Recent developments in the molecular recognition of carbohydrates by artificial receptors
Monika
Mazik
Institut für Organische Chemie der Technischen Universität Freiberg, Leipziger Strasse 29, D-09596, Freiberg, Germany. E-mail: Monika.Mazik@chemie.tu-freiberg.de; Fax: +49-3731-39-3170; Tel: +49-3731-39-2389
First published on 10th January 2012
Abstract
Despite the key role that carbohydrates play in a wide range of biological processes, the molecular details of carbohydrate-mediated recognition events are not fully understood. In this context, artificial receptors using noncovalent interactions for sugar binding provide useful model systems to study the basic principles of carbohydrate-based molecular recognition processes. The studies in this area are also strongly motivated by the belief that carbohydrate-binding agents could be used for the detection and treatment of diseases. This review covers representative examples of carbohydrate receptors operating through noncovalent interactions, with a focus on developments in receptor systems over the last two years.
 Monika Mazik | Monika Mazik received her PhD degree in chemistry from the Silesian Technical University at Gliwice, and did post-doctoral work with Prof. Reiner Sustmann at the University of Essen, Germany. After post-doctoral research, she worked on her habilitation (1998–2000) at the University of Essen. In 2002 she became Professor at the Technical University of Braunschweig, Germany, and since 2011 she has been Professor at the Technical University of Freiberg. Her research interests involve the design and synthesis of receptors for molecular recognition of biorelevant molecules, self-assembly of organic compounds, synthesis of heterocycles, as well as molecular modeling calculations. |
1. Introduction
The development of new receptors for carbohydrate recognition continues to be a fascinating and important area of research. Interest derives from the enormous importance of carbohydrate-mediated recognition processes in biology,1 and the need for new therapeutics (e.g. anti-infective agents) and carbohydrate sensors, which can be developed on the base of carbohydrate-binding agents. Two main strategies have been employed for the design of synthetic carbohydrate receptors. One strategy involves the exploitation of non-natural bonding interactions and relies on the reversible formation of covalent bonds from diol units and boronic acids.2 The second strategy exploits noncovalent interactions for sugar binding and aims at the development of biomimetic receptors.3 The design of biomimetic receptors can be directed towards either a better understanding of recognition phenomena in nature, or towards potential applications in medicine, analytical chemistry and other areas.It should be noted that both effective and selective recognition of carbohydrates by receptors operating through noncovalent interactions is still a challenging goal of artificial receptor chemistry. In particular, the recognition of carbohydrates in aqueous media, in which solvent molecules compete significantly for binding sites of the receptor, remains a challenge. Mimicking the binding motifs observed in the crystal structures of protein–carbohydrate complexes4 (for examples, see Fig. 1) was shown to be very useful for the development of artificial carbohydrate receptors. Although very interesting receptor systems have been developed, the exact prediction of the binding preferences of the artificial receptors is still far away and it is hoped that systematic studies in this area will contribute significantly to the solution of this problem.
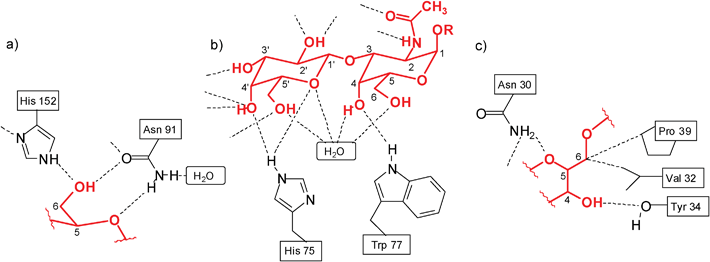 | ||
| Fig. 1 Examples of hydrogen bonds in the complexes of galactose-binding protein with D-glucose (a)4c and Amaranthus caudatus agglutinin with Galβ3GalNAc (b).4a Examples of hydrogen bonds and van der Waals contacts in the complex of Galanthus nivalis agglutinin with Manα3(Manα6)Man (c).4a,4f | ||
This review covers examples of artificial receptors using noncovalent interactions for sugar binding, with a focus on representative developments in receptor systems over the last two years; earlier examples are described in several reviews, which are cited in ref. 3.
2. Binding studies in organic media
Several receptor types based on a macrocyclic and acyclic scaffold have been designed for the binding studies in non-polar and polar organic solvents, although solvent competition usually makes recognition in polar solvents much less effective.5 Particularly, many representatives of hydrogen bonding receptors have been prepared and studied. The receptor molecules have been constructed on the base of both neutral and ionic recognition groups, to form neutral and ionic hydrogen bonds with the sugar substrates. Long-chain alkyl glycosides have usually been investigated as substrates for artificial receptors in homogeneous organic media, whereas sugar derivatives, which are insoluble in non-polar media, have been used for recognition studies in two-phase systems. Such studies involve dissolution of solid carbohydrates in non-polar solvents6 or extraction of carbohydrates from aqueous into non-polar media.7 It should also be noted that oligosaccharides have received far less attention in artificial receptor chemistry than monosaccharides.2.1. Molecular recognition of monosaccharides
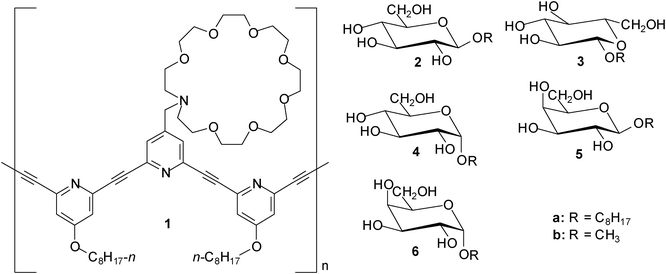
An azacrown-attached meta-ethynylpyridine polymer 1 was investigated for its carbohydrate recognition and the additive effect of triethylene tetramine–trifluoroacetic acid (TETA–TFA) by Abe, Inouye et al. in 2009.8 When octyl β-D- (2a) and L-glucopyranosides (3a) were added to a CH2Cl2 solution of 1, a mirror-image pair of induced circular dichroism (ICD) bands was observed, indicating that 1 formed chiral helical complexes with the guests. These ICDs were significantly enhanced by the addition of oligoammonium cations. The authors concluded that the ICD enhancements arise from the formation of a pseudopolyrotaxane structure between the azacrown and the oligoammonium moieties, which stabilize the chiral helical complexes by cross-linking the side chains. The binding constants were further enhanced by the addition of trifluoroacetic acid; the binding constants for β-glucoside 2 were found to be 800 and 1500 M−1 in the absence and presence of TFA, respectively (in the absence of both TETA and TFA the binding constant amounted to 100 M−1).The synthesis and binding properties of eight porphyrin-based receptors containing urea, carbamate or amide groups were described in 2010 by Hong et al.9 The interactions of these receptors with three octyl glycosides, such as β-D-glucoside (2a) , α-D-glucoside (4a), and β-D-galactoside (5a), were investigated by 1H NMR and UV-Vis titrations, as well as induced circular dichroism (ICD). In chloroform the binding constants ranged from 102 to 105 M−1; the best results were obtained with a urea-appended zinc porphyrin incorporating four benzyl groups. The binding affinity of this receptor decreased in the sequence β-galactoside 5a > β-glucoside 2a > α-glucoside 4a. In a more competitive medium, such as CDCl3–CD3OD (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), a significant drop in binding constants was observed (K11∼102 M−1). The authors concluded that in the case of the urea-appended porphyrins, the urea NHs act as strong hydrogen bonding donors for sugar hydroxyl oxygens and the porphyrin plane is used for mimicking the CH–π interactions10–12 with sugar CHs.
1, v/v), a significant drop in binding constants was observed (K11∼102 M−1). The authors concluded that in the case of the urea-appended porphyrins, the urea NHs act as strong hydrogen bonding donors for sugar hydroxyl oxygens and the porphyrin plane is used for mimicking the CH–π interactions10–12 with sugar CHs.
Our group has continued the studies on acyclic receptors,6g,6h,13–15 such as compounds containing a trisubstituted trialkylbenzene core, which were shown to be particularly interesting objects for systematic studies. Depending on the nature of the recognition units and connecting bridges used as building blocks, a variety of receptors with different binding preferences and affinities could be obtained.
In comparison to the previously described symmetrical, three-armed aminopyridine-based receptor 7,6f which was shown to exhibit high affinity and preference for β-glucoside 2, 8-hydroxyquinoline-based receptors 8 and 913 showed significantly increased binding affinity toward β-galactoside 5 (K11 = 148![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 and K21 = 1580 M−1 for 8•5a16,17 compared to K11 = 3070 and K12 = 470 M−1 for 7•5a in CDCl3). It is noteworthy that the enhancement of the binding affinity of 8/9 towards 5 was achieved through a relatively simple variation of the receptor structure.
700 and K21 = 1580 M−1 for 8•5a16,17 compared to K11 = 3070 and K12 = 470 M−1 for 7•5a in CDCl3). It is noteworthy that the enhancement of the binding affinity of 8/9 towards 5 was achieved through a relatively simple variation of the receptor structure.
It should also be noted that the imidazole/aminopyridine- and indole/aminopyridine-based receptors 10 and 116g were also found to display significantly higher binding affinity for β-galactoside 5 than the symmetrical aminopyridine-based receptor 76f. The design of receptors 10 and 11, including both 4(5)-substituted imidazole or 3-substituted indole units as the entities used in nature, and a 2-aminopyridine group as a heterocyclic analogue of the asparagine/glutamine primary amide side chain, was inspired by hydrogen bonding motifs shown in Fig. 1a,b. Both hydrogen-bonding and interactions of the sugar CHs with the phenyl rings of the receptors, as characterized by 1H NMR spectroscopy and X-ray analysis, were shown to contribute to the stabilisation of the receptor–sugar complexes. Such interactions were also suggested by molecular modelling calculations, as shown for 10•5b in Fig. 2.
| Receptor–sugar complex | Solvent | K 11 [M−1] | K 21 or K12i [M−1] | β 21 = K11K21 or β12 = K11K12 [M−2] |
|---|---|---|---|---|
a Average Ka values from multiple titrations.
b Errors in Ka are less than 20%.
c CDCl3 was stored over activated molecular sieves and deacidified with Al2O3.
d 0.02–0.04% H2O.
e DMSO-d6–CDCl3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 v/v.
f DMSO-d6–CDCl3, 10 95 v/v.
f DMSO-d6–CDCl3, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 v/v.
g DMSO-d6–CDCl3, 20 90 v/v.
g DMSO-d6–CDCl3, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v.
h
K
21 corresponds to 2 80 v/v.
h
K
21 corresponds to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–sugar association constant.
i
K
12 corresponds to 1 1 receptor–sugar association constant.
i
K
12 corresponds to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 receptor–sugar association constant.
j Hostest program indicated “mixed” 1 2 receptor–sugar association constant.
j Hostest program indicated “mixed” 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2 1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–sugar binding model with K11>105 and K21∼104; however, the binding constants were too large to be accurately determined by the NMR method.16,17 1 receptor–sugar binding model with K11>105 and K21∼104; however, the binding constants were too large to be accurately determined by the NMR method.16,17
|
||||
| 8•2a | CDCl3 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1060; h | 7.37 × 107 |
| 5% DMSO-d6–CDCl3 | 4300 | 300; h | 1.29 × 106 | |
| 8•4a | CDCl3 | 6810 | 100; h | 6.81 × 105 |
| 8•5a | CDCl3 | 148![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1580; h | 2.34 × 108 |
| 5% DMSO-d6–CDCl3 | 8600 | 770; h | 6.62 × 106 | |
| 13•2a | CDCl3c | >105 | ||
| H2O-containing CDCl3d | >105 | |||
| 5% DMSO-d6–CDCl3e | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1200; h | 9.40 × 107 | |
| 13•2b | 10% DMSO-d6–CDCl3f | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
340; h | 4.06 × 106 |
| 13•4a | CDCl3c | >105 | ||
| H2O-containing CDCl3d | >105 | |||
| 5% DMSO-d6–CDCl3e | >105 | |||
| 13•4b | 10% DMSO-d6–CDCl3f | 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
2470; h | 2.72 × 108 |
| 20% DMSO-d6–CDCl3g | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070 070 |
650; h | 1.56 × 107 | |
| 13•5a | CDCl3 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
800; h | 1.06 × 107 |
| 18•2a | CDCl3 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
530; h | 1.52 × 107 |
| 5% DMSO-d6–CDCl3e | 2550 | 190; i | 4.85 × 105 | |
| 18•4a | CDCl3 | 4360 | 210 ; i | 9.15 × 105 |
| 18•5a | CDCl3 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 540 540 |
1680; h | 7.48 × 107 |
| 5% DMSO-d6–CDCl3e | 3830 | 300; i | 1.15 × 106 | |
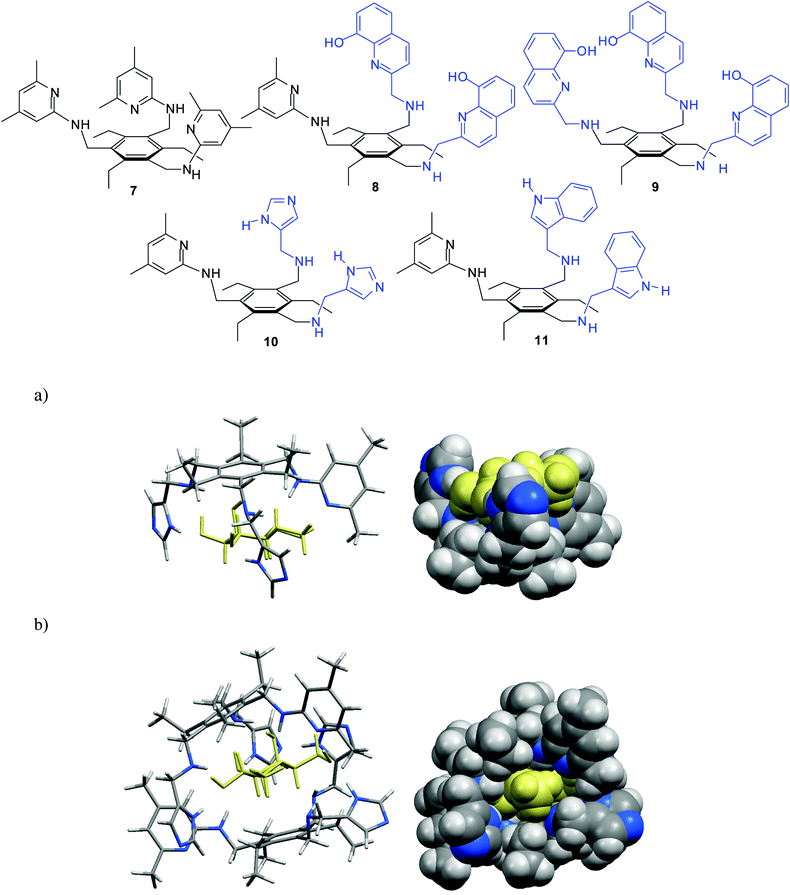 | ||
Fig. 2 Energy-minimized structure of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (a) and 2 1 (a) and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (b) formed between imidazole/aminopyridine-based receptor 10 and β-galactoside 5b (different representations). MacroModel V.8.5, OPLS-AA force field, MCMM, 50 1 complex (b) formed between imidazole/aminopyridine-based receptor 10 and β-galactoside 5b (different representations). MacroModel V.8.5, OPLS-AA force field, MCMM, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps. Color code: receptor C, grey; receptor N, blue; sugar molecule, yellow.6g 000 steps. Color code: receptor C, grey; receptor N, blue; sugar molecule, yellow.6g | ||
In contrast to 8-hydroxyquinoline-based receptors 8/913 and to the indole/imidazole-based receptors 10/11,6g the phenanthroline/aminopyridine-based receptors6h,14a12 and 13 were shown to display a high binding affinity towards α-glucoside 4 and α-galactoside 6 (K11 > 105 M−1 in CDCl3; for examples, see Table 1) as well as a strong α- vs. β anomer binding preference. In comparison to the symmetrical receptor 7, dramatic changes of the binding affinity and selectivity were observed after the replacement of one or two pyridine-based recognition units by phenanthroline-based units; the values of the association constants K11 ranged from 1310 M−1 for 7•4a to K11 > 105 M−1 for 12•4a and 13•4a in CDCl3. According to molecular modelling calculations, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes between receptor 12/13 and α-glucopyranoside 4 were shown to be stabilized by several hydrogen bonds as well as interactions of sugar CHs with the aromatic groups of the receptor molecule (see Fig. 3).
1 complexes between receptor 12/13 and α-glucopyranoside 4 were shown to be stabilized by several hydrogen bonds as well as interactions of sugar CHs with the aromatic groups of the receptor molecule (see Fig. 3).
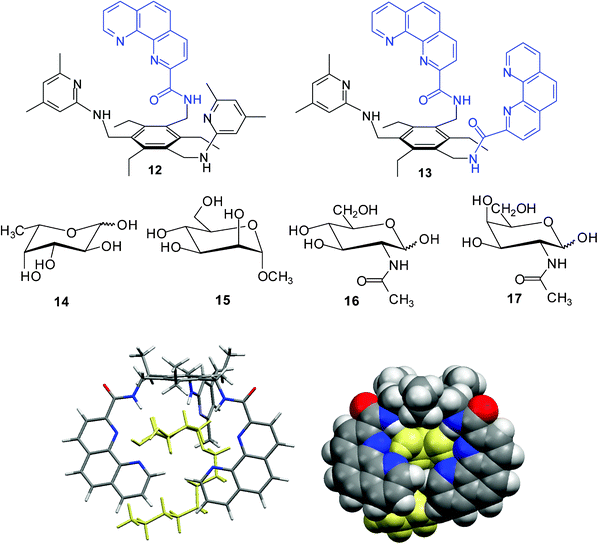 | ||
Fig. 3 Energy-minimized structure of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formed between receptor 13 and octyl α-D-glucopyranoside (4a) (MacroModel V.8.5, OPLS-AA force field, MCMM, 50 1 complex formed between receptor 13 and octyl α-D-glucopyranoside (4a) (MacroModel V.8.5, OPLS-AA force field, MCMM, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps). Color code: receptor C, grey; O, red; N, blue; the sugar molecule is highlighted in yellow.6h 000 steps). Color code: receptor C, grey; O, red; N, blue; the sugar molecule is highlighted in yellow.6h | ||
The binding studies in DMSO-d6–CDCl3 mixtures showed that affinities of 13 for the tested glycosides decrease as solvent polarity increases (see Table 1); however, the determined binding constants were significantly higher than those determined for 12 (for example, K11 = 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 M−1 and K21 = 2470 M−1 for 13•4b, in comparison with K11 = 10
340 M−1 and K21 = 2470 M−1 for 13•4b, in comparison with K11 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 and K21 = 840 M−1 for 12•4a in 10% DMSO-d6 in CDCl3). The binding strength towards the examined glycosides was shown to decrease in the sequence α-glucoside 4 > β-glucoside 2∼α-galactoside 6 > β-galactoside 5.
500 M−1 and K21 = 840 M−1 for 12•4a in 10% DMSO-d6 in CDCl3). The binding strength towards the examined glycosides was shown to decrease in the sequence α-glucoside 4 > β-glucoside 2∼α-galactoside 6 > β-galactoside 5.
Extractions of sugars 2b, 4b, 5b, 6b, and 14–17 from the solid state into a CDCl3 solution of receptor 13 provided further evidence for strong complexation of α-glycosides, in agreement with the results obtained in homogenous solutions by 1H NMR titrations.6h The extractability decreased in the sequence α-glucoside 4b > β-glucoside 2b∼α-galactoside 6 > fucose 14 > β-galactoside 5b∼N-acetyl-galactosamine 16 > α-mannoside 15 > N-acetyl-glucosamine 17. The α- vs. β-anomer binding preference of 13 in the recognition of glucosides indicated by 1H NMR titrations, was also confirmed by the extractions of methyl α-D-glucoside (4b) and methyl β-D-glucoside (2b) from water into chloroform. Compound 13 (1 mM chloroform solution) showed notable selectivity for α-glucoside, extracting 0.4 equiv. of α-glucoside 4b, but only about 0.15 equiv. of β-glucoside 2b, from 1 M aqueous solution of the corresponding sugar. Furthermore, compound 13 showed notable α- vs. β-anomer selectivity in the recognition of galactosides; the receptor was able to extract about 0.3 equiv. of α-galactoside 6 from water into chloroform solution. It should be noted that the observed preference for α- over the β-glycosides differs from those observed for other receptor systems, which usually showed higher affinity for the β-anomers.18,19
It is also noteworthy that interesting hydrogen-bonded complexes with water molecules have been revealed by the X-ray analysis of the phenanthroline-based receptors.6h,14 In the case of compound 12, X-ray crystallographic investigations revealed the presence of three water molecules in the binding pocket of the receptor (the 12·3H2O aggregate is stabilized by NH⋯O, OH⋯N, and OH⋯O hydrogen bonds), whereas in the case of 13 the presence of two water molecules and one ethanol was observed (see Fig. 4).
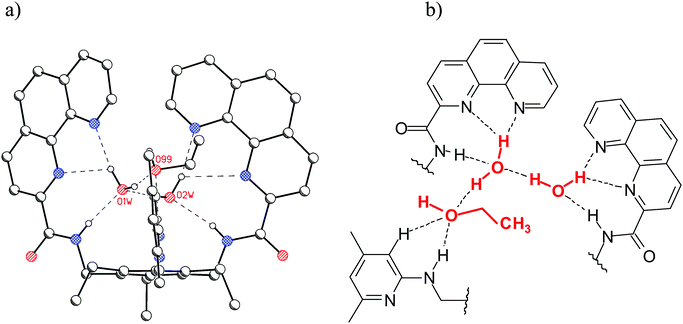 | ||
| Fig. 4 (a) Crystal structure of 13 (C–Hs are omitted for clarity); two hydrogen-bonded water molecules and one ethanol molecule are present in the binding pocket of 13. (b) Schematic representation of the binding motifs in the binding pocket of 13.6h | ||
Binding motifs observed in the crystal structures of protein–carbohydrate complexes, in particular the participation of the primary amide group of asparagine and the isopropyl group of valine (see Fig. 1c) in the formation of hydrogen bonds and van der Waals contacts, respectively, has also inspired the design of artificial receptor 18,15 which was expected to be able to recognise a sugar molecule through a combination of NH⋯O and OH⋯N hydrogen bonds, CH–π interactions10–12 and van der Waals contacts. Instead of the primary amide group shown in Fig. 1c, the 2-aminopyridine unit was used, which can be regarded as a heterocyclic analogue of the asparagine/glutamine primary amide side chain and was shown to be an effective recognition group for carbohydrates. The binding properties of 18 towards selected monosaccharides were compared with those of compounds 19–21.
1H NMR spectroscopic titrations (for examples, see Fig. 5) and binding studies in two-phase systems, such as dissolution of solid carbohydrates in apolar media, revealed effective recognition of neutral carbohydrates by 18, β- vs. α-anomer binding preferences in the recognition of glycosides and significantly increased binding affinity towards β-galactoside 5 in comparison with the previously described symmetrical receptor 76f and other acyclic receptors.20 Although 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes predominated in the solution, the presence of 1
1 complexes predominated in the solution, the presence of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 2
2 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–sugar complexes, depending on the titration conditions, were also detected.
1 receptor–sugar complexes, depending on the titration conditions, were also detected.
![(a,b) Partial 1H NMR spectra (400 MHz; CDCl3) of receptor 18 before (bottom) and after the addition of β-glucoside 2a (a) and β-galactoside 5a (b); [18] = 0.97 mM, equiv. of 2a or 5a: 0.00–4.80. Shown are pyridine CH3 resonances of 18. (c) Partial 1H NMR spectra of sugar 2a before (bottom) and after the addition of receptor 18 (inverse titration); [2a] = 0.78 mM, equiv. of 18: 0.00–4.99.15](/image/article/2012/RA/c2ra01138g/c2ra01138g-f5.gif) | ||
| Fig. 5 (a,b) Partial 1H NMR spectra (400 MHz; CDCl3) of receptor 18 before (bottom) and after the addition of β-glucoside 2a (a) and β-galactoside 5a (b); [18] = 0.97 mM, equiv. of 2a or 5a: 0.00–4.80. Shown are pyridine CH3 resonances of 18. (c) Partial 1H NMR spectra of sugar 2a before (bottom) and after the addition of receptor 18 (inverse titration); [2a] = 0.78 mM, equiv. of 18: 0.00–4.99.15 | ||
Compound 18, containing isopropylamino groups, was shown to be a more effective carbohydrate receptor than the isobutylamino-based compound 19. Liquid–liquid extractions demonstrated its ability to extract monosaccharides from water into chloroform; such ability is interesting, considering that the receptor possesses a very simple, acyclic structure. Compared to the previously described receptor 7, incorporating three aminopyridine-based recognition units,6f receptor 18 showed significantly increased affinity to β-galactoside 5 (about 10 times higher affinity), but decreased affinity towards β-glucoside 2 (about 2 times lower). The affinities of 20 for the tested monosaccharides were shown to be considerably lower than those of 18 and 19. Tighter binding of monosaccharides by 18/19 compared to 20 has been attributed to van der Waals contacts between the monosaccharide substrate and the isopropyl/isobutyl groups, that are absent in 20. The replacement of the aminopyridine group in 18 and 19 by an isopropylamino or isobutylamino unit resulted in a fall in the binding constants. The affinity of the symmetrical isopropylamino-based receptor 21 towards the selected β-glycosides was shown to be similar to that of 20. Compared to the symmetrical aminopyridine-based receptor 7, compound 21, possessing only three NH groups as hydrogen bonding sites, showed a significantly decreased affinity (about 10 times lower) to β-glucoside 2a, but a similar affinity towards β-galactoside 5a. Considering the simple structure of 21, the binding affinity towards β-galactoside is noteworthy.
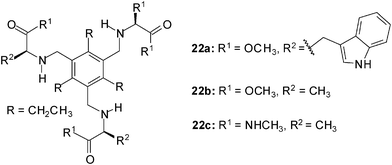
Evaluation of amino acids as chiral ligands for the enantio-differentiation of carbohydrates was reported by Riguera, Fernandez-Megia et al. in 2010.21 The interactions of receptors of type 22 with octyl β-D-glucoside (2a) and octyl β-L-glucoside (3a) were studied by 1H NMR spectroscopy in CDCl3. 1H NMR titrations, which were performed at constant concentration of the corresponding sugar, revealed severe overlapping of carbohydrate and receptor resonances. The authors proved 1D TOCSY (Total Correlation Spectroscopy) NMR experiments to be a useful filtering strategy widening the scope of NMR titrations to systems where overlapping hampers the direct analysis of the carbohydrate resonances. The best fit of the titration data was obtained when a mixed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (receptor to sugar ratio) was used for the calculation of the binding constants (EQNMR software16c). The tested receptors showed only moderate affinity in CDCl3; the binding constants K11 and K21 were in the range of 58–465 M−1 and 51–142 M−1, respectively. Whereas 22a was not able to discriminate between the enantiomers 2a and 3a, receptors 22b and 22c showed a slightly preference for the β-L-glucoside 3a.
1 binding model (receptor to sugar ratio) was used for the calculation of the binding constants (EQNMR software16c). The tested receptors showed only moderate affinity in CDCl3; the binding constants K11 and K21 were in the range of 58–465 M−1 and 51–142 M−1, respectively. Whereas 22a was not able to discriminate between the enantiomers 2a and 3a, receptors 22b and 22c showed a slightly preference for the β-L-glucoside 3a.
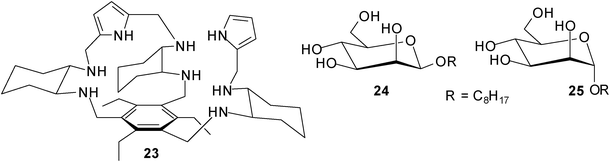
Roelens, Jiménes-Barbero et al. described effective recognition of mannosides by receptor 23, which was developed by combining a chiral diamino building block with pyrrolic hydrogen-bonding units on the triethylbenzene scaffold.22 Receptor 23 was prepared in both enantiomerically pure forms, the R,R,R,R,R,R enantiomer [(R)-23] and the S,S,S,S,S,S enantiomer [(S)-23]. Interestingly, (S)-23 displayed a clear preference for octyl β-D-mannoside (24) over the corresponding α-anomer 25 [K11 = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 and K12 = 1096 M−1 for (S)-23•24 compared to K11 = 3090 M−1 and K21 = 223 M−1 for (S)-23•25; the titration results indicated different binding models for the two systems]. Furthermore, (S)-23 showed stronger binding of β-mannoside 24 than (R)-23 in a polar medium like acetonitrile [K11 = 758 M−1 and K21 = 26 M−1 for (R)-23•24]. It should be noted that 23 did not show any enantio-discrimination in binding to octyl α-mannoside 25. The structural features of the receptor–mannoside complexes were investigated in solution and in the solid state by a combined X-ray, NMR spectroscopy, and molecular modelling approach.22b
000 M−1 and K12 = 1096 M−1 for (S)-23•24 compared to K11 = 3090 M−1 and K21 = 223 M−1 for (S)-23•25; the titration results indicated different binding models for the two systems]. Furthermore, (S)-23 showed stronger binding of β-mannoside 24 than (R)-23 in a polar medium like acetonitrile [K11 = 758 M−1 and K21 = 26 M−1 for (R)-23•24]. It should be noted that 23 did not show any enantio-discrimination in binding to octyl α-mannoside 25. The structural features of the receptor–mannoside complexes were investigated in solution and in the solid state by a combined X-ray, NMR spectroscopy, and molecular modelling approach.22b
2.2. Molecular recognition of disaccharides: di- vs. monosaccharide binding preferences
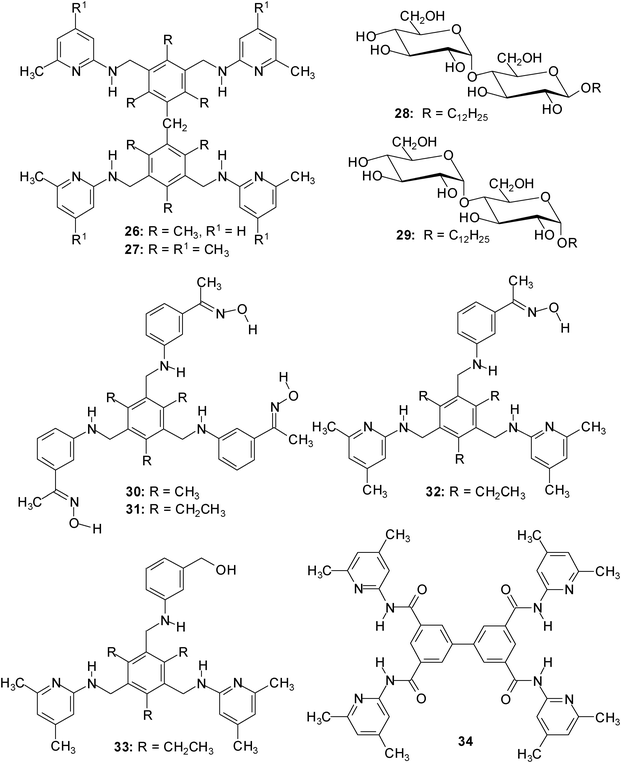
Although some receptors show interesting oligo- vs. monosaccharides preferences, the selective recognition of oligosaccharides by receptors using noncovalent interactions is still rare; some examples published until 2008 are given in ref. 23.In 2009, dimesitylmethane-derived receptors 26/27, incorporating four heterocyclic recognition groups capable of serving as hydrogen bonding sites, were designed to recognize disaccharides.24 It has been shown by 1H NMR and fluorescence spectroscopic titrations (for examples, see Fig. 6 and 7) that compounds 26/27 display high binding affinities towards α- and β-maltoside, 28 and 29, as well as strong di- vs. monosaccharide preferences in organic media (similar to the previously described receptors 30–34;25 examples of association constants are given in Table 2).
![Plot of the observed (×) and calculated (–) chemical shifts of the NH resonances of 27 (1.02 mM) as a function of added β-maltoside 28 (a) or β-glucopyranoside 2a (b) The [receptor]:[sugar] ratio is marked.24](/image/article/2012/RA/c2ra01138g/c2ra01138g-f6.gif) | ||
| Fig. 6 Plot of the observed (×) and calculated (–) chemical shifts of the NH resonances of 27 (1.02 mM) as a function of added β-maltoside 28 (a) or β-glucopyranoside 2a (b) The [receptor]:[sugar] ratio is marked.24 | ||
![Fluorescence titration of receptor 26 with α-maltoside 29 (a) and β-glucopyranoside 2a (b) in CHCl3; [26] = 8.51 × 10−5 and 9.57 × 10−5 M; Equiv. of 29 = 0.00–4.03; Equiv. of 2a = 0.00–18.69. Excitation wavelength 324 nm. Fluorescence intensity increased with increasing sugar concentration.24](/image/article/2012/RA/c2ra01138g/c2ra01138g-f7.gif) | ||
| Fig. 7 Fluorescence titration of receptor 26 with α-maltoside 29 (a) and β-glucopyranoside 2a (b) in CHCl3; [26] = 8.51 × 10−5 and 9.57 × 10−5 M; Equiv. of 29 = 0.00–4.03; Equiv. of 2a = 0.00–18.69. Excitation wavelength 324 nm. Fluorescence intensity increased with increasing sugar concentration.24 | ||
| Receptor–sugar complex | Solvent | K 11 [M−1] | K 21 or K12d [M−1] | β 21 or β12e [M−2] | Methodf |
|---|---|---|---|---|---|
a Average Ka values from multiple titrations.
b Errors in Ka are less than 20%.
c
K
21 corresponds to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–sugar association constant.
d
K
12 corresponds to 1 1 receptor–sugar association constant.
d
K
12 corresponds to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 receptor–sugar association constant.
e
β
21 = K11K21, β12 = K11K12.
f
1H NMR spectroscopic titrations (CDCl3 and DMSO-d6–CDCl3, 1 2 receptor–sugar association constant.
e
β
21 = K11K21, β12 = K11K12.
f
1H NMR spectroscopic titrations (CDCl3 and DMSO-d6–CDCl3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 v/v) or fluorescence titrations (CHCl3 and DMSO/CHCl3, 1 99 v/v) or fluorescence titrations (CHCl3 and DMSO/CHCl3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 v/v).
g The best fit of the titration data was obtained with the “pure” 2 99 v/v).
g The best fit of the titration data was obtained with the “pure” 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor-substrate binding model.16 1 receptor-substrate binding model.16
|
|||||
| 26•28 | CDCl3 | >100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (K21)g 000 (K21)g |
NMR | ||
| CHCl3 | 5.76 × 107 (K21) | fluorescence | |||
| 1% DMSO–CHCl3 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
fluorescence | |||
| 26•29 | CHCl3 | 1.61 × 107 (K21) | fluorescence | ||
| 1% DMSO–CHCl3 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
fluorescence | |||
| 26•2b | CDCl3 | 260 | 630 (K12) | 1.63 × 105 | NMR |
| CHCl3 | 350 | 840 (K12) | 2.94 × 105 | fluorescence | |
| 30•28 | CDCl3 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
NMR | ||
| CHCl3 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
fluorescence | |||
| 30•2b | CDCl3 | 170 | 1730 (K12) | 2.94 × 105 | NMR |
| 34•28 | CDCl3 | >100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (K21)g 000 (K21)g |
NMR | ||
| 34•2b | CDCl3 | 8800 | 300 (K12) | 2.64 × 106 | NMR |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–disaccharide complexes in chloroform solutions (K21 > 105 M−1, see Table 2).24 The addition of dimethyl sulfoxide caused both the change of the binding model and a substantial drop in the binding affinity. The curve fitting of the titration data obtained in the presence of DMSO indicated the formation of complexes with 1
1 receptor–disaccharide complexes in chloroform solutions (K21 > 105 M−1, see Table 2).24 The addition of dimethyl sulfoxide caused both the change of the binding model and a substantial drop in the binding affinity. The curve fitting of the titration data obtained in the presence of DMSO indicated the formation of complexes with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–disaccharide stoichiometry with K11 of 104 M−1 (see Table 2). As expected, relatively low binding constants were obtained upon titrating compounds 26/27 with β-glucopyranoside 2a. The binding studies indicated the formation of complexes with 1
1 receptor–disaccharide stoichiometry with K11 of 104 M−1 (see Table 2). As expected, relatively low binding constants were obtained upon titrating compounds 26/27 with β-glucopyranoside 2a. The binding studies indicated the formation of complexes with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 receptor–monosaccharide stoichiometry with K11 and K12 of 102 M−1 in chloroform (see Table 2). Both 1H NMR and fluorescence titrations clearly showed that the receptor–monosaccharide complexes are much less stable than those formed with the disaccharides 28/29. Receptors 26/27 are thus representatives of a series of acyclic carbohydrate-binding receptors displaying an interesting di- vs. monosaccharide preference. The acyclic architecture is notably easy to prepare and especially suitable for systematic variations.
2 receptor–monosaccharide stoichiometry with K11 and K12 of 102 M−1 in chloroform (see Table 2). Both 1H NMR and fluorescence titrations clearly showed that the receptor–monosaccharide complexes are much less stable than those formed with the disaccharides 28/29. Receptors 26/27 are thus representatives of a series of acyclic carbohydrate-binding receptors displaying an interesting di- vs. monosaccharide preference. The acyclic architecture is notably easy to prepare and especially suitable for systematic variations.
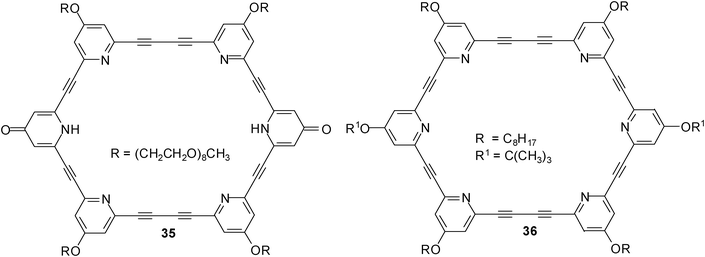
In 2011, Abe, Inouye et al. reported the synthesis of a D2h-symmetrical diacetylenic macrocycle having pyridine–pyridone–pyridine modules (compound 35).26 The binding properties of the macrocyclic receptor towards carbohydrates were studied by CD and UV-vis titration experiments in CH2Cl2 and CH3CN. As substrates for these studies, four monosaccharides, octyl β-D-glucopyranoside (2a), octyl β-D-galactopyranoside (5a), octyl β-D-mannopyranoside (24) and octyl β-D-fructopyranoside were used, and one disaccharide, dodecyl β-D-maltopyranoside (28).
In CH2Cl2, macrocycle 35 showed a much greater affinity for β-maltoside 28 (K11 = 1.4 × 106 M−1) than for the tested monosaccharides (K11 values were estimated to be in the range of 103 M−1 for 2a and 5a, and 104 M−1 for 24). The authors pointed out that the pyridine N–H and pyridine N atoms act as hydrogen bond donors (D) and acceptors (A), respectively, so that alkyl glycosides can be recognized within the cavity of 35. The binding of β-maltoside 28 was less effective in a more polar solvent like acetonitrile (K11 was estimated to be 1.8 × 103 M−1 for 35•28); no association was detected with saccharides in a water solution by CD analyses. The efficiency of the pyridone rings was shown by the comparison with all-pyridine macrocycle 36; the (A–D–A)2-type macrocycle 35 was shown to be more powerful carbohydrate receptor than the (A–A–A)2-type compound 36.
3. Binding studies in aqueous media
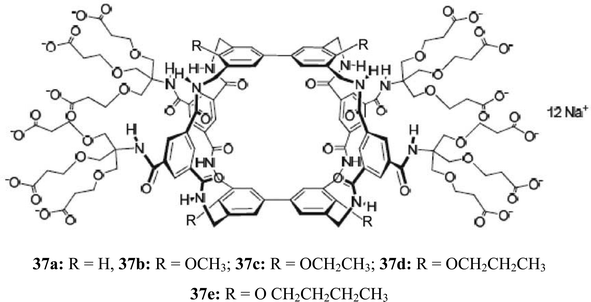
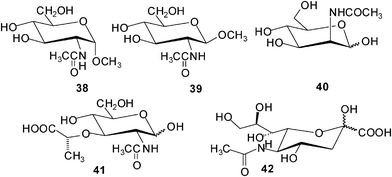
Davis and coworkers have continued their successful studies on macrocyclic receptors, which were inspired by carbohydrate-binding proteins, and were designed to provide both apolar and polar contacts to a saccharide molecule.27,28 The studies focused on binding the β-glucosyl family of saccharides, characterized by “all-equatorial” arrays of polar functional groups. Compound 37a was shown to be a selective receptor for O-linked β-N-acetyl-D-glucosaminyl units.27 The binding of 37a to N-acetylamino carbohydrates, such as methyl glycosides of GlcNAc (38 and 39), N-acetyl-D-galactosamine (17), N-acetyl-D-mannosamine (40), N–acetylmuramic acid (41), and N–acetylneuraminic acid (42), was studied in D2O by using 1H NMR titrations. In some cases the results were checked using isothermal titration calorimetry (ITC) and induced circular dichroism (ICD). The results of the binding studies with N-acetylamino carbohydrates were compared with those obtained for 15 other carbohydrates (among other things, methyl β-D-glucoside, methyl α-D-glucoside, D-cellobiose, D-maltose, and L-fucose). Particularly interesting results were obtained with GlcNAcβ-OMe 39. Receptor 37a showed a remarkable preference for 39 (K11 = 630 M−1) versus other tested carbohydrates, including the α anomer 38 (K11 = 24 M−1) and N-acetylgalactosamine (17) (K11 = 2 M−1). Both GlcNAcα-OMe (38) and methyl β-D-glucoside (2b) (K11 = 28 M−1) were bound with affinities that are more than 20 times lower than that of 39.The binding studies with 37b showed that the incorporation of the methoxy groups produces a general increase in affinities (K11 was determined to be 730 M−1 for GlcNAcβ-OMe 39, 70 M−1 for β-glucoside 2b, and 35 M−1 for D-glucose).28 The only exception was N-acetyl-D-glucosamine (GlcNAc 16; K11 = 41 M−1), for which a small decrease in affinities was observed between compound 37a and 37b. Binding to glucose was enhanced by a factor of about 4.
The replacement of the methoxy substituents by ethoxy or propoxy groups (compounds 37c and 37d) produced a further increase in affinities for such substrates as D-glucose, methyl β-D-glucoside (2b) and D-cellobiose, as well as for the closely related all-equatorial substrates 2-deoxy-D-glucose and D-xylose. By contrast, binding to GlcNAc (16) and GlcNAcβ-OMe (39) was substantially decreased. Finally, the presence of butoxy substituents (37e) caused a drop in binding constants for most substrates.
As mentioned by the authors, particularly interesting results were obtained with 37d. In contrast to 37a, macrotricycle 37d was shown to be a more promising receptor for β-glucosyl units, and for glucose itself.28 In comparison to the 4,4′-unsubstituted receptor 37a, it bound glucose more strongly (by a factor of ca. 6) and considerably more selective (K11 = 60 M−1 for 37d and D-glucose). Receptor 37d showed glucose–glactose selectivity of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and glucose–GlcNAc selectivity of 9
1 and glucose–GlcNAc selectivity of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, whereas the corresponding values for 37a were 4.5
1, whereas the corresponding values for 37a were 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, respectively. It should be noted that receptor 37d was shown to be more glucose selective than the readily available lectins used for this substrate, such as concanavalin A, Lens culinaris agglutinin and Pisum sativum agglutinin.
6, respectively. It should be noted that receptor 37d was shown to be more glucose selective than the readily available lectins used for this substrate, such as concanavalin A, Lens culinaris agglutinin and Pisum sativum agglutinin.
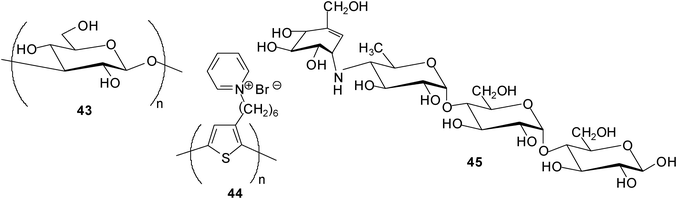
Fukuhara and Inoue tested the combined use of curdlan (43), a linear glucan composed of (1–3)-linked β-D-glucose units, and 2,5-poly[3-(1-pyridinium)hexylthiophene] (44) in saccharide sensing.29 The studies showed that an in situ hybrid complex of curdlan with the water-soluble polythiophene was able to act as a saccharide chemosensor in aqueous media, enabling the discrimination of tetrasaccharide acarbose (45) at 1 μM from 24 mono-, di-, tri-, tetra-, and pentasaccharides. The properties of the curdlan-polythiophene system were examined by UV-vis and CD spectroscopies.
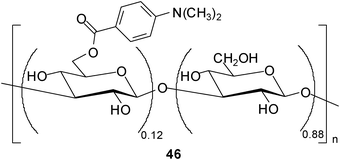
In addition, Fukuhara and Inoue30 reported oligosaccharide sensing with chromophore-modified curdlan, 6-O-[4-(dimethylamino)benzoyl]curdlan, in aqueous media. The degree of substitution of the modified curdlan used for the complexation studies was determined as 0.12 (see structure 46). The authors investigated the ability of 46 for sensing a variety of saccharides in DMSO–H2O mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) by using circular dichroism (CD) spectroscopy. Interestingly, 46 displayed a preference for tetrasaccharides and was shown to be able to discriminate the tetrasaccharide acarbose at a concentration of ≥30 mM from 24 mono-, di-, tri- and tetrasaccharides. As mentioned by the authors, the sensing strategy used utilizes the glucan as a recognition device and the appended chromophore as a reporter.
9 v/v) by using circular dichroism (CD) spectroscopy. Interestingly, 46 displayed a preference for tetrasaccharides and was shown to be able to discriminate the tetrasaccharide acarbose at a concentration of ≥30 mM from 24 mono-, di-, tri- and tetrasaccharides. As mentioned by the authors, the sensing strategy used utilizes the glucan as a recognition device and the appended chromophore as a reporter.
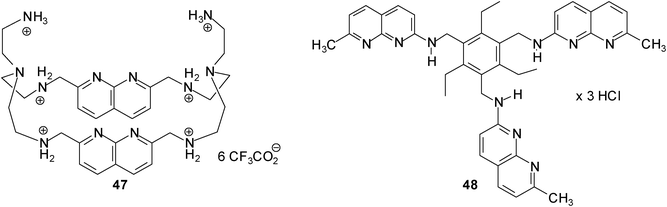
The binding abilities of 1,8-naphthyridine-based macrocyclic receptor 47 against 14 neutral (such as D-galactose, D-glucose, 2-deoxy- and 3-deoxy-D-glucose) and anionic carbohydrates (N-acetylneuraminic acid, muramic acid, D-glucose-1-phosphate and D-glucose-6-phosphate) were tested by Mensah and Cudic.31 The binding affinities were examined by UV/vis and fluorescence titrations in cacodylate buffer at pH 6.5. Among the monosaccharide substrates receptor 47 showed the strongest binding affinity for N-acetylneuraminic acid (42); the binding constant K11 determined on the base of the UV/vis and fluorescence method was found to be ∼1200 M−1 and ∼3000 M−1, respectively. Earlier studies from our group showed that acyclic 1,8-naphthyridine-based receptor 48 was able to recognize N-acetylneuraminic acid (42) with K11 = 3880 M−1 and K12 = 10930 M−1 in a D2O–DMSO-d6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) mixture.32,33
9, v/v) mixture.32,33
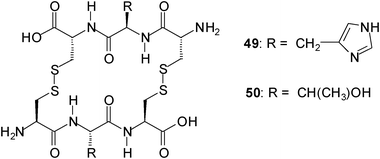
Ravoo and coworkers described a dynamic combinatorial approach to the identification of biomimetic carbohydrate receptors.34 They explored a dynamic combinatorial library (DCL) of cyclic peptides to select receptors that are assembled from tripeptides under thermodynamic equilibrium. To create DCLs from a set of tripeptides under physiological conditions they used the reversible disulfide exchange. In a DCL composed of three tripeptides, for example, an interaction between the cyclic dimer HisHis (49) and N-acetylneuraminic acid (42) was identified, whereas in a DCL of six tripeptides, a selective 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 interaction of the cyclic dimmer TyrTyr (50) with trehalose was found.
1 interaction of the cyclic dimmer TyrTyr (50) with trehalose was found.
4. Conclusion
Recent studies have shown that the area of sugar recognition by receptors operating through noncovalent interactions continues to grow. Strong binding of sugars could be achieved in organic media with both acyclic and macrocyclic receptors. Many of these receptors were inspired by carbohydrate-binding proteins, and were designed to provide both apolar and polar contacts to a sugar molecule. In some cases, studies in two-phase systems, such as dissolution of solid carbohydrates in apolar media and phase transfer of sugars from aqueous into organic solvents, revealed effective recognition of neutral carbohydrates and interesting binding preferences. Most of the binding studies involved the complexation of monosaccharides and, although some receptors showed interesting di- vs. monosaccharides preference, the selective recognition of oligosaccharides by receptors using noncovalent interactions is rare. While many interesting systems have been reported and encouraging results generated, the exact prediction of the binding preferences35 of the receptors still represents an unsolved problem. Systems which operate in water, in which the solvent molecules compete significantly for binding sites of the receptor, are still very rare and their affinities are mostly low; however, very promising results have been reported. Particular powerful carbohydrate receptors, which can be seen as “synthetic lectins”, have been described by Davis and coworkers.It is without doubt that molecular recognition of carbohydrates remains a fascinating area of future research, and it seems to be realistic that studies with well-designed synthetic receptors will significantly contribute to the solution of some unsolved problems. It is hoped that artificial carbohydrate receptors will help to enhance the knowledge on molecular details of carbohydrate-mediated recognition events and will provide a base for the development of systems with interesting applications in medicine and other areas.
References
- (a) H. Osborn, T. Khan, Oligosaccharides. Their synthesis and biological roles. Oxford, University Press, New York, 2000 Search PubMed; (b) T. K. Lindhorst, Essentials of Carbohydrate Chemistry and Biochemistry, Wiley-VCH, Weinheim, 2007 Search PubMed; (c) H. G. Garg, M. K. Cowman, C. A. Hales, ed. Carbohydrate Chemistry, Biology and Medical Applications, Elsevier, Amsterdam, 2008 Search PubMed.
- (a) For reviews on boronic acid-based receptors, see: T. D. James and S. Shinkai, Top. Curr. Chem., 2002, 218, 159–200 CrossRef CAS; (b) T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Angew. Chem., Int. Ed. Engl., 1996, 35, 1910–1922 CrossRef; (c) S. Striegler, Curr. Org. Chem., 2003, 7, 81–102 CrossRef CAS; (d) T. D. James, M. D. Phillips and S. Shinkai, Boronic Acids in saccharide Recognition, Royal Society of Chemistry, Cambridge, 2006 Search PubMed; (e) D. G. Hall, Ed. Boronic Acids. Preparation and Applications in Organic Synthesis and Medicine, Wiley-VCH, 2005 Search PubMed.
- (a) For reviews on carbohydrate recognition with artificial receptors, see: A. P. Davis and T. D. James, In Functional Synthetic Receptors; T. Schrader, A. D. Hamilton, ed., Wiley-VCH: Weinheim, Germany, 2005, 45–109 Search PubMed; (b) A. P. Davis and R. S. Wareham, Angew. Chem. Int. Ed., 1999, 38, 2979–2996 CAS; (c) S. Penadés, ED. Host–Guest Chemistry – Mimetic Approaches to Study Carbohydrate Recognition, Top. Curr. Chem.Vol. 218, Springer-Verlag: Berlin, 2002 Search PubMed; (d) D. B. Walker, G. Joshi and A. P. Davis, Cell. Mol. Life Sci., 2009, 66, 3177–3191 CrossRef CAS; (e) S. Jin, Y. Cheng, S. Reid, M. Li and B. Wang, Med. Res. Rev., 2010, 30, 171–257 CAS; (f) A. P. Davis, Org. Biomol. Chem., 2009, 7, 3629–3638 RSC; (g) S. Kubik, Angew. Chem., Int. Ed., 2009, 48, 1722–1725 CrossRef CAS; (h) M. Mazik, ChemBioChem, 2008, 9, 1015–1017 CrossRef CAS; (i) M. Mazik, Chem. Soc. Rev., 2009, 38, 935–956 RSC; (j) For review on recent advances on the application of NMR methods to study recognition properties of carbohydrates, see: A. Ardá, J. Caňada, J. Jiménez-Barbero, J. P. Ribeiro and M. Morando, Carbohydr. Chem., 2009, 35, 333–355 Search PubMed.
- (a) H. Lis and N. Sharon, Lectins; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003 Search PubMed; (b) H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637–674 CrossRef CAS; (c) F. A. Quiocho, Pure Appl. Chem., 1989, 61, 1293–1306 CrossRef CAS; (d) W. I. Weiss and K. Drickamer, Annu. Rev. Biochem., 1996, 65, 441–473 CrossRef; (e) S. P. Spurlino, G.-Y. Lu and F. A. Quiocho, J. Biol. Chem., 1991, 266, 5202–5219 Search PubMed; (f) C. S. Wright and G. Hester, Structure, 1996, 4, 1339–1352 CrossRef CAS.
- For a discussion on solvent effects in carbohydrate binding by synthetic receptors, see: E. Klein, Y. Ferrand, N. P. Barwell and A. P. Davis, Angew. Chem., Int. Ed., 2008, 47, 2693–2696 CrossRef CAS.
- (a) For examples of receptors, which are able to dissolve solid carbohydrates in apolar media, see reference 3a and: A. Bähr, B. Felber, K. Schneider and F. Diederich, Helv. Chim. Acta, 2000, 83, 1346–1376 CrossRef; (b) M. Inouye, J. Chiba and H. Nakazumi, J. Org. Chem., 1999, 64, 8170–8176 CrossRef CAS; (c) M. Inouye, T. Miyake, M. Furusyo and H. Nakazumi, J. Am. Chem. Soc., 1995, 117, 12416 CrossRef CAS; (d) A. Ardá, C. Venturi, C. Nativi, O. Francesconi, G. Gabrielli, F. J. Caňada, J. Jiménez-Barbero and S. Roelens, Chem.–Eur. J., 2010, 16, 414–418 CrossRef; (e) M. Mazik, W. Radunz and W. Sicking, Org. Lett., 2002, 4, 4579–4582 CrossRef CAS; (f) M. Mazik, W. Radunz and R. Boese, J. Org. Chem., 2004, 69, 7448–7462 CrossRef CAS; (g) M. Mazik and A. Hartmann, Beilstein J. Org. Chem., 2010, 6(9) CAS; (h) M. Mazik, A. Hartmann and P. G. Jones, Chem.–Eur. J., 2009, 15, 9147–9159 CrossRef CAS.
- (a) For examples of macrocyclic receptors, which are able to extract sugars from water into nonpolar organic solutions, see: T. Velasco, G. Lecollinet, T. Ryan and A. P. Davis, Org. Biomol. Chem., 2004, 2, 645–647 RSC; (b) T. Ryan, G. Lecollinet, T. Velasco and A. P. Davis, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4863–4866 CrossRef CAS; (c) Y. Aoyama, Y. Tanaka and S. Sugahara, J. Am. Chem. Soc., 1989, 111, 5397 CrossRef CAS; (d) Y. Aoyama, Y. Tanaka, H. Toi and H. Ogoshi, J. Am. Chem. Soc., 1988, 110, 634 CrossRef CAS.
- H. Abe, S. Takashima, T. Yamamoto and M. Inouye, Chem. Commun., 2009, 2121–2123 RSC.
- J.-D. Lee, Y.-H. Kim and J.-I. Hong, J. Org. Chem., 2010, 75, 7588–7595 CrossRef CAS.
- (a) For recent discussions on the importance of carbohydrate-aromatic interactions, see: R. Lucas, I. Gómez-Pinto, A. Aviňó, J. J. Reina, R. Eritja, C. González and J. C. Morales, J. Am. Chem. Soc., 2011, 133, 1909–1916 CrossRef CAS; (b) J. Wohlert, U. Schnupf and J. W. Brady, J. Chem. Phys., 2010, 133, 155103 CrossRef; (c) K. Ramirez-Gualito, R. Alonso-Rios, B. Quiroz-Garcia, A. Rojas-Aguilar, D. Diaz, J. Jiménez-Barbero and G. Cuevas, J. Am. Chem. Soc., 2009, 131, 18129–18138 CrossRef CAS; (d) S. Tsuzuki, T. Uchimaru and M. Mikami, J. Phys. Chem. B, 2009, 113, 5617–5621 CrossRef CAS; (e) G. Terraneo, D. Potenza, A. Canales, J. Jiménez-Barbero, K. K. Baldridge and A. Bernardi, J. Am. Chem. Soc., 2007, 129, 2890–2900 CrossRef CAS; (f) J. Screen, E. C. Stanca-Kaposta, D. P. Gamblin, B. Liu, N. A. Macleod, L. C. Snoek, B. G. Davis and J. P. Simons, Angew. Chem., Int. Ed., 2007, 46, 3644–3648 CrossRef CAS; (g) Z. R. Laughrey, S. H. Kiehna, A. J. Riemen and M. L. Waters, J. Am. Chem. Soc., 2008, 130, 14625–14633 CrossRef CAS.
- For examples of CH–π interactions in the crystal structures of the complexes formed between artificial receptors, carbohydrates, see: M. Mazik, H. Cavga and P. G. Jones, J. Am. Chem. Soc., 2005, 127, 9045–9052 CrossRef CAS.
- (a) For recent discussions on the nature of the CH–π interactions, see: M. Nishio, Y. Umezawa, K. Honda, S. Tsuboyama and H. Suezawa, CrystEngComm, 2009, 11, 1757–1788 RSC; (b) O. Takahashi, Y. Kohno and M. Nishio, Chem. Rev., 2010, 110, 6049–6076 CrossRef CAS; (c) M. Nishio, Phys. Chem. Chem. Phys., 2011, 13, 13873 RSC.
- M. Mazik and C. Geffert, Org. Biomol. Chem., 2011, 9, 2319–2326 CAS.
- (a) M. Mazik, A. Hartmann and P. G. Jones, Eur. J. Org. Chem., 2010, 458–463 CrossRef CAS; (b) M. Mazik and A. Hartmann, J. Org. Chem., 2008, 73, 7444–7450 CrossRef CAS.
- M. Mazik and C. Sonnenberg, J. Org. Chem., 2010, 75, 6416–6423 CrossRef CAS.
-
(a)
C. S. Wilcox and N. M. Glagovich, Program HOSTEST 5.6; University of Pittsburgh: Pittsburgh, PA, 1994 Search PubMed;
(b)
C. S. Wilcox, in Frontiers in Supramolecular Chemistry and Photochemistry, ed. H.-J. Schneider and H. DürrVCH, Weinheim, 1991, 123 Search PubMed;
(c) M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC;
(d) K21 corresponds to 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 receptor–sugar association constant. K12 corresponds to 1
1 receptor–sugar association constant. K12 corresponds to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 receptor–sugar association constant. β21 = K11K21, β12 =K11K12.
2 receptor–sugar association constant. β21 = K11K21, β12 =K11K12. - For a review discussing the limitations of the NMR method, see: L. Fielding, Tetrahedron, 2000, 56, 6151–6170 CrossRef CAS.
- (a) This preference has been ascribed to the hydrogen-bonding abilities of sugar OH groups. As discussed in ref. 18b and 18c, the axial 1-alkoxy group in α-glucoside 4 can form intramolecular hydrogen bonds with 2-OH group more easily than the equatorial 1-alkoxy substituent in the β-anomer can do. Therefore, the 2-OH in β-glucoside 4 is relatively free from intramolecular hydrogen bonding and can interact with receptor molecules more strongly. The strong binding of the α-anomer 4 by some receptors (see ref. 19) indicates that the intermolecular receptor–substrate interactions are able to compete effectively with the intramolecular H-bonding network in the carbohydrate; (b) S. Tamaru, S. Shinkai, A. B. Khasanov and T. W. Bell, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4972–4976 CrossRef CAS; (c) J. Cuntze, L. Owens, V. Alcásar, P. Seiler and F. Diederich, Helv. Chim. Acta, 1995, 78, 367–390 CrossRef CAS.
- (a) For examples of receptors, which were shown to bind the α-glucoside better than the β-anomer, see ref. 6h, 14b, and P. B. Palde, P. C. Gareiss and B. L. Miller, J. Am. Chem. Soc., 2008, 130, 9566–9573 CrossRef CAS; (b) M. Mazik and W. Sicking, Tetrahedron Lett., 2004, 45, 3117–3121 CrossRef CAS.
- (a) M. Mazik and M. Kuschel, Chem.–Eur. J., 2008, 14, 2405–2419 CrossRef CAS; (b) M. Mazik and M. Kuschel, Eur. J. Org. Chem., 2008, 1517–1526 CrossRef CAS; (c) M. Mazik, M. Kuschel and W. Sicking, Org. Lett., 2006, 8, 855–858 CrossRef CAS; (d) M. Mazik and W. Sicking, Chem.–Eur. J., 2001, 7, 664–670 CrossRef CAS; (e) M. Mazik, H. Bandmann and W. Sicking, Angew. Chem., Int. Ed., 2000, 39, 551–554 CrossRef CAS.
- F. Fernandez-Trillo, E. Fernandez-Megia and R. Riguera, J. Org. Chem., 2010, 75, 3878–3881 CrossRef CAS.
- (a) C. Nativi, O. Francesconi, G. Gabrielli, A. Vacca and S. Roelens, Chem.–Eur. J., 2011, 17, 4814–4820 CrossRef CAS; (b) A. Ardá, F. J. Caňada, C. Nativi, O. Francesconi, G. Gabrielli, A. Ienco, J. Jiménez-Barbero and S. Roelens, Chem.–Eur. J., 2011, 17, 4821–4829 CrossRef.
- (a) For examples of receptors showing oligo- vs. monosaccharide preferences, see ref. 24 and: U. Neidlein and F. Diederich, Chem. Commun., 1996, 1493–1494 RSC; (b) A. S. Droz, U. Neidlein, S. Anderson, P. Seiler and F. Diederich, Helv. Chim. Acta, 2001, 84, 2243–2289 CrossRef CAS; (c) G. Lecollinet, A. P. Dominey, T. Velasco and A. P. Davis, Angew. Chem., Int. Ed., 2002, 41, 4093–4096 CrossRef CAS; (d) V. Král, O. Rusin and F. P. Schmidtchen, Org. Lett., 2001, 3, 873–876 CrossRef; (e) O. Rusin, K. Lang and V. Král, Chem.–Eur. J., 2002, 8, 655–663 CrossRef CAS; (f) R. D. Hubbard, S. R. Horner and B. L. Miller, J. Am. Chem. Soc., 2001, 123, 5810–5811 CrossRef CAS; (g) Y. Ferrand, M. P. Crump and A. P. Davis, Science, 2007, 318, 619–622 CrossRef CAS; (h) M. Mazik and H. Cavga, J. Org. Chem., 2006, 71, 2957–2963 CrossRef CAS.
- M. Mazik and A. C. Buthe, Org. Biomol. Chem., 2009, 7, 2063–2071 CAS.
- (a) M. Mazik and A. C. Buthe, J. Org. Chem., 2007, 72, 8319–8326 CrossRef CAS; (b) M. Mazik and A. C. Buthe, Org. Biomol. Chem., 2008, 6, 1558–1568 RSC; (c) M. Mazik and A. König, J. Org. Chem., 2006, 71, 7854–7857 CrossRef CAS.
- H. Abe, Y. Chida, H. Kurokawa and M. Inouye, J. Org. Chem., 2011, 76, 3366–3371 CrossRef CAS.
- Y. Ferrand, E. Klein, N. P. Barwell, N. P. Crump, J. Jiménez-Barbero, C. Vicent, G.-J. Boons, S. Ingale and A. P. Davis, Angew. Chem., Int. Ed., 2009, 48, 1775–1779 CrossRef CAS.
- N. P. Barwell, M. P. Crump and A. P. Davis, Angew. Chem., Int. Ed., 2009, 48, 7673–7676 CrossRef CAS.
- G. Fukuhara and Y. Inoue, J. Am. Chem. Soc., 2011, 133, 768–770 CrossRef CAS.
- G. Fukuhara and M. Inouye, Chem. Commun., 2010, 46, 9128–9130 RSC.
- A. A. Mensah and P. Cudic, Curr. Org. Chem., 2011, 15, 1097–1104 CrossRef CAS.
- M. Mazik and H. Cavga, Eur. J. Org. Chem., 2007, 3633–3638 CrossRef CAS.
- (a) For further examples of binding studies with N-acetylneuraminic acid, see: M. Mazik and H. Cavga, J. Org. Chem., 2007, 72, 831–838 CrossRef CAS; (b) M. Mazik and A. König, Eur. J. Org. Chem., 2007, 3271–3276 CrossRef CAS , and references therein..
- M. Rauschenberg, S. Bomke, U. Karst and B. J. Ravoo, Angew. Chem., Int. Ed., 2010, 49, 7340–7345 CrossRef CAS.
- (a) For a discussion on selectivity in supramolecular host–guest complexes, see: H.-J. Schneider and A. Yatsimirsky, Chem. Soc. Rev., 2008, 37, 263–277 RSC; (b) For a discussion on binding mechanisms in supramolecular complexes, see: H.-J. Schneider, Angew. Chem., Int. Ed., 2009, 48, 3924–3977 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |