 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A comprehensive review on the environmental applications of graphene–carbon nanotube hybrids: recent progress, challenges and prospects
Jomol P.
John
 a,
Mary Nancy
T. E.
a,
Mary Nancy
T. E.
 b and
Bindu Sharmila
T. K.
b and
Bindu Sharmila
T. K.
 *c
*c
aDepartment of Chemistry, Maharajas College Ernakulam, 682011, Kerala, India. E-mail: jomoljohn2001@gmail.com
bDepartment of Chemistry, Fatima Mata National College, Kollam, 691001, Kerala, India. E-mail: marynancy@fmnc.ac.in
cDepartment of Chemistry, Maharajas College Ernakulam, 682011, Kerala, India. E-mail: drbindusharmilatk@maharajas.ac.in; Tel: 9446892578
First published on 7th September 2021
Abstract
Environmental pollution by water-soluble pollutants, heavy metal ions and harmful greenhouse gases is triggering significant concern worldwide and is affecting the stability of the environment. Hence, it is indispensable to develop novel materials to mitigate environmental pollution. Graphene/carbon nanotube hybrid materials exhibit exceptional potential for environmental applications including sensing and monitoring of contaminants and their remediation. These 3D network materials possess a larger surface area, enhanced electrical conductivity, thermal conductivity, porosity, minimal agglomeration and higher mechanical strength compared to their building blocks, i.e., 1D carbon nanotubes (CNTs) and 2D graphene. Moreover, the porosity and extremely interconnected structures of these hybrid materials yield an accessible interior surface area, efficient mass transport, etc. These outstanding properties combined with the hydrophobicity, stability and conductivity of these materials provide a general platform for the detection and disposal of various pollutants. This review presents the promising environmental applications of 3D graphene/CNT hybrid materials with special focus on the synergistic effects arising from the combination of graphene and CNT. Most of the relevant literature related to the removal of oils and organic solvents, adsorption of dyes, removal of heavy metal ions, gas sensors and the catalytic conversion of pollutants is reviewed to shed light on the current challenges and upcoming opportunities.
1. Introduction
Graphene, a two-dimensional (2D) single layer of sp2-hybridized carbon atoms, has attracted remarkable attention due to its unique combination of high surface area, mechanical properties and electrical conductivity.1–4 However, to minimize the surface free energy, aggregation of the graphene sheets and restacking occur due to their strong van der Waals interactions, thereby greatly hindering the practical applications of graphene.1 Thus, to overcome this drawback, the surface of the graphene layers must be altered through covalent functionalization, non-covalent stabilization or dispersal in specific solvents.5The high aspect ratio of carbon nanotubes (CNTs) together with their remarkable electrical conductivity, mechanical properties, and most importantly, their similar carbon structure enable them to function as an effective spacer between graphene sheets to create 3D nanostructures having enriched functional properties.6–8Fig. 1(a–c) presents images of a 1D CNT, 2D graphene, and 3D graphene–CNT hybrid.
 | ||
| Fig. 1 (a) CNT (1D). (b) Graphene (2D). (c) Graphene–CNT hybrid (3D). Reproduced from ref. 7 with permission from the American Chemical Society. | ||
Remarkable research has been conducted in recent years to combine the extraordinary properties of carbon nanotubes and graphene and integrate them to form 3D hybrid systems.9–12 The integration of CNTs and graphene prevents the re-stacking of graphene sheets and helps to achieve the practical utilization of the synergistic effect between CNTs and graphene. Moreover, CNTs can improve electron transfer by bridging the defects and increasing the layer gap among graphene sheets.
Graphene/CNT hybrid materials possess larger surface area, enhanced electrical conductivity, thermal conductivity, porosity, minimal agglomeration and higher mechanical strength than their building blocks. Moreover, the porosity and extremely interconnected structures of these hybrid materials yield an accessible interior surface area, efficient mass transport, etc.13 Besides being superhydrophobic, they exhibit superoleophilicity, which enables their use as potential materials to remedy oil spills and pollution problems.14 These 3D hybrid materials exhibit excellent electrical properties, which make them suitable for application as electrochemical sensors. The outstanding electrical conductivity of these materials coupled with surface functionalization by various nanoparticles makes them suitable for catalytic applications. Moreover, they possess remarkable mechanical stability, flexibility and strength, which are desirable for various environmental applications.
3D graphene/carbon nanotube networks exhibit exceptional potential in the efficient removal of harmful pollutants from contaminated air and water owing to their following peculiar properties. Firstly, the 3D hybrid structures based on graphene and CNT combine micro-, meso- and macro-pores, where the micro- and mesoporosity provide a high surface, area while their macroporosity guaranties accessibility to this surface, enabling the diffusion and transfer of contaminant molecules and ions.11 Secondly, these 3D hybrids are highly stable, and thus can be applied in various environments including severe acidic conditions15 and a wide temperature range.16 Thirdly, their integrated morphology offers effective contaminant separation and recycling and minimizes possible environmental risks triggered by the accidental release of graphene and CNT.17
Depending on their architecture, graphene/CNT hybrids can be categorised into (i) hybrids of graphene with vertical CNTs (G/vCNT), (ii) hybrids of graphene with horizontal CNTs (G/hCNT) and (iii) CNTs wrapped with graphene (G/wCNT).10,18–21 There are two fundamentally different approaches for the synthesis of these hybrids, which are the assembly method and in situ method.
The assembly method employs various methods such as layer-by-layer assembly, sol–gel synthesis, vacuum filtration, solution processing, and electrophoretic deposition.10,22,23 Because of their simplicity and scalability, these methods are extensively employed to assemble G/hCNT and G/wCNT hybrids through non-covalent interactions such as electrostatic, π–π stacking, van der Waals and covalent interactions between the functional groups of graphene and CNTs. However, the assembly processes has some significant limitations such as multi-step processing as well as poor control of the orientation, density and morphology of the hybrid structures.
The in situ growth of graphene/CNT hybrids can result in better control of the nanoscale architecture than assembly-based methods by adjusting the fabrication conditions. In situ approaches (chemical unzipping, chemical vapor deposition, etc.) guarantee a uniform distribution of carbon allotropes and involve less processing steps.6,24,25 Covalently bonded seamless hybrid structures of G/hCNT, G/vCNT and G/wCNT are produced by these methods.25–27 However, for industrial scale production, the use of toxic chemicals (Ni (CO)4, B2H6, etc.), explosives and high processing temperature limit their practical application, and hence requires further improvements.
Although the synthesis, properties and applications of 3D graphene/CNT hybrids have been reviewed earlier,11–13,28,29 the numerous studies on the applications of these materials in recent years make it necessary to recap the advances in this area. Considering that studies on the environmental applications of 3D graphene/CNT hybrids are crucial for our survival, the present review aims to present promising environmental applications related to the removal of oils and organic solvents, adsorption of dyes, removal of heavy metal ions, gas sensing and the catalytic conversion of pollutants to shed light on the current challenges and upcoming opportunities.
2. Removal of oils and organic solvents
Accidental spills of catastrophic oils and chemical reagents during extraction, storage and transportation cause adverse impacts on the marine and land ecosystems and impose a series of issues related to environmental pollution.30–32 Moreover, oil spills lead to loss of energy resources.33 Hence, it is inevitable to develop efficient remediation methods. To address this severe issue, various techniques including membrane filtration, biological separation, photo decomposition, use of chemical dispersants and skimmers, combustion, wet oxidation and adsorption have been recommended.34–36 Among them, adsorption is considered the most efficient, economical and promising solution due to its low cost, easy operation, simple process and little damage to the environment.31,37 However, although several sorbent materials such as fibres,38 cross-linked polymers and resins,39,40 polymer gels,41,42 organic–inorganic hybrids,43 silica,44 carbon-based materials45 and nanocomposites46 have been established, these materials exhibit certain drawbacks. For example, microporous polymers exhibit high adsorption capacity but their production cost is high. Moreover, even though expanded graphite can efficiently remove crude oil and petroleum products, it cannot be applied for several polar solvents such as toluene and dimethyl sulfoxide. Hence, the development of innovative adsorption materials for the efficient separation of organic solvents and oils from water is of increasing significance.Generally, an efficient oil adsorbent should be highly oil–water selective, i.e., possess high adsorption capacity for oil and strong hydrophobicity, eco-friendly and reusable.47 Extensive research has been done on the development of innovative porous adsorbents having superhydrophobicity and superoleophilicity. In this regard, allotropes of carbon such as CNTs and graphene have emerged as potential materials to deal with oil spill and pollution problems by exploiting their hydrophobic interfacial properties.48–51
Various sorbents remove or retrieve contaminants either by absorption or adsorption, or both. Absorption occurs via the intermolecular penetration of oil into the pores of the sorbent, and hence depends on the porosity of sorbents together with the viscosity and adhesion properties of oil. During adsorption, oil is accumulated or retained on the surface.52 In contrast, the driving force leading to adsorption is the attraction between oil and the outer surface of the sorbents, which is controlled by various physical and chemical interactions such as hydrogen bonding, van der Waals forces, steric interactions, hydrophobicity, and polarity.53
CNT sponges made up of self-assembled interconnected CNT networks with an expected porosity of >99% were synthesised by Gui et al., which could be utilised more than thousand times without substantial changes in their structure, absorption capacity and hydrophobicity. The sponges had excellent structural flexibility (Fig. 2a) and huge strain deformation with nearly full volume recovery. They exhibited a contact angle of about 156° for water droplets, and owing to their low density, could float on water.50 For various oils and solvents, they exhibited high absorption capacities ranging from 80 to 180 times their weight. Vegetable oil absorption by a CNT sponge in a water–oil mixture is presented in Fig. 2b. The high absorption capability and rapid absorption were because of the physisorption of molecules and ability to store them in sponge pores. By utilizing mechanical compression, the absorbed oil or solvent molecules could be recovered.
 | ||
| Fig. 2 (a) CNT sponge bent at a large angle. (b) Vegetable oil absorption by CNT sponge. Reproduced from ref. 50 with permission from John Wiley and Sons. | ||
Graphene–based macrostructures also show excellent applications for the removal of organic solvents and oil because of their vast surface area, ultra-lightness, abundant porosity, hydrophobic nature, ability of surface functionalization and high compressibility.54,55 Moreover, they are more cost effective than CNTs. Aromatic solvents generally exhibit greater affinity for these sorbents compared to aliphatic solvents because of their strong stacking interactions.56
To prepare graphene sheets from graphite, one of the frequently used routes is via graphite oxide. The underlying basic strategy is to completely exfoliate graphite oxide into individual sheets (graphene oxide), followed by their reduction to produce ‘graphene-like sheets’.57,58 Graphene oxide (GO), having a high density of functional groups such as hydroxyl and epoxide groups on its basal planes, in addition to carboxyl and carbonyl groups located presumably at the edges, is considered a potential precursor of graphene.59–64 Although graphene in its oxidized form is nonconductive and sensitive to high temperature, reduced graphene oxide (rGO) can restore these properties.65,66 However, it is difficult to fully restore the structure and properties to that of pristine graphene.
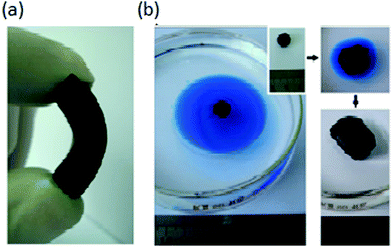
Bi et al. prepared spongy graphene via the reduction of graphene oxide platelets in a suspension followed by shaping via moulding. The macroporous and shape-mouldable material having a density of 12 ± 5 mg cm−3 with a specific surface area of ∼432 m2 g−1 could remove various petroleum products and toxic organic solvents at 20 to 86 times its weight (chloroform: 86.1, lubricating oil: 68.5 and toluene: 54.8).48 For dodecane absorption, it exhibited an absorption rate of 0.57 g g−1 s−1. Interestingly, it could be regenerated more than 10 times through distillation, with a negligible reduction in its oil or organic sorption capacity, and hence can be employed as a competent recyclable sorbent material (Fig. 3).
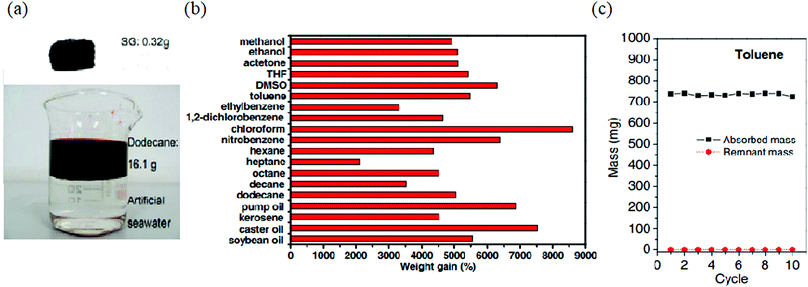 | ||
| Fig. 3 (a) 0.32 g spongy graphene can remove 16.1 g of dodecane. (b) Sorption efficiency for various oils and organic solvents. (c) Recyclability for 10 cycles. Reproduced from ref. 48 with permission from John Wiley and Sons. | ||
In an inspiring work, Zhao et al. synthesised a nitrogen-doped 3D graphene framework, which could adsorb organic solvents and oils up to 200–600 times its own weight, via the hydrothermal reaction of graphene oxide using pyrrole.67 The rich open porous structures offered an ultralow density (2.1 ± 0.3 mg cm−3) (Fig. 4a). Their adsorption efficiency considering weight gain and comparison of the adsorption capacities of common carbon-based materials are shown in Fig. 4b and c, respectively. They exhibited an adsorption rate (41.7 g g−1 s−1) that is higher than that of pure graphene.
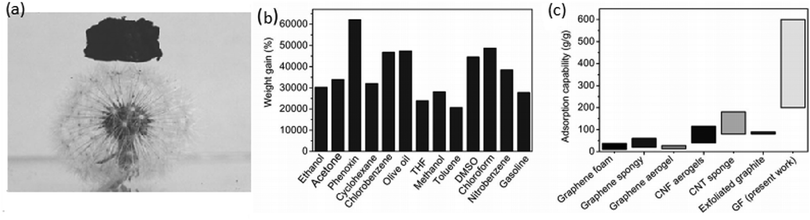 | ||
| Fig. 4 (a) Piece of GF size of 1.8 cm × 1.1 cm × 1.2 cm standing on a dandelion. (b) Adsorption efficiency considering weight gain. (c) Comparison of the adsorption capacities of common carbon-based materials. Reproduced from ref. 67 with permission from John Wiley and Sons. | ||
Despite the hydrophobicity in graphene-based macrostructures, their high cavity content together with appropriate pore size lead to some water adsorption.68 In this context, the synthesis of graphene/carbon nanotube hybrids exhibits promising potential. Carbon nanotubes can be utilised to enhance the robustness and surface roughness of graphene, which is attributed to its better adsorption capacity and hydrophobicity. Superoleophilic and super hydrophobic foam of a graphene/CNT hybrid was synthesised via the chemical vapour deposition method in two steps, which exhibited excellent hydrophobic properties due to its surface roughness, bulk porous structure and hydrophobicity of CNTs.14 This hybrid material can be employed for the elimination of organic solvents and oil in water owing to its macroporous structure and superoleophilicity.
Good efficiency was obtained by an ultra-flyweight aerogel fabricated using CNTs and giant graphene oxide sheets with a very low density (0.75 mg cm−3). The aerogel obtained was highly hydrophobic, having a contact angle of ∼132.9° and surface area of ∼272 m2 g−1 with average pore size of 123 nm. The high porosity of the aerogel (∼99.9%) led to the fast and efficient removal of oils, ranging between 215–913 times its weight depending on the oil density. The rate of absorption exhibited by the aerogel (68.8 g g−1 s−1) was much greater than that of a graphene aerogel (0.57 g g−1 s−1).69
Kabiri and co-workers reported a single-step approach for the synthesis of 3-D graphene/CNT networks by heating an aqueous mixture of graphene oxide and CNTs in the presence of Fe2+ ions.70 The as-prepared aerogels exhibited exceptional adsorption characteristics for the removal of organic solvents, oils and fats in a non-turbulent water–oil system. The entire oil was instantly adsorbed and entirely taken up within 20 s by immersing the graphene–CNT aerogels in the oil. The oil-filled aerogels could float on the surface of water without releasing the oil or penetration of water into their structure. The graphene–CNT aerogels exhibited an adsorption capacity of 21–35 times its own weight. Compared to other adsorbents tested previously (for gasoline adsorption), such as carbon nanofiber (CNF)/carbon foam (16 g g−1),71 CNT–polydimethylsiloxane-coated polyurethane (17.5 g g−1),72 cotton grass (19 g g−1)73 and carbonized pith bagasse (23.86 g g−1),74 the graphene–CNT aerogel exhibited a better adsorption performance of 30 g g−1. Moreover, its adsorption capacity was enhanced with an increase in the density of the organic solvents and oil. This is because liquids having a higher surface tension and viscosity will repel molecular disruption and also avoid diffusion towards the porous aerogel given that surface molecules attempt to close pack together. It was also reported that the graphene/CNT aerogel could continuously remove organic pollutants and oil in an aqueous system via pressure-driven adsorption. Only 350 mg of the graphene/CNT aerogel could separate about 10 L of oil in combination with a vacuum system. Thus, the hybrid aerogel can act as a superior filter for the removal of oil, even after saturation.
Wan et al. reported a green and facile method to synthesize graphene/CNT aerogels having a very low density (6.2–12.8 mg cm−3) via a single-step hydrothermal redox reaction. With an enhanced GO/CNT mass proportion (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3
1 and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the adsorption capacities reached 100–270 times their weight depending on the density of the adsorbed organics. Moreover, the graphene/CNT aerogels retained excellent stability and reusability after continuous adsorption–squeezing experiments.75
1), the adsorption capacities reached 100–270 times their weight depending on the density of the adsorbed organics. Moreover, the graphene/CNT aerogels retained excellent stability and reusability after continuous adsorption–squeezing experiments.75
More interestingly, a hydrothermal process to synthesise a graphene aerogel starting with graphene oxide was reported, which was utilized as a template for growing CNTs.76 The in situ-grown CNTs on graphene sheets provided a hierarchical hybrid structure having a low density, exceptional hydrophobicity and oleophilicity together with improved surface area possessing meso- and micro-scale pores. Thus, the hybrid structure exhibited greater adsorption capacity than reduced graphene oxide itself. The material displayed a steady adsorption capacity throughout repeated adsorption/burn operations and retained more than 90% adsorption capacity over 10 cycles (Fig. 5a and b). The adsorption capacity exhibited by the hybrid aerogel for various organic liquids is shown in Fig. 5c with a maximum capacity of 322.8 ± 8.3 g g−1 for ortho-dichlorobenzene. Hence, it can adsorb various oils with excellent reusability. By employing in situ burning, the adsorbed solvents were removed easily, while the aerogel retained its porous framework without being burnt in air or collapsing, indicating the outstanding fire resistance and thermal stability of the rGO/CNT aerogel (Fig. 5d).
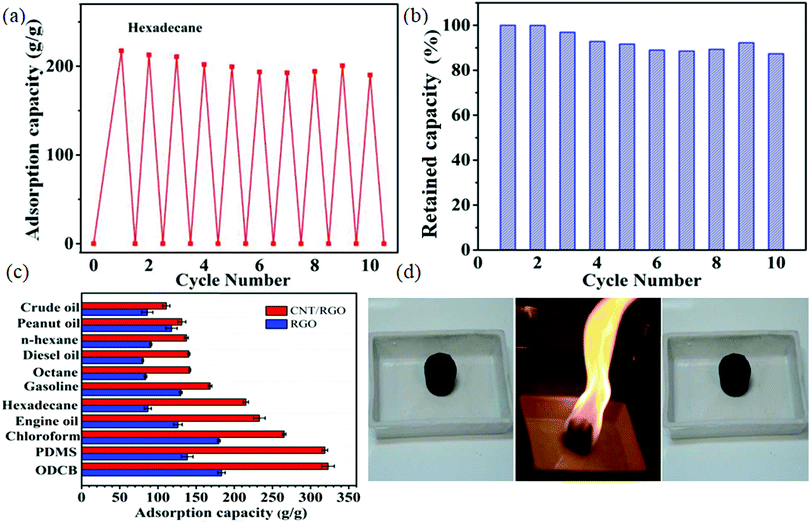 | ||
| Fig. 5 (a) Adsorption recyclability of hybrid aerogel over ten cycles. (b) Retained adsorption capacity exhibited by the sample. (c) Adsorption capacity of hybrid aerogel for different organic liquids. (d) Outstanding thermal stability, and hence reusability of hybrid aerogel via combustion. Reproduced from ref. 76 with permission from Elsevier. | ||
Physical absorption can also be employed as an eco-friendly and efficient route for removing organic contaminants from water. Zhan and co-workers introduced 1D poly-dopamine-functionalized multiwalled carbon nanotubes in a graphene aerogel to prepare a robust and ultra-lightweight composite aerogel for the effective absorption of organic pollutants.77 Environmental pollution was greatly reduced by using this composite, as no reducing agents were used for its formation. The surface area and stability of the composite were significantly enhanced after combining with multi-walled carbon nanotubes (MWCNTs). The reduction in pore size improved the capillary flow and absorption for organic contaminants, ranging from 125 to 533 g g−1, which exceeded that of most of reported absorption materials. Moreover, the composite displayed an outstanding reusable performance in absorption–combustion, absorption–distillation and absorption–squeezing cycles depending on the characteristics of the various organic solvents.
In another interesting study, fluffy spheres of CNTs/graphene/nickel were developed by employing renewable carbon sources.78 Carbon-encapsulated nickel nanoparticles were prepared through the hydrothermal carbonization of potato starch at a low temperature and catalytically graphitized and cracked in biogas at 700 °C. The graphene shells were peeled off to form graphene nanoplatelets and MWCNTs grew and in situ integrated into the platelets. For the removal of organic solvents from water, the prepared nanocomposite showed an exceptional performance with an adsorption capacity ranging from ∼112 to ∼145 g g−1.
Cai et al. reported the synthesis of an aerogel, rGO/amino MWCNT, through the chemical reaction between the –COOH groups on GO and –NH2 groups on amino MWCNT in combination with a freeze-drying and high-temperature annealing process.79 The presence of strong chemical bonding enhanced the hydrophobic property and microstructures of the hybrid aerogel. Oil–water separation experiments and adsorption tests proved the outstanding adsorption capability of the aerogel for various oils and organic solvents such as dichloromethane (242.31 g g−1), acetone (219.54 g g−1), sesame oil (156.35 g g−1), ethyl acetate (152.79 g g−1) and cyclohexane (122.46 g g−1). Moreover, oil–water separation was achieved within 25 s.
Decorating graphene/CNT-based sorbents using magnetic nanoparticles facilitates their easy collection after saturation using a magnetic field. Moreover, they can be employed to ‘mop-up’ oil on the surface of water with the help of a permanent magnet to move them around to track oil spills.80 Even if the sorbent is accidentally smashed, all the broken pieces of sorbent can be safely recovered. Based on their facile synthetic methods, high adsorption capability, stable cyclic performance, etc., graphene–CNT hybrid structures show huge potential in water decontamination and oil remediation. The performance of graphene–CNT hybrids for the removal of various oils and organic solvents is summarized in Table 1. Further research to control the functional groups on graphene surface to couple selective adsorption characteristics and achieve the highest hydrophobicity is essential in this area.
| Sl No. | Type of material | Synthetic method | Target | Removal capacity (g g−1) | Ref. |
|---|---|---|---|---|---|
| 1 | CNT sponge | CVD using 1,2-dichlorobenzene and ferrocene | Various oils and organic solvents | 80–180 | 50 |
| 2 | Spongy graphene | Reducing graphene oxide platelets in suspension followed by shaping via moulding | Petroleum products, fats, toluene, alkanes, and other organic solvents | 20–86 | 48 |
| 3 | Nitrogen-doped 3D graphene | Hydrothermal reaction of graphene oxide using pyrrole | Various oils and organic solvents | 200–600 | 67 |
| 4 | Graphene/CNT hybrid foam | Two-step CVD including CNT growth on graphene–Ni substrate followed by removal of Ni | Compressor oil, sesame oil, toluene, chloroform, dimethylformamide, etc. | 80–130 | 14 |
| 5 | Graphene/CNT aerogel | Freeze drying aqueous solutions of graphene oxide sheets and CNTs followed by reduction | Crude oil, motor oil, vegetable oil, n-hexane, ethanol, toluene, 1,4-dioxane, chloroform, etc. | 215–913 | 69 |
| 6 | Graphene/CNT aerogel | Reduction of graphene oxide in presence of Fe nanoparticles and formation of graphene–CNT hydrogel followed by freeze drying | Various oils (gasoline), fats (vegetable oils and paraffin) and organic solvents (toluene and tetrahydrofuran) | 21–35 | 70 |
| 7 | Graphene–CNT aerogel | Hydrothermal redox reaction involving the addition of ethylenediamine to graphene oxide–CNT dispersion followed by heating and freeze drying | Various oils and organic solvents including n-hexane, toluene, phenixin and lube | 100–270 | 75 |
| 8 | Graphene–CNT aerogel | Preparation of graphene aerogel using GO via a hydrothermal process followed by in situ growth of CNTs | Petroleum products, chloroform, hexane, octane, peanut oil, ortho-chlorobenzene, etc. | 110–330 | 76 |
| 9 | Graphene/MWCNT–PDA composite aerogel | Preparation of GO/MWCNT-PDA hydrogel followed by freeze drying and annealing | Acetone, chloroform, n-dodecane, n-hexane and n-heptane | 125–533 | 77 |
| 10 | CNTs/graphene/Ni fluffy spheres | Catalytical graphitization of carbon encapsulated Ni nanoparticles followed by cracking and in situ growth of MWCNT into the graphene nanoplatelets | Various oils and organic solvents-hexane, gasoline, chloroform, diesel, toluene and dichlorobenzene | 112–145 | 78 |
| 11 | rGO/amino MWCNT aerogel | Reaction between GO and amino MWCNT together with freeze-drying and annealing | Ethyl acetate, cyclohexane, dichloromethane, acetone and sesame oil | 122–242 | 79 |
3. Adsorption of dyes
With the rapid industrial development, water pollution resulting from organic dyes discharged from the textile, leather, food, plastic, paper, cosmetics and pharmaceutical industries without adequate treatment processes has become a serious problem affecting aquatic life, human health and the ecosystem because of their colour and high chemical oxygen demand.81,82 The release of exceedingly coloured waste interferes with the transmission of light, and thus upsets biological processes, causing harm to aquatic systems. Many organic dyes exhibit resistance to bio-degradation. Moreover, they are stable to light, chemical treatments etc., and thus result in high bio-toxicity and carcinogenic effects. Therefore, the development of innovative treatment processes for the efficient removal of dyestuffs has attained great importance and special interest.83 Most dyes are dissolved in cationic (Rhodamine B, methylene blue, malachite green, etc.) or anionic (Bordeaux red, methyl orange, rose Bengal sodium salt, etc.) forms. Thus, the rich porous structure and vast surface area of graphene/CNT hybrids allow the diffusion and provide storage space for dye molecules. Moreover, electrostatic and efficient π–π interactions can be exploited to take full advantage of these materials for the effective adsorption of dyes, particularly those having aromatic structures.In this regard, an graphene/CNT hybrid aerogel obtained via the supercritical CO2 drying of its aqueous gel precursors was reported.84 The ultralight hybrid aerogel exhibited high conductivity (7.5 S m−1), ample pore volume (2.58 cm3 g−1), large surface area (435 m2 g−1) and excellent scavenging ability for basic dyes as a sorbent. It exhibited an adsorption capacity of 145.9 mg g−1 for Rhodamine B, which is higher than that with substances such as Rhizopus oryzae biomass (39.08 mg g−1), O-carboxymethyl chitosan-N-lauryl (38.5 mg g−1)85 and porous graphitic carbon (73.0 mg g−1)86 for the same dye. The acid-treated MWCNT/graphene hybrid aerogel exhibited an adsorption capacity of 190.9 mg g−1 for methylene blue, which is much higher than that with most of the conventional adsorbents under similar conditions for the same dye.87 For the adsorption of acid fuchsin, the MWCNT/graphene and acid-treated MWCNT/graphene hybrid aerogels exhibited an adsorption capacity of 66.4 mg g−1 and 35.8 mg g−1, respectively (Fig. 6). The electrostatic repulsion between the adsorbed acidic dyes and hybrid aerogels caused relatively lower capacities.
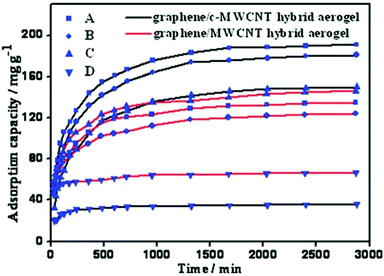 | ||
| Fig. 6 Adsorption of dyes: methylene blue (A), fuchsin (B), Rhodamine B (C), and acid fuchsin (D) by graphene/MWCNT hybrid aerogel. Reproduced from ref. 84 with permission from The Royal Society of Chemistry. | ||
In an interesting work by Ai and Jiang, a graphene–CNT aerogel exhibited an outstanding performance in removing methylene blue from an aqueous solution with a maximum adsorption capacity of 81.97 mg g−1. The removal efficiency was 97% at the initial MB concentration of 10 mg L−1.88 Kotal and Bhowmick reported that hybrid materials based on CNT chemically bonded to reduced graphene oxide exhibited a maximum adsorption capacity of 245 mg g−1 for crystal violet (greater than that using magnetic-modified MWCNT (228 mg g−1)) and 219 mg g−1 for Rhodamine 6G (greater than using cane sugar-reduced graphene (55 mg g−1)).89
With the progress in adsorption research on graphene/CNT hybrid structures, there have been constant efforts to develop all-carbon nanoarchitecture membranes having improved membrane selectivity and superior water permeability. By intercalating surface-functionalized multi-walled carbon nanotubes with a small diameter into rGO sheets, Goh et al. developed exceptionally stable membranes, which could reject almost 100% organic dyes having different charges (cationic: Rhodamine B and methylene blue and anionic: Acid Orange 7). The exceptional stability exhibited by these materials resulted from the van der Waals force and π–π interactions between the rGO sheets and MWCNTs. Moreover, their water permeability of 52.7 L m−2 h−1 bar−1 was 4.8 times that of pristine rGO membrane and 5–10 times greater than that most commercial nanofiltration membranes.90
Lee et al. synthesised a hybrid graphene–CNT aerogel by growing CNTs on the surface of a graphene aerogel using a nickel catalyst.91 The large specific surface area together with mesoporous nature of the hybrid material made it suitable for water treatment. The hybrid aerogel obtained via the chemical vapour deposition of CNTs on graphene metal salts (G-CNT_A) exhibited a superior adsorption capacity for methylene blue of 626 mg g−1. Van der Waals forces together with the π–π interaction among the nanocarbon materials and organic dyes resulted in high adsorption. It could effectively remove anionic and cationic dyes from water. The adsorption capacity of G–CNT_A for methyl orange was 532 mg g−1 and Congo red was 560 mg g−1, which was much greater compared to that of other graphene-based adsorbents (methyl orange: 101.34 mg g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92 and Congo red: 33.66 mg g−1
92 and Congo red: 33.66 mg g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 93). The adsorption capacity for crystal violet of 575 mg g−1 was greater than that obtained with the hybrid material based on CNT chemically bonded to reduced graphene oxide (245 mg g−1). Moreover, owing to its magnetic nature, the aerogel was easy to separate from water and could be readily recycled and reused.
93). The adsorption capacity for crystal violet of 575 mg g−1 was greater than that obtained with the hybrid material based on CNT chemically bonded to reduced graphene oxide (245 mg g−1). Moreover, owing to its magnetic nature, the aerogel was easy to separate from water and could be readily recycled and reused.
Chen et al. reported a promising nanofiltration membrane prepared by loading rGO intercalated with CNTs on an anodic aluminium oxide microfiltration membrane using a facile vacuum-assisted filtration method. The membrane was used to separate dyes, nanoparticles, organophosphates, sugars, proteins, and especially humic acid from water. It had good permeability, good anti-fouling properties and high retention efficiency. The retention was greater than 97.3% for methyl orange and the permeability of the membrane was 20–30 L m−2 h−1 bar−1.94 Ansari and co-workers employed an oxidative polymerisation technique to combine MWCNT-GO composites with polyaniline, and to generate additional functionality, doped them with p-toluene sulfonic acid.95 The composite was successfully employed for the adsorptive removal of Congo red dye and Cr4+, and the maximum adsorption of Congo red dye and Cr4+ was observed at 30 °C in acidic medium. The highly functionalised nanocomposite system enabled the removal of multicomponent pollutants from aqueous solutions.
Huang et al. dispersed GO and modified CNTs (MCNTs) in water, mixed them with toluene, and then porous graphene–CNTs composites were synthesised via hydrothermal reaction.96 By varying the ratio of GO/MCNTs to toluene, the pore size of the composite could be controlled. With a decrease in pore size, the equilibrium adsorption capacity increased and the rate of adsorption decreased. The nanocomposite could maintain good adsorption capacity for methylene blue even after 5 cycles.
Electrochemically enhanced adsorption (electro sorption) can be effectively applied for the superior removal of organic pollutants. For the electrosorption of organic dyes, Yue et al. developed a freestanding porous rGO/SWCNT film as a flexible electrode material by assembling SWCNTs and spherical polystyrene into a graphene film, and subsequent reduction and calcination at 500 °C in Ar for 1 h.97 The 3D porous structure and highly crumpled surface enhanced the diffusion of ions and adsorption. For methylene blue, the film exhibited outstanding recyclability with observed maximum adsorption capacity of 13.01 g g−1 and the capacity retention after the 5th cycle was ∼103%.
α-FeOOH anchored by a GO-CNT aerogel nanocomposite was fabricated via a hydrolysis process and employed as a persulfate activator (α-FeOOH@GCA + K2S2O8) for the decolourisation of Orange II. The decolourisation of the dye was enhanced to ∼99% compared to that of the pristine α-FeOOH (∼44%) or GO-CNTs (∼18%). The enhanced catalytic activity resulted from the formation of a heterojunction by GO-CNTs and α-FeOOH, as confirmed by the presence of Fe–O–C bonds. In the case of recycling, the decolourisation efficiency of the dye by the activated persulfate system dropped from the first to third cycle. However, the addition of a small amount of Fe2+ or ultraviolet irradiation could restore the activity of the system.98
Yao et al. fabricated highly porous graphene/carbon nanotube hybrid beads possessing high mechanical strength through the self-assembly of graphene and CNTs together with a poly-acrylonitrile coating and KOH-activated carbonization.99 The hybrid beads possessed a high specific surface area of 1270 m2 g−1, hierarchical porous structure and low density of 5.7 mg mL−1. Moreover, they exhibited outstanding cyclic resilient property, excellent mechanical stability and the maximum adsorption capacity for methylene blue reached ∼521.5 mg g−1.
Hu et al. reported the preparation of 3D graphene oxide/carbon nanotube nanostructures having a large surface area via a freeze-drying method.100 The prepared nanostructures with GO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CNT = 1
CNT = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 had a surface area of 257.6 m2 g−1 and could adsorb both anionic (methyl orange) and cationic (Rhodamine B) dyes in aqueous solution. The maximum adsorption capacity for methyl orange of 66.96 mg g−1 and Rhodamine B of 248.48 mg g−1 was better than that of modified graphene oxides or CNTs. Hence, the synergistic effect of 1D CNTs on 2D graphene can provide a uniform 3D nano filtration membrane, which results in elevated permeability and separation capability. However, studies regarding the practical separation applications of these hybrid structures are limited, further research to develop smart porous membranes is anticipated to occur in the near future.
5 had a surface area of 257.6 m2 g−1 and could adsorb both anionic (methyl orange) and cationic (Rhodamine B) dyes in aqueous solution. The maximum adsorption capacity for methyl orange of 66.96 mg g−1 and Rhodamine B of 248.48 mg g−1 was better than that of modified graphene oxides or CNTs. Hence, the synergistic effect of 1D CNTs on 2D graphene can provide a uniform 3D nano filtration membrane, which results in elevated permeability and separation capability. However, studies regarding the practical separation applications of these hybrid structures are limited, further research to develop smart porous membranes is anticipated to occur in the near future.
4. Removal of heavy metal ions
As a consequence of urbanization and rapid industrialization over the past few decades, plenty of toxic metal ions have been discharged from industries such as fertilizer production, mining operations, storage batteries, metal processing plants, metal plating, and alloy industries into the environment without adequate treatment processes.101–103 These contaminants, which are not biodegradable, easily accumulate and cause toxic effects to ecosystems and public health. For the removal of various metals, several methods such as ion-exchange, membrane filtration, chemical precipitation, electrochemical treatment, advanced oxidation, reverse osmosis and adsorption have been developed.104,105 Among them, adsorption is widely employed for the removal of metals owing to its high efficiency, simple design, ease of operation, low maintenance cost and capability of metal recovery.Carbon-based nanocomposites have shown promising adsorption performances towards these pollutants. The adsorption process is driven by (i) electrostatic interactions,106,107 (ii) ion exchange process108 and (iii) surface complexation with metal ions.109,110 In the case of electrostatic interactions, heavy metal cations are attracted by the oxygen functionalities (–OH, –O–, –COOH, etc.) on the surface of the adsorbent, which are negatively charged. For ion exchange, metal cations replace the H3O+ groups present in the adsorption sites. Similar to the adsorption of cationic dyes, the adsorption capacity can be improved by increasing the pH for both mechanisms. Conversely, it is also essential to prevent a high enough pH for precipitating metal hydroxides.106 Moreover, the adsorption of metal on graphene is endothermic, an enhanced adsorption capacity can be achieved at a higher temperature.
Hybrid polymer–GO sponges have attracted considerable interest for the removal of metal ions, and in this regard, chitosan–gelatin/GO monoliths with high porosity (>97%) were synthesised using a unidirectional freeze drying method for the adsorption of Pb2+ and Cu2+.111 The introduction of GO considerably enhanced the porous structure and compressive strength of the hybrid monolith. They exhibited high stability, and hence only a slight reduction in adsorption ability was observed even after recycling several times. Moreover, they are nontoxic and biodegradable.
3D graphene–CNT hybrid networks have been deeply investigated on account of their easy separation and large specific surface area. They exhibit outstanding potential for removing heavy metal ions such as Cu2+, Pb2+, As3+ and U6+. A previously mentioned graphene/CNT hybrid aerogel84 showed high scavenging ability for heavy metal ions as binding species. The binding capacity exhibited by the acid-treated MWCNT/graphene hybrid aerogel for various metals including Pb2+ of 0.51 mmol g−1 (104.9 mg g−1), Hg2+ of 0.46 mmol g−1 (93.3 mg g−1), Ag+ of 0.59 mmol g−1 (64 mg g−1) and Cu2+ of 0.53 mmol g−1 (33.8 mg g−1) was much greater than that of the MWCNT/graphene hybrid aerogel for the same metals as follows: Pb2+-0.21 mmol g−1 (44.5 mg g−1), Hg2+-0.38 mmol g−1 (75.6 mg g−1), Ag+-0.43 mmol g−1 (46 mg g−1) and Cu2+-0.15 mmol g−1 (9.8 mg g−1), as shown in Fig. 7. This is because electrostatic interaction mainly controls the binding of ions to porous hosts112 and the acid-treated MWCNT/graphene hybrid aerogel possessed more oxygen-containing groups, which led to the efficient binding of ions.113,114
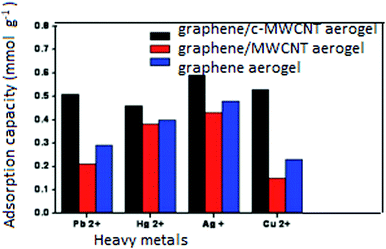 | ||
| Fig. 7 Adsorption capacity of graphene/MWCNT hybrid aerogels for heavy metal ions. The related adsorption capacity of the graphene alone aerogel is also shown for comparison. Reproduced from ref. 84 with permission from The Royal Society of Chemistry. | ||
It is also interesting that the incorporation of iron minerals in the graphene/CNT networks has the advantage of preventing the aggregation of the carbon nanostructures. Moreover, the facile separation of the trapped metals can be easily achieved by exploiting their intrinsic magnetic properties.115 In this regard, Zhang et al. synthesised a graphene–CNT hybrid aerogel via the hydrothermal reduction of GO and CNTs with the addition of ferrous ions.116 Owing to its competent porous structure, it exhibited efficient removal of lead ions of 232–451 mg g−1, which is higher than that of other carbon-based adsorbents, including carbonaceous nanofiber membranes (221 mg g−1) and pure graphene aerogels (374 mg g−1). The amount of CNTs and ferrous sulphate used had a significant influence on the metal removal efficiency, and that prepared using 30% CNTs had a higher efficiency among the samples with same amount of ferrous sulphate. Moreover, the mass production of graphene–CNT–iron oxide hybrid structures was achieved via a one-pot microwave route, which is highly versatile.117 Owing to its open porous nanostructures and high aspect ratio, the hybrid material exhibited efficient removal of arsenic from contaminated water (Fig. 8).
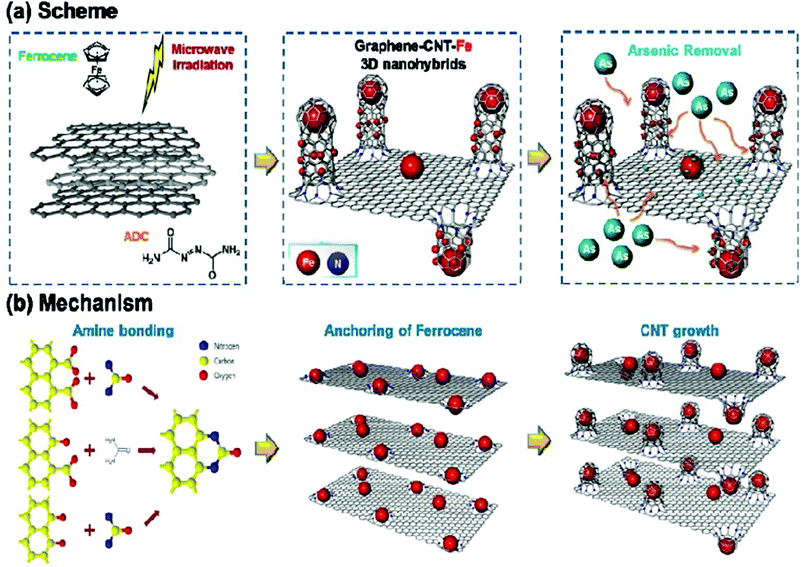 | ||
| Fig. 8 (a) Growth of CNTs on graphene. (b) Amino functionalization of graphene sheets. Reproduced from ref. 117 with permission from the American Chemical Society. | ||
To separate and remove As3+ and Pb2+ from contaminated water, 3D GO membranes bridged with glutathione-conjugated CNT were developed, which could capture As3+ (>96%), As5+ (>92%) and Pb2+ (>98%).118 The excellent removal efficiency is attributed to the high affinity of As3+ and Pb2+ for glutathione, since they can both bind to glutathione using –SH. Moreover, the open pore network of the CNT-bridged GO membrane facilitated the fast diffusion of As3+, As5+ and Pb2+ inside the 3D network, which resulted in a high adsorption capacity (Fig. 9a–c). In contrast, only 12% of As3+, 9% of As5+ and 18% of Pb2+ could be removed using the 3D GO membrane bridged with CNT (without glutathione) (Fig. 9d).
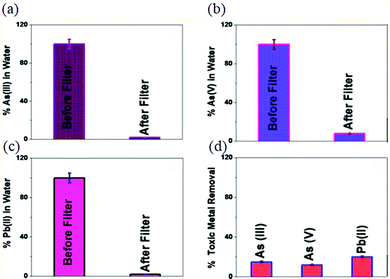 | ||
| Fig. 9 (a) As3+, (b) As5+, and (c) Pb2+ removal efficiency using 3D GO membrane bridged with glutathione-conjugated CNT. (d) Removal efficiency for 3D GO membrane bridged with CNT. Reproduced from ref. 118 with permission from the American Chemical Society. | ||
The removal of uranium has gained remarkable attention owing to the radioactive damage it causes to human health and the ecology. Graphene–CNT aerogels exhibited a high efficiency for the removal of U6+ with a monolayer sorption capacity of 86.1 mg g−1.119 Diethylenetriamine-functionalized CNTs dispersed in GO colloids were synthesised for the selective solid-phase extraction and analysis of trace-level Cr3+, Fe3+, Pb2+, and Mn2+ ions in wastewater.120 The solid-phase sorbent could achieve maximum static adsorption capacities up to 95% for Cr3+ (5.4 mg g−1), Fe3+ (13.8 mg g−1), Pb2+ (6.6 mg g−1) and Mn2+ (9.5 mg g−1) within about 30 min. Moreover, common coexisting ions had no substantial interference on the pre-concentration and separation of these ions at pH 4.
Interestingly, hydrosols of CNTs/GO sealed in dialysis bags showed outstanding efficiency for the elimination of trace Gd3+ from water, without re-pollution.121 The hybrids having an MCNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MGO ratio ranging from 1
MGO ratio ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–1
8–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 showed a remarkable synergistic effect because during the adsorption process, restacking of the GO nanosheets was inhibited by the CNTs. The theoretical maximum Gd3+ adsorption capacity of 534.76 mg g−1 (t = 60 min, pH = 5.9, and T = 303 K) with an 86.42% increase compared to GO was exhibited by the hybrids having MCNTs
2 showed a remarkable synergistic effect because during the adsorption process, restacking of the GO nanosheets was inhibited by the CNTs. The theoretical maximum Gd3+ adsorption capacity of 534.76 mg g−1 (t = 60 min, pH = 5.9, and T = 303 K) with an 86.42% increase compared to GO was exhibited by the hybrids having MCNTs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MGO = 1
MGO = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. Moreover, a sorption capacity of 347.83 mg g−1 was retained in the fourth cycle, indicating its exceptional regeneration performance.
6. Moreover, a sorption capacity of 347.83 mg g−1 was retained in the fourth cycle, indicating its exceptional regeneration performance.
Recently, an ultra-lightweight and robust 3D graphene/polydopamine-modified MWCNT hybrid aerogel with adsorption capacities as large as 318.47 mg g−1 for Cu2+ and 350.87 mg g−1 for Pb2+ was synthesised.122 The synergistic effects of surface complexation and chelation between the active sites in the hybrid aerogel and metal ions caused the exceptionally high adsorption. In another report, a graphene/CNT hybrid was added to the structure of activated carbon (AC) to improve the adsorption of Mn2+ from aqueous solutions.123 The maximum adsorption exhibited for the AC/G/CNT composite of 96.18% was greater than that for AC (89.38%) after 30 min. The introduction of the G/CNT hybrid enhanced the efficiency by 7.5% under same conditions by creating more pores and improving the surface properties of AC.
Graphene/CNT hybrid materials can effectively be employed as an environmentally friendly sensing electrodes for the detection of heavy metal ions, which are much better than either graphene or CNT alone. In this regard, Huang et al. prepared 3D graphene/MWCNT hybrid nanocomposites via the direct electrochemical reduction of GO–MWCNT nanocomposites. The glassy carbon electrode (GCE) modified with the G-MWCNTs exhibited high sensitivity for the electrochemical detection of trace amounts of Cd2+ and Pb2+ ions, with the lowest detection concentration of 0.5 μg L−1 for Cd2+ and 0.5 μg L−1 for Pb2+. The synergistic effect of MWCNTs and graphene improved the preconcentration efficiency of metal ions and accelerated the electron transfer rate at the G-MWCNT/electrolyte interface, which led to an enhanced detection sensitivity.124
Hybrid structures of functionalized CNTs and graphene-based materials exhibit outstanding selectivity and high sensitivity for metal ions. In this regard, self-assembled electrochemically cleaved graphene sheets (EGNs) and xanthate-functionalized CNT hybrids were developed to fabricate an improved carbon paste electrode for an electrochemical sensor with high sensitivity and specificity. EGNs improved the electrical conductivity of the hybrid electrode. Additionally, the introduction of EGNs dispersed the functionalized carbon nanotubes to enlarge the electroactive surface area, which possessed a large number of active reaction sites for electrochemical reactions.125 Owing to their fast electron transfer and much better adsorption, they exhibited good response and specificity for the detection of Cu2+.
Double-walled carbon nanotube-graphene hybrid thin films synthesized on copper foil via chemical vapour deposition under low pressure exhibited high transparency with 94.3% transmittance. This hybrid material when transferred onto the surface of screen-printed electrodes enhanced the surface area by 1.4 times and electrochemical current by 2.4 times, which was employed for the enzymatic electrochemical detection of As5+. The fabricated sensor exhibited good stability, precision and excellent reproducibility.126
Recently, a glassy carbon electrode (GCE) modified with an electrochemically reduced GO–MWCNT–L-cysteine (ErGO–MWNTs–L-cys) nanocomposite using the drop-casting method was successfully employed for the detection of lead ions. The presence of rich –NH2 groups, large surface area and electrical conductivity of the composite significantly improved the selectivity and sensitivity for the detection of lead ions. The voltammograms of from differential pulse anodic stripping voltammetry illustrated that the anodic peak currents were directly proportional to the concentration of lead ions in the range of 0.2–40 μg L−1 with a limit of detection of 0.1 μg L−1 (S/N = 3). Moreover, the nanocomposite-modified GCE exhibited satisfactory reproducibility and stability, and hence is suitable for the fabrication of stable sensors.127 Thus, graphene–CNT hybrid-based sorbents hold great promise for potential applications in the detection and removal of metal ions.
5. Gas sensors
The development of gas sensors that can operate at room temperature has attracted considerable attention owing to their excellence in avoiding high temperature, which leads to easy integration, power consumption at a low level and good stability.128 However, semiconductor metal oxide-based gas sensors demand high temperature for activating their semiconductor properties for the detection of gases, limiting their application for gas sensing at room temperature.129Carbon nanostructures exhibit great potential for gas sensing applications owing to their strong covalent bonds, high stability under diverse environmental conditions, unique electronic properties, low cost and ease of composite formation. Owing to its large surface area and unique electrical properties, graphene is suitable for efficient gas sensing applications. At room temperature, mechanically exfoliated graphene sheets utilized in gas sensing devices exhibited remarkably low detection noise levels.130 However, despite the great potential demonstrated by graphene for application in chemical sensors, its mass production and integration into device architecture are challenging for the viable fabrication of sensors. Even though cost-effective methods for the large-scale fabrication of graphene have been achieved via chemical exfoliation and used for NO2 sensors, its response was unstable and relatively weak.131,132 CNTs also exhibit outstanding potential for the detection of several gases.128,133 Even though a more rapid and larger gas sensing response is shown by CNTs than that of graphene, the integration of CNTs onto flexible substrates is a big challenge owing to their imperfect contact with metal electrodes. Moreover, the slow recovery of CNT-based gas sensors and complexity of their fabrication limit their applications. Accordingly, 3D carbon nanostructures made of CNTs and graphene are suitable candidates for the fabrication of gas sensors operating at room temperature owing to their excellent chemical and physical stability, large surface area, high carrier mobility at room temperature and detectable resistance change after adsorption or desorption.
In this regard, a flexible NO2 sensor employing hybrid films of vertical CNTs and reduced graphene was developed.134 The sensor was prepared using a PI substrate (polyimide), gold electrode, hybrid films of CNTs/reduced graphene and an Ni/Cu micro heater. After 60 min exposure, the reduced graphene films exhibited no noticeable NO2 sensing, whereas the growth of vertical CNT arrays on the surface of reduced graphene increased the sensitivity by 20%. Moreover, an increase in resistance was observed with a 15 mm bending radius. Stable sensing performances even at extreme bending stress were achieved owing to the exceptional flexibility of reduced graphene.
Kaniyoor and Ramaprabhu synthesised G/MWCNT hybrids via the solution phase mixing of graphene suspensions and MWCNT.135 The aggregation of graphene was further prevented by dispersing nanoparticles of Pt into the hybrids. Using Nafion, the ternary hybrid was solubilized and the drop-casting method was used to fabricate a sensor. At room temperature, the sensor coated on an Al2O3 substrate exhibited increased sensitivity to H2. The response time and sensitivity almost linearly increased with the H2 concentration. Moreover, the ternary hybrid structure exhibited remarkable stability and repeatability.
In another report, a graphene/CNT 3D hybrid was developed via two-step CVD under atmospheric pressure.136 The developed monolithic graphene foam conformally covered with a dense CNT mesh could be efficiently employed as electrochemical electrodes in sensors. Modification using horseradish peroxidase and Nafion enabled the detection of H2O2 over a wide range (10 μM–1 mM), exhibiting a high sensitivity of 137.9 mA M−1 cm−2 and detection limit as low as ∼1 μM with S/N ∼17.4.
Graphene materials based on rGO have attracted great attraction for gas sensing owing to the controllable tuning of their semiconductor properties using surface modification, bulk quantity production and low cost.137,138 However, these sensors experience certain inadequacies of long recovery time and low response, limiting their wide applications. Surface modification of rGO using covalent or noncovalent methods enables the fabrication of highly efficient sensing materials by tuning its semiconductor properties. SnO2 was employed to modify rGO139–142 for the detection of nitrogen dioxide, hydrogen sulphide and acetone, but the operating temperature was relatively high. SnO2–rGO hybrids operating at low temperature have also been developed,143–145 but the sensing performances (recovery time, response time and sensitivity) of these sensors require further enhancement for practical applications. Thus to satisfy this requirement, MWCNTs were introduced and rGO–MWCNT–SnO2 was fabricated via the hydrothermal method and a room temperature NO2 sensor was developed, which exhibited good stability, high selectivity, high response, fast response and high recovery rate.146
The integration of CNT and graphene, achieved by coupling through poly(ionic liquid) (PIL) as an inter-linker, resulted in active chemical and temperature sensors.147 For chemical sensing applications, the hybrid composite could function as a chemo resistor for the detection of various volatile organic compounds at room temperature and exhibited a better performance in sensitivity (at ppm level), faster signal response and lower detection limit compared to graphene-only devices. Moreover, a temperature sensor was also fabricated using the hybrid materials, which exhibited a fast response to a small temperature gradient with high stability and sensitivity.
Interestingly, a surface plasmon resonance (SPR)-based fibre optic sensor was developed for methane gas sensing, where four varieties of probes were made up by coating an unclad core of fibre with silver, and then using any of the sensing over-layers of CNT, rGO, G/CNT and G/CNT/PMMA nanocomposite. The interaction of methane molecules with the sensing over-layer changed the dielectric constant of the sensing layer. With an increase in methane concentration, a red shift was observed in the resonance wavelength. The probe coated using the G/CNT/PMMA nanocomposite over-layer showed higher selectivity for methane gas than that with the CNT, rGO, and G/CNT sensing over-layers. Moreover, the doping concentration of 5 wt% G/CNT gave the maximum sensitivity.148
Another significant factor affecting the performance of sensing devices is humidity. A hydrophobic sensing material operating at room temperature was developed by depositing hybrid films of MWCNT/graphene on an Ni substrate employing microwave plasma-enhanced chemical vapour deposition in the temperature range of 500–700 °C and pressure of 20 torr.149 For ammonia sensing, a quick response and recovery time of 96 s were observed for the film deposited at 700 °C. Additionally, the hydrophobic surface of the film enabled gas sensing even a in humid environment.
A flexible NO2 sensor fabricated from a tungsten trioxide nanoparticle-loaded MWCNT/RGO hybrid on a polyimide substrate exhibited a maximum response of 17% to 5 ppm, a short response/recovery time of 7/15 min and low limit of detection of 1 ppm.150 Moreover, outstanding mechanical flexibility was observed, even at a bending angle of 90° and after 106 cycles of bending/relaxing process.
A 3D hybrid structure of MnO2/graphene/CNTs decorated with gold nanoparticles (AuNPs/MnO2/G/CNTs), which was sensitive to H2O2, was developed via a three-step method.151 The MnO2 nanoparticles attached to the G/CNT network served as the active element for sensing H2O2. The uniformly distributed gold nanoparticles on the surface of the MnO2 particles provided further active sites for adsorbing H2O2 molecules and enhanced the electrical conductivity of the hybrid. The AuNP/MnO2/G/CNT hybrids exhibited outstanding electrocatalytic activity for H2O2 with appreciable sensitivity of 452 μA mM−1 cm−2 and low detection limit of 0.1 μM (S/N = 3).
3D graphene–CNT structures synthesized by CVD were decorated with TiO2 nanoparticles via the sparking method and efficiently utilized for toluene sensing at room temperature. The response for toluene was enhanced by more than 7 times upon decoration using TiO2 nanoparticles. 3D TiO2/G-CNT had shown significantly greater selectivity and sensitivity than graphene, CNTs, 3D G-CNT and TiO2–CNT over a concentration range of 50–500 ppm at room temperature.152
An ammonia sensor employing Sn–TiO2/rGO/CNT nanocomposites was fabricated by Seekaew, Pon-On et al., which exhibited outstanding response together with superior selectivity to ammonia among various environmental gases and volatile organic compounds at room temperature.153 The gas sensor having a molar ratio of Sn/Ti = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 exhibited the maximum response to NH3 among the ratios and pure Sn–TiO2 and rGO/CNT gas sensors.
10 exhibited the maximum response to NH3 among the ratios and pure Sn–TiO2 and rGO/CNT gas sensors.
A flexible and fully transparent UV sensor made up of photoactive CNT networks and graphene electrodes was reported with an improved photoresponse (30 times higher) in comparison to a CNT–Au sensor.154 The similar molecular structure of CNT and graphene resulted in low contact resistance between them, and hence enabled efficient charge transfer (Fig. 10). Moreover, the CNT/graphene UV sensor exhibited over 80% optical transparency at 550 nm and exceptional mechanical flexibility without substantial variation in electrical resistance over 500 cycles against external bending.
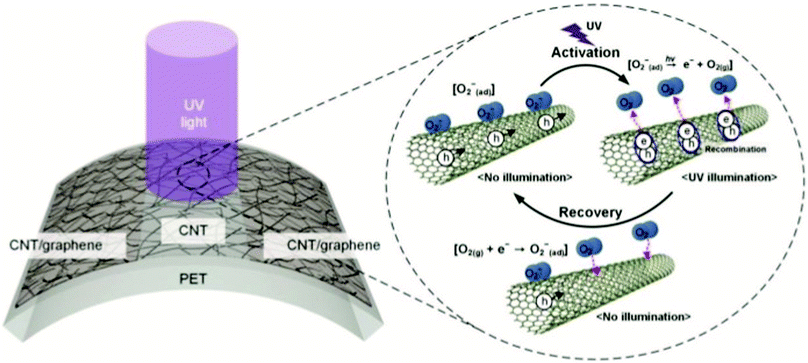 | ||
| Fig. 10 CNT/graphene UV sensor. Photo detection mechanism: CNT networks connect the graphene electrodes. In ambient air, CNT is p-doped by O2 adsorbed on its surface. UV light illumination causes oxygen desorption, and hence reduction in hole concentration in CNT, which leads to an increase in electrical resistance. Reproduced from ref. 154 with permission from John Wiley and Sons. | ||
Recently, an optoelectronic device for NO2 sensing at room temperature was developed using an MoS2/rGO hybrid on vertically aligned CNTs.155 Together with an ohmic contact with the hybrid material, the vertically aligned CNT produced a weak charge impurity scattering in the rGO layers, which improved the response time by 40% and photo responsivity by 236% compared to that of the gold-contacted device. Moreover, under laser illumination, the complete recovery time of ∼150 s and outstanding sensitivity of ∼41% at 100 ppm NO2 were demonstrated, which showed enhancements compared to sensing in the dark.
The adsorption of gases by 3D graphene/CNT networks is a relatively unexplored field of study, with only a few articles published in the past few years reporting graphene foams as adsorbents of CO2,156,157 acetone158 and formaldehyde.159 However, MWCNTs and graphene oxide were individually used as efficient supports for layered double hydroxides (LDH) for enhancing the CO2 sorption capacity of adsorbents, but their stability subjected to TSA (temperature-swing adsorption) cycles required improvement. To satisfy this, GO/MWCNT hybrid systems were prepared with an enhanced surface area, greater stability and compatibility for the active LDH material.160 The incorporated hybrid carbon network enhanced the thermal stability of LDH for over 20 cycles and intensely reduced the sintering observed with either MWCNT or GO separately. Moreover, equal proportions of MWCNT and GO resulted in maximum sorption (Fig. 11).
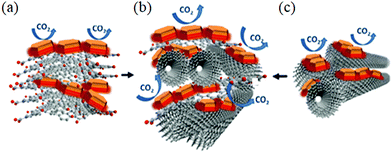 | ||
| Fig. 11 (a) GO-LDH. (b) Hybrid GO/MWNT-LDH. (c) MWNT-LDH. Reproduced from ref. 160 with permission from Elsevier. | ||
An interesting aerogel of carbon nanotube-enhanced amino-functional graphene was synthesised by adding CNTs into graphene oxide solution and reducing them using ethylene diamine.161 The improved microstructure of the aerogel and –NH2 groups on the graphene layer improved the chemical and physical adsorption of formaldehyde. The breakthrough time of the aerogel of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 min g−1 was greater than that of reduced graphene aerogels. Moreover, the aerogel exhibited an adsorption capacity of 27.43 mg g−1. The results showed that together with physical adsorption, chemical adsorption also enhanced the removal of formaldehyde. Thus, in the future, the integration of graphene–CNT hybrids in sensing devices can enable effective environmental monitoring and remediation.
300 min g−1 was greater than that of reduced graphene aerogels. Moreover, the aerogel exhibited an adsorption capacity of 27.43 mg g−1. The results showed that together with physical adsorption, chemical adsorption also enhanced the removal of formaldehyde. Thus, in the future, the integration of graphene–CNT hybrids in sensing devices can enable effective environmental monitoring and remediation.
6. Catalytic conversion of pollutants
For the removal of toxic chemicals, various methods such as adsorption, catalytic reduction and photocatalytic degradation have been used.162,163 To achieve efficient pollutant transformation, high adsorption capacity is inevitable. Upon irradiation of a photocatalyst with visible and/or UV light, their valence band electrons get promoted to the conduction band, thereby generating holes. The as-formed electron–hole pairs move to the surface and initiate redox reactions with the adsorbate. However, the catalytic efficiency of semiconductors is largely limited because of their rapid electron–hole pair recombination. In this regard, 3D graphene/CNT hybrids can be employed as a competent platform for the conversion of various contaminants with metal/metal oxide nanoparticles owing to the following reasons. (i) The 3D network structure can adsorb a large quantity of contaminants, (ii) the hybrid structure can function as a suitable framework for the efficient dispersal of the catalyst, which would otherwise aggregate because of its high surface energy and (iii) the well interconnected 3D network facilitates fast charge transportation, preventing electron–hole recombination. Thus, 3D CNT/graphene hybrids exhibit significant potential for the efficient conversion of pollutants.13Graphene-based materials having good electrical conductivity and large surface area were prepared by pillaring GO and rGO with CNTs via the chemical vapour deposition method, employing acetonitrile as the carbon source and nickel nanoparticles as the catalyst.164 The composite material exhibited outstanding performance under visible light-activated photocatalysis for the degradation of Rhodamine B dye owing to its unique porous structure and the excellent electron transfer property of graphene.
For the degradation of methyl orange in the presence of H2O2 under visible light, 3D CNT/rGO was implanted on Cu2O composite spheres.165 The observed degradation on CNTs/rGO/Cu2O of ∼99.8% was much greater than that on rGO/Cu2O of ∼77.6%, CNTs/Cu2O of ∼72.3% and pure Cu2O spheres of ∼67.9%. Under visible illumination, the electrons in the valence band of Cu2O were excited to the conduction band and transferred to CNTs/rGO, which could collect and transport electrons, leading to electron–hole separation. Meanwhile, the H2O2 molecules were reduced and the generated ˙OH radicals directly decomposed the dye.
Wang et al. synthesised a graphene/MWCNT/TiO2 hybrid via the solvothermal method, which demonstrated improved photocatalytic efficiency over graphene/TiO2 in the degradation of methylene blue and the reduction of Cr6+ under UV irradiation.166 The photocatalytic activity was dependent on the percentage of CNTs in the hybrid and optimal mass ratio observed was MWCNTs: TiO2 = 5%. CNTs served as charge transmitting paths, thereby decreasing the recombination rate of electron–hole pairs. For the degradation of methylene blue, the apparent rate constant was 2.2 times greater and for the reduction of Cr6+, the value was 1.9 times greater than that of the graphene/TiO2 composite (Fig. 12).
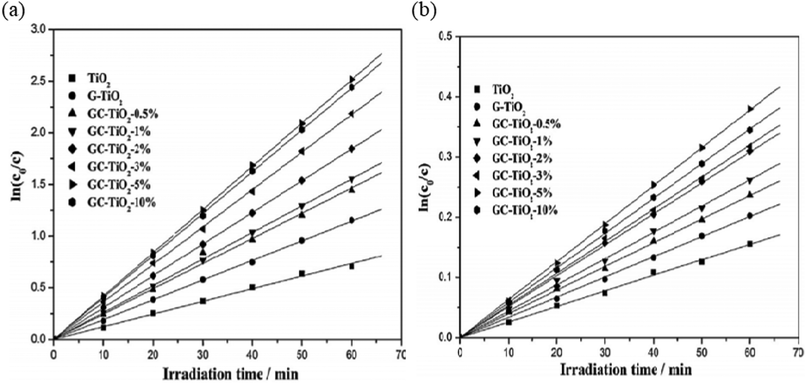 | ||
| Fig. 12 Linear transformed ln(c0/c) = kt of the kinetic curves of (a) methylene blue degradation and (b) photoreduction of Cr6+ for TiO2, graphene/TiO2 and graphene/CNT/TiO2 composites. Reproduced from ref. 166 with permission from Elsevier. | ||
Although adsorption has attracted great attention for the removal of dyes because of its simple design, high efficiency and economic feasibility, it causes second pollution in the course of the recovery of the dyes.167 In this regard, the catalytic oxidation of dyes has gained remarkable interest for treating a huge amount of contaminated water with low energy consumption. Especially, catalytic wet peroxide oxidation is effective for removing various organic pollutants on a large scale.168–170 A ternary hybrid of MnO2@CNT-reduced graphene oxide was prepared, which exhibited superior catalytic activity in comparison to MnO2@CNT in removing Basic Red 18 dye in the presence of H2O2 within a short time.171 Moreover, the hybrid exhibited excellent decolourisation through three successive catalytic wet peroxide oxidations within 30 min.
Although Fe3O4 nanoparticles possess peroxidase-like catalytic activities and high electrocatalytic activity when loaded on CNT supports, their catalytic efficiencies in aqueous systems encounter some challenges owing to the poor dispersion of the hydrophobic CNTs and low stability of the catalysts. In this regard, amphiphilic GO nanosheets were employed to disperse CNTs and a stable G/CNT/Fe3O4 nanocomposite with improved catalytic activity was synthesised.172 The nanocomposite exhibited more efficient peroxidase-like catalytic activities and electrocatalysis to H2O2 and superior aqueous stability compared to Fe3O4 nanoparticles and CNT/Fe3O4. Moreover, the nanocomposite had advantages such as environmental stability, magnetic separation and recyclability.
Interestingly, a composite aerogel of graphene oxide and CNTs decorated with FeOOH nanoparticles was synthesized, which exhibited remarkable catalytic activity for the degradation of methylene blue, Rhodamine B, Orange II, phenol and BPA.173 The 3D porous network enabled effective charge/mass transfer, thereby improving the catalytic performance of FeOOH. The efficient conversion between Fe2+/Fe3+ and synergistic coupling of FeOOH with the GO/CNT aerogel enhanced the photocatalytic activity of the hybrid.
Qu et al. prepared a heterostructured nanocomposite by combining oxygen-modified monolayer graphite-like C3N4 with GO and nitrogen-doped CNTs, exhibiting outstanding activity for the degradation of Rhodamine 6G in comparison to pure O-g-C3N4 and O-g-C3N4/GO under visible light irradiation.174 GO and nitrogen-doped CNT acted as electronic acceptors and inhibited the recombination of electron–hole pairs (Fig. 13). The nanocomposite was further employed for the removal and degradation of tetracycline hydrochloride in water, which confirmed its catalytic activity and recyclable stability.
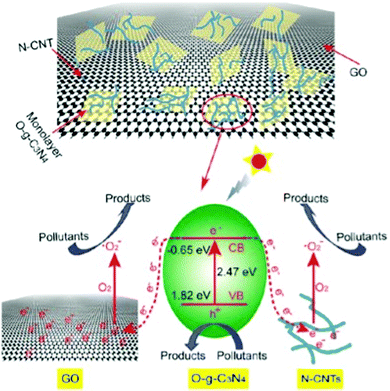 | ||
| Fig. 13 Electron transfer and photocatalytic degradation of pollutants by O-g-C3N4/GO/N-CNT under visible light irradiation. Reproduced from ref. 174 with permission from the American Chemical Society. | ||
In another study, Yi et al. reported the synthesis of 3D graphene/CNT-supported copper nanocubes with platinum skin via simple electrodeposition and galvanic replacement.175 The hybrid having a 3D interconnected network enabled quick mass diffusion and rapid electron transfer. Consequently, the hybrid exhibited outstanding electrocatalytic activity and superior durability compared to the Pt/C catalyst towards the methanol oxidation reaction.
Catalytic reduction has gained widespread attention for the removal of toxic chemicals on account of its efficiency, cost effectiveness, simple preparation and energy saving.176,177 3D N-doped graphene–CNT networks well encapsulated with Ni nanoparticles were synthesised using Ni-based MOFs (metal organic frameworks) and melamine as precursors.178 Among the various products obtained, that pyrolyzed at 800 °C exhibited an outstanding performance of ∼99.6% reduction of Cr6+ with HCOOH, owing to the synergistic effects of the porous 3D network together with high N-doping level and enormous ultrafine Ni nanoparticles (Fig. 14). In addition, its microstructure and catalytic efficiency remained unchanged even after the 10th recycling, signifying its exceptional stability.
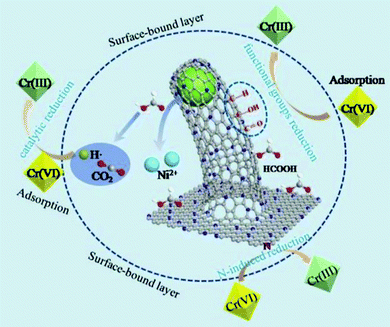 | ||
| Fig. 14 Cr6+ reduction with HCOOH using 3D Ni@N-CNTs/N-G. Reproduced from ref. 178 with permission from Elsevier. | ||
Tran et al. reported the eco-friendly and simple synthesis of a partially reduced GO/CNT/Fe/Ag hybrid within 10–30 s via simple microwave irradiation.179 During the reduction of 4-nitrophenol using NaBH4, the hybrid exhibited exceptional catalytic efficiency with a rate constant of ∼14.66 × 10−3 s−1. By utilizing its large surface area, graphene oxide helped to immobilize more Ag nanoparticles and rGO/CNT promoted electron transfer in the reduction of 4-nitrophenol owing to its high electrical conductivity. Fe nanoparticles and Fe3C enabled easy recovery with the help of an external magnet. Moreover, the hybrid retained stability and catalytic efficiency even after the 5th cycle.
In another interesting study, sorption and catalysis were simultaneously realized for the effective removal of As5+ and Se6+ over magnetic GO/oxidized CNT hydrogels.180 Sorption facilitated the catalytic reactions; meanwhile, catalytic reduction supported the release of the occupied sorption sites, and thus resumed another sorption-catalysis cycle. The insertion of oxidised CNTs increased the pore volume and specific surface area of the GO hydrogel, which enabled a further enhanced decontamination performance. The hybrid exhibited an outstanding decontamination capacity of 258.2 mg g−1 for As5+ and 46.2 mg g−1 for Se6+. Ultra-fast removal equilibriums were identified within 9 min for As5+ and 2 min for Se6+; moreover, remarkable stability was also exhibited in repeated experiments.
Organic pollutants were removed efficiently by employing magnetic CNT/rGO decorated with silver nanoparticles.181 For generating magnetic carbon nanotubes, Fe3O4 nanoparticles were grown and anchored on MWCNT, and then coated with polydopamine to introduce new surface functionalities. Hydrothermal treatment with GO resulted in reduced GO/polydopamine/magnetic CNT, which exhibited outstanding adsorption capability. The deposition of silver nanoparticles on the nanocomposite integrated an efficient catalytic performance in the removal of methylene blue and 4-nitrophenol. Moreover, easy regeneration of the nanocomposite via desorption using ethanol and water (for more than 15 cycles) and easy magnetic separation show great potential in wastewater treatment.
Kotal and coworkers developed a graphene-assisted Co nanoparticle-embedded nitrogen-doped CNT (G/Co/NCNT) hybrid via the controlled annealing of GO-ZIF-67 under an Ar atmosphere for the photocatalytic degradation of Reactive Black 5 under visible light.182 The high surface area of graphene enabled greater ZIF-67 decoration, thereby the growth of NCNTs was higher in the G/Co/NCNT hybrid compared to Co/NCNT. A higher amount of nitrogen doping led to lowering of the band gap and enhanced photocatalytic activity. The hybrid demonstrated 98% degradation ability after 60 min, had wide pH tolerance and recyclability even after 5 cycles under visible light.
7. Environmental impacts of graphene/carbon nanotube hybrids
The environmental impact of nanomaterials is a matter of concern nowadays, which should be evaluated critically. Understanding the total impact of a process or product on the environment is an inevitable part of green manufacturing and a green product. Evaluation of the total life cycle of a product from raw materials, manufacturing, use, and ultimate reuse/disposal should be performed seriously to maintain environmental sustainability. Life cycle analysis (LCA) is a systematic tool that can be used to study and analyse strategies to meet environmental challenges. The life-cycle paradigm necessitates the consideration of not only the immediate impacts of a process or product, but also its long-term response.183 The outcome of LCA can have a positive impact on the ecosystem, human health and natural resources.Both graphene and carbon nanotubes are resistant to harsh environments. Moreover, they offer good recycling capabilities. The regeneration of graphene and CNT has been studied using acids and bases, making the recovery of contaminants feasible. In certain cases, batch methods appear to be more efficient for contaminant adsorption than fixed bed methods. This choice will allow the maximum adsorption with to be obtained with the minimum amount of adsorbent, thereby reducing the quantities of harmful waste to be treated.184 Both graphene and CNT have been investigated to evaluate their environmental impact and toxicity. Studies on zebra fish have revealed that their cardiac rates and growth are affected by exposure to carbon nanotubes.185 It has also been reported that CNT can interact with biomolecules in water and cause toxic effects.186
Even though graphene is considered a chemically stable material, changes in pH affect the protonation of its hydroxyl or carbonyl groups. At low pH, graphene tends to form aggregates, at high pH it dissolves like a salt, while at neutral pH it stays suspended in the solution.187 The stability of graphene oxide depends on its particle size and functional groups on its surface.188 In this scenario, its dose-related toxicity can vary. Overall, the formation of colloids should be absolutely avoided. Owing to the hydrophilic nature of graphene oxide nanosheets, they can be dispersed in aqueous solution, thereby demonstrating different dispersion performances in comparison to pristine graphene and reduced graphene.189 Therefore, insight into the variability of graphene is essential to understand the interaction, adsorption, transformation, and toxicity criteria of graphene. However, the effect of graphene on the human body is still unknown, although graphene oxide is known to accumulate in the lungs of mice when inhaled, but no pathological outcome was further reported.
To the best of our knowledge, there is very little information available on the risks of exposure to graphene/CNT hybrid materials for both humans and animals and their long-lasting environmental problems. Thus, to efficiently utilize these materials in environmental remediation systems, their release and disposal need to be properly planned and evaluated. Life cycle analysis of these hybrid materials is a potential research area that has to be explored.
8. Conclusion and future perspectives
Environmental pollution by water-soluble pollutants, heavy metal ions and harmful greenhouse gases is triggering significant concern worldwide. Immense progress had already achieved in the synthesis, composite formation and potential applications of graphene/CNT hybrid materials for environmental monitoring, sensing and remediation. The adsorption of various pollutants such as oils, organic solvents and dyes by these hybrid materials is appreciable owing to their facile synthetic methods, high adsorption capability, stable cyclic performance, low cost, easy operation, simple processes and little damage to the environment. Moreover, by employing electrostatic interactions, ion exchange process and surface complexation with metal ions, these materials are widely used for removing metals owing to their high efficiency, simple design, ease of operation, low maintenance cost and capability of metal recovery. Furthermore, with their tuneable pore size and huge surface area, these materials are very effective for the capture of gaseous pollutants.Although great progress has been attained in this field, further research in real-life situations is necessary. To promote further research and practical applications, the following challenges need to be the focus. (i) Considering that various adsorption mechanisms simultaneously occur on the surface of GO and CNTs, which vary with changes in surface functionalization and environmental test conditions, more studies regarding these mechanisms are necessary. (ii) Further research to control the functional groups on the surface of graphene to couple selective adsorption characteristics and ultimate hydrophobicity is essential in this area. (iii) Even though these hybrid materials can provide uniform 3D nanofiltration membranes, which result in elevated permeability and separation capability, studies regarding their practical separation applications are limited. (iv) Although graphene/CNT hybrid materials can be effectively employed as environment-friendly sensing electrodes for the detection of heavy metal ions, studies regarding this are limited. (v) Similarly, these 3D hybrid materials have been found to be effective in gas sensing; however, studies on the capture of gaseous pollutants are in their initial stage. Whether they are capable of carbon capture and even take-over of dust in the atmosphere or indoor environment needs to be examined. (vi) The catalytic conversion of pollutants using these hybrid materials requires further investigation relating to some critical issues regarding their activity and stability enhancement mechanisms. Moreover, many techniques presented in this review are on the laboratory level, their commercialization requires further research.
There are numerous environmental pollutants, only a few of which have been explored to date. Thus, further research to develop smart porous membranes and exploitation of the immense sensing applications of these materials to develop efficient sensing devices is anticipated in the near future. Moreover, for the efficient utilization of these materials in environmental remediation systems, their life cycle analysis is inevitable. This is a potential research area to be explored. It is anticipated that the boundless scope of these 3D hybrid materials will enable the control of environmental pollution, resulting in a sustainable world.
Author contributions
Jomol P. John: conceptualization, resources, writing, reviewing and editing. Mary Nancy T. E.: conceptualization, resources. Bindu Sharmila T. K.: conceptualization, supervision, reviewing and editing.Conflicts of interest
There are no conflicts of interest to declare.References
- A. K. Geim and K. S. Novoselov, The rise of graphene, Nat. Mater., 2007, 6(3), 183–191 CrossRef CAS PubMed
.
- C. Rao, H. R. Matte and K. Subrahmanyam, Synthesis and selected properties of graphene and graphene mimics, Acc. Chem. Res., 2013, 46(1), 149–159 CrossRef CAS PubMed
.
- Y. Zhu, H. Ji, H.-M. Cheng and R. S. Ruoff, Mass production and industrial applications of graphene materials, Natl. Sci. Rev., 2018, 5(1), 90–101 CrossRef CAS
.
- K. S. Novoselov, D. Jiang, F. Schedin, T. Booth, V. Khotkevich and S. Morozov,
et al., Two-dimensional atomic crystals, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(30), 10451–10453 CrossRef CAS PubMed
.
- N. Behabtu, J. R. Lomeda, M. J. Green, A. L. Higginbotham, A. Sinitskii and D. V. Kosynkin,
et al., Spontaneous high-concentration dispersions and liquid crystals of graphene, Nat. Nanotechnol., 2010, 5(6), 406–411 CrossRef CAS PubMed
.
- Y. Zhu, L. Li, C. Zhang, G. Casillas, Z. Sun and Z. Yan,
et al., A seamless three-dimensional carbon nanotube graphene hybrid material, Nat. Commun., 2012, 3(1), 1–7 Search PubMed
.
- G. K. Dimitrakakis, E. Tylianakis and G. E. Froudakis, Pillared graphene: a new 3-D network nanostructure for enhanced hydrogen storage, Nano Lett., 2008, 8(10), 3166–3170 CrossRef CAS PubMed
.
- D. Kondo, S. Sato and Y. Awano, Self-organization of novel carbon composite structure: graphene multi-layers combined perpendicularly with aligned carbon nanotubes, Appl. Phys. Express, 2008, 1(7), 074003 CrossRef
.
- S. Azizighannad and S. Mitra, Controlled synthesis of reduced graphene oxide-carbon nanotube hybrids and their aqueous behavior, J. Nanoparticle Res., 2020, 22(6), 130 CrossRef CAS
.
- V. T. Dang, D. D. Nguyen, T. T. Cao, P. H. Le, D. L. Tran and N. M. Phan,
et al., Recent trends in preparation and application of carbon nanotube–graphene hybrid thin films, Adv. Natl. Sci.: Nanosci. Nanotechnol., 2016, 7(3), 033002 Search PubMed
.
- S. Nardecchia, D. Carriazo, M. L. Ferrer, M. C. Gutiérrez and F. del Monte, Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: synthesis and applications, Chem. Soc. Rev., 2013, 42(2), 794–830 RSC
.
- X. Wu, F. Mu and H. Zhao, Recent progress in the synthesis of graphene/CNT composites and the energy-related applications, J. Mater. Sci. Technol., 2020, 55, 16–34 CrossRef
.
- A. Dasgupta, L. P. Rajukumar, C. Rotella, Y. Lei and M. Terrones, Covalent three-dimensional networks of graphene and carbon nanotubes: synthesis and environmental applications, Nano Today, 2017, 12, 116–135 CrossRef CAS
.
- X. Dong, J. Chen, Y. Ma, J. Wang, M. B. Chan-Park and X. Liu,
et al., Superhydrophobic and superoleophilic hybrid foam of graphene and carbon nanotube for selective removal of oils or organic solvents from the surface of water, Chem. Commun., 2012, 48(86), 10660–10662 RSC
.
- B. Yu, J. Xu, J.-H. Liu, S.-T. Yang, J. Luo and Q. Zhou,
et al., Adsorption behavior of copper ions on graphene oxide–chitosan aerogel, J. Environ. Chem. Eng., 2013, 1(4), 1044–1050 CrossRef CAS
.
- Z.-Y. Wu, C. Li, H.-W. Liang, Y.-N. Zhang, X. Wang and J.-F. Chen,
et al., Carbon nanofiber aerogels for emergent cleanup of oil spillage and chemical leakage under harsh conditions, Sci. Rep., 2015, 4(1), 4079 CrossRef PubMed
.
- M. S. Mauter and M. Elimelech, Environmental Applications of Carbon-Based Nanomaterials, Environ. Sci. Technol., 2008, 42(16), 5843–5859 CrossRef CAS PubMed
.
- X. Dong, G. Xing, M. B. Chan-Park, W. Shi, N. Xiao and J. Wang,
et al., The formation of a carbon nanotube–graphene oxide core–shell structure and its possible applications, Carbon, 2011, 49(15), 5071–5078 CrossRef CAS
.
- A. L. Gorkina, A. P. Tsapenko, E. P. Gilshteyn, T. S. Koltsova, T. V. Larionova and A. Talyzin,
et al., Transparent and conductive hybrid graphene/carbon nanotube films, Carbon, 2016, 100, 501–507 CrossRef CAS
.
- C. Zhang, L. Ren, X. Wang and T. Liu, Graphene Oxide-Assisted Dispersion of Pristine Multiwalled Carbon Nanotubes in Aqueous Media, J. Phys. Chem. C, 2010, 114(26), 11435–11440 CrossRef CAS
.
- L. L. Zhang, Z. Xiong and X. Zhao, Pillaring chemically exfoliated graphene oxide with carbon nanotubes for photocatalytic degradation of dyes under visible light irradiation, ACS Nano, 2010, 4(11), 7030–7036 CrossRef CAS PubMed
.
- S. Badhulika, T. Terse-Thakoor, C. M. Chaves Villarreal and A. Mulchandani, Graphene hybrids: synthesis strategies and applications in sensors and sensitized solar cells, Front. Chem., 2015, 3, 38 Search PubMed
.
-
W. Fan, L. Zhang and T. Liu, Strategies for the Hybridization of CNTs with Graphene in Graphene-Carbon Nanotube Hybrids for Energy and Environmental Applications, Springer, 2017, pp. 21–51 Search PubMed
.
- D. V. Kosynkin, A. L. Higginbotham, A. Sinitskii, J. R. Lomeda, A. Dimiev and B. K. Price,
et al., Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons, Nature, 2009, 458(7240), 872–876 CrossRef CAS PubMed
.
-
R. Paul, M. Vincent, V. Etacheri and A. K. Roy, Carbon nanotubes, graphene, porous carbon, and hybrid carbon-based materials: synthesis, properties, and functionalization for efficient energy storage in Carbon Based Nanomaterials for Advanced Thermal and Electrochemical Energy Storage and Conversion, Elsevier, 2019, pp. 1–24 Search PubMed
.
- S. S. J. Aravind, V. Eswaraiah and S. Ramaprabhu, Facile synthesis of one dimensional graphene wrapped carbon nanotube composites by chemical vapour deposition, J. Mater. Chem., 2011, 21(39), 15179–15182 RSC
.
- Y. Li, Z. Li, L. Lei, T. Lan, Y. Li and P. Li,
et al., Chemical vapor deposition-grown carbon nanotubes/graphene hybrids for electrochemical energy storage and conversion, FlatChem, 2019, 15, 100091 CrossRef CAS
.
- A. Kumar, K. Sharma and A. R. Dixit, Carbon nanotube- and graphene-reinforced multiphase polymeric composites: review on their properties and applications, J. Mater. Sci., 2020, 55(7), 2682–2724 CrossRef CAS
.
- Y. Shen, Q. Fang and B. Chen, Environmental Applications of Three-Dimensional Graphene-Based Macrostructures: Adsorption, Transformation, and Detection, Environ. Sci. Technol., 2015, 49(1), 67–84 CrossRef CAS PubMed
.
- Y. Cheng, P. Xu, W. Zeng, C. Ling, S. Zhao and K. Liao,
et al., Highly hydrophobic and ultralight graphene aerogel as high efficiency oil absorbent material, J. Environ. Chem. Eng., 2017, 5(2), 1957–1963 CrossRef CAS
.
- S. Kizil, Bulbul Sonmez H. One-pot fabrication of reusable hybrid sorbents for quick removal of oils from wastewater, J. Environ. Manage., 2020, 261, 109911 CrossRef CAS PubMed
.
- R.-P. Ren, W. Li and Y.-K. Lv, A robust, superhydrophobic graphene aerogel as a recyclable sorbent for oils and organic solvents at various temperatures, J. Colloid Interface Sci., 2017, 500, 63–68 CrossRef CAS PubMed
.
- V. K. Sharma, T. J. McDonald, H. Kim and V. K. Garg, Magnetic graphene–carbon nanotube iron nanocomposites as adsorbents and antibacterial agents for water purification, Adv. Colloid Interface Sci., 2015, 225, 229–240 CrossRef CAS PubMed
.
- S. Hou, X. Wu, Y. Lv, W. Jia, J. Guo and L. Wang,
et al., Ultralight, highly elastic and bioinspired capillary-driven graphene aerogels for highly efficient organic pollutants absorption, Appl. Surf. Sci., 2020, 509, 144818 CrossRef CAS
.
- J. Huang and Z. Yan, Adsorption mechanism of oil by resilient graphene aerogels from oil–water emulsion, Langmuir, 2018, 34(5), 1890–1898 CrossRef CAS PubMed
.
- H. Wang, C. Wang, S. Liu, L. Chen and S. Yang, Superhydrophobic and superoleophilic graphene aerogel for adsorption of oil pollutants from water, RSC Adv., 2019, 9(15), 8569–8574 RSC
.
- W. Wan, F. Zhang, S. Yu, R. Zhang and Y. Zhou, Hydrothermal formation of graphene aerogel for oil sorption: the role of reducing agent, reaction time and temperature, New J. Chem., 2016, 40(4), 3040–3046 RSC
.
- M. Inagaki, A. Kawahara and H. Konno, Sorption and recovery of heavy oils using carbonized fir fibers and recycling, Carbon, 2002, 40(1), 105–111 CrossRef CAS
.
- A. M. Atta, R. A. M. El-Ghazawy, R. K. Farag and A. A. A. Abdel-Azim, Swelling and Network Parameters of Oil Sorbers Based on Alkyl Acrylates and Cinnamoyloxy Ethyl Methacrylate Copolymers, J. Polym. Res., 2006, 13(4), 257–266 CrossRef CAS
.
- V. Janout, S. B. Myers, R. A. Register and S. L. Regen, Self-Cleaning Resins, J. Am. Chem. Soc., 2007, 129(17), 5756–5759 CrossRef CAS PubMed
.
- T. Ono, T. Sugimoto, S. Shinkai and K. Sada, Lipophilic polyelectrolyte gels as super-absorbent polymers for nonpolar organic solvents, Nat. Mater., 2007, 6(6), 429–433 CrossRef CAS PubMed
.
- T. Ono, T. Sugimoto, S. Shinkai and K. Sada, Molecular Design of Superabsorbent Polymers for Organic Solvents by Crosslinked Lipophilic Polyelectrolytes, Adv. Funct. Mater., 2008, 18(24), 3936–3940 CrossRef CAS
.
- J. Yuan, X. Liu, O. Akbulut, J. Hu, S. L. Suib and J. Kong,
et al., Superwetting nanowire membranes for selective absorption, Nat. Nanotechnol., 2008, 3(6), 332–336 CrossRef CAS PubMed
.
- A. Sayari, S. Hamoudi and Y. Yang, Applications of Pore-Expanded Mesoporous Silica. 1. Removal of Heavy Metal Cations and Organic Pollutants from Wastewater, Chem. Mater., 2005, 17(1), 212–216 CrossRef CAS
.
- C. Wu, X. Huang, X. Wu, R. Qian and P. Jiang, Mechanically Flexible and Multifunctional Polymer-Based Graphene Foams for Elastic Conductors and Oil–Water Separators, Adv. Mater., 2013, 25(39), 5658–5662 CrossRef CAS PubMed
.
- Y. Chu and Q. Pan, Three-Dimensionally Macroporous Fe/C Nanocomposites As Highly Selective Oil-Absorption Materials, ACS Appl. Mater. Interfaces, 2012, 4(5), 2420–2425 CrossRef CAS PubMed
.
- H. Liu and H. Qiu, Recent advances of 3D graphene-based adsorbents for sample preparation of water pollutants: a review, Chem. Eng. J., 2020, 393, 124691 CrossRef CAS
.
- H. Bi, X. Xie, K. Yin, Y. Zhou, S. Wan and L. He,
et al., Spongy Graphene as a Highly Efficient and Recyclable Sorbent for Oils and Organic Solvents, Adv. Funct. Mater., 2012, 22(21), 4421–4425 CrossRef CAS
.
- A. B. Dichiara, T. J. Sherwood, J. Benton-Smith, J. C. Wilson, S. J. Weinstein and R. E. Rogers, Free-standing carbon nanotube/graphene hybrid papers as next generation adsorbents, Nanoscale, 2014, 6(12), 6322–6327 RSC
.
- X. Gui, J. Wei, K. Wang, A. Cao, H. Zhu and Y. Jia,
et al., Carbon nanotube sponges, Adv. Mater., 2010, 22(5), 617–621 CrossRef CAS PubMed
.
- F. Hu, S. Chen, C. Wang, R. Yuan, D. Yuan and C. Wang, Study on the application of reduced graphene oxide and multiwall carbon nanotubes hybrid materials for simultaneous determination of catechol, hydroquinone, p-cresol and nitrite, Anal. Chim. Acta, 2012, 724, 40–46 CrossRef CAS PubMed
.
-
C. P. Karan, R. Rengasamy and D. Das, Oil spill cleanup by structured fibre assembly. 2011 Search PubMed
.
- S. Sabir, Approach of cost-effective adsorbents for oil removal from oily water, Crit. Rev. Environ.
Sci. Technol., 2015, 45(17), 1916–1945 CrossRef CAS
.
- S. Yang, L. Chen, L. Mu, B. Hao, J. Chen and P.-C. Ma, Graphene foam with hierarchical structures for the removal of organic pollutants from water, RSC Adv., 2016, 6(6), 4889–4898 RSC
.
- S. Yang, L. Chen, C. Wang, M. Rana and P.-C. Ma, Surface roughness induced superhydrophobicity of graphene foam for oil-water separation, J. Colloid Interface Sci., 2017, 508, 254–262 CrossRef CAS PubMed
.
- Y. Qian, I. M. Ismail and A. Stein, Ultralight, high-surface-area, multifunctional graphene-based aerogels from self-assembly of graphene oxide and resol, Carbon, 2014, 68, 221–231 CrossRef CAS
.
- S. Stankovich, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets, Carbon, 2006, 44(15), 3342–3347 CrossRef CAS
.
- S. Stankovich, D. A. Dikin, G. H. Dommett, K. M. Kohlhaas, E. J. Zimney and E. A. Stach,
et al., Graphene-based composite materials, Nature, 2006, 442(7100), 282–286 CrossRef CAS PubMed
.
- S. Park, J. An, I. Jung, R. D. Piner, S. J. An and X. Li,
et al., Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents, Nano Lett., 2009, 9(4), 1593–1597 CrossRef CAS PubMed
.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes and Y. Jia,
et al., Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide, Carbon, 2007, 45(7), 1558–1565 CrossRef CAS
.
- H. He, T. Riedl, A. Lerf, J. Klinowski and N. M. R. Solid-state, studies of the structure of graphite oxide, J. Phys. Chem., 1996, 100(51), 19954–19958 CrossRef CAS
.
- H. He, J. Klinowski, M. Forster and A. Lerf, A new structural model for graphite oxide, Chem. Phys. Lett., 1998, 287(1–2), 53–56 CrossRef CAS
.
- A. Lerf, H. He, T. Riedl, M. Forster and J. Klinowski,
13C and 1H MAS NMR studies of graphite oxide and its chemically modified derivatives, Solid State Ionics, 1997, 101, 857–862 CrossRef
.
- A. Lerf, H. He, M. Forster and J. Klinowski, Structure of graphite oxide revisited, J. Phys. Chem. B, 1998, 102(23), 4477–4482 CrossRef CAS
.
- A. B. Bourlinos, D. Gournis, D. Petridis, T. Szabó, A. Szeri and I. Dékány, Graphite oxide: chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids, Langmuir, 2003, 19(15), 6050–6055 CrossRef CAS
.
- N. A. Kotov, I. Dékány and J. H. Fendler, Ultrathin graphite oxide–polyelectrolyte composites prepared by self-assembly: transition between conductive and non-conductive states, Adv. Mater., 1996, 8(8), 637–641 CrossRef CAS
.
- Y. Zhao, C. Hu, Y. Hu, H. Cheng, G. Shi and L. Qu, A versatile, ultralight, nitrogen-doped graphene framework, Angew. Chem., Int. Ed., 2012, 51(45), 11371–11375 CrossRef CAS PubMed
.
- H. Li, L. Liu and F. Yang, Covalent assembly of 3D graphene/polypyrrole foams for oil spill cleanup, J. Mater. Chem. A, 2013, 1(10), 3446–3453 RSC
.
- H. Sun, Z. Xu and C. Gao, Multifunctional, ultra-flyweight, synergistically assembled carbon aerogels, Adv. Mater., 2013, 25(18), 2554–2560 CrossRef CAS PubMed
.
- S. Kabiri, D. N. H. Tran, T. Altalhi and D. Losic, Outstanding adsorption performance of graphene–carbon nanotube aerogels for continuous oil removal, Carbon, 2014, 80, 523–533 CrossRef CAS
.
- N. Xiao, Y. Zhou, Z. Ling and J. Qiu, Synthesis of a carbon nanofiber/carbon foam composite from coal liquefaction residue for the separation of oil and water, Carbon, 2013, 59, 530–536 CrossRef CAS
.
- C.-F. Wang and S.-J. Lin, Robust superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from water, ACS Appl. Mater. Interfaces, 2013, 5(18), 8861–8864 CrossRef CAS PubMed
.
- S. Suni, A.-L. Kosunen, M. Hautala, A. Pasila and M. Romantschuk, Use of a by-product of peat excavation, cotton grass fibre, as a sorbent for oil-spills, Mar. Pollut. Bull., 2004, 49(11–12), 916–921 CrossRef CAS PubMed
.
- M. Hussein, A. Amer and I. Sawsan, Oil spill sorption using carbonized pith bagasse: 1. Preparation and characterization of carbonized pith bagasse, J. Anal. Appl. Pyrolysis, 2008, 82(2), 205–211 CrossRef CAS
.
- W. Wan, R. Zhang, W. Li, H. Liu, Y. Lin and L. Li,
et al., Graphene–carbon nanotube aerogel as an ultra-light, compressible and recyclable highly efficient absorbent for oil and dyes, Environ. Sci.: Nano, 2016, 3(1), 107–113 RSC
.
- C. Wang, S. Yang, Q. Ma, X. Jia and P.-C. Ma, Preparation of carbon nanotubes/graphene hybrid aerogel and its application for the adsorption of organic compounds, Carbon, 2017, 118, 765–771 CrossRef CAS
.
- W. Zhan, S. Yu, L. Gao, F. Wang, X. Fu and G. Sui,
et al., Bioinspired Assembly of Carbon Nanotube into Graphene Aerogel with “Cabbagelike” Hierarchical Porous Structure for Highly Efficient Organic Pollutants Cleanup, ACS Appl. Mater. Interfaces, 2018, 10(1), 1093–1103 CrossRef CAS PubMed
.
- T. Tao, G. Li, Y. He and P. Duan, Hybrid carbon nanotubes/graphene/nickel fluffy spheres for fast magnetic separation and efficient removal of organic solvents from water, Mater. Lett., 2019, 254, 440–443 CrossRef CAS
.
- J. Cai, J. Tian, H. Gu and Z. Guo, Amino carbon nanotube modified reduced graphene oxide aerogel for oil/water separation, ES Mater. Manuf., 2019, 6(2), 68–74 Search PubMed
.
- D. P. Hashim, N. T. Narayanan, J. M. Romo-Herrera, D. A. Cullen, M. G. Hahm and P. Lezzi,
et al., Covalently bonded three-dimensional carbon nanotube solids via boron induced nanojunctions, Sci. Rep., 2012, 2(1), 363 CrossRef PubMed
.
- L. Labiadh and A. R. Kamali, 3D graphene nanoedges as efficient dye adsorbents with ultra-high thermal regeneration performance, Appl. Surf. Sci., 2019, 490, 383–394 CrossRef CAS
.
- R. Nasiri and N. Arsalani, Synthesis and application of 3D graphene nanocomposite for the removal of cationic dyes from aqueous solutions: response surface methodology design, J. Cleaner Prod., 2018, 190, 63–71 CrossRef CAS
.
- Y. Liu, Y. Tian, C. Luo, G. Cui and S. Yan, One-pot preparation of a MnO2–graphene–carbon nanotube hybrid material for the removal of methyl orange from aqueous solutions, New J. Chem., 2015, 39(7), 5484–5492 RSC
.
- Z. Sui, Q. Meng, X. Zhang, R. Ma and B. Cao, Green synthesis of carbon nanotube–graphene hybrid aerogels and their use as versatile agents for water purification, J. Mater. Chem., 2012, 22(18), 8767 RSC
.
- S. K. Das, J. Bhowal, A. R. Das and A. K. Guha, Adsorption behavior of rhodamine B on rhizopus o ryzae biomass, Langmuir, 2006, 22(17), 7265–7272 CrossRef CAS PubMed
.
- L. Sun, C. Tian, L. Wang, J. Zou, G. Mu and H. Fu, Magnetically separable porous graphitic carbon with large surface area as excellent adsorbents for metal ions and dye, J. Mater. Chem., 2011, 21(20), 7232–7239 RSC
.
- B. S. Girgis, A. M. Soliman and N. A. Fathy, Development of micro-mesoporous carbons from several seed hulls under varying conditions of activation, Microporous Mesoporous Mater., 2011, 142(2–3), 518–525 CrossRef CAS
.
- L. Ai and J. Jiang, Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene–carbon nanotube hybrid, Chem. Eng. J., 2012, 192, 156–163 CrossRef CAS
.
- M. Kotal and A. K. Bhowmick, Multifunctional hybrid materials based on carbon nanotube chemically bonded to reduced graphene oxide, J. Phys. Chem. C, 2013, 117(48), 25865–25875 CrossRef CAS
.
- K. Goh, W. Jiang, H. E. Karahan, S. Zhai, L. Wei and D. Yu,
et al., All-carbon nanoarchitectures as high-performance separation membranes with superior stability, Adv. Funct. Mater., 2015, 25(47), 7348–7359 CrossRef CAS
.
- B. Lee, S. Lee, M. Lee, D. H. Jeong, Y. Baek and J. Yoon,
et al., Carbon nanotube-bonded graphene hybrid aerogels and their application to water purification, Nanoscale, 2015, 7(15), 6782–6789 RSC
.
- N. Li, M. Zheng, X. Chang, G. Ji, H. Lu and L. Xue,
et al., Preparation of magnetic CoFe2O4-functionalized graphene sheets via a facile hydrothermal method and their adsorption properties, J. Solid State Chem., 2011, 184(4), 953–958 CrossRef CAS
.
- Y. Yao, S. Miao, S. Liu, L. P. Ma, H. Sun and S. Wang, Synthesis, characterization, and adsorption properties of magnetic Fe3O4@ graphene nanocomposite, Chem. Eng. J., 2012, 184, 326–332 CrossRef CAS
.
- X. Chen, M. Qiu, H. Ding, K. Fu and Y. Fan, A reduced graphene oxide nanofiltration membrane intercalated by well-dispersed carbon nanotubes for drinking water purification, Nanoscale, 2016, 8(10), 5696–5705 RSC
.
- M. O. Ansari, R. Kumar, S. A. Ansari, S. P. Ansari, M. A. Barakat and A. Alshahrie,
et al., Anion selective pTSA doped polyaniline@graphene oxide-multiwalled carbon nanotube composite for Cr(VI) and Congo red adsorption, J. Colloid Interface Sci., 2017, 496, 407–415 CrossRef CAS PubMed
.
- Y. Huang, J. Zhu, H. Liu, Z. Wang and X. Zhang, Preparation of porous graphene/carbon nanotube composite and adsorption mechanism of methylene blue, SN Appl. Sci., 2019, 1(1), 37 CrossRef
.
- F. Yue, Q. Zhang, L. Xu, Y. Zheng, C. Yao and J. Jia,
et al., Porous Reduced Graphene Oxide/Single-Walled Carbon Nanotube Film as Freestanding and Flexible Electrode Materials for Electrosorption of Organic Dye, ACS Appl. Nano Mater., 2019, 2(10), 6258–6267 CrossRef CAS
.
- S. Su, Y. Liu, W. He, X. Tang, W. Jin and Y. Zhao, A novel graphene oxide-carbon nanotubes anchored α-FeOOH hybrid activated persulfate system for enhanced degradation of Orange II, J. Environ. Sci., 2019, 83, 73–84 CrossRef PubMed
.
- T. Yao, L. Qiao and K. Du, High tough and highly porous graphene/carbon nanotubes hybrid beads enhanced by carbonized polyacrylonitrile for efficient dyes adsorption, Microporous Mesoporous Mater., 2020, 292, 109716 CrossRef CAS
.
- C. Hu, D. Grant, X. Hou and F. Xu, High rhodamine B and methyl orange removal performance of graphene oxide/carbon nanotube nanostructures, Mater. Today Proc., 2020, S2214785320314772 Search PubMed
.
- T. Liu, B. Gao, J. Fang, B. Wang and X. Cao, Biochar-supported carbon nanotube and graphene oxide nanocomposites for Pb(II) and Cd(II) removal, RSC Adv., 2016, 6(29), 24314–24319 RSC
.
- R. Nasiri, N. Arsalani and Y. Panahian, One-pot synthesis of novel magnetic three-dimensional graphene/chitosan/nickel ferrite nanocomposite for lead ions removal from aqueous solution: RSM modelling design, J. Cleaner Prod., 2018, 201, 507–515 CrossRef CAS
.
- B. Tan, H. Zhao, Y. Zhang, X. Quan, Z. He and W. Zheng,
et al., Amphiphilic PA-induced three-dimensional graphene macrostructure with enhanced removal of heavy metal ions, J. Colloid Interface Sci., 2018, 512, 853–861 CrossRef CAS PubMed
.
- S. Wang, H. Sun, H.-M. Ang and M. Tadé, Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials, Chem. Eng. J., 2013, 226, 336–347 CrossRef CAS
.
- D. Zhao, Y. Wang, S. Zhao, M. Wakeel, Z. Wang and R. S. Shaikh,
et al., A simple method for preparing ultra-light graphene aerogel for rapid removal of U(VI) from aqueous solution, Environ. Pollut., 2019, 251, 547–554 CrossRef CAS PubMed
.
- H. Gao, Y. Sun, J. Zhou, R. Xu and H. Duan, Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification, ACS Appl. Mater. Interfaces, 2013, 5(2), 425–432 CrossRef CAS PubMed
.
- Y. Lei, F. Chen, Y. Luo and L. Zhang, Synthesis of three-dimensional graphene oxide foam for the removal of heavy metal ions, Chem. Phys. Lett., 2014, 593, 122–127 CrossRef CAS
.
- X. Mi, G. Huang, W. Xie, W. Wang, Y. Liu and J. Gao, Preparation of graphene oxide aerogel and its adsorption for Cu2+ ions, Carbon, 2012, 50(13), 4856–4864 CrossRef CAS
.
- M. Liu, C. Chen, J. Hu, X. Wu and X. Wang, Synthesis of magnetite/graphene oxide composite and application for cobalt(II) removal, J. Phys. Chem. C, 2011, 115(51), 25234–25240 CrossRef CAS
.
- G. Zhao, X. Ren, X. Gao, X. Tan, J. Li and C. Chen,
et al., Removal of Pb(II) ions from aqueous solutions on few-layered graphene oxide nanosheets, Dalton Trans., 2011, 40(41), 10945–10952 RSC
.
- N. Zhang, H. Qiu, Y. Si, W. Wang and J. Gao, Fabrication of highly porous biodegradable monoliths strengthened by graphene oxide and their adsorption of metal ions, Carbon, 2011, 49(3), 827–837 CrossRef CAS
.
- B. Xiao and K. Thomas, Competitive adsorption of aqueous metal ions on an oxidized nanoporous activated carbon, Langmuir, 2004, 20(11), 4566–4578 CrossRef CAS PubMed
.
- M. Benitez, D. Das, R. Ferreira, U. Pischel and H. García, Urea-containing mesoporous silica for the adsorption of Fe(III) cations, Chem. Mater., 2006, 18(23), 5597–5603 CrossRef CAS
.
- A. Liu, K. Hidajat, S. Kawi and D. Zhao, A new class of hybrid mesoporous materials with functionalized organic monolayers for selective adsorption of heavy metal ions, Chem. Commun., 2000,(13), 1145–1146 RSC
.
- L. Li, G. Zhou, Z. Weng, X.-Y. Shan, F. Li and H.-M. Cheng, Monolithic Fe2O3/graphene hybrid for highly efficient lithium storage and arsenic removal, Carbon, 2014, 67, 500–507 CrossRef CAS
.
- M. Zhang, B. Gao, X. Cao and L. Yang, Synthesis of a multifunctional graphene–carbon nanotube aerogel and its strong adsorption of lead from aqueous solution, RSC Adv., 2013, 3(43), 21099–21105 RSC
.
- S. Vadahanambi, S.-H. Lee, W.-J. Kim and I.-K. Oh, Arsenic removal from contaminated water using three-dimensional graphene-carbon nanotube-iron oxide nanostructures, Environ. Sci. Technol., 2013, 47(18), 10510–10517 CAS
.
- B. P. Viraka Nellore, R. Kanchanapally, F. Pedraza, S. S. Sinha, A. Pramanik and A. T. Hamme,
et al., Bio-conjugated CNT-bridged 3D porous graphene oxide membrane for highly efficient disinfection of pathogenic bacteria and removal of toxic metals from water, ACS Appl. Mater. Interfaces, 2015, 7(34), 19210–19218 CrossRef CAS PubMed
.
- Z. Gu, Y. Wang, J. Tang, J. Yang, J. Liao and Y. Yang,
et al., The removal of uranium(VI) from aqueous solution by graphene oxide–carbon nanotubes hybrid aerogels, J. Radioanal. Nucl. Chem., 2015, 303(3), 1835–1842 CAS
.
- X. Zhu, Y. Cui, X. Chang and H. Wang, Selective solid-phase extraction and analysis of trace-level Cr(III), Fe(III), Pb(II), and Mn(II) Ions in wastewater using diethylenetriamine-functionalized carbon nanotubes dispersed in graphene oxide colloids, Talanta, 2016, 146, 358–363 CrossRef CAS PubMed
.
- L. Guo, Y. Xu, M. Zhuo, L. Liu, Q. Xu and L. Wang,
et al., Highly efficient removal of Gd(III) using hybrid hydrosols of carbon nanotubes/graphene oxide in dialysis bags and synergistic enhancement effect, Chem. Eng. J., 2018, 348, 535–545 CrossRef CAS
.
- W. Zhan, L. Gao, X. Fu, S. H. Siyal, G. Sui and X. Yang, Green synthesis of amino-functionalized carbon nanotube-graphene hybrid aerogels for high performance heavy metal ions removal, Appl. Surf. Sci., 2019, 467, 1122–1133 CrossRef
.
- A. Ghozatloo and M. Shariaty-Niassar, Graphene/CNT hybrid effect on Absorption of Mn2+ by Activated Carbon, Anal. Methods Environ. Chem. J., 2019, 2(2), 31–36 CrossRef
.
- H. Huang, T. Chen, X. Liu and H. Ma, Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials, Anal. Chim. Acta, 2014, 852, 45–54 CrossRef CAS PubMed
.
- F. Yang, D. He, B. Zheng, D. Xiao, L. Wu and Y. Guo, Self-assembled hybrids with xanthate functionalized carbon nanotubes and electro-exfoliating graphene sheets for electrochemical sensing of copper ions, J. Electroanal. Chem., 2016, 767, 100–107 CrossRef CAS
.
- P. N. D. Duoc, N. H. Binh, T. V. Hau, C. T. Thanh, P. V. Trinh and N. V. Tuyen,
et al., A novel electrochemical sensor based on double-walled carbon nanotubes and graphene hybrid thin film for arsenic(V) detection, J. Hazard. Mater., 2020, 400, 123185 CrossRef CAS PubMed
.
- T. AL-Gahouari, G. Bodkhe, P. Sayyad, N. Ingle, M. Mahadik and S. M. Shirsat,
et al., Electrochemical Sensor: L-Cysteine Induced Selectivity Enhancement of Electrochemically Reduced Graphene Oxide–Multiwalled Carbon Nanotubes Hybrid for Detection of Lead (Pb2+) Ions, Front. Mater., 2020, 7, 68 CrossRef
.
- J. Kong, N. R. Franklin, C. Zhou, M. G. Chapline, S. Peng and K. Cho,
et al., Nanotube molecular wires as chemical sensors, Science, 2000, 287(5453), 622–625 CrossRef CAS PubMed
.
- Q. Wan, Q. Li, Y. Chen, T.-H. Wang, X. He and J. Li,
et al., Fabrication and ethanol sensing characteristics of ZnO nanowire gas sensors, Appl. Phys. Lett., 2004, 84(18), 3654–3656 CrossRef CAS
.
- F. Schedin, A. K. Geim, S. V. Morozov, E. Hill, P. Blake and M. Katsnelson,
et al., Detection of individual gas molecules adsorbed on graphene, Nat. Mater., 2007, 6(9), 652–655 CrossRef CAS PubMed
.
- J. D. Fowler, M. J. Allen, V. C. Tung, Y. Yang, R. B. Kaner and B. H. Weiller, Practical chemical sensors from chemically derived graphene, ACS Nano, 2009, 3(2), 301–306 CrossRef CAS PubMed
.
- G. Lu, L. E. Ocola and J. Chen, Reduced graphene oxide for room-temperature gas sensors, Nanotechnology, 2009, 20(44), 445502 CrossRef PubMed
.
- T. Zhang, S. Mubeen, N. V. Myung and M. A. Deshusses, Recent progress in carbon nanotube-based gas sensors, Nanotechnology, 2008, 19(33), 332001 CrossRef PubMed
.
- H. Y. Jeong, D.-S. Lee, H. K. Choi, D. H. Lee, J.-E. Kim and J. Y. Lee,
et al., Flexible room-temperature NO2 gas sensors based on carbon nanotubes/reduced graphene hybrid films, Appl. Phys. Lett., 2010, 96(21), 213105 CrossRef
.
- A. Kaniyoor and S. Ramaprabhu, Hybrid carbon nanostructured ensembles as chemiresistive hydrogen gas sensors, Carbon, 2011, 49(1), 227–236 CrossRef CAS
.
- X. Dong, Y. Ma, G. Zhu, Y. Huang, J. Wang and M. B. Chan-Park,
et al., Synthesis of graphene–carbon nanotube hybrid foam and its use as a novel three-dimensional electrode for electrochemical sensing, J. Mater. Chem., 2012, 22(33), 17044–17048 RSC
.
- S. Liu, B. Yu, H. Zhang, T. Fei and T. Zhang, Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids, Sens. Actuators, B, 2014, 202, 272–278 CrossRef CAS
.
- W. Yuan, A. Liu, L. Huang, C. Li and G. Shi, High-performance NO2 sensors based on chemically modified graphene, Adv. Mater., 2013, 25(5), 766–771 CrossRef CAS PubMed
.
- S.-J. Choi, B.-H. Jang, S.-J. Lee, B. K. Min, A. Rothschild and I.-D. Kim, Selective detection of acetone and hydrogen sulfide for the diagnosis of diabetes and halitosis using SnO2 nanofibers functionalized with reduced graphene oxide nanosheets, ACS Appl. Mater. Interfaces, 2014, 6(4), 2588–2597 CrossRef CAS PubMed
.
- C. Marichy, P. A. Russo, M. Latino, J.-P. Tessonnier, M.-G. Willinger and N. Donato,
et al., Tin dioxide–carbon heterostructures applied to gas sensing: structure-dependent properties and general sensing mechanism, J. Phys. Chem. C, 2013, 117(38), 19729–19739 CAS
.
- G. Neri, S. G. Leonardi, M. Latino, N. Donato, S. Baek and D. E. Conte,
et al., Sensing behavior of SnO2/reduced graphene oxide nanocomposites toward NO2, Sens. Actuators, B, 2013, 179, 61–68 CrossRef CAS
.
- Z. Zhang, R. Zou, G. Song, L. Yu, Z. Chen and J. Hu, Highly aligned SnO2 nanorods on graphene sheets for gas sensors, J. Mater. Chem., 2011, 21(43), 17360–17365 RSC
.
- Q. Lin, Y. Li and M. Yang, Tin oxide/graphene composite fabricated via a hydrothermal method for gas sensors working at room temperature, Sens. Actuators, B, 2012, 173, 139–147 CrossRef CAS
.
- S. Mao, S. Cui, G. Lu, K. Yu, Z. Wen and J. Chen, Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals, J. Mater. Chem., 2012, 22(22), 11009–11013 RSC
.
- P. A. Russo, N. Donato, S. G. Leonardi, S. Baek, D. E. Conte and G. Neri,
et al., Room-temperature hydrogen sensing with heteronanostructures based on reduced graphene oxide and tin oxide, Angew. Chem., Int. Ed., 2012, 51(44), 11053–11057 CrossRef CAS PubMed
.
- S. Liu, Z. Wang, Y. Zhang, C. Zhang and T. Zhang, High performance room temperature NO2 sensors based on reduced graphene oxide-multiwalled carbon nanotubes-tin oxide nanoparticles hybrids, Sens. Actuators, B, 2015, 211, 318–324 CrossRef CAS
.
- T. T. Tung, C. Pham-Huu, I. Janowska, T. Kim, M. Castro and J.-F. Feller, Hybrid Films of Graphene and Carbon Nanotubes for High Performance Chemical and Temperature Sensing Applications, Small, 2015, 11(28), 3485–3493 CrossRef CAS PubMed
.
- S. K. Mishra, S. N. Tripathi, V. Choudhary and B. D. Gupta, Surface Plasmon Resonance-Based Fiber Optic Methane Gas Sensor Utilizing Graphene-Carbon Nanotubes-Poly(Methyl Methacrylate) Hybrid Nanocomposite, Plasmonics, 2015, 10(5), 1147–1157 CrossRef CAS
.
- A. Bisht, S. Chockalingam, O. Panwar, A. Kesarwani, B. Singh and V. Singh, Structural, field emission and ammonia gas sensing properties of multiwalled carbon nanotube-graphene like hybrid films deposited by microwave plasma enhanced chemical vapor deposition technique, Sci. Adv. Mater., 2015, 7(7), 1424–1434 CrossRef CAS
.
- U. Yaqoob, A. I. Uddin and G.-S. Chung, A high-performance flexible NO2 sensor based on WO3 NPs decorated on MWCNTs and RGO hybrids on PI/PET substrates, Sens. Actuators, B, 2016, 224, 738–746 CrossRef CAS
.
- S.-J. Li, J.-C. Zhang, J. Li, H.-Y. Yang, J.-J. Meng and B. Zhang, A 3D sandwich structured hybrid of gold nanoparticles decorated MnO2/graphene-carbon nanotubes as high performance H2O2 sensors, Sens. Actuators, B, 2018, 260, 1–11 CrossRef CAS
.
- Y. Seekaew, A. Wisitsoraat, D. Phokharatkul and C. Wongchoosuk, Room temperature toluene gas sensor based on TiO2 nanoparticles decorated 3D graphene-carbon nanotube nanostructures, Sens. Actuators, B, 2019, 279, 69–78 CrossRef CAS
.
- Y. Seekaew, W. Pon-On and C. Wongchoosuk, Ultrahigh selective room-temperature ammonia gas sensor based on tin–titanium dioxide/reduced graphene/carbon nanotube nanocomposites by the solvothermal method, ACS Omega, 2019, 4(16), 16916–16924 CrossRef CAS PubMed
.
- S. Pyo, J. Choi and J. Kim, A Fully Transparent, Flexible, Sensitive, and Visible-Blind Ultraviolet Sensor Based on Carbon Nanotube-Graphene Hybrid, Adv. Electron. Mater., 2019, 5(2), 1800737 CrossRef
.
- F. Ghasemi, Vertically aligned carbon nanotubes, MoS2–rGo based optoelectronic hybrids for NO2 gas sensing, Sci. Rep., 2020, 10(1), 11306 CrossRef PubMed
.
- P. M. Sudeep, T. N. Narayanan, A. Ganesan, M. M. Shaijumon, H. Yang and S. Ozden,
et al., Covalently interconnected three-dimensional graphene oxide solids, ACS Nano, 2013, 7(8), 7034–7040 CrossRef CAS PubMed
.
- Z.-Y. Sui, Y. Cui, J.-H. Zhu and B.-H. Han, Preparation of three-dimensional graphene oxide–polyethylenimine porous materials as dye and gas adsorbents, ACS Appl. Mater. Interfaces, 2013, 5(18), 9172–9179 CrossRef CAS PubMed
.
- Y. He, N. Zhang, F. Wu, F. Xu, Y. Liu and J. Gao, Graphene oxide foams and their excellent adsorption ability for acetone gas, Mater. Res. Bull., 2013, 48(9), 3553–3558 CrossRef CAS
.
- J. Liang, Z. Cai, L. Li, L. Guo and J. Geng, Scalable and facile preparation of graphene aerogel for air purification, RSC Adv., 2014, 4(10), 4843–4847 RSC
.
- M. De Marco, R. Menzel, S. M. Bawaked, M. Mokhtar, A. Y. Obaid and S. N. Basahel,
et al., Hybrid effects in graphene oxide/carbon nanotube-supported layered double hydroxides: enhancing the CO2 sorption properties, Carbon, 2017, 123, 616–627 CrossRef CAS
.
- L. Wu, Z. Qin, L. Zhang, T. Meng, F. Yu and J. Ma, CNT-enhanced amino-functionalized graphene aerogel adsorbent for highly efficient removal of formaldehyde, New J. Chem., 2017, 41(7), 2527–2533 RSC
.
- B. Bhaduri, M. Engel, T. Polubesova, W. Wu, B. Xing and B. Chefetz, Dual functionality of an Ag–Fe3O4-carbon nanotube composite material: Catalytic reduction and antibacterial activity, J. Environ. Chem. Eng., 2018, 6(4), 4103–4113 CrossRef CAS
.
- U. Szewzyk, R. Szewzyk, W. Manz and K.-H. Schleifer, Microbiological Safety of Drinking Water, Annu. Rev. Microbiol., 2000, 54(1), 81–127 CrossRef CAS PubMed
.
- L. L. Zhang, Z. Xiong and X. Zhao, Pillaring chemically exfoliated graphene oxide with carbon nanotubes for photocatalytic degradation of dyes under visible light irradiation, ACS Nano, 2010, 4(11), 7030–7036 CrossRef CAS PubMed
.
- B. Zeng, X. Chen, X. Ning, C. Chen, W. Deng and Q. Huang,
et al., Electrostatic-assembly three-dimensional CNTs/rGO implanted Cu2O composite spheres and its photocatalytic properties, Appl. Surf. Sci., 2013, 276, 482–486 CrossRef CAS
.
- C. Wang, M. Cao, P. Wang, Y. Ao, J. Hou and J. Qian, Preparation of graphene–carbon nanotube-TiO2 composites with enhanced photocatalytic activity for the removal of dye and Cr(VI), Appl. Catal., A, 2014, 473, 83–89 CrossRef CAS
.
- L. Zhang, H. Li, Y. Liu, Z. Tian, B. Yang and Z. Sun,
et al., Adsorption-photocatalytic degradation of methyl orange over a facile one-step hydrothermally synthesized TiO2/ZnO–NH2–RGO nanocomposite, RSC Adv., 2014, 4(89), 48703–48711 RSC
.
- N. A. Fathy, S. E. El-Shafey, O. I. El-Shafey and W. S. Mohamed, Oxidative degradation of RB19 dye by a novel γ-MnO2/MWCNT nanocomposite catalyst with H2O2, J. Environ. Chem. Eng., 2013, 1(4), 858–864 CrossRef CAS
.
- N. A. Fathy, S. M. El-Khouly, N. A. Hassan and R. M. Awad, Free-and Ni-doped carbon xerogels catalysts for wet peroxide oxidation of methyl orange, J. Water Process Eng., 2017, 16, 21–27 CrossRef
.
- R. S. Ribeiro, N. A. Fathy, A. A. Attia, A. M. Silva, J. L. Faria and H. T. Gomes, Activated carbon xerogels for the removal of the anionic azo dyes Orange II and Chromotrope 2R by adsorption and catalytic wet peroxide oxidation, Chem. Eng. J., 2012, 195, 112–121 CrossRef
.
- N. A. Fathy, S. E. El-Shafey and O. I. El-Shafey, Synthesis of a novel MnO2 @carbon nanotubes-graphene hybrid catalyst (MnO2@CNT-G) for catalytic oxidation of basic red 18 dye (BR18), J. Water Process Eng., 2017, 17, 95–101 CrossRef
.
- H. Wang, S. Li, Y. Si, Z. Sun, S. Li and Y. Lin, Recyclable enzyme mimic of cubic Fe3O4 nanoparticles loaded on graphene oxide-dispersed carbon nanotubes with enhanced peroxidase-like catalysis and electrocatalysis, J. Mater. Chem. B, 2014, 2(28), 4442–4448 RSC
.
- Y. Liu, X. Liu, Y. Zhao and D. D. Dionysiou, Aligned α-FeOOH nanorods anchored on a graphene oxide-carbon nanotubes aerogel can serve as an effective Fenton-like oxidation catalyst, Appl. Catal., B, 2017, 213, 74–86 CrossRef CAS
.
- L. Qu, G. Zhu, J. Ji, T. Yadav, Y. Chen and G. Yang,
et al., Recyclable visible light-driven Og-C3N4/graphene oxide/N-carbon nanotube membrane for efficient removal of organic pollutants, ACS Appl. Mater. Interfaces, 2018, 10(49), 42427–42435 CrossRef CAS PubMed
.
- G. Yi, Z. Chang, G. Liu and L. Yang,
In situ fabrication of copper nanocubes with platinum skin on 3D graphene-carbon nanotubes hybrid for efficient methanol electrooxidation, Int. J. Electrochem. Sci., 2019, 14, 7232–7240 CrossRef CAS
.
- Z. Mohammadi and M. H. Entezari, Sono-synthesis approach in uniform loading of ultrafine Ag nanoparticles on reduced graphene oxide nanosheets: an efficient catalyst for the reduction of 4-nitrophenol, Ultrason. Sonochem., 2018, 44, 1–13 CrossRef CAS PubMed
.
- Z. Yan, L. Fu, X. Zuo and H. Yang, Green assembly of stable and uniform silver nanoparticles on 2D silica nanosheets for catalytic reduction of 4-nitrophenol, Appl. Catal., B, 2018, 226, 23–30 CrossRef CAS
.
- K. Zhu, C. Chen, S. Lu, X. Zhang, A. Alsaedi and T. Hayat, MOFs-induced encapsulation of ultrafine Ni nanoparticles into 3D N-doped graphene–CNT frameworks as a recyclable catalyst for Cr(VI) reduction with formic acid, Carbon, 2019, 148, 52–63 CrossRef CAS
.
- X. T. Tran, M. Hussain and H. T. Kim, Facile and fast synthesis of a reduced graphene oxide/carbon nanotube/iron/silver hybrid and its enhanced performance in catalytic reduction of 4-nitrophenol, Solid State Sci., 2020, 100, 106107 CrossRef CAS
.
- J. Liang, Z. Ding, H. Qin, J. Li, W. Wang and D. Luo,
et al., Ultra-fast enrichment and reduction of As(V)/Se(VI) on three dimensional graphene oxide sheets-oxidized carbon nanotubes hydrogels, Environ. Pollut., 2019, 251, 945–951 CrossRef CAS PubMed
.
- M. R. Islam, M. Ferdous, M. I. Sujan, X. Mao, H. Zeng and M. S. Azam, Recyclable Ag-decorated highly carbonaceous magnetic nanocomposites for the removal of organic pollutants, J. Colloid Interface Sci., 2020, 562, 52–62 CrossRef CAS PubMed
.
- M. Kotal, A. Sharma, S. Jakhar, V. Mishra, S. Roy and S. C. Sahoo,
et al., Graphene-Templated Cobalt Nanoparticle Embedded Nitrogen-Doped Carbon Nanotubes for Efficient Visible-Light Photocatalysis, Cryst. Growth Des., 2020, 20(7), 4627–4639 CrossRef CAS
.
- F. Field and J. R. Ehrenfeld, Life-cycle analysis: the role of evaluation and strategy, Meas. Environ. Perform. Ecosyst. Cond., 1999, 29–41 Search PubMed
.
- A. B. Dichiara, S. J. Weinstein and R. E. Rogers, On the choice of batch or fixed bed adsorption processes for wastewater treatment, Ind. Eng. Chem. Res., 2015, 54(34), 8579–8586 CrossRef CAS
.
- X. T. Liu, X. Y. Mu, X. L. Wu, L. X. Meng, W. B. Guan and Y. Qiang,
et al., Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos, Biomed. Environ. Sci., 2014, 27(9), 676–683 CAS
.
- R. Das, S. B. Abd Hamid, M. E. Ali, A. F. Ismail, M. Annuar and S. Ramakrishna, Multifunctional carbon nanotubes in water treatment: the present, past and future, Desalination, 2014, 354, 160–179 CrossRef CAS
.
- C.-J. Shih, S. Lin, R. Sharma, M. S. Strano and D. Blankschtein, Understanding the pH-dependent behavior of graphene oxide aqueous solutions: a comparative experimental and molecular dynamics simulation study, Langmuir, 2012, 28(1), 235–241 CrossRef CAS PubMed
.
- S. Kashyap, S. Mishra and S. K. Behera, Aqueous colloidal stability of graphene oxide and chemically converted graphene, J. Nanoparticles, 2014, 2014, 640281 Search PubMed
.
- J. Zhao, Z. Wang, J. C. White and B. Xing, Graphene in the aquatic environment: adsorption, dispersion, toxicity and transformation, Environ. Sci. Technol., 2014, 48(17), 9995–10009 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2021 |



