 Open Access Article
Open Access ArticleCan we make color switchable photovoltaic windows?
Josephine L.
Surel
 ab and
Jeffrey A.
Christians
ab and
Jeffrey A.
Christians
 *a
*a
aDepartment of Engineering, Hope College, Holland, MI 49423, USA. E-mail: christians@hope.edu
bDepartment of Physics, University of Oxford, Clarendon Laboratory, Parks Road, Oxford OX1 3PU, UK
First published on 3rd July 2023
Abstract
The development of smart windows could enhance the functionality of the large glass facades found in modern buildings around the globe. While these facades offer occupants views and natural light, the poor insulating qualities of glass cut against the desire for more efficient use of energy resources. In this perspective article, we explore recent developments for next-generation smart window technologies that can offer improved energy management through dynamic color switching, reducing heating and cooling loads, while also generating electricity through the photovoltaic effect. Approaches with chromogenic organic dyes and halide perovskite semiconductors have been developed for switchable photovoltaic windows, but each of these comes with unique challenges. These approaches are briefly discussed and evaluated with an eye to their future prospects. We hope that this perspective will spur other researchers as they think about the various materials and chemical design challenges associated with color switchable photovoltaic windows. Perhaps these initial demonstrations and research ideas can then become marketable products that efficiently use space to improve occupant comfort and reduce the energy demand of the built environment.
Introduction
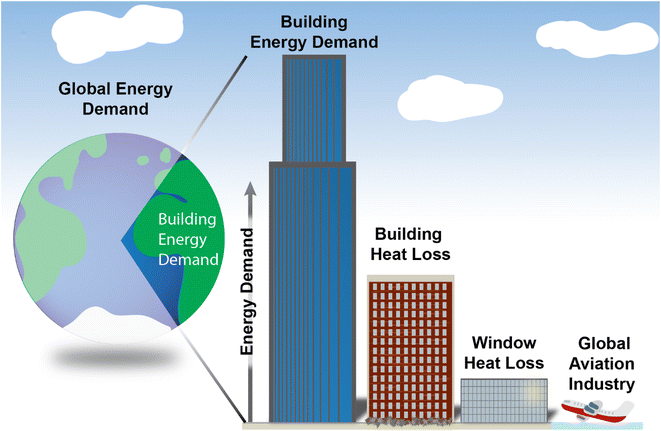
Transitioning from a fossil fuel to a renewable energy global economy requires a dramatic reimagining of how we generate, use, and conserve energy. One key aspect of this transition is reducing energy losses through improved efficiency in the built environment. The Intergovernmental Panel on Climate Change estimates that improved energy efficiency technology and practices have the potential to mitigate approximately 42% of building emissions by 2050.1 Moreover, improved efficiency means reduced material, capital, and land use for renewable infrastructure, and, given the supply and implementation constraints of renewables globally, a faster and smoother energy transition.Buildings accounted for 31% of total global energy demand in 2019 and offer potential for large reductions in energy use through improved design.1–3 Work toward this end will grow in impact as the world continues to urbanize. Heat loss through windows in particular is a large source of energy loss. Cooling and heating of buildings accounts for approximately 40%1 of their total energy demand, and of this, 25–30% is heat loss/gain through windows.1 In sum, heat loss through windows represents something like 3–4% of total global energy demand (Fig. 1). For perspective, the global aviation industry accounts for ∼2% of global energy demand.4–6 Therefore, improved window technologies offer a large potential for energy savings.
Photovoltaically active windows offer the potential to improve the energy and capital payback of more thermally efficient windows by generating energy.3 Nevertheless, standard photovoltaic (PV) windows are fundamentally limited in efficiency because of the need for high visible light transparency; a good solar cell absorbs light while a good window transmits light. The detailed balance limit for semitransparent semiconductor-based PVs has been investigated in detail across a range of visible transmittance values, but is only ∼16.5% at 60% average visible transmittance (for a semiconductor with a ∼2.3 eV band gap).7 This theoretical performance will be lessened with efforts to control infrared transmission and reduce the solar heat gain coefficient (SHGC) of the window, as well as by color management of the PV layer/device so that it is a neutral color and aesthetically pleasing for window applications. Despite the fundamental limits in efficiency, PV windows still have the potential for a similar or higher annual energy production rate than rooftop solar for tall buildings with large glass facades and small roof areas.8
One promising concept is a PV window that, under an external stimulus, can change color from optically dark to highly transparent. A color changing PV window could balance the need for light harvesting and optical transparency with the ability to adjust transmission depending on the building design, the weather, or even a user's desires. This approach would also dynamically change the SHGC of the window depending on cooling/heating needs. In this way, it could balance the tradeoffs necessary to become both a good solar cell and a good window.
In a simulation of various window glazing technologies performed by Wheeler et al.,9 several somewhat non-intuitive findings and design rules have been uncovered. First, the possible energy savings from improvements in window glazing is largest in climates with a significant heating load, such as New York City, versus a less seasonal but sunnier location, such as San Diego. This finding was true for traditional low emissivity double or triple pane windows, as well as photovoltaically active windows (dynamic or not). This analysis also demonstrated that dynamic PV windows do offer energy savings benefits when compared with conventional double pane window construction or static semi-transparent PV windows, and could even result in net-zero buildings for tall buildings in some locations because of their energy production.9 This analysis, and the prevalence of large glass facades throughout modern architecture, therefore motivates the continued investigation of dynamic PV window technologies.
At the heart of the switchable PV window concept is an active PV cell with controllable optical absorption. Most of the development in smart windows has been around achieving reliable color changing windows that change optical properties upon some stimulus.10 Taking this same concept, but then making the material a functioning PV device, requires a deeper look at device design, the mechanism used to achieve a color change, and the possibilities for long lifetimes without optical degradation. Aesthetics are key in window design, making smart window products extremely sensitive to even slight changes in color, and windows require significant effort to replace, much more so than PV modules in most other scenarios, necessitating long term stability in visual appearance as well as performance. Therefore, the design criteria for switchable PV windows is more complicated than the “Stability, Cost, Efficiency” target for typical photovoltaics. While performance metrics and measurement best practices have been developed for transparent and semi-transparent PV,11 these guidelines are not universally applicable to switchable PV window devices. Light utilization and color rendering are key factors in device performance,11–13 and building modeling will be critical to understanding overall design targets and predicting performance.9,14,15 These complexities are only briefly mentioned in this present work.
This perspective addresses the various methods so far explored to achieve precisely controllable optical absorption and identifies the key materials design hurdles for each method. Key technologies under development that are highlighted here include wireless electrochromics, liquid crystals in luminescent solar concentrators, chromogenic organic dyes for dye sensitized and organic solar cells, and chromogenic halide perovskite solar cells. These designs for switchable PV windows are all still in their infancy, but there exist several promising avenues for further investigation and development that could take this technology out of the lab and into the real world.
Wireless electrochromic windows
The initial attempt to fabricate something like a switchable PV window used a dye-sensitized solar cell (DSC) powering a WO3 electrochromic counter electrode (Fig. 2a).16 Present dynamic window technologies rely on electrochromics like WO3,17 but this wireless electrochromic architecture achieves the same transmission control wirelessly, operating essentially as a photochromic device. While this design has the desired chromic properties, it does not provide any electrical output and does not offer the same degree of user control as a standard electrochromic. That said, continued development of this technique and the demonstration of practical prototype devices represent an interesting integration of photovoltaics and electrochromics which have the benefits of electrochromics, but the installation cost and requirements of traditional windows.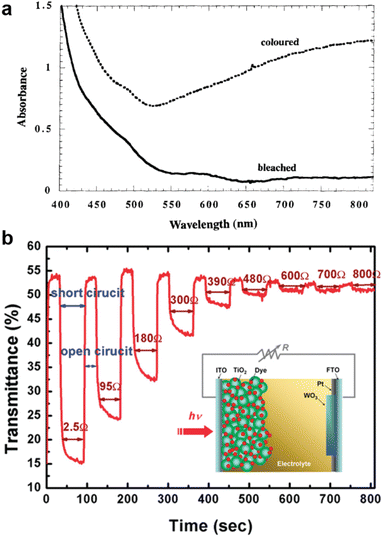 | ||
| Fig. 2 (a) Absorption spectrum of a dye-sensitized photoelectrochromic cell before (bleached) and after (colored) illumination at an intensity of ∼75 mW cm−2 for 1 min. The absorption of the dye/TiO2 dominates the “bleached” state, while LixWO3 is responsible for most of the absorbance in the colored state. Reprinted with permission from ref. 16. Copyright 1996, Springer Nature. (b) Transient optical transmittance at 788 nm of a photoelectrochromic cell with the structure shown in the inset. The cell is placed under a variable load and illuminated with a white-light LED source (4 mW cm−2). Reprinted with permission from ref. 19. Copyright 2009, American Chemical Society. | ||
This approach has been extended to other solar cell technologies, such as organic solar cells, and demonstrated in platforms which could be relatively low cost.18 One interesting further development of this idea demonstrated tunable transmission by the addition of a variable load between the DSC and the electrochromic counter electrode. Using this method, transmission was shown to be controllable between 15% and 50% on timescales of tens of seconds (Fig. 2b).19 More precise control of window transparency offers the potential for system optimization to maximize savings from cooling energy, lighting energy, and heating energy.17 Another interesting approach to achieve wireless electrochromic windows, which may be easier to develop into a workable product, is to use an opaque photovoltaic cell coupled to a separate electrochromic window. By locating an opaque PV cell in an unobtrusive location, perhaps along the window frame, the need for a more specialized transparent PV device would be removed. For instance, Ling et al. coupled3 an efficient metal halide perovskite solar cell with a 4,4′-(thiophene-2,5-diyl)bis(1-ethylpyridin-1-ium)diiodide gel electrochromic to achieve an integrated electrochromic device which did not require external power input.20 A similar approach can also be taken using liquid crystals, where the alignment of the liquid crystal is controlled by a PV device and is used to control transmissivity.21
These PV-powered electrochromic, or photovoltachromic cells, may not provide electrical output, but their ability to dynamically alter their transmission properties wirelessly, still makes them an interesting candidate for next generation window glazing, much like traditional electrochromics. By separating the photochromic and photovoltaic tasks into two distinct devices, this approach offers high flexibility to fine tune the aesthetic design, chromic properties, and stimulus response of a final product. This combination of traits makes this design a promising avenue for the future of electrochromic installations.
Liquid crystals and luminescent solar concentrators
Luminescent solar concentrators (LSC) are materials which absorb incident irradiation and then reemit spectrally-shifted photons, such that some fraction of the emitted photons are trapped by total internal reflection and reflected out to the edges of the device. This is typically an emitting species, such as quantum dots or organic dyes, embedded in a transparent matrix. When coupled with photovoltaic cells at the edges of the device, these devices can act as both windows and solar cells with tunable color and transparency. These architectures have been under development for over four decades as both a way to reduce the area of PV needed, an application obviated by low PV costs in this last decade, and as a device design which can open up new installation opportunities.22 The primary limitation of LSC has historically been their low efficiency (low to mid single digit power conversion efficiencies) even when coupled with highly efficient solar cells.22,23In the context of this discussion, of particular note are luminescent solar concentrators which feature liquid crystals as the transparent matrix. By using liquid crystals in an LSC, it is possible to design a window in which the host liquid crystal responds to an external stimulus, such as voltage, temperature, or lighting conditions, to change from a transparent (aligned) state to an opaque (unaligned) state, similar to privacy glass. A smart window utilizing this method was first described by Debije in 2010,24 who proposed a device that could vary transmission by 31% as well as generate electricity. Application of an external voltage to a matrix of liquid crystals caused the crystals to reorient, and fluorescent dyes embedded in the matrix were consequently reoriented. Individual fluorescent dye molecules are dipole absorbers and so have a preferred axis for absorption, so by altering the orientation via an external voltage the absorption of the smart window device could hence be varied.
Sol, Debije and coworkers later enhanced this approach, changing LC alignment by 90 degrees to enable a third “tinted” state. They developed an LSC based on a fluorescent dye embedded in a liquid crystal cell which could be switched between three color states: transparent, tinted, and opaque, based on applied voltage and the alignment of the liquid crystals (Fig. 3).25 Coupling these color changes with relevant photovoltaic performance will, in the view of these authors, remain quite challenging due to the optics of LSCs, but wireless liquid crystal windows (where the device is powered by small PV cells integrated at the LSC edges) with multiple transparency states could be an interesting application.
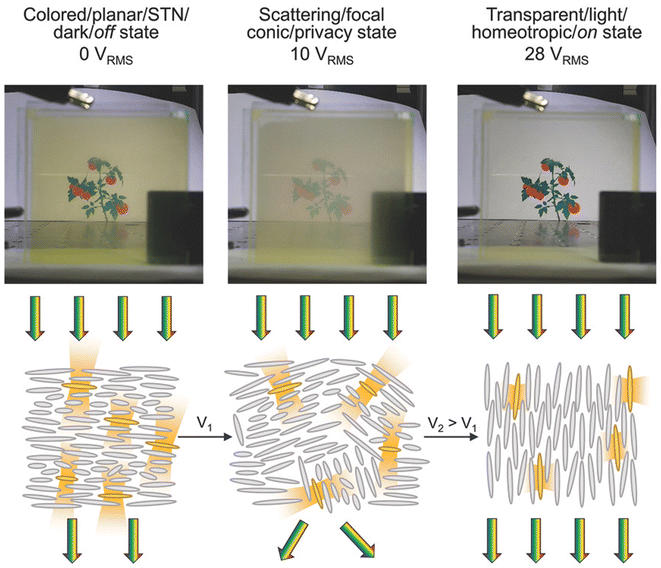 | ||
| Fig. 3 Photographs and schematics of light transmission through a liquid crystal LSC. The optical state of the liquid crystal is manipulated by applied voltage. Reprinted with permission from ref. 25. Copyright 2018, John Wiley and Sons. | ||
Chromogenic organic molecules in solar cells
To combine PV output with the color changing properties of an electrochromic, photochromic or thermochromic organic molecules have been used in dye-sensitized solar cells and organic photovoltaics. This design has distinct benefits versus traditional static windows or even electrochromic windows, but also comes with the challenges of integrating multiple functions into a single device. Color changing organic dyes have long been a mainstay of materials and devices that change color upon the application of a stimulus. While the earliest photochromic systems, utilizing an anthracene–dianthracene photochromic cycle, were described in the 1860s, the first phenomenon more closely related to modern industrial photochromism was described in 1876 by ter Meer, who described the reversible color change of the potassium salt of dinitroethane from colorless in the dark to red in sunlight.26–28 With early commercial potential identified in the 1950s, by 1969 over 800 photochromic spiropyrans belonging to 46 subclasses had been investigated, yet degradation upon multiple color change cycles remained challenging for commercial applications.26,27 More recently, stimuli-responsive polymers have been designed which are controlled by an array of stimulants, including temperature, light, pH, and voltage.29 These responsive polymers are under investigation across a diverse set of applications, but, despite the long and widely varied investigation, most of the commercial value of photochromic dyes is still in their use in eyeglasses which dynamically change their color state, and novelty items.27The dye-sensitized solar cell (DSC) architecture, with dye molecules anchored to nanostructured semiconductor thin films,30 is of particular note for switchable PV because it can combine the chromism of organic molecules with the electrical output of a DSC. While the basics of such an architecture appear relatively straightforward, it has proven difficult to achieve both PV output and a large change in light transmission.
An early demonstration of a switchable DSC utilized spirooxazine dyes.31 These dye molecules are photochromic and change color under UV illumination. Specifically, a center spiro ring is closed initially, but opens with UV exposure, extending the conjugation across the dye molecule. The carboxylic acid-functionalized spirooxazine dyes attach to TiO2 nanoparticle surfaces and are regenerated by conventional iodide/triiodide electrolytes; however, they demonstrated relatively low photovoltage (∼0.5 V) and very low photocurrent in the colored state (∼0.1 mA) in the prototype device. Furthermore, the change in visible transmission was relatively modest (Fig. 4). The challenge of combining sufficient chromic changes with high, or even modest, photovoltaic performance has also been proven significant in attempts by other researchers.32
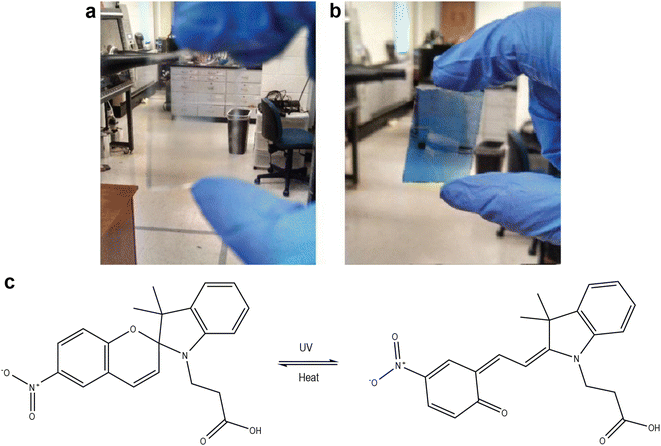 | ||
| Fig. 4 Color change of a spirooxazine dye on a TiO2 film (a) before and (b) after exposure to sunlight. DSCs fabricated using this dye showed power conversion efficiencies of 0.007% (clear) and 0.028% (colored). (c) Chemical structures of the dyes shown above. Figure adapted with permission from ref. 31. Copyright 2015 by Johnson et al., licensee AIMS Press. | ||
In one of the most successful attempts to date of a photochromic dye-sensitized solar cell, Huaulmé and coworkers incorporated several different photochromic dyes based on the diphenyl-naphthopyran photochromic unit (one of which is termed NPI).33 Two key problems that had plagued earlier studies were addressed through this work: (i) the relatively slow kinetics of the color change, and (ii) the poor sensitization characteristics of other photochromic dyes. Poor charge injection from the dye molecules into the TiO2 scaffold resulted in low photocurrent values and overall low performance.31 By extending the conjugation of the photochromic dye's colored state onto the anchoring functional group, charge injection from the dye into the TiO2 was improved dramatically. Additionally, the authors sought to design photochromic dyes with the type of push–pull electronic structure seen in high-performing DSCs.34 The result of designing the photochromic dyes around the requirements of DSCs was a dramatically improved photocurrent of over 12 mA cm−2, and open circuit voltages of slightly more than 0.5 V (Fig. 5). A more recent publication from the same research group built on this work and improved device performance to approximately 4% using a co-sensitized DSC.35 The dyes tested in this work showed a change in average visible transmittance (AVT) from 20.6–44% in the active, colored state to 59–61% in the non-activated state. While performance is still modest in comparison to state-of-the-art DSCs, this work shows that the lessons learned over the past decades in dye development for DSCs can be transferred to photochromic dye development. While these results portend further improvements in photochromic DSCs, the ability of these structures to modulate their transmittance is still only modest. This likely limits their usefulness, at least for the moment, in building integrated photovoltaics.
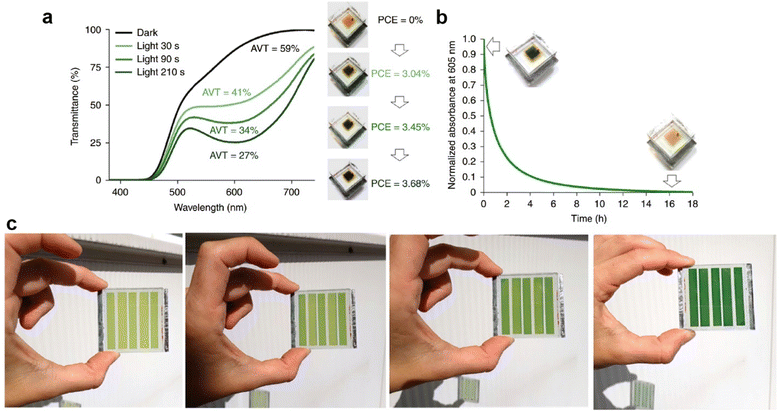 | ||
| Fig. 5 Characterization of NPI-based semi-transparent DSC. (a) Evolution of the average visible transmission (AVT) (measured between 380 and 740 nm), PCE and pictures of a semi-transparent NPI-based solar cell as a function of light exposure time (under standard irradiation conditions). (b) Bleaching curve (at 25 °C) registered at λmax of a complete semi-transparent NPI-based solar cell, with pictures of the cell before and after decoloration. (c) Evolution of the color of an NPI-based solar semi-transparent mini-module when exposed to natural light at 20 °C. Interval times between pictures (from left to right) of 30, 30 and 240 s cumulatively. Adapted with permission from ref. 21. Copyright 2020 Nature Publishing Group. | ||
A largely unexplored application of chromogenic organics is in organic photovoltaic (OPV) devices. The design of organic light absorber molecules for an OPV device differs significantly from a DSC, as does the operating mechanism. In an OPV, organic molecules behave as both light absorber and charge transport network. The ability to more easily tune the energy alignment of OPV devices, as well as significant developments in the molecular design of the OPV components, has led to recent record power conversion efficiencies of 19.2%.36 On the other hand, because charge transport occurs within the sensitizing dye, instead of within a separate semiconductor and redox couple as in DSCs (e.g., in TiO2 and I−/I3−), the design of photochromic dyes for OPV devices is somewhat more challenging.
As existing photochromic dyes do not have conductivities which are sufficiently high to allow for use in OPV devices, new photochromic dyes are needed to enable this device architecture. To date, this goal has yet to be achieved. However, in an initial demonstration, Saes et al. fabricated an OPV device with poly-TPD and PC61BM as the donor and acceptor pair and then inserted a photochromic diarylethene dye into the device to provide the chromogenic behavior.37 Because both poly-TPD and PC61BM absorb visible light weakly, the photochromic behavior of the diarylethene dyes can be observed. While this architecture did show some small changes in device transmission under UV illumination, the overall performance of the devices decreased with increasing diarylethene concentration. This decrease occurred from an already low baseline seen with a simple poly-TPD and PC61BM device, due to their low absorption. This work highlights the need for new chromogenic dyes which can act as either the donor or acceptor in the OPV device.
Switchable PSCs
Metal halide perovskite (MHP) solar cells have surpassed both DSC and OPV performance by a significant margin.36 Since the earliest demonstrations of halide perovskites in photovoltaics,38 however, researchers have struggled against various instabilities. The instabilities in these intriguing semiconductors are typically the undesired consequence of the low formation energy of MHPs.39,40 Certain instabilities which lead to large color changes have been explored as potential chromogenic pathways. The development of chromogenic semiconductors with the high electronic quality of MHPs could open the door for a new generation of color changing devices, with color changing PV windows as a potential cornerstone application. Somewhat unique to MHPs is the range of mechanisms which provide large changes in transmission, with some material compositions exhibiting several different chromogenic mechanisms. To date, the mechanisms investigated include thermally driven crystal phase transformations,41,42 hydration43–45 or complexation with other intercalant molecules,46–52 changes in structural dimensionality,53 and other mechanisms besides.54 This wide range of mechanisms and stimuli has led researchers to imagine many other applications beyond color changing photovoltaic windows,46,54,55 but those most relevant to windows are discussed here.One of the early demonstrations of the chromogenic nature of MHP materials was the hydration of methylammonium lead iodide to form colorless hydrates, such as H2O·CH3NH3PbI3.43–45 It was identified in these early studies that this process could, under certain circumstances, be reversible, but it could also lead to color changes for the CH3NH3PbI3 hydrate.43–45 While the initial demonstrations of CH3NH3PbI3 hydration were focused on the relevance of this mechanism to the degradation of MHP solar cells when exposed to water,44,45 the hydration was taken advantage of by Halder et al. in 2015 through a change in material stoichiometry.43 In this study, a colorless, wide bandgap hydrate, (CH3NH3)4PbI6·2H2O was favored below 60 °C while a brown film was present at temperatures above 60 °C due to the reversible hydration/dehydration mechanism:
The color change was found to be quite stable over 10 cycles, and there was a distinct difference in the photovoltaic performance between the two states, but the power conversion efficiency was only 1.2% in the colored state. A similar hydration mechanism was displayed with a 2D/3D MHP device which achieved a power conversion efficiency of 0.5% in the colored state and a switching temperature of >60 °C, although the kinetics were a few hours at these lower temperatures and minute timescales required ∼100 °C.56 However, the larger issue with this color change mechanism is that the reliance on water hydration will lead to the degradation of the CH3NH3PbI3 under operational conditions.39,57
In 2017, Wheeler et al. improved on this previous work with the change from water to methylamine.50 When CH3NH3PbI3 films were kept under a low partial pressure of methylamine (CH3NH2) gas, a similar intercalation/deintercalation reaction was observed
This change in mechanism led to a photothermal phase transformation window that could be tuned with different partial pressures of CH3NH3 gas. However, the key issue revealed in this initial switchable photothermal PV window was the morphological changes of the MHP films with continued color switches (Fig. 6d).
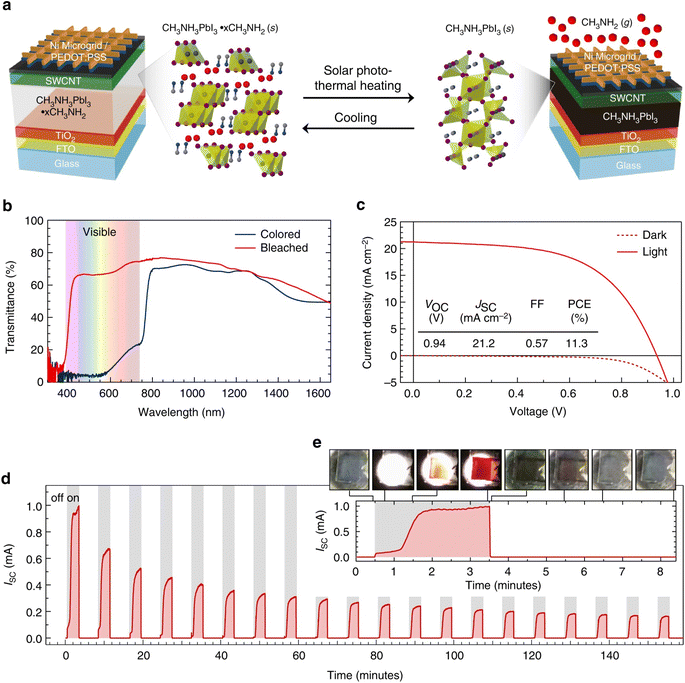 | ||
| Fig. 6 Composition and performance of switchable photovoltaic window devices. (a) Schematic of PV window device architecture and switching process. (b) Transmittance of PV devices in the bleached (red) and colored (blue) states as a function of wavelength. (c) Current density as a function of voltage of the champion switchable PV device in the dark (dashed) and under illumination (solid). The inset table shows PV performance metrics of the device before being bleached. (d) Short-circuit current as a function of time for 20 cycles of 3 min of illumination followed by 5 min of cooling in the dark. (e) Short-circuit current as a function of time for the first illumination cycle. The optical images show the transition from bleached to colored and back to bleached at the indicated times during the cycling process. Reproduced with permission from ref. 50. Copyright 2017 Springer Nature. | ||
More recent work by ourselves, along with coworkers, exploring the interaction between CH3NH2 and MHPs has improved the understanding of this interaction chemistry and outlined initial guidelines for improving reversibility and limiting morphological changes. Using a variety of 2D MHPs, we demonstrated that 2D MHPs also display similar chromogenic behavior to CH3NH3PbI3, but that there are an array of different possible interactions with CH3NH2 that can lead to the formation of secondary phases.52 The control of the interactions between the A-site cation and the PbI62− octahedral sheets of the structure was shown to be a useful tool for developing more robust compounds for chromogenic MHPs. It was demonstrated that secondary phase formation can be suppressed through careful design of the long-chain A-site cations. Tuning the cation–cation and cation–PbI62− interactions (at both the cation–NH3+ “head” and the “tail”) was shown to lead to dramatically improved reversibility of the methylamine-induced color change, in structural and morphological studies, with HO-PEA2PbI4 (4-hydroxy-phenethylammonium lead iodide) showing promising reversibility (Fig. 7). Future efforts may be able to incorporate these same design rules to improve the reversibility of chromogenic MHPs with methylamine or other intercalation compounds.
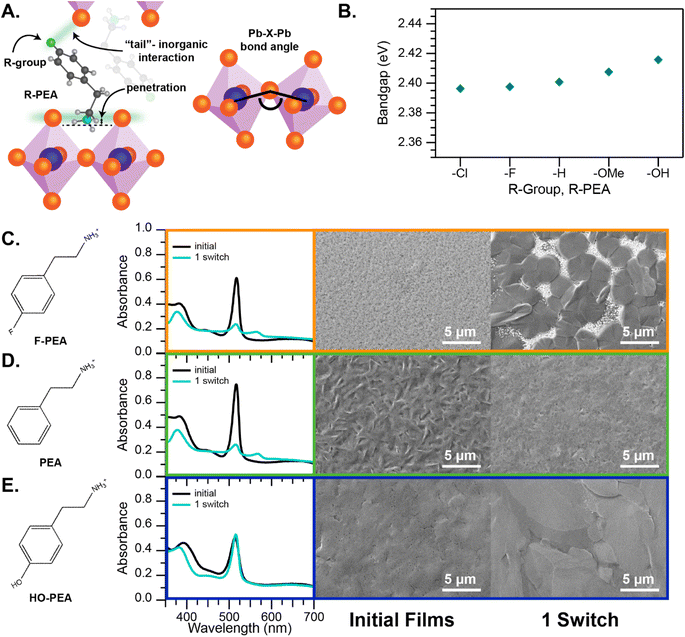 | ||
| Fig. 7 Intercalation/deintercalation in R-PEA2PbI4 materials. (A) Diagram showing how organic cation penetration into the perovskite layer can lead to Pb–I–Pb bond distortions and interactions with cation “tail” and adjacent axial halide. (B) Band gap energy calculated from the absorption excitonic peak plotted for various R-groups in R-PEA2PbI4 films. (C–E) Results of MA intercalation/deintercalation across three select R-PEA2PbI4 materials. From left to right is shown, the structure of the A-site cation, UV-visible absorbance spectra of the R-PEA2PbI4 film before and after one switch, and SEM images of film morphology for these materials before and after one switch. Reproduced with permission from ref. 52. Copyright 2022, American Chemical Society. | ||
In addition to these mechanisms, thermochromic MHPs have been demonstrated based upon materials which are metastable in their dark, perovskite crystal phase. This was first reported in a device in 2018 by Lin and coworkers with CsPbIBr2 as a model system,41 with a similar system proposed and illustrated in a patent by one of us and several coworkers.42 A key development of the work by Lin et al. was a more thermally robust transparent device architecture.41 This enabled devices that showed stability in their photovoltaic output over 10 color change cycles. However, the phase transition temperature for these materials is outside of the range that can be accessed in standard PV window applications, as this initial demonstration showed a thermochromic switching temperature of 150–190 °C between the visibly transparent non-perovskite phase and the light-absorbing perovskite phase. The kinetics of this phase change can become quite slow (hours) as the temperature of the phase change is lowered below this level because of the decrease in the thermodynamic driving force. Current evidence indicates that the nucleation of the non-perovskite phase plays an important role in the kinetics of the phase transition, and that water plays a part in initiating these non-perovskite nucleation sites.41,58,59 As such, developing this mechanism further, and increasing the kinetics to a point where it could be technologically relevant, will likely require the more precise control of nucleation site formation.
A final notable challenge for all of the MHP PV window approaches is the attainable color space of a completed device.8 While research to date has focused on the color change mechanisms and the development of higher performance or more robust systems, future research will need to carefully consider the aesthetic aspects of the technology if it is to be used in either building facades or consumer products. Although some basic mechanisms for controlling the color of semi-transparent MHP solar cells have been explored,60,61 this has not received enough attention from researchers developing these devices.
Technology outlook
Switchable PV windows integrate two separate technologies to provide unique functionality that enables both energy savings and energy generation in a single device. With the continued architectural preference for large glass facades, this technology can improve the thermal performance of the window glazing through dynamic changes in optical absorption, while enabling energy generation in existing spaces. While these features make switchable PV windows attractive, the combination of functions makes it a difficult technology from a materials and device design perspective. Rosales et al. have shown that 20–27.5 °C is the optimal switching temperature range across climate zones because it reduces the cooling load of the building when the device is in the colored state, and the heating load when the device is transparent.62 This provides a target for thermochromic materials and demonstrates their advantage over photochromic mechanisms which prioritize PV output, but would be poorly tuned to reduce heating/cooling needs for the building.Ideal thermal PV window coatings would have strong absorption in the near infrared portion of the solar spectrum, not just in the visible. Significant absorption in the near IR is important to limit the SHGC of the window glazing to reduce cooling load for the building. Near IR modulation driven by a thermochromic mechanism would allow for heightened SHGC during colder weather, and reduced SHGC during warm weather, improving building performance in both conditions. However, achieving strong optical changes in both the visible and near infrared is an especially challenging task. While this is an interesting long-term design target, a hybrid approach where the dynamic changes of the window glazing occur in the visible, and standard static near IR absorbing coatings are tuned to the climatic location of the building, may prove to be the most tractable in the near-term. Chromogenic dyes have a long history and have proven to be a rather reliable technology in applications where extremely long lifespans are not required (viz., eyeglasses, some textile use, actinometry, and thermoplastics). However, current chromogenic dyes do not appear promising with regard to their PV properties, making the design of new classes of photochromic and thermochromic dyes necessary, especially for OPV devices. As discussed previously, a dynamic SHGC driven by a thermochromic mechanism has distinct advantages over photochromic windows. For dye systems, demonstrations have, to date, used photochromism. The use of photochromism puts a premium on PV efficiency for system performance, especially in more temperate climates where building heating load is higher.62 Moreover, the photostability of current commercial photochromic dyes is not sufficient for applications in window glazing.27 In these two respects, we can learn from past developments in OPV and DSC dye design, but there remain significant hurdles. The optical absorbance of the common DSC electrolytes is a complication, but semi-transparent DSCs have been demonstrated.63,64 More challenging for dye-based systems will be the design of dyes with strong absorbance modulation across the entire visible spectrum while achieving a neutral color state. Further development of co-sensitization with multiple chromogenic dye molecules could enable this.35 For OPV architectures specifically, the design of new classes of chromogenic dye molecules or polymers that have high charge mobility and improved photostability is needed. Overall, for these systems the stability (PV performance and color stability) of the chromogenic dyes developed is likely to be the most significant challenge, especially for dyes with “black” absorption.
Luminescent solar concentrators also have a long history of research, and incorporation of fluorescent dyes (or semiconductors) in liquid crystal matrices have been studied for a range of optical and electronic devices. Like with chromogenic dyes, the photostability of the dye used in liquid crystal LSCs is vital for their implementation in switchable energy-generating windows.65 For this type of device, coupling the color change with significant PV performance will also be a challenge, as the efficiencies of even the best LSCs are quite low because of the optical mechanism of the device.22 That said, the ability of liquid crystal LSCs to provide multiple desirable color states (clear, tinted, and opaque), makes them rather unique.
Metal halide perovskites have been touted by researchers as the “next-big-thing” across a huge range of semiconductor device applications. This optimism is in large part because of the high initial performance of MHP devices and their potential for large area processing. While high photovoltaic performance has been difficult to achieve with photochromic dye/organic solar cells, many of the early demonstration devices featuring MHPs achieved respectable initial performances.41,50 This is especially relevant as the majority of research efforts in this space have been focused on the chromogenic mechanisms rather than device efficiency. That said, while MHPs offer a promising material system that addresses some of the challenges faced with organic chromogenic dyes, they come with their own unique set of challenges around material and device stability. One challenge for MHPs not seen to the same degree in the other approaches discussed is the toxicity of lead. This risk must be weighed and designed against. Possibilities exist to build encapsulation strategies into the device design,66 and even “worst-case” complete failure will likely not pose a significant environmental risk if an adequate sorbing phase (e.g., soil) is present.67 Thus, in BIPV applications with double-pane windows, lead exposure risk currently appears quite small, although disposal of the windows becomes more challenging than traditional glass.
Final MHP products could be largely similar to standard argon filled double pane windows, although the requirements for the edge sealant will change because it must now be chemically compatible with the intercalation molecule. Use of the intercalation mechanism has enabled ideal switching temperatures and fast kinetics for thermochromic halide perovskites.62 However, two main problems are readily apparent for this mechanism. First, there are a number of different side reactions which can occur between the intercalation molecule and the halide perovskite (or the other layers in the device stack). Most notable of these is protonation of the iodide in the halide perovskite to HI, which then can volatilize and cause irreversible material degradation.68,69 This is especially pertinent for intercalation compounds with acidic hydrogens (e.g., water, methanol, etc.). These reactions can be initiated by light or temperature which makes them especially problematic in PVs and therefore necessitates testing of material switching and stability in the light and the dark, measurements which are currently scarce in the published literature. The second major instability of this design is the mechanical instability caused by repeated expansion/contraction of the materials from the intercalation/deintercalation process that drives the color change. This expansion/contraction can lead to significant morphological changes after very few color change cycles.50,52 Both of these instabilities remain challenging, but work is being done to template both materials52 and device structures62 to help relieve these stresses and offer improved device lifetime. Because of these two issues, while it addresses many aspects necessary for switchable photovoltaic windows, this switching mechanism's particular stability issues are likely to be a major hurdle to its development into a product.
Devices which take advantage of the phase instability of MHPs are able to reduce the mechanical stresses associated with a color change, likely simplifying the design of reliable devices and, in the authors' opinion, providing a more straightforward pathway to commercially relevant photostability and cycling stability. However, the low switching temperatures required of thermochromic materials makes devices reliant on these phase changes difficult because of the slow kinetics as the temperature is reduced.41 This is not an insignificant materials design challenge. Pushing this phase transition temperature from ∼150 °C closer to 30 °C, while maintaining switching rates of minutes, will only be possible with better control of the material kinetics, and may require material compositions which are currently beyond the reach of standard thin film solution processing.70 Therefore, while this approach remains interesting, there does not exist a demonstrated pathway to lower switching temperature to the desired range (just above room temperature) while maintaining the necessary kinetics.
Conclusion
We believe that switchable photovoltaic windows have large commercial potential because they address an area of need while providing new functionality in building design. These products would provide a sought-after combination of revenue generation (energy production), reduced operating costs (better insulating properties), and improved user experience (a reduction of sun glare without completely sacrificing the view). While this makes switchable PV windows very desirable, each of the potential technologies explored present significant challenges to overcome on the road to commercial viability.When comparing the number of publications on these topics to the amount of work that has been done on standard opaque solar cells using these technologies, or even semi-transparent devices for BIPV, all of the approaches discussed herein are still in their infancy (Table 1).
Because of this relative lack of research, many challenges in characterization still exist and the future of the technology as a whole is still very uncertain. The several mechanisms thus far explored for switchable photovoltaics all offer their own advantages and challenges, with the linked difficulties of robust photostability and color cycling stability operating as the common hurdles. These are likely to remain two dominant challenges for the field moving forward. Any smart window technology necessitates very long product lifetimes because window production and installation costs are both high. Therefore, we anticipate that product lifetimes would need to be at least as long as current commercial utility-scale photovoltaics, perhaps even longer. One slight caveat, however, is that product lifetime for a smart window is more directly related to the optical appearance than photovoltaic production. So, while degradation in PV performance is important, retention of the “look” of the window is a more important aspect of product durability than simply performance. Even so, overcoming long-term stability issues will require a significant research effort, making commercialization of switchable PV windows a long-term pursuit in our view.
A third challenge common to all of these approaches which should not be overlooked is the difficulty in achieving a suitable color.11,13,61,71 Because implementation of color changing PV windows would be in architectural spaces, adoption will most certainly not be due to functionality and cost/energy savings alone. Achieving a desirable (or at least avoiding an undesirable) aesthetic has proven to be a substantial challenge even for static BIPV technologies that have received more significant research and commercialization effort. Adopting the reporting protocols used for semi-transparent PV, such as those outlined by Lunt and coworkers,11 (understanding that these need to be modified/expanded for switchable PV windows) will be an important step to make common progress on this point. However, future work needs to consider the specific reporting metrics and targets for switchable PV windows (e.g., color rendering index in transparent and opaque states, AVT, light utilization efficiency, PV stability, color stability, switching kinetics, and more). While these topics has been largely avoided thus far for color changing PV windows, this area must rise to the fore, and soon.
Because of the challenges still remaining for color changing PV windows, combination PV and electrochromic or liquid crystal windows appear to be worth continued pursuit in the near- and mid-term. Such wireless chromogenic windows would integrate two more mature technologies and enable some of the same benefits, excepting energy production, as switchable PV windows. Moreover, combined with a battery this could offer the user, or product designer, precise control of window transparency at all light conditions.
Smart window technologies, including switchable PV windows, are an important technological tool to allow architects and designers to continue to build major buildings with large glass facades while significantly reducing the energy footprint of those structures. Given the continued urbanization occurring globally and the necessity to reduce the energy impact of new and existing structures, the time is ripe to invest in the research and development of next-generation window technologies that can make an impact later this century, while pushing to accelerate the adoption of first-generation smart window technologies that will provide desirable functionality and save energy now.
Author contributions
JC conceived of the project. JS and JC both wrote and edited the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors acknowledge support in part by the Hope College Dean of Natural and Applied Sciences and the Hope College Department of Engineering. JC acknowledges support by the Towsley Research Scholars Program grant from the Towsley Foundation of Midland, Michigan. JS acknowledges support by the Clare Boothe Luce Research Scholar program. Research reported in this publication was supported in part by funding provided by the National Aeronautics and Space Administration (NASA), under award number 80NSSC20M0124, Michigan Space Grant Consortium (MSGC), and from the National Science Foundation (DMR-2128632).References
- L. F. Cabeza, Q. Bai, P. Bertoldi, J. M. Kihila, A. F. P. Lucena, É. Mata, S. Mirasgedis, A. Novikova and Y. Saheb, Buildings, in IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. P. R. Shukla, J. Skea, R. Slade, A. Al Khourdajie, R. van Diemen, D. McCollum, M. Pathak, S. Some, P. Vyas, R. Fradera, M. Belkacemi, A. Hasija, G. Lisboa, S. Luz and J. Malley, Cambridge University Press, Cambridge, UK and New York, NY, USA, 2022, DOI:10.1017/9781009157926.011.
- T. Ibn-Mohammed, R. Greenough, S. Taylor, L. Ozawa-Meida and A. Acquaye, Operational vs. Embodied Emissions in Buildings—A Review of Current Trends, Energy Build., 2013, 66, 232–245, DOI:10.1016/j.enbuild.2013.07.026.
- T. Salameh, M. E. H. Assad, M. Tawalbeh, C. Ghenai, A. Merabet and H. F. Öztop, Analysis of Cooling Load on Commercial Building in UAE Climate Using Building Integrated Photovoltaic Façade System, Sol. Energy, 2020, 199, 617–629, DOI:10.1016/j.solener.2020.02.062.
- P. Jaramillo, S. K. Ribeiro, P. Newman, S. Dhar, O. E. Diemuodeke, T. Kajino, D. S. Lee, S. B. Nugroho, X. Ou, A. H. Strømman and J. Whitehead, Transport, in IPCC, 2022: Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. P. R. Shukla, J. Skea, R. Slade, A. Al Khourdajie, R. van Diemen, D. McCollum, M. Pathak, S. Some, P. Vyas, R. Fradera, M. Belkacemi, A. Hasija, G. Lisboa, S. Luz and J. Malley, Cambridge University Press, Cambridge, UK and New York, NY, USA, 2022, DOI:10.1017/9781009157926.012.
- M. S. Ryerson, Future Energy Demands on the Global Aviation Industry, Kleinman Center for Energy Policy, https://kleinmanenergy.upenn.edu/research/publications/future-energy-demands-on-the-global-aviation-industry/ Search PubMed.
- IEA, Aviation, IEA, Paris, 2022, https://www.iea.org/reports/aviation, License: CC BY 4.0 Search PubMed.
- L. M. Wheeler and V. M. Wheeler, Detailed Balance Analysis of Photovoltaic Windows, ACS Energy Lett., 2019, 4(9), 2130–2136, DOI:10.1021/acsenergylett.9b01316.
- J. Bing, L. G. Caro, H. P. Talathi, N. L. Chang, D. R. Mckenzie and A. W. Y. Ho-Baillie, Perovskite Solar Cells for Building Integrated Photovoltaics—Glazing Applications, Joule, 2022, 6(7), 1446–1474, DOI:10.1016/j.joule.2022.06.003.
- V. M. Wheeler, J. Kim, T. Daligault, B. A. Rosales, C. Engtrakul, R. C. Tenent and L. M. Wheeler, Photovoltaic Windows Cut Energy Use and CO2 Emissions by 40% in Highly Glazed Buildings, One Earth, 2022, 5(11), 1271–1285, DOI:10.1016/j.oneear.2022.10.014.
- A. S. R. Bati, Y. L. Zhong, P. L. Burn, M. K. Nazeeruddin, P. E. Shaw and M. Batmunkh, Next-Generation Applications for Integrated Perovskite Solar Cells, Commun. Mater., 2023, 4(1), 1–24, DOI:10.1038/s43246-022-00325-4.
- C. Yang, D. Liu, M. Bates, M. C. Barr and R. R. Lunt, How to Accurately Report Transparent Solar Cells, Joule, 2019, 3(8), 1803–1809, DOI:10.1016/j.joule.2019.06.005.
- C. J. Traverse, R. Pandey, M. C. Barr and R. R. Lunt, Emergence of Highly Transparent Photovoltaics for Distributed Applications, Nat. Energy, 2017, 2(11), 849–860, DOI:10.1038/s41560-017-0016-9.
- N. Lynn, L. Mohanty and S. Wittkopf, Color Rendering Properties of Semi-Transparent Thin-Film PV Modules, Build. Environ., 2012, 54, 148–158, DOI:10.1016/j.buildenv.2012.02.010.
- B. K. Koyunbaba, Z. Yilmaz and K. Ulgen, An Approach for Energy Modeling of a Building Integrated Photovoltaic (BIPV) Trombe Wall System, Energy Build., 2013, 67, 680–688, DOI:10.1016/j.enbuild.2011.06.031.
- J. E. Goncalves, T. van Hooff and D. Saelens, A Physics-Based High-Resolution BIPV Model for Building Performance Simulations, Sol. Energy, 2020, 204, 585–599, DOI:10.1016/j.solener.2020.04.057.
- C. Bechinger, S. Ferrere, A. Zaban, J. Sprague and B. A. Gregg, Photoelectrochromic Windows and Displays, Nature, 1996, 383(6601), 608–610 CrossRef CAS.
- A. Cannavale, U. Ayr, F. Fiorito and F. Martellotta, Smart Electrochromic Windows to Enhance Building Energy Efficiency and Visual Comfort, Energies, 2020, 13(6), 1449, DOI:10.3390/en13061449.
- N. Davy, M. Sezen-Edmonds and J. Gao, et al., Pairing of near-ultraviolet solar cells with electrochromic windows for smart management of the solar spectrum, Nat. Energy, 2017, 2, 17104, DOI:10.1038/nenergy.2017.104.
- J. J. Wu, M. D. Hsieh, W. P. Liao, W. T. Wu and J. S. Chen, Fast-Switching Photovoltachromic Cells with Tunable Transmittance, ACS Nano, 2009, 3(8), 2297–2303, DOI:10.1021/nn900428s.
- H. Ling, J. Wu, F. Su, Y. Tian and Y. J. Liu, High Performance Electrochromic Supercapacitors Powered by Perovskite-Solar-Cell for Real-Time Light Energy Flow Control, Chem. Eng. J., 2022, 430, 133082, DOI:10.1016/j.cej.2021.133082.
- J. Murray, D. Ma and J. N. Munday, Electrically Controllable Light Trapping for Self-Powered Switchable Solar Windows, ACS Photonics, 2017, 4(1), 1–7, DOI:10.1021/acsphotonics.6b00518.
- M. G. Debije and P. P. C. Verbunt, Thirty Years of Luminescent Solar Concentrator Research: Solar Energy for the Built Environment, Adv. Energy Mater., 2012, 2(1), 12–35, DOI:10.1002/aenm.201100554.
- L. H. Slooff, E. E. Bende, A. R. Burgers, T. Budel, M. Pravettoni, R. P. Kenny, E. D. Dunlop and A. Büchtemann, A Luminescent Solar Concentrator with 7.1% Power Conversion Efficiency, Phys. Status Solidi RRL, 2008, 2(6), 257–259, DOI:10.1002/pssr.200802186.
- M. G. Debije, Solar Energy Collectors with Tunable Transmission, Adv. Funct. Mater., 2010, 20(9), 1498–1502, DOI:10.1002/adfm.200902403.
- J. A. H. P. Sol, G. H. Timmermans, A. J. van Breugel, A. P. H. J. Schenning and M. G. Debije, Multistate Luminescent Solar Concentrator “Smart” Windows, Adv. Energy Mater., 2018, 8(12), 1702922, DOI:10.1002/aenm.201702922.
- H. G. Heller, Photochromics for the Future, in Electronic Materials: From Silicon to Organics, ed. L. S. Miller and J. B. Mullin, Springer US, Boston, MA, 1991, pp. 471–483, DOI:10.1007/978-1-4615-3818-9_31.
- A. Towns, Photochromic Dyes, Phys. Sci. Rev., 2021, 6, 477–511, DOI:10.1515/psr-2020-0191.
- E. ter Meer, Ueber Dinitroverbindungen der Fettreihe, Justus Liebigs Ann. Chem., 1876, 181(1) DOI:10.1002/jlac.18761810102.
- F. D. Jochum and P. Theato, Temperature- and Light-Responsive Smart Polymer Materials, Chem. Soc. Rev., 2013, 42(17), 7468–7483, 10.1039/C2CS35191A.
- B. O'Regan and M. Grätzel, A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films, Nature, 1991, 353(6346), 737–740, DOI:10.1038/353737a0.
- N. M. Johnson, Y. Y. Smolin, C. Shindler, D. Hagaman, M. Soroush, K. K. S. Lau and H. F. Ji, Photochromic Dye-Sensitized Solar Cells, AIMS Mater. Sci., 2015, 2(4), 503–509, DOI:10.3934/matersci.2015.4.503.
- S. Ma, H. Ting, Y. Ma, L. Zheng, M. Zhang, L. Xiao and Z. Chen, Smart Photovoltaics Based on Dye-Sensitized Solar Cells Using Photochromic Spiropyran Derivatives as Photosensitizers, AIP Adv., 2015, 5, 057154, DOI:10.1063/1.4921880.
- Q. Huaulmé, V. M. Mwalukuku, D. Joly, J. Liotier, Y. Kervella, P. Maldivi, S. Narbey, F. Oswald, A. J. Riquelme, J. A. Anta and R. Demadrille, Photochromic Dye-Sensitized Solar Cells with Light-Driven Adjustable Optical Transmission and Power Conversion Efficiency, Nat. Energy, 2020, 5(6), 468–477, DOI:10.1038/s41560-020-0624-7.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Dye-Sensitized Solar Cells with 13% Efficiency Achieved through the Molecular Engineering of Porphyrin Sensitizers, Nat. Chem., 2014, 6(3), 242–247, DOI:10.1038/nchem.1861.
- V. M. Mwalukuku, J. Liotier, A. J. Riquelme, Y. Kervella, Q. Huaulmé, A. Haurez, S. Narbey, J. A. Anta and R. Demadrille, Strategies to Improve the Photochromic Properties and Photovoltaic Performances of Naphthopyran Dyes in Dye-Sensitized Solar Cells, Adv. Energy Mater., 2023, 13(8), 2203651, DOI:10.1002/aenm.202203651.
- NREL, Research Cell Record Efficiency Chart, https://www.nrel.gov/pv/cell-efficiency.html, accessed, 6/12/ 2023 Search PubMed.
- B. W. H. Saes, M. M. Wienk and R. A. J. Janssen, Photochromic Organic Solar Cells Based on Diarylethenes, RSC Adv., 2020, 10(50), 30176–30185, 10.1039/d0ra04508j.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Novel Photoelectrochemical Cell with Mesoscopic Electrodes Sensitized by Lead-Halide Compounds (11), in 212th ECS Meeting, 2007, p. 397 Search PubMed.
- J. S. Manser, M. I. Saidaminov, J. A. Christians, O. M. Bakr and P. V. Kamat, Making and Breaking of Lead Halide Perovskites, Acc. Chem. Res., 2016, 49(2), 330–338, DOI:10.1021/acs.accounts.5b00455.
- N. Park, M. Grätzel, T. Miyasaka, K. Zhu and K. Emery, Towards Stable and Commercially Available Perovskite Solar Cells, Nat. Energy, 2016, 1(11), 16152, DOI:10.1038/nenergy.2016.152.
- J. Lin, M. Lai, L. Dou, C. S. Kley, H. Chen, F. Peng, J. Sun, D. Lu, S. A. Hawks, C. Xie, F. Cui, A. P. Alivisatos, D. T. Limmer and P. Yang, Thermochromic Halide Perovskite Solar Cells, Nat. Mater., 2018, 17(3), 261–267, DOI:10.1038/s41563-017-0006-0.
- L. M. Wheeler, J. M. Luther, J. A. Christians and J. J. Berry, Energy-Harvesting Chromogenic Devices, US Pat., US20210087877A1, November 24, 2020, https://patents.google.com/patent/US20210087877A1/en, accessed, 2023-02-14.
- A. Halder, D. Choudhury, S. Ghosh, A. S. Subbiah and S. K. Sarkar, Exploring Thermochromic Behavior of Hydrated Hybrid Perovskites in Solar Cells, J. Phys. Chem. Lett., 2015, 6(16), 3180–3184, DOI:10.1021/acs.jpclett.5b01426.
- J. A. Christians, P. A. Miranda Herrera and P. V. Kamat, Transformation of the Excited State and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air, J. Am. Chem. Soc., 2015, 137(4), 1530–1538, DOI:10.1021/ja511132a.
- A. M. A. Leguy, Y. Hu, M. Campoy-Quiles, M. I. Alonso, O. J. Weber, P. Azarhoosh, M. van Schilfgaarde, M. T. Weller, T. Bein, J. Nelson, P. Docampo and P. R. F. Barnes, Reversible Hydration of CH3NH3PbI3 in Films, Single Crystals and Solar Cells, Chem. Mater., 2015, 27(9), 3397–3407, DOI:10.1021/acs.chemmater.5b00660.
- Y. Zhao and K. Zhu, Optical Bleaching of Perovskite (CH3NH3)PbI3 through Room-Temperature Phase Transformation Induced by Ammonia, Chem. Commun., 2014, 50(13), 1605–1607, 10.1039/c3cc48522f.
- S.-H. Kim, A. Kirakosyan, J. Choi and J. H. Kim, Detection of Volatile Organic Compounds (VOCs), Aliphatic Amines, Using Highly Fluorescent Organic-Inorganic Hybrid Perovskite Nanoparticles, Dyes Pigm., 2017, 147, 1–5, DOI:10.1016/j.dyepig.2017.07.066.
- D. Bogachuk, L. Wagner, S. Mastroianni, M. Daub, H. Hillebrecht and A. Hinsch, The Nature of the Methylamine–MAPbI3 Complex: Fundamentals of Gas-Induced Perovskite Liquefaction and Crystallization, J. Mater. Chem. A, 2020, 8(19), 9788–9796, 10.1039/D0TA02494E.
- T. Zhao, S. T. Williams, C.-C. Chueh, D. W. DeQuilettes, P.-W. Liang, D. S. Ginger and A. K.-Y. Jen, Design Rules for the Broad Application of Fast (<1 s) Methylamine Vapor Based, Hybrid Perovskite Post Deposition Treatments, RSC Adv., 2016, 6(33), 27475–27484, 10.1039/C6RA03485C.
- L. M. Wheeler, D. T. Moore, R. Ihly, N. J. Stanton, E. M. Miller, R. C. Tenent, J. L. Blackburn and N. R. Neale, Switchable Photovoltaic Windows Enabled by Reversible Photothermal Complex Dissociation from Methylammonium Lead Iodide, Nat. Commun., 2017, 8(1), 1722, DOI:10.1038/s41467-017-01842-4.
- B. A. Rosales, L. E. Mundt, L. T. Schelhas and L. M. Wheeler, Reversible Methanolation of Metal Halide Perovskites, J. Am. Chem. Soc., 2022, 144(2), 667–672, DOI:10.1021/jacs.1c10942.
- J. L. Surel, E. V. Cutlip, J. R. Mandeville and J. A. Christians, Switchable Color Semiconductors: Methylamine Intercalation, Deintercalation, and Retention in Two-Dimensional Halide Perovskites, ACS Appl. Energy Mater., 2022, 5(10), 12029–12038, DOI:10.1021/acsaem.2c01352.
- B. A. Rosales, L. E. Mundt, T. G. Allen, D. T. Moore, K. J. Prince, C. A. Wolden, G. Rumbles, L. T. Schelhas and L. M. Wheeler, Reversible Multicolor Chromism in Layered Formamidinium Metal Halide Perovskites, Nat. Commun., 2020, 11(1), 1–12, DOI:10.1038/s41467-020-19009-z.
- A. A. Zhumekenov, M. I. Saidaminov, O. F. Mohammed and O. M. Bakr, Stimuli-Responsive Switchable Halide Perovskites: Taking Advantage of Instability, Joule, 2021, 5(8), 2027–2046, DOI:10.1016/j.joule.2021.07.008.
- Z. Li and Y. Yin, Stimuli-Responsive Optical Nanomaterials, Adv. Mater., 2019, 31(15), 1807061, DOI:10.1002/adma.201807061.
- Y. Zhang, Z. Wang, S. Hu, P. Yan, H. Li and C. Sheng, Robust and Swiftly Reversible Thermochromic Behavior of a 2D Perovskite of (C6H4(CH2NH3)2)(CH3NH3)[Pb2I7] for Smart Window and Photovoltaic Smart Window Applications, ACS Appl. Mater. Interfaces, 2021, 13(10), 12042–12048, DOI:10.1021/acsami.1c00163.
- S. Cheng and H. Zhong, What Happens When Halide Perovskites Meet with Water?, J. Phys. Chem. Lett., 2022, 13(10), 2281–2290, DOI:10.1021/acs.jpclett.2c00166.
- Z. Lin, Y. Zhang, M. Gao, J. A. Steele, S. Louisia, S. Yu, L. N. Quan, C.-K. Lin, D. T. Limmer and P. Yang, Kinetics of Moisture-Induced Phase Transformation in Inorganic Halide Perovskite, Matter, 2021, 4(7), 2392–2402, DOI:10.1016/j.matt.2021.04.023.
- J. A. Christians, J. Outen, R. M. Campagna, Z. R. Wylie and P. Ruffolo, Passivating Surface Iodide Defects Slows the CsPbI3 Phase Transformation, in 2022 IEEE 49th Photovoltaics Specialists Conference (PVSC), 2022, p. 0283, DOI:10.1109/PVSC48317.2022.9938665.
- R. E. Beal, D. J. Slotcavage, T. Leijtens, A. R. Bowring, R. A. Belisle, W. H. Nguyen, G. F. Burkhard, E. T. Hoke and M. D. McGehee, Cesium Lead Halide Perovskites with Improved Stability for Tandem Solar Cells, J. Phys. Chem. Lett., 2016, 7(5), 746–751, DOI:10.1021/acs.jpclett.6b00002.
- G. E. Eperon, V. M. Burlakov, A. Goriely and H. J. Snaith, Neutral Color Semitransparent Microstructured Perovskite Solar Cells, ACS Nano, 2014, 8(1), 591–598, DOI:10.1021/nn4052309.
- B. A. Rosales, J. Kim, V. M. Wheeler, L. E. Crowe, K. J. Prince, M. Mirzokarimov, T. Daligault, A. Duell, C. A. Wolden, L. T. Schelhas and L. M. Wheeler, Thermochromic Halide Perovskite Windows with Ideal Transition Temperatures, Adv. Energy Mater., 2023, 13, 2203331, DOI:10.1002/aenm.202203331.
- K. Zhang, C. Qin, X. Yang, A. Islam, S. Zhang, H. Chen and L. Han, High-Performance, Transparent, Dye-Sensitized Solar Cells for See-Through Photovoltaic Windows, Adv. Energy Mater., 2014, 4(11), 1301966, DOI:10.1002/aenm.201301966.
- W. Naim, V. Novelli, I. Nikolinakos, N. Barbero, I. Dzeba, F. Grifoni, Y. Ren, T. Alnasser, A. Velardo, R. Borrelli, S. Haacke, S. M. Zakeeruddin, M. Graetzel, C. Barolo and F. Sauvage, Transparent and Colorless Dye-Sensitized Solar Cells Exceeding 75% Average Visible Transmittance, JACS Au, 2021, 1(4), 409–426, DOI:10.1021/jacsau.1c00045.
- A. M. Kendhale, A. P. H. J. Schenning and M. G. Debije, Superior Alignment of Multi-Chromophoric Perylenebisimides in Nematic Liquid Crystals and Their Application in Switchable Optical Waveguides, J. Mater. Chem. A, 2012, 1(2), 229–232, 10.1039/C2TA00400C.
- S. Y. Park, J.-S. Park, B. J. Kim, H. Lee, A. Walsh, K. Zhu, D. H. Kim and H. S. Jung, Sustainable Lead Management in Halide Perovskite Solar Cells, Nat. Sustain., 2020, 3(12), 1044–1051, DOI:10.1038/s41893-020-0586-6.
- F. Schmidt, L. Ledermann, A. Schäffer, H. J. Snaith and M. Lenz, Rapid Sequestration of Perovskite Solar Cell-Derived Lead in Soil, J. Hazard. Mater., 2022, 436, 128995, DOI:10.1016/j.jhazmat.2022.128995.
- J. M. Frost, K. T. Butler, F. Brivio, C. H. Hendon, M. Van Schilfgaarde and A. Walsh, Atomistic Origins of High-Performance in Hybrid Halide Perovskite Solar Cells, Nano Lett., 2014, 14(5), 6, DOI:10.1021/nl500390f.
- J. Huang, S. Tan, P. D. Lund and H. Zhou, Impact of H 2 O on Organic–Inorganic Hybrid Perovskite Solar Cells, Energy Environ. Sci., 2017, 10(11), 2284–2311, 10.1039/C7EE01674C.
- A. Hazarika, Q. Zhao, E. A. Gaulding, J. A. Christians, B. Dou, A. R. Marshall, T. Moot, J. J. Berry, J. C. Johnson and J. M. Luther, Perovskite Quantum Dot Photovoltaic Materials beyond the Reach of Thin Films: Full-Range Tuning of A-Site Cation Composition, ACS Nano, 2018, 12(10), 10327–10337, DOI:10.1021/acsnano.8b05555.
- C. Yang, W. Sheng, M. Moemeni, M. Bates, C. K. Herrera, B. Borhan and R. R. Lunt, Ultraviolet and Near-Infrared Dual-Band Selective-Harvesting Transparent Luminescent Solar Concentrators, Adv. Energy Mater., 2021, 11, 2003581, DOI:10.1002/aenm.202003581.
| This journal is © The Royal Society of Chemistry 2023 |



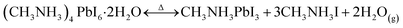
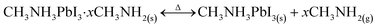
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000+
000+