Recent progress in low dimensional (quasi-2D) and mixed dimensional (2D/3D) tin-based perovskite solar cells
Malouangou Maurice
Davy
,
Tsiba Matondo
Jadel
,
Chen
Qin
,
Bai
Luyun
and
Guli
Mina
 *
*
Beijing Key Laboratory of Novel Thin Film Solar Cells, School of Renewable Energy, North China Electric Power University, Beijing 102206, China. E-mail: gulimina@ncepu.edu.cn
First published on 2nd November 2020
Abstract
Low dimensional (quasi-2D) and mixed dimensional (2D/3D) halide perovskites have emerged in the field of perovskite solar cells. They are more stable than their 3D homologs. However, most of the high efficiency perovskite solar cells contain lead, which is toxic; this toxicity impedes the deployment of this technology through mass production and commercialization. Much effort has been made to reduce the toxicity in perovskite solar cells, which is conducive to the development of environmentally friendly halide perovskite solar cells. Moreover, Sn is currently the most promising element to replace lead and develop lead free perovskite solar cells. Indeed, the high sensitivity of Sn2+ cations towards moisture and oxygen was a huge challenge for preparing stable solar cells. However, this issue has been solved by incorporating organic ammonium cations with different sizes and functional groups, which not only reduce the dimensions from 3D to quasi-2D but also improve their environmental stability. Quasi-2D perovskites have been actively explored to overcome the poor environmental stability of perovskite solar cells, owing to the hydrophobic nature of the large organic cations. Another important approach consisted of appropriately tuning the number of alkyl ammonium cations in the 3D perovskite precursor solution and providing mixed 2D/3D perovskites, which was also shown to be an effective method to produce stable and efficient perovskite solar cells by remarkably blocking the intrusion of moisture from the external atmosphere into the vulnerable 3D perovskite part. In this paper, we provide a brief review of recent advancements in two (quasi 2D) and mixed dimensional (2D/3D) Sn-based perovskite solar cells.
1. Introduction
Organic–inorganic hybrid halide perovskites have demonstrated great potential in optoelectronic applications, such as light emitting diodes and solar cells, due to their excellent optoelectronic properties and ease of fabrication.1 The power conversion efficiency of perovskite solar cells has increased rapidly from 3.8% in 2009 to 25.2% in 2019.2 This great success essentially arises from their extraordinary properties, mainly a high optical absorption coefficient, a tunable bandgap, long carrier recombination lifetimes, and a high electron/hole mobility and transmission quality, along with small electron/hole effective masses and small exciton binding energy.3–11 One more important advantage of halide perovskites is the possibility of processing the materials using a number of simple solution-based or evaporative thin-film techniques with low cost.12–15Most of the high performance halide perovskite devices contain pure lead, which is a highly toxic metal for humans and the environment.16 The high toxicity and chemical instability of lead in perovskite materials limit its commercialization and represent the drawback of lead halide perovskite materials.17 Furthermore, it is harmful to many different tissues and organs, including the heart, bones, and kidneys, and the reproductive and nervous systems. Additionally, it affects the brain, which is the most sensitive organ.18 Thus, children are particularly vulnerable to the neurotoxic effects of lead, and even relatively low levels of exposure can cause serious and, in some cases, irreversible neurological damage.19 Many alternatives with lower toxicity have been proposed in the literature, including Sn, Ge, Sb, Bi, Cu, etc.20–23 A promising alternative to lead based perovskite solar cells is tin based perovskite solar cells, which have shown encouraging results for achieving high performance. Besides, Sn based photovoltaic devices exhibit great potential because of their wider visible absorption spectrum, which is relative to their narrower optical bandgap (1.55 eV for MAPbI3 and 1.30 eV for pure MASnI3)24 and their higher charge carrier mobility than that of Pb-based perovskites.25,26 However, the high sensitivity of Sn2+ cations towards moisture and oxygen makes it challenging to design stable solar cells.27 Sn2+ in tin-based perovskites is unstable and easily oxidized to Sn4+, which deteriorates the optoelectronic properties and morphology of the perovskite film and reduces the efficiency and stability of these materials.27,28
Several research groups have focused on stabilizing Sn based perovskite solar cells by developing some strategies such as additive engineering (SnF2 and SnCl2), in which the additive effects can be related to their great electronegativity, for example F and Cl which interact with the neighboring Sn2+ in the perovskite lattice to prevent the Sn2+ species from losing its electron pair in further oxidation.29–31 Solvent engineering (DMF, DMSO, diethyl ether, toluene…) leads to a dense and uniform film32–34 and also compositional engineering (A-site and B-site).35–38 To further increase the stability of Sn based perovskites, researchers have introduced large spacer cations; these larger organic cations do not fit into the vacancies of the cubic structure, and so allow the formation of new materials and modify the perovskite properties, thus generating low (quasi-2D) and mixed (2D/3D) dimensional perovskite structures.39,40 The main interest of studying low and mixed dimensional perovskites for photovoltaic applications has been to improve their stability against moisture due to the hydrophobic nature of the employed bulky cations.41–43 The introduction of different amounts of organic amine molecules leads to adjustment of the optical bandgap of perovskite for the optimal energy level, which expands the application of perovskite materials. Compared with the rapid development of lead-based PSCs, the performance of tin-based PSCs has been improved much more slowly due not only to the poor chemical stability of tin (Sn2+) in the perovskite lattice and the difficulty in controlling the film morphology, but also to the Sn-defects, particularly Sn-vacancies, in tin-based perovskites,26,44 which result in undesirable non-recombination of photo-carriers and the loss of open circuit voltage (VOC). In addition, using quasi-2D Sn-based perovskite as a light absorber leads to improved device stability45 and reduced density of Sn-vacancies and other defects. An additional approach consists of introducing a small amount of large organic cations into 3D precursor which ensures the formation of mixed dimensional (2D/3D) Sn-based perovskite, which also allows an important improvement in performance and stability compared to those of the pure quasi-2D ones.46 2D/3D hetero-structures provide the characteristics of 3D structures while preserving the advantages of 2D structures. Moreover, there are many other advantages of quasi-2D and 2D/3D mixed perovskites, such as a large bang gap leading to a high open circuit voltage, a high quality film deposited through one step spin coating without high temperature annealing, and more tunable structures than their 3D counterparts. On the other hand quasi-2D hybrid perovskites have greater flexibility in chemical structures than 3D perovskites because they do not need to respect the Goldschmidt factor for the organic cation R-NH3+. Hence, they offer greater freedom of composition than 3D perovskites. Quasi-2D perovskite is far more moisture resistant and devices can be fabricated under a humid atmosphere because of their unique properties like larger formation energy and hydrophobicity. Owing to these outstanding physical and chemical properties, quasi-2D perovskites have been shown to have an important role in improving the stability of PSCs under ambient conditions.47–50
In this paper, we discuss the recent progress of quasi-2D and 2D/3D Sn based perovskite solar cells from the viewpoint of dimensional engineering. First, we present the preparation methods, structure and optoelectronic properties of quasi-2D perovskites, including the band structure, optical properties, and charge transport. We then point out recent achievements of quasi-2D and 2D/3D Sn-based perovskite solar cells.
2. Preparation methods of quasi-2D perovskite solar cells
The quasi-2D Sn based perovskite preparation methods are quite similar to those of quasi-2D Pb based perovskites for thin films, using a solution-processing technique whereby stoichiometric precursors are dissolved in a solution and deposited on a surface and assembled into the 2D perovskite structure. There are two main methods typically used for perovskite material deposition: solution processing and vacuum processing.51 For this review the focus is on the first method which is relative to solution processing; generally, the spin coating process is widely used in optoelectronic thin film preparation. It is a low cost and convenient method and is divided into one-step and two-step methods.52,53In one-step solution processing, all of the precursors are dissolved in the same solution. The perovskite film is formed by depositing the precursor solution on the substrate using, for example, spin coating, slot die coating, or drop-casting techniques. The solvent evaporates and the perovskite crystallizes. Besides, to synthesize a pure quasi-2D perovskite structure, a solution of metal halide and a long organic cation (which does not fit the octahedral inorganic framework) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio is necessary. Otherwise, when a metal halide is mixed with both short and long organic cations, two possibilities can occur: (1) the organic cations may be locally arranged into separate structures or (2) the precursors may be arranged into a more complex layered hybrid structure in which the long organic cations separate the slabs of the 3D perovskites.51 However, the one-step spin-coating has been shown to produce poor film quality due to the difficulty of controlling the reaction kinetics. To control the reaction kinetics, the anti-solvent method has been introduced, and a further improvement in film quality has also been observed by using the intermediate phase. The role of these different techniques is to slow down both the nucleation and crystal growth rate, which decrease the impurity phase in the perovskite film formation and the quality of the final perovskite film. The two step method can also be used to synthesize quasi-2D perovskite materials. In this method, the metal halide precursor is deposited onto the substrate in the first step and then in the second step the metal halide electrode is dipped in a solution that contains the desired ammonium-based cations or the desired ammonium salt is spin-coated on the halide film.54 This method offers the advantage of allowing a better film cover on the substrate and control of the perovskite film morphologies. The perovskite film preparations using these two methods are illustrated in Fig. 1. Besides these two common methods, there are also other methods which are relative to the scalability of perovskite devices. These methods are blade coating,55,56 spray-coating,57 slot-die-coating and so on, and they allow not only the controlled growth of high quality perovskite films via one step, but also reduced material wastage.58
2 molar ratio is necessary. Otherwise, when a metal halide is mixed with both short and long organic cations, two possibilities can occur: (1) the organic cations may be locally arranged into separate structures or (2) the precursors may be arranged into a more complex layered hybrid structure in which the long organic cations separate the slabs of the 3D perovskites.51 However, the one-step spin-coating has been shown to produce poor film quality due to the difficulty of controlling the reaction kinetics. To control the reaction kinetics, the anti-solvent method has been introduced, and a further improvement in film quality has also been observed by using the intermediate phase. The role of these different techniques is to slow down both the nucleation and crystal growth rate, which decrease the impurity phase in the perovskite film formation and the quality of the final perovskite film. The two step method can also be used to synthesize quasi-2D perovskite materials. In this method, the metal halide precursor is deposited onto the substrate in the first step and then in the second step the metal halide electrode is dipped in a solution that contains the desired ammonium-based cations or the desired ammonium salt is spin-coated on the halide film.54 This method offers the advantage of allowing a better film cover on the substrate and control of the perovskite film morphologies. The perovskite film preparations using these two methods are illustrated in Fig. 1. Besides these two common methods, there are also other methods which are relative to the scalability of perovskite devices. These methods are blade coating,55,56 spray-coating,57 slot-die-coating and so on, and they allow not only the controlled growth of high quality perovskite films via one step, but also reduced material wastage.58
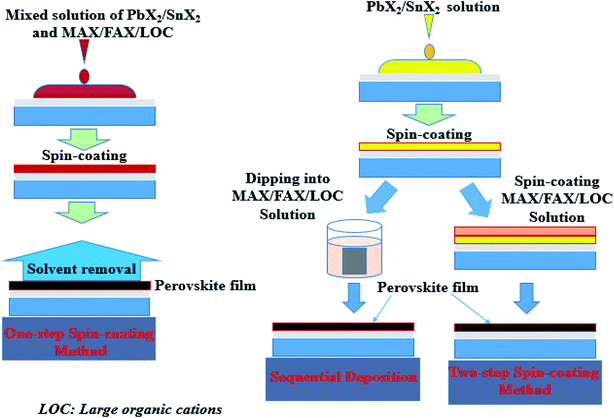 | ||
| Fig. 1 Schematic illustration of the diverse fabrication methods for perovskite solar cells.59 (Reproduced from ref. 59 with permission from the Royal Society of Chemistry, copyright 2015.) | ||
The important role of these different preparation methods is to improve the quality of perovskite layers with high coverage, by reducing the defect density and the carrier recombination, and also to achieve a high power conversion efficiency. The PCE of perovskite solar cells depends on the quality of the film; hence, the preparation methods are the key factors to achieving a high PCE in perovskite solar cells.59
3. Structure of quasi-2D perovskite solar cells
Sn2+ metal cations are the most likely substitute for Pb2+ in the perovskite structure since they both belong to group 14 in the periodic table which leads to them having similar chemical properties and they also possess similar ionic radii.60,61In this section, the structure of quasi-2D perovskite materials based on Sn or Pb is presented; these two compounds show similar behavior in quasi-2D layer structures. However, the inorganic layers of quasi-2D perovskites resemble the parent 3D perovskite structures, such as MAPbI3 and MASnI3. The quasi-2D perovskite materials can be described as the components obtained from the reduction of the dimensions of the 3D perovskite lattice. The quasi-2D perovskites are usually represented by the general formula (A′)m(A)n−1BnX3n+1, where A′ can be divalent (m = 1) or monovalent (m = 2) cations that form a bilayer or monolayer connecting the inorganic (A)n−1BnX3n+1 2D sheets, where n is considered the thickness of the inorganic layer and can be adjusted by tuning the precursor composition. In general, n = 1 corresponds to a pure two-dimensional layered structure,62n = ∞ constitutes a three-dimensional structure, and when n is another integer, it corresponds to a quasi-two-dimensional layered structure,63 as shown in Fig. 2a. Based on the orientation of the crystalline layer slice in the 3D framework, quasi-2D perovskites can be divided into three types: (100), (110) and (111) oriented perovskites;64,65 these different crystal orientations are illustrated in Fig. 2b.
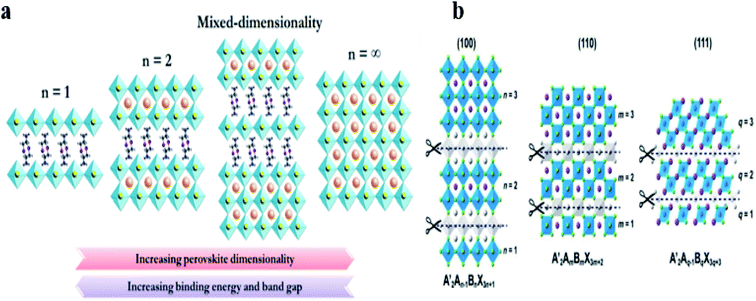 | ||
| Fig. 2 (a) Schematic illustration showing the crystalline structure of 2D perovskites (n = 1 and 2, where n represents the metal halide lattices), mixed dimensionality perovskites and 3D perovskites (n = ∞),63 (b) Schematic of differently oriented families of 2D perovskites: the <100> plane, A′2An−1BnX3n+1; the <110> plane, A′2AmBmX3m+2; and the <111> plane, A′2Aq−1BqX3q+2. Cuts along <100>, <110> and <111> directions (grey parts) result in the corresponding different types of quasi-2D perovskites.64 Reproduced from ref. 63 with permission from the American Chemical Society, copyright 2019 and ref. 64 from Wiley-VCH, copyright 2019. | ||
Among these three orientations, (100) is the most commonly studied in quasi-2D perovskite materials. The structural characteristic of quasi-2D perovskite is expressed by the general formula: A′2An−1BnX3n+1, where the inorganic sheets are obtained by taking n layers along the 100 direction of the 3D perovskites.65 The perovskite materials obtained with the above formula present a great tolerance to the organic and inorganic components, this allows them to have a high degree of compositional.
The (110)-oriented perovskite layers are often highly distorted, showing interesting behaviors such as the formation of self-trapped excitons and broad-band/white-light emission at room temperature. In this structure, it is difficult to modulate the thickness of inorganic layers, and so these materials are not used in perovskite solar cells. The (111) orientation is obtained by cutting along the diagonal of a 3D cubic lattice and has a general formula of A′2Aq−1BqX3q+2, where the members have q > 1. The (111)-oriented perovskites are attractive solar cell absorbers due to their p-type character and relatively small effective masses for both holes and electrons; however, their strong excitonic nature has appeared to limit the performance in solar cells thus far in their development.
Generally, quasi-2D perovskite materials are divided into two great families: the Ruddlesden–Popper (RP) type and the Dion Jacobson (DJ) type,63 and the difference between these two is due to the nature of the large organic cations. The RP compositions are generally described as A′2An−1BnX3n+1, where A′ is an aryl ammonium or alkyl cation (examples are phenyl-ethyl ammonium (PEA) and butylammonium (BA)); the small A cation is typically Cs, FA, or MA; the B site is Sn or Pb; and the X site is I, Br, or Cl. The DJ compositions are represented by the general formula A′An−1BnX3n+1, where A′ is a diammonium cation (H3N-R-NH3). Besides these two great families, there is another family with alternating cations in the interlayer space; in this family the most common cation used is guanidinium (GA), and it is represented by the general formula A′AnBnX3n+1.64 These different structures are represented in Fig. 3a–c.
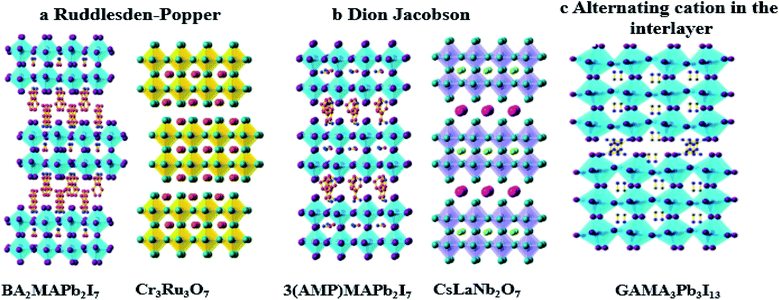 | ||
| Fig. 3 Crystal structure of: (a) Ruddlesden–Popper, (b) Dion Jacobson, (c) alternating cations in the interlayer space.64 Reproduced from ref. 64 with permission from Wiley-VCH, copyright 2019. | ||
4. Optoelectronic properties of quasi-2D perovskites
4.1 Band structure
In the layer-structured quasi-2D perovskites, the large-sized organic cation interlayers can limit charge carriers within a two-dimensional range. These interlayers also act as dielectric regulators, determining the electrostatic force on the electron–hole pairs.66 The alternating arrangement of metal halide inorganic sheets and bulky organic interlayers results in a multiple-quantum-well (MQW) electronic structure (Fig. 4a).67 The high organic and inorganic dielectric constant leads to a huge electron–hole binding energy (Eb) in quasi-2D perovskites.68,69 The optical Eg of A′2An−1BnX3n+1 perovskite generally decreases as the n value increases (Fig. 4b); this leads to modulation of the band gap for an appropriate application. For example, in the series of (PEA)2A2n−1PbnI3n+1, with n from 1 to 3, the difference in the light absorption and the color of the films obtained can be observed, and the perovskite film with n = 1 based on (PEA)2PbI4 absorbs blue and appears orange, while the perovskite film with n = 2 (PEA)2(MA)Pb2I7 absorbs green and appears red and finally, the perovskite film with n = 3 based on (PEA)2(MA)2Pb3I10 absorbs the entire visible spectrum and appears black. This is due to the decrease of the bandgap from 2.36 eV (n = 1) to 1.94 eV (n = ∞).70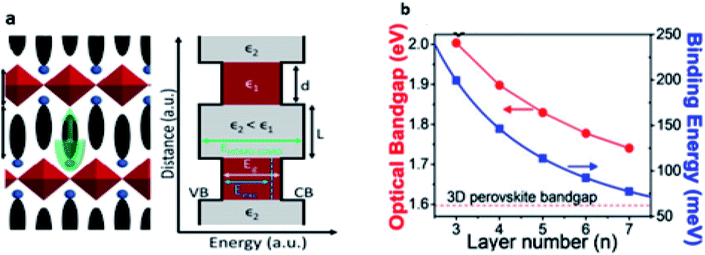 | ||
| Fig. 4 (a) Energy diagram corresponding to the quasi-2D structures. Valence band VB, conduction band CB, electronic bandgap (Eg), and optical bandgap Eexc68 (b) the optical bandgap as a function of the n value derived from the photoluminescence (PL) spectra.70 (Reproduced from ref. 68 with permission from the American Chemical Society, copyright 2019, and reproduced from ref. 70 from Wiley-VCH under the terms of the Creative Commons CC-BY license, copyright 2019). | ||
4.2 Quantum confinement
Quasi-2D perovskites are considered sheet structures which induce quantum confinement effects.Quantum confinement effects describe electrons in terms of energy levels, potential wells, valence bands, conduction bands, and electron energy band gap.71 These effects were reported in the literature and we can see, for example, a quasi-2D perovskite obtained with phenyl-ethyl ammonium. The BV and BC of the inorganic octahedral layers form very fine potential wells (about 0.5 nm in length) separated by potential barriers. The latter are formed by the border orbital HOMO and LUMO (HOMO = highest occupied molecular orbital and LUMO = lowest unoccupied molecule orbital) of organic molecules whose energy difference (between 5 and 6 eV) is typically very large in front of the gap of the inorganic part (approximately 2.4 eV for a PbI2-based perovskite).72 This succession of potential wells separated by almost infinite barriers forms what is called a type I multi-quantum well structure.
In the quantum multi-well system, the electronic wave function is confined inside the sheets of inorganic octahedra. It is possible to modulate this confinement, as well as the gap, by varying the width of the potential wells. This is notably achieved thanks to the RP perovskites with the formula (R-NH3)2An–1BnX3n+1, where the number n corresponds to the number of octahedral sheets. As n increases, the quantum confinement decreases, as does the energy gap73,74 as described in Fig. 4b.
4.3 Excitonic effects
Quasi-2D perovskites have been shown to have strong excitonic effects and have relatively mobile Wannier–Mott type excitons with a Bohr radius in the layer plane between 10 and 20 Å.Excitons have an essential influence on charge transport in semiconductors.75 The quasi-2D structure generally shows a large exciton binding energy (Eb) of several hundred meV which significantly enhances the interaction between electrons and holes compared to that in 3D perovskites.76 To address this challenge, significant effort has been focused on controlling the growth of the inorganic perovskite framework perpendicular to the substrate to facilitate vertical charge transport for efficient charge collection in PV devices.77–80 Although the large Eb in low-n 2D perovskites may be detrimental for charge separation in solar cells, it can be beneficial for other optoelectronic applications beyond solar cells. For instance, the excitonic effect can significantly promote radiative recombination, which leads to a higher photoluminescence quantum yield (PLQY) in perovskite-based LED devices, making them excellent candidates for high-efficiency LEDs. Additionally, the excitonic character in quasi-2D perovskites also results in interesting and tunable exciton–phonon,81 exciton–photon,82 and exciton–exciton coupling,83 as well as in the formation of self-trapped excitons (which leads to broadband emission) and many other paths for relevant optoelectronic applications.
4.4 Anisotropy and transport properties
The particular structure of quasi-2D perovskites only allows the conduction of charges in the direction parallel to the planes of the octahedra, perpendicular conduction being prevented by the presence of organic potential barriers. The charge-transfer behavior of quasi-2D perovskite also shows a strong dependence on the n value. In quasi-2D perovskite films, the holes transfer from high-n QWs or the bulk to the low-n phase along with electrons flowing in the opposite direction.84,85 An optimized n distribution of the QWs will enable more efficient charge transfer across quasi-2D structures with mixed n values. Besides, as the ratio of the number of inorganic perovskite plates per organic spacer increases, the mobility increases significantly as a continuous inorganic pathway is achieved. The orientation of the quasi-2D perovskite layers is the key to the device performance and it is dependent on the distance between the organic and inorganic layers: when the inorganic plates are arranged parallel to the charge-collection direction, better device performance can usually be obtained.86,87The control of the optoelectronic quality of quasi-2D and mixed 2D/3D perovskite films is an important point to obtain high-efficiency perovskite solar cells. Besides this, the control of the crystal growth is also important for charge transport and extraction. It is reasonable to minimize the disordered structure in the film formation by appropriate selection of the organic cations, which leads to ensuring both performance and stability.89 The PCE of quasi-2D and mixed 2D/3D perovskite solar cells is particularly dependent on the orientation and the homogeneous energy alignment of the perovskite film vertically to the substrate to ensure the good charge transport and extraction as shown in Fig. 5a. In contrast, Fig. 5b illustrates the random orientation, which impedes the charge transport and extraction.88
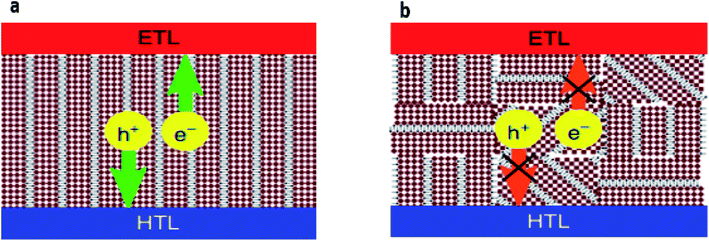 | ||
| Fig. 5 Charge transport: (a) the vertical orientation provides a direct pathway for electron and hole extraction and (b) partial random orientation hinders charge extraction.88 (Reproduced from ref. 88 with permission from Nature Communications under the terms of the Creative Commons CC-BY license, copyright 2018.) | ||
5. Two-dimensional (quasi-2D) Sn-based perovskite solar cells
Quasi-2D perovskites have been proposed in perovskite cells solar thanks to their long hydrophobic organic chains which protect them from moisture and humidity and improve their environmental stability. In the following sections we attempt to cover some of the most remarkable recent achievements in the field of quasi-2D tin based perovskite solar cells. The section is divised into two subsections: RP and DJ. The LD perovskite interlayer has a trap passivation effect to help suppress the accumulation and recombination of carriers, which, in turn, leads to efficient extraction of carriers.45.1 Ruddlesden–Popper (RP) quasi-2D Sn based perovskite solar cells
RP layered perovskites are widely studied in quasi-2D perovskites, in which two layers of monovalent ammonium cations (butylamine, phenylamine, etc.) are inserted between the halide inorganic layers and form the van der Waals gap between the adjacent unit cells.90–93 Here some of studies using RP structures for quasi-2D Sn-based perovskite solar cells are presented.| (BA)2(MA)n−1SnnI3n+1 (n = 4) |
Inspired by the previous work using large organic cations in lead-based perovskite solar cells, Cao and coworkers94 were the first to introduce butylammonium in Sn based perovskite to form quasi-2D Sn perovskite materials. The perovskite film obtained in this study presented the feasibility of being used as a light absorber for solar cells with an optical band gap of 1.42 eV for n = 4. However, they discovered that the film orientation was dependent on the precursor solvent, in which dimethyl sulfoxide (DMSO) allowed parallel orientation while dimethyl formamide (DMF) allowed perpendicular orientation to the substrate. To further improve the film quality, triethyl phosphine was used as an antioxidant to reduce the oxidation of Sn, which provided a dense and uniform perovskite film. As a result, a PCE of 2.53% was obtained for the champion device. More importantly, quasi-2D Sn-based perovskite solar cells showed outstanding stability compared to the 3D analogs and retained more than 90% of their efficiency after 1 month. Later, Qui and coworkers95 used the synergy effect of Lewis adducts and the ion exchange process using a mixture of the polar aprotic solvent dimethyl sulfoxide (DMSO) and the ionic liquid solvent methylammonium acetate (MAAc) to manage the crystallization kinetics of quasi-2D Sn based perovskites; the crystal formation is described in Fig. 6a. Dense and smooth BA2MA3Sn4I13 perovskite films were obtained which presented a large grain size close to 9 μm, high carrier mobility, and low defect density (Fig. 6g and h). As a result, a PCE of 4.03% was achieved (Fig. 6d–f) and the devices showed good stability in a nitrogen atmosphere for 94 days without any degradation (Fig. 6i–j). More recently, Li et al.96 studied the effects of alkyl chain length on crystal growth and the oxidation process of quasi-2D Sn-based perovskite solar cells by employing different alkyl ammonium spacer cations [butylamine (BA: CH3(CH2)3NH3+), octylamine (OA: CH3(CH2)7NH3+), and dodecylamine (DA: CH3(CH2)11NH3+)]. This study revealed that the phase distribution of quasi-2D Sn-based perovskite crystals became disordered and less oriented with the increase of the alkyl chain length. Therefore, organic spacer cations with shorter chain lengths are favorable for inducing oriented crystal growth and ordered phase distribution. The advantages of applying short alkyl chains in the organic spacer cation (e.g., BA) mitigated the oxidation process of Sn2+ and delivered superior photovoltaic device performance.
| (PEA)2(FA)n−1SnnI3n+1 (n = 5) |
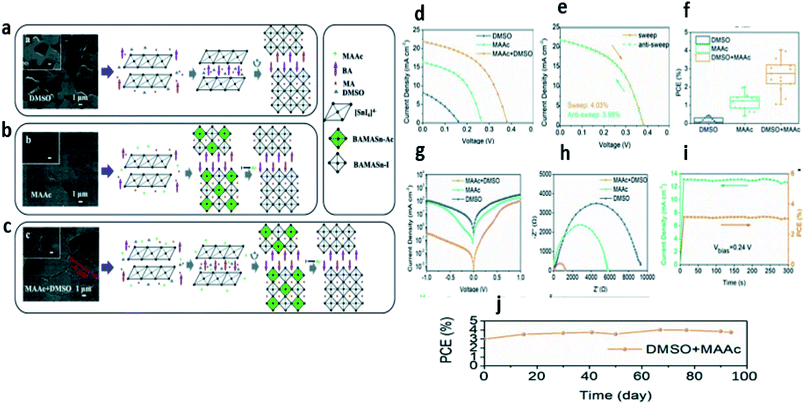 | ||
| Fig. 6 SEM images and schematic diagram of the crystallization process of LD RP BA2MA3Sn4I13 perovskite films fabricated from different solvents: (a) DMSO, (b) MAAc, and (c) DMSO + MAAc, (d) photocurrent density versus voltage curve, (e) hysteresis effect test, (f) PCE distribution statistics of the studied devices, (g) dark J–V curves, (h) electrical impedance spectrum, (i) stable output of the perovskite device, and (j) stability of the device stored in N2.95 (Reproduced from ref. 95 with permission from Wiley-VCH under the terms of the Creative Commons CC-BY license, copyright 2019.) | ||
Phenethylammonium iodide is the most common large organic cation used in 2D Pb-based perovskite solar cells. Liao et al.97 were the first to study quasi-2D tin perovskite solar cells by using PEA; they investigated the film formation and found that with 20% PEA in the precursor solution, the film grew perpendicular to the substrate. The perpendicular crystal growth in quasi-2D perovskite is beneficial for charge transfer and extraction. However, the photovoltaic parameters were investigated by fabricating the device with an inverted planar architecture based on ITO/NiOx/perovskite/PCBM/Al, and the highest PCE reached 5.94% with an open circuit voltage (VOC) of 0.59 V, a short-circuit current density (JSC) of 14.44 mA cm2, and a fill factor (FF) of 69%. In addition, the device retained its performance for over 100 hours without encapsulation, and the enhancement in photovoltaic parameters was due to the growth of compact and smooth films. From the results, the authors demonstrated that the dimensional reduction from 3D FASnI3 to quasi-2D (PEA)2(FA)4Sn5I16 significantly improved the material stability and effectively suppressed oxidation disproportionation, resulting in stable device performance.
Qiu et al.98 in their work investigated the mixed spacer organic cations n-butylamine (BA) and phenylethylamine (PEA) in quasi-2D RP Sn perovskite to control the crystallization process. They discovered that when BA+ and PEA+ were combined to form (BA0.5PEA0.5)FA3Sn4I13 quasi-2D RP perovskites, the intermediate phase hindered the homogeneous and ordered nucleation of the crystal and the Sn oxidation was suppressed (Fig. 7a); as a result, high-quality film morphology and improved crystal orientation were obtained (Fig. 7b and c). The device showed an efficiency of 8.82% with a VOC of 0.60 V, JSC of 21.82 mA cm−2, and FF of 66.73%, whereas the individual BA− (PEA−) based device exhibited an efficiency of 5.55% (6.42%) with a VOC of 0.55 V (0.58 V), JSC of 16.92 mA cm−2 (16.57 mA cm−2), and FF of 59.57% (66.56%) (Fig. 7d–f). Furthermore, the device with mixed BA–PEA showed significantly improved stability compared to that with the individual BA or PEA (Fig. 7g and h). More recently, Li and coworkers99 used phenyl ethyl ammonium chloride (PEACl) as an additive to grow pure phase quasi-2D crystals of Sn-perovskite with excellent vertical crystal orientation. The role of phenyl ethyl ammonium chloride was to act as a barrier layer at the surface of the crystals protecting tin from oxidation. The utilization of PEACl also helped to improve device performance. The device prepared with this strategy showed an excellent PCE (9.1%) with less hysteresis.
| AVA2FAn−1SnnI3n+1 (n = 5) |
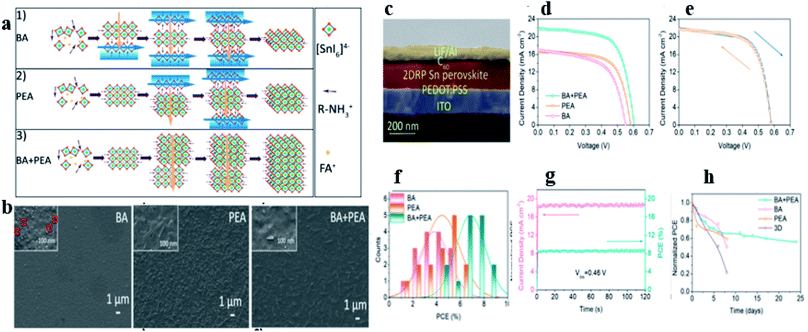 | ||
| Fig. 7 (a) Schematic illustrations of the crystal growth process in 2D RP Sn perovskites based on (1) BA, (2) PEA, and (3) BA + PEA, (b) top-view SEM images of 2D RP Sn perovskite films, (c) cross-sectional SEM image of the 2D RP Sn PSC, and (d) J−V curves of 2D RP Sn perovskite devices based on BA, PEA, and BA + PEA. (e) Hysteresis effect of the 2D RP Sn perovskite device based on BA + PEA, (f) histogram of PCEs of BA-based, PEA-based, and BA + PEA-based 2D RP Sn PSCs, (g) stable output of BA + PEA-based 2D RP Sn PSCs at Vbis = 0.46 V for 120 s, (h) stability of 2D RP (BA, PEA, and BA + PEA) and 3D Sn perovskite devices in a N2 atmosphere.98 (Reproduced from ref. 98 with permission from the American Chemical Society, copyright 2019.) | ||
To further improve the stability and the PCE of quasi-2D Sn based perovskite solar cells, Xu and coworkers100 developed a new quasi-2D Sn compound by using 5-ammoniumvaleric acid (5-AVA+) as the organic spacer. In order to enhance the vertical orientation of crystal growth, the authors added an appropriate amount of ammonium chloride (NH4Cl) as an additive, and this approach led to highly vertically oriented tin-based quasi-2D perovskites. The role of the highly ordered vertically oriented perovskite films was to significantly improve the charge collection efficiency between two electrodes. The solar cells employing AVA2FA4Sn5I16, as light absorbers delivered a power conversion efficiency of up to 8.71%. The superior crystal quality of perovskite thin-films facilitated the separation and extraction of carriers and prevented the thin film from attacking oxygen and moisture leading to better stability.
In short, quasi-2D RP Sn-based perovskite solar cells showed excellent environmental stability compared to their 3D counterparts, and their PCEs were much improved due to the high quality of perovskite films with lower defect densities and enhanced charge transfer to the charge transport layers.
5.2 Dion Jacobson (DJ) quasi-2D Sn based perovskite solar cells
This class is based on alkyl diammonium cations, leading to the formation of only one layer in quasi-2D perovskite which allows a more rigid structure than the RP one and reduces the interlayer distance. The shortened interlayer distance might reduce the barrier of charge transfer between inorganic [SnI6]4− slabs, which will benefit the charge transport and extraction.| (4AMP) (FA)3Sn4I13 (n = 4) |
Chen and coworkers101 were the first to investigate the quasi-2D DJ Sn-based perovskite solar cells. In their study, they used 4-(aminomethyl)piperidinium to form DJ perovskite (Fig. 8a) and found that the PL peak suggests an optical bandgap of 1.47 eV. However, they further investigated the effect of n number of inorganic layers on the optical absorption of these DJ perovskites, by varying n from 1 to 4, which results in a systematic red-shift of the absorption edge and the PL peak (Fig. 8b). After preparing a device without a HTL (Fig. 8c), a PCE of 4.22% was achieved, with a JSC of 14.90 mA cm−2, VOC of 0.64 V, and FF of 0.443 (Fig. 8d), and they discovered that only 9% decay of the initial PCE was observed after the device was exposed to 1 sun illumination in a N2 atmosphere at 45 °C for 100 hours (Fig. 8e). More recently, Chen et al.102 demonstrated the presence of 4AMP both at the surface and in the bulk of the tin-based thin film, which indicated the encapsulation in situ by 4AMP molecules of the film. This structure led to a possible passivation of both the grain surfaces and grain boundaries, which not only allowed a delay in the O2/H2O ingression into the bulk for improved stability, but also suppressed the generation of Sn-defects and reduced non-radiative recombination of photo-carriers. This approach led to a slightly increased VOC of 0.69 V and a much improved PCE: the champion device showed a PCE of 10.26%, with a VOC of 0.69 V, JSC of 21.15 mA cm−2, and FF of 0.703. In addition, the perovskite devices retained 77% of their initial PCE after 500 hours under continuous one sun illumination.
| (BEA)FA2Sn3I10 (n = 3) |
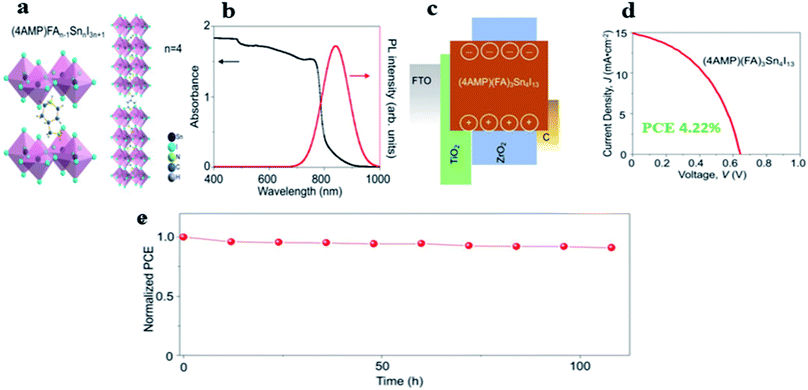 | ||
| Fig. 8 DJ Sn-based halide perovskite (4AMP) (FA)n−1SnnI3n+1 (n = 4): (a) schematic crystal structure, (b) absorption and PL characteristics, (c) energy-levels diagram for DJ, (d) V curves, (e) stability as a function of storage duration (in a N2 atmosphere, at 45 °C under 1-sun illumination).101 (Reproduced from ref. 101 with permission from the American Chemical Society, copyright 2018.) | ||
More recently, Li et al.103 introduced 1,4-butane diamine (BEA) into FASnI3 to develop DJ quasi-2D Sn perovskites, and they investigated the film quality and found that the (BEA)FA2Sn3I10 film exhibited high crystal symmetry, resulting in good absorption and carrier separation. The device achieved a PCE up to 6.43%, which was much higher than that of the 3D FASnI3 device (4.20%). This enhancement was attributed to the better film quality and reduced defects due to the inhibition of Sn2+ oxidation in the (BEA)FA2Sn3I10 film. In addition, the (BEA)FA2Sn3I10 devices showed more durability against oxidation, illumination, and humidity than 3D FASnI3 devices and lost only 9% of their initial PCE; this long-term stability was attributed to the stable structure and strong hydrophobicity of BEA.
These different studies showed that the introduction of ammonium cations featuring large organic residues leads to the formation of quasi-2D Sn-based perovskites which demonstrate that these large organic cations can efficiently block water from reacting with the perovskite layer, leading to a strongly enhanced stability of the material. This means that the organic cations prevent the oxidation of Sn2+ to Sn4+ and slow down self-doping effects. However, the formation of Sn4+ leads to the introduction of defect sites into the perovskite structures, which act as recombination centers for photo-excited charge carriers. Due to the quantum well structure the carrier transport properties of two dimensional metal halide-based perovskites are poor. Therefore, their PCE is still low due to the presence of the insulator part of organic cations, which hinders the charge transport and extraction.
The inclusion of quasi-2D perovskites impacts carrier transport and causes a decrease in PCE. To further improve the charge transport, mixed dimensional perovskites (2D/3D) have been explored which combine the synergetic effect of these two types of structures (2D which is more stable and 3D which is more efficient) and then further increase the stability without sacrificing the performance. Mixed 2D/3D Sn-based perovskite solar cells are presented in the next section, to show the advantage of using these structures to improve the performance of solar cells.
6. Mixed dimensional (3D/2D) Sn-based perovskite solar cells
The PCE of quasi-2D tin is still low compared to those of 3D ones; to further increase the PCE in Sn based perovskite, researchers have developed mixed dimensional perovskite by reducing the content of large organic cations in the precursor solution to obtain 2D/3D structures.104 Considering the advantages of this complex structure, Shao et al.105 incorporated a small concentration of PEA (0.08 M) in the precursor solution and obtained 2D/3D Sn perovskite solar cells. They argued that the extended ordering and packing of crystal planes improved the robustness and integrity of the perovskite structure and helped to suppress the formation of the tin vacancies and therefore the background carrier density. The high degree of crystallinity and the preferential crystal orientation are key factors for improving the efficiency of perovskite solar cells. The 2D/3D champion device showed a VOC of 0.525 V, a JSC of 24.1 mA cm−2 and an FF of 0.71 resulting in a PCE of 9.0%.The 2D/3D Sn-based perovskite solar cells demonstrated much higher stability upon exposure to light and ambient conditions due to the enhanced robustness of the perovskite films. Kim et al.106 incorporated formamidinium thiocyanate (FASCN) as an additive into the mixed 2D/3D tin-based perovskites to improve the degree of crystallinity in the out-of-plane direction (Fig. 9a–c). They discovered that FASCN protected perovskites from oxidation during film formation, due to the strong chemical interactions with the tin component (Sn2+). With the additive, the 2D/3D champion PSCs achieved a PCE of 8.17% with a JSC of 22.5 mA cm−2, VOC of 0.53 V and FF of 68.3% which are much higher than those for the control device which shows a JSC of 19.9 mA cm−2, VOC of 0.50 V, FF of 57.5%, and PCE of 5.74% (Fig. 9d–f). More remarkably, the perovskite device did not show any degradation after exposure to solar light for 2 hours and retained more than 90% of its initial performance after storing in a nitrogen environment for 1000 hours as illustrated in Fig. 9h. The improved ambient stability of the 2D/3D-based device is probably due to higher resistance to oxygen and moisture as a result of the improved crystallinity and the higher hydrophobicity of the perovskite film. Jokar et al.107 in their study utilized ethylene diammonium diiodide (EDAI2) and butylammonium iodide (BAI) as additives in FASnI3 films to passivate the surface defect states and to prevent Sn2+/Sn4+ oxidation. The addition of BAI improved the PCE from 4.0% (3D) to 5.5%. The addition of EDAI2 into the perovskite film further improved the initial PCE to 7.4%, which continuously increased to 8.9% when the device was stored in a glovebox for over 1400 hours. In the same trend of using EDAI2 as an additive in 2D/3D Sn based perovskite solar cells108 Jokar et al. in 2019 incorporated GAI cations into the FASnI3 precursor solution with ethylene diammonium diiodide (EDAI2) as the additive to enhance both the photovoltaic performance and the stability of tin-based perovskites; they demonstrated that the PCE of the device was continuously increased from 8.5% in the fresh sample to 9.6% after storage in a glovebox environment for 2000 hours. Ran and coworkers109 developed a bilateral interfacial engineering strategy to fabricate efficient 2D/3D Sn based PSCs; this strategy was based on a multichannel inter-diffusion two-step film fabrication protocol which involved evaporating PEA on the top of the FA film to form a PEAI/FAI mixture, and a 2D/3D (PEA, FA)SnI3 bulk heterojunction film was obtained after evaporation of SnI2. They found that the film structure was able successfully to improve the performance and stability of the PSC devices. As a result, a champion efficiency of 6.98% was achieved.
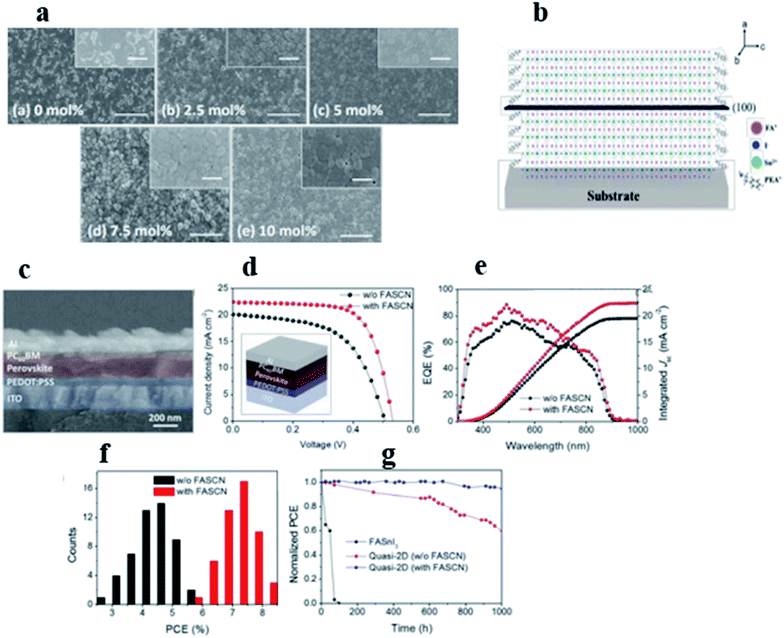 | ||
| Fig. 9 (a) SEM images of quasi-2D tin-based perovskite films (10% PEAI) with FASCN additive amounts, (b) schematic crystal structure of a quasi-2D tin-based perovskite film (10% PEAI), (c) cross-sectional SEM image of a fabricated device, (d) J–V curves under AM 1.5 G illumination for the best-performing quasi-2D tin-based perovskite solar cell with the FASCN additive compared to the reference cell (inset shows the schematic illumination of device configuration), (e) EQE spectra and integrated JSC calculated from the EQE, (f) distribution of PCEs for 50 devices in each case, and (g) stability represented by the normalized PCEs of unencapsulated devices in a nitrogen-filled glove box for over 1000 hours (H2O < 2.2 ppm, O2 < 5 ppm).106 (Reproduced from ref. 106 with permission from the Royal Society of Chemistry, copyright 2018.) | ||
Some strategies like the triple cation strategy has also been developed for mixed 2D/3D Sn based perovskite solar cells to stabilize Sn2+. Rath et al.110 used a triple cation MA0.75FA0.15PEA0.1SnI3 to develop mixed dimensional Sn based perovskite. In their study, they combined compositional engineering with a double anti-solvent dripping process using chlorobenzene and a preheated substrate at 70 °C to obtain a homogeneous and pinhole free absorber layer. The photovoltaic parameters are listed in Table 1. Furthermore, the perovskite devices retained 87% of their initial PCE after storage in a glovebox environment for 5000 hours and further stability tests under active load and continuous illumination also revealed exceptional stability of these solar cells in operation.
| Perovskites | Device structure | V OC (mV) | J SC (mA cm−2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| Quasi-2D RP | ||||||
| (BA)2(MA)4Sn5I16 | FTO/c-TiO2/perovskite/PTAA/AU | 0.23 | 24.1 | 45.7 | 2.53 | 94 |
| (BA)2(FA)2Sn3I10 | FTO/c-TiO2/m-TiO2/perovskite/PTAA/AU | 0.42 | 23.98 | 40.21 | 4.04 | 96 |
| (PEA)2(FA)4Sn5I16 | ITO/NiOx/perovskite/PCBM/Al | 0.59 | 14.44 | 69 | 5.9 | 97 |
| (BA0.5PEA0.5)2FA3Sn4I13 | ITO/PEDOT:PSS/perovskite/C60/LiF/Al | 0.60 | 21.82 | 66.73 | 8.82 | 98 |
| Bn2FASn2I7 | FTO/c-TiO2/m-TiO2/perovskite/AU | 0.40 | 10.55 | 55.1 | 2.35 | 116 |
| FASnI3:PEACl | ITO/NiOx/perovskite/PCBM/Al | 0.59 | 22.06 | 69 | 9.1 | 99 |
| AVA2FA4Sn5I16 | ITO/PEDOT:PSS/perovskite/PCBM/BCP/Ag | 0.61 | 21.0 | 68.0 | 8.71 | 100 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Quasi-2D DJ | ||||||
| (4AMP) (FA)3Sn4I13 | FTO/TiO2/ZrO2/perovskite/C | 0.64 | 14.90 | 44.3 | 4.22 | 101 |
| (BEA)FA2Sn3I10 | ITO/PEDOT:PSS/perovskite/PCBM/BCP/Ag | 0.62 | 18.85 | 55 | 6.43 | 103 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| 2D/3D | ||||||
| PEA0.08FA0.92SnI3 | ITO/PEDOT:PSS/perovskite/PCBM/BCP/Ag | 0.52 | 24.1 | 71 | 9.0 | 105 |
| PEA0.1FA0.9SnI3 | ITO/PEDOT:PSS/perovskite/PCBM/Al | 0.53 | 22.5 | 68.3 | 8.17 | 106 |
| EDA0.1FA0.9SnI3 | ITO/PEDOT:PSS/perovskite/C60/BCP/Ag | 0.58 | 21.3 | 71.8 | 8.9 | 107 |
| GA0.2FA0.78SnI3 | ITO/PEDOT:PSS/perovskite/C60/BCP/Ag | 0.61 | 21.2 | 72 | 9.6 | 108 |
| (PEA, FA) SnI3 | ITO/PEDOT:PSS/perovskite/C60/BCP/Ag | 0.47 | 20.07 | 74 | 6.98 | 109 |
| MA0.75FA0.15PEA0.1SnI3 | ITO/PEDOT:PSS/perovskite/C60/BCP/Ag | 0.45 | 18.4 | 61 | 5.0 | 110 |
| PEA0.15FA0.85SnI3 | ITO/NiOx/perovskite/PCBM/CBP/Ag | 0,61 | 22.0 | 70.1 | 9.41 | 112 |
| PEA0.15FA0.85SnI3 | ITO/PEDOT:PSS/perovskite/IBCA/BCP/Ag | 0.94 | 17.4 | 75 | 12.4 | 113 |
Kayesh and coworkers111 in their study investigated the effect of 5-ammonium valeric acid iodide (5-AVAI) on grain boundaries, film formation and crystallinity of FASnI3 based perovskite with a large area. After morphological, structural and elemental characterization, they found that 5-AVAI affected the crystal growth of the perovskite film through its hydrogen bonds with I- of the SnI64− octahedra with lower Sn4+ content. In addition, the PCE of PSCs was not only improved from 3.4% to 7.0% in a 0.25 cm2 area, but also remained stable under 1 sun continuous illumination at maximum power point tracking for 100 hours. Indeed, the existence of 5-AVAI molecules at the grain boundaries and the formation of highly crystalline FASnI3 films effectively enhanced their air stability.
In the same trend of controlling the orientation of crystal growth and phase distribution in mixed dimensional perovskites, Wang et al.112 reported that the incorporation of ammonium thiocyanate (NH4SCN) into the 2D/3D Sn perovskite considerably improved the film quality and pushed the PCE close to that of the 3D Sn based perovskite solar cells. An efficiency of 9.41% was achieved for mixed 2D/3D Sn-based perovskite solar cells, which was due to the increased carrier mobility and reduced background carrier density. The device based on this technique showed much enhanced stability and retained 90% of its initial PCE for nearly 600 hours.
More recently, Jiang and coworkers113 developed a new design of ETMs through replacing PCBM with ICBA and performing additive engineering to reduce the interface carrier recombination. In their study, they used PEAI as a spacer cation and NH4SCN as an additive and found that the film obtained by this engineering exhibited diminished defect density (Fig. 10a). The reduced density of defects of the SCN-treated film led to an increased open circuit voltage and efficiency of the device (Fig. 10g and h); as a result, the champion device gave a record power conversion efficiency of 12.4% with a VOC = 0.94 V, JSC = 17.4 mA cm−2, and FF = 75% (Fig. 10b–d). In addition, the device demonstrated excellent shelf stability by maintaining 90% of its initial efficiency for over 3800 hours (Fig. 10e and f).
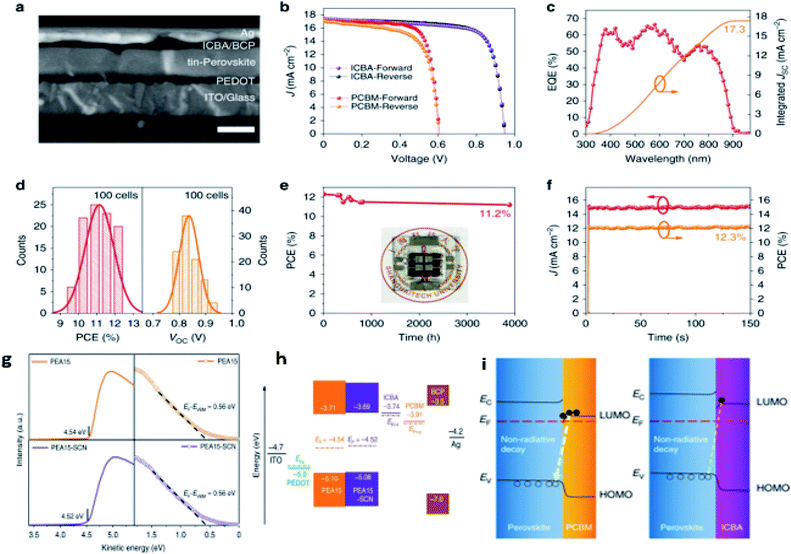 | ||
| Fig. 10 (a) Cross-sectional SEM image of the PEA15-SCN device, the scale bar is 200 nm, (b) J–V curves of the certified PEA15-SCN device with ICBA and the champion device of the PEA15-SCN film with PCBM, (c) EQE curve and integrated JSC of the certified PEA15-SCN device, (d) histograms for PCE and VOC of the PEA15-SCN device, (e) the stability of the encapsulated PEA15-SCN device stored in a N2 atmosphere, (f) stabilized power output for the PEA15-SCN device (at 0.81 V) under simulated AM 1.5 G solar illumination at 100 mW cm−2, (g) UPS spectra of the secondary electron cutoff and valence band of perovskite films, (h) schematic illustration of energy levels (dashed lines represent the quasi-Fermi level of ICBA (EFn–I), PCBM (EFn–P), and PEDOT (EFp)), (i) the schematic diagrams of interface recombination for the two samples.113 (Reproduced from ref. 113 with permission from Nature Communications under the terms of the Creative Commons CC-BY license, copyright 2020.) | ||
Table 1 summarizes the PCEs of quasi-2D and 2D/3D Sn based perovskite solar cells and Table 2 shows some additives used in the control of film formation. All of these efforts have been developed to improve the stability and efficiency of Sn based perovskite solar cells; however, the efficiency is still lower compared to that of the Pb based perovskite solar cells.
| Co-additives | Role of co-additives |
|---|---|
| Ammonium thiocyanate (NH4SCN) | Control the crystal growth and the phase distribution |
| Ammonium chloride (NH4Cl) | Control the orientation and the morphology of crystal growth |
| Formamidinium thiocyanate (FASCN) | Control the orientation of crystal growth |
| Ethylene diammonium diiodide (EDAI2) | Control the film morphology and induce defect passivation |
Furthermore, additional efforts are still required not only to protect the oxidation of Sn but also to improve the efficiency while having a particular effect on charge transport layers and the homogeneous energy landscape. The homogeneous energy landscape decreases the defect density and reduces the film's energy disorder but also allows an increase in the open circuit voltage, which is the big obstacle to obtaining high efficiency in Sn based perovskite solar cells. In mixed dimensional perovskites, high n values provide better sunlight absorption and higher carrier mobility; in contrast low n values provide moisture resistance, which can be attributed to the hydrophobicity of the organic layers and higher-quality films.
The enhanced stability of the mixed dimensional based perovskites might be attributed to the hydrophobicity of the long cation chain, the highly oriented and dense nature of the 2D perovskite films, and the suppressed ion migration in 3D perovskites.114,115
7. Conclusion
In this report, we have reviewed recent advances in quasi-2D and 2D/3D Sn-based perovskite solar cells. They have great potential to replace Pb based perovskite solar cells, as illustrated by the continuous increase in the PCE from 2.5% for the pure quasi-2D in the first report to 12% for the mixed 2D/3D currently with excellent stability. This improvement in performance and stability was due to the more enhanced film quality and crystal orientation parallel to the substrate, ensuring a balance between the performances, bandgaps, charge transport properties, and ambient stability, which are critical for photovoltaic applications, but above all due to inhibiting tin oxidation from Sn2+ to Sn4+. However, the current results of solar cell efficiency show that the Sn-based perovskites are even more inferior to the Pb-based perovskites, and the main reason for the relatively low efficiency of Sn-based perovskite solar cells is the low open circuit voltage (VOC), which is due to the high rate of recombination instead of charge transfer. Much effort should be made to push the PCE of Sn-based PSCs near that of the Pb based perovskite solar cells.Researchers should focus on (1) the design of new hydrophobic cations combined with co-additives and co-cations to develop mixed dimensional (2D/3D) Sn-based perovskite solar cells, which can at the same time improve both the stability and the photovoltaic performance of Sn-based perovskites by reducing the rate of oxidation of Sn2+ to Sn4+ as much possible. The mixed dimensional solar cell has shown the possibility of controlling the crystal growth orientation on the substrate and avoiding self-oxidation of Sn. More research has to be undertaken (2) to design new charge transport materials to better achieve charge transport across the different interfaces and increase VOC as well as reducing the defect concentration associated with interface recombination and tuning the energy level alignment. Additional efforts should be made (3) to develop advanced device structures to further improve the PCE of quasi-2D and 2D/3D Sn-based PSCs. Particular effort should be made (4) to develop an environmentally friendly solvent which will contribute to decreasing the degree of toxicity in perovskite materials and to find the optimum between crystal orientation and charge transport. In addition, the solvent is of great importance in the control of crystallization and crystal growth.
Current research on quasi-2D and 2D/3D Sn perovskite is still in the early stage, and there is a long way to go to realize high-performance. Besides, we believe that 2D/3D Sn-based perovskite materials have the most promising prospects in the future of photovoltaic applications.
Conflicts of interest
“There are no conflicts to declare”.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. 2017YQ003 & 2014MS32), Beijing outstanding talents cultivation funding project (No. 2013A009005000003) and the National Natural Science Foundation of China (No. 51102092).References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS.
- NREL Best-Research-Cell-Efficiencies.20201026.pdf., https://www.nrel.gov/pv/cell-efficiency.html.
- Q. Zhang, F. Hao, J. Li, Y. Zhou, Y. Wei and H. Lin, Perovskite solar cells: must lead be replaced - and can it be done?, Sci. Technol. Adv. Mater., 2018, 19(1), 425–442 CrossRef CAS.
- H. Yao, F. Zhou, Z. Li, Z. Ci, L. Ding and Z. Jin, Strategies for Improving the Stability of Tin-Based Perovskite (ASnX3) Solar Cells, Adv. Sci., 2020, 1903540 CrossRef CAS.
- X. Zhao, H. Shen, Y. Zhang, X. Li, X. Zhao, M. Tai, J. Li, J. Li, X. Li and H. Lin, Aluminum-Doped Zinc Oxide as Highly Stable Electron Collection Layer for Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2016, 8, 7826–7833 CrossRef CAS.
- E. Li, Y. Guo, T. Liu, W. Hu, N. Wang, H. He and H. Lin, Preheating-assisted deposition of solution-processed perovskite layer for an efficiency-improved inverted planar composite heterojunction solar cell, RSC Adv., 2016, 6(37), 30978–30985 RSC.
- M. Gratzel, The light and shade of perovskite solar cells, Nat. Mater., 2014, 13(9), 838–842 CrossRef CAS.
- J. Burschka, N. Pellet, S. J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Gratzel, Sequential deposition as a route to high-performance perovskite-sensitized solar cells, Nature, 2013, 499(7458), 316–319 CrossRef CAS.
- P. P. Boix, K. Nonomura, N. Mathews and S. G. Mhaisalkar, Current progress and future perspectives for organic/inorganic perovskite solar cells, Mater. Today, 2014, 17(1), 16–23 CrossRef CAS.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites, Science, 2012, 18, 65–72 Search PubMed.
- N.-G. Park, Perovskite solar cells: an emerging photovoltaic technology, Mater. Today, 2015, 18(2), 65–72 CrossRef CAS.
- Z. Song, C. L. McElvany, A. B. Phillips, I. Celik, P. W. Krantz, S. C. Watthage, G. K. Liyanage, D. Apul and M. J. Heben, A technoeconomic analysis of perovskite solar module manufacturing with low-cost materials and techniques, Energy Environ. Sci., 2017, 10(6), 1297–1305 RSC.
- M. Cai, Y. Wu, H. Chen, X. Yang, Y. Qiang and L. Han, Cost-Performance Analysis of Perovskite Solar Modules, Adv. Sci., 2017, 4(1), 1600269 CrossRef.
- H. Zhang, M. K. Nazeeruddin and W. C. H. Choy, Perovskite Photovoltaics: The Significant Role of Ligands in Film Formation, Passivation, and Stability, Adv. Mater., 2019, 31(8), e1805702 CrossRef.
- D. B. Mitzi, K. Chondroudis and C. R. Kagan, Organic–inorganic electronics, IBM J. Res. Dev., 2001, 45, 29–45 CAS.
- A. H. Slavney, T. Hu, A. M. Lindenberg and H. I. Karunadasa, A Bismuth-Halide Double Perovskite with Long Carrier Recombination Lifetime for Photovoltaic Applications, J. Am. Chem. Soc., 2016, 138(7), 2138–2141 CrossRef CAS.
- A. M. Elseman, A. E. Shalan, S. Sajid, M. M. Rashad, A. M. Hassan and M. Li, Copper-Substituted Lead Perovskite Materials Constructed with Different Halides for Working (CH3NH3)2CuX4-Based Perovskite Solar Cells from Experimental and Theoretical View, ACS Appl. Mater. Interfaces, 2018, 10(14), 11699–11707 CrossRef CAS.
- J. Balmes, K. M. Cecil, C. J. Brubaker, C. M. Adler, K. N. Dietrich, M. Altaye, J. C. Egelhoff, S. Wessel, I. Elangovan, R. Hornung, K. Jarvis and B. P. Lanphear, Decreased Brain Volume in Adults with Childhood Lead Exposure, PLoS Med., 2008, 5(5), e112 CrossRef.
- A. Babayigit, A. Ethirajan, M. Muller and B. Conings, Toxicity of organometal halide perovskite solar cells, Nat. Mater., 2016, 15(3), 247–251 CrossRef CAS.
- Z. Shi, J. Guo, Y. Chen, Q. Li, Y. Pan, H. Zhang, Y. Xia and W. Huang, Lead-Free Organic-Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives, Adv. Mater., 2017, 29(16), 1605005 CrossRef.
- D. Cortecchia, H. A. Dewi, J. Yin, A. Bruno, S. Chen, T. Baikie, P. P. Boix, M. Gratzel, S. Mhaisalkar, C. Soci and N. Mathews, Lead-Free MA2CuCl(x)Br(4-x) Hybrid Perovskites, Inorg. Chem., 2016, 55(3), 1044–1052 CrossRef CAS.
- I. Kopacic, B. Friesenbichler, S. F. Hoefler, B. Kunert, H. Plank, T. Rath and G. Trimmel, Enhanced Performance of Germanium Halide Perovskite Solar Cells through Compositional Engineering, ACS Appl. Energy Mater., 2018, 1(2), 343–347 CrossRef CAS.
- W. Zi, X. Ren, X. Ren, Q. Wei, F. Gao and S. F. Liu, Perovskite/germanium tandem: A potential high efficiency thin film solar cell design, Opt. Commun., 2016, 380, 1–5 CrossRef CAS.
- F. Hao, C. C. Stoumpos, D. H. Cao, R. P. H. Chang and M. G. Kanatzidis, Lead-free solid-state organic–inorganic halide perovskite solar cells, Nat. Photonics, 2014, 8(6), 489–494 CrossRef CAS.
- C. R. Kagan, D. B. Mitzi and C. D. Dimitrakopoulos, Organic-Inorganic Hybrid Materials as Semiconducting Channels in Thin-Film Field-Effect Transistors, Science, 1999, 286, 945–947 CrossRef CAS.
- M. Konstantakou and T. Stergiopoulos, A critical review on tin halide perovskite solar cells, J. Mater. Chem. A, 2017, 5(23), 11518–11549 RSC.
- W. Ke and M. G. Kanatzidis, Prospects for low-toxicity lead-free perovskite solar cells, Nat. Commun., 2019, 10(1), 965 CrossRef.
- Y. Ogomi, A. Morita, S. Tsukamoto, T. Saitho, N. Fujikawa, Q. Shen, T. Toyoda, K. Yoshino, S. S. Pandey, T. Ma and S. Hayase, <CH3NH3SnxPb(1−x)I3 Perovskite Solar Cells Covering up to 1060 nm, J. Phys. Chem. Lett., 2014, 5, 1004–1011 CrossRef CAS.
- W. Liao, D. Zhao, Y. Yu, C. R. Grice, C. Wang, A. J. Cimaroli, P. Schulz, W. Meng, K. Zhu, R. G. Xiong and Y. Yan, Lead-Free Inverted Planar Formamidinium Tin Triiodide Perovskite Solar Cells Achieving Power Conversion Efficiencies up to 6.22, Adv. Mater., 2016, 28(42), 9333–9340 CrossRef CAS.
- S. J. Lee, S. S. Shin, Y. C. Kim, D. Kim, T. K. Ahn, J. H. Noh, J. Seo and S. I. Seok, Fabrication of Efficient Formamidinium Tin Iodide Perovskite Solar Cells through SnF(2)-Pyrazine Complex, J. Am. Chem. Soc., 2016, 138(12), 3974–3977 CrossRef CAS.
- T.-B. Song, T. Yokoyama, S. Aramaki and M. G. Kanatzidis, Performance Enhancement of Lead-Free Tin-Based Perovskite Solar Cells with Reducing Atmosphere-Assisted Dispersible Additive, ACS Energy Lett., 2017, 2(4), 897–903 CrossRef CAS.
- X. Liu, K. Yan, D. Tan, X. Liang, H. Zhang and W. Huang, Solvent Engineering Improves Efficiency of Lead-Free Tin-Based Hybrid Perovskite Solar Cells beyond 9%, ACS Energy Lett., 2018, 3(11), 2701–2707 CrossRef CAS.
- R. M. I. Bandara, K. D. G. I. Jayawardena, S. O. Adeyemo, S. J. Hinder, J. A. Smith, H. M. Thirimanne, N. C. Wong, F. M. Amin, B. G. Freestone, A. J. Parnell, D. G. Lidzey, H. J. Joyce, R. A. Sporea and S. R. P. Silva, Tin(iv) dopant removal through anti-solvent engineering enabling tin based perovskite solar cells with high charge carrier mobilities, J. Mater. Chem. C, 2019, 7(27), 8389–8397 RSC.
- G. Liu, C. Liu, Z. Lin, J. Yang, Z. Huang, L. Tan and Y. Chen, Regulated Crystallization of Efficient and Stable Tin-Based Perovskite Solar Cells via a Self-Sealing Polymer, ACS Appl. Mater. Interfaces, 2020, 1700204 Search PubMed.
- Z. Zhao, F. Gu, Y. Li, W. Sun, S. Ye, H. Rao, Z. Liu, Z. Bian and C. Huang, Mixed-Organic-Cation Tin Iodide for Lead-Free Perovskite Solar Cells with an Efficiency of 8.12, Adv. Sci., 2017, 4(11), 1700204 CrossRef.
- Z. Lin, C. Liu, G. Liu, J. Yang, X. Duan, L. Tan and Y. Chen, Preparation of efficient inverted tin-based perovskite solar cells via the bidentate coordination effect of 8-hydroxyquinoline, Chem. Commun., 2020, 4007–4010 RSC.
- W. Gao, C. Ran, J. Li, H. Dong, B. Jiao, L. Zhang, X. Lan, X. Hou and Z. Wu, Robust Stability of Efficient Lead-Free Formamidinium Tin Iodide Perovskite Solar Cells Realized by Structural Regulation, J. Phys. Chem. Lett., 2018, 9(24), 6999–7006 CrossRef CAS.
- S. Tsarev, A. G. Boldyreva, S. Y. Luchkin, M. Elshobaki, M. I. Afanasov, K. J. Stevenson and P. A. Troshin, Hydrazinium-assisted stabilisation of methylammonium tin iodide for lead-free perovskite solar cells, J. Mater. Chem. A, 2018, 6(43), 21389–21395 RSC.
- S. Zhang, P. Audebert, Y. Wei, A. Al Choueiry, G. Lanty, A. Bréhier, L. Galmiche, G. Clavier, C. Boissière, J.-S. Lauret and E. Deleporte, Preparations and Characterizations of Luminescent Two Dimensional Organic-inorganic Perovskite Semiconductors, Materials, 2010, 3(5), 3385–3406 CrossRef CAS.
- T. C.-J. Yang, P. Fiala, Q. Jeangros and C. Ballif, High-Bandgap Perovskite Materials for Multijunction Solar Cells, Joule, 2018, 2(8), 1421–1436 CrossRef CAS.
- I. C. Smith, E. T. Hoke, D. Solis-Ibarra, M. D. McGehee and H. I. Karunadasa, A layered hybrid perovskite solar-cell absorber with enhanced moisture stability, Angew. Chem., Int. Ed., 2014, 53(42), 11232–11235 CrossRef CAS.
- L. Dou, Emerging two-dimensional halide perovskite nanomaterials, J. Mater. Chem. C, 2017, 5(43), 11165–11173 RSC.
- Y. Wei, P. Audebert, L. Galmiche, J. S. Lauret and E. Deleporte, Synthesis, optical properties and photostability of novel fluorinated organic–inorganic hybrid (R-NH3)2PbX4 semiconductors, J. Phys. D: Appl. Phys., 2013, 46(13), 135105 CrossRef.
- Q. Tai, J. Cao, T. Wang and F. Yan, Recent advances toward efficient and stable tin-based perovskite solar cells, EcoMat, 2019, 1(1), e12004 CrossRef.
- J. Wang, J. Dong, F. Lu, C. Sun, Q. Zhang and N. Wang, Two-dimensional lead-free halide perovskite materials and devices, J. Mater. Chem. A, 2019, 7(41), 23563–23576 RSC.
- C. Gai, J. Wang, Y. Wang and J. Li, The Low-Dimensional Three-Dimensional Tin Halide Perovskite: Film Characterization and Device Performance, Energies, 2019, 13(1), 1–26 CrossRef.
- D. H. Cao, C. C. Stoumpos, O. K. Farha, J. T. Hupp and M. G. Kanatzidis, 2D Homologous Perovskites as Light-Absorbing Materials for Solar Cell Applications, J. Am. Chem. Soc., 2015, 137(24), 7843–7850 CrossRef CAS.
- J. Qiu, Y. Zheng, Y. Xia, L. Chao, Y. Chen and W. Huang, Rapid Crystallization for Efficient 2D Ruddlesden–Popper (2DRP) Perovskite Solar Cells, Adv. Funct. Mater., 2018, 29(47), 1806831 CrossRef.
- X. Dai, K. Xu and F. Wei, Recent progress in perovskite solar cells: the perovskite layer, Beilstein J. Nanotechnol., 2020, 11, 51–60 CrossRef CAS.
- C. Li, Y. Pan, J. Hu, S. Qiu, C. Zhang, Y. Yang, S. Chen, X. Liu, C. J. Brabec, M. K. Nazeeruddin, Y. Mai and F. Guo, Vertically Aligned 2D/3D Pb–Sn Perovskites with Enhanced Charge Extraction and Suppressed Phase Segregation for Efficient Printable Solar Cells, ACS Energy Lett., 2020, 5(5), 1386–1395 CrossRef CAS.
- R. K. Misra, B. E. Cohen, L. Iagher and L. Etgar, Low-Dimensional Organic-Inorganic Halide Perovskite: Structure, Properties, and Applications, ChemSusChem, 2017, 10(19), 3712–3721 CrossRef CAS.
- Y. Wang, W. Fu, J. Yan, J. Chen, W. Yang and H. Chen, Low-bandgap mixed tin–lead iodide perovskite with large grains for high performance solar cells, J. Mater. Chem. A, 2018, 6(27), 13090–13095 RSC.
- Z. Cheng and J. Lin, Layered organic–inorganic hybrid perovskites: structure, optical properties, film preparation, patterning and templating engineering, CrystEngComm, 2010, 12, 2646–2662 RSC.
- K. Liang, D. B. Mitzi and M. T. Prikas, Synthesis and Characterization of Organic-Inorganic Perovskite Thin Films Prepared Using a Versatile Two-Step Dipping Technique, Chem. Mater., 1998, 10, 403–411 CrossRef CAS.
- Y. Zhong, R. Munir, J. Li, M.-C. Tang, M. R. Niazi, K. Zhao and A. Amassian, Blade-Coated Organolead Triiodide Perovskite Solar Cells with Efficiency >17%: An In Situ Investigation, ACS Energy Lett., 2018, 3(5), 1078–1085 CrossRef CAS.
- F. Guo, S. Qiu, J. Hu, H. Wang, B. Cai, J. Li, X. Yuan, X. Liu, K. Forberich, C. J. Brabec and Y. Mai, A Generalized Crystallization Protocol for Scalable Deposition of High-Quality Perovskite Thin Films for Photovoltaic Applications, Adv. Sci., 2019, 6(17), 1901067 CrossRef.
- A. T. Barrows, A. J. Pearson, C. K. Kwak, A. D. F. Dunbar, A. R. Buckley and D. G. Lidzey, Efficient planar heterojunction mixed-halide perovskite solar cells deposited via spray-deposition, Energy Environ. Sci., 2014, 7(9), 2944–2950 RSC.
- F. Guo, W. He, S. Qiu, C. Wang, X. Liu, K. Forberich, C. J. Brabec and Y. Mai, Sequential Deposition of High-Quality Photovoltaic Perovskite Layers via Scalable Printing Methods, Adv. Funct. Mater., 2019, 29(24), 1900964 CrossRef.
- L. Zheng, D. Zhang, Y. Ma, Z. Lu, Z. Chen, S. Wang, L. Xiao and Q. Gonga, Morphology Control of the Perovskite Film for Efficient Solar Cells, Dalton Trans., 2015, 10582–10593 RSC.
- D. G. Billing and A. Lemmerer, Inorganic–organic hybrid materials incorporating primary cyclic ammonium cations: The lead iodide series, CrystEngComm, 2007, 9(3), 236–244 RSC.
- Q. Chen, N. De Marco, Y. Yang, T.-B. Song, C.-C. Chen, H. Zhao, Z. Hong, H. Zhou and Y. Yang, Under the spotlight: The organic–inorganic hybrid halide perovskite for optoelectronic applications, Nano Today, 2015, 10(3), 355–396 CrossRef CAS.
- T. M. Koh, V. Shanmugam, J. Schlipf, L. Oesinghaus, P. Muller-Buschbaum, N. Ramakrishnan, V. Swamy, N. Mathews, P. P. Boix and S. G. Mhaisalkar, Nanostructuring Mixed-Dimensional Perovskites: A Route Toward Tunable, Efficient Photovoltaics, Adv. Mater., 2016, 28(19), 3653–3661 CrossRef CAS.
- L. Mao, C. C. Stoumpos and M. G. Kanatzidis, Two-Dimensional Hybrid Halide Perovskites: Principles and Promises, J. Am. Chem. Soc., 2019, 141(3), 1171–1190 CrossRef CAS.
- E. Shi, Y. Gao, B. P. Finkenauer, Akriti, A. H. Coffey and L. Dou, Two-dimensional halide perovskite nanomaterials and heterostructures, Chem. Soc. Rev., 2018, 47(16), 6046–6072 RSC.
- C. Ortiz-Cervantes, P. Carmona-Monroy and D. Solis-Ibarra, Two-Dimensional Halide Perovskites in Solar Cells: 2D or not 2D?, ChemSusChem, 2019, 12(8), 1560–1575 CrossRef CAS.
- K. Zheng and T. Pullerits, Two dimensions are better for perovskites, J. Phys. Chem. Lett., 2019, 10(19), 5881–5885 CrossRef CAS.
- X. Hong, T. Ishihara and A. V. Nurmikko, Dielectric confinement effect on excitons in PbI4-based layered semiconductors, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45(12), 6961–6964 CrossRef CAS.
- Z. Guo, X. Wu, T. Zhu, X. Zhu and L. Huang, Electron-Phonon Scattering in Atomically Thin 2D Perovskites, ACS Nano, 2016, 10(11), 9992–9998 CrossRef CAS.
- D. B. Straus and C. R. Kagan, Electrons, Excitons, and Phonons in Two-Dimensional Hybrid Perovskites: Connecting Structural, Optical, and Electronic Properties, J. Phys. Chem. Lett., 2018, 9(6), 1434–1447 CrossRef CAS.
- C. M. M. Soe, G. P. Nagabhushana, R. Shivaramaiah, H. Tsai, W. Nie, J.-C. Blancon, F. Melkonyan, D. H. Cao, B. Traoré, L. Pedesseau, M. Kepenekian, C. Katan, J. Even, T. J. Marks, A. Navrotsky, A. D. Mohite, C. C. Stoumpos and M. G. Kanatzidis, Structural and thermodynamic limits of layer thickness in 2D halide perovskites, Proc. Natl. Acad. Sci., 2019, 116(1), 58–66 CrossRef.
- L. Polavarapu, B. Nickel, J. Feldmann and A. S. Urban, Advances in Quantum-Confined Perovskite Nanocrystals for Optoelectronics, Adv. Energy Mater., 2017, 7(16), 1700267 CrossRef.
- C. Katan, N. Mercier and J. Even, Quantum and Dielectric Confinement Effects in Lower-Dimensional Hybrid Perovskite Semiconductors, Chem. Rev., 2019, 119(5), 3140–3192 CrossRef CAS.
- B. Cheng, T.-Y. Li, P. Maity, P.-C. Wei, D. Nordlund, K.-T. Ho, D.-H. Lien, C.-H. Lin, R.-Z. Liang, X. Miao, I. A. Ajia, J. Yin, D. Sokaras, A. Javey, I. S. Roqan, O. F. Mohammed and J.-H. He, Extremely reduced dielectric confinement in two-dimensional hybrid perovskites with large polar organics, Commun. Phys., 2018, 1(1), 80 CrossRef.
- J. Even, L. Pedesseau and C. Katan, Understanding quantum confinement of charge carriers in layered 2D hybrid perovskites, Chemphyschem, 2014, 15(17), 3733–3741 CrossRef CAS.
- Q. Zhang, R. Su, W. Du, X. Liu, L. Zhao, S. T. Ha and Q. Xiong, Advances in Small Perovskite-Based Lasers, Small Methods, 2017, 1(9), 1700163 CrossRef.
- J. S. Manser, J. A. Christians and P. V. Kamat, Intriguing Optoelectronic Properties of Metal Halide Perovskites, Chem. Rev., 2016, 116(21), 12956–13008 CrossRef CAS.
- H. Tsai, W. Nie, J. C. Blancon, C. C. Stoumpos, R. Asadpour, B. Harutyunyan, A. J. Neukirch, R. Verduzco, J. J. Crochet, S. Tretiak, L. Pedesseau, J. Even, M. A. Alam, G. Gupta, J. Lou, P. M. Ajayan, M. J. Bedzyk and M. G. Kanatzidis, High-efficiency two-dimensional Ruddlesden-Popper perovskite solar cells, Nature, 2016, 536(7616), 312–316 CrossRef CAS.
- F. Li, Y. Pei, F. Xiao, T. Zeng, Z. Yang, J. Xu, J. Sun, B. Peng and M. Liu, Tailored dimensionality to regulate the phase stability of inorganic cesium lead iodide perovskites, Nanoscale, 2018, 10(14), 6318–6322 RSC.
- X. Zhang, X. Ren, B. Liu, R. Munir, X. Zhu, D. Yang, J. Li, Y. Liu, D.-M. Smilgies, R. Li, Z. Yang, T. Niu, X. Wang, A. Amassian, K. Zhao and S. Liu, Stable high efficiency two-dimensional perovskite solar cells via cesium doping, Energy Environ. Sci., 2017, 10(10), 2095–2102 RSC.
- Y. Jiang, X. He, T. Liu, N. Zhao, M. Qin, J. Liu, F. Jiang, F. Qin, L. Sun, X. Lu, S. Jin, Z. Xiao, T. Kamiya and Y. Zhou, Intralayer A-Site Compositional Engineering of Ruddlesden–Popper Perovskites for Thermostable and Efficient Solar Cells, ACS Energy Lett., 2019, 4(6), 1216–1224 CrossRef CAS.
- D. B. Straus, S. Hurtado Parra, N. Iotov, J. Gebhardt, A. M. Rappe, J. E. Subotnik, J. M. Kikkawa and C. R. Kagan, Direct Observation of Electron-Phonon Coupling and Slow Vibrational Relaxation in Organic-Inorganic Hybrid Perovskites, J. Am. Chem. Soc., 2016, 138(42), 13798–13801 CrossRef CAS.
- A. Brehier, R. Parashkov, J. S. Lauret and E. Deleporte, Strong exciton-photon coupling in a microcavity containing layered perovskite semiconductors, Appl. Phys. Lett., 2006, 89(17), 171110 CrossRef.
- M. Braun, W. Tuffentsammer, W. Tuffentsammer and H. C. Wolf, Tailoring of energy levels in lead chloride based layered perovskites and energy transfer between the organic and inorganic planes, Chem. Phys. Lett., 1999, 157–164 CrossRef CAS.
- A. H. Proppe, R. Quintero-Bermudez, H. Tan, O. Voznyy, S. O. Kelley and E. H. Sargent, Synthetic Control over Quantum Well Width Distribution and Carrier Migration in Low-Dimensional Perovskite Photovoltaics, J. Am. Chem. Soc., 2018, 140(8), 2890–2896 CrossRef CAS.
- J. Liu, J. Leng, K. Wu, J. Zhang and S. Jin, Observation of Internal Photoinduced Electron and Hole Separation in Hybrid Two-Dimensional Perovskite Films, J. Am. Chem. Soc., 2017, 139(4), 1432–1435 CrossRef CAS.
- Y. Chen, S. Yu, Y. Sun and Z. Liang, Phase Engineering in Quasi-2D Ruddlesden-Popper Perovskites, J. Phys. Chem. Lett., 2018, 9(10), 2627–2631 CrossRef CAS.
- C. Ma, D. Shen, T. W. Ng, M. F. Lo and C. S. Lee, 2D Perovskites with Short Interlayer Distance for High-Performance Solar Cell Application, Adv. Mater., 2018, 30(22), e1800710 CrossRef.
- A. Z. Chen, M. Shiu, J. H. Ma, M. R. Alpert, D. Zhang, B. J. Foley, D.-M. Smilgies, S.-H. Lee and J. J. Choi, Origin of vertical orientation in two-dimensional metal halide perovskites and its effect on photovoltaic performance, Nat. Commun., 2018, 9(1), 1336 CrossRef.
- O. Voznyy, B. R. Sutherland, A. H. Ip, D. Zhitomirsky and E. H. Sargent, Engineering charge transport by heterostructuring solution-processed semiconductors, Nat. Rev. Mater., 2017, 2(6), 17026 CrossRef CAS.
- I. Poli, S. Eslava and P. Cameron, Tetrabutylammonium cations for moisture-resistant and semitransparent perovskite solar cells, J. Mater. Chem. A, 2017, 5(42), 22325–22333 RSC.
- G. Liu, Z. Liu, F. Zeng, X. Wang, S. Li and X. Xie, High performance two-dimensional perovskite solar cells based on solvent induced morphology control of perovskite layers, Chem. Phys. Lett., 2020, 743, 137186 CrossRef CAS.
- C. Zhao, W. Tian, J. Leng, Y. Zhao and S. Jin, Controlling the Property of Edges in Layered 2D Perovskite Single Crystals, J. Phys. Chem. Lett., 2019, 10(14), 3950–3954 CrossRef CAS.
- X. Zhang, G. Wu, W. Fu, M. Qin, W. Yang, J. Yan, Z. Zhang, X. Lu and H. Chen, Orientation Regulation of Phenylethylammonium Cation Based 2D Perovskite Solar Cell with Efficiency Higher Than 11%, Adv. Energy Mater., 2018, 8(14), 1702498 CrossRef.
- D. H. Cao, C. C. Stoumpos, T. Yokoyama, J. L. Logsdon, T.-B. Song, O. K. Farha, M. R. Wasielewski, J. T. Hupp and M. G. Kanatzidis, Thin Films and Solar Cells Based on Semiconducting Two-Dimensional Ruddlesden–Popper (CH3(CH2)3NH3)2(CH3NH3)n−1SnnI3n+1 Perovskites, ACS Energy Lett., 2017, 2(5), 982–990 CrossRef CAS.
- J. Qiu, Y. Xia, Y. Chen and W. Huang, Management of Crystallization Kinetics for Efficient and Stable Low-Dimensional Ruddlesden-Popper (LDRP) Lead-Free Perovskite Solar Cells, Adv. Sci., 2019, 6(1), 1800793 CrossRef.
- F. Li, Y. Xie, Y. Hu, M. Long, Y. Zhang, J. Xu, M. Qin, X. Lu and M. Liu, Effects of Alkyl Chain Length on Crystal Growth and Oxidation Process of Two-Dimensional Tin Halide Perovskites, ACS Energy Lett., 2020, 5(5), 1422–1429 CrossRef CAS.
- Y. Liao, H. Liu, W. Zhou, D. Yang, Y. Shang, Z. Shi, B. Li, X. Jiang, L. Zhang, L. N. Quan, R. Quintero-Bermudez, B. R. Sutherland, Q. Mi, E. H. Sargent and Z. Ning, Highly Oriented Low-Dimensional Tin Halide Perovskites with Enhanced Stability and Photovoltaic Performance, J. Am. Chem. Soc., 2017, 139(19), 6693–6699 CrossRef CAS.
- J. Qiu, Y. Xia, Y. Zheng, W. Hui, H. Gu, W. Yuan, H. Yu, L. Chao, T. Niu, Y. Yang, X. Gao, Y. Chen and W. Huang, 2D Intermediate Suppression for Efficient Ruddlesden–Popper (RP) Phase Lead-Free Perovskite Solar Cells, ACS Energy Lett., 2019, 4(7), 1513–1520 CrossRef CAS.
- M. Li, W.-w. Zuo, Y.-G. Yang, M. H. Aldamasy, Q. Wang, S. H. T. Cruz, S.-L. Feng, M. Saliba, Z.-K. Wang and A. Abate, Tin halide perovskite films made of highly oriented 2D crystals enable more efficient and stable lead-free perovskite solar cells, ACS Energy Lett., 2020, 1923–1929 CrossRef CAS.
- H. Xu, Y. Jiang, T. He, S. Li, H. Wang, Y. Chen, M. Yuan and J. Chen, Orientation Regulation of Tin-Based Reduced-Dimensional Perovskites for Highly Efficient and Stable Photovoltaics, Adv. Funct. Mater., 2019, 29(47), 1807696 CrossRef CAS.
- M. Chen, M.-G. Ju, M. Hu, Z. Dai, Y. Hu, Y. Rong, H. Han, X. C. Zeng, Y. Zhou and N. P. Padture, Lead-Free Dion−Jacobson Tin Halide Perovskites for Photovoltaics, ACS Energy Lett., 2018, 4, 276–277 CrossRef.
- M. Chen, Q. Dong, F. T. Eickemeyer, Y. Liu, Z. Dai, A. D. Carl, B. Bahrami, A. H. Chowdhury, R. L. Grimm, Y. Shi, Q. Qiao, S. M. Zakeeruddin, M. Grätzel and N. P. Padture, High-Performance Lead-Free Solar Cells Based on Tin-Halide Perovskite Thin Films Functionalized by a Divalent Organic Cation, ACS Energy Lett., 2020, 2223–2230 CrossRef CAS.
- P. Li, X. Liu, Y. Zhang, C. Liang, G. Chen, F. Li, M. Su, G. Xing, X. Tao and Y. Song, Low-Dimensional Dion-Jacobson-Phase Lead-Free Perovskites for High-Performance Photovoltaics with Improved Stability, Angew. Chem., Int. Ed., 2020, 6909–6914 CrossRef CAS.
- L. Zeng, Z. Chen, S. Qiu, J. Hu, C. Li, X. Liu, G. Liang, C. J. Brabec, Y. Mai and F. Guo, 2D-3D heterostructure enables scalable coating of efficient low-bandgap Sn–Pb mixed perovskite solar cells, Nano Energy, 2019, 66, 104099 CrossRef CAS.
- S. Shao, J. Liu, G. Portale, H.-H. Fang, G. R. Blake, G. H. ten Brink, L. J. A. Koster and M. A. Loi, Highly Reproducible Sn-Based Hybrid Perovskite Solar Cells with 9% Efficiency, Adv. Energy Mater., 2018, 8(4), 1702019 CrossRef.
- H. Kim, Y. H. Lee, T. Lyu, J. H. Yoo, T. Park and J. H. Oh, Boosting the performance and stability of quasi-two-dimensional tin-based perovskite solar cells using the formamidinium thiocyanate additive, J. Mater. Chem. A, 2018, 6(37), 18173–18182 RSC.
- E. Jokar, C.-H. Chien, A. Fathi, M. Rameez, Y.-H. Chang and E. W.-G. Diau, Slow surface passivation and crystal relaxation with additives to improve device performance and durability for tin-based perovskite solar cells, Energy Environ. Sci., 2018, 11(9), 2353–2362 RSC.
- E. Jokar, C. H. Chien, C. M. Tsai, A. Fathi and E. W. Diau, Robust Tin-Based Perovskite Solar Cells with Hybrid Organic Cations to Attain Efficiency Approaching 10, Adv. Mater., 2019, 31(2), e1804835 Search PubMed.
- C. Ran, W. G. un Xi, F. Yuan, T. Lei, B. Jiao, X. Hou and Z. Wu, Bilateral Interface Engineering Towards Efficient 2D-3D Bulk Heterojunction Tin Halide Lead-Free Perovskite Solar Cells, ACS Energy Lett., 2018, 713–721 CAS.
- T. Rath, J. Handl, S. Weber, B. Friesenbichler, P. Fürk, L. Troi, T. Dimopoulos, B. Kunert, R. Resel and G. Trimmel, Photovoltaic properties of a triple cation methylammonium/formamidinium/phenylethylammonium tin iodide perovskite, J. Mater. Chem. A, 2019, 7(16), 9523–9529 CAS.
- M. E. Kayesh, K. Matsuishi, R. Kaneko, S. Kazaoui, J.-J. Lee, T. Noda and A. Islam, Coadditive Engineering with 5-Ammonium Valeric Acid Iodide for Efficient and Stable Sn Perovskite Solar Cells, ACS Energy Lett., 2018, 4(1), 278–284 CrossRef.
- F. Wang, X. Jiang, H. Chen, Y. Shang, H. Liu, J. Wei, W. Zhou, H. He, W. Liu and Z. Ning, 2D-Quasi-2D-3D Hierarchy Structure for Tin Perovskite Solar Cells with Enhanced Efficiency and Stability, Joule, 2018, 2, 2732–2743 CrossRef CAS.
- X. Jiang, F. Wang, Q. Wei, H. Li, Y. Shang, W. Zhou, C. Wang, P. Cheng, Q. Chen, L. Chen and Z. Ning, Ultra-high open-circuit voltage of tin perovskite solar cells via an electron transporting layer design, Nat. Commun., 2020, 11(1), 1245 CrossRef CAS.
- Z. Wang, Q. Lin, F. P. Chmiel, N. Sakai, L. M. Herz and H. J. Snaith, Efficient ambient-air-stable solar cells with 2D–3D heterostructured butylammonium-caesium-formamidinium lead halide perovskites, Nat. Energy, 2017, 2(9), 17135 CrossRef CAS.
- W. Ke, C. Chen, I. Spanopoulos, L. Mao, I. Hadar, X. Li, J. M. Hoffman, Z. Song, Y. Yan and M. G. Kanatzidis, Narrow-Bandgap Mixed Lead/Tin-Based 2D Dion-Jacobson Perovskites Boost the Performance of Solar Cells, J. Am. Chem. Soc., 2020, 142(35), 15049–15057 CrossRef CAS.
- I. Zimmermann, S. Aghazada and M. K. Nazeeruddin, Lead and HTM Free Stable Two-Dimensional Tin Perovskites with Suitable Band Gap for Solar Cell Applications, Angew. Chem., Int. Ed., 2019, 58(4), 1072–1076 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |