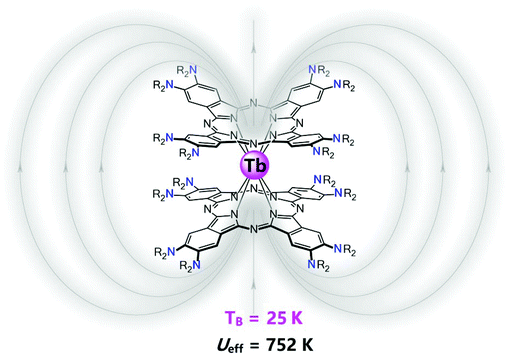
Marriage of phthalocyanine chemistry with lanthanides: a single-ion magnet with a blocking temperature up to 25 K
Jun-Long
Zhang

Beijing National Laboratory of Molecular Science State Key Laboratory of Rare Earth Materials Chemistry and Applications, College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, China. E-mail: zhangjunlong@pku.edu.cn
First published on 13th September 2017
Abstract
A combination of rich phthalocyanine chemistry such as peripheral substitution and the unique properties of lanthanides to enhance organic radical-f interaction is expected to afford new SIMs with a higher working temperature.
Magnetic phenomena and materials are everywhere and significantly improve our lives. From the brilliant perspective of molecule-based information storage and quantum computing, the field of magnetic materials has been undergoing a shift of focus away from bulky magnets and nanomagnets toward molecular magnets.1 Since the discovery of dodecametallic manganese-acetate (Mn12) in the early 1990s,2 multifunctional single-molecule magnets (SMMs) or single-ion magnets (SIMs) with slow magnetic relaxation have attracted increasing attention, not only deepening the study of magnetism but also forming the chemical basis for molecular spintronics.
One of the most challenging issues to make SMMs practicable is how to enhance the working temperature for the SMMs to exhibit magnetic bistability properties. Previous attempts to enhance the spin value of the ground state have been focused on simply scaling molecules to contain more spin-bearing atoms.3 However, the increase of total spins is usually accompanied by more symmetrical molecular structures that frequently counteract the anisotropies, creating a small energy barrier.3 Until 2003, mononuclear lanthanide complexes, (Bu4N)[Ln(Pc)2] (Ln = TbIII or DyIII, Bu4N = tetrabutylammonium),4 provided a way to understand and control the single-ion magnetic anisotropy, and in turn to enhance the magnetic behavior in single-ion magnets (SIMs).5 This was a landmark for the marriage of phthalocyanine chemistry with lanthanides, which offered a “golden” opportunity to address the critical issue of SMMs.
Toward this goal, tremendous progress has been made in the synthesis of bis(tetrapyrrole) lanthanide double-decker SIMs to disclose the structural and electronic effects on the magnetic performance,6–9 however the electronic effect of the phthalocyanine periphery on the SIM properties still remains unclear. Jiang and co-workers have provided an important answer to this scientific question.10 They incorporated the dialkylamino substituent, one of the strongest electron-donating groups known so far, onto the phthalocyanine periphery in bis(phthalocyaninato) rare earth complexes, resulting in the homoleptic bis[2,3,9,10,16,17,23,24-octakis (dibutylamino)phthalocyaninate] rare earth complexes M{Pc[N(C4H9)2]8}2 {Pc[N(C4H9)2]8 = 2,3,9,10,16,17,23,24-octakis(dibutylamino)phthalocyanine, M = Y, Tb} (Scheme 1). The electron-donating effect of the sixteen dialkylamino groups was clearly revealed by the significant red shift in the Q absorption bands and the significant shift in both the first oxidation and the first reduction potentials to the negative direction, relative to those of M(Pc)2.11 In particular, the electrostatic potential around the terbium ion gets significantly increased to −11.59 a.u. for Tb{Pc[N(C2H5)2]8}2 from −3.75 a.u for Tb(Pc)2 (Fig. 1), which results in a significant enhancement over the ligand coordination field around the terbium ion and therefore intensifies the molecular magnetic anisotropy. Their design also depends on the steric effect of the bulky dialkylamino groups, which leads to a square-antiprismatic coordination polyhedron for the terbium ion, which in turn ensures the good SMM properties of the terbium double-decker complex in terms of the coordination geometry. Their bis(phthalocyaninato) terbium double-decker SIM did indeed show the significantly intensified SIM performance hoped-for, with a spin reversal energy barrier of 752 ± 8 K and a blocking temperature of 25 K. In view of the previous highest blocking temperature for bis(tetrapyrrole) lanthanide SIMs, 10 K, it is a good breakthrough. It is worth noting that bis(tetrapyrrole) lanthanide-based SIMs with higher energy barriers have been reported by T. Torres8 and R. Sessoli,9 however, the blocking temperature of these SIMs is much lower than that of Tb{Pc[N(C2H5)2]8}2, revealing the complicated relationship between the energy barrier and the blocking temperature. Nevertheless, by comparison of the dynamic magnetic properties of these SIMs, it is found that the quantum tunneling of magnetization (QTM) seems to be more significant in the SIMs with higher energy barriers, which may be the reason why the higher energy barriers do not result in a higher blocking temperature for these bis(tetrapyrrole) lanthanide-based SIMs. Therefore, in order to increase the blocking temperature, the QTM should be suppressed and the energy barrier should be increased as well. As evidenced by Tb{Pc[N(C2H5)2]8}2, the suppressed QTM is correlated to the near-perfect square-antiprismatic polyhedron coordination mode around Tb3+ arising from the steric effect of the bulky dialkylamino groups. This provides an alternative way to increase the blocking temperature.
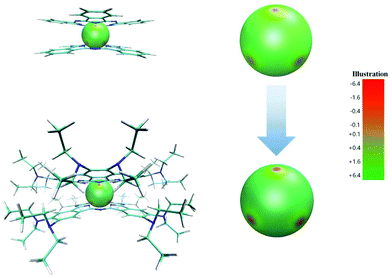 | ||
| Fig. 1 Electrostatic potential projection on a 2 Å radius sphere centered at the Tb3+ position with the two phthalocyanine ligands in Tb(Pc)2 and Tb{Pc[N(C4H9)2]8}2. | ||
The study by Jiang and co-workers not only reports a record blocking temperature for bis(tetrapyrrole) lanthanide double-decker SIMs, but also has clearly clarified the significant effect of the electron-donating peripheral substituents on the SIM functionality of bis(phthalocyaninato) terbium SIMs, offering an efficient method for the design and synthesis of sandwich-type tetrapyrrole lanthanide SIMs with enhanced magnetic behavior. Finally, it is worth noting that there are surely other structural factors which affect the sandwich-type tetrapyrrole lanthanide SIM performance. In particular, unsymmetrical peripheral substitution has been proven to be able to elevate the spin-reversal energy barrier of the sandwich-type tetrapyrrole Tb SMM, leading to the highest effective energy barrier of 939 K for bis(tetrapyrrole) lanthanide-based SIMs reported to date.8 As a consequence, the combination of rich phthalocyanine chemistry such as unsymmetrical peripheral substitution and the unique properties of lanthanides to enhance organic radical-f interaction in the bis(tetrapyrrole) lanthanide SIMs is expected to afford new SIMs with a higher working temperature, making a step forward towards the practical application of SMMs.
Conflicts of interest
There are no conflicts to declare.References
- L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179 CrossRef CAS PubMed.
- R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS.
- (a) L. M. C. Beltran and J. R. Long, Acc. Chem. Res., 2005, 38, 325 CrossRef CAS PubMed; (b) R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011 RSC; (c) M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353 RSC; (d) G. Aromí, D. Aguilà, P. Gamez, F. Luis and O. Roubeau, Chem. Soc. Rev., 2012, 41, 537 RSC; (e) G. A. Timco, T. B. Faust, F. Tuna and R. E. P. Winpenny, Chem. Soc. Rev., 2011, 40, 3067 RSC.
- N. Ishikawa, M. Sugita, T. Ishikawa, S. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS PubMed.
- Y.-S. Meng, S.-D. Jiang, B.-W. Wang and S. Gao, Acc. Chem. Res., 2016, 49, 2381 CrossRef CAS PubMed.
- (a) H. Wang, B. Wang, Y. Bian, S. Gao and J. Jiang, Coord. Chem. Rev., 2016, 306, 195 CrossRef CAS; (b) J. Liu, Y. Chen, F. Guo and M. Tong, Coord. Chem. Rev., 2014, 281, 26 CrossRef CAS; (c) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef CAS PubMed; (d) J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC.
- (a) H. Wang, K. Wang, J. Tao and J. Jiang, Chem. Commun., 2012, 48, 2973 RSC; (b) J. Kan, H. Wang, W. Sun, W. Cao, J. Tao and J. Jiang, Inorg. Chem., 2013, 52, 8505 CrossRef CAS PubMed; (c) H. Wang, K. Qian, K. Wang, Y. Bian, J. Jiang and S. Gao, Chem. Commun., 2011, 47, 9624 RSC.
- C. R. Ganivet, B. Ballesteros, G. de la Torre, J. M. Clemente-Juan, E. Coronado and T. Torres, Chem. – Eur. J., 2013, 19, 1457 CrossRef CAS PubMed.
- M. Mannini, F. Bertani, C. Tudisco, L. Malavolti, L. Poggini, K. Misztal, D. Menozzi, A. Motta, E. Otero, P. Ohresser, P. Sainctavit, G. G. Condorelli, E. Dalcanale and R. Sessoli, Nat. Commun., 2014, 5, 4582 CAS.
- Y. Chen, F. Ma, X. Chen, B. Dong, K. Wang, S. Jiang, C. Wang, X. Chen, D. Qi, H. Sun, B. Wang, S. Gao and J. Jiang, Inorg. Chem. Front., 2017, 4, 1465–1471 RSC.
- P. Zhu, F. Lu, N. Pan, D. P. Arnold, S. Zhang and J. Jiang, Eur. J. Inorg. Chem., 2004, 2004, 510 CrossRef.
| This journal is © the Partner Organisations 2017 |