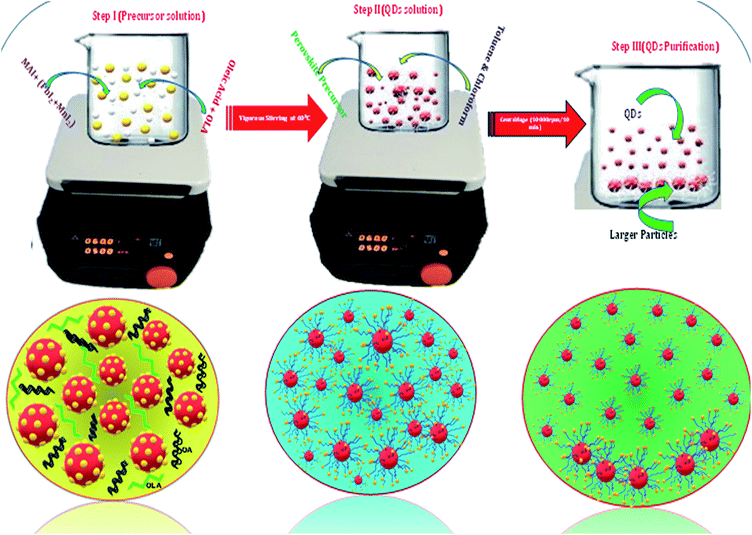
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rapid synthesis of hybrid methylammonium lead iodide perovskite quantum dots and rich MnI2 substitution favouring Pb-free warm white LED applications†
Rajan Kumar
Singh
 ab,
Sudipta
Som
ab,
Sudipta
Som
 b,
Somrita
Dutta
b,
Somrita
Dutta
 c,
Neha
Jain
a,
Mei-Tsun
Kuo
b,
Jai
Singh
c,
Neha
Jain
a,
Mei-Tsun
Kuo
b,
Jai
Singh
 a,
Ranveer
Kumar
*a and
Teng-Ming
Chen
a,
Ranveer
Kumar
*a and
Teng-Ming
Chen
 *c
*c
aDepartment of Physics, Dr. Harisingh Gour Central University, Sagar, 470003, M. P., India. E-mail: ranveerssi@yahoo.com; Fax: +91 88635723764; Tel: +91 88635731695
bDepartment of Chemical Engineering, National Taiwan University, Taipei, Taiwan, Republic of China
cDepartment of Applied Chemistry, National Chiao Tung University, 1001 University Road, Hsinchu 30010, Taiwan. E-mail: tmchen@mail.nctu.edu.tw
First published on 7th June 2019
Abstract
We present a facile room temperature synthesis of CH3NH3Pb1−xMnxI3 perovskite quantum dots (PQDs) substituting manganese (Mn2+) at the lead (Pb2+) sites to minimize environmental pollution and make it commercially feasible. By varying the concentration of Mn2+ from 0 to 60%, the PQDs exhibit strong color tunability from red to orange color suggesting successful energy transfer due to Mn2+ inclusion. We observed a high external photoluminescence quantum yield (PLQY) of 98% for unsubstituted CH3NH3PbI3 and >50% for up to 15% Mn2+ substituted PQDs. The average lifetime of PQDs was found to shorten with increasing Mn2+ replacement. We demonstrate a white LED prototype by employing the CH3NH3Pb1−xMnxI3 PQDs with green QDs on a blue LED chip. The CRI and CCT value varying from 92 to 80 and 5100 K to 2900 K, respectively, indicate the usability of the Mn2+ substituted PQDs as efficient warm white LEDs with a promising CRI and good stability.
Introduction
The discovery of hybrid perovskite quantum dots (HPQDs) (CH3NH3PbX3, X = Cl, Br, I) has opened a new era of research and development in new generation lighting technology as quantum dot light emitting diodes (QDLEDs) and backlight applications owing to their superior narrow emission, high photoluminescence quantum yield (PLQY), wide color range, and long diffusion length with high absorption coefficients.1–4 Moreover, the combination of organic/inorganic characteristics and an easy solution based synthesis approach at low temperature imparts a unique property to these materials. However, the presence of toxic Pb2+ at the B site in an ABX3 structure has restricted its commercialization.5,6 The use of heavy metals, including lead in an electronic device has already been restricted in the European Union, and other countries are also planning to introduce similar regulations in the near future.7 Therefore, the development of Pb2+ free or less Pb2+ based HPQDs that retain the excellent features of the original PQDs is obligatory. Cation exchange or substitution of Pb with divalent cations, such as, Cu2+, Zn2+, Sn2+, and Mn2+, could be a promising approach to modulate the optical and electronic properties of HPQDs.8,9 The forbidden 4T1 → 6A1 transition of Mn2+ makes this cation a suitable dopant to act as an economical colour emitter as well as decreasing the toxic level of the Pb-based perovskites due to its intense orange emission which remains independent of the physical and electronic configuration of the host.6,9The lead-free mixed halides CsSnX3 (X = Cl, Br, I) fabricated by Jellicoe and group had stability issues under ambient conditions and had a low PLQY (<10%) due to the easy oxidation of Sn(II) to Sn(IV).10 Liu et al. reported the partial replacement of Pb2+ with Mn2+ in CsPb1−xMnxCl3 PQDs (x = 0.3 ≤ x ≤ 0.4) with maximum PLQYs up to 54% via a hot injection route.11 Additionally, Mn2+ doping was also reported with CsPbBr3, CsPbCl3−xBrx and CsPbI3 inorganic perovskite QDs by different groups.12,13 On the other hand, no reports are found on Pb substituted HPQDs and most of the studies were found on MAPbBr3 QDs due to their high stability and PLQY. The first solution based HPQDs were fabricated by the Pérez-Prieto group in 2014 with a PLQY of 20%.14 After that, Zhang et al. reported CH3NH3PbBr3 HPQDs in 2015 with an absolute quantum yield of up to 70% with the modification of the ligands in a room temperature process15 and recently the PLQY reached up to 100% by a spray synthesis route. However, obtaining high QY with CH3NH3PbI3 is still challenging with the maximum being 56% due to the higher sensitivity of iodine to moisture and air.16,17 Therefore, we need to work on increasing the PLQY for red QDs that can be used for red LEDs and perovskite quantum dot solar cells because for photovoltaic devices a MAPbI3 based material was found to be the best absorber.
To the best of our knowledge, Pb2+ substitution with Mn2+ in CH3NH3PbI3 PQDs by a room temperature synthesis method has not been reported to date. Therefore, in the present work, we present a novel approach to obtain CH3NH3PbI3 HPQDs with a PLQY of up to 98% and CH3NH3Pb1−xMnxI3 HPQDs via Mn2+ substitution to reduce the toxicity of PQDs. We further demonstrate a white LED prototype by employing the as-prepared best CH3NH3Pb1−xMnxI3 PQDs as color conversion materials, with green QDs on a blue LED chip to prove the probable commercialization of the present materials in the future for general lighting applications such as QDLEDs and backlight systems.
Experimental section
Synthesis methods
Similarly, for synthesis of CH3NH3Pb1−xMnxI3 QDs, a mixture of 0.01 mmol (0.0159 gm) of CH3NH3I and (0.1 − x) mmol of PbI2 and (0.1x) [here x = 0.05 to 0.60] mmol of MnI2 was dissolved in 1 ml of DMF. The rest of the process was the same as the CH3NH3PbI3 QD synthesis. Further details of precursors and conditions are provided in Table S1.† The reason behind the selection of solvent is explained in the ESI.† In the case of iodine-based HPQDs, the selection of solvent is very important because such types of perovskite are very sensitive to the atmosphere and they are also very reactive due to the presence of I− ions. Therefore, a weak (non-polar) solvent is selected which can react very fast with the perovskite material. The reactivity of chloroform is higher than that of toluene due to the high dielectric constant of chloroform (ε = 4.81). Hence only chloroform or a mixture of chloroform and toluene is used for CH3NH3PbI3 HPQDs to promote better and fast nucleation. On the other hand, in the case of toluene the reaction kinetics is comparably slow and may lead to the destruction of the QDs during the centrifugation because of the higher sensitivity and instability of iodine-based perovskites.
Characterization
The XRD of the perovskite QDs was measured using a Bruker D8 powder XRD with Cu Kα radiation over the range of 10 < 2θ < 60° with a step size of 0.02 and operating at 40 kV to 40 mA. TEM images and HRTEM patterns were recorded using a JEOL high-resolution transmission electron microscope (HR-TEM) equipped with a LaB6 filament and CCD camera. Samples of different PQD samples for TEM analysis were prepared by casting 10 μl of colloidal solution onto a standard copper grid. The size distribution and particle size of PQDs were obtained from the TEM images and the d value was calculated from the HRTEM patterns with ImageJ software. Optical UV-vis absorption spectra were measured using a Hitachi U-2900. Photoluminescence (PL) spectra of PQDs were recorded using an FS-5 Fluorescence Spectrophotometer at 420 nm excitation wavelength in the wavelength range of 500 to 820 nm. The photoluminescence decay time curves were measured using a time correlated single photon counting (TCSPC) system on an FS-5 Fluorescence Spectrophotometer PL system equipped with a 150 W xenon lamp and a 360 nm laser source respectively. The absolute quantum yield (QY) of each PQD sample was determined using a Horiba Jobin Yvon Fluorolog according to the given equation:| PLQY (%) = (number of photon emitted/number of photon absorbed) × 100. |
For luminescent materials, PLQY characterization is very important for a deep understanding of molecular and light absorbing/emitting properties. Mostly, PLQY is measured using an integrating sphere. From this technique, PLQY can be determined directly. In this tool, a sphere is coated with all reflective surfaces to capture all the light going in and out of the sphere. The PLQY measurement helps to find the fluorescence emission (Ec) and the scattering (Lc) of the sample and also the emission and scattering of a blank i.e. Ea and La respectively. So with the help of an integral sphere setup, spectral measurements of PLQY can be measured using the given formula;
| PLQY = ((Ec − Ea)/(La − Lc)) × 100 |
Results and discussion
A series of Mn substituted CH3NH3PbI3 based HPQDs were synthesized by a ligand assisted room temperature approach. The synthesis procedure, the selection of solvent and the thorough description are included in the ESI and the detailed chemical compositions are also given in Table S1.†Fig. 2(a–g) show the phase identification, microscopic characterization, luminescence and time resolved spectroscopy of CH3NH3PbI3 HPQDs. Typical TEM images of the obtained HPQDs showed the homogeneous distribution of tiny spherical HPQDs with an average particle size of ∼3 nm and distribution of 0.03 nm as shown in Fig. 2(a) and (b). The HRTEM image of CH3NH3PbI3 QDs (bottom part of Fig. 2(a)) showed a good crystalline structure with an interplanar distance of 0.31 Å, which corresponds to the (004) plane of CH3NH3PbI3.18
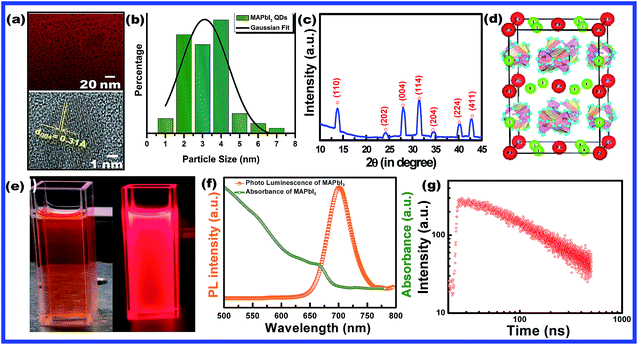 | ||
| Fig. 2 (a) TEM and HR-TEM images (the high resolution image is available in the ESI (Fig. S1†)), (b) particle size-distribution, (c) X-ray diffraction pattern, and (d) corresponding crystal structure of CH3NH3PbI3 (MAPbI3) hybrid perovskite quantum dots synthesized via a modified low temperature route. (e) Digital photograph of CH3NH3PbI3 PQDs in toluene solution under visible and 365 nm UV light, (f) UV-visible absorbance and photoluminescence spectra and (g) time resolved decay kinetics of CH3NH3PbI3 PQDs. | ||
Fig. 2(c) shows the XRD pattern of the synthesized CH3NH3PbI3 HPQDs. All diffraction peaks matched well with the reported peaks of CH3NH3PbI3 HPQDs indicating the presence of a tetragonal structure with space group I4/mcm.19 VESTA software was utilized to draw the crystal structure of the obtained HPQDs fused with one Pb2+, one CH3NH3+, and three iodine anions in the unit cell as shown in Fig. 2(d). The structural and morphological analyses indicated the formation of a CH3NH3PbI3 perovskite structure in QD form via the present synthesis route.
The visual appearance of chloroform/toluene solution of CH3NH3PbI3 HPQDs under a 365 nm UV-light source shows a bright red emission (Fig. 2(e)). The reason behind the selection of solvent is explained in the ESI.†Fig. 2(f) shows the steady-state absorption and photoluminescence (PL) spectra of CH3NH3PbI3 HPQDs. Broad and strong absorption in the visible region and near-infrared region revealed the promising light absorbing quality of QDs. Additionally, the sharp absorption edge of the sample also suggested a direct bandgap nature.20 The PL emission of the obtained PQDs was also observed at 700 nm with a high intensity and color purity. The absolute PLQY of CH3NH3PbI3 HPQDs was estimated to be 98%. To the best of our knowledge, it is the highest value reported for CH3NH3PbI3 HPQDs prepared under ambient conditions to date. The PL decay curve can be fitted well with a single exponential function for CH3NH3PbI3 PQDs to obtain an average lifetime of 98.29 ns (Fig. 2(g)). The above discussion indicates an efficient fabrication of red HPQDs with PLQY > 98% and efficient lifetimes.21
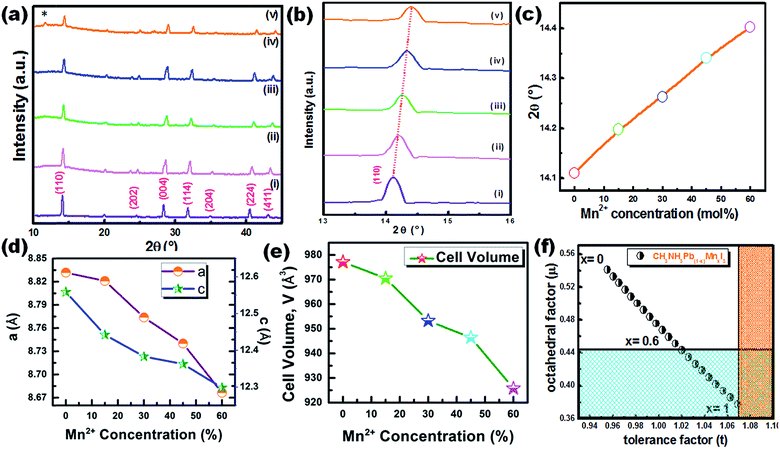
The XRD pattern of CH3NH3Pb1−xMnxI3 PQDs with different concentrations of Mn2+ substituting Pb2+ ions is shown in Fig. 3(a). The XRD pattern indicates the presence of a tetragonal phase for all the synthesized samples even after Mn doping. However, a minimal shifting towards higher 2θ was observed for the Mn incorporated perovskites. The higher angle shifting is clear from the variation of the enlarged (110) XRD peak position with different Mn concentrations from 0% to 60% as shown in Fig. 3(b and c). The shifting can be ascribed to the substitution of Mn2+ ions with smaller ionic radii compared to Pb2+ ions (see Fig. 3(b and c)). The smaller ionic radius of Mn is also expected to cause a reduction of perovskite lattices and the cell volume of the resultant samples as shown in Fig. 3(d and e). According to Vegard's law, the lattice constant and cell volume of the compound have a linear relationship and our experimental data also follow Vegard's law. These results also correlate with previous research studies based on the substitution of Mn with small ionic radii.19
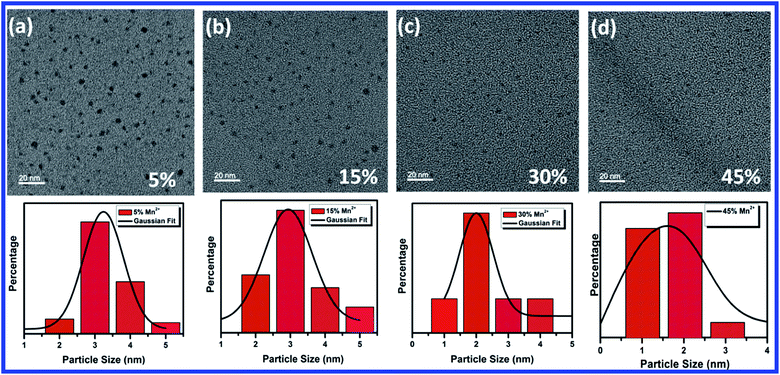
TEM images of 5%, 15%, 30% and 45% Mn2+ ion doped CH3NH3Pb1−xMnxI3 HPQDs are shown in Fig. 4(a–d) respectively. TEM images of all HPQDs showed spherical dots with an average particle size in the range of ∼1 to 3 nm. The distribution density of particles decreased with the substitution of Mn2+ ions with Pb2+ ions in CH3NH3Pb1−xMnxI3 PQDs owing to the sensitivity of Mn2+ under ambient conditions compared to Pb2+.22 It is well known that agglomeration in QDs will be less owing to the high surface energy. As we explained in the structural section, according to Vegard's law, the lattice constants and cell volume are directly proportional to the ionic radii of cations present in the compound. Hence, with the substitution of Mn2+ ions instead of Pb2+ ions the lattice constant is observed to decrease resulting in cell volume contraction. Such reduction in cell volume due to Mn2+ substitution in CH3NH3Pb1−xMnxI3 PQDs further results in the reduction of particle size.
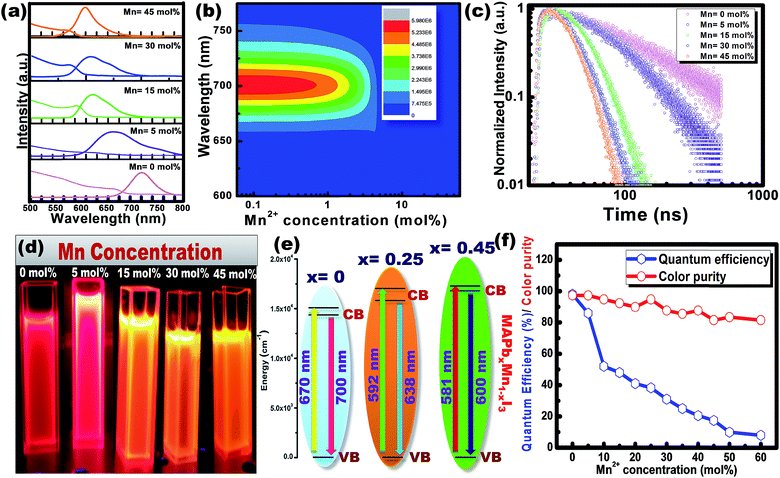
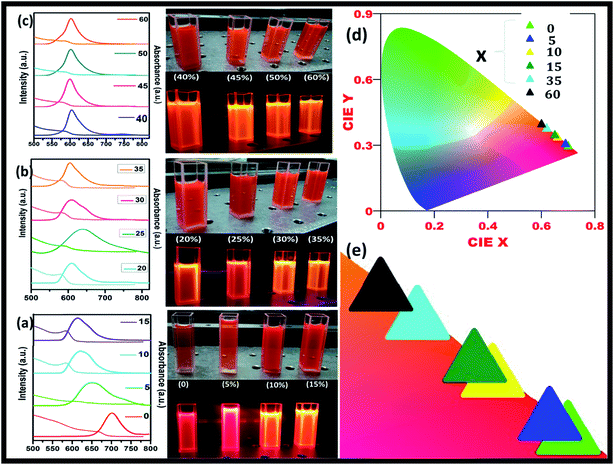
Fig. 5(a) shows the steady-state absorption and PL of CH3NH3Pb1−xMnxI3 HPQDs with the variation of Mn2+ from 0 mol% to 45 mol%. Fig. 6(a–c) show the variation of absorption and PL spectra of CH3NH3Pb1−xMnxI3 PQDs with continuous variation of Mn2+ ion concentration from 0 mol% to 60 mol%. The absorption spectra showed a blue shift after the replacement of Pb2+ with Mn2+. The emission peaks of CH3NH3Pb1−xMnxI3 HPQDs also shifted from 700 to 600 nm indicating successful replacement of Pb2+ in CH3NH3PbI3 HPQDs.23a The changes in the color of HPQDs suggest the successful energy transfer from PQDs to Mn2+ dopants in the CH3NH3Pb1−xMnxI3 perovskite samples as shown in Fig. 4(e). Fig. 4(b) displays the 3D scan of the variation of PL emission intensity and wavelength with the increase in Mn2+ concentration. PL emission intensity was found to decrease along with a blue shift in color emission as clearly visible in the photographs of the resulting CH3NH3Pb1−xMnxI3 HPQD colloidal solutions (Fig. 5(d) and Fig. 6(a–c)). Furthermore, to better enable the comparison of color variation, the influence of Pb2+ to Mn2+ cation exchange on the color coordinates of CH3NH3Pb1−xMnxI3 PQDs is shown in the ESI† and Fig. 6(d and e).
The PL emission spectra reveal an interesting broadening for Mn content along with an obvious blue shift due to the combined effect of quantum confinement and Mn incorporation. It is well known that agglomeration in QDs will be less owing to the high surface energy. Moreover, as the ionic radius of Mn2+ ions is lower than that of Pb2+ ions, the lattice volume starts to shrink with the incorporation of Mn2+ ions into the CH3NH3PbI3 host. And hence the particle size starts to decrease with the incorporation of Mn2+ ions in place of Pb2+ ions as is clear from the TEM image and corresponding statistical distribution. As the particle size starts to decrease from approximately 4 nm to 1.5 nm, the quantum confinement plays a role for the blue shifting of PL emission. Fig. 6(a) shows the steady-state absorption and PL of CH3NH3Pb1−xMnxI3 HPQDs with the variation of Mn2+ from 0 mol% to 60 mol%. The absorption spectra showed a blue shift after the replacement of Mn2+ in Pb2+ sites. However, careful examinations of PL emission spectra reveal an interesting broadening with the increase in Mn concentration as shown in Fig. S3.† The emission spectra of CH3NH3Pb1−xMnxI3 with x = 0, 0.05, 0.15, 0.30 and 0.60 are shown in Fig. S3(i–v)† respectively. The emission spectra of CH3NH3PbI3 shows a broad band around 700 nm which is blue shifted compared to its bulk counterpart (Fig. S2†). With the increase in Mn2+ concentration, the peak position blue shifted due to the quantum confinement effect as discussed earlier. Hassan et al. have also observed similar results to ours.23b However, the PL emission peak can be deconvoluted into two distinguished peaks centred at 634 and 600 nm. According to the results of Hassan et al., in the present research, 634 nm emissions can be assigned to the characteristic emission of the CH3NH3PbI3 perovskite host. Zou et al.23c have reported the dominant broad emission band peaking at ∼600 nm of the perovskite host, which can be readily ascribed to the 4T1 → 6A1 transition of Mn2+ doped in CH3NH3Pb1−xMnxI3. When the Mn2+ concentration is more than 30%, the contribution from Mn2+ is dominant over the host perovskite and hence at 60% Mn2+ doping only a 600 nm peak is visible. Furthermore, CH3NH3Pb1−xMnxI3 (x = 0) and CH3NH3Pb1−xMnxI3 (x = 0.3) PQDs exhibit almost the same PL excitation spectra when monitoring their excitonic or Mn2+-related emissions. The similar excitation features suggested a clear energy transfer from the CH3NH3PbI3 host Mn2+.23
Time-resolved PL spectroscopy was used to correlate the variation of the PL emission efficiency of the CH3NH3Pb1−xMnxI3 (x = 0 to 0.60) as shown in Fig. 5(c) and S4(a–c).†
For CH3NH3PbI3 and CH3NH3Pb0.95Mn0.05I3 PQDs, single exponential PL decay was observed reflecting the homogeneous distribution of the elements. The result was similar to PL decay in Mn2+: ZnSe and Mn2+:CdS/ZnS nano-crystals as reported earlier.24,25 However, for more than 5% Mn2+ doping, the PL decay curve could be fitted well with a biexponential function. For more than 5% Mn2+ doping, the PL decay curve could be fitted well with a bi-exponential function as follows
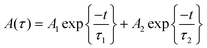 | (1) |
| τav = A1τ12 + A2τ22/A1τ1 + A2τ2 | (2) |
The average lifetime of 0 to 60% Mn2+ doped CH3NH3PbI3 PQDs gradually decreases as the emission shifts to a shorter wavelength because of the increased content of Mn2+ (Mn2+ has a lower atomic no. than Pb2+). CH3NH3PbI3 and CH3NH3Pb0.95Mn0.05I3 PQDs have a longer lifetime that can be attributed to the lower non-radiative energy transfer, trap-defects and surface states of the QDs. The decrease in τav with increase in Mn2+ concentration in CH3NH3Pb1−xMnxI3 PQDs is shown in Fig. S4.†
The maximum color purity (details are given in the ESI†) was estimated to be 97% for CH3NH3PbI3 and it continually decreased with the elevation of the doping concentration of Mn2+ (Fig. 5(f)). The effect on PLQY of Mn2+ concentration in CH3NH3PbI3 PQDs is shown in Fig. 5(f). The total external PLQY of CH3NH3PbI3 and CH3NH3Pb0.95Mn0.05I3 was observed to be 98% and 86%, respectively, and then decreased with further increase in Mn2+ concentration. Promising PLQY has been obtained at even low concentrations of Mn2+ (up to 20%). It is the highest concentration when compared to analogous results for Mn2+ doped nanocrystals.26–29 The absolute PLQY, average lifetime and color purity of PQDs with different concentrations of MnI2 are summarized in Table 1 and a plot between the PLQY and color purity is also shown in Fig. S5.†
| PQDs | PLQY (%) | τ 1 (ns) | (A1) | τ 2 (ns) | (A2) | Average lifetime (ns) | Color purity (%) |
|---|---|---|---|---|---|---|---|
| CH3NH3PbI3 | 98.00 | 98.29 | 2.28 | — | — | 98.29 | 85.28871 |
| CH3NH3Pb0.90Mn0.05I3 | 86.00 | 69.09 | 2.31 | — | — | 69.09 | 99.12048 |
| CH3NH3Pb0.90Mn0.10I3 | 52.00 | 30.26 | 2.29 | 44.50 | 4.13 | 36.48 | 96.53949 |
| CH3NH3Pb0.85Mn0.15I3 | 48.00 | 17.53 | 1.86 | 29.83 | 2.12 | 24.64 | 95.38515 |
| CH3NH3Pb0.80Mn0.20I3 | 41.00 | 15.39 | 2.32 | 26.84 | 3.37 | 21.44 | 94.05758 |
| CH3NH3Pb0.75Mn0.25I3 | 38.30 | 13.32 | 1.77 | 21.10 | 1.00 | 19.05 | 99.12048 |
| CH3NH3Pb0.70Mn0.30I3 | 31.00 | 13.13 | 1.86 | 20.93 | 2.65 | 17.28 | 91.68279 |
| CH3NH3Pb0.65Mn0.35I3 | 25.00 | 13.17 | 1.92 | 18.34 | 1.13 | 16.81 | 91.68279 |
| CH3NH3Pb0.60Mn0.40I3 | 20.50 | 11.47 | 3.24 | 15.23 | 1.63 | 14.20 | 89.42367 |
| CH3NH3Pb0.55Mn0.45I3 | 17.50 | 12.20 | 1.84 | 14.93 | 1.15 | 14.00 | 85.28871 |
| CH3NH3Pb0.50Mn0.50I3 | 10.0 | 11.14 | 3.24 | 13.13 | 1.63 | 12.53 | 87.28919 |
| CH3NH3Pb0.40Mn0.60I3 | 8.0 | 11.21 | 1.92 | 12.23 | 1.13 | 11.88 | 85.28871 |
It is evident that with the Mn2+ ion substitution in CsMn1−xPbxI3 PQDs, the PLQY is observed to drastically reduce from 98% to 8%. The reason for such a decrease could be attributed to the change in the energy band structure due to Mn2+ ion substitution. In the case of manganese ion substitution, the electrons are distributed between tg2 and eg bands according to crystal field stabilization theory. However, for Mn2+ ions the unpaired electrons in the d orbital remain distributed in the low energy tg2 band leaving behind empty eg bands. In the case of excitation, the electrons in the tg2 band may get excited to the eg band, which is essentially a non-radiative transformation owing to its low energy difference, and this mechanism is shown in Fig. S6.† In such a scenario, the excitation of electrons leads to a radiation quenching effect. Thus low photo luminescence quantum yields (PLQYs) and reduced lifetimes are observed in the case of Mn2+ ion substitution.
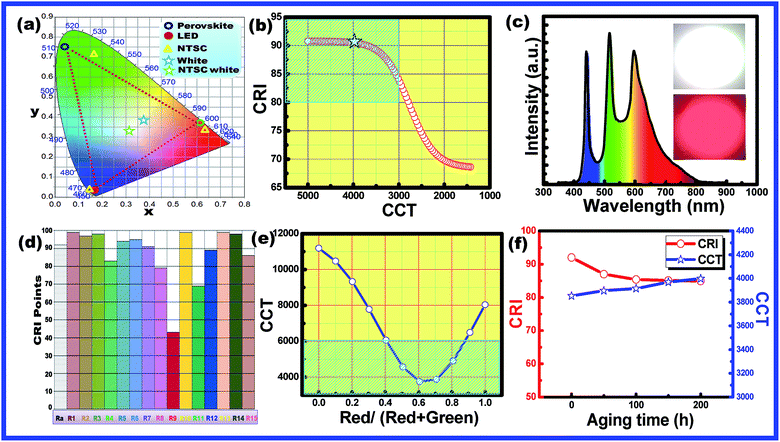
The obtained CH3NH3Pb1−xMnxI3 PQDs were utilized to fabricate LED devices. In the present case, the perovskite emitters were integrated as color converting layers combined with blue LEDs and the color parameters of the obtained W-LEDs are shown in Fig. 7. From the CIE chromaticity diagram (Fig. 7(a)) it is clear that the connection lines of the color point of blue LED with green and red PQDs always pass through the white emission and hence the combination can produce white light consistently. Therefore, white light emissions were simulated by the linear combination of the emission of green and red PQDs with blue LEDs. Color correlated temperature (CCT) and color rendering index (CRI) values of the corresponding white emission were evaluated with the help of methods reported earlier.30,31
The evaluated data were used to plot a CCT–CRI diagram as shown in Fig. 7(b). The simulation results indicated that warm white light with CCT < 5000 K and CRI > 80 (blue shaded area) can be simply realized in the current combination. In the present simulation, a high CRI of 92 was obtained at a CCT of 5100 K and the lowest CCT value of 2900 K with a CRI of 80 was obtained. The simulation results indicated that the present combination can produce an efficient warm white light with a promising CRI.32 Therefore, a series of WLEDs were fabricated by encapsulating a blue InGaN LED chip with various amounts of green and red PQDs to validate the simulation results. The electroluminescence (EL) spectra of the fabricated WLED are shown in Fig. 7(c). The inset of Fig. 7(c) presents the fabricated WLED with high brightness. WLEDs with chromaticity coordinates at (0.34, 0.37), a CRI of 91 and a CCT of 4000 K were obtained and are shown as star points in Fig. 7(a). Different color rendering parameters for the fabricated WLEDs are shown in Fig. 7(d). The figure indicated the promising CRI nature of the obtained WLEDs. The CCT values can be tuned with the change in red to green ratios from cool light (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 K) to warm light (3800 K) as shown in Fig. 7(e).
000 K) to warm light (3800 K) as shown in Fig. 7(e).
The shaded region in Fig. 7(e) confirmed the presence of warm white light emission from the present materials by controlling the amount of green and red emitting PQDs.33 The results are consistent with the simulation results. The combination of a blue LED chip and green and red PQDs results in efficient warm white light (4000 K) with a high CRI (91) along with an improved stability since only ∼10% of the initial intensity is lost after 200 h under accelerated aging conditions (85 °C and 85% relative humidity) as can be observed in Fig. 7(f). From the above discussion it is clear that, the present PQDs can act as an efficient red emitting material for warm white light emitting applications.
Conclusion
In conclusion, we have offered an inexpensive and speedy room temperature synthesis method for the fabrication of CH3NH3Pb1−xMnxI3 PQDs with spherical shape and a particle size of ∼1.5 to 3 nm. Absorbance and PL emission studies show that with increasing Mn2+ substitution of Pb2+, in CH3NH3PbI3, the PQDs produce an emission colour varying from red to orange due to efficient energy transfer from QDs to Mn2+ ions. The unsubstituted PQDs showed PLQYs of nearly 98% while with 5% Mn2+ substitution the PLQY decreases to 86% and further declines with increasing concentration of Mn2+. The average lifetime of pure (CH3NH3PbI3) and PQDs with very low concentration of Mn2+ was observed to be longer than that of the PQDs with higher Mn2+ concentration. The fabricated series of WLEDs combining a blue InGaN LED chip with various amounts of green and red PQDs produced warm white light with CCT < 5000 K and CRI > 80. The best WLED with chromaticity coordinates at (0.34, 0.37), a CRI of 91 and a CCT of 4000 K was obtained with an improved stability since only ∼10% of the initial intensity is lost after 200 h under accelerated aging conditions (85 °C and 85% relative humidity). These results confirm the commercial usability of the present PQDs for general lighting applications as environment friendly QD-LEDs and backlight systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to Sophisticated Instrument Laboratory of the Dr Harisingh Gour Central University and National Chiao Tung University for providing characterization facilities. Rajan acknowledges the Senior Research Fellowship (SRF) provided by University Grants Commission (UGC), Govt. of India.References
- X. Yang, X. Zhang, J. Deng, Z. Chu, Q. Jiang, J. Meng, P. Wang, L. Zhang, Z. Yin and J. You, Nat. Commun., 2018, 9, 570 CrossRef PubMed.
- M. V. Kovalenko, L. Protesescu and M. I. Bodnarchuk, Science, 2017, 358, 745–750 CrossRef CAS PubMed.
- R. K. Singh, R. Kumar, A. Kumar, N. Jain, R. K. Singh and J. Singh, J. Alloys Compd., 2018, 743, 728–736 CrossRef CAS.
- F. Liu, Y. Zhang, C. Ding, S. Kobayashi, T. Izuishi, N. Nakazawa, T. Toyoda, T. Ohta, S. Hayase, T. Minemoto, K. Yoshino, S. Dai and Q. Shen, ACS Nano, 2017, 11, 10373–10383 CrossRef CAS PubMed.
- Z. Liu, Y. Zhang, Y. Fan, Z. Chen, Z. Tang, J. Zhao, Y. lv, J. Lin, X. Guo, J. Zhang and X. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 13053–13061 CrossRef CAS PubMed.
- A. Babayigit, D. Duy Thanh, A. Ethirajan, J. Manca, M. Muller, H.-G. Boyen and B. Conings, Sci. Rep., 2016, 6, 18721 CrossRef CAS PubMed.
- S. Chakraborty, W. Xie, N. Mathews, M. Sherburne, R. Ahuja, M. Asta and S. G. Mhaisalkar, ACS Energy Lett., 2017, 2, 837–845 CrossRef CAS.
- M. Leng, Y. Yang, K. Zeng, Z. Chen, Z. Tan, S. Li, J. Li, B. Xu, D. Li, M. P. Hautzinger, Y. Fu, T. Zhai, L. Xu, G. Niu, S. Jin and J. Tang, Adv. Funct. Mater., 2018, 28, 1704446 CrossRef.
- S. Zou, Y. Liu, J. Li, C. Liu, R. Feng, F. Jiang, Y. Li, J. Song, H. Zeng, M. Hong and X. Chen, J. Am. Chem. Soc., 2017, 139, 11443–11450 CrossRef CAS PubMed.
- T. C. Jellicoe, J. M. Richter, H. F. J. Glass, M. Tabachnyk, R. Brady, S. E. Dutton, A. Rao, R. H. Friend, D. Credgington, N. C. Greenham and M. L. Böhm, J. Am. Chem. Soc., 2016, 138, 2941–2944 CrossRef CAS PubMed.
- H. Liu, Z. Wu, J. Shao, D. Yao, H. Gao, Y. Liu, W. Yu, H. Zhang and B. Yang, ACS Nano, 2017, 11, 2239–2247 CrossRef CAS PubMed.
- L. Fei, X. Yuan, J. Hua, M. Ikezawa, R. Zeng, H. Li, Y. Masumoto and J. Zhao, Nanoscale, 2018, 10, 19435–19442 RSC.
- Q. A. Akkerman, D. Meggiolaro, Z. Dang, F. De Angelis and L. Manna, ACS Energy Lett., 2017, 2, 2183–2186 CrossRef CAS PubMed.
- L. C. Schmidt, A. Pertegás, S. González-Carrero, O. Malinkiewicz, S. Agouram, G. Mínguez Espallargas, H. J. Bolink, R. E. Galian and J. Pérez-Prieto, J. Am. Chem. Soc., 2014, 136, 850–853 CrossRef CAS PubMed.
- F. Zhang, H. Zhong, C. Chen, X. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS PubMed.
- F. Zhang, S. Huang, P. Wang, X. Chen, S. Zhao, Y. Dong and H. Zhong, Chem. Mater., 2017, 29, 3793–3799 CrossRef CAS.
- I. Levchuk, P. Herre, M. Brandl, A. Osvet, R. Hock, W. Peukert, P. Schweizer, E. Spiecker, M. Batentschuk and C. J. Brabec, Chem. Commun., 2017, 53, 244–247 RSC.
- Y. Hassan, Y. Song, R. D. Pensack, A. I. Abdelrahman, Y. Kobayashi, M. A. Winnik and G. D. Scholes, Adv. Mater., 2016, 28, 566–573 CrossRef CAS PubMed.
- O. Vybornyi, S. Yakunin and M. V. Kovalenko, Nanoscale, 2016, 8, 6278–6283 RSC.
- R. K. Singh, R. Kumar, N. Jain, S. R. Dash, J. Singh and A. Srivastava, J. Taiwan Inst. Chem. Eng., 2019, 96, 538–542 CrossRef CAS.
- X. Yuan, S. Ji, M. C. De Siena, L. Fei, Z. Zhao, Y. Wang, H. Li, J. Zhao and D. R. Gamelin, Chem. Mater., 2017, 29, 8003–8011 CrossRef CAS.
- D. Gao, B. Qiao, Z. Xu, D. Song, P. Song, Z. Liang, Z. Shen, J. Cao, J. Zhang and S. Zhao, J. Phys. Chem. C, 2017, 121, 20387–20395 CrossRef CAS.
- (a) W. Liu, Q. Lin, H. Li, K. Wu, I. Robel, J. M. Pietryga and V. I. Klimov, J. Am. Chem. Soc., 2016, 138, 14954–14961 CrossRef CAS PubMed; (b) Y. Hassan, et al. , J. Am. Chem. Soc., 2019, 141, 1269–1279 CrossRef CAS PubMed; (c) S. Zou, et al. , J. Am. Chem. Soc., 2017, 139, 11443–11450 CrossRef CAS PubMed.
- Z. Hu, S. Xu, X. Xu, Z. Wang, Z. Wang, C. Wang and Y. Cui, Sci. Rep., 2015, 5, 14817 CrossRef CAS PubMed.
- S. Cao, J. Zheng, J. Zhao, L. Wang, F. Gao, G. Wei, R. Zeng, L. Tian and W. Yang, J. Mater. Chem. C, 2013, 1, 2540–2547 RSC.
- Q. Wei, M. Li, Z. Zhang, J. Guo, G. Xing, T. C. Sum and W. Huang, Nano Energy, 2018, 51, 704–710 CrossRef CAS.
- S. Das Adhikari, A. Dutta, S. K. Dutta and N. Pradhan, ACS Energy Lett., 2018, 3, 1247–1253 CrossRef CAS.
- G. Fang, D. Chen, S. Zhou, X. Chen, L. Lei, J. Zhong and Z. Ji, J. Mater. Chem. C, 2018, 6, 5908–5915 RSC.
- K. Xu and A. Meijerink, Chem. Mater., 2018, 30, 5346–5352 CrossRef CAS PubMed.
- Y. Liu, D. Tu, H. Zhu and X. Chen, Chem. Soc. Rev., 2013, 42, 6924–6958 RSC.
- M. Shang, C. Li and J. Lin, Chem. Soc. Rev., 2014, 43, 1372–1386 RSC.
- C. Feldmann, T. Jüstel, C. R. Ronda and P. J. Schmidt, Adv. Funct. Mater., 2003, 13, 511–516 CrossRef CAS.
- R. Zheng, Q. Zhang, J. Ding, C. Liu, J. Lv, K. Xu, L. Fu and W. Wei, Opt. Mater. Express, 2018, 8, 639–647 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9na00330d |
| This journal is © The Royal Society of Chemistry 2019 |