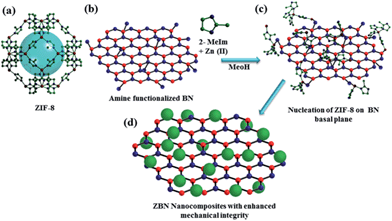
Functionality preservation with enhanced mechanical integrity in the nanocomposites of the metal–organic framework, ZIF-8, with BN nanosheets†
Ram
Kumar
a,
Devraj
Raut
b,
Irshad
Ahmad
c,
Upadrasta
Ramamurty
b,
Tapas Kumar
Maji
a and
C. N. R.
Rao
*a
aInternational Centre for Materials Science, Chemistry and Physics of Materials Unit and Sheikh Saqr Laboratory, Jawaharal Nehru Centre for Advanced Scientific Research, Jakkur, P.O., Bangalore-560064, India. E-mail: cnrrao@jncasr.ac.in
bDepartment of Materials Engineering, Indian Institute of Science, Bangalore-560012, India
cDepartment of Arts and Sciences, American University of Ras Al Khaimah, P.O.Box 10021, United Arab Emirates
First published on 3rd July 2014
Abstract
Metal–organic frameworks (MOFs) and boron nitride both possess novel properties, the former associated with microporosity and the latter with good mechanical properties. We have synthesized composites of the imidazolate based MOF, ZIF-8, and few-layer BN in order to see whether we can incorporate the properties of both these materials in the composites. The composites so prepared between BN nanosheets and ZIF-8 have compositions ZIF–1BN, ZIF–2BN, ZIF–3BN and ∼ZIF–4BN. The composites have been characterized by PXRD, TGA, XPS, electron microscopy, IR, Raman and solid state NMR spectroscopy. The composites possess good surface areas, the actual value decreasing only slightly with the increase in the BN content. The CO2 uptake remains nearly the same in the composites as in the parent ZIF-8. More importantly, the addition of BN markedly improves the mechanical properties of ZIF-8, a feature that is much desired in MOFs. Observation of microporous features along with improved mechanical properties in a MOF is indeed noteworthy. Such manipulation of properties can be profitably exploited in practical applications.
Conceptual insightsMetal–organic frameworks (MOFs) are an important class of materials with potential applications in gas storage and catalysis. One of the key challenges for their practical applications is low mechanical robustness and stability as they contain significant structural porosity and are typically fragile in nature. One possible way of overcoming this drawback is through compositing with a two-dimensional nanomaterial such as BN which has high mechanical strength. Such an effort to improve the mechanical properties of MOFs has not been reported hitherto. We have investigated, for the first time, the possibility of enhancing the mechanical properties of a MOF, in the case of microporous zeolitic imidazolate framework ZIF-8. For this purpose, we prepared composites where ZIF-8 is chemically bonded to few-layer BN. Incorporating the properties of both BN and the MOF, especially to improve the mechanical strength of the latter, is indeed novel. |
Single and few-layer graphene and their analogues exhibit many novel physical, mechanical and adsorption properties.1 Recently, layered inorganic compounds, of which hexagonal boron nitride (BN) is an important member with high thermal and chemical stabilities as well as good mechanical properties, have attracted considerable attention.2 Nanosheets of BN containing few-layers have been prepared by mechanical exfoliation, chemical exfoliation using ultrasonication in a polar solvent and chemical vapour deposition.2c,3 Metal–organic frameworks (MOFs) are an important class of compounds for potential applications in gas storage and catalysis.4 One of the key challenges that needs to be overcome for industrial-scale deployment of these compounds will be their low mechanical robustness and stability as these materials, which contain significant structural porosity within them, tend to exhibit low mechanical properties and typically are fragile in nature.5 Furthermore, zeolites and several other MOFs undergo amorphization under pressure.6 We felt that a possible way of overcoming such a drawback would be through compositing MOFs with two-dimensional nanomaterials such as graphene and BN.2b,7 The advantages of composites of MOFs prepared with nanoparticles has been nicely described by Doherty et al.,8 and multifunctional nanoparticle–MOF core–shell structures with interesting properties such as gas sensing and catalysis are reported by Tang et al.9 Nanocomposites can also exhibit synergistic interactions between the MOF particles and the layered nanosheets, as demonstrated in the case of few-layer graphene and nanodiamond reinforced polymer composites.10 In the literature MOF–graphene nanocomposites have been prepared and their adsorption and electrical properties have been examined.11 There has been no study on mechanical properties of the composites or effort to improve the mechanical properties of MOFs. We have investigated, for the first time, the possibility of enhancing the mechanical performance of a microporous zeolitic imidazolate framework (ZIF-8) which possesses good chemical and thermal stabilities,12 by compositing it with few-layer BN. Incorporating the properties of both BN and the MOF, especially the mechanical strength of the former in the composite, is a much desired objective. We have used prototypical ZIF-8 (Zn(MeIM)2, MeIM = 2-methylimidazolate) with the sodalite zeolitic structure, possessing large cavities (∼11.6 Å diameter) and interconnected by narrow windows (3.4 Å), for this purpose (Scheme 1). Recognizing that physical mixing of ZIF-8 with BN may not impart the necessary mechanical stability to ZIF-8, we have studied the ZBN nanocomposites where ZIF-8 attaches to BN by chemical bonds prepared in situ in the colloidal dispersion of few-layer BN. Few-layer BN was prepared by the urea route in an ammonia atmosphere, and has a reasonably high surface area.2a Formation of the nanocomposite is favoured by the amine groups present on the BN basal plane, resulting from the urea route which creates an NH3 atmosphere in the reaction medium.13 The mole ratio of BN in the composite with respect to ZIF-8 was varied between 1 and 4, and the composites so-obtained with increasing BN content are denoted by ZBN-1, ZBN-2, ZBN-3 and ZBN-4 (see the Experimental section, ESI†).
The phase purity of the ZBN composites was examined by powder X-ray diffraction. The patterns of the ZBN composites are consistent with the cubic space group of ZIF-8 (Fig. S1, ESI†). ZIF-8 nanocrystals are present on the BN basal plane with the Zn(II) centres having the same coordination environment as ZIF-8. Hence, no significant change in powder X-ray diffraction patterns is observed except for a slight broadening in the patterns and a decrease in intensity due to the decrease in particle size. Thermogravimetric analysis of the composites in an oxygen atmosphere shows high thermal stability up to ca. 260 °C before decomposition of the framework structure (Fig. 1). ZIF-8 on heating in an oxygen atmosphere decomposes to ZnO leaving 35.4 wt% of ZnO as the residue (experimental weight loss 35%). Pure BN does not show any weight loss up to high temperatures. The nanocomposites ZBN-1, 2, 3 and 4 show weight losses giving ZnO–BN, ZnO–2BN, ZnO–3BN and ∼ZnO–4BN, respectively, as products. XRD patterns and other measurements confirm that the product residues contain only ZnO and BN (Fig. S6, ESI†). Elemental analysis also confirms the composition of the ZBN composites (Table S1†).
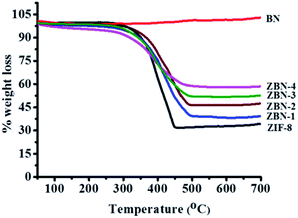 | ||
| Fig. 1 Thermogravimetric profile of ZIF-8 (black), ZBN-1 (blue), ZBN-2 (wine), ZBN-3 (olive), ZBN-4 (magenta) and BN (red) nanocomposites in an oxygen atmosphere. | ||
In Fig. 2, a typical X-ray photoelectron spectrum (XPS) in the case of ZBN-2 is displayed, which shows signals due to B (1s) and Zn (2p) with the expected B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratio of ∼2. Fig. 3 shows the infrared (IR) spectra of BN, ZIF-8 and ZBNs. The IR spectrum of BN shows bands at 803 and 1378 cm−1 attributed to the out-of-plane B–N–B bending mode and the in-plane B–N transverse optical mode, respectively, along with the N–H stretching band due to the amine groups (Fig. S8a, ESI†).13a,14 The N 1s XPS spectrum of BN showed features at 398.7 and 400.5 eV corresponding to N–B and N–H bonds, respectively (Fig. S8b, ESI†).15
Zn ratio of ∼2. Fig. 3 shows the infrared (IR) spectra of BN, ZIF-8 and ZBNs. The IR spectrum of BN shows bands at 803 and 1378 cm−1 attributed to the out-of-plane B–N–B bending mode and the in-plane B–N transverse optical mode, respectively, along with the N–H stretching band due to the amine groups (Fig. S8a, ESI†).13a,14 The N 1s XPS spectrum of BN showed features at 398.7 and 400.5 eV corresponding to N–B and N–H bonds, respectively (Fig. S8b, ESI†).15
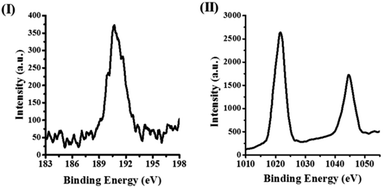 | ||
| Fig. 2 (I) High resolution B (1s) X-ray photoelectron spectrum of ZBN-2; (II) high resolution Zn (2p) X-ray photoelectron spectrum of ZBN-2. | ||
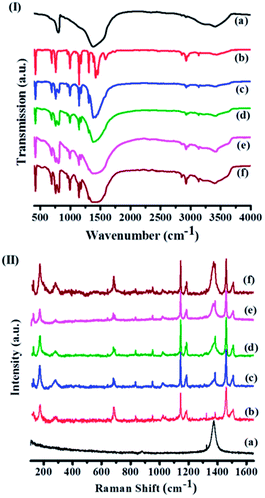 | ||
| Fig. 3 (I) Infrared spectra and (II) Raman spectra of (a) few-layer BN, (b) ZIF-8, (c) ZBN-1, (d) ZBN-2, (e) ZBN-3 and (f) ZBN-4. | ||
The IR spectrum of ZIF-8 shows a band at 421 cm−1 due to Zn–N stretching. The bands at 2854, 2927, 2958 and 3136 cm−1 arise from aliphatic and aromatic C–H stretching of 2-methylimidazole. The IR spectra of ZBNs have bands corresponding to ZIF-8 besides those at 807 and near 1377 cm−1 due to BN. Raman spectra collected at several different locations of ZBNs show bands at 1373 cm−1 corresponding to the E2g tangential mode of BN besides bands due to ZIF-8 (Fig. 3(II)).16 The spectrum of ZIF-8 has bands at 174, 686, 1146 and 1459 cm−1 corresponding to Zn–N stretching, imidazole ring puckering, C–N stretching and methyl bending, respectively.17 Bands corresponding to aliphatic and aromatic C–H stretching is observed at 2928, 3114 and 3135 cm−1 (Fig. S7, ESI†). The 11B solid state NMR spectra of the BN and ZBN-2 prepared by us were recorded at 11 kHz MAS and 160.47 MHz frequency (Fig. S2, ESI†). The BN sample shows a 11B spectrum different from that of pure hexagonal BN18 due to the presence of amine groups. Thus our sample of BN shows a strong signal at 1.96 ppm with much weaker signals in the 11–24 ppm region. The 11B spectrum of ZBN-2 is different with strong signals at 1.8 and 24.5 ppm with weaker features at 18.5 and 12.9 ppm.
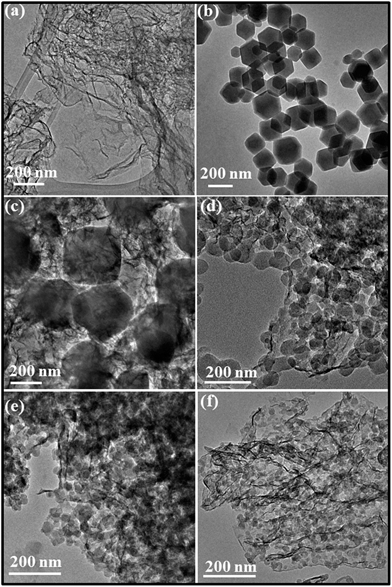
The morphology and homogeneity of ZBNs were characterized using transmission electron microscopy (TEM). Typical TEM images of few-layer BN and ZBNs and particle size distributions are displayed in Fig. 4 and S9 (ESI†). ZIF-8 nanocrystals have a hexagonal morphology with most particles having a size in the range of 150–220 nm. ZBN-1 has an irregular morphology embedded in the few-layer BN matrix with a particle size in the same range as ZIF-8. With increasing BN content in the composites, the particle size decreases and a change from hexagonal to spherical morphology occurs. Thus, the image of ZBN-2 shows dense and uniformly decorated spherical ZIF-8 nanocrystals. Most of the particles have a size in the range of 18–35 nm for ZBN-2 and ZBN-3. The size of the particles decreases to 14–20 nm in ZBN-4. A similar role of graphene in controlling the particle size and morphology of the composites to a MOF and photoactive zeolite has been reported.11a,c,19 The decrease in particle size and change in morphology of ZIF-8 nanocrystals are attributed to the interaction of Zn(II) with the amine functional group present on the BN basal plane. It is to be noted that boron in BN being electron deficient can interact with the nitrogen of 2-methylimidazole to stabilize ZIF-8 nanocrystals on the BN basal plane.20 FESEM images confirm the presence of ZIF-8 nanoparticles on BN sheets (Fig. S3, ESI†).
Surface areas of ZIF-8, BN and ZBNs were determined using N2 adsorption at 77 K (Fig. 5(I)). Few layer BN prepared under an ammonia atmosphere has a surface area of 537 m2 g−1 calculated using the BET model (Fig. S5, ESI†). The BET surface areas of ZIF-8, ZBN-1, ZBN-2, ZBN-3 and ZBN-4 are 1450, 1379, 1272, 1196 and 1110 m2 g−1, respectively. The adsorption profile has microporous type-I adsorption behaviour with small hysteresis in the case of ZBN-3 and ZBN-4. Interestingly, the uptake of CO2 at 195 K and 1 atm of the ZBN nanocomposites is essentially the same as that of ZIF-8, the actual values of ZBN-1, 2, 3 and 4 being 27, 24, 23 and 23 wt% compared to 25 wt% of ZIF-8 (Fig. 5(II)).
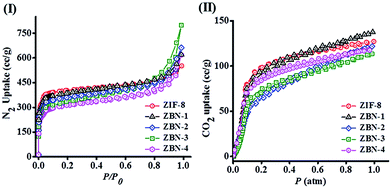 | ||
| Fig. 5 (I) N2 adsorption profile at 77 K and (II) CO2 adsorption profile at 195 K of ZIF-8 (red circle), ZBN-1 (black triangle), ZBN-2 (blue diamond), ZBN-3 (green square) and ZBN-4 (magenta circle). | ||
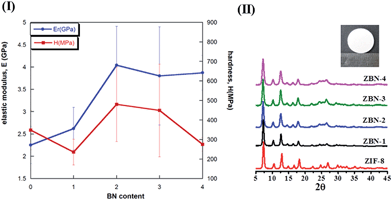
Fig. 6 shows the variation of the elastic modulus, E, and hardness, H, with the BN content in the nanocomposite. The error bars correspond to the standard deviations on 30 indents that were made on each pellet (see the Experimental section, ESI†). The measured values of E and H for the pure ZIF-8 are 2.25 GPa and 343 MPa. These values are ∼25 and 35% lower than the respective E and H values measured on {110} faces of single crystals by Tan et al.21 Since, the sintered pellets are polycrystalline, it is to be expected that the measured values are somewhat lower as they are ‘volume averaged’ properties of different crystals with each oriented randomly. In addition, the size of the crystals is much smaller in the present case. The addition of one BN to ZIF-8 as in ZBN-1 enhances E marginally (by ∼10%) whereas the hardness gets reduced, which is somewhat surprising. However, the nanocomposite with 2 BN units, ZBN-2, shows a remarkable improvement in both the properties, with E nearly-double that of the bare ZIF-8 while H is ∼30% greater. A further increase in the BN content to ZIF-8 does not lead to additional enhancement in E and H; in fact H decreases markedly whereas E remains invariant. The reduction in H at higher BN contents, especially in ZBN-4, is possibly due to incomplete compaction during pelletizing. We have also examined the mechanical properties of nanocomposite pellets wherein the ZIF-8 and BN were physically mixed before pelletizing. While these mixtures also showed property enhancements, the scatter in data is significantly more. This observation is on expected lines as significant heterogeneity is inevitable in pellets made by physical mixing of the components. Enhancement in the elastic modulus is attributed to synergistic interaction between ZIF-8 nanocrystals and BN sheets. Maximum synergy is observed up to a specific BN content with no property enhancement on increasing the BN content. This behaviour is similar to that of polymer matrix composites reinforced with nanocarbons where maximum enhancement is observed up to a certain concentration, with further addition leading to no improvement.10 If there were to be a matrix, the matrix material will provide for such synergy as both the particles and sheets would interact with each other through the matrix. Since there is no matrix the chemical interaction between ZIF-8 nanocrystals and BN basal plane ensures synergy. The above results confirm that the presence of BN in the ZIF-8–BN composites markedly enhances the mechanical properties of ZIF-8 and bestows the much needed structural integrity. The approach used in the present study – namely chemical synthesis of nanocomposites can be utilized to engineer framework materials with not only desired functional properties but also mechanical robustness required in practical situations.
We have successfully prepared a nanocomposite wherein an imidazolate based metal–organic framework (ZIF-8) is chemically bonded with nanosheets of BN. It is noteworthy that the nanocomposites retain the microporous characteristics, good surface areas and CO2 adsorption, while at the same time possessing improved mechanical properties, afforded by the presence of BN sheets. Such manipulation of properties of MOF composites may have useful applications.
Acknowledgements
Thanks are due to S. Sasidhara for assistance in preparing the pellets.Notes and references
- (a) A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 Search PubMed; (b) C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752 CrossRef CAS PubMed; (c) C. N. R. Rao, H. S. S. Ramakrishna Matte and U. Maitra, Angew. Chem., Int. Ed., 2013, 52, 13162 Search PubMed.
- (a) A. Nag, K. Raidongia, K. P. S. S. Hembram, R. Datta, U. V. Waghmare and C. N. R. Rao, ACS Nano, 2010, 4, 1539 Search PubMed; (b) M. S. R. N. Kiran, K. Raidongia, U. Ramamurty and C. N. R. Rao, Scr. Mater., 2011, 64, 592 CrossRef CAS PubMed; (c) C. Zhi, Y. Bando, C. Tang, H. Kuwahara and D. Golberg, Adv. Mater., 2009, 21, 2889 CrossRef CAS.
- (a) A. Pakdel, Y. Bando and D. Golberg, Chem. Soc. Rev., 2014, 43, 934 RSC; (b) K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451 Search PubMed.
- (a) S. Kitagawa, R. Kitaura and S.-I. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334 CrossRef CAS PubMed; (b) L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294 RSC; (c) A. K. Cheetham, C. N. R. Rao and R. K. Feller, Chem. Commun., 2006, 4780 RSC; (d) D. Bradshaw, J. B. Claridge, E. J. Cussen, T. J. Prior and M. J. Rosseinsky, Acc. Chem. Res., 2005, 38, 273 Search PubMed.
- J. C. Tan and A. K. Cheetham, Chem. Soc. Rev., 2011, 40, 1059 RSC.
- K. W. Chapman, G. J. Halder and P. J. Chupas, J. Am. Chem. Soc., 2009, 131, 17546 CrossRef CAS PubMed.
- D. Barun, K. E. Prasad, U. Ramamurty and C. N. R. Rao, Nanotechnology, 2009, 20, 125705 CrossRef PubMed.
- C. M. Doherty, D. Buso, A. J. Hill, S. Furukawa, S. Kitagawa and P. Falcaro, Acc. Chem. Res., 2013, 47, 396 CrossRef PubMed.
- (a) L. He, Y. Liu, J. Liu, Y. Xiong, J. Zheng, Y. Liu and Z. Tang, Angew. Chem., Int. Ed., 2013, 52, 3741 CrossRef CAS PubMed; (b) Y. Liu and Z. Tang, Adv. Mater., 2013, 25, 5819 CrossRef CAS PubMed; (c) M. Zhao, K. Deng, L. He, Y. Liu, G. Li, H. Zhao and Z. Tang, J. Am. Chem. Soc., 2014, 136, 1738 CrossRef CAS PubMed.
- K. E. Prasad, B. Das, U. Maitra, U. Ramamurty and C. N. R. Rao, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 13186 CrossRef CAS PubMed.
- (a) M. Jahan, Q. Bao, J.-X. Yang and K. P. Loh, J. Am. Chem. Soc., 2010, 132, 14487 CrossRef CAS PubMed; (b) C. Petit and T. J. Bandosz, Adv. Mater., 2009, 21, 4753 CAS; (c) R. Kumar, K. Jayaramulu, T. K. Maji and C. N. R. Rao, Chem. Commun., 2013, 49, 4947 RSC.
- K. S. Park, Z. Ni, A. P. Côté, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186 Search PubMed.
- (a) C. Zhi, Y. Bando, C. Tang, S. Honda, K. Sato, H. Kuwahara and D. Golberg, Angew. Chem., Int. Ed., 2005, 44, 7932 CrossRef CAS PubMed; (b) C. Zhi, Y. Bando, C. Tang and D. Golberg, J. Am. Chem. Soc., 2005, 127, 17144 Search PubMed; (c) C. Zhi, Y. Bando, C. Tang and D. Golberg, J. Phys. Chem. B, 2006, 110, 8548 CrossRef CAS PubMed; (d) T. Ikuno, T. Sainsbury, D. Okawa, J. M. J. Fréchet and A. Zettl, Solid State Commun., 2007, 142, 643 CrossRef CAS PubMed.
- Y. Gu, M. Zheng, Y. Liu and Z. Xu, J. Am. Ceram. Soc., 2007, 90, 1589 CrossRef CAS PubMed.
- (a) T. Sainsbury, T. Ikuno, D. Okawa, D. Pacilé, J. M. J. Fréchet and A. Zettl, J. Phys. Chem. C, 2007, 111, 12992 CrossRef CAS; (b) T. Ramanathan, F. T. Fisher, R. S. Ruoff and L. C. Brinson, Chem. Mater., 2005, 17, 1290 CrossRef CAS.
- S. Saha, D. V. S. Muthu, D. Golberg, C. Tang, C. Zhi, Y. Bando and A. K. Sood, Chem. Phys. Lett., 2006, 421, 86 CrossRef CAS PubMed.
- (a) G. Kumari, K. Jayaramulu, T. K. Maji and C. Narayana, J. Phys. Chem. A, 2013, 117, 11006 CrossRef CAS PubMed; (b) L. M. Markham, L. C. Mayne, B. S. Hudson and M. Z. Zgierski, J. Phys. Chem., 1993, 97, 10319 CrossRef; (c) D. A. Carter and J. E. Pemberton, J. Raman Spectrosc., 1997, 28, 939 CrossRef CAS.
- P. S. Marchetti, D. Kwon, W. R. Schmidt, L. V. Interrante and G. E. Maciel, Chem. Mater., 1991, 3, 482 CrossRef CAS.
- Z. Ren, E. Kim, S. W. Pattinson, K. S. Subrahmanyam, C. N. R. Rao, A. K. Cheetham and D. Eder, Chem. Sci., 2012, 3, 209 RSC.
- S. Pal, S. R. C. Vivekchand, A. Govindaraj and C. N. R. Rao, J. Mater. Chem., 2007, 17, 450 RSC.
- J.-C. Tan, B. Civalleri, C.-C. Lin, L. Valenzano, R. Galvelis, P.-F. Chen, T. D. Bennett, C. Mellot-Draznieks, C. M. Zicovich-Wilson and A. K. Cheetham, Phys. Rev. Lett., 2012, 108, 095502 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4mh00065j |
| This journal is © The Royal Society of Chemistry 2014 |