Design of hydrogel-based wearable EEG electrodes for medical applications
Ju-Chun
Hsieh†
 a,
Yang
Li†
a,
Yang
Li†
 b,
Huiqian
Wang
c,
Matt
Perz
a,
Qiong
Tang
d,
Kai Wing Kevin
Tang
a,
Ilya
Pyatnitskiy
b,
Huiqian
Wang
c,
Matt
Perz
a,
Qiong
Tang
d,
Kai Wing Kevin
Tang
a,
Ilya
Pyatnitskiy
 a,
Raymond
Reyes
a,
Hong
Ding
a and
Huiliang
Wang
a,
Raymond
Reyes
a,
Hong
Ding
a and
Huiliang
Wang
 *a
*a
aDepartment of Biomedical Engineering, The University of Texas at Austin, Austin, TX 78712, USA. E-mail: evanwang@utexas.edu
bDepartment of Chemical Engineering, Polytechnique Montréal, Montréal, Québec H3C3J7, Canada
cDepartment of Mathematics, The University of Texas at Austin, Austin, TX 78712, USA
dDepartment of Aerospace Engineering and Engineering Mechanics, The University of Texas at Austin, Austin, TX 78712, USA
First published on 9th June 2022
Abstract
The electroencephalogram (EEG) is considered to be a promising method for studying brain disorders. Because of its non-invasive nature, subjects take a lower risk compared to some other invasive methods, while the systems record the brain signal. With the technological advancement of neural and material engineering, we are in the process of achieving continuous monitoring of neural activity through wearable EEG. In this article, we first give a brief introduction to EEG bands, circuits, wired/wireless EEG systems, and analysis algorithms. Then, we review the most recent advances in the interfaces used for EEG recordings, focusing on hydrogel-based EEG electrodes. Specifically, the advances for important figures of merit for EEG electrodes are reviewed. Finally, we summarize the potential medical application of wearable EEG systems.
1. Introduction
1.1 Overview of EEG
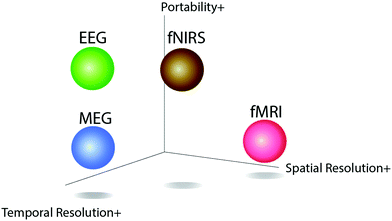
The electroencephalogram (EEG) is a widely used method for monitoring an individual's brain activity. With electrodes that are highly sensitive to electrical activity, the electrical potential of neuron cells that carries physiological information is recorded. The electrical action potential, the so-called neural spike, is the basic unit of all kinds of brain neural activity, that is important for understanding and monitoring brain functions and neurological diseases.To understand the information on brain structure and neural activity, the four main measures are Functional Magnetic Resonance Imaging (fMRI), Magnetoencephalography (MEG), Functional Near-Infrared Spectroscopy (fNIRS), and EEG. The fMRI or fNIRS is a neuroimaging system that measures the changes in blood flow to determine brain function and neural activity. The blood flow is correlated with the electrical neuron activity due to neurovascular coupling. The strength of fMRI and fNIRS are their spatial resolution but lower temporal resolution than EEG due to the direct measurement of neural activity. MEG, on the other hand, maps brain activity by magnetic fields generated from electrical currents occurring in the brain. In comparison to fMRI, MEG could have improved spatial and temporal resolution but both fMRI and MEG are limited by the immobility of the instruments: these equipments are too large to be used as an ambulatory measuring tool. This is hard for user cases in hospitals, not to mention the chance of transferring these techs to home-use health monitoring systems. EEG, on the other hand, has essentially the same signal source as MEG but it is more capable of being a movable measuring instrument through direct measurement of electrical neural activity. Thus, EEG can be a promising method to approach neurological applications, especially considering its high temporal resolution, high versatility, and cost-efficiency. Fig. 1 shows the comparison between these measures of brain activity for their portability, spatial resolution, and temporal resolution.
EEG has been a powerful and popular tool for brain-computer interface (BCI) and event-related potentials (ERPs) research ever since these fields thrived. In recent years, thanks to the blossoming of artificial intelligence and big data in this era and the dramatic evolution of microelectronics as well, EEG applications have expanded from research-oriented tools to more practical use. EEG has become one of the main evaluation tools of brain disease including sleep disorders1–6 and epileptic seizures7–11 clinically, potential applications in stroke recovery12–15 and head trauma.16–19 To date, developers have further extended EEG applications’ reach outside of medical use to other fields such as sports training and condition monitoring for athletes,20–22 robot controls,23–26 evaluation of driver vigilance.27,28 Even though these applied technologies have not yet developed to a very mature stage, it shows that more advanced EEG applications are one of the most promising technologies soon.
Conventionally, wired EEG systems are still a mainstay approach to conduct EEG monitoring, both in laboratories or clinics. The current EEG system setup and configuration are elaborated in the following section. Since the wired EEG recording requires numerous wiring connections, the frequent cable disconnections from both ends of electrodes and instrumentation can be extremely frustrating. Furthermore, a wired system must increase the total volume of the system that is directly connected to a subject. Lastly, a conventional EEG system needs a long-winded wet electrode preparation by a trained specialist to ensure the best signal quality of the measurement. As a result, the development of wireless and wearable systems seems to be a reasonable solution to this current situation. A wireless and wearable system also enables experiments and clinical applications that a wired one can never do, for instance, some motion-rich experiments or long-term and continuous disease monitoring while not taking patients out from their daily lives to a hospital.
Bearing all that in mind, developing a wireless and wearable EEG system is a favorable and promising orientation in this field. Prior to this review, numerous independent studies have been done to approach this aim from various angles, which are the driving force of this article. In particular, we focus on the development of wearable EEG hydrogel electrodes that enables advanced wearable EEG recording for medical applications. We start with the conventional setup of EEG systems in medical applications, including the meaning of different EEG bands, circuit setup for EEG system, algorithm, and classification. Next, in the “Wearable, hydrogel-based EEG electrode system: a cutting-edge solution”, we discuss the important part of data acquisition technology – the electrodes, including over the different types of electrodes including hydrogel electrodes, and figure of merits for designing the electrodes. We will then report the developed wearable EEGs that target the most widely applied brain-related diseases and disorders. For example, BCIs,29–31 epilepsy diagnosis,7–11,32,33 sleep disorder diagnosis,1–6 and mental health evaluation.34,35
1.2 Introduction to EEG bands
EEG is the language of the brain, telling the stories of our bodies with electrical signals. For a long time, raw EEG data has been depicted as consisting of several different frequency bands, which are Delta (0.1 to 4 Hz), Theta (4 to 8 Hz), Alpha (8 to 13 Hz), Beta (13 to 30 Hz), and Gamma (greater than 30 Hz). The delta-band is the dominant frequency band during sleep. It helps humans to decrease their awareness toward the outside world, allowing us to enter a deeply relaxed state and enabling us to access the unconscious corner in our brain. The delta-band generally declines during our time of focusing and concentrating states. A disease that is largely related to this band is called Attention Deficit Disorder (ADD). Patients with ADD suffer from focusing because of the increase of delta-band amplitude instead of decrease. Theta-band is more related to the subconsciousness state of the brain and hence often related to the research of memory and emotion36,37 and sleep monitoring. The alpha-band is the bridge between consciousness and subconsciousness, working as a critical role in the coalescence of brain activity in different frequencies. Alpha-band plays an active role in cognitive processes of brain activity, such as knowledge access and information processing.38 Beta activity has a long history of relation to Parkinson's disease. The neurodegenerative nature of Parkinson's disease is associated with the neuronal activity in the beta-band. In addition to observing Parkinson's disease by patients’ motor impairments, the decreased dopamine release and increased beta-band oscillations are also important indicators. High-frequency Gamma band oscillation in EEG is thought to be a diagnostic biomarker in Alzheimer's disease (AD) and mild cognitive impairment (MCI). The increased gamma-band power has been reported in AD and MCI patients compared to healthy control subjects.39 To obtain these biomarkers that are hidden in the brainwaves, the EEG measuring system has already been developed for decades and is still being improved and innovative. Next, we introduce the conventional wired measurement system that has been used for a long time in clinics, and the more recent EEG sensing platform, the wireless systems.1.3 Circuit setup for EEG measurements
An EEG circuit measures the electrical potentials caused by postsynaptic potentials generated by neurons.40,41 Two electrodes placed on the surface of the skin function together in a manner similar to that of a voltmeter, measuring the strength of the dipole.40,41 Naturally, the potential encounters several sources of impedance along its journey from the cortex to a processor, with most impedance coming from the outermost layer of the skin, the stratum corneum, in the case of most EEG circuit designs. Contact impedance between EEG electrodes must be greater than 1 kΩ, lest a shortcut is created between them, and below 10 kΩ to ensure a signal can be acquired.40 The cables connecting the electrodes to the rest of the circuit are often twisted and ultimately blended together to improve electromagnetic compatibility and shielded with a driven guard to reduce current leakage. Of course, a full EEG system could include hundreds of electrodes, so groups of electrodes may feed into a multiplexer, allowing for a selection of inputs, reducing demands on other circuit components.Ideally, as much of the EEG circuit is housed within a metal container to minimize electronic noise. The use of differential measurements helps to eliminate noise from the signal that would otherwise be caused by electromagnetic interference. A differential amplifier with a common-mode rejection ratio greater than 80 dB is frequently employed to eliminate any signals that appear in both electrodes. Consequently, the inclusion of a differential amplifier, typically an instrumentation amplifier, in an EEG circuit serves to increase the strength of signals of interest while removing noise.42 An EEG system normally will have a set of amplifiers and band-pass filters in the analog front-end circuit for providing robust and amplified EEG signals to the following steps in the data preprocessing.43–45 Data acquired via the skin electrode(s) will normally first be amplified by analog amplifiers, then processed with a low-pass filter (LPF) and high-pass filter (HPF) to filter out the unwanted signal. In tandem, these filters are used to create a specific range of possible frequencies for data, typically between 0.1 Hz and 100 Hz, but no narrower than 0.5 Hz to 70 Hz. This upper bound is chosen to eliminate the movement artifacts caused by muscles, which normally vary above 100 Hz. The lower bound is chosen to remove artifacts caused by the accumulation of sweat and any minor electrode movements. With the raw data sufficiently attenuated, the data after filtration must now pass through an analog-digital converter (ADC). If the system has a wireless transmission feature, a wireless transmitter module such as Bluetooth is needed for transmitting the collected data to the processors. If the data is in alternating current, an ADC with a 10–12 bit digital resolution may suffice, though a direct current circuit may necessitate a 20-bit digital resolution. The digital signal can be delivered to a processor for analysis such as a laptop or a smartphone in more recent devices.
1.4 Wired and wireless EEG systems
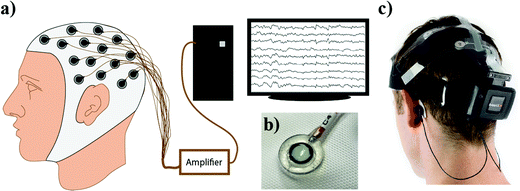
The conventional EEG recording systems in clinical settings are traditionally composed of an EEG headset with metal or Ag/AgCl (silver/silver chloride) electrodes on the corresponding channels. The number of channels on a multichannel EEG recording system can range from 8 to 256 or more (Fig. 2(a)). To collect the EEG signals, the conductive gel will be filled in the space between the scalp and the electrode46 (Fig. 2(b)). Then, the electrodes are connected to the circuit board with metal wires. These wire connections can be complicated and messy, and the entire system is far from portable. From wired and cumbersome systems to wireless and portable one, wireless systems allow EEG signals to be acquired with a further shortened preparation time and in a much easier way compared to the traditional system. To date, there are quite a few wireless EEG monitoring systems on the market such as B-Alert X24 and Mindwave, etc. As a multi-channel EEG system, the configuration of a wireless system is simpler than the conventional system. Table 1 shows the advantages and disadvantages of wired and wireless systems (Fig. 2(c)). While Mindwave is using dry electrodes for data acquisition, B-Alert X24 is using wet electrodes instead. This is because of the different targeting consumers, while B-Alert is more like a medical/research-grade instrument, Mindwave is targeting daily use or education and entertainment. Owing to the difference in electrodes, the two systems have contrasting setup times. As expected, only 3 minutes to set up for a dry-electrode-use system (Mindwave), and >20 minutes to set up for a wet-electrode-use system (B-Alert X-24).47 Even though a wireless system can largely save time for setting up all the wires, we generally still need gels for the electrodes to operate, which is both very time-consuming and does not last for a long time. That brings us to the next topic of this review, cutting-edge technology for the electrodes.| Advantage(s) | Disadvantage(s) | |
|---|---|---|
| Wired systems | • Connecting to the power system instead of a battery, long-term EEG monitoring can be achieved. | • Complicated wiring. |
| • Data security is better than wireless systems. | • Systems can be sometimes bulky and cumbersome. | |
| • Longer setup time. | ||
| • Not able to be a portable daily-use device but a medical center instrument, which is not conducive to its widespread development. | ||
| Wireless systems | • More portable and compact size. | • More external interferences. |
| • No wire connection issues. | • The battery capacity and power consumption can largely affect the recording time. | |
| • Less noise from the wiring. | • Data security can be an issue. | |
| • Less setup time. | ||
| • Has a great chance to monitor/record brain activity without being in the medical center (less interruption to daily life). |
1.5 Data processing and classification
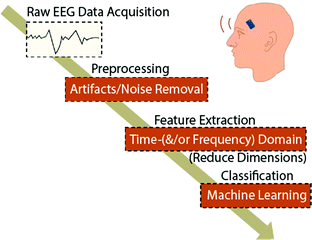
EEG signals are often subjected to a variety of interferences resulting in a heavily noisy bioelectrical signal. Preprocessing steps are necessary to isolate the EEG signal from these noises in order to achieve the most ideal information for post-processing in many applications. However, the removal of all noise in the measurement is impossible. Thus, the best we can do is to remove the major noise components that jeopardize the following steps of signal processing via feature extraction and classification.Typically, the process of EEG-based algorithms on disease prediction and monitoring can be represented in Fig. 3. When the raw EEG data is acquired from a subject, some common noise and artifacts are induced. Fortunately, a well-established foundation of prior studies has already developed a multitude of ways to remove artifacts in EEG data.48–50 While all these preprocessing methods and toolboxes are well-built and perform greatly in removing artifacts from EEG data, independent component analysis (ICA) has shown to be a common solution to noise/artifact removal. Besides the analysis methods without prior knowledge of a signal such as ICA, the prior-knowledge-based signal decomposition methods are also frequently used in EEG artifacts removal. By having prior knowledge and/or assumptions about the signal of interest and artifacts through physiological understanding in previous studies, the artifacts can be extracted by the design of the signal separation function of an algorithm. For example, wavelet decomposition is regarded as an ideal approach for motion artifact and ocular artifact suppression in EEG.51–53 With preprocessing being vital in noise removal and signal of interest isolation, postprocessing follows subsequently in extracting meaningful data dependent on the aim of the application. Therefore, with feature extraction being a basis in determining feature vector from a regular one in EEG signal analysis, the methods can be mostly, if not all, classified into four categories: (1) non-linear methods,54 (2) time-domain methods,55 (3) frequency domain methods56 and (4) time-frequency domain methods.56–58
After feature extraction, diverse EEG classification algorithms will be applied for different medical/clinical applications of EEG systems. All classifiers fall under the category of supervised and unsupervised learning. In supervised learning, such as support vector machines (SVM),59–62 decision tree (DT),61,63 random forest (RF),64,65 and K-nearest neighbor (KNN),66 the input data is provided along with labelled output data for training the classifier in making accurate predictions. Contrarily, unsupervised learning, such as neural network (NN),67–70 involves only the input data provided for training the classifier via the innate differences in feature vectors extracted from the input.
2. Wearable, hydrogel-based EEG electrode system: a cutting-edge solution
2.1 Categories of EEG electrodes
In order to obtain a high-quality, continuous recording of EEG signals over time, the materials of electrodes are the first priority. Generally, EEG electrodes need to provide good and constant contact with the skin or scalp to lower the impedance. In this section, different types of EEG electrodes are introduced briefly.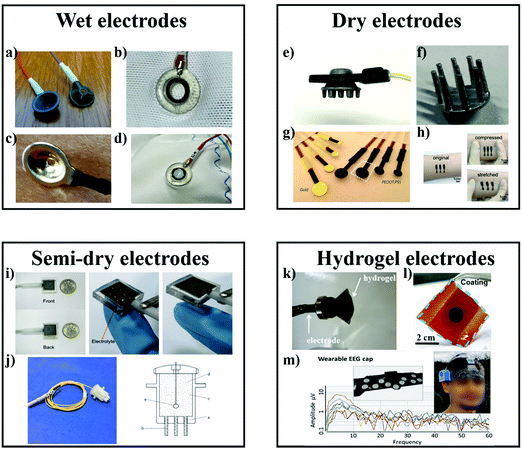 | ||
| Fig. 4 Different types of EEG electrodes. (a)–(d) Wet electrodes: (a) disposable and (b) reusable silver/silver chloride (Ag/AgCl) passive wet EEG electrodes.75 (c) Gold cup wet electrode.73 (d) Close-up of an Ag/AgCl electrode contacting skin via a conductive gel. (e)–(h) Dry electrodes: (e) a dry and through-hair EEG sensor. (f) 3D printed EEG electrode coated with silver paint.75 (g) Photograph of dry PEDOT:PSS electrodes with different diameters.76 (h) The conformal contact at the skin-rGO electrode interface during compression and stretch.77 (i) and (j) Semi-dry electrodes: (i) the passive Ti electrode as well as the electrode under pressure and after the pressure removal.78 (j) Photo of a single semi-dry electrode and schematic diagram of the semi-dry electrode prototype, including flexible electrode tips (a), porous ceramic wicks (b), a built-in reservoir (c), 3.5% saline solution (d), and sintered silver/silver chloride (Ag/AgCl) electrode (e).79 (k)–(m) Hydrogel electrodes: (k) alginate-based hydrogel electrodes.80 (l) Photograph of a hydrogel coating on non-woven fabric (commercial bioelectrode with electrolyte hydrogel removed).81 (m) Headband for signal acquisition with hydrogel electrodes integrated on the textile with printed silver interconnect on the other side of the textile and EEG signals acquired by the headband during sleep.82 | ||
Wet electrodes have previously been adopted to study the overnight sleep EEG signals, while the replacement of the electrodes after each measurement is indispensable, as the dry-out of conducting gel will significantly hamper the recording of high-quality EEG signals.83 In addition, conducting gel may bring some allergic problem, as skin irritation or rashes sometimes happens to the patients after wearing the wet electrodes for a few hours.84 As a result, wet electrodes are not suitable for prolonged EEG monitoring for wearable devices due to the high cost of electrodes replacements and issues related to stability and biocompatibility.
Up to now, wet electrodes are still considered as a benchmark of quality to which novel electrodes designs are compared. However, the inconvenience of their application has driven research into alternative electrode designs.85
Other conducting materials, such as gold and microporous titanium,94–96 conducting polymer poly(3,4-ethylenedioxythiophene) doped with poly(styrene sulfonate) (PEDOT:PSS),97–99 carbon nanotubes (CNTs),100 and graphene,101–103 have also been investigated for their possible use in EEG electrodes. For example, Leleux et al. fabricated a flexible EEG electrode via depositing PEDOT:PSS on a polyimide substrate and obtain a signal-to-noise ratio (SNR) of 24.4 dB when measuring the somatosensory evoked potentials (Fig. 4(g)).76 Li et al. reported the ultrathin, conformable, and breathable reduced graphene oxide (rGO) electrodes prepared by simultaneous room temperature reduction and patterning process, and achieve an EEG recording with a high fidelity due to the conformal contact with skin (Fig. 4(h)).77 Yang et al. developed an ultrathin (150 nm), ultralightweight (0.24 mg cm−2), self-adhesive, and transparent Ag nanowire/thermoplastic elastomer electrode, which exhibited a maximum SNR of 51 dB over the wide frequency range of 0–22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Hz.104 Unfortunately, these innovations have yet to consistently match the quality of wet electrodes.
000 Hz.104 Unfortunately, these innovations have yet to consistently match the quality of wet electrodes.
2.2 Figure of merit in hydrogel EEG electrodes
In order to understand the advantages and disadvantages of different electrodes, the qualities of interest and the techniques used to measure them must be defined. For the EEG devices, especially for prolonged monitoring, the impedance, adhesion, stability as well as biocompatibility are considered as the most important figures of merit.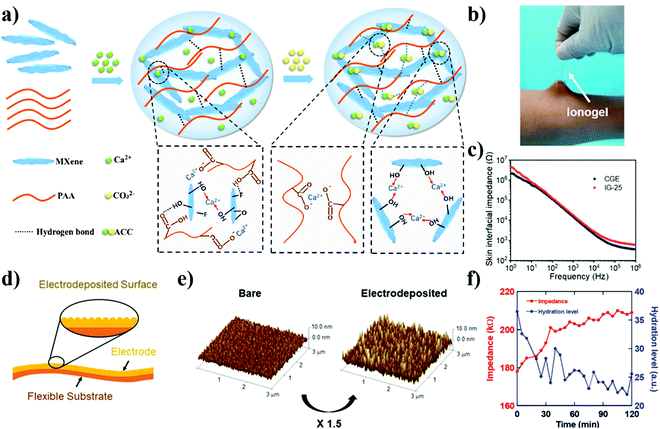 | ||
| Fig. 5 EEG electrodes with decreased impedance. (a) Schematic illustration of the fabrication of the MXene-PAA-ACC hydrogel.115 (b) The photos of ionogel electrode adhered to human skin. (c) Skin interfacial impedance of ionogel electrode IG-25 and commercial gel electrode.119 (d) Schematic of the proposed flexible skin electrode. A gold layer is electrodeposited to maximize the surface area. (e) Atomic force microscopy (AFM) images of bare gold (left) and electrodeposited gold (right) electrodes. The surface area was increased 1.5 times by the electrodeposition process.120 (f) The relationship between the skin impedance and the hydration level after application of skin lotion.121 | ||
Another method to lower the impedance relies on the incorporation of ions, either by soaking hydrogels in the electrolyte solution (two-step solvent exchange) or adding salts into the precursor of hydrogels during synthesis (one-pot synthesis). For instance, increasing the ionic strength by including ionic compounds like salt in that hydrogel electrode lower the impedance.122 To improve the ionic conductivity, Pan et al. introduced 5 wt% lithium chloride (LiCl) into the PAAm-alginate hydrogel.123 The resulting hydrogel had an intrinsic impedance of 270 Ω (the impedance of the hydrogel without LiCl is ≈MΩ at 1 Hz), which is similar to commercial electrodes of 210 Ω at 1 Hz. Besides the salts, ionic liquids or polymerized ionic liquids are often adopted to enhance the ionic strength in the electrodes. Yu et al. designed a high-performance and adhesive ionogel by one-step free radical polymerization (Fig. 5(b)).119 The obtained ionogel electrodes exhibited similar impedance and ECG recording performance as commercial electrodes and provided a continuous and stable ECG monitoring in the aquatic environment due to the strong adhesion to human skin (Fig. 5(c)).
In terms of EEG monitoring, it is worth noting that the outermost layer of the skin, the stratum corneum, is responsible for much of the impedance encountered at the electrode-skin interface in most electrode designs. As a result, skin contact impedance dominates the quality of EEG signals. Of course, skin contact impedance depends on certain factors of skin as well, including the presence of hair follicles and sweat glands, the patient's hydration, temperature, and external force.124–126
Electrodes with greater areas of contact with skin typically have a lower impedance.127,128 This greater surface area of contact manifests in the overall size of the electrode's face as well as its texture-a rougher surface affords a greater surface area. For example, Yun et al. increased the surface area of flexible polyimide substrate by 1.54 times via the electrodeposition of gold nanoparticles (Fig. 5(d) and (e)). Due to the improvement in the surface area, the optimized electrodes demonstrate a higher SNR in ECG and EMG compared to commercial wet Ag/AgCl electrodes.120 In addition, the surface moisture affects the skin-contact impedance significantly, as high water-ratio hydrogel electrodes can generally hydrate the skin, thus leading to a lower contact impedance. Matsukawa et al. studied the variation of the impedance of gold nanomesh electrodes under different hydration levels, showing that the measured skin impedance was negatively correlated with the hydration level (Fig. 5(f)).121 Similarly, ions or ionic species released from the electrodes can penetrate the pores of the skin, providing a more conductive skin and better EEG signals. Leleux et al. compared the performance of conformal electrodes made of Au and PEDOT:PSS in a dry state and in conjunction with an ionic liquid gel, discovering that the ionic liquid decreased impedance at the interface with human skin to levels that are similar to those of commercial electrodes (at 1 kHz).129 In addition, the ionic liquid gel did not dry out and the electrodes continued to show a low impedance over the course of 3 days, while the commercial electrode gave up after only 20 hours. However, high ion concentration inside the recording electrodes may cause skin irritation for patients.
Some preparation methods, such as abrading the skin, using a gel, or wiping the skin with ethanol, may be employed to help reduce the impedance of the skin-electrode interface by partial removal of the stratum corneum. Nevertheless, these methods will generally cause pain for the patients, which is not recommended for long-term monitoring. In the previous reports, additional treatments may make use of penetration enhancers like surfactants, terpenes, and Azones, to reduce the impedance of the skin by altering the hydration of stratum corneum or packing structure of the ordered lipids in the intercellular channels.130
The ways promoting adhesion could be categorized into three directions: (i) functional design of materials, (ii) biomimetic structural design of electrodes, (iii) post-treatment. The adhesion strengthened by these three ways is summarized in Table 2. The functional design aims at creating strong chemical cross-links between electrodes and skin. Among all bonds, covalent bonds,133 hydrogen bonds,134,135 and electrostatic interactions,136 are commonly used for the enhancement of on-skin adhesion. A covalent bond has quite high energy, while static covalent bonds are not irreversible, namely, it couldn’t reform once the bonds are broken. Dynamic covalent bonds could compensate for such weakness since it is able to reform by pH, light, and heat stimulus. Although a single hydrogen bond is weak, two or more polymer chains can form hydrogen-bonded complexes to strengthen adhesion.137 Yuk et al. proposed a dry double-sided tape (DST) by a combination of gelatin or chitosan and acrylic acid (AA) grafted with N-hydroxysuccinimide ester, which removes water from the surface by hydration and forms rapid and robust adhesion with the tissue surface within 5 seconds under pressure at around 1 kPa.138 Ji et al. copolymerized dopamine methacrylamide (DMA), AA, and methoxyethyl acrylate (MEA) monomers to obtain a random copolymer p(DMA-co-AA-co-MEA) (pDAM) (Fig. 6(a)).139 Due to the strong adhesion of dopamine-containing motif and ionic conductivity provided by AA, the electrophysiological signals recorded using these water-resistant electrodes were stable and insensitive to the impact of water flow.
| Fabrication method | Materials | Energy toughness (J m−2) | Adhesion (kPa) | Ref. |
|---|---|---|---|---|
| Functional design | DST | >710 | 120 (Normal) | 138 |
| Ca2+ doped PAAm-alginate hydrogel | >1000 | 140 | ||
| PAAm-alginate hydrogel | 1250–1500 | 141 | ||
| PAAm + PDMS | 866.9 | 133 | ||
| Biomimetic structural design | PAA | >750 | 134 | |
| PDMS | 10–50 | 45–70 | 142 | |
| PDMS | >120 | 143 | ||
| 108 (Normal),147 (Shear) | 144 | |||
| PDMS | 25–200 (Normal) | 145 | ||
| Polyunsaturated aldehyde (PUA) | ||||
| Post treatment | PUA | 210 (Shear) | 146 | |
| Polystyrene | 350–1050 | >1000 (Shear) | 147 | |
| Polypropylene (PP) | 148 |
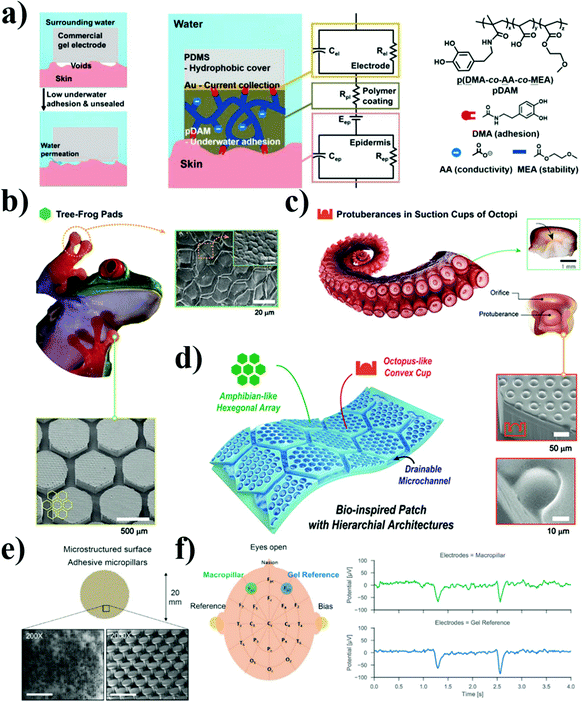 | ||
| Fig. 6 Possible methods to improve the adhesion of EEG electrodes. (a) Voids exist at the commercial gel electrode–skin interface due to skin surface structure, which allows water in and cause decreased adhesion. Schematic showing the water-resistant pDAM polymer coating bridging the Au/Polydimethylsiloxane (PDMS) electrode and skin, and the corresponding circuit. Chemical structures of the dopamine-containing ionic-conductive random copolymer pDAM.139 (b) Photograph of pads of a tree frog and scanning electron microscopy (SEM) images of hexagonal structures on its pads and the hierarchical structures (400 μm thickness, PDMS) of frog-inspired hexagonal microchannels (200 μm in width, 300 μm in height, and 600 μm spacing). (c) Photographic images of Octopus vulgaris tentacle and suction cup with protuberance and SEM images of the hierarchical structures with octopus-like convex structures (15 μm in radius and 18 in height) on hexagonal microchannels. (d) Schematic illustration of the amphibian and octopus-like hierarchical architectures.142 (e) Soft electrode with grasshopper inspired microstructured surface for improved dry adhesion to the skin (thickness = 100 μm). The scale bars are 200 μm (200×) and 20 μm (2000×). (f) The alpha waves disappear when the eyes are kept open. The peaks in the time signal correspond to typical eye blink effects.149 | ||
Biomimetic structural design is gaining more and more focus in recent years due to its wide application and universality for all materials.142,150 According to the functions of adhesives in different environments, such a design is divided into wet adhesives inspired by amphibians and marine creatures such as octopus,142,151 and dry adhesives from insects and lizards like beetles, flies, spiders, geckos, and anole.144,152,153 For wet adhesives, it has significant adhesive performance as a result of obvious suction force underwater and moisture environment.145 For example, Kim et al. designed adhesive skin patches with hexagonal micropatterns inspired by the hierarchical microchannel network in the toe pads of tree frogs and convex cup architectures in the suckers of octopi (Fig. 6(b)–(d)).142 The amphibian- and octopus-like adhesives possess enhanced pulling adhesion and omnidirectional peel resistance against various dynamic wet skins, hence enabling the stable monitoring of vital biosignals. As far as dry adhesives are concerned, the adhesion is enhanced through van der Waals interactions, and the adhesion strength is affected by the tilt angle and the aspect ratio. Stauffer et al. reported super-soft and self-adhesive microstructured electrodes inspired by grasshopper feet, and it could adhere repeatedly to the skin with a force of up to 1 kPa without further attachment even during strong movement or deformation of the skin (Fig. 6(e)).149 This electrode could obtain excellent alpha activity signals in the EEG recording from the back of the head through dense hair (Fig. 6(f)).
Post-treatment could be classified into two types: chemical modification and plasma treatment. Chemical modification of polymers is aiming to create new chemical groups or moieties that could optimize the polymer matrix to strengthen the adhesion.154 For instance, alkaline treatment is one of the most common chemical treatments of natural fiber polymer composites. Polymers are dipped into the sodium hydroxide solution for several hours to increase the adhesion by removing impurities like lignin, wax, or oil covering.155 Plasma treatment is another way to promote surface adhesion between two layers by adjusting the parameters like gas flow, pressure, and treatment time.148 Dirk et al. used different discharge of gases such as Ar, He, and N2 to etch, and found that the gas type and plasma conditions have to be adjusted on the polymer type to minimize the aging effects.156
Encapsulating the hydrogel in an elastomer like polyurethane or latex has been found to reduce dehydration and improve general mechanical stability.159 Liu et al. sandwich the PAAm-alginate hydrogel in the elastomer PDMS or Ecoflex and found that the water retention of the hydrogel was around 70% after 6 days still high enough to maintain its function (Fig. 7(a) and (b)).160 However, the inability to establish a strong bond between the elastomer coating and the hydrogel has prompted exploration into alternatives. As the ions of dissolved salts are known to alter the material properties of gels by interacting with the water molecules, a variety of salts (i.e. CaCl2, NaCl, LiCl, LiBr, et al.) have been explored as additives.161–163 Bai and colleagues have found that the use of greater salt concentrations and certain salt species such as LiCl, potassium acetate (KAc), and MgCl2 can extend hydrogel electrode lifespan to several days.164 The best water retention was observed for 12 M LiCl with a cumulative water loss of 11% after 5 days (rel. humidity of environment 20%) (Fig. 7(c)). The higher ionic hydration degree of LiCl enables stronger bond strength between cation/anion-water molecule pairs and more bonded water molecules, resulting in more difficulty of water molecules evaporation and better water retention capacity (Fig. 7(d)).
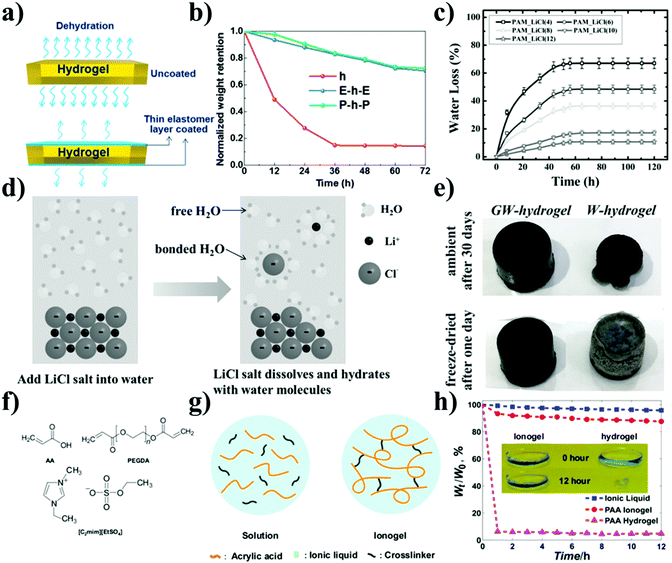 | ||
| Fig. 7 Possible methods to improve the stability of EEG electrodes. (a) Schematic diagram of the antidehydration of stacked elastomer/hydrogel. (b) Normalized weight retention of the uncoated hydrogel, Ecoflex–hydrogel–Ecoflex (E-h-E), and PDMS–hydrogel–PDMS (P-h-P) kept at a dry environment with a humidity of 26% at 30 °C.160 (c) Evolution of water loss with time for hydrogel with added LiCl kept in the chamber at 25 °C and relative humidity of 30%. (d) Schematic of the hydration of LiCl in water.164 (e) Comparison of GW-hydrogels and hydrogels after being placed in air for 30 d or after 1 d of freezing dry.165 (f) Chemical structure of the hydrogel precursor AA, the crosslinker poly(ethylene glycol) diacrylate (PEGDA), and the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate. (g) The schematic image of the matrix of the ionogel. (h) Change of weight of the ionic liquid, ionogel and hydrogel in a thermostatic chamber at 100 °C. Inset: Photos of the ionogel, and hydrogel before and after being in the thermostatic chamber for 12 h.166 | ||
Water loss was also found to be affected by atmospheric humidity.167 The fragility and temperature sensitivity of these designs can be mitigated by instead employing an organohydrogel, essentially a hydrogel but formed with a binary solvent consisting of an organic solvent, such as ethylene glycol or glycerol, mixed with water.168 The inclusion of an organic solvent confers a greater mechanical strength and a greater temperature resistance to the material. Han et al. obtained mussel-inspired glycerol–water hydrogel (GW-hydrogel) with CNTs as conducting nanofillers, via a gelation process in glycerol–water binary-solvent system.165 The GW-hydrogel exhibited no performance degradation after prolonged storage in normal condition (25 °C) for 30 days (Fig. 7(e)). Similarly, via the exchange of water, ionic liquids can be employed to form ionogels. By radical polymerization of AA in the ionic liquid 1-ethyl-3-methylimidazolium ethylsulfate, Chen et al. synthesized a transparent, stretchable ionogel that might have promise in EEG applications (Fig. 7(f) and (g)).166 Due to the non-volatility, the ionic liquid and ionogel show much less weight loss and better stability compared to the hydrogels after being in the thermostatic chamber for 12 h (Fig. 7(h)).
All of these solutions have been relatively successful in their endeavor to retain the benefits inherent to hydrogel's flexibility while improving upon its stability. However, further testing is needed to identify if one stands out as clearly superior for the purposes of EEG signal acquisition.169
However, those designs that rely on breaking the stratum corneum present the risk of damaging the skin or causing pain if designed or applied incorrectly. Pogo probes have been employed to ensure that probes remain in contact without harm, though they can still cause discomfort.172 Unfortunately, some metal electrode designs prevent water vapor from leaving the skin, causing irritation and inflammation.121 Gas-permeable nanomeshes have been found to not cause these symptoms by allowing the patient's skin to breathe freely.173 With this improvement, metal dry electrode designs represent an unequivocal improvement over wet electrodes.
While hydrogel-based designs offer a method to circumvent the need for the conductive gels used in wet electrodes, they offer advantages in other aspects as well. Naturally, hydrogel electrodes offer greater pliability, helping them stay in contact with the skin for longer periods and provide more conduction and improved comfort, as it affords more motion for patients.174 Furthermore, to improve the biocompatibility, many biocompatible polymers (i.e., polyvinyl alcohol, polyethylene glycol, PAA, chitosan, collagen, etc.) have been exploited for either hydrogel-based on-skin applications, causing only mild and even no irritation no reaction at all in either in vivo or in vitro testing, so it is unlikely that major issues will be encountered in using them.175–181 Unfortunately, all of the salt species discussed above have the potential to harm the human body. Although KAc and MgCl2 only present a threat of mild irritation, LiCl, the most promising species can severely burn skin, induce dehydration, and even damage kidneys.182–184 If salts are to be pursued as a method to improve hydrogel electrode conductivity, special care needs to be taken to either identify less dangerous species or ensure that salts in the hydrogel have no chance of coming into contact with the skin. Of course, the same also applies to any other additives being investigated for use in hydrogel electrodes. While ionogels have not been explored to a similar depth within the context of EEG electrode applications, biocompatible ionogels using compounds like SiO2 are available and might be employed in future research.185
2.3 Potential medical applications of hydrogel-based wearable EEG systems
With more and more advancements in hydrogel-based electrodes, the development of wearable EEG systems based on hydrogel-based electrodes has started to accelerate. Due to the advancement of electrode technology, the quality of the signal is improving over time for applications in wearable EEG systems. We report several potential medical applications that utilized single channel/multichannel EEG systems in the following sections. These applications would benefit from the development of advanced wearable hydrogel-based EEG electrodes. In particular, most of the EEG applications with wet electrodes mentioned need to recalibrate repeatedly in the hospital. The development of hydrogel electrodes will allow wearable EEG systems to be utilized for continuous, long-term EEG monitoring and diagnostics of disease at home.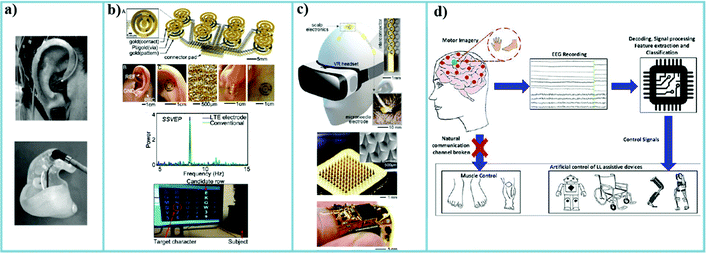 | ||
| Fig. 8 (a) Images of a subject wearing an earplug Ear-EEG.29 (b) The schematic diagram of the design of a soft and curved electrode system for an SSVEP-based BCI text speller system.30 (c) An illustration of a subject wearing the motor-imagery-based scalp EEG electronics with microneedle electrodes and a closeup of stretchable interconnectors.31 (d) Generic concept diagram of EEG-based BCI controlling assistive devices.25 | ||
Most recently, Mahmood et al. reported a wireless scalp electronic system for motor imagery(MI)-based BCI.31 With the imperceptible microneedle electrodes (Fig. 8(c)), the study showed an advantageous contact surface area and the reduced electrode impedance density, resulting in great classification accuracy. Combining with the convolutional neural network (CNN), the system realized real-time, continuous motor imagery-based BCI.
To operate a BCI, the user must create various brain activity patterns that the system will recognize through the classification of EEG signals and translate into specific commands. There are also different paradigms of EEG-based BCI such as P300, SSVEP, and MI. The MI paradigm is especially popular in the stroke rehabilitation field,186–188 enabling text-to-speech, robotic control, smart wheelchairs, BCI prosthetics, and more,25,189 as shown in Fig. 8(d).
According to a study from Lotte et al., in the case of performance, adaptive classification techniques should be favored over static techniques, both for classifiers and spatial filters.190 Adaptive classifiers are those whose parameters are routinely updated online based on the new data to deal with EEG non-stationarity which helps follow changes in EEG features over time and calibration of the BCI system to a specific user. All linear classifiers may be made adaptive, and adaptive SVM has been employed successfully and more frequently in the last decade.189
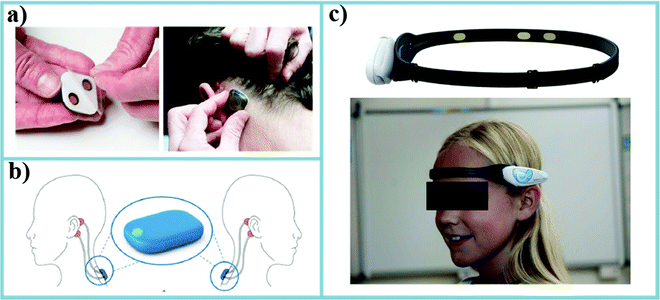 | ||
| Fig. 9 Some examples of the newest epilepsy monitoring devices. (a) The Epilog™ miniature wearable EEG sensor uses an adhesive and conductive hydrogel that serves as the interface between Epilog and the scalp when used below the hairline.191 (b) Four silver/silver chloride standard electrodes (in orange) are placed behind the ears of the patient and connected to Sensor Dot, which is attached to the upper back via an adhesive (in blue). An enlarged image of the SD is given in the circle.192 (c) The BrainLink EEG epileptic seizure monitoring system uses a headband to acquire bipolar signals from the dry electrodes on FP1 and F7.193 | ||
Alongside the wearable system, there have been tremendous efforts dedicated to developing generalizable machine learning (ML) algorithms to extract information of epileptic activity in EEG data as well. Recently, many ML methods are applied in the detection of seizures in epilepsy from EEG signals which may be more efficient and consistent than the diagnosis of human physicians. According to the literature, studies related to epilepsy EEG classification tend to use SVM the most, similar to the situation in automatic sleep stage classification (ASSC). Researchers choose other classifiers as well, including ANN, KNN, RF, Naïve-Bayesian (NB), DT, etc. In the study by Acharya et al. in 2012, the researchers presented a method for automatically detecting normal, pre-ictal, and ictal conditions in recorded EEG signals from the Bonn University dataset.55 They extracted four entropy-based non-linear features: ApEn, SampEn, S1, and S2 from full time-series EEG data and trained seven classifiers, including FSC (Fuzzy Sugeno Classifier), SVM, KNN, PNN (Probabilistic Neural Network), DT, GMM (Gaussian Mixture Model), and NB. The FSC classifier turned out to outperform the others, which presented the highest accuracy of 98.1%, highest sensitivity of 99.4%, and specificity of 100%. In the case of accuracy, SVM also gained a relatively high performance of 95.9%. We have summarized the classifications that used in epilepsy EEG in Table 3.
| Ref. | Dataset | Classification/accuracy |
|---|---|---|
| Lu et al.8 | Bonn University (five subsets denoted as Z, O, F, N and S) | SVM: 99.00% |
| Wang et al.9 | Bonn University (five subsets denoted as Z, O, F, N and S, each group 100 samples) | RF: 96.7% |
| Siuly and Li7 | Bonn University (five subsets denoted as Z, O, F, N and S, each group 100 samples) | SVM: 99.96–100% |
| NB: 99.24% | ||
| KNN: 98.82% | ||
| San-Segundo et al.10 | Bonn University (five subsets denoted as Z, O, F, N and S, each group 100 samples) | NN: 95.6–99.8% |
| Note: they also test on another multichannel dataset | ||
| Zhang et al.11 | Bonn University (five subsets denoted as Z, O, F, N and S, each group 100 samples) | SVM: 80.43% |
| Note: the samples were grouped as A, B, C, D, E. | ||
| Wen and Zhang32 | Bonn University (five subsets denoted as Z, O, F, N and S, each group 100 samples) | KNN: 97.3–98.0% (KNN) |
| DT: 89.7–96.0% (DT) | ||
| NN: 90.0–97.6% (NN) | ||
| NB: 82.3–96.7% | ||
| Note: the samples were grouped as A, B, C,D, E. | ||
| Buettner, Frick, and Rieg33 | Bonn University (five subsets denoted as Z, O, F, N and S, each group 100 samples) | RF: 99.0% |
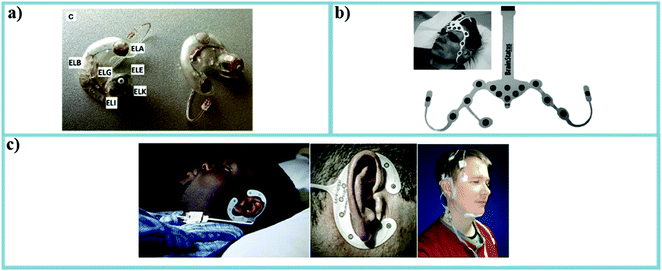 | ||
| Fig. 10 (a) Based on similar ideas, several works have been developing different types of in-ear EEG-based sleep sensors. This in-ear EEG was first developed by Mikkelsen et al. in 2015.200 (b) A forehead EEG-based sleep sensor.212 (c) A set of subjects wearing the recently developed flex-printed around-the-ear electrode array. The figure on the right was the gold standard PSG measurement setup.214 | ||
The system-level progress has benefited from the blossom of the ML algorithms, which boost the developments in the ASSC approach, which would minimize the time requirement of clinicians, increase the diagnostic precision in the classification of sleep stages and improve the treatment for sleep disorders.216 Here, we give a summary of the most recent and popular classification algorithms in Table 4.
| Ref. | Dataset | Sleep stages | Channel | Classification/accuracy |
|---|---|---|---|---|
| Tsinalis et al.2 | Sleep PSG 20 healthy subjects 20 hour recording per subject | Wake (W), REM (R), non-R stages 1–4 (N1, N2, N3), Movement | Fpz Cz | CNN: 82% |
| Yücelbaş et al.6 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758 epochs of 28 subjects (21 healthy subjects and 7) obstructive sleep apnea (OSA) patients 758 epochs of 28 subjects (21 healthy subjects and 7) obstructive sleep apnea (OSA) patients |
Wake (W), non-rapid eye movement (NREM) and rapid eye movement (REM) | Single channel | RF: 78.08% |
| ANN: 70.53% | ||||
| DT: 58.74% | ||||
| NB: 57.31% | ||||
| da Silveira, Kozakevicius, and Rodrigues4 | Sleep-EDF database two-night sleep analysis of 10 male and 10 female subjects | All five possible sleep stage arrangements (2–6 state) | Pz-Oz | RF: 90.5–97.3% |
| Sharma, Pachori, and Upadhyay3 | Sleep-EDF database eight objects (four healthy, four unhealthy) | All five possible sleep stage arrangements (2–6 state) | Pz-Oz | 2 and 6 class state: |
| RF: 98.02% and 89.74% | ||||
| DT: 96.67% and 85.85% | ||||
| KNN: 95.91% and 83.56% | ||||
| NB: 89.01% and 71.8% | ||||
| Qureshi and Vanichayobon5 | 25 subjects, 21 males and 4 females | W/S1/S2/S3/S4/REM | C4-A1 | RF: 97.73% |
| SVM: 93.28% | ||||
| Zhu, Li, and Wen1 | Sleep-EDF database 8 subjects | AWA, S1, S2, S3, S4, REM | Pz-Oz | SVM: |
| AWA-REM 96.1% | ||||
| AWA-REM 96.7% | ||||
| S1-REM 75.5% | ||||
| (S1–S2)-SWS 90.6% | ||||
| S1–S2 89.2% | ||||
| S3–S4 77.0% |
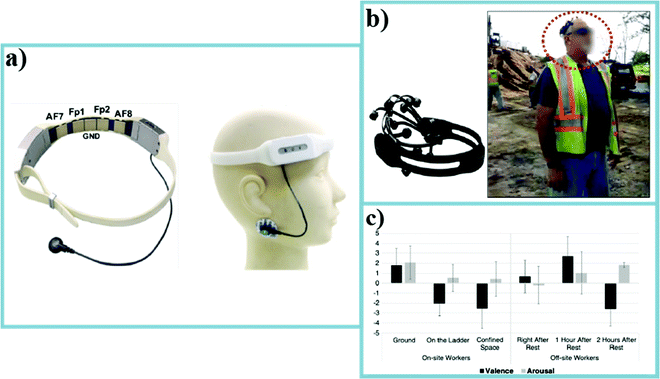 | ||
| Fig. 11 (a) The wearable forehead EEG device for identifying ketamine responses in treatment-resistant depression.35 (b) A image of a construction worker wearing a wireless EEG sensor for emotional state measuring.34 (c) The emotional state monitoring results in different work conditions for on-site and off-site workers after the different amount of working hours: valence and arousal.34 | ||
3. Conclusions and perspectives
Currently, the clinical EEG electrodes are still wet-electrode-based to achieve high-quality recording of EEG signals. However, to maintain prolonged monitoring with wearable EEG devices, the impedance, adhesion, stability and biocompatibility are all considered as very important figures of merits. In this review, an emerging type of biopotential electrodes based on hydrogels is discussed. The tunability of hydrogels allows the possibility for the replacement of clinically used Ag/AgCl wet electrodes. With more and more advancements in hydrogel-based electrodes, the hydrogel-based electrodes can be made more versatile according the clinical needs. Here, besides the advancement in the electrodes materials, we also report several different types of EEG electrode systems used for several important medical applications. Majority of these systems are still using standard wet or dry electrodes, but these applications would benefit from the development of advanced wearable hydrogel-based EEG electrodes. Although the challenges of applying hydrogel electrodes to clinical settings still remains, the promising future of the hydrogel-based electrode is undeniable. With the continuous development of the important parameters for hydrogel electrodes technology (including impedance, adhesion, stability and biocompatibility), we believe that hydrogel electrodes based wearable EEG systems will be widely utilized for continuous, long-term EEG monitoring and diagnostics of disease at home.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
H. W. acknowledges funding support from UT Austin Startup Funds, NIH Mentored Research Scientist Career Development Award (National Institute of Mental Health 1K01MH117490-01), Texas Health Catalyst Award and Robert A. Welsh Foundation Grant (No. F-2084-20210327).References
- G. Zhu, Y. Li and P. Wen, IEEE J. Biomed. Health Inform., 2014, 18, 1813–1821 Search PubMed.
- O. Tsinalis, P. M. Matthews, Y. Guo and S. Zafeiriou, arXiv preprint arXiv:1610.01683, 2016.
- R. Sharma, R. B. Pachori and A. Upadhyay, Neural. Comput. Appl., 2017, 28, 2959–2978 CrossRef.
- T. L. da Silveira, A. J. Kozakevicius and C. R. Rodrigues, Med. Biol. Eng. Comput., 2017, 55, 343–352 CrossRef PubMed.
- S. Qureshi and S. Vanichayobon, Evaluate different machine learning techniques for classifying sleep stages on single-channel EEG, In 2017 14th International Joint Conference on Computer Science and Software Engineering (JCSSE), IEEE, 2017, pp. 1–6 Search PubMed.
- Ş. Yücelbaş, C. Yücelbaş, G. Tezel, S. Özşen and Ş. Yosunkaya, Expert Syst. Appl., 2018, 102, 193–206 CrossRef.
- S. Siuly and Y. Li, Comput. Methods Programs Biomed., 2015, 119, 29–42 CrossRef PubMed.
- Y. Lu, Y. Ma, C. Chen and Y. Wang, Technology and Health Care, 2018, 26, 337–346 Search PubMed.
- X. Wang, G. Gong, N. Li and S. Qiu, Front. Hum. Neurosci., 2019, 13, 52 CrossRef PubMed.
- R. San-Segundo, M. Gil-Martín, L. F. D'Haro-Enríquez and J. M. Pardo, Computers in biology and medicine, 2019, 109, 148–158 CrossRef.
- Y. Zhang, J. Dong, J. Zhu and C. Wu, IEEE Access, 2019, 7, 127600 Search PubMed.
- F. Cincotti, F. Pichiorri, P. Aricò, F. Aloise, F. Leotta, F. de Vico Fallani, J. D. R. Millán, M. Molinari and D. Mattia, EEG-based Brain-Computer Interface to support post-stroke motor rehabilitation of the upper limb, In 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE, 2012, pp. 4112–4115 Search PubMed.
- K. K. Ang, K. S. G. Chua, K. S. Phua, C. Wang, Z. Y. Chin, C. W. K. Kuah, W. Low and C. Guan, Clin. EEG Neurosci., 2015, 46, 310–320 CrossRef PubMed.
- K. K. Ang and C. Guan, IEEE Trans. Neural Syst. Rehabilitation Eng., 2016, 25, 392–401 Search PubMed.
- R. Foong, K. K. Ang, C. Quek, C. Guan, K. S. Phua, C. W. K. Kuah, V. A. Deshmukh, L. H. L. Yam, D. K. Rajeswaran and N. Tang, IEEE Trans. Biomed. Eng., 2019, 67, 786–795 Search PubMed.
- J. D. Lewine, S. Plis, A. Ulloa, C. Williams, M. Spitz, J. Foley, K. Paulson, J. Davis, N. Bangera and T. Snyder, Clin. Neurophysiol., 2019, 36, 298–305 CrossRef PubMed.
- L. L. Popa, H. Dragos, C. Pantelemon, O. V. Rosu and S. Strilciuc, J Med Life, 2020, 13, 8 Search PubMed.
- Y. Kubota, H. Nakamoto, S. Egawa and T. Kawamata, J. Intensive Care, 2018, 6, 1–8 CrossRef.
- A. Sanz-García, M. Pérez-Romero, J. Pastor, R. G. Sola, L. Vega-Zelaya, F. Monasterio, C. Torrecilla, G. Vega, P. Pulido and G. J. Ortega, J. Neural Eng., 2018, 15, 066029 CrossRef PubMed.
- K. Thompson, K. Celoch, F. Pizzo, A. I. Fins and J. Tartar, NeuroSports, 2020, 1, 11 Search PubMed.
- D. R. Seshadri, R. T. Li, J. E. Voos, J. R. Rowbottom, C. M. Alfes, C. A. Zorman and C. K. Drummond, NPJ Digit. Med., 2019, 2, 1–16 CrossRef PubMed.
- E. Butkevičiūtė, L. Bikulčienė, T. Sidekerskienė, T. Blažauskas, R. Maskeliūnas, R. Damaševičius and W. Wei, IEEE Access, 2019, 7, 7206–7217 Search PubMed.
- T. F. Bastos-Filho, Introduction to Non-Invasive EEG-Based Brain-Computer Interfaces for Assistive Technologies, CRC Press, 2020 Search PubMed.
- J. H. Cho, J. H. Jeong, K. H. Shim, D. J. Kim and S. W. Lee, Classification of hand motions within EEG signals for non-invasive BCI-based robot hand control, In 2018 IEEE International Conference on Systems, Man, and Cybernetics (SMC), IEEE, 2018, pp. 515–518 Search PubMed.
- M. Tariq, P. M. Trivailo and M. Simic, Front. Hum. Neurosci., 2018, 312 CrossRef PubMed.
- A. N. Belkacem and A. Lakas, A Cooperative EEG-based BCI Control System for Robot–Drone Interaction, In 2021 International Wireless Communications and Mobile Computing (IWCMC), IEEE, 2021, pp. 297–302 Search PubMed.
- W.-L. Zheng, K. Gao, G. Li, W. Liu, C. Liu, J.-Q. Liu, G. Wang and B.-L. Lu, IEEE Trans. Intell. Transp. Syst., 2019, 21, 170–184 Search PubMed.
- G. Zhang and A. Etemad, IEEE Trans. Neural Syst. Rehabilitation Eng., 2021, 29, 1138–1149 Search PubMed.
- P. Kidmose, D. Looney, M. Ungstrup, M. L. Rank and D. P. Mandic, IEEE Trans. Biomed. Eng., 2013, 60, 2824–2830 Search PubMed.
- J. J. Norton, D. S. Lee, J. W. Lee, W. Lee, O. Kwon, P. Won, S.-Y. Jung, H. Cheng, J.-W. Jeong and A. Akce, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 3920–3925 CrossRef CAS PubMed.
- M. Mahmood, S. Kwon, H. Kim, Y. S. Kim, P. Siriaraya, J. Choi, B. Otkhmezuri, K. Kang, K. J. Yu and Y. C. Jang, Adv. Sci., 2021, 8, 2101129 CrossRef PubMed.
- T. Wen and Z. Zhang, Medicine, 2017, 96(19) DOI:10.1097/MD.0000000000006879.
- R. Buettner, J. Frick and T. Rieg, High-performance detection of epilepsy in seizure-free EEG recordings: A novel machine learning approach using very specific epileptic EEG sub-bands, In ICIS, 2019 Search PubMed.
- S. Hwang, H. Jebelli, B. Choi, M. Choi and S. Lee, J. Constr. Eng. Manag., 2018, 144, 04018050 CrossRef.
- Z. Cao, C.-T. Lin, W. Ding, M.-H. Chen, C.-T. Li and T.-P. Su, IEEE Trans. Biomed. Eng., 2018, 66, 1668–1679 Search PubMed.
- J. Zheng, R. F. Stevenson, B. A. Mander, L. Mnatsakanyan, F. P. Hsu, S. Vadera, R. T. Knight, M. A. Yassa and J. J. Lin, Neuron, 2019, 102, 887–898.e885 CrossRef CAS PubMed.
- T. Korotkova, A. Ponomarenko, C. K. Monaghan, S. L. Poulter, F. Cacucci, T. Wills, M. E. Hasselmo and C. Lever, Neurosci. Biobehav. Rev., 2018, 85, 65–80 CrossRef PubMed.
- W. Klimesch, Trends Cognit. Sci., 2012, 16, 606–617 CrossRef PubMed.
- J. Van Deursen, E. Vuurman, F. Verhey, V. van Kranen-Mastenbroek and W. Riedel, J. Neural Transm., 2008, 115, 1301–1311 CrossRef CAS PubMed.
- A. B. Usakli, Comput. Intell. Neurosci., 2010, 2010 DOI:10.1155/2010/630649.
- M. A. Lopez-Gordo, D. Sanchez-Morillo and F. P. Valle, Sensors, 2014, 14, 12847–12870 CrossRef CAS PubMed.
- C. S. Wang, J. Biomed. Biotechnol., 2012, 2012 DOI:10.1155/2012/274939.
- J. H. Hong, M. C. Liang, M. Y. Haung, T. H. Tsai, Q. Fang and S. Y. Lee, Analog front-end circuit with low-noise amplifier and high-pass sigma-delta modulator for an EEG or ECoG acquisition system, In International Symposium on Bioelectronics and Bioinformations, IEEE, 2011, pp. 17–20 Search PubMed.
- S. Patki, B. Grundlehner, T. Nakada and J. Penders, Low power wireless EEG headset for BCI applications, In International Conference on Human-Computer Interaction, Springer, Berlin, Heidelberg, 2011, pp. 481–490 Search PubMed.
- J. Xu, S. Mitra, A. Matsumoto, S. Patki, C. Van Hoof, K. A. Makinwa and R. F. Yazicioglu, IEEE, J. Solid State Circ., 2014, 49, 2005–2016 Search PubMed.
- A. J. Casson, Biomed. Eng. Lett., 2019, 9, 53–71 CrossRef PubMed.
- E. Ratti, S. Waninger, C. Berka, G. Ruffini and A. Verma, Front. Hum. Neurosci., 2017, 11, 398 CrossRef PubMed.
- A. Delorme and S. Makeig, J. Neurosci. Methods, 2004, 134, 9–21 CrossRef PubMed.
- L. J. Gabard-Durnam, A. S. Mendez Leal, C. L. Wilkinson and A. R. Levin, Front. Neurosci., 2018, 12, 97 CrossRef PubMed.
- A. Pedroni, A. Bahreini and N. Langer, NeuroImage, 2019, 200, 460–473 CrossRef PubMed.
- G. Inuso, F. La Foresta, N. Mammone and F. C. Morabito, Wavelet-ICA methodology for efficient artifact removal from Electroencephalographic recordings, In 2007 international joint conference on neural networks, IEEE, 2007, pp. 1524–1529 Search PubMed.
- P. S. Kumar, R. Arumuganathan, K. Sivakumar and C. Vimal, Int. J. Open Probl. Comput. Math., 2008, 1, 188–200 Search PubMed.
- F. C. Robertson, T. S. Douglas and E. M. Meintjes, IEEE Trans. Biomed. Eng., 2010, 57, 1377–1387 CAS.
- U. R. Acharya, S. V. Sree, G. Swapna, R. J. Martis and J. S. Suri, Knowl. Based Syst., 2013, 45, 147–165 CrossRef.
- U. R. Acharya, F. Molinari, S. V. Sree, S. Chattopadhyay, K.-H. Ng and J. S. Suri, Biomed. Signal Process, 2012, 7, 401–408 CrossRef.
- A. S. Al-Fahoum and A. A. Al-Fraihat, Int. Sch. Res. notices, 2014, 2014 DOI:10.1155/2014/730218.
- K. Rasheed, A. Qayyum, J. Qadir, S. Sivathamboo, P. Kwan, L. Kuhlmann, T. O’Brien and A. Razi, IEEE Rev. Biomed. Eng., 2020, 14, 139–155 Search PubMed.
- A. Prochazka, J. Kukal and O. Vysata, Wavelet transform use for feature extraction and EEG signal segments classification, In 2008 3rd International symposium on communications, control and signal processing, IEEE, 2008, pp. 719–722 Search PubMed.
- V. Vapnik, The nature of statistical learning theory, Springer science & business media, 1999 Search PubMed.
- S. K. Satapathy and D. Loganathan, SN Computer Science, 2021, 2(3), 1–16 CrossRef.
- S. Motamedi-Fakhr, M. Moshrefi-Torbati, M. Hill, C. M. Hill and P. R. White, Biomed. Signal Process, 2014, 10, 21–33 CrossRef.
- M. A. Hearst, S. T. Dumais, E. Osuna, J. Platt and B. Scholkopf, IEEE Intell. Syst. Appl., 1998, 13, 18–28 Search PubMed.
- J. R. Quinlan, Mach. Learn., 1986, 1, 81–106 Search PubMed.
- B. Şen, M. Peker, A. Çavuşoğlu and F. V. Çelebi, J. Med. Syst., 2014, 38, 1–21 CrossRef PubMed.
- Q. Wang, D. Zhao, Y. Wang and X. Hou, Med. Biol. Eng. Comput., 2019, 57, 1693–1707 CrossRef PubMed.
- D. M. Atallah, M. Badawy and A. El-Sayed, SN Appl. Sci., 2019, 1, 1–17 Search PubMed.
- M. Hajinoroozi, Z. Mao, T.-P. Jung, C.-T. Lin and Y. Huang, Signal Process. Image Commun., 2016, 47, 549–555 CrossRef.
- R. Boostani, F. Karimzadeh and M. Nami, Comput. Meth. Prog. Bio., 2017, 140, 77–91 CrossRef.
- J. Han, J. Pei and M. Kamber, Data mining: concepts and techniques, Elsevier, 2011 Search PubMed.
- J. Schmidhuber, Neural networks, 2015, 61, 85–117 CrossRef PubMed.
- G. Li, J. Wu, Y. Xia, Y. Wu, Y. Tian, J. Liu, D. Chen and Q. He, J. Neural Eng., 2020, 17, 026001 CrossRef PubMed.
- A. J. Casson, M. Abdulaal, M. Dulabh, S. Kohli, S. Krachunov and E. Trimble, in Seamless Healthcare Monitoring: Advancements in Wearable, Attachable, and Invisible Devices, ed. T. Tamura and W. Chen, Springer International Publishing, Cham, 2018, pp. 45–81 DOI:10.1007/978-3-319-69362-0_2.
- G. B. Tseghai, B. Malengier, K. A. Fante and L. V. Langenhove, Autex Res. J., 2021, 21, 63–70 CrossRef CAS.
- G. Li, S. Wang and Y. Y. Duan, Sens. Actuators, B, 2018, 277, 250–260 CrossRef CAS.
- P. Salvo, R. Raedt, E. Carrette, D. Schaubroeck, J. Vanfleteren and L. Cardon, Sens. Actuators, A, 2012, 174, 96–102 CrossRef CAS.
- P. Leleux, J.-M. Badier, J. Rivnay, C. Bénar, T. Hervé, P. Chauvel and G. G. Malliaras, Adv. Healthcare Mater., 2014, 3, 490–493 CrossRef CAS PubMed.
- Z. Li, W. Guo, Y. Huang, K. Zhu, H. Yi and H. Wu, Carbon, 2020, 164, 164–170 CrossRef CAS.
- H.-L. Peng, L. Jing-Quan, H.-C. Tian, Y.-Z. Dong, B. Yang, X. Chen and C.-S. Yang, Sens. Actuators, B, 2016, 226, 349–356 CrossRef CAS.
- G. Li, D. Zhang, S. Wang and Y. Y. Duan, Sens. Actuators, B, 2016, 237, 167–178 CrossRef CAS.
- P. Pedrosa, P. Fiedler, L. Schinaia, B. Vasconcelos, A. C. Martins, M. H. Amaral, S. Comani, J. Haueisen and C. Fonseca, Sens. Actuators, B, 2017, 247, 273–283 CrossRef CAS.
- Q. Wang, X. Pan, C. Lin, X. Ma, S. Cao and Y. Ni, Chem. Eng. J., 2020, 396, 125341 CrossRef CAS.
- F. M. Carvalho, P. Lopes, M. Carneiro, A. Serra, J. Coelho, A. T. de Almeida and M. Tavakoli, ACS Appl. Electron. Mater., 2020, 2, 3390–3401 CrossRef CAS.
- S. Leach, K. Y. Chung, L. Tüshaus, R. Huber and W. Karlen, Front. Neurosci., 2020, 14, 586 CrossRef PubMed.
- R. Hajare and S. Kadam, Global Transitions Proceedings, 2021, 2, 467–475 CrossRef.
- E. H. T. Shad, M. Molinas and T. Ytterdal, IEEE Sens. J., 2020, 20, 14565–14577 CAS.
- P. Fiedler, R. Mühle, S. Griebel, P. Pedrosa, C. Fonseca, F. Vaz, F. Zanow and J. Haueisen, IEEE Trans. Neural Syst. Rehabilitation Eng., 2018, 26, 750–757 Search PubMed.
- P. Fiedler, C. Fonseca, E. Supriyanto, F. Zanow and J. Haueisen, Hum. Brain Mapp., 2022, 43, 1295–1308 CrossRef PubMed.
- R. J. Gentili, K. J. Jaquess, I. M. Shuggi, E. P. Shaw, H. Oh, L.-C. Lo, Y. Y. Tan, C. A. Domingues, J. A. Blanco, J. C. Rietschel, M. W. Miller and B. D. Hatfield, Psychophysiology, 2018, 55, e13059 CrossRef PubMed.
- H. Hinrichs, M. Scholz, A. K. Baum, J. W. Y. Kam, R. T. Knight and H.-J. Heinze, Sci. Rep., 2020, 10, 5218 CrossRef CAS PubMed.
- G. Di Flumeri, P. Aricò, G. Borghini, N. Sciaraffa, A. Di Florio and F. Babiloni, Sensors, 2019, 19, 1365 CrossRef.
- G. Pei, J. Wu, D. Chen, G. Guo, S. Liu, M. Hong and T. Yan, Sensors, 2018, 18, 3396 CrossRef PubMed.
- L. Yang, Q. Liu, Z. Zhang, L. Gan, Y. Zhang and J. Wu, Adv. Mater. Technol., 2022, 2100612 CrossRef CAS.
- A. Harati and A. Jahanshahi, Sens. Actuators, A, 2021, 326, 112727 CrossRef CAS.
- H. Yuan, Y. Li, J. Yang, H. Li, Q. Yang, C. Guo, S. Zhu and X. Shu, Micromachines, 2021, 12, 1521 CrossRef PubMed.
- W. Liu, W. Zhou, S. Liu, C. Zhang, S. Huang, Y. Li and K. S. Hui, Sens. Actuators, A, 2018, 269, 515–523 CrossRef CAS.
- G. B. Tseghai, B. Malengier, K. A. Fante and L. V. Langenhove, IEEE Sens. J., 2021, 21, 22077–22085 CAS.
- D. Khodagholy, J. N. Gelinas, T. Thesen, W. Doyle, O. Devinsky, G. G. Malliaras and G. Buzsáki, Nat. Neurosci., 2015, 18, 310–315 CrossRef CAS PubMed.
- D. Khodagholy, T. Doublet, P. Quilichini, M. Gurfinkel, P. Leleux, A. Ghestem, E. Ismailova, T. Hervé, S. Sanaur, C. Bernard and G. G. Malliaras, Nat. Commun., 2013, 4, 1575 CrossRef PubMed.
- G. B. Tseghai, B. Malengier, K. A. Fante, A. B. Nigusse and L. Van Langenhove, Sensors, 2020, 20, 1742 CrossRef CAS.
- F. La Foresta, F. C. Morabito, S. Marino and S. Dattola, Electronics, 2019, 8, 1031 CrossRef.
- L. Shao, Y. Guo, W. Liu, T. Sun and D. Wei, Mater. Res. Express, 2019, 6, 085619 CrossRef CAS.
- L.-W. Ko, C.-H. Su, P.-L. Liao, J.-T. Liang, Y.-H. Tseng and S.-H. Chen, J. Neural Eng., 2021, 18, 046060 CrossRef.
- P. Zhai, X. Xuan, H. Li, C. Li, P. Li and M. Li, Carbon, 2022, 189, 71–80 CrossRef CAS.
- X. Yang, L. Li, S. Wang, Q. Lu, Y. Bai, F. Sun, T. Li, Y. Li, Z. Wang, Y. Zhao, Y. Shi and T. Zhang, Adv. Electron. Mater., 2020, 6, 2000306 CrossRef CAS.
- G.-L. Li, J.-T. Wu, Y.-H. Xia, Q.-G. He and H.-G. Jin, J. Neural Eng., 2020, 17, 051004 CrossRef PubMed.
- H. Hua, W. Tang, X. Xu, D. D. Feng and L. Shu, Micromachines, 2019, 10, 518 CrossRef PubMed.
- N. M. El Ters, A. M. Mathur, S. Jain, Z. A. Vesoulis and J. M. Zempel, Clin. Neurophysiol., 2018, 129, 1366–1371 CrossRef PubMed.
- G. Shen, K. Gao, N. Zhao, Z. Yi, C. Jiang, B. Yang and J. Liu, J. Neural Eng., 2021, 18, 066047 CrossRef PubMed.
- Y. Li, X. Zhou, B. Sarkar, N. Gagnon-Lafrenais and F. Cicoira, Adv. Mater., 2022, 2108932 CrossRef CAS PubMed.
- X. Sheng, Z. Qin, H. Xu, X. Shu, G. Gu and X. Zhu, Sci. China: Technol. Sci., 2021, 64, 273–282 CrossRef CAS.
- X. Zhou, A. Rajeev, A. Subramanian, Y. Li, N. Rossetti, G. Natale, G. A. Lodygensky and F. Cicoira, Acta Biomater., 2022, 139, 296–306 CrossRef CAS.
- B. Lu, H. Yuk, S. Lin, N. Jian, K. Qu, J. Xu and X. Zhao, Nat. Commun., 2019, 10, 1043 CrossRef PubMed.
- H. Yuk, B. Lu and X. Zhao, Chem. Soc. Rev., 2019, 48, 1642–1667 RSC.
- Y. Ohm, C. Pan, M. J. Ford, X. Huang, J. Liao and C. Majidi, Nat. Electron., 2021, 4, 185–192 CrossRef CAS.
- X. Li, L. He, Y. Li, M. Chao, M. Li, P. Wan and L. Zhang, ACS Nano, 2021, 15, 7765–7773 CrossRef CAS PubMed.
- S. Carli, M. Bianchi, E. Zucchini, M. Di Lauro, M. Prato, M. Murgia, L. Fadiga and F. Biscarini, Adv. Healthcare Mater., 2019, 8, 1900765 CrossRef PubMed.
- X. Wu, W. Pei, H. Zhang, Y. Chen, X. Guo, H. Chen and S. Wang, J. Electroanal. Chem., 2015, 758, 26–32 CrossRef CAS.
- K. Wang, C. L. Frewin, D. Esrafilzadeh, C. Yu, C. Wang, J. J. Pancrazio, M. Romero-Ortega, R. Jalili and G. Wallace, Adv. Mater., 2019, 31, 1805867 CrossRef PubMed.
- Z. Yu and P. Wu, Adv. Funct. Mater., 2021, 31, 2107226 CrossRef CAS.
- I. Yun, J. Jeung, H. Lim, J. Kang, S. Lee, S. Park, S. Seong, S. Park, K. Cho and Y. Chung, ACS Appl. Electron. Mater., 2021, 3, 1842–1851 CrossRef CAS.
- R. Matsukawa, A. Miyamoto, T. Yokota and T. Someya, Adv. Healthcare Mater., 2020, 9, 2001322 CrossRef CAS.
- T. Shay, O. D. Velev and M. D. Dickey, Soft Matter, 2018, 14, 3296–3303 RSC.
- L. Pan, P. Cai, L. Mei, Y. Cheng, Y. Zeng, M. Wang, T. Wang, Y. Jiang, B. Ji, D. Li and X. Chen, Adv. Mater., 2020, 32, 2003723 CrossRef CAS.
- E. S. Kappenman and S. J. Luck, Psychophysiology, 2010, 47, 888–904 Search PubMed.
- B. H. Cornish, B. J. Thomas and L. C. Ward, Appl. Radiat. Isot., 1998, 49, 475–476 CrossRef CAS PubMed.
- A. Albulbul, Bioengineering, 2016, 3, 20 CrossRef PubMed.
- G. Dijk, H. J. Ruigrok and R. P. O'Connor, Adv. Mater. Interfaces, 2020, 7, 2000675 CrossRef CAS.
- J. W. Haverkort, Electrochim. Acta, 2019, 295, 846–860 CrossRef CAS.
- P. Leleux, C. Johnson, X. Strakosas, J. Rivnay, T. Hervé, R. M. Owens and G. G. Malliaras, Adv. Healthcare Mater., 2014, 3, 1377–1380 CrossRef CAS PubMed.
- P. Karande, A. Jain and S. Mitragotri, J. Controlled Release, 2006, 110, 307–313 CrossRef CAS PubMed.
- Y. Niu, H. Liu, R. He, Z. Li, H. Ren, B. Gao, H. Guo, G. M. Genin and F. Xu, Mater. Today, 2020, 41, 219–242 CrossRef.
- N. V. de Camp, G. Kalinka and J. Bergeler, Sci. Rep., 2018, 8, 14041 CrossRef PubMed.
- Q. Liu, G. Nian, C. Yang, S. Qu and Z. Suo, Nat. Commun., 2018, 9, 846 CrossRef PubMed.
- Y. Wang, K. Jia, C. Xiang, J. Yang, X. Yao and Z. Suo, ACS Appl. Mater. Interfaces, 2019, 11, 40749–40757 CrossRef CAS PubMed.
- P. Song and H. Wang, Adv. Mater., 2020, 32, 1901244 CrossRef CAS PubMed.
- E. A. Appel, M. W. Tibbitt, J. M. Greer, O. S. Fenton, K. Kreuels, D. G. Anderson and R. Langer, ACS Macro Lett., 2015, 4, 848–852 CrossRef CAS PubMed.
- J. Yang, R. Bai, B. Chen and Z. Suo, Adv. Funct. Mater., 2020, 30, 1901693 CrossRef CAS.
- H. Yuk, C. E. Varela, C. S. Nabzdyk, X. Mao, R. F. Padera, E. T. Roche and X. Zhao, Nature, 2019, 575, 169–174 CrossRef CAS PubMed.
- S. Ji, C. Wan, T. Wang, Q. Li, G. Chen, J. Wang, Z. Liu, H. Yang, X. Liu and X. Chen, Adv. Mater., 2020, 32, 2001496 CrossRef CAS PubMed.
- J. Li, A. D. Celiz, J. Yang, Q. Yang, I. Wamala, W. Whyte, B. R. Seo, N. V. Vasilyev, J. J. Vlassak, Z. Suo and D. J. Mooney, Science, 2017, 357, 378–381 CrossRef CAS PubMed.
- H. Yuk, T. Zhang, S. Lin, G. A. Parada and X. Zhao, Nat. Mater., 2016, 15, 190–196 CrossRef CAS.
- D. W. Kim, S. Baik, H. Min, S. Chun, H. J. Lee, K. H. Kim, J. Y. Lee and C. Pang, Adv. Funct. Mater., 2019, 29, 1807614 CrossRef.
- H. Zhang, C. Bian, J. K. Jackson, F. Khademolhosseini, H. M. Burt and M. Chiao, ACS Appl. Mater. Interfaces, 2014, 6, 9126–9133 CrossRef CAS PubMed.
- H. E. Jeong, M. K. Kwak and K. Y. Suh, Langmuir, 2010, 26, 2223–2226 CrossRef CAS PubMed.
- S. Baik, J. Kim, H. J. Lee, T. H. Lee and C. Pang, Adv. Sci., 2018, 5, 1800100 CrossRef.
- H. E. Jeong, J.-K. Lee, H. N. Kim, S. H. Moon and K. Y. Suh, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 5639–5644 CrossRef CAS.
- A. Rantell, Trans. IMF, 1969, 47, 197–202 CrossRef CAS.
- C. Mühlhan, S. Weidner, J. Friedrich and H. Nowack, Surf. Coat. Technol., 1999, 116-119, 783–787 CrossRef.
- F. Stauffer, M. Thielen, C. Sauter, S. Chardonnens, S. Bachmann, K. Tybrandt, C. Peters, C. Hierold and J. Vörös, Adv. Healthcare Mater., 2018, 7, 1700994 CrossRef.
- S. Kim, J. Wu, A. Carlson, S. H. Jin, A. Kovalsky, P. Glass, Z. Liu, N. Ahmed, S. L. Elgan, W. Chen, P. M. Ferreira, M. Sitti, Y. Huang and J. A. Rogers, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 17095–17100 CrossRef CAS PubMed.
- M. K. Choi, O. K. Park, C. Choi, S. Qiao, R. Ghaffari, J. Kim, D. J. Lee, M. Kim, W. Hyun, S. J. Kim, H. J. Hwang, S.-H. Kwon, T. Hyeon, N. Lu and D.-H. Kim, Adv. Healthcare Mater., 2016, 5, 80–87 CrossRef CAS PubMed.
- Y. Ma, S. Ma, Y. Wu, X. Pei, S. N. Gorb, Z. Wang, W. Liu and F. Zhou, Adv. Mater., 2018, 30, 1801595 CrossRef.
- W. R. Hansen and K. Autumn, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 385–389 CrossRef CAS PubMed.
- S. Vecchiato, J. Ahrens, A. Pellis, D. Scaini, B. Mueller, E. Herrero Acero and G. M. Guebitz, ACS Sustainable Chem. Eng., 2017, 5, 6456–6465 CrossRef CAS.
- R. Agrawal, N. S. Saxena, K. B. Sharma, S. Thomas and M. S. Sreekala, Mater. Sci. Eng., A, 2000, 277, 77–82 CrossRef.
- D. Hegemann, H. Brunner and C. Oehr, Nucl. Instrum. Methods Phys. Res., Sect. B, 2003, 208, 281–286 CrossRef CAS.
- A. Markovic, M. Kaess and L. Tarokh, Sci. Rep., 2020, 10, 15935 CrossRef CAS PubMed.
- Y. Wang, Z. Qu, W. Wang and D. Yu, Colloids Surf., B, 2021, 208, 112088 CrossRef CAS PubMed.
- H. Yuk, T. Zhang, G. A. Parada, X. Liu and X. Zhao, Nat. Commun., 2016, 7, 12028 CrossRef PubMed.
- T. Liu, M. Liu, S. Dou, J. Sun, Z. Cong, C. Jiang, C. Du, X. Pu, W. Hu and Z. L. Wang, ACS Nano, 2018, 12, 2818–2826 CrossRef CAS PubMed.
- X.-F. Zhang, X. Ma, T. Hou, K. Guo, J. Yin, Z. Wang, L. Shu, M. He and J. Yao, Angew. Chem., Int. Ed., 2019, 58, 7366–7370 CrossRef CAS PubMed.
- X. P. Morelle, W. R. Illeperuma, K. Tian, R. Bai, Z. Suo and J. J. Vlassak, Adv. Mater., 2018, 30, 1801541 CrossRef PubMed.
- Z. Wang, J. Cheng, J. Zhou, J. Zhang, H. Huang, J. Yang, Y. Li and B. Wang, Nano Energy, 2018, 50, 106–117 CrossRef CAS.
- Y. Bai, B. Chen, F. Xiang, J. Zhou, H. Wang and Z. Suo, Appl. Phys. Lett., 2014, 105, 151903 CrossRef.
- L. Han, K. Liu, M. Wang, K. Wang, L. Fang, H. Chen, J. Zhou and X. Lu, Adv. Funct. Mater., 2018, 28, 1704195 CrossRef.
- B. Chen, J. J. Lu, C. H. Yang, J. H. Yang, J. Zhou, Y. M. Chen and Z. Suo, ACS Appl. Mater. Interfaces, 2014, 6, 7840–7845 CrossRef CAS PubMed.
- X. Zhao, F. Chen, Y. Li, H. Lu, N. Zhang and M. Ma, Nat. Commun., 2018, 9, 3579 CrossRef PubMed.
- Z. Zhao, K. Zhang, Y. Liu, J. Zhou and M. Liu, Adv. Mater., 2017, 29, 1701695 CrossRef PubMed.
- D. Zhou, F. Chen, S. Handschuh-Wang, T. Gan, X. Zhou and X. Zhou, Chem. Phys. Chem., 2019, 20, 2139–2154 CrossRef CAS PubMed.
- J. A. Chiong, H. Tran, Y. Lin, Y. Zheng and Z. Bao, Adv. Sci., 2021, 8, 2101233 CrossRef CAS.
- C. F. Guo, Q. Liu, G. Wang, Y. Wang, Z. Shi, Z. Suo, C.-W. Chu and Z. Ren, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 12332–12337 CrossRef CAS.
- S. Lin, J. Liu, W. Li, D. Wang, Y. Huang, C. Jia, Z. Li, M. Murtaza, H. Wang, J. Song, Z. Liu, K. Huang, D. Zu, M. Lei, B. Hong and H. Wu, Nano Lett., 2019, 19, 6853–6861 CrossRef CAS PubMed.
- A. Miyamoto, S. Lee, N. F. Cooray, S. Lee, M. Mori, N. Matsuhisa, H. Jin, L. Yoda, T. Yokota, A. Itoh, M. Sekino, H. Kawasaki, T. Ebihara, M. Amagai and T. Someya, Nat. Nanotechnol., 2017, 12, 907–913 CrossRef CAS PubMed.
- J. Wu, Z. Wu, S. Han, B.-R. Yang, X. Gui, K. Tao, C. Liu, J. Miao and L. K. Norford, ACS Appl. Mater. Interfaces, 2019, 11, 2364–2373 CrossRef CAS.
- N. Alexandre, J. Ribeiro, A. Gärtner, T. Pereira, I. Amorim, J. Fragoso, A. Lopes, J. Fernandes, E. Costa, A. Santos-Silva, M. Rodrigues, J. D. Santos, A. C. Maurício and A. L. Luís, J. Biomed. Mater. Res., Part A, 2014, 102, 4262–4275 Search PubMed.
- X. Jing, H.-Y. Mi, X.-F. Peng and L.-S. Turng, Carbon, 2018, 136, 63–72 CrossRef CAS.
- P. Moutsatsou, K. Coopman and S. Georgiadou, Polymers, 2017, 9, 687 CrossRef PubMed.
- S. Zhang, Y. Li, G. Tomasello, M. Anthonisen, X. Li, M. Mazzeo, A. Genco, P. Grutter and F. Cicoira, Adv. Electron. Mater., 2019, 5, 1900191 CrossRef.
- Y. Li, X. Li, S. Zhang, L. Liu, N. Hamad, S. R. Bobbara, D. Pasini and F. Cicoira, Adv. Funct. Mater., 2020, 30, 2002853 CrossRef CAS.
- Y. Li, S. Zhang, X. Li, V. R. N. Unnava and F. Cicoira, Flexible Printed Electron., 2019, 4, 044004 CrossRef CAS.
- Y. Li, S. Zhang, N. Hamad, K. Kim, L. Liu, M. Lerond and F. Cicoira, Macromol. Biosci., 2020, 20, 2000146 CrossRef CAS PubMed.
- K. Feron, R. Lim, C. Sherwood, A. Keynes, A. Brichta and P. C. Dastoor, Int. J. Mol. Sci., 2018, 19, 2382 CrossRef.
- D. Gao, K. Parida and P. S. Lee, Adv. Funct. Mater., 2020, 30, 1907184 CrossRef CAS.
- M. Torculas, J. Medina, W. Xue and X. Hu, ACS Biomater. Sci. Eng., 2016, 2, 1211–1223 CrossRef CAS.
- F. Wang, S. Zhang, Y. Zhang, Q. Lin, Y. Chen, D. Zhu, L. Sun and T. Chen, Nanomaterials, 2019, 9, 343 CrossRef CAS PubMed.
- M. A. Cervera, S. R. Soekadar, J. Ushiba, J. d R. Millán, M. Liu, N. Birbaumer and G. Garipelli, Annals of clinical and translational neurology, 2018, 5, 651–663 CrossRef PubMed.
- A. Biasiucci, R. Leeb, I. Iturrate, S. Perdikis, A. Al-Khodairy, T. Corbet, A. Schnider, T. Schmidlin, H. Zhang and M. Bassolino, Nat. Commun., 2018, 9, 1–13 CrossRef CAS PubMed.
- L. Tonin and J. d R. Millán, Annu. Rev. Control Robot. Auton. Syst., 2021, 4, 191–214 CrossRef.
- R. Janapati, V. Dalal, N. Govardhan and R. S. Gupta, Review on EEG-BCI classification techniques advancements, in IOP Conference Series: Materials Science and Engineering, IOP Publishing, 2020, vol. 981, no. 3, p. 032019 Search PubMed.
- F. Lotte, L. Bougrain, A. Cichocki, M. Clerc, M. Congedo, A. Rakotomamonjy and F. Yger, J. Neural Eng., 2018, 15, 031005 CrossRef CAS PubMed.
- M. A. Frankel, M. J. Lehmkuhle, M. Watson, K. Fetrow, L. Frey, C. Drees and M. C. Spitz, Clin. Neurophysiol Pract., 2021, 6, 172–178 CrossRef PubMed.
- L. Swinnen, C. Chatzichristos, K. Jansen, L. Lagae, C. Depondt, L. Seynaeve, E. Vancaester, A. Van Dycke, J. Macea and K. Vandecasteele, Epilepsia, 2021, 62, 2741–2752 CrossRef PubMed.
- G. Japaridze, D. Loeckx, T. Buckinx, S. Armand Larsen, R. Proost, K. Jansen, P. MacMullin, N. Paiva, S. Kasradze and A. Rotenberg, Epilepsia, 2022 DOI:10.1111/epi.17200.
- S. A. Imtiaz and E. Rodriguez-Villegas, Ann. Biomed. Eng., 2014, 42, 2344–2359 CrossRef.
- T. Svensson, U.-i Chung, S. Tokuno, M. Nakamura and A. K. Svensson, J. Psychosom. Res., 2019, 126, 109822 CrossRef.
- S. Kwon, H. Kim and W.-H. Yeo, iScience, 2021, 24, 102461 CrossRef PubMed.
- M. Yoshida, K. Kashiwagi, H. Kadotani, K. Yamamoto, S. Koike, M. Matsuo, N. Yamada, M. Okawa and Y. Urade, J. Oral Sleep Med., 2015, 1, 140–147 Search PubMed.
- Z. Liang and M. A. Chapa Martell, J. Healthc. Inform. Res., 2018, 2, 152–178 CrossRef PubMed.
- B. Koley and D. Dey, Comput. Biol. Med., 2012, 42, 1186–1195 CrossRef CAS PubMed.
- K. B. Mikkelsen, S. L. Kappel, D. P. Mandic and P. Kidmose, Front. Neurosci., 2015, 9, 438 Search PubMed.
- A. Stochholm, K. Mikkelsen and P. Kidmose, Automatic sleep stage classification using ear-EEG, In 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), IEEE, 2016, pp. 4751–4754 Search PubMed.
- D. Looney, V. Goverdovsky, I. Rosenzweig, M. J. Morrell and D. P. Mandic, Ann. Am. Thorac. Soc., 2016, 13, 2229–2233 CrossRef PubMed.
- K. B. Mikkelsen, D. B. Villadsen, M. Otto and P. Kidmose, Biomed. Eng. Online, 2017, 16, 1–15 CrossRef PubMed.
- T. Nakamura, V. Goverdovsky, M. J. Morrell and D. P. Mandic, IEEE J. Transl. Eng. Health Med., 2017, 5, 1–8 Search PubMed.
- T. Nakamura, Y. D. Alqurashi, M. J. Morrell and D. P. Mandic, IEEE Trans. Biomed. Eng., 2019, 67, 203–212 Search PubMed.
- K. B. Mikkelsen, Y. R. Tabar, S. L. Kappel, C. B. Christensen, H. O. Toft, M. C. Hemmsen, M. L. Rank, M. Otto and P. Kidmose, Sci. Rep., 2019, 9, 1–12 CrossRef CAS PubMed.
- Y. D. Alqurashi, T. Nakamura, V. Goverdovsky, J. Moss, M. I. Polkey, D. P. Mandic and M. J. Morrell, Nat. Sci. Sleep, 2018, 10, 385 CrossRef PubMed.
- C.-T. Lin, C.-H. Chuang, Z. Cao, A. K. Singh, C.-S. Hung, Y.-H. Yu, M. Nascimben, Y.-T. Liu, J.-T. King and T.-P. Su, IEEE Access, 2017, 5, 10612–10621 Search PubMed.
- D. J. Levendowski, L. Ferini-Strambi, C. Gamaldo, M. Cetel, R. Rosenberg and P. R. Westbrook, J. Clin. Sleep Med., 2017, 13, 791–803 CrossRef PubMed.
- S. Blum, R. Emkes, F. Minow, J. Anlauff, A. Finke and S. Debener, J. Neural Eng., 2020, 17, 034003 CrossRef PubMed.
- M. R. Carneiro, A. T. de Almeida and M. Tavakoli, IEEE Sens. J., 2020, 20, 15107–15116 CAS.
- S. Myllymaa, A. Muraja-Murro, S. Westeren-Punnonen, T. Hukkanen, R. Lappalainen, E. Mervaala, J. Töyräs, K. Sipilä and K. Myllymaa, J. Sleep Res., 2016, 25, 636–645 CrossRef PubMed.
- M. G. Bleichner and S. Debener, Front. Hum. Neurosci., 2017, 11, 163 Search PubMed.
- A. Sterr, J. K. Ebajemito, K. B. Mikkelsen, M. A. Bonmati-Carrion, N. Santhi, C. Della Monica, L. Grainger, G. Atzori, V. Revell and S. Debener, Front. Hum. Neurosci., 2018, 452 CrossRef PubMed.
- K. B. Mikkelsen, J. K. Ebajemito, M. A. Bonmati-Carrion, N. Santhi, V. L. Revell, G. Atzori, C. Della Monica, S. Debener, D. J. Dijk and A. Sterr, J. Sleep Res., 2019, 28, e12786 CrossRef PubMed.
- K. A. I. Aboalayon, M. Faezipour, W. S. Almuhammadi and S. Moslehpour, Entropy, 2016, 18, 272 CrossRef.
- D. V. Iosifescu, S. Greenwald, P. Devlin, D. Mischoulon, J. W. Denninger, J. E. Alpert and M. Fava, European Neuropsychopharmacology, 2009, 19, 772–777 CrossRef CAS PubMed.
- M. Arns, W. H. Drinkenburg, P. B. Fitzgerald and J. L. Kenemans, Brain Stimul., 2012, 5, 569–576 CrossRef PubMed.
- S. Olbrich and M. Arns, Int. Rev. Psychiatry, 2013, 25, 604–618 CrossRef.
- X. Li, B. Hu, J. Shen, T. Xu and M. Retcliffe, J. Med. Syst., 2015, 39, 1–6 CrossRef PubMed.
- M. Balconi, G. Fronda and D. Crivelli, Stress, 2019, 22, 200–209 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |