioavailable in humans.86,87 Lipinski's rule states that, in general, an orally active drug has no more than one violation of the following criteria: a molecular weight (MW) less than 500 Daltons, no more than 5 hydrogen bond donors (HBDs), no more than 10 hydrogen bond acceptors (HBAs), and an octanol–water partition coefficient log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P not greater than 5. Although the rule cannot guarantee a molecule compliant with all criteria is druggable, it provides a guide for medicinal chemists for better design of compounds with satisfactory pharmacokinetics. So far, the criteria have spawned many extensions. In particular, compounds that meet the two criteria of 10 or fewer rotatable bonds (ROTB) and polar surface area (PSA) equal to or less than 140 Å2 are predicted to have good oral bioavailability.88
P not greater than 5. Although the rule cannot guarantee a molecule compliant with all criteria is druggable, it provides a guide for medicinal chemists for better design of compounds with satisfactory pharmacokinetics. So far, the criteria have spawned many extensions. In particular, compounds that meet the two criteria of 10 or fewer rotatable bonds (ROTB) and polar surface area (PSA) equal to or less than 140 Å2 are predicted to have good oral bioavailability.88
Neurodegenerative diseases such as PD, AD, HD, and others share common features at cellular and subcellular levels as well as sharing common molecular signaling pathways that may lead to apoptosis, necroptosis, and inflammation.89 Compounds with neuroprotective properties must possess certain physico-chemical properties to allow brain penetration and exposure.
Herein, we analyze the four Lipinski properties MW, HBD, HBA, and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P along with ROTB and PSA of the above 84 TCM compounds, and those of current anti-PD and anti-AD drugs and candidates in clinical trials. We then compare these three sets to evaluate the likelihood of the TCM components being useful CNS active drugs.
P along with ROTB and PSA of the above 84 TCM compounds, and those of current anti-PD and anti-AD drugs and candidates in clinical trials. We then compare these three sets to evaluate the likelihood of the TCM components being useful CNS active drugs.
3.1 Physicochemical properties of anti-PD drugs, anti-AD drugs and TCM compounds
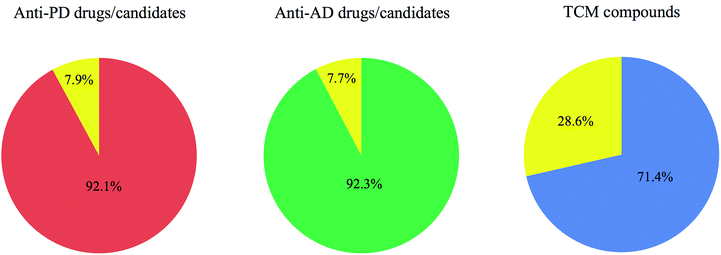
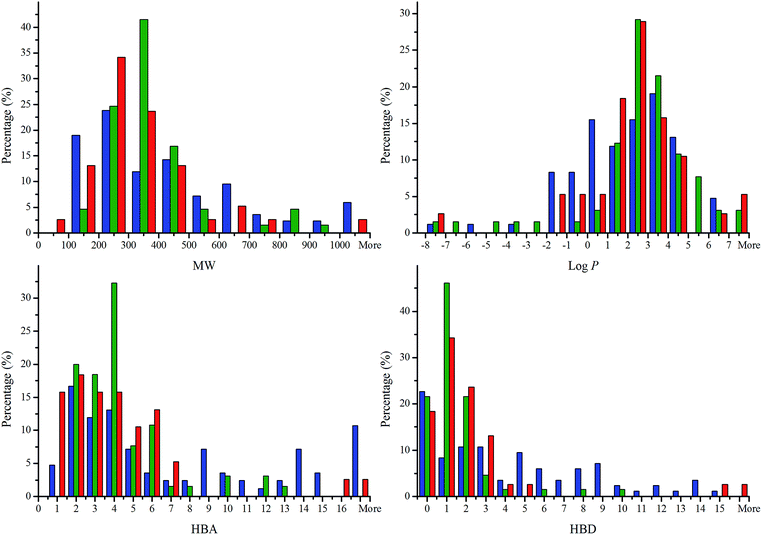
Searching Parkinson's disease and Parkinson's disease dementia in Thomson Reuters Cortellis identified a total of 38 small molecules. Similarly, searching Alzheimer's disease in Thomson Reuters Cortellis resulted in a total of 65 small molecules. Memantine and GM-600 are two drugs in Phase II clinical trial now with indications for both Parkinson's and Alzheimer's diseases.The structures of the 38 anti-PD drugs, the 65 anti-AD drugs and the 84 TCM compounds were converted into SMILES format and imported into Instant JChem to calculate the physicochemical parameters of each molecule.90 The calculated data for anti-PD and anti-AD drugs and candidates in clinical trials are shown in Tables 2 and 3, respectively, and those for 84 TCM compounds in Table 4. The percentages of anti-PD drugs, anti-AD drugs and TCM compounds compliant with Lipinski's rule of five are depicted in Fig. 1. The histograms for molecular weight, calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, and hydrogen bond acceptors and donors for three sets of compounds are shown in Fig. 2.
P, and hydrogen bond acceptors and donors for three sets of compounds are shown in Fig. 2.
| Name | MW | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
HBA | HBD | ROTB | PSA | Ro5 | No. violations |
|---|---|---|---|---|---|---|---|---|
| a The physicochemical data of memantine and GM-600 are given in Table 2 and not duplicated in Table 3. | ||||||||
| Levodopa | 197.19 | −1.79 | 5 | 4 | 3 | 103.78 | Pass | 0 |
| Melevodopa | 211.21 | 0.62 | 4 | 3 | 4 | 92.78 | Pass | 0 |
| Pergolide | 314.49 | 4.23 | 1 | 1 | 4 | 19.03 | Pass | 0 |
| Apomorphine | 267.32 | 2.87 | 3 | 2 | 0 | 43.70 | Pass | 0 |
| Bromocriptine | 654.60 | 3.89 | 6 | 3 | 5 | 118.21 | Pass | 1 |
| Ropinirole | 260.37 | 3.06 | 2 | 1 | 7 | 32.34 | Pass | 0 |
| Cabergoline | 451.60 | 2.58 | 4 | 2 | 8 | 71.68 | Pass | 0 |
| Aplindore | 310.35 | 2.01 | 4 | 2 | 4 | 59.59 | Pass | 0 |
| Pramipexole | 211.33 | 1.76 | 3 | 2 | 3 | 50.94 | Pass | 0 |
| Entacapone | 305.29 | 1.63 | 6 | 2 | 5 | 127.70 | Pass | 0 |
| Tolcapone | 273.24 | 3.28 | 5 | 2 | 3 | 100.67 | Pass | 0 |
| Opicapone | 385.12 | 2.33 | 7 | 2 | 3 | 149.46 | Pass | 0 |
| Rasagiline | 171.24 | 2.30 | 1 | 1 | 2 | 12.03 | Pass | 0 |
| Selegiline | 223.74 | 2.85 | 1 | 0 | 4 | 3.24 | Pass | 0 |
| Budipine | 293.45 | 4.95 | 1 | 0 | 3 | 3.24 | Pass | 0 |
| Carbidopa | 226.23 | −1.21 | 6 | 5 | 4 | 115.81 | Pass | 0 |
| Altropane | 429.27 | 3.95 | 2 | 0 | 5 | 29.54 | Pass | 0 |
| 123I-ioflupane | 431.28 | 3.70 | 2 | 0 | 6 | 29.54 | Pass | 0 |
| Aemantine | 179.3 | 2.07 | 1 | 1 | 0 | 26.02 | Pass | 0 |
| Amantadine | 151.25 | 1.47 | 1 | 1 | 0 | 26.02 | Pass | 0 |
| GM1 ganglioside | 1602.93 | 3.76 | 31 | 20 | 56 | 540.58 | Fail | 3 |
| Pardoprunox | 233.27 | 1.29 | 4 | 1 | 1 | 44.81 | Pass | 0 |
| Rotigotine | 315.47 | 4.34 | 2 | 1 | 6 | 23.47 | Pass | 0 |
| Istradefylline | 384.43 | 2.42 | 5 | 0 | 6 | 76.90 | Pass | 0 |
| Tozadenant | 406.50 | 1.48 | 6 | 2 | 3 | 87.16 | Pass | 0 |
| V-81444 | 321.34 | 2.22 | 6 | 2 | 3 | 121.67 | Pass | 0 |
| 123I-MNI-420 | 573.36 | 4.07 | 7 | 1 | 5 | 106.54 | Pass | 1 |
| AAD-2004 | 325.28 | 2.56 | 4 | 3 | 6 | 69.56 | Pass | 0 |
| IRX-4204 | 366.54 | 6.61 | 2 | 1 | 4 | 37.30 | Pass | 1 |
| GM-600 | 798.89 | −7.00 | 16 | 15 | 24 | 373.08 | Fail | 3 |
| Talipexole | 209.31 | 1.65 | 3 | 1 | 2 | 42.15 | Pass | 0 |
| Baicalein | 272.25 | 2.84 | 5 | 3 | 1 | 86.99 | Pass | 0 |
| Mitoquinone | 678.81 | 8.71 | 4 | 0 | 16 | 52.60 | Fail | 2 |
| Nicotine | 162.23 | 1.16 | 2 | 0 | 1 | 16.13 | Pass | 0 |
| Monosodium alpha luminol | 200.15 | −0.06 | 3 | 3 | 0 | 84.22 | Pass | 0 |
| Vatiquinone | 440.66 | 7.81 | 3 | 1 | 12 | 54.37 | Pass | 1 |
| Fampridine | 94.11 | −0.07 | 2 | 1 | 0 | 38.91 | Pass | 0 |
| Zonisamide | 212.23 | 0.11 | 3 | 1 | 2 | 86.19 | Pass | 0 |
| Name | MW | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
HBA | HBD | ROTB | PSA | Ro5 | No. violations |
|---|---|---|---|---|---|---|---|---|
| Huperzine A | 242.32 | 0.62 | 2 | 2 | 0 | 55.12 | Pass | 0 |
| Bryostatin-1 | 905.03 | 5.04 | 12 | 4 | 13 | 240.11 | Fail | 3 |
| Galantamine | 287.35 | 1.16 | 4 | 1 | 1 | 41.93 | Pass | 0 |
| Rivastigmine | 250.34 | 2.41 | 2 | 0 | 5 | 32.78 | Pass | 0 |
| Posiphen | 337.42 | 4.25 | 4 | 1 | 3 | 44.81 | Pass | 0 |
| Pioglitazone | 356.44 | 3.33 | 4 | 1 | 7 | 68.29 | Pass | 0 |
| PRX-3140 | 384.51 | 1.23 | 4 | 2 | 7 | 69.64 | Pass | 0 |
| ABT-126 | 313.42 | 2.71 | 4 | 0 | 3 | 38.25 | Pass | 0 |
| TTP-488 | 532.12 | 8.22 | 3 | 0 | 14 | 39.52 | Fail | 2 |
| Idalopirdine | 394.41 | 5.17 | 2 | 2 | 9 | 37.05 | Pass | 1 |
| Roflumilast | 403.21 | 4.45 | 4 | 1 | 7 | 60.45 | Pass | 0 |
| Encenicline | 320.84 | 3.04 | 2 | 1 | 2 | 32.34 | Pass | 0 |
| Ladostigil | 272.34 | 2.38 | 2 | 1 | 5 | 41.57 | Pass | 0 |
| ARC-100 | 869.99 | 4.65 | 10 | 3 | 17 | 211.68 | Pass | 1 |
| Laquinimod | 356.80 | 2.55 | 3 | 1 | 3 | 60.85 | Pass | 0 |
| Risperidone | 410.48 | 2.63 | 4 | 0 | 4 | 61.94 | Pass | 0 |
| MK-8931 | 338.43 | 2.53 | 4 | 2 | 3 | 69.08 | Pass | 0 |
| Circadin | 232.28 | 1.15 | 2 | 2 | 4 | 54.12 | Pass | 0 |
| IRX-4204 | 366.54 | 6.61 | 2 | 1 | 4 | 37.30 | Pass | 1 |
| NSI-189 | 366.50 | 3.95 | 4 | 1 | 7 | 48.47 | Pass | 0 |
| Donepezil | 379.49 | 4.21 | 4 | 0 | 6 | 38.77 | Pass | 0 |
| DSP-8658 | 417.50 | 5.86 | 4 | 1 | 9 | 68.53 | Pass | 1 |
| AZD-3293 | 361.46 | 3.67 | 4 | 1 | 4 | 60.50 | Pass | 0 |
| T-817MA | 291.41 | 2.29 | 3 | 1 | 7 | 32.70 | Pass | 0 |
| Shionogi | 347.41 | 2.97 | 4 | 1 | 4 | 67.92 | Pass | 0 |
| Zibotentan | 424.43 | 1.01 | 8 | 1 | 5 | 132.99 | Pass | 0 |
| Idebenone | 338.44 | 3.57 | 5 | 1 | 12 | 72.83 | Pass | 0 |
| ANAVEX-2-73 | 281.39 | 3.50 | 2 | 0 | 4 | 12.47 | Pass | 0 |
| Velusetrag | 504.64 | −0.10 | 6 | 2 | 7 | 110.26 | Pass | 1 |
| Masitinib | 498.64 | 4.97 | 6 | 2 | 7 | 73.39 | Pass | 0 |
| S-38093 | 449.59 | 2.88 | 6 | 1 | 9 | 65.37 | Pass | 0 |
| Saracatinib | 542.03 | 3.20 | 10 | 1 | 8 | 90.44 | Pass | 1 |
| T3D-959 | 419.47 | 4.53 | 5 | 1 | 9 | 81.79 | Pass | 0 |
| ELND-005 | 180.16 | −3.78 | 6 | 6 | 0 | 121.38 | Pass | 1 |
| Apabetalone | 370.40 | 2.49 | 6 | 2 | 6 | 89.38 | Pass | 0 |
| CHF-5074 | 325.16 | 5.24 | 2 | 1 | 3 | 37.30 | Pass | 1 |
| SUVN-502 | 448.38 | 3.56 | 4 | 0 | 3 | 45.55 | Pass | 0 |
| Pinocembrin | 256.25 | 3.14 | 4 | 2 | 1 | 66.76 | Pass | 0 |
| GLN-1062 | 391.46 | 3.66 | 4 | 0 | 4 | 48.00 | Pass | 0 |
| TAK-070 | 385.54 | 6.64 | 2 | 0 | 7 | 12.47 | Pass | 1 |
| Pozanicline | 192.26 | 0.83 | 3 | 1 | 3 | 34.15 | Pass | 0 |
| Davunetide | 824.92 | −7.48 | 13 | 10 | 22 | 355.85 | Fail | 3 |
| MSDC-0160 | 370.42 | 3.15 | 5 | 1 | 7 | 85.36 | Pass | 0 |
| Nimodipine | 418.44 | 2.54 | 6 | 1 | 10 | 117.00 | Pass | 0 |
| Clioquinol | 305.50 | 3.36 | 2 | 1 | 0 | 33.12 | Pass | 0 |
| Acetyl-L-carnitine | 203.24 | −4.45 | 3 | 0 | 6 | 66.43 | Pass | 0 |
| Octohydroaminoacridine | 202.30 | 2.58 | 2 | 1 | 0 | 38.91 | Pass | 0 |
| AAD-2004 | 325.28 | 2.56 | 4 | 3 | 6 | 69.56 | Pass | 0 |
| Moclobemide | 268.74 | 1.45 | 3 | 1 | 4 | 41.57 | Pass | 0 |
| ST-101 | 236.27 | 1.84 | 3 | 0 | 0 | 32.67 | Pass | 0 |
| PU-H71 | 494.33 | 2.67 | 7 | 2 | 7 | 100.11 | Pass | 0 |
| Nilvadipine | 385.37 | 2.24 | 6 | 1 | 7 | 131.56 | Pass | 0 |
| Bisnorcymserine | 379.50 | 5.50 | 4 | 1 | 4 | 44.81 | Pass | 1 |
| GTS-21 | 308.37 | 2.96 | 4 | 0 | 4 | 43.71 | Pass | 0 |
| Cutamesine dihydrochloride | 368.51 | 4.19 | 4 | 0 | 9 | 24.94 | Pass | 0 |
| Piromelatine | 312.32 | 1.59 | 4 | 2 | 5 | 80.42 | Pass | 0 |
| HF-0220 | 306.44 | 2.46 | 3 | 2 | 0 | 57.53 | Pass | 0 |
| (−)Clausenamide | 297.35 | 1.47 | 3 | 2 | 3 | 60.77 | Pass | 0 |
| Oxiracetam | 158.16 | −2.60 | 3 | 2 | 2 | 83.63 | Pass | 0 |
| Tacrine | 202.30 | 2.58 | 2 | 1 | 0 | 38.91 | Pass | 0 |
| SEMAX | 813.92 | −6.65 | 12 | 8 | 21 | 286.32 | Fail | 3 |
| Resveratrol | 228.24 | 3.40 | 3 | 3 | 2 | 60.69 | Pass | 0 |
| Rilapladib | 735.81 | 8.08 | 5 | 0 | 13 | 53.09 | Fail | 2 |
| Lovastatin | 404.54 | 3.90 | 3 | 1 | 7 | 72.83 | Pass | 0 |
| Minaprine | 298.38 | 2.19 | 5 | 1 | 5 | 50.28 | Pass | 0 |
| No | MW | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
HBA | HBD | ROTB | PSA | Ro5 | No. violations | In Fig. 3c | In Fig. 3d | In Fig. 3e |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1109.29 | −1.55 | 23 | 15 | 16 | 377.29 | Fail | 3 | |||
| 2 | 1079.27 | −0.92 | 22 | 14 | 15 | 357.06 | Fail | 3 | |||
| 3 | 1079.27 | −0.92 | 22 | 14 | 16 | 357.06 | Fail | 3 | |||
| 4 | 947.15 | 0.22 | 18 | 12 | 13 | 298.14 | Fail | 3 | |||
| 5 | 947.15 | −0.04 | 18 | 12 | 12 | 298.14 | Fail | 3 | |||
| 6 | 801.01 | 0.68 | 14 | 10 | 10 | 239.22 | Fail | 3 | |||
| 7 | 801.01 | 0.68 | 14 | 10 | 10 | 239.22 | Fail | 3 | |||
| 8 | 622.87 | 3.76 | 8 | 6 | 7 | 139.84 | Fail | 2 | |||
| 9 | 785.01 | 1.73 | 13 | 9 | 9 | 218.99 | Fail | 3 | |||
| 10 | 396.43 | −0.22 | 8 | 6 | 11 | 139.84 | Pass | 1 | √ | √ | √ |
| 11 | 480.46 | −0.39 | 10 | 5 | 7 | 164.37 | Pass | 0 | √ | √ | √ |
| 12 | 480.46 | −1.12 | 9 | 5 | 7 | 172.21 | Pass | 0 | √ | √ | √ |
| 13 | 496.46 | −0.69 | 11 | 6 | 7 | 184.60 | Fail | 2 | √ | √ | √ |
| 14 | 584.57 | 2.11 | 10 | 4 | 10 | 170.44 | Pass | 1 | √ | ||
| 15 | 406.38 | 0.83 | 9 | 7 | 5 | 160.07 | Pass | 1 | √ | √ | √ |
| 16 | 270.24 | 3.82 | 5 | 3 | 0 | 94.83 | Pass | 0 | √ | √ | √ |
| 17 | 284.26 | 3.97 | 5 | 2 | 1 | 83.83 | Pass | 0 | √ | √ | √ |
| 18 | 254.24 | 4.12 | 4 | 2 | 0 | 74.60 | Pass | 0 | √ | √ | √ |
| 19 | 284.22 | 3.27 | 6 | 3 | 1 | 111.90 | Pass | 0 | √ | √ | √ |
| 20 | 270.24 | 2.84 | 5 | 3 | 1 | 94.83 | Pass | 0 | √ | √ | √ |
| 21 | 362.33 | −3.43 | 10 | 6 | 4 | 158.30 | Pass | 1 | √ | ||
| 22 | 524.47 | −5.20 | 15 | 9 | 7 | 237.45 | Fail | 3 | |||
| 23 | 686.61 | −7.39 | 20 | 13 | 10 | 327.60 | Fail | 3 | |||
| 24 | 624.59 | 0.82 | 14 | 9 | 11 | 245.29 | Fail | 3 | |||
| 25 | 286.28 | −1.37 | 7 | 5 | 4 | 119.61 | Pass | 0 | √ | √ | √ |
| 26 | 124.14 | 0.90 | 2 | 2 | 1 | 40.46 | Pass | 0 | √ | √ | √ |
| 27 | 384.47 | 2.61 | 4 | 1 | 5 | 67.87 | Pass | 0 | √ | √ | √ |
| 28 | 384.47 | 2.61 | 4 | 1 | 5 | 67.87 | Pass | 0 | √ | √ | √ |
| 29 | 190.24 | 2.73 | 1 | 0 | 2 | 26.30 | Pass | 0 | √ | √ | √ |
| 30 | 190.24 | 3.36 | 1 | 0 | 3 | 26.30 | Pass | 0 | √ | √ | √ |
| 31 | 380.48 | 4.69 | 2 | 0 | 4 | 52.60 | Pass | 0 | √ | √ | √ |
| 32 | 194.27 | 3.39 | 1 | 0 | 3 | 26.30 | Pass | 0 | √ | √ | √ |
| 33 | 192.25 | 3.07 | 1 | 0 | 3 | 26.30 | Pass | 0 | √ | √ | √ |
| 34 | 194.18 | 1.67 | 4 | 2 | 3 | 66.76 | Pass | 0 | √ | √ | √ |
| 35 | 180.16 | 1.53 | 4 | 3 | 2 | 77.76 | Pass | 0 | √ | √ | √ |
| 36 | 168.15 | 1.17 | 4 | 2 | 2 | 66.76 | Pass | 0 | √ | √ | √ |
| 37 | 136.19 | 0.06 | 2 | 0 | 0 | 25.78 | Pass | 0 | √ | √ | √ |
| 38 | 276.29 | 4.00 | 2 | 0 | 0 | 47.28 | Pass | 0 | √ | √ | √ |
| 39 | 294.34 | 4.53 | 2 | 0 | 0 | 47.28 | Pass | 0 | √ | √ | √ |
| 40 | 310.34 | 3.25 | 3 | 1 | 1 | 67.51 | Pass | 0 | √ | √ | √ |
| 41 | 296.36 | 3.89 | 3 | 0 | 0 | 43.37 | Pass | 0 | √ | √ | √ |
| 42 | 312.32 | 1.92 | 4 | 2 | 0 | 87.74 | Pass | 0 | √ | √ | √ |
| 43 | 278.30 | 3.36 | 3 | 0 | 0 | 43.37 | Pass | 0 | √ | √ | √ |
| 44 | 276.29 | 4.00 | 2 | 0 | 0 | 47.28 | Pass | 0 | √ | √ | √ |
| 45 | 296.36 | 3.51 | 2 | 0 | 0 | 47.28 | Pass | 0 | √ | √ | √ |
| 46 | 138.12 | 1.08 | 3 | 2 | 1 | 57.53 | Pass | 0 | √ | √ | √ |
| 47 | 154.12 | 1.02 | 4 | 3 | 1 | 77.76 | Pass | 0 | √ | √ | √ |
| 48 | 180.16 | 1.53 | 4 | 3 | 2 | 77.76 | Pass | 0 | √ | √ | √ |
| 49 | 198.17 | 0.58 | 5 | 4 | 3 | 97.99 | Pass | 0 | √ | √ | √ |
| 50 | 360.31 | 3.00 | 7 | 5 | 7 | 144.52 | Pass | 0 | √ | √ | √ |
| 51 | 538.46 | 3.75 | 11 | 7 | 9 | 211.28 | Fail | 3 | √ | ||
| 52 | 494.45 | 4.74 | 9 | 7 | 9 | 184.98 | Pass | 1 | √ | ||
| 53 | 718.61 | 4.99 | 14 | 9 | 14 | 278.04 | Fail | 3 | √ | ||
| 54 | 208.25 | 2.62 | 3 | 0 | 4 | 27.69 | Pass | 0 | √ | √ | √ |
| 55 | 208.25 | 2.62 | 3 | 0 | 4 | 27.69 | Pass | 0 | √ | √ | √ |
| 56 | 208.25 | 2.62 | 3 | 0 | 4 | 27.69 | Pass | 0 | √ | √ | √ |
| 57 | 178.23 | 2.78 | 2 | 0 | 3 | 18.46 | Pass | 0 | √ | √ | √ |
| 58 | 178.23 | 2.76 | 2 | 0 | 4 | 18.46 | Pass | 0 | √ | √ | √ |
| 59 | 178.23 | 2.78 | 2 | 0 | 3 | 18.46 | Pass | 0 | √ | √ | √ |
| 60 | 680.82 | 1.66 | 12 | 8 | 6 | 214.44 | Fail | 3 | |||
| 61 | 754.69 | 0.30 | 17 | 8 | 17 | 279.05 | Fail | 3 | √ | ||
| 62 | 568.48 | −1.49 | 14 | 9 | 6 | 245.29 | Fail | 3 | |||
| 63 | 668.60 | 0.08 | 15 | 8 | 14 | 260.59 | Fail | 3 | √ | ||
| 64 | 1045.21 | 0.15 | 21 | 11 | 10 | 314.83 | Fail | 3 | |||
| 65 | 1207.35 | −1.63 | 26 | 14 | 13 | 393.98 | Fail | 3 | |||
| 66 | 608.54 | −1.68 | 15 | 9 | 7 | 245.29 | Fail | 3 | |||
| 67 | 534.69 | 4.29 | 5 | 3 | 8 | 99.77 | Pass | 1 | √ | √ | √ |
| 68 | 295.38 | 3.39 | 3 | 0 | 2 | 21.70 | Pass | 0 | √ | √ | √ |
| 69 | 282.46 | 6.78 | 2 | 1 | 15 | 37.30 | Pass | 1 | √ | √ | √ |
| 70 | 280.45 | 6.42 | 2 | 1 | 14 | 37.30 | Pass | 1 | √ | √ | √ |
| 71 | 278.43 | 6.06 | 2 | 1 | 13 | 37.30 | Pass | 1 | √ | √ | √ |
| 72 | 304.47 | 6.59 | 2 | 1 | 14 | 37.30 | Pass | 1 | √ | √ | √ |
| 73 | 416.38 | −0.03 | 9 | 6 | 3 | 156.91 | Pass | 1 | √ | √ | √ |
| 74 | 254.24 | 2.73 | 4 | 2 | 1 | 66.76 | Pass | 0 | √ | √ | √ |
| 75 | 416.38 | 0.46 | 9 | 5 | 4 | 145.91 | Pass | 0 | √ | √ | √ |
| 76 | 460.69 | 3.59 | 4 | 4 | 1 | 80.92 | Pass | 0 | √ | √ | |
| 77 | 444.69 | 4.59 | 3 | 3 | 1 | 60.69 | Pass | 0 | √ | √ | √ |
| 78 | 490.72 | 2.69 | 5 | 5 | 2 | 101.15 | Pass | 0 | √ | √ | |
| 79 | 504.70 | 3.16 | 6 | 5 | 2 | 118.22 | Pass | 1 | √ | √ | |
| 80 | 444.69 | 4.51 | 3 | 3 | 1 | 60.69 | Pass | 0 | √ | √ | √ |
| 81 | 636.60 | −1.18 | 14 | 8 | 10 | 234.29 | Fail | 3 | √ | ||
| 82 | 474.46 | 1.09 | 9 | 5 | 7 | 155.14 | Pass | 0 | √ | √ | √ |
| 83 | 606.57 | 0.22 | 13 | 8 | 8 | 225.06 | Fail | 3 | √ | ||
| 84 | 368.38 | 4.12 | 6 | 2 | 8 | 93.06 | Pass | 0 | √ | √ | √ |
It was found that 92% of anti-PD drugs and 92% of anti-AD drugs had less than two violations of the Lipinski's parameters, with 82% and 77% having no violations, respectively. These values were decreased in the TCM data set, which had 71% and 57% in these categories, respectively.
The histogram of molecular weight (Fig. 2a) showed that about 84% of the anti-PD drugs, 88% of the anti-AD drugs and 69% of the TCM compounds were distributed between molecular weights of 100–500 Da, with the anti-PD drugs and TCM compounds peaking at 200–300 Da while the anti-AD drugs peaked at 300–400 Da. About 13% of the anti-PD drugs and 12% of the anti-AD drugs had molecular weights over 500 Da while 31% for the TCM compounds had molecular weights over 500.
The calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (Fig. 2b) of the majority of all sets (90% for anti-PD, 86% for anti-AD and 92% for TCM) fell into the same region from −2 to 6 but the distribution maximum was at 2–3 for the anti-PD and anti-AD drugs while at 3–4 for the TCM compounds. The overall percentage of compounds satisfying the log
P (Fig. 2b) of the majority of all sets (90% for anti-PD, 86% for anti-AD and 92% for TCM) fell into the same region from −2 to 6 but the distribution maximum was at 2–3 for the anti-PD and anti-AD drugs while at 3–4 for the TCM compounds. The overall percentage of compounds satisfying the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P criteria was enhanced for the TCM compounds (95%) as compared to the anti-PD (92%) and anti-AD (86%) drugs.
P criteria was enhanced for the TCM compounds (95%) as compared to the anti-PD (92%) and anti-AD (86%) drugs.
The histogram of hydrogen bond acceptors (HBA) (Fig. 2c) for the anti-PD and anti-AD drugs showed a narrower distribution compared with that of the TCM compounds. About 95% of the anti-PD drugs and 91% of the anti-AD drugs concentrated in a range of 1–7 while only 60% of the TCM compounds fall into the same range. The HBA values of the TCM compounds fluctuated from 1 to more than 16. The percentage of the TCM compounds with acceptable HBA (no more than 10) was 73% as compared to 95% for both anti-PD and anti-AD drugs.
The HBD histogram distribution showed a maximum at zero donors for the TCM compounds, in contrast to 1 for the anti-PD and anti-AD drugs. About 95% of the anti-PD and anti-AD drugs had no more than 5 hydrogen bond donors while the number of the TCM compounds compliant with Ro5 was 65%.
The histograms of the four Lipinski's parameters showed more similarities among these three datasets on the properties of MW and the calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P than the hydrogen bond acceptors and donors. This is probably due to the natural products having more O-containing functionalities and hydroxyl groups. The percentages of the TCM compounds were lower than those of the anti-PD and anti-AD drugs in all Ro5 parameters except for the calculated log
P than the hydrogen bond acceptors and donors. This is probably due to the natural products having more O-containing functionalities and hydroxyl groups. The percentages of the TCM compounds were lower than those of the anti-PD and anti-AD drugs in all Ro5 parameters except for the calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P. The results for the TCM compounds are quite similar to the two previous analyses of natural products.91,92
P. The results for the TCM compounds are quite similar to the two previous analyses of natural products.91,92
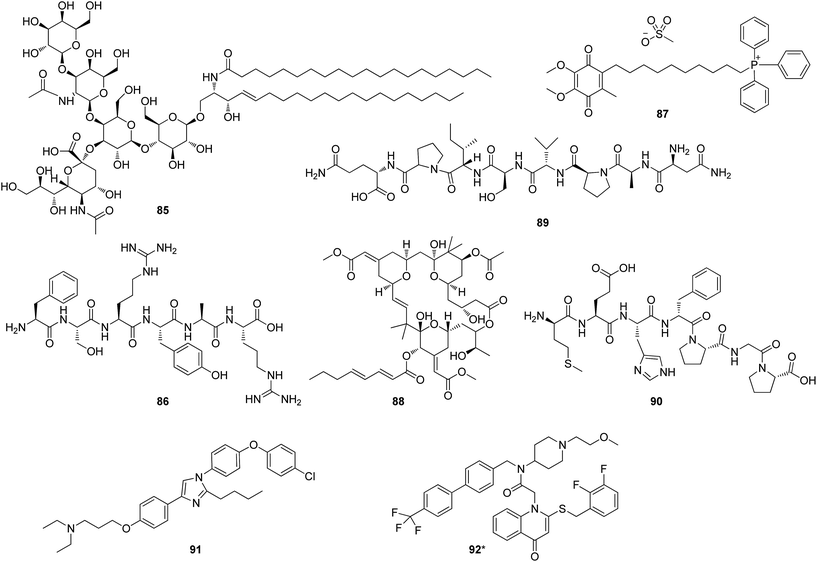
Considering the structural aspects some conclusions are presented. For anti-PD drugs, GM1 ganglioside (85), GM-600 (86) and mitoquinone (87) have two or three violations of Lipinski's rules. GM1 ganglioside is now an approved drug while the other two are drug candidates in Phase II clinical trial. GM-600 is a peptide used in intravenous formulation. Mitoquinone, a mitochondria-targeted antioxidant, is used with mitoquinol as a redox mixture in the treatment of Parkinson's disease, liver disease, etc. The anti-AD drugs, bryostatin-1 (88), davunetide (89) and SEMAX (90) violated three of the Lipinski's parameters, and TTP-488 (91) and rilapladib (92) had two violations. Bryostatin-1 is a natural product in Phase II clinical trial used in intravenous formulation. Davunetide and SEMAX are two peptides used as biological therapeutics. They are not drugs in oral formulation but in nasal or buccal systemic formulation.
With respect to the TCM set, 24 of 84 compounds had two or more violations of the Lipinski's parameters. The structural survey of these compounds further revealed that 22 of them contained at least one sugar moiety in the molecule, e.g. ginsenosides (1–9), rehmannioside A (22), rehmannioside D (23), and jujubosides A (64) and B (65). The existence of sugar moiety provides more O-containing functionalities and hydroxyl groups, which might account for the violations of HBA and HBD values, as well as molecular weights, rotatable bonds and polar surface area.
These analyses revealed one fact that nearly all launched drugs or candidates are compliant with the Ro5 or only have one violation. A majority of the TCM set follows this rule although the percentage is lower than for the drug sets. Those failing in the Ro5 are mostly compounds with one or more sugar moieties.
3.2 Comparison of physicochemical space of anti-PD drugs, anti-AD drugs and TCM compounds
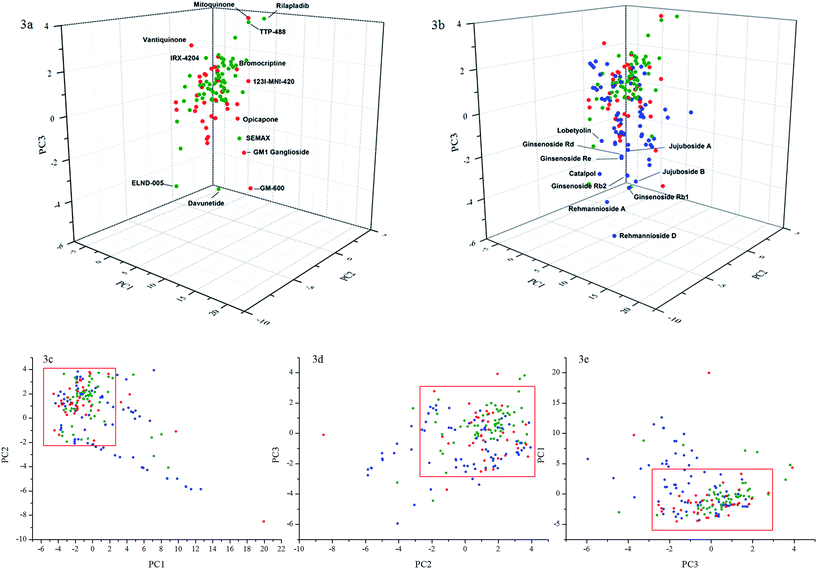
In contrast to Lipinski's parameters focusing on a restricted set of drug-like properties generated directly from the molecular structures, ChemGPS-NP93 is a tool tuned for identifying volumes of chemical space to allow correlation to biological activities. ChemGPS-NP has been designed to handle the chemical diversity of natural products. It can discover physiochemical properties not directly discernible from structural data, and can chart biologically relevant chemical space and provide an efficient mapping device for prediction of properties and activities of groups of compounds. Therefore, ChemGPS-NP was used for principle component analysis (PCA) of anti-PD drugs, anti-AD drugs and TCM compounds to compare their distribution in physicochemical space. The first three principle components that explained 71% of the variance are plotted in Fig. 3a and b. Size, shape and polarizability were described in PC1, aromaticity- and conjugation-related properties of the compounds were explained in PC2, and lipophilicity, polarity and H-bond capacity were expressed in PC3.Fig. 3a demonstrates the score plot of 38 anti-PD drugs and 65 anti-AD drugs. The graph shows that the majority of these molecules concentrated in a relatively narrow area of physicochemical space, which means that these compounds shared similarities in the described parameters. GM1 ganglioside, GM-600, mitoquinone, devunetide, SEMAX, TTP-488 and rilapladib, violating more than two Lipinski's parameters, were found to be a relatively far distance from the core of the area. Bryostatin-1 was also at such a distance but cannot be discerned from this perspective. Other compounds with one violation of the Lipinski's rules such as vatiquinone, bromocriptine, 123I-MNI-420, opicapone, and IRX-4204 were also observed in the skirt area of the space.
Fig. 3b displayed the score plot comparison of physicochemical spaces of the current drugs and the TCM compounds. The majority of the TCM compounds fall into the drug-like physicochemical space defined by anti-PD and anti-AD drugs. The cluster of the spots, which were found below the core space, represented ginsenosides Rb1, Rb2, Rd, and Re, jujubosides A and B, rehmanniosides A and D, catalpol, and etc. All these compounds, containing at least one sugar moiety, possesses higher molecular weights than 500, and the HBA and HBD values exceed the Lipinski's rule of five while having a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value within the range. They were quite close to GM1 ganglioside and peptides like GM-600, davunetide and SEMAX in physicochemical space.
P value within the range. They were quite close to GM1 ganglioside and peptides like GM-600, davunetide and SEMAX in physicochemical space.
It is noticeable that more than 30 compounds containing sugar moieties were identified as the major bioactive constituents from the 15 selected traditional herbal medicines. A large number of saponins including ginsenosides are biologically active and have favourable ADME/T properties (absorption, distribution, metabolism, excretion, and toxicology), despite the fact that they do not satisfy the proposed “drug-likeness” criteria like the Ro5. The launched anti-PD drug GM1 ganglioside is an example.
In summary, the majority of the current anti-PD and anti-AD drugs and candidates are compliant with the Lipinski's rules and concentrated in a narrow area of physicochemical space. The 2D plots identify a narrow area of physicochemical space for the anti-PD and anti-AD group (enclosed within the rectangles) that also contains many TCM derived compounds. The cluster of compounds containing sugar moieties is the exceptions and may constitute a special class. Details of the TCM spots in the red rectangles of three 2D plots are indicated also in Table 4.
3.3 Prediction of blood–brain barrier permeation of TCM compounds
The ability to permeate across the blood brain barrier (BBB) is essential for drugs acting on the central nervous system. It is determined by their molecular size and physicochemical properties, as well as by complex binding and elimination processes occurring in the circulation, peripheral organs, at the BBB and in the brain parenchyma and its fluids.94 A number of methods and models are available for examining BBB permeation in vivo, in vitro, and in silico. A combination of techniques can give valuable information on ways to optimize permeation, and implications for drug discovery, delivery and toxicity.95,96In silico prediction of the BBB permeability of 84 TCM compounds was carried out. 3D structures of all TCM compounds were generated by LigPrep (version 2.3, Schrödinger, LLC, New York, NY, 2009) with the selected OPLS_2005 force field, neutralized functional groups, retained specified chiralities, and other parameters default. The generated 3D structures and QikProp (version 3.2, Schrödinger, LLC, New York, NY, 2009) were then used to calculate three descriptors QPPCaco, QPlogBB and QPPMDCK, which can reflect directly the ability of a molecule to permeate the BBB. Table 5 showed the recommended values of three descriptors for CNS drugs with good BBB permeability.
| Descriptor | Description | Recommended values |
|---|---|---|
| QPPCaco | Predicating Caco-2 cell permeability in nm s−1 | <25 poor, >500 great |
| QPlogBB | Predicating brain/blood coefficient | −3.0 to 1.2 |
| QPPMDCK | Predicating MDCK cell permeability in nm s−1 | <25 poor, >500 great |
The calculation results of 84 TCM compounds are showed in Table 6. Up to 58 of 84 compounds (69%) had QPlogBB values falling into the recommended range, and 54 and 46 compounds had QPPCaco and QPPMDCK values greater than 25, respectively, while 24 and 20 had values greater than 500. Taking all three descriptors into consideration, 46 of 84 compounds (54.8%) gave both ideal QPlogBB values and reasonable QPPCaco and QPPMDCK values (greater than 25). Among them, 20 of 46 compounds had QPPCaco and QPPMDCK values greater than 500, that is, 23.8% of 84 TCM compounds showed good BBB permeability in the in silico model. Compared with the fact that more than 98% of all small molecule drugs do not cross the BBB,97 the ratio of 23.8% of TCM compounds having good predicated BBB permeability is impressive. It might be ascribed to the fact that all these are bioactive compounds coming from the selected herbs that had definite therapeutic effects on brain disorders.
| No | QPPCaco | QPlogBB | QPPMDCK | No | QPPCaco | QPlogBB | QPPMDCK |
|---|---|---|---|---|---|---|---|
| 1 | 0.081 | −7.756 | 0.019 | 43 | 1580.237 | −0.183 | 811.233 |
| 2 | 0.154 | −7.038 | 0.038 | 44 | 1624.592 | −0.142 | 835.872 |
| 3 | 0.104 | −7.455 | 0.024 | 45 | 1557.722 | −0.159 | 798.746 |
| 4 | 1.704 | −5.192 | 0.503 | 46 | 197.54 | −1.002 | 85.711 |
| 5 | 1.561 | −5.088 | 0.458 | 47 | 26.733 | −1.237 | 12.549 |
| 6 | 6.096 | −4.425 | 1.996 | 48 | 33.041 | −1.359 | 15.778 |
| 7 | 4.468 | −4.582 | 1.427 | 49 | 12.457 | −1.823 | 5.497 |
| 8 | 209.676 | −2.047 | 91.416 | 50 | 1.534 | −3.62 | 0.571 |
| 9 | 31.67 | −3.325 | 11.85 | 51 | 0.085 | −4.249 | 0.032 |
| 10 | 11.159 | −3.336 | 29.957 | 52 | 0.624 | −4.083 | 0.216 |
| 11 | 73.028 | −2.156 | 29.236 | 53 | 0.05 | −4.76 | 0.018 |
| 12 | 16.988 | −2.616 | 6.044 | 54 | 9906.038 | −0.058 | 5899.293 |
| 13 | 19.024 | −2.808 | 6.831 | 55 | 9906.038 | −0.068 | 5899.293 |
| 14 | 35.924 | −2.784 | 13.58 | 56 | 9906.038 | −0.068 | 5899.293 |
| 15 | 16.523 | −3.062 | 5.865 | 57 | 9906.038 | 0.102 | 5899.293 |
| 16 | 79.729 | −1.532 | 32.146 | 58 | 9906.038 | 0.078 | 5899.293 |
| 17 | 263.149 | −1.084 | 116.858 | 59 | 9906.038 | 0.108 | 5899.293 |
| 18 | 262.357 | −0.979 | 116.477 | 60 | 0.589 | −3.117 | 0.258 |
| 19 | 6.468 | −1.969 | 2.707 | 61 | 3.432 | −4.466 | 1.073 |
| 20 | 80.467 | −1.586 | 32.468 | 62 | 4.102 | −4.024 | 1.301 |
| 21 | 36.491 | −2.155 | 13.812 | 63 | 5.792 | −3.942 | 1.889 |
| 22 | 7.37 | −3.417 | 2.451 | 64 | 6.108 | −4.615 | 2.001 |
| 23 | 1.026 | −4.855 | 0.291 | 65 | 0.355 | −6.534 | 0.092 |
| 24 | 1.881 | −5.141 | 0.56 | 66 | 2.508 | −4.066 | 0.764 |
| 25 | 71.41 | −2.057 | 28.536 | 67 | 142.653 | −0.306 | 190.75 |
| 26 | 919.525 | −0.463 | 451.821 | 68 | 2268.275 | 0.807 | 1326.462 |
| 27 | 276.504 | −0.309 | 136.387 | 69 | 228.773 | −1.609 | 127.756 |
| 28 | 182.992 | −0.435 | 87.298 | 70 | 299.059 | −1.165 | 170.664 |
| 29 | 2169.133 | −0.244 | 1142.446 | 71 | 228.77 | −1.287 | 127.754 |
| 30 | 2302.987 | −0.176 | 1218.833 | 72 | 270.453 | −1.191 | 153.09 |
| 31 | 1152.492 | −0.814 | 576.731 | 73 | 17.867 | −2.766 | 6.383 |
| 32 | 2367.728 | −0.186 | 1255.91 | 74 | 391.215 | −0.903 | 179.39 |
| 33 | 2334.953 | −0.178 | 1237.129 | 75 | 36.51 | −2.536 | 13.819 |
| 34 | 67.212 | −1.139 | 33.994 | 76 | 510.616 | −0.93 | 239.241 |
| 35 | 22.483 | −1.537 | 10.407 | 77 | 928.264 | −0.624 | 456.464 |
| 36 | 80.015 | −0.862 | 41.044 | 78 | 270.196 | −1.291 | 120.244 |
| 37 | 5483.01 | 0.344 | 3112.735 | 79 | 45.367 | −1.356 | 22.227 |
| 38 | 1529.531 | −0.195 | 783.133 | 80 | 1043.056 | −0.578 | 517.772 |
| 39 | 1657.431 | −0.164 | 854.149 | 81 | 6.021 | −3.761 | 1.97 |
| 40 | 690.871 | −0.628 | 331.709 | 82 | 31.585 | −2.569 | 11.816 |
| 41 | 1713.375 | −0.153 | 885.354 | 83 | 2.969 | −3.78 | 0.917 |
| 42 | 328.906 | −0.942 | 148.718 | 84 | 178.589 | −2.159 | 76.859 |
An in-depth investigation into chemical structures revealed that most saponins with two or more sugar units such as ginsenosides (1–7) showed very bad BBB permeability. The small molecules with good BBB permeability included lactones (29–33) and ligustrazine (37) from Chuanxiong, diterpene quinones (38, 39, 41, 44, 45) and phenolic acid (46) from Danshen, and sesquiterpenes (54–59) from the volatile oil of Shichangpu. These results were consistent with those obtained on in vitro and in vivo models. Yuan et al. established a BBB model of ECV304/C6 cell co-culture, on which the Danshen extract was screened. The results indicated that 16 compounds could permeate over this model and four of them, tanshinone I (38), cryptotanshinone (41), danshensu (49) and protocatechuic acid (47) were identified.98 α-Asarone (55), β-asarone (54), and elemicin, the major compounds of the volatile oil of Shichangpu were found to pass through the blood brain barrier in pharmacokinetics investigations on rat.99,100 Senkyunolide I was detectable in the cerebrospinal fluid in a study to find the absorbed constituents of the Chuanxiong active fraction of migraine following oral administration.101
A matching of the BBB permeability with the physicochemical properties of the TCM compounds revealed that the 23.8% of TCM compounds with good predicated BBB permeability also obeyed Lipinski's rules. Saponins, especially those with two or more sugar units are a special class, which had bad predicted BBB permeability and did not obey the Lipinski's rule of five, either. The saponins (11, 21, 25 and 73) with molecular weight less than 500 and only one sugar unit, however, showed ideal BBB permeability and physicochemical properties. Given metabolism in the body, the biological function of saponins might be ascribed to their metabolites. As a large portion of the TCM natural products, saponins require further investigation to become drug leads.
4 Conclusions
Natural products have been a major resource of new drugs due to their highly diversified structures and specific biological activities. Traditional Chinese Medicines not only embrace these characteristics but also have thousands of years of clinical practice. Artemisinin from Artemisia annua and depside salts from Salvia miltiorrhiza are two successful examples of the use of compounds contained in TCMs. These constituents can explain, at least partially, the traditional therapeutic effects of the respective herbs.Our investigation concentrated on TCMs which have long been used to treat neurodegenerative diseases. The biological activities of the main constituents from the selected TCMs were reviewed and the results showed that they have a close relationship with brain disorders. The physico-chemical analyses of these TCM compounds showed that many TCM compounds obey Lipinski's rule of five, and fall into similar physico-chemical space, following PCA analysis, as occupied by current anti-PD and anti-AD drugs or candidates. In other words, these TCM compounds showed an excellent matching with the launched drugs or drug candidates from the physico-chemical perspective. On the other hand, the predicted BBB permeability showed that some TCM constituents had good BBB permeability. These results indicate that a high ratio of TCM compounds have the requisite physico-chemical properties and BBB permeability to have potential to be CNS drug leads.
The analysis reveals a remarkable convergence of thousands of years of traditional use and modern medicinal chemistry developments to front-lead physicochemical space in the design of new therapeutics. While, the combination of molecules as practiced in TCM remains to be developed into western medicinal design, the convergence between TCM components and modern medicinal chemistry understanding of physico-chemical properties is amazing.
This convergence, which reveals a common physico-chemical base for TCM and western medicine, is pivotal to further development of ancient TCM in an understandable and generally acceptable way. Herbal materials with distinct chemical constitution, known mechanism of action and identified targets will contribute to the modernization, standardization and globalization of TCM. Screening TCMs for lead compounds taking into account the physico-chemical properties and predicted BBB properties is likely to be a productive method to discover new leads for neurodegenerative diseases, in particular.
Notwithstanding the above, TCM still confronts many challenges. Generally accepted regulations for raw materials are urgently required. More bioactive constituent(s) separated from TCMs need to have their mechanism of action and targets identified. Synergistic effects of combinations provide another area that needs to be addressed.
Our review provides evidence that supports the view of Dr Margaret Chan, WHO Director-General, given in her opening remarks at the International Forum on Traditional Medicine, held in Macao SAR on 19 August 2015, “Countries aiming to integrate the best from traditional and modern medicine would do well to look not at the many differences between the two approaches. Instead, they should look at those areas where both converge to help tackle the unique health challenge of the 21st century.”
5 Acknowledgments
The work was financially supported by Griffith University International Postgraduate Research Scholarship (GUIPRS) and Griffith University Postgraduate Research Scholarship (GUPRS) to CT, and partially supported by the National Science and Technology Major Project “Key New Drug Creation and Manufacturing Program” (No. 2012ZX09301001-001) in China.6 Notes and references
- Y. Y. Tu, Nat. Med., 2011, 17, 1217–1220 CrossRef CAS PubMed.
- X. C. Li, C. Yu, Y. L. Lu, Y. L. Gu, J. Lu, W. Xu, L. J. Xuan and Y. P. Wang, Drug Metab. Dispos., 2007, 35, 234–239 CrossRef CAS PubMed.
- Hepeng Jia, Nat. Biotechnol., 2005, 23, 771–772 CrossRef.
- J. D. Adams, Traditional Chinese Medicine: Scientific Basis for Its Use, RSC Publishing, 2013 Search PubMed.
- J. C. He and W. W. Wang, J. Tradit. Chin. Med., 2010, 51, 1024–1027 Search PubMed.
- Z. Y. Hu, Y. Huang, G. Liu, F. Liu, W. X. Zhou and Y. X. Zhang, Pharmacol. Clin. Chin. Mater. Med., 2012, 28, 252–256 Search PubMed.
- Q. Z. Su, Y. Z. Sun, L. Zeng and L. X. Fu, Guangming Zhongyi, 2008, 23, 542–543 Search PubMed.
- W.-Z. Yang, Y. Hu, W.-y. Wu, M. Ye and D.-A. Guo, Phytochemistry, 2014, 106, 7–24 CrossRef CAS PubMed.
- C. H. Lee and J.-H. Kim, J. Ginseng Res., 2014, 38, 161–166 CrossRef CAS PubMed.
- L. Peng, S. Sun, L.-H. Xie, S. M. Wicks and J.-T. Xie, Cardiovasc. Ther., 2012, 30, e183–e188 CrossRef CAS.
- J.-H. Kim, J. Ginseng Res., 2012, 36, 16–26 CrossRef CAS PubMed.
- J.-M. Lü, Q. Yao and C. Chen, Curr. Vasc. Pharmacol., 2009, 7, 293–302 CrossRef.
- H. J. Kim, P. Kim and C. Y. Shin, J. Ginseng Res., 2013, 37, 8–29 CrossRef CAS PubMed.
- S.-Y. Nah, D.-H. Kim and H. Rhim, CNS Drug Rev., 2007, 13, 381–404 CAS.
- S.-Y. Nah, Front. Physiol., 2014, 5, 1–13 Search PubMed.
- Y. Cheng, L. H. Shen and J. T. Zhang, Acta Pharmacol. Sin., 2005, 26, 143–149 CrossRef CAS PubMed.
- J. H. Yang, S. J. Han, J. H. Ryu, I. S. Jang and D. H. Kim, Biol. Pharm. Bull., 2009, 32, 1710–1715 CAS.
- P. C. Shieh, C. W. Tsao, J. S. Li, H. T. Wu, Y. J. Wen, D. H. Kou and J. T. Cheng, Neurosci. Lett., 2008, 434, 1–5 CrossRef CAS.
- G. Z. Zhang, A. L. Liu, Y. B. Zhou, X. San, T. W. Jin and Y. Jin, J. Ethnopharmacol., 2012, 142, 754–761 CrossRef PubMed.
- I. Smith, E. M. Williamson, S. Putnam, J. Farrimond and B. J. Whalley, Nutr. Rev., 2014, 72, 319–333 CrossRef PubMed.
- H. Sungwon, Y. Yong, K. Kang, S. Y. Shin, Y. H. Lee and Y. Lim, J. Microbiol. Biotechnol., 2009, 19, 556–559 Search PubMed.
- S. Xu, L. Yang, Q. Lin, Z. Liu, Q. Feng, L. Ma and M. Liu, Chromatographia, 2008, 68, 459–462 CAS.
- D. Wang, Q.-R. Tan and Z.-J. Zhang, J. Mol. Neurosci., 2013, 51, 581–590 CrossRef CAS PubMed.
- R. Sun, K. Wang, D. Wu, X. Li and Y. Ou, Folia Neuropathol., 2012, 50, 270–276 CrossRef CAS.
- B.-Y. Cao, Y.-P. Yang, W.-F. Luo, C.-J. Mao, R. Han, X. Sun, J. Cheng and C.-F. Liu, J. Ethnopharmacol., 2010, 131, 122–129 CrossRef CAS PubMed.
- K. Wang, L. Zhu, X. Zhu, K. Zhang, B. Huang, J. Zhang, Y. Zhang, L. Zhu, B. Zhou and F. Zhou, Cell. Mol. Neurobiol., 2014, 34, 227–234 CrossRef CAS.
- O. A. Heikal, T. Akao, S. Takeda and M. Hattori, Biol. Pharm. Bull., 1997, 20, 517–521 CAS.
- S. L. Hsiu, Y. T. Lin, K. C. Wen, Y. C. Hou and P. D. L. Chao, Planta Med., 2003, 69, 1113–1118 CrossRef CAS PubMed.
- X. Li, Y. Li, J. Chen, J. Sun, X. Li, X. Sun and X. Kang, Neurosci. Lett., 2010, 483, 1–5 CrossRef CAS PubMed.
- F.-L. Sun, L. Zhang, R.-Y. Zhang and L. Li, Eur. J. Pharmacol., 2011, 660, 283–290 CrossRef CAS PubMed.
- F. Zhang, Y.-Y. Wang, J. Yang, Y.-F. Lu, J. Liu and J.-S. Shi, Oxid. Med. Cell. Longevity, 2013, 2013, 680545 Search PubMed.
- G. Y. Lv, Z. H. Lou, S. H. Chen, H. Gu and L. T. Shan, J. Ethnopharmacol., 2011, 137, 449–456 CrossRef CAS.
- H. Gong, Z. He, A. Peng, X. Zhang, B. Cheng, Y. Sun, L. Zheng and K. Huang, Sci. Rep., 2014, 4, 5648 CAS.
- M. Convertino, R. Pellarin, M. Catto, A. Carotti and A. Caflisch, Protein Sci., 2009, 18, 792–800 CAS.
- M. Pickhardt, Z. Gazova, M. von Bergen, I. Khlistunova, Y. P. Wang, A. Hascher, E. M. Mandelkow, J. Biernat and E. Mandelkow, J. Biol. Chem., 2005, 280, 3628–3635 CrossRef CAS.
- Y.-Y. Tian, L.-J. An, L. Jiang, Y.-L. Duan, J. Chen and B. Jiang, Life Sci., 2006, 80, 193–199 CrossRef CAS PubMed.
- B. Jiang, J. Du, L. Jian-hui, Y.-M. Bao and L.-J. An, Brain Res., 2008, 1188, 139–147 CrossRef CAS PubMed.
- Y.-Y. Tian, B. Jiang, L.-J. An and Y.-M. Bao, Eur. J. Pharmacol., 2007, 568, 142–148 CrossRef CAS PubMed.
- J.-N. Dai, Y. Zong, L.-M. Zhong, Y.-M. Li, W. Zhang, L.-G. Bian, Q.-L. Ai, Y.-D. Liu, J. Sun and D. Lu, PLoS One, 2011, 6(7), e21891 CAS.
- G. Jiang, H. Wu, Y. Hu, J. Li and Q. Li, Cell. Mol. Neurobiol., 2014, 34, 591–602 CrossRef CAS PubMed.
- H. Kumar, I. S. Kim, S. V. More, B. W. Kim, Y. Y. Bahk and D. K. Choi, J. Evidence-Based Complementary Altern. Med., 2013, 2013, 514095 Search PubMed.
- Q. Wang, G. Chen and S. Zeng, J. Pharm. Biomed. Anal., 2008, 46, 399–404 CrossRef CAS PubMed.
- Y.-F. Xian, Z.-X. Lin, Q.-Q. Mao, Z. Hu, M. Zhao, C.-T. Che and S.-P. Ip, J. Evidence–Based Complementary Altern. Med., 2012, 2012, 802625 Search PubMed.
- D. Yuan, B. Ma, J.-y. Yang, Y.-Y. Xie, L. Wang, L.-J. Zhang, Y. Kano and C.-F. Wu, Int. Immunopharmacol., 2009, 9, 1549–1554 CrossRef CAS PubMed.
- Y. Song, R. Qu, S. Zhu, R. Zhang and S. Ma, Phytother. Res., 2012, 26, 1528–1533 CAS.
- X. M. Wu, Z. M. Qian, L. Zhu, F. Du, W. H. Yung, Q. Gong and Y. Ke, Br. J. Pharmacol., 2011, 164, 332–343 CrossRef CAS PubMed.
- Y. Peng, J. Sun, S. Hon, A. N. Nylander, W. Xia, Y. Feng, X. Wang and C. A. Lemere, J. Neurosci., 2010, 30, 8180–8189 CrossRef CAS.
- S. Ma, S. Xu, B. Liu, J. Li, N. Feng, L. Wang and X. Wang, Naunyn–Schmiedeberg's Arch. Pharmacol., 2009, 379, 565–574 CrossRef CAS PubMed.
- M. Srinivasan, A. R. Sudheer and V. P. Menon, J. Clin. Biochem. Nutr., 2007, 40, 92–100 CrossRef CAS PubMed.
- T. O. Cheng, Int. J. Cardiol., 2007, 121, 9–22 CrossRef PubMed.
- L. M. Zhou, Z. Zuo and M. S. S. Chow, J. Clin. Pharmacol., 2005, 45, 1345–1359 CrossRef CAS PubMed.
- X. Chen, J. Guo, J. Bao, J. Lu and Y. Wang, Med. Res. Rev., 2014, 34, 768–794 CrossRef CAS PubMed.
- L. Chen, Y. Lu and S. Z. Zheng, Chin. Tradit. Herb. Drugs, 2009, 40, 476–479 CAS.
- J. Y. Han, J. Y. Fan, Y. Horie, S. Miura, D. H. Cui, H. Ishii, T. Hibi, H. Tsuneki and I. Kimura, Pharmacol. Ther., 2008, 117, 280–295 CrossRef CAS PubMed.
- L. Cui, W. Chan, J.-L. Wu, Z.-H. Jiang, K. Chan and Z. Cai, Talanta, 2008, 75, 1002–1007 CrossRef CAS PubMed.
- C. H. Liu, X. J. Liu and H. S. Yang, Chin. Arch. Tradit. Chin. Med., 2006, 24, 1280–1281 Search PubMed.
- Y. Chen, G. Wei, H. Nie, Y. Lin, H. Tian, Y. Liu, X. Yu, S. Cheng, R. Yan, Q. Wang, D. H. Liu, W. Deng, Y. Lai, J. H. Zhou, S. X. Zhang, W. W. Lin and D. F. Chen, Brain Res., 2014, 1552, 41–54 CrossRef CAS PubMed.
- Z.-T. Mo, Y.-Q. Fang, Y.-P. He and S. Zhang, Acta Pharmacol. Sin., 2012, 33, 737–742 CrossRef CAS PubMed.
- S. Manikandan and R. S. Devi, Pharmacol. Res., 2005, 52, 467–474 CrossRef CAS.
- Q. Chen, Y. G. Cao and C. H. Zhang, Acta Pharmacol. Sin., 2002, 37, 913–917 CAS.
- Y. Hu, J. Li, P. Liu, X. Chen, D.-H. Guo, Q.-S. Li and K. Rahman, J. Biomed. Biotechnol., 2012, 2012, 1–5 Search PubMed.
- Y. Hu, M.-Y. Liu, P. Liu, X. Dong and A. D. W. Boran, J. Mol. Neurosci., 2014, 53, 600–607 CrossRef CAS.
- Y. Ikeya, K. Sugama, M. Okada and H. Mitsuhashi, Chem. Pharm. Bull., 1991, 39, 6 CrossRef.
- Y. Ikeya, S. Takeda, M. Tunakawa, H. Karakida, K. Toda and T. Yamaguchi, Biol. Pharm. Bull., 2004, 27, 1081–1085 CAS.
- J. W. Zhang and Q. Zhao, China J. Chin. Med., 2013, 28, 550–553 CAS.
- B. Q. Chen, G. J. Du and Q. T. Xu, J. Chin. Med. Mater., 2002, 25, 429–430 CrossRef.
- S. M. Guo, X. W. Fan and J. W. He, J. Chin. Med. Mater., 1998, 21, 578 Search PubMed.
- J. W. Fu, W. Qiao and Z. H. Chen, Journal of Tianjin Medical University, 2005, 11, 52–54 Search PubMed.
- B. L. Li, C. T. Xia and B. X. Yuan, Journal of Xi'an Jiaotong University (Medical Sciences), 2008, 29, 227–229 Search PubMed.
- C. Wang, Z. L. You, Q. Xia, T. Xiong, Y. Xia and D. Z. Yao, Acta Pharmacol. Sin., 2007, 28, 334–338 CrossRef CAS PubMed.
- S. Z. Li and X. P. Wang, Chin. Tradit. Pat. Med., 1999, 21, 88–89 Search PubMed.
- L. J. Sun, J. Hebei Norm. Univ., Nat. Sci. Ed., 2001, 25, 217–218 CAS.
- N. Nishiyama, Y.-L. Wang, J. Y. Kaimori, A. Ishihara and H. Saito, Phytother. Res., 2006, 6, 289–293 CrossRef.
- K. A. Youdim, A. Martin and J. A. Joseph, Int. J. Dev. Neurosci., 2000, 18, 383–399 CrossRef CAS.
- Y. Dong, B. Xu, L. Lin and Q. Za, Food & Machinery, 2005, 21, 85–88 Search PubMed.
- H. Zhang, Y. Liu, M. Lao, Z. Ma and X. Yi, Exp. Gerontol., 2011, 46, 30–37 CrossRef PubMed.
- Q. Zhang, W.-d. Huang, X.-y. Lv and Y.-m. Yang, Cell Biol. Int., 2012, 36, 419–426 CrossRef CAS PubMed.
- G. Wang, L. Zhou, Y. Zhang, M. Dong, X. Li, J. Liu and Y. Niu, Neurosci. Lett., 2011, 487, 88–93 CrossRef CAS PubMed.
- Y. Zou, B. Hong, L. Fan, L. Zhou, Y. Liu, Q. Wu, X. Zhang and M. Dong, Free Radical Res., 2013, 47, 55–63 CrossRef CAS PubMed.
- H.-Q. Chen, X.-J. Wang, Z.-Y. Jin, X.-M. Xu, J.-W. Zhao and Z.-J. Xie, Neurosci. Res., 2008, 62, 123–130 CrossRef CAS PubMed.
- C.-M. Lin, R.-D. Lin, S.-T. Chen, Y.-P. Lin, W.-T. Chiu, J.-W. Lin, F.-L. Hsu and M.-H. Lee, Phytochemistry, 2010, 71, 2147–2156 CrossRef CAS.
- J. K. Prasain, K. Jones, N. Brissie, R. Moore, J. M. Wyss and S. Barnes, J. Agric. Food Chem., 2004, 52, 3708–3712 CrossRef CAS PubMed.
- O. Pancharoen, U. Prawat and P. Tuntieachwuttiul, in Studies in Natural Products Chemistry, ed. Atta-ur-Rahman, Elsevier Science B. V., The Netherlands, 2000, pp. 797–865 Search PubMed.
- T. Ahmed and A.-H. Gilani, Phytother. Res., 2014, 28, 517–525 CrossRef CAS PubMed.
- M. Metzler, E. Pfeiffer, S. I. Schulz and J. S. Dempe, BioFactors, 2013, 39, 14–20 CrossRef CAS PubMed.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 2001, 46, 3–26 CrossRef CAS.
- C. A. Lipinski, Drug Discovery Today: Technol., 2004, 1, 337–341 CrossRef CAS PubMed.
- D. F. Veber, S. R. Johnson, H. Y. Cheng, B. R. Smith, K. W. Ward and K. D. Kopple, J. Med. Chem., 2002, 45, 2615–2623 CrossRef CAS PubMed.
- M. Rasool, A. Malik, M. S. Qureshi, A. Manan, P. N. Pushparaj, M. Asif, M. H. Qazi, A. M. Qazi, M. A. Kamal, S. H. Gan and I. A. Sheikh, J. Evidence-Based Complementary Altern. Med., 2014, 2014, 1–7 Search PubMed.
- Instant JChem, ChemAxon Ltd., 6.2.1. edn, 2014 Search PubMed.
- R. J. Quinn, A. R. Carroll, N. B. Pham, P. Baron, M. E. Palframan, L. Suraweera, G. K. Pierens and S. Muresan, J. Nat. Prod., 2008, 71, 464–468 CrossRef CAS PubMed.
- M. Feher and J. M. Schmidt, J. Chem. Inf. Comput. Sci., 2003, 43, 218–227 CrossRef CAS PubMed.
- J. Larsson, J. Gottfries, S. Muresan and A. Backlund, J. Nat. Prod., 2007, 70, 789–794 CrossRef CAS PubMed.
- G. F. Ecker and C. R. Noe, Curr. Med. Chem., 2004, 11, 1617–1628 CrossRef CAS PubMed.
- N. J. Abbott, J. Inherited Metab. Dis., 2013, 36, 437–449 CrossRef CAS PubMed.
- D. B. Stanimirovic, M. Bani-Yaghoub, M. Perkins and A. S. Haqqani, Expert Opin. Drug Discovery, 2014, 10, 1–15 CrossRef PubMed.
- W. M. Pardridge, Neurotherapeutics, 2005, 2, 3–14 CrossRef PubMed.
- J. Yuan, J. Cui, W. Shang, J. Liu and Z. Zhang, Sci. Sin.: Chim., 2010, 40, 717–724 Search PubMed.
- H. Wu and Y. Fang, Acta Pharmacol. Sin., 2004, 39, 836–838 Search PubMed.
- H. Tang and R. Li, J. Tradit. Chin. Med., 2002, 18, 40–41 Search PubMed.
- Y. Yuan, X. Lin, Y. Feng, D.-S. Xu and Y.-H. Wang, Chin. Pharm. J., 2010, 45, 694–697 CAS.
Footnote |
| † The structures with an asterisked number means their stereochemistry was unidentified or unclear in the publications. |
| This journal is © The Royal Society of Chemistry 2016 |