Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tunable emission from H-type supramolecular polymers in optical nanocavities†
Giulia
Lavarda
 *a,
Anton M.
Berghuis
b,
Kripa
Joseph
a,
Joost J. B.
van der Tol
a,
Shunsuke
Murai
*a,
Anton M.
Berghuis
b,
Kripa
Joseph
a,
Joost J. B.
van der Tol
a,
Shunsuke
Murai
 c,
Jaime
Gómez Rivas
b and
E. W.
Meijer
c,
Jaime
Gómez Rivas
b and
E. W.
Meijer
 *a
*a
aInstitute for Complex Molecular Systems and Laboratory of Macromolecular and Organic Chemistry, Eindhoven University of Technology, Eindhoven 5600 MB, The Netherlands. E-mail: g.lavarda@tue.nl; e.w.meijer@tue.nl
bEindhoven Hendrik Casimir Institute and Institute for Complex Molecular Systems, Eindhoven University of Technology, Eindhoven 5600 MB, The Netherlands
cDepartment of Material Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo, Kyoto 6158510, Japan
First published on 9th February 2024
Abstract
H-type supramolecular polymers with preferred helicity and highly efficient emission have been prepared from the self-assembly of chiral tetraphenylene-based monomers. Implementation of the one-dimensional fibers into dielectric nanoparticle arrays allows for a significant reshaping of fluorescence due to weak light–matter coupling.
Tunable emission in optically active materials is a desirable feature for a wide range of applications, from optoelectronics to biomedicine.1–4 Due to their structural and electronic properties, π-conjugated chromophores are ideal building blocks for the preparation of functional materials for optical technologies.5,6 By exploiting the supramolecular interactions between π-surfaces, the molecular arrangement and aggregate morphology can be precisely controlled at the microscopic scale.7 Nevertheless, drastic quenching of fluorescence is often observed in stacked architectures of emissive chromophores, limiting optical performance.
The proper molecular design of organic building blocks provides however an effective strategy for the preparation of luminescent assemblies. Recently, this phenomenon is often referred to as aggregation-induced emission (AIE), but it has been known for much longer.8,9 The emission in these cases results from the suppression of non-radiative deactivation pathways through the restriction of intramolecular rotational or vibrational modes in the aggregate state, with tetraphenylethylene (TPE) serving as a prototypical example.10 The optical properties of these emissive materials make them interesting candidates for implementation in light-emitting, sensing and imaging technologies, among others.8,11–13
Therefore, the study of fluorescent H-type aggregates – where molecular units are stacked in a face-to-face arrangement – is critical to the advancement of emission-based applications. In this context, long-range ordered supramolecular polymers can be conveniently used as model systems to gain insights into the fundamental effects underlying optoelectronic materials.14,15 Despite numerous reports on TPE aggregates, this core has rarely been employed in the preparation of H-type one-dimensional supramolecular polymers.16–19 Instead, C3-symmetric discotics decorated with amide motifs are typically used for the latter, ensuring the formation of helical fibers.20
On the other hand, light–matter coupling has attracted much attention in the last decades as a tool to engineer the optical properties of materials, showing significant potential for improving the performance of organic electronics.21,22 In the weak coupling regime, excitonic transitions exchange energy with resonant photonic modes, resulting in the modification of emission properties.23,24 Although this research area is rapidly expanding, the effect of light–matter coupling on the optical properties of H-type supramolecular polymers received little attention.25
In this communication, we report the tuning of emission from one-dimensional H-type assemblies via weak coupling to the surface lattice resonances in arrays of dielectric TiO2 nanoparticles. To induce self-assembly into fluorescent supramolecular polymers, we designed a monomer featuring a TPE core decorated at the para-position of the phenyl rings with amide functionalities and solubilizing (S)-3,7-dimethyloctyl chains (S-TPE, Fig. 1). The target compound was synthesized by an amidation reaction between tetrakis(4-carboxy)-tetraphenylethylene precursor and 3,7-dimethyloctylamine using COMU as the coupling reagent. The product was purified by conventional laboratory techniques and fully characterized by 1H-NMR, 13C-NMR and mass spectrometry (see ESI† for further details).
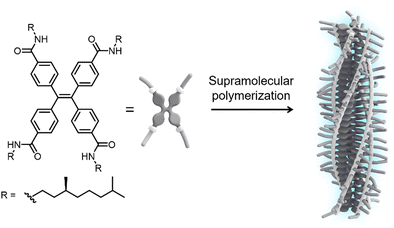 | ||
| Fig. 1 Chemical structure of the S-TPE monomer and schematic representation of its assembly into emissive H-type supramolecular polymers with preferred helicity. | ||
UV-vis measurements of S-TPE solutions in 1,1,2,2-tetrachloroethane (TCE) show the typical absorption profile of TPE chromophores, with maxima at 333 and 296 nm (Fig. S6a, ESI†). The linear dependence of absorbance on concentration and the absence of any Cotton effect indicate that the compound is monomerically dissolved at room temperature in the concentration range studied (namely 5–200 μM, Fig. S6b and d, ESI†). The absence of significant emission under these experimental conditions is attributed to the activation of competitive decay pathways resulting from the free rotation of the phenyl moieties in the monomer (Fig. S6c, ESI†). In this context, the presence of a weak emission around 500 nm at concentrations higher than 30 μM can be attributed to the formation of excimer species.26
Next, the ability of S-TPE to assemble in decalin/TCE mixtures under dilute conditions (i.e., 20 μM) was investigated. The addition of apolar solvent to polar TCE resulted in the appearance and gradual increase of a CD signal in the 260–400 nm region for decalin ratios higher than 85%, with a minimum at 322 nm and a maximum at 270 nm (Fig. S7a, ESI†). At the same time, a marked improvement in emission is observed with increasing amounts of alkane solvent (Fig. S7b, ESI†).
Variable temperature (VT) UV-vis, CD and emission spectroscopy studies were performed on 20 μM solutions in decalin/TCE 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
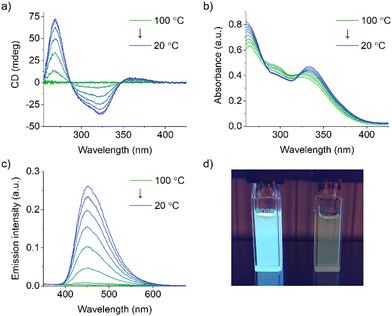
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) to further investigate the self-assembly behavior of S-TPE. While the absence of any CD signal at 100 °C indicates that S-TPE is present in solution as a monomeric species, the appearance and successive enhancement of a Cotton effect upon controlled cooling to 20 °C at a rate of 1 °C min−1 indicates an ongoing self-assembly process (Fig. 2a). These observations are consistent with previous reports on stacked aggregates of chiral TPE species, indicating the formation of H-type supramolecular polymers with a preferred helicity.18 In parallel, spectroscopic changes are observed in the corresponding UV-vis spectra, including a bathochromic shift (namely 8 nm) of the low-energy absorption (Fig. 2b).27 The restriction of intramolecular rotation of the phenyl rings within the stacks activates a radiative decay channel, as evidenced by the rapid increase in TPE emission upon cooling (Fig. 2c and d).
1 (v/v) to further investigate the self-assembly behavior of S-TPE. While the absence of any CD signal at 100 °C indicates that S-TPE is present in solution as a monomeric species, the appearance and successive enhancement of a Cotton effect upon controlled cooling to 20 °C at a rate of 1 °C min−1 indicates an ongoing self-assembly process (Fig. 2a). These observations are consistent with previous reports on stacked aggregates of chiral TPE species, indicating the formation of H-type supramolecular polymers with a preferred helicity.18 In parallel, spectroscopic changes are observed in the corresponding UV-vis spectra, including a bathochromic shift (namely 8 nm) of the low-energy absorption (Fig. 2b).27 The restriction of intramolecular rotation of the phenyl rings within the stacks activates a radiative decay channel, as evidenced by the rapid increase in TPE emission upon cooling (Fig. 2c and d).
The self-assembly is likely driven by hydrogen-bond interactions between the amide functionalities, as indicated by the rupture of the supramolecular structures upon addition of an hydrogen-bond scavenger at the end of the cooling ramp (Fig. S8, ESI†). As such, a 0.4% volume fraction of MeOH is sufficient to cause the complete disappearance of the CD signal attributable to the supramolecular polymers. Stacking interactions between the monomeric units likely contribute to drive the assembly, with the latter being triggered in relatively apolar environments where the extended aromatic core experiences poor solvation. However, we speculate that the twisted conformation of the TPE core prevents extended π–π stacking between adjacent monomers in the assembly, ensuring a strong emissive character of the aggregates.
Although the non-sigmoidal shape of the CD cooling curves suggests a nucleation–elongation mechanism, simulations with a mass-balance model for cooperative polymerization do not provide an accurate fit in the nucleation phase (Fig. S9, ESI†).28 This is likely due to the coexistence of different aggregated species.29 Since TPE emission results from the restriction of intramolecular rotational modes, regardless of the nature of the aggregate, it also cannot serve as a readout to follow the supramolecular polymerization.
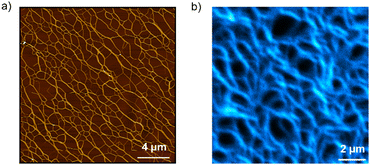
Atomic force microscopy (AFM) and emission microscopy experiments were performed on spin-coated and drop-casted samples prepared by deposition of 20 μM solutions of S-TPE in decalin/TCE 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) on freshly cleaved mica or glass substrates, respectively. AFM studies revealed the formation of a network of tens of micrometers one-dimensional structures whose height profile (namely, 2 nm) corresponds to that expected for single fibers (Fig. 3a and Fig. S10, S11, ESI†). The formation of one-dimensional supramolecular structures was further demonstrated by emission spectroscopy, which revealed the highly fluorescent behavior of the assemblies (Fig. 3b and Fig. S12, ESI†).
1 (v/v) on freshly cleaved mica or glass substrates, respectively. AFM studies revealed the formation of a network of tens of micrometers one-dimensional structures whose height profile (namely, 2 nm) corresponds to that expected for single fibers (Fig. 3a and Fig. S10, S11, ESI†). The formation of one-dimensional supramolecular structures was further demonstrated by emission spectroscopy, which revealed the highly fluorescent behavior of the assemblies (Fig. 3b and Fig. S12, ESI†).
Overall, the results obtained from the study of the self-assembly of non-symmetric, propeller-shaped S-TPE monomer are consistent with the knowledge gained from the study of hydrogen-bonded supramolecular polymers made of C3-symmetric discotics.20
Next, we investigated the emissive properties of S-TPE supramolecular polymers embedded in optical nanocavities. The latter consist of dielectric nanoparticles arranged in a square lattice with a spacing close to the wavelength of visible light. These cavities exhibit high-quality optical resonances, known as Mie Surface Lattice Resonances (M-SLRs), resulting from the interaction between the localized Mie resonances of the individual nanoparticles and the in-plane diffraction orders (DOs) of the array.30–35 The M-SLRs concentrate the electromagnetic fields near the array surface. Consequently, when an equilibrated solution of supramolecular TPE assemblies is drop casted onto the nanocavity surface (Fig. S13, ESI†),36 the strong fields enhance the interaction of (the excitons in the) TPE fibers with light, resulting in enhanced emission at the resonant wavelengths of the cavity. These resonant wavelengths, which control the directional emission from the TPE fibers, can be tuned by modifying the cavity properties such as particle size, particle material, lattice symmetry, or lattice period.
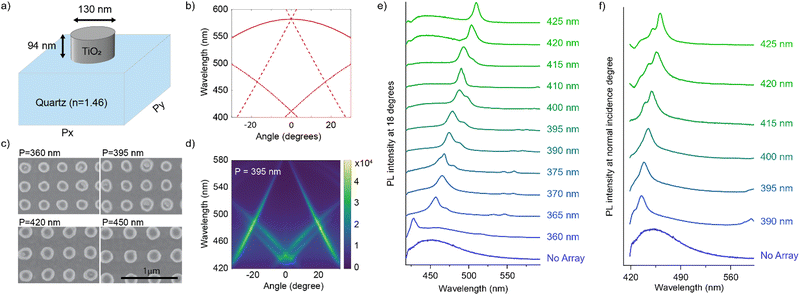
In this connection, we designed an optical nanocavity consisting of TiO2 nanodisks with a diameter of 130 nm and a height of 94 nm on a quartz substrate (Fig. 4a), and varied the lattice period between 360 nm and 425 nm to control the emission spectrum of the TPE fibers on the cavity (Fig. 4c for SEM images and ESI† for fabrication details). The resonant energies of the in-plane diffraction orders of these arrays can be calculated using the 2D lattice equation:
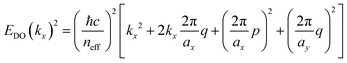 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ is the incident wave vector along the x direction, neff is the effective refractive index of the surrounding medium, (p, q) are the grating orders along the x and y directions and ax and ay are the lattice vectors along the x and y directions.
θ is the incident wave vector along the x direction, neff is the effective refractive index of the surrounding medium, (p, q) are the grating orders along the x and y directions and ax and ay are the lattice vectors along the x and y directions.
We plot the calculated (±1, 0), (0, ±1) and (±1, ±1) diffraction orders for an array with a lattice period of 395 nm in Fig. 4b. With TPE fibers placed on the array, angle-resolved emission is measured using a Fourier microscope (see ESI† for further details). The observed emission from the coupled system closely matches the dispersion of the calculated diffraction orders (Fig. 4d). Thus, the lattice design provides direct control over the emission spectrum of the TPE fibers on the array. It should be noted that due to the interaction of the DOs with the Mie resonances of the individual particles and excitons in the fibers, the actual resonances are slightly shifted with respect to the calculated DOs.37 The emission spectra at an angle of 18 degrees for different lattice constants (ax) are plotted in Fig. 4e, showing the tunability of the emission peak from 430 up to 510 nm. In Fig. 4f the emission in the normal direction is plotted, where the redistribution of fluorescence is controlled by the resonant wavelength (λres) of the first diffraction order of the cavity. For these cases, eqn (1) simplifies to:
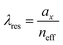 | (2) |
In summary, we have investigated the modulation of emission from H-type supramolecular polymers based on TPE chromophores by exploiting the surface lattice resonances of dielectric TiO2 nanoparticles. Spectroscopic studies in dilute decalin/TCE solutions revealed the hydrogen bonding-driven assembly of TPE-based monomers into one-dimensional assemblies with preferred helicity. Within the stacks, restriction of intramolecular rotation of the phenyl moieties allows emission. AFM and confocal microscopy revealed the formation of tens of micrometers long fibers. Implementation of the latter into optical nanocavities composed of TiO2 nanoparticle arrays resulted in a drastic redistribution of emission as a consequence of weak coupling to the M-SLR. The insights gained from this work pave the way for further studies on exciton-photon coupling in aggregated emissive systems and materials, opening new possibilities for the implementation of well-designed supramolecular assemblies in optoelectronics and photonics. The properties of H-type supramolecular polymers under different regimes of light–matter coupling and within chiral cavities will be investigated in the future in our laboratories.38,39
The authors acknowledge the support of the ICMS microscopy facilities, Dr Yuyang Wang for assistance with emission microscopy measurements, Stef Jansen for the computational modeling of supramolecular polymerization, Dr Stefan Meskers for fruitful discussion and the ICMS Animation Studio for the cartoon of the assembly. This work received funding from the European Research Council (H2020-EU.1.1., SYNMAT project, ID 788618), the Dutch Ministry of Education, Culture and Science for the Gravitation Program Functional Molecular Systems (024.001.035) and the Japan Society for the Promotion of Science for a Bilateral Joint Research Project (JPJSBP120239921). G. L. acknowledges a Marie Skłodowska-Curie Postdoctoral Individual Fellowship (101026072) for financial support. J. G. R. acknowledges the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) for financial support through the Vici Grant No. 680-47-628.
Conflicts of interest
There are no conflicts to declare.Notes and references
- H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke and Y. Urano, Chem. Rev., 2010, 110, 2620–2640 CrossRef CAS PubMed.
- J. Han, S. Guo, H. Lu, S. Liu, Q. Zhao and W. Huang, Adv. Opt. Mater., 2018, 6, 1800538 CrossRef.
- X. Dai, Y. Deng, X. Peng and Y. Jin, Adv. Mater., 2017, 29, 1607022 CrossRef PubMed.
- M. Pan, W.-M. Liao, S.-Y. Yin, S.-S. Sun and C.-Y. Su, Chem. Rev., 2018, 118, 8889–8935 CrossRef CAS PubMed.
- J. Li and K. Pu, Chem. Soc. Rev., 2019, 48, 38–71 RSC.
- A. Mateo-Alonso, Chem. Mater., 2023, 35, 1467–1469 CrossRef CAS.
- Q. Li and Z. Li, Acc. Chem. Res., 2020, 53, 962–973 CrossRef CAS PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- F. Würthner, Angew. Chem., Int. Ed., 2020, 59, 14192–14196 CrossRef PubMed.
- J. Liu, H. Zhang, L. Hu, J. Wang, J. W. Y. Lam, L. Blancafort and B. Z. Tang, J. Am. Chem. Soc., 2022, 144, 7901–7910 CrossRef CAS PubMed.
- F. Hu, S. Xu and B. Liu, Adv. Mater., 2018, 30, 1801350 CrossRef PubMed.
- Z. Zhao, H. Zhang, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9888–9907 CrossRef CAS PubMed.
- J. Li, J. Wang, H. Li, N. Song, D. Wang and B. Z. Tang, Chem. Soc. Rev., 2020, 49, 1144–1172 RSC.
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071–4097 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- H. Li, B. S. Li and B. Z. Tang, Chem. – Asian J., 2019, 14, 674–688 CrossRef CAS PubMed.
- S. K. Saraswathi and J. Joseph, ACS Appl. Nano Mater., 2022, 5, 3018–3027 CrossRef CAS.
- R. Liu, Z. Feng, X. Yan, Y. Lv, J. Wei, J. Hao and Z. Yang, J. Am. Chem. Soc., 2023, 145(31), 17274–17283 CrossRef CAS PubMed.
- J. S. Oh, K. Y. Kim, M. Kim, S. Kim, S. H. Jung and J. H. Jung, Angew. Chem., Int. Ed., 2023, 62, e202300913 CrossRef CAS PubMed.
- S. Cantekin, T. F. A. de Greef and A. R. A. Palmans, Chem. Soc. Rev., 2012, 41, 6125–6137 RSC.
- D. S. Dovzhenko, S. V. Ryabchuk, Y. P. Rakovich and I. R. Nabiev, Nanoscale, 2018, 10, 3589–3605 RSC.
- Y. Xu, D. Ji, H. Song, N. Zhang, Y. Hu, T. D. Anthopoulos, E. M. Di Fabrizio, S. Xiao and Q. Gan, Adv. Opt. Mater., 2018, 6, 1800444 CrossRef.
- E. M. Lindh, P. Lundberg, T. Lanz, J. Mindemark and L. Edman, Sci. Rep., 2018, 8, 6970 CrossRef PubMed.
- C. Zang, S. Liu, M. Xu, R. Wang, C. Cao, Z. Zhu, J. Zhang, H. Wang, L. Zhang, W. Xie and C.-S. Lee, Light: Sci. Appl., 2021, 10, 116 CrossRef CAS PubMed.
- J. Ren, Q. Liao, H. Huang, Y. Li, T. Gao, X. Ma, S. Schumacher, J. Yao, S. Bai and H. Fu, Nano Lett., 2020, 20, 7550–7557 CrossRef CAS PubMed.
- T. Takeda, S. Yamamoto, M. Mitsuishi and T. Akutagawa, Org. Biomol. Chem., 2016, 14, 8922–8926 RSC.
- Although exciton coupling theory predicts a hypsochromic shift of the absorption band for H-type aggregates, additional factors (such as geometric changes of the monomer core upon assembly or gas-to-crystal shifts) may contribute to the displacement of the absorption features of the aggregate relative to the monomeric state.
- D. H. Zhao and J. S. Moore, Org. Biomol. Chem., 2003, 1, 3471–3491 RSC.
- Since the used mass-balance model for cooperative polymerization is based on a two species (monomer ⇆ polymer) equilibrium, the discrepancy between the experimental data and the optimized fit suggests the coexistence of additional processes affecting the concentration of monomeric units involved in the (monomer ⇆ polymer) process.
- V. E. Babicheva and A. B. Evlyukhin, Laser Photonics Rev., 2017, 11, 1700132 CrossRef.
- J. Li, N. Verellen and P. Van Dorpe, J. Appl. Phys., 2018, 123, 083101 CrossRef.
- G. W. Castellanos, P. Bai and J. Gómez Rivas, J. Appl. Phys., 2019, 125, 213105 CrossRef.
- G. W. Castellanos, S. Murai, T. V. Raziman, S. Wang, M. Ramezani, A. G. Curto and J. Gómez Rivas, ACS Photonics, 2020, 7, 1226–1234 CrossRef CAS.
- R. Heilmann, A. I. Väkeväinen, J.-P. Martikainen and P. Törmä, Nanophotonics, 2020, 9, 267–276 CrossRef CAS.
- F. Todisco, R. Malureanu, C. Wolff, P. A. D. Gonçalves, A. S. Roberts, N. A. Mortensen and C. Tserkezis, Nanophotonics, 2020, 9, 803–814 CrossRef CAS.
- Equilibrated solutions were obtained upon heating at 100 °C for 20 minutes and subsequent cooling to 20 °C at a rate of 1 °C min−1.
- J. Bohn, T. Bucher, K. E. Chong, A. Komar, D.-Y. Choi, D. N. Neshev, Y. S. Kivshar, T. Pertsch and I. Staude, Nano Lett., 2018, 18, 3461–3465 CrossRef CAS PubMed.
- C. Genet, ACS Photonics, 2022, 9, 319–332 CrossRef CAS.
- H. Hübener, U. De Giovannini, C. Schäfer, J. Andberger, M. Ruggenthaler, J. Faist and A. Rubio, Nat. Mater., 2021, 20, 438–442 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, synthetic procedures and characterization data of new compounds, self-assembly studies and dispersion of the arrays. See DOI: https://doi.org/10.1039/d3cc05877h |
| This journal is © The Royal Society of Chemistry 2024 |