Open Access Article
Open Access ArticleRetracted Article: Recent developments in energy storage systems for marine environment
Jaya
Verma
* and
Deepak
Kumar
Centre for Automotive Research and Tribology, Indian Institute of Technology Delhi, New Delhi – 110016, India. E-mail: jayaverma@iitd.ac.in
First published on 29th September 2021
Abstract
Marine batteries are designed specifically for marine vehicles with heavier plates and robust construction to withstand the vibration and pounding that can occur on board any powerboat. These batteries are specially developed to meet the potential and futuristic needs of sea vehicle applications. This paper reviews several types of energy storage systems for marine environments, which have been extensively used to improve the overall performance of marine vehicles. Key technological developments and scientific challenges are considered for a broad range of marine batteries. The primary and secondary marine battery technologies are discussed, and the corresponding outputs are reported in terms of energy capacity, charging–discharging rates, cycle life, energy and power density, shelf life, and their environmental impact. The performance comparison is analyzed for various batteries such as lead-acid, lithium-ion, nickel–cadmium, silver–zinc, and open water-powered batteries for marine applications. After a brief discussion on these technologies, the global scenario of the marine battery market is reported, which is segmented by regions, applications, and ship types. Further, we summarize the eco-marine power system, and the future directions of marine energy storage systems are highlighted, followed by advanced Al-battery technology and marine energy storage industry outlooks up to 2025.
1. Introduction
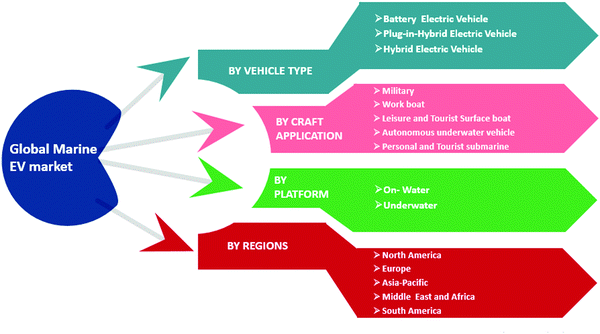
In recent years, concerns about severe environmental pollution and fossil fuel consumption have grabbed the attention of the transportation industry, particularly in marine vessels. Electrification in commercial and military ships has been a trend in the recent past in order to reduce emissions and to improve efficiency.1–4 The International Marine Organization (IMO) in 2012 stated that global SOx and NOx emissions from shipping exhibited about 13% and 15% of global SOx and NOx, respectively.5 It further states that for international shipping, total CO2 emissions are around 796 million tons, which are approximately 2.2% of the global CO2 emissions. The CO2 emissions from ships all over the globe were found to be 2.6% of the global CO2 emissions. Moreover, IMO predicts that by 2050, CO2 emissions in the case of international shipping could increase to between 50% and 250%. IMO announced guidelines and regulations in January 2015 for Emission Controlled Areas (ECA) as a consequence of modifications applied in the International Convention for the Prevention of Pollution from ships.6 The European Commission set forth a novel climate agreement (the Paris Protocol) with the extended goal of diminishing global emissions by up to 60% by 2050 as compared to 2010 levels.7 In the past, the cost of energy and environmental concerns were not of great importance in marine power systems. In order to save fuel and decrease emissions, several solutions have been proposed. For instance, substituting alternative fuels, exhaust gases after treatment, and using hybrid propulsions are the frequently implemented applied approaches to achieve the environmental guidelines imposed by IMO. Therefore, novel concepts such as hybrid energy storage systems (HESS) should be investigated to reduce emissions (SOx, NOx, CO2, etc.) for marine environment vehicles.The fast growth of electric and hybrid transportation systems opens doors for further developments in ESS. Currently, the solutions are not technologically and commercially adequate in several features, causing barriers to their broader usage. The ESS technologies vary from each other in terms of expense and technical aspects such as power density, energy density, charge and discharge time, operating temperature, lifetime, environmental impact, and maintenance requirements. Several studies have been conducted in recent years, especially in the last decade, to improve the ESS capacity. A typical single ESS technology that can provide higher power and energy density, greater lifetime, and other such specifications is not likely to be developed in the near future. Therefore, in order to improve the capabilities, two or more ESS technologies can be hybridized. The global marine electric vehicle production market depends on various parameters such as the requirements of the vehicle type according to the application, whether it is an on-water or underwater vehicle, as well as the demand in particular regions for these marine EV vehicles (Fig. 1).
The Energy Storage System (ESS) for marine or sea vehicles is a combination of dissimilar energy storage technologies that have different characteristics with regard to energy capacity, cycle life, charging and discharging rates, energy and power density, response rate, shelf life, and so on. Mainly two types of batteries are used for sea vehicles: primary and secondary. Primary batteries are single-use batteries because they cannot be recharged. These batteries are combinations of certain metals, such as copper and zinc, which will produce electrical activity when placed in special solutions called electrolytes. The two metals form the electrodes. The electrolyte creates a chemical action that causes the zinc to form positive ions and the copper to form negative ions. These ions are freely flowing in the electrolyte. No current flow can occur until the electrode terminals are connected to a circuit. The electrons then flow from the zinc electrode through the external circuit to the copper electrode. The chemical reaction between the zinc and the electrolyte continues, and the zinc is eventually used up in the process. Secondary batteries can be discharged and recharged again and again. The discharge and recharge happen through an electric current, where a reverse current then helps to restore the electrons to their original composition. We outline the comparison of electrode materials, energy density, power density, temperature range, shelf life, etc., for primary marine batteries in Table 1, and the characteristics of secondary batteries are covered in Table 3, which are mostly used at present for sea vehicles. Marine ESS technologies can be categorized into higher energy and power technologies. Higher energy devices such as batteries, fuel cells, pumped hydro, and CAES can supply energy for a longer duration but their power is low. On the other hand, higher power devices such as flywheels, super-capacitors, SMES, and higher power batteries can supply very high power but for a shorter duration. Battery technology can be employed in both categories due to their wide characteristic range. Hence, the hybridization of higher energy density devices with higher power density devices will yield a better ESS. In this way, high-energy devices will provide long-term power needs, whereas higher power devices will cater to shorter durations but higher power needs.8,9
| Leclanche’ | Alkaline | Mercury | Magnesium | Organic electrolyte Li/SO2-PC-AN/C | Li/SOCl2 | |
|---|---|---|---|---|---|---|
| Negative electrode | Zn | Zn | Zn | Mg | Li | Li |
| Positive electrode | MnO2 | MnO2 | HgO | MnO2 | SO2/C | SOCl2/C |
| Electrolyte | NH4CI/H2O | KOH/H2O | KOH/H2O | MgCl2/H2O | SO2-PC-AN/LiBr | LiAICl4SOCl2 |
| Energy density (W h kg−1) | 66 | 77 | 99 | 143 | 330 | 450–680 |
| Energy density (W h cu cm−1) | 0.12 | 0.18 | 0.43 | 0.24 | 0.49 | 0.91–1.5 |
| Open circuit voltage (V) | 1.5 | 1.5 | 1.35 | 1.7 | 2.9 | 3.7 |
| Voltage stability (0–90%) | 1.5–0.7 | 1.5–0.8 | 1.3–1.1 | 1.7–0.9 | 2.7–2.0 | 3.6–3.2 |
| Power density (W kg−1) | 55 | 66 | 11 | 88 | 110 | 550–2200 |
| Power density (W cu cm−1) | 0.09 | 0.09 | 0.04 | 0.18 | 0.16 | 0.3–2.7 |
| Temperature range (°C) | −6 to 54 | −29 to 71 | −6 to 71 | 5–71 | −40 to 74 | −40 to 100 |
| Operating voltage (V) at 1 mA cm−2 | 1.3 | 1.3 | 1.2 | 1.7 | 2.7 | 3.6 |
| Shelf life (years) at 21 °C | 1 | 2 | >2 | 2 | >2 | >2 |
2. Energy storage for maritime industries
From international shipping to local passenger transport, the maritime industry is largely dependent on fossil fuels to power its vessels. Even though shipping is a relatively climate-friendly transport method, the combined greenhouse gas emissions from maritime transport across the globe are still enormous. In order to lower emissions, a push towards electric and hybrid vessels is oncoming. Electrification, however, relies on a range of factors, including infrastructure upgrades, technological advances, operational and regulatory frameworks, as well as issues of cost.11 At present, various companies and projects view electric propulsion as a primary consideration. In these vessels, the battery storage system is of paramount importance as it works as the main source of power for all the electrical and electronic systems. Even as the main power source for propulsion, in the case of electric propulsion vessels, the battery system also generates propulsion power.12 Therefore, a more detailed presentation of the different types of batteries is being included herein, as shown in Fig. 2. Starting with the battery types that are already used in the maritime industry according to previous studies,13–19 a draft of the different marine battery types is presented. In recent times, lithium-ion batteries have positioned themselves at the forefront of battery energy storage technology for many applications.20 This disruptive creation will shake up many industries, from consumer electronics to the energy, oil, and gas sectors, to transport and the maritime industry.21,222.1 Specific parameters and requirements for the marine battery system
The selection of a power source for a specific marine application with a defined energy requirement is usually done by comparing the performances of completely neutrally buoyant battery sections, given a defined available volume and a design depth.23Electrochemical power sources for marine vehicle applications are classified into four different groups:
(1) standard batteries inside a pressure hull and working at normal pressure;
(2) pressure-compensated batteries working at ambient pressure, but electrically insulated from the seawater;
(3) seawater batteries;
(4) fuel cells.
Typical examples from the three battery groups are lead/acid batteries operating at normal pressure in conventional submarines, pressure compensated batteries in the US Navy deep-sea rescue vehicle (DSRV), and magnesium/silver chloride seawater batteries in torpedoes such as the UK Stingray lightweight torpedo. In the magnesium/silver chloride battery, seawater is used as the battery electrolyte and the internal pressure of the battery is equal to the external (ambient) pressure, given by the water depth and seawater density. In the DSRV, the battery is based on silver/zinc cells. where the voids in the cell are filled with oil and the pressure of the electrolyte is kept equal to the external pressure via a flexible member between the oil and the seawater.24
When comparing battery systems, several factors should be considered in addition to the specific energy content and power capability, with typical factors being cost, battery life (both in terms of cycle and calendar life), maintenance requirements, and safety. This also holds for marine batteries. In addition, pressure-compensated batteries may have variations in buoyancy with depth or the degree of discharge. Variation in buoyancy with the depth of discharge is always the case for seawater batteries. For shallow diving underwater vehicles (UWV's), the net mean density of the pressure vessel is low and the simplest and probably the most efficient battery solution is to use a battery system with a high energy density and put the battery and electronic systems together inside the pressure vessel. In this case, the only concern is to keep a safe atmosphere within the pressure vessel at all times; with safe meaning non-explosive, non-combustible, and non-corrosive. Using typical battery data, Table 2 shows the expected performance.25
| Chemistry | Energy density of cell (W h kg−1) | Energy density of neutrally buoyant system (W h kg−1) | System energy (kW h) |
|---|---|---|---|
| Ni/Cd 4.5 A h D-cell | 36 | 14 | 4.2 |
| Ni/MH 9 A h D-cell | 60 | 23 | 6.9 |
| Ag/Zn | 120 | 46 | 14 |
| Li-ion MP176065 | 120 | 46 | 14 |
| Li/SOCl2 | 450–680 | 153–230 | 47–70 |
In the future, lithium-ion batteries developed and optimised for low-rate, room temperature discharge, should be available with much higher energy density than indicated above. The availability of large EV cells should decrease the cost and increase the performance of the Li-ion marine batteries. In terms of safety, however, the authors prefer many small cells, as it is our experience with lithium batteries that battery incidents mostly start with only one cell. Thus, even though increasing the cell number increases the probability of an incident, keeping the cell size small may reduce the severity of the incident.26
Most battery chemistries can be used in pressure compensated designs. The main requirement is that the battery does not contain voids that may be compressed. Typically, the cell is composed of solid or liquid phases, with a flexible member between the liquid electrolyte and the outside water. This flexible member compensates for differences in the compressibility of the different materials, allowing volume change with pressure and also allowing for small amounts of compressible gas in the battery. If a larger amount of gas is expected, a gas separation system is mandatory.27,28
2.2 Challenges and opportunities
Over the last ten years, the general consensus has shifted to the position that there is enough lithium to meet the exponential growth of electric propulsion, due to the growing exploitation of mineral deposits around the world. Furthermore, manufacturing experience is rapidly making lithium batteries cheaper, even against rising lithium prices. It is likely that by 2030, even the majority of grid storage requirements, a market previously thought to be fertile ground for cheaper batteries with lower outright performance, will be most economically met by lithium batteries; the question of which battery to use remains. Lithium-ion batteries are a work in progress characterized mainly by cathode choice. The most popular cathode in marine and automotive applications is nickel manganese cobalt (NMC), which provides reasonable safety combined with very good cell-specific energy.29 However, NMC has intrinsic issues with thermal stability, and depends on cobalt, which is scarce, expensive, and has ethical issues with its supply chain. All of the current lithium-ion batteries use flammable organic electrolytes, and NMC, in particular, is vulnerable to thermal runaway, where the cell temperature increases rapidly and leads to fires that are very difficult to extinguish. Since an uncontrolled fire on board is not an option in marine applications, comprehensive fire extinguishing systems are required, as well as continuous cooling during normal operation.30 Even so, fire control relies on limiting the thermal runaway to a small number of cells, and so the cells are contained in waterproof metal cases and arranged in racks that are separated by a significant air gap. These design factors cause the fully installed energy density of lithium NMC battery packs to be around half of that for automotive battery packs – 86 W h kg−1 for the Ellen Eferry vs. 160 W h kg−1 for a Tesla Model 3.31,32Because lithium NMC marine battery installations require so much compromise to be made safe, there is significant scope for using lower performance but safer chemistry to achieve similar or better overall performance. A good example is lithium iron phosphate (LFP), which has around 65% of the specific energy of NMC but is far safer and replaces expensive and problematic nickel and cobalt with highly abundant iron and phosphorous. The increased safety means that the next generation of Chinese electric vehicles will feature lithium iron phosphate packs with specific energy only 12.5% less than Tesla/Panasonic's lithium nickel cobalt aluminum (NCA) packs, which are themselves more energy-dense than NMC. Because the safety requirements are even more onerous for marine applications, it is likely that LFP offers near parity-specific energy to NMC, though this could not be confirmed from the literature. With more uptake of the technology for marine applications, the LFP price should eventually drop markedly below that of NMC. LFP has already been approved for marine use and is currently in use on boats.33,34
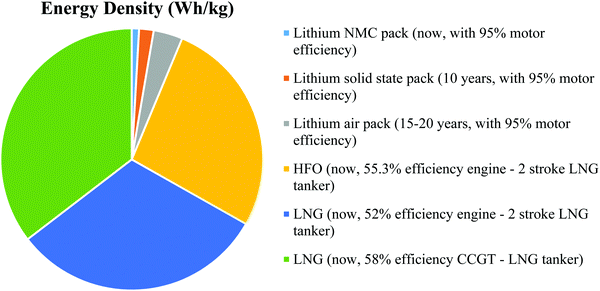
The next 10 years are also expected to see a series of incremental advancements in cathode materials, including high nickel (Fig. 3) and lithium-rich cathodes, both of which offer higher voltages and capacities than today's cathodes.35 There is a trade-off in terms of cathode stability, but this might be sufficiently mitigated by the inherent safety of a solid electrolyte. The trend is to reduce or eliminate cobalt while raising the specific energy and voltage. One cathode that seems unlikely to feature in marine applications is lithium–sulfur. Despite having double the specific energy of today's lithium-ion batteries, it cannot improve on the volumetric energy density of lithium NMC.36 It also has cycle life issues that are unlikely to be completely resolved and is considered a suitable battery for aeronautical applications, i.e., drones where weight is critical. Sodium is touted as a potential alternative to lithium due to its abundance (in seawater), with lots of crossovers from lithium ions in terms of manufacturing, and electrode and electrolyte technology; however, in reality, it is a larger, heavier ion with a lower electrochemical potential. Apart from decreased specific energy, the cycle life is shortened by the shuttling of larger ions into the electrodes. Other alternatives tend to fall around the lead-acid performance level at under 50 W h kg−1, including flow batteries (which have been commercialized for grid storage) and aluminum batteries (which remain in the laboratory).37 These are discussed in some detail in this paper but from a practical standpoint, they can be largely discounted from future marine applications up until 2050. The lithium–air batteries (or other metal–air batteries) may appear in the 15–20 year timeframe. While the theoretical specific energy of lithium–air is 3500 W h kg−1, 950 W h kg−1 has been stated as a realistic maximum in practice. Metal–air batteries may still suffer the same problems as fuel cells with slow oxygen reactions and might also need the support of a high-power battery type.38 However, while this battery type remains in the laboratory with little evidence of an impending breakthrough, the elimination of the cathode and the quadrupling of the specific energy of today's battery cells make lithium–air an important potential contributor to future transport energy storage.39 The cell-level specific energy of the different battery types discussed is shown in Fig. 4 along with the expected timeframe for their arrival.
 | ||
| Fig. 3 The specific energy of cell chemistries expected to be used in marine applications over the next 5–20 years. | ||
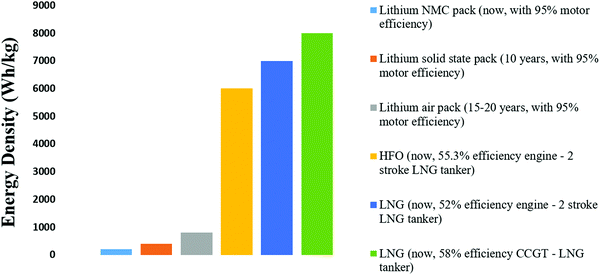 | ||
| Fig. 4 Estimated pack-level specific energy of different battery types vs. HFO (heavy fuel oil) and LNG (liquefied natural gas). | ||
An estimation of the maximum range of battery-electric boats is provided. Today's lithium NMC technology has allowed battery-electric boats with a range up to about 50 km, which in the case of Ellen have already substituted all of their ballasts for batteries. Solid-state batteries, when mature in around 10 years, may triple the specific energy (at the pack level, mostly through improved safety) and allow a range up to 150 km. Lithium–air might double this specific energy again, taking the range to 300 km. Therefore, even with a large margin of error, battery-electric boats are unlikely to significantly exceed a 500 km range and will probably never sail further than 1000 km. On longer journeys than this, a hybrid solution will be required. Fig. 4 shows the relative specific energy of marine fuels as compared to today's battery packs, those predicted for solid-state lithium in 10 years, and for lithium–air in 15–20 years, showing clearly why fuel is required on longer journeys.40,41
2.3 Advancement in lithium-ion batteries for marine environments
For over a century, lead-acid batteries have been the standard source of stored energy for marine vehicles; they are utilized to power the vehicle's main propulsion, or as a stand-by battery. However, more is required of submarines – they must demonstrate increased endurance and cope with greater speed demands. A new generation of energy storage technology is required, based on lithium-ion batteries (LIBs).42,43Lithium-ion batteries could be a game-changer in marine applications, with the potential to be a primary source of power not just for submarines, but also unmanned underwater vehicles (UUVs) and torpedoes. Although LIBs are already found in exercise torpedoes and some UUVs, the technology is maturing; it is still relatively new as compared to lead-acid batteries, which have been in production since the late 19th century.44,45 However, their compact design means that LIBs are more efficient and have more excellent reliability than their lead-acid counterparts.46 Similar to conventional Li-ion batteries, marine lithium-ion batteries are also comprised of the anode, cathode, separator, and the supporting solution in which the progression of lithium ions occurs from the cathode to anode and vice versa during the charge/discharge process.47–50 The materials that are typically used for fabricating the anode are metallic lithium,51,52 graphitic carbon,53,54 hard carbon,55,56 synthetic graphite,57 lithium titanate,53,58 tin-based alloys,59 and silicon-based materials.60 The materials used for making the cathode are an oxide of lithium manganese,61 lithium cobalt oxide,62 FeS2,63 V2O5,63 lithium nickel cobalt manganese oxide,64 lithium-ion phosphate,65,66 and electronic conducting polymers.67,68 The materials used as electrolytes include LiPF6,69 LiClO4,70 LiAsF671 and LiCF3SO3.72,73 Apart from these main components, there are other components such as a binder, flame retardant, gel precursor, and electrolyte solvent. Lithium-ion batteries (LIBs) have been extensively used to power a variety of marine vehicles because of their higher energy density and eco-friendly nature. Despite these benefits, the cycle life and power density still need to be upgraded for their use in electrically driven vehicles (EVs), large-scale energy storage, and other wide-range applications towards marine vehicles.74
Researchers are continuously advancing battery technologies to make them useful for marine environments. Advanced lithium-ion (Li-ion) battery technology offers interesting new possibilities for the creation of highly efficient and cost-effective marine propulsion and auxiliary systems.75 Advanced Li-ion technology is of particular interest for fully electric and hybrid propulsion systems, where the batteries work in conjunction with diesel, or possibly gas turbine, generators, and electric motors.76 Its specific advantages will vary according to the type of application. For workboats such as harbor tugs, which spend most of their time at sea moving into position and only operate at full power for very short periods, hybrid power will significantly improve fuel efficiency and reduce CO2 emissions. Rather than sizing a diesel engine for peak power, it can be specified at a more economical size for average power, with the extra power drawn from the batteries when required.77 They offer substantial enhancements over their predecessors; they have a higher energy density, a long service life, and require less maintenance. Li-ion batteries can be characterized as energy storage systems that rely on insertion reactions from both electrodes where lithium ions act as the charge carriers.78 Given this broad definition, several different cell chemistries make up the Li-ion battery family. Most Li-ion batteries use a negative electrode,79 principally made from carbon (e.g., graphite) or lithium titanate (Li4Ti5O12), with some novel materials under development, namely, Li metal and Li(Si) alloys. The electrolyte used varies based on the choice of electrode materials but is typically composed of a mixture of lithium salts (e.g., LiPF6) and an organic solvent (e.g., diethyl carbonate) to allow for ion transfer.80 A separating membrane is used to allow lithium ions to pass between the electrodes while preventing an internal short circuit.21,22 For the transport aspects of the battery when operating as an energy source (i.e., a galvanic device) the electrons travel from the negative electrode to the positive electrode while the Li+ ions simultaneously travel from the negative electrode through the electrolyte to the positive electrode to maintain electroneutrality. When the system is operated in charge mode (i.e., as an electrolytic device) the electron current and Li+-ion flow is reversed.81
The widespread deployment of solid-state lithium batteries will almost certainly be the next significant advancement in energy storage, and this is expected within 5 years, with the second generation of solid-state batteries providing results closer to their theoretical performance available within 10 years for marine applications. A solid-state electrolyte prevents the risk of dendrites shorting the battery and facilitates the use of a lithium metal anode, the ‘holy grail’, with the highest specific energy of any anode. Solid-state batteries could provide up to 75% better specific energy as compared to the best lithium-ion batteries today. However, the safety impact might be even greater: with the fire risk and even the cooling requirement all but eliminated, marine battery packs might triple in specific energy overall within 10 years, with similar increases in achievable ranges.81
2.4 Military and civilian uses of lithium-ion batteries
The civil marine industry needs to adapt quickly to meet ever-tighter environmental regulations. Designers play a vital part in this evolution by integrating new concepts in ship architecture with cutting-edge battery technologies to reduce emissions and increase efficiency and profitability.82 LIBs are a promising technology for several civil and defense applications. They have the potential to be one of the best sources of power for defense underwater applications because their compact designs bring higher efficiency and strengthen reliability for underwater applications. Although less than 1% of the total demand for LIBs currently comes from the defense sector, this is expected to increase as their potential is realized.20LIBs will make a real impact in submarines, thanks to their long service life and low maintenance requirements. Big-name designers such as Spanish shipbuilder Navantia, high-tech naval defense company Naval Group, and Swedish defense company Saab Kockums have already designed LIBs for submarines.82 The Japanese Navy employs the technology in the Sōryū-class submarine, a diesel-electric attack sub, and South Korea has utilized them for their next-generation attack submarines. The US Special Forces delivery vehicle, the Dry Combat Submersible (DCS), and the Russian Surrogat unmanned mini-sub are both powered, or will be, by LIBs. LIBs are also of value in civil maritime, namely in unmanned underwater vehicles (UUVs). Thyssen Krupp Marine Systems, a German defense company, intend to power their Modifiable Underwater Mothership (MUM), a modular underwater vehicle, which aims to ‘conquer the deep sea’, with LIBs. MUM will aid the transport and deployment of payloads, have applications in the offshore wind and oil and gas industries, as well as the ability to explore hard-to-reach areas such as the Arctic ice regions.83–85
2.5 The future of LIBs for the marine environment
Lithium-ion batteries are emerging as crucial for energy storage. They will have a decisive role in electric mobility, i.e., our ability to travel using electrically powered, rechargeable vehicles, whether on land or underwater. It can be seen that the evolution of LIB-powered electric vehicles has not only improved lithium-ion-based technologies but has also resulted in a steady decline in the prices of lithium-based batteries.Lithium-ion batteries have the potential to change how energy is stored and used, but as with any developing technologies, there are still improvements that can be made. Introducing graphene, a single layer of carbon-based material, could transform how energy storage technology is used. As reported by Qian, the use of a carbon material called graphene-like-graphite (GLG) as the anode material for lithium-ion batteries can deliver a high capacity of 608 mA h g−1 and provide superior rate capability. The morphology and crystal structure of GLG are quite similar to those of graphite, which is currently used as the anode material for lithium-ion batteries. Therefore, it is expected to be used in the same manner as conventional graphite materials to fabricate the cells. Based on the data obtained from various spectroscopic techniques, they propose a structural GLG model in which nanopores and pairs of C–O–C units are introduced within the carbon layers stacked with three-dimensional regularity. Three types of highly ionic lithium ions are found in fully charged GLG and stored between its layers. The oxygen atoms introduced within the carbon layers seem to play an important role in accommodating a large number of lithium ions in GLG. Moreover, the large increase in the interlayer spacing observed for fully charged GLG is ascribed to the migration of oxygen atoms within the carbon layer introduced in the state of C–O–C to the interlayer space maintaining one of the C–O bonds. As such, this material can be used to create lightweight and durable batteries with a high capacity for energy storage and quick charging. Researchers from the Samsung Advanced Institute of Technology (SAIT) and Seoul National University's School of Chemical and Biological Engineering also designed a graphene coating for lithium-ion batteries that will increase their capacity by 45% and make their charging speed five times faster. The batteries will also last longer and can maintain temperatures approaching 60 °C, making them ideal for electric cars; it remains to be seen if they can develop a version for on water/underwater applications.86
3. Global marine battery market scenario
Maritime transport is considered the backbone of global trade and economy; shipping carried more than 85% of the world's goods, by volume, in 2018. Seaborne trade continues to expand, bringing benefits to consumers across the world through competitive freight costs. This is also expected to drive the growth of the global marine battery market during the forecast period. The global marine battery market has witnessed high growth in recent years owing to an increase in seaborne trade and implementation of the IMO Sulfur 2020 Regulation. Moreover, the growing maritime tourism industry is driving the growth of the market. Furthermore, factors such as the use of renewable sources of energy for battery charging and advancements in hybrid propulsion technology are expected to offer lucrative opportunities for market growth. The global marine battery market is projected to register a CAGR of 48.1% from 2020 to 2025. Batteries are the crucial components of the ships; they are used to provide starting service and deep cycle service. Flooded lead-acid, gelled-electrolyte batteries, Li-ion batteries, and absorbed glass mat (AGM) batteries are commonly used for marine applications but an increase in the electrification of ships requires advanced batteries.87A flooded battery uses a reservoir of liquid sulfuric acid. When charging, it produces hydrogen and oxygen. Hydrogen may explode, which is why its storage needs to have holes or any form of ventilation so that the element may be emitted safely. This type of battery can also better overcome overcharging as compared to other types.
A gel battery is maintenance- and leakage-free, submersible, and spill-proof. It has also the longest charging cycles. Manufacturers of gel batteries have completely eliminated the gassing; therefore, they can be safely used next to sensitive electronics and in public areas.
The Thin Plate Pure Lead (TPPL) and Lithium Manganese Battery is among the newest innovations batteries have gone through. It uses 99% lead that is rolled, not cast, into thin plates. Because of the structure, it can accept higher charging amperage. This battery also charges quickly and has 400 charge and discharge cycles.
The AGM battery is made with fine, highly porous microfiber glass separators tightly compressed between its positive and negative plates, endowing it with good starting power and deep-cycling capability.88
3.1 Market segmentation
• Deep cycle service: this segment accounted for the largest market share in 2019. A deep cycle battery is used to deliver an extended, slow discharge of fewer amps for several minutes or hours. The demand for these batteries has been with the electrification of ships.
• Dual purpose service: this segment is expected to grow at the highest CAGR during the forecast period. Dual-purpose batteries are used in applications for both starting and deep cycle service. It delivers powerful cranking amperage for easy starting and low amp draw service for consistent auxiliary power.
• Defense: this segment is expected to register a higher growth rate during the forecast period. The defense segment includes destroyers, corvettes, frigates, offshore support vessels, aircraft carriers, and submarines. Increasing military spending on the development of new and advanced combat vessels is expected to increase the demand for high-performance marine batteries.
• Europe: Europe is the second-largest market for marine batteries. This trend is projected to continue during the forecast period due to investments by prominent players such as Saft Groupe SA and Systems Sunlight SA in the development of marine batteries.
• Asia-Pacific: Asia-Pacific accounted for the largest market share in 2019. The market in the region is also projected to register the highest CAGR during the forecast period. Increasing defense expenditure in India and China and the rising demand for new and advanced naval vessels are driving the regional market growth.
• Middle East and Africa: the increase in defense expenditure in Saudi Arabia, the UAE, and Israel is driving market growth in the Middle East and Africa.
• Latin America: in Latin America, the increase in naval expenditure in Brazil for the procurement of advanced naval vessels is driving the growth of the market.88
4. Evolution of marine battery systems
Current lithium batteries used in marine applications are reaching maturity and predictions are that further performance improvements will be small due to the limitations of the chemistry. In contrast, Li–sulfur (Li–S) has the potential to surpass Li-ion by being able to store more capacity than many other battery storage systems without any safety issues. Autonomous underwater vehicles are energy limited; this restricts their operational envelope, and so speeds are usually low (2–4 knots) and endurance is restricted. By significantly increasing the energy available within the vehicle, the operational envelope can be expanded, thereby increasing the speed and range.Lithium–sulfur (Li–S) cells have five times the theoretical maximum specific energy of lithium-ion. The increased specific energy and improved lower density of the cells mean that they could be an excellent replacement for the current lithium rechargeable cells used in marine autonomous systems. Li–S is predicted to have a 'neutrally buoyant' specific energy up to 3–4 times that of the current lithium-ion cells used by state-of-the-art deep-diving underwater systems, thus reducing or eliminating the need for highly expensive buoyancy material in the vehicle, and increasing the endurance, speed, and payload. It was thought that the availability of such technology was many years away, yet innovative technology companies such as OXIS Energy are proving that the chemistry is far closer than anticipated. Working with an experienced battery assembly partner such as Steatite means that lithium–sulfur battery packs are progressing development beyond proof of concept. Steatite and OXIS are well placed to deliver battery packs designed for the most challenging environments. Li–S batteries offer distinct advantages over conventional lithium rechargeable technologies; they are considerably lighter and safer. Steatite is developing battery packs complete with a battery management system that will not require the heavy and bulky pressure housings that are currently used in many underwater autonomous systems. This modular battery design offers a scalable high-energy solution that can be utilized in a variety of marine applications, including, seafloor systems, nodes, and underwater vehicles.89
Conventional Li-ion batteries have alkyl carbonate-based electrolytes that have unresolved safety issues. The thermal energy and flammability issues of the solvent vapour will start to occur at around 160 °C, which is likely to result in venting and cell disassembly. The OXIS Li–S technology is safer than Li-ion due to a higher flash point of electrolyte and the protective lithium sulfide layer on the anode. OXIS cells today offer a significant improvement over the best performing Li-ion solution available. At the research and development level with long-life cells, they are already achieving 220 W h kg−1. Projections for the ultra-light cells predicted a gravimetric energy density of 400 W h kg−1 early in 2017. Lithium–sulfur cells offer wider benefits for marine autonomous systems, the cells have increased specific energy as compared to current lithium cells used in AUV applications, and the cells are significantly less dense as compared to the conventional lithium polymer cells, with a density of 900 kg m−3 compared to 2100 kg m−3. This decrease in density produces a substantial benefit for AUV applications as it is necessary to float the batteries. It is considered that the specific energy of a battery pack for an AUV must include the mass of the foam required to float the batteries. Assuming that the batteries are floated with one of the best available 6000 m rated syntactic foams and taking the nominal density, we can calculate the amount of foam required to float a 1 kg pack and hence calculate the specific energy of a neutrally buoyant battery system. Reducing or eliminating the need for expensive syntactic foam will greatly reduce the overall cost as compared with other solutions. This benefit of the lithium–sulfur battery pack facilitates additional research equipment, longer deployment time, and increased speed.90
4.1 Open water power battery
The open water power battery (OWP's) that “drinks” in seawater to operate is safer and cheaper, and provides a tenfold increase in range as compared to traditional lithium-ion batteries used for unpiloted underwater vehicles.Most UUVs use lithium-based batteries that have several issues. They are known to catch fire, so UUV-sized batteries are generally not shippable by air. Also, their energy density is limited, meaning expensive service ships chaperone UUVs to sea, recharging the batteries as necessary. The batteries also need to be encased in expensive metal pressure vessels; in short, they are rather short-lived and unsafe. In contrast, the OWP's power system is safer, cheaper, and longer-lasting. It consists of an alloyed aluminum, a cathode alloyed with a combination of elements (primarily nickel), and an alkaline electrolyte that is positioned between the electrodes. When a UUV equipped with the power system is placed in the ocean, seawater is pulled into the battery, and it is split at the cathode into hydroxide anions and hydrogen gas. The hydroxide anions interact with the aluminum anode, creating aluminum hydroxide and releasing electrons. These electrons travel back toward the cathode, donating energy to a circuit along the way to begin the cycle anew. Both the aluminum hydroxide and hydrogen gas are jettisoned as harmless waste. Components are only activated when flooded with water. Once the aluminum anode corrodes, it can be replaced at a low cost.91
4.2 Batteries for marine ships
In recent years, considerable research and development work has been done on advanced power sources for marine electric and hybrid vehicles as described in the following sections:Besides the 4300 kW h Leclanché battery, the ship has a record-breaking 4 MW charging rate, allowing for nearly 1 C charging. Following Ellen's example, ferry operator Stena Line is working towards installing a 1000 kW h battery system on the ‘Stena Jutlandica’, which operates between Gothenburg, Sweden and Frederikshavn, Denmark. Once this first step is taken, Stena plans to connect a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kW h battery pack to the propellers allowing for a 10 mile pure electric range. Later on, the battery capacity will be expanded to 50
000 kW h battery pack to the propellers allowing for a 10 mile pure electric range. Later on, the battery capacity will be expanded to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kW h, enabling roughly 50 miles of pure-electric range or approximately the distance between Gothenburg and Frederikshavn.
000 kW h, enabling roughly 50 miles of pure-electric range or approximately the distance between Gothenburg and Frederikshavn.
In China, the first pure electric container ship in the world was launched in November 2017 to transport coal. IDTechEx's review reads as follows: “according to China News, the powertrain is equipped with a mixture of super-capacitors and lithium-ion batteries for a total energy capacity of 2400 kW h; the powertrain reportedly enables a range of 50 miles on a single charge. The vessel currently travels inland down the Pearl River in Guangdong Province, where new emission control areas came into force in January 2019”. Also in Asia, Asahi Tanker is developing the e5 project for Japan. This would be the first sea-going pure electric and autonomous tanker. It is expected that the 60 metre long ship, whose five e's stand for electrification, environment, evolution, economy, and efficiency, hits the water by 2022 with a 4000 kW h battery enabling an 80 mile range.92
On all hybrid ships, the battery is an essential part of the power system but in most cases, it will be secondary to the main engines, which presently are most likely to be running on conventional fuels, or be able to switch to one of the greener alternatives now being developed. In these ships, battery systems come in two distinct types. That on the Viking Lady was mainly a prototype designed to store any excess power generated by the ship's engines and use it as needed for any purpose. In contrast, the system on Ampere was specifically designed to provide all the necessary propulsion power, as well as all other requirements of the vessel. On the Carolyn Dorothy, the system was also specifically designed for adapting to the changing requirements of the vessel type.93
Energy storage in batteries also optimizes the entire propulsion solution since it provides smoother power for the main engines. Batteries avoid so-called transient engine loads, a major advantage for larger vessels such as cruise ships, which have a constant need for considerable amounts of energy. Batteries provide instant power, in contrast to diesel gensets that can take up to half a minute to fire up; in other words, the batteries help prevent blackouts.94
4.3 Secondary electrochemical marine batteries
There are some other secondary electrochemical batteries used for marine applications (Table 3). Silver–zinc cells have been used extensively in AUVs, for example in the Odyssey vehicle.94 However, batteries formed from silver–zinc cells require very careful maintenance and record-keeping regarding the state of charge to ensure safe operation. Standard silver–zinc cells also have a limited number of charge–discharge cycles, a limited life once the electrolyte has been added to the cell, and a high capital cost. These limitations have led several AUV manufacturers to seek alternative rechargeable power sources, or, in the case of Autosub, the higher energy requirements have been met using primary batteries.95,96 Nevertheless, significant research and development efforts have been directed towards augmenting the silver–zinc chemistry in order to improve the energy density and the number of charge–recharge cycles. These efforts have involved modified anodes, for example adding bismuth, cadmium, or lead oxide to the zinc oxide and using new electro-permeable membranes to almost double the cycle life.97| Type | W h kg−1 | W h l−1 | W kg−1 | W l−1 | Temp. range (°C) | Cycle life | Energy efficiency (%) |
|---|---|---|---|---|---|---|---|
| Lead–acid | 20–30 | 60–80 | 100 | 230 | −40 to 55 | 700 | 68 |
| Lithium ion | 90–150 | 150–200 | 250 | 275 | –20 to 45 | 600–1000 | — |
| Lithium–solid polymer | 130–190 | 170–240 | — | — | 120 | 300–3000 | — |
| Nickel–cadmium | 40–55 | 60–90 | 100 | 180 | −40 to 60 | 1500 | 70 |
| Nickel–metal hydride | 50–70 | 100–150 | 145 | 330 | Ambient | 1500 | 75 |
| Silver–zinc | 100–120 | 180–200 | 400 | 660 | −48 to 71 | 100 | 75 |
| Sodium–sulfur | 90–120 | 120–130 | 150 | 136 | 350 | 800 | 70 |
| ZEBRA: sodium–nickel chloride | 110–120 | 110–120 | 120 | 160 | 300 | 700 | — |
| Aluminum–oxygen | — | 260 | — | — | Ambient | — | 81 |
| RAM: secondary manganese alkaline | 40–80 | 110–220 | — | — | Ambient | >25 | — |
Nickel–cadmium (∼55 W h kg−l) presents one commercially available proven alternative to lead–acid and silver–zinc. The higher energy density and longer life of the nickel–cadmium, however, comes at a price that is ten times that of the lead–acid. Careful thermal management is necessary when charging nickel–cadmium cells in situ. During charging, the recombination reaction is exothermic; in large packs, the heat generated must be dissipated to avoid an excessive temperature increase that will reduce the charging efficiency. Repetitive shallow discharges of nickel–cadmium cells lead to a (reversible) decrease in capacity, the so-called ‘memory effect’. The cell's full capacity can be restored through a deep discharge followed by a normal recharge. Disposal of used nickel-cadmium cells requires special considerations due to the significant amount of toxic heavy metals present. Notwithstanding these complications, nickel–cadmium cells have been used in several AUVs, including those from Florida Atlantic University.98
For marine applications, sodium–sulfur cells also have high energy densities and long life at a reasonable cost, but they operate at 295–350 °C, which presents some handling and operational problems. However, BAE Systems demonstrated that these problems could be overcome with proper engineering.99,100 The support systems that needed to be installed in the vehicle to manage the cells led to a substantial decrease in the energy density. The battery itself is comprised of ABB type B120 12 V 800 A h batteries, each weighing 13 kg and rated at 9.6 kW h (738 W h kg−1), which is close to the theoretical energy density of 790 W h kg−1.41 However, with the battery management unit, the rigid metal framework, the electrical heating element, all inside a double-walled stainless steel outer container with fiberglass insulation in vacuo between the walls, the total system providing 38 kW h weighed 550 kg, equivalent to an energy density of 69 W h kg−1.39 In-water tests were successful and the need to maintain the battery temperature above 295 °C was not a significant operational problem.
Zebra batteries are also a useful development for marine ships. It is a class of cells that use liquid sodium metal as the anode and chlorides of various transition metals as the cathode. Cells using iron II, copper II, cobalt, nickel, and chromium II chloride have been developed. The nickel chloride cell, for example, has an open-circuit voltage of 2.58 V and a theoretical energy density of 750 W h kg−1.40 Extensive trials of these batteries, with capacities in the region of 13–17 kW h, have taken place in electric vehicles.100,101
4.4 Battery systems for electric leisure boats
In the over one hundred electric vehicle sectors tracked by IDTechEx, they predominantly foresee a transition partly or completely to a traction battery over the next two decades. The case is not so simple for the marine sector; due to the sheer scale of the power, energy, and distance requirements for many vessels, reducing maritime emissions will require solutions varying from batteries and fuel cells to premium fuels, scrubbers, and slow-steaming. Today, batteries have mainly emerged in leisure boating, ferries, and short-sea vessels, where they have enjoyed a steady uptake due to small vessel sizes or well-defined cyclical routes (that allow for opportunity charging). In larger deep-sea vessels, uptake is slow but unprecedented global emissions regulations are driving change, and shortages of traditional solutions on the horizon are creating new opportunities for energy storage start-ups in the arena.102By volume, electric leisure boating is the largest market, with tens of thousands sold yearly; electric leisure boats can be thought of as cars of the marine world as they are privately owned, have short-range requirements, and can jump straight to a pure electric powertrain. In contrast, there are less than 20 hybrid deep-sea vessels in service, yet the sector has the largest market value and demand for maritime batteries due to the vessel sizes and high energy requirements involved.
The new report ‘Electric Leisure & Sea-going Boats and Ships 2021–2040’ provides historical data from 2016 and forecasts up to 2040 for the number of electric vessels, battery demand (MW h), and market value ($ billion) broken down by pure electric and hybrid powertrain, as well as by each marine sector: leisure boats, fishing, cruise ships, ferries, offshore support, tugboats and deep-sea. The report delves into key underlying technologies and draws parallels and differences with the auto industry. All results are underpinned by primary research and interviews undertaken around the globe, from Seoul, South Korea to San Diego, USA.
Williams Advanced Engineering is working with a fellow Oxfordshire-based firm, OXIS Energy Ltd, on the production of a world-first battery system, designed for an all-new 40 ft electric luxury boat. The craft, from Yachts de Luxe (YdL) of Singapore and designed by the renowned boat designer, Jean Jacques Coste, will be the world's first-ever luxury boat to be powered by lithium–sulfur (Li–S) cell and battery systems technology. The objective is to achieve a range between 70 and 100 nautical miles at cruising speed, setting new standards for range in electric boats. Williams Advanced Engineering will be responsible for the state-of-the-art 400 kW h battery system, which comprises ultra-light, high-power/high energy density Li-S cells, provided by OXIS Energy Ltd, and the battery management system.102
(i) No incumbents on the market. The incumbents that make and sell the most outboards, Yamaha, Honda, and Mercury, have made no moves to develop electric versions. This is common in the more niche electric vehicle categories, which are often overlooked by policymakers in favor of cars and commercial vehicles. The result is that start-ups like Torqeedo have literally created and driven the electric leisure boating market almost single-handedly. This also means they have an unchallenged reign: Torqeedo's high level of experience and developed product line-up makes it difficult for other start-ups to compete. Incumbents, backed by large balance sheets and market experience, would be the first real challenge. In short, incumbents entering the market would improve competition, improve awareness of electric boats as an option, and likely increase overall market sales.
(ii) A lack of emissions regulation. This challenge goes hand in hand with the last point. Policymakers' apathetic attitude towards emissions regulation for outboards and inland vessels has allowed incumbents to easily develop and sell products that increase overall boating emissions, rather than reduce them.
(iii) High battery prices. System-level maritime battery prices are several times higher than those of other industries such as electric cars and stationary energy storage. The new IDTechEx report “Li-ion Batteries 2020–2030” gives a detailed overview of how we expect Li-ion battery prices to evolve over the next decade.
The new IDTechEx report, “Electric Leisure & Sea-going Boats and Ships 2021–2040”, provides historical data from 2016 and forecasts up to 2040 for the number of electric vessels, battery demand (MW h), and market value ($ billion) broken down by pure electric and hybrid powertrain as well as by each marine sector: leisure boats, fishing, cruise ships, ferries, offshore support, tugboats and deep-sea. The report delves into key underlying technologies and draws parallels and differences with the auto industry. All results are underpinned by primary research and interviews undertaken around the globe. This report also forms a part of the broader electric vehicle and energy storage research at IDTechEx, where they track the adoption of electric vehicles, battery trends, and demand across more than 100 different mobility sectors, summarized in one master report, “Electric Vehicles 2020–2030: 2nd Edition”.103
Torqeedo, the market-leading supplier of electric outboard and inboard drives for leisure boats and small fishing boats, recently surpassed 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 electric boat drive sales. It is a milestone that marks steady progress for the industry.104 What is remarkable about this achievement is that it has largely been met with little to no financial incentives or regulation on outboard or inland vessel emissions, which are both key drivers for other electric vehicle segments (the limited emissions regulation that does exist is highly local: a handful of lakes in Germany and Amsterdam's central canals). As a result, growth has been steady but also slow, and it is not taking off in the same way as other electric vehicle markets like passenger cars (or even categories within the marine sector, such as offshore support vessels). Part of the apathy towards restricting boating emissions is down to prioritizing the largest problems first. While emissions per outboard can be over 30 times the emissions per car, the scale of the problem is still much smaller: no more than 500
000 electric boat drive sales. It is a milestone that marks steady progress for the industry.104 What is remarkable about this achievement is that it has largely been met with little to no financial incentives or regulation on outboard or inland vessel emissions, which are both key drivers for other electric vehicle segments (the limited emissions regulation that does exist is highly local: a handful of lakes in Germany and Amsterdam's central canals). As a result, growth has been steady but also slow, and it is not taking off in the same way as other electric vehicle markets like passenger cars (or even categories within the marine sector, such as offshore support vessels). Part of the apathy towards restricting boating emissions is down to prioritizing the largest problems first. While emissions per outboard can be over 30 times the emissions per car, the scale of the problem is still much smaller: no more than 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 outboards are sold yearly as compared with roughly 80 million cars (OICA). Still, allowing boats a free pass on emissions undermines the massive effort from other industries, and will ultimately lead to a more difficult transition down the line.103
000 outboards are sold yearly as compared with roughly 80 million cars (OICA). Still, allowing boats a free pass on emissions undermines the massive effort from other industries, and will ultimately lead to a more difficult transition down the line.103
4.5 Eco-marine power system
Some additional renewable energy power source vehicles are discussed in this section targeted to green technology systems for marine environments.12An interesting example is the Auriga Leader, a car carrier ship for Toyota, back in 2009. The vessel is about 200 m long, weighs around 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons, and carries up to 6200 cars, transporting them from Toyota Motor Corporation factories in Japan to the Port of Long Beach. On the Auriga Leader, 328 solar panels were installed on its top, providing a maximum power output of 40 kW.44 This was the first time that a carrier ship used solar energy to cover part of its electricity needs, substituting the auxiliary diesel engines. On that occasion, the panel's installation not only make the Auriga Leader greener by reducing the pollutants freed to the atmosphere, it also made it more economical and efficient by reducing the vessel's diesel consumption.105
000 tons, and carries up to 6200 cars, transporting them from Toyota Motor Corporation factories in Japan to the Port of Long Beach. On the Auriga Leader, 328 solar panels were installed on its top, providing a maximum power output of 40 kW.44 This was the first time that a carrier ship used solar energy to cover part of its electricity needs, substituting the auxiliary diesel engines. On that occasion, the panel's installation not only make the Auriga Leader greener by reducing the pollutants freed to the atmosphere, it also made it more economical and efficient by reducing the vessel's diesel consumption.105
5. Future perspectives of marine energy storage systems
Research on sustainability in the maritime industry is a relatively new and growing interdisciplinary research field. The technologies in the focus of this analysis can be implemented as total BE, or hydrogen solutions, or as hybrid solutions where they are combined, or with conventional engines. Fully electric ships depend on charging infrastructure in harbors, which requires access to the electricity grid.107 Currently, hydrogen is mainly produced from natural gas; however, there is potential for the extensive Norwegian production of fossil-free hydrogen through the electrolysis of water (using renewable energy). Hydrogen propulsion solutions include fuel cells that convert hydrogen fuel into electricity. Since many technologies can fulfil similar needs (Sandén and Hillman, 2011), such as energy, the various BE and hydrogen solutions can be either complementary to each other, or competitors. Different versions of BE and hydrogen solutions have particular advantages and disadvantages regarding emission reductions, fuel, investment costs, and so forth (Table 4).| Electric (full) | Electric hybrid | Hydrogenb | |
|---|---|---|---|
| a The environmental benefits of electric power (battery) and hydrogen depends on the source of electricity used. b Hydrogen here refers to hydrogen produced via electrolysis from renewable energy. | |||
| Reduction of NOxa | Very high | Moderate | Very high |
| Reduction of GHGa | Very high | Moderate-High | Very high |
| Reduction of SOxa | Very high | Moderate | Very high |
| Investment cost (on vessels) | High | Moderate-High | High |
| Fuel cost | Low | Moderate | High |
| Vessel adaptation | Very high | Low-Moderate | High |
| Availability (incl. infrastructure) | Moderate | Moderate | Low |
| Infrastructure adaptation (incl. fuel production/energy conversion) | Moderate-high | Low-high | Very high |
| Importance of regularity | High | Low-high | Low |
| Market segment suitability | Vessels – short routes (e.g. ferries) | All – esp. variable energy demand | All |
5.1 Exploring aluminum battery technology for marine applications
According to the report of The Maritime Executive, Yara Marine Technologies, which currently specializes in complete emission control systems for the maritime industry, announced the selection of new battery technology as the first company for its accelerator program. The maritime industry has shown strong interest in the potential of batteries as one element to achieve the goal of reducing emissions. After initial discussions, Yara believes the new battery technology being pursued by the start-up company holds strong potential due to its ability to generate power, unique properties that make it rechargeable with minimal lost time, and its space efficiency.The start-up company Phoenician Energy is seeking to adapt aluminium–air battery technology for marine applications. The company licenses the developing battery technology and from that has developed a 4.8 MW h system enclosed in a 20-foot shipping container. “Phoenician Energy's use of aluminium–air battery technology for the maritime industry triggered our scientists’ curiosity,” says Thomas Koniordos, CEO of Yara Marine Technologies. “Their container battery is especially interesting. The concept taps into several recent trends and developments, such as circular economy and the electrification of marine vessels. We believe this technology may have an important role to play in a greener maritime industry for future generations.”
Jesper, Head of Research and Development at Yara Marine, explains how the system works and what makes it attractive for further development. The charging of an aluminium–air battery happens at an aluminum production plant, he explains, and then the battery in effect consumes aluminum, which is the most abundant metal in the earth's crust. As the aluminum depletes, it generates alumina, which is also a valued commodity. The alumina can be recycled back to aluminum at the aluminum plant, but it also has uses in other industries.
“Al–air batteries have one of the highest energy densities of all batteries, with more than four times the capacity of the conventional lithium-ion battery.51 Higher density means that longer ranges can be achieved, and a smaller footprint means more space for revenue-producing cargo,” says Udi Erell, Founder and President of Phoenician Energy. “In addition, they do not recharge their system on board, instead, they replace it with a fully charged one, eliminating downtime that would be required for recharging conventional batteries. The consumed battery is then re-equipped with new aluminum. Furthermore, Aluminum-air batteries do not lose capacity when not in use, nor do they degrade over time. They are also inherently safe, with no danger of temperature runaway”.108
Together with Yara Marine Technologies, Phoenician Energy will move into its next phase. The company expects to access Yara's experience with engineering and procurement as well as with production and installation. The accelerator is a six-month program, after which Yara Marine Technologies may choose to invest in the company and its technology. Launched in 2020, the program's goal is to identify promising new green technologies for the maritime industry and assist those startup companies.109
5.2 Global marine battery industry outlook to 2025
The “Marine Battery Market by Battery Type (lithium, fuel cell, lead-acid), Propulsion Type (fully electric, hybrid, conventional), Application, Sales Channel, Ship Range, Nominal Capacity, Battery Design, Battery Function, and Region – Global Forecast to 2025” report has been added to the offering of ResearchAndMarkets.com. The global marine battery market is projected to grow from USD 250 million in 2020 to USD 812 million by 2025, at a CAGR of 48.1% between the 2020 and 2025 period. The increase in seaborne trade across the globe and the development of lithium batteries are anticipated to drive the growth of the marine battery market. However, the limited range and capacity of fully electric ships are limiting the overall growth of the market.110Based on the sales channel, the aftermarket segment is anticipated to grow at a higher CAGR than the OEM segment during the forecast period. Ship owners are updating or retrofitting the equipment installed on their existing vessels to increase efficiency and durability. Ships can be retrofitted with batteries and electric motors to reduce the load demand on engines for propulsion. A growing number of offshore vessel owners/operators are upgrading their diesel-electric propulsion systems to hybrid configurations to provide greater operational flexibility and minimize fuel consumption across their fleets.
Based on propulsion type, the fully electric segment is projected to witness the highest CAGR during the forecast period. Fully electric-driven ships have a positive effect on the environment since the inclusion of high energy storage in batteries and optimized power control can reduce fuel consumption, maintenance, and emissions. The growth of the fully electric segment can be attributed to the increasing demand for fully electric small and medium passenger and cargo ships. Several manufacturers are jointly investing in the manufacturing of ships adhering to the IMO 2020 rule.50
6. Conclusion
This paper reviews different hybrid combinations of energy storage systems for marine environments, which are applied in the literature. It was observed that among the batteries, the Li-ion is at present the most used battery for sea vehicle applications on-water, as well as under-water because of its higher energy density, a long service life, and it requires less maintenance. Solid-state batteries are also under research for marine applications. According to the research study, It may offer up to 75% better specific energy of the best lithium-ion batteries and the safety impact might be even greater with the fire risk and the cooling requirement. We are moving towards green and clean environment-friendly methodologies for energy generation, so eco-power systems are also a considerable approach to reducing the environmental pollution in marine environments using solar/wind energy. A great advancement in the marine battery technology is considered through the development of an open water power battery (OWP) that drinks in sea water to operate, which is safer and cheaper, and provides a tenfold increase in range as compared to the traditional lithium-ion batteries used for unpiloted underwater vehicles. Future research is targeted at developing the Al-battery technology for marine applications because it can generate four times the energy capacity as compared to the conventional lithium-ion battery. Continuous research and development are underway by various marine industries and the global marine battery market is projected to grow USD 812 million by 2025, at a CAGR of 48.1% up to the year 2025.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the CART, IIT Delhi.References
- T. J. McCoy, IEEE Electron. Mag., 2015, 3, 4–11 Search PubMed.
- B. Zahedi, L. E. Norum and K. B. Ludvigsen, J. Power Sources, 2014, 255, 341–354 CrossRef CAS.
- N. Doerry, IEEE Electron. Mag., 2015, 3, 12–21 Search PubMed.
- N. Doerry, J. Amy and C. Krolick, Proc. IEEE, 2015, 103, 2243–2251 Search PubMed.
- K. Lim, International Maritime Organization (IMO), Third IMO Greenhouse Gas Study, International Maritime Organization (IMO), London, UK, 2014 Search PubMed.
- International Maritime Organization: Prevention of Air Pollution from Ships, 2018, available online: http://www.imo.org/en/OurWork/Environment/PollutionPrevention/AirPollution/Pages/AirPollution.aspx.
- EU Commission, The Paris Protocol–A Blueprint for Tackling Global Climate Change Beyond 2020, 2018, available online: https://www.eesc.europa.eu/sites/default/files/resources/docs/15_362-ppaper_changementclim_en.pdf.
- D. Cericola and R. Kötz, Electrochim. Acta, 2012, 72, 1–17 CrossRef CAS.
- G. Lloyd, DNV, Shipping Industry Eyeing Hydrogen Fuel Cells as Possible Pathway to Emissions Reduction, 2018, available online: http://www.greencarcongress.com/2012/09/h2shipping-20120907.html.
- P. K. Shen, A. C. C. Tseung and C. Kuo, J. Power Sources, 1994, 47, 119–127 CrossRef CAS.
- Energy storage for maritime industry, 2020, available online: https://www.theexplorer.no/solutions/energy-storage-for-the-maritime-industry.
- A. G. Koumentakos, Appl. Syst. Innov., 2019, 2, 1–21 CrossRef.
- C. S. Chin, J. Jia, J. H. K. Chiew, W. D. Toh, Z. Gao, C. Zhang and C. McCann, J. Energy Storage, 2019, 21, 724–740 CrossRef.
- DeepSea Power & Light. SeaBattery Power Module, 2019, available online: https://seatronics-group.com/files/6114/1811/8353/DeepSea_Power__Light_SeaBattery_-_Datasheet.pdf.
- Furukawa Battery, Valve Regulated Type Stationary Lead-Acid Batteries, 2019, available online: https://www.furukawadenchi.co.jp/english/products/indust/ub.htm.
- General Dynamics Mission Systems Inc. Bluefin 1.5 kWh Subsea Battery, 2019, available online: https://gdmissionsystems.com/en/products/underwater-vehicles/bluefin-robotics/1--5-kwh-subseabattery.
- PlanB E Storage Ltd. PBES Specification Sheet, Available online: http://www.pbes.com/wp-content/uploads/2018/07/PBES-Overview-NMC-LTO_2018--07--11.pdf, 2019.
- Y. Wang, S. Song, C. Xu, N. Hu, J. Molenda and L. Lu, Nano Mater. Sci., 2019, 1, 91–100 CrossRef.
- SWE Advanced Battery Solutions-SWE SeaSafe Subsea Battery Modules, 2019, available online: https://www.swe.com/seasafe-subsea-modules.
- Lithium-ion Batteries and their Future in Underwater Applications, 2020, available online: https://www.azom.com/article.aspx?articleid=19664.
- A. R. Dehghani-Sanij, E. Tharumalingam, M. B. Dusseault and R. Fraser, Energy Rev., 2019, 104, 192–208 Search PubMed.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- Ø. Hasvold and N. Størkersen, J. Power Sources, 2001, 96, 252–258 CrossRef.
- Ø. Hasvold, International Power Sources Symposium, Leatherhead, UK, vol. 14, 1993, p. 243 Search PubMed.
- N. Størkersen, K. Kristensen, A. Indereide, J. Seim and T. Glancey, Sea Technol., 1998, 39, 99 Search PubMed.
- P. E. Hagen, Dual use development: the HUGIN untethered underwater vehicle, in Proceedings of the SMi conference on Naval Mines, London, 2000 Search PubMed.
- A. Zolla, J. Westenberge and D. Noll, Proceeedings of the 39th Power Sources Conference, Cherry Hill, USA, 1999, pp. 64–68 Search PubMed.
- P. H. Smith, et al. , Power Sources, 1993, 14, 257–265 CAS.
- https://www.southampton.ac.uk/˜assets/doc/the%20future%20of%20batteries%20in%20the%20marine%20sector.pdf .
- S. Solem, J. Mar. Sci. Technol., 2015, 20, 406–416 CrossRef.
- M. Soleymani, A. Yoosofi and M. Kandi-D, J. Mar. Sci. Technol, 2015, 4, 739–751 CrossRef.
- B. Craig, et al. , Renewable Sustainable Energy Rev., 2020, 133, 1–18 CrossRef.
- D. Stampatori, P. P. Raimondi and M. Noussan, Energies, 2020, 13, 2638 CrossRef CAS.
- J. F. Peters, et al. , Renewable Sustainable Energy Rev., 2017, 67, 491–506 CrossRef CAS.
- D. Lin, Y. Liu and Y. Cui, Nat. Nanotechnol., 2017, 12, 194–206 CrossRef CAS PubMed.
- A. Manthiram, J. Phys. Chem. Lett., 2011, 2, 176–184 CrossRef CAS.
- A. R. Dehghani-Sanij, et al. , Renewable Sustainable Energy Rev., 2019, 104, 192–208 CrossRef.
- D. P. Finegan, et al. , Nat. Commun., 2015, 6, 6924 CrossRef CAS PubMed.
- M. Rana, J. Kim and L. Peng, et al. , Nanoscale, 2021, 25, 11086–11092 RSC.
- T. Nemeth, et al. , J. Energy Storage, 2020, 31, 101656 CrossRef.
- S. Choudhury, Nat. Commun., 2019, 10, 4398 CrossRef PubMed.
- M. Rana, J. Kim, L. Peng and H. Lim, et al. , ACS Appl. Energy Mater., 2020, 3, 5523–5532 CrossRef CAS.
- Y. Kim, T. Park, N. Jongbeom, J. W. Yi and J. Kim, et al. , Nanoscale, 2020, 12, 8608–8625 RSC.
- H. Peng, S. Wang, M. Kim and J. Kim, et al. , Energy Storage Mater., 2020, 25, 313–323 CrossRef.
- P. Mei, J. Lee, M. Pramanik, A. Alshehri and J. Kim, et al. , ACS Appl. Mater. Interfaces, 2018, 10, 19739–19745 CrossRef CAS PubMed.
- GlobalData, Lithium-ion batteries are potential game-changer in underwater applications, 2020, available online: https://www.globaldata.com/lithium-ion-batteries-potential-game-changer-underwater-applications.
- Y. Luo, Y. Tang, S. Zheng, Y. Yan, H. Xue and H. Pang, J. Mater. Chem. A, 2018, 6, 4236–4259 RSC.
- M. A. Hannan, M. M. Hoque, A. Hussain, Y. Yusof and P. J. Ker, IEEE Access, 2018, 6, 19362–19378 Search PubMed.
- M. S. Islam and C. A. Fisher, Chem. Soc. Rev., 2014, 43, 185–204 RSC.
- Y. Wang, P. He and H. Zhou, Adv. Energy Mater., 2012, 2, 770–779 CrossRef CAS.
- W. Xu, J. Wang, F. Ding, X. Chen, E. Nasybulin, Y. Zhang and J. G. Zhang, Energy Environ. Sci., 2014, 7, 513–537 RSC.
- L. A. Selis and J. M. Seminario, RSC Adv., 2018, 8, 5255–5267 RSC.
- N. Nitta, F. Wu, J. T. Lee and G. Yushin, Mater. Today, 2015, 18, 252–264 CrossRef CAS.
- Z. Yan, Q. Hu, G. Yan, H. Li, K. Shih, Z. Yang, X. Li, Z. Wang and J. Wang, Chem. Eng. J., 2017, 321, 495–501 CrossRef CAS.
- H. Zheng, Q. Qu, L. Zhang, G. Liu and V. S. Battaglia, RSC Adv., 2012, 2, 4904–4912 RSC.
- K. Wang, Y. Jin, S. Sun, Y. Huang, J. Peng, J. Luo, Q. Zhang, Y. Qiu, C. Fang and J. Han, ACS Omega, 2017, 2, 1687–1695 CrossRef CAS PubMed.
- C. Ma, Y. Zhao, J. Li, Y. Song, J. Shi, Q. Guo and L. Liu, Carbon, 2013, 64, 553–556 CrossRef CAS.
- S. Chauque, F. Oliva, A. Visintin, D. Barraco, E. Leiva and O. Cámara, J. Electroanal. Chem., 2017, 799, 142–155 CrossRef CAS.
- O. Crosnier, T. Brousse and D. Schleich, Ionics, 1999, 5, 311–315 CrossRef CAS.
- A. Casimir, H. Zhang, O. Ogoke, J. C. Amine, J. Lu and G. Wu, Nano Energy, 2016, 27, 359–376 CrossRef CAS.
- Y. Yang, D. Shu, H. Yu, X. Xia and Z. Lin, J. Power Sources, 1997, 65, 227–230 CrossRef CAS.
- J. Shu, M. Shui, F. Huang, Y. Ren, Q. Wang, D. Xu and L. Hou, J. Phys. Chem. C, 2010, 114, 3323–3328 CrossRef CAS.
- D. T. Tran, H. Dong, S. D. Walck and S. S. Zhang, RSC Adv., 2015, 5, 87847–87854 RSC.
- Y. Yang, L. Li, H. Fei, Z. Peng, G. Ruan and J. M. Tour, ACS Appl. Mater. Interfaces, 2014, 6, 9590–9594 CrossRef CAS PubMed.
- Y. Kim, ACS Appl. Mater. Interfaces, 2012, 4, 2329–2333 CrossRef CAS PubMed.
- L. H. Hu, F. Y. Wu, C. T. Lin, A. N. Khlobystov and L. J. Li, Nat. Commun., 2013, 4, 1687 CrossRef PubMed.
- C. T. Hsieh, C. T. Pai, Y. F. Chen, I. L. Chen and W. Y. Chen, J. Taiwan Inst. Chem. Eng., 2014, 45, 1501–1508 CrossRef CAS.
- P. Sengodu and A. D. Deshmukh, RSC Adv., 2015, 5, 42109–42130 RSC.
- T. M. Higgins, S. H. Park, P. J. King, C. Zhang, N. McEvoy, N. C. Berner, D. Daly, A. Shmeliov, U. Khan and G. Duesberg, ACS Nano, 2016, 10, 3702–3713 CrossRef CAS PubMed.
- S. Zhang, T. Jow, K. Amine and G. Henriksen, J. Power Sources, 2002, 107, 18–23 CrossRef CAS.
- G. G. Botte, R. E. White and Z. Zhang, J. Power Sources, 2001, 97, 570–575 CrossRef.
- S. I. Tobishima, M. Arakawa and J. I. Yamaki, Electrochim. Acta, 1988, 33, 239–244 CrossRef CAS.
- I. Gunawan and B. Sugeng, Materials Science and Engineering, IOP Publishing, 2017, p. 012039 Search PubMed.
- Q. Li, J. Chen, L. Fan, X. Kong and Y. Lu, Green Energy Environ., 2016, 1, 18–42 CrossRef.
- G. A. Elia, J. B. Park, Y. K. Sun, B. Scrosati and J. Hassoun, ChemElectroChem, 2014, 1, 47–50 CrossRef.
- D. Zhang, S. Wang, Y. Ma and S. Yang, Two-dimensional nanosheets as building blocks to construct three-dimensional structures for lithium storage, J. Energy Chem., 2017, 27, 128–145 CrossRef.
- A. Mishra, A. Mehta and S. Basu, et al. , Mater. Sci. Energy Technol., 2018, 1, 182–187 Search PubMed.
- T. Horiba, Proc. IEEE, 2014, 102, 939–950 CAS.
- J. M. Tarascon, Electrochem. Soc. Interface, 2016, 25, 79–83 CrossRef CAS.
- M. A. Hannan, M. M. Hoque and A. Hussain, et al. , IEEE Access, 2018, 6, 19362–19378 Search PubMed.
- Y. Miao and P. Hynan, et al. , Energies, 2019, 12, 1–20 Search PubMed.
- https://www.saftbatteries.com/market-sectors/transportation/marine .
- NavalTechnology, Lithium-ion Batteries for Under Water Use: Technology Trends, 2020, NavalTechnology, available online: https://www.naval-technology.com/comment/lithium-ion-battery-technology.
- C. Woodford, Lithium-ion batteries, 2020, ExplainThatStuff, available online: https://www.explainthatstuff.com/how-lithium-ion-batteries-work.html.
- EDROnline, Marine Systems presents new modular underwater vehicle: MUM project to set new standard for unmanned underwater operations, 2020, available online: https://www.edrmagazine.eu/thyssenkrupp-marine-systems-presents-new-modular-underwater-vehicle-mum-project-to-set-new-standard-for-unmanned-underwater-operations.
- Q. Cheng, Y. Okamoto and N. Tamura, et al. , Sci. Rep., 2017, 7, 1–18 CrossRef PubMed.
- Global Marine Battery Market Report, by Type, 2021, available online: https://www.marketresearchfuture.com/reports/marine-battery-market-9530.
- The next generation of lithium batteries for marine autonomous systems, 2017, available online: https://www.solidstateplc.com/wpcontent/uploads/Next_Generation_of_Lithium_Batteries_Marine_Applications-Lithium_Sulfur_07_2017.pdf.
- Marine battery, 2014, available online: https://www.upsbatterycenter.com/blog/important-points-know-marine-battery.
- MIT News – Batteries that “drink” seawater could power long-range underwater vehicles, 2017, available online: https://news.mit.edu/2017/batteries-drink-seawater-long-range-autonomous-underwater-vehicles-0615.
- Electric ships – a new target for Li-ion battery producers, 2020, available online: https://www.mining.com/electric-ships-a-new-target-for-li-ion-battery-producers.
- Battery and hybrid ships, 2020, available online: https://www.shipinsight.com/articles/battery-and-hybrid-ships-how-do-they-work-and-how-many-are-in-operation.
- Marine hybrid battery applications, 2020, available online: https://www.deif.com/marine-offshore/applications/battery-for-marine-hybrid.
- J. G. Bellingham, C. A. Goudey, T. R. Consi, J. W. Bales, O. K. Atwood, J. J. Leonard and C. Chryssostomidis, Proceedings of the 1994 Symposium on Autonomous Underwater Vehicle Technology, 1994, vol. 168, pp. 148–155 Search PubMed.
- P. H. Smith, S. O. James and P. B. Keller, IEEE Symp. AUV Technol., 1996, 7, 441–447 Search PubMed.
- G. Griffiths, P. Stevenson, A. T. Webb, N. W. Millard, S. O. McPhail, M. Pebody and J. R. Perrett, Proceedings 11th Unmanned Untethered Submersible Technology, 1999, vol. 25, pp. 1–12 Search PubMed.
- S. M. Smith and S. E. Ounn, Proceedings of the 1994 Symposium on Autonomous Underwater Vehicle Technology, 1994, vol. 26, pp. 139–147 Search PubMed.
- J. Cockburn, Power Sources 14: The 18th International Power Sources Symposium, Stratford-upon-Avon, 1993, vol. 14, pp. 327–353 Search PubMed.
- A. M. Tonge and J. Cockburn, Sea Technol., 1993, 34, 39–43 Search PubMed.
- M. Abu Sharkh and G. Griffiths, J. Soc. Underwater Technol., 2003, 25, 143–148 CrossRef.
- Battery System for a Luxury Electric Boat, 2020, available online: https://www.electricvehiclesresearch.com/articles/22493/battery-system-for-a-luxury-electric-boat.
- The Challenges Ahead for Electric Leisure Boating, 2020, available online: https://www.idtechex.com/de/research-article/the-challenges-ahead-for-electric-leisure-boating/20589.
- Afloat.ie Team. Afloat, 2019, available online: https://afloat.ie/port-news/ferry-news/item/41662-electric-ferrytakes-to-the-water-with-volvo-penta-in-sweden.
- G. Atkinson, J. Binns and H. Nguyen, Cogent Eng., 2018, 5, 15435–15464 Search PubMed.
- H. Hoffmeister, ECO FLETTNER Rotor Sail Stands the Test, DNV GL, Piraeus, Greece, 2018, https://www.dnv.com/expert-story/maritime-impact/ECO-FLETTNER-rotor-sail-stands-the-test.html.
- H. Bach, A. Bergek and O. Bjørgum, et al. , Transp. Res. Part D: Transp. Environ., 2020, 87, 1–22 CrossRef.
- Electrical Installations – Guidance for Safe Design, Installation and Operation of Lithium-ion Batteries, in Marine Guidance Note, 2016, Maritime and Coastguard Agency, Southampton, UK.
- Exploring Aluminum Battery Technology for Marine Applications, 2021, available online https://www.maritime-executive.com/article/exploring-aluminum-battery-technology-for-marine-applications.
- Global Marine Battery Industry Outlook, 2020, available online: https://www.globenewswire.com/news-release/2020/07/22/2066021/0/en/Global-Marine-Battery-Industry-Outlook-2020-2025-Increase-in-Seaborne-Trade-Across-the-Globe-and-Development-of-Lithium-Batteries-are-Driving-Market-Growth.html.
- Aluminum–air battery innovator, 2021, https://www.freightwaves.com/news/aluminum-air-battery-innovator-wins-spot-in-yara-marine-x-program.
| This journal is © The Royal Society of Chemistry 2021 |