A new approach for recycling waste rubber products in Li–S batteries†
Byeong-Chul
Yu
,
Ji-Won
Jung
,
Kyusung
Park
and
John B.
Goodenough
*
Texas Materials Institute, University of Texas at Austin, Texas 78712, USA. E-mail: jgoodenough@mail.utexas.edu
First published on 10th November 2016
Abstract
Vulcanized rubber products contain polymer backbones crosslinked with sulfur to improve mechanical strength. Burning of waste rubber products emits toxic gases, and recycling of rubber by breaking the C–S bond with costly reagents and heat is also haunted by environmental concerns. The crosslinked polymers can be extracted chemically at room temperature and, by a simple solution-drop method, used to prepare a bifunctional cathode layer on the cathode current collector of a Li–S battery. The C–S bond of the crosslinking sulfur can be broken reversibly in a discharge/recharge cycle to provide a sulfur source, and the broken carbon bond can capture a lithium polysulfide soluble in a liquid organic electrolyte by forming a C–Li–S bond during discharge. During charge, the Li is extracted and the C–S bond is reformed. However, added sulfur powder is also needed in the cathode of a Li–S battery. The data also provide a low-cost way to recycle waste rubber electrochemically.
Broader contextThe lithium sulfur battery based on the electrochemical lithiation/delithiation of sulfur is one of the most promising energy storage systems as a post lithium-ion battery. However, there is a critical challenge in the development of advanced Li/S cells. When sulfur reacts with lithium during discharge/charge, the intermediate polysulfides (Li2Sx, 4 ≤ x ≤ 8) are dissolved in the organic electrolyte, leading to a shuttle phenomenon. To overcome this problem, various approaches have been employed to address polysulfide shuttle challenges in Li–S cells. In this work, we propose recycling waste rubber in a Li–S battery. We can easily obtain a vulcanized rubber solution with CS2 from the waste rubber. We coat a vulcanized rubber polymer onto a freestanding CNF current collector by the solution-drop method. The simple room-temperature fabrication replaces the commonly used carbon and sulfur mixing followed by heating to diffuse the sulfur into the carbon. Interestingly, the C–S bond in vulcanized rubber is broken by lithiation on discharge, and the broken C site bonds with lithium in the lithium polysulfides as evidence by XPS, which leads to a significant capacity increase and stable cycle life. This means that vulcanized rubber from various rubber products can provide alternative active and binder material for the cathode of a Li–S battery. |
The lithium–sulfur rechargeable battery cell (Li–S) offers a theoretical cathode specific capacity of 1675 mA h g−1,1–3 but its practical use has been frustrated by the existence of lithium polysulfide intermediates of the cathode reaction that are soluble in the liquid electrolyte of the cell;4 the soluble polysulfides diffuse to the anode and corrode it or to surfaces where they are not recycled on charge. Various approaches have been employed to overcome this problem,5–23 but none have proven commercially viable. An alternative approach is to use the vulcanized polymers of waste rubber products as a source of the sulfur in the cathode of a Li–S cell.24–34 Since toxic gases are emitted from the burning of waste rubber, alternative uses of waste rubber have been explored; one of these has been as a carbon source for an electrochemical capacitor or a Li-ion battery.35–37
In this paper, we demonstrate that vulcanized polymers from either used tires or rubber stopper can be cheaply extracted and used as bifunctional cathode layers of a Li–S cell; the polymers act both as a source of the sulfur and, by a C–Li bond, as a trap of the lithium polysulfides on a substrate connected electronically to the cathode current collector.
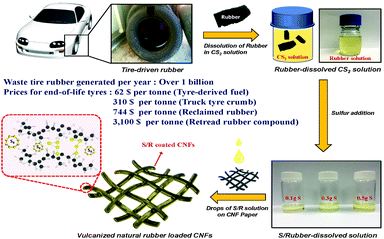
Fig. 1 displays a schematic of the preparation of the rubber solution from a used tire and the cathode electrode. There were impurities in the initial solution because the tire consisted of rubbers, carbon, filler and other additives. Fig. S1 (ESI†) shows the XRD of the amorphous rubber solution before and after filtering to remove impurities. Al2Si2O5(OH)4, which was used for modifying the properties of the rubber upon vulcanization, can be removed by filtering. The pure rubber polymer was identified by FT-infrared spectroscopy (FTIR), Fig. S2(a)–(c) (ESI†), with different wavenumber ranges. The 3450 cm−1 is from the OH− group from water and the wavenumbers from 2800 to 3000 cm−1 can be attributed to C–H stretching modes. The absorption peaks at 1630 and 1520 cm−1 are due to C–C or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C; the two peaks at 1455 and 1380 cm−1 to the C–H bending. The peaks at 840 and 470 cm−1 are related to the C–S and S–S vibrations of the sulfur crosslinked polymers.38 A typical tire contains four kinds of natural rubber, polybutadiene, styrene butadiene rubber, and butyl rubber.39 The FT-IR spectrum indicates the tire contained vulcanized natural rubber.40
C; the two peaks at 1455 and 1380 cm−1 to the C–H bending. The peaks at 840 and 470 cm−1 are related to the C–S and S–S vibrations of the sulfur crosslinked polymers.38 A typical tire contains four kinds of natural rubber, polybutadiene, styrene butadiene rubber, and butyl rubber.39 The FT-IR spectrum indicates the tire contained vulcanized natural rubber.40
Commercial sulfur powder was added to the rubber solution to increase the amount of active sulfur. The final wt% sulfur of the R/xS samples was obtained by the weight loss of sulfur in a thermogravimetric analysis (TGA), Fig. S3 (ESI†), which gave 18, 47, 63, and 85 wt% sulfur, respectively, for R, R/1S, R/3S, and R/5S. Elemental analysis (EA) was performed to reconfirm the sulfur content of the R/xS samples. The EA reveals sulfur loading of 21.34, 49.23, 64.84 and 86.2 wt% for R, R/1S, R/3S, and R/5S. The carbon disulfide (CS2) solution was loaded by drops onto a 3/8-in-diameter CNF film, see experimental, and the CS2 was evaporated at room temperature. A light carbon flexible nanofiber (CNF) film replaced the Al-foil cathode current collector normally employed. Adopting a porous carbonaceous current collector to the sulfur cathode can enhance the electrochemical performance compared to Al foil. Fig. S4 (ESI†) shows real images of CNF films and electrodes. The simple room-temperature fabrication replaces the commonly used carbon and sulfur mixing followed by heating to diffuse the sulfur into the carbon.41,42 The interstitial space of the R/xS cathode allows penetration of the liquid electrolyte to the bridging molecules. This design improves the volumetric as well as the specific energy density of a Li–S cell. Fig. S5 (ESI†) displays the X-ray diffraction (XRD) spectra of the different cathodes. The sulfur powder was crystalline, the CNF and CNF/R samples were amorphous. The sulfur peaks of the CNF/R/S were significantly decreased compared to those of the CNF/S samples because the sulfur was covered by a rubber layer, see discussion in next section. X-ray photoelectronspectroscopy (XPS), Fig. S6 (ESI†), was conducted for elemental analysis. The C1s peaks of the CNF of Fig. S6(a) (ESI†) are from the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.5 eV), the C–N (285.4 eV), and C–O (286.3 eV) bonds of the PAN precursor, which can enhance the electrical conductivity and be effective trapping the polysulfides.43 The new peak at 286 eV of the CNF/R and CNF/S of Fig. S6(b) and (c) (ESI†) reveals the C–S bond of the vulcanized natural rubber.28 Fig. S6(d)–(f) (ESI†) displays the S2p peaks of each cathode. We confirmed that there were C–S peaks (165.1 eV and 163.7 eV) and elemental sulfur peaks (2p1/2 162.7 eV and 2p3/2 161.5 eV) in the CNF/R sample from the crosslinking S8 molecules. The sulfur peaks increase with the sulfur additives in the CNF/R/S samples.
C (284.5 eV), the C–N (285.4 eV), and C–O (286.3 eV) bonds of the PAN precursor, which can enhance the electrical conductivity and be effective trapping the polysulfides.43 The new peak at 286 eV of the CNF/R and CNF/S of Fig. S6(b) and (c) (ESI†) reveals the C–S bond of the vulcanized natural rubber.28 Fig. S6(d)–(f) (ESI†) displays the S2p peaks of each cathode. We confirmed that there were C–S peaks (165.1 eV and 163.7 eV) and elemental sulfur peaks (2p1/2 162.7 eV and 2p3/2 161.5 eV) in the CNF/R sample from the crosslinking S8 molecules. The sulfur peaks increase with the sulfur additives in the CNF/R/S samples.
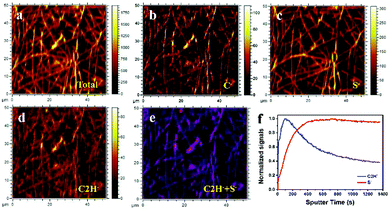
Field emission scanning-electron-microscopy (FESEM) images, Fig. S7 (ESI†), reveal the CNF current collector is a web of fibers of 500 nm width; the sulfur of the CNF/S after CS2 evaporation forms large particles of aggregated S8 molecules, but the aggregation is reduced by the rubber on the CNF/R/S sample. To confirm surface and element distribution of CNF/R/S samples, scanning transmission electron microscopy (STEM) mode with bright field (BF) and dark field (DF) was employed. Fig. S8 (ESI†) shows the R/S material is well-coated on the CNF. Since there were two sources of the sulfur, the powder and the vulcanized rubber, time-of-flight secondary ion mass spectrometry (TDF-SIMS) was made; Fig. 2 presents the mapping images and depth profile of the CNF/R/S electrode. The mapping size of the high-resolution images was 50 μm × 50 μm and the C−, S− and C2H− were confirmed by element analysis. The C− and S− are mainly from the CNF electrode and sulfur powder, respectively. The C2H− originates from the vulcanized natural rubber. The combined image with C2H−(blue) and S−(red) is shown in Fig. 2e. The two active materials were well distributed on the CNF electrode. The surface of CNF/R/S electrode was examined by depth profiles of S− and C2H− elements in Fig. 2f. The C2H− signal was higher than that of S− in the first stage (<300 s). The signal of C2H− continuously decreased and that of S− was saturated inside of surface, which indicates that sulfur powder was coated by a vulcanized rubber layer a few nm thick (the rate of sputtering was 0.01 nm s−1). The various sulfur cathodes were galvanostatically tested with 1 M LiCF3SO3 (LITF) in DOL/DME/0.5 M LiNO3 as electrolyte, a celgard 2500 separator, and Li metal as counter electrode. We used LITF salt instead of LIFTSI because the cycle stability of both salt systems in DME/DOL electrolyte is similar. The voltage range was set from 1.8 V to 2.8 V to avoid LiNO3 decomposition below 1.7 V. All electrochemical tests used total weight of rubber and sulfur, but not that of the current collector, to calculate current values and capacities. The voltage profiles at 1C rate (=1673 mA g−1) of the various cathodes are shown in Fig. S9 (ESI†). The CNF/5S electrode without rubber, Fig. S9(a) (ESI†), showed two regions of voltage V versus capacity during discharge and a capacity fade on the second cycle. The vulcanized sulfur of the CNF/R sample, Fig. S9(b) (ESI†), involved breaking and reforming (according to ex situ XPS) crosslinking of the sulfur; the charging voltage of 2.4 V is similar to that of the CNF/5S. The differential capacity plot of the CNF/R cathode, Fig. S9(c) (ESI†), shows small peaks at 2.45 V on discharge that have been attributed to the conversion of the crosslinking sulfur of the vulcanized rubber to Li-ion polysulfides.28,44 These data show that vulcanized rubber from a used tire can provide an alternative sulfur source for a Li–S battery that operates reversibly during discharge/charge with a capacity close to that of the sulfur in the vulcanized rubber. The voltage profiles of CNF/R/1S, CNF/R/3S and CNF/R5S with different sulfur ratios are shown in Fig. S9(d)–(f) (ESI†). As the amount sulfur increased, not only the capacity dramatically increased, but also the overpotential between discharge and charge voltages decreased. The large overpotential of the CNF/R/1S electrode was due to a thick coating layer of vulcanized rubber. Therefore, a thin coating layer is needed to get a better electrochemical performance because the rubber is an electric insulator.
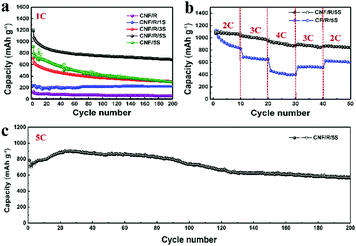
Fig. 3 displays the electrochemical performances of the various sulfur cathodes operating at different rates. Fig. 3a compares cycle performances at 1C rate during 200 cycles. Compared to CNF/R/5S, the capacity of CNF/5S electrode at first cycle was low and dropped continuously over 200 cycles due to dissolution of polysulfides during discharge/charge. The CNF/R/5S electrode showed a higher capacity and good retention with over 800 mA h g−1 after 200 cycles. The rate capabilities are shown in Fig. 3b. A commercial carbon paper (CP) with active R/5S material was used to compare with our freestanding CNF electrode. The SEM images of CP and CP/R/5S electrodes are shown in Fig. S10 (ESI†). The rate performance of the CNF/R/5S electrode was enhanced because the 3D network of nano-sized carbon fiber facilitates both electron and ion transport. The voltage profiles at different current rates from 0.2C to 4C of the CNF/R/5S electrode are presented in Fig. S11 (ESI†). Fig. 3c presents the cycle performance of CNF/R/5S at a high rate (=5C). The first discharge and charge capacities were 784.5 mA h g−1 and 708.9 mA h g−1, showing >90% coulombic efficiency. The cycle retention over 200 cycles was 81.1% despite the high current rate.
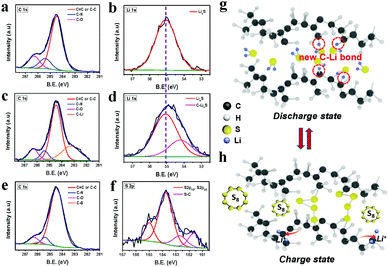
An ex situ XPS was employed to prove the Li bonding and reaction mechanism of the CNF/R/5S electrode during discharge/charge. Cycled CNF/R/5S cells were disassembled in an Ar-filled glove box and the electrodes were washed with DME to remove lithium salt and organic materials. Fig. 4a–f displays ex situ XPS of CNF/5S and CNF/R/5S electrodes. The C1s peaks of CNF/5S after discharge were the same as those of the pristine electrode shown in Fig. S6 (ESI†). The peak at 55.06 eV in Fig. 4b was assigned to the Li2S product, which agrees with previous papers.13 Interestingly, a new peak of C1s appeared when the CNF/R/5S was discharged in the 1st cycle. This 183.25 eV peak of C1s is from the C–Li bond45–48 while the C–S peak of vulcanized rubber disappeared. Based on peak changes of C1s, the C–S bond is broken by the reaction of lithium with the crosslinked sulfur of vulcanize rubber, as is confirmed in Fig. S10 (ESI†) and Fig. 3. Xu group reported the lithiation mechanism of sulfurized polyacrylonitrile.12 In this paper, the authors already knew the sulfur content in the composite and calculated the theoretical capacity based on the sulfur weight in their material. They assumed that all the sulfur atoms were reduced to S2−via the reaction, 2Li + S → Li2S because the experimental specific capacity was close to the calculated theoretical capacity. However, there was no evidence to determine whether the C–S bond in the sulfurized polyacrylonitrile composite is broken during discharge. The sites of the broken C bond in the rubber matrix would be unstable, which can provide an adsorption site. Therefore, lithium from the Li2Sx polysulfides after discharge is attracted by the broken C bond to form a C–Li2S bond (54.2 eV), which accounts for the shift in Fig. 4d to a lower S2p binding energy compared to that of the CNF/5S electrode. This result indicates that the broken carbon bond in the polymer backbone releases electronic charge to lithium in the lithium polysulfides to form a C–Li–S bond. At the atomic level, pure sulfur S8 molecules should interact via van der Waals attraction, but after lithiation of the terminated sulfurs in Li2Sx (4 ≤ x ≤ 8), electrostatic interactions between lithium and sulfur or asymmetric bonding by a covalent component between lithium and surrounding chemical environments become more important. Therefore, the improved electrochemical properties in Fig. 3 can be attributed to a capture of soluble polysulfides by the formation of Li–C bonds on the polymers. This new finding contradicts the assumption made by most workers with vulcanized polymers for a Li–S battery who have suggested that the C–S bond is not broken during cycling.27–30 After cycling, the XPS C1s peak of the C–Li bond disappeared and that of the C–S bond reappeared showing that the crosslinking sulfur molecules reformed on removal of the Li during charge, as is also evident from the S-2p1/2 and S-2p3/2 peaks in Fig. 4f. The mechanism of the sulfur reduction/oxidation of the crosslinking sulfur by reaction with lithium is depicted schematically in Fig. 4g and h.
We have compared the use of vulcanized rubber from a laboratory used stopper with that from a used tire as a coating layer on the CNF current collector. Fig. S12(a) and (b) (ESI†) shows the waste rubber stopper used and the rubber solution obtained from it. The voltage profile and cycle performance of the electrode prepared from stopper rubber is shown in Fig. S12(c) and (d) (ESI†). The first discharge/charge cycle at 0.2 C showed a large irreversible capacity (1295 to 906 mA h g−1). However, after the second cycle at 0.5 C, the capacity saturated at 610 mA h g−1 over 200 cycles.
Finally, these demonstrations not only show that various used rubber products can provide alternative active and binder material for the cathode of a Li–S battery. They also show that recycling of rubber products electrochemically can overcome the principal recycling limitation of breaking the C–S bond with costly reagents and heat.
Conclusions
Vulcanized rubber products can be used in the cathode of a Li–S battery as a bifunctional layer on a carbon nanofiber (CNF) current collector to provide both a source of sulfur and a trap for Li polysulfides that are soluble in an organic–liquid electrolyte. The sulfur of a vulcanized rubber product crosslinks polymer backbones, and the mechanism of reversible electrochemical insertion/extraction of lithium into the crosslinking sulfur has been shown to break the C–S bond between the polymer and the sulfur crosslinking molecule on discharge to create a C–Li–S bond; on charge, the lithium is removed and the C–S bond reforms. This observation has important implications not only for fabrication of a low-cost cathode of a Li–S battery, but also for recycling of rubber products.Acknowledgements
This research was supported by the Hyundai Research Foundation (UTA15-000746).References
- A. Manthiram, Y. Fu and Y.-S. Su, Acc. Chem. Res., 2013, 46, 1125–1134 CrossRef CAS PubMed.
- Y. Yang, G. Y. Zheng and Y. Cui, Chem. Soc. Rev., 2013, 42, 3018–3032 RSC.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- Y. V. Mikhaylik and J. R. Akridge, J. Electrochem. Soc., 2004, 151, A1969–A1976 CrossRef CAS.
- G. Zheng, Y. Yang, J. J. Cha, S. S. Hong and Y. Cui, Nano Lett., 2011, 11, 4462–4467 CrossRef CAS PubMed.
- B. Zhang, X. Qin, G. R. Li and X. P. Gao, Energy Environ. Sci., 2010, 3, 1531–1537 CAS.
- N. Jayaprakash, J. Shen, S. S. Moganty, A. Corona and L. A. Archer, Angew. Chem., Int. Ed., 2011, 50, 5904–5908 CrossRef CAS PubMed.
- J. Guo, Y. Xu and C. Wang, Nano Lett., 2011, 11, 4288–4294 CrossRef CAS PubMed.
- X. Ji, K. T. Lee and F. A. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- G. M. Zhou, Y. B. Zhao and A. Manthiram, Adv. Energy Mater., 2015, 5, 1402263 CrossRef.
- W. D. Zhou, X. C. Xiao, M. Cai and L. Yang, Nano Lett., 2014, 14, 5250–5256 CrossRef CAS PubMed.
- J. L. Wang, J. Yang, J. Y. Xie and N. X. Xu, Adv. Mater., 2002, 14, 963–965 CrossRef CAS.
- K. Park, J. H. Cho, J.-H. Jang, B.-C. Yu, A. T. De La Hoz, K. M. Miller, C. J. Ellison and J. B. Goodenough, Energy Environ. Sci., 2015, 8, 2389–2395 CAS.
- Y. Yang, G. Yu, J. J. Cha, H. Wu, M. Vosgueritchian, Y. Yao, Z. Bao and Y. Cui, ACS Nano, 2011, 5, 9187–9193 CrossRef CAS PubMed.
- W. Li, Q. Zhang, G. Zhng, Z. W. Seh, H. Yao and Y. Cui, Nano Lett., 2013, 13, 5534–5540 CrossRef CAS PubMed.
- W. D. Zhou, X. C. Xiao, M. Cai and L. Yang, Nano Lett., 2014, 14, 5250–5256 CrossRef CAS PubMed.
- K. T. Lee, R. Black, T. Yim, X. L. Ji and L. F. Nazar, Adv. Energy Mater., 2012, 2, 1490 CrossRef CAS.
- Z. W. Seh, W. Li, J. J. Cha, G. Zheng, Y. Yang, M. T. McDowel, P.-C. Hsu and Y. Cui, Nat. Commun., 2013, 4, 1331 CrossRef PubMed.
- X. Tao, J. Wang, C. Liu, H. Wang, Q. Cai, W. Li, G. Zhou, C. Zu and Y. Cui, Nat. Commun., 2016, 7, 11203 CrossRef CAS PubMed.
- X. Tao, F. Chen, Y. Xia, H. Huang, Y. Gan, X. Chen and W. Zhang, Chem. Commun., 2013, 49, 4513–4515 RSC.
- J. M. Zheng, J. Tian, D. Wu, M. Gu, W. Xu, C. Wang, F. Gao, M. H. Engelhard, J.-G. Zhang, J. Liu and J. Xiao, Nano Lett., 2014, 14, 2345–2352 CrossRef CAS PubMed.
- J. Jiang, J. Zhu, W. Ai, X. Wang, Y. Wang, C. Zou, W. Huang and T. Yu, Nat. Commun., 2015, 6, 8622 CrossRef CAS PubMed.
- Z. Cui, C. Zu, W. Zhou, A. Manthiram and J. B. Goodenough, Adv. Mater., 2016, 28, 6926–6931 CrossRef CAS PubMed.
- W. J. Chung, J. J. Giebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wiw, N. A. Nguyen, B. W. Guralnick, J. Park, A. Somogyi, P. Theato, M. E. Mckay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- H. Kim, J. Lee, H. Ahn, O. Kim and M. J. Park, Nat. Commun., 2015, 6, 7278 CrossRef CAS PubMed.
- B. Zhang, S. Wang, M. Xiao, D. Han, S. Song, G. Chen and Y. Meng, RSC Adv., 2015, 5, 38792 RSC.
- A. G. Simmonds, J. J. Griebel, J. Park, K. R. Kim, W. J. Chung, V. P. Pleshko, J. Kim, E. T. Kim, R. S. Glass, C. L. Soles, Y.-E. Sung, K. Char and J. Pyun, ACS Macro Lett., 2014, 3, 229–232 CrossRef CAS.
- C. Fu, G. Li, J. Zhang, B. Cornejo, S. S. Piao, K. N. Bozhilov, R. C. Haddon and J. Guo, ACS Energy Lett., 2016, 1, 115–120 CrossRef CAS.
- S. Wei, L. Ma, K. E. Hendrickson, Z. Tu and L. A. Archr, J. Am. Chem. Soc., 2015, 137, 12143–12152 CrossRef CAS PubMed.
- S.-C. Zhang, L. Zhang, W.-K. Wang and W.-J. Cue, Synth. Met., 2010, 160, 2041–2044 CrossRef CAS.
- H. Chen, C. Wang, C. Hu, J. Zhang, S. Gao, W. Lu and L. Chen, J. Mater. Chem. A, 2015, 3, 1392–1395 CAS.
- S. H. Je, T. H. Hwang, S. N. Talapaneni, O. Buyukcakir, H. J. Kim, J.-S. Yu, S.-G. Woo, M. C. Jang, B. K. Son, A. Coskun and J. W. Choi, ACS Energy Lett., 2016, 1, 566–572 CrossRef CAS.
- S. S. Zhang, Front. Energy Res., 2013, 1, 10 Search PubMed.
- S. S. Zhang, Energies, 2014, 7, 4588–4600 CrossRef.
- A. K. Naskar, Z. Bi, Y. Li, S. K. Akato, D. Saha, M. Chi, C. A. Bridges and M. P. Paranthaman, RSC Adv., 2014, 4, 38213 RSC.
- M. Boora, M. P. Paranthaman, A. K. Naskar, Y. Li, K. Akato and Y. Gogotsi, ChemSusChem, 2015, 8, 3576–3581 CrossRef PubMed.
- Y. Li, M. P. Parnathaman, K. Akato, A. K. Naskar, A. M. Levine, R. J. Lee, S.-O. Kim, J. Zhang, S. Dai and A. Manthiram, J. Power Sources, 2016, 316, 232–238 CrossRef CAS.
- G. Socrates, Infrared and Raman Characteristic Group Frequencies, John Wiley & Sons Ltd., Chichester, 3rd edn, 2004, p. 366, 0470093072 Search PubMed.
- A. Quek and R. Balasubramanian, J. Anal. Appl. Pyrolysis, 2013, 101, 1–16 CrossRef CAS.
- P. Dey, K. Naskar, B. Dash, S. Nair, G. Unnikrishnan and G. B. Nando, RSC Adv., 2015, 5, 31886 RSC.
- Z. Yuan, H.-J. Peng, J.-Q. Huang, X.-Y. Liu, D.-W. Wang, X.-B. Cheng and Q. Zhang, Adv. Funct. Mater., 2014, 24, 6105–6112 CrossRef CAS.
- L. Ji, M. Rao, S. Aloni, L. Wang, E. J. Cairns and Y. Zhang, Energy Environ. Sci., 2011, 4, 5053 CAS.
- H. Song, T. Xu, M. L. Gordin, P. Zhu, D. Lv, Y.-B. Jiang, Y. Chen, Y. Duan and D. Wang, Adv. Funct. Mater., 2014, 24, 1243–1250 CrossRef.
- H. S. Ryu, Z. Guo, H. J. Ahn, G. B. Cho and H. Liu, J. Power Sources, 2009, 189, 1179–1183 CrossRef CAS.
- M.-S. Song, R.-H. Kim, S.-W. Baek, K.-S. Lee, K. Park and A. Benayad, J. Mater. Chem. A, 2014, 2, 631 CAS.
- C. Xu, B. Sun, T. Gustafsson, K. Edstrom, D. Brandell and M. Hahin, J. Mater. Chem. A, 2014, 2, 7256 CAS.
- D. Aurbach, E. Pollak, R. Elazri, G. Salitra, C. S. Kelley and J. Affinito, J. Mater. Chem. A, 2014, 2, 7256 Search PubMed.
- C. Zu and A. Manthiram, Adv. Energy Mater., 2014, 4, 1400897 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ee02770a |
| This journal is © The Royal Society of Chemistry 2017 |