L-Valine methyl ester-containing tetraphenylethene: aggregation-induced emission, aggregation-induced circular dichroism, circularly polarized luminescence, and helical self-assembly†
Hongkun
Li
abc,
Juan
Cheng
d,
Yihua
Zhao
c,
Jacky W. Y.
Lam
bc,
Kam Sing
Wong
d,
Hongkai
Wu
c,
Bing Shi
Li
*a and
Ben Zhong
Tang
*bc
aDepartment of Chemistry and Chemical Engineering, Shenzhen University, Shenzhen, 518060, China. E-mail: phbingsl@google.com; Fax: +86-755-26536141; Tel: +86-755-26558094
bThe Hong Kong University of Science & Technology (HKUST)-Shenzhen Research Institute, No. 9 Yuexing 1st RD, South Area, Hi-tech Park, Nanshan, Shenzhen 518057, China. E-mail: tangbenz@ust.hk; Fax: +852-2358-1594; Tel: +852-2358-7375
cDepartment of Chemistry, Institute for Advanced Study, Institute of Molecular Functional Materials and State Key Laboratory of Molecular Neuroscience, HKUST, Clear Water Bay, Kowloon, Hong Kong, China
dDepartment of Physics, HKUST, Clear Water Bay, Kowloon, Hong Kong, China
First published on 6th June 2014
Abstract
L-Valine methyl ester-containing tetraphenylethene (Val-TPE) has been designed and synthesized. This novel molecule exhibits aggregation-induced emission (AIE), aggregation-induced circular dichroism (AICD), circularly polarized luminescence (CPL) and the capacity to self-assemble into helical nanofibers.
Conceptual insightsThis work provides a novel strategy for the construction of high efficiency fluorescent helical nanofibers through self-assembling of aggregation-induced emission (AIE) molecules. By introducing an L-valine methyl ester pendant into the typical AIE molecule tetraphenylethene, the AIE molecule (Val-TPE) is further endowed with chiral polarized luminescence and the ability to self-assemble into helical fibers. Though AIE is well-known, fabricating AIE molecules into novel functional architectures is a less-touched area. This work represents a successful example of hybridizing AIE, chirality and self-assembling ability. This kind of molecule is of particular interest for the increasing demand for the miniaturization of electronic devices. |
The construction of desirable functional architectures by molecular self-assembly has been a hot research topic in chemistry, biology and material science in recent years.1 In this approach, π-conjugated luminescent materials have attracted considerable attention due to their unique optical and electrical properties, and potential applications in optoelectronic devices and sensors.2 Unfortunately, most π-conjugated molecules emit intensively in solution, but become weakly fluorescent or even non-emissive in the aggregation or solid state. This is the notorious aggregation-caused quenching (ACQ) effect,3 which greatly limits the scope of the applications of the self-assembled luminescent materials. Therefore, development of new luminogens with high efficiency in the solid state is highly demanded.
The discovery of the AIE property of fluorescent molecules offers a straightforward strategy to solve the ACQ problem.4 Typical AIE molecules are characterized by propeller-shaped structures, which show very weak fluorescence in the solution. Upon aggregation they emit intensively mainly due to the restriction of intramolecular motions (RIMs) in the aggregates.5 The aggregates show a morphology distribution from nanoparticles to microfibers, which are formed via the noncovalent interactions of molecules. The novel AIE property not only solves the ACQ problem, but also provides a simple way to fabricate novel luminescent micro/nano-architectures by deliberately designing the peripheries of the AIE scaffold. This method is effective.6 For instance, Tian and coworkers constructed nanorings with high emission efficiency using the conjugated oligocarbazoles with an anthracene core.7 Zheng reported the fabrication of hollow micro-/nanospheres with porous channels by the self-assembly of TPE macrocycles. The spheres could be further transformed into bird nests consisting of nanorods under ultrasound.8 Our group synthesized biphenyl-containing TPE derivatives, which can self-assemble into highly fluorescent micro/nanofibers upon aggregation.9
Recently, we have also exploited the possibility of endowing the AIE architectures with optical activity by introducing chiral attachments into the peripheries of typical AIE structures, such as silole.10 With an introduction of chiral sugar moieties to a silole derivative, we fabricated right-handed helical nanoribbons that preserved the AIE property and emitted right-handed circularly polarized luminescence as well.10a Chiral amino acids are also ideal candidates for chirality induction. They have strong supramolecular self-assembling capabilities and can lead to the formation of judicious assembled structures. Our previous research has confirmed that the incorporation of chiral amino acid units into the pendants of polyphenylacetylene can efficiently induce the polymers to form helical conformation and superhelical assemblies.11 With respect to the combination of amino acid attachments with the AIE system, it is a less touched area.12 Hereby, we demonstrate a strategy of hybridizing valine with TPE to fabricate helical assemblies which have both AIE and CPL properties.
Val-TPE was synthesized in a high yield through the copper-catalyzed azide–alkyne “click” reaction (Scheme 1) and the experimental details are provided in the ESI.† The molecular structure of Val-TPE was characterized by spectroscopy methods, from which satisfactory results were obtained (Fig. S1–S3, ESI†).
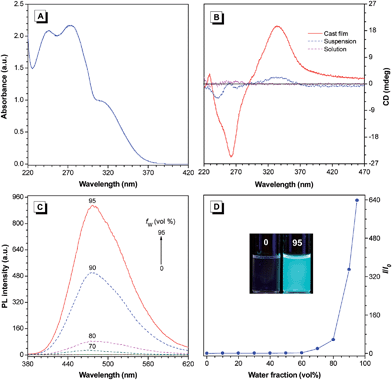
Val-TPE displayed an absorption maximum at 315 nm in THF solution, which corresponded to the π–π* transition of Val-TPE (Fig. 1A). We further investigated its optical activity using circular dichroism (CD) spectroscopy. As can be seen from Fig. 1B, in solution, Val-TPE only exhibited a very weak CD signal at the short-wavelength (<300 nm in solution), which may be owing to the random orientation of chromophores.13 CD peaks at 240, 272 and 333 nm were observed in the CD spectrum of Val-TPE in a DCE–hexane (1/9, v/v) mixture, an indication of aggregation-induced circular dichroism (AICD). The cast film of Val-TPE prepared by evaporation of its DCE solution, showed strong cotton effects at the wavelengths of 263 and 335 nm. Since the valine-containing unit (2) is CD silent at any wavelength longer than 300 nm,14 the peaks at 333 and 335 nm are considered to stem from the absorption of the TPE moiety, implying a successful chirality transfer from the valine-containing pendants to the TPE moieties in the aggregate state.
The photoluminesence (PL) spectra of Val-TPE in THF and THF–water mixtures were also recorded to check if the modified TPE molecule still maintained the AIE property. As shown in Fig. 1C, the PL curves of Val-TPE in the THF are flat lines parallel to the abscissa. The same trend is observed for THF–water mixtures with water fractions (fw) lower than 60%. The PL intensities of Val-TPE increase significantly when the fw is higher than 70% and reach the highest at the fw of 95%, which is 640 times higher than that in THF solution (Fig. 1D). Because Val-TPE is insoluble in water, the higher water content must have led to the aggregation of the molecules in the THF–water mixture. Thus, Val-TPE still preserves the AIE feature.
CPL is the emission analog of CD that reflects the chiroptical properties of the luminescent molecules upon excitation. The performance of CPL-active materials is generally evaluated by the emission dissymmetry factor (gem), which is defined as gem = 2(IL − IR)/(IL + IR), where IL and IR are the intensities of the left- and right-handed polarized emissions, respectively. Recently, CPL-active organic materials have received considerable attention for their potential application in biosensing and optoelectronic devices.15 Since Val-TPE has strong CD signals and intensive fluorescence in the film state, it is thus anticipated to show good CPL performance. According to our previous finding that micropatterning the chiral luminescent materials can enhance their CPL activities,10a Val-TPE sample was also micropatterned on quartz substrates using a Teflon-based stamp containing microfluidic channels for CPL measurement,16 each channel of which is 10 μm wide and 10 μm deep rectangular. The experimental details are provided in the ESI.† Various evaporation rates of solvent were attempted to control the morphology of micropatterned Val-TPE, but bundles of fiber morphology rather than normal films were always formed (Fig. S4†). This may be due to the strong self-assembling property of Val-TPE molecules and the confinement effect in microfluidic channels. The bundles of fibers emit intensive blue fluorescence, which is consistent with that observed in the PL spectra. Val-TPE self-assembles into similar fibers in the cast film, but they do not have a uniform coverage on the substrate and are only rich in the surrounding region due to the evaporation effect of the solvent. With respect to the micropatterned film, when the evaporation of Val-TPE solution takes place in a confined space, molecules tend to cover the substrate in a more uniform manner. This is likely the reason that the CPL property of the micropatterned film is more significant than that of the cast film. When the sample was in the powder state, the CD signal was extremely low (not shown), so its CPL property is anticipated to be even lower. It implies that the self-assembled structures, namely the fibers formed upon the evaporation of solvent were crucial for the CPL property. We then investigated the CPL behavior of the fluorescent fibers formed by Val-TPE with a home-built CPL measurement system.10 The microfibers of Val-TPE show a positive signal in its fluorescence bands and an average gem of 0.03 at the wavelength of 400–600 nm in the CPL spectrum (Fig. 2). The gem also exhibits less dependence on the emission wavelength. In contrast, most of the “conventional” organic molecules show small gem values (<0.01) in the solutions,17 and even worse CPL properties in the condensed state owing to the ACQ effect.
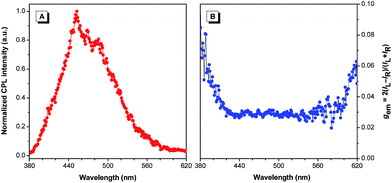 | ||
| Fig. 2 Plots of (A) CPL and (B) CPL dissymmetry factor (gem) versus wavelength of fibers formed by Val-TPE, λex: 325 nm. | ||
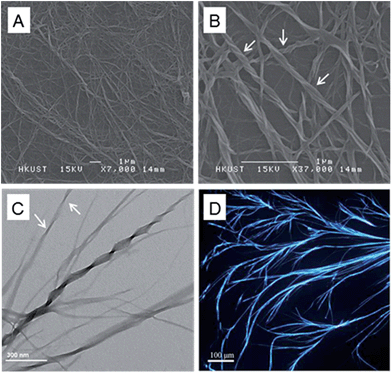
The fine structures of the fluorescent fibers formed by Val-TPE were further studied by imaging the sample with the microscopy techniques. SEM images provide more detailed information of the morphology of the fibers. As shown in Fig. 3A, Val-TPE forms thickly packed entangled fibers with elementary fibers further twisting round each other to form thicker ones. The amplified image in Fig. 3B further indicates that the elementary fibers are actually “nanofibers”. Each nanofiber clearly exhibits a left-handed screw sense, as most clearly shown by the labeled area in Fig. 3B, which is consistent with the positive CPL signal in Fig. 2. The nanofibers have an average width of ∼35–55 and a helical pitch ∼190 nm. The TEM image in Fig. 3C shows a co-existence of helical fibers, nanoribbons, and combined structures with one of the ends bearing the morphology of a helical fiber (labeled with arrows) and the other end having the appearance of a ribbon. It provides an important clue that the nanofibers are likely wrapped up by the ribbons. A fluorescence image, as exemplified by Fig. 3D, further indicated that the nano/microfibers reached the length of more than 1 mm and they were highly luminescent. While considering the dimension of a single VAL-TPE molecule is only ∼1 nm, the molecules exhibited extraordinary capacity to self-assemble into such long fibers. This kind of self-assembling manner is typical for amphiphilic molecules, such as chiral amphiphiles and chiral amphiphilic polymers to form helical fibers.18 The general steps involve the formation of a ribbon-like or a film-like structure owing to the amphiphilicity of the molecules, which is followed by a wrapping up of the ribbon or film due to the asymmetric force exerted by the chiral centers of the amino acid attachments. Hydrogen bonds between the amino acid attachments and π–π stacking of the TPE scaffold help to stabilize the helical conformation and supramolecular assemblies.
In summary, we have put forward a novel strategy for the construction of functional nanofibers through self-assembling of chiral AIE molecules. The combination of desirable architectures with novel AIE and CPL properties has made this kind of molecule an ideal candidate in many applications, including optoelectronic displays, chiral recognition and chemical sensors. The fabrication of helical nanofibers formed by amino acid-functionalized AIE compounds with AIE and CPL properties provides a successful example in this approach. It is of particular interest for the increasing demand for the miniaturization of electronic devices. Precise control over chirality and fluorescence in the self-assembling process is still a challenging task in the molecular design. Deciphering the substituent effect on the light emission and self-assembling process is also crucial to optimize the molecular design and the potential application of the molecules. Our future work involves expansion of the amino acid substituents and exploration of the fine tuning of the self-assembling structures. Hopefully, it will provide important insights into the rational design of AIE-based functional materials.
Acknowledgements
This work was partially supported by the National Natural Science Foundation of China (21104046), the Outstanding Youth Foundation of Shenzhen (JC201005250038A), the National Basic Research Program of China (973 program, 2013CB834701), the Research Grants Council of Hong Kong (604711, 604913, HKUST2/CRF/10 and N_HKUST620/11) and the University Grants Committee of Hong Kong (AoE/P-03/08).Notes and references
- G. M. Whitesides and B. Grzybowski, Science, 2002, 295, 2418 CrossRef CAS PubMed.
- Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC.
- J. B. Birks, Photophysics of Aromatic Molecules, Wiley, New York, 1970 Search PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740 RSC.
- (a) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC; (b) Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC; (c) E. P. J. Parrott, N. Y. Tan, R. Hu, J. A. Zeitler, B. Z. Tang and E. Pickwell-MacPherson, Mater. Horiz., 2014, 1, 251 RSC.
- (a) Z. Zhao, J. W. Y. Lam and B. Z. Tang, Soft Matter, 2013, 9, 4564 RSC; (b) B. K. An, J. Gierschner and S. Y. Park, Acc. Chem. Res., 2012, 45, 544 CrossRef CAS PubMed.
- B. Xu, J. He, Y. Dong, F. Chen, W. Yu and W. Tian, Chem. Commun., 2011, 6602 RSC.
- (a) S. Song and Y. S. Zheng, Org. Lett., 2013, 15, 820 CrossRef CAS PubMed; (b) H. T. Feng, S. Song, Y. C. Chen, C. H. Shen and Y. S. Zheng, J. Mater. Chem. C, 2014, 2, 2353 RSC.
- (a) Z. Zhao, S. Chen, X. Shen, F. Mahtab, Y. Yu, P. Lu, J. W. Y. Lam, H. S. Kwok and B. Z. Tang, Chem. Commun., 2010, 686 RSC; (b) W. Z. Yuan, F. Mahtab, Y. Gong, Z. Q. Yu, P. Lu, Y. Tang, J. W. Y. Lam, C. Zhu and B. Z. Tang, J. Mater. Chem., 2012, 22, 10472 RSC.
- (a) J. Liu, H. Su, L. Meng, Y. Zhao, C. Deng, J. C. Y. Ng, P. Lu, M. Faisal, J. W. Y. Lam, X. Huang, H. Wu, K. S. Wong and B. Z. Tang, Chem. Sci., 2012, 3, 2737 RSC; (b) J. C. Y. Ng, J. Liu, H. Su, Y. Hong, H. Li, J. W. Y. Lam, K. S. Wong and B. Z. Tang, J. Mater. Chem. C, 2014, 2, 78 RSC.
- (a) B. S. Li, J. W. Y. Lam, Z. Yu and B. Z. Tang, Langmuir, 2012, 28, 5770 CrossRef CAS PubMed; (b) B. S. Li, K. K. L. Cheuk, F. Salhi, J. W. Y. Lam, J. A. K. Cha, X. Xiao, C. Bai and B. Z. Tang, Nano Lett., 2001, 1, 323 CrossRef CAS.
- H. Liu, Z. Lv, K. Ding, X. Liu, L. Yuan, H. Chen and X. Li, J. Mater. Chem. B, 2013, 1, 5550 RSC.
- P. K. Sukul, P. K. Singh, S. K. Maji and S. Malik, J. Mater. Chem. B, 2013, 1, 153 RSC.
- B. S. Li, K. K. L. Cheuk, L. Ling, J. Chen, X. Xiao, C. Bai and B. Z. Tang, Macromolecules, 2003, 36, 77 CrossRef CAS.
- (a) H. Maeda, Y. Bando, K. Shimomura, I. Yamada, M. Naito, K. Nobusawa, H. Tsumatori and T. Kawai, J. Am. Chem. Soc., 2011, 133, 9266 CrossRef CAS PubMed; (b) T. Ikeda, T. Masuda, T. Hirao, J. Yuasa, H. Tsumatori, T. Kawai and T. Haino, Chem. Commun., 2012, 6025 RSC; (c) X. Liu, J. Jiao, X. Jiang, J. Li, Y. Cheng and C. Zhu, J. Mater. Chem. C, 2013, 1, 4713 RSC.
- K. Ren, W. Dai, J. Zhou, J. Su and H. Wu, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 8162 CrossRef CAS PubMed.
- (a) J. Kumar, T. Nakashima, H. Tsumatori and T. Kawai, J. Phys. Chem. Lett., 2014, 5, 316 CrossRef CAS; (b) R. Carr, N. H. Evans and D. Parker, Chem. Soc. Rev., 2012, 41, 7673 RSC; (c) T. Kaseyama, S. Furumi, X. Zhang, K. Tanaka and M. Takeuchi, Angew. Chem., Int. Ed., 2011, 50, 3684 CrossRef CAS PubMed.
- (a) H. Cao, Q. Yuan, X. Zhu, Y.-P. Zhao and M. Liu, Langmuir, 2012, 28, 15410 CrossRef CAS PubMed; (b) C. C. Lee, C. Grenier, E. W. Meijer and A. P. H. J. Schenning, Chem. Soc. Rev., 2009, 38, 671 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, 1H, 13C NMR and HRMS spectra (Fig. S1–S3), and microfibers of Val-TPE prepared by evaporation of its DMF solution (Fig. S4). See DOI: 10.1039/c4mh00078a |
| This journal is © The Royal Society of Chemistry 2014 |