Open Access Article
Open Access ArticleWater-stable metal–organic frameworks (MOFs): rational construction and carbon dioxide capture
Cao
Xiao
ab,
Jindou
Tian
a,
Qihui
Chen
 *ab and
Maochun
Hong
*ab and
Maochun
Hong
 *ab
*ab
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, 350002, P. R. China. E-mail: chenqh@fjirsm.ac.cn; hmc@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing, 100049, P. R. China
First published on 10th January 2024
Abstract
Metal–organic frameworks (MOFs) are considered to be a promising porous material due to their excellent porosity and chemical tailorability. However, due to the relatively weak strength of coordination bonds, the stability (e.g., water stability) of MOFs is usually poor, which severely inhibits their practical applications. To prepare water-stable MOFs, several important strategies such as increasing the bonding strength of building units and introducing hydrophobic units have been proposed, and many MOFs with excellent water stability have been prepared. Carbon dioxide not only causes a range of climate and health problems but also is a by-product of some important chemicals (e.g., natural gas). Due to their excellent adsorption performances, MOFs are considered as a promising adsorbent that can capture carbon dioxide efficiently and energetically, and many water-stable MOFs have been used to capture carbon dioxide in various scenarios, including flue gas decarbonization, direct air capture, and purified crude natural gas. In this review, we first introduce the design and synthesis of water-stable MOFs and then describe their applications in carbon dioxide capture, and finally provide some personal comments on the challenges facing these areas.
1. Introduction
Metal–organic frameworks (MOFs) are a kind of organic–inorganic hybrid material with intramolecular pores formed by coordination self-assembly of organic ligands and metal ions/clusters.1 Compared with traditional porous materials (such as porous carbons and zeolite molecular sieves), the pore size and pore surface of MOFs can be precisely regulated by reasonable design of building elements and modification of pores.2–17 In addition, the structure of MOFs is long-range ordered, which enables the structure–activity relationships of MOFs easy to be revealed by single crystal X-ray diffraction (SCXRD).18–21 In this context, MOFs have been widely used in various fields, such as gas adsorption and separation,22–37 pollutant capture,38–40 electrical/ionic conduction,41–43 selective recognition,44–46 and heterogeneous catalysis.47–50 At the initial stage, scientists mainly focused on the synthesis of MOFs with novel structures and the exploration of new functions of MOFs. However, if MOFs are to be used in the real world as practical materials rather than just as potential materials, they must meet the requirements of practical applications, such as economy, processability and stability.51 Given the ubiquity of water vapor in the real world, water stability is the first hurdle to be overcome in the practical applications of MOFs. Over the past decade, hundreds of water-stable MOFs have been prepared (Scheme 1 and Table 1), such as famous MILs, UiOs, PCNs, BUTs, ZIFs, and MAFs. With the successful synthesis of more and more water-stable MOFs, the understanding of the water stability of MOFs has also been deepened.52–62 For instance, the selection of suitable metals and ligands with matching softness/hardness can effectively construct water-stable MOFs, and the introduction of hydrophobic groups into the pores or surfaces of MOFs can further improve their water stability. Recently, a series of water-stable MOFs have been successfully constructed, and a timely summary can provide a guide for the controllable construction of more stable MOFs that can be used in a harsher environment.| MOFs | Metal | Ligands | Testing condition | Time | Ref. |
|---|---|---|---|---|---|
| a Linkers are abbreviated as: BDC = terephthalate; BDC-NH2 = 2-aminoterephthalate; BTC = benzene-1,3,5-tricarboxylate; H2NDC(SO3H)2 = 4,8-disulfonaphthalene-2,6-dicarboxylatlate; H4tcpp = 2,3,5,6-tetrakis(4-carboxyphenyl)pyrazine; BPDC = biphenyl-4,4′-dicarboxylate; BCQD = 5′,5′′′-bis(4-carboxyphenyl)-[1,1′:3′,1′′:4′′,1′′′:3′′′,1′′′′-quinquephenyl]-4,4′′′′-dicarboxylate, PBPTT = 4,4′,4′′,4′′′-(4,4′-(1,4-phenylene)bis(pyridine-6,4,2-triyl))tetrabenzoate, H4BTEB = 4,4′,4′′,4′′′-(benzene-1,2,4,5-tetrayltetrakis(ethyne-2,1-diyl)) tetrabenzoic acid, H4CPTTA = 5′-(4-carboxyphenyl)-[1,1′:3′,1′′-terphenyl]-3,4′′,5-tricarboxylic acid, TPDC-2CH3 = 2′,5′-dimethyl-terphenyl-4,4′′-dicarboxylic acid, TPDC-4CH3 = 2′,3′,5′,6′-tetramethyl-terphenyl-4,4′′-dicarboxylic acid, TPDC-4CH2N3- = 2′,3′,5′,6′-tetra(azidomethyl)-[1,1′:4′,1′′-terphenyl]- 4,4′′-dicarboxylate, H2dmcapz = 3,5-dimethyl-4-carboxypyrazole, H2TCPP = tetrakis(4-carboxyphenyl)porphyrin, H3TZBPDC = 4′-(1H-tetrazol-5-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid, Hetz = 3,5-diethyl-1,2,4-triazole, H2btm = bis(5-methyl-1H-1,2,4-triazol-3-yl)methane; btz = 1,4-bis(4H-1,2,4-triazol-4-yl)benzene; H3BTN = 1,3,5-tri(6-hydroxycarbonylnaphthalen-2-yl)benzene); H3BTB = 1,3,5-tris(4-carboxyphenyl)benzene); H2imPim = 2-(1H-imidzol-2-yl)-3H-imidazo[4,5-c]pyridine; 1,4-BDP = 1,4-benzenedi(4′-pyrazolyl); 1,3-BDP = 1,3-benzenedi(4′-pyrazolyl); H2bdpb = 1,4-bis[(3,5-dimethyl)-pyrazol-4-yl]benzene; H3BTP = 1,3,5-tris(1H-pyrazol-4-yl)benzene; H3TPTA = 2,4,6-tris(4-(pyrazolate-4-yl)phenyl)-1,3,5-triazine; H3BTTri = 1,3,5-tris(1H-1,2,3-triazol-5-yl)benzene; H2fbdim = 2,6-ditrifluoromethyl-benzodiimidazole; H2DTBDA = 3′,5′-di(1H-1,2,4-triazol1-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid; H3CTTA = dimethyl-5′-(4-(methoxycarbonyl)phenyl)-2′,4′,6′-trimethyl-[1,1′:3′,1′′- terphenyl]-4,4′′-dicarboxylic acid; BPDC-4Me = 3,3′,5,5′-tetramethyl-1,1′-biphenyl; TPDC-2CH2N3 = 2′,5′-bis(azidomethyl)-[1,1′:4′,1′′-terphenyl]-4,4′′-dicarboxylic acid; H3TTNA = 6,6′,6′′-(2,4,6-trimethylbenzene-1,3,5-triyl)tris(2-naphthoic acid)); H2BDB = [1,1′:3′,1′′-terphenyl]-4,4′′-dicarboxylic acid; H4TCPB-Br2 = (1,4-dibromo-2,3,5,6-tetrakis(4-carboxyphenyl)benzene; BTBA = biphenyl-3,4,5-tricarboxylic acid; H2FTZB = 2-fluoro-4-(1H-tetrazol-5-yl)benzoic acid; 1,4-H2NDC = 1,4-naphthalenedicarboxylate; MeIM = 2-methyl-1H-imidazole; bIM = 1H-benzo[d]imidazole; IM = 2H-imidazole; nIM = 2-nitro-1H-imidazole; cbIM = 6-chloro-1H-benzo[d]imidazole; ICA = imidazolate-2-carboxyaldehyde; H2bbta = 1H,5H-benzo(1,2-d:4,5-d′)bistriazole); TPMA = tris(pyridin4-ylmethyl)amine; pyz-NH2 = 2-aminopyrazine; H4TPP = 5,10,15,20-tetra(1H-pyrazol-4-yl)porphyrin; H4TPPP = 5,10,15,20-tetra(pyrazolate-4-yl)porphyrin; TBPZ = 3,3′,5,5′-tetraethyl-4,4′-bipyrazolate; o-H2mpba = 2-(3,5-dimethyl-1H-pyrazol-4-yl)benzoic acid; 1,2,4-TAZT = 1,2,4-triazolate; H4DH3PhDC = 2′,5′-dimethyl-3,3′′-dihydroxy-[1,1′:4′,1′′-terphenyl]-4,4′′-dicarboxylic acid; dtp = 3,6-di(4-pyridyl)-1,2,4,5-tetrazine; pyr = pyrazine; HQc = quinoline-5-carboxylic acid; dobpdc = 4,4′-dioxido-3,3′-biphenyldicarboxylate; TABZ = 3,3′,5,5′-tetramethyl-1H,1′H-4,4′-bipyrazole; F-pymo = 5-fluoropyrimidin-2-olate, HINO = isonicotinic acid N-oxide; H4TBAPy-2 = 1,2,7,8-tetrakis(p-benzoic-acid)pyrene]; L4H = 4-[2-[3,6,8-tris[2-(4-carboxyphenyl) ethynyl]-pyren-1-yl]ethynyl]-benzoic acid; 5-Aa-IPA = 5-acetamidoisophthalate. | |||||
| MIL-101(Cr) | Cr(III) | BDC | Aqueous solution (pH = 0–12) | 2 months | 76 |
| MIL-101(Fe) | Fe(III) | BDC | Aqueous solution (pH = 1–12) | 1 day | 77 |
| MIL-53(Cr) | Cr(III) | BDC | Water | 2 days | 78 |
| 0.07 M HCl aqueous solution | |||||
| 0.07 M NaOH aqueous solution | |||||
| MIL-53(Al) | Al(III) | BDC | Water | 2 months | 78 |
| Aqueous solution (pH = 4–12) | 3 days | ||||
| In2S3@MIL-101 | Cr(III) | BDC | Water | — | 79 |
| Aqueous solution (pH = 3–8) | |||||
| NH2-MIL-53(Al) | Al(III) | BDC-NH2 | Water | 2 months | 80 |
| Aqueous solution (pH = 4, 12) | |||||
| MIL-101-Fe-NH2 | Fe(III) | BDC-NH2 | Aqueous solution (pH = 1–12) | 1 day | 81 |
| MIL-100(Cr) | Cr(III) | BTC | Water | 12 months | 82 |
| MIL-100(Fe) | Fe(III) | BTC | Water | — | 83 |
| MIL-100(Al) | Al(III) | BTC | Water | — | 84 |
| BUT-18 | Al(III) | H3CTTA | Water, boiling water | 24 h | 85 |
| Aqueous solution (pH = 1) | |||||
| Aqueous solution (pH = 10) | |||||
| BUT-8(Cr) | Cr(III) | H2NDC(SO3H)2 | 10 M H2SO4 aqueous solution | 1 week | 86 |
| Concentrated HCl solution | |||||
| UiO-66 | Zr(IV) | BDC | Aqueous solution (pH = 0–12) | 2 months | 87 |
| NH2-UiO-66 | Zr(IV) | BDC | Water | 2 months | 80 |
| 12 M HCl and 16 M HNO3 | |||||
| UiO-67 | Zr(IV) | BPDC | Water | 2 months | 87 |
| Aqueous solution (pH = 4) | 3 days | ||||
| Aqueous solution (pH = 12) | 2 months | ||||
| UiO-67-4Me | Zr(IV) | BPDC-4Me | Water | 14 days | 88 |
| Aqueous solution (pH = 2, 12) at 80 °C | |||||
| UiO-67-4Me–NH2-38% | Zr(IV) | H2DABPDC | Water | 14 days | 88 |
| Aqueous solution (pH = 2, 12) at 80 °C | |||||
| PCN-56(Zr) | Zr(IV) | TPDC-2CH3 | Aqueous solution (pH = 2) | 2 days | 89 |
| Aqueous solution (pH = 11) | 1 day | ||||
| PCN-57(Zr) | Zr(IV) | TPDC-4CH3 | Aqueous solution (pH = 2) | 4.5 days | 89 |
| Aqueous solution (pH = 11) | 2 days | ||||
| PCN-58(Zr) | Zr(IV) | TPDC-2CH2N3 | Water | 24 h | 89 |
| Aqueous solution (pH = 2) | 24 h | ||||
| Aqueous solution (pH = 11) | 15 h | ||||
| PCN-59(Zr) | Zr(IV) | TPDC-4CH2N3 | Water | 72 h | 89 |
| Aqueous solution (pH = 2) | 20 h | ||||
| Aqueous solution (pH = 11) | 24 h | ||||
| PCN-223 | Zr(IV) | H2TCPP | Aqueous solution (pH = 0–10) | 24 h | 90 |
| PCN-225(Zr) | Zr(IV) | H2TCPP | Boiling water | 12 h | 146 |
| Aqueous solution (pH = 0–12) | |||||
| PCN-228(Zr) | Zr(IV) | H4TCP-1 | Water | 1 day | 91 |
| Aqueous solution (pH = 0) | |||||
| Aqueous solution (pH = 12) | |||||
| PCN-229(Zr) | Zr(IV) | H4TCP-2 | Water | 1 day | 91 |
| Aqueous solution (pH = 0) | |||||
| Aqueous solution (pH = 12) | |||||
| PCN-230(Zr) | Zr(IV) | H4TCP-2 | Aqueous solution (pH = 0–12) | 1 day | 91 |
| OPA-PCN-222(Zr) | Zr(IV) | H2TCPP | Water | 1 month | 92 |
| Aqueous solution (pH = 11) | 7 days | ||||
| BUT-12 | Zr(IV) | H3CTTA | Boiling water | 24 h | 93 |
| HCl solutions (2 M, 6 M, and concentrated HCl) | |||||
| Aqueous solution (pH = 10) | |||||
| BUT-13 | Zr(IV) | H3TTNA | Boiling water | 24 h | 93 |
| HCl solutions (2 M, 6 M, and concentrated HCl) | |||||
| Aqueous solution (pH = 10) | |||||
| BUT-14 | Zr(IV) | BCQD | Boiling water | 48 h | 94 |
| Concentrated HCl aqueous solution | |||||
| Aqueous solution (pH = 10) | |||||
| BUT-15 | Zr(IV) | PBPTT | Boiling water | 48 h | 94 |
| Concentrated HCl aqueous solution | |||||
| Aqueous solution (pH = 10) | |||||
| BUT-17 | Zr(IV) | H4CPTTA | Boiling water | — | 95 |
| Concentrated HCl aqueous solution | |||||
| Aqueous solution (pH = 12) | |||||
| BUT-44 | Zr(IV) | H4BTEB | Water | 72 h | 96 |
| Aqueous solution (pH = 10) | 1 day | ||||
| Aqueous solutions (1 M HCl) | 1 day | ||||
| BUT-66 | Zr(IV) | H2BDB | Water | 1 month | 97 |
| Aqueous solution (pH = 1–10) | |||||
| BUT-67 | Zr(IV) | H2NDB | Water | 48 h | 97 |
| Aqueous solution (pH = 1–10) | |||||
| MOF-808 | Zr(IV) | BTC | Boiling water | 24 h | 98 |
| NU-1100 | Zr(IV) | L4H | Water | 24 h | 99 |
| H2BPDC | |||||
| ISO-NU-1000 | Zr(IV) | H4TBAPy-2 | Water | 24 h | 100 |
| MIP-207 | Ti(IV) | BTC | Water | 3 days | 101 |
| MIP-208 | Ti(IV) | 5-Aa-IPA | Boiling water | 8 h | 102 |
| Aqueous solution (pH = 0–10) | |||||
| BUT-172 | In(III) | H3CTTA | Boiling water | 1 day | 103 |
| Aqueous solution (pH = 3, 10) | |||||
| BUT-173 | In(III) | H3CTTA | Water | 1 day | 103 |
| Aqueous solution (pH = 3, 10) | |||||
| In(tcpp) | In(III) | H4TCPP | Aqueous solution (pH = 2, 12) | 24 h | 104 |
| Water | |||||
| USTC-8(In) | In(III) | H2TCPP | Aqueous solution (pH = 0–11) | 12 h | 105 |
| Y-BTC | Y(III) | BTC | Water | — | 106 |
| Y-pek-MOF-1 | Y(III) | BTBA | Water at 85 °C | 24 h | 107 |
| [(CH3)2NH2]2[Tb6(μ3-OH)8(FTZB)6(H2O)6]·(H2O)22 | Y(III) | H2FTZB | Water | 24 h | 108 |
| Tb-BTC | Tb(III) | H2FTZB | Water | — | 106 |
| Tb-pek-MOF-1 | Tb(III) | BTBA | Water at 85 °C | 24 h | 107 |
| [(CH3)2NH2]2[Y6(μ3-OH)8(FTZB)6(H2O)6]·(H2O)52 | Tb(III) | H2FTZB | Water | 24 h | 108 |
| La-BTN | La(III) | H3BTN | Water | 1 day | 109 |
| Aqueous solution (pH = 2) at 100 °C | |||||
| Aqueous solution (pH = 12) | |||||
| La-BTB | La(III) | H3BTB | Water at 60 °C and 100 °C | 3 days | 110 |
| Aqueous solution (pH = 2–14) | |||||
| Eu-1,4-NDC-fcu-MOF | Eu(III) | 1,4-H2NDC | Boiling water | 24 h | 111 |
| Aqueous solution (pH = 3.5–10) | |||||
| PCN-202 | Ni(II) | 1,4-H2NDC | Aqueous solution (pH = 12) | 1 day | 112 |
| PCN-600(Fe) | Fe(III) | 1,4-H2NDC | Aqueous solution (pH = 2–11) | 24 h | 113 |
| ZIF-8/MAF-4 | Zn(II) | MeIM | Boiling water | 7 days | 114 and 115 |
| MAF-23 | Zn(II) | H2btm | Water | 7 days | 116 |
| MAF-stu-1 | Zn(II) | H2imPim | Boiling water | 9 days | 117 |
| Aqueous solution (pH = 2, 11) | |||||
| Zn(1,4-BDP) | Zn(II) | 1,4-BDP | Water at 40 °C | — | 118 |
| Zn(1,3-BDP) | Zn(II) | 1,3-BDP | Boiling water | 3 days | 118 |
| ZIF-11 | Zn(II) | bIM | Water | 7 days | 119 |
| ZIF-61 | Zn(II) | IM, MeIM | Boiling water | 7 days | 120 |
| ZIF-68 | Zn(II) | bIm, nIM | Boiling water | 7 days | 120 |
| ZIF-69 | Zn(II) | cbIM, nIM | Boiling water | 7 days | 120 |
| ZIF-70 | Zn(II) | IM, nIM | Boiling water | 7 days | 120 |
| ZIF-90 | Zn(II) | ICA | Water | 7 days | 120 |
| ZIF-67 | Co(II) | MeIM | Boiling water | 7 days | 120 |
| MFU-1 | Co(II) | H2bdpb | Water | 6 months | 121 |
| MAF-X27-Cl | Co(II) | H2bbta | Acidic solution (0.001 M HCl) | 1 week | 122 |
| Alkaline solution (1.0 M KOH) | |||||
| FJI-H30 | Co(II) | TPMA | Water | 5 days | 123 |
| Aqueous solution (pH = 2–12) | |||||
| ZJU-75 | Co(II) | pyz-NH2 | Water | 3 days | 124 |
| Aqueous solution (pH = 1–12) | |||||
| Co-btz-ht | Co(II) | btz | Aqueous solution (pH = 1–12) | 20 h | 125 |
| PCN-601 | Ni(II) | H4TPP | Saturated NaOH solution at 100 °C | 24 h | 126 |
| 0.1 mM HCl aqueous solution | |||||
| PCN-602(Ni) | Ni(II) | H4TPPP | 0.1 mM HCl aqueous solution | 24 h | 127 |
| 1 M NaOH aqueous solution | |||||
| Ni3(BTP)2 | Ni(II) | H3BTP | Boiling water | 2 weeks | 128 |
| Boiling aqueous solution (pH = 2–14) | |||||
| Ni-btz-ht | Ni(II) | btz | Aqueous solution (pH = 1–12) | 20 h | 125 |
| BUT-32 | Ni4 clusters | H3TPTA | Boiling water | 24 h | 129 |
| Aqueous solution (pH = 3) | |||||
| NaOH aqueous solution (4 M) | |||||
| BUT-33 | Ni4 clusters | H3TPTA | Boiling water | 24 h | 129 |
| Aqueous solution (pH = 3) | |||||
| NaOH aqueous solution (4 M) | |||||
| Cu-BTTri | Cu(II) | H3BTTri | Boiling water | 3 days | 130 |
| Aqueous solution (pH = 3) | 1 day | ||||
| MAF-2 | Cu(I) | Hetz | Water | — | 131 |
| MAF-41 | Cu(I) | H2fbdim | Water | One week | 132 |
| Boiling water | One week | ||||
| Aqueous solution (pH = 3–14) | 3 days | ||||
| Cu2TBPZ | Cu(I) | TBPZ | Water | 24 h | 133 |
| Aqueous solution (pH = 3, 12) | |||||
| [Ag2(o-Hmpba)2(o-H2mpba)2] | Ag(I) | o-H2mpba | Water | 8 months | 135 |
| Aqueous solution (pH = 2–13) | 1 week | ||||
| FJI-H14 | Cu(II) | H2BTTA | Aqueous solution (pH = 2–12) at 100 °C | 24 h | 136 |
| FJI-H25Fe | Fe(III) | H2BTTA | Aqueous solution (pH = 8–12) | — | 137 |
| FJI-H29 | Zn(II) | H2DTBDA | Water | 2 days | 138 |
| Aqueous solution (pH = 2–13) | |||||
| [Zn4(μ4-O)-(μ4-4-carboxy-3,5-dimethyl-4-carboxy-pyrazolato)3] | Zn(II) | H2dmcapz | Water | — | 139 |
| 0.001 M HNO3 aqueous solution | |||||
| USTC-7 | Zn(II) | H3TZBPDC | Aqueous solution (pH = 2–12) | 12 h | 140 |
| FJI-H36 | Ni(II) | H2DTBDA | Water | — | 141 |
| Aqueous solution (pH = 2–12) | |||||
The continued increase of atmospheric carbon dioxide (CO2) has led to a number of serious climate problems, including rising sea levels and rising temperatures. In addition to causing climate change, high levels of atmospheric CO2 can also cause a range of health problems for humans in confined spaces (e.g., submarines and airplanes). Furthermore, as an undesired concomitant of many important fine chemicals (e.g., natural gas), CO2 has greatly affected their production and transportation. Therefore, there is an urgent need to develop efficient and economical carbon capture technologies to trap unwanted CO2 from air and fine chemicals. Due to their high adsorption capacity and relatively low recovery energy, MOFs have proven to be a kind of promising adsorbent for carbon capture and have been widely used in a variety of scenarios, including flue gas decarbonization, direct air capture, and purified natural gas.63–75
Considering that water vapor is widely present in flue gas, air, and crude natural gas, a practical MOF-based CO2 adsorbent should first have good water stability. To date, many water-stable MOFs that can realize efficient CO2 capture have been reported; and a timely collation of these advances can help scientists design and synthesize MOFs that can be used to capture CO2 in industry.
In this review, we begin with a brief introduction to the importance of water-stable MOFs and the urgency of carbon capture. Then, the definition of stability (including thermodynamic stability and kinetic stability), synthetic strategies (from universal strategies to specific approaches), and important progress (e.g., classic examples and recent advances) of water-stable MOFs are introduced in detail. In addition, the applications of water-stable MOFs in different scenarios (e.g., flue gas decarbonization, direct air capture, and purified natural gas) are systematically discussed. Finally, some personal comments on the challenges facing these areas are presented. Despite our best efforts, we have only been able to cover a small fraction of the many studies on water-stable MOFs. However, we also hope that our review will provide a useful reference for researchers interested in designing water-stable MOFs for CO2 capture.
2. Design and synthesis of water-stable MOFs
2.1. The definition and characterization of water stability of MOFs
Water stability is used to describe the extent to which a substance is affected by water, also known as water resistance. With regard to MOFs, their water stability refers to the ability to maintain their structure when exposed to water. For a given MOF, if it is exposed to water for a long time, its ordered structure will not be damaged, and its porous properties can be maintained, and it can be considered water-stable. In this context, the water stability of MOFs is usually evaluated by powder X-ray diffraction (PXRD) tests and gas adsorption experiments; in which PXRD analysis is used to determine whether the ordered structure of MOFs is destroyed, and a gas adsorption experiment is used to evaluate whether the porosity of MOFs is effectively maintained. It must be pointed out that the characterization of the water stability of MOFs is directly related to their application scenarios. For example, the use of MOFs in aqueous solutions may lead to the dissolution of partial MOFs. At this time, other tests (e.g., nuclear magnetic resonance (NMR) and inductive coupled plasma emission spectrometer (ICP)) are needed to accurately evaluate their water stability.2.2. The thermodynamic stability of MOFs
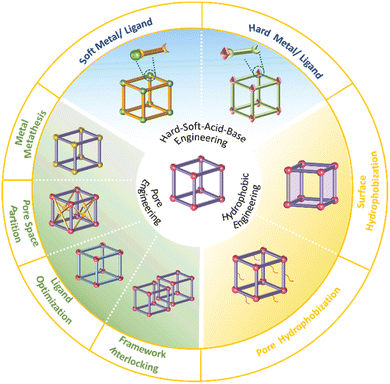
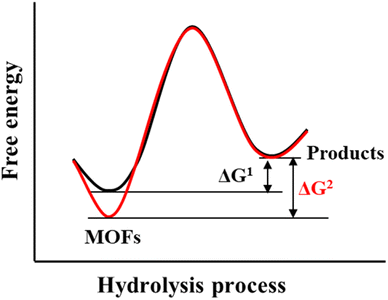
The hydrolysis of MOFs is a complex chemical transformation, in which the nucleophilic H2O will attack the electrophilic metal ions, and the incompletely dissociated organic ligands will in turn continue to bond with the metal ions; only after a series of complex reactions, the hydrolytic products which are favourable both in kinetics and thermodynamics will be formed. For a given MOF, its thermodynamic stability to water refers to the ΔG of the hydrolysis reaction (Scheme 2). If the ΔG is greater than 0, then its hydrolysis cannot occur spontaneously and it can be considered water-stable. According to the Gibbs equation ΔG = ΔH − TΔS, if ΔG is to be greater than 0, it must be ensured that ΔH is much greater than 0, because hydrolysis of MOFs is a process of entropy increasing (ΔS > 0). This means that the metal–ligand interactions of a water-stable MOF should be greater than the corresponding metal–oxygen interactions formed by hydrolysis. Thus, only the coordination elements with strong enough interaction (generally greater than the corresponding metal–water interaction) can be used to synthesize water-stable MOFs.To optimize the thermodynamic stability of MOFs in water, it is necessary to increase the bonding strength of metal ions and organic ligands as much as possible. For any MOF, the organic ligand acts as a Lewis base that provides electrons, while the metal ion acts as a Lewis acid that accepts electrons. According to Hard–Soft-Acid–Base (HSAB) theory, Lewis acids/bases can be divided into three categories, including hard acids/bases, soft acids/bases, and borderline acids/bases. Hard acids/bases refer to particles with a high charge density and small radius, which have low polarizability but high polarity. Soft acids/bases are those particles with a low charge density and large radius, which have high polarizability but low polarity. Thermodynamically stable complexes are preferentially constructed from hard acids and hard bases or from soft acids and soft bases. In this regard, water-stable MOFs can be directly constructed from hard metals (e.g., Al3+, Zr4+, In3+, Ti4+, Hf4+, Fe3+, and Cr3+)/ligands (carboxylic acid ligands or other ligands that contain free oxygen atoms) and soft metals (e.g., Zn2+ and Cu+)/ligands (e.g., imidazolyl derivatives, triazolyl derivatives, and tetrazolyl derivatives). In addition, post-synthesis modification (PSM) can also be used to improve the water stability of the as-prepared MOFs.
2.3. The kinetic stability of MOFs
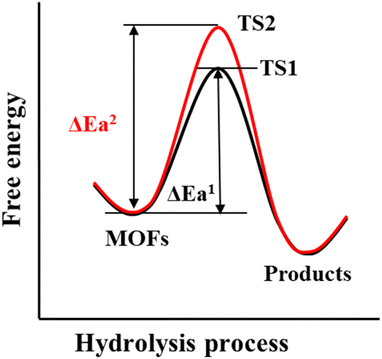
The thermodynamics of a given reaction can only reveal whether it can occur spontaneously. Whether the reaction can proceed smoothly also depends on its kinetics. For a thermodynamically unstable MOF, the hydrolysis reaction is also difficult to occur if the activation energy of hydrolysis is very high (Scheme 3). Therefore, the kinetic stability of a given MOF to water is related to its hydrolytic activation energy.To improve the kinetic stability of a MOF to water, the activation energy of hydrolysis should be increased as much as possible. The hydrolysis of the MOF is so complex that its activation energy is difficult to calculate.142,143 In this case, inhibition of the hydrolysis kinetics of a MOF can only be qualitatively analyzed by reaction rate theory. According to such theory, the hydrolysis reaction of MOFs can be inhibited by preventing the effective collision between H2O molecules and the metal ions of MOFs. In this regard, introducing enough hydrophobic groups on the surface of a MOF can effectively prevent the contact between H2O and the MOF. In addition, the introduction of appropriate hydrophobic groups into the pores of MOFs can effectively prevent the diffusion of water in the pores.
2.4. Reported water-stable MOFs
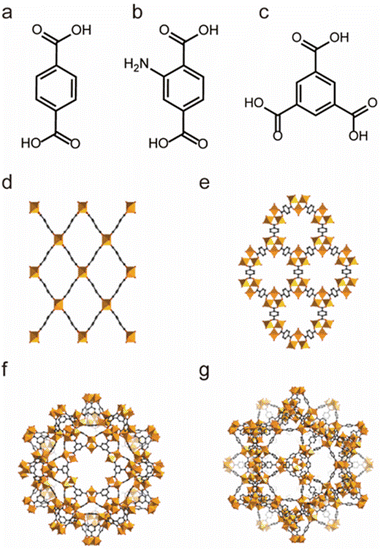 | ||
| Fig. 1 The structures of the MIL series and the used ligands. (a) The H2BDC ligand. (b) The H2BDC-NH2 ligand. (c) The 1,3,5-BTC ligand. (d) MIL-53. (e) MIL-88. (f) MIL-100. (g) MIL-101. Reproduced from ref. 144 with permission from Wiley, Copyright 2018. | ||
The Zr–O unit with very strong bond energy is another building block that is widely used in the synthesis of water-stable MOFs. UiO-66, as a representative example, constructed from 12-coordinated Zr6O4(OH)4(CO2)12 clusters and linear terephthalic acid (H2BDC) ligands, was reported by Lillerud and co-workers in 2008 (Fig. 2).87,145 Due to the extremely strong interactions between Zr4+ ions and O anions, UiO-66 not only displayed excellent water/acid/base resistance but also resisted various common organic solvents. Then other water-stable UiO series (e.g., UiO-67 and UiO-68) based on the same strategy were prepared by using other linear carboxylate ligands. Very recently, through the modification of ligands and the mixing of modified ligands, Su's group has achieved the controllable construction of UiO-67-4Me-R2-x% with excellent acid–base stability and adjustable hydrophilicity and hydrophobicity.88
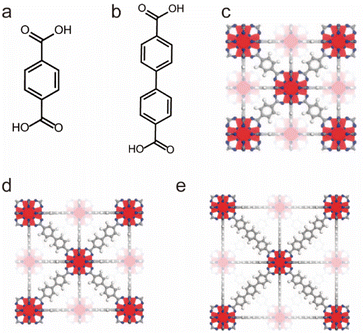 | ||
| Fig. 2 The structures of the UiO series and the used ligands. (a) The H2BDC ligand. (b) The H2BPDC ligand. (c) UiO-66. Reproduced from ref. 87 with permission from the American Chemical Society, Copyright 2008. (d) UiO-67. Reproduced from ref. 87 with permission from the American Chemical Society, Copyright 2008. (e) UiO-68. Reproduced from ref. 87 with permission from the American Chemical Society, Copyright 2008. | ||
Based on suitable Zr–O clusters and different carboxylate ligands, Zhou's group also reported a series of water-stable MOFs (PCN series) (Fig. 3). They first used single or mixed linear dicarboxylic acids bearing methyl or azide groups (Fig. 3a–c) to prepare three isoreticular MOFs, PCN-56, PCN-57 and PCN-58 with different functional groups.89 Notably, such as-prepared functionalized MOFs could keep their framework and crystallinity well for a long time in diluted HCl solution (pH = 2) and NaOH solution (pH = 11). Then they synthesized a series of more stable MOFs (PCN-225, PCN-228, PCN-229 and PCN-230) using porphyrin-derived tetracarboxylate ligands and zirconium salts. These newly prepared materials had better acid tolerance and their framework could be stabilized in 1 M HCl solution. This may be because porphyrin groups can also react with hydrogen ions.91,146,147
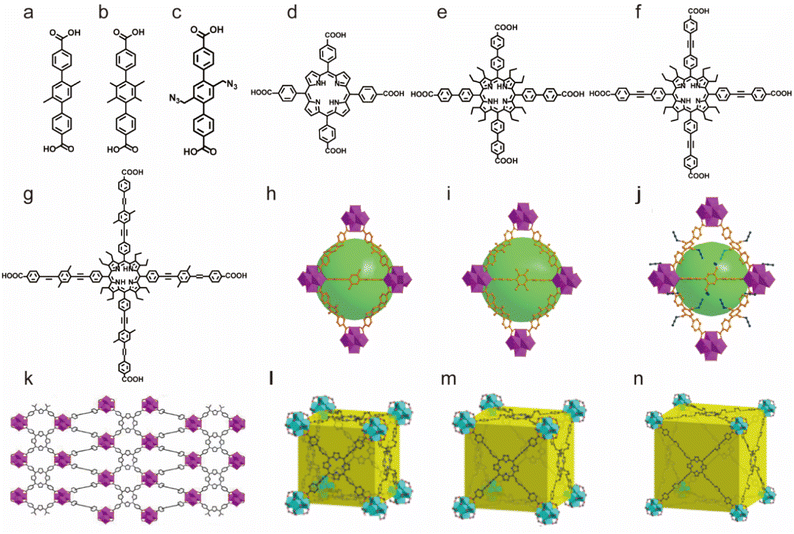 | ||
| Fig. 3 The structures of the PCN series and the used ligands. (a) The TPDC-2CH3 ligand. (b) The TPDC-4CH3 ligand. (c) The TPDC-2CH2N2 ligand. (d) The H2TCPP ligand. (e) The H4TCP-1 ligand. (f) The H4TCP-2 ligand. (g) The H4TCP-3 ligand. (h) PCN-56. Reproduced from ref. 89 with permission from the American Chemical Society, Copyright 2012. (i) PCN-57. Reproduced from ref. 89 with permission from the American Chemical Society, Copyright 2012. (j) PCN-58. Reproduced from ref. 89 with permission from the American Chemical Society, Copyright 2012. (k) PCN-225. Reproduced from ref. 146 with permission from the American Chemical Society, Copyright 2013. (l) PCN-228. Reproduced from ref. 91 with permission from the American Chemical Society, Copyright 2015. (m) PCN-229. Reproduced from ref. 91 with permission from the American Chemical Society, Copyright 2015. (n) PCN-230. Reproduced from ref. 91 with permission from the American Chemical Society, Copyright 2015. | ||
Li's group also prepared a series of water-stable Zr-based MOFs based on suitable polycarboxylate ligands (Fig. 4). All these reported porous materials possessed excellent chemical stability. For example, BUT-17 not only displayed strong stability in boiling water but also resisted concentrated HCl solution and NaOH aqueous solution (pH = 12).95 Notably, several of them (e.g., BUT 12–15 and 17) could resist concentrated HCl, which makes them one of the most acid-resistant MOFs.93,96,97
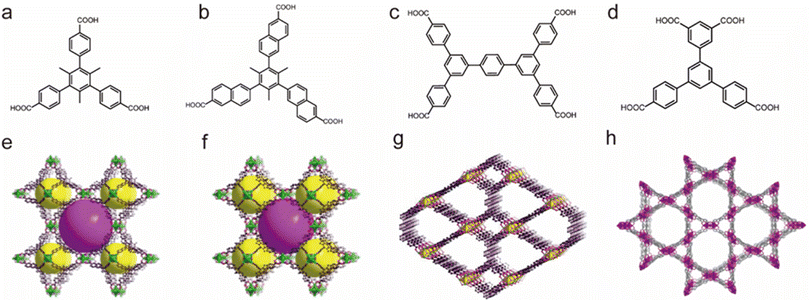 | ||
| Fig. 4 The structures of the BUT series and the used ligands. (a) The H3CTTA ligand. (b) The H3TTNA ligand. (c) The BCQD ligand. (d) The H4CPTTA ligand. (e) BUT-12. Reproduced from ref. 93 with permission from the American Chemical Society, Copyright 2016. (f) BUT-13. Reproduced from ref. 93 with permission from the American Chemical Society, Copyright 2016. (g) BUT-14. Reproduced from ref. 94 with permission from the American Chemical Society, Copyright 2017. (h) BUT-17. Reproduced from ref. 95 with permission from Springer Nature, Copyright 2019. | ||
In addition to Zr4+, Ti4+ ions can also be used to construct water-stable Ti-based MOFs. Since the ionic radius of Ti4+ is smaller than that of Zr4+, it can react with various oxygen-containing ligands more vigorously, making the preparation of crystalline Ti-based MOFs more challenging. Recently, the Serre team used pre-prepared titanium-oxygen clusters (Ti8AF) to self-assemble with H3BTC ligands to achieve the controlled synthesis of Ti-MOF (MIP-207) (Fig. 5a and c), which could be stable in water for more than 3 days.101 By reacting a two-arm carboxylate ligand 5-acetamidoisophthalate (5-Aa-IPA) with Ti8AF clusters, they synthesized a more stable MOF (MIP-208) (Fig. 5b and d), which could be stable in boiling water and aqueous solutions with pH 0–10, representing the most stable Ti-MOFs reported to date.102 The enhanced stability was thought to come from the infinite titanium–oxygen chains in its structure.
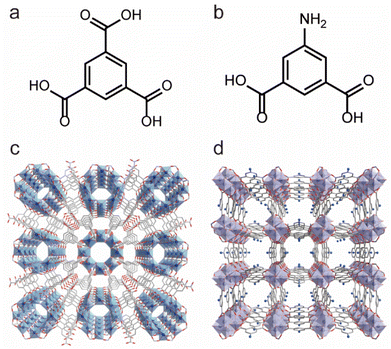 | ||
| Fig. 5 The structures of Ti-MOFs and the used ligands. (a) The 1,3,5-BTC ligand. (b) 5-Aa-IPA ligand. (c) The structures of MIP-207. Reproduced from ref. 101 with permission from Elsevier, Copyright 2020. (d) The structures of MIP-208. Reproduced from ref. 102 with permission from Elsevier, Copyright 2020. | ||
Besides tetravalent Zr4+/Ti4+, trivalent In3+ can also be used to construct water-stable MOFs (Fig. 6).103–105 Based on tritopic carboxylate ligands, Li et al. successfully obtained two isomeric In(III)-MOFs, BUT-172 and BUT-173, which had slightly different three-dimensional (3D) open framework structures but the same secondary building units (SBUs) (Fig. 6a, d and e). These two In(III)-MOFs were highly stable in water at room temperature due to the relatively strong In–O bonds. However, only BUT-172 could maintain its structural integrity in aqueous solutions with pH between 3 and 10 at ambient temperature or in boiling water. Such unusual stability discrepancy may result from their distinct structural symmetries. Using a π-conjugated ligand, tetrakis (4-carboxyphenyl)porphyrin (TCPP), an indium-based porphyrinic MOF, USTC-8, was successfully synthesized by Jiang's group (Fig. 6c and g).105 As anticipated, USTC-8 proved its exceptional water stability by maintaining the framework integrity in pH = 1–12 aqueous solutions for 12 h. To detect the pollutant in the aqueous solution, Li's group reported a novel luminous MOF (In(tcpp)) by combining the chromophore ligand 2,3,5,6-tetrakis(4-carboxyphenyl)pyrazine (H4tcpp) and In(III) salts (Fig. 6b and f).104 The as-prepared sample could remain stable in water, acidic solution (pH = 2), and alkaline solution (pH = 12), confirming its practicability for detecting pollutants in water.
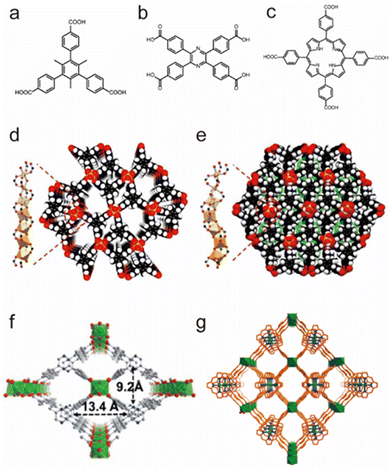 | ||
| Fig. 6 The structures of representative In(III)-MOFs and the used ligands. (a) The H3CTTA ligand. (b) The H4TCPP ligand. (c) The H2TCPP ligand. (d) BUT-172. Reproduced from ref. 103 with permission from the Royal Society of Chemistry, Copyright 2020. (e) BUT-173. Reproduced from ref. 103 with permission from the Royal Society of Chemistry, Copyright 2020. (f) In(tcpp). Reproduced from ref. 104 with permission from the Royal Society of Chemistry, Copyright 2021. (g) USTC-8. Reproduced from ref. 105 with permission from the American Chemical Society, Copyright 2018. | ||
Due to the high coordination numbers and the strong metal–oxygen interaction, rare-earth (RE) salts are another class of metal ions that are widely used for the synthesis of water-stable MOFs.148 By employing 1,3,5-benzenetricarboxylate (BTC) as the organic linker, a series of microporous lanthanide-organic framework enantiomers (Y-BTC and Tb-BTC) were prepared by Jiang and co-workers (Fig. 7a and g).106 The as-synthesized Ln-BTC not only could retain its framework in water but also exhibit excellent thermal stability (450 °C). Kitagawa's group synthesized a La(III)-framework (La-BTB) based on the H3BTB (H3BTB = 1,3,5-tris(4-carboxyphenyl)benzene) ligand (Fig. 7e and i), and it could maintain its framework integrity even after being soaked in boiling water, in hot (60 °C) HCl solution (pH = 2) and NaOH solution (pH = 14) for three days.110 Based on the 1,3,5-tri(6-hydroxycarbonylnaphthalen-2-yl)benzene) (H3BTN) ligand, another La(III)-MOF(La-BTN) was further reported by Kitagawa et al. (Fig. 7f and h).109 La-BTN was able to retain its structural integrity not only in a boiling acidic solution (pH = 2) but also in a strongly alkaline solution (pH = 12) for over 1 day. Eddaoudi et al. prepared a series of RE-fcu-MOFs (RE = Y(III) or Tb(III)) with fcu topology based on the 2-fluoro-4-(1H-tetrazol-5-yl)benzoic acid (H2-FTZB) ligand (Fig. 7c and l),108 in which an unusual metal cluster [RE6(μ3-OH)8(O2C–)6(N4C–)6]2− was in situ generated and served as a 12-connected metal node for MOFs' construction. Both isomeric MOFs show favourable water and thermal stability. After that, they reported another two La(III)-MOFs (pek-MOF-1 and 2) that were constructed from biphenyl-3,4,5-tricarboxylic acid (Fig. 7d) and 5-(4-carboxybenzyloxy)isophthalic acid, in which two different metal nodes (Fig. 7k), including a 12-connected nonnuclear and an 8-connected hexanuclear RE(III)-cluster, were also in situ formed.107 Both of them exhibited good water resistance and thermal stability. Furthermore, they used commercial 1,4-naphthalenedicarboxylic acid to prepare a series of isostructural fcu-MOFs (Fig. 7b and j).111 Owing to the 12-connected [RE6(μ3-OH)8(O2C–)12] node, Eu-1,4-NDC-fcu-MOF could maintain its structural integrity in boiling water for at least 24 h.
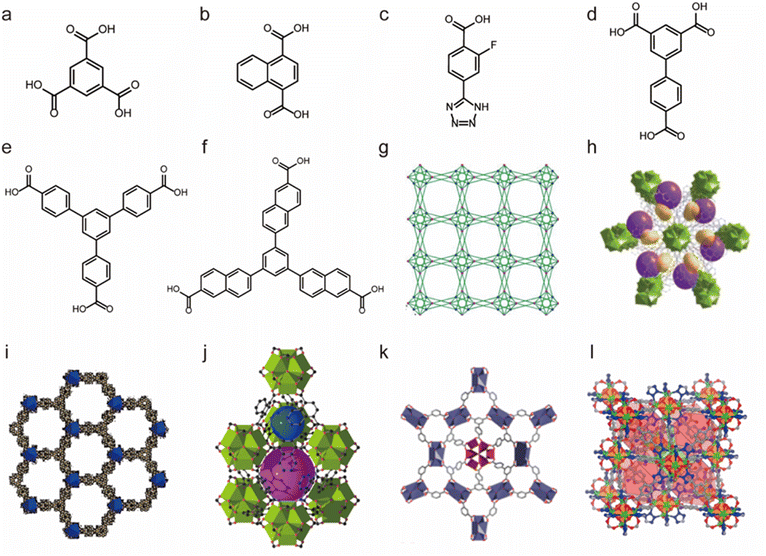 | ||
| Fig. 7 The structures of representative rare-earth MOFs and the used ligands. (a) The 1,3,5-BTC ligand. (b) The 1,4-H2NDC ligand. (c) The H2FTZB ligand. (d) The BTBA ligand. (e) The BTB ligand. (f) The BTN ligand. (g) Y-BTC. Reproduced from ref. 106 with permission from the American Chemical Society, Copyright 2010. (h) La-BTN. Reproduced from ref. 109 with permission from the Royal Society of Chemistry, Copyright 2014. (i) La-BTB. Reproduced from ref. 110 with permission from Wiley, Copyright 2013. (j) Eu-1,4-NDC-fcu-MOF. Reproduced from ref. 111 with permission from the American Chemical Society, Copyright 2015. (k) pek-MOF-1. Reproduced from ref. 107 with permission from the American Chemical Society, Copyright 2015. (l) [(CH3)2NH2]2[Tb6(μ3-OH)8(FTZB)6(H2O)6]·(H2O)22. Reproduced from ref. 108 with permission from the American Chemical Society, Copyright 2013. | ||
In short, many water-stable MOFs were successfully prepared from various hard metal ions and carboxylate-based ligands, including famous MILs, UIOs, PCNs, and BUTs. Due to the very strong metal–oxygen interaction, many of them also exhibit excellent acid/base resistance and thermal stability. It is noteworthy that BUT-8(Cr) even can retain its structural integrity in concentrated H2SO4.
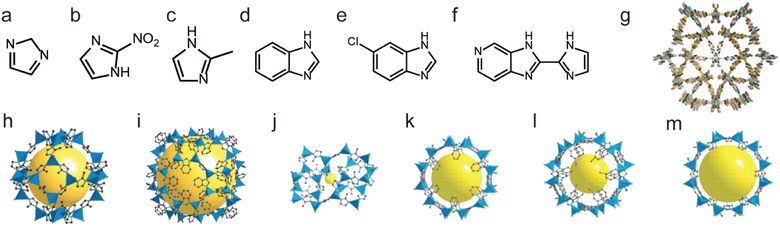 | ||
| Fig. 8 The structures of representative Zn(II)-MOFs and the used ligands. (a) The IM ligand. (b) The nIM ligand. (c) The MeIM ligand. (d) The PhIM ligand. (e) The cbIM ligand. (f) The H2imPim ligand. (g) MAF-stu-1. Reproduced from ref. 117 with permission from Wiley, Copyright 2019. (h) ZIF-8 (MAF-4). Reproduced from ref. 111 with permission from the National Academy of Sciences of the USA, Copyright 2006. (i) ZIF-11. Reproduced from ref. 111 with permission from the National Academy of Sciences of the USA, Copyright 2006. (j) ZIF-61. (k) ZIF-68. (l) ZIF-69. (m) ZIF-70. Reproduced from ref. 120 with permission from The American Association for the Advancement of Science, Copyright 2008. | ||
Not limited to Zn2+, other low-valent metal ions such as Co2+, Ni2+, Cu2+, and Ag+ can also be regarded as soft acids to prepare water-stable MOFs with appropriate N-containing linkers (soft bases). For instance, Yaghi et al. prepared a water-stable Co(II)-MOF (ZIF-67) based on Co(II) salt and the 2-methylimidazolate (MeIM) ligand (Fig. 9a and g).120 ZIF-67 could retain its structure after being soaked in water for 7 days. Based on Co(II) salts and 1,4-bis[(3,5-dimethyl)-pyrazol-4-yl]benzene (H2bdpb), Volkmer et al. developed a novel MOF (MFU-1). Due to the inert [CoON3] units, MFU-1 did not decompose even when exposed to humid air for more than six months (Fig. 9b and h).121 In 2016, Chen et al. found that MAF-X27-Cl, composed of 1H,5H-benzo-(1,2-d:4,5-d)bistriazole and Co(II) (Fig. 9d and i), showed exceptional chemical stability and could retain its original crystallinity in acidic (0.001 M HCl) or strongly alkaline (1.0 M KOH) solution for at least 1 week.122 Recently, we prepared a flexible framework (FJIH-30) (Fig. 9e and j) through combining the tris(pyridin4-ylmethyl)amine (TPMA) ligand with Co(SCN)2. Its framework could be retained not only in water for 5 days but also in aqueous solution with a wide pH range (from 2 to 12).123 Very recently, Li's group developed a microporous framework (ZJU-75) by using the pyz-NH2 ligand, Co(NO3)2·6H2O and K2[Ni(CN)4]·nH2O (Fig. 9c and f).124 ZJU-75 also displayed high chemical stability; it could maintain its structural integrity after being submerged in water and aqueous solutions of pH 1 and 12 for three days or exposed to air for three months.
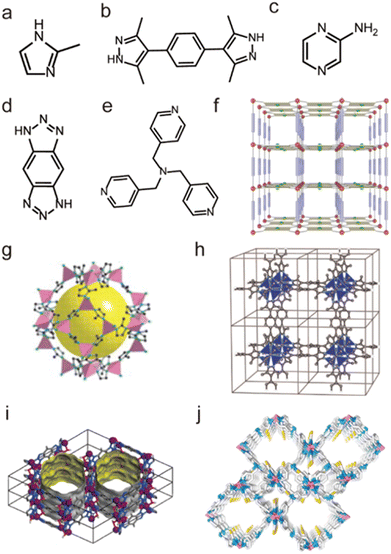 | ||
| Fig. 9 The structures of representative Co(II)-MOFs and the used ligands. (a) The MeIM ligand. (b) The H2bdpb ligand. (c) The pyz-NH2 ligand. (d) The H2bbta ligand. (e) The TPMA ligand. (f) ZJU-75. Reproduced from ref. 124 with permission from Wiley, Copyright 2023. (g) ZIF-67. Reproduced from ref. 120 with permission from The American Association for the Advancement of Science, Copyright 2008. (h) MFU-1. Reproduced from ref. 121 with permission from Wiley, Copyright 2009. (i) MAF-X27-Cl. Reproduced from ref. 122 with permission from the American Chemical Society, Copyright 2016. (j) FJI-H30. Reproduced from ref. 123 with permission from the American Chemical Society, Copyright 2020. | ||
The self-assembly based on Ni2+ and N-containing linkers can also lead to water-stable MOFs. In 2011, Long and co-workers used 1,3,5-tris(1H-pyrazol-4-yl)benzene (H3BTP) and Ni(II) salt to prepare a microporous pyrazolate-bridged MOF (Ni3(BTP)2) (Fig. 10c and i).128 Remarkably, the framework of Ni3(BTP)2 was stable in boiling water, boiling HCl or HNO3 aqueous solutions at pH 2, and a boiling NaOH aqueous solution at pH 14, and maintained both its crystallinity and porosity after 14 days of uninterrupted tests. By isoreticular expansion, another two water-stable Ni(II)-pyrazolate MOFs (BUT-32 and BUT-33) were synthesized from a conformation-matched elongated pyrazolate ligand, 2,4,6-tris(4-(pyrazolate-4-yl)phenyl)-1,3,5-triazine (H3TPTA) (Fig. 10d, g and h) by Li's group in 2020.129 Given the network interpenetration in BUT-32, the two MOFs possessed the same topology but had different pore sizes. Both the thermodynamic product BUT-32 and the kinetic product BUT-33 could retain their structure in boiling water for 24 hours, a HCl aqueous solution (pH = 3), and NaOH aqueous solution (4 M) at room temperature, respectively. Guided by a top-down topological analysis, Zhou's group rationally synthesized a water-stable Ni(II)-MOF (PCN-601) from a pyrazolate-based 5,10,15,20-tetra(1H-pyrazol-4-yl)porphyrin ligand (H4TPP) (Fig. 10a and e).126 PXRD and N2 adsorption further revealed its extraordinary base resistance; PCN-601 was the first identified MOF that could retain its crystallinity and porosity in saturated NaOH solution (∼20 mol L−1) both at room temperature and 100 °C. Such excellent chemical stability was believed to come from the high connectivity numbers between the porphyrinic ligands and ([Ni8(OH)4(H2O)2Pz12], Pz = pyrazolate) clusters, robust Ni(II)–N bonds and a relatively short length of the ligand. Based on a longer 5,10,15,20-tetrakis(4-(pyrazolate-4-yl)phenyl)porphyrin (H4TPPP) ligand and the same 12-connected [Ni8(OH)4(H2O)2Pz12] cluster, Zhou's group synthesized another water-stable Ni(II)-MOF (PCN-602) (Fig. 10b and f).127 Although PCN-602 has the same secondary building units and topology as PCN-601, its base stability was significantly reduced and it could only remain stable in 1 M NaOH solution. This may be because its pore size is larger than that of PCN-601, which is conducive to the diffusion of hydroxide anions.
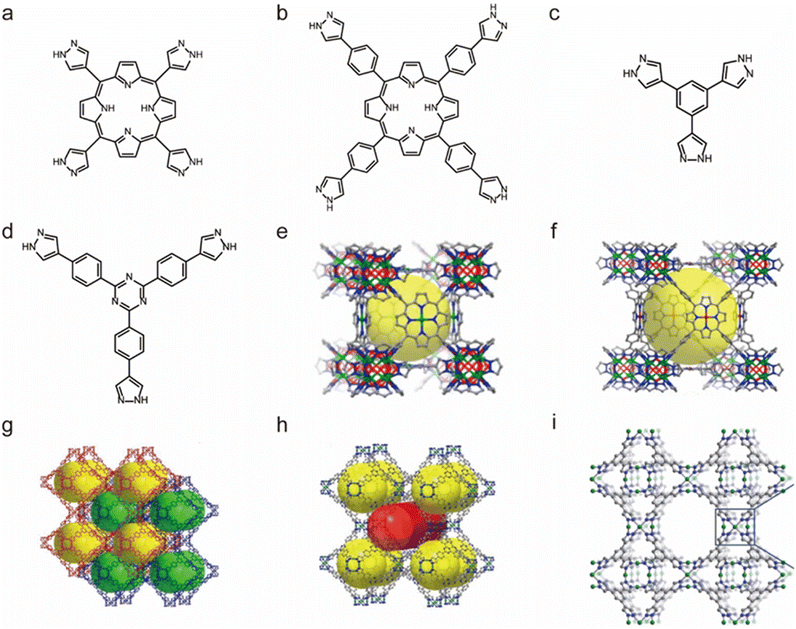 | ||
| Fig. 10 The structures of representative Ni(II)-MOFs and the used ligands. (a) The H4TPP ligand. (b) The H4TPPP ligand. (c) The H3BTP ligand. (d) The H3TPTA ligand. (e) PCN-601. Reproduced from ref. 126 with permission from the American Chemical Society, Copyright 2016. (f) PCN-602. Reproduced from ref. 127 with permission from the American Chemical Society, Copyright 2017. (g) BUT-32. Reproduced from ref. 129 with permission from the American Chemical Society, Copyright 2020. (h) BUT-33. Reproduced from ref. 129 with permission from the American Chemical Society, Copyright 2020. (i) Ni3(BTP)2. Reproduced from ref. 128 with permission from the Royal Society of Chemistry, Copyright 2011. | ||
Additionally, there are also many water-stable MOFs constructed from Cu(I), Cu(II), and Ag(I) ions and N-containing ligands. For example, based on an azolate ligand Hetz (Hetz = 3,5-diethyl-1,2,4-triazole), Chen's group reported a NbO type Cu(I)-framework (MAF-2), which showed good water vapor tolerance (Fig. 11c and i).131 Another water-stable Cu(I)-MOF, namely Cu2TBPZ (TBPZ = 3,3′,5,5′-tetraethyl-4,4′-bipyrazolate) (Fig. 11b and h), was synthesized through a stepwise method by Li's group.133 Cu2TBPZ could maintain its structural integrity after being soaked in various boiling solvents (e.g., THF, toluene, hexane, and DMSO), water, and even in acidic (0.001 M HCl) or basic (0.001 M NaOH) aqueous solutions for 24 h. In 2019, Zhang and co-workers synthesized a new Cu(I)-MOF(MAF-41) based on a linear ligand, 2,6-ditrifluoromethyl-benzodiimidazole (H2fbdim) (Fig. 11d and j).132 After complete guest removal below 150 °C, MAF-41 displayed good chemical stability in boiling water for at least one week, in aqueous solutions with a wide range of pH (3–14) at room temperature for at least 3 days. In addition, the structural integrity of MAF-41 could be maintained for at least 6 months when exposed to humid air (∼70 RH%). Their team also developed a water-stable Ag(I)-MOF[Ag2(o-Hmpba)2(o-H2mpba)2] by using the 2-(3,5-dimethyl-1H-pyrazol-4-yl)benzoic acid (o-H2mpba) ligand (Fig. 11e and f).135 Due to its hydrophobicity, the as-prepared Ag(I)-MOF could remain intact and keep its color unchanged in the air for at least 8 months, and also in aqueous solution with a wide range of pH (2–13) for at least 1 week. Based on the Cu(II) ion and triazolate ligand 1,3,5-tris(1H-1,2,3-triazol-5-yl)benzene (H3BTTri), Long's group prepared a water-stable MOF (Cu-BTTri) (Fig. 11a and g),130 which also exhibited strong resistance for both acidic solutions (pH = 3) and boiling water.
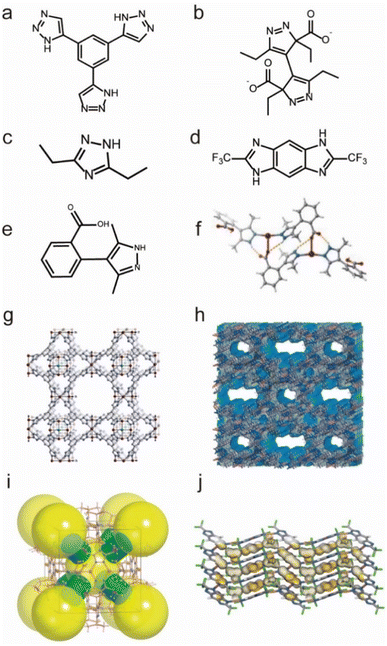 | ||
| Fig. 11 The structures of representative Cu/Ag-MOFs and the used ligands. (a) The H3BTTri ligand. (b) The TBPZ ligand (TBPZ = 3,3′,5,5′-tetraethyl-4,4′-bipyrazolate). (c) The Hetz ligand. (d) The H2fbdim ligand. (e) The o-H2mpba ligand. (f) [Ag2(o-Hmpba)2(o-H2mpba)2]. Reproduced from ref. 135 with permission from Wiley, Copyright 2020. (g) Cu-BTTri. Reproduced from ref. 130 with permission from the American Chemical Society, Copyright 2009. (h) Cu2TBPZ. Reproduced from ref. 133 with permission from Wiley, Copyright 2014. (i) MAF-2. Reproduced from ref. 131 with permission from the American Chemical Society, Copyright 2008. (j) MAF-41. Reproduced from ref. 132 with permission from Springer Nature, Copyright 2019. | ||
As previously indicated, chemically stable MOFs can be rationally prepared from soft low-valent metal ions and soft N-containing ligands. However, due to the very strong coordination interaction, the assembly between these soft coordination units usually leads to powder (e.g., microcrystals and amorphous solids) unsuitable for SCXRD analysis. Due to the reversibility of coordination bonds, high-quality single crystals of MOFs can be self-assembled by selecting coordination units with weak coordination (e.g., selecting soft acids and hard bases), but MOFs constructed from weak coordination units are usually less stable. By self-assembling the ligands containing both soft and hard coordination atoms with soft metal ions, both the crystallinity and stability of MOFs can be balanced well. In this context, many water-stable MOFs based on ligands containing both N and O atoms were prepared. For example, Navarro and co-workers prepared a water-stable MOF [Zn4(μ4-O)-(μ4-4-carboxy-3,5-dimethyl-4-carboxy-pyrazolato)3] based on a N-containing carboxylate ligand 3,5-dimethyl-4-carboxypyrazole (H2dmcapz) ligand and Zn(II) ion, which could retain its framework integrity in water and boiling MeOH/benzene for 24 hours (Fig. 12a and e).139 Then Jiang's group synthesized a water-stable MOF (USTC-7) by using the N-containing carboxylate ligand 4′-(1H-tetrazol-5-yl)-[1,10-biphenyl]-3,5dicarboxylic acid (H3TZBPDC) and Zn(II) salt (Fig. 12b and f).140 USTC-7 exhibited its exceptional stability not only in various boiling solvents but also in pH = 2–12 aqueous solutions for 12 h. Based on N-containing carboxylate ligands, our group designed and synthesized a series of water-stable MOFs with good crystallinity, such as FJI-H14, FJI-H25Fe, FJI-H29 and FJI-H36.136,141 Through the reaction of 2,5-di(1H-1,2,4-triazol-1-yl)terephthalic acid (H2BTTA) with Cu(NO3)2, a water-stable MOF (FJI-H14) was first prepared (Fig. 12c and g). FJI-H14 not only could retain its framework in the pH = 2–12 aqueous solutions at 373 K for 24 hours but also displayed excellent thermal stability (503 K). Structural analyses demonstrate that such excellent stability results from its CuN2O3 units with strong coordination and abundant free N atoms that can react with hydrogen ions. Combining the same ligand (H2BTTA) with other metal nodes, [Fe3(μ3-O)(CH3COO)6] clusters and Co-doped [Fe2Co(μ3O)(CH3COO)6] clusters, two isomeric MOFs (FJI-H25Fe and FJI-H25FeCo) with distinct water stability were further prepared (Fig. 12c and h).137 The Fe-MOF could retain its framework even in a pH = 12 aqueous solution while FeCo-MOF would quickly hydrolyse in the pH = 10 aqueous solution. The base stability of FeCo-MOF is lower than that of Fe-MOF due to partial substitution of Co; since the weaker Co–O units made the Co substituted framework more vulnerable to be deconstructed by OH− anions. Based on a longer 3′,5′-di(1H-1,2,4-triazol1-yl)-[1,1′-biphenyl]-3,5-dicarboxylic acid (H2DTBDA) ligand, another two water-stable MOFs (FJI-H29 and FJI-H36) were further synthesized (Fig. 12d, i and j).138 Both of them displayed excellent acid/base resistance and good thermal stability. FJI-H29 exhibited better base stability (pH = 13) but less thermal stability (423 K), while FJI-H36 showed better thermal stability (473 K) but less base resistance (pH = 12). This means that the chemical stability (e.g., base resistance and thermal stability) of MOFs can be controllably regulated by the selection of metal ions and ligands with different hardness.
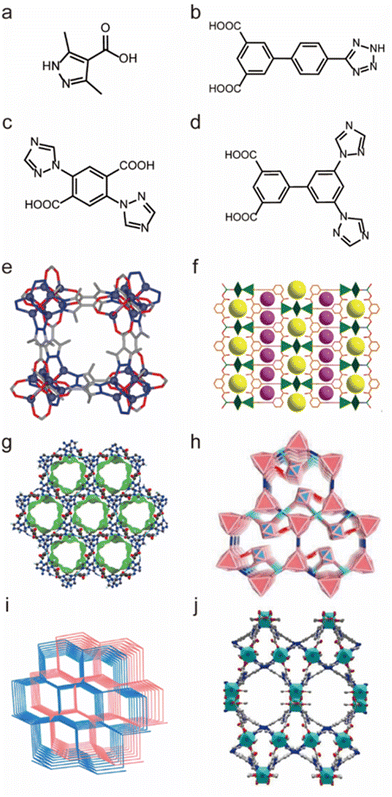 | ||
| Fig. 12 The structures of representative MOFs based on N-containing carboxylate ligands and the used ligands. (a) The H2dmcapz ligand. (b) The H3TZBPDC ligand. (c) The H2BTTA ligand. (d) The H2DTBDA ligand. (e) [Zn4(μ4-O)-(μ4-4-carboxy-3,5-dimethyl-4-carboxy-pyrazolato)3]. Reproduced from ref. 139 with permission from the American Chemical Society, Copyright 2011. (f) USTC-7. Reproduced from ref. 140 with permission from the Royal Society of Chemistry, Copyright 2016. (g) FJI-H14. Reproduced from ref. 136 with permission from Springer Nature, Copyright 2017. (h) FJI-H25Fe. Reproduced from ref. 137 with permission from Wiley, Copyright 2020. (i) FJI-H29. Reproduced from ref. 138 with permission from the American Chemical Society, Copyright 2020. (j) FJI-H36. Reproduced from ref. 141 with permission from Wiley, Copyright 2023. | ||
In a word, many water-stable MOFs based on various soft metal ions and N-containing ligands were reported, including ZIFs, MAFs, PCNs, BUTs, and FJI-Hs. Due to the strong metal–nitrogen interaction, most of the reported MOFs also displayed good acid/base stability, especially base stability.
Notably, PCN-601 could retain its structural integrity in saturated NaOH solution (about 20 mol L−1). This means that MOFs can be used normally even in a strongly alkaline environment.
2.4.3.1. Enhancing stability through interlocking of frameworks. In addition to the weak coordination nodes, the relatively large pores of MOFs can also lead to their instability; this is because large pores facilitate the diffusion of small molecules that can break down their framework. For a specific MOF, the interlocking of its independent framework can not only enhance the interactions between interlocked frameworks but also reduce its pore size, so the interlocking of the framework can be used to enhance the water stability of MOFs. For example, Walton's group used the interpenetration of the framework to enhance the water stability of MOFs.149 They prepared two isostructural MOFs DMOF [Zn(BDC)(DABCO)0.5] and MOF-508 [Zn(BDC)(BPY)0.5] by using different pillared ligands. The DMOF was a non-catenated pillared layer structure while MOF-508 was a two-fold interpenetrated pillared layer structure. Even though the pKa of DABCO is significantly higher than that of BPY, the interlocked MOF-508 also exhibited better water stability than the non-interlocked DMOF. This enhanced stability is thought to be due to the higher thermodynamic stability and lower water absorption of the interlocked structure. The same enhanced effect can also be found in other interlocked MOFs (e.g., Zn-BTTB-DMBPY and Co-BTTB-DMBPY).150
Interestingly, the water stability of MOFs can also be influenced by the direction of interpenetration. Chen and co-workers synthesized three isomeric MOFs([Zn(Hmpba)2]) through a solvothermal reaction of 4-(3,5-dimethyl-1H-pyrazol-4-yl)benzoic acid (H2mpba) and Zn(NO3)2.151 Although three isomers had 4-fold interpenetrated structures, they exhibited entirely different water stability due to the different directions of interpenetration. Here, two nanoporous metastable isomers would spontaneously convert into the most stable nonporous isomer. This not only confirms that the interpenetration of the framework can improve the thermodynamic stability of MOFs but also indicates that the interpenetration with small pores is more stable than that with large pores.
2.4.3.2. Enhancing stability through pore space partition. As mentioned previously, for a metastable MOF containing large pores, its stability can be improved by the interlocking of the framework. This is because interlocking can effectively improve its thermodynamic stability and reduce its pore size. In fact, matched ligands also can be used to enhance the thermodynamic stability of a metastable MOF through pore space partition (PSP).7 For example, Du's group prepared two water-stable MOFs (1 and 2) with comparable structural properties but distinct water stability based on an MIL-88 type Co(II)-MOF through the PSP strategy.152 After introducing two size-matching triangular blocks [Co2(ina)3(H2O)2]+ and 2,4,6-tri(4-pyridyl)-1,3,5-triazine (tpt) into the pores, the moisture stability of the MOFs with tpt substitution rose dramatically. Recently, Bu and colleagues prepared a family of acid/base-resistant Cr-MOFs by dividing the large pore of the original Cr-MOFs into interconnected small pores employing triangle ligands (Fig. 13).153 The newly formed Cr-MOFs (CPM-243(F)) could maintain their crystallinity even under very severe conditions such as HCl aqueous solution and 10 M NaOH aqueous solution. Such exceptional stability was due to the presence of the multi-binding pore-partitioning ligand, framework rigidity, and highly connected Cr3O nodes.
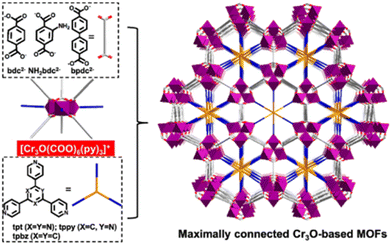 | ||
| Fig. 13 The structural components, together with the framework viewed along the c axis. NH2bdc2− = 2-aminoterephthalate, bpdc2− = biphenyl-4,4′-dicarboxylate), tpt = 2,4,6-tri(4-pyridyl)-1,3,5triazine, tpbz = 1,3,5-tri(4-pyridyl)-benzene, and tppy = 2,4,6-tris(4-pyridyl)pyridine. Reproduced from ref. 153 with permission from the American Chemical Society, Copyright 2021. | ||
Chen, Xiang, and co-workers also used the PSP strategy to realize the improvement of MOFs' stability.154 As shown in Fig. 14, by a triangular ligand (Tripp = 2,4,6-tris(4-pyridyl)pyridine), every metal cluster in the original FJU-88 increased three coordination bonds. Due to the increased connection numbers of metal clusters, the thermal stability of the newly generated MOF (FJU-90) (up to 300 °C) was noticeably higher than that of the initial FJU-88 (durable only below 50 °C). Moreover, FJU-90 could maintain its structure even after being submerged in water for a day or exposed to air for a month, but the mother MOF (FJU-88) could not. The same improved effect also can be found in [Cu2(obb)2(bpy)0.5(DMF)]·2DMF and [Co2(INT)3(H2O)2].155,156
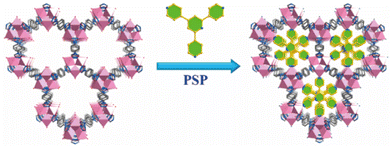 | ||
| Fig. 14 Pore space partition (PSP) through symmetry- and size-matching-regulated ligand insertion. Reproduced from ref. 154 with permission from the American Chemical Society, Copyright 2019. | ||
2.4.3.3. Enhancing stability through increasing the rigidity of the ligand. In some situations, the rigidity of organic linkers tends to reduce the structural diversity but is beneficial to construct chemically stable MOFs. For instance, Zhou, Li, and co-workers prepared 13 Zr-MOFs by combining three ligands with the same topology but different rigidity with Zr6O4(OH4)(CO2)n units (n = 8 or 12) (Fig. 15).157 The MOFs constructed from the less rigid ligand LB1 were labile while those with rigid ligands LB2 and LB3 were chemical stable, which could maintain intact in water, acidic solution (pH = 1), or weakly basic (pH = 11) aqueous solution. The enhancement of stability may be due to the fact that rigid ligands have higher deformation energy barriers than flexible ligands and tend to maintain their conformation in the framework, which significantly restricts the dissociation of M-L and improves its repair. The same improvements also could be found in other isostructural MOFs (e.g., BUT-109(Zr)/BUT-110(Zr), and CoBTTBBPY/CoBTTBAZPY, and ZnBTTBBPY/ZnBTTBAZPY).158,159
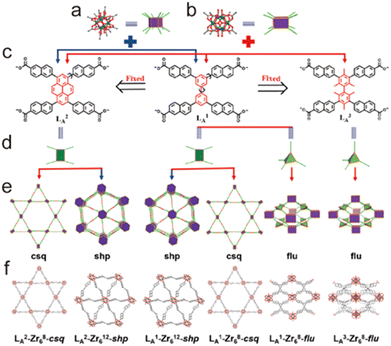 | ||
| Fig. 15 Construction of a series of Zr-MOFs by using naphthoate-based tetracarboxylate ligands. (a) 12-Connected Zr6 clusters; (b) 8-connected Zr6 clusters; (c) different ligands LA1, LA2, and LA3; (d) configuration of ligands; different topologies (e) and structures (f) of LA2-Zr68-csq (5), LA2-Zr612-shp (4), LA1-Zr612-shp (3), LA1-Zr68-csq (2), LA1-Zr68-flu (1), and LA3-Zr68-flu (6) MOFs, respectively. Reproduced from ref. 157 with permission from the American Chemical Society, Copyright 2019. | ||
2.4.3.4. Enhancing stability through ligand charge-separation. In addition to the strategies given above, the charge density of organic ligands also plays a significant role in framework stability. Bu's group developed a ligand charge-separation (LCS) strategy for the stabilization of MOFs with high-charge-density ligands (Fig. 16).160 By breaking down the ligand 2,5-dioxido-1,4-benzenedicarboxylate (dobdc4−) of MOF-74-Zn [Zn2(obpdc)] into OH− and obdc3− (H3obdc = 2-hydroxyterephthalic acid), two new MOFs CPM-74 [Zn2(OH)(obpdc)] and CPM-75 [Zn2(OH)(obpdc)] could be obtained. Compared with parent MOF-74-Zn, the two newly prepared MOFs had the same density of metal sites and similar Zn–O chains, so they could be considered quasi-MOF-74-Zn isomers. Although MOF-74-Zn was sensitive to water, both CPM-74 and CPM-75 could stably exist in aqueous solutions with pH from 3 to 10, exceeding most reported Zn(II)-MOFs based on carboxylate ligands. This significant enhancement may be because the separated low-charge-density ligands can coordinate with Zn(II) ions more flexibly, resulting in more stable coordination configurations.
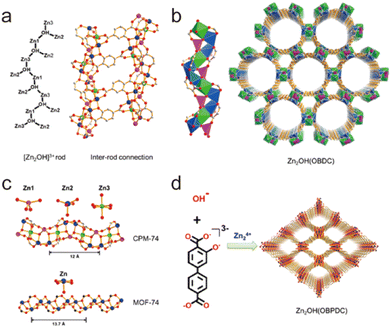 | ||
| Fig. 16 (a) ChemDraw representation of [Zn2(OH)]3+ rod and ball-and-stick view of inter-rod connection. (b) Polyhedral view of the rod and the framework of CPM-74. (c) Comparison between the chains in CPM-74 and MOF-74. (d) Building scheme of CPM-75. Zn1, pink; Zn2, blue; Zn3, green; C, gray; O, red. Reproduced from ref. 160 with permission from the American Chemical Society, Copyright 2019. | ||
2.4.3.5. Enhancing stability through metal metathesis. Theoretically, replacing the metal ions in a labile MOF with suitable metal ions that can form stronger coordination bonds and lead to an isomeric but more stable MOF. This means that the metal metathesis can effectively improve the stability of a metastable MOF without changing its framework and pore. Through metal metathesis and oxidation, Zhou and co-workers realized the enhancement of stability of a labile Mg-based MOF (PCN-426-Mg) (Fig. 17).161 After PSM, the as-prepared isostructural PCN-426-Cr possessed the stronger Cr(III)–O units. Although PCN-426-Mg was particularly vulnerable to humidity, the newly formed PCN-426-Cr could maintain its crystallinity even in an extremely acidic solution (4 M HCl) or a pH = 12 aqueous solution. Furthermore, Zhang and Wang developed a mild and efficient method for one-step synthesizing Cr(III)-MOFs from Fe(III)-MOFs through metal metathesis, namely solvent-assisted metal metathesis. They found that acetone could effectively improve the efficiency of metal exchange by coordinating with the Fe(III) ions of MOFs. Assisted by acetone, a series of isostructural Cr(III)-MOFs could be directly prepared from their Fe(III)-based precursors through the exchange of metal ions, and all the modified MOFs displayed enhanced stability.162
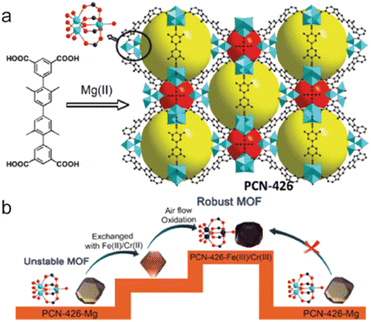 | ||
| Fig. 17 (a) The ligand and the structure of PCN-426-Mg; (b) the metal exchange routes for PCN-426. Reproduced from ref. 161 with permission from the American Chemical Society, Copyright 2014. | ||
2.4.4.1. Enhancing the kinetic stability by directly using hydrophobic ligands. Directly using hydrophobic ligands is the most commonly used method to improve the kinetic stability of MOFs in water. For example, employing hydrophobic polymer ligands and pyridine linkers, Cohen's group synthesized a series of polymer–metal–organic frameworks (polyMOFs) (Fig. 18).163 In contrast to the parent MOF without hydrophobic units, polyMOFs could retain their crystallinity after being exposed to boiling water for 24 hours due to the cross-linking of polymer chains in the pores of polyMOFs. Apart from the polymer ligands, other hydrophobic units such as nonpolar alkyl chains and fluorine-containing groups can be introduced into organic linkers to prepare water-resistant MOFs. For instance, with the help of methyl-functionalized ligands, Li and co-workers could realize the controllable construction of a water-stable MOF based on water-unstable Cu(II) paddle wheel units (Fig. 19).164 Through combining a ligand (H8tdhb) functionalized with six methyl groups with Cu(II) ions, they prepared a microporous MOF (BUT-155a) with Cu(II) paddle wheel units. Even though the Cu(II) paddle wheel units are highly sensitive to water, the as-prepared BUT-155a also displayed excellent water stability due to the introduction of hydrophobic methyl groups. It could maintain structural integrity even in boiling water for 24 hours and the aqueous solution with a wide pH range (4s–10).
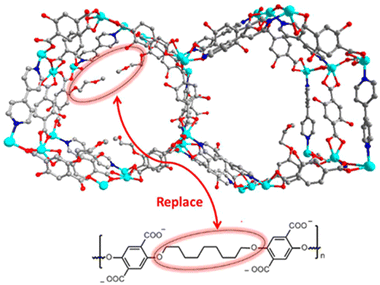 | ||
| Fig. 18 Packing diagram of MOF 1 along the c-axis direction (top); design concept for creating a polyMOF analogue of MOF 1 via replacing dangling groups by polymer chains (bottom). Reproduced from ref. 163 with permission from the American Chemical Society, copyright 2016. | ||
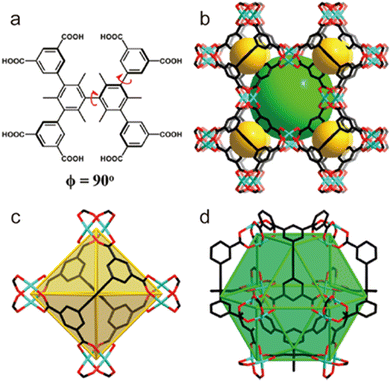 | ||
| Fig. 19 (a) Structure of the ligand H8tdhb, (b) crystal structure of BUT-155a, and (c) octahedral cage (cage A) and (d) cuboctahedral cage (cage B) in BUT-155a (color code: C, black; O, red; Cu, blue; H atoms on ligands are omitted for clarity). Reproduced from ref. 164 with permission from the American Chemical Society, copyright 2017. | ||
Another water-stable Cu(II)-MOF (USTC-6) was prepared by Jiang's group through using a fluorine-containing ligand (4,4′-(per-fluoropropane-2,2-diyl)diphthalic acid). Due to the strong hydrophobicity of the CF3 groups, USTC-6 demonstrated extraordinary endurance to water and aqueous solutions (pH = 2–10), despite the fact that the majority of Cu–O coordination bonds in MOFs are susceptible to hydrolysis.165 Similarly, Li and co-workers realized the construction of a water-stable Zn(II) paddle wheel-based MOF by using fluorine-containing ligands. Through separately reacting the ligand 2-(trifluoromethyl) terephthalic acid (BDC-CF3) and 2, 5-bis(trifluoromethyl) terephthalic acid (BDC-(CF3)2) with Zn(II) salt, they constructed two fluorinated Zn-DMOFs (named FDMOF-1 and FDMOF-2) (Fig. 20).166 FDMOF-1 and FDMOF-2 exhibited ultrahigh structural stability due to the introduction of fluorinated functional groups into the confined pore space. Even at 70% relative humidity, they could maintain their structural integrity well for a period of six months.
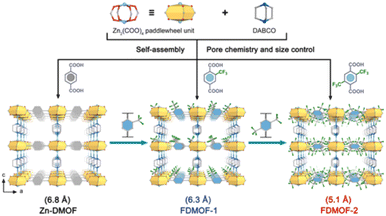 | ||
| Fig. 20 Fluorinated functional group introduction into the confined pore space. Reproduced from ref. 166 with permission from Wiley, Copyright 2023. | ||
In order to explore the effect of the length of hydrophobic chains on the water stability of MOFs, a series of isostructural NbO-type MOFs were synthesized by Zhou's group.167 They found that the water stability of the resulting Cu(II)-MOFs increased significantly while their thermal stability reduced as the length of the hydrophobic chains increased. Besides the length of hydrophobic groups, the distance between metal nodes and hydrophobic groups is another key parameter for influencing the chemical stability of MOFs. A reasonable comparison between MOF-508 and its two analogues (SCUTC-18 and SCUTC-19) was made by Ma's group.168 In contrast to the unsubstituted bipyridine ligands of MOF-508, two methyl groups were introduced into the ortho- and meso-sites of the bipyridine ligands in SCUTC-18 and SCUTC-19, respectively. Only SCUTC-18 protected by the closer methyl groups could retain its porous structure after being exposed to humid air for 30 days.
2.4.4.2. Enhancing the kinetic stability by bonding hydrophobic groups. As mentioned above, since hydrophobic groups can effectively block the contact between water and metal nodes, kinetically stable MOFs can be directly constructed by using hydrophobic ligands. Besides directly using hydrophobic ligands, introducing suitable hydrophobic groups through PSM also can be used to stabilize water-sensitive MOFs. In 2010, Cohen's team successfully proved that the hydrophobicity of MOFs could be introduced by PSM.171 According to the PSM strategy, they prepared two types of isoreticular MOFs (IRMOF series and MIL series). For IRMOFs, their water contact angles could increase to 116° after being modified with long alkyl substituents, and this value could be compatible with common hydrophobic materials. After being modified with different alkyl substituents, the water contact angles of MILs could even reach 150°, demonstrating that their hydrophobicity can be comparable with that of superhydrophobic materials. Based on the PSM method, Bradshaw and co-workers also realized the enhancement of the structural stability of UiO-66-NH2.172 They used the diazotization reaction of UiO-66-NH2 to prepare a more stable MOF (UiO-66-N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-ind). The modified UiO-66-N
N-ind). The modified UiO-66-N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-ind could retain its structural integrity in a pH = 12 aqueous solution, while the unmodified UiO-66-NH2 only resisted a weaker basic solution (pH = 9).
N-ind could retain its structural integrity in a pH = 12 aqueous solution, while the unmodified UiO-66-NH2 only resisted a weaker basic solution (pH = 9).
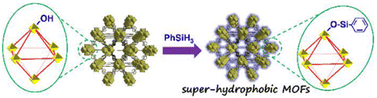
In addition to organic ligands, the hydrophobic groups also can be introduced into the metal nodes of MOFs through the PSM strategy. For example, different hydrophobic groups were introduced into the Zr6-oxo nodes of NU-1000 through the solvent-assisted ligand incorporation (SALI) method by Farha, Hupp, and co-workers.173,174 The water resistance of Zr6-oxo nodes could be efficiently improved after being modified with organic carboxylates. Notably, the perfluorodecanoic acid functionalized NU-1000 exhibited remarkable water stability; it could maintain the crystallinity and porosity even after 20 cycles of water vapor adsorption. By introducing hydrophobic phenylsilane into the metal-oxo nodes of NH2-UiO-66, Kim's group realized the controllable construction of a super-hydrophobic MOF NH2-UiO-66(Zr)-shp (Fig. 21).169 NH2-UiO-66(Zr)-shp not only retained the acid resistance of NH2-UiO-66(Zr) (e.g., resisted 12 M HCl and 16 M HNO3) but also exhibited highly improved base stability (resisted 0.1 M NaOH solution at least for 5 hours).
 | ||
| Fig. 21 Preparation of super-hydrophobic NH2-UiO-66(Zr)-shp. Reproduced from ref. 169 with permission from Wiley, Copyright 2019. | ||
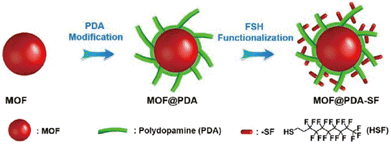
2.4.4.3. Enhancing the kinetic stability by forming hybrid composites. Introducing hydrophobic groups into the pores of MOFs indeed can significantly improve their kinetic stability toward water. However, additional hydrophobic groups in the pores usually reduce the porosity of MOFs. Direct compositing of metastable MOFs and hydrophobic materials can also effectively improve the water stability of MOFs but can maintain their pore structure as much as possible. Many water-stable hybrid composites based on water-instable MOFs have been reported. For example, by coating a thin hydrophobic polydimethylsiloxane (PDMS) layer on the surface of initial MOFs, Yu, Jiang, and colleagues developed a universal approach to improve the water-resistance of MOFs. In addition to improving their water resistance, the fragile MOFs (MOF-5, HKUST-1, and ZnBT) coated with PDMS also inherited their original crystalline structure and pore properties.175 In addition, Queen's team constructed a series of extremely hydrophobic and stable MOF composites utilizing a post-synthetic polymerization strategy (Fig. 22).170 Here, polydopamine was first coated on the surface of MOFs and hydrophobic molecules were introduced by Michael addition. Notably, a range of MOFs (e.g., HKUST-1 (Cu), Cu-TDPAT (Cu), and Mg-MOF-74 (Mg)) after modification demonstrated outstanding stability even under strongly acidic or basic conditions. In 2018, Cohen's group successfully incorporated UiO-66-NH2 into nylon (PA-66) polymer fibers to give a hybrid composite by an interfacial polymerization method, which displayed improved stability.176 Through interfacial polymerization, the UiO-66-NH2 MOF was copolymerized with polyamide fiber (PA-66) to produce a hybrid composite with 29% MOF (mass fraction). Compared to MOF particles that were merely physically confined in the polymer, the covalently linked hybrid material exhibited better stability.
 | ||
| Fig. 22 The modification process used to prepare hydrophobic MOF@PDA-SF composites. Reproduced from ref. 170 with permission from the Royal Society of Chemistry, Copyright 2019. | ||
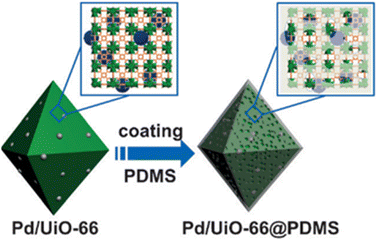
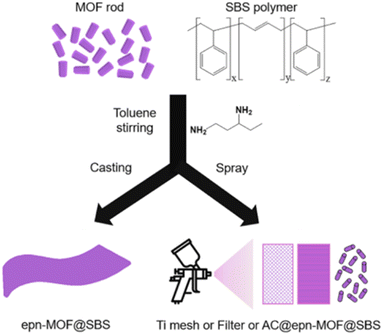
Recently, the Pd/UiO-66 nanocomposite surface was successfully covered with a thin layer of hydrophobic polydimethylsiloxane (PDMS) using a chemical vapor deposition (CVD) method by Jiang's group (Fig. 23).177 The enhanced stability and retained porosity of Pd/UiO-66@PDMS were further confirmed by PXRD and N2 sorption analysis. Moreover, the modified catalyst (Pd/UiO-66@PDMS) exhibited significantly enhanced surface wettability and recycling stability, paving a new way for developing new catalysts with high activity and stability. Similarly, by combining hydrophobic polymer polystyrene-block-polybutadiene-block-polystyrene (SBS) with epn-functionalized Mg2(dobpdc) (epn-MOF) and various supports (e.g., Ti mesh, HEPA filter, and granular AC), Hong and co-workers prepared a series of epn-MOF@SBS composites on different supports, including Ti mesh@epn-MOF@SBS, Filter@epn-MOF@SBS, and AC@epn-MOF@SBS (Fig. 24).178 The as-prepared hybrid materials could maintain their stability and activity even after a long time of exposure to 60% RH.
 | ||
| Fig. 23 Preparative route for Pd/UiO-66@PDMS. Reproduced from ref. 177 with permission from Wiley, Copyright 2016. | ||
 | ||
| Fig. 24 Fabrication of epn-MOF@SBS, Ti mesh@epn-MOF@SBS, Filter@epn-MOF@SBS, and AC@epn-MOF@SBS composites. Reproduced from ref. 178 with permission from Elsevier, Copyright 2022. | ||
To encapsulate HKUST-1 within polystyrene, Maspoch et al. developed a facile spray-drying technique, which could produce water-resistant HKUST-1@polystyrene spheres.179 Although HKUST-1 is very sensitive to water, the as-prepared hybrid composite (HKUST-1@polystyrene) could also maintain its water uptake capability even after three consecutive cycles. Very recently, Zhao's group realized the controllable construction of a super hydrophobic MOF membrane on polymer substrates through controlling the growth of ZIF-8 and using the surface coating technique.180 In comparison to its pristine, the as-prepared hybrid composite (PDMS/MOF-NS/PVDF) displayed significantly enhanced hydrophobicity.
Besides hydrophobic polymers, carbon nanotubes could also be incorporated into MOFs, affording a hybrid composite with significantly improved water stability. The growth of Hf12-porphyrin metal–organic frameworks on carbon nanotubes (CNTs) was reported by Lin's group in 2018.181 The as-synthesized Hf12-CoDBP/CNT hybrid composite could remain inert even in a pH = 1 aqueous solution. The same improved effects could also be achieved in other hybrid composites (e.g., MOFMC, C-CNT, IM-CNT, and LM-CNT) based on MOFs and carbon nanotubes.182,183
3. Water-stable MOFs for carbon dioxide capture
3.1. Background of MOFs for carbon capture
Due to the rapid increase of population and the acceleration of urbanization, human's demand for fossil energy has increased dramatically, which leads to excessive emissions of CO2. It is reported that the concentration of CO2 in the air has increased from 310 ppm in 1960 to 415 ppm in 2021. Excess CO2 in the atmosphere leads to a series of serious environmental problems, such as global warming, sea level rise, ocean acidification, and extreme weather. More than half of global CO2 emissions come from the flue gas of power plants; thus, CO2 emissions should be greatly reduced if unwanted CO2 can be effectively captured from flue gas. At present, organic amine solution is mainly used in power plants to capture CO2 from flue gas, but the desorption of CO2 from organic amine requires a lot of energy; in addition, organic amine can cause serious corrosion of equipment. Therefore, there is an urgent need to develop more energy-efficient and green technologies for capturing CO2 from flue gas.Although CO2 from flue gas can be captured by decarbonizing devices installed in power plants, about 27% of global CO2 emissions are difficult to capture effectively. So, it is necessary to develop technologies that can be used to capture trace CO2 from the air. Direct air capture (DAC) is a negative emission technology in which chemical processes are used to capture CO2 from ambient air, which can be used to not only reduce the excessive CO2 (>415 ppm) in the atmosphere to the optimal level below 350 ppm, but also compensate for the CO2 emissions from economic activities that are difficult to decarbonize. Currently, there are a few absorbents available for DAC, including alkaline aqueous solutions (such as NaOH and KOH) and porous solids loaded with amines. These technologies also have the problem of a high recovery energy, and more energy-efficient DAC technologies should be developed.
Natural gas has been considered as a promising clean energy due to its high energy-conversion efficiency and low environmental impact. Unfortunately, crude natural gas usually contains a large amount of CO2, which not only sharply reduces the burning heat of methane, but also causes serious corrosion to the transmission pipelines. In order to capture CO2 from crude natural gas, alkali adsorption technology has been widely used in industry, which also requires a high recovery energy. Thus, more energy-efficient technologies for purifying natural gas are urgently needed.
Alkali solution can indeed efficiently capture unwanted CO2, but it requires a large amount of energy to recover and tends to corrode equipment. Using suitable porous materials to capture CO2 can overcome these challenges and achieve more energy-efficient carbon capture. Compared with traditional porous materials such as activated carbon, mesoporous silica, and zeolite, the pore size and pore environment of MOFs can be precisely regulated, which enables them to capture CO2 more efficiently and selectively. To achieve efficient carbon capture of MOFs in various scenarios (e.g., flue gas decarbonization, direct air capture, and natural gas upgrading), many MOFs with different active sites and pore sizes have been designed and synthesized. Ideally, a practical carbon capture adsorbent should have not only excellent adsorption performance, such as high adsorption capacity, high adsorption selectivity, and low adsorption enthalpy; but also excellent chemical stability, recyclability, and high cost-effectiveness. However, developing such a MOF remains a huge challenge, as it is very difficult to achieve these functions simultaneously through one MOF.
3.2. Physical and chemical properties of carbon dioxide
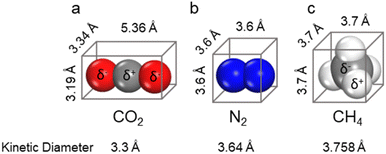
To achieve efficient and selective capture of CO2 by MOFs, it is necessary to fully understand the physical and chemical properties of CO2 and its competing gases (e.g., N2 and CH4) (Scheme 4). CO2 is a linear molecule with strong polarity, composed of one sp hybrid carbon atom and two sp2 hybrid oxygen atoms. The as-formed carbon–oxygen bond has the characteristics of both carbon–oxygen double bond and carbon–oxygen triple bond. The carbon atom in the middle is electrically deficient, while the oxygen atoms at both ends are electrically rich. The size and kinetic diameter of CO2 are 3.19 × 3.34 × 5.39 Å3 and 3.3 Å respectively. Due to its specific structure, CO2 can not only coordinate with metal ions providing empty orbitals, but also generate dipole interactions with various polar atoms (e.g., H, N, and O) as well as π–π interactions with conjugated molecules (e.g., benzene ring). In this regard, by introducing suitable active sites that can interact with CO2 into a MOF, its CO2 adsorption performance can be improved thermodynamically, and the construction of pores matched with CO2 (about 3.3 Å) is kinetically conducive to CO2 capture. N2, the main component of flue gas and air, may compete with CO2 in the process of flue gas decarbonization and direct air capture; therefore, inhibiting the competitive adsorption of N2 is conducive to improving the efficiency of CO2 capture. N2 is a spherical, non-polar molecule with a size and kinetic diameter of 3.6 × 3.6 × 3.6 Å3 and 3.64 Å, respectively. As the main component of natural gas, CH4 is a spherical molecule with weak polarity, whose size and kinetic diameter are 3.7 × 3.7 × 3.7 Å3 and 3.758 Å, respectively. By inhibiting the competitive adsorption of CH4, the purification efficiency of natural gas can be significantly improved. Therefore, by increasing the pore polarity of MOFs and controlling its pore size (smaller than the kinetic diameter of N2/CH4), the competitive adsorption of N2/CH4 can be significantly inhibited.3.3. The adsorption mechanisms and characterization of MOFs for carbon dioxide
When a fluid comes into contact with a porous solid, its components accumulate on the surface of the solid, which is called adsorption. If the interactions between the adsorbate and the adsorbent are weak intermolecular interactions (e.g., van der Waals forces, hydrophobic interactions, electrostatic forces, and hydrogen bonding), this process can be defined as physical adsorption, also known as physisorption, which usually exhibits low adsorption heat but fast adsorption and high desorption kinetics. When an adsorption process involves one or more chemical reactions, it can be defined as chemical adsorption (chemisorption), which is an irreversible adsorption process with a high adsorption enthalpy. If the interaction between a specific MOF and CO2 is relatively weak (e.g., the adsorption enthalpy is less than 40 kJ mol−1), then its adsorption of CO2 is undoubtedly physical adsorption. In contrast, the adsorption between CO2 and a MOF that can react with CO2 while producing new species should be a chemical adsorption process. Despite having a strong interaction with CO2 (e.g., the adsorption enthalpy is greater than 50 kJ mol−1), a particular MOF does not react with the adsorbed CO2 or generate new species. We still discuss it according to physical adsorption, even though the real situation may be the coexistence of physical and chemical adsorption.When CO2 enters the pores of MOFs and interacts with the active sites, not only will the charge distribution or strength of carbon–oxygen bonds be changed, but potential new species may also be formed. All of these can be detected by NMR and infrared absorption spectroscopy (IR). In addition, when the adsorbed CO2 molecules are orderly distributed in the pores of MOFs, their adsorption sites can be accurately located through SCXRD, PXRD, and neutron powder diffraction (NPD) analyses. Therefore, the adsorption character of MOFs for CO2 can be analyzed in detail. In addition, due to the determinacy of the structure of MOFs, molecular simulation (MS) is also a useful supplement to understanding the adsorption mechanism of MOFs for CO2. Next, we briefly introduce the reported adsorption mechanisms and characterization of MOFs for CO2, hoping to provide a useful guidance for designing MOF-based CO2 adsorbents with better performance.
 | ||
| Fig. 25 Carbon dioxide adsorption on open metal ions through coordination interactions. (a) The Ni(II)–CO2 interactions in CPO-27-Ni. Reproduced from ref. 184 with permission from the Royal Society of Chemistry, Copyright 2008. (b) The Co(II)–CO2 interactions in Co-MOF-74. Reproduced from ref. 185 with permission from the Royal Society of Chemistry, Copyright 2017. (c) The Zn(II)–CO2 interactions in UTSA-74. Reproduced from ref. 186 with permission from the American Chemical Society, Copyright 2016. (d) The Cu(II)–CO2 interactions in FJI-H14. Reproduced from ref. 136 with permission from Springer Nature, Copyright 2017. | ||
In addition to various open metal sites, polar atoms that can form strong dipole interactions with CO2 are another type of adsorption site for CO2, which have also been widely introduced into MOFs for carbon capture, and a variety of polar groups are proved to stabilize the adsorbed CO2 molecules. For instance, in the MOF (Zn2(Atz)2(ox), Atz = 3-amino-1,2,4-triazole, ox = oxalate) containing aromatic amine groups, Shimizu and co-workers observed the bonding of adsorbed CO2 and aromatic amine groups for the first time, where the electrically deficient C atoms could be stabilized by the electrically rich N atoms. The distance between them was about 3.152 Å, less than the sum of van der Waals radii of N and C atoms (Fig. 26a).187 Based on the uncoordinated triazole groups, Chen and Zhang also observed strong N⋯C dipole interactions (Fig. 26b),188 where the adsorbed CO2 molecules were synergistically stabilized by two adjacent N atoms (C15–N8, 3.178(8) Å; C15–N2, 3.011(8) Å; C16–N11, 3.15(1) Å; C16–N5, 3.26(1) Å), resulting in a high adsorption enthalpy. Polar oxygen atoms can also be served as adsorption sites for CO2 capture. For example, Schröder and Yang used nitro groups to achieve an efficient promotion of CO2 adsorption. Through NPD analyses, they further found that there were strong C⋯O dipole interactions between the adsorbed CO2 and nitro groups with a distance of 2.88 Å and 3.21 Å, respectively (Fig. 26c).189 Recently, our group proved that the carboxylic acid group could well adsorb and stabilize CO2 through SCXRD. In the structure of CO2@FJI-H38, the adsorbed CO2 is simultaneously stabilized by two O atoms of the carboxylic acid group, with the shortest C–O distance of only 2.78 Å, far below the sum of van der Waals radii of C and O atoms (Fig. 26d).190 Like N and O atoms, the more electronegative fluorine atoms can also adsorb CO2 effectively. As confirmed by Eddaoudi's group, the CO2 adsorbed in the pores of NbOFFIVE-1-Ni can be efficiently stabilized by four different (NbOF5)2− with a C⋯F distance of 3.050(8) Å, lower than the sum of the van der Waals radii of C and F atoms (Fig. 26e).191 In another isomorphic MOF (dptz-CuTiF6), the CO2 located in the pore was also stabilized by the surrounding TiF62− ions, where the distance between the F and C atoms was only 2.512 Å, indicating that a stronger C⋯F dipole interaction is formed (Fig. 26f).192
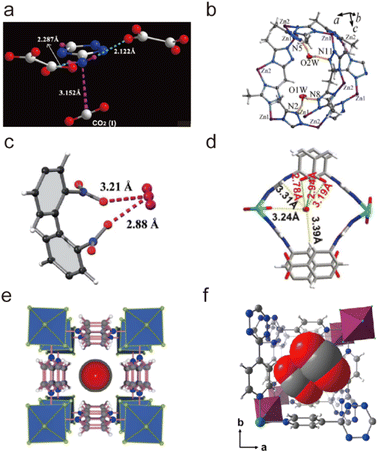 | ||
| Fig. 26 Carbon dioxide adsorption on electrically rich atoms through dipole interactions. (a) The N⋯CO2 dipole interactions in Zn2(Atz)2(ox). Reproduced from ref. 187 with permission from the American Association for the Advancement of Science, Copyright 2010. (b) The N⋯CO2 dipole interactions in [Zn2(btm)2]. Reproduced from ref. 188 with permission from the American Chemical Society, Copyright 2012. (c) The O⋯CO2 dipole interactions in MFM-102-NO2. Reproduced from ref. 189 with permission from the Royal Society of Chemistry, Copyright 2020. (d) The O⋯CO2 dipole interactions in FJI-H38. Reproduced from ref. 190 with permission from Wiley, Copyright 2023. (e) The F⋯CO2 dipole interactions in NbOFFIVE-1-Ni. Reproduced from ref. 191 with permission from the American Chemical Society, Copyright 2016. (f) The F⋯CO2 dipole interactions in dptz-CuTiF6. Reproduced from ref. 192 with permission from Elsevier, Copyright 2019. | ||
Since electron-rich oxygen atoms easily form hydrogen bonding with electron-deficient hydrogen atoms, active hydrogen atoms can also serve as adsorption sites for CO2. In 2012, Chen's group found that a MOF (UTSA-16) without OMS could also efficiently capture CO2. Through SCXRD analysis, they found that the coordinated H2O on the framework was the adsorption site of CO2 (O3w–H⋯O72 = 2.971(1) Å and O3w–H⋯O71 = 3.067(1) Å) (Fig. 27a).193 In the same year, Schröder and Yang confirmed that the hydroxyl group was also an efficient adsorption site for CO2 through PXRD. In the structure of CO2@NOTT-300, the adsorbed CO2 and hydroxyl group formed a stronger hydrogen-bonding interaction than that between CO2 and UTSA-16, with a distance of only 2.298 Å between H and O atoms (Fig. 27b).194 In 2017, they confirmed that the active hydrogen on the amide group and phenyl group could also form strong hydrogen-bonding interactions with adsorbed CO2 through NPD experiments. The distance of the adsorbed CO2 from the H atoms of the amide group and the phenyl group was 2.22 and 1.97 Å, respectively (Fig. 27c).195 In the structure of CO2@MFM-102-NO2, they further found that the adsorbed CO2 was only 1.91 and 1.74 Å away from the H atoms of the phenyl group, implying the formation of stronger hydrogen-bonding based on CH and CO2 (Fig. 27d).189
 | ||
| Fig. 27 Carbon dioxide adsorption on electron-deficient H atoms through hydrogen-bonding interactions. (a) The OH⋯O hydrogen-bonding interactions in UTSA-16. Reproduced from ref. 193 with permission from Springer Nature, Copyright 2012. (b) The OH⋯O hydrogen-bonding interactions in NOTT-300. Reproduced from ref. 194 with permission from Springer Nature, Copyright 2012. (c) The NH/CH⋯O hydrogen-bonding interactions in MFM-188a. Reproduced from ref. 195 with permission from Springer Nature, Copyright 2017. (d) The CH⋯O hydrogen-bonding interactions in MFM-102-NO2. Reproduced from ref. 189 with permission from the Royal Society of Chemistry, Copyright 2020. | ||
Due to the presence of π bonds and p electrons, π–π or p–π interactions can be formed between CO2 and suitable conjugated molecules. Therefore, molecules containing conjugated groups (e.g., phenyl groups) can also serve as adsorption sites of CO2. Both Walton and Smit's group used MS to confirm that the phenyl rings could indeed serve as an effective adsorption site of CO2 (Fig. 28a and b).196,197 However, due to its weak strength and the lack of clear directionality, it is difficult to directly characterize the π–π interactions between adsorbed CO2 and MOFs. So far, only very limited examples that can directly reveal the π–π interactions between CO2 and MOFs have been reported. In 2013, Li and Paris first observed the π–π/p–π interactions between adsorbed CO2 and phenyl groups through SXCRD analysis, where the adsorbed CO2 was located between two centroids of the phenyl groups, with an average distance of 3.81 Å (Fig. 28c).198 Later, Schröder and Yang discovered another type of interaction between adsorbed CO2 and phenyl groups through NPD analysis. In the structure of the CO2-adsorbed MFM-300(VIV), CO2 bound with the two adjacent phenyl rings in a ‘sandwich’ form, and the distance between the O atom of CO2 and the phenyl group was 3.069(2) and 3.146(3) Å respectively (Fig. 28d).199
 | ||
| Fig. 28 Carbon dioxide adsorption on phenyl groups through π–π interaction. (a) and (b) The potential π–π interaction between adsorbed CO2 and phenyl rings based on MS. Reproduced from ref. 196 with permission from the American Chemical Society, Copyright 2013. Reproduced from ref. 197 with permission from Springer Nature, Copyright 2019. (c) The π–π/p–π interactions between adsorbed CO2 and phenyl groups in CaSDB. Reproduced from ref. 198 with permission from Wiley, Copyright 2013. (d) The π–π/p–π interactions between adsorbed CO2 and phenyl groups in MFM-300(VIV). Reproduced from ref. 199 with permission from Springer Nature, Copyright 2017. | ||
In short, OMSs that can provide empty orbitals for CO2, polar atoms (e.g., N, O, and F) that can generate dipole interactions with CO2, H atoms that can form hydrogen-bonding with CO2, and conjugated molecules can all serve as effective sites for the physical adsorption of CO2. It should also be pointed out that multiple CO2 adsorption sites can exist simultaneously in MOFs, and adsorbed CO2 molecules can be synergistically stabilized by multiple adsorption sites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 would be formed (Fig. 29d).203
1 would be formed (Fig. 29d).203
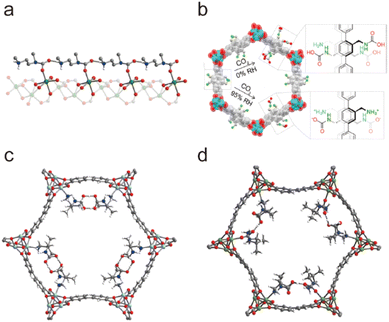 | ||
| Fig. 29 Carbon dioxide adsorption on nitrogen atoms through the formation of ammonium carbamate/carbamic acid. (a) The ammonium carbamate chain in mmen-Mn2(dobpdc). Reproduced from ref. 200 with permission from Springer Nature, Copyright 2015. (b) The carbamic acid and ammonium carbamate in IRMOF-74-III-(CH2NH2)2. Reproduced from ref. 201 with permission from the American Chemical Society, Copyright 2017. (c) The carbamate pairs in dmpn-Zn2(dobpdc). Reproduced from ref. 202 with permission from the American Chemical Society, Copyright 2017. (d) The mixture of carbamic acid and ammonium carbamate in dmpn-Mg2(dobpdc). Reproduced from ref. 203 with permission from the American Chemical Society, Copyright 2018. | ||
In addition to aliphatic amines, O-containing alkaline groups (e.g., hydroxyl anions and alcohol anions) can also react reversibly with CO2 to produce carbonates. Thus, suitable O-containing groups that can react reversibly with CO2 can also be introduced into MOFs as chemisorption sites of CO2. Due to the strong alkalinity of O-containing alkaline groups, only MOFs with an excellent base tolerance can introduce them. So only very limited examples of CO2 adsorption based on O-containing alkaline groups have been reported. In 2011, Stoddart and Yaghi used CD-MOF-2 to achieve the chemisorption of CO2 in MOFs for the first time, where free primary alcohol groups could react with adsorbed CO2 to form alkyl carbonates, which can be monitored through 13C solid-state NMR (the signal at 158 ppm) (Fig. 30a).204 In 2015, Zhang and Chen achieved another mode of CO2 chemisorption using a MOF (MAF-X25ox, [MnIIMnIII(OH)Cl2(bbta)], H2bbta = 1H,5H-benzo(1,2-d:4,5-d′)bistriazole) containing monodentate hydroxide. The newly formed bicarbonate could be determined by IR spectroscopy, where multiple new adsorption peaks (the peaks at 3682/1224/1050 cm−1) attributed to bicarbonate were generated simultaneously (Fig. 30c).206 By mimicking the active centre of carbonic anhydrase, both Wade and Dincǎ’s groups synthesized different MOFs containing active ZnN3OH units by introducing hydroxide anions into Zn(II) metal nodes, separately realizing their chemical adsorption of CO2.209 Here, the Zn–OH group also reacts with the adsorbed CO2 to produce bicarbonate, which can be confirmed by in situ IR and diffuse reflectance infrared Fourier transform spectroscopy respectively (Fig. 30d).207 Recently, Milner and collaborators also achieved the chemical adsorption of CO2 using a MOF (KOH CD-MOF), which was isomorphic to CD-MOF-2 but less alkaline. Here, the adsorbed CO2 reacted with the hydroxyl anions in the pores of the MOF to form bicarbonate, which could be confirmed using solid 13C NMR (the signal at 159.6 ppm) and IR spectra (the peak at 1663 cm−1) simultaneously (Fig. 30b).205
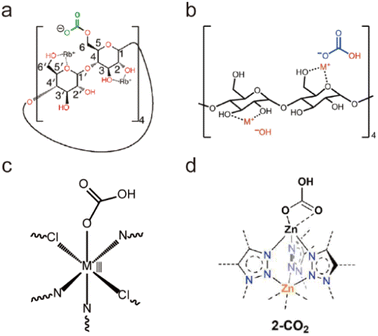 | ||
| Fig. 30 Carbon dioxide adsorption on oxygen atoms through the formation of carbonate. (a) The alkyl carbonates in CD-MOF-2. Reproduced from ref. 204 with permission from the American Chemical Society, Copyright 2011. (b) The bicarbonate in KOH CD-MOF. Reproduced from ref. 205 with permission from Wiley, Copyright 2022. (c) The bicarbonate in MAF-X25ox. Reproduced from ref. 206 with permission from the Royal Society of Chemistry, Copyright 2015. (d) The bicarbonate in [Zn(ZnOH)4(bibta)3]. Reproduced from ref. 207 with permission from the American Chemical Society, Copyright 2018. | ||
In short, suitable alkaline groups (e.g., aliphatic amines, hydroxyl anions, and alcohol anions) that can react reversibly with CO2 can be used as chemisorption sites of CO2, and the newly formed species based on adsorbed CO2 can be characterized through solid-state NMR and IR spectroscopy. It is worth mentioning that physical adsorption can also coexist even if an MOF has a high density of chemisorption sites.
3.4. Carbon capture based on water stable MOFs
As mentioned previously, since the pore size and pore environment of MOFs can be precisely controlled through structural design, they have better prospects in the field of carbon capture than traditional porous materials such as mesoporous silicon and zeolite. Indeed, the carbon capture performance of some MOFs has surpassed that of commercial zeolite materials. Considering that water vapor is widely present in flue gas, air, and crude natural gas, a practical carbon adsorbent should first have a good water vapor resistance. Although there have been many reviews on the progress of MOFs in the field of carbon capture, these articles are mainly focused on carbon capture performance and mechanisms. Here, we focus on the progress of water-stable (at least moisture stable) MOFs in flue gas decarbonization, direct air capture, and natural gas purification, hoping to provide a useful reference for the development of practical and excellent carbon adsorbents.| MOFs | Stability | CO2 adsorption amount at 1 bar (mmol g−1) | CO2 adsorption amount at 0.15 bar (mmol g−1) | IAST selectivity at 1 bar (15/85) CO2/N2 | Q st (kJ mol−1) | Temperature (K) | Ref. |
|---|---|---|---|---|---|---|---|
| a 1.2 bar. b At 40 mbar. c At 20 bar. d 0.06 bar. e Calculated from Henry's law constant. f 800 torr. g Dry air. h RH 10%. i 0.1 bar. | |||||||
| UiO-66 | pH = 0–12 | 1.79 | 0.37 | 19.4 | 24.5 | 298K | 234 |
| UiO-66-NH2 | Water-stable | 3 | ∼0.11 | ∼57 | 28 | 298 | 235 |
| UiO-66-(OPr)2 | pH = 1–11 | ∼2.6 | 1.19 | 39.1 | ∼34.5 | 298 | 236 |
| UiO-66-(OH)2 | Water-stable | ∼4.6 | 1.92 | 66 | 34.5 | 298 | 236 |
| UiO-66-SO3H-0.15 | pH = 1–12 | 2.23 | 0.51 | 16.3 | 26.6 | 298 | 234 |
| UiO-66-SO3Li-0.15 | pH = 1–12 | 3.28 | 0.93 | 39.7 | 25.4 | 298 | 234 |
| UiO-66-(COOLi)4-EX | Water-stable | 2.34 | 0.7 | 26.62 | 27.5 | 298 | 237 |
| UiO-66(Zr)-(COOLi)2 | Water-stable | 1.36 | 0.44 | 50.8 | 34.7 | 298 | 238 |
| UiO-66-EA | Water-stable | 1.7 | 1.05 | 365 | 66 | 298 | 239 |
| UiO-66(Hf)-(OH)2 | pH = 1–10 | 4.06 | 1.81 | 93 | 28.4 | 298 | 240 |
| NOTT-300 | Water-stable | 7.0 | 2.64 | 815e | 27–30 | 273 | 194 |
| Al(HCOO)3 | (HCl 12M-pH 14) | ∼4 | 2.7 | 368 | 47.9 | 320 | 210 |
| MIP-207 | Water-stable | 2.11 | 0.89 | 52.8 | — | 298 | 101 |
| CPM-74 | pH = 3–10 | ∼2.91 | — | 72–77 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) 90) |
33 | 198 | 160 |
| NOTT-140 | Water-stable | 20.72c | — | — | 24.7 | 283 | 246 |
| La-BTN | pH = 2–12 | 3.904 | — | 93 | 28.5 | 273 | 109 |
| NKMOF-3-Eu | Moisture-stable (90% RH) | 1.9 | — | ∼160 | 47 | 298 | 212 |
| NJU-Bai53 | pH = 2–11 | 5.01 | — | — | 88.3 | 298 | 233 |
| ZIF-301 | Moisture-stable (80% RH) | 1.47f | — | 19e | ∼30 | 298 | 247 |
| ZIF-300 | Moisture-stable (80% RH) | 1.32f | — | 22e | ∼28 | 298 | 247 |
| ZIF-1001 | pH = 3–14 | ∼0.67 | 0.328 | 19.7 | 26.4 | 298 | 248 |
| ZIF-1002 | pH = 3–14 | ∼0.50 | 0.268 | 21.5 | 26.9 | 298 | 248 |
| ZIF-1003 | pH = 3–14 | ∼0.49 | 0.265 | 21.6 | 28.4 | 298 | 248 |
| ZIF-1004 | pH = 3–14 | ∼0.65 | 0.348 | 24.4 | 28.1 | 298 | 248 |
| ZnF(daTZ) | pH = 1–12 | ∼1.8 | 0.96 | 120 | 33 | 298 | 249 |
| ZnF(aTZ) | pH = 2–12 | ∼1.85 | 0.56 | 46 | 32.3 | 298 | 249 |
| ZnF(TZ) | pH = 3–12 | ∼1.63 | 0.27 | 13 | 23.6 | 298 | 249 |
| SIFSIX-3-Zn | Moisture-stable (74% RH) | 2.55 | — | 1818 | 45 | 298 | 214 |
| SIFSIX-2-Cu-i | Moisture-stable (95% RH) | 5.41 | — | 140 | 31.9 | 298 | 213 |
| UTSA-120 | Moisture-stable (80% RH) | 5.0 | 3.56 | ∼600 | 27–31 | 296 | 215 |
| dptz-CuTiF6 | Moisture-stable | 4.52 | 2.93i | — | 38.2 | 298 | 192 |
| Cu-BTTri | Water-stable | 3.24 | 0.277d | 21 | 21 | 298 | 130 |
| SIFSIX-3-Cu | Moisture-stable (74% RH) | ∼2.50 | — | — | 54 | 298 | 214 |
| Qc-5-Cu-sql-β | Water-stable | 2.16 | — | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
36 | 293 | 216 |
| Cu–F-pymo | pH = 1–14 | 1.19 | — | >107 | 29.1 | 298 | 250 |
| TIFSIX-Cu-TPA | Water-stable | 2.1 | — | 910 | 39.2 | 298 | 251 |
| SIFSIX-18-Ni-β | Moisture-stable (90% RH) | ∼3 | ∼2.8 | — | 52 | 298 | 230 |
| SIFSIX-3-Ni | Moisture-stable (75% RH) | 2.7 | ∼2.5i | — | 50.9 | 298 | 231 |
| TIFSIX-3-Ni | Moisture-stable (75% RH) | ∼2.42 | 2.39 | — | 50 | 298 | 229 |
| NbOFFIVE-1-Ni | Moisture-stable (95% RH) | 2.2 | — | — | 54 | 298 | 191 |
| CALF-20 | Water-stable | 4.07a | — | 230 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) 90) |
39 | 293 | 217 |
| FJI-H14 | pH = 2–12 | 6.52 | 2.3 | 51 | 26.6 | 298 | 136 |
| FJI-H29 | pH = 2–13 | 1.79 | 0.92 | 146 | 34 | 298 | 138 |
| ZU-301 | pH = 2–12 | 2.44 | — | ∼850 | 38 | 298 | 252 |
| PCN-200 | pH = 2–12 | ∼1.56 | ∼1.69 | 60 | 38.5 | 273 | 253 |
| MOF-808-DL-Lys | Water-stable | — | 1.04 | — | 80 | 298 | 211 |
| MOF-808-Gly | Water-stable | — | 0.54g | — | 46 | 298 | 211 |
| 0.69h | |||||||
| een-Mg2(dobpdc) | Moisture-stable (100% RH) | 5.05 | 4.04 | — | 70–74 | 313 | 226 |
| en-Mg2(dobpdc) | Moisture-stable (100% RH) | 4.57 | 3.62 | 230 | 49–51 | 298 | 219 |
| ipen-Mg2(dobpdc) | Moisture-stable (100% RH) | 4.05 | 3.47 | — | 76–85 | 313 | 226 and 242 |
| een-MOF/Al–Si–C17-200 | Moisture-stable | ∼3.47 | 2.82 | 342(15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75) 75) |
52−66 | 313 | 220 |
| Mg2(dobpdc)(3-4-3) | Moisture-stable | ∼3.4b | — | — | 99 | 363 | 243 |
| en-CuBTTri | Water-stable | 1.27 | 0.366d | 25 | 90 | 298 | 130 |
| MAF-X25ox | Water-stable | 7.14 | 4.08 | 250 | 120 | 298 | 206 |
| MAF-X27ox | Water-stable | 6.70 | 4.06 | 262 | 124 | 298 | 206 |
| KHCO3 CD-MOF | Water-stable | 2.50 | 0.63 | 81 | 49.5 ± 1.8 | 313 | 205 |
| PN@MOF-5 | Moisture-stable (40% RH) | 3.48 | — | 212(14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86) 86) |
29 | 273 | 222 |
As mentioned above, water-stable MOFs can be directly constructed from hard metal ions and carboxylate ligands. Then, using this strategy, many water-stable MOFs have been synthesized and used for carbon capture. For example, based on the cheap Al(III) ions and formate ligands, Cheetham, Zhao, and co-workers prepared a chemically stable MOF (ALF), which could retain its framework in both strong base solutions (1 M NaOH) and acidic solutions (12 M HCl) (Fig. 31a).210 Due to its polar ultramicro-pores, ALF could selectively capture CO2 from N2 with a high CO2/N2 (85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) selectivity of 368 (Fig. 31b). Notably, ALF could be used to effectively extract CO2 from simulated flue gas (15/85 CO2/N2 binary gas at 323 K) (Fig. 31c) with a high adsorption capacity (0.80 mmol g−1) and adsorption selectivity (75) (Fig. 31c), and such excellent separation performance could remain for at least 130 cycles (Fig. 31d). Furthermore, ALF could be prepared on a large scale, making it a potential carbon capture reagent for flue gas decarbonization.
15) selectivity of 368 (Fig. 31b). Notably, ALF could be used to effectively extract CO2 from simulated flue gas (15/85 CO2/N2 binary gas at 323 K) (Fig. 31c) with a high adsorption capacity (0.80 mmol g−1) and adsorption selectivity (75) (Fig. 31c), and such excellent separation performance could remain for at least 130 cycles (Fig. 31d). Furthermore, ALF could be prepared on a large scale, making it a potential carbon capture reagent for flue gas decarbonization.
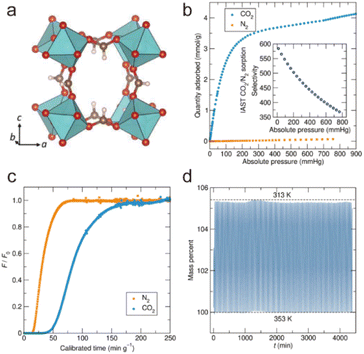 | ||
| Fig. 31 The structure and CO2 adsorption performance of ALF. Reproduced from ref. 210 with permission from the Advancement of Science, Copyright 2022. (a) The structure of ALF. (b) The CO2/N2 sorption isotherms of ALF at 298 K. (c) The breakthrough curves of ALF at 323 K. (d) 130 cycles of adsorption and desorption. | ||
Due to the excellent water stability of Zr–O clusters, many functionalized Zr-MOFs have been synthesized for carbon capture.234–240 For example, Yaghi's group prepared a series of amino acid (AA) functionalized MOFs (MOF-808-AA) based on a highly stable MOF-808 [Zr6O4(OH)4(BTC)2(HCOO)6], which could effectively capture CO2 from simulated flue gas in the presence of water.211 The CO2 uptake performances of MOF-808AAs varied from 0.277 to 1.040 mmol g−1 at 15 kPa (15% CO2 in 1 atm gas mixture, relevant to flue gas). According to the binary CO2/H2O adsorption isotherms, the CO2 uptake of MOF-808-Gly in the presence of water was significantly higher than that under dry conditions. This might be because the amine residues in the pores enabled MOF-808-AA to have enough affinity for CO2 to form bicarbonate. Moreover, the adsorbed CO2 could be released via vacuum swing adsorption instead of heating. By introducing ethanolamine (EA) into water-stable UiO-66, Zhang and co-workers also realized an efficient CO2 capture under humid conditions.239 Due to the strong interactions between amino groups and CO2, the newly formed UiO-66-EA displayed higher adsorption enthalpy (66 kJ mol−1) and adsorption selectivity (356) than the original UiO-66 (30 kJ mol−1 and 15), and such excellent CO2 adsorption performance could be completely maintained even at 82% RH.
Water-stable lanthanide MOFs can also serve as efficient CO2 adsorbents. For example, Zhang's team synthesised a series of robust ultramicroporous Ln-MOFs (NKMOF-3-Ln) (Fig. 32a), which could maintain their framework intact after being exposed to humid air with 90% RH for 7 days.212 Due to its enzyme-like channels, NKMOF-3-Ln could be used to realize molecular sieving of CO2 from N2 (Fig. 32b and c), in which the CO2 adsorption capacity and adsorption enthalpy would be enhanced gradually with the contraction of the ionic radius of lanthanide elements (Fig. 32d).
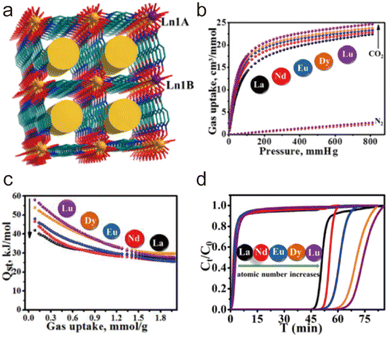 | ||
| Fig. 32 (a) The structure of NKMOF-3-Ln. (b)–(d) The CO2/N2 adsorption isotherms, CO2 adsorption enthalpy, and breakthrough curves of CO2/N2 (15/85) mixtures at 298 K. Reproduced from ref. 212 with permission from the American Chemical Society, Copyright 2019. | ||
As described previously, thermodynamically stable MOFs could also be constructed using soft metal ions and soft N-containing ligands. Many water/moisture-stable MOFs based on this strategy have been used for carbon capture. For example, Zaworotko, Eddaoudi and co-workers used moisture-stable ultramicroporous frameworks (SIFSIX-2-Cu-i and SIFSIX-3-Zn) to realize an efficient and selective CO2 capture (Fig. 33a). Due to the matched pore size (5.15 Å and 3.84 Å) and strong polar F− anions, both of them could be used to effectively and selectively capture CO2 at a relatively low CO2 partial pressure (0.1 bar) (Fig. 33c and d).213,214 Notably, SIFSIX-2-Cu-i could maintain its CO2 adsorption capacity in the presence of water vapour (Fig. 33b). Such excellent CO2 adsorption performance makes SIFSIX-2-Cu-i a promising absorbent for capturing CO2 from flue gas.
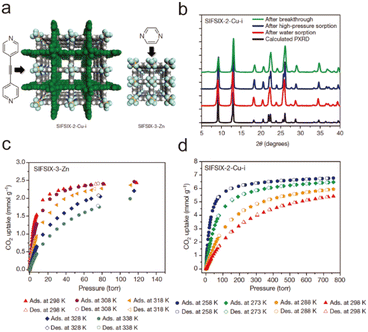 | ||
| Fig. 33 (a) The structure of SIFSIX-2-Cu-i and SIFSIX-3-Zn. (b) PXRD patterns of SIFSIX-2-Cu-i after breakthrough experiments, high-pressure absorption, and water adsorption. (c) and (d) The CO2 sorption isotherms of SIFSIX-3-Zn (c) and SIFSIX-2-Cu-i at different temperatures. Reproduced from ref. 213 and 214 with permission from Springer Nature, Copyright 2015. | ||
To further enhance CO2 capture, Chen et al. synthesized another ultramicroporous MOF (UTSA-120) using 3, 6-di(4-pyridyl)-1,2,4,5-tetrazine ligand, which had a similar pore to SIFSIX-2-Cu-i but with abundant N atoms.215 Compared with SIFSIX-2-Cu-i, UTSA-120 not only displayed a better CO2 sorption capacity (3.56 mmol g−1 at 296 K) at a low pressure (0.15 bar), but also exhibited a higher CO2/N2 adsorption selectivity. Notably, the IAST selectivity of UTSA-120 for simulated flue gas was 4 times higher than that of SIFSIX-2-Cu-i. Such an enhanced CO2 sorption capacity at a low pressure was thought to have resulted from the high density of nitrogen sites. Even after an exposure of the UTSA-120 sample to air for 1 month, its carbon capture performance could be completely maintained.
As mentioned previously, the crystallinity and stability of MOFs can be optimized using self-assembling ligands containing both soft and hard coordination atoms with soft metal ions. Based on this strategy, many water-stable MOFs have been developed and used for CO2 capture. For instance, Zaworotko, Zhang and co-workers prepared a water-stable MOF (Qc-5-Cu-sql-β) based on quinoline-5-carboxylic acid (HQc) and Cu(II) salts,216 which had a very small pore that could match with CO2 very well (3.3 Å). Notably, Qc-5-Cu-sql-β could maintain its structural integrity not only in water for 21 days but also in air with 75% RH for 14 days. Due to the well-matched pores, Qc-5-Cu-sql-β could be used to realize the molecular sieving of CO2 with a very high IAST selectivity of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Dynamic breakthrough experiments further demonstrated that the CO2 separation capacity of Qc-5-Cu-sql-β could also be maintained even at 75% RH.
000. Dynamic breakthrough experiments further demonstrated that the CO2 separation capacity of Qc-5-Cu-sql-β could also be maintained even at 75% RH.
Based on the H2BTTA ligand containing both N and O atoms, our group also realized the controlled construction of an acid/base-resistant MOF (FJI-H14) (Fig. 12g) and its efficient carbon capture.136 FJI-H14 exhibited a very high CO2 adsorption capacity of 6.52 mmol g−1 while only with a relatively low adsorption enthalpy (the Qst at a low coverage is 26.6 kJ mol−1) (Fig. 34a and b). Its IAST selectivity for a CO2/N2 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) mixture was calculated to be 51 at 298 K and 1 bar (Fig. 34c). Even after 5 cycles, FJI-H14 still retained almost 100% adsorption capacity, suggesting its excellent CO2 tolerance (Fig. 34d). Mechanistic studies indicated that such excellent CO2 adsorption performance (e.g., very high adsorption capacity and high adsorption selectivity) came from the synergy of the confined pores and high density of open metal sites.
85) mixture was calculated to be 51 at 298 K and 1 bar (Fig. 34c). Even after 5 cycles, FJI-H14 still retained almost 100% adsorption capacity, suggesting its excellent CO2 tolerance (Fig. 34d). Mechanistic studies indicated that such excellent CO2 adsorption performance (e.g., very high adsorption capacity and high adsorption selectivity) came from the synergy of the confined pores and high density of open metal sites.
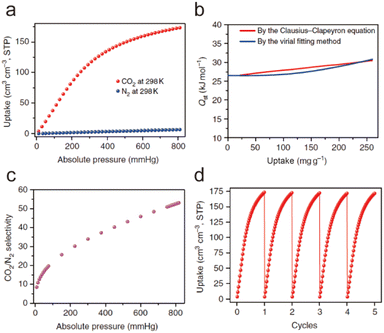 | ||
Fig. 34 (a) The CO2 and N2 adsorption isotherms for FJI-H14 at 298 K. (b) The isosteric heat of CO2 adsorption (Qst) for FJI-H14. (c) The IAST selectivity of FJI-H14 for CO2/N2 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85). (d) 5 Cycles of CO2 adsorption for FJI-H14 at 298 K. Reproduced from ref. 136 with permission from Springer Nature, Copyright 2017. 85). (d) 5 Cycles of CO2 adsorption for FJI-H14 at 298 K. Reproduced from ref. 136 with permission from Springer Nature, Copyright 2017. | ||
Using 1,2,4-triazolate, oxalate, and Zn(II) as coordination units, Shimizu and co-workers prepared another highly stable MOF ([Zn2(1,2,4-triazolate)2(oxalate)], CALF-20) (Fig. 35a).217 Even after being exposed to steam at 150 °C for a week, CALF-20 could well maintain its structure. The uptake of CALF-20 for CO2 was up to 4.07 mmol g−1 at 1.2 bar and 293 K, and its IAST selectivity for a CO2/N2 mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) was calculated to be 230 (Fig. 35b). Such excellent CO2 adsorption capacity could also be retained in the presence of water vapor due to different adsorption sites of CO2 and H2O. Furthermore, CALF-20 was resistant to wet acid gases and could be reused (>450
90) was calculated to be 230 (Fig. 35b). Such excellent CO2 adsorption capacity could also be retained in the presence of water vapor due to different adsorption sites of CO2 and H2O. Furthermore, CALF-20 was resistant to wet acid gases and could be reused (>450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) without the loss of adsorption capacity. In addition, the kg-scale of CALF-20 could be mildly prepared. All of these make CAL-20 a potential adsorbent for decarbonization in power plants.
000 cycles) without the loss of adsorption capacity. In addition, the kg-scale of CALF-20 could be mildly prepared. All of these make CAL-20 a potential adsorbent for decarbonization in power plants.
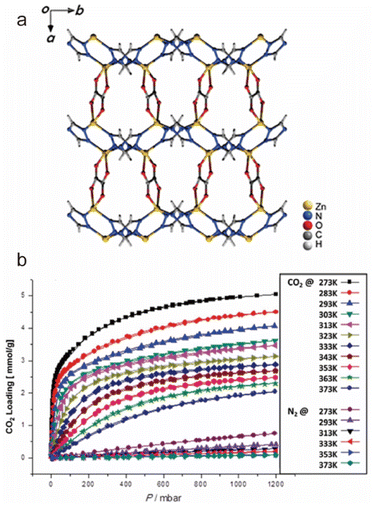 | ||
| Fig. 35 (a) The structure of CALF-20. (b) The CO2 and N2 isotherms of CALF-20 at different temperatures. Reproduced from ref. 217 with permission from the Advancement of Science, Copyright 2021. | ||
Active sites (e.g., amines and hydroxides) that can strongly interact with CO2 are often also able to coordinate with metal ions, so it is difficult to directly synthesize MOFs containing these active sites. By introducing these functional groups into water-stable MOFs, CO2 adsorption can be promoted while also maintaining their stability. For example, through introducing ethylenediamine (en) into a water-stable MOF (CuBTTri) constructed from soft N-containing ligands, Long and co-workers successfully prepared an en-functionalized MOF (en-CuBTTri) and realized an enhanced CO2 capture.130 After ethylenediamine grafting, the selectivity of CO2 over N2 increased from 21 to 25 at 1 bar; and the adsorption enthalpy of CO2 significantly rose from 21 kJ mol−1 to 90 kJ mol−1, especially at extremely low loadings. Such enhanced adsorption performance could be attributed to the affinity of alkylamine for carbon dioxide. To further improve CO2 adsorption capacity, N,N′-dimethylethylenediamine (mmen) was also introduced into the framework of CuBTTri by Long's group.218 In comparison to unmodified CuBTTri, modified mmen-CuBTTri was able to adsorb 4.2 mmol g−1 of CO2 at 298 K and 1 bar, showing an increase of 15% in adsorption capacity. According to ideal adsorbed solution theory, mmen-CuBTTri exhibited a relatively high selectivity of 327 for the CO2/N2 gas mixture (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) at 298 K. The extremely high Qst for CO2 (96 kJ mol−1 at zero coverage) was considered to be the main drive for the enhanced adsorption capacity and adsorption selectivity. Furthermore, even after 72 cycles of use, mmen-CuBTTri could also retain its adsorption capacity for CO2. Besides Long's group, Hong and co-workers also reported several amine-functionalized MOFs, which exhibited enhanced CO2 adsorption capacity.219,220
85) at 298 K. The extremely high Qst for CO2 (96 kJ mol−1 at zero coverage) was considered to be the main drive for the enhanced adsorption capacity and adsorption selectivity. Furthermore, even after 72 cycles of use, mmen-CuBTTri could also retain its adsorption capacity for CO2. Besides Long's group, Hong and co-workers also reported several amine-functionalized MOFs, which exhibited enhanced CO2 adsorption capacity.219,220
Through introducing a hydroxide group into water-resistant MOFs (MAF-X25/27) based on N-containing ligands,221 Chen's group also realized enhanced CO2 adsorption by synthesizing two hydroxide-functionalized isostructural frameworks [MnIIMnIII(OH)Cl2(bbta)] (MAF-X25ox, 1′) and [CoIICoIII(OH)Cl2(bbta)] (MAF-X27ox, 2′).206 In comparison to unmodified precursors, their CO2 adsorption capacities at 1 bar and 298 K increased from 5.4 and 4.2 mmol g−1 to 7.1 and 6.7 mmol g−1, respectively. At 0.15 bar and 298 K, the adsorption capacity of 1′ and 2′ was 410% and 540% larger than that of their precursors, reaching 4.08 and 4.06 mmol g−1, respectively. Moreover, the CO2/N2 selectivity of 1′ and 2′ at 298 K was calculated to be 250 and 264, 8.6 and 9.2 times higher than that of their precursors, respectively. The significant increase in Qst (from 38 and 28 kJ mol−1 to 99 and 110 kJ mol−1) demonstrated the ultrahigh CO2 affinity of 1′ and 2′. Notably, the breakthrough curves of 2′ were nearly the same in both dry and high-humidity environments, while its precursor almost could not capture CO2 from the wet mixture of CO2 and N2. Based on a hydroxide-functionalized MOF (KHCO3 CD-MOF), Milner and co-workers also realized the enhancement of CO2 adsorption capacity.205
In addition to enhancing the kinetic water stability of MOFs, hydrophobic engineering can also be used to improve their CO2 capture performance. For instance, Wang's group successfully realized the improvement of water stability and CO2 adsorption by introducing hydrophobic polynaphthylene (PN) into MOF-5 (Fig. 36a).222 In contrast to water-sensitive MOF-5, hydrophobically modified PN@MOF-5 could retain its crystallinity and porosity upon exposure to humid air (40% RH) for 40 h. Due to the smaller pores and the hydrophobic aromatic groups, PN@MOF-5 exhibited significantly enhanced CO2 capture performance (Fig. 36b). Compared with the original MOF-5, the adsorption capacity was doubled and the adsorption selectivity for CO2/N2 (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86) at 1 bar and 273 K increased by 23-fold (Fig. 36c). Such excellent CO2 adsorption performance could be largely maintained at 65% RH.
86) at 1 bar and 273 K increased by 23-fold (Fig. 36c). Such excellent CO2 adsorption performance could be largely maintained at 65% RH.
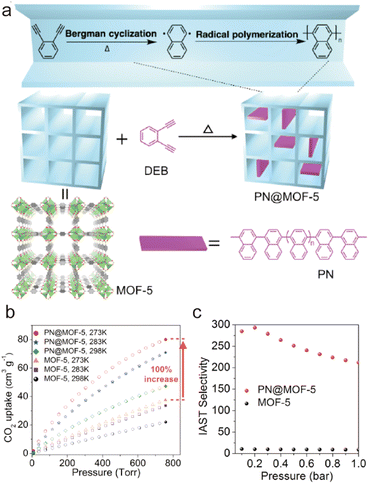 | ||
Fig. 36 (a) Introducing hydrophobic polynaphthylene (PN) into MOF-5 through the polymerization of DEB. (b) The CO2 adsorption isotherms of MOF-5 and PN@MOF-5 at different temperatures. (c) The IAST selectivity of MOF-5 and PN@MOF-5 for a CO2/N2 mixture (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86) at 273 K. Reproduced from ref. 222 with permission from the American Chemical Society, Copyright 2016. 86) at 273 K. Reproduced from ref. 222 with permission from the American Chemical Society, Copyright 2016. | ||
To achieve efficient decarbonization of flue gas, many water-stable MOFs based on different synthetic strategies were prepared. Water-stable MOFs based on the Hard–Soft-Acid–Base strategy can not only be directly used for CO2 adsorption (e.g., ALF), but also be used as potential precursors for PSM to achieve more efficient CO2 capture (e.g., MOF 808 and CuBTTri). In addition, molecular sieving of CO2 can be achieved by using MOFs with matched pores (e.g., SIFSIX-2-Cu-I and UTSA-120), exhibiting very high adsorption selectivity but relatively low adsorption enthalpy. The self-assembly of ligands containing both N and O atoms with soft metals is beneficial for constructing MOFs with high performance, stability, and economy, such as CLAF-20. It is worth mentioning that CLAF-20 can be directly used for the decarbonization of flue gas and can be also mass-produced by BASF, making it a promising industrial CO2 adsorbent.
| MOFs | Stability | CO2 uptake at different pressures (mmol g−1) | Q st (kJ mol−1) | Temperature (K) | Ref. | |
|---|---|---|---|---|---|---|
| 390 ppm | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm 000 ppm |
|||||
| a 400 ppm. b 500 ppm. c 1000 ppm. | ||||||
| NJU-Bai51 | pH = 1–12 | 0.13 | — | 33.3 | 298 | 269 |
| NJU-Bai53 | pH = 2–11 | 0.64a | — | 88.3 | 298 | 233 |
| [Zn(ZnOH)4(bibta)3] | Moisture-stable | 2.2 | — | 71 | 298 | 207 |
| SIFSIX-3-Cu | Moisture-stable (74% RH) | 1.24a | 2.34 | 54 | 298 | 214 |
| dptz-CuTiF6 | Moisture-stable | — | 2.1 | 38.2 | 298 | 192 |
| TIFSIX-3-Co | Moisture-stable | 1.05a | 2.63 | — | 298 | 228 |
| NbOFFIVE-1-Ni | Moisture-stable (95% RH) | 1.30a | ∼2.15 | 54 | 298 | 191 |
| TIFSIX-3-Ni | Moisture-stable (75% RH) | 1.12a | 1.75 | 50.9 | 298 | 229 |
| SIFSIX-18-Ni-β | Moisture-stable (90% RH) | 0.4b | 2.2 | 52 | 298 | 230 |
| SIFSIX-3-Ni | Moisture-stable (75% RH) | 0.35a | 1.9 | 45 | 298 | 231 |
| GeFSIX-3-Ni | Moisture-stable | 1.07a | 2.41 | 55.5 | 298 | 270 |
| FJI-H38 | pH = 2–12 | — | 2.33 | 27 | 298 | 190 |
| Mg2(dobdc)(N2H4)1.8 | Moisture-stable (82% RH) | 3.89 | — | 118 | 298 | 223 |
| en-Mg2(dobpdc) | Moisture-stable (100% RH) | 2.83 | 49–51 | 298 | 219 | |
| ED-Mg-MOF-74 | Moisture-stable | 1.5 | — | — | 298 | 268 |
| epn-MOF80@SBS | Moisture-stable (60% RH) | — | 2.8c | 67 | 298 | 178 |
Based on hard metals/ligands, Bai's group successfully synthesised two isostructural MOFs, [Fe3O(TPBTM6−)(Cl)(H2O)2]n (NJU-Bai52) and [Sc3O(TPBTM6−)(OH)(H2O)2]n(NJU-Bai53) (TPBTM = N,N′,N′′-tris(isophthalyl)-1,3,5-benzenetricarboxamide) (Fig. 37a).233 Due to the strong Fe–O/Sc–O units, both of them could maintain their structural integrity in H2O for two months. Notably, NJU-Bai53 could not only preserve its framework in aqueous solutions (pH = 2 to 11) but also maintain its porosity after water treatment (Fig. 37b). Due to the presence of monodentate hydroxide, NJI-Bai53 displayed higher CO2 adsorption capacity than NJI-Bai52. At 298 K and 0.4 mbar, it could adsorb 0.64 mmol g−1 of CO2, which was 50 times higher than that of NJU-Bai52 (0.013 mmol g−1) (Fig. 37c). At zero-coverage, the adsorption enthalpy of NJU-Bai53 for CO2 was up to 88.3 kJ mol−1, demonstrating the presence of strong chemisorption interactions (Fig. 37d). According to the simple selectivity equation S = (q1/q2)/(p1/p2), the CO2/N2 (0.4/800) selectivity of NJU-Bai53 at 298 K was calculated to be 6397. All of these make NJU-Bai53 a potential adsorbent for CO2 capture from the air.
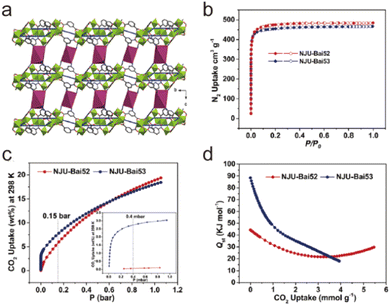 | ||
| Fig. 37 (a) The structure of NJU-Bai52 and NJU-Bai53. (b) The N2 sorption isotherms of NJU-Bai53 (77 K) before and after water treatment. (c) The CO2 adsorption isotherms of NJU-Bai52 and NJU-Bai53 at 298 K. (d) The adsorption enthalpy of NJU-Bai52 and NJU-Bai53 for CO2. Reproduced from ref. 233 with permission from the American Chemical Society, Copyright 2019. | ||
Besides hard metals/ligands, soft metals/ligands are also widely used to construct water/moisture MOFs for direct air capture. For instance, Eddaoudi's group prepared a moisture-stable ultramicroporous MOF (SIFSIX-3-Cu) based on the pyrazine ligand and CuSiF6.214 SIFSIX-3-Cu had not only very narrow pores (3.5 Å) suitable for CO2 but also high-density F− anions that could form strong interactions with CO2. Due to its polar ultramicroporous pores, SIFSIX-3-Cu displayed not only a very high trace CO2 adsorption capacity (1.24 mmol g−1 at 0.4 mbar and 298 K) but also extremely high adsorption selectivity (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 for CO2/N2 (1/999)). Further breakthrough experiments demonstrated that such excellent adsorption performance could be retained under humid conditions (74% RH). By replacing (SiF6)2− with (NbOF5)2−, Eddaoudi's group synthesized another hydrolytically stable ultramicroporous MOF (NbOFFIVE-1-Ni) with a similar but smaller pore (about 3.2 Å) than SIFSIX-3-Cu (Fig. 38a).191 Due to the more matched pore (pore size and pore environment). NbOFFIVE-1-Ni displayed a higher trace CO2 adsorption capacity (1.3 mmol g−1 at 0.4 mbar and 298 K) than SIFSIX-3-Cu, meaning that NbOFFIVE-1-Ni was the most efficient physical adsorbent for direct air capture (Fig. 38b). Single-crystal X-ray diffraction studies demonstrated that such excellent adsorption capacity resulted from the good match between the adsorbed CO2 and the pores of NbOFFIVE-1-Ni (Fig. 38c).
500 for CO2/N2 (1/999)). Further breakthrough experiments demonstrated that such excellent adsorption performance could be retained under humid conditions (74% RH). By replacing (SiF6)2− with (NbOF5)2−, Eddaoudi's group synthesized another hydrolytically stable ultramicroporous MOF (NbOFFIVE-1-Ni) with a similar but smaller pore (about 3.2 Å) than SIFSIX-3-Cu (Fig. 38a).191 Due to the more matched pore (pore size and pore environment). NbOFFIVE-1-Ni displayed a higher trace CO2 adsorption capacity (1.3 mmol g−1 at 0.4 mbar and 298 K) than SIFSIX-3-Cu, meaning that NbOFFIVE-1-Ni was the most efficient physical adsorbent for direct air capture (Fig. 38b). Single-crystal X-ray diffraction studies demonstrated that such excellent adsorption capacity resulted from the good match between the adsorbed CO2 and the pores of NbOFFIVE-1-Ni (Fig. 38c).
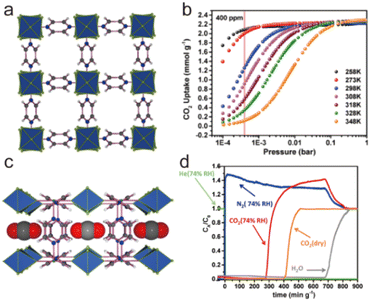 | ||
| Fig. 38 (a) The structure of NbOFFIVE-1-Ni. (b) The CO2 sorption isotherms of NbOFFIVE-1-Ni at different temperatures. (c) The CO2 adsorption sites in NbOFFIVE-1-Ni. (d) The breakthrough curves with the mixed-gas CO2/N2 (1/99) at 1 bar and 298 K in both dry and humid environments. Reproduced from ref. 191 with permission from the American Chemical Society, Copyright 2016. | ||
Furthermore, the adsorption capacity of NbOFFIVE-1-Ni for CO2 could be maintained even at high humidity (Fig. 38d) and the adsorbed CO2 could be completely desorbed at 328 K under vacuum. All of these make NbOFFIVE-1-Ni a promising adsorbent for capturing CO2 in the atmosphere.
Using a methylated dipyrazolyl ligand (3,3′,5,5′-tetramethyl-1H,1′H-4,4′-bipyrazole) and NiSiF6 as coordination units, Zaworotko's group realized the controlled construction of a more hydrophobic ultramicroporous framework (SIFSIX-18Ni-β).230 Due to its ultramicropores, SIFSIX-18-Ni-β also exhibited high adsorption capacity for trace CO2 (e.g., 0.4 mmol g−1 at 500 ppm and 2.2 mmol g−1 at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm). More importantly, due to the introduction of hydrophobic methyl groups, SIFSIX-18-Ni-β displayed a very low H2O uptake (1.6 mmol g−1) even at 95% RH, which allowed SIFSIX-18-Ni-β to preferentially adsorb CO2 from nitrogen and water in any case. This work not only provides a potential trace CO2 adsorbent for confined spaces but also offers a useful strategy for developing efficient trace CO2 adsorbents resisting water vapor.
000 ppm). More importantly, due to the introduction of hydrophobic methyl groups, SIFSIX-18-Ni-β displayed a very low H2O uptake (1.6 mmol g−1) even at 95% RH, which allowed SIFSIX-18-Ni-β to preferentially adsorb CO2 from nitrogen and water in any case. This work not only provides a potential trace CO2 adsorbent for confined spaces but also offers a useful strategy for developing efficient trace CO2 adsorbents resisting water vapor.
Very recently, through the reaction of a soft N-containing ligand (3, 5-di(4H-1, 2, 4-triazol-4-yl) benzoic acid, HDTBA) with Co(II) salts, our group reported an acid/base-resistant ultramicroporous MOF (FJI-H38), which could capture trace CO2 from humid air (Fig. 39a).190 Due to the stable CoN4O nodes, FJI-H38 could retain its framework in aqueous solutions with a pH of 2–12. It could capture trace CO2 from air with the high adsorption capacity (0.57 mmol g−1 at 0.5 mbar and 2.33 mmol g−1 at 0.01 bar) and the very high adsorption selectivity (6469 at 0.5 mbar) but a relatively low adsorption enthalpy (27 kJ mol−1) (Fig. 39b and c). Such outstanding CO2 adsorption performance could be largely maintained even at high humidity (75 RH%) (Fig. 39d). Mechanistic studies demonstrated that this excellent adsorption performance came from the synergism of flexible frameworks, ultramicropores, and different adsorption sites of H2O and CO2. Furthermore, FJI-H38 could be prepared on a large scale and reused without losing adsorption capacity, making it a potential adsorbent for trace CO2 in industry.
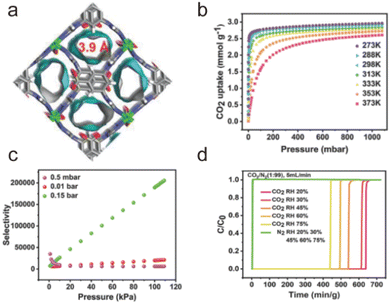 | ||
Fig. 39 (a) The structure of FJI-H38. (b) The CO2 sorption isotherms of FJI-H38 at different temperatures. (c) The IAST selectivity of FJI-H38 for CO2/N2 at various partial pressures. (d) The breakthrough curves of FJI-H38 for CO2/N2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) under different humid conditions. Reproduced from ref. 190 with permission from Wiley, Copyright 2023. 99) under different humid conditions. Reproduced from ref. 190 with permission from Wiley, Copyright 2023. | ||
By introducing amino groups into MOFs through pore engineering not only can their CO2 capture capacity be improved, but their water resistance is also enhanced. For example, Chen, Zhang and co-workers introduced a very small amine molecule (N2H4) into the pores of Mg2(dobdc), preparing a amine-functionalized MOF (Mg2(dobdc)(N2H4)1.8) (Fig. 40a).223 Due to the extremely high concentration of amine groups (7.08 mmol cm−3 or 6.01 mmol g−1), the as-synthesized Mg2(dobdc)(N2H4)1.8 could capture 3.89 mmol g−1 of CO2 at 0.4 mbar and 298 K, demonstrating its excellent trace CO2 adsorption capacity (Fig. 40b). Even at high humidity, such excellent CO2 adsorption performance could also be retained (Fig. 40c), which might be because the grafted N2H4 was not substituted by H2O. In addition to (Mg2(dobdc)(N2H4)1.8), there were several amine-functionalized MOFs (e.g., dmpn-Mg2(dobpdc) and nmen-Mg2(dobpdc)) that could realize efficient capture of trace CO2.202,226
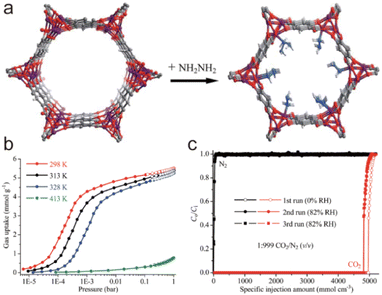 | ||
Fig. 40 (a) Introducing N2H4 into pores of Mg2(dobdc). (b) The CO2 sorption isotherms of (Mg2(dobdc)(N2H4)1.8) at different temperatures. (c) The breakthrough curves of (Mg2(dobdc)(N2H4)1.8) for CO2/N2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999) under different humid conditions. Reproduced from ref. 223 with permission from the Royal Society of Chemistry, Copyright 2016. 999) under different humid conditions. Reproduced from ref. 223 with permission from the Royal Society of Chemistry, Copyright 2016. | ||
Hydrophobic engineering can be used to simultaneously improve the water stability and trace CO2 adsorption of MOFs. For example, based on water-resistant Mg2(dobpdc) hybrids after hydrophobic modification (Fig. 24), Hong's group realized enhanced trace CO2 capture performance.178 Especially, epn-MOF80@SBS demonstrated an impressive CO2 adsorption of 2.8 mmol g−1 at 1000 ppm. Under the conditions with 60% RH, it could maintain consistent CO2 removal performance after over 10 cycles and even 7 days of exposure.
Through polymer coating, Zaworotko's group also improved the water resistance of SIFSIX-3-Ni and its adsorption for trace CO2 adsorption (Fig. 41a). Compared with uncoated SIFSIX-3-Ni, the HPMCP-coated HPMCP/SIFSIX-3-Ni displayed a significantly enhanced water resistance. After coating, the water contact angle increased by 8 times, and the framework could be preserved after being exposed to moisture for a long time. In high humid environments, HPMCP/SIFSIX-3-Ni could also display excellent gas separation performance while SIFSIX-3-Ni could not (Fig. 41b). Such excellent CO2 separation performance could be retained for at least 5 cycles (Fig. 41c), suggesting that it could be used to control indoor air quality.254
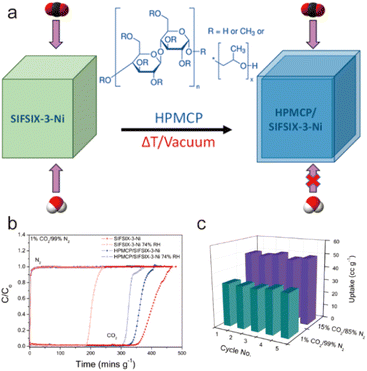 | ||
Fig. 41 (a) Coating HPMCP on SIFSIX-3-Ni. (b) The breakthrough curves of SIFSIX-3-Ni and HPMCP/SIFSIX-3 for CO2/N2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99) under different humid conditions. (c) The HPMCP/SIFSIX-3-Ni recyclability tests for humid 1% CO2/99% N2 and 15% CO2/85% N2 gas mixtures (regeneration at 80 °C). Reproduced from ref. 254 with permission from the American Chemical Society, Copyright 2020. 99) under different humid conditions. (c) The HPMCP/SIFSIX-3-Ni recyclability tests for humid 1% CO2/99% N2 and 15% CO2/85% N2 gas mixtures (regeneration at 80 °C). Reproduced from ref. 254 with permission from the American Chemical Society, Copyright 2020. | ||
Compared to flue gas decarbonization, there are very few water-stable MOFs available for direct air capture. These MOFs either have strong active sites (e.g., NJI-Bai53, and Mg2(dobdc)(N2H4)1.8) that can chemically adsorb CO2 or have ultramicropores that match well with CO2 (e.g., SIFSIX-3-Cu, NbOFFIVE-1-Ni, SIFSIX-18-Ni-β, and FJI-H38). Compared with chemisorption, physical adsorption based on matched pores has lower recovery energy and is more conducive to the recycling of adsorbents.244 It is worth mentioning that the capture capacities of SIFSIX-3-Cu, NbOFFIVE-1-Ni, and FJI-H38 exceed that of commercial zeolite 13X. Among these materials, FJI-H38 has lower adsorption enthalpy and better stability, indicating that it is a more practical adsorbent for trace CO2 capture.
| MOFs | Stability | CO2 adsorption amount at 1 atm (mmol g−1) | IAST selectivity at 1 bar (50/50) CO2/CH4 | Q st (kJ mol−1) | Temperature (K) | Ref. |
|---|---|---|---|---|---|---|
| a Calculated from Henry's law constant. | ||||||
| UiO-66-SO3Li-0.15 | pH = 1–12 | 3.28 | 12.7 | 25.4 | 298 | 234 |
| UiO-66-SO3Rb-1 | pH = 1–12 | 0.54 | 54.2 | 35.0 | 298 | 234 |
| UiO-66-SO3H-0.64 | pH = 1–12 | 1.54 | 15.2 | 33.2 | 298 | 234 |
| UiO-66-SO3Na-0.64 | pH = 1–12 | 0.94 | 46.2 | 35.1 | 298 | 234 |
| UiO-66-SO3K-1 | pH = 1–12 | 0.91 | 18 | 31.9 | 298 | 234 |
| UiO-66(Hf) | Water-stable | 1.50 | 12 | 22.8 | 298 | 240 |
| UiO-66(Hf)-(OH)2 | pH = 1–10 | 4.06 | 30 | 28.4 | 298 | 240 |
| UiO-66(Hf)-NH2 | Water-stable | 2.80 | 15 | 25.6 | 298 | 240 |
| UiO-66(Hf)-(COOH)2 | Water-stable | 1.20 | 13 | 28.2 | 298 | 240 |
| UiO-66(Hf)-(F)4 | Water-stable | 0.82 | 16 | 23.4 | 298 | 240 |
| PCN-222 | Water-stable | 1.16 | 4.3 | 18.3 | 298 | 134 |
| NJU-Bai52 | Water-stable | 5.3 | 13.5 | 44.2 | 298 | 233 |
| NJU-Bai51 | pH = 1–12 | — | 10.7 | 33.3 | 298 | 269 |
| NJU-Bai50 | pH = 1–12 | — | 4.4 | 26.6 | 298 | 269 |
| CPM-231 | Moisture-stable (75% RH) | 6.77 | 2.65 | 20.4 | 298 | 258 |
| ee-2-Mg2(dobpdc) | Water-stable | ∼4.5 | — | −65 ± 1 | 298 | 260 |
| UTSA-280 | Water-stable | 3.0 | — | 42.9 | 298 | 267 |
| [Zn3(OH)2(pzdc)(atz)] | pH = 2–12 | 2.1 | 1.3 × 107 | 48 | 273 | 263 |
| SIFSIX-3-Zn | Moisture-stable | 2.55 | 231 | 45 | 298 | 214 |
| UTSA-49 | Moisture-stable | 3.08 | 33.7 | 27 | 298 | 273 |
| UTSA-120 | Moisture-stable | 5.0 | 100 | 27–31 | 296 | 215 |
| UTSA-121 | Moisture-stable | 2.65 | 10 | 21.9 | 298 | 274 |
| Qc-5-Cu-sql-β | Water-stable | 2.16 | 3300 | 36 | 293 | 216 |
| SIFSIX-2-Cu-i | Moisture-stable | 5.41 | 33 | 31.9 | 298 | 213 |
| Cu(MTABA) | Water-stable | 1.5 | 9.9 | 38.4 | 298 | 276 |
| Cu–F-pymo | pH = 1–14 | 1.19 | >107 | 29.1 | 298 | 250 |
| Co-btz-ht | pH = 1–12 | 2.16 | 63 | 38 | 273 | 125 |
| Ni-btz-ht | pH = 1–12 | 4.24 | 54 | ∼38 | 273 | 125 |
| GeFSIX-3-Co | Moisture-stable | 2.65 | 160 | 39.1 | 298 | 270 |
| GeFSIX-3-Ni | Moisture-stable | 2.61 | 930 | 55.5 | 298 | 270 |
| WOFOUR-1-Ni | pH = 2–13 | 2.32 | 26 | 65.5 | 298 | 277 |
| NbOFFIVE-1-Ni | Moisture-stable (95% RH) | 2.2 | 6528 | 54 | 273 | 191 |
| CSMCRI-9 | pH = 4–10 | 3.34 | 5.39 | 28.21 | 273 | 278 |
| CSMCRI-3 | pH = 5–10 | 2.4 | 11.7 | 27 | 273 | 279 |
| MUF-16 | Water-stable | 2.13 | 6690 | 32.3 | 293 | 265 |
| MUF-16(Ni) | Water-stable | 2.14 | 1220 | 37.3 | 293 | 265 |
| MUF-16(Mn) | Water-stable | 2.25 | 470 | 36.6 | 293 | 265 |
| IITKGP-6 | Water-stable | 1.67 | 5.1 | 23 | 295 | 280 |
| [Co2(4,4′-bpy)(STBZ)] | Water-stable | 1.63 | 9.9 | 29–31 | 298 | 281 |
| FJI-H29 | pH = 2–13 | 1.79 | 25 | 34 | 298 | 138 |
| ZJNU-27 | Water-stable | 3.54 | 5.4 | 26.7 | 298 | 282 |
| ZJNU-28 | Water-stable | 3.43 | 9.6 | 32.1 | 298 | 282 |
| ZJNU-26 | Water-stable | 3.06 | 4.3 | 30.4 | 298 | 282 |
| SNNU-52 | pH = 3–11 | 2.64 | 7.3 | 28.3 | 298 | 257 |
| SNNU-53 | pH = 3–11 | 1.96 | 7.1 | 34.2 | 298 | 257 |
| SNNU-55 | pH = 3–11 | 1.49 | 10 | 33.6 | 298 | 257 |
| SNNU-56 | pH = 3–11 | 2.49 | 6.2 | 32.5 | 298 | 257 |
| SNNU-54 | Water-stable | 2.31 | 7.5 | 38 | 298 | 257 |
| SNNU-51 | Water-stable | 2.4 | 15.2 | 23.3 | 298 | 257 |
| MOF-508b | Water-stable | 1.85 | 3 | 19.2 | 303 | 283 |
| Zn(BDC)(BPY)0.5 | Water-stable | 0.32 | 12.3 | 10.0–37.2 | 298 | 284 |
| QI-Cu | Water-stable | 4.56 | 6.1 | 23.75 ± 0.49 | 293 | 271 |
| UTSA-16GO | Water-stable | 3.62 | 114.4 | 42.1 | 296 | 259 |
| MOF-505@GO | Moisture-stable | 3.94 | 8.6 | — | 298 | 275 |
Like flue gas decarbonization, many water-stable MOFs based on hard metal/carboxylate ligands have been prepared and used to purify natural gas. For instance, based on the hard Ca(II) ion and squaric acid ligand, Chen's group also achieved the controlled synthesis of a water-stable MOF ([Ca(C4 O4)(H2O)] UTSA-280) and its efficient purification of CH4 (Fig. 42a).264,265 Due to the strong Ca–O bonds, the as-synthesized UTSA-280 could remain its framework and porosity even after being immersed in water for 1 month. Due to its matched pore, UTSA-280 exhibited a relatively high CO2 adsorption capacity (3.0 mmol g−1) but almost no CH4 adsorption (Fig. 42b). Dynamic breakthrough experiments demonstrated that UTSA-280 could capture 1.918 mmol g−1 of CO2 from a CO2/CH4 (50/50) mixture at 298 K, and such excellent separation performance could be completely retained for over 5 cycles (Fig. 42c and d). All of these make UTSA-280 a potential adsorbent for natural gas purification.
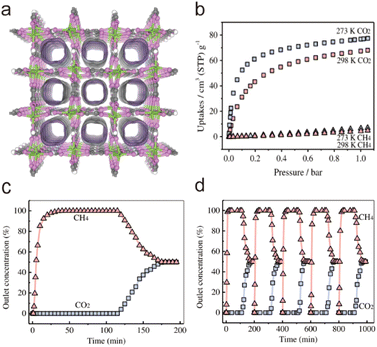 | ||
Fig. 42 (a) The structure of UTSA-280. (b) The CO2/CH4 sorption isotherms of UTSA-280 at 273 and 298 K. (c) and (d) The breakthrough curves and cycles of UTSA-280 for CO2/CH4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Reproduced from ref. 245 with permission from Wiley, Copyright 2020. 1). Reproduced from ref. 245 with permission from Wiley, Copyright 2020. | ||
Water-stable MOFs based on soft metals/ligands have also been widely prepared and used to purify crude natural gas. For example, Eddaoudi's group prepared a moisture stable MOF (AlFFIVE-1-Ni, KAUST-8) based on the pyrazine ligand and Ni(NO3)2, which could maintain its framework integrity even after being exposed to humid air with 95% RH (Fig. 43a).266,267 Due to its specific pore environment, AlFFIVE-1-Ni could effectively and equally adsorb CO2 and H2S from gas mixtures containing CO2, H2S and CH4 in a wide temperature range. This unusual co-adsorption of CO2 and H2S was further proved by temperature-dependent desorption (Fig. 43b). Moreover, such excellent separation performance could be retained not only in the presence of water vapour, but also during multiple cycling tests (Fig. 43c and d). This work represents a simple and efficient method for the simultaneous removal of acid gases (e.g., H2S and CO2) from crude natural gas.
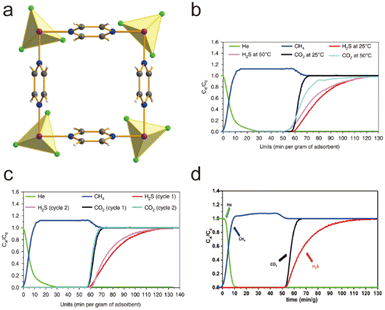 | ||
| Fig. 43 (a) The framework of AlFFIVE-1-Ni. (b) The breakthrough experiments using a CO2/H2S/CH4:5/5/90 gas mixture on AlFFIVE-1-Ni at 298 K and 323 K (1 bar). (c) The breakthrough trials using a fresh sample under the same parameters (298 K) after optimal activation (105 °C) and after five breakthrough cycles. (d) The breakthrough experiments under humid conditions (65% RH). Reproduced from ref. 266 with permission from the American Association for the Advancement of Science, Copyright 2017. Reproduced from ref. 267 with permission from Springer Nature, Copyright 2018. | ||
Based on soft Cu(II) ions and 5-fluoropyrimidin-2-olate ligands, Chen's group also achieved the synthesis and natural gas purification of a water-stable MOF (Cu–F-pymo). Due to the stable CuN4 nodes, Cu–F-pymo could preserve its framework in aqueous solutions with a pH of 1–14.250 Due to its polar ultramicropores (about 3.3 Å), Cu–F-pymo could exclusively capture CO2 over CH4 and N2. At 298 K and 1 atm, Cu–F-pymo exhibited a relatively high CO2 adsorption capacity of 1.6 mmol g−1 but very low adsorption capacities for both CH4 (0.042 mmol g−1) and N2 (0.0075 mmol g−1). Such unusual size-sieving separation performance could be further confirmed by their IAST selectivity for both CO2/CH4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) (>107) and CO2/N2 (15
50) (>107) and CO2/N2 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85) (>107) mixtures. Such excellent separation performance could be maintained for at least 5 cycling tests.
85) (>107) mixtures. Such excellent separation performance could be maintained for at least 5 cycling tests.
Through selecting soft N-containing ligands (5-aminoisophthalic acid, H2aip), Telfer's team prepared a series of water-stable microporous MOFs (M(Haip)2; M = Co, (MUF-16); M = Ni, MUF-16(Ni); M = Mn, MUF-16(Mn)), all of which could be used to purify natural gas effectively (Fig. 44a).265 They could remain their frameworks after being immersed to water for two weeks. At 1 atm and 293 K, the CO2 adsorption capacity of isomeric MUF-16, MUF-16(Ni) and MUF-16(Mn) was about 2.13 mmol g−1, 2.13 mmol g−1 and 2.25 mmol g−1, respectively (Fig. 44b). Meanwhile, MUF-16 could only capture a very small amount of CH4 (0.054 mmol g−1) under the same conditions. Notably, the IAST selectivity of MUF-16 for the mixture of CO2 and CH4 (50/50) was up to 6690 at 293 K and 1 bar (Fig. 44c). Mechanistic analysis showed that such excellent selectivity came from the match of its pore and CO2 (from size to polarity). Moreover, such an excellent CO2 adsorption capacity could be maintained even after being soaked in water for 24 h and being exposed to humid air (80% RH) for 12 months (Fig. 44d).
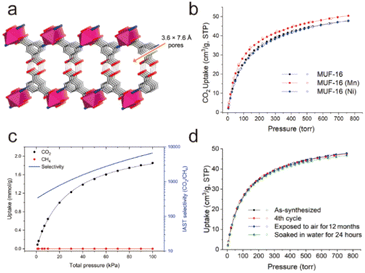 | ||
Fig. 44 (a) The structure of MUF-16. (b) The CO2 adsorption isotherms of MUF-16s at 293 K. (c) The IAST selectivity of MUF-16 for CO2/CH4 (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50) at 293 K. (d) The CO2 adsorption isotherms of MUF-16 at 293 K, after 4 consecutive sorption cycles, 12 months of exposure to air with 80% RH, and 24 hours of immersion in water. Reproduced from ref. 265 with permission from Springer Nature, Copyright 2021. 50) at 293 K. (d) The CO2 adsorption isotherms of MUF-16 at 293 K, after 4 consecutive sorption cycles, 12 months of exposure to air with 80% RH, and 24 hours of immersion in water. Reproduced from ref. 265 with permission from Springer Nature, Copyright 2021. | ||
Based on another soft N-containing ligand (3,5-pyrazoledicarboxylic acid, H3pzdc), Zhang and co-workers also realized the controllable preparation and natural gas purification of a water-stable MOF ([Zn3(OH)2(pzdc)(atz)], Hatz = 3-amino-1,2,4-trizole).263 It could retain its framework in aqueous solutions (pH 3–12) and boiling water. Due to its polar ultramicropores, the activated [Zn3(OH)2(pzdc)(atz)] displayed a relatively high CO2 adsorption capacity of 2.1 mmol g−1 but a very low adsorption capacity for CH4 (0.056 mmol g−1), leading to a very high single-component selectivity for CO2/CH4 (50/50) (1.3 × 107). This means that [Zn3(OH)2(pzdc)(atz)] is a very rare adsorbent, which can realize molecular sieving of CO2 from CO2/CH4.
As mentioned above, hydrophobic engineering could enhance the water stability and CO2 capture performance of MOFs simultaneously. Through introducing hydrophobic groups, Zhao and co-worker also realized the improvement of the water stability and CH4 purification capacity of NOTT-101.271 They prepared a new MOF QI-Cu through incorporating methyl and methylol groups into a famous MOF NOTT-101. Notably, the modified QI-Cu could remain its framework in water for at least 1 month while the framework of NOTT-101 would completely collapse in water within 24 h. Due to the reduced pores and increased groups, QI-Cu exhibited better CO2 adsorption capacity and higher CO2/CH4 adsorption selectivity than NOTT-101.
Compared to direct air capture, there are more water-stable MOFs available for purifying natural gas. Due to the similar polarity and size of CH4 and N2, MOFs can be used for decarbonization of flue gas, as well as for purifying natural gas. Purifying natural gas is mainly to remove CO2 impurities from CH4, so the adsorption selectivity of MOFs for CO2/CH4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is the most important indicator, and many recently reported MOFs display very high CO2/CH4 adsorption selectivity (e.g., [Zn3(OH)2(pzdc)(atz)], MUF-16, UTSA-280, and AlFFIVE-1-Ni). Among these MOFs, UTSA-280 and MUF-16 can be prepared from inexpensive building blocks and are expected to be used as potential adsorbents for natural gas purification.
1) is the most important indicator, and many recently reported MOFs display very high CO2/CH4 adsorption selectivity (e.g., [Zn3(OH)2(pzdc)(atz)], MUF-16, UTSA-280, and AlFFIVE-1-Ni). Among these MOFs, UTSA-280 and MUF-16 can be prepared from inexpensive building blocks and are expected to be used as potential adsorbents for natural gas purification.
4. Summary and perspective
In order to develop practical MOFs for the real world, increased studies have been carried out on the design and synthesis of water-resistant MOFs, with a series of water-stable MOFs constructed reasonably. In this review, we first briefly introduce the definition of water stability of MOFs and how to characterize their stability. To discuss the water stability of MOFs more fully, we divide it into thermodynamic stability and kinetic stability, and then introduce a method to improve their thermodynamic and kinetic stability. According to Hard–Soft-Acid–Base theory, thermodynamically stable MOFs can be reasonably constructed through directly selecting metal ions and ligands with matched softness/hardness. Therefore, we first discuss the reported water-stable MOFs directly constructed from coordination units with matched hardness, including (1) water-stable MOFs based on hard metal ions and ligands and (2) water-stable MOFs based on soft metal ions and ligands. In addition, other specific strategies that can be used to improve the thermodynamic stability of MOFs (e.g., interlocking of frameworks and improving their rigidity) are also introduced. The kinetic stability of MOFs to water can be improved by reducing the kinetics of their hydrolysis reaction. Therefore, a series of strategies that can inhibit the kinetics of MOF hydrolysis, including the direct use of hydrophobic ligands, the introduction of hydrophobic groups into the pores of MOFs, the coating of hydrophobic films on the surface of MOFs, and the increase of steric hindrance around metal nodes, are discussed in detail.Although great advance has been made in this area, there are several challenges that still need to be overcome. For example, because the currently used PXRD technique only reflects the state of MOFs before and after exposure to water, the interactions between MOFs and water remain unclear at the microscopic level. If such microscopic interactions between water and MOFs can be revealed by combining various in situ characterization and computational simulations,272 the water-stable MOFs that meet different application requirements (e.g., carbon capture at high humidity and radioactive metal capture under strong acids) can be precisely designed and synthesized. In addition, balancing the water stability and activity of MOFs is another challenge. Metal ions and ligands with matched hardness can be used to directionally construct water-stable MOFs. However, the functions of MOFs are also achieved by introducing specific Lewis acid or base sites. Therefore, it is necessary to not only avoid the competitive active sites interfering with the assembly of MOFs, but also ensure that the active sites can be reasonably introduced into the channels of MOFs, which is a contradiction. The hydrophobic modification of MOFs may also affect the performance of MOFs, because the channels of MOFs can be changed. More importantly, if water-stable MOFs are to be widely used in industry, both their economy and processability need to be solved. For economy, first of all, cheap organic ligands and metal salts should be selected as the raw materials for a target MOF, and then large-scale synthesis should be realized through cheap reaction media (such as water and ethanol). Finally, the recovery of unreacted ligands/metals and reaction media should be achieved. In terms of processability, the controllable preparation of a target MOF with different sizes should be achieved first, and then appropriate devices (e.g., fixed bed, thin film, etc.) should be made according to different application scenarios.
The controllable preparation of water-stable MOFs further expands the application scenarios of MOFs. Due to the necessity and urgency of carbon capture, we only introduce the progress of water-resistant MOFs in carbon capture in this review. We begin with a brief introduction to the background of carbon capture and then discuss the applications of water-stable MOFs to flue gas decarbonization, direct air capture, and the purification of crude natural gas. Considering that the carbon capture capacity of some reported MOFs has been superior to that of commercial molecular sieves, it is very possible to use appropriate MOFs to achieve carbon capture in industry. Before realizing industrialization, the following problems need to be solved. (1) The target MOFs need to be tested and cycled for a long time under real working conditions; (2) it is also necessary to solve the processability and economy of the target MOFs.
In a word, more and more water-stable MOFs are synthesized, and the understanding of the relationship between the water stability and structure of MOFs is getting deeper and deeper, and the use of water-stable MOFs is more and more extensive. Notably, the carbon capture capacity of some reported MOFs has been better than that of commercial molecular sieves. So, we believe that MOFs will eventually be widely used as a type of porous material for carbon capture in the real world. We hope that this review could accelerate the achievement of this goal.
Author contributions
Conceptualization: M. Hong and Q. Chen; writing – original draft: C. Xiao; validation: J. Tian; writing – review & editing: M. Hong and Q. Chen.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We gratefully acknowledge the financial support from the National Key Research and Development Program of China (2021YFA1500400 and 2018YFA0704500) and the Natural Science Foundation of Fujian Province (2023J06046).References
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. Paik Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CrossRef CAS.
- M. Eddaoudi, D. B. Moler, H. L. Li, B. L. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319–330 CrossRef CAS PubMed.
- R. J. Hill, D. L. Long, N. R. Champness, P. Hubberstey and M. Schroder, Acc. Chem. Res., 2005, 38, 335–348 CrossRef CAS PubMed.
- Y. He, B. Li, M. O'Keeffe and B. Chen, Chem. Soc. Rev., 2014, 43, 5618–5656 RSC.
- L. Feng, K.-Y. Wang, X.-L. Lv, T.-H. Yan and H.-C. Zhou, Natl. Sci. Rev., 2020, 7, 1743–1758 CrossRef CAS PubMed.
- J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2012, 112, 1001–1033 CrossRef CAS PubMed.
- Q.-G. Zhai, X. Bu, X. Zhao, D.-S. Li and P. Feng, Acc. Chem. Res., 2017, 50, 407–417 CrossRef CAS PubMed.
- Y. Bai, Y. Dou, L.-H. Xie, W. Rutledge, J.-R. Li and H.-C. Zhou, Chem. Soc. Rev., 2016, 45, 2327–2367 RSC.
- S. Furukawa, J. Reboul, S. Diring, K. Sumida and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5700–5734 RSC.
- G. Ferey, C. Mellot-Draznieks, C. Serre and F. Millange, Acc. Chem. Res., 2005, 38, 217–225 CrossRef CAS.
- J. J. I. V. Perry, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC.
- V. Guillerm and M. Eddaoudi, Acc. Chem. Res., 2021, 54, 3298–3312 CrossRef CAS PubMed.
- Z. Chen, K. O. Kirlikovali, P. Li and O. K. Farha, Acc. Chem. Res., 2022, 55, 579–591 CrossRef CAS PubMed.
- H. Assi, G. Mouchaham, N. Steunou, T. Devic and C. Serre, Chem. Soc. Rev., 2017, 46, 3431–3452 RSC.
- L. Chen, Q. Chen, M. Wu, F. Jiang and M. Hong, Acc. Chem. Res., 2015, 48, 201–210 CrossRef CAS PubMed.
- S. M. Cohen, Chem. Rev., 2012, 112, 970–1000 CrossRef CAS PubMed.
- T. Islamoglu, S. Goswami, Z. Li, A. J. Howarth, O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2017, 50, 805–813 CrossRef CAS PubMed.
- J.-P. Zhang, P.-Q. Liao, H.-L. Zhou, R.-B. Lin and X.-M. Chen, Chem. Soc. Rev., 2014, 43, 5789–5814 RSC.
- T. L. Easun, F. Moreau, Y. Yan, S. Yang and M. Schroeder, Chem. Soc. Rev., 2017, 46, 239–274 RSC.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461–466 CrossRef CAS.
- R. Kubota, S. Tashiro, M. Shiro and M. Shionoya, Nat. Chem., 2014, 6, 913–918 CrossRef CAS.
- Y. Xie, Y. Shi, E. M. Cedeno Morales, A. El Karch, B. Wang, H. Arman, K. Tan and B. Chen, J. Am. Chem. Soc., 2023, 145, 2386–2394 CrossRef CAS PubMed.
- Y. Xiao, Y. Chen, A. N. Hong, X. Bu and P. Feng, Angew. Chem., Int. Ed., 2023, 62, e202300721 CrossRef CAS PubMed.
- X. W. Gu, E. Wu, J. X. Wang, H. M. Wen, B. Chen, B. Li and G. Qian, Sci. Adv., 2023, 9, eadh0135 CrossRef CAS PubMed.
- C. Yu, Z. Guo, L. Yang, J. Cui, S. Chen, Y. Bo, X. Suo, Q. Gong, S. Zhang, X. Cui, S. He and H. Xing, Angew. Chem., Int. Ed., 2023, 62, e202218027 CrossRef CAS.
- Z. Niu, Z. Fan, T. Pham, G. Verma, K. A. Forrest, B. Space, P. K. Thallapally, A. M. Al-Enizi and S. Ma, Angew. Chem., Int. Ed., 2022, 61, e202117807 CrossRef CAS PubMed.
- J. Pei, H.-M. Wen, X.-W. Gu, Q.-L. Qian, Y. Yang, Y. Cui, B. Li, B. Chen and G. Qian, Angew. Chem., Int. Ed., 2021, 60, 25068–25074 CrossRef CAS PubMed.
- Q. Dong, Y. Huang, J. Wan, Z. Lu, Z. Wang, C. Gu, J. Duan and J. Bai, J. Am. Chem. Soc., 2023, 145, 8043–8051 CrossRef CAS PubMed.
- J. W. Wang, S. C. Fan, H. P. Li, X. Bu, Y. Y. Xue and Q. G. Zhai, Angew. Chem., Int. Ed., 2023, 62, e202217839 CrossRef CAS.
- Y. Jiang, L. Wang, T. Yan, J. Hu, W. Sun, R. Krishna, D. Wang, Z. Gu, D. Liu, X. Cui, H. Xing and Y. Zhang, Chem. Sci., 2023, 14, 298–309 RSC.
- B. Zhu, J.-W. Cao, S. Mukherjee, T. Pham, T. Zhang, T. Wang, X. Jiang, K. A. Forrest, M. J. Zaworotko and K.-J. Chen, J. Am. Chem. Soc., 2021, 143, 1485–1492 CrossRef CAS.
- M. Bonneau, C. Lavenn, J. J. Zheng, A. Legrand, T. Ogawa, K. Sugimoto, F. X. Coudert, R. Reau, S. Sakaki, K. I. Otake and S. Kitagawa, Nat. Chem., 2022, 14, 816–822 CrossRef CAS.
- V. I. Nikolayenko, D. C. Castell, D. Sensharma, M. Shivanna, L. Loots, K. A. Forrest, C. J. Solanilla-Salinas, K. I. Otake, S. Kitagawa, L. J. Barbour, B. Space and M. J. Zaworotko, Nat. Chem., 2023, 15, 542–549 CrossRef CAS.
- S. Zhou, O. Shekhah, A. Ramirez, P. Lyu, E. Abou-Hamad, J. Jia, J. Li, P. M. Bhatt, Z. Huang, H. Jiang, T. Jin, G. Maurin, J. Gascon and M. Eddaoudi, Nature, 2022, 606, 706–712 CrossRef CAS PubMed.
- D. E. Jaramillo, A. Jaffe, B. E. R. Snyder, A. Smith, E. Taw, R. C. Rohde, M. N. Dods, W. DeSnoo, K. R. Meihaus, T. D. Harris, J. B. Neaton and J. R. Long, Chem. Sci., 2022, 13, 10216–10237 RSC.
- W. Gong, Y. Xie, X. Wang, K. O. Kirlikovali, K. B. Idrees, F. Sha, H. Xie, Y. Liu, B. Chen, Y. Cui and O. K. Farha, J. Am. Chem. Soc., 2023, 145, 2679–2689 CrossRef CAS PubMed.
- H. Zeng, M. Xie, T. Wang, R.-J. Wei, X.-J. Xie, Y. Zhao, W. Lu and D. Li, Nature, 2021, 595, 542–548 CrossRef CAS PubMed.
- T. He, X. J. Kong, Z. X. Bian, Y. Z. Zhang, G. R. Si, L. H. Xie, X. Q. Wu, H. Huang, Z. Chang, X. H. Bu, M. J. Zaworotko, Z. R. Nie and J. R. Li, Nat. Mater., 2022, 21, 689–695 CrossRef CAS PubMed.
- S. Sharma, A. V. Desai, B. Joarder and S. K. Ghosh, Angew. Chem., Int. Ed., 2020, 59, 7788–7792 CrossRef CAS PubMed.
- X. Wang, F. Ma, S. Liu, L. Chen, S. Xiong, X. Dai, B. Tai, L. He, M. Yuan, P. Mi, S. Gong, G. Li, Y. Tao, J. Wan, L. Chen, X. Sun, Q. Tang, L. He, Z. Yang, Z. Chai and S. Wang, J. Am. Chem. Soc., 2022, 144, 13634–13642 CrossRef CAS PubMed.
- T. Chen, J.-H. Dou, L. Yang, C. Sun, J. J. Oppenheim, J. Li and M. Dinca, J. Am. Chem. Soc., 2022, 144, 5583–5593 CrossRef CAS PubMed.
- G. Skorupskii, B. A. Trump, T. W. Kasel, C. M. Brown, C. H. Hendon and M. Dinca, Nat. Chem., 2020, 12, 131–136 CrossRef CAS PubMed.
- S. Kim, B. Joarder, J. A. Hurd, J. Zhang, K. W. Dawson, B. S. Gelfand, N. E. Wong and G. K. H. Shimizu, J. Am. Chem. Soc., 2018, 140, 1077–1082 CrossRef CAS PubMed.
- J. Tian, L. Liu, K. Zhou, Z. Hong, Q. Chen, F. Jiang, D. Yuan, Q. Sun and M. Hong, Chem. Sci., 2020, 11, 9818–9826 RSC.
- L. Li, L. Guo, D. H. Olson, S. Xian, Z. Zhang, Q. Yang, K. Wu, Y. Yang, Z. Bao, Q. Ren and J. Li, Science, 2022, 377, 335–339 CrossRef CAS.
- Y. Han, Y. Chen, Y. Ma, J. Bailey, Z. Wang, D. Lee, A. M. Sheveleva, F. Tuna, E. J. L. McInnes, M. D. Frogley, S. J. Day, S. P. Thompson, B. F. Spencer, M. Nikiel, P. Manuel, D. Crawshaw, M. Schroder and S. Yang, Chem, 2023, 9, 738–754 Search PubMed.
- L. Yang, P. Cai, L. Zhang, X. Xu, A. A. Yakovenko, Q. Wang, J. Pang, S. Yuan, X. Zou, N. Huang, Z. Huang and H.-C. Zhou, J. Am. Chem. Soc., 2021, 143, 12129–12137 CrossRef CAS PubMed.
- Y. Wu, X. Wang, K. O. Kirlikovali, X. Gong, A. Atilgan, K. Ma, N. M. Schweitzer, N. C. Gianneschi, Z. Li, X. Zhang and O. K. Farha, Angew. Chem., Int. Ed., 2022, 61, e202117528 CrossRef CAS PubMed.
- S. Wang, H. G. T. Ly, M. Wahiduzzaman, C. Simms, I. Dovgaliuk, A. Tissot, G. Maurin, T. N. Parac-Vogt and C. Serre, Nat. Commun., 2022, 13, 1–8 Search PubMed.
- Q. Mo, L. Zhang, S. Li, H. Song, Y. Fan and C.-Y. Su, J. Am. Chem. Soc., 2022, 144, 22747–22758 CrossRef CAS PubMed.
- A. J. Howarth, Y. Y. Liu, P. Li, Z. Y. Li, T. C. Wang, J. Hupp and O. K. Farha, Nat. Rev. Mater., 2016, 1, 15018 CrossRef CAS.
- K. Wang, Y. Li, L.-H. Xie, X. Li and J.-R. Li, Chem. Soc. Rev., 2022, 51, 6417–6441 RSC.
- T. He, X.-J. Kong and J.-R. Li, Acc. Chem. Res., 2021, 54, 3083–3094 CrossRef CAS PubMed.
- L.-H. Xie, M.-M. Xu, X.-M. Liu, M.-J. Zhao and J.-R. Li, Adv. Sci., 2020, 7, 1901758 CrossRef CAS.
- N. C. Burtch and K. S. Walton, Acc. Chem. Res., 2015, 48, 2850–2857 CrossRef CAS PubMed.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed.
- X. Zhang, B. Wang, A. Alsalme, S. Xiang, Z. Zhang and B. Chen, Coord. Chem. Rev., 2020, 423, 213507 CrossRef CAS.
- M. Ding, X. Cai and H.-L. Jiang, Chem. Sci., 2019, 10, 10209–10230 RSC.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H. C. Zhou, Adv. Mater., 2018, 30, e1704303 CrossRef PubMed.
- J. Duan, W. Jin and S. Kitagawa, Coord. Chem. Rev., 2017, 332, 48–74 CrossRef CAS.
- K. Jayaramulu, F. Geyer, A. Schneemann, S. Kment, M. Otyepka, R. Zboril, D. Vollmer and R. A. Fischer, Adv. Mater., 2019, 31, e1900820 CrossRef PubMed.
- S. Wang and C. Serre, ACS Sustain. Chem. Eng., 2019, 7, 11911–11927 CAS.
- H. Lin, Y. Yang, Y.-C. Hsu, J. Zhang, C. Welton, I. Afolabi, M. Loo and H.-C. Zhou, Adv. Mater., 2023, 35, e2209073 CrossRef.
- X. Yao, K. E. Cordova and Y. B. Zhang, Small Struct., 2022, 3, 2100209 CrossRef CAS.
- R. Sahoo, S. Mondal, D. Mukherjee and M. C. Das, Adv. Funct. Mater., 2022, 32, 2207197 CrossRef CAS.
- R. Custelcean, Chem. Sci., 2021, 12, 12518–12528 RSC.
- X. Shi, H. Xiao, H. Azarabadi, J. Song, X. Wu, X. Chen and K. S. Lackner, Angew. Chem., Int. Ed., 2020, 59, 6984–7006 CrossRef CAS PubMed.
- D.-D. Zhou, X.-W. Zhang, Z.-W. Mo, Y.-Z. Xu, X.-Y. Tian, Y. Li, X.-M. Chen and J.-P. Zhang, EnergyChem, 2019, 1, 100016 CrossRef.
- R. L. Siegelman, P. J. Milner, E. J. Kim, S. C. Weston and J. R. Long, Energy Environ. Sci., 2019, 12, 2161–2173 RSC.
- J. Liu, Y. Wei and Y. Zhao, ACS Sustain. Chem. Eng., 2019, 7, 82–93 CrossRef CAS.
- Z. G. Hu, Y. X. Wang, B. B. Shah and D. Zhao, Adv. Sustainable Syst., 2019, 3, 1800080 CrossRef.
- M. Ding, R. W. Flaig, H.-L. Jiang and O. M. Yaghi, Chem. Soc. Rev., 2019, 48, 2783–2828 RSC.
- Y. Belmabkhout, V. Guillerm and M. Eddaoudi, Chem. Eng. J., 2016, 296, 386–397 CrossRef CAS.
- Z. Zhang, Z.-Z. Yao, S. Xiang and B. Chen, Energy Environ. Sci., 2014, 7, 2868–2899 RSC.
- G. Ferey, C. Serre, T. Devic, G. Maurin, H. Jobic, P. L. Llewellyn, G. De Weireld, A. Vimont, M. Daturi and J.-S. Chang, Chem. Soc. Rev., 2011, 40, 550–562 RSC.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef PubMed.
- Z. Li, X. Liu, W. Jin, Q. Hu and Y. Zhao, J. Colloid Interface Sci., 2019, 554, 692–704 CrossRef CAS PubMed.
- I. J. Kang, N. A. Khan, E. Haque and S. H. Jhung, Chem.–Euro. J., 2011, 17, 6437–6442 CrossRef CAS PubMed.
- L. Liang, L. Liu, F. Jiang, C. Liu, D. Yuan, Q. Chen, D. Wu, H.-L. Jiang and M. Hong, Inorg. Chem., 2018, 57, 4891–4897 CrossRef CAS PubMed.
- K. Leus, T. Bogaerts, J. De Decker, H. Depauw, K. Hendrickx, H. Vrielinck, V. Van Speybroeck and P. Van Der Voort, Microporous Mesoporous Mater., 2016, 226, 110–116 CrossRef CAS.
- L. Z. Qin, X. H. Xiong, S. H. Wang, L. Zhang, L. L. Meng, L. Yan, Y. N. Fan, T. A. Yan, D. H. Liu, Z. W. Wei and C. Y. Su, ACS Appl. Mater. Interfaces, 2022, 14, 45444–45450 CrossRef CAS PubMed.
- K. A. Cychosz and A. J. Matzger, Langmuir, 2010, 26, 17198–17202 CrossRef CAS PubMed.
- P. Horcajada, S. Surble, C. Serre, D. Y. Hong, Y. K. Seo, J. S. Chang, J. M. Greneche, I. Margiolaki and G. Ferey, Chem. Commun., 2007, 43, 2820–2822 RSC.
- C. Volkringer, D. Popov, T. Loiseau, G. Férey, M. Burghammer, C. Riekel, M. Haouas and F. Taulelle, Chem. Mater., 2009, 21, 5695–5697 CrossRef CAS.
- J. Lv, B. Wang, Y. B. Xie, P. L. Wang, L. Shu, X. O. Su and J. R. Li, Environ. Sci.: Nano, 2019, 6, 2759–2766 RSC.
- F. Yang, G. Xu, Y. Dou, B. Wang, H. Zhang, H. Wu, W. Zhou, J.-R. Li and B. Chen, Nat. Energy, 2017, 2, 877–883 CrossRef CAS.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- N.-X. Zhu, Z.-W. Wei, C.-X. Chen, X.-H. Xiong, Y.-Y. Xiong, Z. Zeng, W. Wang, J.-J. Jiang, Y.-N. Fan and C.-Y. Su, Angew. Chem., Int. Ed., 2022, 61, e202112097 CrossRef CAS PubMed.
- H. L. Jiang, D. W. Feng, T. F. Liu, J. R. Li and H. C. Zhou, J. Am. Chem. Soc., 2012, 134, 14690–14693 CrossRef CAS PubMed.
- D. W. Feng, Z. Y. Gu, Y. P. Chen, J. Park, Z. W. Wei, Y. J. Sun, M. Bosch, S. Yuan and H. C. Zhou, J. Am. Chem. Soc., 2014, 136, 17714–17717 CrossRef CAS PubMed.
- T. F. Liu, D. W. Feng, Y. P. Chen, L. F. Zou, M. Bosch, S. Yuan, Z. W. Wei, S. Fordham, K. C. Wang and H. C. Zhou, J. Am. Chem. Soc., 2015, 137, 413–419 CrossRef CAS PubMed.
- Y. X. Sun, Q. Sun, H. L. Huang, B. Aguila, Z. Niu, J. A. Perman and S. Q. Ma, J. Mater. Chem. A, 2017, 5, 18770–18776 RSC.
- B. Wang, X.-L. Lv, D. Feng, L.-H. Xie, J. Zhang, M. Li, Y. Xie, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 6204–6216 CrossRef CAS PubMed.
- B. Wang, Q. Yang, C. Guo, Y. X. Sun, L. H. Xie and J. R. Li, ACS Appl. Mater. Interfaces, 2017, 9, 10286–10295 CrossRef CAS PubMed.
- B. Wang, P. Wang, L.-H. Xie, R.-B. Lin, J. Lv, J.-R. Li and B. Chen, Nat. Commun., 2019, 10, 3861 CrossRef PubMed.
- X.-J. Kong, Y.-Z. Zhang, T. He, X.-Q. Wu, M.-M. Xu, S.-N. Wang, L.-H. Xie and J.-R. Li, CrystEngComm, 2018, 20, 6018–6025 RSC.
- L. H. Xie, X. M. Liu, T. He and J. R. Li, Chem, 2018, 4, 1911–1927 CAS.
- W. Liang, H. Chevreau, F. Ragon, P. D. Southon, V. K. Peterson and D. M. D'Alessandro, CrystEngComm, 2014, 16, 6530–6533 RSC.
- O. V. Gutov, W. Bury, D. A. Gomez-Gualdron, V. Krungleviciute, D. Fairen-Jimenez, J. E. Mondloch, A. A. Sarjeant, S. S. Al-Juaid, R. Q. Snurr, J. T. Hupp, T. Yildirim and O. K. Farha, Chem.–Euro. J., 2014, 20, 12389–12393 CrossRef CAS PubMed.
- Z. Lu, J. Duan, H. Tan, L. Du, X. Zhao, R. Wang, S. Kato, S. Yang and J. T. Hupp, J. Am. Chem. Soc., 2023, 145, 4150–4157 CrossRef CAS PubMed.
- S. Wang, H. Reinsch, N. Heymans, M. Wahiduzzaman, C. Martineau-Corcos, G. De Weireld, G. Maurin and C. Serre, Matter, 2020, 2, 440–450 CrossRef CAS.
- S. Wang, M. Cabrero-Antonino, S. Navalon, C.-c. Cao, A. Tissot, I. Dovgaliuk, J. Marrot, C. Martineau-Corcos, L. Yu, H. Wang, W. Shepard, H. Garcia and C. Serre, Chem, 2020, 6, 3409–3427 CAS.
- W.-B. Zhong, R.-X. Li, J. Lv, T. He, M.-M. Xu, B. Wang, L.-H. Xie and J.-R. Li, Inorg. Chem. Front., 2020, 7, 1161–1171 RSC.
- H.-Q. Yin, K. Tan, S. Jensen, S. J. Teat, S. Ullah, X. Hei, E. Velasco, K. Oyekan, N. Meyer, X.-Y. Wang, T. Thonhauser, X.-B. Yin and J. Li, Chem. Sci., 2021, 12, 14189–14197 RSC.
- F. C. Leng, H. Liu, M. L. Ding, Q. P. Lin and H. L. Jiang, ACS Catal., 2018, 8, 4583–4590 CrossRef CAS.
- H. L. Jiang, N. Tsumori and Q. Xu, Inorg. Chem., 2010, 49, 10001–10006 CrossRef CAS PubMed.
- D. Alezi, A. M. Peedikakkal, L. J. Weselinski, V. Guillerm, Y. Belmabkhout, A. J. Cairns, Z. Chen, L. Wojtas and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 5421–5430 CrossRef CAS PubMed.
- D. X. Xue, A. J. Cairns, Y. Belmabkhout, L. Wojtas, Y. Liu, M. H. Alkordi and M. Eddaoudi, J. Am. Chem. Soc., 2013, 135, 7660–7667 CrossRef CAS PubMed.
- J. Duan, M. Higuchi, R. Krishna, T. Kiyonaga, Y. Tsutsumi, Y. Sato, Y. Kubota, M. Takata and S. Kitagawa, Chem. Sci., 2014, 5, 660–666 RSC.
- J. G. Duan, M. Higuchi, S. Horike, M. L. Foo, K. P. Rao, Y. Inubushi, T. Fukushima and S. Kitagawa, Adv. Funct. Mater., 2013, 23, 3525–3530 CrossRef CAS.
- D. X. Xue, Y. Belmabkhout, O. Shekhah, H. Jiang, K. Adil, A. J. Cairns and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 5034–5040 CrossRef CAS PubMed.
- S. Yuan, J. S. Qin, J. Li, L. Huang, L. Feng, Y. Fang, C. Lollar, J. Pang, L. Zhang, D. Sun, A. Alsalme, T. Cagin and H. C. Zhou, Nat. Commun., 2018, 9, 808 CrossRef PubMed.
- K. Wang, D. Feng, T.-F. Liu, J. Su, S. Yuan, Y.-P. Chen, M. Bosch, X. Zou and H.-C. Zhou, J. Am. Chem. Soc., 2014, 136, 13983–13986 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- X. C. Huang, Y. Y. Lin, J. P. Zhang and X. M. Chen, Angew. Chem., Int. Ed., 2006, 45, 1557–1559 CrossRef CAS PubMed.
- Y. Wang, N.-Y. Huang, X.-W. Zhang, H. He, R.-K. Huang, Z.-M. Ye, Y. Li, D.-D. Zhou, P.-Q. Liao, X.-M. Chen and J.-P. Zhang, Angew. Chem., Int. Ed., 2019, 58, 7692–7696 CrossRef CAS PubMed.
- Z. S. Wang, M. Li, Y. L. Peng, Z. Zhang, W. Chen and X. C. Huang, Angew. Chem., Int. Ed., 2019, 58, 16071–16076 CrossRef CAS PubMed.
- H. J. Choi, M. Dinca, A. Dailly and J. R. Long, Energy Environ. Sci., 2010, 3, 117–123 RSC.
- A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939–943 CrossRef CAS PubMed.
- M. Tonigold, Y. Lu, B. Bredenkotter, B. Rieger, S. Bahnmuller, J. Hitzbleck, G. Langstein and D. Volkmer, Angew. Chem., Int. Ed., 2009, 48, 7546–7550 CrossRef CAS PubMed.
- X.-F. Lu, P.-Q. Liao, J.-W. Wang, J. X. Wu, X.-W. Chen, C.-T. He, J.-P. Zhang, G.-R. Li and X.-M. Chen, J. Am. Chem. Soc., 2016, 138, 8336–8339 CrossRef CAS PubMed.
- J. Tian, C. Shi, C. Xiao, F. Jiang, D. Yuan, Q. Chen and M. Hong, Inorg. Chem., 2020, 59, 18264–18275 CrossRef CAS PubMed.
- D. Liu, J. Pei, X. Zhang, X. W. Gu, H. M. Wen, B. Chen, G. Qian and B. Li, Angew. Chem., Int. Ed., 2023, 62, e202218590 CrossRef CAS PubMed.
- S. Li, S. Zeng, Y. Tian, X. Jing, F. Sun and G. Zhu, Nano Res., 2021, 14, 3288–3293 CrossRef CAS.
- K. Wang, X.-L. Lv, D. Feng, J. Li, S. Chen, J. Sun, L. Song, Y. Xie, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2016, 138, 914–919 CrossRef CAS PubMed.
- X.-L. Lv, K. Wang, B. Wang, J. Su, X. Zou, Y. Xie, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2017, 139, 211–217 CrossRef CAS PubMed.
- V. Colombo, S. Galli, H. J. Choi, G. D. Han, A. Maspero, G. Palmisano, N. Masciocchi and J. R. Long, Chem. Sci., 2011, 2, 1311–1319 RSC.
- T. He, Z. H. Huang, S. Yuan, X. L. Lv, X. J. Kong, X. D. Zou, H. C. Zhou and J. R. Li, J. Am. Chem. Soc., 2020, 142, 13491–13499 CrossRef CAS PubMed.
- A. Demessence, D. M. D'Alessandro, M. L. Foo and J. R. Long, J. Am. Chem. Soc., 2009, 131, 8784–8786 CrossRef CAS PubMed.
- J.-P. Zhang and X.-M. Chen, J. Am. Chem. Soc., 2008, 130, 6010–6017 CrossRef CAS PubMed.
- D. D. Zhou, P. Chen, C. Wang, S. S. Wang, Y. Du, H. Yan, Z. M. Ye, C. T. He, R. K. Huang, Z. W. Mo, N. Y. Huang and J. P. Zhang, Nat. Mater., 2019, 18, 994–998 CrossRef CAS PubMed.
- J.-H. Wang, M. Li and D. Li, Chem.–Euro. J., 2014, 20, 12004–12008 CrossRef CAS PubMed.
- D. Lv, R. Shi, Y. Chen, Y. Chen, H. Wu, X. Zhou, H. Xi, Z. Li and Q. Xia, Ind. Eng. Chem. Res., 2018, 57, 12215–12224 CrossRef CAS.
- Z. M. Ye, X. W. Zhang, P. Q. Liao, Y. Xie, Y. T. Xu, X. F. Zhang, C. Wang, D. X. Liu, N. Y. Huang, Z. H. Qiu, D. D. Zhou, C. T. He and J. P. Zhang, Angew. Chem., Int. Ed., 2020, 59, 23322–23328 CrossRef CAS PubMed.
- L. Liang, C. Liu, F. Jiang, Q. Chen, L. Zhang, H. Xue, H. L. Jiang, J. Qian, D. Yuan and M. Hong, Nat. Commun., 2017, 8, 1233 CrossRef PubMed.
- J. Tian, F. Jiang, D. Yuan, L. Zhang, Q. Chen and M. Hong, Angew. Chem., Int. Ed., 2020, 59, 13101–13108 CrossRef CAS PubMed.
- D. Wu, C. Liu, J. Tian, F. Jiang, D. Yuan, Q. Chen and M. Hong, Inorg. Chem., 2020, 59, 13542–13550 CrossRef CAS PubMed.
- C. Montoro, F. Linares, E. Q. Procopio, I. Senkovska, S. Kaskel, S. Galli, N. Masciocchi, E. Barea and J. A. R. Navarro, J. Am. Chem. Soc., 2011, 133, 11888–11891 CrossRef CAS PubMed.
- Y. Hu, M. Ding, X.-Q. Liu, L.-B. Sun and H.-L. Jiang, Chem. Commun., 2016, 52, 5734–5737 RSC.
- J. Tian, Q. Chen, F. Jiang, D. Yuan and M. Hong, Angew. Chem., Int. Ed., 2023, 62, e202215253 CrossRef CAS PubMed.
- C. Chen, Z. Yu, D. S. Sholl and K. S. Walton, J. Phys. Chem. Lett., 2022, 13, 4891–4896 CrossRef CAS PubMed.
- A. J. Rieth, A. M. Wright and M. Dinca, Nat. Rev. Mater., 2019, 4, 708–725 CrossRef CAS.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar, Y. Sun, J. Qin, X. Yang, P. Zhang, Q. Wang, L. Zou, Y. Zhang, L. Zhang, Y. Fang, J. Li and H.-C. Zhou, Adv. Mater., 2018, 30, e1704303 CrossRef PubMed.
- A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Euro. J., 2011, 17, 6643–6651 CrossRef CAS PubMed.
- H. L. Jiang, D. W. Feng, K. C. Wang, Z. Y. Gu, Z. W. Wei, Y. P. Chen and H. C. Zhou, J. Am. Chem. Soc., 2013, 135, 13934–13938 CrossRef CAS PubMed.
- S. C. Li, C. P. Liu, Q. H. Chen, F. L. Jiang, D. Q. Yuan, Q. F. Sun and M. C. Hong, Chem. Sci., 2022, 13, 9016–9022 RSC.
- F. Saraci, V. Quezada-Novoa, P. R. Donnarumma and A. J. Howarth, Chem. Soc. Rev., 2020, 49, 7949–7977 RSC.
- H. Jasuja and K. S. Walton, Dalton Trans., 2013, 42, 15421–15426 RSC.
- H. Jasuja, Y. Jiao, N. C. Burtch, Y. G. Huang and K. S. Walton, Langmuir, 2014, 30, 14300–14307 CrossRef CAS PubMed.
- C.-T. He, P.-Q. Liao, D.-D. Zhou, B.-Y. Wang, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Chem. Sci., 2014, 5, 4755–4762 RSC.
- D.-M. Chen, N.-N. Zhang, J.-Y. Tian, C.-S. Liu and M. Du, J. Mater. Chem. A, 2017, 5, 4861–4867 RSC.
- H. Yang, F. Peng, A. N. Hong, Y. Wang, X. Bu and P. Feng, J. Am. Chem. Soc., 2021, 143, 14470–14474 CrossRef CAS PubMed.
- Y. Ye, Z. Ma, R.-B. Lin, R. Krishna, W. Zhou, Q. Lin, Z. Zhang, S. Xiang and B. Chen, J. Am. Chem. Soc., 2019, 141, 4130–4136 CrossRef CAS PubMed.
- Y. X. Tan, Y. P. He and J. Zhang, Inorg. Chem., 2012, 51, 9649–9654 CrossRef CAS PubMed.
- D. M. Chen, J. Y. Tian, C. S. Liu and M. Du, Chem. Commun., 2016, 52, 8413–8416 RSC.
- X.-L. Lv, S. Yuan, L.-H. Xie, H. F. Darke, Y. Chen, T. He, C. Dong, B. Wang, Y.-Z. Zhang, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2019, 141, 10283–10293 CrossRef CAS PubMed.
- X.-J. Kong, T. He, J. Zhou, C. Zhao, T.-C. Li, X.-Q. Wu, K. Wang and J.-R. Li, Small, 2021, 17, 2005357 CrossRef CAS PubMed.
- J. R. Karra, H. Jasuja, Y.-G. Huang and K. S. Walton, J. Mater. Chem. A, 2015, 3, 1624–1631 RSC.
- H. Yang, F. Peng, C. Dang, Y. Wang, D. Hu, X. Zhao, P. Feng and X. Bu, J. Am. Chem. Soc., 2019, 141, 9808–9812 CrossRef CAS PubMed.
- T. F. Liu, L. F. Zou, D. W. Feng, Y. P. Chen, S. Fordham, X. Wang, Y. Y. Liu and H. C. Zhou, J. Am. Chem. Soc., 2014, 136, 7813–7816 CrossRef CAS PubMed.
- J.-H. Wang, Y. Zhang, M. Li, S. Yan, D. Li and X.-M. Zhang, Angew. Chem., Int. Ed., 2017, 56, 6478–6482 CrossRef CAS PubMed.
- Z. J. Zhang, H. T. H. Nguyen, S. A. Miller, A. M. Ploskonka, J. B. DeCoste and S. M. Cohen, J. Am. Chem. Soc., 2016, 138, 920–925 CrossRef CAS PubMed.
- Y. Chen, B. Wang, X. Q. Wang, L. H. Xie, J. P. Li, Y. B. Xie and J. R. Li, ACS Appl. Mater. Interfaces, 2017, 9, 27027–27035 CrossRef CAS PubMed.
- Z. R. Jiang, J. Ge, Y. X. Zhou, Z. Y. U. Wang, D. X. Chen, S. H. Yu and H. L. Jiang, NPG Asia Mater., 2016, 8, e253 CrossRef CAS.
- Y. Wang, T. Li, L. Li, R. B. Lin, X. Jia, Z. Chang, H. M. Wen, X. M. Chen and J. Li, Adv. Mater., 2023, 35, 2207955 CrossRef CAS PubMed.
- T. A. Makal, X. Wang and H. C. Zhou, Cryst. Growth Des., 2013, 13, 4760–4768 CrossRef CAS.
- D. Y. Ma, Y. W. Li and Z. Li, Chem. Commun., 2011, 47, 7377–7379 RSC.
- D. Sun, P. R. Adiyala, S. J. Yim and D. P. Kim, Angew. Chem., Int. Ed., 2019, 58, 7405–7409 CrossRef CAS PubMed.
- S. Yang, L. Peng, D. T. Sun, M. Asgari, E. Oveisi, O. Trukhina, S. Bulut, A. Jamali and W. L. Queen, Chem. Sci., 2019, 10, 4542–4549 RSC.
- J. G. Nguyen and S. M. Cohen, J. Am. Chem. Soc., 2010, 132, 4560–4561 CrossRef CAS PubMed.
- J. Aguilera-Sigalat and D. Bradshaw, Chem. Commun., 2014, 50, 4711–4713 RSC.
- P. Deria, Y. G. Chung, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Sci., 2015, 6, 5172–5176 RSC.
- J. Liu, R. Anderson, K. M. Schmalbach, T. R. Sheridan, Z. Wang, N. M. Schweitzer, A. Stein, N. A. Mara, D. Gomez-Gualdron and J. T. Hupp, J. Mater. Chem. A, 2022, 10, 17307–17316 RSC.
- W. Zhang, Y. Hu, J. Ge, H.-L. Jiang and S.-H. Yu, J. Am. Chem. Soc., 2014, 136, 16978–16981 CrossRef CAS.
- M. Kalaj, M. S. Denny Jr, K. C. Bentz, J. M. Palomba and S. M. Cohen, Angew. Chem., Int. Ed., 2019, 58, 2336–2340 CrossRef CAS.
- G. Huang, Q. H. Yang, Q. Xu, S. H. Yu and H. L. Jiang, Angew. Chem., Int. Ed., 2016, 55, 7379–7383 CrossRef CAS.
- Y. Seok Chae, S. Park, D. Won Kang, D. Won Kim, M. Kang, D. San Choi, J. Hyeak Choe and C. Seop Hong, Chem. Eng. J., 2022, 433, 133856 CrossRef CAS.
- A. Carne-Sanchez, K. C. Stylianou, C. Carbonell, M. Naderi, I. Imaz and D. Maspoch, Adv. Mater., 2015, 27, 869–873 CrossRef CAS PubMed.
- L.-H. Xu, S.-H. Li, H. Mao, Y. Li, A.-S. Zhang, S. Wang, W.-M. Liu, J. Lv, T. Wang, W.-W. Cai, L. Sang, W.-W. Xie, C. Pei, Z.-Z. Li, Y.-N. Feng and Z.-P. Zhao, Science, 2022, 378, 308–313 CrossRef CAS PubMed.
- D. Micheroni, G. X. Lan and W. B. Lin, J. Am. Chem. Soc., 2018, 140, 15591–15595 CrossRef CAS PubMed.
- S. J. Yang, J. Y. Choi, H. K. Chae, J. H. Cho, K. S. Nahm and C. R. Park, Chem. Mater., 2009, 21, 1893–1897 CrossRef CAS.
- J. Noh, W. Chen, P. Wu, Y. Huang, J. Tan, H. C. Zhou and C. Yu, Adv. Funct. Mater., 2021, 31, 2104899 CrossRef CAS.
- P. D. C. Dietzel, R. E. Johnsen, H. Fjellvag, S. Bordiga, E. Groppo, S. Chavan and R. Blom, Chem. Commun., 2008, 44, 5125–5127 RSC.
- M. I. Gonzalez, J. A. Mason, E. D. Bloch, S. J. Teat, K. J. Gagnon, G. Y. Morrison, W. L. Queen and J. R. Long, Chem. Sci., 2017, 8, 4387–4398 RSC.
- F. Luo, C. Yan, L. Dang, R. Krishna, W. Zhou, H. Wu, X. Dong, Y. Han, T.-L. Hu, M. O'Keeffe, L. Wang, M. Luo, R.-B. Lin and B. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684 CrossRef CAS PubMed.
- R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, P. G. Boyd, S. Alavi and T. K. Woo, Science, 2010, 330, 650–653 CrossRef CAS PubMed.
- P. Q. Liao, D. D. Zhou, A. X. Zhu, L. Jiang, R. B. Lin, J. P. Zhang and X. M. Chen, J. Am. Chem. Soc., 2012, 134, 17380–17383 CrossRef CAS PubMed.
- T. D. Duong, S. A. Sapchenko, I. da Silva, H. G. W. Godfrey, Y. Cheng, L. L. Daemen, P. Manuel, M. D. Frogley, G. Cinque, A. J. Ramirez-Cuesta, S. Yang and M. Schroeder, Chem. Sci., 2020, 11, 5339–5346 RSC.
- D. Song, F. Jiang, D. Yuan, Q. Chen and M. Hong, Small, 2023, 19, 2302677 CrossRef CAS PubMed.
- P. M. Bhatt, Y. Belmabkhout, A. Cadiau, K. Adil, O. Shekhah, A. Shkurenko, L. J. Barbour and M. Eddaoudi, J. Am. Chem. Soc., 2016, 138, 9301–9307 CrossRef CAS PubMed.
- W. Liang, P. M. Bhatt, A. Shkurenko, K. Adil, G. Mouchaham, H. Aggarwal, A. Mallick, A. Jamal, Y. Belmabkhout and M. Eddaoudi, Chem, 2019, 5, 950–963 CAS.
- S. Xiang, Y. He, Z. Zhang, H. Wu, W. Zhou, R. Krishna and B. Chen, Nat. Commun., 2012, 3, 954 CrossRef PubMed.
- S. H. Yang, J. L. Sun, A. J. Ramirez-Cuesta, S. K. Callear, W. I. F. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schroder, Nat. Chem., 2012, 4, 887–894 CrossRef CAS PubMed.
- F. Moreau, I. da Silva, N. H. Al Smail, T. L. Easun, M. Savage, H. G. Godfrey, S. F. Parker, P. Manuel, S. Yang and M. Schroder, Nat. Commun., 2017, 8, 14085 CrossRef CAS PubMed.
- N. C. Burtch, H. Jasuja, D. Dubbeldam and K. S. Walton, J. Am. Chem. Soc., 2013, 135, 7172–7180 CrossRef CAS PubMed.
- P. G. Boyd, A. Chidambaram, E. Garcia-Diez, C. P. Ireland, T. D. Daff, R. Bounds, A. Gladysiak, P. Schouwink, S. M. Moosavi, M. M. Maroto-Valer, J. A. Reimer, J. A. R. Navarro, T. K. Woo, S. Garcia, K. C. Stylianou and B. Smit, Nature, 2019, 576, 253–256 CrossRef CAS PubMed.
- A. M. Plonka, D. Banerjee, W. R. Woerner, Z. J. Zhang, N. Nijem, Y. J. Chabal, J. Li and J. B. Parise, Angew. Chem., Int. Ed., 2013, 52, 1692–1695 CrossRef CAS PubMed.
- Z. Z. Lu, H. G. W. Godfrey, I. da Silva, Y. Q. Cheng, M. Savage, F. Tuna, E. J. L. McInnes, S. J. Teat, K. J. Gagnon, M. D. Frogley, P. Manuel, S. Rudic, A. J. Ramirez-Cuesta, T. L. Easun, S. H. Yang and M. Schroder, Nat. Commun., 2017, 8, 14212 CrossRef CAS PubMed.
- T. M. McDonald, J. A. Mason, X. Kong, E. D. Bloch, D. Gygi, A. Dani, V. Crocella, F. Giordanino, S. O. Odoh, W. S. Drisdell, B. Vlaisavljevich, A. L. Dzubak, R. Poloni, S. K. Schnell, N. Planas, K. Lee, T. Pascal, L. F. Wan, D. Prendergast, J. B. Neaton, B. Smit, J. B. Kortright, L. Gagliardi, S. Bordiga, J. A. Reimer and J. R. Long, Nature, 2015, 519, 303–308 CrossRef CAS PubMed.
- R. W. Faig, T. M. O. Popp, A. M. Fracaroli, E. A. Kapustin, M. J. Kalmutzki, R. M. Altamimi, F. Fathieh, J. A. Reimer and O. M. Yaghi, J. Am. Chem. Soc., 2017, 139, 12125–12128 CrossRef PubMed.
- P. J. Milner, R. L. Siegelman, A. C. Forse, M. I. Gonzalez, T. Runcevski, J. D. Martell, J. A. Reimer and J. R. Long, J. Am. Chem. Soc., 2017, 139, 13541–13553 CrossRef CAS PubMed.
- A. C. Forse, P. J. Milner, J. H. Lee, H. N. Redfearn, J. Oktawiec, R. L. Siegelman, J. D. Martell, B. Dinakar, L. B. Porter-Zasada, M. I. Gonzalez, J. B. Neaton, J. R. Long and J. A. Reimer, J. Am. Chem. Soc., 2018, 140, 18016–18031 CrossRef CAS PubMed.
- J. J. Gassensmith, H. Furukawa, R. A. Smaldone, R. S. Forgan, Y. Y. Botros, O. M. Yaghi and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 15312–15315 CrossRef CAS PubMed.
- M. E. Zick, S. M. Pugh, J. H. Lee, A. C. Forse and P. J. Milner, Angew. Chem., Int. Ed., 2022, 61, e202206718 CrossRef CAS PubMed.
- P.-Q. Liao, H. Chen, D.-D. Zhou, S.-Y. Liu, C.-T. He, Z. Rui, H. Ji, J.-P. Zhang and X.-M. Chen, Energy Environ. Sci., 2015, 8, 1011–1016 RSC.
- C. E. Bien, K. K. Chen, S.-C. Chien, B. R. Reiner, L.-C. Lin, C. R. Wade and W. S. W. Ho, J. Am. Chem. Soc., 2018, 140, 12662–12666 CrossRef CAS PubMed.
- A. Demessence, D. M. D'Alessandro, M. L. Foo and J. R. Long, J. Am. Chem. Soc., 2009, 131, 8784–8786 CrossRef CAS PubMed.
- A. M. Wright, Z. Wu, G. Zhang, J. L. Mancuso, R. J. Comito, R. W. Day, C. H. Hendon, J. T. Miller and M. Dinca, Chem, 2018, 4, 2894–2901 CAS.
- H. A. Evans, D. Mullangi, Z. Deng, Y. Wang, S. B. Peh, F. Wei, J. Wang, C. M. Brown, D. Zhao, P. Canepa and A. K. Cheetham, Sci. Adv., 2022, 8, eade1473 CrossRef CAS PubMed.
- H. Lyu, O. I.-F. Chen, N. Hanikel, M. I. Hossain, R. W. Flaig, X. Pei, A. Amin, M. D. Doherty, R. K. Impastato, T. G. Glover, D. R. Moore and O. M. Yaghi, J. Am. Chem. Soc., 2022, 144, 2387–2396 CrossRef CAS PubMed.
- L. Han, T. Pham, M. J. Zhuo, K. A. Forrest, S. Suepaul, B. Space, M. J. Zaworotko, W. Shi, Y. Chen, P. Cheng and Z. J. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 23192–23197 CrossRef CAS PubMed.
- P. Nugent, Y. Belmabkhout, S. D. Burd, A. J. Cairns, R. Luebke, K. Forrest, T. Pham, S. Q. Ma, B. Space, L. Wojtas, M. Eddaoudi and M. J. Zaworotko, Nature, 2013, 495, 80–84 CrossRef CAS PubMed.
- O. Shekhah, Y. Belmabkhout, Z. Chen, V. Guillerm, A. Cairns, K. Adil and M. Eddaoudi, Nat. Commun., 2014, 5, 4228 CrossRef CAS PubMed.
- H.-M. Wen, C. Liao, L. Li, A. Alsalme, Z. Alothman, R. Krishna, H. Wu, W. Zhou, J. Hu and B. Chen, J. Mater. Chem. A, 2019, 7, 3128–3134 RSC.
- K.-J. Chen, D. G. Madden, T. Pham, K. A. Forrest, A. Kumar, Q.-Y. Yang, W. Xue, B. Space, J. J. Perry, J.-P. Zhang, X.-M. Chen and M. J. Zaworotko, Angew. Chem., Int. Ed., 2016, 55, 10268–10272 CrossRef CAS PubMed.
- J.-B. Lin, T. T. T. Nguyen, R. Vaidhyanathan, J. Burner, J. M. Taylor, H. Durekova, F. Akhtar, R. K. Mah, O. Ghaffari-Nik, S. Marx, N. Fylstra, S. S. Iremonger, K. W. Dawson, P. Sarkar, P. Hovington, A. Rajendran, T. K. Woo and G. K. H. Shimizu, Science, 2021, 374, 1464–1469 CrossRef CAS PubMed.
- T. M. McDonald, D. M. D'Alessandro, R. Krishna and J. R. Long, Chem. Sci., 2011, 2, 2022–2028 RSC.
- W. R. Lee, S. Y. Hwang, D. W. Ryu, K. S. Lim, S. S. Han, D. Moon, J. Choi and C. S. Hong, Energy Environ. Sci., 2014, 7, 744–751 RSC.
- J. H. Choe, H. Kim, M. Kang, H. Yun, S. Y. Kim, S. M. Lee and C. S. Hong, J. Am. Chem. Soc., 2022, 144, 10309–10319 CrossRef CAS PubMed.
- P.-Q. Liao, X.-Y. Li, J. Bai, C.-T. He, D.-D. Zhou, W.-X. Zhang, J.-P. Zhang and X.-M. Chen, Chem.–Euro. J., 2014, 20, 11303–11307 CrossRef CAS PubMed.
- N. Ding, H. Li, X. Feng, Q. Wang, S. Wang, L. Ma, J. Zhou and B. Wang, J. Am. Chem. Soc., 2016, 138, 10100–10103 CrossRef CAS PubMed.
- P.-Q. Liao, X.-W. Chen, S.-Y. Liu, X.-Y. Li, Y.-T. Xu, M. Tang, Z. Rui, H. Ji, J.-P. Zhang and X.-M. Chen, Chem. Sci., 2016, 7, 6528–6533 RSC.
- H. Li, K. Wang, D. Feng, Y.-P. Chen, W. Verdegaal and H.-C. Zhou, Chemsuschem, 2016, 9, 2832–2840 CrossRef CAS PubMed.
- T. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef CAS PubMed.
- W. R. Lee, J. E. Kim, S. J. Lee, M. Kang, D. W. Kang, H. Y. Lee, V. Hiremath, J. G. Seo, H. Jin, D. Moon, M. Cho, Y. Jung and C. S. Hong, ChemSusChem, 2018, 11, 1694–1707 CrossRef CAS PubMed.
- Z. Zhang, Q. Ding, S. B. Peh, D. Zhao, J. Cui, X. Cui and H. Xing, Chem. Commun., 2020, 56, 7726–7729 RSC.
- Z. Zhang, Q. Ding, J. Cui, X. Cui and H. Xing, Sci. China Mater., 2021, 64, 691–697 CrossRef.
- A. Kumar, C. Hua, D. G. Madden, D. O'Nolan, K. J. Chen, L. J. Keane, J. J. Perry and M. J. Zaworotko, Chem. Commun., 2017, 53, 5946–5949 RSC.
- S. Mukherjee, N. Sikdar, D. O'Nolan, D. M. Franz, V. Gascon, A. Kumar, N. Kumar, H. S. Scott, D. G. Madden, P. E. Kruger, B. Space and M. J. Zaworotko, Sci. Adv., 2019, 5, eaax9171 CrossRef CAS PubMed.
- K.-J. Chen, H. S. Scott, D. G. Madden, T. Pham, A. Kumar, A. Bajpai, M. Lusi, K. A. Forrest, B. Space, J. J. Perry and M. J. Zaworotko, Chem, 2016, 1, 753–765 CAS.
- M. Jiang, B. Li, X. Cui, Q. Yang, Z. Bao, Y. Yang, H. Wu, W. Zhou, B. Chen and H. Xing, ACS Appl. Mater. Interfaces, 2018, 10, 16628–16635 CrossRef CAS PubMed.
- X. H. Song, M. X. Zhang, C. Chen, J. G. Duan, W. W. Zhang, Y. Pan and J. F. Bai, J. Am. Chem. Soc., 2019, 141, 14539–14543 CrossRef CAS PubMed.
- Z. G. Hu, K. Zhang, M. Zhang, Z. G. Guo, J. W. Jiang and D. Zhao, Chemsuschem, 2014, 7, 2791–2795 CrossRef CAS PubMed.
- G. E. Cmarik, M. Kim, S. M. Cohen and K. S. Walton, Langmuir, 2012, 28, 15606–15613 CrossRef CAS PubMed.
- Y. X. Wang, Z. G. Hu, T. Kundu, Y. D. Cheng, J. Q. Dong, Y. H. Qian, L. Z. Zhai and D. Zhao, ACS Sustainable Chem. Eng., 2018, 6, 11904–11912 CrossRef CAS.
- Z. G. Hu, S. Faucher, Y. Y. Zhuo, Y. Sun, S. N. Wang and D. Zhao, Chem.–Euro. J., 2015, 21, 17245–17255 Search PubMed.
- Z. G. Hu, M. Khurana, Y. H. Seah, M. Zhang, Z. G. Guo and D. Zhao, Chem. Eng. Sci., 2015, 124, 61–69 CrossRef CAS.
- L.-J. Li, P.-Q. Liao, C.-T. He, Y.-S. Wei, H.-L. Zhou, J.-M. Lin, X.-Y. Li and J.-P. Zhang, J. Mater. Chem. A, 2015, 3, 21849–21855 RSC.
- Z. Hu, A. Nalaparaju, Y. Peng, J. Jiang and D. Zhao, Inorg. Chem., 2016, 55, 1134–1141 CrossRef CAS PubMed.
- W. L. Queen, M. R. Hudson, E. D. Bloch, J. A. Mason, M. I. Gonzalez, J. S. Lee, D. Gygi, J. D. Howe, K. Lee, T. A. Darwish, M. James, V. K. Peterson, S. J. Teat, B. Smit, J. B. Neaton, J. R. Long and C. M. Brown, Chem. Sci., 2014, 5, 4569–4581 RSC.
- J. Park, J. R. Park, J. H. Choe, S. Kim, M. Kang, D. W. Kang, J. Y. Kim, Y. W. Jeong and C. S. Hong, ACS Appl. Mater. Interfaces, 2020, 12, 50534–50540 CrossRef CAS PubMed.
- E. J. Kim, R. L. Siegelman, H. Z. H. Jiang, A. C. Forse, J.-H. Lee, J. D. Martell, P. J. Milner, J. M. Falkowski, J. B. Neaton, J. A. Reimer, S. C. Weston and J. R. Long, Science, 2020, 369, 392–396 CrossRef CAS PubMed.
- A. Kumar, D. G. Madden, M. Lusi, K.-J. Chen, E. A. Daniels, T. Curtin, J. J. Perry and M. J. Zaworotko, Angew. Chem., Int. Ed., 2015, 54, 14372–14377 CrossRef CAS PubMed.
- A. R. Millward and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 17998–17999 CrossRef CAS PubMed.
- C. Tan, S. Yang, N. R. Champness, X. Lin, A. J. Blake, W. Lewis and M. Schroder, Chem. Commun., 2011, 47, 4487–4489 RSC.
- N. T. T. Nguyen, H. Furukawa, F. Gandara, H. T. Nguyen, K. E. Cordova and O. M. Yaghi, Angew. Chem., Int. Ed., 2014, 53, 10645–10648 CrossRef CAS PubMed.
- X. Zha, X. Li, A. A. Al-Omari, S. Liu, C. C. Liang, A. Al-Ghourani, M. Abdellatief, J. Yang, H. L. Nguyen, B. Al-Maythalony, Z. Shi, K. E. Cordova and Y. B. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202207467 CrossRef CAS PubMed.
- Z. Shi, Y. Tao, J. Wu, C. Zhang, H. He, L. Long, Y. Lee, T. Li and Y.-B. Zhang, J. Am. Chem. Soc., 2020, 142, 2750–2754 CrossRef CAS PubMed.
- Y. Shi, Y. Xie, H. Cui, Z. A. Alothman, O. Alduhaish, R.-B. Lin and B. Chen, Chem. Eng. J., 2022, 446, 137101 CrossRef CAS.
- Y. Hu, Y. Jiang, J. Li, L. Wang, M. Steiner, R. F. Neumann, B. Luan and Y. Zhang, Adv. Funct. Mater., 2023, 33, 2213915 CrossRef CAS.
- C. Yu, Q. Ding, J. Hu, Q. Wang, X. Cui and H. Xing, Chem. Eng. J., 2021, 405, 126937 CrossRef CAS.
- M. Wriedt, J. P. Sculley, A. A. Yakovenko, Y. Ma, G. J. Halder, P. B. Balbuena and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 9804–9808 CrossRef CAS PubMed.
- D. G. Madden, A. B. Albadarin, D. O'Nolan, P. Cronin, J. J. Perry, S. Solomon, T. Curtin, M. Khraisheh, M. J. Zaworotko and G. M. Walker, ACS Appl. Mater. Interfaces, 2020, 12, 33759–33764 CrossRef CAS PubMed.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed.
- Q. G. Zhai, X. Bu, C. Mao, X. Zhao, L. Daemen, Y. Cheng, A. J. Ramirez-Cuesta and P. Feng, Nat. Commun., 2016, 7, 13645 CrossRef CAS PubMed.
- Y.-Y. Xue, S.-N. Li, Y.-C. Jiang, M.-C. Hu and Q.-G. Zhai, J. Mater. Chem. A, 2019, 7, 4640–4650 RSC.
- R. L. Siegelman, J. A. Thompson, J. A. Mason, T. M. McDonald and J. R. Long, Chem. Sci., 2022, 13, 11772–11784 RSC.
- Y. Shen, Z. Li, L. Wang, Y. Ye, Q. Liu, X. Ma, Q. Chen, Z. Zhang and S. Xiang, J. Mater. Chem. A, 2015, 3, 593–599 RSC.
- J. Lu, C. Perez-Krap, M. Suyetin, N. H. Alsmail, Y. Yan, S. Yang, W. Lewis, E. Bichoutskaia, C. C. Tang, A. J. Blake, R. Cao and M. Schroder, J. Am. Chem. Soc., 2014, 136, 12828–12831 CrossRef CAS PubMed.
- X.-W. Zhang, D.-D. Zhou and J.-P. Zhang, Chem, 2021, 7, 1006–1019 CAS.
- O. T. Qazvini, R. Babarao and S. G. Telfer, Nat. Commun., 2021, 12, 197 CrossRef CAS PubMed.
- J.-B. Lin, W. Xue, J.-P. Zhang and X.-M. Chen, Chem. Commun., 2011, 47, 926–928 RSC.
- R.-B. Lin, L. Li, A. Alsalme and B. Chen, Small Struct., 2020, 1, 2000022 CrossRef.
- R. B. Lin, L. Li, H. L. Zhou, H. Wu, C. He, S. Li, R. Krishna, J. Li, W. Zhou and B. Chen, Nat. Mater., 2018, 17, 1128–1133 CrossRef CAS PubMed.
- A. Cadiau, Y. Belmabkhout, K. Adil, P. M. Bhatt, R. S. Pillai, A. Shkurenko, C. Martineau-Corcos, G. Maurin and M. Eddaoudi, Science, 2017, 356, 731–735 CrossRef CAS.
- Y. Belmabkhout, P. M. Bhatt, K. Adil, R. S. Pillai, A. Cadiau, A. Shkurenkol, G. Maurin, G. P. Liu, W. Koros and M. Eddaoudi, Nat. Energy, 2018, 3, 1059–1066 CrossRef CAS.
- S. Choi, T. Watanabe, T. H. Bae, D. S. Sholl and C. W. Jones, J. Phys. Chem. Lett., 2012, 3, 1136–1141 CrossRef CAS PubMed.
- X. Song, M. Zhang, C. Chen, J. Duan, W. Zhang, Y. Pan and J. Bai, Chem. Commun., 2019, 55, 3477–3480 RSC.
- Z. Q. Zhang, Q. Ding, S. B. Peh, D. Zhao, J. Y. Cui, X. L. Cui and H. B. Xing, Chem. Commun., 2020, 56, 7726–7729 RSC.
- C. Wang, L. Li, S. Tang and X. Zhao, ACS Appl. Mater. Interfaces, 2014, 6, 16932–16940 CrossRef CAS PubMed.
- N. C. Burtch, I. M. Walton, J. T. Hungerford, C. R. Morelock, Y. Jiao, J. Heinen, Y.-S. Chen, A. A. Yakovenko, W. Xu, D. Dubbeldam and K. S. Walton, Nat. Chem., 2020, 12, 186–192 CrossRef CAS PubMed.
- S. Xiong, Y. Gong, H. Wang, H. Wang, Q. Liu, M. Gu, X. Wang, B. Chen and Z. Wang, Chem. Commun., 2014, 50, 12101–12104 RSC.
- O. Alduhaish, R.-B. Lin, H. Wang, B. Li, H. D. Arman, T.-L. Hu and B. Chen, Cryst. Growth Des., 2018, 18, 4522–4527 CrossRef CAS.
- Y. Chen, D. Lv, J. Wu, J. Xiao, H. Xi, Q. Xia and Z. Li, Chem. Eng. J., 2017, 308, 1065–1072 CrossRef CAS.
- P. Das and S. K. Mandal, ACS Appl. Mater. Interfaces, 2020, 12, 37137–37146 CrossRef CAS PubMed.
- M. H. Mohamed, S. K. Elsaidi, T. Pham, K. A. Forrest, B. Tudor, L. Wojtas, B. Space and M. J. Zaworotko, Chem. Commun., 2013, 49, 9809–9811 RSC.
- N. Seal, M. Singh, S. Das, R. Goswami, B. Pathak and S. Neogi, Mater. Chem. Front., 2021, 5, 979–994 RSC.
- R. Goswami, N. Seal, S. R. Dash, A. Tyagi and S. Neogi, ACS Appl. Mater. Interfaces, 2019, 11, 40134–40150 CrossRef CAS PubMed.
- A. Pal, S. Chand and M. C. Das, Inorg. Chem., 2017, 56, 13991–13997 CrossRef CAS PubMed.
- V. Gupta and S. K. Mandal, Dalton Trans., 2019, 48, 415–425 RSC.
- L. Fan, S. Lin, X. Wang, L. Yue, T. Xu, Z. Jiang and Y. He, Inorg. Chem., 2021, 60, 2704–2715 CrossRef CAS PubMed.
- L. Bastin, P. S. Bárcia, E. J. Hurtado, J. A. C. Silva, A. E. Rodrigues and B. Chen, J. Phys. Chem. C, 2008, 112, 1575–1581 CrossRef CAS.
- H. Liu, Y. Zhao, Z. Zhang, N. Nijem, Y. J. Chabal, H. Zeng and J. Li, Adv. Funct. Mater., 2011, 21, 4754–4762 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |