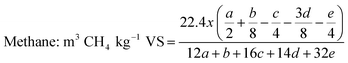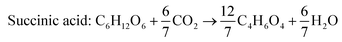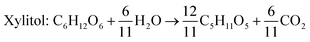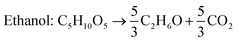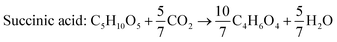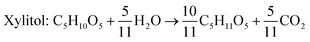Sugarcane bagasse based biorefineries in India: potential and challenges
Kakasaheb S.
Konde
a,
Sanjay
Nagarajan
 b,
Vinod
Kumar
c,
Sanjay V.
Patil
*a and
Vivek V.
Ranade
*b
b,
Vinod
Kumar
c,
Sanjay V.
Patil
*a and
Vivek V.
Ranade
*b
aDepartment of Alcohol Technology and Biofuels, Vasantdada Sugar Institute, Manjari (Bk.), Pune, 412307, India. E-mail: sv.patil@vsisugar.org.in
bSchool of Chemistry and Chemical Engineering, Queen's University of Belfast, Stranmillis Road, Belfast BT95AG, UK. E-mail: v.ranade@qub.ac.uk
cSchool of Water, Energy and Environment, Cranfield University, Cranfield, MK43 0AL, UK
First published on 10th November 2020
Abstract
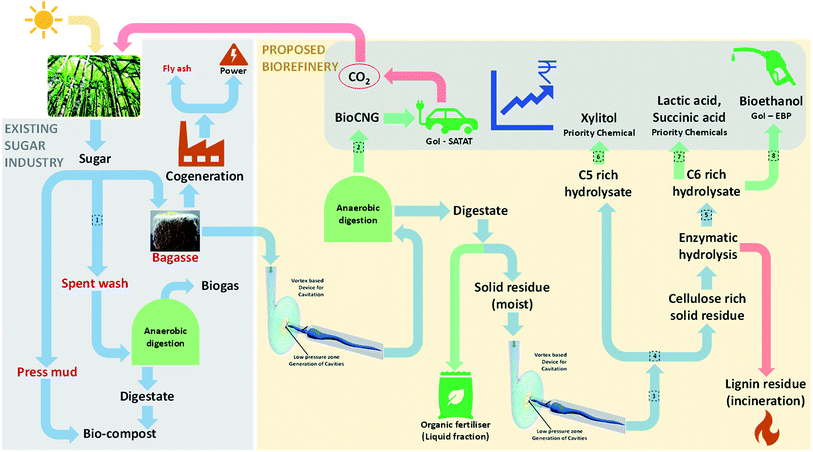
Sugarcane bagasse (SCB) is one of the world's most abundant agricultural residues and in an Indian context, ∼100 million tonnes per annum is produced. The current use of SCB is restricted to the cogeneration of steam and power; however considering its potential, cogeneration is not the best valorisation route. Furthermore, with falling electricity prices and reducing global sugar prices due to excess sugar stock, it is inevitable that the waste generated (SCB) by sugar mills are utilised for generating revenue sustainably. With this background, this review aims to put forth a biorefinery perspective based on SCB feedstock. Biogas and bioethanol are the Government of India's current focus with policies and subsidies clearly pointing towards a sizeable future market. Therefore, alongside these biofuels, high-value chemicals such as xylitol, succinic acid and lactic acid were identified as other desired products for biorefineries. This review firstly discusses SCB pre-treatment options based on end applications (saccharification or anaerobic digestion, AD). Next, state-of-the-art for each of these aspects was reviewed and our perspective on a profitable biorefinery is presented. We propose an AD based biorefinery where vortex-based hydrodynamic cavitation was found to be the best choice for pre-treatment. AD is considered not only a bioprocess for energy production here but also a ‘pre-treatment’, where partial conversion of holocellulose leads to a digestate rich in a loosened fibre matrix. This digestate rich in cellulose can be enzymatically hydrolysed and further valorised biochemically. This approach would be cost effective and provide a sustainable waste management route for sugar mills.
Introduction
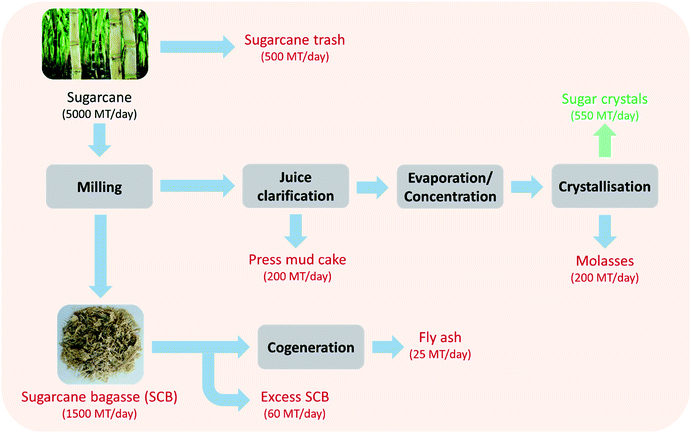
Sugarcane is a rich source of sucrose (∼10%) and accounts for approximately 80% of the global sugar production. India is one of the leading sugarcane (and sugar) producing countries in the world. The Indian sugar industry has witnessed tremendous growth in the past 60 years without any impediments. As a result, the area under sugarcane cultivation and sugar production have been continuously increasing over the last six decades. India currently ranks second in the cultivation area (5.11 million ha) and sugarcane production (303.83 million tonnes) next to Brazil. Since 1930–31, the number of sugar mills in operation in India increased from 29 to 520, thereby leading to an increase in sugar production from 0.12 million to 32.82 million tonnes per year in 2018–19.1 Today, the Indian sugar industry's annual output is worth approximately ₹800 billion (∼$12.5 billion). With this impeccable net worth, the sugar industry plays an important role in the socio-economic development of India by contributing to rural infrastructure like roads, education, medicine, access to finance, other facilities, etc. The Indian sugar industry being the second largest agro-based industry after cotton plays a vital role in the rural livelihood of ∼60 million sugarcane farmers and ∼0.5 million workers directly employed in sugar mills. Employment is also generated in various ancillary activities relating to transportation, trade servicing of machinery and supply of agriculture inputs.While this increased the overall wellbeing of sugarcane farmers, the growth of the sugarcane industry also led to associated waste related issues. The major solid waste streams generated in the sugar manufacturing process include sugarcane trash, sugarcane bagasse (SCB), press mud cake (PMC) and SCB fly ash (Fig. 1). The key characteristics of these solid wastes are summarized in Table 1. Although cogeneration for steam and electricity production is the most harnessed industrial route for SCB valorisation,2 high value co-product and biofuel production from SCB and other wastes has also been explored.3 For example, SCB is also used as a raw material in agro-residue based pulp and paper mills. Other SCB based products with added value are furfural and animal feed. Another example is PMC that is rich in organic content, phosphate, calcium and magnesium and therefore is used as a fertilizer after bio-composting with biomethanated spent wash (distillery wastewater). Attempts have been made to utilise PMC as a fuel in sugar mills either alone or in combination with SCB; however it leads to the generation of 25% more ash than SCB.4 Sugarcane trash is conventionally disposed by burning in the fields. SCB fly ash is typically disposed in pits; however it is also applied on land for soil amendment in some areas or used for brick manufacturing. In addition to the solid wastes generated during sugar production, a significant portion of mother liquor left behind after crystallisation of sugars from juice ends up as a liquid waste – molasses (4% of sugarcane crushed). Molasses is typically used to produce ethanol via fermentation. Many sugar mills also run distilleries as their subsidiaries for producing fuel ethanol and extra neutral alcohol to maximise profit margins. The additional profits become critical especially because, in the recent years, due to the excess production of sugarcane, the sugar inventory has been piling up in India leading to a crisis. In addition, due to decreasing international prices of sugar, paying a fair and remunerative price (FRP) to the farmers has also become a challenge. In view of the sugar crises, the Government of India (GoI) took a policy decision to implement the ethanol blending programme (EBP) and to go for 10% blending (in petrol) throughout the country and cut oil imports (20% by 2030). Furthermore, to increase profits with molasses based ethanol, efficient waste management is required to valorise the waste streams into value added products.
| Solid waste | Description | Fraction of sugarcane plant production | Composition | References |
|---|---|---|---|---|
| Sugarcane trash | Leaves and tops obtained upon sugarcane harvesting | 8–10% | 40–44% cellulose | Bhardwaj et al., 2019 |
| 30–33% hemicellulose | Franco et al., 2013 | |||
| 17–22% lignin | Singh et al., 2008 (ref. 9–11) | |||
| 4–5% ash | ||||
| Sugarcane bagasse | Dry fibrous residue obtained after sugarcane crushing and juice extraction | 30% | 40–50% cellulose | Abhilash & Singh, 2008 |
| 19–25% hemicellulose | Ezhumalai & Thangavelu, 2010 | |||
| 17–25% lignin | Ingle et al., 2017 (ref. 12–14) | |||
| 2–4% ash | ||||
| Press mud cake | Solid residue obtained upon sugarcane juice clarification | 4% | 5–14% crude wax | Velmurugan & Partha, 2006 (ref. 15) |
| 15–30% fibre | ||||
| 5–15% crude protein | ||||
| 5–15% sugar | ||||
| 4–10% SiO | ||||
| 1–4% CaO | ||||
| 1–3% PO | ||||
| 0.5–1.5% MgO | ||||
| 9–10% ash | ||||
| Sugarcane bagasse fly ash | Generated after the combustion of SCB | 0.005–0.066 tons of fly ash per ton SCB crushed | 65% SiO2 and other metal oxides | Umamaheswaran & Batra, 2008 (ref. 16) |
Amongst the waste generated by the sugar industry, SCB contributes to a significant proportion (∼100 million tonnes per annum). It is also one of the largest agriculture residues in the world.5–7 This waste does not just present a challenge, but also creates an opportunity where proper waste resource management can lead to additional revenue. This revenue will help in tackling the problems faced by the Indian sugar industry such as the falling sugar prices, surplus sugar production and payment of FRP. A preliminary review of the literature indicates that SCB can serve as a potential feedstock for low cost production of green chemicals and fuels. This is mainly attributed to its abundant availability making its supply constant and stable.
The current SCB utilization approach in India is restricted to cogeneration (of steam & power). Considering the valorisation potential of SCB, cogeneration is not the best option (although mature). There is limitation on use of SCB for cogeneration due to the diminishing market price of electricity. There will be a high demand for ethanol [>9 billion litres by 2030 (ref. 8)] with excellent differential pricing offered by the GoI based on use of different intermediates/by-products of sugarcane processing. In future, the economics of the sugar industry will not only depend on sugar, ethanol or cogeneration but will also depend on the optimal use of (wastes) resources available within the sugar industry. Meghana and Shastri's recent review on the global sugar industry with a strong focus on environmental impacts suggested that monetising the environmental impacts will also act as a driver towards utilising these wastes as resources 2. This paradigm shift to waste-based biorefineries with a circular economy approach is therefore the way forward for the Indian sugar industries.
With this background, this review aims to examine innovative value-added products in addition to fuels that can be obtained from the transformation of SCB in an Indian context. Such initiatives towards a biorefinery are expected to promote alternative approaches to steam and power. This work presents the state-of-the-art in this field especially focusing on high value products. The manuscript is structured in a way to first describe the potential of SCB as a feedstock for biorefineries, followed by the importance of pre-treatment in enhancing product yields. Finally, routes and processes for gaseous and liquid products, reported yields and challenges in adopting these SCB valorisation solutions in a biorefinery are highlighted.
Sugarcane bagasse (SCB) and its potential as a feedstock for biorefineries
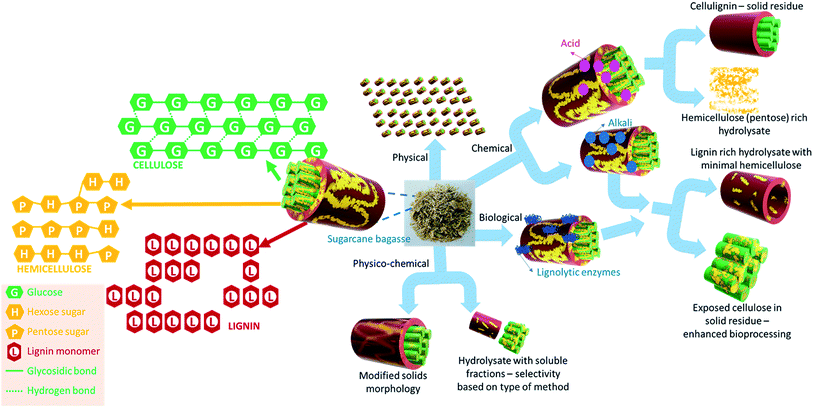
SCB is a lignocellulosic biomass that contains cellulose, hemicellulose and lignin which together form a complex and recalcitrant structure. Cellulose is a linear homopolymer of glucose while hemicellulose is a heteropolymer containing a variety of hexose and pentose sugars and sugar acids. The sugars present in hemicellulosic fractions are typically glucose, galactose, mannose, arabinose and xylose with xylose being the most abundant sugar (∼90%). Thus, cellulose and hemicellulose present in SCB are sources of fermentable sugars. The final fermentable sugar concentration in hydrolysate depends on the cellulosic and hemicellulosic content (Table 1). The composition of SCB also affects its combustion characteristics and energy yield. The proximate and ultimate analysis of SCB as reported in the literature is summarised in Table 2. The fixed carbon was found to be in the range of 75.8% to 85.5%, volatile matter varied between 11% and 20% and the ash content varied between 2% and 5%. The data indicate that the composition of bagasse varies depending on its source and sugar processing. The average gross calorific value (GCV) of SCB was found to be 3990 kcal kg−1 and is comparable to that of lignite coal and wood. Therefore, a significant quantity of bagasse is typically used in biomass boilers.| Proximate analysis (%) on dry basis | Elemental analysis (%) | GCV (kcal kg−1) | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fixed carbon | Volatile matter | Ash | C | H | N | S | Cl | O | ||
| 20.01 | 75.85 | 4.14 | 48.67 | 6.70 | 0.45 | 0.08 | — | 44.10 | 4014.2 | Islam et al., 2010 (ref. 18) |
| 18.00 | 79.90 | 2.20 | 44.60 | 5.80 | 0.60 | 0.10 | 0.00 | 44.50 | 4322.3 | Leal et al., 2013 (ref. 19) |
| 11.26 | 84.50 | 4.24 | 38.84 | 6.85 | 0.02 | 0.39 | — | 53.90 | 4124.1 | Gonçalves et al., 2017 (ref. 20) |
| 12.39 | 85.49 | 2.12 | 49.84 | 6.00 | 0.20 | 0.06 | — | 43.90 | — | Kumar et al., 2017 (ref.21) |
| 13.05 | 84.41 | 2.45 | 49.20 | 4.70 | 0.20 | 0.04 | 0.16 | 43.00 | 3558.9 | Shukla & Kumar, 2017 (ref. 22) |
| 11.95 | 84.78 | 3.28 | 44.86 | 5.87 | 0.24 | 0.06 | — | 48.97 | 4298.4 | Varma & Mondal, 2017 (ref. 23) |
| 14.84 | 82.42 | 2.75 | 41.90 | 5.50 | 0.29 | 0.01 | — | 52.20 | — | Ghorbannezhad et al., 2018 (ref. 24) |
| 16.09 | 79.09 | 4.90 | 32.5 | 5.01 | 0.38 | 0.56 | — | 61.55 | 3948.2 | Kanwal et al., 2019 (ref. 25) |
| 14.26 | 83.46 | 2.17 | 46.37 | 6.29 | 0.55 | 0.11 | — | 46.79 | 3422.7 | Manatura, 2020 (ref. 26) |
In the crushing season 2018–19, sugarcane crushing, sugar and SCB production was 303.83 million tonnes, 32.82 million tonnes and 91.15 million tonnes in India respectively.1 SCB cogeneration in India picked up pace after the ‘National programme on promotion of biomass power/bagasse-based cogeneration’ was implemented by the GoI in 1992. As it leads to environmental and economic sustainability, the GoI formulated many promotional policies for setting up more combined heat and power (CHP) plants, which further encouraged such sugar plants to adopt cogeneration technology.17 During the last two decades, many sugar mills have installed co-generation (power/steam) plants from 10 MW h−1 to above 50 MW h−1 capacities. It helped in earning additional revenue and allowed sugar mills to pay a higher cane price to farmers. The current total installed capacity of these cogeneration plants in India is 9200 MW. Despite its use in boilers, a significant amount of SCB still remains as waste. As mentioned previously, the SCB based cogeneration electricity prices are going down, as cheap electricity is now available from other sources. Therefore, there is a need to identify other valorisation routes that can help in effective SCB management with a surplus economic gain.
In the present scenario, the production of chemicals is completely reliant on fossil fuels which is non-sustainable and has a negative impact on the environment. As a result, there is a growing demand for clean, greener and sustainable technologies to manufacture fuels and chemicals that requires a paradigm shift from petrochemical based synthesis towards bio-based production.27 The main bottleneck in the commercial success of biorefineries is high cost which stems from feedstocks and their pre-treatment. Profitable biorefineries could be realized by making use of waste streams rich in renewable carbon and substantially reduce the production cost and spare edible feedstocks.28 As mentioned earlier, India being the second largest producer of sugarcane crop in the world generates massive amounts of SCB as waste. SCB is an inexpensive source of fermentable sugars and a potential feedstock for second-generation biorefineries. It provides a significant opportunity for biofuel/biochemical/chemical industries in India and has potential for rural economy development. Being a lignocellulosic feedstock with a low nutritional value, the use of SCB precludes concerns about the food vs. fuel debate, especially in a country like India with a huge population. The current use of SCB for power generation or CHP is a low grade application and does not utilize its full potential. The annexation of second generation (2 G) biorefineries into existing sugar mills through more efficient use of waste streams such as SCB will lead to revitalisation and sustainability of sugar industries.28,29
In 2017, the GoI announced a call, in association with UK, on the “Industrial Waste Challenge” to find green and sustainable solutions for waste streams generated by five major industries in India and sugar mills were one of them. Our consortium (vWa) was funded under this call and we proposed valorisation of SCB to five products of huge commercial value: bioCNG, xylitol, n-butanol, lactic and succinic acid and are working towards it. Potential products from SCB and their theoretical yields are listed Table 3. The key challenges in the valorisation of SCB via a biochemical route are: development of a cost-effective pre-treatment method to make the biomass amenable to further biotransformation, simple & cost effective detoxification, effective hydrolysis (without or in house low cost enzymes) and biotransformation of sugar in hydrolysates to the desired products which meet customer specifications without compromising the safety, environment and economics.
| Productb | Glucose | Xylose | Cellulose | Hemicellulose | SCBa |
|---|---|---|---|---|---|
| a SCB composition: 42% cellulose 31% hemicellulose and balance other constituents. b see appendix I for calculations of yield. | |||||
| Biogas (m3 kg−1) | 0.37 | 0.37 | 0.41 | 0.42 | 0.44 |
| Ethanol (kg kg−1) | 0.51 | 0.51 | 0.57 | 0.58 | 0.42 |
| n-Butanol (kg kg−1) | 0.41 | 0.41 | 0.46 | 0.46 | 0.34 |
| Lactic acid (kg kg−1) | 1.00 | 1.00 | 1.11 | 1.13 | 0.82 |
| Succinic acid (kg kg−1) | 1.12 | 1.12 | 1.25 | 1.27 | 0.92 |
| Xylitol (kg kg−1) | 0.92 | 0.92 | 1.02 | 1.04 | 0.75 |
Pre-treatment of sugarcane bagasse
Making a complex lignocellulosic biomass amenable for further transformations is the critical first step in maximising its utilisation potential. The extent of biomass conversion is dependent on its superficial and supramolecular structure, lignin, cellulose and hemicellulose content, particle size and elemental composition. Specifically, with the structural composition of SCB, recalcitrance lies in the encapsulating lignin-hemicellulose structure shielding the valuable cellulose, cellulose crystallinity and lignin-holocellulose interlinks hindering its extent of utilisation. To enhance the bioavailability and thereby increase the valorisation potential of SCB, pre-treatment methods are essential. An efficient pre-treatment method should not only be effective in terms of enhancing the sugar yields or making the biomass more bioavailable, but also be scalable and economical. Pre-treatment is considered to be the most expensive process step in a lignocellulosic biorefinery and can contribute to about 20% of the total cost, with the combined costs of pre-treatment, enzymatic production and hydrolysis accounting for up to 40%.30 Since it is the first step, it has a pervasive impact on the cost of all biological processing operations downstream. Hence the pre-treatment must be advanced and fully integrated with the rest of the process to harvest the complete potential of lignocellulosic biomass.30 Therefore, the choice of the pre-treatment method becomes crucial for a SCB biorefinery. A range of SCB pre-treatment methods are reported in the literature for two important applications, namely, enhancing saccharification (and bioethanol/value added product fermentation) and intensifying biogas generation. These methods are discussed in this section and summarised in Table 4.| Pre-treatment type | Method and operating conditions | Particle size (mm) | SCB concentration, g L−1 (volume, L) | CH4 yield | Reference |
|---|---|---|---|---|---|
| (a) For biogas production | |||||
| Physical | Milled to <0.85 mm | 5 | — | Increase – 33% | Kivaisi & Eliapenda, 1994 (ref. 37) |
| Milled to 1 mm | >10 | — | Increase – 15% (∼310 mL CH4 per g VS) | Leite et al., 2015 (ref. 38) | |
| Chemical | Ca(OH)2, 0.47 g lime per g dry matter, 90 °C, 150 rpm, 90 h | — | 40–80 (0.1) | 148–183 mL CH4 per g COD (solid residue) & 3.5 L CH4 per L liquor | Rabelo et al., 2011 (ref. 39) |
| Alkaline H2O2, 7.36% (v/v), pH 11.5, 25 °C, 150 rpm, 1 h | — | 40–150 (0.1) | 84–127 mL CH4 per g COD (solid residue) & 6.5 L CH4 per L liquor | ||
| HCl, 0.63–1.97 M, 103–137 °C, 6–74 min | 1 | 100 (0.5) | Increase – 36 to 122 mL CH4 per g | Costa et al., 2014 (ref. 40) | |
| NaOH, 0.8–1.8 M, 116–184 °C, 13–47 min | Increase – 36 to 139 mL CH4 per g | ||||
| Physico-chemical | Vortex based hydrodynamic cavitation, 0–117 passes, 3.9 barg | 2 | 10 (15) | Increase – 175 to 229 mL CH4 per g VS | Nagarajan & Ranade, 2019 (ref. 41) |
| Pre-treatment type | Method and operating conditions | Initial particle size (mm) | SCB concentration, g L−1 (volume, L) | Inhibitors generated | Effect of pre-treatment | Enhancement upon enzymatic hydrolysis | Reference | |
|---|---|---|---|---|---|---|---|---|
| Glucose yield | Xylose yield | |||||||
| (b) For sugar based fermentative products | ||||||||
| Physical | Planetary ball milling, 1–4 h | <1 | 50 g in 0.5 L milling cup | Acetic acid | Complete conversion to amorphous cellulose | 89% | 77% | Buaban et al., 2010 (ref. 35) |
| Wet disk milling, 0–143 min | <2 | 50 (20) | Acetic acid | Insignificant change in cellulose crystallinity, ∼20 μm defibrillated structure | 22% to 49% | ∼10% to 37% | da Silva et al., 2010 (ref. 31) | |
| Wet disk milling, 0–71 min | 2 | 20 (3) | — | 14-Fold increase in the specific surface area | 13% to 62% | 10% to 43% | Barros et al., 2013 (ref. 34) | |
| Soaked in glycerol for 24 h, and then microwaved (2450 MHz) for 5 min | 3 | 333 (0.03) | — | Partial removal of hemicellulose (corresponding increase in cellulose%) | 2% to 35% | — | de Cassia Pereira et al., 2015 (ref. 42) | |
| Biological | Pleurotus florida, Coriolopsis caperata RCK 2011 & Ganoderma sp. rckk-02, 0.5 g L−1 inoculum, 30 °C, 25 days, pH 5.5 | 1–2 | 40 (0.5) | — | 5–8% removal of lignin | Increase in total reducing sugars from 20% to 49% | Deswal et al., 2014 (ref. 43) | |
| Ceriporiopsis subvermispora 0.5 g kg−1 SCB inoculum, 27 °C, 7–60 days, pH 5.5 | — | 25 g soaked in water & autoclaved | — | 48% removal of cellulose | Increase from 23%–55% | — | Machado & Ferraz, 2017 (ref. 44) | |
| 47% removal of hemicellulose | ||||||||
| Physico-chemical | SO2/H2SO4 catalysed steam explosion, 130–205 °C, 5–60 min | — | 25–111 g L−1 impregnated with ∼2% SO2 and/or ∼1% H2SO4 | Furfural, acetic acid & 5-HMF | 50–60% solubilisation of hemicellulose | 92% | 82% | Carrasco et al., 201045, |
| Steam explosion, 190 °C, 15 min | — | 80 (250) | — | ∼75% solubilisation of hemi-cellulose | — | — | Rocha et al., 2020 (ref. 46) | |
Pre-treatment methods are generally classified into four categories; physical, chemical, biological and physico-chemical pre-treatment (Fig. 2). Comminution is the most common physical pre-treatment where SCB is milled to a desirable particle size range. Milling not only helps in reducing the particle size or increasing the specific surface area, but concomitantly also affects the cellulose crystallinity. The combined effect of an increased surface area and reduced crystallinity in turn facilitates enhanced enzymatic hydrolysis thereby improving the saccharification rate and yield. This in turn facilitates enhanced enzyme binding to the cellulose fractions of the biomass thereby improving the saccharification rate and yield.31,32 Milling performance and the specific milling energy required are dictated by parameters such as the moisture content of SCB, required comminution ratio (ratio of initial particle size to final particle size) and the type of mill used. An increased moisture content leads to an increased energy draw and plugs the meshes. An increase in the comminution ratio (decrease in the final particle size) also leads to an increase in the specific milling energy. For instance, using a bench scale knife mill, Miao et al. reported that ∼720 kW h per tonne dry weight is required to grind SCB (∼40% moisture content) from an initial size of 20–25.4 mm to a final particle size of 2 mm.33 When the final particle size was 8 mm, the energy requirement reduced to ∼390 kW h per tonne dry weight. The aforementioned factors affect dry milling, whereas an alternative wet milling approach has also been exploited in the literature to pre-treat SCB. For instance, da Silva et al. reported the use of wet disk milling as a method to pre-treat SCB for enhancing the saccharification yield.31 Lab scale milling pre-treatment for enhanced saccharification of SCB is often reported;34,35 however, the most important parameter, the specific milling energy requirement, is hardly reported. In any case, the energy consumption values based on lab scale milling will not be relevant for industrial scale milling. The data on energy requirements for large scale milling are not readily available.
Chemical pre-treatment typically utilises an acid or an alkali to hydrolyse or delignify the biomass. They may be used in conjunction with compressed hot water or saturated steam, so that the chemical pre-treatment method becomes a physico-chemical method. Chemical pre-treatment methods often end up producing inhibitors (of fermentation) such as 5-hydroxymethylfurfural, acetic acid, formic acid or furfural, under acidic conditions. The hydrolysates when not detoxified appropriately will negatively influence the subsequent fermentation. Furthermore, as a general rule, biomass slurries upon chemical pre-treatment need to be neutralised prior to fermentation/biogas generation and therefore may incur additional costs at scale.
Unlike chemical pre-treatment, biological pre-treatment involves the use of enzymes or microbes to delignify or hydrolyse SCB. A class of fungi known as the brown rot fungi produces extracellular lignolytic enzymes such as lignin peroxidases or laccases that help in delignification. A close ally, white rot fungi on the other hand can produce lignocellulolytic enzymes that can also hydrolyse cellulose (cellulase), in addition to breaking down lignin.36 While biological methods have proven to be advantageous for delignification with low input energy requirements, the slow rate of pre-treatment is often seen as a significant disadvantage. On the other hand, the process also becomes expensive when highly pure enzymes are utilised for targeted pre-treatment and fractionation.
However, it may be possible to recover and recycle enzymes to reduce the overall pre-treatment cost.47
Physico-chemical methods are attractive pre-treatment options as they potentially overcome the disadvantages posed by other techniques. Steam explosion with a mild acid or alkali has particularly gained interest for SCB pre-treatment to enhance its saccharification potential and in turn bioethanol yields.46,48–50 Furthermore, it has also been proposed to be an energy efficient pre-treatment method for bioethanol production.51 The rapid depressurisation during steam explosion facilitates physical pre-treatment by breaking the fibres while the acid/alkali will catalyse hydrolysis or delignification of the biomass. The process conditions can be tuned to obtain C5 sugar rich hydrolysate and cellulose rich residue. Physico-chemical pre-treatment seems a promising method in terms of scalability and energy efficiency, not only for saccharification or bioethanol production but also for enhancing biogas yields via anaerobic digestion (AD) of SCB. For instance, the use of hydrodynamic cavitation (HC) as a biomass pre-treatment method is increasing rapidly.41,52,53 HC is the phenomenon of formation, growth and implosion of vaporous cavities. HC occurs in a flowing fluid as a result of a local drop in pressure due to the design geometry. During HC pre-treatment, reactive radical species generated in situ from the cleavage of water molecules chemically pre-treats the biomass, whereas the collapsing cavities induce shear and physically pre-treat the biomass, affecting the particle size and morphology.
Various pre-treatment categories were introduced earlier in the section; however, it is important to match specific methods to the end applications. For example, anaerobic digestion being a robust technology can handle toxins well when compared to classic sugar fermentation; therefore, detoxification maybe required prior to fermentation, whereas they may not be necessary for biogas generation applications. Similarly, breaking open the biomass structure would be sufficient to enhance biogas yields, whereas fractionation is required for enhanced saccharification and fermentation. Therefore, a few examples from the literature are specifically presented for these two main applications, namely (i) biogas production (Table 4a) and (ii) saccharification (both C5 and C6 sugars) leading to fermentation (Table 4b).
The simplest pre-treatment that can be performed for enhancing biogas generation is comminution. K. Kivaisi et al. reported an enhanced biogas generation of up to 33% for a comminution ratio of 5.8 with the final SCB particle size being 0.85 mm.37 A. F. Leite et al. on the other hand reported an enhancement of up to 15% in biogas yield for a comminution ratio of 10 for a final particle size of 1 mm.38 Although the final particle sizes were similar in both cases, with the comminution ratio for Leite et al. being almost twice as much as the former, the improvement in biogas observed was almost half as much. While both the groups used SCB that had a VS content of over 90% (on dry basis), the lignin content differed significantly (Kivaisi & Eliapenda reported a lignin content of SCB 9% as compared to Leite et al. at ∼16%), which may have influenced the variation in the enhancement observed.
Chemical methods have been reported extensively to improve biogas generation. For instance, Rabelo et al. (2011) utilised calcium hydroxide (lime) to pre-treat SCB at 90 °C for 90 h.39 They used a lime loading of 0.47 g g−1 SCB and observed a decrease in lignin and hemicellulose content from 23% to 20% and 25% to 13%, respectively. A corresponding increase in the cellulose content in the solid residue up to 66% (from 38%) was also reported. When alkaline hydrogen peroxide treatment was opted (7.36% v/v) at room temperature for 1 h to pre-treat SCB, a much higher increase in the cellulose content was observed in the residue (81%). Both these methods favoured enhanced biogas yields from the solid residue upon pre-treatment. Additionally, to test the possibility of a biorefinery, the solid residue rich in cellulose was utilised for saccharification and the liquor was subjected to biomethanation potential (BMP) tests. Alkaline peroxide treatment was favourable for supporting biogas generation from the liquor due to the higher quantity of hemicellulose solubilised from SCB (corresponding xylan content in solids reduced from 25% to 8%). Costa et al. (2014) utilised conventional sodium hydroxide pre-treatment to delignify SCB (1 mm particle size).40 They reported a 26% lignin removal that resulted in a 3.8 fold increase in biomethane production to 139 L CH4 per kg substrate. While alkali pre-treatment is generally favoured for delignification, acid hydrolysis is exploited for solubilising hemicellulose (minimal acid soluble lignin) to produce cellulignin (solid residue rich in cellulose and lignin). However, the type of acid influences the outcome upon pre-treatment. For example, for enhancing biogas production, when H2SO4 is used to pre-treat SCB, the sulfates formed during the neutralisation stage have to be removed; otherwise, the biomethane yields will be compromised. This is due to the action of a class of bacteria known as the sulfate reducing bacteria (SRB) present in the digester. SRB utilises sulfate and compete with methanogens for protons from the feedstock/volatile fatty acids, thereby reducing the available protons for methane generation. Costa et al. therefore reported the use of HCl for the pre-treatment of 1 mm SCB particles for enhancing the biomethane production. They achieved >50% solubilisation of holocellulose that favoured an increase in BMP up to 122 L CH4 per kg substrate (3.4 times increase). The increase in BMP upon acid pre-treatment was lower as compared to alkali pre-treatment, reiterating the fact that lignin induced recalcitrance hinders holocellulose bioavailability and therefore must be removed.
Nagarajan and Ranade (2019) reported a novel vortex based HC pre-treatment to enhance the biogas yields from SCB. The novel vortex based device used by S. Nagarajan et al.41 harnessed HC using a vortex flow based device invented by.54 It has eminent advantages over conventional linear flow devices, such as no small constrictions and cavitation occurring in the core and away from the walls leading to no device erosion and hence requiring minimum maintenance. Additionally, the energy required for vortex based HC pre-treatment of SCB was reported to be 140 kW h per tonne dry weight (for a low biomass loading of 1%), which was significantly lower than the milling energy mentioned earlier and other HC devices used for agricultural residue pre-treatment.55,56
Mechanical grinding is known to improve the enzymatic saccharification efficiency. Accordingly, milling pre-treatment for enhanced saccharification of SCB is often reported.31,34,35 da Silva et al. compared ball milling and wet disk milling of 2 mm SCB particles based on the saccharification efficiency. With the untreated SCB yielding 22% glucan upon enzymatic hydrolysis, ball milled SCB yielded >80% and wet disk milled SCB yielded 49%. The ability of ball milling to completely convert the crystalline cellulose in SCB to amorphous cellulose was attributed as the reason for such a high saccharification yield.
Deswal et al. (2014) utilised three white rot fungi, namely, Pleurotus florida, Coriolopsis caperata RCK 2011 and Ganoderma sp. rckk-02, to delignify SCB.43 The highest delignification of 8% was observed when P. florida was used to treat SCB for 25 days. The solid residue upon recovery was subjected to enzymatic saccharification that yielded 49% reducing sugars as compared to untreated SCB (20%). More recently, Machado and Ferraz reported delignification of SCB using Ceriporiopsis subvermispora.44 The autoclaved material when subjected to fungal fermentation facilitated the removal of 47% lignin and 48% hemicellulose in 60 days. This favoured the enzymatic saccharification of cellulose rich residue and increased the glucan yield to 55% from 23%.
Carrasco et al.(2010) performed steam explosion of SO2 impregnated SCB under their optimised conditions of 190 °C for 5 min to obtain a xylose yield of ∼60%.45 Fermentation inhibitors such as acetic acid, furfural and 5-hydroxymethylfurfural were also observed in the hydrolysate along with xylose and xylo-oligomers. The solid residue upon pre-treatment was saccharified using an enzyme cocktail that yielded 90% glucose at 2% solid loading. Carvalho et al. (2018) were able to achieve similar xylose yields by using a higher concentration of SO2 in combination with a lower temperature (3%, 150 °C and 30 min).48 Swapping SO2 with 0.5% H2SO4 and reducing the time by half also yielded similar concentrations of xylose in the hydrolysate. They also demonstrated that autocatalytic steam explosion (without an added catalyst) is selective towards xylo-oligosaccharides whereas in the presence of an acid catalyst, xylose production was favoured. More recently, autocatalytic steam explosion at 190 °C for 15 min was performed with 80 g L−1 SCB in a 250 L pressure reactor by G. J. M. Rocha et al.46 to recover hemicellulose rich hydrolysate (∼90%). The solid residue (cellulignin) was further treated with 1% NaOH aqueous solution under oxygen pressure to solubilise lignin and leave behind a pure cellulose rich solid residue. With tuneable parameters paving way for selective fractionation and inhibitor minimisation, steam explosion is a suitable candidate for pilot and industrial trials aimed at bioethanol production. Accordingly, there have been a few studies at the demo scale level to produce xylose rich hydrolysates continuously from SCB (e.g. DBT-ICT technology and Praj Industries, India).57–59
Other pre-treatment methods have also been utilised for SCB pre-treatment such as microwave,42 compressed hot water,60 organosolv,61 ionic liquids,62 ammonia fibre explosion,63 supercritical CO2,64 acoustic cavitation,65 photocatalysis,66 gamma radiation,67 Fenton's reagent68 and ensiling pre-treatment.69 These methods are however not discussed in this review as they are mainly restricted to the lab scale, expensive, energy intensive or not scalable at this point of time. The readers are henceforth directed to the references directly to know more on these methods. Amongst the pre-treatment methods discussed, (catalytic) steam explosion and hydrodynamic cavitation for bioethanol production and biogas generation respectively seem promising options for scale up with economic feasibility and therefore may be used to realise a bagasse based biorefinery.
SCB to biogas
Biogas is an admixture of methane and carbon dioxide predominantly. Negligible quantities of ammonia, hydrogen sulphide, hydrogen, nitrogen and water vapour may also be present. Biogas when upgraded to compressed biogas (CBG) containing >90% CH4 can be utilised as a transportation fuel and is analogous to compressed natural gas (CNG). Biogas is primarily produced by anaerobic digestion (AD) of organic matter. AD is a complex synergistic microbial process that occurs in the absence of oxygen. The carbon and electron source to sustain microbial growth and metabolism comes from the organic matter being fed. AD has the capability to utilise complex feedstocks such as agricultural residue for biogas generation (e.g. SCB).Neither AD nor biogas is alien to India. In fact, the world's first biogas plant was built in Bombay (now Mumbai), India in 1859 that utilised sewage sludge as the feedstock.70 Currently, the total biogas production in India is 2.07 billion m3 per year. This is quite low compared to its potential, which is estimated to be in the range of 29–48 billion m3 per year.71 In the current context, with the policy drivers in India clearly pointing towards a biogas market, it is important that the current subsidies provided by the GoI are leveraged. To build base and boost the capacity for biogas generation, the Ministry of Petroleum and Natural Gas initiated the Sustainable Alternative Toward Affordable Transportation (SATAT) scheme in late 2018.72 The crux of this scheme was to create a buy in for CBG (compressed biogas) produced from waste biomass sources (₹46 per kg CBG)73 and utilise it as a sustainable transportation fuel. The initial expression of interest call aimed at supporting the phased setup of 5000 biogas plants spanning across India. Recently, the Reserve Bank of India (RBI) has also included CBG plants under priority sector lending. With India being the world's second largest producer of sugarcane (303.83 million tonnes in 2019) and ∼30% ending up as SCB (91.15 million tonnes) and operating over 500 sugar mills, the availability of SCB as a feedstock for biogas generation via AD fits the purpose precisely. As per the notification from the Department of Agriculture and Farmers Welfare, GoI (13th July 2020), the fermented organic digestate coming out from AD of SCB/other agro-wastes is now approved as organic manure in Fertilizer Control Order (FCO). The enhanced and readily available nitrogen in the digestate when used as an organic fertilizer would partly or fully offset the need for chemical fertilizers which in itself has a high energy demand during production.74 This will immensely help the biogas and CBG sector in future.
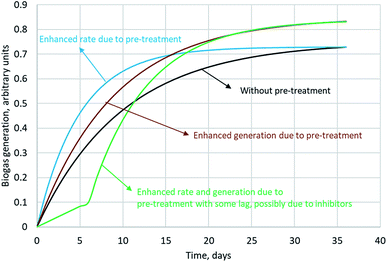
Biogas generation from SCB is dependent on its composition, particle size and morphology. The cellulose and hemicellulose fractions together in SCB constitute about 60–70% of its dry weight (or total solids, TS) and can be predominantly utilised towards biogas generation. Lignin generally remains unaffected; however it can hinder the effective utilisation of polysaccharides. Therefore, complete conversion of the holocellulose fraction is unlikely. Inherent variability in the composition of SCB can occur due to the species of cane utilised, time of harvest or varying efficiencies of juice extraction. For instance, Janke et al. (2015) sampled SCB from plants located in two different Brazilian states in two different seasons and tested the biochemical methanation potential (BMP) of these variants. With the volatile solids (VS) content not overly different (96 ± 2.7% of TS), a huge variation in BMP was observed between the two samples, namely 236 and 326 mL CH4 per g VS. They attributed this difference to the varying quantities of easily utilisable residual non-structural carbohydrates in SCB present upon juice extraction. Using the elemental composition of SCB, theoretical BMP can be calculated using the Buswell equation. The theoretical maximum varies in the range of 425–487 mL CH4 per g VS for SCB.41 Theoretical BMP is calculated based on the assumption that all VS can be utilised towards biogas generation. In reality, however this can never be achieved. The experimental BMP of SCB reported in the literature spans over a broad range from 37 (assuming a VS content of 96% of TS)40 to 326 mL CH4 per g VS,75 while the typical range is 170–250 mL CH4 per g VS. Pre-treatment has the capability to improve the BMP. The influence of pre-treatment on biogas production with typically observed biogas generation profiles is captured in Fig. 3. A variety of studies reporting an increase in BMP upon SCB pre-treatment are summarised in Table 5.
| SCB initial particle size (mm) | Pre-treatment and operating conditions | BMP upon pre-treatment (mL CH4 per g VS) | Increase in BMP (%) | Reference |
|---|---|---|---|---|
| a mL CH4 per g COD. | ||||
| ≤1 | 10 g SCB soaked in 140 g aqueous ethanolic ammonia (10% ammonia +25%) ethanol, 70 °C for 12 h, solids used for BMP | 249 | 135% | Sajad Hashemi et al., 2019 (ref. 76) |
| 5 | Milled to <0.85 mm | — | 33% | Kivaisi & Eliapenda, 1994 (ref. 37) |
| ≤2 | 1 M NaOH for 30 days | — | 44% | |
| 1 M HCl for 30 days | — | 32% | ||
| 1 M NH4OH for 30 days | — | 22% | ||
| ≤2 | Vortex based hydrodynamic cavitation – 9 passes | 229 | 24% | Nagarajan & Ranade, 2019 (ref. 41) |
| ≤3 | 100 g L−1 SCB, hydrothermal pre-treatment, 180 °C, 1931 kPa, 20 min followed by 8.5% lime treatment for 4 days | 220 | 61% | Mustafa et al., 2018 (ref. 77) |
| ≤10 (>68% SCB in the range of 0.18–1.6 mm) | 18.6 g L−1 SCB, 2% (w/v) H2SO4, autoclaved, 121 °C, 15 min | 200 | 34% | Badshah et al., 2012 (ref. 78) |
| 18.6 g L−1 SCB, 2% (w/v) H2SO4, autoclaved, 121 °C, 15 min, solid residue enzymatically saccharified – Accellerase® 1500, 60 h | 173 | 16% | ||
| >10 | Milled to 1 mm | ∼310 | ∼15% | Leite et al., 2015 (ref. 38) |
| 1.7 | 240 g L−1 SCB, hydrothermal pre-treatment, 178.6 °C, 43.6 min, hemicellulose rich hydrolysate for AD, OLR – 2.4 g COD per L per day | 270a | — | Ribeiro et al., 2017 (ref. 79) |
Sajad Hashemi et al. (2019) subjected milled SCB (1 mm) to ethanolic ammonia pre-treatment to enhance the BMP.76 An equal weight of 10% v/v aqueous ammonia was mixed with a range of ethanol–water mixtures (5–50%) and 10 g milled SCB was soaked to the resulting ethanol–ammonia aqueous solution. Pre-treatment was performed in a closed reagent bottle at either 50 or 70 °C for 12 or 24 h. The BMP of the untreated milled SCB, neutralised solid fraction, ethanol-ammonia free liquid and slurry were utilised for batch mesophilic BMP tests. The highest BMP was obtained from the solid (249 mL CH4 per g VS) and liquid (298 mL CH4 per g VS) fractions (pre-treated at 70 °C for 12 h with 10% ammonia and 25% ethanol). In the case of the slurry, an increase in BMP was observed with an increase in pre-treatment severity (299.3 mL CH4 per g VS upon pre-treatment at 70 °C for 24 h with 10% ammonia and 50% ethanol). The obtained methane yield was over 2-fold higher than that observed with untreated milled SCB. The delignification and breakdown of lignin-carbohydrate bonds were ascribed to be the reasons contributing towards an enhanced BMP.
Leite et al. (2015) performed batch BMP experiments with raw SCB (∼10 mm particle size), milled SCB (1 mm) and lime treated milled SCB (10% w/w, lime/SCB in 24 mL water for 24 h).38 They achieved a 20% increase in biomethane yield upon milling pre-treatment, whereas an alkali pre-treatment step post milling improved the yield by 50%. They suggested that lime treatment might be economically feasible upon recycling the liquid phase; however, no cost analysis was presented. Mustafa et al. (2018) also utilised lime pre-treatment as a strategy to enhance the BMP of 3 mm SCB.77 They used 8.5% lime and pre-treated SCB for 4 days to achieve an increase in biogas yield by 23%. They attributed the enhancement to the delignification observed as a result of lime pre-treatment. When hydrothermal pre-treatment (compressed hot water) was opted at 180 °C for 20 min, a >35% increase in biogas yield was observed. The methane content in biogas for all the cases was observed to be over 65%. Upon hydrothermal pre-treatment, a diauxic digestion trend was observed which indicates that there may have been production of inhibitors during pre-treatment. When hydrothermal pre-treatment was combined with lime pre-treatment sequentially, the biomethane yield enhancement observed was over 61%. In contrast to hydrothermal pre-treatment, a single digestion stage was observed upon sequential pre-treatment indicating that the inhibitors that might have been produced did not interfere with the microbial metabolism. The enhanced energy that was produced upon sequential pre-treatment was calculated to be 366 kW h per tonne dry weight (difference of energy in methane obtained from pre-treated SCB and untreated SCB). Without taking into account the energy required for milling, considering the energy required to heat water for hydrothermal pre-treatment (210 kW h per tonne dry weight), the sequential pre-treatment yielded biomethane with a net energy gain of 156 kW h per tonne dry weight.
Nagarajan and Ranade (2019) used a vortex based HC device to pre-treat 2 mm SCB particles at a solid loading of 1% (w/w) for 9 passes through the device.41 The energy required for pre-treatment was 140 kW h per tonne dry weight (for 1% solid loading) and the net energy gain achieved was 398 kW h per tonne dry weight from the enhanced biomethane yield achieved (24%). The modified surface morphology, particle size reduction and chemical compositional alternation due to cavitation pre-treatment were attributed to be the reasons for an enhanced biomethane production. Assuming that the energy gain due to the enhanced biomethane generation stayed constant at 537 kW h per tonne dry weight for various solid loading, the net energy gain will increase with increased solid loading. For example, 5% solid loading (based on the dry weight of SCB) will enhance the net energy gain to more than 500 kW h per tonne of dry SCB with 9 pass HC pre-treatment. HC therefore appears to be a promising method to pre-treat SCB for enhancing the biomethane production. Additionally, hybrid treatment methods such as alkali in combination with HC may also be tested to specifically improve the delignification of SCB for further enhancing the BMP.
While batch BMP tests are indicative of the biomethane potential of pre-treated (and untreated) SCB, they do not effectively present the long term effect of pre-treatment on digestion performance. Continuous AD tests are therefore important to assess this criterion. A proper understanding of the industrial scale anaerobic digesters' behaviour can be studied by conducting lab scale continuous/semi-continuous AD using relevant operating conditions.80 One of the crucial operating conditions that needs to be considered for continuous AD is the organic loading rate (OLR). An optimal OLR must be identified for a stable continuous AD performance.81,82 A sub-optimal OLR can cause digester failure, whereas a high OLR can cause digester acidification. For instance, Ribeiro et al. (2017) hydrothermally pre-treated 240 g L−1 SCB at 178.6 °C for 43.6 min and utilised the hemicellulose rich hydrolysate for continuous biogas production. During their investigation, they varied the OLR from 1.2–4.8 g COD per L per day and reported that the highest COD conversion of 86% was observed at an OLR of 2.4 g COD per L per day. When the OLR was further doubled, the conversion fell to 74%. The conversion of COD to biogas was lower at a higher OLR due to the increased formation of volatile fatty acids and increased accumulation of inhibitors such as HMF that may have altered the microbial synergy required for optimum biogas generation. Another parameter that needs attention in continuous AD is the quantity of inoculum added to the digester during start-up. Batch BMP utilises an inoculum to substrate ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on the basis of VS. However, for continuous digesters, this might not be the case and therefore, during digester start up, the proportion of the substrate added to the inoculum should be thoroughly thought through.
1 on the basis of VS. However, for continuous digesters, this might not be the case and therefore, during digester start up, the proportion of the substrate added to the inoculum should be thoroughly thought through.
AD when compared to other renewable energy technologies requires the least capital cost.83 To further add value, AD should not only be considered for biogas generation, but also be considered as the base of a viable biorefinery. SCB may be partially converted in AD and the resulting digestate would contain the unconverted holocellulose fraction with a loosened fibre matrix due to microbial activity.84 This digestate may be used for further value addition without needing expensive chemical or enzymatic pre-treatment. Thus, AD may be used as a base for the biorefinery not only to recover energy in the form of biogas/CBG, but also to open up the lignocellulosic matrix of unconverted biomass in the digestate which can be converted to sugars and other value added products (see the next section).
SCB to alcohols and acids
Efficient hydrolysis of SCB and hassle-free production of renewable 2G sugars create a compelling commercial opportunity for fermentative production of chemicals. Like any other lignocellulosic material, the two major sugars present in SCB are glucose (C6) and xylose (C5). Most of the studies carried out using SCB as the feedstock have focussed on the production of ethanol and xylitol. Cellulosic fraction was utilized for ethanol production while the hemicellulosic part was employed for xylitol accumulation.28,85Bioethanol
The current global ethanol supply is produced mainly from edible (sugar and starch) feedstocks and is however not sustainable. Therefore, second generation lignocellulosic ethanol production has received significant attention,86 as it promotes energy sustainability and decreases net greenhouse gas emissions. SCB is the most investigated lignocellulosic material for 2G ethanol production.28Saccharomyces cerevisiae, a conventional yeast, is the most studied and commercial microorganism for fermentative ethanol production. It shows high resistance to ethanol and inhibitors present in hydrolysate. However, it cannot ferment C5 sugars released from the hemicellulosic fraction to ethanol due to the absence of required genes for the assimilation of these sugars.87S. cerevisiae has been metabolically engineered to enable xylose based ethanol production. However, there are no commercially used strains capable of converting C5 sugars into ethanol efficiently.88,89 Different pre-treatments, methods of hydrolysis and fermentation modes have varying impacts on glucose release and eventually on ethanol production. The maximum theoretical yield of ethanol on glucose is 0.51 g g−1 and the yield obtained with separate hydrolysis and fermentation (SHF) is in the range of 0.40–0.46 g g−1. However, lower yields (0.31–0.38 g g−1) are obtained with simultaneous saccharification and fermentation (SSF).28Neves et al. (2016) investigated cellulosic ethanol production from native as well as ethanol extracted SCB.49 The extraction with ethanol was performed to reduce the total extractive content for the removal of inhibitory compounds. They made use of the steam explosion method with three different configurations for the pre-treatment of native and ethanol extracted SCB: autohydrolysis and dilute acid hydrolysis with H2SO4 and H3PO4. The pre-treated SCB was subjected to enzymatic hydrolysis for glucose release using commercial cellulase preparation Cellic CTec2 followed by fermentation with an industrial strain of S. cerevisiae. Similar results were obtained for glucose recovery and ethanol production with three pre-treatment methods. The ethanol production in terms of titer and yield was better with SHF than with SSF. The highest ethanol titer (27.1 g L−1) and yield (0.47 g g−1) were obtained during SHF using SCB pre-treated by autohydrolysis. On the other hand, the total reaction time was lower in SSF (48 h) than in SHF (84 h) leading to high volumetric productivities. The maximum ethanol productivity was achieved (0.58 g L−1 h−1) when SSF was carried out after pre-hydrolysis with a total processing time of 24 h. Though ethanol extraction removed 80% of organic solvent extractable materials from SCB, this extraction had no impact on ethanol production regardless of the pre-treatment method and fermentation strategy. In a recent study, de Araujo Guilherme et al. (2019) employed different strategies to optimize the ethanol production from SCB through SSF in batch and fed-batch modes.90 They made use of four pre-treatment methods (acid, alkali, hydrothermal and hydrogen peroxide) and four microbial strains for ethanol production. In addition, the enzyme dose and inoculum size were optimized. The highest cellulose content of 65% was achieved with acid-alkali pre-treatment and was most favourable towards ethanol production. The synergistic effect of two pre-treatment favoured the removal of lignin and hemicellulose by acid and alkaline steps, respectively. Furthermore, the combined pre-treatment resulted in the reduction of crystallinity of cellulose. Among the four strains used, the maximum ethanol yield was obtained using S. cerevisiae PE-2 and cellulosic carbon after acid-alkali pre-treatment. The batch SSF process using optimized parameters (inoculum size: 1 g L−1, enzyme dose: 15 FPU cellulase per g cellulose and initial cellulose: 6%) resulted in an ethanol titer, yield and productivity of 31.5 g L−1, 0.47 g g−1 and 1.75 g L−1 h−1. The fed-batch cultivation with three times less enzyme dose in comparison to the batch process yielded 29.8 g L−1 in 40 h with 0.45 g g−1 yield. To overcome the limitations of SSF, Liu et al. (2016) included pre-hydrolysis of alkali pre-treated SCB, i.e. after 24 h of pre-hydrolysis at 50 °C, the temperature was reduced to 37 °C and inoculated with yeast for SSF.91 With this approach, they were able to enhance the solid loading up to 36%. Ethanol fermentation was conducted in batch and fed-batch modes with pre-hydrolysis SSF. The maximum ethanol titer of 66.9 g L−1 was achieved with a conversion efficiency of 72.9% in 96 h in fed-batch mode with 30% (w/v) loading. Despite much efforts, the use of SCB as the feedstock for ethanol synthesis is still far from commercialisation due to expensive pre-treatments, moderate titers and low volumetric productivities.92,93
To incentivize the 2G ethanol sector and support and create a suitable ecosystem for setting up commercial projects and increasing Research & Development, the Government of India on 28-Feb-2019 launched the “Pradhan Mantri JI-VAN (Jaiv Indhan Vatavaran Anukool Fasal Awashesh Nivaran) Yojana” as a tool to create 2G ethanol capacity in the country and attract investments in this new sector. The said scheme has been notified on 08-Mar-2019 in the Extraordinary Gazette of India. The scheme objective is to support 12 Commercial Scale and 10 demonstration scale 2G ethanol projects with a viability gap funding with a total financial outlay of ₹1969.50 crores for the period 2018–19 to 2023–24. Out of the ₹1969.50 crores, ₹1800 crores have been allocated for supporting 12 commercial projects, ₹150 crores have been allocated for supporting 10 demonstration projects and the remaining ₹19.50 crores will be provided to the Centre for High Technology (CHT) as administrative charges.
Xylitol
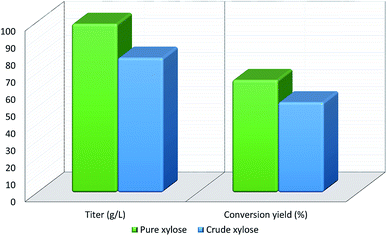
Xylitol is a polyol with applications in the food, odontological, and pharmaceutical industries. Xylitol is a platform chemical and according to the US Department of Energy, it is amongst the 12 renewable added-value chemicals which can be manufactured using biomass. Xylitol has been granted the GRAS (Generally Regarded As Safe) status by the US Food and Drug Administration for application in both food and beverage industries, a factor which will further stimulate its commercial growth. Xylitol has huge commercial potential and the market is expected to reach $1.37 billion by 2025.27,94 Xylose to xylitol conversion is a single step reduction which can be carried out chemically or biochemically. The chemical route involves catalytic hydrogenation of purified xylose from hemicellulosic hydrolysates while biochemically, xylose is reduced to xylitol mediated by xylose reductase and NAD(P)H. The biological production of xylitol has received significant attention due to its sustainability and eco-friendly nature. On the other hand, the chemical route is energy intensive, requires extensive purification, suffers from low product recovery and involves catalyst deactivation making it expensive.95,96 Significant research has been done in the last two decades to enhance the economic viability of bioproduction of xylitol and to this end, hemicellulosic hydrolysate from different biomass rich in xylose including SCB has been utilized for the bioproduction of xylitol by a number of research groups.Our consortium worked towards xylitol production using SCB from Indian sugar mills. The xylose rich hemicellulosic hydrolysate for this purpose was provided by our industrial partner Nova Pangaea Technologies. We used two different yeast strains with the GRAS status for the biotransformation of xylose into xylitol: Yarrowia lipolytica and Pichia fermentans. Y. lipolytica is a non-conventional oleaginous yeast lacking the ability to grow on xylose; however, the cell mass of yeast can transform xylose into xylitol. The high cell density of yeast was accumulated on glucose/pure glycerol/crude glycerol followed by the transformation of xylose into xylitol and the conversion yields obtained were ≥90%. When pure xylose was replaced with hemicellulosic hydrolysate in an optimized medium, the yield dropped to 54% (ref. 97) (Fig. 4). The observed drop in yield might have been due to the inhibitors in hydrolysate which would have been overcome with suitable detoxification. Although this preliminary work was positive, more work is required to achieve superior performance. In another study98 (manuscript under review), a xylose eating and xylitol accumulating yeast P. fermentans was isolated. The wild type strain was subjected to random mutagenesis and a mutant strain outperforming the wild type was identified. The culture medium was optimized using a statistical method and the process was scaled up from a shake flask (250 mL with a working volume of 50 mL) to a bioreactor level (2.5 L with a working volume of 1.0 L). After media optimization, the mutant strain produced a maximum xylitol titer and yield of 70.5 g L−1 and 0.49 g g−1 respectively from pure xylose. While on xylose rich hydrolysate, the strain was able to accumulate 62.3 g L−1 xylitol with a conversion yield of 0.43 g g−1. Upon scaling up using an optimized media composition, the xylitol titer and yield improved to 98.9 g L−1 and 0.67 g g−1, respectively with pure xylose. In the case of hydrolysate, the xylitol concentration and conversion yield enhanced to 79 g L−1 and 0.54 g g−1 respectively (Fig. 5).
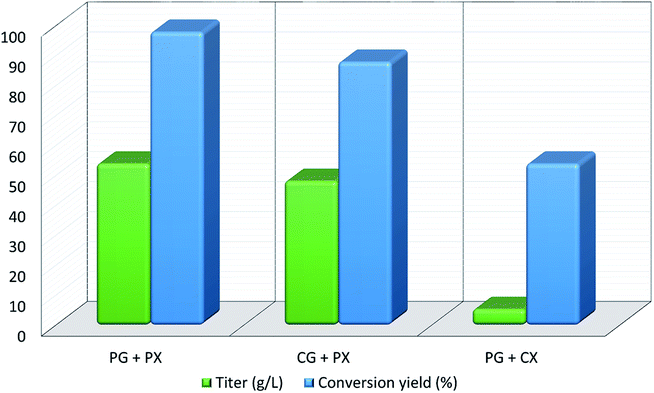 | ||
| Fig. 4 Xylitol production and yield from co-fermentation of glycerol and xylose by Y. lipolytica (PG: pure glycerol; CG: crude glycerol; PX: pure xylose; CX: crude xylose). | ||
 | ||
| Fig. 5 Comparison of xylitol accumulation by newly isolated yeast P. fermentans using pure and crude xylose. | ||
The integrated biorefineries for simultaneous synthesis of ethanol and xylitol could enhance the profitability of cellulosic ethanol production by 2.3-fold.94 Therefore, the co-production of ethanol and xylitol from cellulosic and hemicellulosic fractions has been attempted to improve the process economics of SCB-based biorefineries. Xylitol has a higher market value than ethanol; therefore, an integrated SCB processing for co-production would create economic benefits making the overall process feasible.99 Two different approaches have been employed for the co-production: use of a single organism for two products, one strain for each metabolite. da Silva et al. (2015) and Dasgupta et al. (2017) used Kluyveromyces marxianus for co-production.95,100K. marxianus produced ethanol (titer: 12 g L−1; yield: 0.22 g g−1; productivity: 0.08 g L−1 h−1) from cellulosic hydrolysate. While with hemicellulosic hydrolysate, xylitol (titer: 9.4 g L−1; yield: 0.40 g g−1; productivity: 0.10 g L−1 h−1) was obtained as the main product with ethanol (1.31 g L−1) as a by-product (da Silva et al. 2015). In a second approach, Castañón-Rodríguez et al. (2015) utilised two different yeasts, S. cerevisiae and C. tropicalis which are best known for their ability to ferment glucose and xylose to ethanol and xylitol, respectively.101 These two yeast strains were co-cultured using a simulated medium of SCB hydrolysate instead of separate fermentation for ethanol and xylitol. The best condition for co-culture was sequential addition where fermentation was initiated with S. cerevisiae and 24 h later, C. tropicalis was inoculated. As a result, the glucose concentration reduced to a low level to allow efficient utilization of xylose. The fed-batch cultivation with sequential co-culture resulted in enhanced production of both metabolites, ethanol (titer: 11.6 g L−1; yield: 0.44 g g−1; productivity: 0.87 g L−1 h−1) and xylitol (titer: 8.5 g L−1; yield: 0.57 g g−1; productivity: 0.27 g L−1 h−1). In another study by Unrean and Ketsub, 2018, S. cerevisiae and C. tropicalis were cultured separately for the production of ethanol and xylitol. The fed-batch cultivation resulted in the highest concentrations of ethanol by S. cerevisiae and xylitol by C. tropicalis at 56.1 g L−1 and 24 g L−1 with product yields of 0.44 and 0.50 g g−1 respectively.
Significant research has been done in the last few decades on bioproduction of xylitol and substantial improvement in the titer, yield and productivity of xylitol has been achieved using metabolic and process engineering approaches. Despite all this, the bioprocess is still far from industrial level production. Even today, the chemical route for xylitol production remains the dominant one.94,96 According to a simulation study by Mountraki et al. (2017), the amount of xylitol crystals which can be obtained from 1 kg of xylose via chemical and biotechnological routes was 0.87 and 0.73 kg, respectively.102 In order to make the biological route competitive to its chemical counterpart, several hurdles need to be overcome to cut down the high capital and operational cost of various steps such as pre-treatment, detoxification, fermentation and downstream processing. Table 6 summarises the xylitol production from SCB by different microorganisms and the comparison reveals that our results outperformed other previous reports, making it competitive. Most of the studies in Table 6 have detoxified the hydrolysate using methods such as over-liming, activated charcoal, ion exchange resin adsorption treatment or a combination of these methods for improving fermentation performance. Detoxification adds to the operational costs and may also lead to loss of sugars. Thus, a cost effective and convenient pre-treatment with minimum or no release of inhibitors eliminating the detoxification step along with efficient recovery of xylitol is highly desirable. This in combination with the design of a hyper and robust xylitol producer with the aid of advanced metabolic engineering and synthetic biology, process design, intensification and integration with sugar mills can enhance the commercial feasibility of xylitol production at the bulk level.
| Organism | Pre-treatment | Detoxification method | Fermentation mode | Titer (g L−1) | Yield (g g−1) | Productivity (g L−1 h−1) | Reference |
|---|---|---|---|---|---|---|---|
| a The value in parenthesis refers to ethanol production from cellulosic hydrolysate. | |||||||
| Candida guilliermondii FTI 20037 | Acid hydrolysis | pH adjustment, activated charcoal, vacuum filtration | Batch | 36.3 | 0.64 | 0.76 | Rodrigues et al., 2003 (ref. 109) |
| Candida guilliermondii FTI 20037 | Acid hydrolysis | pH adjustment, activated charcoal | Batch; immobilized | 47.5 | 0.81 | 0.40 | Carvalho et al., 2004 (ref. 110) |
| Candida tropicalis | Acid hydrolysis | pH adjustment, activated charcoal, ion exchange | Batch | 19.5 | 0.65 | — | Rao et al., 2006 (ref. 111) |
| Candida tropicalis JH030 | Acid hydrolysis | pH adjustment | Batch | 12.5 | 0.51 | — | Huang et al., 2011 (ref. 112) |
| Debaryomyces hansenii | Steam explosion, acid hydrolysis | Activated charcoal, ion exchange | Batch | 13.8 | 0.69 | 0.28 | Prakash et al., 2011 (ref. 113) |
| Candida guilliermondii FTI20037 | Acid hydrolysis | Vacuum concentration, pH adjustment, activated charcoal | Batch | 36.1 | 0.65 | 0.75 | Hernández-Pérez et al., 2016 (ref. 85) |
| Candida tropicalis | Hot water autohydrolysis | Vacuum concentration, over-liming, activated charcoal | Batch | 32.0 | 0.46 | 0.27 | Vallejos et al., 2016 (ref. 114) |
| Candida guilliermondii FTI20037 | Acid hydrolysis | Vacuum concentration, pH adjustment, activated charcoal | Batch | 41.8 | 0.66 | 0.29 | de Arruda et al., 2017 (ref. 115) |
| Yarrowia lipolytica | Hydrothermal | pH adjustment | Batch | 5.4 | 0.54 | 0.11 | Prabhu et al., 2020c (ref. 97) |
| Pichia fermentans | Hydrothermal | pH adjustment | Batch | 79.0 | 0.54 | 0.47 | Prabhu et al., 2020a (ref. 98) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Co-production of xylitol and ethanol | |||||||
| Kluyveromyces marxianus ATCC 36907 | Acid–alkaline hydrolysis | Over-liming | Batch | 9.4 (12.0) | 0.40 (0.22) | 0.10 (0.08) | da Silva et al., 2015 (ref. 100) |
| Saccharomyces cerevisiae/Candida tropicalis | — | pH adjustment | Fed-batch | 8.5 (11.6) | 0.57 (0.44) | 0.27 (0.87) | Castañón-Rodríguez et al., 2015 (ref. 101) |
| Kluyveromyces marxianus IIPE453 | Acid and steam hydrolysis | Over-liming | Batch with cell recycling | 11.1 (21.6) | 0.32 (0.45) | 0.19 (0.90) | Dasgupta et al., 2017 (ref. 95) |
| Saccharomyces cerevisiae/Candida tropicalis | Acid hydrolysis | pH adjustment | Fed-batch | 24.0 (56.1) | 0.50 (0.44) | 0.25 (0.58) | Unrean & Ketsub, 2018 (ref. 99) |
Succinic acid
According to the US Department of Energy, SA is a top platform chemical which can be produced from biomass. The presence of two carboxyl groups makes it a versatile precursor molecule for manufacturing a large number of industrially important products such as tetrahydrofuran, 1,4-butanediol, γ-butyrolactone, adipic acid, aliphatic esters, etc.103 The global production of SA was reported to be 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons in 2016 and is expected to double by 2025.104 Due to growing interest towards greener production of chemicals in recent times, there has been a paradigm shift from petrochemical synthesis towards bio-based production of SA, a potential alternative. The bio-based SA production also contributes to reduction in carbon emission as it requires carbon dioxide, a primary greenhouse gas, as a co-substrate. The commercial potential of bioproduction of SA is impeded by its higher cost in comparison to chemically synthesized SA.105,106 The cost of production can be reduced by the use of crude renewable sources such as SCB. There are handful of studies using SCB as the feedstock for bioproduction of SA (Table 7). Borges and Pereira (2011) used Actinobacillus succinogenes for SA production from xylose.107 The culture medium was optimized using a central composite rotational design with four variables NaHCO3, MgSO4, yeast extract and KH2PO4. The optimized medium composition resulted in a SA production of 14.2 g L−1, yield of 0.64 g g−1 and productivity of 0.67 g L−1 h−1. When pure xylose was replaced with SCB hemicellulosic hydrolysate containing 52 g L−1 xylose, SA accumulated was 22.5 g L−1 with a yield and productivity of 0.43 g g−1 and 1.01 g L−1 h−1. Though all the xylose was consumed and the production rate was faster, the conversion efficiency was lower in comparison to pure xylose, probably due to the presence of fermentation inhibitors. Xi et al. (2013) combined acid hydrolysis and ultrasonic pre-treatment which improved the sugar yield by 29.5%.108 The highest sugar concentration of 43.9 g L−1 was obtained when ultrasonication was carried out for 40 minutes in comparison to 33.9 g L−1 without ultrasonic pre-treatment. This allowed the maximum use of hemicellulosic carbon and reduced the yeast extract requirement by 60%.
000 metric tons in 2016 and is expected to double by 2025.104 Due to growing interest towards greener production of chemicals in recent times, there has been a paradigm shift from petrochemical synthesis towards bio-based production of SA, a potential alternative. The bio-based SA production also contributes to reduction in carbon emission as it requires carbon dioxide, a primary greenhouse gas, as a co-substrate. The commercial potential of bioproduction of SA is impeded by its higher cost in comparison to chemically synthesized SA.105,106 The cost of production can be reduced by the use of crude renewable sources such as SCB. There are handful of studies using SCB as the feedstock for bioproduction of SA (Table 7). Borges and Pereira (2011) used Actinobacillus succinogenes for SA production from xylose.107 The culture medium was optimized using a central composite rotational design with four variables NaHCO3, MgSO4, yeast extract and KH2PO4. The optimized medium composition resulted in a SA production of 14.2 g L−1, yield of 0.64 g g−1 and productivity of 0.67 g L−1 h−1. When pure xylose was replaced with SCB hemicellulosic hydrolysate containing 52 g L−1 xylose, SA accumulated was 22.5 g L−1 with a yield and productivity of 0.43 g g−1 and 1.01 g L−1 h−1. Though all the xylose was consumed and the production rate was faster, the conversion efficiency was lower in comparison to pure xylose, probably due to the presence of fermentation inhibitors. Xi et al. (2013) combined acid hydrolysis and ultrasonic pre-treatment which improved the sugar yield by 29.5%.108 The highest sugar concentration of 43.9 g L−1 was obtained when ultrasonication was carried out for 40 minutes in comparison to 33.9 g L−1 without ultrasonic pre-treatment. This allowed the maximum use of hemicellulosic carbon and reduced the yeast extract requirement by 60%.
| Organism | Pre-treatment | Feedstock | Detoxification method | Fermentation mode | Titer (g L−1) | Yield (g g−1) | Productivity (g L−1 h−1) | Reference |
|---|---|---|---|---|---|---|---|---|
| Actinobacillus succinogenes | Acid hydrolysis | Hemicellulosic hydrolysate | pH adjustment | Batch | 22.5 | 0.43 | 1.01 | Borges & Pereira, 2011 (ref. 107) |
| Actinobacillus succinogenes | Acid hydrolysis with ultrasonication | Hemicellulosic hydrolysate | Activated charcoal | Batch | 23.7 | 0.79 | 0.99 | Xi et al., 2013 (ref. 108) |
| Actinobacillus succinogenes | Alkali hydrolysis | Cellulosic/hemicellulosic hydrolysate | pH adjustment | Fed-batch | 70.8 | 0.82 | 1.42 | Chen et al., 2016 (ref. 116) |
| Actinobacillus succinogenes | Alkali hydrolysis | Cellulosic/hemicellulosic hydrolysate | pH adjustment | Repeated-batch | 120 | 0.81 | 1.65 | Chen et al., 2016 (ref. 116) |
| Yarrowia lipolytica | Alkali hydrolysis | Cellulosic/hemicellulosic hydrolysate | pH adjustment | Batch | 33.2 | 0.58 | 0.33 | Ong et al., 2019 (ref. 122) |
| Yarrowia lipolytica | Hydrothermal | Hemicellulosic hydrolysate | pH adjustment | Batch | 5.6 | 0.14 | 0.093 | Prabhu et al., 2020b (ref. 123) |
The batch fermentation of non-detoxified hemicellulosic hydrolysate containing 22.5 g L−1 xylose, 3.6 g L−1 glucose and 3.9 g L−1 arabinose was performed in a bioreactor. All the sugars were consumed, and SA achieved at the end of fermentation was 23.7 g L−1. The yield and productivity were 0.79 g g−1 and 0.99 g L−1 h−1. The hydrolysate contained 2.84 g L−1 total soluble phenolic compounds, 0.42 g L−1 HMF, and 0.71 g L−1 furfural. Despite this, surprisingly, the results obtained with non-detoxified hydrolysate were better than those of the detoxified one. The SA titer and yield achieved were 20.9 and 20.2% higher in comparison to detoxified hydrolysate showing that detoxification was not required. No specific explanation was offered.
In another study by Chen et al. (2016), SCB-based SA production by using A. succinogenes was evaluated.116 They employed two different pre-treatment methods for SCB with different concentrations of acid/alkali and found that alkali pre-treatment was better than its acid counterpart. The lignin removal was more than 90% with NaOH treatment, while in the case of H2SO4 treatment, it was between 40 and 70%. The best results were obtained with 0.25 M NaOH with a cellulose and hemicellulose retention of 97.9 and 87.3%, respectively, and a lignin removal of 93.3%. The composition of enzyme cocktail was optimized using an orthogonal design and the optimal conditions for saccharification of pre-treated SCB was 15, 9, 0.2 and 20% v/w biomass of cellulase, xylanase, glucanase and pectinase concentration, respectively. This optimal cocktail yielded a reducing sugar concentration of 55 g L−1 with glucose and xylose in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The fed-batch cultivation was carried out using an initial reducing sugar concentration of 55 g L−1 (41 g L−1 glucose & 14 g L−1 xylose) and the culture was fed when the sugar level dropped below 20 g L−1. At the end of the experiment, 70.8 g L−1 of SA accumulated with a yield and productivity of 0.82 g g−1 and 1.42 g L−1 h−1. They also conducted repeated batch fermentation using cell immobilized on the surface of SCB residue (SBR) after alkaline pre-treatment and enzymatic hydrolysis. Three batches were performed and at the end of each batch fermentation, spent media were pumped out and a fresh medium was added for cell growth and SA production. NaHCO3 was used to modulate pH and served as a source of CO2. The sugar consumption profile and SA production in all three batches were similar. The amount of sugars consumed and SA synthesized after the three batches in a total time of 64 h was 149 and 120 g L−1. The overall yield and productivity were 0.81 g g−1 and 1.65 g L−1 h−1. Acetic acid was obtained as the by-product in all the experiments (4–10 g L−1).
1. The fed-batch cultivation was carried out using an initial reducing sugar concentration of 55 g L−1 (41 g L−1 glucose & 14 g L−1 xylose) and the culture was fed when the sugar level dropped below 20 g L−1. At the end of the experiment, 70.8 g L−1 of SA accumulated with a yield and productivity of 0.82 g g−1 and 1.42 g L−1 h−1. They also conducted repeated batch fermentation using cell immobilized on the surface of SCB residue (SBR) after alkaline pre-treatment and enzymatic hydrolysis. Three batches were performed and at the end of each batch fermentation, spent media were pumped out and a fresh medium was added for cell growth and SA production. NaHCO3 was used to modulate pH and served as a source of CO2. The sugar consumption profile and SA production in all three batches were similar. The amount of sugars consumed and SA synthesized after the three batches in a total time of 64 h was 149 and 120 g L−1. The overall yield and productivity were 0.81 g g−1 and 1.65 g L−1 h−1. Acetic acid was obtained as the by-product in all the experiments (4–10 g L−1).
Bacteria are extremely sensitive towards low pH and require moderate pH for their growth, resulting in the consumption of large quantities of the neutralizing agent that affects the productivity cost.117 Further at neutral pH, SA is obtained in the form of succinate salts and needs an additional step of acidification to bring it back to the acid form leading to accumulation of by-products such as gypsum, in-turn making the downstream process more critical. This problem can be resolved by carrying out the fermentation at a low pH value.118 Obtaining SA in an unionized form will simplify the downstream processing and lead to an economical process as it will avoid neutralization during fermentation and acidification during product recovery.104 Yeasts are potential host to produce organic acid because of their high tolerance to low pH and are naturally predisposed to grow under low pH, below 4.119Yarrowia lipolytica is an oleaginous and non-conventional yeast and excellent cell factory for synthesizing a large variety of commercially important products.120 It has an amazing ability to grow perfectly well over a wide pH range without significant change in growth parameters.121 In a recent report by Ong et al. (2019), the feasibility of SA production from co-fermentation of glucose and xylose by Y. lipolytica was investigated.122 They utilised pure as well as crude glucose and xylose from SCB as the feedstock. To maximize sugar release from pre-treated SCB, enzymatic hydrolysis was optimized through three parameters: pH, temperature and enzyme dosage. They reported 33.2 g L−1 SA by Y. lipolytica using SCB hydrolysate containing 47.3 g L−1 glucose and 20.2 g L−1 xylose during bioreactor cultivation. Glucose was completely consumed by the yeast; however, a large fraction of xylose remained unutilized (50–70%). Our consortium also produced SA from SCB hemicellulosic hydrolysate by Y. lipolytica without the control of pH.123 The yeast can utilize a variety of carbon sources but lacks the ability to metabolize xylose. Y. lipolytica was engineered for xylose assimilation and SA production. The batch cultivation of recombinant strain in the bioreactor resulted in a SA titer of 11.2 g L−1 with a yield of 0.18 g g−1. The experiment was repeated with crude xylose (40 g L−1) rich hydrolysate derived from SCB. Hydrolysate after pre-treatment often contains inhibitors which can negatively impact the performance of microorganisms. The cell growth was unaffected as the biomass yield was similar in both the cases. The strain accumulated 5.6 g L−1 of SA titer with a yield of 0.14 g g−1 (Fig. 6). Acetic acid was obtained as the major product at higher quantities (8.3 g L−1) than the desired product SA. Further work to divert produced acetic acid towards SA is underway.
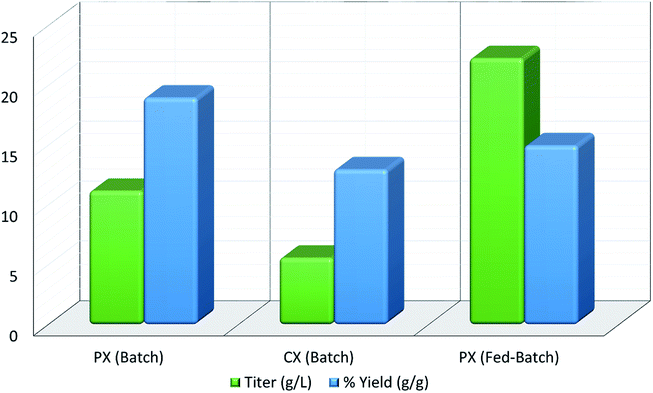 | ||
| Fig. 6 Succinic acid production from pure and crude xylose by engineered Y. lipolytica during batch and fed-batch fermentation (PX: pure xylose; CX: crude xylose). | ||
It is important to discuss two studies in recent times related to cost analysis for SA production from SCB. The sugar yield during pre-treatment has a significant impact on the commercial viability of LCB-based biorefineries. Nieder-Heitmann et al. (2020) investigated the profitability of different pre-treatment methods for SCB through simulation work using Aspen Plus.124 They screened available pre-treatment technologies, and identified nine methods which were simulated and compared for the co-production of succinic acid and electricity in a SCB and trash biorefinery. The nine pre-treatment methods were as follows: dilute acid treatment (DAT) with enzymatic hydrolysis (EH); DAT without EH; NaOH with EH; organosolv with EH; ammonia fibre expansion with EH; steam explosion (STEX) with EH; STEX with SO2 and EH; STEX with NaOH and EH; wet oxidation with EH. Except organosolv and wet oxidation, all other pre-treatment methods were found to be profitable. The most profitable was steam explosion with a SA yield, capital cost and internal rate of return (IRR) of 45.7 kg SA per 100 ton dry mass, $ 384.2 million and 28% respectively. The two challenges identified were proper mixing for efficient mass and heat transfer during pre-treatment and scale up of EH to the commercial level.
In another study, Klein et al. (2017) carried out simulation studies using Aspen Plus for the integration of SA production from SCB to optimized SCB biorefineries with the production of first generation (1G) ethanol and electricity.125 SCB was pre-treated using dilute H2SO4 and hemicellulosic hydrolysate (C5 liquor) obtained was fermented to SA by using wild type A. succinogenes after detoxification. The cellulignin fraction was utilized for electricity generation via CHP. The downstream step was designed and simulated based on literature data and resulted in a recovery of SA of up to 99%. The calculated cost of SA production was $2.32 per kg with sugarcane and capital cost being the major contributors. The calculated cost was similar to that of sugar-based SA production, $2.26 per kg SA from sucrose. Furthermore, the internal rate of return for the integrated SA biorefinery (15.8%) was lower than that of an ethanol distillery (17%). The 1G ethanol production is a mature and well-established technology; on the other hand, 2G SA production is in infancy and an emerging technology with lots of scope for improvement at the process as well as at the strain level.
Lactic acid (LA)
LA is an industrially important chemical and has been included in the revised list of platform chemicals prepared by the US Department of Energy.126 LA finds its applications in food, chemical, textile, pharmaceutical, and other industries127,128 and its worldwide demand is estimated to be 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–150
000–150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonne per year.129 LA can be produced commercially either chemically or by fermentation. Chemical synthesis results in a racemic mixture of two isomers, whereas microbial fermentation can lead to an optically pure isomer depending on the strain, raw materials and conditions used during fermentation.130,131 Most of the LA (∼90%) worldwide comes from microbial fermentation of carbohydrates and the remaining from chemical synthesis (∼10%). The major fermentative manufacturers of LA are Nature Works LLC (USA), Purac (Netherlands) and Galactic (Belgium).132
000 tonne per year.129 LA can be produced commercially either chemically or by fermentation. Chemical synthesis results in a racemic mixture of two isomers, whereas microbial fermentation can lead to an optically pure isomer depending on the strain, raw materials and conditions used during fermentation.130,131 Most of the LA (∼90%) worldwide comes from microbial fermentation of carbohydrates and the remaining from chemical synthesis (∼10%). The major fermentative manufacturers of LA are Nature Works LLC (USA), Purac (Netherlands) and Galactic (Belgium).132
LA has also been receiving great attention as a feedstock for the manufacture of polylactic acid (PLA), a biodegradable polymer used as a raw material in packaging as well as fibres and foams. On an industrial scale, PLA production is considered a relatively immature technology as compared with petrochemical raw materials, mainly due to the high production cost of LA which is the raw material for PLA. The high costs of LA production are due to expensive sugar & nitrogen sources required for fermentation along with the downstream recovery and purification process. A major concern in LA fermentation is reducing the cost of the raw materials. This problem can be resolved through fermentative production of LA from low cost materials such as wastes from agricultural and agro-industrial residues. SCB can be the choice of raw material for LA production due to its low cost and well-established supply chain. However, most starchy and lignocellulose materials must be pre-treated by physicochemical and enzymatic methods as discussed in earlier sections. Also, LA from SCB will be economically attractive and competitive with LA production from pure sugars, if their productivities and yields are similar to those of pure sugar fermentation. It can only be accomplished when enzymatic liquefaction is performed at a high solid loading of pre-treated lignocellulosic biomass with uncompromised and concentrated sugar yields. Some of the factors that have a direct impact on enzyme hydrolysis are the type of pre-treatment133,134 origin of the cellulases complex and accessory enzymes associated with it, pH, water availability and substrate feeding strategies.135,136
LA production from SCB reported in the literature is summarised in Table 8. Patel et al. (2005) used Bacillus sp. 36D1 (isolate) for LA production. Batch SSF of 20 g L−1 Solka Floc (commercial cellulose powder) with 10 FPU of Spezyme CE per g cellulose was carried out for 96 h by varying pH (4.5 to 7 at 50 °C) and temperature (30 to 60 °C at pH 5).137 The volumetric productivity of LA was optimal between fermentation pH values of 4.5 and 5.5 at 50 °C. At a constant pH of 5, s LA titer of 18.3 g L−1 with s maximal LA volumetric productivity of 0.17 g L−1 h−1 was observed at 55 °C with L(+) isomer optical purity more than 95%. Furthermore, they also investigated the co-fermentation of cellulose-derived glucose and sugarcane bagasse hemicellulose derived xylose simultaneously (SSCF). In a batch SSCF of 40% acid pre-treated hemicellulose hydrolysate (over-limed) and 20 g L−1 Solka Floc cellulose, Bacillus sp. 36D1 produced about 35 g L−1 LA in about 144 h with 15 FPU of Spezyme CE per g cellulose.
| Microorganism | Pre-treatment | Detoxification method | Fermentation mode | Titer (g L−1) | Yield (g g−1) | Productivity (g L−1 h−1) | Reference |
|---|---|---|---|---|---|---|---|
| Bacillus sp. 36D1 | Acid | Acid hydrolysate treated with lime | Batch SSF | 35 | — | 0.24 | Patel et al., 2005 (ref. 137) |
| Lactobacillus delbreuckii mutant Uc-3 | Steam–alkali | — | Batch SSF | 67.0 | 0.83 | 0.93 | Adsul et al., 2007 (ref. 138) |
| Lactobacillus casei | Acid–solvent | Solid fraction water wash | Batch | 25.7 | 1.00 | 0.27 | Jonglertjunya et al., 2014 (ref. 143) |
| Bacillus sp. P38 | Acid–alkali | Solid fraction water wash | Fed-batch | 185.0 | 0.99 | 1.93 | Peng et al., 2014 (ref. 144) |
| Bacillus coagulans | Acid–steam explosion | Solid fraction water wash | Batch SSF | 70.4 | 0.90 | 1.14 | van der Pol et al., 2016 (ref. 139) |
| Lactobacillus pentosus | Acid–steam | Solid fraction water wash | Fed-batch SSF | 72.7 | 0.61 | 1.01 | Unrean, 2018 (ref. 145) |
| Bacillus coagulans | Acid | Acid hydrolysate pH adjustment | Batch | 55.9 | 0.87 | 1.7 | de Oliveira et al., 2019 (ref. 131) |
| Lactobacillus pentosus | Acid–alkali | Acid hydrolysate pH adjustment | Batch | 65.0 | 0.93 | 1.01 | Wischral et al., 2019 (ref. 146) |
| Lactobacillus pentosus | Acid | Acid hydrolysate pH adjustment followed by filtration | Batch | 55.4 | 0.72 | 0.43 | González-Leos et al., 2019 (ref. 147) |
| Bacillus coagulans | Alkali | Solid fraction water wash | Batch | 68.7 | 0.92 | 2.86 | Nalawade et al., 2020b (ref. 141) (manuscript under review) |
| Acid–alkali | 66.9 | 0.88 | 2.79 | ||||
| Alkali–acid | 71.8 | 0.90 | 2.99 | ||||
| Cavitation with alkali | 62.5 | 0.92 | 2.60 |
To improve the LA titer and productivity, Adsul et al. (2007) have used steam and alkali pre-treated SCB at a higher solid loading and Lactobacillus delbrueckii mutant Uc-3.138 Batch SSF of 80 g L−1 pre-treated SCB was conducted using a cellulase preparation (10 FPU per g of pre-treated SCB) derived from a mutant strain of Penicillium janthinellum. A LA titer of 67 g L−1 was obtained in 96 h from steam-alkali pre-treated SCB. In another study, Van der Pol et al. (2016) employed multiple strategies for the optimization of batch SSF of SCB obtained after acid pre-treatment and steam explosion.139 They have investigated whether furfural addition (one of the inhibitors generated during pre-treatment) to precultures of Bacillus coagulans had a beneficial effect on the LA fermentation of pre-treated and enzyme hydrolysed SCB. The preculture was cultivated in PYPD medium with 1 g L−1 furfural. The pre-treated SCB solid fraction was hydrolysed with either a liquid fraction obtained from pre-treatment of SCB or demineralized water. Pre-treated SCB was hydrolysed with the enzyme cocktail GC220 and fermented by using B. coagulans DSM 2315 at pH 5.8 and 50 °C. For batch SSF of SCB with the liquid fraction from pre-treatment of SCB, a LA titer of 74.6 g L−1 and a productivity of 0.92 g L−1 h−1 were obtained for preculture in the PYPD medium with furfural as compared to a LA titer of 64.1 g L−1 and a productivity of 0.78 g L−1 h−1 for preculture in the PYPD medium. It was found that pre-cultivation in the presence of furfural was beneficial for LA fermentation. For batch SSF of pre-treated SCB, solid in water and preculture in the PYPD medium with furfural, the LA productivity increased to 1.14 g L−1 h−1 with 70.4 g L−1 of LA. The better productivity in the latter case was due to reduction in the lag phase from 40 h to 32 h.
Increasing the sugar concentration in hydrolysates can improve the productivity and yield of fermentation products. To achieve higher sugar concentrations in SCB hydrolysate, evaporation after hydrolysis or pre-treatment has been exploited as a strategy. Peng et al. (2014) concentrated enzyme hydrolysate of acid-alkali treated SCB. In their fed-batch fermentation experiments, an L(+) LA titer of 185 g L−1 with a productivity of 1.93 g L−1 h−1 was achieved with Bacillus sp. P38. In another study, de Oliveira et al. (2019) concentrated the hemicellulosic hydrolysate after acid pre-treatment of SCB five times.131 For B. coagulans fermentation, an L(+) LA titer of 55.9 g L−1 with a yield of 0.87 g g−1 and a productivity of 1.7 g L−1 h−1 was obtained.
A range of bacterial strains such as Lactobacillus delbreuckii, Lactobacillus pentosus, Bacillus sp. and B. coagulans have been reported for LA fermentation (Table 8). The optimal growth conditions for these bacteria are spread over the temperature range of 35–50 °C, but around neutral pH. The enzymatic hydrolysis conditions required to produce sugar rich hydrolysates however require radically different conditions, namely, temperature in the range of 45–55 °C and pH in the range of 4.5–5.5. Therefore, SSF as an approach for LA production from SCB would not be a feasible option as it compromises the operating parameters such as temperature and pH for either the hydrolysis stage or the fermentation stage. Hence, SHF can be a preferred approach over SSF especially for LA fermentation.
Along these lines, two strategies were evaluated for LA production in our consortium,140 wherein pre-treatment of 12.5% SCB loading, Cellic CTec2 enzyme and thermophilic Bacillus coagulans NCIM 5648 were used. In first strategy, when Cellic CTec2 was dosed at 30 FPU g−1 of pre-treated SCB, it hydrolysed 75.8% cellulose and 88.6% xylan in 24 h. However, when the enzyme loading was changed to 25 mg protein per g glucan in the second case, it hydrolysed 72.3% and 68% cellulose and xylan respectively. The valorisation of glucose rich filtrates obtained from strategies 1 and 2 using two different media resulted in 50.4 g L−1 and 51.2 g L−1 of LA production from 54.7 to 62.7 g L−1 of glucose respectively. Despite opting for two alternative strategies during high solid SHF, an optically pure LA production of around 50 g L−1 was achieved within a short duration of 45–54 h from SCB hydrolysate.
In continuation to the above work in our consortium, a comparative study of four pre-treatments – alkali, acid–alkali, alkali–acid and HC in the presence of alkali – were conducted for SCB and their impact was evaluated based on sugar released from pre-treated SCB after enzymatic hydrolysis and subsequent LA production in a separate fermenter (SHF) by B. coagulans NCIM 5648 (ref. 141 manuscript under review). The material balance from 100 g of untreated SCB to LA for alkali (A), acid–alkali (B), alkali–acid (C) and HC with alkali (D) pre-treatment routes is shown in Fig. 7. After pre-treatment of SCB, 59 g, 32.4 g, 31.2 g and 62 g of pre-treated SCB were obtained for cases (A) through to (D) respectively. 17.5% (w/v) pre-treated SCB loading was used for enzymatic hydrolysis using the Cellic CTec2 enzyme. The enzyme hydrolysed SCB medium was used for LA fermentation using B. coagulans. Unlike most of the Bacillus strains reported earlier such as B. coagulans GKN316 and B. coagulans NL01,142B. coagulans NCIM 5648 was unable to valorise xylose to LA.
 | ||
| Fig. 7 Mass balance of LA production by B. coagulans using SCB pre-treated with different methods, (a) SCB to LA mass balance in numbers and (b) SCB to LA mass balance in bar charts. | ||
For the alkali pre-treatment (A), the recovery of 35.58 g cellulose, 8.6 g hemicellulose, and 8.1 g lignin in 59 g alkali treated SCB was achieved from 100 g untreated SCB. For the enzyme hydrolysis, 17.5 g of alkali treated SCB (17.5% w/v) was used. This resulted in a glucose release of 84.3 g L−1 with a hydrolysis efficiency of 72%. The enzyme hydrolysate was used for lactic acid fermentation using B. coagulans NCIM 5648. An L(+) LA titer, productivity and yield of 68.7 g L−1, 2.86 g L−1 h−1 and 0.92 g g−1 were achieved respectively. From this route, 28.43 g of glucose was released during hydrolysis and 26.16 g of L(+) LA was produced. For the sequential alkali–acid pre-treatment (C), 23.4 g cellulose recovery in 31.2 g of alkali–acid treated SCB was obtained from 100 g untreated SCB. As mentioned earlier in (A), 17.5 g of alkali-acid treated SCB was used for enzyme hydrolysis. The highest sugar release of 89.3 g L−1 was achieved for the alkali–acid pre-treated SCB. After fermentation of enzyme hydrolysate, an L(+) LA titer, productivity and yield of 71.8 g L−1, 2.99 g L−1 h−1 and 0.90 g g−1 were achieved respectively. This corresponded to 14.33 g L(+) LA. With cavitation in the presence of alkali (D), an L(+) LA titer of 62.5 g L−1, productivity of 2.60 g L−1 h−1 and yield of 0.92 g g−1 were achieved. While (D) was similar to (A), sequential acid-alkali pre-treatment (B) was similar to (C). Considering the overall approach (Fig. 7 and Table 8), alkali pre-treatment was found to be the best method for an enhanced L(+) LA yield. The product yield achieved was over 80% higher in comparison to the sequential pre-treatment. The material balance of multiple routes clearly shows that the substantial loss of the materials during any pre-treatment step may result in a lower economic efficiency of the overall process.
Summary and path forward
This brief review outlines the need and the potential of valorisation of waste biomass streams produced by sugar industries (in India) in line with the sentence attributed to Mahatma Gandhi “Waste is a resource in the wrong place”.148 The current situation of large national stocks of sugar and the overall price trends of sugar in world markets are driving down the prices of sugar in India. Therefore, transforming the so-called waste biomass streams of sugar mills to higher value products is needed more than ever. The crux of transforming waste biomass streams into resource is to ensure that the cost of pre-treating these biomass streams (for making them amenable for further value addition) and the cost of extracting value added products from pre-treated waste streams are lower than the potential revenue generated by the recovered products. An attempt is made here to recap the discussion and provide specific recommendations based on the earlier discussion.SCB, the predominant solid waste generated during sugarcane processing is a complex lignocellulosic biomass containing valuable components in cellulose and hemicellulose (interlinked to lignin) which requires significant pre-treatment. The pre-treatment of SCB is broadly grouped into two classes: first is for opening up the lignocellulosic matrix and enhancing the bioavailability of SCB. This is mainly used for recovering biogas–biomethane from SCB. HC is the most economical and recommended option for this class of pre-treatment.41 The second class of pre-treatment is focussed on enhancing the enzymatic saccharification yield from SCB (C5 and C6 sugars). Mild acid hydrolysis in the presence of steam is the recommended option when C5 rich hydrolysate is the desired entity.57,58 Enzymatic hydrolysis of the obtained cellulignin or subjecting it to alkali hydrolysis prior to enzymatic hydrolysis will yield a cellulose rich residue.
In an integrated biorefinery approach, C6 sugar (glucose) from SCB can be utilized for LA or ethanol production and C5 sugar (xylose) in hydrolysate may be utilized for SA or xylitol production or biogas production using anaerobic digestion. An integrated approach for the co-production of ethanol (from C6) and xylitol (from C5) from SCB is a promising starting point;94 however further investigative efforts are required to progress further for realising these approaches on an industrial scale.
It is necessary to make use of microorganism(s), which can metabolize C6 as well as C5 sugars to maximize the bioconversion of lignocellulosic sugars.87 Unlike glucose, xylose valorisation through a biochemical route is largely ignored, as most of the industrial microbes lack an efficient metabolic pathway for their assimilation. Furthermore, those microbial strains which can assimilate xylose suffer from a major setback due to preference for glucose as a carbon substrate over xylose. This suppresses xylose utilization during co-fermentation due to carbon catabolite repression (CCR).97,149 Hence, more attention needs to be paid to the rewiring of metabolic networks of microbial strains to metabolize multiple carbon sources, especially glucose and xylose, from the feedstock which will be essential for de-risking the commercial viability of the bioprocesses.150,151 In the last two decades, efforts on xylose valorisation have intensified.152 To improve the efficacy of xylose-based fermentation, efficient xylose-utilizing as well as non-xylose/inefficient xylose-utilizing strains have been engineered to expand the available substrate range, enable rapid assimilation of xylose and eliminate CCR for manufacturing fuels and chemicals from LCB-based feedstock.123,153,154 Despite advances in metabolic engineering and synthetic biology tools, xylose is still an inferior carbon source in comparison to glucose and more efforts are required to design efficient microbial cell factories for xylose-based production. Besides, the development of robust strains with a high tolerance level against inhibitors released during pre-treatment and end products can result in a high product yield which will eventually benefit the overall process economics.151,152
Hydrolysis and fermentation processes need to be intensified for enhancing the productivity of SCB based biorefineries. The optimal conditions for hydrolysis and fermentation may be different and will vary for the enzyme complex used for hydrolysis and microbes used for fermentative production of desired products. Therefore, to achieve better combined productivity (hydrolysis & fermentation), selection of SHF vs. SSF is critical. For a specific case of LA production, SHF is recommended over SSF for better productivity.140,141
The current state-of-the-art processes for transforming SCB into the value added products discussed here are still not yet economically feasible.92–94,96,124,125 Major drivers for the deployment of biorefineries are sustainable & renewable energy supply, inclusive economic growth to save foreign exchange, less dependency on imported crude petroleum, low carbon footprint and green environment. Despite having high potential, the establishment of a biorefinery faces the following challenges. Firstly, round the year availability of lignocellulosic feedstock at a low price is a major concern for biorefineries. It is crucial to establish a proper mechanism for collection, transportation and handling of biomass feedstock. Secondly, 2G biorefinery investments are capital-intensive, involve large risks, and take a long time to become market ready. Finally, as discussed previously, pre-treatment and enzymatic hydrolysis can account for up to 40% of the total operational costs. Regarding the sugar industry in India, as far as the feedstock and supply-chain are concerned, the existing sugar mills have a well-established supply chain for sugarcane and hence readily available SCB on site. A typical sugar plant consists of a sugar unit, an ethanol production unit, a cogeneration plant (steam and power) and an anaerobic digestion unit (when a distillery is attached). These units facilitate the use of surplus steam, electricity and water on site. Typical equipment and process machinery required for expanding the plant are not different to those required for setting up biorefineries. They include fermenters, distillation columns, the evaporation plant, anaerobic digesters, etc. which can be used to setup biorefinery facilities. Saccharification modules required for the production of C5 or C6 sugars from polysaccharides may be the only new addition that is currently alien to sugar industries. Such a project can be a ‘bolt-on’ project which will be much cheaper than a Greenfield project. We believe that an AD centric biorefinery may overcome some of the limitations of the state-of-the-art and make the valorisation techno-economically viable. AD is an accepted technology in the sugar industry as it is used to operate digesters for processing spent wash from associated distilleries. AD is a robust technology and can handle fluctuations in feed quality and quantity. It hosts a consortium of microbes working in synergy and therefore can handle a wider range of pH and temperature. The capex requirements of AD are also one of the lowest among the competing technology platforms. The disadvantages of AD are the underlying transformation processes that are usually slow as they require large residence time, leading to larger digester volumes. It also offers limited control over substrate fraction selectivity. HC based pre-treatment can be used to intensify AD operation. It has been shown that HC based pre-treatment improves the rate as well as yield of biogas generation. One of the promising paths forward that we envisage is the use of AD not only as a bioprocess step for energy recovery but also as ‘pre-treatment’. In addition to recovering energy in the form of biogas–biomethane, it will also open up the lignocellulosic matrix of biomass (SCB). In the envisaged biorefinery, AD is used for only partially converting the organic carbon in the feed to biogas/biomethane. The digestate is processed further by separating the solid and liquid fractions, where the former will be subjected to pre-treatment for recovering C5 and C6 sugars and the latter can be sold as a fertiliser. C5 and C6 sugars produced from digestate can be used for generating high value products such as alcohols (ethanol and xylitol) and acids (LA and SA). It has to be ensured that the high value products produced from the ‘digestate’ should be of intended purity determined by the industry (e.g., 80–90% purity required for food grade lactic acid and > 90% for pharmaceutical grade155) and free from contaminants. This proposed biorefinery is shown in Fig. 8. The intended idea of using AD for partial conversion offers several advantages like smaller digesters due to a shorter residence time (required for partial conversion) and an inexpensive way to distort the lignocellulosic matrix for further bioprocessing. This has potential of substantially reducing the cost of conversion to sugars and thereby improving the overall economic viability. This approach may eliminate the need for harsh thermo-chemical treatments or expensive enzymatic treatment.
Further research, specifically on identifying optimal HC based pre-treatment suitable for partial conversion of organic carbon to biogas in AD, further cavitation based pre-treatment of digestate to make it amenable for extracting sugars and subsequent fermentation of sugars to desired products (alcohols and acids) are needed to realise the proposed biorefinery. We hope that this review and the proposed biorefinery will stimulate potential translation of ongoing research to practice in near future.
Declaration of interests
One of the authors, Professor Vivek Ranade, is a co-founder and Director of Vivira Process Technologies Pvt. Ltd., India, which commercially offers hydrodynamic cavitation devices.Appendix 1
where biomass is represented with the chemical formula CaHbOcNdSe
Stoichiometric equation for different products from glucose:
| Ethanol: C6H12O6 → 2C2H6O + 2CO2 |
| Lactic acid: C6H12O6 → 2C3H6O3 |
Stoichiometric equation for different products from xylose:
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Innovate UK, Newton Fund, BBSRC UK and Department of Biotechnology, Government of India (vWa Project, Grant BB/S011951/1).References
- National Federation of Co-operative Sugar Factories Limited, Cooperative Sugar, National Federation of Cooperative Sugar Factories Ltd., New Delhi, 2019 Search PubMed.
- M. Meghana and Y. Shastri, Bioresour. Technol., 2020, 303, 122929 CrossRef CAS.
- S. Solomon, Sugar Tech, 2011, 13, 255–265 CrossRef CAS.
- P. A. Ochoa George, J. J. C. Eras, A. S. Gutierrez, L. Hens and C. Vandecasteele, Waste Biomass Valorization, 2010, 1, 407–413 CrossRef CAS.
- J. M. Hernández-Salas, M. S. Villa-Ramírez, J. S. Veloz-Rendón, K. N. Rivera-Hernández, R. A. González-César, M. A. Plascencia-Espinosa and S. R. Trejo-Estrada, Bioresour. Technol., 2009, 100, 1238–1245 CrossRef.
- A. Pandey, C. R. Soccol, P. Nigam and V. T. Soccol, Bioresour. Technol., 2000, 74, 69–80 CrossRef CAS.
- M. R. Trejo-Hernández, A. Ortiz, A. I. Okoh, D. Morales and R. Quintero, Chemosphere, 2007, 68, 848–855 CrossRef.
- P. Purohit and S. Dhar, Promoting low carbon transport in India. Biofuel roadmap for India, United Nations Environment Programme-DTU Partnership, Climate and Sustainable Development, Technical University of Denmark, Magnum Custom Publishing, New Delhi, India, 2015, ISBN: 978-87-93130-66-1 Search PubMed.
- N. K. Bhardwaj, D. Kaur, S. Chaudhry, M. Sharma and S. Arya, J. Cleaner Prod., 2019, 217, 225–233 CrossRef CAS.
- H. C. J. Franco, M. T. B. Pimenta, J. L. N. Carvalho, P. S. G. Magalhães, C. E. V. Rossell, O. A. Braunbeck, A. C. Vitti, O. T. Kölln and J. Rossi Neto, Sci. Agric., 2013, 70, 305–312 CrossRef.
- P. Singh, A. Suman, P. Tiwari, N. Arya, A. Gaur and A. K. Shrivastava, World J. Microbiol. Biotechnol., 2008, 24, 667–673 CrossRef CAS.
- P. C. Abhilash and N. Singh, Bioresour. Technol., 2008, 99, 8961–8966 CrossRef CAS.
- S. Ezhumalai and V. Thangavelu, Bioresources, 2010, 5, 1879–1894 CAS.
- S. Ingle, P. Ashtavinayak, D. Amol and P. Sanjay, J. Environ. Chem. Eng., 2017, 5, 2861–2868 CrossRef.
- S. Velmurugan and N. Partha, Indian Chem. Eng., 2006, 48, 160–163 Search PubMed.
- K. Umamaheswaran and V. S. Batra, Fuel, 2008, 87, 628–638 CrossRef CAS.
- Ministry of New and Renewable Energy – Government of India, Programme/Scheme Wise Physical Progress in 2020–21 & Cumulative up to June, 2020, https://mnre.gov.in/the-ministry/physical-progress, accessed 04 Sep, 2020 Search PubMed.
- M. R. Islam, H. Haniu, M. N. Islam and M. S. Uddin, J. Therm. Sci. Technol., 2010, 5, 11–23 CrossRef CAS.
- M. R. L. V. Leal, M. V. Galdos, F. V. Scarpare, J. E. A. Seabra, A. Walter and C. O. F. Oliveira, Biomass Bioenergy, 2013, 53, 11–19 CrossRef.
- E. V. Gonçalves, F. L. Seixas, L. R. de Souza Scandiuzzi Santana, M. H. N. O. Scaliante and M. L. Gimenes, Can. J. Chem. Eng., 2017, 95, 1269–1279 CrossRef.
- D. Kumar, S. A. Soomro, H. U. Mahar, S. Aziz, I. N. Unar, A. R. Memon, A. A. Malik and S. Hussain, Technical Journal, 2017, 22(2), 113–117 Search PubMed.
- A. Shukla and S. Y. Kumar, Int. J. Appl. Eng. Res., 2017, 12, 14739–14745 Search PubMed.
- A. K. Varma and P. Mondal, Ind. Crops Prod., 2017, 95, 704–717 CrossRef CAS.
- P. Ghorbannezhad, M. D. Firouzabadi, A. Ghasemian, P. J. de Wild and H. J. Heeres, J. Anal. Appl. Pyrolysis, 2018, 131, 1–8 CrossRef CAS.
- S. Kanwal, N. Chaudhry, S. Munir and H. Sana, Waste Manag., 2019, 88, 280–290 CrossRef CAS.
- K. Manatura, Case Stud. Therm. Eng., 2020, 19, 100623 CrossRef.
- L. Venkateswar Rao, J. K. Goli, J. Gentela and S. Koti, Bioresour. Technol., 2016, 213, 299–310 CrossRef CAS.
- T. L. Bezerra and A. J. Ragauskas, Biofuels, Bioprod. Biorefin., 2016, 10, 634–647 CrossRef CAS.
- S. Farzad, M. A. Mandegari, M. Guo, K. F. Haigh, N. Shah and J. F. Görgens, Biotechnol. Biofuels, 2017, 10, 87 CrossRef.
- B. Yang and C. E. Wyman, Biofuels, Bioprod. Biorefin., 2008, 2, 26–40 CrossRef CAS.
- A. S. A. da Silva, H. Inoue, T. Endo, S. Yano and E. P. S. Bon, Bioresour. Technol., 2010, 101, 7402–7409 CrossRef.
- S. Park, J. O. Baker, M. E. Himmel, P. A. Parilla and D. K. Johnson, Biotechnol. Biofuels, 2010, 3, 10 CrossRef.
- Z. Miao, T. E. Grift, A. C. Hansen and K. C. Ting, Ind. Crops Prod., 2011, 33, 504–513 CrossRef CAS.
- R. d. R. O. d. Barros, R. d. S. Paredes, T. Endo, E. P. d. S. Bon and S.-H. Lee, Bioresour. Technol., 2013, 136, 288–294 CrossRef.
- B. Buaban, H. Inoue, S. Yano, S. Tanapongpipat, V. Ruanglek, V. Champreda, R. Pichyangkura, S. Rengpipat and L. Eurwilaichitr, J. Biosci. Bioeng., 2010, 110, 18–25 CrossRef CAS.
- Y. Sun and J. Cheng, Bioresour. Technol., 2002, 83, 1–11 CrossRef CAS.
- A. K. Kivaisi and S. Eliapenda, Renewable Energy, 1994, 5, 791–795 CrossRef CAS.
- A. F. Leite, L. Janke, H. Harms, J. W. Zang, W. Fonseca-Zang, W. Stinner and M. Nikolausz, Energy Fuels, 2015, 29, 4022–4029 CrossRef CAS.
- S. C. Rabelo, H. Carrere, R. Maciel Filho and A. C. Costa, Bioresour. Technol., 2011, 102, 7887–7895 CrossRef CAS.
- A. G. Costa, G. C. Pinheiro, F. G. C. Pinheiro, A. B. Dos Santos, S. T. Santaella and R. C. Leitão, Chem. Eng. J., 2014, 248, 363–372 CrossRef CAS.
- S. Nagarajan and V. V. Ranade, Ind. Eng. Chem. Res., 2019, 58, 15975–15988 CrossRef CAS.
- J. de Cassia Pereira, N. Paganini Marques, A. Rodrigues, T. Brito de Oliveira, M. Boscolo, R. da Silva, E. Gomes and D. A. Bocchini Martins, J. Appl. Microbiol., 2015, 118, 928–939 CrossRef CAS.
- D. Deswal, R. Gupta, P. Nandal and R. C. Kuhad, Carbohydr. Polym., 2014, 99, 264–269 CrossRef CAS.
- A. d. S. Machado and A. Ferraz, Bioresour. Technol., 2017, 225, 17–22 CrossRef CAS.
- C. Carrasco, H. M. Baudel, J. Sendelius, T. Modig, C. Roslander, M. Galbe, B. Hahn-Hägerdal, G. Zacchi and G. Lidén, Enzyme Microb. Technol., 2010, 46, 64–73 CrossRef CAS.
- G. J. M. Rocha, L. P. Andrade, C. Martín, G. T. Araujo, V. E. Mouchrek Filho and A. A. S. Curvelo, Ind. Crops Prod., 2020, 147, 112227 CrossRef CAS.
- A. M. Lali, A. A. Odaneth, S. H. Birhade, J. J. Victoria and S. C. Sawant, Process for production of soluble sugars from biomass, US Pat. US9862980B2, 2018.
- A. F. A. Carvalho, W. F. Marcondes, P. de Oliva Neto, G. M. Pastore, J. N. Saddler and V. Arantes, Bioresour. Technol., 2018, 250, 221–229 CrossRef CAS.
- P. V. Neves, A. P. Pitarelo and L. P. Ramos, Bioresour. Technol., 2016, 208, 184–194 CrossRef CAS.
- T. A. L. Silva, H. D. Z. Zamora, L. H. R. Varão, N. S. Prado, M. A. Baffi and D. Pasquini, Waste Biomass Valorization, 2018, 9, 2191–2201 CrossRef CAS.
- P. Silva Ortiz and S. de Oliveira, Energy, 2014, 76, 130–138 CrossRef.
- S. Nagarajan and V. V. Ranade, Bioresource Technology Reports, 2020, 100480, DOI:10.1016/j.biteb.2020.100480.
- K. Nakashima, Y. Ebi, N. Shibasaki-Kitakawa, H. Soyama and T. Yonemoto, Ind. Eng. Chem. Res., 2016, 55, 1866–1871 CrossRef CAS.
- V. V. Ranade, A. A. Kulkarni and V. M. Bhandari, Vortex diodes as effluent treatment devices, US Pat. US9422952B2, 2016.
- M. Langone, M. Soldano, C. Fabbri, F. Pirozzi and G. Andreottola, Appl. Biochem. Biotechnol., 2018, 184, 1200–1218 CrossRef CAS.
- M. Zieliński, P. Rusanowska, A. Krzywik, M. Dudek, A. Nowicka and M. Dębowski, Energies, 2019, 12, 525 CrossRef.
- S. Pal, S. Joy, P. Kumbhar, K. D. Trimukhe, R. Gupta, R. C. Kuhad, A. J. Varma and S. Padmanabhan, Biomass Convers. Biorefin., 2017, 7, 179–189 CrossRef.
- S. Pal, W. J. Satyendra and R. P. Ravikumar, Preparation of hydrolysate of lignocellulosic materials, WIPO Patent WO2014203271A2, 2014.
- M. H. L. Silveira, A. K. Chandel, B. A. Vanelli, K. S. Sacilotto and E. B. Cardoso, Bioresource Technology Reports, 2018, 3, 138–146 CrossRef.
- M. E. Vallejos, M. D. Zambon, M. C. Area and A. A. d. S. Curvelo, Ind. Crops Prod., 2015, 65, 349–353 CrossRef CAS.
- L. Mesa, E. González, C. Cara, M. González, E. Castro and S. I. Mussatto, Chem. Eng. J., 2011, 168, 1157–1162 CrossRef CAS.
- A. S. A. da Silva, S.-H. Lee, T. Endo and E. P. S. Bon, Bioresour. Technol., 2011, 102, 10505–10509 CrossRef.
- T. Mokomele, L. da Costa Sousa, V. Balan, E. Van Rensburg, B. E. Dale and J. F. Görgens, Biotechnol. Biofuels, 2018, 11, 127 CrossRef.
- T. Benazzi, S. Calgaroto, V. Astolfi, C. Dalla Rosa, J. V. Oliveira and M. A. Mazutti, Enzyme Microb. Technol., 2013, 52, 247–250 CrossRef CAS.
- M. J. Madison, G. Coward-Kelly, C. Liang, M. N. Karim, M. Falls and M. T. Holtzapple, Biomass Bioenergy, 2017, 98, 135–141 CrossRef CAS.
- O. Jafari and H. Zilouei, Bioresour. Technol., 2016, 214, 670–678 CrossRef CAS.
- K. Kapoor, N. Garg, R. K. Diwan, L. Varshney and A. K. Tyagi, Radiat. Phys. Chem., 2017, 141, 190–195 CrossRef CAS.
- T. Zhang and M.-J. Zhu, Bioresour. Technol., 2016, 214, 769–777 CrossRef CAS.
- M. Ambye-Jensen, R. Balzarotti, S. T. Thomsen, C. Fonseca and Z. Kádár, Biotechnol. Biofuels, 2018, 11, 336 CrossRef CAS.
- P. J. Meynell, Methane: Planning a Digester, Schocken Books, New York, 1976 Search PubMed.
- S. Mittal, E. O. Ahlgren and P. R. Shukla, Energy Policy, 2018, 112, 361–370 CrossRef CAS.
- Ministry of Petroleum Natural Gas Government of India, Energizing and Empowering India - Annual report 2018-19, 2019, http://petroleum.nic.in/sites/default/files/AR_2018-19.pdf Search PubMed.
- Indian Oil Corporation Limited, Bharat Petroleum Corporation Limited and Hindustan Petroleum Corporation Limited, Expression of Interest from entrepreneurs/sole proprietorships/partnerships/limited liability partnerships/companies/cooperative societies/technology providers for production and supply of compressed biogas (CBG) under, SATAT, 2018, https://www.bharatpetroleum.com/pdf/EOICBGFINAL-46978-4fd490.pdf Search PubMed.
- H. Katuwal and A. K. Bohara, Renewable Sustainable Energy Rev., 2009, 13, 2668–2674 CrossRef.
- L. Janke, A. Leite, M. Nikolausz, T. Schmidt, J. Liebetrau, M. Nelles and W. Stinner, Int. J. Mol. Sci., 2015, 16, 20685 CrossRef CAS.
- S. Sajad Hashemi, K. Karimi and A. Majid Karimi, Fuel, 2019, 248, 196–204 CrossRef CAS.
- A. M. Mustafa, H. Li, A. A. Radwan, K. Sheng and X. Chen, Bioresour. Technol., 2018, 259, 54–60 CrossRef CAS.
- M. Badshah, D. M. Lam, J. Liu and B. Mattiasson, Bioresour. Technol., 2012, 114, 262–269 CrossRef CAS.
- F. R. Ribeiro, F. Passos, L. V. A. Gurgel, B. E. L. Baêta and S. F. de Aquino, Sci. Total Environ., 2017, 584–585, 1108–1113 CrossRef CAS.
- L. Janke, A. F. Leite, M. Nikolausz, C. M. Radetski, M. Nelles and W. Stinner, J. Waste Manag., 2016, 48, 199–208 CrossRef CAS.
- M. Hassan, W. Ding, M. Umar and G. Rasool, Bioresour. Technol., 2017, 230, 24–32 CrossRef CAS.
- N. Aramrueang, J. Rapport and R. Zhang, Biosyst. Eng., 2016, 147, 174–182 CrossRef.
- P. V. Rao, S. S. Baral, R. Dey and S. Mutnuri, Renewable Sustainable Energy Rev., 2010, 14, 2086–2094 CrossRef CAS.
- C. Sawatdeenarunat, K. C. Surendra, D. Takara, H. Oechsner and S. K. Khanal, Bioresour. Technol., 2015, 178, 178–186 CrossRef CAS.
- A. F. Hernández-Pérez, I. A. L. Costa, D. D. V. Silva, K. J. Dussán, T. R. Villela, E. V. Canettieri, J. A. Carvalho, T. G. Soares Neto and M. G. A. Felipe, Bioresour. Technol., 2016, 200, 1085–1088 CrossRef.
- A. W. Bhutto, K. Harijan, K. Qureshi, A. A. Bazmi and A. Bahadori, J. Cleaner Prod., 2015, 95, 184–193 CrossRef CAS.
- L. Canilha, A. K. Chandel, T. S. S. Milessi, F. A. F. Antunes, W. L. C. Freitas, M. G. A. Felipe and S. S. da Silva, J. Biomed. Biotechnol., 2012, 2012 Search PubMed.
- J. T. Cunha, P. O. Soares, A. Romaní, J. M. Thevelein and L. Domingues, Biotechnol. Biofuels, 2019, 12, 20 CrossRef.
- R. E. Hector, J. A. Mertens, M. J. Bowman, N. N. Nichols, M. A. Cotta and S. R. Hughes, Yeast, 2011, 28, 645–660 CrossRef CAS.
- A. de Araujo Guilherme, P. V. F. Dantas, C. E. d. A. Padilha, E. S. dos Santos and G. R. de Macedo, J. Environ. Manage., 2019, 234, 44–51 CrossRef.
- Y. Liu, J. X. Xu, Y. Zhang, M. He, C. Liang, Z. Yuan and J. Xie, BioResources, 2016, 11, 2548–2556 CAS.
- O. Rosales-Calderon and V. Arantes, Biotechnol. Biofuels, 2019, 12, 240 CrossRef.
- S. Vieira, M. V. Barros, A. C. N. Sydney, C. M. Piekarski, A. C. de Francisco, L. P. d. S. Vandenberghe and E. B. Sydney, Bioresour. Technol., 2020, 299, 122635 CrossRef CAS.
- A. F. Hernández-Pérez, P. V. de Arruda, L. Sene, S. S. da Silva, A. Kumar Chandel and M. d. G. de Almeida Felipe, Crit. Rev. Biotechnol., 2019, 39, 924–943 CrossRef.
- D. Dasgupta, D. Ghosh, S. Bandhu and D. K. Adhikari, Microbiol. Res., 2017, 200, 64–72 CrossRef CAS.
- Y. Xu, P. Chi, M. Bilal and H. Cheng, Appl. Microbiol. Biotechnol., 2019, 103, 5143–5160 CrossRef CAS.
- A. A. Prabhu, D. J. Thomas, R. Ledesma-Amaro, G. A. Leeke, A. Medina, C. Verheecke-Vaessen, F. Coulon, D. Agrawal and V. Kumar, Microb. Cell Fact., 2020, 19, 121 CrossRef CAS.
- A. A. Prabhu, E. Bosakornranut, Y. Amraoui, D. Agarwal, F. Coulon, V. Vivekanand, V. K. Thakur and V. Kumar, Biotechnol. Biofuels, 2020 Search PubMed , 10.21203/rs.3.rs-45506/v1, accepted.
- P. Unrean and N. Ketsub, Ind. Crops Prod., 2018, 123, 238–246 CrossRef CAS.
- D. D. V. da Silva, P. V. de Arruda, F. M. C. F. Vicente, L. Sene, S. S. da Silva and M. das Graças de Almeida Felipe, Ann. Microbiol., 2015, 65, 687–694 CrossRef.
- J. F. Castañón-Rodríguez, J. M. Domínguez-González, B. Ortíz-Muñiz, B. Torrestiana-Sanchez, J. A. R. de León and M. G. Aguilar-Uscanga, Eng. Life Sci., 2015, 15, 96–107 CrossRef.
- A. D. Mountraki, K. R. Koutsospyros, B. B. Mlayah and A. C. Kokossis, Waste Biomass Valorization, 2017, 8, 2283–2300 CrossRef CAS.
- K.-K. Cheng, X.-B. Zhao, J. Zeng, R.-C. Wu, Y.-Z. Xu, D.-H. Liu and J.-A. Zhang, Appl. Microbiol. Biotechnol., 2012, 95, 841–850 CrossRef CAS.
- M. Babaei, K. Rueksomtawin Kildegaard, A. Niaei, M. Hosseini, S. Ebrahimi, S. Sudarsan, I. Angelidaki and I. Borodina, Front. Bioeng. Biotechnol., 2019, 7, 361 CrossRef.
- J. Akhtar, A. Idris and R. A. Aziz, Appl. Microbiol. Biotechnol., 2014, 98, 987–1000 CrossRef CAS.
- E. Stylianou, C. Pateraki, D. Ladakis, M. Cruz-Fernández, M. Latorre-Sánchez, C. Coll and A. Koutinas, Biotechnol. Biofuels, 2020, 13, 72 CrossRef CAS.
- E. R. Borges and N. Pereira, J. Ind. Microbiol. Biotechnol., 2011, 38, 1001–1011 CrossRef CAS.
- Y.-l. Xi, W.-y. Dai, R. Xu, J.-h. Zhang, K.-q. Chen, M. Jiang, P. Wei and P.-k. Ouyang, Bioprocess Biosyst. Eng., 2013, 36, 1779–1785 CrossRef CAS.
- R. C. L. B. Rodrigues, M. G. A. Felipe, I. C. Roberto and M. Vitolo, Bioprocess Biosyst. Eng., 2003, 26, 103–107 CrossRef CAS.
- W. Carvalho, J. C. Santos, L. Canilha, J. B. Almeida e Silva, M. G. A. Felipe, I. M. Mancilha and S. S. Silva, Process Biochem., 2004, 39, 2135–2141 CrossRef CAS.
- R. S. Rao, C. P. Jyothi, R. S. Prakasham, P. N. Sarma and L. V. Rao, Bioresour. Technol., 2006, 97, 1974–1978 CrossRef CAS.
- C.-F. Huang, Y.-F. Jiang, G.-L. Guo and W.-S. Hwang, Bioresour. Technol., 2011, 102, 3322–3329 CrossRef CAS.
- G. Prakash, A. J. Varma, A. Prabhune, Y. Shouche and M. Rao, Bioresour. Technol., 2011, 102, 3304–3308 CrossRef CAS.
- M. E. Vallejos, M. Chade, E. B. Mereles, D. I. Bengoechea, J. G. Brizuela, F. E. Felissia and M. C. Area, Ind. Crops Prod., 2016, 91, 161–169 CrossRef CAS.
- V. P. de Arruda, J. C. dos Santos, R. de Cássia Lacerda Brambilla Rodrigues, D. D. V. da Silva, C. K. Yamakawa, G. J. de Moraes Rocha, J. N. Júnior, J. G. da Cruz Pradella, C. E. Vaz Rossell and M. das Graças de Almeida Felipe, J. Ind. Eng. Chem., 2017, 47, 297–302 CrossRef.
- P. Chen, S. Tao and P. Zheng, Bioresour. Technol., 2016, 211, 406–413 CrossRef CAS.
- W. Dessie, F. Xin, W. Zhang, Y. Jiang, H. Wu, J. Ma and M. Jiang, Appl. Microbiol. Biotechnol., 2018, 102, 9893–9910 CrossRef CAS.
- M. Sauer, D. Porro, D. Mattanovich and P. Branduardi, Trends Biotechnol., 2008, 26, 100–108 CrossRef CAS.
- E. Cavallo, H. Charreau, P. Cerrutti and M. L. Foresti, FEMS Yeast Res., 2017, 17, fox084 CrossRef.
- H.-H. Liu, X.-J. Ji and H. Huang, Biotechnol. Adv., 2015, 33, 1522–1546 CrossRef CAS.
- M. Egermeier, H. Russmayer, M. Sauer and H. Marx, Front. Microbiol., 2017, 8, 49 Search PubMed.
- K. L. Ong, C. Li, X. Li, Y. Zhang, J. Xu and C. S. K. Lin, Biochem. Eng. J., 2019, 148, 108–115 CrossRef CAS.
- A. A. Prabhu, R. Ledesma-Amaro, C. S. K. Lin, F. Coulon, V. K. Thakur and V. Kumar, Biotechnol. Biofuels, 2020, 13, 113 CrossRef CAS.
- M. Nieder-Heitmann, K. Haigh, J. Louw and J. F. Görgens, Biofuels, Bioprod. Biorefin., 2020, 14, 55–77 CrossRef CAS.
- B. C. Klein, J. F. L. Silva, T. L. Junqueira, S. C. Rabelo, P. V. Arruda, J. L. Ienczak, P. E. Mantelatto, J. G. C. Pradella, S. V. Junior and A. Bonomi, Biofuels, Bioprod. Biorefin., 2017, 11, 1051–1064 CrossRef CAS.
- J. J. Bozell and G. R. Petersen, Green Chem., 2010, 12, 539–554 RSC.
- R. A. Abd Alsaheb, A. Aladdin, N. Z. Othman, R. Abd Malek, O. M. Leng, R. Aziz and H. A. El Enshasy, J. Chem. Pharm. Res., 2015, 7, 729–735 CAS.
- C. Rodrigues, L. P. S. Vandenberghe, A. L. Woiciechowski, J. de Oliveira, L. A. J. Letti and C. R. Soccol, in Current Developments in Biotechnology and Bioengineering, ed. A. Pandey, S. Negi and C. R. Soccol, Elsevier, 2017, pp. 543–556, DOI:10.1016/B978-0-444-63662-1.00024-5.
- T. Ghaffar, M. Irshad, Z. Anwar, T. Aqil, Z. Zulifqar, A. Tariq, M. Kamran, N. Ehsan and S. Mehmood, J. Radiat. Res. Appl. Sci., 2014, 7, 222–229 CrossRef CAS.
- B. Oonkhanond, W. Jonglertjunya, N. Srimarut, P. Bunpachart, S. Tantinukul, N. Nasongkla and C. Sakdaronnarong, J. Environ. Chem. Eng., 2017, 5, 2533–2541 CrossRef CAS.
- A. R. de Oliveira, R. Schneider, C. E. Vaz Rossell, R. Maciel Filho and J. Venus, Bioresource Technology Reports, 2019, 6, 26–31 CrossRef.
- R. P. John, K. M. Nampoothiri and A. Pandey, Appl. Microbiol. Biotechnol., 2007, 74, 524–534 CrossRef CAS.
- L. J. Jonsson and C. Martin, Bioresour. Technol., 2016, 199, 103–112 CrossRef.
- A. K. Kumar and S. Sharma, Bioresour. Bioprocess, 2017, 4, 7 CrossRef.
- M. R. Mukasekuru, J. Hu, X. Zhao, F. F. Sun, K. Pascal, H. Ren and J. Zhang, ACS Sustainable Chem. Eng., 2018, 6, 12787–12796 CrossRef CAS.
- D. H. Kim, H. M. Park, Y. H. Jung, P. Sukyai and K. H. Kim, Bioresour. Technol., 2019, 284, 391–397 CrossRef CAS.
- M. A. Patel, M. S. Ou, L. O. Ingram and K. T. Shanmugam, Biotechnol. Prog., 2005, 21, 1453–1460 CrossRef CAS.
- M. G. Adsul, A. J. Varma and D. V. Gokhale, Green Chem., 2007, 9, 58–62 RSC.
- E. C. van der Pol, G. Eggink and R. A. Weusthuis, Biotechnol. Biofuels, 2016, 9, 248 CrossRef.
- K. Nalawade, P. Baral, S. Patil, A. Pundir, A. K. Kurmi, K. Konde, S. Patil and D. Agrawal, Renewable Energy, 2020, 157, 708–717 CrossRef CAS.
- K. Nalawade, P. Saikia, S. Behera, K. S. Konde and S. Patil, Biomass Convers. Biorefin., 2020, under review Search PubMed.
- T. Jiang, H. Qiao, Z. Zheng, Q. Chu, X. Li, Q. Yong and J. Ouyang, PLoS One, 2016, 11, e0149101 CrossRef.
- W. Jonglertjunya, N. Srimarut, N. Lukkanakul, C. Sakdaronnarong and N. Nasongkla, Adv. Mater. Res., 2014, 931–932, 1597–1601 Search PubMed.
- L. Peng, N. Xie, L. Guo, L. Wang, B. Yu and Y. Ma, PLoS One, 2014, 9, e107143 CrossRef.
- P. Unrean, Ind. Crops Prod., 2018, 111, 660–666 CrossRef CAS.
- D. Wischral, J. M. Arias, L. F. Modesto, D. de França Passos and N. Pereira Jr, Biotechnol. Prog., 2019, 35, e2718 CrossRef.
- A. González-Leos, M. G. Bustos-Vázquez, G. C. Rodríguez-Castillejos, L. V. Rodríguez-Durán and A. Del Ángel-Del Ángel, Rev. Mex. Ing. Quim., 2019, 19, 377–386 Search PubMed.
- D. Schneider and A. Ragossnig, Waste Manage. Res., 2014, 32, 563–564 CrossRef.
- C. Sievert, L. M. Nieves, L. A. Panyon, T. Loeffler, C. Morris, R. A. Cartwright and X. Wang, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 7349–7354 CrossRef CAS.
- F. M. Gírio, F. Carvalheiro, L. C. Duarte and R. Bogel-Łukasik, in D-xylitol: Fermentative Production, Application and Commercialization, ed. S. S. da Silva and A. K. Chandel, Springer Berlin Heidelberg, Berlin, Heidelberg, 2012, pp. 3–37, DOI:10.1007/978-3-642-31887-0_1.
- V. Kumar, P. Binod, R. Sindhu, E. Gnansounou and V. Ahluwalia, Bioresour. Technol., 2018, 269, 443–451 CrossRef CAS.
- S. Kwak, J. H. Jo, E. J. Yun, Y. S. Jin and J. H. Seo, Biotechnol. Adv., 2019, 37, 271–283 CrossRef CAS.
- R. G. de Paula, A. C. C. Antonieto, L. F. C. Ribeiro, N. Srivastava, A. O'Donovan, P. K. Mishra, V. K. Gupta and R. N. Silva, Biotechnol. Adv., 2019, 37, 107347 CrossRef CAS.
- S. Kwak and Y. S. Jin, Microb. Cell Fact., 2017, 16, 82 CrossRef.
- A. Ahmad, F. Banat and H. Taher, Environ. Technol. Innov., 2020, 20, 101138 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |